b. University of Chinese Academy of Sciences, 100049 Beijing, China
Magnolia sinica (Law) Noot. (Magnoliaceae), a rare tree species endemic to Southeast Yunnan, China, was first described as Manglietiastrum sinicum Law in 1979 (Law, 1979). Most Chinese botanists often call this species M. sinicum and also use its common name huagaimu (Sun et al., 2012; Wang et al., 2016). Its other synonyms are Manglietia sinica (Chen and Nooteboom, 1993) and Pachylarnax sinica (Xia et al., 2008). Because the species has been referred to as M. sinica internationally, M. sinica is also used in the present study.
M. sinica distributes in south subtropical monsoon broadleaved evergreen forests and scatters at altitudes between 1339 and 1707 m (Wang et al., 2016). Recent anthropogenic activities, including deforestation for commercial cultivation (e.g. Amomum tsao-ko Crevost et Lem. and Cunninghamia lanceolata) and habitat destruction, have lead both to the reduction of its population size and to serious habitat fragmentation. This species is categorized as Critically Endangered on the IUCN Red List (Cicuzza et al., 2007; Rivers et al., 2016) and has been identified as a “Plant Species with Extremely Small Populations (PSESP)” (State Forestry Administration of China, 2012; Ren et al., 2012; Ma et al., 2013; Volis, 2016) for priority conservation in China. It has also been targeted as one of the 20 species approved by the Yunnan government for urgent rescue action before 2015 (Wang et al., 2016). In the field, a total of 52 individuals of M. sinica were isolated in eight isolated populations. Of these 52 individuals, nine are young trees with a DBH (diameter at breast height) of less than 22.5 cm (Wang et al., 2016).
Although M. sinica flowers well, its fruit/seed set is low and seedlings are rarely found in the wild. Conservation via ex situ cultivation in gardens and reinforcement/reintroduction in the wild have been conducted by Kunming Botanical Garden (Sun, 2013). However, the rarity of seedling/saplings in the wild limits the potential for the natural regeneration of this species. Therefore, despite ex situ and in situ conservation trials, effective conservation measures based on scientific studies are imperative.
Knowledge of reproductive biology is essential for the effective protection of endangered plants, especially for species with small populations (Spira, 2001; Evans et al., 2004; Xiao and Xu, 2006). Successful reproduction is crucial in maintaining a viable population size, which is of critical concern to highly endangered taxa facing extinction (Pandit and Babu, 2003; Gong et al., 2014). In species relying on seeds to recruit new individuals into populations, population viability may be closely related to seed dynamics and conservation measures may depend on understanding the factors that limit seed production (Pavlik et al., 1993; Zhao and Sun, 2009) and dispersal. When seed production is mediated by pollinators, it can be influenced by pollinator abundance or behavior (Bierzychudek, 1981; Larson and Barrett, 2000). It may be limited by pollen, because self-pollen may cause reduction in seed production through inbreeding depression (Bosch and Waser, 1999; Brown and Kephart, 1999; Zhao and Sun, 2009). Seed production in particular, as a seed source for offspring, may directly affect seed dispersal. Seed dispersal may also be influenced by disperser abundance or behavior, if seed dispersal is mediated by dispersers.
Studies on the reproductive biology of threatened species with low reproductive capabilities have been the focus of recent research and have become an important aspect of conservation management. Despite extensive attention and critical conservation status, little information is available on the reproduction of M. sinica. The current study was undertaken to gain knowledge of the reproductive characteristics of M. sinica and aims to address the following issues: (1) its floral biology, especially the flowering process; (2) its pollination biology and the role of pollinators in fruit set; (3) its breeding system; (4) the characteristics of seed dispersal and seed germination of the species in the natural habitats.
1. Methods and materials 1.1. Study sitesThe pollination biology of M. sinica was investigated from 2014 to 2015 in Jingping County of Honghe Hani-Yi Autonomous Prefecture. The population there comprises four individuals. One is located beside a mountain trail in Zhongliang village and the other three are located in the thick forest on Luoguoping Mountain. Reproductively mature individuals of M. sinica are usually tall trees. The tree in Zhongliang village was chosen as the experimental subject because of its convenience and easy access, and a 17 m high stand was built around it for experiments. All observations and experiments on the reproduction of the species were carried out on this tree, in the middle of March when M. sinica began flowering.
