b. University of Chinese Academy of Sciences, Beijing, 100049, China;
c. Kunming City Center for Disease Control and Prevention, Kunming, 650034, China
Mosquitoes, which are the main vector of mosquito-borne diseases such as dengue fever, yellow fever, malaria and chikungunya fever, seriously threaten human health (Brown and Hebert, 1997). The use of insecticides and repellents represent major strategies for preventing mosquito-borne diseases (Ma et al., 2019). Insecticides obtained by chemical synthesis are usually characterized by their high efficiency and speed. However, the widespread use of synthetic insecticides has increased environmental pollution and insect resistance (Ascher, 1993; Govindarajan et al., 2012; Raguraman and Singh, 1997). Insect repellents are an alternative strategy to control mosquitoes. The best-known repellent on the market is N, N-diethyl-3-methylbenzamide (DEET). However, studies have found that DEET produces a number of side effects, including urticaria syndrome, anaphylaxis, hypotension, and neurotoxicity (Adeiran and Fabiyi, 2012; Brown and Hebert, 1997; Corbel et al., 2009). By contrast, plant-derived repellents and insecticides, especially plant essential oils, have shown significant advantages such as rapid biodegradability, eco-friendliness, and safety for insect and mosquito control (Ali et al., 2015; Bedini et al., 2018; Wang et al., 2015; Yang et al., 2005). Therefore, it is essential to identify new plant-derived repellents and insecticides for mosquito control.
One potential source for plant-derived mosquito repellent is Zingiber cassumunar Roxb (Zingiberaceae), synonym of Zingiber purpureum Rosc., recorded in Flora of Yunnan, and commonly known as cassumunar ginger (Kunming Institute of Botany, 1997). Z. cassumunar, known in Thailand as Zingiber montanum (J.Koenig) Link ex A.Dietr (Sanatombi and Sanatombi, 2017; Verma et al., 2018) is called 'bu lei' by the Dai people living in Yunnan province of China, where it is an essential raw material in Dai medicine, one of China's four major ethnic medicines. For example, it has been used as the main material of the Dai patent medicine called "pills of shuangjiang weitong" (Wei and Tang, 2018). The plant rhizome has been widely used in folk medicine to treat inflammations, sprains, rheumatism, muscular pain, wounds, asthma, coughs, skin diseases, dyspepsia, stomach bloating and also has the folk usage of mosquito repellent (Mektrirat et al., 2020; Pongprayoon et al., 1997; Sharifi-Rad et al., 2017; Singh et al., 2015; Talukder et al., 2015; Verma et al., 2018). Z. cassumunar has also shown extensive pharmacological activities, including anti-inflammatory, analgesic, antimicrobial, anti-allergic, antiarrhythmatic, antihistaminic, anticancer, and antioxidant activities (Cotchakaew and Soonwera, 2019; Sanatombi and Sanatombi, 2017; Singh et al., 2015). Our recent study showed that Z. cassumunar possesses anti-aging, skin-whitening, and anti-inflammatory properties, indicating its potential value for natural cosmetic products (Li et al., 2019).
Previous studies have reported that Zingiber cassumunar essential oil or extracts exhibit larvicidal and repellent activities against Aedes aegypti mosquitoes and insecticide activity against several pests (Bandara et al., 2005; Boonyuan et al., 2014; Jantan et al., 2003; Nugroho et al., 1996; Phasomkusolsil and Soonwera, 2010; Phukerd and Soonwera, 2014; Talukder et al., 2015; Wang et al., 2015). Recent research has also shown that combinations of Alpinia galanga, Curcuma zedoaria, and Z. cassumunar with Eucalyptus globulus essential oils possess ovicidal and adulticidal activities against Ae. albopictus and Anopheles minimus (Cotchakaew and Soonwera, 2019). However, to date, the constituents of Z. cassumunar essential oil responsible for its repellent, larvicidal and adulticidal activity against Ae. albopictus are still unclear.
Aedes albopictus, known as the Asian tiger mosquito, is a dangerous vector of arboviruses that causes dengue fever, chikungunya fever, and yellow fever (Labbé et al., 2010). In this study we evaluated the repellent, larvicidal, and adulticidal activities of the Z. cassumunar essential oil against Ae. albopictus and identified the active constituents of the essential oil.
2. Materials and methods 2.1. General experimental proceduresNuclear magnetic resonance (NMR) spectra were recorded on a Bruker AV-500 at 500 MHz for the 1H NMR and 13C NMR (Bruker, Bremen, Germany). Chemical shifts (δ) were given in parts per million (ppm) with reference to the solvent signals, and coupling constants were measured in hertz. Optical rotation was recorded on an Autopol Ⅵ polarimeter (Rudolph, US). Column chromatography (CC) was done using silica gel (200–300 mesh, Qingdao Marine Chemical Co. Ltd., China). Thin layer chromatography (TLC) was performed on silica gel GF254 (Qingdao Marine Chemical Co. Ltd., China), and spots were visualized by heating silica gel plates sprayed with 1% vanillin and 10% H2SO4 in ethanol.
2.2. Plant materialsFresh rhizomes of Zingiber cassumunar were collected from the Dai Hospital in Dai Autonomous Prefecture of Xishuangbanna, Yunnan Province, China. A voucher specimen (KUN 1415653) was deposited in the Herbarium of Kunming Institute of Botany, Chinese Academy of Sciences (KUN).
2.3. Breeding of mosquitoesAedes albopictus were reared in the mosquito insectary at Kunming City Center for Disease Control and Prevention. The insectary was maintained at 27 ± 2 ℃ and 60 ± 10% relative humidity under a cycle of light and dark (14 h: 10 h). Mosquito breeding went through four stages: egg, larva, pupa, and adult. Eggs attached to filter paper were placed in a ceramic bowl containing dechlorinated water. About a week later, larvae began to hatch out. Larvae were fed with a 2% suspension of rat food (Specialty Feeds, Specialty Feeds Pty Ltd., Perth, Australia). When larvae pupated, pupae were transferred from the bowl to a cup containing dechlorinated water and placed in a screened cage where adults began to breed. Adults were fed with a ball of cotton soaked in a 10% sucrose solution. First instar larvae were used for the larvicidal bioassay and 4- to 5-day-old adults were used for the repellent and adulticidal bioassay.
