b. College of Traditional Chinese Medicine, Yunnan Key Laboratory of Dai and Yi Medicines, Yunnan University of Chinese Medicine, Kunming, 650500, Yunnan, China;
c. Key Laboratory of Economic Plants and Biotechnology, Kunming Institute of Botany, Chinese Academy of Sciences, Yunnan Key Laboratory for Wild Plant Resources, Kunming 650201, Yunnan, China;
d. School of Natural Sciences, University of Tasmania, Private Bag 55, Hobart, Tasmania 7001, Australia
The frequency and intensity of extreme climatic events caused by global climate change have increased, exposing plants to unprecedented pressures with regard to survival (Adams et al., 2017). Previous studies have demonstrated strong effects of drought on plant production from the flowering to fruiting stages of reproductive development (Boyer and Westgate, 2004; Passioura, 2006). Recent findings have indicated that cavitation in xylem conduits of water transport systems is associated with plant mortality under water stress (Brodribb and Cochard, 2009; Barigah et al., 2013; Anderegg et al., 2016; Zhang and Brodribb, 2017; Rodriguez-Dominguez et al., 2018; Brodribb et al., 2020). Consequently, the ability of plants to resist cavitation formation in the xylem is a major component of plant drought tolerance and survival (Barigah et al., 2013; Anderegg et al., 2016). However, relatively few studies have investigated cavitation resistance in flowers (Zhang and Brodribb, 2017; Bourbia et al., 2020). It is necessary to address this fundamental aspect of flower water physiology for predicting hydraulic failure of plant reproductive organs under drought (Brodribb et al., 2020).
The extent to which flowering plants successfully reproduce is closely associated with the unique structures of their floral organs (Memmott and Waser, 2002; van der Niet and Johnson, 2012), which are typically located at the periphery of plants, and are thus similarly exposed as leaves to abiotic stress. For example, flowers release more water than leaves by transpiration during drought (Lambrecht, 2013). An understanding of the physiological traits of floral water transport systems in the broader context of whole-plant physiology would, therefore, provide new insights into the ecology and evolution of flowers (Roddy et al., 2016, 2018; Zhang et al., 2018). Despite this importance, research on the structures of water-transporting xylem tissue, the capacity of flowers to resist drought, and the relationships between these factors is scarce (Zhang and Brodribb, 2017; Bourbia et al., 2020).
Xylem embolism during water stress is probably the primary source of injury to droughted plants (Bréda et al., 2006; Brodribb and Cochard, 2009; Barigah et al., 2013; Anderegg et al., 2016; Adams et al., 2017). To understand the capacity of a species to resist embolism, it is important to first examine the thresholds of hydraulic failure within plant organs (Zimmermann, 1978; Zhang and Brodribb, 2017; Rodriguez-Dominguez et al., 2018), because the ability of plants to resist cavitation appears to be organ-specific (Zhang and Brodribb, 2017; Rodriguez-Dominguez et al., 2018). The variation in vulnerability among different organs within species is largely a result of hydraulic 'segmentation' (Zimmermann, 1978). It has been hypothesized that central tissues (costly organs like stems) are the last to succumb to cavitation under acute drought stress, which further protects resource-dense tissues, such as those in stems (Zimmermann, 1983; Tyree and Ewers, 1991). Previous studies have shown that the more distal tissues in woody plants are usually more vulnerable than stems under drought conditions, supporting the 'segmentation' hypothesis (Choat et al., 2005; Johnson et al., 2011; Hochberg et al., 2017; Zhang and Brodribb, 2017). However, because of hydraulic methodological and technical limitations, few studies have explored the variation in the degree of segmentation between vegetative and reproductive organs within a species (Zhang and Brodribb, 2017; Bourbia et al., 2020). Recent advances in optical techniques to measure the embolism vulnerability of xylem have provided new opportunities to investigate the formation and spread of embolisms along the continuous water column within xylem from roots, stems, leaves, to flowers (Brodribb et al., 2016; Zhang and Brodribb, 2017; Rodriguez-Dominguez et al., 2018; Skelton et al., 2018).
Hydraulic vulnerability under acute water stress probably results from a suite of anatomical characteristics. Previous studies on the relationships of xylem vulnerability with xylem anatomical or structural traits of leaves and stems have demonstrated the association between the structures of bordered pits and the capacity for resistance to cavitation in leaves and stems (Hargrave et al., 1994; Sperry, 2003; Hacke et al., 2009; Jansen et al., 2009; Blackman et al., 2010; Lens et al., 2011; Bouche et al., 2014; Li et al., 2016). For instance, the cavitation resistance was negatively correlated with pit aperture diameter, but not with tracheid lumen diameter, torus diameter and pit membrane diameter for 115 conifer species (Bouche et al., 2014). Flowers are functionally distinct from leaves and stems. Flowers may also differ from leaves and stems with respect to the xylem structure. For instance, compared with leaves, petal xylem is more vulnerable to embolism in several woody species (Zhang and Brodribb, 2017). In contrast to the xylem in leaves and stems, the xylem conduit in flowers may not be well reinforced due to less mechanical support. To date, no studies have yet demonstrated whether the structural traits of floral xylem conduits, such as bordered pit sizes, are related to flower drought resistance.