The seed dispersal experiment was conducted in November 2014 in a natural habit of M. sinica in Chinese fir seed orchards (label DLS-T), located in Dalishu Township of Maguan County in Wenshan Zhuang-Miao Autonomous Prefecture.
Further seed germination experiments were conducted in November 2014, at three natural localities: DLS-T, Zhongliang village of Jingping County in Honghe Hani-Yi Autonomous Prefecture (label ZL-V), and Miechang Township of Maguan County in Wenshan Zhuang-Miao Autonomous Prefecture (two plots, label MC-T-1 and MC-T-2).
1.2. Pollen viability and stigma receptivityThe examination of pollen viability and stigma receptivity was conducted on three flowers per day over five consecutive days at full-bloom stage in both 2014 and 2015.
Pollen viability was examined using the MTT method (Dafni, 1992). After pollen has been mixed with MTT solution and left for 10 min, viable pollen will turn dark purple in color whereas inviable pollen will turn tawny-yellow or remain unchanged. Pollen was taken from anthers at different flowering stages and mixed evenly with 1‰ MTT on two slides. The numbers of viable and inviable pollen grains were counted over three separate views per slide, using a light microscope.
Stigma receptivity was examined by the benzidine-hydrogen peroxide method (Dafni, 1992). After soaking in a benzidinehydrogen peroxide solution, a receptive stigma will show peroxidase activity and turn blue with a mass of bubbles surrounding it. The depth of the blue color indicates the intensity of receptivity. At different flowering stages, two stigmas per flower were picked and assessed for mucus secretion. They were then soaked in the benzidine-hydrogen peroxide solution. The changes of color and occurrences of bubbles were observed by eye.
1.3. Flowering dynamicsBased on primary observations, flower buds that are about to open can easily be recognized by the softness of their bud tips and their stronger fragrance, and some mature flower buds will open simultaneously at the same time every dusk over the flowering duration of the tree. In every flowering-season over two consecutive years, five flowers were labeled and their flowering stages were observed continuously until the tepals wilted.
1.4. Observation of floral visitorsObservations of floral visitors were carried out from 8:00 AM to 22:00 PM over four consecutive days, spreading across two flowering seasons: March 2014 and April 2015. During observations, five or six flowers were randomly labeled per day. Visitors to these flowers were caught using tweezers and kept in 70% ethyl alcohol for subsequent identification in the laboratory.
1.5. Breeding systemTo evaluate the breeding system of M. sinica, flower buds that would definitely blossom at dusk were selected randomly, marked and caged with sulfate paper bags (30 × 25 cm) every day in the morning at the full-bloom stage in both 2014 and 2015. Five pollination treatments were assigned at the moment when the flowers started to open. Autonomous self-pollination (label SPON, n=12) was tested by bagging flowers to exclude visitors. Selfcompatibility was tested by bagging emasculated flowers and transferring their own pollen by hand (label SELF-S, n=22) and bagging emasculated flowers and being hand-pollinated with pollen from other flowers at the same tree (label SELF-D, n=62). To test for xenogamy (label CROSS, n=47), flowers were treated similarly but were hand-pollinated with pollen from other trees. Control flowers were unmanipulated (label OPEN, n=150).
The marked flowers were harvested in early November, when fruits were collected to assess fruit and seed set.
The self-compatibility index (SCI) was used to determine the breeding system of M. sinica and was obtained as mean percentage fruit set from hand self-pollination over that from hand crosspollination. Species with SCI ratios≤0.2 are considered to be selfincompatible, otherwise, they are considered to be selfcompatible (Jhumur et al., 2008).
