Globally, 'tropical lowland evergreen rainforests' are regarded as the most diverse terrestrial ecosystems, having both high species richness per unit area and high equitability in abundance sharing (Whitmore, 1984). These forests occur around the equator and extend between the Tropic of Cancer and the Tropic of Capricorn in three major chunks, namely, the Amazon basin of South America, the Congo River basin of Central Africa, and the ever-wet peninsula and islands of Southeast Asia (cf. figures 1.1 and 1.4 in Corlett and Primack, 2011). In addition, there are two smaller and very distinctive rainforest regions on the giant islands of Madagascar and New Guinea (Corlett and Primack, 2011). In northeastern India, northern Myanmar and southwestern China, 'tropical lowland evergreen rainforests' occur around the Tropic of Cancer (Corlett, 2009). In southwestern China, the climate is extreme for tropical rainforests, with a mean annual rainfall between 1200 and 1800 mm and a mean annual temperature of about 21-22.8 ℃ (Zhu, 1997). Northeastern India has a relatively wetter climate (rainfall 2000-12, 000 mm) than southwestern China, and is therefore more conducive for the growth of lowland rainforests.

|
| Fig. 1 a) Geographical location of sampled sites in the State of Meghalaya in India; and b) map of rainfall distribution. The horizontal dashed line indicates the Tropic of Cancer. |
The available information on the structure and floristics of lowland rainforests north of the Tropic of Cancer (around 27°30'N) stems mainly from Arunachal Pradesh in northeastern India (Proctor et al., 1998; Bhuyan et al., 2003; Nath et al., 2005; Deb et al., 2009), and near the Tropic of Cancer (around 21-22°N) from Xishuangbanna in southern Yunnan province (Zhu, 1997; Shanmughavel et al., 2001; Lü et al., 2010; Lan et al., 2012) and from Hainan Island in China (Meng et al., 2011). The State of Meghalaya represents the westernmost limit of the lowland rainforests north of the Tropic of Cancer. These scarcely known rainforests patchily occupy rolling hills on the southern slopes of the Shillong plateau (also referred to as the Meghalaya plateau since 1971), below 1000 m altitude, where the world's heaviest rainfall is received, especially in the Cherrapunji-Mawsynram sector. This region has been virtually ignored on world maps of rainforests.
According to the seminal work of Champion and Seth (1968), the lowland rainforests of Meghalaya would closely correspond to 'Cachar tropical evergreen forests'. Indeed, Champion and Seth considered this vegetation as 'climatic climax' with fewer giant trees and infrequent climbers. They remarked that: "… after shifting cultivation on hill slopes, fallows are restocked with the deciduous species and if protected from slashing-and-burning, will revert to a closed evergreen type". Champion and Seth (1968) further remarked that the natural regeneration of evergreen species was better in these forests than in dipterocarpus lowland evergreen rainforests in the Deomali Division of Assam. While forest types, such as the lowland 'Khasi hill sal' forests (Tripathi and Shankar, 2014), montane evergreen rainforests (Khiewtam and Ramakrishnan, 1993; Jamir and Pandey, 2003; Upadhaya et al., 2003) and subtropical pine forests (Tripathi et al., 2003) have been extensively investigated, the floristics, structure and regeneration of the lowland rainforests of Meghalaya have yet to be studied.
In order to generate a dataset from the western edge of lowland rainforests north of the Tropic of Cancer, we initiated a study of the rainforests of Meghalaya. The data shall not only fill the gap in understanding of rainforests in northern limits, but also facilitate fine cross-continental comparisons. We examined: (1) if lowland rainforests of Meghalaya exhibit the characteristic physiognomy, life-form spectrum and floristic composition typical of equatorial and tropical seasonal rainforests; (2) if diversity of woody species declines moving away from the equator towards the northern edges of rainforests, corroborating the globally recognized latitudinal diversity gradient (Brown, 2014); (3) if floristic richness of landscape (gamma diversity) and of samples (alpha diversity) as well as compositional heterogeneity of samples (beta diversity) are competitive with those of other rainforests; and (4) if equitability of species abundances follows a lognormal distribution, shaping a high value of Shannon's diversity index (H').
We supplement our results by comparing them with the equatorial lowland rainforests predominated by dipterocarps, and lowland seasonal rainforests near the Tropic of Cancer in Xishuangbanna in southwestern China (Zhu, 1997; Lü et al., 2010), as this region belongs to the same biogeographical entity. Finally, we compare our results with lowland rainforests in extreme northern limits in the world, which occur in Namdapha National Park in Arunachal Pradesh (Proctor et al., 1998; Nath et al., 2005; Deb et al., 2009) and Deomali Forest Division in Assam (Bhuyan et al., 2003) in northeastern India. The comparisons affirm that the lowland rainforests of Meghalaya yield the highest value of H' among the rainforests north of the Tropic of Cancer, even while these values are lower than the equatorial lowland rainforests.
2. Materials and methods 2.1. Study areaThe hilly State of Meghalaya abounds between 24°02' and 26°07'N latitude and 89°48' and 92°51'E longitude and covers a geographical area of 22, 429 km2 (Fig. 1a). Meghalaya is included in the 'Indo-Burma' global biodiversity hotspot (Myers, 2003). The east-west series of mountains ascends from 100 m in the north to about 1965 m towards the south, attaining the highest peak at Upper Shillong and a second peak at Tura before descending further south to nearly 50 m, carving an archway (cf. figure 1 in Shankar et al., 1993). The low elevations all along the periphery of Meghalaya plateau experience fairly high temperature, whereas the higher elevations in the centre of the plateau benefit from moderate temperatures. The minimum temperature (℃) in the coldest month, (January), mean annual temperature (℃) and annual rainfall (mm) at some sites on southern slopes are: 10.2, 22.0 and 5011 at Mawlynnong (517 m), 7.8, 17.6 and 9963 at Cherrapunji (1274 m), 11.7, 24.4 and 3704 at Dawki (82 m), 10.2, 21.7 and 5629 at Ranikor (602 m) and 12.0, 24.2 and 3127 at Tura (246 m) (www.climate-data.org). Frost may occur at the table land of Cherrapunji, but is unrecorded for our study sites below 900 m.
The Cherrapunji-Mawsynram sector on the southern slopes of Meghalaya receives exceptionally high levels of rainfall (Fig. 1b). Cherrapunji holds two world records: (1) greatest 48-hour rainfall (2493 mm) during June 15-16, 1995; and (2) greatest 12-month rainfall (26, 470 mm) during August, 1860 and July, 1861 (WMO, 2014). The southwest monsoon moving from the Bay of Bengal causes heavy rainfall on abruptly rising southern slopes of the Shillong plateau and rapidly diminishes northward, creating a steep gradient in rainfall (ca. 10, 000 mm) within a distance of only 100 km. About 80% of rain falls during the rainy season and most of the remainder falls in spring, rendering the winter cool and dry (Shankar et al., 1991). The climate is controlled by the Asia-Pacific monsoon with the following distinct seasons: spring (March-April), rainy (May-September), autumn (October-mid-November) and winter (mid-November-February).
The rainforests of Meghalaya occur mainly on lateritic soil developed from siliceous rocks, such as gneisses, schists and granite rocks of Archaean age. The surface of the highest rainfall area of the Shillong plateau is largely flat and dissected by canyons up to 1000 m deep. The steep slopes of the canyons harbour stunted evergreen vegetation. The plateau is composed of thick horizontally bedded sandstones and siltstones passing south into limestone facies that support patches of the rainforest. The landscape is characterized by either exposed bedrock or the remains of lateritic cover, armoured by a surface layer of coarse gravely residual debris with very sparse grass cover (Shankar et al., 1991). The underlying, weathered regolith permits very low infiltration and subsurface runoff, but speeds up overland flow and soil erosion (Soja and Starkel, 2006). Long-term human intervention in these globally extreme pluvial conditions has destroyed a dense vegetation cover and thick soil of the primary ecosystem (Ramakrishnan, 2001). Soil pH ranges between 4.6 and 6.1 and soil texture is predominantly sandy loam.
2.2. Field samplingSix patches of tropical lowland evergreen rainforest were sampled in Meghalaya. The sampled transects were 10 m wide and up to 500 m in length. To determine species frequency, transects encompassed contiguous subplots of 50 m in length. In all, 2.45 ha were sampled. All stems≥10 cm girth at breast height (1.37 m above ground level) were included in enumeration. Each stem was measured for girth (cm), height (m), phenophase (leaf flush, flowering, fruiting) and damage (top broken, lopping, disease) following Murali et al. (1996). The voucher specimens of species were put up on herbarium sheets for identification. The plant species were identified and their habits verified from the regional floras (Hooker, 1872-1897; Kanjilal et al., 1934-1940; Balakrishnan, 1981-1983; Haridasan and Rao, 1985-1987). The herbarium of the Botanical Survey of India, Shillong was consulted for identification. The accepted botanical names were adopted from The Plant List (2013) and the plant families follow APG Ⅲ (2009).
The species were classified into the following growth forms: large tree, medium tree, small tree, shrub, scandent shrub and liana following Shankar (2001). The tree species which occupy the upper-canopy (≥20- < 25 m height) and emergent strata (≥25 m height) were large trees; the species which occur in middle-canopy (≥10- < 20 m) were medium-trees; and the species which restrict themselves to understory ( < 10 m) were small trees. Multistemmed species predominant in understory were considered shrub, and the species climbing on the trunks of other trees were designated as liana. The stragglers were classified as scandent shrubs. In some tables, the data for scandent shrubs and liana were considered together as 'liana' because of the negligible presence of the latter. We assigned a phanerophytic life-form (Raunkiaer, 1934) to each species following measurements of maximum height: megaphanerophyte (>20 m), mesophanerophyte (>10-20 m), microphaerophyte (5- < 10 m), nanophanerophyte ( < 5 m) and liana (all heights).
2.3. Data analysisThe phytosociological dataset was analysed individually for six sites and as an assemblage by pooling all six samples. The occurrences of species in contiguous subplots of 500 m2 were taken into account for calculation of frequency (Mueller-Dombois and Ellenberg, 1974). A frequency distribution was developed in five frequency classes of Raunkiaer (1934). The density of a species in a hectare was determined by dividing the count of individuals in all samples by the total area sampled. The stand density was found by summing the densities of all species in the assemblage. The basal area of each individual was calculated from its respective girth and, in multi-stemmed individuals, basal area of each stem was calculated separately (Shankar, 2001). The basal areas of all individuals of a species were summed to arrive at the total basal area of the species. The stand basal area was found by summing the basal areas of all species. For each species, the frequency, density and basal area were converted into relative values by dividing respectively by the sums of frequencies, densities and basal areas of all species. The Importance Value Index (IVI) of a species was computed by summing relative density, relative frequency and relative basal area of the respective species (Curtis and McIntosh, 1950). Here we refer to the sums of individuals, basal areas and importance values of all species belonging to the respective family as the numbers of individuals, basal area and importance value of a family (FIV).
PAST software Version 3.14 (Hammer et al., 2001) was used for clustering of samples, ordination of species and abundance modelling for plotting species abundances in descending rank order. Unweighted pair-group average (UPGMA) algorithm and BrayeCurtis similarity measure based on abundance data were selected for clustering samples. An ordination of species was performed, using counts of individuals of species in samples, in PCA module of PAST software and the locations of species in PCA space were recognized from consequential PCA scores. A scree plot of eigenvalues was examined for significance of the principal components.
Four classical models of rank-abundance plots described by Whittaker (1972) and Magurran (1988) were fitted to species abundances (importance values): (1) the geometric series (Motomura's niche pre-emption hypothesis), (2) Fisher's logarithmic series, (3) the broken-stick series (MacArthur's random niche boundary hypothesis), and (4) May's truncated lognormal distribution which is modified from Preston's octave frequency distribution. Here, we include results on lognormal distribution.
The alpha diversity (α) is the mean number of species in samples and the gamma diversity (γ) is the total number of species in all samples (Whittaker, 1972). The beta diversity (βw), which refers to compositional heterogeneity among sampling units, was calculated following Whittaker (1972), as follows:
| $ {{\beta }_{\text{w}}}=\left(\gamma /\bar{\alpha } \right)-1 $ |
where, γ is total species diversity at regional or landscape level and α=the mean species diversity at the local or within-habitat scale. The beta diversity is zero if all species in all samples are the same and it is maximum (number of samples-1) if there is no overlap of species among all samples. To allow comparisons among sites, Harrison's beta-one index (β-1) was computed as follows (Harrison et al., 1992):
| $ {{\beta }_{-1}}=100\frac{\left(\gamma /\bar{\alpha } \right)-1}{N-1} $ |
where, N is the number of samples. Beta-one ranges from 0 (complete similarity) to 100 (complete dissimilarity).