In the present study, we used relatively new optical techniques to investigate patterns of peduncle-, petiole-, and stem xylem vulnerability to embolism in an important ornamental species, Magnolia grandiflora. The peduncle is the stalk of a flower or an inflorescence, the petiole is the stalk that attaches a leaf to the stem. Both peduncles and petioles play an important role in water transport to flowers and leaves. We hypothesized that peduncles will be most vulnerable to cavitation, and stems will be least vulnerable. Furthermore, we tested whether the bordered pit properties of xylem in peduncles, petioles, and stems are correlated with their cavitation resistance.
2. Materials and methods 2.1. Plant materialMagnolia grandiflora L. is an evergreen tree that grows to a maximum height of 30 m, native to the southeastern United States of America, and blooms from May to July. This species is widely cultivated as an ornamental tree. Considering the sample accessibility, all plants used in this study were older than 20 years, approximately 40 cm in diameter at breast height and approximately 9 m in height. To minimize native embolism, stems were taken from well-watered plants (plants were watered 1–2 times per week as needed) at the Kunming Botanical Garden, Kunming, Yunnan, China (25°08′42″N, 102°44′31″E; elevation 1990 m). Samples for optical experiments and scanning electron microscopy were collected in June 2018.
2.2. Optical measurement of xylem vulnerabilityStems of approximately 1.5 m in length were cut from healthy plants at predawn to minimize native embolism and were then immediately transported to the laboratory in plastic bags with damp paper towels to prevent water loss. Stems with flowers and leaves were re-cut under water and then hydraulically equilibrated for ca. 30 min. We employed an optical xylem vulnerability method to capture embolism events in the peduncles, petioles, and stems of six individual plants under drought conditions using a microscope (Leica S8APO, Leica Microsystems Vertrieb GmbH, Wetzlar, Germany) as previous studies described (Brodribb et al., 2017; Zhang and Brodribb, 2017; Rodriguez-Dominguez et al., 2018). The samples were firmly attached using adhesive tape and padded clamps to ensure no movement of the sample. The sections of the peduncles, petioles and stems of ca. 1 cm in length (this length was found to be appropriate for monitoring the cavitation based on our previous experiments) were prepared by carefully removing the epidermis and the bark using the sharp blades to expose the xylem (Brodribb et al., 2017). Diameters of peduncles, petioles and stems were approximately 8 mm, 2 mm and 7 mm, respectively. Samples were then secured under a microscope, and images were continuously collected (scanning interval of every 3 min) for 3–5 days. Meanwhile, a stem psychrometer (ICT, Armidale, Australia) was attached to the stem adjacent to the monitored area to continuously measure the water potentials of peduncles, petioles, and stems. To test the validity of stem psychrometer readings, a Scholander-type pressure chamber (PMS Instrument Company, Model1505D-EXP, USA) was periodically used to measure leaf water potentials. We found that the relationship between leaf water potentials measured using the pressure chamber and stem water potentials measured with the ICT stem psychrometer for Magnolia grandiflora was highly close to a 1:1 regression line (Fig. S1, R2 = 0.95, P < 0.001, n = 10), confirming the accuracy of stem psychrometer readings.
2.3. Image capture and analysisImages of the peduncles, petioles, and stems were collected using reflected light every 3 min during the drying process until all embolism events ceased, at which point the organs were considered to be completely embolized. The optical events were recorded using an image subtraction method (www.opensourceov.org) that highlights rapid changes in light transmission caused by rapid bubble expansion while filtering out all other, slower movements associated with drying (Brodribb et al., 2016; Zhang and Brodribb, 2017; Rodriguez-Dominguez et al., 2018; Skelton et al., 2018; Bourbia et al., 2020). Bubble expansion in conduits should be very fast when samples are under significant tension. Slower movements are interpreted as being due to water draining from fibres or immature vessels. These elements did not contribute to hydraulic conductivity and were eliminated from the analysis by only considering changes occurring between subsequent images. The embolism area of each image was calculated as the sum of non-zero pixels and expressed as cumulative embolisms. The percentage of the total cumulative embolism area (the cumulative total area per image/max cumulative total area × 100) was plotted against stem water potentials to make the vulnerability curves, which was then fitted with an exponential sigmoidal equation (Pammenter and Van der Willigen, 1998; Rodriguez-Dominguez et al., 2018). Water potential that induced 50% embolism (P50) was calculated from the curves (Rodriguez-Dominguez et al., 2018). It is worth noting that calculations of P50 based on optical and centrifuge methods are in strong agreement (Brodribb et al., 2017). A recent study also confirmed that the optical method can provide a reasonable estimation on xylem vulnerability (Chen et al., 2020). Specifically, spatiotemporal color maps were created using an image subtraction method in ImageJ (Schindelin et al., 2012). Firstly, the stack of original images and the stack of processed events were opened in ImageJ. After thresholding, a function 'Colour Slices' was used, a cumulative 'Z-projection' of all the image sequences produced a color map of the sum of all embolisms (Brodribb et al., 2016). Finally, spatiotemporal maps of cavitation propagation were produced by coloring the embolism area in each image combined with the water potential data measured by the stem psychrometer.