2.4. Extraction of essential oilFive kilograms of fresh Zingiber cassumunar rhizome was cut into small pieces and subjected to hydrodistillation for 7 h as described in the Chinese Pharmacopeia (State Pharmacopeia Commission, 2015). Z. cassumunar essential oil was separated from aqueous solution and dried over anhydrous sodium sulfate with an extraction ratio of 1.13%.
2.5. Gas chromatography and gas chromatography-mass spectrometry analysisZingiber cassumunar essential oil was analyzed using an Agilent 7890A gas chromatograph with an HP-5 (Agilent 19091J-115, 5% diphenyl polysiloxane, 50 m × 0. 32 mm i.d., 0.52 μm film thickness) capillary column and a flame ionization detector (GC-FID). The oven temperature was programmed to rise from 50 to 250 ℃ at the rate of 5 ℃/min and then hold isothermally for 10 min at 250 ℃. Finally, oven temperature was increased to 300 ℃ for 3 min post-run with helium as the carrier gas at a flow rate of 1.5 mL/min. The injector and the detector temperatures were set at 250 ℃, and all injections were performed in split mode adjusted at 40:1. The relative percentages of chemical compositions in the Z. cassumunar essential oil were calculated using integration of the peak areas in the GC-FID chromatograms.
Zingiber cassumunar essential oil was analyzed by gas chromatography-mass spectrometry (GC–MS). The capillary column was a HP-5 (Agilent 19091J-115, 5% diphenyl polysiloxane, 50 m × 0. 32 mm i.d., 0.52 μm film thickness). The oven temperature was programmed to rise from 50 to 250 ℃ at a rate of 5 ℃/min and hold isothermally for 10 min at 250 ℃. The injector temperature was set at 250 ℃. Helium was used as the carrier gas with a flow rate of 1.5 mL/min. The mass spectrum was a quadrupole mass spectrometer with an electron impact ionization at 70 eV. Ion source and quadrupole mass analyzer temperatures were set at 230 ℃ and 150 ℃, respectively. Electron ionization mass spectra were recorded over the mass range from 50 to 500 m/z.
Chemical composition of Zingiber cassumunar essential oil was tentatively identified by comparing fragmentation patterns in the mass spectra with those listed in a commercial mass spectral library (NIST 14) and finally confirmed by comparing the relative retention index (RRI) determined relative to the retention times of the homologous series of n-alkanes (C8–C30) with the literature retention index (RIlit) reported by Adams (Adams, 2007). The isolated compounds were confirmed by comparing their NMR data with those of previously reported data.
2.6. Bioassay-guided fractionation and purification of active compoundsZingiber cassumunar essential oil (8.17 g) was subjected to a gradient elution of petroleum ether (PE)-ethyl acetate (EtOAc) (100:0, 50:1, 20:1, 7:1) on a silica gel column to yield four fractions: Fr.1, Fr.2, Fr.3 and Fr.4. Based on the repellent bioassay, Fr.2 (2.25 g) was further fractionationed by a silica gel column, eluting with PE-CHCl3 (10:1, 9:1, 8:1, 7:1) to successively give 1 ((−)-terpinen-4-ol, 435 mg) and 2 ((E)-1-(3, 4-dimethoxyphenyl) butadiene, 330 mg).
2.7. In-cage mosquito repellent bioassayWe determined the minimum effective dosage (MED) of Zingiber cassumunar essential oil required for repellent activity using an in-cage mosquito repellent model described by Ma et al. (2019). These experiments were conducted at the Kunming Center for Disease Control and Prevention. Bioassays were performed using five concentrations of each tested sample (1.50, 0.75, 0.38, 0.19, 0.1, 0.05, 0.02 mg/cm2), which were prepared as follows. Tested sample including Z. cassumunar essential oil, fractions and compounds isolated from Z. cassumunar essential oil was dissolved with anhydrous ethanol to yield a 2 mL solution. Then, absorbent cotton gauze (5 cm × 10 cm) was treated with 1 mL of solution. The remaining 1 mL of solvent was diluted by adding 1 mL anhydrous ethanol and absorbent cotton gauze was treated with 1 mL of this solution. This process was repeated for three additional dilutions. The treated gauze was placed in a ventilated area for 3–5 min to allow the anhydrous ethanol to evaporate. Once dried, treated gauze was placed on the back of the hands of volunteers. First, the hands and arms of volunteers were wrapped in two layers of polyethylene film to protect them from mosquito bites. A window was cut in the plastic film (4 cm × 8 cm), which was covered by dry treated gauze. Volunteers placed treated hands into cages that contained three hundred 4- to 5-day-old adult mosquitoes. The number of mosquitoes that perched on the gauze was recorded during a 1-min test period. We regarded fewer than three bites as evidence of an effective dosage; fractions that showed repellent activity at high concentrations were subsequently tested at lower concentrations until the MED was determined. If three bites or more were recorded at a given concentration, a higher concentration was tested until a MED was determined. If a concentration of 1.5 mg/cm2 was ineffective, the fraction was deemed ineffective. DEET was used as a positive control and ethanol was used as the solvent control.
2.8. Larvicidal bioassayWe evaluated the larvicidal activity of Zingiber cassumunar essential oil and its major constituents against Aedes albopictus by using the impregnation method described by Ma et al. (2019). Bioassays were performed using five concentrations of tested sample (104, 52, 26, 13, 6.5 μg/mL), which were prepared as follows. Briefly, 2.5 mg of each tested sample was dissolved in 15 μL dimethyl sulfoxide (DMSO) and diluted with deionized water to 12 mL sample solution. Then, 6 mL of sample solution was added to 0.6 mL of a 2% suspension of rat feed and was diluted with deionized water to 12 mL larvae test solution. The remaining 6 mL sample solution was diluted by using the above dilution method, which was then repeated for three additional concentrations. We used an 8-cm Pasteur pipet to transfer first instar larvae (in 1 mL of test solution) to a 24-well plate. We placed five larvae in each well and used five wells. We then added 5 mL of larvae test solution to each well. We used DMSO (2.5 mg) as a blank control group, which consisted of five duplicate wells. Larval mortality was recorded after 24 h. Larvae that showed no movement in the well after manual disturbance of water by a pipet tip were recorded as dead.