1.6. Seed dispersal and seed germination in the wildField trials were conducted to determine the seed-dispersal strategy of M. sinica. A total of 900 seeds were assigned to the following six treatments (five replicates of 30 seeds each): seeds with red arils were distributed: (a) on a dehiscent fruit shell which was bound above the ground on a branch, (b) in a circle (diameter, ca.1 m) on the ground. Seeds with the arils removed, and half seeds with red arils and half seeds with the aril removed were arranged the same ways. In the treatments where seeds were placed on the ground, every seed was labeled with a numbered plastic tag (1 cm × 3 cm) attached by a thin stainless-steel wire (diameter, ca. 0.2 mm) 10 cm long, similar to the procedures reported by Xiao et al. (2006). This seed-tagging method allowed us to follow the exact fate of the seed over time. The seed-carrying behavior of rodents and birds was monitored continuously by infrared cameras. During seven consecutive days, numbers of seeds remaining were recorded, and, during each visit, seeds that had been removed were retrieved from around seeds stations (diameter of searching area, ca. 10e15 m) at dusk.
To test the effects of removing red arils from seeds on germination rates, germination assays were performed on a total of 800 seeds, 400 seeds of which the red arils were removed. A total of 100 seeds with red arils and 100 seeds with arils removed were paired and sowed in ZL-V. Other seeds were arranged in the same way in MC-T-1, MC-T-2 and DLS-T. All seeds were buried in a circle (diameter, ca. 1 m) under the surface soil at a depth of 6 cm. Conditions of seed germination were recorded, and the germination rates of seeds with red arils and seeds with arils removed were compared in 2015.
2. Results 2.1. Flowering processTwo consecutive years of observation of M. sinica at the experimental site showed that flowering started around mid-March and lasted until the middle of April. The peak flowering time was from late March until early April. The terminal, bisexual flowers opened and closed in a two-day rhythm and the flowering period for a single flower was three to four days. Based on data gathered from pollen viability and stigma receptivity experiments described below, five different flowering stages were distinguished to clarify floral processes and pollination.
Before a flower opened, several whorls of green bracts detached, and, after the last whorl of a red bract detached, the pink-purple tepals were exposed. The pre-pistillate stage commenced in the morning (ca. 8:00 AM) and ended at dusk (ca. 18:30 PM) (Fig. 1A). During this stage, the flower bud became soft and emitted a strong fragrance, and this was the indication that the tepals would open soon and the anthesis of this flower would be initiated. The pistillate stage commenced at ca. 18:30 to 19:00 PM and continued until ca. 20:30 PM. The tepals started to separate and moved outwards to completely open within 10e15 min (Fig. 1B-C), revealing the gynoecium. The green stigmas were brilliant with viscous, nonodorous exudates, and the anthers were closed tightly at the basal part of the torus. Beetle visitors were found present and active in the flower. Tepals of the flower in the pistillate stage started closing approximately 1 h later (ca. 20:10 PM) within 15e20 min (Fig. 1D), indicating that the pistillate stage ended and the pre-staminate stage initiated. The pre-staminate stage continued throughout the whole night until ca. 12: 00 AM of the next day, when the anthers started dehiscencing introrsely. The re-closed flower in this stage could be easily distinguished from the pre-pistillate flower by the extended outer tepals (Fig. 1E). The staminate stage lasted from ca. 12:00 AM to 17:30 PM of the second afternoon. The flower began to re-open approximately 3.5 h (ca. 15:30 PM) after the commencement of the staminate stage and was completely open after 10e15 min (Fig. 1F). Stigmas gradually turned yellowish without obvious exudates. The wilting stage commenced at ca. 17:30 PM and lasted for 1e2 days until the stigmas turned black and wilted, and anthers and tepals gradually detached from the receptacle.