The Shannon's diversity index was calculated following Shannon (1948) as:
| $ H'=-\sum\limits_{i=1}^{S}{\frac{{{n}_{i}}}{N}}{{\log }_{e}}\frac{{{n}_{i}}}{N} $ |
The Simpson's index of dominance was calculated following Simpson (1949) as:
| $ D=\sum\limits_{i=1}^{S}{\frac{{{n}_{i}}\left({{n}_{i}}-1 \right)}{N\left(N-1 \right)}} $ |
The Pielou's index of evenness was calculated following Pielou (1975) as:
| $ E=\frac{H'}{H{{'}_{\max }}} $ |
Where, ni=importance value of ith species, N=sum of importance values of all species, S=number of species in the assemblage and H' max is loge S.
3. ResultsOf 2408 individuals sampled, 99% were live and 1% dead (Table 1). About 0.5% of live individuals were partially damaged by wind throws. The coefficient of variation for the count of individuals per sample was 27.8%. About 7.6% of live individuals were multi-stemmed. Hence, 2385 live individuals were of≥10 cm girth, 1145 individuals were of≥30 cm girth, and 262 individuals were of≥90 cm girth. The live individuals comprised 184 taxa (species) in 142 genera and 66 families (Table 2). Of these, 173 taxa could be identified up to the species level with certainty. Seven taxa were identified up to the genus level, one taxon up to the family level and three taxa remained unidentified.
| Attribute | Sample sites | All samples | |||||
| T1 | T2 | T3 | T4 | T5 | T6 | ||
| Altitude (m) | 863 | 531 | 812 | 319 | 750 | 577 | 642±205 |
| Transect | |||||||
| Width (m) | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Length (m) | 500 | 300 | 400 | 350 | 400 | 500 | 2450 |
| Area (ha) | 0.5 | 0.3 | 0.4 | 0.35 | 0.4 | 0.5 | 2.45 |
| Count of species | 40 | 41 | 48 | 51 | 61 | 51 | 184 |
| Count of individuals | |||||||
| Live | 349 | 344 | 606 | 314 | 428 | 344 | 2385 |
| Dead | 5 | 3 | 10 | 4 | 1 | 0 | 23 |
| Total (live + dead) | 354 | 347 | 616 | 318 | 429 | 344 | 2408 |
| Multi-stemmed | 29 | 25 | 33 | 25 | 57 | 13 | 182 |
| Species | Family | Growth form | Occurrence | Density (ha-1) | Basal area (cm2 ha-1) | IVI |
| 1. Schima wallichii Choisy | Theaceae | LT | 21 | 40.0 | 32, 277 | 18.7 |
| 2.Macropanax undulatus (Wall. ex G. Don) Seem. | Araliaceae | ST | 20 | 58.8 | 19, 960 | 16.0 |
| 3. Cinnamomum tamala (Buch.-Ham.) T. Nees & Eberm. | Lauraceae | MT | 15 | 38.8 | 13, 889 | 11.0 |
| 4. Castanopsis armata (Roxb.) Spach | Fagaceae | MT | 7 | 22.4 | 18, 655 | 10.0 |
| 5. Boehmeria glomeruliferaMiq. | Urticaceae | SH | 16 | 61.2 | 1989 | 9.1 |
| 6. Syzygium tetragonum (Wight) Wall. ex Walp. | Myrtaceae | MT | 17 | 34.7 | 1988 | 6.5 |
| 7. Persea odoratissima (Nees) Kosterm. | Lauraceae | MT | 7 | 9.0 | 12, 046 | 6.2 |
| 8. Oreocnide integrifolia (Gaudich.) Miq. | Urticaceae | ST | 17 | 30.2 | 2249 | 6.1 |
| 9. Itea macrophylla Wall. | Iteaceae | ST | 16 | 26.5 | 2965 | 5.9 |
| 10. Toona ciliata M. Roem. | Meliaceae | LT | 6 | 11.0 | 10, 816 | 5.9 |
| 11. Stereospermum chelonoides (L. f.) DC. | Bignoniaceae | LT | 5 | 6.9 | 11, 247 | 5.5 |
| 12. Sarcosperma griffithii Hook. f. ex C.B. Clarke | Sapotaceae | MT | 14 | 23.3 | 3393 | 5.5 |
| 13. Castanopsis lanceifolia (Oerst.) Hickel & A. Camus | Fagaceae | LT | 13 | 23.3 | 3579 | 5.4 |
| 14. Xanthophyllum flavescens Roxb. | Polygalaceae | LT | 6 | 10.6 | 9595 | 5.4 |
| 15. Helicia nilagirica Bedd. | Proteaceae | MT | 13 | 20.0 | 2915 | 4.8 |
| 16. Macaranga indica Wight | Euphorbiaceae | MT | 17 | 14.3 | 3050 | 4.8 |
| 17. Symplocos sumuntia Buch.-Ham. ex D. Don | Symplocaceae | ST | 14 | 25.3 | 496 | 4.6 |
| 18. Castanopsis indica (Roxb. ex Lindl.) A. DC. | Fagaceae | LT | 10 | 11.0 | 5879 | 4.6 |
| 19. Magnolia hodgsonii (Hook. f. & Thomson) H. Keng | Magnoliaceae | LT | 6 | 5.3 | 6312 | 3.6 |
| 20. Claoxylon longipetiolatum Kurz | Euphorbiaceae | ST | 7 | 18.4 | 2241 | 3.6 |
| 21. Ostodes paniculata Blume | Euphorbiaceae | MT | 12 | 12.2 | 1799 | 3.5 |
| 22. Cinnamomum bejolghota (Buch.-Ham.) Sweet | Lauraceae | LT | 9 | 15.9 | 1796 | 3.5 |
| 23. Garcinia ellipticaWall. ex Wight | Clusiaceae | ST | 6 | 20.0 | 1567 | 3.4 |
| 24. Aidia cochinchinensis Lour. | Rubiaceae | MT | 8 | 7.3 | 4040 | 3.3 |
| 25. Mallotus tetracoccus (Roxb.) Kurz | Euphorbiaceae | MT | 6 | 5.3 | 4459 | 3.0 |
| 26. Saurauia roxburghii Wall. | Actinidiaceae | ST | 5 | 12.7 | 2166 | 2.7 |
| 27. Calamus erectus Roxb. | Arecaceae | SH | 5 | 17.6 | 245 | 2.5 |
| 28. Caryota urens L. | Arecaceae | MT | 7 | 5.3 | 2852 | 2.5 |
| 29. Bischofia javanica Blume | Phyllanthaceae | LT | 5 | 3.7 | 3962 | 2.5 |
| 30. Aporosa octandra (Buch.-Ham. ex D. Don) Vickery | Phyllanthaceae | ST | 3 | 9.8 | 2837 | 2.4 |
| 31. Trevesia palmata (Roxb. ex Lindl.) Vis. | Araliaceae | ST | 10 | 10.2 | 191 | 2.4 |
| 32. Garuga pinnata Roxb. | Burseraceae | LT | 2 | 0.8 | 5682 | 2.4 |
| 33. Dillenia indica L. | Dilleniaceae | LT | 6 | 3.3 | 3469 | 2.4 |
| 34. Hydnocarpus kurzii(King) Warb. | Achariaceae | MT | 5 | 9.0 | 2215 | 2.4 |
| 35. Lithocarpus elegans (Blume) Hatus. ex Soepadmo | Fagaceae | LT | 9 | 9.8 | 516 | 2.4 |
| 36. Eriobotrya bengalensis(Roxb.) Hook. f. | Rosaceae | LT | 5 | 7.8 | 2506 | 2.4 |
| 37. Leea indica (Burm. f.) Merr. | Vitaceae | ST | 10 | 8.6 | 347 | 2.3 |
| 38. Engelhardtia spicata Lechen ex Blume | Juglandaceae | LT | 8 | 4.9 | 1853 | 2.2 |
| 39. Callicarpa arborea Roxb. | Lamiaceae | MT | 7 | 5.7 | 1817 | 2.2 |
| 40. Macaranga denticulata (Blume) Mull.Arg. | Euphorbiaceae | MT | 7 | 5.3 | 1875 | 2.1 |
| 41. Artocarpus lakoochaRoxb. | Moraceae | LT | 5 | 4.1 | 2726 | 2.1 |
| 42. Duabanga grandiflora(DC.) Walp. | Lythraceae | LT | 7 | 4.5 | 1849 | 2.1 |
| 43. Styrax serrulatusRoxb. | Styracaceae | ST | 7 | 9.4 | 443 | 2.0 |
| 44. Syzygium diospyrifolium (Wall. ex Duthie) S.N. Mitra | Myrtaceae | ST | 10 | 6.1 | 169 | 2.0 |
| 45. Wendlandia ligustrina Wall. ex G. Don | Rubiaceae | ST | 9 | 6.1 | 194 | 1.9 |
| 46. Ficus hirtaVahl | Moraceae | ST | 8 | 6.9 | 258 | 1.9 |
| 47. Alangium chinense (Lour.) Harms | Cornaceae | ST | 7 | 7.3 | 481 | 1.8 |
| 48. Castanopsis tribuloides(Sm.) A. DC. | Fagaceae | MT | 7 | 5.3 | 770 | 1.7 |
| 49. Xerospermum glabratum Radlk. | Sapindaceae | MT | 2 | 4.1 | 2900 | 1.7 |
| 50. Crypteronia paniculataBlume | Crypteroniaceae | LT | 2 | 0.8 | 3640 | 1.7 |
| 51. Litsea laeta(Nees) Hook. f. | Lauraceae | ST | 7 | 5.3 | 532 | 1.7 |
| 52. Antidesma montanum Blume | Phyllanthaceae | ST | 4 | 9.0 | 422 | 1.6 |
| 53. Lithocarpus thomsonii (Miq.) Rehder | Fagaceae | MT | 3 | 5.3 | 1692 | 1.6 |
| 54. Phoebe lanceolata (Nees) Nees | Lauraceae | ST | 7 | 3.7 | 490 | 1.5 |
| 55. Quercus glauca Thunb. | Fagaceae | LT | 4 | 5.7 | 972 | 1.5 |
| 56. Drimycarpus racemosus (Roxb.) Hook. f. ex Marchand. | Anacardiaceae | LT | 6 | 3.7 | 729 | 1.4 |
| 57. Polyalthia simiarum(Buch.-Ham. ex Hook. f. & Thomson) Benth. | Annonaceae | LT | 3 | 3.7 | 1796 | 1.4 |
| 58. Litsea salicifolia (Roxburgh ex Nees) Hook. f. | Lauraceae | ST | 6 | 5.3 | 197 | 1.4 |
| 59. Euonymus attenuatus Wall. ex M.A. Lawson | Celastraceae | ST | 5 | 6.5 | 203 | 1.4 |
| 60. Terminalia myriocarpa Van Heurck & Mull. Arg. | Combretaceae | LT | 1 | 0.4 | 3304 | 1.4 |
| 61. Turpinia pomifera(Roxb.) DC. | Staphyleaceae | MT | 1 | 1.2 | 3073 | 1.4 |
| 62. Gmelina arborea Roxb. | Lamiaceae | MT | 4 | 2.9 | 1462 | 1.4 |
| 63. Mallotus paniculatus (Lam.) Mull.Arg. | Euphorbiaceae | ST | 7 | 2.9 | 337 | 1.3 |
| 64. Ficus cyrtophylla (Wall. ex Miq.) Miq. | Moraceae | ST | 5 | 4.5 | 586 | 1.3 |
| 65. Pterospermum acerifolium (L.) Willd. | Malvaceae | LT | 4 | 3.3 | 1010 | 1.2 |
| 66. Ardisia thomsonii Mez | Primulaceae | SH | 5 | 5.3 | 64 | 1.2 |
| 67. Viburnum odoratissimumKer Gawl. | Adoxaceae | ST | 4 | 5.7 | 305 | 1.2 |
| 68. Myristicasp. | Myristicaceae | ST | 3 | 6.9 | 311 | 1.2 |
| 69. Artocarpus chaplasha Roxb. | Moraceae | LT | 4 | 2.9 | 1094 | 1.2 |
| 70. Eurya acuminataDC. | Pentaphylacaceae | ST | 5 | 4.9 | 157 | 1.2 |
| 71. Elaeocarpus lanceifolius Roxb. | Elaeocarpaceae | MT | 5 | 3.7 | 334 | 1.2 |
| 72. Knema linifolia(Roxb.) Warb. | Myristicaceae | LT | 5 | 4.1 | 120 | 1.1 |
| 73.Lepisanthes rubiginosa (Roxb.) Leenh. | Sapindaceae | ST | 3 | 1.6 | 1507 | 1.1 |
| 74. Morinda angustifolia Roxb. | Rubiaceae | SH | 5 | 4.1 | 58 | 1.1 |
| 75. Amblyanthus glandulosus (Roxb.) A. DC. | Primulaceae | SH | 5 | 4.1 | 41 | 1.1 |
| 76. Archidendron clypearia(Jack) I.C. Nielsen | Leguminosae | ST | 5 | 3.3 | 159 | 1.0 |
| 77. Macropanax dispermus (Blume) Kuntze | Araliaceae | ST | 4 | 3.7 | 399 | 1.0 |
| 78. ML081T32 | Indeterminate | LT | 1 | 1.2 | 1895 | 0.9 |
| 79. Saraca indicaL. | Leguminosae | MT | 4 | 3.7 | 93 | 0.9 |
| 80. Camellia kissi Wall. | Theaceae | SH | 4 | 3.7 | 42 | 0.9 |
| 81. Ficus benjamina L. | Moraceae | LT | 1 | 0.4 | 2029 | 0.9 |