2.9. Adulticidal bioassaysWe evaluated the adulticidal activity of Zingiber cassumunar essential oil against Aedes albopictus by using airtight fumigation in conical flasks as described by Yang et al. (2005). Each 250 mL conical flask was placed into ten 4- to 5-day-old female mosquitoes. Filter paper (1 cm × 3 cm) was glued to the bottle of a rubber stopper. The rubber stopper was tightly stuffed into the conical flask to ensure a good seal. The test samples contained the Z. cassumunar essential oil, (−)-terpinen-4-ol, and (E)-1-(3, 4-dimethoxyphenyl) butadiene. The Z. cassumunar essential oil was diluted with acetone to give five concentration (1.6, 3.2, 6.4, 12.8 and 25.6%). The testing concentrations of (−)-terpinen-4-ol, and (E)-1-(3, 4-dimethoxyphenyl) butadiene were 0.4, 0.8, 1.6, 3.2 and 6.4%. A volume of 10 μL of testing solution was immediately dropped on filter paper and the rubber stopper quickly stuffed into the conical flask. In the control groups, acetone (10 μL) was used to replace the test sample under the same conditions. Each sample was tested three times. The mosquitoes were constantly fumigated and the mortality was recorded at 0.5, 1, 2, 4, 8 and 24 h. Mosquitoes were considered dead when they did not move in response to flask shaking. The fumigating adulticidal activity of the testing samples against Ae. albopictus was evaluated by LC50 (50% lethal concentration) and LT50 (50% lethal time). LT50 was obtained by the airtight fumigation model in conical flasks and tested under the concentration of the LC95 at 0.5 h. The number of deaths was checked and recorded every minute for 30 min.
2.10. Statistical analysisWhen mortality of the blank control was greater than 20%, larvicidal and adulticidal bioassays were discarded and repeated. LC50, LC90, LC95, LT50, 95% confidence intervals, and regression equation were analyzed to evaluate the larvicidal and adulticidal activities of Zingiber cassumunar essential oil against Aedes albopictus.
3. Results and discussion 3.1. Chemical composition of essential oilChemical constituents of essential oil from Zingiber cassumunar rhizomes were analyzed by GC-FID, GC–MS and NMR. Twenty-three components were identified, accounting for 92.75% of the overall composition. Sabinene (49.90%), (−)-terpinen-4-ol (13.51%, 1) and (E)-1-(3, 4-dimethoxyphenyl) butadiene (DMPBD, 10.31%, 2) were major compounds in Z. cassumunar essential oil (Table 1). Z. cassumunar essential oil was characterized by monoterpene hydrocarbons (63.88%), oxygenated monoterpenes (27.02%) and sesquiterpene hydrocarbons (1.85%).
| NO. | RRIa | RIlitb | compounds | relative content (%)c | identification |
| 1 | 933 | 924 | α-thujene | 0.47 | MS, RI |
| 2 | 942 | 932 | α-pinene | 1.33 | MS, RI |
| 3 | 982 | 969 | sabinene | 49.90 | MS, RI |
| 4 | 988 | 974 | β-pinene | 2.60 | MS, RI |
| 5 | 993 | 988 | myrcene | 1.60 | MS, RI |
| 6 | 1025 | 1014 | α-terpinene | 1.89 | MS, RI |
| 7 | 1032 | 1022 | o-cymene | 0.28 | MS, RI |
| 8 | 1037 | 1024 | limonene | 0.47 | MS, RI |
| 9 | 1040 | 1025 | β-phellandrene | 0.84 | MS, RI |
| 10 | 1042 | 1026 | cineole | 0.17 | MS, RI |
| 11 | 1067 | 1054 | γ-terpinene | 3.79 | MS, RI |
| 12 | 1077 | 1065 | trans-4-thujanol | 0.91 | MS, RI |
| 13 | 1098 | 1086 | terpinolene | 0.71 | MS, RI |
| 14 | 1109 | 1098 | trans-4-thujanol | 0.80 | MS, RI |
| 15 | 1133 | 1118 | cis-p-menth-2-en-1-ol | 0.44 | MS, RI |
| 16 | 1151 | 1136 | trans-p-menth-2-en-1-ol | 0.25 | MS, RI |
| 17 | 1192 | 1174 | (−)-terpinen-4-ol | 13.51 | MS, RI, NMR |
| 18 | 1203 | 1186 | α-terpineol | 0.26 | MS, RI |
| 19 | 1220 | 1207 | trans-piperitol | 0.12 | MS, RI |
| 20 | 1361 | 1346 | α-terpinyl acetate | 0.25 | MS, RI |
| 21 | 1508 | 1493 | α-zingiberene | 0.26 | MS, RI |
| 22 | 1540 | 1521 | β-sesquiphellandrene | 1.59 | MS, RI |
| 23 | 1643 | (E)-1-(3, 4-dimethoxyphenyl)butadiene | 10.31 | NMR | |
| Total | 92.75 | ||||
| Monoterpene hydrocarbons | 63.88 | ||||
| Oxygenated monoterpenes | 27.02 | ||||
| Sesquiterpene hydrocarbons | 1.85 | ||||
| The essential oil was diluted with n-hexane as a concentration of 2%. a Relative retention index calculated using a series of n-alkanes (C8–C30) on the HP-5 capillary column. b Literature relative index. c Relative content calculated using peak normalization method by GC-FID. |
|||||
Previous research on the chemical composition of Zingiber cassumunar essential oil from China, the identified sabinene, terpinen-4-ol, and γ-terpinen, but not DMPBD, likely because NIST 14 and retention index methods fail to identify this compound (Wang et al., 2015). In this study, DMPBD was isolated and identified by NMR. In addition, the optical rotation of (−)-terpinen-4-ol was firstly measured in present study with [α]D20-15.88 (c 0.17, acetone). Terpinen-4-ol has been reported to exist as two optical isomers, which show different bioactivities in different plants (Li et al., 2015).