|
| Fig. 1 Flowering process, insects visitors and fruits of M. sinica. (A) A bud tip at the pre-pistillate stage; (B) e (C) Tepals separating and moving outwords to a fully open at the pistillate stage; (D) Tepals re-closing at the end of the pistillate stage; (E) Tepals except for the outer ones remaining closed during the pre-staminate stage; (F) The tepals completely re-opening at the staminate stage; (G) e (H) Beetles entering the open chamber at the pistillate stage. (I) e (J) Beetles leaving the re-opening chamber at the staminate stage; (K) e (L) Beetles (Pleocomidae) visiting the open flower, touching the exudate-secreting stigma; (M) Tepals partly eaten on the inside by visiting beetles. (N) Bees (A. mellifera) visiting a reopening flower during the staminate stage; (O) Young fruit; (P) Mature fruit with red aril on seeds. |
The examination of pollen viability revealed that during the prepistillate stage and the pistillate stage, pollen was immature and inviable. Some pollen began to show viability (42.03±3.98%) the following morning (about 8:30 AM) and the overall pollen viability increased to a peak (93.93±0.71%) 30 min after the anthers dehisced at about 12:00 AM. After that, pollen viability declined to 80% at the beginning of the wilting stage when the anthers started to fall from the flower.
Stigmas receptivity was weak during the pre-pistillate stage, though it increased up until the opening of the flowers at the pistillate stage. Stigma receptivity then declined until the end of staminate stage.
2.3. Observation of flower visitorsVisitors observed during flowering period were unidentified species of beetles belonging to the taxa Pleocomidae, Cetoniidae, Curculionidae, Geotrupidae, Carabidae, Elateridae and Scarabaeidae and bees identified as Apis mellifera (Apidae). Based on observations made over two years, the beetles and bees showed different visitation patterns.
Coleoptera beetles were observed to be relatively abundant between about 18:30 and 20:30 PM. The beetles entered the flowers either from the tip of the tepals or across the base of tepals (Fig. 1G-H) and became trapped inside in the floral chamber when the tepals re-closed tightly in the pre-staminate stage and the staminate stage. Inside the re-closed flowers, beetles continued actively moving and feeding on tepal tissue. Upon the re-opening of the flowers, the beetles were finally released (Fig. 1I-J). During the visitation, only two species of Pleocomidae (Fig. 1K-L) and Curculionidae beetles were observed to enter and leave the chamber carrying pollen grains, suggesting that these two beetles might be effective pollinators, while the role of other species remained unclear. Beetle excrement was sometimes found filling the interior tepals of flowers that had been visited (Fig. 1M), although the stigmas of these flowers suffered no damage. A. mellifera visited only when the flowers re-opened with peak abundance at 15:30-16:30 PM, and with a visiting frequency of 28.3±1.47 h-1. As the bees only occurred at the staminate stage to gather pollen grains, they might not be the effective pollinators. Some flowers had their androecia severely damaged (or detached) as a result of vigorous pollen gathering by A. mellifera (Fig. 1N).
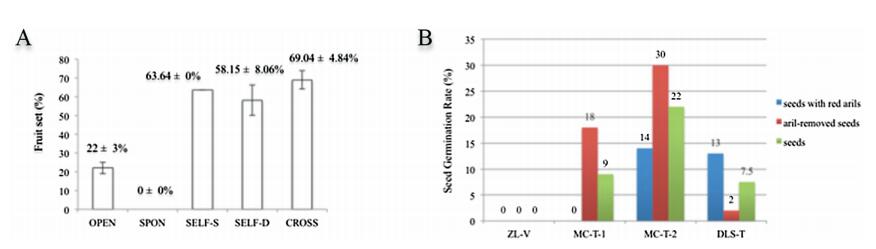
2.4. Breeding systemThe mean percentage fruit set for the five pollination treatments are given in Fig. 2A. The fruit set resulting from natural pollination (OPEN) was 22±3%. Bagging-flowers without emasculation (SPON) yielded no fruits, indicating that autonomous self-pollination was not possible in this species. The hand-pollinated within flower (SELF-S) mean percentage fruit set was 63.64±0.00%, which was marginally higher than that under hand-pollinated within the plant treatments (SELF-D) (58.15±8.06%). The fruit set resulting from outcrossed hand-pollinations was recorded as 69.04±4.84%. ANOVAs manifested differences between hand-pollinations and pollination under controlled conditions were significant (F1, 8=6.47, p=0.003). Nevertheless, fruit set under hand-pollination within the same plant was not significantly different from that under outcrossed hand-pollinations (F1, 6=0.33, p=0.586). The SCI ratio was calculated as 0.86. This showed that M. sinica was mostly self-compatible. The seed set from hand-pollination was 31.77±1.10%, but the seed set from natural pollination was 16.97±0.19%. The difference between the two treatments was significant (F27, 39=8.51, p=0.004).
|
| Fig. 2 Fruit sets and seed germination rates of M. sinica (A) Fruit sets (mean percentage and standard error) with different pollination treatments; (B) Seed germination rates in different plots. |
Our study did not record birds, rodents or other animals taking M. sinica seeds, either with or without red arils, in the wild. This may indicate that the experimental site lacked effective animal populations for M. sinica seed dispersal, or that frequencies of animal vector visitation were rather low.
