b. Key Laboratory of Biological Resources and Ecology of Pamirs Plateau in Xinjiang Uygur Autonomous Region, Kashi University, Kashi, Xinjiang, 844000, China
Morphological or spatial–temporal variation of stigma or stamen movement is important in the sexual interference and reproduction of flowering plants (Dole, 1990; Lloyd, 1992; Bynum and Smith, 2001; He et al., 2006; Abdusalam and Tan, 2014; Wang et al., 2017; Ye et al., 2019). The degree of spatial–temporal separation of floral sexual organs (herkogamy and dichogamy, respectively) can enhance male and female fitness in hermaphroditic flowers by preventing self-pollination and reducing interference between the functions of male and female organs (Barrett, 2002; Ren and Tang, 2012; Leite et al., 2016; Wang et al., 2017). The dramatic movements of floral structures that can separate male and female organs, such as flexistyly (e.g., the two phenotypes of Alpinia Roxb.), slow, or up and down, stamen movement in Ruta graveolens L., are thought to have evolved to promote successful reproduction in flowering plants (Li et al., 2001; Barrett, 2002; Yu and Huang, 2004; Von Hase et al., 2006; Wang et al., 2018).
Floral movements include both passive (non-directional and independent of ecological factors) and initiated (oriented response) movements (Ichmura and Suto, 1998; He et al., 2006; Ren and Tang, 2012; Wang et al., 2017), and they differ in function and adaptation in male-female reproduction and fitness (Ren and Tang, 2012; Abdusalam and Tan, 2014; Leite et al., 2016). Movement of flower parts can reduce inter- or intra-sexual interference (Ren and Bu, 2014; Wang et al., 2017; Ye et al., 2019), influence mating patterns (increase the delay of self-pollination or cross-pollination) (Grant et al., 1979; Taylor et al., 2006; Du et al., 2012; Ren and Tang, 2012; Abdusalam and Tan, 2014; Wang et al., 2018), increase pollen transfer (Lewis, 1982; Lloyd, 1992; He et al., 2006; Song et al., 2013), and protect male and female organs under harsh environmental conditions (Schlindwein and Wittmann, 1997; Ren and Tang, 2012; Abdusalam and Tan, 2014). However, even in open flowers, the effectiveness of male organ functions may be increased by stamen movement (Ren and Tang, 2012; Wang et al., 2017; Ye et al., 2019).
Stamen movement is a key floral trait in reproductive fecundity, and it prevents interference between male-female sexual organs during the lifespan of hermaphroditic flowers (Schlindwein and Wittmann, 1997; Sandvik and Totland, 2003; Ren and Tang, 2012; Wang et al., 2017). Some plant stamen movement is induced by the filament or anther moving in response to pollinator activities or petal movement (Abdusalam and Tan, 2014; Wang et al., 2017), and is controlled by light and temperature (Henning and Weigend, 2012), growth of epidermal cells in the filament (Du et al., 2012) and changes in the calcium content of cells that regulate water retention (Lechowski and Bialczyk, 1992; Cota-Sánchez et al., 2013).
Most flowering plant stamen movement (stimulated, simultaneous and slow, quick and explosive, and cascade) is induced by turgor pressure (energy from the flower) of cells in the filaments (Schlindwein and Wittmann, 1997; Taylor et al., 2006; Ren and Tang, 2012; Henning and Weigend, 2013). Stimulated and simultaneous stamen movement can result in self-pollen being deposited on the stigma, which increases self-pollination and female-male interference, e.g., in Berberidaceae (Lechowski and Bialczyk, 1992), and Calycanthaceae (Azuma et al., 2005). In contrast, quick, explosive and cascading (including one-by-one or group-by-group) stamen movements can increase pollen dispersal and out-crossing. For example, in Loasaceae, Moraceae, Parnassiaceae, Rutaceae and Tropaeolaceae, one-by-one stamen movements have an effect on pollen presentation in each flower (Taylor et al., 2006; Ren and Tang, 2012). R. graveolens L. (Rutaceae), Tropaeolum majus L. (Tropaeolaceae), Parnassia epunctulata J. T. Pan and Parnassia palustis L. (Parnassiaceae) have stamens that move one-by-one, thereby increasing pollen transfer and avoiding inter- and intra-sexual organ interferences (Henning and Weigend, 2012; Ren and Tang, 2012; Ren and Bu, 2014; Armbruster et al., 2014). However, in some species, stamen use group-by-group (slow) movement, for example, in Geranium L., but the effect of stamen group-by-group movement on anther-stigma spatial–temporal separation in these flowers has not been experimentally determined.
The subject of this study is Geranium pratense L., a plant with temporal floral closure. Our preliminary field observations on this species found that flowers have two whorls of five stamens each that dehisce group by group (move into the center of the flowers) during the first day (herkogamy with stamens longer than the pistil) of flowering before the stigma opens (becomes receptive) and temporal flower closure (Fig. 1). These flowers close temporarily in the evening, which reduces petal and sexual organ movement. We predicted that group-by-group stamen movement in flowers of G. pratense would provide spatial–temporal separation of the anthers and stigma, thereby preventing self-pollination and interference at male and female stages, while increasing pollen removal. To test this hypothesis, we asked the following specific questions: (1) How do the different stages of the floral sexual organs influence pollinator activities? (2) What are the effects of stamen group-by-group movement on pollen removal, avoidance of sexual organ interference and mating pattern? Information on the effects of stamen group-by-group movement on the reproductive success of G. pratense will increase our understanding of the adaptive mechanisms of related species to changing conditions.

