b. The Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201, Yunnan, PR China;
c. Kunming Botanical Garden, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201, Yunnan, PR Chinaa
China is one of the richest countries in the world in terms of plant diversity. Third only to Brazil and Columbia, China harbours over 30, 000 plant species (Yang et al., 2005). However, at least 200 plant species have become extinct in the last 50 years and c.5000 more are currently threatened or on the brink of extinction, making China one of the highest priorities for global biodiversity conservation (Volis, 2016).
Among this rich diversity of plants in China, there are 120 wild plant species that were identified in 2012 as the first group for urgent protection nationally. These species have the following features: 1) estimated to have < 5000 mature individuals in the wild; 2) distribution restricted to a limited range with a few locations; 3) recognition as national or regional endemic species of China; and 4) potential for economic development or scientific value. Reflecting these attributes, a descriptor is now used for this cluster of species: 'Plant Species with Extremely Small Populations (PSESP)' (Ma et al., 2013; Sun, 2013). As a result of a new policy framework, several national and regional-level conservation strategies and actions for conserving China's PSESP are being implemented. Such an approach is particularly important as a recent spatial distribution analysis of 33 species from the 120 PSESP list revealed that only 12 of these are considered to be well protected in the National Nature Reserves (Wang et al., 2016a, b). Importantly, significant progress has been made to increase the coverage of threatened species in China's botanic gardens. For example, Xishuangbanna Tropical Botanical Garden, Yunnan has an extensive collection of nationally red-listed species. Nonetheless, only around 60 PSESP (i.e., about half) have been propagated and cultivated exsitu in China's botanic gardens (Sun, We-B., pers comm.).
The global need for botanical gardens to protect threatened species, e.g., trees, in dedicated conservation collections is recognised (Cavender et al., 2015). The broad aim of establishing and managing such living ex situ collections should be to maintain the greatest biodiversity at the greatest economic and logistic efficiency (Cibrian-Jaramillo et al., 2013). Yet the problem of limited genetic diversity of individual species in these collections is well known. Part of the solution is to establish a combined management strategy for the acquisition of living collections among botanic gardens and other organizations interested in plant conservation (Cibrian-Jaramillo et al., 2013). In the case of the cycad Zamia decumbens, collections can better conserve the genetic diversity of in situ populations as long as multiple accession are made over more than one year (Griffith et al., 2015).
Conservation efforts with the world's most threatened species is also hindered by gaps in fundamental biological information, e.g., on trees (Cavender et al., 2015) and on their seed biology (Pritchard et al., 2014). Two biological traits are of particular importance when considering the efficient and effective utilisation of seeds and fern spores in both the ex situ and in situ environment: desiccation tolerance and dormancy/germination. Storing seeds is one of the main means of ex situ conservation and involves drying as thefirst step in the preservation process. Tolerance of drying opens up the opportunity for reduced temperature storage, which is the main means of conserving millions of accessions of plant genetic resources (http://www.fao.org/agriculture/crops/core-themes/theme/seeds-pgr/sow/sow2/en/), and underpins the global market for seeds, which was valued at US$53.76 billion in 2014 (http://www.marketsandmarkets.com/MarketReports/seed-market-126130457.html). Fern spores are a ready source of germplasm to aid re-establishment of waning fern populations (Pennisi, 2010). Since they tolerate high levels of desiccation (Ballesteros, 2010), dry storage of fern spores could provide simple and economical ex situ conservation of genetic diversity in a relatively small space (Pence, 2008; Ballesteros, 2010; Ibars and Estrelles, 2012). Germination timing, which is often strongly dependent on temperature (for dormancy loss and germination speed), is a key stage in species regeneration in situ and in the assessment of seed viability in ex situ collections (Smith et al., 2003; Suo et al., 2015).
Here we review what is known about the seed and fern spore biology (storage and germination) of at-risk species, using China's PSESP as a case study, and highlight some areas of future research that need addressing.
2. Materials and methodsThe list of PSESP in Ma et al. (2013) and Sun (2013) was consulted and the plant name and authority checked against The Plant List (http://www.theplantlist.org/). Data searches were run on the 2008 Seed Information Database (http://data.kew.org/sid/), using the terms 'seed storage' and 'seed germination.' Individual species records from the published literature were summarised when available, particularly to bring the analysis up to date. Otherwise a seed biology perspective is given on either the genus or family basis, including some perceived scientific challenges.
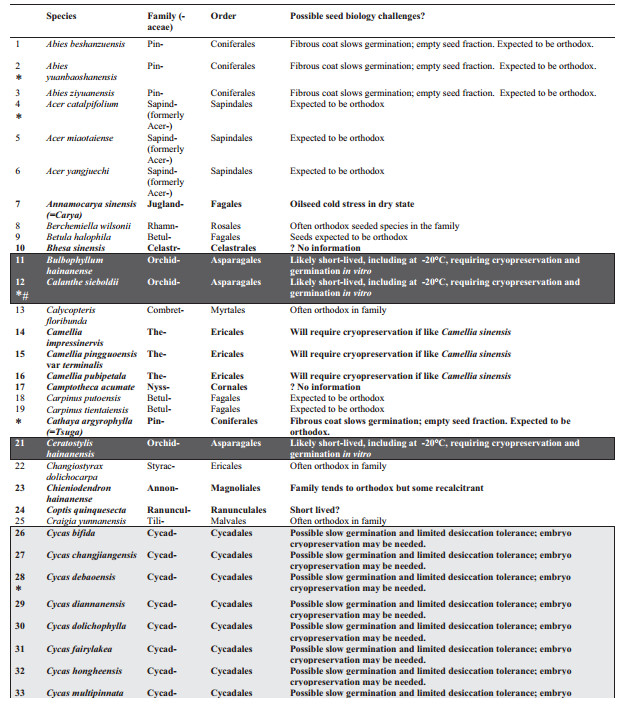
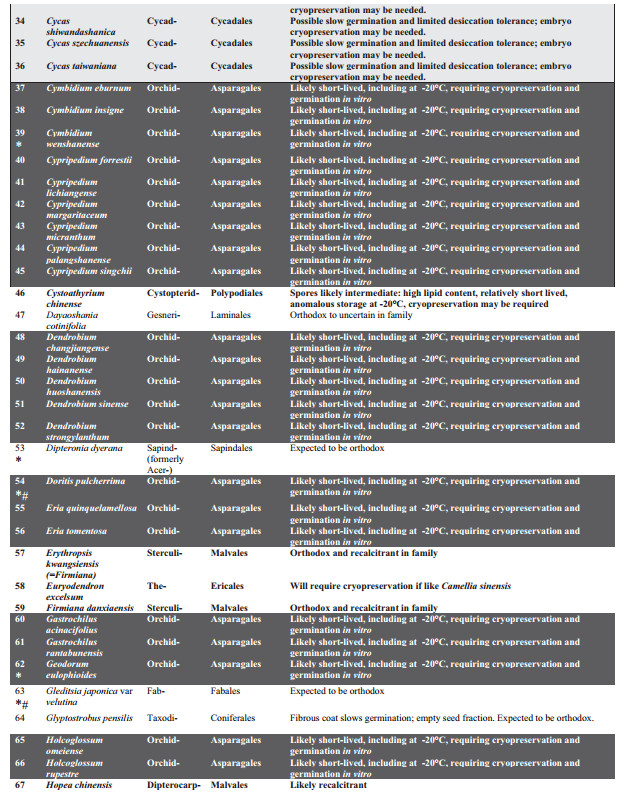
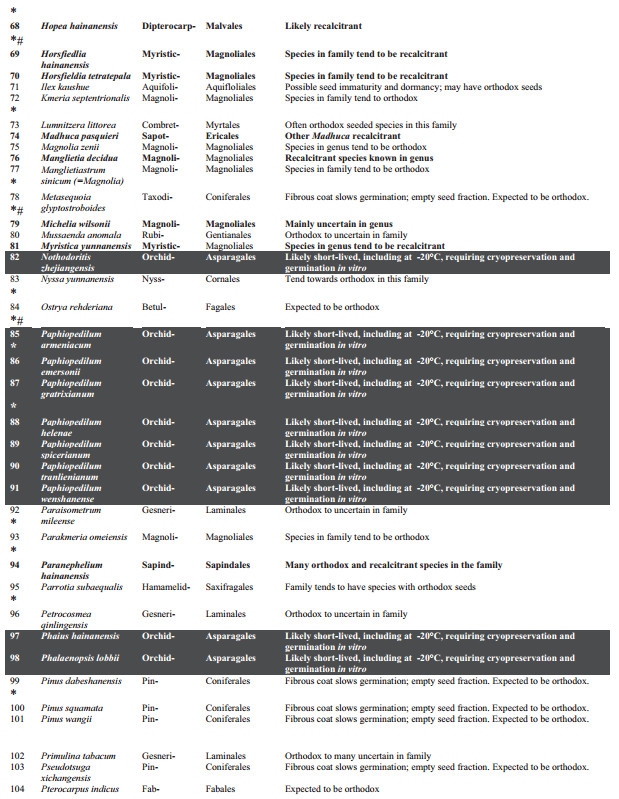
3. Results and discussionThe taxonomic spread of the 120 PSESP extends to 76 genera from 33 families (Table 1) of mainly seed plants. Ferns (sensu lato, i.e. monilophytes) are represented by just one family and species. The species are not evenly spread among seed-bearing families with significant clustering in the Orchidaceae (37 species) and Cycadaceae (11 species). These two over-represented groups contribute 40% of PSESP species and should provide an urgent focus for future seed biology studies.