2.4. Scanning electron microscopyA conventional scanning electron microscope (Zeiss Sigma 300, Germany) with a voltage of 10 kV was used to measure inter-vessel pit and vessel parameters. Longitudinal sections of the peduncle, petiole, and stem tissues were examined. Specimens were split in a tangential plane, fixed to aluminum stubs with an electron-conductive carbon sticker, and coated with gold using a sputter coater (Cressington 108 Auto, UK) for 2 min (Jansen et al., 2009; Lens et al., 2011). Nine pit parameters were measured for at least 53 different pits from 2 to 3 vessels on each peduncle, petiole and stem from six different trees: long diameter of inner pit aperture, short diameter of inner pit aperture, inner pit aperture area, long diameter of outer pit aperture, short diameter of outer pit aperture, outer pit aperture area, long diameter of pit membrane, short diameter of pit membrane, and pit membrane area (Fig. S2).
2.5. Statistical analysisDifferences in P50 and pit traits between peduncles, petioles and stems were evaluated by one-way ANOVA (P < 0.05) after testing for the normality and homogeneity of variances. Comparison of means was then performed by post-hoc Tukey's test (P < 0.05). The relationships of P50 with pit traits were tested using the linear mixed-effect model. In the model, pit trait and organ interactions were set as fixed factors and individual as a random factor to account for repeat observations within individual. All statistical analyses were conducted in R v.4.0.0 (R Core Team, 2020).
3. ResultsXylem embolism propagation events in the peduncles, petioles, and stems of Magnolia grandiflora were easily observed using the optical method (Fig. 1). The plots of xylem embolism events vs. stem water potentials indicated considerable variation in the water potential at which 50% of the cavitation events occurred (P50) among peduncles, petioles, and stems (Fig. 2). The mean P50 ranged from −0.39 to −0.98 MPa for peduncles, from −1.96 to −3.32 MPa for petioles, and from −2.60 to −3.80 MPa for stems. The mean P50 in peduncles (−0.64 ± 0.09 MPa) was significantly higher than P50 in petioles (−2.70 ± 0.21 MPa) (F = 79.81, P < 0.001) and the P50 of peduncles was also significantly higher than that of stems (−3.12 ± 0.21 MPa) (F = 121.16, P < 0.001). However, there was no significant difference in P50 between petioles and stems (F = 2.05, P = 0.18). Based on the values of P50, the most vulnerable organ measured in Magnolia grandiflora was the peduncle, followed by the petiole; the stem was most resistant to cavitation (Fig. 3).
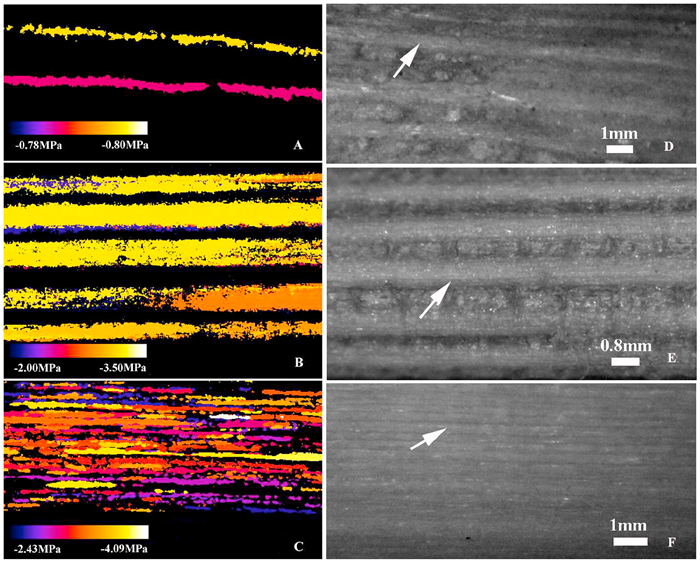
|