|
| Fig. 1 Spatial interference between stamens of the inner and outer whorls and between stamens of both whorls and stigma in naturally pollinated (A–E) and bagged (no pollinator) flowers (F–J) of Geranium pratense at five floral stages: (A and F) just-opened flower; (B and G) outer whorl stamens dehisced; (C and H), inner whorl stamens dehisced; (D and I) style elongated; and (E and J) stigma lobes expanded. |
Our experiments were conducted in a natural population of Geranium pratense growing in Bostanterak of Wucha County (39°25ʹ20–25″N, 75°09ʹ28–36″E, elevation: 2000–2500 m) in Xinjiang province, China, in July 2017 and 2019.
Geranium pratense is a summer-flowering herbaceous species that grows in grassland and alpine habitats at 1400–4000 m in Xinjiang Province, China. It produces a single umbellate inflorescence with 30–36 flowers that bloom in mid-June, while its fruits mature in early-August. Flowers of G. pratense have five sepals and the stigma at the center of the flower is surrounded by two whorls of five stamens each. These flowers respond to changes in light conditions, closing temporarily at dark, and reopening when light levels increase. In natural populations, stamen movement occurs in each flower at five floral stages (Fig. 1).
2.2. Movement of floral organs causes spatial and temporal separation of male and female floral functionsTo estimate variation in anther and stigma spatial separation, the length of each stamen in both whorls and the pistil, and the vertical distance between each whorl of stamens and the stigma was measured in 30 flowers in each of the five floral stages: just-opened flower, outer whorl stamens dehisced, inner whorl stamens dehisced, style elongated, and stigma lobes expanded. All measurements were made (to the nearest 0.01 mm) on a sunny day using SE 2000 electronic calipers (Holy Instrument and Technology Co., Ltd; Guilin, China). To determine the duration of the five floral stages, 30 flower buds were marked, and the flowering stage was observed and recorded at 1-h intervals.
To determine temporal variation in pollen viability and stigma receptivity, 30 flower buds were covered with mesh netting to exclude pollinators. After the flowers opened, pollen viability at different floral stages was measured under a light microscope using the MTT method (Rodriguez-Riano and Dafni, 2000). To examine stigma receptivity, 100 flowers with non-dehisced anthers were selected, the anthers were removed, and flowers were then covered with mesh netting to exclude pollinators. At each of the five floral stages, 20 emasculated flowers were cross-pollinated by hand and then bagged with mesh bags. After a 3-week period during which the fruits matured, fruit and seed set were compared.
2.3. Pollinator type, visitation frequency and rewardsPollinator visitation frequency, visitation duration, and behavior was determined at each floral stage. On a sunny day, 30 flower buds were randomly selected. After the flowers opened, insect visiting frequency and each insect during a foraging bout were observed for 10 flowers each in the outer male stage (outer whorl of stamens dehisced), inner male stage (inner whorl of stamens dehisced) and female (style open) stages of flowering. We photographed and caught 3–4 insects of each type for taxonomic identification. The following day, 20 flower buds were selected and covered with a mesh bag. After the flowers opened, the nectar volume was determined for the three flower stages according to the methods described by Dafni et al. (2005).
2.4. Effect of stamen movement on pollen removal rateTo assess the influence of group-by-group movement on pollen removal, we selected 30 plants with four mature flowers (flowers opened but anthers not dehisced) of the same size and at the same position of inflorescence. To establish a baseline level of pollen in these flowers, one flower from each inflorescence was selected, and the two whorls of stamens from each flower were placed together in separate 2-mL glass bottles (one bottle per flower) and taken to the laboratory. The other three flowers were assigned to one of three treatments: (1) filaments of the two whorls of stamens tied together with style; (2) filaments of the two whorls of stamens tied together with the petals; and (3) filaments and stamens unmanipulated. After two days, the two whorls of stamens from these flowers were placed together in separate 2-mL glass bottles and taken to the laboratory. The number of pollen grains remaining on all anthers of each flower was counted under the Olympus BH-2 optical microscope (Nikon SMZ1000, Nikon, Japan) following the method described by Abdusalam et al. (2020).
2.5. Relationships between pollen removal rate and sexual interferenceTo determine the influence of stamen movement on the level of stamen–stamen interference and stamen-style position (interference), we selected 40 just-opened flowers (anthers not dehisced) in the natural population. Twenty flowers were allowed to be pollinated naturally and 20 flowers were covered with mesh bags to exclude insects. The position of the inner and outer whorls stamens, and the distance between the style and the inner and outer whorls stamens at different floral stages for each bagged and naturally pollinated flowers were measured using SE 2000 electronic calipers and photographs were taken. We recorded the angle and speed of stamen movement for bagged and naturally pollinated flowers. A protractor was used to measure the filament angle of the stamens in the inner and outer whorls at five floral stages (just-opened flower; outer whorl stamens dehisced, when outer whorl stamens move to floral center; inner whorl stamens dehisced, when inner whorl stamens move to floral center; inner and outer whorl stamens dehisced; style elongated; and stigma lobes expanded) for each treated flower. When the filament angle was < 10°, the stamens were considered moved away from the floral center (Ren and Bu, 2014).