| 82. Lasianthus hookeriC.B. Clarke ex Hook. f. | Rubiaceae | SH | 4 | 3.7 | 38 | 0.9 |
| 83. Schoepfiasp. | Schoepfiaceae | ST | 4 | 2.0 | 486 | 0.9 |
| 84. Artocarpus heterophyllusLam. | Moraceae | MT | 2 | 3.3 | 836 | 0.9 |
| 85. Photinia integrifolia Lindl. | Rosaceae | ST | 3 | 2.0 | 785 | 0.9 |
| 86. Melia dubia Cav. | Meliaceae | LT | 1 | 0.4 | 1763 | 0.8 |
| 87. Cyathea khasyanaDomin | Cyatheaceae | ST | 3 | 3.3 | 224 | 0.8 |
| 88. Pterygota alata(Roxb.) R.Br. | Malvaceae | LT | 4 | 2.4 | 66 | 0.8 |
| 89. Actinodaphne obovata (Nees) Blume | Lauraceae | MT | 3 | 2.4 | 353 | 0.8 |
| 90. Ficus hispida L. f. | Moraceae | ST | 4 | 2.0 | 96 | 0.8 |
| 91. Gynocardia odorataR.Br. | Achariaceae | LT | 3 | 1.6 | 557 | 0.8 |
| 92. ML081T48 | Lauraceae | LT | 3 | 1.6 | 557 | 0.8 |
| 93. Tarenna asiatica (L.) Kuntze ex K. Schum. | Rubiaceae | ST | 4 | 2.0 | 59 | 0.8 |
| 94. Ligustrum robustum (Roxb.) Blume | Oleaceae | SH | 3 | 1.2 | 646 | 0.8 |
| 95. Goniothalamus sesquipedalis(Wall.) Hook. f. & Thomson | Annonaceae | SH | 3 | 2.9 | 158 | 0.7 |
| 96. Psychotria symplocifoliaKurz | Rubiaceae | SH | 3 | 3.3 | 31 | 0.7 |
| 97. Pandanus furcatus Roxb. | Pandanaceae | ST | 3 | 2.0 | 355 | 0.7 |
| 98. Protium serratum(Wall. ex Colebr.) Engl. | Burseraceae | LT | 3 | 2.4 | 231 | 0.7 |
| 99. Xylosma controversaClos | Salicaceae | ST | 3 | 2.9 | 43 | 0.7 |
| 100. Carallia brachiata(Lour.) Merr. | Rhizophoraceae | ST | 4 | 1.6 | 30 | 0.7 |
| 101. Mallotus philippensis(Lam.) Mull.Arg. | Euphorbiaceae | ST | 4 | 1.6 | 20 | 0.7 |
| 102. Eurya japonicaThunb. | Pentaphylacaceae | SH | 3 | 2.9 | 25 | 0.7 |
| 103. Lindera melastomaceaFern.-Vill. | Lauraceae | ST | 2 | 3.7 | 114 | 0.7 |
| 104. Magnolia champaca (L.) Baill. ex Pierre | Magnoliaceae | LT | 3 | 1.2 | 393 | 0.7 |
| 105. Microcos paniculata L. | Malvaceae | ST | 2 | 2.0 | 474 | 0.6 |
| 106. Brassaiopsis glomerulata(Blume) Regel | Araliaceae | ST | 2 | 3.3 | 129 | 0.6 |
| 107. Careya arborea Roxb. | Lecythidaceae | MT | 1 | 0.4 | 1260 | 0.6 |
| 108. Fagraea ceilanica Thunb. | Gentianaceae | SH | 3 | 1.2 | 281 | 0.6 |
| 109. Bridelia glauca Blume | Phyllanthaceae | MT | 3 | 1.6 | 141 | 0.6 |
| 110. Albizia chinensis(Osbeck) Merr. | Leguminosae | LT | 2 | 0.8 | 685 | 0.6 |
| 111. Schefflera pueckleri (K. Koch) Frodin | Araliaceae | SS | 2 | 2.0 | 336 | 0.6 |
| 112. Tetrameles nudiflora R. Br. | Tetramelaceae | LT | 1 | 0.4 | 1135 | 0.6 |
| 113. Ficus auriculataLour. | Moraceae | SS | 2 | 1.2 | 534 | 0.6 |
| 114. Ficus heteropleura Blume | Moraceae | SS | 3 | 1.2 | 158 | 0.6 |
| 115. MesuaferreaL. | Calophyllaceae | LT | 2 | 2.0 | 281 | 0.6 |
| 116. Ceriscoides campanulata (Roxb.) Tirveng. | Rubiaceae | ST | 2 | 2.9 | 42 | 0.6 |
| 117. Ulmus lanceifolia Roxb. ex Wall. | Ulmaceae | LT | 2 | 1.6 | 383 | 0.6 |
| 118. Kayea floribundaWall. | Calophyllaceae | MT | 3 | 1.2 | 138 | 0.6 |
| 119. Sapindus attenuatusWall. | Sapindaceae | ST | 3 | 1.6 | 20 | 0.6 |
| 120. Vitex quinata(Lour.) F.N. Williams | Lamiaceae | LT | 1 | 0.8 | 906 | 0.5 |
| 121. Dalbergia assamicaBenth. | Leguminosae | MT | 2 | 2.4 | 54 | 0.5 |
| 122. Plectocomia himalayana Griff. | Arecaceae | SS | 2 | 2.4 | 26 | 0.5 |
| 123. Magnolia insignis Wall. | Magnoliaceae | LT | 1 | 0.4 | 927 | 0.5 |
| 124. Parkia timoriana(DC.) Merr. | Leguminosae | LT | 2 | 1.2 | 335 | 0.5 |
| 125. Firmiana colorata (Roxb.) R.Br. | Malvaceae | ST | 2 | 1.6 | 171 | 0.5 |
| 126. Phoebe attenuata (Nees) Nees | Lauraceae | LT | 2 | 0.8 | 374 | 0.5 |
| 127. Vitex pinnata L. | Lamiaceae | LT | 2 | 1.6 | 110 | 0.5 |
| 128. Aglaia elaeagnoidea(A. Juss.) Benth. | Meliaceae | ST | 2 | 0.8 | 291 | 0.5 |
| 129. Trema orientalis (L.) Blume | Cannabaceae | MT | 1 | 0.4 | 760 | 0.5 |
| 130. Micromelum integerrimum(Buch.-Ham. ex DC.) | Rutaceae | ST | 2 | 1.6 | 22 | 0.4 |
| Wight & Arn. ex M. Roem. | ||||||
| 131. Maesa indica (Roxb.) A. DC. | Primulaceae | ST | 2 | 1.6 | 21 | 0.4 |
| 132. Heritiera macrophylla Wall. ex Kurz | Malvaceae | LT | 2 | 1.6 | 19 | 0.4 |
| 133. Dasymaschalon longiflorum (Roxb.) Finet & Gagnep. | Annonaceae | ST | 2 | 1.6 | 18 | 0.4 |
| 134. Toddalia asiatica(L.) Lam. | Rutaceae | SS | 2 | 1.6 | 16 | 0.4 |
| 135. Antidesma sp. | Phyllanthaceae | ST | 2 | 1.2 | 85 | 0.4 |
| 136. Alstonia scholaris (L.) R. Br. | Apocynaceae | LT | 1 | 0.4 | 636 | 0.4 |
| 137. Syzygium cumini (L.) Skeels | Myrtaceae | LT | 1 | 0.4 | 618 | 0.4 |
| 138. Xantolis assamica (C. B. Clarke) P. Royen | Sapotaceae | ST | 2 | 1.2 | 24 | 0.4 |
| 139. Olax acuminata Wall. ex Benth. | Olacaceae | ST | 2 | 1.2 | 24 | 0.4 |
| 140. Symplocos pyrifoliaWall. ex G. Don | Symplocaceae | ST | 1 | 2.4 | 31 | 0.4 |
| 141. Cinnamomumsp. | Lauraceae | LT | 1 | 0.4 | 583 | 0.4 |
| 142. Memecylon cerasiforme Kurz | Melastomataceae | ST | 2 | 0.8 | 90 | 0.4 |
| 143. Randia sp. | Rubiaceae | MT | 1 | 0.4 | 503 | 0.4 |
| 144. Bauhinia variegata L. | Leguminosae | ST | 2 | 0.8 | 28 | 0.4 |
| 145.Sterculia hamiltonii (Kuntze) Adelb. | Malvaceae | ST | 2 | 0.8 | 16 | 0.4 |
| 146. Gomphandra tetrandra(Wall.) Sleumer | Stemonuraceae | SH | 2 | 0.8 | 14 | 0.4 |
| 147. Reevesia wallichii R.Br. | Malvaceae | MT | 1 | 1.6 | 107 | 0.3 |
| 148. Zanthoxylum ovalifolium Wight | Rutaceae | ST | 1 | 1.2 | 72 | 0.3 |
| 149. Elaeocarpussp. | Elaeocarpaceae | LT | 1 | 0.4 | 293 | 0.3 |
| 150.ML071T37 | Indeterminate | MT | 1 | 0.4 | 257 | 0.3 |
| 151. Clerodendrum hastatum(Roxb.) Lindl. | Lamiaceae | SH | 1 | 1.2 | 26 | 0.3 |
| 152. Tectona grandis L. f. | Lamiaceae | LT | 1 | 0.4 | 240 | 0.3 |
| 153.ML081T18 | Indeterminate | LT | 1 | 0.4 | 218 | 0.3 |
| 154. Litsea monopetala(Roxb.) Pers. | Lauraceae | MT | 1 | 0.4 | 198 | 0.2 |
| 155. Dracaena elliptica Thunb. & Dalm. | Asparagaceae | SH | 1 | 0.8 | 70 | 0.2 |
| 156. Cinnamomum glaucescens(Nees) Hand.-Mazz. | Lauraceae | LT | 1 | 0.4 | 150 | 0.2 |
| 157. Glochidion lanceolarium (Roxb.) Voigt | Phyllanthaceae | ST | 1 | 0.8 | 19 | 0.2 |
| 158. Nerium oleander L. | Apocynaceae | SH | 1 | 0.8 | 16 | 0.2 |
| 159. Benkara griffithii (Hook. f.) Ridsdale | Rubiaceae | SH | 1 | 0.8 | 15 | 0.2 |
| 160. Ficus pyriformis Hook. & Arn. | Moraceae | SH | 1 | 0.8 | 15 | 0.2 |
| 161. Clerodendrum bracteatum Wall. ex Walp. | Lamiaceae | SH | 1 | 0.8 | 12 | 0.2 |
| 162. Psychotria adenophyllaWall. | Rubiaceae | SH | 1 | 0.8 | 11 | 0.2 |
| 163. Hiptage acuminata Wall. ex A. Juss. | Malpighiaceae | SS | 1 | 0.8 | 8 | 0.2 |
| 164. Ardisia pedunculosaWall. | Primulaceae | SH | 1 | 0.8 | 6 | 0.2 |
| 165. Baccaurea ramifloraLour. | Phyllanthaceae | MT | 1 | 0.4 | 115 | 0.2 |
| 166. Erythrina strictaRoxb. | Leguminosae | MT | 1 | 0.4 | 81 | 0.2 |
| 167. Dendrocnide sinuata (Blume) Chew | Urticaceae | SH | 1 | 0.4 | 48 | 0.2 |
| 168. Cynometra ramiflora L. | Leguminosae | SH | 1 | 0.4 | 32 | 0.2 |
| 169. Ficussp.1 | Moraceae | MT | 1 | 0.4 | 31 | 0.2 |
| 170. Oroxylum indicum(L.) Kurz | Bignoniaceae | ST | 1 | 0.4 | 30 | 0.2 |
| 171. Calliandra umbrosa (Wall.) Benth. | Leguminosae | ST | 1 | 0.4 | 29 | 0.2 |
| 172. Elaeocarpus prunifoliusWall. ex Mull.Berol. | Elaeocarpaceae | ST | 1 | 0.4 | 27 | 0.2 |
| 173. Actinodaphne angustifolia Nees | Lauraceae | MT | 1 | 0.4 | 25 | 0.2 |
| 174. Olea salicifolia Wall. ex G. Don | Oleaceae | ST | 1 | 0.4 | 17 | 0.2 |
| 175. Garcinia lanceifolia var.oxyphylla (Planch. & Triana) Laness. | Clusiaceae | SH | 1 | 0.4 | 13 | 0.2 |
| 176. Alchornea tiliifolia(Benth.) Müll.Arg. | Euphorbiaceae | ST | 1 | 0.4 | 9 | 0.2 |
| 177. Litsea lancifolia (Roxb. ex Nees) Benth. & Hook. f. ex Villar | Lauraceae | ST | 1 | 0.4 | 9 | 0.2 |
| 178. Machilus gamblei King ex Hook. f. | Lauraceae | MT | 1 | 0.4 | 8 | 0.2 |
| 179. Bauhinia rufa (Bong.) Steud. | Leguminosae | LI | 1 | 0.4 | 7 | 0.2 |
| 180. Flueggea virosa(Roxb. ex Willd.) Royle | Phyllanthaceae | SH | 1 | 0.4 | 5 | 0.2 |
| 181. Areca catechuL. | Arecaceae | MT | 1 | 0.4 | 5 | 0.2 |
| 182. Sapindus erectusHiern | Sapindaceae | ST | 1 | 0.4 | 5 | 0.2 |
| 183. Boehmeria macrophylla Hornem. | Urticaceae | SH | 1 | 0.4 | 3 | 0.2 |
| 184. Mahonia napaulensisDC. | Berberidaceae | SH | 1 | 0.4 | 3 | 0.2 |
| Total for all species | 49 | 973.5 | 273, 378 | 300.0 |
The identified species belonged to 65 families and the three unidentified species were placed in an 'indeterminate' family (Table 2). Thirty-two families had a single species each, 13 families had two species each, seven families had three species each, and 14 families had four or more species each. The most speciose families were Lauraceae (16 species), Moraceae (11), Leguminosae (10), Rubiaceae (10), Euphorbiaceae (8) and Phyllanthaceae (8). At the generic level, Leguminosae and Rubiaceae topped the list with nine genera each and Lauraceae followed with eight genera (Table 3).