| Fig. 1 Spatiotemporal color maps showing the propagation of cavitation in the peduncles (A), petioles (B), and stems (C) of Magnolia grandiflora using the optical vulnerability method. The grey images indicate the vascular bundles in the peduncles (D), petioles (E), and stems (F). Scale bars show the water potentials and each embolism event. Arrows indicate the vascular bundles. |
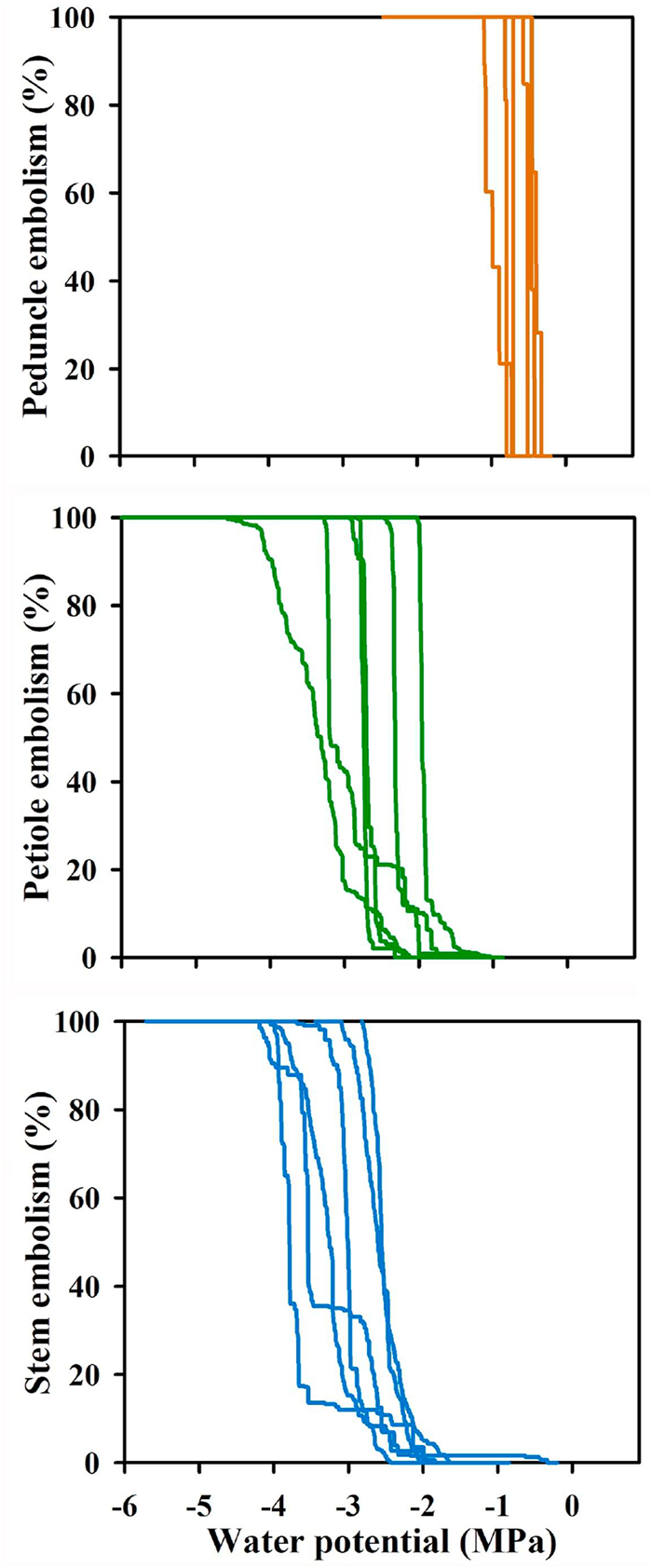
|
| Fig. 2 Optical vulnerability curves for peduncles, petioles, and stems of Magnolia grandiflora, n = 6. The lines are made from the beginning of embolization every 3 min to the end of the embolization. |
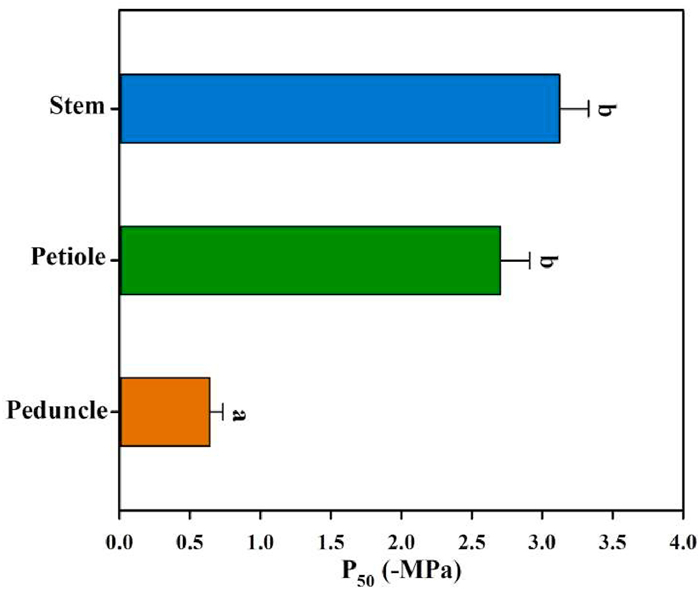
|
| Fig. 3 Differences among the average P50 of peduncles, petioles and stems in Magnolia grandiflora derived by fitting a sigmoidal exponential equation to an optical vulnerability curve for each organ from six individuals. Different letters indicate significant difference in P50 between organs. |
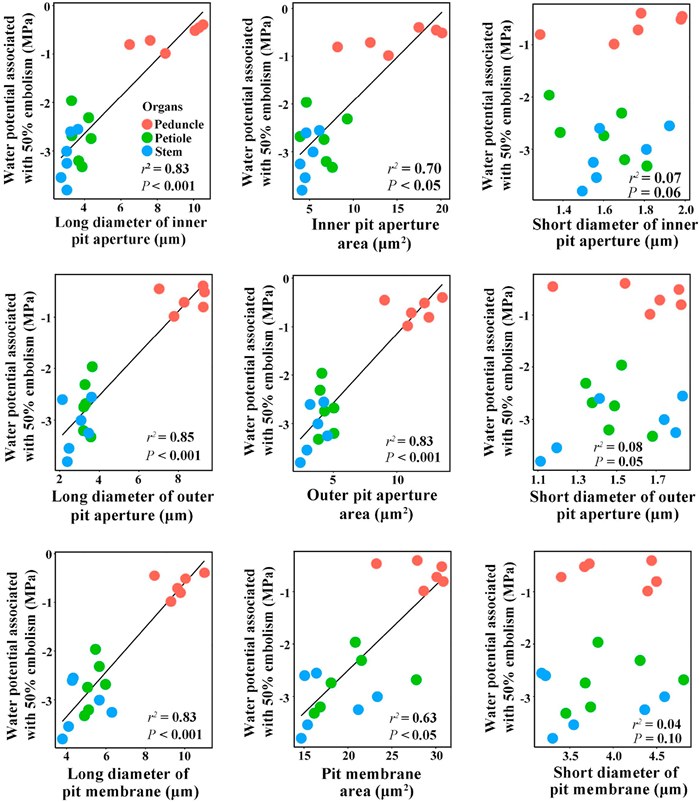
Considerable variation in the sizes of the outer and inner pit apertures and pit membranes was observed in peduncles, petioles, and stems (Fig. 4 and Table 1). Specifically, the long diameter of inner pit aperture, inner pit aperture area, long diameter of outer pit aperture, outer pit aperture area, long diameter of pit membrane, and pit membrane area were all significantly larger in peduncles than in petioles and stems, but there were no significant differences in these pit traits between petioles and stems. There were no significant differences in the short diameter of inner pit aperture, short diameter of outer pit aperture and short diameter of pit membrane among peduncles, petioles and stems (Table 1). Across all organs, P50 was significantly correlated with long diameters of both inner and outer pit apertures, both inner and outer pit aperture area, long diameter of pit membrane, and the pit membrane area, but not significantly correlated with short diameters of both inner and outer pit apertures, and the short diameter of pit membrane (Fig. 5 and Table S1).
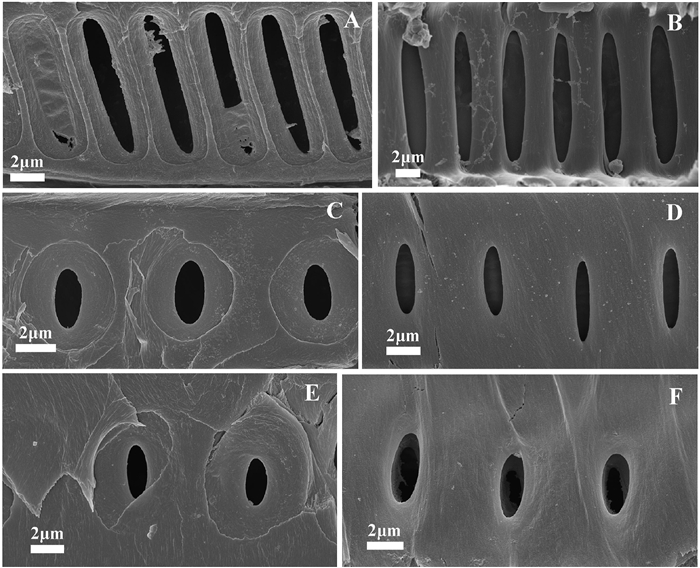
|
| Fig. 4 Scanning electron microscopy images of the outer pit aperture, inner pit aperture, and pit membrane in Magnolia grandiflora. (A) outer pit apertures in peduncles, (B) inner pit apertures in peduncles, (C) outer pit apertures in petioles, (D) inner pit apertures in petioles, (E) outer pit apertures in stems, and (F) inner pit apertures in stems. |
| Pit traits | Peduncles | Petioles | Stems |
| Long diameter of inner pit aperture (μm) | 8.85±0.36a | 3.79±0.15b | 3.13±0.12b |
| Short diameter of inner pit aperture (μm) | 1.73±0.05a | 1.57±0.06a | 1.65±0.07a |
| Inner pit aperture area (μm2) | 14.94±0.93a | 6.33±0.44b | 4.79±0.32b |