 |
 |
 |
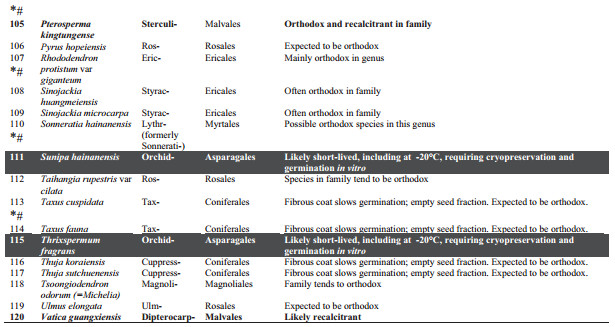 |
Of the 120 PSESP, there is readily available information on seed germination for 28 species (23% of PSESP), but storage characteristics are accessible for only 8% of PSESP (i.e., 10 species), for which germination protocols are also known. These 28 species are profiled below. Our interpretation of the published literature for the listed species, or close relatives, suggests that storage under international gene bank standards (i.e., drying to 15% RH, plus hermetic storage at -20 ℃) may only be realistic for 40% of the PSESP (i.e. 48 species). The other 72 species (60%) may have to be cryopreserved for long-term storage, as spores, seeds or embryonic axes (Li and Pritchard, 2009; Walters et al., 2013).
3.1. Ferns in PSESPThere is only one fern species in PSESP for China, Cystoathyrium chinense Ching. This species was believed to be extinct in the wild but was recently rediscovered (Wei and Zhang, 2014). It is a critically endangered species endemic to China with no more than 40 extant individuals, closely related to North American taxa of the genus Cystopteris (Wei and Zhang, 2014).
To our knowledge there is no published information on the spore biology of C. chinense. However, fern spore storage and germination have been well studied (reviewed in Ballesteros, 2010; Suo et al., 2015) including related species, such as Cystopteris fragilis (e.g. Ballesteros et al., 2012). In terms of storage and conservation, spores of C. fragilis died at room conditions within 3 years, but initial germination percentage and rate was preserved after cryogenic storage at -80 and -196 ℃. In general, fern spores appear to be tolerant to desiccation, showing increased longevity as storage temperature decreases (e.g. Quintanilla et al., 2002; Aragón and Pangua, 2004; Li et al., 2010; Ballesteros et al., 2011, 2012; Li and Shi, 2014). However faster than expected ageing of fern spores from some species at temperatures between -10 ℃ and -30 ℃ (Ballesteros, 2010, and references therein; Li et al., 2010; Ballesteros et al., 2012; Li and Shi, 2014) have led to the suggestion that fern spores may have a storage physiology similar to intermediate seeds (Pence, 2008; Ballesteros, 2010). Cryopreservation is feasible for many fern spores and may be necessary to maximize spore longevity for long-term ex situ conservation (Pence, 2008; Ballesteros, 2010; Li et al., 2010; Ballesteros et al., 2011, 2012; Li and Shi, 2014; Mikula et al., 2015).
Most fern spores germinate on diverse mineral culture medium at temperatures between 20 and 25 ℃, however optimal light intensity and illumination time for spore germination are different among species (Suo et al., 2015). For C. fragilis spores, germination is successful in mineral culture medium, at 20 ℃ with a 12 h photoperiod (common fluorescent tubes, photon irradiance 25-50 μmol m-2 s-1 in the 400-700 nm region). Additionally, some spores may require specific metal ions, pH, or other specificities during the in vitro culture.
3.2. Over-represented groups in PSESPThere are two over-represented groups in PSESP for China constituting 40% of the species: cycads and orchids.
3.3. CycadsOf the 26 gymnosperms (22% of species) listed, 11 species (9%) are from a single genus, Cycas, in the family Cycadaceae. Globally cycads (Cycadaceae, Stangeriaceae, Zamiaceae) are the most threatened group of plant species (Donaldson, 2003), with 196 out of 339 taxa (58%) recently listed by IUCN (IUCN, 2015). Cycads are also included in Appendix Ⅰ or Ⅱ of CITES (Rutherford et al., 2013).
There appears to be no published information on the seed biology of the 11 Cycas species on the China PSESP list, other than Jian et al. (2006) noting that all five populations of Cycas fairylakea 'have an opposite pyramid age structure, few coning plants, little seed production, and a low level of seed germination rate or ste rility.' Across the genus as a whole, i.e., just over 100 species, there is seed biology information for only four species. On the basis of the seeds tolerating sun-drying (Langkamp and Plaisted, 1987), Cycas angulata R.Br., Cycas armstrongii Miq. and Cycas media R.Br. are proposed to be orthodox for desiccation tolerance (http://data.kew.org/sid/). In contrast, the storage response of large seeds (8.2 g per seed) of Cycas revoluta Thunb is described as being uncertain. The seed is not likely to be recalcitrant (i.e., highly desiccation sensitive) as Dehgan and Schutzman (1989) found that about half the seeds lost germinability when stored open at 22 ℃, compared to >90% after 6 months air-dry storage at 2 ℃. Our recent assessment indicated that commercially-available C. revoluta seeds showed some desiccation tolerance with viability being lost around 15% moisture content, and opportunities for cryopreserving the isolated embryo is now being considered (Nadarajan, J., Pritchard, H.W., pers comm).
Seeds of most cycads require several months to germinate (Dehgan and Schutzman, 1989). There are at least three features that could contribute to delayed germination: a fleshy sarcotesta; a thick and hard sclerotesta; and, in some species, an immature embryo at the time of seed abscission. Again for the 'model' cycad, C. revoluta, germination has been stimulated by mechanical removal of the sarcotesta, scarification of the sclerotesta with H2SO4 and seed treatment with GA3 as a means of reducing any physiological dormancy has also been attempted with varying success (Dehgan and Schutzman, 1989).
Although studies mainly on C. revoluta are informing future options for the handling of cycad seed and pollen, there is clearly an requirement for investigations on the seed storage and germination biology of PSESP cycads and other species in this important group of ancient plants.