| Plant family | Growth form | Total | FIV | ||||
| Large tree | Medium tree | Small tree | Shrub | Lianaa | |||
| 1. Achariaceae | 4 | 22 | 262 | 1.0 | |||
| 2. Actinidiaceae | 31 | 31 | 0.9 | ||||
| 3. Adoxaceae | 14 | 14 | 0.4 | ||||
| 4. Anacardiaceae | 9 | 9 | 0.5 | ||||
| 5. Annonaceae | 9 | 4 | 7 | 203 | 0.9 | ||
| 6. Apocynaceae | 1 | 2 | 32 | 0.2 | |||
| 7. Araliaceae | 1864 | 5 | 1915 | 6.9 | |||
| 8. Arecaceae | 142 | 43 | 6 | 634 | 1.9 | ||
| 9. Asparagaceae | 2 | 2 | 0.1 | ||||
| 10. Berberidaceae | 1 | 1 | 0.1 | ||||
| 11. Bignoniaceae | 17 | 1 | 182 | 1.9 | |||
| 12. Burseraceae | 82 | 82 | 1.1 | ||||
| 13. Calophyllaceae | 5 | 3 | 82 | 0.4 | |||
| 14. Cannabaceae | 1 | 1 | 0.2 | ||||
| 15. Celastraceae | 16 | 16 | 0.5 | ||||
| 16. Clusiaceae | 49 | 1 | 502 | 1.2 | |||
| 17. Combretaceae | 1 | 1 | 0.5 | ||||
| 18. Cornaceae | 18 | 18 | 0.6 | ||||
| 19. Crypteroniaceae | 2 | 2 | 0.6 | ||||
| 20. Cyatheaceae | 8 | 8 | 0.3 | ||||
| 21. Dilleniaceae | 8 | 8 | 0.8 | ||||
| 22. Elaeocarpaceae | 1 | 9 | 1 | 113 | 0.5 | ||
| 23. Euphorbiaceae | 914 | 574 | 148s | 6.4 | |||
| 24. Fagaceae | 1224 | 813 | 2037 | 9.1 | |||
| 25. Gentianaceae | 3 | 3 | 0.2 | ||||
| 26. Iteaceae | 65 | 65 | 2.0 | ||||
| 27. Juglandaceae | 12 | 12 | 0.7 | ||||
| 28. Lamiaceae | 73 | 212 | 52 | 337 | 1.8 | ||
| 29. Lauraceae | 475 | 1266 | 455 | 21816 | 9.8 | ||
| 30. Lecythidaceae | 1 | 1 | 0.2 | ||||
| 31. Leguminosae | 52 | 163 | 113 | 1 | 1 | 3410 | 1.6 |
| 32. Lythraceae | 11 | 11 | 0.7 | ||||
| 33. Magnoliaceae | 173 | 173 | 1.6 | ||||
| 34. Malpighiaceae | 2 | 2 | 0.1 | ||||
| 35. Malvaceae | 183 | 4 | 113 | 337 | 1.4 | ||
| 36. Melastomataceae | 2 | 2 | 0.1 | ||||
| 37. Meliaceae | 282 | 2 | 303 | 2.4 | |||
| 38. Moraceae | 183 | 92 | 333 | 2 | 62 | 6811 | 3.5 |
| 39. Myristicaceae | 10 | 17 | 272 | 0.8 | |||
| 40. Myrtaceae | 1 | 85 | 15 | 1013 | 3.0 | ||
| 41. Olacaceae | 3 | 3 | 0.1 | ||||
| 42. Oleaceae | 1 | 3 | 42 | 0.3 | |||
| 43. Pandanaceae | 5 | 5 | 0.2 | ||||
| 44. Pentaphylacaceae | 12 | 7 | 192 | 0.6 | |||
| 45. Phyllanthaceae | 9 | 52 | 514 | 1 | 668 | 2.7 | |
| 46. Polygalaceae | 26 | 26 | 1.8 | ||||
| 47. Primulaceae | 4 | 253 | 294 | 1.0 | |||
| 48. Proteaceae | 49 | 49 | 1.6 | ||||
| 49. Rhizophoraceae | 4 | 4 | 0.2 | ||||
| 50. Rosaceae | 19 | 5 | 242 | 1.1 | |||
| 51. Rubiaceae | 192 | 273 | 315 | 7710 | 3.3 | ||
| 52. Rutaceae | 72 | 4 | 113 | 0.4 | |||
| 53. Salicaceae | 7 | 7 | 0.2 | ||||
| 54. Sapindaceae | 10 | 93 | 194 | 1.2 | |||
| 55. Sapotaceae | 57 | 3 | 602 | 2.0 | |||
| 56. Schoepfiaceae | 5 | 5 | 0.3 | ||||
| 57. Staphyleaceae | 3 | 3 | 0.5 | ||||
| 58. Stemonuraceae | 2 | 2 | 0.1 | ||||
| 59. Styracaceae | 23 | 23 | 0.7 | ||||
| 60. Symplocaceae | 682 | 682 | 1.7 | ||||
| 61. Tetramelaceae | 1 | 1 | 0.2 | ||||
| 62. Theaceae | 98 | 9 | 1072 | 6.5 | |||
| 63. Ulmaceae | 4 | 4 | 0.2 | ||||
| 64. Urticaceae | 74 | 1523 | 2264 | 5.2 | |||
| 65. Vitaceae | 21 | 21 | 0.8 | ||||
| 66. Indeterminate family | 42 | 1 | 53 | 0.5 | |||
| Total | 52249 | 62738 | 91563 | 29727 | 247 | 2385184 | 100 |
| a liana include scandent shrubs. | |||||||
Individually, no single family was incredibly dominant in count of individuals (Table 3). The top seven families accounted for < 10% of individuals each: Urticaceae (9.5%), Lauraceae (9.1%), Fagaceae (8.5%), Araliaceae (8%), Euphorbiaceae (6.2%), Theaceae (4.5%) and Myrtaceae (4.2%). The next 20 families, each with≥1% but < 4% of individuals, contributed 37% of the individuals. The remaining 39 families, each with < 1% of individuals, shared only 13% of individuals. Five families (Berberidaceae, Cannabaceae, Combretaceae, Lecythidaceae and Tetramelaceae) had only one individual each and another five families (Asparagaceae, Crypteroniaceae, Malpighiaceae, Melastomataceae and Stemonuraceae) had only two individuals each.
In terms of basal area, no single family was exceedingly dominant as the top seven families, each with < 12% basal area, accounted for 56.5% of the basal area: Theaceae (11.8%), Fagaceae (11.7%), Lauraceae (11.5%), Araliaceae (7.7%), Euphorbiaceae (5%), Meliaceae (4.7%) and Bignoniaceae (4.1%). The next 20 families, each with≥1% but < 4% of the basal area, contributed to 33.7% basal area. The remaining 39 families, each with < 1% of the basal area, shared only 9.7% of the basal area.
In terms of the importance value index (IVI), no single family was dominant as the top eight families, each with < 10% of IVI, accounted for 50.7% of IVI: Lauraceae (9.8%), Fagaceae (9.1%), Araliaceae (6.9%), Theaceae (6.5%), Euphorbiaceae (6.4%), Urticaceae (5.2%), Moraceae (3.5%) and Rubiaceae (3.3%). The next 19 families, each with≥1% but < 3% of the importance value, contributed 32.8% importance value. The remaining 39 families, each with < 1% of the importance value, shared 16.4% of the importance value.
3.2. Physiognomy and life-form spectrumThe height of the canopy is low, i.e., below 30 m. The emergent trees occur between 25 and 30 m, upper-canopy between 20 and 25 m, middle-canopy between 10 and 20 m and understory below 10 m. The forest exhibits a pyramidal packing of species and individuals in rather inconspicuous vertical stratification. The general physiognomy of the forest is evergreen. The overall deciduousness in the assemblage was only 16.3%, i.e., only 30 out of 184 species were deciduous. These species accounted for only 7.5% of individuals, 17.7% of the basal area and 12.1% of the IVI. Some species were facultative deciduous (retaining leaves in rainforests, but not in dry forests during winter season) and these were considered as evergreen. Among deciduous species, 26 were trees, three were shrubs and one was a liana.
Partitioning of deciduous species among canopy strata showed that the deciduousness was minimum in the understory (girth < 30 cm), which increased through lower and middle-canopy (girth≥30 cm, but < 90 cm) and was maximum in upper-canopy and emergent strata (girth≥90 cm) in count of species (Fig. 2a), count of individuals (Fig. 2b), accumulation of basal area (Fig. 2c) and importance value index (Fig. 2d). Leguminosae, Lamiaceae (with inclusion of Verbenaceae vide APG Ⅲ), Malvaceae (with inclusion of Sterculiaceae vide APG Ⅲ), Meliaceae and Rubiaceae were the principal families contributing to deciduousness in Meghalaya. All species with an IVI≥3 were distinctively evergreen with the exception of Stereospermum chelonoides and Toona ciliata. Some other deciduous species include the following: Bischofia javanica, Garuga pinnata, Engelhardita spicata, Artocarpus lakoocha, Alangium chinense, Gmelina arboreaand Pterygota alata.

|
| Fig. 2 Deciduousness in canopy of lowland rainforest of Meghalaya. The black bars depict proportions of deciduous species in different strata and grey horizontal line depicts the average value for entire canopy. |
The majority of species were 'small tree', followed by 'large tree', 'medium tree', 'shrub', 'scandent shrub' and 'liana' (Table 4). Small trees included a single tree fern, Cyathea khasyana (Table 2). The count of individuals was maximum for 'small tree' followed by 'medium tree', 'large tree', 'shrub', 'scandent shrub' and 'liana' (Table 4). In terms of accumulation of basal area, 'large tree' ranked at the top followed by 'medium tree', 'small tree', 'shrub', 'scandent shrub' and 'liana' (Table 4). In terms of IVI, the 'tree' was the most dominant growth form in the assemblage with competitive proportions of 'large tree', 'medium tree' and 'small tree'. The IVI for shrubs was small, while for scandent shrubs and liana it was almost negligible (Table 4).
| Growth form | Species | Density | Basal area | IVI | |||||||
| (#) | (%) | (ha-1) | (%) | (m2 ha-1) | (%) | Out of 300 | (%) | ||||
| Large trees | 49 | 26.6 | 213.1 | 21.9 | 13.21 | 48.3 | 95.7 | 31.9 | |||
| Medium trees | 38 | 20.7 | 255.9 | 26.3 | 9.02 | 33.0 | 84.5 | 28.2 | |||
| Small trees | 63 | 34.2 | 373.5 | 38.4 | 4.61 | 16.9 | 93.0 | 31.0 | |||
| Shrubs | 27 | 14.7 | 121.2 | 12.4 | 0.38 | 1.4 | 23.7 | 7.9 | |||
| Liana including scandent shrubs | 7 | 3.8 | 9.8 | 1.0 | 0.11 | 0.4 | 3.1 | 1.0 | |||
| All species | 184 | 100 | 973.5 | 100 | 27.33 | 100 | 300 | 100 | |||
The rainforests of Meghalaya exhibited a phanerophytic lifeform spectrum. Mega and mesophanerophytes were dominant in the species count (Fig. 3a), count of individuals (Fig. 3b), basal area (Fig. 3c) and importance value index (Fig. 3d). Micro-and nanophanerophytes were more prominent in species count and in count of individuals as compared to accumulation of basal area and IVI. There were few liana phanerophytes. The proportion of deciduous species was much smaller than the proportion of evergreen species in all life forms.