The chemical composition of the Zingiber cassumunar essential oil in various countries and regions has been reported. Previous studies (Bua-in and Paisooksantivatana, 2009; Leelarungrayub et al., 2017; Sukatta et al., 2009; Taroeno et al., 1991; Verma et al., 2018; Yingngam and Brantner, 2018) have shown that rhizome of Z. cassumunar essential oil from Thailand, India, and Indonesia are mainly composed of sabinene, terpinen-4-ol, and DMPBD, which is consistent with the results of our study. However, other studies have reported major components of Z. cassumunar essential oil that are inconsistent with our findings. For example, previous studies have reported that the main constituents of the Z. cassumunar essential oil from Thailand are sabinene, terpinen-4-ol, and γ-terpinen, respectively (Chaiyana et al., 2017; Okonogi, 2012). The chemical compositions of the Z. cassumunar essential oil from Malaysia and Bangladesh also differ from Z. cassumunar essential oil from China and Thailand; specifically, the main components of Z. cassumunar essential oil from Malaysia has been reported as 2, 6, 9, 9-tetramethyl-2, 6, 10- cycloundecatrien-1-one and α-caryophyllene, whereas in Bangladesh these components have been reported as triquinacene 1, 4-bis (methoxy), (Z)-ocimene and terpinen-4-ol (Bhuiyan et al., 2008; Kamazeri et al., 2012). These differences of chemical composition of the Z. cassumunar essential oil may be caused by variation in growth environment, harvesting period, geographical distribution (Yang et al., 2005).
3.2. Isolation and identification of compounds 1 and 2 from Zingiber cassumunar essential oilTo identify the active constituents of Zingiber cassumunar essential oil that repel mosquitoes, we used silica gel column chromatography to fractionate the essential oil into four fractions (Fr.1, Fr.2, Fr.3 and Fr.4). Our bioassay indicated that Fr.2 had mosquito repellant properties and was therefore further separated by silica gel column chromatography to successively obtain compounds 1 and 2, which are the major components of the Z. cassumunar essential oil. After comparing their NMR data with previous reports, we identified compound 1 as (−)-terpinen-4-ol and compound 2 as (E)-1-(3, 4-dimethoxyphenyl) butadiene (DMPBD) (Ngo and Brown, 1998; Tangyuenyongwatana and Gritsanapan, 2008). The structures of compounds 1 and 2 are shown in Fig.1.
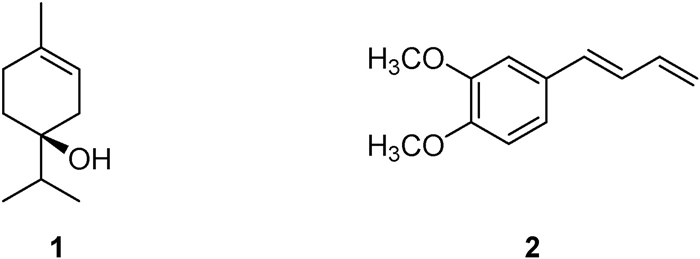
|
| Fig. 1 The structures of isolated compounds 1 and 2. |
(−)-Terpinene-4-ol (1): colorless oil, [α]D25-15.88 (c 0.170, acetone); Rf = 0.65 (silica, PE/CHCl3 1:1), Vanillin as chromogenic agent; 1H NMR (500 MHz, CDCl3) δ: 5.30 (1H, dtt, J = 4.9, 3.5, 1.7 Hz, H-2), 2.17(1H, m, H-6a), 2.14(1H, m, H-3a), 1.92 (2H, m, H-3b, 6b), 1.68 (3H, s, H-10), 1.65 (2H, m, H-7, 5a), 1.55 (1H, ddd, J = 13.4, 11.3, 6.0 Hz, H-5b), 0.94 (3H, d, J = 6.9 Hz, H-8 or 9), 0.92 (3H, d, J = 6.9 Hz, H-8 or 9); 13C-NMR (125 MHz, CDCl3) δ: 134.0 (C-1), 118.6 (C-2), 71.9 (C-4), 36.9 (C-7), 34.7 (C-3), 30.9 (C-5), 27.2 (C-6), 23.4 (C-10), 17.0(C-8), 17.0(C-9).
(E)-1-(3, 4-Dimethoxyphenyl) butadiene (DMPBD, 2): white solid; Rf = 0.58 (silica, PE/CHCl3 1:1), Vanillin as chromogenic agent; 1H-NMR (CDCl3, 500 MHz) δ: 6.96 (1H, d, J = 2.0 Hz, H-2′), 6.94 (1H, dd, J = 8.0, 2.0 Hz, H-6′), 6.82 (1H, d, J = 8.0 Hz, H-5′), 6.67 (1H, dd, J = 15.3, 10.0 Hz, H-3), 6.49 (2H, m, H-1), 5.30 (1H, d, J = 15.3 Hz, H-4), 5.13 (1H, d, J = 10.0 Hz, H-2), 3.91 (3H, s), 3.88 (3H, s); 13C NMR (CDCl3, 125 MHz) δ: 149.0 (C-3′), 148.9 (C-4′), 137.2 (C-2), 132.6 (C-4), 130.2 (C-1′), 127.9 (C-3), 119.8 (C-6′), 116.7 (C-1), 111.1 (C-5′), 108.6 (C-2′), 55.9 (OCH3), 55.8 (OCH3).