3.4. OrchidsSeed storage can be challenging for some species of orchids. For the >90 orchid species' (and/or hybrids) seed storage responses studied so far, c. 69%, 9% and 22% have been assigned, respectively, as orthodox, uncertain and intermediate, i.e., partially desiccation tolerant, but with sub-zero temperature sensitivity that appears to be species-specific (so-called Type Ⅱ species (Pritchard, 2004)). Thus the challenge is not so much in relation to tolerance of drying (there are no known recalcitrant seeded orchids, to date) but with regards to storage lifespan. Since the first comparative assessments of dry seed storage noted that orchid seeds often have lifespans around 10% of the longest lived seeds under the same conditions (Pritchard et al., 1999; Pritchard and Dickie, 2003), confirmatory results have been presented on another >10 species in the family (Hay et al., 2010; Seaton et al., 2013). Overall, these studies indicate that about a third of the tested orchid species have seeds that undergo precipitous loses in germinability over relatively short times in dry, cold storage, a response that might relate to the physical transformation of the seed lipids (e.g. Cattleya aurantiaca; Pritchard and Seaton, 1993). However, cryopreservation of mature orchid seeds has been successful for a wide range of terrestrial and tropical species; 36 and 21 species respectively (see a listing for Popova et al., 2016). Such studies are limited usually to storage times of≤2 years. Only longer-term studies at a range of seed moisture contents and storage temperatures will provide an adequate prospect for species ex situ conservation in this important and extremely diverse family (>25, 000 species).
As the seeds are extremely small (dust-like and often weighing c. 3 mg), orchid seeds in nature rely on a symbiotic host to provide an external source of carbon for the completion of germination, protocorm formation and seedling growth (Arditti, 1992). This nutrient requirement can be replicated in vitro on asymbiotic media, with optimal composition varying with species. Readers are referred to Arditti (1984) as a starting point.
3.5. Species seed biology profiles1) Abies yuanbaoshanensis Y.J.Lu & L.K.Fu (Pinaceae)
The natural regeneration ability of this species is poor, with variable cone production per plant, number of seeds per fruit, and seed quality (Tang et al., 2001). Seeds absorb water readily and full seeds germinate better in the incubator (19%) than in the nursery/field garden (7%). Such low seed germination may make any assisted expansion of the population difficult and hinder natural regeneration. Seed storage information is not available.
2) Acer catalpifolium Rehder (Sapindaceae)
Seeds collected from two locations vary in seed mass from an average of 19-28 mg (Yu et al., 2008). Seeds readily absorb water (imbibe), and can germinate at 25 ℃ to 42-49%, depending on location. Germination is equally good in the light or the dark. A seed storage assessment is needed on this species, as variable levels of desiccation tolerance is known in species of this genus.
3) Berchemiella wilsonii (C.K.Schneid.) Nakai var pubipetiolata H. Qian (Rhamnaceae)
Seed quality in this species can be low, based on only 32% of seeds becoming red after tetrazolium chloride (viability) staining (Dang et al., 2005). Forty percent of full seeds did not stain and the rest (28%) were found to be empty. Treatment with 98% sulphuric acid increases the rate and total uptake of water, indicating some barrier to permeability. Germination inhibitors are present in the embryo and the pericarp, and the highest germination (14%) is achieved by soaking the seeds for 24 h in 300 mg-1 GA3 before sowing for germination. A seed storage assessment is yet to be made.
4) Calanthe sieboldii Decne. ex Regel (Orchidaceae)
For storage, information on this species is limited to the hybrid Calanthe discolor × sieboldii and it seems likely that this will not affect greatly the interpretation. The seed storage behaviour is thought to be intermediate, meaning that the seeds tolerate at least air-drying, but then store poorly at conventional seed bank temperature (i.e., -20 ℃). This was the case for C. discolor × sieboldii seed as air-dry storage over a one year period at both 20 ℃ and -15 to -17 ℃ resulted in a germination decrease from 72% to c. 20%; non-dried seed failed to germinate after about 8 months storage (Hasegawa et al., 1978).
Immature seeds of C. sieboldii from unripe capsules at 80 days post pollination (comparedto 120DPP for fullmaturity) can begerminated on sterilized media (Park et al., 2000). Capsules can be surfacesterilized in 2% sodium hypochlorite for 20 min, washed several times with sterile, distilled water, and the capsules cut open and the seeds removed. The most appropriate medium for germination is modified Murashige and Skoog (MSH) with seed encapsulated in calcium alginate beads before incubation in the dark, resulting in 18% germination and protocorm formation in 8 weeks. Adding 1 mg l-1 putrescine to the medium increases germination and protocorm formation in the dark to c. 44%. Supplementing MSH medium with 25 mg l-1 adenine sulphate increases germination and protocorm formation to 66%. Generally, germination was lower in the light (16 h photoperiod) than the dark (Park et al., 2000). However, for seeds of C. discolor × sieboldii isolated from capsules nearly 6 months after pollination, germination on a Hyponex-Bactopeptone medium reaches 72% at 25 ℃ on a 16 h photoperiod (Hasegawa et al., 1978). The physiological needs for in vitro germination rates for terrestrial orchids varyamong species and response levels can be low, possibly as a result of seed dormancy (Rasmussen, 1996).
5) Cathaya argyrophylla Chun & Kuang (Pinaceae)
The mean seed weight is 19.7 mg, but varies from 12 to 28 mg with mother trees (Xie and Li, 2000). Seeds stained with tetrazolium chloride indicate 50% viability (Cao et al., 2010), with about 16% empty seed fraction (Xie and Li, 2000). But to achieve this level of germination, seeds stored moist at 4 ℃ for 30 days require soaking for 24 h in 100 mg-1 of NAA or IAA, yielding 55% germination. Moist storage at 4 ℃ over a longer period of 90 days results in decreasing germination, accompanied by a fall in enzyme activity, e.g. superoxide dismutase (SOD). Nonetheless, refrigerated storage of moisture seed has been recommended for these seeds. The tolerance of the seeds to drying is not clear, although seeds at 11% moisture content retain 50% viability.
6) Cycas debaoensis Y.C.Zhong & C.J.Chen (Cycadaceae)
Seeds of C. debaoensis can germinate between temperatures of 20 and 35 ℃, with 87-93% germinating at 25-30 ℃ (Wang et al., 2014). Peeled seeds have a higher germination potential, and overall the germination rate is quick, suggesting that germination factors are not the main cause of endangerment in this species. Seed storage information appears not to be available.
7) Cymbidium wenshanense YS Wu & FY Liu (Orchidaceae)
The in vitro germination of young seed of this orchid is low, but prominently higher when seed is harvested from 6 month old capsules (Ding et al., 2005). Although seeds can germinate on a basic nutrient medium, adding coconut milk stimulates germination. Plantlets development is relatively slow, taking 7 months from seed sowing. A seed storage assessment is yet to be made.
8) Dipteronia dyeriana Henry (Sapindaceae)
With the testa removed, germination at 6 ℃ reaches 98% in 5 months, perhaps indicative of the presence of some physiological dormancy. Seeds sown in the wild germinate to 17% after the first winter, increasing to 58% after two winters and one summer when kept in pots. Warmer (15-30 ℃) temperatures result in no germination and there is no light requirement for germination. Based on tetrazolium chloride (vital) staining, seed tolerates storage at 4 ℃ for 510 days with 96% vitality (Ouyang et al., 2006), but a full assessment of seed storage response is needed.
9) Doritis pulcherrima Lindl. (Orchidaceae)
The seed storage behaviour for this species is thought to be intermediate, as < 20% germination was observed after 2 months hermetic storage at -10 ℃ (Bowling and Thompson, 1972). The seeds of 3-month-old pods can, however, tolerate cryopreservation by plunging into liquid nitrogen (Thammasiri, 2000). After chemical dehydration in plant vitrification solution 2 (PVS2) at 25 ℃ for 50 min, cryopreservation and rapid warming, 62% of seeds can develop into normal seedlings. Germination is achieved on Vacin and Went medium. Similarly, D. pulcherrima seeds 90 days after pollination containing 95% globular embryos germinate to 90% on asymbiotic medium (Wu et al., 2005). Adding the phytohormones NAA (0.5 mg l-1) greatly improve germination. Glutamine (400 mg l-1) supports protocorm growth and peptone (2000 mg l-1) promotes seedlings formation (Wu et al., 2005).