2.6. Seed germinationThe germination rates of the seeds with red arils and seeds with arils removed as well as the seed germination rates in ZL-V, MC-T-1, MC-T-2 and DLS-T are shown in Fig. 2B. Because of inappropriate sowing-plot (a path to the farmland) in ZL-V, the seed germination rate here was zero and was excluded from the following analysis. In addition, the differences in the seed germination rates between MC-T-1, MC-T-2 and DLS-T were small in ANOVAs (F2, 3=1.09, p=0.441). The germination rate of seeds with red arils sowed in MC-T-1, MC-T-2 and DLS-T was calculated as 9±2.13%, much lower than that of seeds with the aril removed (16.67±3.82%). However, ANOVAs suggested that the differences in germination rates between seeds with red arils and seeds with arils removed were not significant (F1, 4=0.68, p=0.455). Thus, red arils did not greatly affect seed germination in this species.
3. DiscussionBased on the relatively high SCI and fruit sets resulting from SELF-S and SELF-D treatments, we concluded that M. sinica was self-compatible. Significant differences in fruit sets between pollinator exclusion (SPON) and control treatment (OPEN) indicated that pollinators were necessary for the reproductive success of M. sinica. In addition, the significant differences between handpollinations and pollination under controlled conditions further suggested that hand-pollinations could increase fruit set and this species was pollinator dependent. Thus, we hypothesized that M. sinica was encountering pollination limitation. Pollination limitation, including pollinator limitation and pollen limitation, which is of great concern for seed production, may occur in small, isolated populations (Huang and Guo, 2002). Using hand-pollination, the quantity and quality of pollen received have been shown to be important factors affecting the seed production in certain species (Zimmerman and Pyke, 1988; Johnston, 1991). Limited pollen flow resulting from pollination limitation in the population may decrease the effective population size and reduce progeny fitness (Barrett and Kohn, 1991; Washitani, 1996). Individuals of M. sinica are known to be only sporadically distributed through its small and fragmented range. Pollen resources for pollination might therefore be limited.
M. sinica was found to be protogynous and displayed a two-day rhythm of sexual presentation, with a few hours (approx. 21 h, during which time stigmas were receptive) being of critical importance for successful fruit production. Protogyny is a characteristic of beetle-pollinated plants (Bertin and Newman, 1993; Momose et al., 1998; Azuma et al., 1999) and many species in the Magnoliaceae are protogynous and beetle-pollinated (Dieringer and Espinosa, 1994; Gottsberger et al., 2012; Dieringer et al., 2015). Furthermore, for the eight species of temperate Magnolia studied by Thien (1974), flowers frequently exhibited diurnal petal movements over 2 d of anthesis, and were visited by several taxa of Coleoptera. However, compared to these species, M. sinica appeared to possess a much shorter opening period (1e1.5 h) for nocturnal beetle visits. In this system, the re-closed tepals formed wide floral chambers and the plants forming such chambers are mostly beetlepollinated (Dieringer et al., 2015). The floral chambers have been suggested to induce long beetle visits by providing shelter, food and sometimes potential mating places (Dieringer et al., 2015). The beetles may stay inside for a longer period to be protected from predation and to some extent from adverse ambient conditions, thus increasing potential pollination for such species. We found that the tepals re-opened and exposed dehiscent anthers on the second afternoon, releasing trapped beetles. Two species of Pleocomidae and Curculionidae beetles were observed to enter and leave the chamber carrying pollen grains, indicating that M. sinica was certainly beetle-pollinated and the two species of Pleocomidae and Curculionidae beetles were effective pollinators of M. sinica.