3.3. Mosquitoes repellent activityWe evaluated the mosquito repellent activity of Zingiber cassumunar essential oil and its major constituents on Aedes albopictus using an in-cage mosquito repellent model (Table 2). Compared to the reference standard N, N-diethyl-3-methylbenzamide (DEET) (0.03 ± 0.01 mg/cm2), Z. cassumunar essential oil showed moderate repellent activity with a MED of 0.16 ± 0.01 mg/cm2. To identify the major active components in theZ. cassumunar essential oil, we evaluated the mosquito repellent activity of all four fractions of Z. cassumunar essential oil. Fr.1, Fr.3, and Fr.4 showed weak mosquito repellent activity. In contrast, Fr.2 showed moderate repellent activity with a MED of 0.25 ± 0.01 mg/cm2. As mentioned in the previous section, Fr.2 was subsequently purified to obtain two major components, compounds 1 and 2, which were then tested for their mosquito repellent activity. Compound 1 exhibited moderate mosquito repellent activity with a MED of 0.19 ± 0 mg/cm2. Compound 2 showed weak repellent activity with a MED of 0.75 ± 0 mg/cm2. These results suggest that the major mosquito repellent ingredient in Z. cassumunar essential oil is compound 1. The results of repellent bioassay were summarized in Table 2.
| Samples | Minimum effective dosage (MED) (mg/cm2) | Repellent activity |
| DEETa | 0.03±0.01 | active |
| essential oil | 0.16±0.01 | moderate |
| Fr.1b | 0.63±0.03 | weak |
| Fr.2 | 0.25±0.01 | moderate |
| Fr.3 | 0.63±0.03 | weak |
| Fr.4 | 0.63±0.03 | weak |
| (−)-terpinen-4-olc | 0.19±0 | moderate |
| DMPBD | 0.75±0 | weak |
| a DEET was postive control. b Fr.1-Fr.4 were four fractions of Zingiber cassumunar essential oil. c (−)-terpinen-4-ol and DMPBD were isolated from Fr.2. |
||
Previous research has indicated that the combination of Zingiber cassumunar and Sweet basil (Ocimum basilicum) essential oil can be used as both a repellent and a feeding deterrent against mosquitoes (Phukerd and Soonwera, 2014). In the present work, we firstly identified that the active constituent of Z. cassumunar essential oil that repels mosquitoes is (−)-terpinen-4-ol (1). This finding was consistant with previous studies that found that (−)-terpinen-4-ol (1) derived from Cryptomeria japonica repels Ae. aegypti and Ae. albopictus (Gu et al., 2009).
3.4. Larvicidal activityWe evaluated toxicities of Zingiber cassumunar essential oil and its major components against first instar larvae of Aedes albopictus by larvicidal bioassay (Table 3). The larvicidal assay indicated that Z. cassumunar essential oil is strongly toxic to first instar larvae of Ae. albopictus, with a LC50 of 44.86 μg/L after 24 h treatment. Compounds 1 and 2 showed no toxicity under the highest tested dosage (104 mg/mL). We also evaluated the toxicity of Fr. 1, which contained 70% sabinene. However, Fr.1 showed no toxicity under the highest tested dosage (104 mg/mL). The blank control group (DMSO) also showed no toxicity. Therefore, larvicidal activity of the Zingiber cassumunar essential oil was not due to its three major components. Other minor constituents of the Z. cassumunar essential oil were probably responsible for the larvicidal activity.
| Regression equation | LC50 (95%CI) μg/L | LC90 (95%CI) μg/L | |
| Essential oil | Y=0.04X-1.58 | 44.86 (36.85–55.94) |
81.33 (67.34–107.30) |
| LC50 and LC90 were the concentration of causing 50% and 90% mortality against larvae after 24 h; 95%CI: 95% confidence intervals. | |||
Previous studies have identified several chemical constituents of Zingiber cassumunar essential oil (α-terpinene, γ-terpinene, β-myrcene, terpinolene, β-pinene, (+)-limonene, and limonene) that show larvicidal activities against Aedes aegypti, Ae. albopictus, and Culex quinquefasciatus (Cheng et al., 2009a, b, Dias et al., 2014, Govindarajan et al., 2012, Haribalan et al., 2009). In addition, plenylbutanoids isolated from Z. cassumunar essential oil also exhibited larvicidal effects againstKaempferia rotunda in a chronic feeding bioassay (Nugroho et al., 1996). DMPBD isolated from Z. cassumunar has shown larvicidal activity against the second instar of Ae. aegypti and ovicidal activity against the bruchid Callosobruchus maculatus (Bandara et al., 2005). However, Z. cassumunar essential oil obtained from Malaysia shows no activity against the four instar of Ae. aegypti, likely because the chemical composition of the rhizome oil from Malaysia differs from those of other countries (Jantan et al., 2003).
3.5. Adulticidal activityWe evaluated the adulticidal activities of the Zingiber cassumunar essential oil, compounds 1, 2, and sabinene (substituted by Fr.1, which contained more than 70% sabinene) against adults of Aedes albopictus using a fumigating bioassay. Compound 2 and sabinene did not result in mosquito death at the testing concentration of 6.4% after fumigating 24 h. The blank control (acetone) showed no toxicity after fumigating 24 h. Z. cassumunar essential oil showed medium adulticidal activity, while compound 1 exhibited potent adulticidal activity (Table 4). Essential oil concentrations of 25.6% (v/v) led to 100% mortality of mosquitoes after fumigating 4 h. Compound 1 concentrations of 6.4% led to 100% mortality after fumigating 1 h. After fumigating 24 h, the LC50 of Z. cassumunar essential oil and compound 1 were 5.44% and 2.10%, respectively.