|
| Fig. 3 Life-form spectrum of lowland rainforest of Meghalaya. The woody species were categorized into five phanerophytic classes: megaphanerophyte, mesophanerophyte, microphanerophyte, nanophanerophyte and liana. The black bars indicate evergreen species and grey bars indicate deciduous species. |
The variation in count of species among samples was low, between 40 and 61 with a mean of 48.6 and a coefficient of variation of 15.8% (Table 1). The most species common to any two samples was 23, between T4 and T5, and the least was four, between T1 and T6. Nearly 63% of species occurred in a single sample, 22.3% of species in two samples, 8.2% of species in three samples, 6% of species in four samples and 0.5% of species in five samples (Fig. 4). No single species occurred in all six samples.
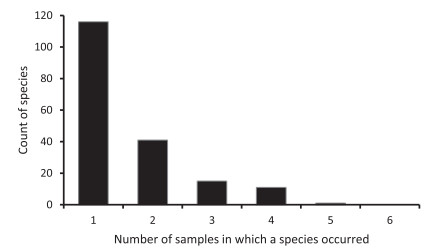
|
| Fig. 4 Frequency of occurrences of species in samples of lowland rainforest of Meghalaya. |
Furthermore, 53 species (28%) appeared in a single subplot (500 m2) out of 49 subplots of six samples. Of these, 37 species were singletons (with one individual). Another 16 species with more than one individual also tended to aggregate within one subplot: 10 species with two individuals each, four species with three individuals each (Clerodendrum hastatum, Turpinia pomifera, Zanthoxylum ovalifolium, ML081T32), one species with four individuals (Reevesia wallichii) and another one species with six individuals (Symplocos pyrifolia). Altogether, 32 species appeared in two subplots with a range of 2-10 individuals, and 23 species appeared in three subplots with a range of 3-24 individuals. In terms of Raunkiaer's frequency spectrum, 91% of species belonged to class 'A' with≥20% frequency of occurrence (Fig. 5). Only 8.2% species belonged to class 'B' (>20-≥40% frequency) and 1.1% of species belonged to class 'C' (>40-≥60% frequency). No single species occurred in class 'D' (>60≥80% frequency) or class 'E' (>80-100% frequency).
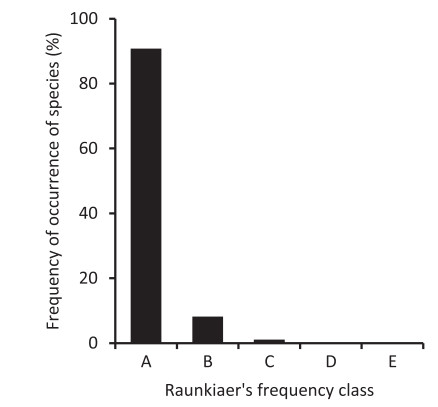
|
| Fig. 5 Frequency of occurrence of species in subplots of 500 m2 size, modelled on Raunkiaer's frequency classes, lowland rainforest of Meghalaya. |
Paired-group cluster analysis based on Bray-Curtis similarity showed a principal cluster of five samples with the T4 sample the most distinct at < 50% similarity to all other samples (Fig. 6). The Cophenetic correlation was 0.9203. Altitudinal location had an influence on clustering of samples. The T4 sample at the lowest altitude (319 m) exhibited the least similarity (33.9%-48.5%) to other samples. The T1 and T3 samples, both between 800 and 900 m, clustered with maximum similarity (72.4%). The T2 and T6 samples, between 500 and 600 m, clustered with 61.1% similarity. The T5 sample, at 750 m, was closer to the sub-cluster of T1 and T3 (66.8%) as compared to the sub-cluster of T2 and T6 (59.7%).
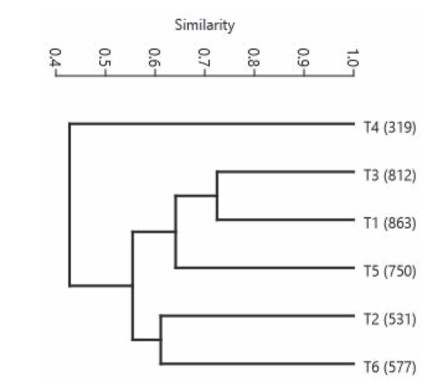
|
| Fig. 6 A cluster analysis of six samples of lowland rainforests of Meghalaya. The sample names are indicted as T1, T2, T3, T4, T5 and T6. The values of elevation (m) of the sites are given in parentheses. |
Stand density was 973.5 ha-1 and stand basal area was 27.34 m2 ha-1 for girth≥10 cm (Table 2). These values were 467.3 ha-1 and 26.06 m2 ha-1, respectively, for girth≥30 cm. The basal area of an average individual was 280.8 cm2 (=59.4 cm girth) for girth≥10 cm and 557.6 cm2 (=83.7 cm girth) for girth≥30 cm.
Eleven species with≥50 individuals each, accounted for 39.5% of the individuals: Boehmeria glomerulifera(150 individuals), Macropanax undulatus (144), Schima wallichii (98), Cinnamomum tamala (95), Syzygium tetragonum (85), Oreocnide integrifolia (74), Itea macrophylla (65), Symplocos sumuntia (62), Sarcosperma griffithii (57), Castanopsis lanceifolia (57) and Castanopsis armata (55). The next 49 species, with≥10 but < 50 individuals each, accounted for 41.5% of individuals. The remaining 124 species, with < 10 individuals each, accounted for 19% of individuals. Most species were rare: 159 species with < 1% of total individuals, 94 species with two or less than two individuals ha-1 and 36 species with one or less than one individual ha-1 (Table 2). No single species endemic to Meghalaya made the list, but Kayea floribunda of Calophyllaceae (arising out of Clusiaceae in APG Ⅲ) is possibly endemic to northeast India.
Twelve species, each with at least 1 m2 basal area, constituted 55.2% of the total basal area: S. wallichii (7.91 m2), M. undulatus (4.89 m2), C. armata (4.57 m2), C. tamala (3.40 m2), Persea odoratissima (2.95 m2), S. chelonoides (2.76 m2), T. ciliata (2.65 m2), Xanthophyllum flavescens(2.35 m2), Magnolia hodgsonii (1.55 m2), Castanopsis indica (1.44 m2), G. pinnata (1.39 m2) and Mallotus tetracoccus(1.09 m2). The next 67 species, each with basal area between≥0.1 and < 1 m2, comprised 40.3% of the basal area. The remaining 105 species, each with basal area < 0.1 m2, accounted for 4.5% basal area (Table 2).
Fourteen species, each with IVI≥5, represented 39.1% of total IVI: S. wallichii (18.7), M. undulatus (16), C. tamala (11), C. armata (10), B. glomerulifera (9.1), S. tetragonum (6.5), P. odoratissima (6.2), O. integrifolia (6.1), I. macrophylla (5.9), T. ciliata (5.9), S. chelonoides (5.5), S. griffithii (5.5), C. lanceifolia(5.4) and X. flavescens (5.4). The next 63 species, each with IVI between≥1 and < 5, constituted 44.2% IVI. The remaining 107 species, each with IVI < 1, shared 16.7% IVI (Table 2).
3.5. Species diversity, abundance modelling and ordinationThe gamma diversity (γ) was 184 species in 2.45 ha. The Whittaker's alpha diversity (α) was 30.7 species per sample. The compositional heterogeneity among samples, in terms of Whittaker's beta diversity (βw), was 2.79 and Harrison's beta-one value was 55.8%. Shannon's diversity (H'), based on the count of individuals with≥10 cm girth, was 4.402 nats (=6.351 bits). The maximum diversity (H'max) was 5.215 nats, Pielou's evenness or homogeneity index (E) was 0.844 and Simpson's dominance index (D) was 0.021. For individuals with≥30 cm girth, H' was 4.254, E was 0.869 and D was 0.029, and for individuals≥90 cm girth, H' was 3.549, E was 0.870 andD was 0.051.
Four classical models of species abundance distribution were applied to rank-abundance data. The observed pattern followed a truncated lognormal distribution in Preston's octaves of abundance (black bars in Fig. 7). The truncation point, or veil line, occurred at 0.125 octave (red line). An expected distribution in octaves of abundance (green bars) was close to the observed abundance distribution (χ2=17.48, df=4, p=0.00016). An estimate of hidden species behind the truncation point, or veil line, yielded 22 species (blue bar).

|
| Fig. 7 A plot of truncated lognormal distribution of species abundances in Preston's octaves (abundance classes) following Pielou's (1975) method of fitting. The observed frequencies of species in octaves are shown in black bars, expected frequencies in green bars and the frequency of hidden species in the blue bar (HS). The red line marked with TP indicates the truncation point. |
The PCA ordination revealed that the first two principal components were important and none of them were exceedingly overriding (Fig. 8a). Nine of the top ten dominant species in upper and middle canopies successfully separated along Component 1, between PCA score 20 and 80 (Fig. 8b). The most dominant species in the understory, B. glomerulifera, distinctly separated along Component 2 (Fig. 8b). Nearly 94% of species concentrated near the centroid in PCA space of Components 1 and 2, indicating that all these species required similar habitat and environmental conditions for growth.
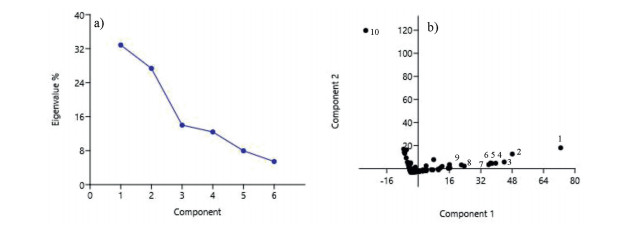
|
| Fig. 8 A PCA ordination of species in lowland rainforest of Meghalaya: a) the scree plot of eigenvalues of the principal components, and b) the dispersion of species in two dimensional space of component 1 and component 2. The top ten species are numbered as: 1) Macropanax undulatus, 2) Schima wallichii, 3) Castanopsis armata, 4) Symplocos sumuntia, 5) Castanopsis lanceifolia, 6) Itea macrophylla, 7) Syzygium tetragonum, 8) Helicia nilagirica, 9) Sarcosperma griffithii and 10) Boehmeria glomerulifera. |
Aerial views of 'tropical lowland evergreen rainforests' appear alike across regions due to striking similarity in physiognomy, but are dissimilar in floristic composition and patterns of tree diversity (Whitmore, 1984; Corlett and Primack, 2011). While an appreciable number of phytosociological studies are available from rainforests in the equatorial region of Asia-Pacific, there are a limited number of studies which examine the northern limits of rainforests around the Tropic of Cancer. The known lowland rainforests north of the Tropic of Cancer are situated up to 27°40'N in Namdapha National Park in northeastern India (Kaul and Haridasan, 1987; Proctor et al., 1998; Deb et al., 2009) and up to 27°20'N in Myanmar (Kingdon-Ward, 1945; Davis, 1960). In China, tropical areas near the Tropic of Cancer are situated disjunctively in southeastern Xizang (Tibet), southern parts of Yunnan, Gunangxi, Guangdong, Taiwan and Hainan Island (Corlett, 2014). The largest tropical area is in Yunnan province, which occupies a transitional position between northeastern India at the junction of the Indian and Burmese plates of Gondwanaland and the Eurasian plate of Laurasia (Audely-Charles, 1987). A vegetation map of China depicts rainforests up to 28°28'N in the eastern vicinity of Namdapha National Park, presumably with the same biogeographical formation (Hou, 1979; Wu, 1980), but phytosociological studies from these forests are not available (Proctor et al., 1998; Corlett, 2014). The westernmost limit of the lowland rainforests north of the Tropic of Cancer occurs in Khasi and Garo hills of Meghalaya, and this paper presents the first dataset on lowland rainforests from this region. The lowland rainforests situated at the northern limits, especially in Meghalaya around 24°00'N, are restricted mainly to moist depressions in rolling mountains below 1000 m in altitude, primarily in patches experiencing tropical climate governed by the Asia-Pacific monsoon. During his visit to Khasi Hills in 1850, J. D. Hooker noticed these rainforests (Hooker, 1854). The rainforests in Khasi Hills tend to grade gradually into 'tropical montane evergreen rainforests' at higher altitudes, between 1200 and 1800 m, and tend to degrade as 'tropical lowland semievergreen forests' following clear-cutting for shifting agriculture (Tripathi and Shankar, 2016).
4.1. Physiognomy, life-form spectrum and frequency of speciesDisplay of an evergreen physiognomy is the characteristic of rainforests despite modest shrinkage of foliage during winter. Deciduous species principally contribute to the reduction in foliage. However, these species flush newer leaves shortly after senescence in spring to sustain an evergreen appearance. Hence, species with long periods of leaflessness (>2 months), as is common in temperate forests, do not occur in tropical rainforests. At our sites, deciduousness increased from the sapling layer through understory and middle-canopy strata and reached a maximum in uppercanopy and emergent strata. Previous research has recorded the distinctive presence of deciduous trees in the canopy of rainforests near the Tropic of Cancer. Notably, at Xishuangbanna, deciduous trees account for one-half of the individuals in the canopy and onethird of the species in the emergent layer (Zhu, 1997). While we found that Xishuangbanna and Meghalaya shared some deciduous trees in common (B. javanica, Tetrameles nudiflora, Ulmus lanceifolia), the levels of deciduousness were lower in Meghalaya compared to Xishuangbanna despite the former being at higher latitudes compared to the latter. In rainforests near the equator, deciduousness is insignificant (Poore, 1968). Increasing deciduousness in rainforests, from the equator towards the Tropic of Cancer, is attributed to distinctively seasonal climate. The levels of deciduousness are presumably governed by annual quantities of rainfall and the period and intensity of dryness during winter.