To examine the effects of stamen and petal movement on stamen-stigma interference, four just-opened flowers (anthers not dehisced) on each of 40 randomly selected plants were assigned to one of four treatments: (1) filaments of outer whorl stamens were tied together with the style after removal of the inner whorl stamens (OF); (2) filaments of the inner whorl stamens were tied together with the style after removal of the outer whorl stamens (IF); and (3) filaments of both whorls stamens were tied together with the style (AF); or (4) no manipulation of floral organs (natural experiment, N). Twenty flowers for each treatment were selected and covered with mesh bags, and the other 20 flowers for each treatment were not bagged but anthers (and pollen) were stained a blue color following the procedure of Mamut et al. (2014). Two days after the initiation of each treatment, the stigma of each flower was placed in a separate 1-mL centrifuge tube, and the number of pollen grains deposited on the stigma was counted under a compound microscope in the laboratory following the method of Abdusalam et al. (2020).
2.6. Mating patternsTo determine the effect of stamen movement on mating pattern, we used five pollination treatments on 100 randomly selected just-opened flowers. Twenty flowers were assigned to each of five treatments: (1) natural pollination; (2) hand cross-pollination (i.e., all flowers were covered with paper bags after removal of all stamens, and the opened flowers were hand-crossed with pollen from plants > 10 m away); (3) hand self-pollination (all flowers were covered with paper bags after the opened flowers were hand self-pollinated); (4) self-pollination (flower buds were kept covered with paper bags and flowers were allowed to self-pollinate); and (5) controlled self-pollination (filaments of all stamen were tied together with the style and flowers bagged with paper bags). Seed production by flowers in each treatment was compared at fruit maturation.
2.7. Statistical analysesAll data were tested for normality of variance prior to analysis to fulfill requirements of a one-way ANOVA or independent samples t-test. Percentage data (deposited self-pollen; removed pollen, fruit and seed set) were arcsine transformed before statistical analysis to ensure homogeneity of variance. Normal and homogeneous data were subjected to further analysis, while abnormal and non-homogeneous data were square root (percentage data, deposited or removed pollen, filament angle and seed set) transformed before analysis to ensure homogeneity of variance. Stamen and pistil length, stamen-stigma distance, pollen viability, stigma receptivity, insect visiting frequency, duration, nectar volume at different floral stage and deposited self-pollen and seed set for different treatment flowers were compared using One-way ANOVA. If ANOVA indicated significant differences, Tukey's HSD test was performed for multiple comparisons to determine significant differences (p < 0.05) among different floral stages. The length of the two whorls of stamens and pistil, stamen-stigma distance, inner-outer whorl of stamens distance, filament angle and deposited self-pollen in the two flower treatments were compared using independent samples t-test. Fruit set for different flower treatments was evaluated by the generalized linear model (GLM) with Poisson distribution and log linear-link function (Abdusalam et al., 2020). All statistical analyses were performed using SPSS 19.0 software (SPSS Inc., Chicago, USA). Non-transformed data appear in all figures.
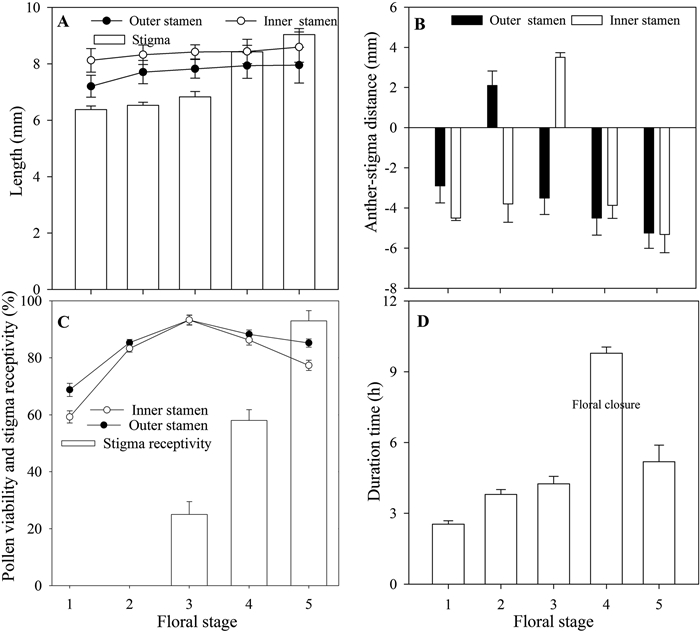
3. Results 3.1. Spatial–temporal variation of male and female organ separationWhen centralized in the flower, the inner whorl stamens (8.59 ± 0.53 mm) were longer than the outer whorl stamens (7.96 ± 0.51 mm) (t = 7.514, p < 0.01, Fig. 2A). Stamen length in the inner (F = 2.615, p > 0.05) and outer (F = 0.545, p > 0.05) whorls did not differ significantly at different floral stages, but pistil length differed significantly at different floral stages (F = 10.103, p < 0.01, Fig. 2A). Inner whorl stamens were significantly longer than the pistil at each flowering stage (F = 10.562, p < 0.01, Fig. 2A), as were the outer whorl stamens (F = 4.653, p < 0.05, Fig. 2A). Male and female organs were spatially isolated at different floral stages (Fig. 2). The distance between both the inner and outer whorl stamens and the stigma differed significantly at different floral stages (t = 8.821, p < 0.01, Fig. 2B); however, inner and outer whorl stamen length did not differ significantly at the female stage (t = 2.041, p > 0.05, Fig. 2B).