10) Geodorum eulophioides (Lam.) Schltr. (Orchidaceae)
The optimal cultural medium for seed germination of G. eulophioides is 1.0 g l-1 Hyponex1 + 1 g l-1 Hyponex2 + 1 mg l-1 6-benzylaminopurine + 0.2 mg l-1 zeatin þ100 mg l-1 coconut milk + 20 g l-1 sucrose + 1 g l-1 activated charcoal (Lan et al., 2014). Optimal culture media have also been devised for protocorm proliferation and plantlet rooting, providing a rapid propagation technology for this endangered species. Nothing is known about seed storage in this species.
11) Gleditsia japonica Miq.1 (Fabaceae).
As with the majority of Fabaceae species' seeds, those of G. japonica tolerate drying at 15% RH and 15 ℃ and storage at c. -20 ℃, with survival recorded after at least four years in the Royal Botanic Gardens, Kew, Millennium Seed Bank (http://data.kew.org/sid/). Since the seed coat of dried G. japonica is extremely hard, few animal species are thought able to feed on it. However, observations in Japan have identified two specialist seed predators in the field, which cause physical damage to >99% of the seeds (Takakura, 2002). Indeed, all germinating seeds found in the field contained bean weevil larvae, indicating that the physical damage facilitated germination. Germination at 25 ℃ (16 h photoperiod) can be enabled through artificially damaging the seeds by drilling a hole c. 1 mm in diameter in the seed coats, subsequently resulting in c. 80% germination (Takakura, 2002). Seeds can also be germinated by chipping with a scalpel (scarifying) and sowing on 1% agar-water at 21 ℃ (12 h photoperiod), resulting in 86% germination (http://data.kew.org/sid/). The hard seed coat can also be scarified to enable water uptake, and germination, using hot water (75 ℃) for 10 min or concentrated sulphuric acid for 60 min (Liu, 2012).
12) Hopea chinensis (Merr.) Hand.-Mazz. (Dipterocarpaceae)
The viable seed rain from H. chinensis is 51%; the remaining seeds suffering from necrosis (36%), damage by insect larvae (10%) or immaturity (3%) (Tang et al., 2009). No seed dormancy is evident, germination reaches 94%, with larger seeds germinating better. Nothing is known about seed storage in this species, although other species in the genus tend to be highly desiccation sensitive (recalcitrant).
13) Hopea hainanensis Merr. & Chun (Dipterocarpaceae)
Seeds of this species take about 170 days from anthesis to maturity (Lan et al., 2007), at which point the seed is c. 34% moisture content, has a mean seed weight of 0.97±0.20 g and an initial germination of 97% (Wang et al., 2004). Seed germination is efficient at 15-20 ℃, and does not require light as there is no obvious difference in germination between seeds sown in full darkness and under light (2800 lx, 14 h photoperiod) (Wen et al., 2002). Although viability measurements and electrical conductivity analyses after drying treatment show gradually increasing desiccation tolerance of seeds/embryonic axes from 122 days after anthesis (Lan et al., 2007), these changes are relative and the seed retains a high level of desiccation sensitivity. Seeds held under room conditions in South Yunnan lose germination ability completely within 3 weeks (Wen et al., 2002). Moreover, seeds are inviable on drying to 20% MC (Song et al., 1984, 1986), with a midpoint moisture content for viability loss of 23-25% (Wang et al., 2004). Moist storage of the seeds (fruits) is possible for many months, but is temperature dependent. At best, 80% germination is possible after 1 year's moist storage (35-38% MC) in coconut dust at 18 ℃ (Song et al., 1984, 1986), or about 30% germination after 9 months at 15 ℃ and c. 34% MC (Wang et al., 2004). The period for viability to fall to 50% (P50) is c. 7, 4 and 3 months for the wet storage of seeds (fruits) at 15 ℃, 5 ℃ and 25 ℃, respectively (Wang et al., 2004). Such moist storage lifespan is quite similar to the recalcitrant seeds of other species in the Dipterocarpaceae, e.g., 3-5 months at 16 ℃ for Hopea odorata, Neobalanocarpus heimii, Shorea assamica, Shorea henryana, Shorea leprosula, Shorea macroptera, Shorea roxburghii and Vatica astrotricha (Pritchard et al., 2004).
14) Kmeria septentrionalis Dandy (Magnoliaceae)
The red aril may inhibit seed germination and should be rubbed off before sowing (Lai et al., 2007). Seeds germinate well in sand. Cold storage in wet sand for 130 days results in 59% of seed being able to germinate; but placing seeds at room temperature with dry sand decreases germination to 12% (Wang et al., 2012). A seed storage assessment is yet to be made.
15) Manglietiastrum sinicum Y.W.Law [is a synonym of Magnolia sinica (Y.W.Law) Noot.] (Magnoliaceae)
Seeds of this species are dormant (Zheng and Sun, 2009). Pretreating seeds with 500 mg l-1 GA3 seeds for 24 h results in 66% germination subsequently at 25 ℃ (12 h photoperiod; fluorescent lights providing 25 mmol m2 s-1). Moist chilling at 4 ℃ for 3 weeks is also effective at breaking seed dormancy, resulting in 56% seed germination after 30 days at 25 ℃. A seed storage assessment is needed on this species.
16) Metasequoia glyptostroboides Hu & W.C.Cheng (Cuppressaceae)
The storage behaviour of this species is suggested to be intermediate (http://data.kew.org/sid/), with Gordon (1992) advising maximum storage for c. 3 years at c.10% moisture content and -10 ℃. Xin et al. (2004) stored seeds at -20 ℃ before some germination experiments. The seeds seem quite robust in storage, with viability maintained for 2 years in hermetic storage at room temperature (Campbell, 1980) and for >2 years in hermetic air-dry storage at 3 ℃ (Gordon, 1992). Dirr and Heuser (1987) recommend hermetic air-dry storage at cool temperatures (c. 5 ℃).
Seeds of this species have a thin and fragile coat, and seeds can be of poor quality with over 90% empty seed fraction (Xin et al., 2004). For germination, pre-treatment at c. 4 ℃ for 6 weeks is recommended (Gosling, 2007). Based on a temperature gradient study, the optimum temperature for germination is c. 24 ℃, with good germination between 19 ℃ and 28 ℃ and no germination below 10 ℃ (Xin et al., 2004). Seed germination can be greatly inhibited by light [65 μmol m2 s-1 (12 h photoperiod)], suggesting that the seeds prefer dark conditions (Xin et al., 2004). Also seed mass and germination rate of restored populations can be significantly lower than those in natural populations, indicating decreased regeneration ability in the restored populations, possibly due to inbreeding depression (Li et al., 2012a).
17) Nyssa yunnanensis W.Q.Yin ex H.N.Qin & Phengklai (Nyssaceae)
The optimum seed germination temperature is 25 ℃, yielding 68% germination (Yuan et al., 2013). When the light conditions are changed from continuous to dark, germination is reduced to 20%. Before sowing seeds are disinfected with 30% H2O2 for c. 6 min and rinsed in distilled water four times. This is potentially a seed dormancy breaking treatment and soaking seeds in 200 mg l-1 GA3 is known to improve germination performance (Yuan et al., 2013). But a full characterisation of seed dormancy in this species is needed, as is an assessment of seed storage capability.
18) Ostrya rehderiana Chun (Betulaceae)
The storage behaviour of this species is uncertain. This is based on the evidence that the seed lifespan is short, with germination falling from 16 to 5% after open storage at room temperature from collection through to the following spring (Zhang et al., 1988). As open storage would have likely resulted in considerable drying of the seeds and some germination was still possible after many months, the seeds are not likely to be recalcitrant (desiccation sensitive). Some seeds of Ostrya carpinifolia germinated after 13 years air-dry storage at 40% r. h. and 4 ℃ (Wildrechner, 1990), and appear to be orthodox in storage behaviour. It is recommended that Ostrya virginiana seeds are dried before storage (Schopmeyer and Leak, 1974). The prospects are, therefore, that seeds of O. rehderiana may be storable.