Mature fruits of M. sinica finally split into an irregular star-like structure, exposing the seeds, which have red fleshy arils. (Fig. 1OeP). This indicates that the red color may act as an attractant to potential dispersal by birds (Duan et al., 2014). However, our field experiments did not observe animals, nor consequently seed dispersers in the typically fragmented habitats of M. sinica. Anthropogenic factors can have cascading effects on seed dispersal (Hamann and Curio, 1999; Meehan et al., 2002; Oppel, 2010). Habitat fragmentation may reduce the abundance of a suite of frugivorous fauna, which in turn reduces seed dispersal of the plant species (Moran et al., 2009). In modern times, as has occurred in Southeast Asia (Bennett et al., 2000; Corlett, 2007; Harrison, 2011), increased human population and a switch to modern technology, for example firearms and nylon mist nets, have lead to increased hunting pressure and the consequent extinction of many species (Zhang et al., 2014).
Natural habitats for M. sinica have been greatly altered by reforestation of C. lanceolata. Also, since the ornamental value of M. sinica was recognized in the early 1980s, local villagers have been collecting its seeds every fruit season, which they then sell at a high price in the market. Our field investigations found that at least some 10, 000 cultivated seedlings and saplings have been cultivated in nurseries, which may contribute to ex situ conservation of germplasm resources of M. sinica. But the massive collection of seeds will also aggravate the lack of seeds dispersing in the wild and make it more difficult for population regeneration.
Therefore, conservation strategies for this PSESP species should focus on the following aspects. First, in order to diminish pollen limitation, saplings should be recruited into the extant populations to increase the population density and provide more pollen resources. Second, small nature reserves should be established to control the damage to natural habitats of M. sinica and promote the recovery of its original habitats, so that it can enhance the pollinator/disperser assemblage. Though the seeds of M. sinica can germinate in the wild, our investigations rarely found seedlings. Thus, seedling morphogenesis may be another limitation to the recruitment of M. sinica and should also be given attention to in the future.
4. AcknowledgementsThis study was supported financially by the NSFC-Yunnan joint fund to support key projects to W.B. Sun (Grant no. U1302262) and the Young Academic and Technical Leader Raising Foundation of Yunnan Province to G. Chen (2015HB091). The authors thank Guoyun Li, Zhiyong Yu and appreciate the cooperation of the Honghe and Wenshan forestry authorities. The first author wishes particularly to thank Rongli Liao and Bin Wang for helping carry out the experiments and Lingzeng Meng for the identification of the insects.
Azuma H., Thien L.B., Kawano S., et al, 1999. Floral scents, leaf volatiles and thermogenic flowers in Magnoliaceae. Plant Species Biol, 14: 121-127. DOI:10.1046/j.1442-1984.1999.00015.x |
Barrett, S.C.H., Kohn, J.R., 1991. The genetic and evolutionary consequences of small population size in plants: implications for conservation. In: Falk, D.,Holsinger, K.E. (Eds.), Genetics and Conservation of Rare Plants. Oxford University Press, Oxford, pp. 3-30.
|
Bennett E.L., Nyaoi A.J., Sompud J., 2000. Saving Borneo's bacon: the sustainability of hunting is Sarawak and Sabah. In: Robinson J.G., Bennett, E.L. (Eds.), Hunting for Sustainability in Tropical Forests. New York: Columbia University Press: 305-324.
|
Bosch M., Waser N.M., 1999. Effects of local density on pollination and reproduction in Delphinium nuttallianum and Aconitum columbianum (Ranunculaceae). Am. J. Bot, 86: 871-879. DOI:10.2307/2656707 |
Brown E., Kephart S., 1999. Variability in pollen load: implication for reproduction and seedling vigor in a rare plant, Silene douglasii var. oraria. Int. J. Plant Sci, 160: 1145-1152. DOI:10.1086/314198 |
Bertin R.I., Newman C.M., 1993. Dichogamy in angiosperms. Bot. Rev, 59: 113-137. |
Chen B.L., Nooteboom H.P., 1993. Notes on Magnoliaceae III: the Magnoliaceae of China. Ann. Mo. Bot. Gard, 80: 999-1104. DOI:10.2307/2399942 |
Cicuzza D., Newton A., Oldfield S., 2007. The Red List of Magnoliaceae. Cambridge: Lavenham Press.