|
| Fig. 2 Floral stamen-stigma spatial–temporal separation (A, C, D) and stamen-stigma distance (B) in Geranium pratense. (A) Spatial change of stamen-style length; (B) spatial change of stamen-style distance; (C) time-dependent pollen viability and stigma receptivity; and (D) duration of different flower stages at floral life span. |
Temporal variation in male and female viability is protandrous (Fig. 2C). Pollen viability of outer (F = 13.19, p < 0.01, Fig. 2C) and inner (F = 6.27, p < 0.01, Fig. 2C) stamens differed significantly from stigma receptivity (F = 16.28, p < 0.01, Fig. 2C) at different floral stages. Although pollen viability is higher than stigma receptivity at male floral stage, stigma receptivity increases at floral closure and the female stage. The duration of each floral stage differed significantly (F = 125.19, p < 0.001, Fig. 2D). The duration of the female stage (5.21 ± 0.75 h) was shorter than that of the male stages (8.05 ± 0.26 h). In addition, the inner male stage (4.25 ± 0.31 h) was shorter than the outer male stages (3.80 ± 0.21 h).
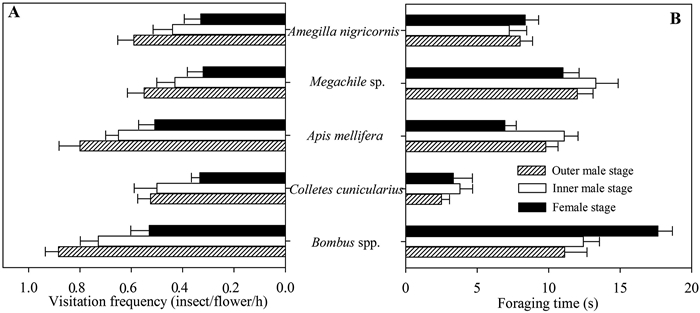
3.2. Pollinator type, visitation frequency and rewardsEight types of insects visited Geranium pratense flowers (Fig. 3). Most insect visitors probed the flower at the base of the pistil, presumably seeking nectar, and often touched the floral center (stigma or anthers). Pollinators included Apis mellifera, Bombus spp., Megachile sp., Colletes cunicularius, Amegilla nigricorni. Non-pollinating visitors included Sideridiis simpiex, Syrphidae sp., and Katamenes sesquicinctus. There were significant variations in pollinator assemblages and their visitation frequencies at different floral stages. A. mellifera, Bombus spp., and Megachile sp. were the most common pollinators, and visitation frequencies of these three pollinators were higher at male stage than at the female stage (Fig. 4A).

|
| Fig. 3 The mainly generalist visitors of Geranium pratense and their visitation behavior in the center of the flower. (A) Apis mellifera; (B) Colletes cunicularius; (C) Bombus spp.; (D) Megachile sp.; (E) Amegilla nigricornis; (F) Syrphidae sp.; (G) Katamenes sesquicinctus; and (H) Sideridiis simpiex. |
|
| Fig. 4 Visitation frequency (A) and visitation duration (B) of floral visitors to natural male (both whorls of stamens dehisced) and female (stigma open) stage flowers of Geranium pratense. |
Nectar volume differed significantly at the three floral stages (F = 3.911, p < 0.05). Flowers produce more nectar during the female stage (0.136 ± 0.009 vl/h) than during the outer (0.087 ± 0.014 vl/h) and inner (0.099 ± 0.015 vl/h) male stages. Pollinator visitation frequency and foraging duration were significantly affected by pollinator type (Fvisiting frequency = 8.06, p < 0.001; Fforaging time = 32.180, p < 0.001) and floral stage (Fvisiting frequency = 9.870, p < 0.001; Fforaging time = 12.012, p < 0.001, Fig. 4). Visitation frequency of all pollinators was higher at the outer male stage than at the inner male stage (Fig. 4A). Foraging duration of pollinators was significantly affected by pollinator type (F = 5.845, p < 0.01) and floral stage (F = 3.732, p < 0.05). Foraging time of Bombus spp. and A. nigricorni was longer at female stages than at both male stages.
3.3. Effects of stamen movement on pollen removal rateThe mean number of pollen grains in flowers prior to experimental treatments was 3717 ± 174. Stamen group-by-group movement had significant positive effects on the percentage of removed pollen in the three treatment flowers (F = 19.478, p < 0.001). The amount of pollen removed was higher for stamens tied around the style (3489 ± 56) and flowers with unmanipulated stamens (2990 ± 39) than in flowers with stamens tied together with the petals (1229 ± 158).