| Long diameter of outer pit aperture (μm) | 8.52±0.19a | 3.32±0.12b | 2.84±0.15b |
| Short diameter of outer pit aperture (μm) | 1.63±0.04a | 1.48±0.04a | 1.50±0.07a |
| Outer pit aperture area (μm2) | 11.61±0.40a | 4.35±0.15b | 3.48±0.21b |
| Long diameter of pit membrane (μm) | 9.67±0.15a | 5.35±0.12b | 4.82±0.20b |
| Short diameter of pit membrane (μm) | 4.02±0.13a | 3.97±0.14a | 3.74±0.14a |
| Pit membrane area (μm2) | 28.52±0.67a | 20.14±0.88b | 17.91±0.99b |
| Different lowercase letters indicate significant differences in traits among organs (P < 0.05). The values represent means ± SE, n = 6. | |||
|
| Fig. 5 Relationships of water potential associated with 50% of embolism with dimensions of inner pit, outer pit, and pit membrane in peduncles, petioles, and stems of Magnolia grandiflora. The fitted line in each panel was derived according to the linear mixed-effect model. Each cycle with the same color represents a measurement on each organ per individual. |
Water that flows through xylem vessels can be blocked by embolisms under stress, thus ultimately cutting the plant off from soil water supply. Because xylem embolism affects plant productivity and survival (Brodribb and Cochard, 2009; Choat et al., 2012), the identification of embolism in the water transporting tissues is important to elucidate plant vulnerability to drought. In the present study, we used the optical method to detect significant differences in the water potentials associated with embolism formation among peduncles, petioles, and stems. We found that the peduncles were more vulnerable to embolism than the petioles and stems, supporting the hydraulic segmentation hypothesis (Zimmermann, 1983; Tyree and Ewers, 1991; Choat et al., 2005; Bourbia et al., 2020). The most likely explanation for our observations is that flowers are hydrated via the xylem of the peduncles, which are more vulnerable to cavitation than the xylem of petioles and stems.
Hydraulic segmentation theory predicts that low-cost structures, such as flowers, will cavitate and be lost before more expensive structures (Zimmermann, 1983). For herbaceous species, there is no hydraulic segmentation among flowers and leaves (Skelton et al., 2017; Zhang and Brodribb 2017). However, flowers of Magnolia grandiflora are big and costly, but more vulnerable to embolism. Bourbira et al. (2020) argued that it is not the cost of the flower that is important, but the cost in water loss that makes it preferable to shed flowers. The flowers of basal angiosperms, such as Magnolia species, have traits associated with higher rates of water loss compared with flowers of monocot and eudicot angiosperms (Roddy et al., 2016). It seems that