Fruits of this species ripen in SeptembereOctober after a development period about 160-180 days (Zhang et al., 1990), but the seed produced is of inferior viability with only about 50% sound seed (Zhang et al., 1988). Seeds imbibe efficiently over 4 h and appear not to have physical dormancy (Le et al., 2013). However, in the wild the seeds germinate in April (Zhang et al., 1990), suggesting that the seed might benefit from cold stratification over winter. However, longer-term (16 weeks) low temperature treatment reduces cumulative germination, indicating the potential for dormancy re-inforcement (Le et al., 2013). Relatively long times (c. 50 days) at 15 ℃ are needed to break dormancy, and seeds can be treated with 400 or 600 mg l-1 GA before transfer to an alternating temperature regime of 15/25 ℃ (Le et al., 2013). Seeds have also been germinated in common gardens and successfully transplanted to the field (Li et al., 2012b).
19) Paphiopedilum armeniacum S.C.Chen & F.Y.Liu (Orchidaceae)
Seed extracted from capsules harvested 120 days after pollination can be germinated in vitro on R medium (see Ardtiii, 1982) reaching 25% (Chen et al., 2004). The preferred conditions are 3 weeks in the dark at 25 ℃, followed by transfer to the light (12 h/d). Coconut milk stimulates germination and activated charcoal (2 g l-1) and banana homogenate (100 g l-1) benefit the growth and development of seedlings (Chen et al., 2004). Black seeds removed from mature capsules at 180 days after pollination do not germinate. This suggests the late onset of seed dormancy, which is known in other species in this genus. Nothing is known about seed storage in this species.
20) Paphiopedilum gratrixianum Rolfe (Orchidaceae)
Seed extracted from capsules developing for 210 days have the highest in vitro germination level (93%) at 25 ℃ in the light (16 h/d) on ½ Robert Ernst (RE) medium + NAA 0.5 g l-1 + BA 0.2 mg l-1 + coconut milk 50 ml l-1 + casein hydrolysate 1 g l-1 (Zhou et al., 2012). In contrast, seeds extracted from capsules after 300 days of development only germinate to 26%, indicating the possible onset of seed dormancy. There appears to be no information about seed storage in this species.
21) Paraisometrum mileense W.T. Wang (Gesneriaceae)
Freshly matured seeds germinate readily in the light (12 h/d) on 1% agar-water at constant temperatures of 20 ℃ and 25 ℃ (100%) and at alternating temperatures (night/day) of 15/25 ℃ (97%) and 20/30 ℃ (100%) (Liu et al., 2015). The fastest germination is at 25 ℃, with a mean germination time of c. 8 days. As the seeds benefitted from cold stratification, enabling subsequent germination over a wider range of temperatures, the seeds are classified as having nondeep physiological dormancy (Liu et al., 2015). A seed storage assessment is yet to be made.
22) Parakmeria omeiensis W.C.Cheng [is a synonym of Magnolia omeiensis (W.C.Cheng) Dandy] (Magnoliaceae).
Seed are contained in a fleshy, red aril which is removed by soaking in water for 1 day, followed by scrubbing off. The slightly flattened, oval seeds germinate to a low level in vitro. Seeds germinate when sown on Murashige and Skoog medium at 25 ℃, and kept in the dark first followed by transfer to light (14/d; 2000-2500 lx) (Yu et al., 2011). An assessment of seed storage capability is required.
23) Parrotia subaequalis (Hung T. Chang) R.M. Hao & H.T. Wei (Hamamelidaceae)
The long spindle-shaped seeds weighing 46 mg each can germinate to 74% at 20-25 ℃ on sand after pre-treatment with 600 mg l-1 GA3 (Hu et al., 2012). Germination reaches 48-60% under a range of lighting conditions (total light, semi-light, and dark). The seeds for germination testing can be air-dried and held at either 3-5 ℃ or room temperature. After 2 years storage at room temperature germination is c. 12%. A full assessment of seed storage capability is now needed.
24) Pinus dabeshanensis W.C.Cheng & Y.W.Law [is a synonym of Pinus armandii var. dabeshanensis (W.C.Cheng & Y.W.Law) Silba] (Pinaceae).
Seeds of this species are large, weighing about 313 mg each. Removing the thick episperm benefits germination, as does immersing seeds in 0.2 mg l-1 GA3 for 12 h (Han et al., 2014). There appears to be no published information on the seed storage of this species.
25) Pterocarpus indicus Willd. (Fabaceae)
Seeds of P. indicus are 6-8 mm long and weigh 422 mg on average (Jøker, 2000). The seed storage behaviour is thought to be orthodox, as seeds are known to tolerate desiccation to 4% MC. Also long-term storage in liquid nitrogen seems feasible as seeds dried to 4-6% MC, or with excised embryos at 5% MC, had good recovery after cryopreservation (Krishnapillay et al., 1994). The bean-shaped seeds have a brown papery testa and do not appear to be physiologically dormant. However, the pericarp can pose a physical restraint to germination and so the seeds are extracted and sown directly in pots or in germination beds. Germination can start within about a week and seedling establishment might take up to about 3 months (Jøker, 2000). As many fruits are empty, the germination count for non-extracted seeds can be low. Therefore, it is recommended that a cut test is applied to a representative sample of fruits to reveal how many seeds there are per fruit (possibly 0, but usually 1-2).
26) Rhododendron protistum var. giganteum (Forrest ex Tagg) D.F. Chamb. (Ericaceae)
When seeds are air-dried to c. 40% RH and stored in sealed polyethylene bags at 4 ℃ for 360 days, germinability is still 70%. Comparable data for storage at room temperature is 59%. As for the seeds of other Rhododendron species, the seed is desiccation tolerant and seed of R. protistum var. giganteum appears to be relatively long-lived (Shen et al., 2015).
Seed germination is highest (c. 80%; mean time to germinate around 10 days) at 15 ℃ and 20 ℃ in the light (14 h/d), a response that can be replicated in the nursery by sowing the seeds on the soil surface. There is a requirement for light, as sowing in the dark (24 h) results in only 2% germination (Shen et al., 2015).
27) Sonneratia × hainanensis W.C. Ko, E.Y. Chen & W.Y. Chen (Lythraceae)
Seeds of this species do not germination in the dark. When incubated at 28/23 ℃ alternating temperature, seeds germinate to 69% when the photoperiod is 12 h/d, but longer photoperiods reduce germination, indicating the possibility of a high irradiance reaction (HIR). Germination reaches about 90% at temperatures from 20 ℃ to 40 ℃, with the optimum being 35 ℃ (Zhang et al., 2012). Nothing appears to have been published on seed storage for this species.
28) Taxus cuspidata Sieb. et Zucc. (Taxaceae)
The embryo of T. cuspidata seed collected in autumn is very small (Qin et al., 2014). Nonetheless, the seed storage behaviour of this species is highly likely to be orthodox, as seed viability has been maintained for 5-6 years in hermetic air-dry storage at c. 3 ℃ (Schopmeyer, 1974).
The seed has a deep dormancy that can last for 2-3 years (Qin et al., 2014) and needs post-maturation (Cheng et al., 2004). Airdry seeds contain ABA and have a hard coat, preventing water and gas exchange and imposing dormancy on the young embryos. Dormancy relief can be achieved by stratifying seeds at different temperatures: 15-20 ℃ for 4-6 months, to facilitate embryo development, followed by 3-5 ℃ for 3-4 months, to shorten the dormancy period. For example, a two-step treatment of 20 ℃/10 ℃ (day/night) followed by 4 ℃ cold (Liao et al., 2010) enables 15% germination and is better than following warm (20 ℃) e cold (4 ℃) [WC] and cold (10 ℃) e cold (4 ℃) [CC] treatments (Liao et al., 2010). Other methods to stimulate germination include the soaking of seeds in a saturated solution of sodium carbonate for 20-30 min, grinding part of the seed coat with coarse river sand, and using a solution of 1.80 mg l-1 6-BA, 2.20 mg l-1 GA3 or 0.30 mg l-1 2, 4-D for 36 h. The seeds can be incubated for 155 d at an alternating temperature of 23/15 ℃ (10 h day/14 h night), to enable the full differentiation of the embryo and germination to 95% (Gu et al., 2015).