3.4. Relationships between pollen removal rate and sexual interferenceThe inner-outer stamen horizontal distance (F = 6.345, p < 0.001) and filament angle (F = 11.039, p < 0.001) at different floral stages differed significantly for natural (open-pollinated) and no-pollinator (covered with mesh bags) flowers (Fig. 5). The horizontal distance of inner whorl stamen-stigma (F = 8.321, p < 0.001) and outer whorl stamen-stigma (F = 7.359, p < 0.001) and filament angle between outer and inner whorl of stamens (F = 8.532, p < 0.001) differed significantly for naturally pollinated flowers and those covered by mesh bags. The horizontal distances of outer and inner whorl stamens were spatially isolated at different floral stages in naturally pollinated flowers; however, during the inner floral stage, there was interference between both the inner and outer whorl stamens in bagged flowers (Fig. 5C and D).

|
| Fig. 5 The mean spatial distance (A, B) and filament angle (C, D) of each whorl stamen at five floral stages (just-opened flower; outer whorl stamens dehisced, when outer whorl stamens move to floral center; inner whorl stamens dehisced, when inner whorl stamens move to floral center; inner and outer whorl of stamens dehisced; style elongated; and stigma lobes expanded) for naturally pollinated and control (bagged) treated flowers of Geranium pratense in a natural population. |
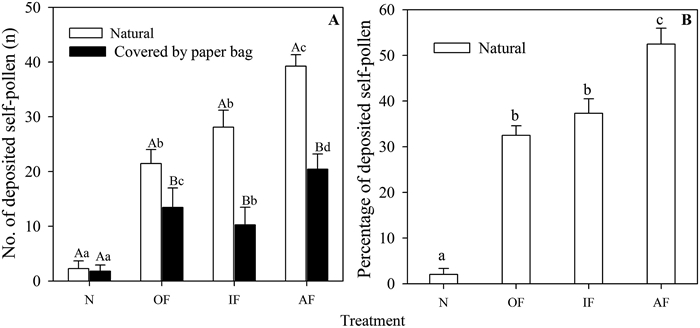
The number of self-pollen grains deposited on the stigma differed significantly in bagged and naturally pollinated flowers (t = 12.257, p < 0.001, Fig. 6); more total pollen was deposited on naturally pollinated flowers than on bagged flowers (Fig. 6A). Flowers in which the outer whorl stamens were tied together with the style had less deposited self-pollen and less total pollen than flowers in which outer whorl stamens were tied together with the style (Fig. 6A and B). When the anthers were tied together with the style, naturally pollinated flowers from the three treatments (OF, IF, and AF) had more deposited self-pollen grains than did bagged flowers from the three treatments (t = 13.269, p < 0.001, Fig. 6A). Fewer self-pollen grains were deposited on the stigma in unmanipulated flowers (i.e., stamens were not tied) than in treated flowers (i.e., anthers were tied with the style) that were bagged (F = 10.156, p < 0.01) or naturally pollinated (F = 8.992, p < 0.01, Fig. 6A). Stamen-style position and petal movement had significant effects on the percentage of deposited self-pollen, and the percentage was lower for unmanipulated flowers (i.e., stamens not tied) than for any of the three flower treatments (Fig. 6B). The percentage of deposited self-pollen on naturally (N) pollinated flowers was significantly (F = 17.482, p < 0.001) lower than that of the three flower treatments (OF, IF, and AF, Fig. 6B).
|
| Fig. 6 Number (A) and percentage (B) of deposited self-pollen grains in two flower treatments after stamens were tied together with the styles. Bars with different lowercase letters indicate significant differences between same type of flower for different treatments, and bars with different uppercase letters indicate significant differences between different types of flowers for the same treatment. N, natural pollination; OF, outer whorl stamen tied together with the style after removal of inner whorl stamens; IF: inner whorl of stamen tied together with the style after removal of outer whorl of stamens; AF: all stamens of flowers tied together with the style. |
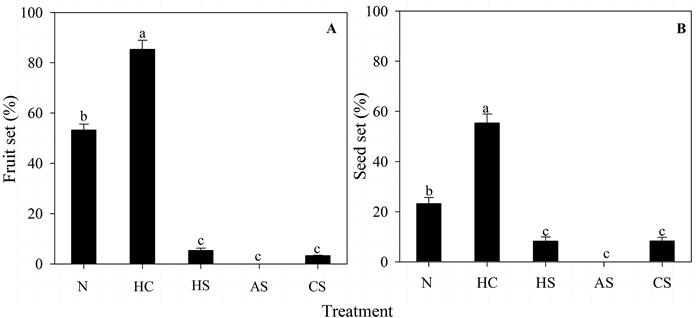
The fruit (χ2 = 45.143, p < 0.001) and seed (χ2 = 15.861, p < 0.01) sets for the five flower treatments differed significantly; they were larger for naturally pollinated and hand cross-pollinated flowers than for the three types of self-pollinated flowers (Fig. 7). However, the fruit (χ2 = 3.045, p > 0.05) and seed sets (χ2 = 2.191, p > 0.05) of the hand self-pollinated, automatic self-pollinated, and control self-pollinated (covered with a paper bag after the stamen was tied together with the style) flowers did not differ significantly. Automatic self-pollinated flowers did not set any fruit, which indicates the absence of spontaneous autogamy.