the cost of water loss in Magnolia grandiflora, with large petals and sepals, makes it preferable to shed flowers under acute water stress (Feild et al., 2009). The shedding of flowers is therefore an effective strategy to reduce water loss. Due to early cavitation in a peduncle of the woody plant, such as Magnolia grandiflora, xylem segmentation may be an effective way to avoid further water loss from leaves and stems. Although we did not measure the cavitation impacts on plants, some droughted plants have been shown to be unable to recover after rewatering if they lose 50% or more of xylem functions as a result of cavitation (Brodribb and Cochard, 2009; Urli et al., 2013). Therefore, drought-stressed plants are likely to lose productivity while investing in repair (Brodribb et al., 2010).
Our results suggest that the size of pits is significantly correlated with cavitation resistance in different organs of this magnolia species, suggesting that a peduncle with larger pit aperture sizes was less resistant to cavitation. However, this result was not unexpected considering that the size of bordered pits has been found to be correlated with vulnerability in other studies (Choat et al., 2008; Lens et al., 2011). This species may, through natural selection, have acquired anatomical adaptations of different tissues for survival in arid environments, and the size of the bordered pits may play a role in resisting cavitation during drought stress.
5. ConclusionThe novel information presented here regarding the ability of xylem in flowers and other organs to resist embolism under drought conditions is helpful for a deeper understanding of the evolution of flowering plants and water transport in different plant organs. In the present study, we addressed a fundamental question of vulnerability of peduncles to drought, with a focus on the relationship between peduncle xylem embolism and its vascular structures, which are functionally important in petioles and stems as well (Blackman et al., 2010; Brodribb et al., 2017; Skelton et al., 2018). We found that the capacity of Magnolia grandiflora to resist xylem embolism is organ-specific. Furthermore, our data showed that the dimension of bordered pits in vessels is significantly correlated with cavitation resistance among peduncles, petioles and stems in Magnolia grandiflora. Such research in woody species is particularly important since many woody angiosperms (e.g., Magnolia grandiflora) are not only important ornamental plants, but they play key roles in ecological systems. Further investigations of these relationships and their underlying mechanisms will advance our understanding of the responses of flowers to drought stress.