4. ConclusionA strong case has been made for the conservation status of the remaining 94% of the world's plant species to be evaluated (Corlett, 2016). Yet, any delay in assessing how to handle the seeds or spores of vascular plants increases the risk of species loss. Based our analysis for China's PSESP (the first identified group) moving beyond species listing to improve both conservation and sustainable use options will require a significant effort to characterise the seed and spore biology of wild species. There has been a concerted effort recently to enhance the populations of six PSESP through several successful reintroduction programmes, including on M. sinicum, Acer yangbiense, Quercus(Cyclobalanopsis) sichourensis, P. armeniacum, P. mileense, D. pulcherrima, C. debaoensis, Euryodendron excelsum and N. yunnanensis (Ren et al., 2012; Sun, 2013, Sun et al., 2016; Wang et al., 2016a, b). Similar, and other, species-based studies in the future should ensure that a full assessment of the seed and spore biology of the species is carried out as a matter of urgency.
It is highly likely that the gaps in knowledge for China's PSESP is replicated for other groups of challenging species, for example, the so-called 'exceptional' species that either produce recalcitrant seeds or few or no seeds (Pence, 2013a). These require the combined application of in vitro conservation and propagation, for reintroduction and research when traditional propagation methods are not adequate, and tissue cryopreservation (Pence, 2013b). Such methods in cryobiotechnology are known for a large range of species, e.g. orchids (Popova et al., 2016), and there have been recent innovations in the delivery of cryoprotectants into seed tissues (Nadarajan and Pritchard, 2014). Currently, research and training in this area of science are decentralised and a more coordinated effort (human and financial resources) to conserve ex situ threatened species of the tropics is recommended.
AcknowledgementsFunding (No.U1302262) to W.B. Sun from the NSFC-Yunnan joint fund on key projects is gratefully acknowledged. The Royal Botanic Gardens, Kew receives grant-in-aid from Defra. This joint work was undertaken under the Memorandum of Agreement (2014-24) on Plant and Fungal Science between the Chinese Academy of Sciences and the Trustees of the Royal Botanic Gardens, Kew. HWP thanks Darwin Initiative Project 21-003 on cycads for funding.
Aragón C.F., Pangua E., 2004. Spore viability under different storage conditions in four rupicolous Asplenium L.taxa. Amer. Fern J, 941: 28-38. |
Arditti J., 1992. Fundamentals of Orchid Biology. New York: John Wiley and Sons: 691 pp.
|
Arditti J., 1982. Orchid Biology: Reviews and Perspectives. USA: Cornell University Press, vol. 2: 271-275.
|
Arditii, J. (Ed.), 1984. Orchid Biology: Reviews and Perspectives, vol. 3. Cornell University Press, USA, 432 pp.
|
Ballesteros, D., 2010. Conservation of fern spores. In: Kumar, A., Fernández, H., Revilla-Bahillo, A. (Eds.), Working with Ferns. Issues and Applications. Springer, New York, pp. 165-172.
|
Ballesteros D., Estrelles E., Walters C., et al, 2011. Effect of storage temperature on green spore longevity for the ferns Equisetum ramosissimum and Osmunda regalis. CryoLetters, 32: 89-98. |
Ballesteros D., Estrelles E., Walters C., et al, 2012. Effects of temperature and desiccation on ex situ conservation of non-green fern spores. Amer. J. Bot, 99: 721-729. DOI:10.3732/ajb.1100257 |
Bowling J.C., Thompson P.A., 1972. On storing orchid seed. Orchid. Rev, 80: 120-121. |
Campbell, M.W., 1980. Plant Propagation for Reforestation in Nepal. Technical Note 1/80. Department of Forestry, Australian National University.
|
Cao J.W., Liu C.L., Zhang B., et al, 2010. Seed germination of endangered Cathaya argyrophylla Chun & Kuang. Acta Ecol. Sin, 30: 4027-4034. |
Cavender N., Westwood M., Bechtoldt C., et al, 2015. Strengthening the conservation value of ex situ tree collections. Oryx, 49: 416-424. DOI:10.1017/S0030605314000866 |
Chen Z.L., Ye X.L., Liang C.Y., et al, 2004. Seed germination in vitro of Paphiopedilum armeniacum and P. micranthum. Acta Hort. Sinica, 31: 540-542. |
Cheng G.Y., Tang X.J., Gao H.B., et al, 2004. Dormancy mechanism and relieving techniques of seeds of Taxus cuspidata Sieb et Zucc. J. Beijing For. Univ, 26: 5-10. |
Cibrian-Jaramillo A., Hird A., Oleas N., et al, 2013. What is the conservation value of a plant in a botanic garden? Using indicators to improve management of ex situ collections. Bot. Rev, 79: 559-577. DOI:10.1007/s12229-013-9120-0 |
Corlett R.T., 2016. Plant diversity in a changing world: status, trends and conservation needs. Plant Divers, 38: 10-16. DOI:10.1016/j.pld.2016.01.001 |
Dang H.S., Zhang Y.J., Jiang M.X., et al, 2005. A preliminary study on dormancy and germination physiology of endangered species, Berchemiella wilsonii (Schneid) Nakai var. pubipetiolata H. Qian seeds. J. Wuhan. Bot. Res, 23: 327-331. |
Dehgan B., Schutzman B., 1989. Embryo development and germination of cycas seeds. J. Amer. Soc. Hortic. Sci, 114: 125-129. |
Ding C.C., Zhang T., Liu F.Y., 2005. Rapid propagation of Cymbidium wenshanense Y. S.Wu et F.Y.Liu. J. Wenshan Teach. Coll, 18: 225-226. |
Dirr M.A., Heuser C.W., 1987. The Reference Manual of Woody Plant Propagation. Athens: Varsity Press.
|
IUCN/SSC Cycad Specialist Group. IUCN, Gland, Switzerland and Cambridge, UK. ix þ 86 pp. In: Donaldson, J.S. (Ed.), 2003. Cycads. Status Survey and Conservation Action Plan.
|
Gordon, A.G., 1992. Seed Manual for Forest Trees. Forestry Commission Bulletin 83. Her Majesty's Stationery Office, London.
|
Gosling, P., 2007. Raising Trees and Shrubs from Seed Collection. Forestry Commission, Edinburgh.
|
Griffith M.P., Calonje M., Meerow A.W., Tut F., Kramer A.T., Hird A., 2015. Can a botanic garden cycad collection capture the genetic diversity in a wild population?. Int. J. Plant Sci, 176: 1-10. DOI:10.1086/678466 |
Gu, D.Z., Shen, H.M., Yan, Z.X., et al., 2015. Seedling and control technology of morphological development of seed embryonic of Taxus cuspidata Sieb. et Zucc. Hubei Agric. Sci. 2015-07.
|
Hasegawa A., Goi M., Sato M., et al, 1978. Fundamental studies on the asymbiotic seed germination of Calanthe. Tech. Bull. Fac. Agric. Kagawa Univ, 29: 251-259. |
Han J.W., Zhang Z.Y., Wang E.M., et al, 2014. Pinus dabeshanensis seed characteristics and the promotion of seed germination. Chin. Agric. Sci. Bull, 30: 5-10. |
Hay F.R., Merritt D.J., Soanes J.A., et al, 2010. Comparative longevity of Australian orchid (Orchidaceae) seeds under experimental and low temperature storage conditions. Bot. J. Linn. Soc, 164: 26-41. DOI:10.1111/boj.2010.164.issue-1 |
Hu G.W., Lu Y.J., Li H.P., et al, 2012. Study on seed germination characters of Parrotia subaequalis. J. Zhejiang For. Sci. Tech, 6: 48-51. |
Ibars A.M., Estrelles E., 2012. Recent developments in ex situ and in situ conservation of ferns. Fern Gaz, 19: 67-86. |
IUCN, 2015. The IUCN Red List of Threatened Species. http://www.iucnredlist.org.