| time (h) | regression equation | LC50 (%) | 95%CI (%) | Chi square | |
| ZCEO | 0.5 | Y=0.04X-1.58 | 23.60 | 19.76–30.24 | 10.9 |
| (−)-Terpinen-4-ol | Y=0.40X-2.26 | 5.63 | 4.86–6.80 | 13.7 | |
| ZCEO | 1 | Y=0.09X-1.66 | 18.80 | 15.84–23.13 | 13.8 |
| (−)-Terpinen-4-ol | Y=1.16X-3.77 | 3.26 | 2.92–3.84 | 2.9 | |
| ZCEO | 2 | Y=0.10X-1.51 | 15.79 | 13.24–19.17 | 14.7 |
| (−)-Terpinen-4-ol | Y=1.29X-3.96 | 3.07 | 2.76–3.48 | 2.7 | |
| ZCEO | 4 | Y=0.19X-2.03 | 10.61 | 9.10–12.71 | 6.0 |
| (−)-Terpinen-4-ol | Y=1.23X-3.57 | 2.91 | 2.60–3.30 | 1.9 | |
| ZCEO | 8 | Y=0.21X-1.97 | 9.54 | 8.17–11.38 | 4.2 |
| (−)-Terpinen-4-ol | Y=1.47X-3.73 | 2.53 | 2.25–2.82 | 3.3 | |
| ZCEO | 24 | Y=0.30X-1.63 | 5.44 | 4.51–6.62 | 4.8 |
| (−)-Terpinen-4-ol | Y=1.06X-2.23 | 2.10 | 1.82–2.25 | 6.0 |
The effect of fumigation time of Zingiber cassumunar essential oil and compound 1 were evaluated by LT50 (Table 5). The LT50 of Z. cassumunar essential oil and compound 1 were 14.33 min and 15.05 min under the concentration of LC95 at 0.5 h. These findings show that compound 1 possesses remarkable adulticidal activity and is one of the major that shows adulticidal activity against Ae. albopictus.
| Regression equation | LT50/mina 95%CI | LC95/%b | Chi square | |
| ZCEO | Y=0.19X-2.76 | 14.33 13.70–14.99 |
43.92 | 23.7 |
| (−)-Terpinen-4-ol | Y=0.23X-3.40 | 15.05 14.48–15.62 |
9.72 | 38.7 |
| a LT50 was the 50% lethal time under the concentration of LC95. b LC95 was the 95% lethal concentration at the fumigating time of 0.5 h. |
||||
Previous studies have reported that Zingiber cassumunar essential oil and compound 1 show fumigant and contact toxicities against Tribolium castaneum and Lasioderma serricorne adults (Wang et al., 2015). Additional main constituents of Z. cassumunar essential oil have been suggested to possess fumigant toxicity against Ae. aegypti, including 1, 8-cineole, p-cymene, γ-terpinene, 4-terpineol, and α-pinene (Lucia et al., 2013). We speculate that Z. cassumunar essential oil possesses insecticidal and adulticidal activities for mosquitoes and insects, and compound 1 may be the major active constituent responsible for its activities. Other minor constituents of Z. cassumunar essential oil, such as α-pinene and γ-terpinene, may also play a synergistic role in adulticidal activity.
4. ConclusionIn this work, we verified that Zingiber cassumunar essential oil acts as a mosquito repellent against Aedes albopictus and identified the main active compound as (−)-terpinen-4-ol. These findings are consistent with the traditional uses of Z. cassumunar essential oil in Dai medicine. This study firstly find that Z. cassumunar essential oil shows larvicidal activity against the first instar of Ae. albopictus. Moreover, we found that Z. cassumunar essential oil and its major components exhibit fumigant toxicity against Ae. albopictus adults, and that (−)-terpinen-4-ol is responsible for this adulticidal activity. These results suggest that Z. cassumunar essential oil possesses potential to be applied in plant-derived repellents and adulticides. (−)-Terpinen-4-ol can also be used in prevention and control of mosquitoes and insects as an important repellent and adulticidal active substance.
The present study found that chemical constituents of essential oil of Zingiber cassumunar from different regions are very different. In our previous study of non-volatile chemical components of this plant, some new compounds with bio-activity were also discovered (Li et al., 2019). That is to say, the secondary metabolites of Z. cassumunar vary from place to place. These bio-activities of Z. cassumunar from Yunnan may be related to the specificity of its chemical compositions. So it is important to protect plants in different habitats.
Author contributionMing-Xiang Li conducted experiments, validation, formal analysis, investigation, and wrote the manuscript. Yong-Peng Ma conducted visualization, methodology, and investigation. Hong-Xia Zhang conducted methodology and investigation. Hong-Zheng Sun and Hong-Hai Su were in charge of breeding and identification of the mosquitoes. Sheng-Ji Pei collected and identified the plant material. Zhi–Zhi Du conducted supervision, conceptualization, writing-Review & Editing, project administration, and funding acquisition.
Declaration of competing interestThe authors declare no conflict of interest.
AcknowledgmentsThis study was supported by grant from National Natural Science Foundation of China (31670337) and Research Project (ZYS2016-001) funded by Yunnan Key Laboratory for Wild Plant Resources.
Adams, R.P., 2007. Identification of essential oil components by gas chromatography/mass spectrometry, 4th edition. Allured Publishing Corporation, Carol stream, Illinois.