The rainforests of Meghalaya exhibited a phanerophytic lifeform spectrum. Species richness can be principally ascribed to tree diversity as there are few species of shrubs or liana. Among woody life-forms, mega-and mesophanerophytes are predominant, commanding up to three-fourths of IVI. The micro-and nanophanerophytes contribute more to species richness than to IVI, and the contribution of liana phanerophytes is negligible. These patterns substantiate the observations of Champion and Seth (1968), and Whitmore (1984) that the dominance of trees over other growth forms is common in lowland forests in India. Notably, similar proportions of trees, shrubs and liana have been recorded in sal-dominated lowland forests in Darjeeling (Shankar, 2001) and in Meghalaya (Tripathi and Shankar, 2014). The dominance of phanerophytes in Meghalaya also conforms to findings in the equatorial rainforests of Malaya (Poore, 1968; Lee et al., 2002). However, Zhu (1997) found that liana phanerophytes attained higher proportions (up to one-fourth) in rainforests of Xishuangbanna. Although the seasonality of climate in Meghalaya is quite similar with that in Xishuangbanna, the quantity of annual rainfall is greater in the former than in the latter, which most likely promotes the dominance of mega-and mesophanerophytes.
Tropical rainforests are usually teeming with an abundance of species with a low frequency of occurrence (Pitman et al., 1999; Small et al., 2004). Previous research at Jengka revealed that certain species, including the most abundant ones, exhibited aggregation (Poore, 1968). For instance, all individuals of Hopea griffithii occurred within 100 m of each other. Many species are distributed nearly at random, and probably no species are uniformly dispersed. We found that in the rainforests of Meghalaya a large number of species showed aggregation. Abundance of species with high aggregation testifies to the fact that the rainforests contain plenty of rare species, which remain confined to specific microhabitats (subplots). Only a few species show moderate levels of dispersion in the forest. This is one of the reasons why 'rainforests appear similar in physiognomy, but differ in floristic composition'. This is also the reason why 'individuals-to-species ratio' is low in rainforests, which means the frequency of newer species added with increasing count of individuals is very high. In other words, beta diversity is high.
4.2. Familial dominanceThe basic framework of the lowland rainforests of Meghalaya is strikingly similar to those near the equator (Poore, 1968; Proctor et al., 1983; Lee et al., 2004; Manokaran et al., 2004) and in Xishuangbanna (Zhu, 1997; Lan et al., 2012). The familial composition is predominantly tropical and many top-ranked families are similar, viz., Euphorbiaceae, Lauraceae, Meliaceae, Moraceae, Myristicaceae, Myrtaceae and Rubiaceae (Table 5). However, some conspicuous differences prevail. Dipterocarpaceae was predominant near the equator and in the Mengla plot in Xishuangbanna (Lan et al., 2012), but was absent in Meghalaya and in other plots in Xishuangbanna (Zhu, 1997; Lü et al., 2010). Dipterocarpaceae is present in some (Deb et al., 2009; Nath et al., 2005), but is absent in many patches of rainforests, both in northeastern India and in southwestern China.
| Family | Meghalaya, India (this study) | Xishuangbanna, China (Zhu, 1997) | Jengka, Malaysia (Poore, 1968) | |||||
| IVI | Rank | IVI | Rank | IVI | Rank | |||
| 1. Lauraceae | 29.4 | 1 | 39.1 | 1 | 43 | 16 | ||
| 2. Euphorbiaceae | 27.4 | 2 | 23.3 | 5 | 155 | 4 | ||
| 3. Fagaceae | 27.2 | 3 | present | < 20 | 41 | 18 | ||
| 4. Araliaceae | 20.7 | 4 | * | * | absent | absent | ||
| 5. Theaceae | 19.6 | 5 | * | * | absent | absent | ||
| 6. Urticaceae | 15.6 | 6 | * | * | absent | absent | ||
| 7. Moraceae | 10.6 | 7 | 26.4 | 2 | 82 | 9 | ||
| 8. Rubiaceae | 10.0 | 8 | 11.6 | 9 | 13 | < 20 | ||
| 9. Myrtaceae | 8.9 | 9 | 7.0 | 14 | 113 | 6 | ||
| 10. Meliaceae | 7.1 | 10 | 16.9 | 6 | 18 | < 20 | ||
| 11. Iteaceae | 5.9 | 11 | * | * | absent | absent | ||
| 12. Sapotaceae | 5.9 | 12 | * | * | 56 | 12 | ||
| 13. Arecaceae | 5.7 | 13 | * | * | absent | absent | ||
| 14. Bignoniaceae | 5.7 | 14 | * | * | 4 | < 20 | ||
| 15. Polygalaceae | 5.4 | 15 | * | * | 52 | 13 | ||
| 16. Leguminosae (Fabaceae) | 4.7 | 21 | 7.7 | 13 | 174 | 3 | ||
| 17. Sapindaceae | 3.6 | 23 | 15.0 | 7 | 44 | 15 | ||
| 18. Clusiaceae (Guttiferae) | 3.6 | 24 | 11.3 | 10 | 33 | < 20 | ||
| 19. Burseraceae | 3.2 | 26 | * | * | 370 | 2 | ||
| 20. Annonaceae | 2.6 | 30 | 24.3 | 4 | 89 | 7 | ||
| 21. Myristicaceae | 2.3 | 32 | 10.8 | 11 | 84 | 8 | ||
| 22. Rutaceae | 1.2 | 47 | 5.0 | 15 | absent | absent | ||
| 23. Ulmaceae | 0.6 | 59 | 26.1 | 3 | 1 | < 20 | ||
| 24. Lecythidaceae# | absent | absent | 11.9# | 8# | absent | absent | ||
| 25. Datiscaceae | absent | absent | 8.1 | 12 | absent | absent | ||
| 26. Dipterocarpaceae | absent | absent | 771 | 1 | ||||
| 27. Sterculiaceae | absent | absent | 132 | 5 | ||||
| The * indicates 'probably absent or in meagre quantities'. #Includes Barringtoniaceae at Xishuangbanna. | ||||||||
Fagaceae occurs in most rainforests from the equator to the northern limits, but it was far more important (with third rank) in Meghalaya than in Xishuangbanna as well as near the equator. Fagaceae is absent from peninsular India, Islands of Andaman and Sri Lanka and appears east of Wallace's line principally in IndoBurma, southern China and western Malesia (Ashton, 1988). The flora of Meghalaya is rich in Fagaceae with 15 species predominating in lower and upper montane forests between 1000 and 2000 m, although some species have previously been found to occur between 400 and 1000 m (Tripathi and Shankar, 2014).
The occurrence of Araliaceae, Theaceae, Urticaceae and Iteaceae, which rank 4, 5, 6, and 11, respectively, was distinct in Meghalaya. These families were probably absent or occurred in meagre quantities in both Xishuangbanna and in many other forests near the equator, including Jengka (Table 5), Batang Gadis (Kartawinata et al., 2004) and Wanariset (Kartawinata et al., 2008). In Meghalaya, Araliaceae is predominant in middle-canopy and Urticaceae in understory. This is not surprising as Theaceae (S. wallichii) has been known to predominate in upper-canopy and emergent strata in almost all forests of Meghalaya (Tripathi and Shankar, 2014, 2016) and other locations in northeastern India (Shankar et al., 1998). Theaceae may occur near the equator, but in meagre quantities. The rainforests of Meghalaya also differed with rainforests of Jengka as well as Xishuangbanna in having much lesser importance of Annonaceae, Lecythidaceae (absent at Jengka), Burseraceae (absent at Xishuangbanna), Clusiaceae, Leguminosae, Sapindaceae and Ulmaceae (Table 5).
4.3. Patterns of species richness, stand density and basal areaThe records of maximum species count emanate from the richest known equatorial lowland rainforests of Malaya Peninsula, Sumatra and Borneo between latitudes 0° and 4° N. Long-term vegetation dynamics studies in large-sized plots (up to 52 ha) as well as individual one-hectare plots of primary rainforests have revealed several instances of more than 200 tree species of≥10 cm dbh in a hectare (Corlett, 2014). Lee et al. (2002) claimed the rainforests of Lambir Hills in Sarawak, Malaysia were the most diverse with 247 tree species per hectare (Table 6). However, Sukardjo et al. (1990) recorded 276 tree species in just a hectare from Sebulu, Indonesia. From studies in East Kalimantan, Indonesia, Kartawinata et al. (2008) claimed the rainforests of Malinau were the most species-rich and those of Wanariset Samboja the second highest species-rich in the world. Future exploration will likely provide ample opportunity for the revision of these records. The rainforests of Meghalaya exhibit only moderate levels of species richness in comparison to species-rich equatorial rainforests. Nonetheless, it is amply clear from Table 6 that: (1) the Islands of Sumatra and Borneo harbour the most species-rich equatorial dipterocarpus rainforests in the world; (2) moving away from the equator towards the Tropic of Cancer and beyond, richness of tree species declines in lowland rainforests; (3) the richness of tree species varies within a narrow range around the Tropic of Cancer and it is comparable between southwestern China and northeastern India; and (4) the maximum richness of large tree species (≥30 cm dbh) does probably occur in Lambir Hills and is followed by Jengka Forest Reserve, Malaysia with 375 species in 23 ha.
| Site | DBH class (cm) | Area sampled (ha) | Species sampled (S) | Shannon's diversity index (H') | Average per hectare | ||
| N | S | BA | |||||
| North of the Tropic of Cancer (Northeastern India) | |||||||
| Meghalaya, this study | ≥10 | 2.45 | 133 | 4.25 | 467 | 89 | 26.1 |
| Namdapha1 | ≥10 | 1 | 116 | ? | 333 | 116 | 29.6 |
| Namdapha2 | ≥10 | 2.4 | 77 | 3.36 | 610 | ? | 98.6 |
| Namdapha-Hornbill3 | ≥10 | 1.2 | 98 | 3.85 | 418 | 88 | 45.5 |
| Namdapha-Gibbonland3 | ≥10 | 1 | 54 | 3.55 | 390 | 54 | 49.7 |
| Deomali4 | ≥3.1 | 0.9 | 47 | 2.02 | 5452 | ? | 104.6 |
| Near the Tropic of Cancer (Southwestern China) | |||||||
| Jinghong SRF5 | ≥10 | 1.04 | 125 | ? | 386 | 120 | 30.03 |
| Menglum6 | ≥10 | 1 | 106 | 4.08 | 393 | 106 | 30.2 |
| Mengla6 | ≥10 | 1 | 94 | 3.93 | 423 | 94 | 39.7 |
| Manyang6 | ≥10 | 1 | 84 | 3.45 | 467 | 84 | 31.3 |
| Mengla7 | ≥10 | 20 | 339 | ? | 617 | 123 | 36.6 |
| Xishuangbanna SRF8 | ≥5 | 1.25 | 131 | ? | 763 | ? | ? |
| Xishuangbanna MRF8 | ≥5 | 1.46 | 140 | ? | 478 | ? | ? |
| Xishuangbanna DRF8 | ≥5 | 1.04 | 125 | ? | 858 | ? | ? |
| Tietahe9 | ≥5 | 1 | 145 | ? | 730 | 145 | 31.3 |
| Caiyanghe9 | ≥5 | 1 | 86 | ? | 489 | 86 | 32.8 |
| Equatorial rainforests of Asia-Pacific | |||||||
| Sarawak Alluvial10 | ≥10 | 1 | 223 | ? | 615 | 223 | 28.0 |
| Sarawak Dipterocarp10 | ≥10 | 1 | 214 | ? | 778 | 214 | 57.0 |
| Sarawak Heath10 | ≥10 | 1 | 123 | ? | 708 | 123 | 43.0 |
| Sarawak Limestone10 | ≥10 | 1 | 73 | ? | 644 | 74 | 37.0 |
| Sarawak Lambir Hill11 | ≥10 | 52 | 1003 | 5.96 | 637 | 247 | 37.8 |
| Pasoh, Malaysia12 | ≥10 | 50 | 678 | 5.64 | 531 | 206 | 25.7 |
| Sebulu, Indonesia13 | ≥10 | 1 | 276 | ? | 592 | 276 | 37.3 |
| Bukit Lawang, Indonesia14 | ≥10 | 1 | 216 | ? | 453 | 216 | ? |
| Kalimantan, Indonesia15 | ≥10 | 15 | 1298 | ? | 584 | 218 | ? |
| Batang Gadis, Indonesia16 | ≥10 | 1 | 184 | ? | 583 | 184 | 40.6 |
| Wanariset, Indonesia17 | ≥10 | 10.5 | 553 | ? | 557 | ? | 33.3 |
| Temburong, Brunei18 | ≥10 | 1 | 231 | ? | 550 | 231 | ? |
| Kuala Belalong, Brunei19 | ≥10 | 1 | 197 | ? | 422 | 197 | 31.4 |
| Comparison of large diameter trees (≥30 cm dbh) | |||||||
| Meghalaya, this study | ≥30 | 2.45 | 59 | 3.55 | 107 | 44 | 17.7 |
| Mengla, Xishuangbanna9 | ≥30 | 20 | 215 | ? | 112 | 43 | 24.8 |
| Lambir Hill, Sarawak11 | ≥30 | 52 | 574 | 5.25 | 119 | 67 | 26.3 |
| Pasoh, Malaysia12 | ≥30 | 50 | 375 | 4.90 | 76 | 47 | 15.9 |
| Jengka, Malaysia20 | ≥30 | 23 | 375 | 5.04 | 120 | ? | 24.2 |
| Jengka, Malaysia21 | ≥30 | 11.7 | 261 | 4.72 | 104 | ? | 23.3 |
| N is number of individuals, S is number of species, BA is basal area in m2 ha-1, H' is Shannon's diversity index in nats, Some values of H' were converted from decs to nats by multiplying with 2.302585, ? means that the value cannot be deduced from the source study. Sources: 1 Proctor et al. (1998), 2 Nath et al. (2005), 3 Deb et al. (2009), 4 Bhuyan et al. (2003), 5 Shanmughavel et al. (2001), 6 Lü et al. (2010), 7 Lan et al. (2012), 8 Zhu (1997), 9 Zheng et al. (2006), 10 Proctor et al. (1983), 11 Lee et al. (2004), 12 Manokaran et al. (2004), 13 Sukardjo et al. (1990), 14 Polosakan (2001), 15 Wilkie et al. (2004), 16 Kartawinata et al. (2004), 17 Kartawinata et al. (2008), 18 Poulsen et al. (1996), 19 Small et al. (2004), 20 Poore (1968), 21 Ho et al. (1987). | |||||||
Among the most species-rich equatorial rainforests, stand density (ha-1) of trees≥10 cm dbh ranges between 422 and 778 (Table 6). In seasonal rainforests of southwestern China, the range is lower, from 386 to 617, and it varies from 478 to 858 for trees≥5 cm dbh (Table 6). North of the Tropic of Cancer, lowland rainforests in northeastern India exhibit a range of 333-610, excluding a study from Deomali, which included individuals of smaller diameter (Table 6). A density of 467 trees ha-1 in rainforests of Meghalaya is lower in comparison to equatorial rainforests, but it is intermediate in the range for rainforests around the Tropic of Cancer. Interestingly, among all rainforests, the density of trees≥10 cm dbh varies by less than two-fold only. Although limited datasets are available, the range of density of trees≥30 cm dbh is also narrow, i.e., from 76 to 120 per hectare and the sites from Meghalaya crop up at the upper end of this range (Table 6).