|
Corlett R.T., 2007. The impact of hunting on the mammalian fauna of tropical Asian forest. Biotropica, 39: 392-303. |
Dafni A., 1992. Pollination Ecology. New York: Oxford Unit Press.
|
Dieringer G., Espinosa S.J.E., 1994. Reproductive ecology of Magnolia schiedeana (Magnoliaceae), a threatened cloud forest tree species in Veracruz, Mexico. Torrey Bot. Soc, 121: 154-159. DOI:10.2307/2997167 |
Dieringer G., Cabrera R.L., Lara M., et al, 2015. Beetle pollination and floral thermogenicity in Magnolia tamaulipana (Magnoliaceae). Int. J. Plant Sci, 160: 64-71. |
Duan Q., Goodale E., Quan R., 2014. Bird fruit preferences match the frequency of fruit colours in tropical Asia. Sci. Rep, 4: 1-8. |
Evans M.E.K., Menges E.S., Gordon D.R., 2004. Mating system and limits to seed production in two Dicerandra mints endemic to Florida scrub. Biodivers. Conserv, 13: 1819-1832. DOI:10.1023/B:BIOC.0000035869.12388.0f |
Gong W.C., Chen G., Vereecken N.J., et al, 2014. Floral scent composition predicts bee pollination system in five butterfly bush (Buddleja, Scrophulariaceae) species. Plant Biol, 17: 245-255. |
Gottsberger G., Gottsberger I.S., Seymourb R.S., et al, 2012. Pollination ecology of Magnolia ovata may explain the overall large flower size of the genus. Flora, 207: 107-118. DOI:10.1016/j.flora.2011.11.003 |
Hamann A., Curio E., 1999. Interactions among frugivores and fleshy fruit trees in a Philippine submontane rain forest. Conserv. Biol, 13: 766-773. DOI:10.1046/j.1523-1739.1999.97420.x |
Harrison R.D., 2011. Emptying the Forest: hunting and the extirpation of wildlife from tropical nature reserves. Bioscience, 61: 919-924. DOI:10.1525/bio.2011.61.11.11 |
Huang S.Q., Guo Y.H., 2002. Variation of pollination and resource limitation in a low seed-set tree, Liriodendron chinense (Magnoliaceae). Bot. J. Linn. Soc, 140: 31-38. DOI:10.1046/j.1095-8339.2002.00080.x |
Jhumur U.S., Dötterl S., Jürgens A., 2008. Floral odors of Silene otites: their variability and attractiveness to mosquitoes. J. Chem. Ecol, 34: 14-25. DOI:10.1007/s10886-007-9392-0 |
Johnston M.O., 1991. Pollen limitation of female reproduction in Lobelia cardinalis and L. siphilitica. Ecology, 72: 1500-1503. DOI:10.2307/1941123 |
Larson B.M.H., Barrett S.C.H., 2000. A comparative analysis of pollen limitation in flowering plants. Biol. J. Linn. Soc, 69: 503-520. DOI:10.1111/j.1095-8312.2000.tb01221.x |
Law Y.W., 1979. A new genus of Magnoliaceae from China. Acta Phytotaxon. Sin, 17: 72-74. |
Ma Y.P., Chen G., Grumbine R.E., et al, 2013. Conservation plant species with extremely small populations (PSESP) in China. Biodivers. Conserv, 22: 803-809. DOI:10.1007/s10531-013-0434-3 |
Meehan H.J., Mcconkey K.R., Drake D.R., 2002. Potential disruptions to seed dispersal mutualisms in Tonga, Western Polynesia. J. Biogeogr, 29: 695-712. DOI:10.1046/j.1365-2699.2002.00718.x |
Momose K., Yumoto T., Nagamitsu T., et al, 1998. Pollination biology in a lowland dipterocarp forest in Sarawak, Malaysia. I. Characteristics of the plant-pollinator community in a lowland dipterocarp forest. Am. J. Bot, 85: 1477-1501. |