|
Adeiran O.I, Fabiyi E., 2012. A cream formulation of an effective mosquito repellent: a topical product from lemongrass oil (Cymbopogon citratus) Stapf. J. Nat. Prod. Plant Resour., 2: 322-327. |
Ali A., Tabanca N., Demirci B., et al, 2015. Chemical composition and biological activity of four salvia essential oils and individual compounds against two species of mosquitoes. J. Agric. Food Chem., 63: 447-456. DOI:10.1021/jf504976f |
Ascher K.R.S., 1993. Nonconventional insecticidal effects of pesticides available from the Neem tree, Azadirachta indica. Arch. Insect Biochem. Physiol., 22: 433-449. DOI:10.1002/arch.940220311 |
Bandara K.A.N.P., Kumar V., Saxena R.C., et al, 2005. Bruchid (Coleoptera: Bruchidae) ovicidal phenylbutanoid from Zingiber purpureum. J. Econ. Entomol., 98: 1163-1169. DOI:10.1603/0022-0493-98.4.1163 |
Bedini S., Flamini G., Ascrizzi R., et al, 2018. Essential oils sensory quality and their bioactivity against the mosquito Aedes albopictus. Sci. Rep., 8: 17857-17867. DOI:10.1038/s41598-018-36158-w |
Bhuiyan M.N.I., Chowdhury J.U., Begum J., 2008. Volatile constituents of essential oils isolated from leaf and rhizome of Zingiber cassumunar Roxb. Bangladesh J. Pharmacol., 3: 69-73. DOI:10.3329/bjp.v3i2.844 |
Boonyuan W., Grieco J.P., Bangs M.J., et al, 2014. Excito-repellency of essential oils against an Aedes aegypti (L.) field population in Thailand. J. Vector Ecol., 39: 112-122. DOI:10.1111/j.1948-7134.2014.12077.x |
Brown M., Hebert A.A., 1997. Insect repellents: An overview. J. Am. Acad. Dermatol., 36: 243-249. DOI:10.1016/S0190-9622(97)70289-5 |
Bua-in S., Paisooksantivatana Y., 2009. Essential oil and antioxidant activity of Cassumunar ginger (Zingiberaceae: Zingiber montanum (Koenig) Link ex Dietr.) collected from various parts of Thailand. Kasetsart J (Nat Sci), 43: 467-475. |
Chaiyana W., Anuchapreeda S., Leelapornpisid P., et al, 2017. Development of microemulsion delivery system of essential oil from Zingiber cassumunar Roxb. rhizome for improvement of stability and anti-inflammatory activity. AAPS Pharm. Sci. Tech., 18: 1332-1342. DOI:10.1208/s12249-016-0603-2 |
Cheng S.S., Chang H.T., Lin C.Y., et al, 2009a. Insecticidal activities of leaf and twig essential oils from Clausena excavata against Aedes aegypti and Aedes albopictus larvae. Pest Manag. Sci., 65: 339-343. DOI:10.1002/ps.1693 |
Cheng S.S., Huang C.G., Chen Y.J., et al, 2009b. Chemical compositions and larvicidal activities of leaf essential oils from two eucalyptus species. Bioresour. Technol., 100: 452-456. DOI:10.1016/j.biortech.2008.02.038 |
Corbel V., Stankiewicz M., Pennetier C., et al, 2009. Evidence for inhibition of cholinesterases in insect and mammalian nervous systems by the insect repellent deet. BMC Biol., 7: 47-57. DOI:10.1186/1741-7007-7-47 |
Cotchakaew N., Soonwera M., 2019. Toxicity of several botanical essential oils and their combinations against females of Aedes albopictus (Skuse) and Anopheles minimus (Theobald): Oviposition deterrent, ovicidal and adulticidal efficacies. Asian Pac. J. Trop. Biomed., 9: 29. DOI:10.4103/2221-1691.250267 |
Dias, C.N., Fernandes, D., Moraes, C., 2014. Essential oils and their compounds as Aedes aegypti L. (Diptera: Culicidae) larvicides: review. Parasitol. Res. 113, 565-592. https://doi.org/10.1007/s00436-013-3687-6
|
Govindarajan M., Sivakumar R., Rajeswari M., et al, 2012. Chemical composition and larvicidal activity of essential oil from Mentha spicata (Linn.) against three mosquito species. Parasitol. Res., 110: 2023-2032. DOI:10.1007/s00436-011-2731-7 |
Gu H.J., Cheng S.S., Lin C.Y., et al, 2009. Repellency of essential oils of Cryptomeria japonica (Pinaceae) against adults of the mosquitoes Aedes aegypti and Aedes albopictus (Diptera: Culicidae). J. Agric. Food Chem., 57: 11127-11133. DOI:10.1021/jf9024486 |
Haribalan P., Nam-Jin K., Young-Joon A., 2009. Larvicidal activity of compounds isolated from Asarum heterotropoides against Culex pipiens pallens, Aedes aegypti, and Ochlerotatus togoi (diptera: culicidae). J. Med. Entomol., 6: 1420-1423. DOI:10.1603/033.046.0624 |
Jantan I., Ping W.O., Visuvalingam S.D., et al, 2003. Larvicidal activity of the essential oils and methanol extracts of Malaysian plants on Aedes aegypti. Pharm. Biol., 41: 234-236. DOI:10.1076/phbi.41.4.234.15665 |
Kamazeri T.S.A.T., Samah O.A., Taher M., et al, 2012. Antimicrobial activity and essential oils of Curcuma aeruginosa, Curcuma mangga, and Zingiber cassumunar from Malaysia. Asian Pac. J. Trop. Med., 5: 202-209. DOI:10.1016/S1995-7645(12)60025-X |
Kunming Institute of Botany, Chinese Academy of Sciences, 1997. Flora of Yunnan, Vol. eighth. Science Press, Beijing.