The basal area (m2 ha-1) of trees≥10 cm dbh ranges between 25.7 and 57 in equatorial rainforests, between 30 and 39 in seasonal rainforests of southwestern China, and between 26.1 and 49.7 in northeastern India (Table 6). Broadly, the basal area of trees≥10 cm dbh varies by nearly two-fold. The basal area in rainforests of Meghalaya is on the lower side of the range of all rainforests. The exceptionally high values of basal area, reported from Deomali by Bhuyan et al. (2003) and from Namdapha by Nath et al. (2005) remain difficult to interpret, as the sampling methods of these studies appear to be unorthodox and inconsistent. Future studies should resolve these unrealistic figures. For large diameter trees (≥30 cm dbh), basal area ranges from 15.9 to 26.3 m2 ha-1 and the sites from Meghalaya occur at the lower end of this range (Table 6). The large trees (≥30 cm dbh) contribute around two-thirds of the basal area to the basal area of all trees≥10 cm dbh. The proportional contribution of the basal area of large trees was comparable between Meghalaya (67.8%), Mengla (67.8%) and Lambir (69.6%), although it was slightly smaller at Pasoh (61.9%).
4.4. Patterns of diversity and equitability of abundancesA pattern of diversity, in terms of Shannon's entropy (H'), is visible along a gradient in latitude, from the equator to the northern limits (Table 6). The value of H' combines both species richness and equitability of abundance and it is not on a linear scale. For trees≥10 cm dbh, equatorial rainforests exhibited maximum H' from Lambir Hills (5.96), which is followed by Pasoh (5.64). The value of H' declines towards northern limits and ranges between 3.5 and 4.0 in rainforests of southwestern China and northeastern India. In this study, H' was 4.25, which is maximum among all rainforests around the Tropic of Cancer, although values for some sites are not available (Table 6). A declining gradient in annual rainfall (5000 to 1500 mm) from the equator to the northern limits probably shapes the patterns of diversity, but excessively high quantity of rainfall (>5000 mm) in Meghalaya appears unutilized, as it does not contribute to enhance diversity and productivity of vegetation (Shankar et al., 1993).
The high value of H' for rainforests of Meghalaya is due to high equitability of species abundances. Clearly, low abundance of the most abundant species and nearly comparable abundance of the top four species distinguishes rainforests of Meghalaya from those in southwestern China wherein a single species is clearly dominant ( Lü et al., 2010; Lan et al., 2012). The value of H' is also sensitive to the quantum of sampling area, as with an increase in area, the number of species increases and adds to the value of H'. The high values of H' from Lambir and Pasoh result from large plots (nearly 50 ha) and these values from the rainforests around Tropic of Cancer result from nearly 1 to 2 ha only. Hence, these comparisons should be applied with prudence. For large trees≥30 cm dbh, H' is smaller for Meghalaya compared to equatorial rainforests (Table 6).
The equitability of species abundances was marginally higher (Pielou's E=0.876) in Meghalaya than at Jengka (E=0.850). A comparison of rank-abundance plot between Meghalaya and Jengka exhibited striking similarity and followed a closely matching lognormal pattern of abundance distribution (Fig. 9). Presumably, with increased sampling efforts more species would be added to the list, resulting in a relatively moderate slope at the intermediate-to-tail region of the curve for Meghalaya, which would be in accord with the Jengka curve. Neither curve exhibits a 'tail off' phenomenon in the abundances of rare species that is common in tropical dry forest in Costa Rica, but not in tropical wet forest in Brazil (Hubbell, 1979). The lognormal abundance distributions prevail in closed, stable and undisturbed assemblages with a high proportion of species with intermediate abundances, i.e., an assemblage of 'a truly middle class' (Magurran and Henderson, 2003). The lognormal distributions indicate that an increasingly greater number of species is getting packed into the assemblage, with the result that more species inevitably random walk to rarity, increasing the risk of local extinction due to stochastic disturbance events (Hubbell, 1979).
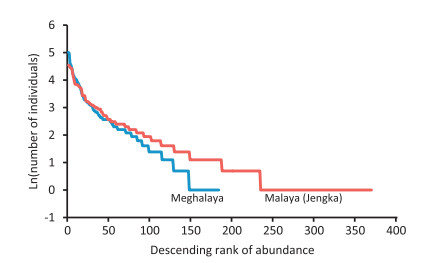
|
| Fig. 9 A comparison of rank-abundance plots of lowland rainforest of Meghalaya (blue) with equatorial rainforest of Jengka (red). Both the curves are based on the count of individuals per species. For Jengka, data were obtained from Poore (1968). |
For all individuals≥10 cm in girth, compositional heterogeneity (βw) of the rainforests of Meghalaya is 'high' and nearly 55.8% of species indicate a very high horizontal patchiness or turnover within the assemblage. The heterogeneity of a landscape is a function of the differentiation in species composition across samples and of the distances among the samples. Due to the lack of data from sites shown in Table 6, it is difficult to make comparisons. By Simpson's measure of D, the probability that the two trees drawn at random from the sample population belong to the same species is smaller at Jengka (1.2%) than in Meghalaya (5.1%).
5. ConclusionsThis study reveals phytosociological attributes of lowland rainforests of Meghalaya, which are situated in the westernmost limits north of the Tropic of Cancer. The rainforests of Meghalaya differ with equatorial rainforests in low stature restricted to 30 m height, inconspicuous multi-layering in vertical stratification, paucity of lianas, absence of Dipterocarpaceae, prominence of Fagaceae and Theaceae, smaller basal area, and lower levels of alpha and gamma diversities. Although the floristic composition is largely native to the Indo-Malaya biogeographical realm, there are a negligible number of species endemic to the local geography. These differences are most likely due to increased seasonality of climate and relatively lower annual mean temperature. Exceptionally high quantities of rainfall do not add to the vigour of the rainforests of Meghalaya, presumably due to poor substratum and impoverished soils. Nonetheless, the rainforests of Meghalaya exhibit striking similarities to seasonal rainforests in southwestern China, exhibiting evergreen physiognomy interspersed by deciduous emergent trees, life-form spectrum, familial composition and levels of stem densities and beta diversity. The equitability of species abundances appears greater in Meghalaya than in southwestern China, resulting in higher H'. These similarities are generally due to seasonal climate, which is broadly similar, i.e. Asia-Pacific monsoondependent around the Tropic of Cancer. The rainforests in Namdapha and Deomali in northeastern India differ from the rainforests of Meghalaya in harbouring Dipterocarpaceae, larger basal area and lower equitability of species abundances. The rainforests of Meghalaya survive largely due to community-based conservation practices. The local tribes have a rich culture of preserving forests as 'sacred groves' not only in inaccessible pockets, but also in the vicinity of hamlets. Enquiries with locals reveal that the rainforests have remained free from grazing, fire and commercial logging, and strong anecdotal religious beliefs and taboos continue to remain popular among the tribes. Government control of forest landholding is minimal and hence legal intervention is not possible. As long as anthropogenic disturbances are under control, the rainforests of Meghalaya shall survive.
AcknowledgementsThe Department of Biotechnology, New Delhi provided principal funding through a grant to US (BT/PR7928/NDB/52/9/2006). AKT received a fellowship from the DBT project and from UGC's meritorious fellowship programme. The authors are grateful to Prof. K.N. Ganeshaiah, UAS, Bengaluru, for encouragement, the custodians of forests in Meghalaya for permission, the Botanical Survey of India, Shillong for access to herbarium, and the Head, Department of Botany, NEHU for logistics. The help rendered by K. Nongrum, D. Kumar, S. Borah and Mrs. Shilpi Agrawal is thankfully acknowledged. AKT led the field work. US developed test hypotheses and study design, reviewed literature, analyzed data and carried out manuscript writing. US is studying phytosociology and regeneration of forest ecosystems in northeastern India under the doctor of science programme at the North-Eastern Hill University, Shillong. US dedicates this paper to Professor Duncan Poore who revealed quantitative facts of the dipterocarpus lowland rainforests of Malaya as early as in 1960s and worked for their conservation until he breathed his last in March, 2016 when this paper was being written (http://www.itto.int/news_releases/id=4777). The authors declare that they have no competing interests.
APG Ⅲ, 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants, APG Ⅲ. Bot. J. Linn. Soc. 161 (2), 105-121. http://dx.doi.org/10.1111/j.1095-8339.2009.00996.x.
|
Ashton P.S., 1988. Dipterocarp biology as a window to the understanding of tropical forest structure. Ann. Rev. Ecol. Syst, 19: 347-370. DOI:10.1146/annurev.es.19.110188.002023 |
Audley-Charles, M. G. , 1987. Dispersal of Gondwanaland, relevance to evolution of the angiosperms. In: Whitmore, T. C. (Ed. ), Biogeographical Evolution of the Malay Archipelago. Clarendon Press, Oxford, pp. 5-21.
|
Balakrishnan, N. P. , 1981-1983. Flora of Jowai and Vicinity, Meghalaya, vols. Ⅰ & Ⅱ. Botanical Survey of India, Howrah, India.
|
Bhuyan P., Khan M.L., Tripathi R.S., 2003. Tree diversity and population structure in undisturbed and human-impacted stands of tropical wet evergreen forest in Arunachal Pradesh, Eastern Himalayas, India. Biodivers. Conserv, 12: 1753-1773. DOI:10.1023/A:1023619017786 |
Brown J.H., 2014. Why are there so many species in the tropics? J. Biogeogr, 41: 8-22. DOI:10.1111/jbi.12228 |
Champion H.G., Seth S.K., 1968. A Revised Survey of the Forest Types of India.. Government of India, New Delhi. |
Corlett, R. T. , 2009. The Ecology of Tropical East Asia. Oxford University Press, Oxford, UK.
|
Corlett, R. T. , 2014. The Ecology of Tropical East Asia, second ed. Oxford U. P. , UK, p. 336.
|
Corlett, R. T. , Primack, R. B. , 2011. Tropical Rain Forests, an Ecological and Biogeographical Comparison, second ed. Wiley-Blackwell, USA, p. 336.
|
Curtis J.T., McIntosh R.P., 1950. The interrelations of certain analytic and synthetic phytosociological characters. Ecology, 31: 435-455. |
Davis, J. , 1960. The forests of Burma. Department of Botany, University of Florida, p. 23.