Moran C., Catterall C.P., Kanowski J., 2009. Reduced dispersal of native plant species as a consequence of the reduced abundance of frugivore species in fragmented rain forest. Biol. Conserv, 142: 541-552. DOI:10.1016/j.biocon.2008.11.006 |
Oppel S., 2010. Bird assemblage and visitation pattern at fruiting Elmerrillia tsiampaca (Magnoliaceae) trees in Papua New Guinea. Biothopica, 42: 229-235. |
Pandit M.K., Babu C.R., 2003. The effects of loss of sex in clonal populations of an endangered perennial Coptis teeta (Ranunculaceae). Bot. J. Linn. Soc, 143: 47-54. DOI:10.1046/j.1095-8339.2003.00192.x |
Pavlik B.M., Ferguson N., Nelson M., 1993. Assessing limitations on the growth of endangered plant populations. Biol. Conserv, 65: 257-265. DOI:10.1016/0006-3207(93)90058-9 |
Ren H., Zhang Q.M., Lu H.F., et al, 2012. Wild plant species with extremely small populations require conservation and reintroduction in China. Ambio, 41: 913-917. DOI:10.1007/s13280-012-0284-3 |
Rivers M., Beech E., Murphy L., et al, 2016. The Red List of Magnoliaceae (Revised and Extended). Lavenham Press Cambridge. Spira, T.P.. 2001. Plant-pollinator interactions: a threatened mutualism with implications for the ecology and management of rare plants. Nat. Areas J, 21: 78-88. |
State Forestry Administration of China, 2012. The Saving and Conservation Program on Extremely Small Populations in China. Unpublished Report. State Forestry Administration of China, China.
|
Sun W.B., Zhou Y., Li X.Y., et al, 2012. Population Reinforcing Program for Magnolia sinica, a Critically Endangered Endemic Tree in Southeast Yunnan Province, China. Washington DC: Island Press: 65-69.
|
Sun, W.B., 2013. Conserving Plant Species with Extremely Small Populations (PSESP) in Yunnan: practice and exploration. Yunnan Science and Technology Press, Kunming, Yunnan.
|
Thien L.B., 1974. Floral biology of Magnolia. Am. J. Bot, 61: 1037-1045. DOI:10.2307/2441921 |
Volis S., 2016. How to conserve threatened Chinese plant species with extremely small populations. Plant Divers, 1: 53-62. |
Wang, B., Ma, Y.P., Chen, G., et al., 2016. Rescuing Magnolia sinica (Magnoliaceae), a Critically Endangered Species Endemic to Yunnan, China. Fauna & Flora International, pp. 1-4.
|
Washitani I., 1996. Predicted genetic consequences of strong fertility selection due to pollinator loss in an isolated population of Primula sieboldii. Conserv. Biol, 10: 59-64. DOI:10.1046/j.1523-1739.1996.10010059.x |
Xia, N.H., Liu, Y.h., Nooteboom, H.P., 2008. Magnoliaceae. In: Flora of China. Beijing :Science Press. St. Louis: China & Missouri Botanical Garden Press, pp. 48-91.
|
Xiao D.X., Xu F.X., 2006. Megasporogenesis and development of female gametophyte in Manglietia decidua (Magnoliaceae). Ann. Bot. Fenn, 43: 437-444. |
Zhang K., Woan T.S., Li J., et al, 2014. Shifting baselines on a tropical forest frontier: extirpations drive declines in local ecological knowledge. PLoS One, 9: 1-8. |
Zhao X.F., Sun W.B., 2009. Abnormalities in sexual development and pollinator limitation in Michelia coriacea (Magnoliaceae), a critically endangered endemic to Southeast Yunnan, China. Flora, 204: 463-470. DOI:10.1016/j.flora.2008.07.001 |
Zimmerman M., Pyke G.H., 1988. Reproduction in Polemonium: assessing the factors limiting seed set. Am. Nat, 131: 723-738. DOI:10.1086/284815 |