|
Jian S., Liu N., Gao Z., et al, 2006. Biological characteristics of wild Cycas fairylakea population in Guangdong Province, China. Front. Biol. China, 1: 430-433. DOI:10.1007/s11515-006-0058-z |
Jøker, D., 2000. Pterocarpus Indicus. Seed Leaflet 37. University of Copenhagen. http://curis.ku.dk/ws/files/20648510/pterocarpus_indicus_int.pdf.
|
Krishnapillay B., Marzalina M., Alang Z.C., 1994. Cryopreservation of whole seeds and excised embryos of Pterocarpus indicus. J. Trop. For. Sci, 7: 313-322. |
Lai J.Y., Huang K.X., Pan C.L., et al, 2007. Study on the seed storage and germination of Kmeria septentrionalis. Guangxi For. Sci, 36: 19-21. |
Lan Q.Y., Jiang X.C., Song S.Q., et al, 2007. Changes in germinability and desiccation-sensitivity of recalcitrant Hopea hainanensis Merr. et Chun seeds during development. Seed Sci. Technol, 35: 21-31. DOI:10.15258/sst |
Lan Y.T., Wei X.L., Huang L., et al, 2014. Study on technology of aseptic sowing and proliferation of protocorms in wild Geodorum eulophioides. J. Anhui Agri. Sci, 42: 395-397, 418. |
Langkamp, P., Plaisted, M., 1987. Appendix 1. Native plant seed usage by the mining industry -a survey. In: Langkamp, P. (Ed.), Germination of Australian Native Plant Seed. Inkata Press, Melbourne, Sydney, pp. 135-181.
|
Le X.W., Cui M.Y., Yang S.Z., et al, 2013. Characters on the seed dormancy and germination of an endangered species, Ostrya rehderiana, in Tianmu Mountain, China. J. East China Norm. Univ. Nat. Sci, 6: 150-158. |
Li D.Z., Pritchard H.W., 2009. The science and economics of ex situ plant conservation. Trends Plant Sci, 14: 614-621. DOI:10.1016/j.tplants.2009.09.005 |
Li Y., Zhang Y.L., Jiang C.D., et al, 2010. Effect of storage temperature on spore viability and early gametophyte development of three vulnerable species of Alsophila (Cyatheaceae). Australian. J. Bot, 58: 89-96. DOI:10.1071/BT09180 |
Li Y., Shi L., 2014. Effect of desiccation level and storage temperature on green spore viability of Osmunda japonica. Cryobiology, 68: 446-450. DOI:10.1016/j.cryobiol.2014.03.002 |
Li Y.Y., Guan S.M., Yang S.Z., et al, 2012b. Genetic decline and inbreeding depression in an extremely rare tree. Conserv. Gen, 13: 343-347. DOI:10.1007/s10592-011-0286-x |
Li Y.Y., Tsang E.P.K., Cui M.Y., et al, 2012a. Too early to call it a success: an evaluation of the natural regeneration of the endangered Metasequoia glyptostroboides. Biol. Cons, 150: 1-4. DOI:10.1016/j.biocon.2012.02.020 |
Liao, Y.J., Li, X., Dong, X.H., 2010. Effect of different temperature stratifications on physiological and biochemical characteristic and seed embryos development of Taxus cuspidata Sieb.et Zucc. J. China Agric. Univ. 2010-01.
|
Liu, C., 2012. Effects of hot water treatment on germination and seedling growth of Gleditsia japonica. For. Sci. Technol. 2012-01.
|
Liu C., Qin S.F., Hu X.J., 2015. Dormancy and germination of Paraisometrum mileense and their ecological implications. Plant Div. Res, 37: 278-282. |
Ma Y.P., Chen G., Grumbine R.E., et al, 2013. Conserving plant species with extremely small populations (PSESP) in China. Biodiv. Cons, 22: 803-809. DOI:10.1007/s10531-013-0434-3 |
Mikula A., Tomiczak K., Makowski D., et al, 2015. The effect of moisture content and temperature on spore aging in Osmunda regalis. Acta Physiol. Plant, 37: 229-240. DOI:10.1007/s11738-015-1985-6 |
Nadarajan, J., Pritchard, H.W., 2014. Biophysical characteristics of successful oilseed embryo cryoprotection and cryopreservation using vacuum infiltration vitrification, an innovation in plant cell preservation. PLoS One. http://dx.doi.org/10.1371/journal.pone.0096169 e0096169.
|
Ouyang Z.Q., Su W.H., Zhang G.F., 2006. Studies on character of seed germination of rare plant Dipteronia dyeriana. Acta Bot. Yunnanica, 28: 509-514. |
Park S.Y., Hosakatte N., Murthy H.N., et al, 2000. In-vitro seed germination of Calanthe sieboldii, an endangered orchid species. J. Plant Biol, 43: 158-161. DOI:10.1007/BF03030493 |
Pence, V.C., 2008. Ex situ conservation of ferns and lycophytes d Approaches and techniques. Pp. 284 e 300. In: Ranker, T.A., Haufler, C.H. (Eds.), Biology and Evolution of Ferns and Lycophytes. Cambridge University Press, Cambridge, UK.
|
Pence V.C., 2013a. In vitro methods and the challenge of exceptional species for Target 8 of the Global Strategy for Plant Conservation. Ann. Mo. Bot. Gard, 99: 214-220. DOI:10.3417/2011112 |
Pence V.C., 2013b. In vitro methods and cryopreservation: tools for endangered exceptional species preservation and restoration. Acta Hort, 1039: 73-79. |
Pennisi E., 2010. Tending the global garden. Science, 329: 1274-1277. DOI:10.1126/science.329.5997.1274 |
Popova E., Sylvestre I., Kim H.H., et al, 2016. Frozen beauty: the cryobiotechnology of orchid diversity. Biotechnol. Adv, 34: 380-403. DOI:10.1016/j.biotechadv.2016.01.001 |
Pritchard, H.W., 2004. Classification of seed storage 'types' for ex situ conservation in relation to temperature and moisture. In: Guerrant, E.O., Havens, K., Maunder, M. (Eds.), Ex Situ Plant Conservation e Supporting Species Survival in the Wild. Island Press, Washington DC, USA, pp. 139-161.
|
Pritchard, H.W., Dickie, J.B., 2003. Predicting seed longevity: use and abuse of seed viability equations. In: Smith, R.D., Dickie, J.B., Linington, S.H., et al. (Eds.), Seed Conservation e Turning Science into Practice. Royal Botanic Gardens, Kew, UK, pp. 653-722.
|
Pritchard H.W., Moat J., Ferraz J.B.S., Marks T.R., Camargo J.L.C., Nadarajan J., Ferraz I.D.K., 2014. Innovative approaches to the preservation of tree species. For. Ecol Manag, 33: 88-98. |
Pritchard H.W., Poynter A.C., Seaton P.T., 1999. Interspecific variation in orchid seed longevity in relation to ultra-drying and cryopreservation. Lindleyana, 14: 92-101. |
Pritchard H.W., Seaton P.T., 1993. Orchid seed storage: historical perspective, current status, and future prospects for long-term conservation. Selbyana, 14: 89-104. |
Pritchard, H.W., Sacandé, M., Berjak, P., 2004. Biological aspects of tropical tree seed desiccation and storage responses. In: Sacandé, M., Jøker, D., Dulloo, M.E., et al. (Eds.), Comparative Storage Biology of Tropical Tree Seeds, pp. 319-341 (IPGRI, Danida).
|
Qin Y.T., Li X., Zhai Z.X., et al, 2014. Research advances in biological characteristics of Taxus cuspidata seeds. North. Hortic: 2014-2023. |
Quintanilla L.G., Amigo J., Pangua E., et al, 2002. Effect of storage method on sporeviability in five globally threatened fern species. Ann. Bot, 904: 461-467. |
Rasmussen H.N., 1996. Terrestrial Orchids e from Seed to Mycotrophic Plant. UK: Cambridge University Press.