|
| Fig. 7 Fruit (A) and seed (B) set in different flower treatments. N, natural pollination; HC, hand cross-pollination; HS, hand self-pollination; AS, automatic self-pollination; and CS, covered with a paper bag after all stamen were tied together with the style. |
Our results indicate that stamen movement and pistil secondary growth in Geranium pratense flowers can greatly reduce the spatial–temporal separation between the two whorls of stamens and between the stamens and pistil. Stamen movement also decreases the separation between both whorls of stamens and the stigma attachment level. Spatial (herkogamy) and temporal (dichogamy) separation of the stamen and stigma can reduce interference between male and female functions in hermaphroditic flowers (Webb and Lloyd, 1986; Barrett, 2002; Ren and Tang, 2012; Armbruster et al., 2014). In G. pratense flowers, the lengths of stamens in both whorls remained the same and the position of each whorl changed at different floral stages, but the length of the pistil increased (secondary growth) during the floral lifespan (Fig. 2A). In addition, stigma receptivity and pollen viability were higher during the female than during the male flowering stages (Fig. 2C). The function of pollen viability and stigma in G. pratense was weakly separated temporally, and movement herkogamy (pistil) can be increased by sexual interference; that is, stamen group-by-group movement can decrease the interference between stamen-pistil temporal functions (pollen viability and stigma receptivity).
Our results suggest that in Geranium pratense there is increased interference between the inner and outer whorls of stamens when pollinator activity is low or anthers are not dehisced, and floral temporal closure occurs (Fig. 1, Fig. 2C), as seen in other species. One-by-one stamen movement in Parnassia palustris and R. graveolens may be delayed if the pollen is not removed (Ren and Tang, 2012; Ren and Bu, 2014). If no pollinator exists, there could be a reduced amount of self-pollen deposited on the stigma after petal movement, and stamen movement decreases the amount of self-pollen deposited in the floral female stage (Fig. 1, Fig. 2C). Thus, stamen group-by-group movement appears to be an important floral characteristic that can increase pollen dispersal and anther-stigma spatial–temporal separation, as well as prevent viability of pollen deposits on the stigma of the same G. pratense flowers. As such, stamen group-by-group movement is likely an adaptation to the harsh grassland environment of this species that increases the pollen removal rate.
4.2. Effects of stamen movement on pollen removal rateOur data indicate that stamen group-by-group movement to the center of Geranium pretanse flowers in the first flowering day is crucial for pollen removal. Since potential pollen and nectar rewards are located in the central part of the flower, stamen movement to the floral center can increase pollen removal rate (pollen removal was greater for flowers with stamens tied to the pistil than for flowers with stamens tied to the petal). The floral center is the optimal position for stigma attachment to insect bodies and for the pollen reception rate, and stamen movement dramatically increases the accuracy of male function (Fig. 4), as seen in other species. In P. palustris and P. epunctulata, stamen one-by-one movement has also been shown to increase the amount of pollen removed (Ren and Bu 2014; Armbruster et al., 2014). In addition, most insect visitors probe the base of the pistil and often touch the floral center in G. pratense. In a controlled field experiment, the percentage of removed pollen was higher in flowers with the filament tied to the stigma than in those with no stamen movement. The group-by-group movement of the anthers can dramatically increase male function in G. pratense. For example, previous studies have shown that stimulated stamen movement can increase pollen removal to the insect body and the amount of pollen landing directly on the stigma rather than on the stamens (Grant et al., 1979). In addition, one-by-one movement of stamens not only decreases pollen discounting but also increases pollen removal in R. graveolens, P. epunctulata and P. palustris (Ren and Tang, 2012; Armbruster et al., 2014; Ren and Bu, 2014).
4.3. Relationships between pollen removal rate and sexual interferenceStamen group-by-group movement and pistil secondary growth (pistil movement) promote spatial–temporal separation of sexual organs in Geranium pratense flowers and prevent stamen-pistil interference. Floral temporal closure on the first day of flowering may reduce stamen–stamen and pistil-stamen interference. Some research has suggested that one-by-one stamen movement is a selective adaptation to prevent sexual interference in hermaphroditic flowers (Ren and Tang, 2012; Ren and Bu, 2014). Stamen movement to the floral central position of G. pratense after anther dehiscence reduces stamen-pistil interference, although low pollinator activity may be reduced between the inner and outer whorls of stamens. Often the stamens of the outer whorl will bend out of the floral center before those of the inner whorl. Successive stamen movements and patterns of style opening in the generalist pollinated G. pratense flowers may promote pollen removal by presenting pollen gradually to pollinators, and can prevent functional interferences between the stamen and pistil and between the inner and outer whorls of stamens. Therefore, group-by-group movement of stamens prevents stamen-pistil spatial–temporal interference; in contrast, one-by-one stamen movement, as reported previously in Loasaceae, Parnassiaceae, Rutaceae, and Tropaeolaceae, prevents anther–anther interference (Ren, 2010; Henning and Weigend, 2012). The evolution of pistil movement and stamen group-by-group movement-dependent sexual spatial–temporal separation should be recognized as an important selective adaptation in G. pratense to the harsh grassland environment.