|
Deb P., Sundriyal R.C., Shankar, Uma, 2009. Tree diversity and population structure in a lowland tropical rainforest in the eastern Himalayas, India. Ind. For, 135: 1526-1544. |
Hammer, O. , Harper, D. A. T. , Ryan, P. D. , 2001. PAST, paleontological statistics software package for education and data analysis. Palaeontola Electron 41, 9. http://palaeo-electronica.org/2001_1/past/issue1_01.htm.
|
Haridasan, K. , Rao, R. R. , 1985-1987. Forest flora of Meghalaya, vols. Ⅰ & Ⅱ. Bishen Singh Mahendra Pal Singh, Dehra Dun, India.
|
Harrison S., Ross S.J., Lawton J.H., 1992. Beta diversity on geographic gradients in Britain. J. Anim. Ecol, 61: 151-158. DOI:10.2307/5518 |
Ho C.C., Newbery McC., Poore M.E.D., 1987. Forest composition and inferred dynamics in Jengka forest Reserve, Malaysia. J. Trop. For. Sci, 3: 25-56. |
Hooker, J. D. , 1854. The Himalayan Journals, Notes of a Naturalist in Bengal, the Sikkim and Nepal Himalayas, the Khasia Mountains etc, vol. 2. John Murray, Albemarle Street, London.
|
Hooker, J. D. , 1872-1897. Flora of British India, vols. IeVII. L. Reeve and Company, London.
|
Hou, H. Y. , 1979. The Vegetation Map of China. Chinese Academy of Sciences, Institute of Botany, Peking.
|
Hubbell S.P., 1979. Tree dispersion, abundance and diversity in a tropical dry forest. Science, 203: 1299-1309. DOI:10.1126/science.203.4387.1299 |
Jamir S.A., Pandey H.N., 2003. Vascular plant diversity in the sacred groves of Jaintia Hills in northeast India. Biodivers. Conserv, 12: 1497-1510. DOI:10.1023/A:1023682228549 |
Kanjilal, U. N. , Kanjilal, P. C. , Das, A. , 1934-1940. Flora of Assam, vols. Ⅰ-Ⅳ. Government of Assam, India.
|
Kartawinata K., Samsoedin I., Heriyanto M., Afriastini J.J., 2004. A tree species inventory in a one-hectare plot at the Batang Gadis National Park, North Sumatra, Indonesia. Reinwardtia, 12: 129-204. |
Kartawinata K., Purwaningsih, Partomihardjo T., Yusuf R., Abdulhadi R., Riswan S., 2008. Floristics and structure of a lowland dipterocarp forest at Wanariset Samboja, East Kalimantan, Indonesia. Reinwardtia, 12(4): 301-323. |
Kaul R.N., Haridasan K., 1987. Forest types of Arunachal Pradesh e a preliminary study. J. Econ. Taxon. Bot, 9: 379-389. |
Khiewtam R.S., Ramakrishnan P.S., 1993. Litter and fine root dynamics of relic sacred grove forest of Cherrapunjee in northeastern India. For. Ecol. Manage, 60: 327-344. DOI:10.1016/0378-1127(93)90087-4 |
Kingdon-Ward F., 1945. A sketch of the botany and geography of north Burma. J. Bombay Nat. Hist. Soc, 45: 16-30. |
Lan G., Zhu H., Cao M., 2012. Tree species diversity of a 20-ha plot in a tropical seasonal rainforest in Xishuangbanna, Southwest China. J. For. Res, 17: 432-439. DOI:10.1007/s10310-011-0309-y |
Lee H.S., Davies S.J., Lafrankie J.V., Tan S., Yamakura T., Itoh A., Ohkubo T., Ashton P.S., 2002. Floristic and structural diversity of mixed dipterocarp forest in Lambir hills National Park, Sarawak, Malaysia. J. Trop. For. Sci, 14: 379-400. |
Lee, H. S. , Sylvester, T. , Davies, S. J. , Lafrankie, J. V. , Ashton, P. S. , Yamakura, T. , Itoh, A. , Ohkubo, T. , Harrison, R. , 2004. In: Losos, E. C. , Leigh, E. G. (Eds. ), Lambir Forest Dynamics Plot, Sarawak, Malaysia. Tropical Forest Diversity and Dynamism: Findings from a Large-scale Plot Network. University of Chicago Press, Chicago, pp. 527-539.
|
Lü X.T., Yin J.X., Tang J.W., 2010. Structure, tree species diversity and composition of tropical seasonal rainforests in Xishuangbanna, South-west China. J. Trop. For. Sci, 22: 260-270. |
Magurran A.E., 1988. Ecological Diversity and its Measurement. New Jersey, USA: Princeton University Press.
|
Magurran, A. E. , Henderson, P. A. , 2003. Explaining the excess of rare species in natural species abundance distributions. Nature 422 (6933), 714-716. http://dx.doi.org/10.1038/nature01547.
|
Manokaran, N. , Seng, Q. E. , Ashton, P. S. , et al. , 2004. Pasoh forest dynamics Plot, Peninsular Malaysia. In: Losos, E. C. , Leigh, E. G. (Eds. ), Lambir Forest Dynamics Plot, Sarawak, Malaysia. Tropical Forest Diversity and Dynamism: Findings from a Large-scale Plot Network. University of Chicago Press, Chicago, pp. 585-598.
|
Meng J., Lu Y., Lei X., Liu G., 2011. Structure and floristics of tropical forests and their implications for restoration of degraded forests of China's Hainan Island. Trop. Ecol, 52: 177-191. |
Muller-Dombois, D. , Ellenberg, H. , 1974. Aims and Methods of Vegetation Ecology. John Wiley and Sons, Inc.
|
Murali K.S., Shankar Uma, Uma Shaanker R., Ganeshaiah K.N., Bawa K.S., 1996. Extraction of non-timber forest products in the forest of Biligiri Rangan Hills, India. 2. Impact of NTFP extraction on regeneration, population structure and species composition. Econ. Bot, 50: 252-269. DOI:10.1007/BF02907329 |
Myers N., 2003. Biodiversity hotspots revisited. BioScience, 53: 916-917. DOI:10.1641/0006-3568(2003)053[0916:BHR]2.0.CO;2 |
Nath P.C., Arunachalam A., Khan M.L., Arunachalam K., Barbhuiya A.R., 2005. Vegetation analysis and tree population structure of tropical wet evergreen forests in and around Namdapha National Park, Northeast India. Biodivers. Conserv, 14: 2109-2136. DOI:10.1007/s10531-004-4361-1 |
Pielou, E. C. , 1975. Ecological Diversity. Wiley, New York.
|
Pitman N.C.A., Terbirgh J., Silman M.R., Nunez V.P., 1999. Tree species distribution in an upper Amazonian forest. Ecology, 80: 2651-2661. DOI:10.1890/0012-9658(1999)080[2651:TSDIAU]2.0.CO;2 |
Polosakan, R. , 2001. Komposisi jenis pohon di hutan kawasan Taman Nasional Bukit Tigapuluh, Propinsi Riau. In: Sunaryo, et al. (Eds. ), Laporan Teknik 2001. LIPI, Bogor, pp. 31-40. Proyek Inventarisasi Dan Karakterisasi Sumberdaya Hayati, Pusat Penelitian Biologi.
|
Poore M.E.D., 1968. Studies in Malaysian rain forest, Ⅰ. The forest on Triassic Sediments in Jengka forest Reserve. J. Ecol, 56: 143-196. |
Poulsen, A. D. , Neilsen, I. C. , Tan, S. , Balslev, H. , 1996. A quantitative inventory of trees in one hectare of mixed dipterocarp forest in Temburong, Brunei Darussalam. In: Edwards, D. S. , Booth, W. E. , Choy, S. (Eds. ), Tropical Rainforest Research: Current Issues. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 139-150.
|
Proctor J., Anderson J.M., Chai P., Vallack H.W., 1983. Ecological studies in four contrasting lowland rain forests in Gunung Mulu National Park, Sarawak: Ⅰ. Forest environment, structure and floristics. J. Ecol, 71: 237-260. |
Proctor J., Haridasan K., Smith G.W., 1998. How far north does lowland evergreen tropical rain forest go? Glob. Ecol. Biogeogr. Lett, 7: 141-146. DOI:10.2307/2997817 |
Ramakrishnan, P. S. , 2001. Ecology and Sustainable Development. National Book Trust, India, p. 198.
|
Raunkiaer C., 1934. The Life Forms of Plants and Statistical Plant Geography. Oxford: Clarendon Press.
|
Shankar Uma, 2001. A case of high tree diversity in sal (Shorea robusta-dominated lowland forest of eastern Himalaya, floristic composition, regeneration and conservation. Curr. Sci, 81: 776-786. |
Shankar Uma, Tripathi R.S., Pandey H.N., 1991. Structure and seasonal dynamics of humid tropical grasslands in Meghalaya, India. J. Veg. Sci, 2: 711-714. DOI:10.2307/3236181 |
Shankar Uma, Pandey H.N., Tripathi R.S., 1993. Phytomass dynamics and primary productivity in humid grasslands along altitudinal and rainfall gradients. Acta Oecol, 14: 197-209. |
Shankar Uma, Lama S.D., Bawa K.S., 1998. Ecosystem reconstruction through 'taungya' plantations following commercial logging of a dry, mixed deciduous forest in Darjeeling Himalaya. For. Ecol. Manage, 102: 131-142. DOI:10.1016/S0378-1127(97)00152-7 |
Shanmughavel P., Zheng Z., Liqing S., Min C., 2001. Floristic structure and biomass distribution of a tropical seasonal rain forest in Xishuangbanna, southwest China. Biomass Bioenergy, 21: 165-175. DOI:10.1016/S0961-9534(01)00023-X |
Shannon C.E., 1948. A mathematical theory of communication. Bell Syst. Tech. J, 27: 379-423 and 623-656. DOI:10.1002/bltj.1948.27.issue-3 |
Simpson E.H., 1949. Measurement of diversity. Natur, 163: 688. DOI:10.1038/163688a0 |
Small A., Martin T.G., Kitching R.L., Wong K.M., 2004. Contribution of tree species to the biodiversity of a 1 ha Old World rainforest in Brunei, Borneo. Biodivers. Conserv, 13: 2067-2088. DOI:10.1023/B:BIOC.0000040001.72686.e8 |
Soja R., Starkel L., 2006. Extreme rainfalls in Eastern Himalaya and southern slope of Meghalaya Plateau and their geomorphologic impacts. Geomorphology, 84: 170-180. |
Sukardjo S., Hagihara A., Yamakura T., Ogawa H., 1990. Floristic composition of a tropical rain forest in Indonesian Borneo. Bull. Nogoya Univ. For, 10: 1-44. |
The Plant List 2013. Version 1. 1. Published on the Internet; http//www.theplantlist.org/(Accessed 10 July 2014).
|
Tripathi, A. K. , Shankar, Uma, 2014. Patterns of species dominance, diversity and dispersion in 'Khasi hill sal' forest ecosystem in northeast India. For. Ecosyst. 1, 23. http://dx.doi.org/10.1186/s40663-014-0023-2.
|
Tripathi, A. K. , Shankar, Uma, 2016. Rainforests North of the Tropic of Cancer: Recovery Patterns 40-years after Shifting Cultivation in Lowland Rainforests of Meghalaya In Review.
|
Tripathi O.P., Pandey H.N., Tripathi R.S., 2003. Distribution, community characteristics and tree population structure of subtropical pine forest of Meghalaya, northeast India. Int. J. Ecol. Env. Sci, 29: 207-214. |
Upadhaya K., Pandey H.N., Law P.S., Tripathi R.S., 2003. Tree diversity in sacred groves of Jaintia Hills in Meghalaya, northeast India. Biodivers. Conserv, 12: 583-597. DOI:10.1023/A:1022401012824 |
Whitmore, T. C. , 1984. Tropical Rain Forests of the Far East, second ed. Clarendon Press, Oxford, p. 352.
|
Whittaker R.H., 1972. Evolution and measurement of species diversity. Taxon, 21: 213-251. DOI:10.2307/1218190 |
Wilkie P., Argent G.C.G., Campbell E.J.F., Saridan A., 2004. The diversity of 15 ha of lowland mixed dipterocarp forest, Central Kalimantan. Biodivers. Conserv, 13: 695-708. DOI:10.1023/B:BIOC.0000011721.04879.79 |
WMO, 2014. Cherrapunji, India, Holds New Record for 48-hour Rainfall. Press Release No. 988. World Meteorological Organization, Switzerland. http://www.wmo.int/pages/mediacentre/news/index_en.html.
|
Wu, C. Y. , 1980. Vegetation of China. Beijing Science Press, Beijing, pp. 363-379 in Chinese.
|
Zheng Z., Feng Z., Cao M., Li Z., Zhang J., 2006. Forest structure and biomass of a tropical seasonal rain forest in Xishuangbanna, southwest China. Biotropica, 38: 318-327. DOI:10.1111/btp.2006.38.issue-3 |
Zhu H., 1997. Ecological and biogeographical studies on the tropical rain forest of south Yunnan, SW China with a special reference to its relation with rain forests of tropical Asia. J. Biogeogr, 24: 647-662. DOI:10.1111/jbi.1997.24.issue-5 |