Author contributionsF.P.Z., J.L.Z. and H.H. designed the study. F.P.Z. performed the experiments. F.P.Z. and J.L.Z. analyzed the data. F.P.Z., J.L.Z. and T.J.B. wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Declaration of competing interestThe authors declare no conflict of interest.
AcknowledgmentsWe thank Rong-Fei Chen and Jing-Qiu Feng for assistance with measurements of anatomical traits and water potentials. We thank Zhi-Jia Gu for providing technical support with electron microscopy. This study was supported by the National Natural Science Foundation of China (31670415, 31870385, 31960224), the "Young Top Talents" Ten Thousands Plan in Yunnan Province (YNWR-QNBJ-2018-337), Science research of Yunnan Provincial Department of Education (2019J1068) and open funding from the CAS Key Laboratory of Tropical Forest Ecology to F.P. Zhang.
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1111/nph.16927.
Adams H.D., Zeppel M.J.B., Anderegg W.R.L., et al, 2017. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol., 1: 1285-1291. DOI:10.1038/s41559-017-0248-x |
Anderegg W.R.L., Klein T., Bartlett M., et al, 2016. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl. Acad. Sci. U.S.A., 113: 5024-5029. DOI:10.1073/pnas.1525678113 |
Barigah T.S., Charrier O., Douris M., et al, 2013. Water stress-induced xylem hydraulic failure is a causal factor of tree mortality in beech and poplar. Ann. Bot, 112: 1431-1437. DOI:10.1093/aob/mct204 |
Blackman C.J., Brodribb T.M., Jordan G.J., 2010. Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytol., 188: 1113-1123. DOI:10.1111/j.1469-8137.2010.03439.x |
Bouche P.S., Larter M., Domec J.C., et al, 2014. A broad survey of hydraulic and mechanical safety in the xylem of conifers. J. Exp. Bot., 65: 4419-4431. DOI:10.1093/jxb/eru218 |
Bourbia I., Carins-Murphy M.R., Gracie A., et al, 2020. Xylem cavitation isolates leaky flowers during water stress in pyrethrum. New Phytol., 227: 146-155. DOI:10.1111/nph.16516 |
Bréda N., Huc R., Granier A., et al, 2006. Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci., 63: 625-644. DOI:10.1051/forest:2006042 |
Brodribb T.J., Cochard H., 2009. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol., 149: 575-584. DOI:10.1104/pp.108.129783 |
Brodribb T.J., Bowman D.J.M.S., Nichols S., et al, 2010. Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit. New Phytol., 188: 533-542. DOI:10.1111/j.1469-8137.2010.03393.x |
Brodribb, T.J., Skelton, R.P., McAdam, S.A.M., et al., 2016. Visual quantification of embolism reveals leaf vulnerability to hydraulic failure, New Phytol. 209, 1403-1409.
|
Brodribb T.J., Carriqui M., Delzon S., et al, 2017. Optical measurement of stem xylem vulnerability. Plant Physiol., 174: 2054-2061. DOI:10.1104/pp.17.00552 |
Brodribb T.J., Powers J., Cochard H., et al, 2020. Hanging by a thread? Forests and drought. Science, 368: 261-266. DOI:10.1126/science.aat7631 |
Boyer I.S., Westgate M.E., 2004. Grain yields with limited water. J. Exp. Bot., 55: 2385-2394. DOI:10.1093/jxb/erh219 |
Chen, Y.J., Maenpuen, P., Zhang, Y.J., et al., 2020. Quantifying vulnerability to embolism in tropical trees and lianas using five methods: Can discrepancies be explained by xylem structural traits? New Phytol. https://www.R-project.org/.