4.4. Stamen movement and mating patternsSelf-pollination and sexual spatial–temporal interference are achieved by flower non-morphological trait movements for some species (Abdusalm and Tan, 2014; Wang et al., 2018). For example, stamen movements result in automatic self-pollination in Sanguinaria canadensis L. (Lloyd, 1992) and R. graveolens (Ren and Tang, 2012). Petal closure (permanent closure) results in automatic self-pollination and ensures the reproductive success of Chilopsis linearis (Cav.) Sweet (Richardson, 2004; Von Hase et al., 2006). Our data indicate that Geranium pratense is a self-incompatible species (Fig. 6) and that group-by-group movement of stamens during the male stage before style opening can prevent self-pollination on the first day of flowering during floral temporal closure. Spatial and temporal separation of paternal-maternal functions can decrease deposition of self-pollen on the stigma and dramatically increase cross-pollination (Fig. 4). As the filament angle and the stamen-stigma distance decrease, the chance of self-pollen deposition on the stigma by insects decreases. Since self-pollinated and non-pollinated bagged flowers produced few mature fruits and seeds, we can conclude that seed set requires cross-pollination. If the anthers still contain pollen during the female stage, stamen movement to the floral center deposits the remaining pollen grains on the stigma. However, temporal separation of stigma opening can decrease the percentage of self-pollen deposited on the stigma; and if there is temporal and spatial separation of the stamen and stigma, sexual organ interference decreases and male and female reproduction increases.
5. ConclusionsOur study demonstrates that a staggered pollen removal level can reduce sexual interference and improve pollination accuracy. We found that temporal functions of male and female organs in Garanium pratense flowers were weakly isolated, but the function of both whorls of stamens was not temporally isolated. These findings indicate that group-by-group movement of the dehisced anthers, and spatial–temporal separation of the function of male and female sexual organs may increase pollen removal, prevent interference between whorls of stamens and between stamens and stigma, and decrease pollen discounting and self-pollen deposition, which may be selectively advantageous.
Author contributionsA.A. designed the study, analyzed the data, and drafted the manuscript. R.M. conducted field work, data collection and extraction. H.H. analyzed the data. G.A. conducted insect taxonomic identification work.
Declaration of competing interestWe declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled, "Pollination adaptations of group-by-group stamen movement in a meadow plant with temporal floral closure".
AcknowledgmentsThis study was supported by the High-level Personnel Training Program of the Xinjiang Uygur Autonomous Region (QN2016BS0597).
Abdusalam A., Tan D.Y., 2014. Contribution of temporal floral closure to reproductive success of the spring-flowering Tulipa iliensis. J. Syst. Evol., 52: 186-194. DOI:10.1111/jse.12036 |
Abdusalam A., Liao W.J., Zhang Z.Q., et al, 2020. Pollinator shifts along an elevation gradient mediate different response in self-pollination in heterostylous Primula nivalis. J. Syst. Evol.. DOI:10.1111/jse.12639 |
Armbruster W.S., Corbet S.A., Vey A.J.M., et al, 2014. In the right place at the right time: Parnassia resolves the herkogamy dilemma by accurate repositioning of stamens and stigmas. Ann. Bot., 113: 97-103. DOI:10.1093/aob/mct261 |
Azuma H., Toyota M., Asakawa Y., 2005. Floral scent chemistry and stamen movement of Chimonanthus praecox (L.) Link (Calycanthaceae). Acta Phytotaxon.. Geobot, 56: 197-201. |
Barrett S.C.H., 2002. Sexual interference of the floral kinds. Heredity, 88: 154-159. DOI:10.1038/sj.hdy.6800020 |
Bynum M.R., Smith W.K., 2001. Floral movements in response to thunder storms improve reproductive effort in the alpine species Gentiana algida (Gentianaceae). Am. J. Bot., 88: 1088-1095. DOI:10.2307/2657092 |
Cota-Sánchez J.H., Almeida O.J.G., Falconer D.J., et al, 2013. Intriguing thigmonastic (sensitive) stamens in the plains prickly pear Opuntia polyacantha (Cactaceae). Flora, 208: 381-389. DOI:10.1016/j.flora.2013.04.009 |
Dafni, A., Kevan, P.G., Husband, B.C., 2005. Practical Pollination Biology. Canada: Enviroquest, Ltd. Cambridge: Ontario, 130-141.