|
Ren H., Zhang Q., Lu H., et al, 2012. Wild plant species with extremely small populations require conservation and reintroduction in China. AMBIO, 41: 913-917. DOI:10.1007/s13280-012-0284-3 |
Rutherford, C., Donaldson, J., Hudson, A., et al., 2013. CITES and Cycads e a User's Guide. Royal Botanic Gardens, Kew, UK, p. 114, 1 compact disc.
|
Schopmeyer, C.S. (Ed.), 1974. Seeds of Woody Plants in the United States. Forest Service of the United States Department of Agriculture, Handbook Number, vol. 450, 883 pp.
|
Schopmeyer, C.S., Leak, W.B., 1974. Ostrya virginiana (mill.). In: Koch, K. (Ed.), Seeds of Woody Plants in the United States. Agriculture Handbook No 450. USDA, Washington DC, Forest Service, pp. 564-565.
|
Seaton P., Kendon J.P., Pritchard H.W., et al, 2013. Orchid conservation: the next ten years. Lankesteriana, 13: 93-101. |
Shen S.K., Wu F.Q., Yang G.S., et al, 2015. Seed germination and seedling emergence in the extremely endangered species Rhododendron protistum var. giganteum-the world's largest Rhododendron. Flora, 216: 65-70. DOI:10.1016/j.flora.2015.08.006 |
Smith, R.D., Dickie, J.B., Linington, S.H., et al., 2003. In: Seed Conservation: Turning Science into Practice. Royal Botanic Gardens, Kew, UK, 1023 pp.
|
Song X., Chen Q., Wang D., et al, 1984. A study on the principal storage conditions of Hopea hainanensis seeds. Sci. Silvae Sin, 20: 225-236. |
Song X., Chen Q., Wang D., et al, 1986. A further study on ultrastructural changes in radicle-tip cells of Hopea hainannensis during deterioration resulted from losing water. Trop. For, 4: 1-6. |
Sun W.B., 2013. Conserving Plant Species with Extremely Small Populations (PSESP) in Yunnan: Practice and Exploration. Kunming, Yunnan: Yunnan Sci. & Technol. Press: 1-100.
|
Sun W.B., Zhou Z.K., Chen W.Y., et al, 2016. Rescuing plant species with extremely small populations in China: the case of the Xichou oak (Quercus sichourensis). Int. Oaks, 27: 163-170. |
Suo J., Chen S., Zhao Q., et al, 2015. Fern spore germination in response to environmental factors. Front. Biol, 10: 358-376. DOI:10.1007/s11515-015-1342-6 |
Takakura K., 2002. The specialist seed predator Bruchidius dorsalis (Coleoptera: Bruchidae) plays a crucial role in the seed germination of its host plant, Gleditsia japonica (Leguminosae). Func. Ecol, 16: 252-257. DOI:10.1046/j.1365-2435.2002.00619.x |
Tang R.Q., Li X.K., Ou Z.L., et al, 2001. The fruiting characteristics and reproductive capacity of seeds of Abies yuanbaoshanensis. Bull. Bot. Res, 3: 403-408. |
Tang W.X., Mao S.Z., Pan B., et al, 2009. Spatial distribution pattern of seed rain and seed germination characteristics of endangered plant Hopea chinensis. J. Fujian Coll. For, 2: 149-154. |
Thammasiri K., 2000. Cryopreservation of seeds of a Thai orchid (Doritis pulcherrima Lindl.) by vitrification. CryoLetters, 21: 237-244. |
Volis S., 2016. How to conserve threatened Chinese plant species with extremely small populations?. Plant Divers, 1: 53-62. |
Walters C., Berjak P., Pammenter N., et al, 2013. Preservation of recalcitrant seeds. Sci, 339: 915-916. DOI:10.1126/science.1230935 |
Wang B., Ma Y.P., Chen G., et al, 2016a. Rescuing Magnolia sinica (Magnoliaceae), a critically endangered species endemic to Yunnan, China. Oryx, 50(3): 446-449. DOI:10.1017/S0030605315000435 |
Wang, C.J., Zhang, J., Wan, J.Z., et al., 2016b. The spatial distribution of threats to plant species with extremely small populations. Fron. Earth Sci. 1-10. http://dx.doi.org/10.1007/s11707-016-0550-y. April 2016.
|
Wang L.F., Ou M.W., Tan Y.F., 2014. The study on the seed germination of endangered species Cycas debaoensis. Seed, 33: 26-29. |
Wang S.T., Zhao Y., Wang X.R., 2012. Study on the dormancy and accelerating germination technology for Kmeria septenrionalis Dandy seed. J. Anhui Agri. Sci, 40: 4502-4503, 4559. |
Wang, X.F., Zhao, T., Yan, M., et al., 2004. Desiccation and storage of Vatica astrotricha, Hopea hainanensis, Podocarpus nagi and Acmena acuminatissma seeds. In:Sacandé, M., Jøker, D., Dulloo, M.E., et al. (Eds.), Comparative Storage Biology of Tropical Tree Seeds, pp. 165-173 (IPGRI, Danida).
|
Wei R., Zhang X.C., 2014. Rediscovery of Cystoathyrium chinense Ching (Cys topteridaceae): Phylogenetic placement of the critically endangered fern spe cies endemic to China. J. Syst. Evol, 52: 450-457. DOI:10.1111/jse.v52.4 |
Wen B., Lan Q., He H., 2002. Effects of illumination, temperature and soil miosture content on seed germination of Hopea hainanensis. J. Trop. Subtrop. Bot, 10: 258-262. |
Wildrechner M.P., 1990. Seed storage for the commercial propagator. Combined Proceedings Inter. Plant Prop. Soc, 40: 571-575. |
Wu C.H., Ye X.L., Liang C.Y., 2005. In vitro seed germination in Doritis pulcherrima. Guihaia, 25: 149-152. |
Xie Z.Q., Li Q.M., 2000. Seed characteristics of endangered plant Cathaya argyrophylla. Acta Phytoecol. Sin, 24: 82-86. |
Xin X., Jing X.M., Sun H.M., et al, 2004. Ecophysiological charactersitcs of seed germination of the relict plant Metasequoia glyptostroboides. Biodivers. Sci, 12: 572-577. |
Yang Q.E., Zhu G.H., Hong D.Y., et al, 2005. World's largest flora completed. Sci, 309: 2163. |
Yu D.P., Li C.H., Ding J., et al, 2011. Preliminary report on character of seed and seed culture in vitro of Parakmeria omeiensis Cheng. Res. Devel, 27: 197-199. |
Yu D.P., Peng D.X., Li C.H., et al, 2008. Research on the biological characters of the seeds of Acer catalpifolium Rehd. Chin. Wild Plant Resour, 6: 30-33. |
Yuan R.L., Xiang Z.Y., Yang W.Z., et al, 2013. Seed dormancy and germination traits of Nyssa yunnanensis. For. Res, 26: 384-388. |
Zhang M.W., Li W.Q., Wu Q.S., et al, 2012. The relationship between environ mental factors and seed germination of Sonneratia hainanensis, an endangered species in Hainan. Pract. For. Techol, 12: 3-6. |
Zhang R.H., Shen X.-K., Yang F.-C., 1990. Study on growth rhythm of Ostrya rehderiana Chun. J. Zhejiang For. Coll, 1990(1). |
Zhang R.H., Gong G.W., Shen X.K., et al, 1988. A study of pollen, seed and seedling of Ostrya rehderiana Chun. J. Zhejiang For. Sci. Technol, 8(4): 7-11. |
Zheng Y.L., Sun W.B., 2009. Seed germination of Huagaimu, a critically endangered plant endemic to southeastern yunnan, China. HortTechnol, 19(2): 427-431. |
Zhou L., Li S.K., Deng K.Y., et al, 2012. Aseptic sowing and tissue culture propagation of Paphiopedilum gratrixianum. J. Anhui Agri. Sci, 40: 9590-9592. |