|
Labbé G.M.C., Nimmo D.D., Alphey L., 2010. Piggybac- and PhiC31-mediated genetic transformation of the Asian tiger mosquito, Aedes albopictus (Skuse). PLoS Negl. Trop. Dis., 4: e788. DOI:10.1371/journal.pntd.0000788 |
Leelarungrayub J., Manorsoi J., Manorsoi A., 2017. Anti-inflammatory activity of niosomes entrapped with Plai oil (Zingiber cassumunar Roxb.) by therapeutic ultrasound in a rat model. Int. J. Nanomedicine , 12: 2469-2476. DOI:10.2147/IJN.S129131 |
Li M., Bai X., Ma Y., et al, 2019. Cosmetic potentials of extracts and compounds from Zingiber cassumunar Roxb. rhizome. Ind. Crop. Prod., 141: 111764. DOI:10.1016/j.indcrop.2019.111764 |
Li L., Xiong X., Ma S., et al, 2015. Comparison of the insecticidal activities of terpinen-4-ol optical isomers and racemate against the housefly, Musca domestica. Acta Entomol. Sin., 58: 761-766. DOI:10.16380/j.kcxb.2015.07.008 |
Lucia A., Zerba E., Masuh H., 2013. Knockdown and larvicidal activity of six monoterpenes against Aedes aegypti (Diptera: Culicidae) and their structure-activity relationships. Parasitol. Res., 112: 4267-4272. DOI:10.1007/s00436-013-3618-6 |
Ma Y., Li M., Zhang H., et al, 2019. Bioassay-guided isolation of active compounds from Adenosma buchneroides essential oil as mosquito repellent against Aedes albopictus. J. Ethnopharmacol., 231: 386-393. DOI:10.1016/j.jep.2018.11.031 |
Mektrirat R., Yano T., Okonogi S., 2020. Phytochemical and safety evaluations of volatile terpenoids from Zingiber cassumunar Roxb. on mature carp peripheral blood mononuclear cells and embryonic zebrafish. Molecules, 25: 613-624. DOI:10.3390/molecules25030613 |
Ngo K., Brown G.D., 1998. Stilbenes, monoterpenes, diarylheptanoids, labdanes and chalcones from Alpinia Katsumadia. Phytochemistry, 47: 1117-1123. DOI:10.1016/S0031-9422(98)80083-6 |
Nugroho B.W., Schwarz B., Wray V., et al, 1996. Insecticidal constituents from rhizomes of Zingiber cassumunar and Kaempferia rotunda. Phytochemistry, 41: 129-132. DOI:10.1016/0031-9422(95)00454-8 |
Okonogi, 2012. Enhancement of anti-cholinesterase activity of Zingiber cassumunar essential oil using a microemulsion technique. Drug Discov. Ther., 6: 249-255. DOI:10.5582/ddt.2012.v6.5.249 |
Phasomkusolsil, S., Soonwera, M., 2010. Insect repellent activity of medicinal plant oils against Aedes aegypti (Linn.); Anopheles minimus (Theobald) and Culex quinquefasciatus Say based on protection time and biting rate. Southeast Asian J. Trop. Med. Public Health 41, 831-840. https://doi.org/10.1371/journal.pntd.0003829
|
Phukerd U., Soonwera M., 2014. Repellency of essential oils extracted from Thai native plants against Aedes aegypti (Linn.) and Culex quinquefasciatus (Say). Parasitol. Res., 113: 3333-3340. DOI:10.1007/s00436-014-3996-4 |
Pongprayoon U., Tuchinda P., Claeson P., et al, 1997. Topical antiinflammatory activity of the major lipophilic constituents of the rhizome of Zingiber cassumunar. Part Ⅱ: Hexane extractives. Phytomedicine, 3: 323-326. DOI:10.1016/S0944-7113(97)80004-9 |
Raguraman S., Singh D., 1997. Biopotentials of Azadirachta indica and Cedrus deodara oils on Callosobruchus chinensis. Pharm. Biol., 35: 344-348. DOI:10.1080/09251619708951280 |
Sanatombi R., Sanatombi K., 2017. Biotechnology of Zingiber montanum (Koenig) link ex A. Dietr. : a review. J. Appl. Res. Med. Aromat. Plants, 4: 1-4. DOI:10.1016/j.jarmap.2016.09.001 |
Sharifi-Rad M., Varoni E.M., Salehi B., et al, 2017. Plants of the genus Zingiber as a source of bioactive phytochemicals: From tradition to pharmacy. Molecules, 22: 1-20. DOI:10.3390/molecules22122145 |
Singh C., Manglembi N., Swapana N., et al, 2015. Ethnobotany, phytochemistry and pharmacology of Zingiber cassumunar Roxb. (Zingiberaceae). J. Pharmacogn. Phytochemistry, 4: 1-6. |
State Pharmacopeia Commission, 2015. Pharmacopeia of the People's Republic of China, 2015 edition. China Medical Science and Technology Press, Beijing, China, pp. 203.
|
Sukatta U., Rugthaworn P., Punjee P., et al, 2009. Chemical composition and physical properties of oil from plai (Zingiber cassumunar Roxb.) obtained by hydro distillation and hexane extraction. Kasetsart J (Nat Sci), 43: 212-217. |
Talukder D., Haque A.B.M.H., Zaman S., et al, 2015. Insecticidal activity of different fractions of petroleum ether extract of Zingiber cassumunar rhizome against Tribolium castaneum. Bangladesh J. Sci. Ind. Res., 50: 143-152. DOI:10.3329/bjsir.v50i2.24355 |
Tangyuenyongwatana P., Gritsanapan W., 2008. A study on artifacts formation in the Thai traditional medicine Prasaplai. Planta Med., 74: 1403-1405. DOI:10.1055/s-2008-1081302 |
Taroeno, Brophy J.J., Zwaving J.H., 1991. Analysis of the essential oil of Zingiber cassumunar Roxb. from Indonesia. Flavour Fragr. J., 6: 161-163. DOI:10.1002/ffj.2730060214 |
Verma R.S., Joshi N., Padalia R.C., et al, 2018. Chemical composition and antibacterial, antifungal, allelopathic and acetylcholinesterase inhibitory activities of cassumunar-ginger. J. Sci. Food Agric., 98: 321-327. DOI:10.1002/jsfa.8474 |
Wang Y., You C.X., Yang K., et al, 2015. Bioactivity of essential oil of Zingiber purpureum rhizomes and its main compounds against two stored product insects. J. Econ. Entomol., 108: 925-932. DOI:10.1093/jee/tov030 |
Wei, Q.L., Tang, L., 2018. Research summary on traditional Dai medicine of Zingiber purpureum Posc. Chinese J. Ethnomedicine Ethnopharmacy 27, 11-13. https://doi.org/CNKI:SUN:MZMJ.0.2018-17-014
|
Yang P., Ma Y., Zheng S., 2005. Adulticidal activity of five essential oils against Culex pipiens quinquefasciatus. J. Pestic. Sci., 30: 84-89. DOI:10.1584/jpestics.30.84 |
Yingngam B., Brantner A., 2018. Boosting the essential oil yield from the rhizomes of cassumunar ginger by an eco-friendly solvent-free microwave extraction combined with central composite design. J. Essent. Oil Res., 30: 409-420. DOI:10.1080/10412905.2018.1503099 |