|
Dole J.A., 1990. Role of corolla abscission in delayed self-pollination of Mimulus guttatus (Scrophulariaceae). Am. J. Bot., 77: 1505-1507. DOI:10.1002/j.1537-2197.1990.tb12562.x |
Du W., Qin K.Z., Wang X.F., 2012. The mechanism of stamen movement in Chimonanthus praecox (Calycanthaceae): differential cell growth rates on the adaxial and abaxial surfaces of filaments after flower opening. Plant Syst. Evol., 298: 561-576. DOI:10.1007/s00606-011-0566-4 |
Grant V., Grant K.A., Hurd P.D., 1979. Pollination of Opuntia lindheimeri and related species. Plant Syst. Evol., 132: 313-320. DOI:10.1007/BF00982393 |
He Y.P., Duan Y.W., Liu J.Q., et al, 2006. Floral closure in response to temperature and pollination in Gentiana straminea Maxim (Gentianaceae), an alpine perennial in the Qinghai-Tibetan Plateau. Plant Syst. Evol., 256: 17-33. |
Henning T., Weigend M., 2012. Total control-pollen presentation and floral longevity in Loasaceae (Blazing Star Family) are modulated by light, temperature and pollinator visitation rates. PLoS ONE, 8: e41121. DOI:10.1371/journal.pone.0041121 |
Henning, T., Weigend, M., 2013. Beautiful, complicated-and intelligent? Novel aspects of the thigmonastic stamen movement in Loasaceae. Plant Signal. Behav. 8, 6, e24605
|
Ichmura K., Suto K., 1998. Environmental factors controlling flower opening and closing in a Portulaca hybrid. Ann. Bot., 82: 67-70. |
Lechowski Z., Bialczyk J., 1992. Effect of external calcium on the control of stamen movement in Berberis vulgaris L.. Biol. Plant, 34: 121-130. DOI:10.1007/BF02925805 |
Leite A.V., Nadia T., Machado I.C., 2016. Pollination of Aosa rupestris (Hook.) Weigend (Loasaceae): are stamen movements induced by pollinators?. Braz. J. Bot., 39: 559-567. DOI:10.1007/s40415-016-0258-y |
Lewis D., 1982. Incompatibility, stamen movement and pollen economy in a heterostyled tropical forest tree, Cratoxylum formosum (Guttiferae). Philos. Trans. R. Soc. Lond. B., 214: 273-283. |
Li Q.J., Xu Z.F., Kress W. J., et al, 2001. Flexible style that encourages outcrossing. Nature, 410: 432. DOI:10.1038/35068635 |
Lloyd D.G., 1992. Self- and cross-fertilization in plants: Ⅱ The selection of self-fertilization. Int. J. Plant Sci., 153: 370-380. DOI:10.1086/297041 |
Mamut J., Xiong Y.Z., Tan D.Y., et al, 2014. Pistillate flowers experience more pollen limitation and less geitonogamy than perfect flowers in a gynomonoecious herb. New Phytol., 201: 670-677. DOI:10.1111/nph.12525 |
Ren M.X., 2010. Stamen movements in hermaphroditic flowers: diversity and adaptive significance. Chinese J. Plant Ecol., 34: 867-875. |
Ren M.X., Bu Z.J., 2014. Is there 'anther-anther interference' within a flower? evidences from one-by-one stamen movement in an insect-pollinated plant. PLoS ONE, 9: e86581. DOI:10.1371/journal.pone.0086581 |
Ren M.X., Tang J.Y., 2012. Up and down: stamen movements in Ruta graveolens (Rutaceae) enhance both outcrossing and delayed selfing. Ann. Bot., 110: 1017-1025. DOI:10.1093/aob/mcs181 |
Richardson S.C., 2004. Benefits and costs of floral visitors to Chilopsis linearis: pollen deposition and stigma closure. Oikos, 107: 363-375. DOI:10.1111/j.0030-1299.2004.12504.x |
Rodriguez-Riano T., Dafni A., 2000. A new procedure to assess pollen viability. Sex. Plant Reprod., 12: 241-244. DOI:10.1007/s004970050008 |
Sandvik S.M., Totland O., 2003. Quantitative importance of staminodes for female reproductive success in Parnassia palustris under contrasting environmental conditions. Can. J. Bot., 81: 49-56. DOI:10.1139/b03-006 |
Schlindwen C., Wittmann D., 1997. Stamen movement in flowers of Opuntia favour (Cactaceae) oligolectic pollinators. Plant Syst. Evol., 204: 179-193. DOI:10.1007/BF00989204 |
Song B., Zhang Z.Q., Stocklin J., et al, 2013. Multifunctional bracts enhance plant fitness during flowering and seed development in Rheum nobile (Polygonaceae), a giant herb endemic to the high Himalayas. Oecologia, 172: 359-370. DOI:10.1007/s00442-012-2518-2 |
Taylor P.E., Card G., House J., et al, 2006. High-speed pollen release in the white mulberry tree, Morus alba L. Sex. Plant Reprod., 19: 19-24. DOI:10.1007/s00497-005-0018-9 |
Von Hase A., Cowling R.M., Ellis A.G., 2006. Petal movement in cape wild flowers protects pollen from exposure to moisture. Plant Ecol., 184: 75-87. DOI:10.1007/s11258-005-9053-8 |
Wang, L.Y., Bao, Y., Wang, H.X., et al., 2017. Slow stamen movement in a perennial herb decreases male-male and male-female interference. AoB Plants 9, plx018. doi: 10.1093/aobpla/plx018.
|
Wang S., Fu W.L., Du W., et al, 2018. Nectary tracks as pollinator manipulators: The pollination ecology of Swertia bimaculata (Gentianaceae). Ecol. Evol., 8: 3187-3207. DOI:10.1002/ece3.3838 |
Webb, C.J., Lloyd, D.G., 1986. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. Ⅱ. Herkogamy. New Zeal. J. Bot. 24, 163-178.
|
Ye, Z.M., Jin, X.F., Yang, J., et al., 2019. Accurate position exchange of stamen and stigma by movement in opposite direction resolves the herkogamy dilemma in a protandrous plant, Ajuga decumbens (Labiatae). AoB Plants 11, plz052. doi: 10.1093/aobpla/plz052.
|
Yu Q., Huang S.Q., 2004. Flexible stigma presentation assists context-dependent pollination in a wild columbine. New Phytol., 169: 237-241. |