|
Choat B., Ball M.C., Luly J.G., et al, 2005. Hydraulic architecture of deciduous and evergreen dry rainforest tree species from north-eastern Australia. Trees, 19: 305-311. DOI:10.1007/s00468-004-0392-1 |
Choat B., Cobb A.R., Jansen S., 2008. Structure and function of bordered pits: new discoveries and impacts on whole-plant hydraulic function. New Phytol., 177: 608-626. DOI:10.1111/j.1469-8137.2007.02317.x |
Choat B., Jansen S., Brodribb T.J., et al, 2012. Global convergence in the vulnerability of forests to drought. Nature, 491: 752-755. DOI:10.1038/nature11688 |
Feild T.S., Chatelet D.S., Brodribb T.J., 2009. Giant flowers of Southern magnolia are hydrated by the xylem. Plant Physiol., 150: 1587-1597. DOI:10.1104/pp.109.136127 |
Hacke U.G., Jacobsen A.L., Pratt R.B., 2009. Xylem function of arid-land shrubs from California, USA: an ecological and evolutionary analysis. Plant Cell Environ., 32: 1324-1333. DOI:10.1111/j.1365-3040.2009.02000.x |
Hargrave K.R., Kolb K.J., Ewers F.W., et al, 1994. Conduit diameter and drought-induced embolism in Salvia mellifera Greene (Labiatae). New Phytol., 126: 695-705. DOI:10.1111/j.1469-8137.1994.tb02964.x |
Hochberg U., Windt C.W., Ponomarenko A., et al, 2017. Stomatal closure, basal leaf embolism and shedding protect the hydraulic integrity of grape stems. Plant Physiol., 174: 764-775. DOI:10.1104/pp.16.01816 |
Jansen S., Choat B., Pletsers A., 2009. Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. Am. J. Bot., 96: 409-419. DOI:10.3732/ajb.0800248 |
Johnson D.M., McCulloh K.A., Meinzer F.C., et al, 2011. Hydraulic patterns and safety margins, from stem to stomata, in three eastern U. S. tree species. Tree Physiol., 31: 659-668. DOI:10.1093/treephys/tpr050 |
Lambrecht S.C., 2013. Floral water costs and size variation in the highly selfing Leptosiphon bicolor (Polemoniaceae). Int. J. Plant Sci., 174: 74-84. DOI:10.1086/668230 |
Lens F., Sperry J.S., Christman M.A., et al, 2011. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytol., 190: 209-723. |
Li S., Lens F., Espino S., et al, 2016. Intervessel pit membrane thickness as a key determinant of embolism resistance in angiosperm xylem. IAWA J., 37: 152-171. DOI:10.1163/22941932-20160128 |
Memmott J., Waser N.M., 2002. Integration of alien plants into a native flower-pollinator visitation web. Proc. Roy. Soc. B Biol. Sci., 269: 2395-2399. DOI:10.1098/rspb.2002.2174 |
Pammenter N.W., Van der Willigen C., 1998. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiol., 18: 589-593. DOI:10.1093/treephys/18.8-9.589 |
Passioura J., 2006. Increasing crop productivity when water is scarce from breeding to field management. Agr. Water Manage., 80: 176-196. DOI:10.1016/j.agwat.2005.07.012 |
Roddy A.B., Brodersen C.R., Dawson T.E., 2016. Hydraulic conductance and the maintenance of water balance in flowers. Plant Cell Environ., 41: 2123-2132. |
Roddy A.B., Simonin K.A, McCulloh K.A., et al, 2018. Water relations of Calycanthus flowers: hydraulic conductance, capacitance, and embolism resistance. Plant Cell Environ., 41: 2250-2262. DOI:10.1111/pce.13205 |
Rodriguez-Dominguez C.M., Carins Murphy M.R., Lucani C., et al, 2018. Mapping xylem failure in disparate organs of whole plants reveals extreme resistance in olive roots. New Phytol., 218: 1025-1035. DOI:10.1111/nph.15079 |
Schindelin, J., Arganda-Carreras, I., Frise, E., et al., 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676-682.
|
Skelton R.P., Brodribb T.J., Choat B., 2017. Casting light on xylem vulnerability in an herbaceous species reveals a lack of segmentation. New Phytol., 214: 561-569. DOI:10.1111/nph.14450 |
Skelton R.P., Dawson T.E., Thompson S.E., et al, 2018. Low vulnerability to xylem embolism in leaves and stems of North American Oaks. Plant Physiol., 77: 1066-1077. |
Sperry J.S., 2003. Evolution of water transport and xylem structure. Int. J. Plant Sci., 164: S115-S127. DOI:10.1086/368398 |
Tyree, M.T., Ewers, F.W., 1991. The hydraulic architecture of trees and other woody plants, New Phytol. 119, 345-360.
|
Urli M., Porte A.J., Cochard H., et al, 2013. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiol., 33: 672-683. |
van der Niet T., Johnson S.D., 2012. Phylogenetic evidence for pollinator-driven diversification of angiosperms. Trends Ecol. Evol., 27: 353-361. DOI:10.1016/j.tree.2012.02.002 |
Zhang F.P., Brodribb T.J., 2017. Are flowers vulnerable to xylem cavitation during drought?. Proc. Roy. Soc. B Biol. Sci., 284: 0162642. |
Zhang F.P., Carins Murphy M.R., Cardoso A.A., et al, 2018. Similar geometric rules govern the distribution of veins and stomata in petals, sepals and leaves. New Phytol., 219: 1224-1234. DOI:10.1111/nph.15210 |
Zimmermann M.H., 1978. Hydraulic architecture of some diffuse-porous trees. Can. J. Bot., 56: 2286-2295. DOI:10.1139/b78-274 |
Zimmermann, M.H., 1983. Xylem Structure and the Ascent of Sap. Springer Berlin Heidelberg, Berlin/Heidelberg, Germany.
|
R Core Team, 2020. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Retrieved from. https://www.R-project.org/. (Accessed 10 January 2020).
|



