b. College of Ecology and Environment, Yunnan University, Kunming, 650504, China
Community assemblages are often studied using traditional taxon-based approaches. However, these approaches neglect to consider evolutionary history, which is an important factor in structuring communities (Tucker et al., 2016). A deeper understanding of the mechanisms that regulate species coexistence can be achieved by examining both phylogenetic and functional community structures (Shooner et al., 2015). For example, the relationships between the flora of East Asia and North America, which share common woody genera but almost no common woody species, will differ depending on whether researchers examine phylogenetic diversity or use classic taxon-based approaches (Qian, 2001).
Phylogenetic and functional community structure can be assessed by comparing the mean phylogenetic and functional distances of co-occurring species to a null model expectation by random sampling in the local species pool (Webb, 2000; Webb et al., 2002). Based on the functional traits conserved within lineages, the phylogenetic structures of communities can be used to detect the causes of assembly (Prinzing et al., 2001). Phylogenetic clustering results from abiotic filtering when filtering is the major force in structuring community assembly, indicating that the shared habitat tolerance and preference of closely related species are the strongest forces that determine species coexistence (Webb, 2000). Phylogenetic overdispersion indicates that biotic filtering is the dominant force, excluding several taxa among closely related species, leading to a community of distantly related species (Webb, 2000; Webb et al., 2002).
In alpine meadows on the southeast edge of the Qinghai-Tibet Plateau, Himalayan marmots (Marmota himalayana) form mounds with excavated soil at the entrance of their burrows. These mounds destroy the natural vegetation of the grassland, and initiate the process of succession. During the first stage of succession excavated soil covers plants and greatly changes niches. Over time, the habitat gradually recovers and plants again occupy the mounds. Despite this frequent and common occurrence, few studies have examined plant community.
One approach to identifying the biotic and abiotic factors that underlie successional systems is to treat succession as a community assemblage that changes along a temporal gradient (Lebrija-Trejos et al., 2010; Lohbeck et al., 2014). Along this temporal gradient, changes in species assemblages alter phylogenetic and functional diversities of plant communities. On marmot mounds, plant species composition changes significantly at different successional stages. During early succession, species that survive must struggle against adverse environmental filters, such as intense sunlight, the stress of desiccation and infertile soils (Carson and Schnitzer, 2008; Bhaskar et al., 2014). As community succession progresses, environmental factors are alleviated, and biotic interactions become major determinants (Leibold et al., 2004; Carson and Schnitzer, 2008).
To understand how plant communities growing on marmot mounds change, we assessed their phylogenetic and functional community structure at different stages of succession. We used community successional composition data sets to address the following issues: (1) do phylogenetic and functional community structures change along a successional series on marmot mounds; (2) do the phylogenetic and functional community structures change in areas surrounding marmot mounds along a succession series; (3) do plant communities on marmot mounds have different phylogenetic and functional community structures than neighboring plant communities along a succession series; and (4) what causes changes in community assembly during succession? The results of this study provide new evidence regarding community assembly processes during succession in alpine zones on the northeastern Qinghai–Tibet Plateau, and provide a new understanding of the maintenance of biodiversity in alpine meadows.
2. Materials and methods 2.1. Study area and sitesThe study site is located in an alpine meadow, 30°11′N, 99°58′E, around 4200 m, in Litang County, northwestern Sichuan Province, on the northeastern edge of the Qinghai–Tibet Plateau. The annual average temperature is approximately 3.0 ℃ and the monsoon season is from May to September.
We divided the community succession process into four stages: early, medium, late and last. In the early stage, mounds are covered with soil newly dug by marmots; in the medium stage, alpine plants cover less than 1/3 of the mounds; in the late stage, alpine plants cover between 1/3-2/3 of the mounds; in the last stage, alpine plants cover more than 2/3 of the mounds, which appears similar to the surrounding area (Fig. 1). We established 24 meadow plots along the succession gradient. Twelve plots each were established on marmot mounds and the surrounding areas, with three plots per stage in each area. To ensure that the mounds were completely covered, each plot was 2 × 2 m. The distance between plots was 20–50 m. The identity of vascular plant species and the number of each species in each plot were recorded to analyze changes in plant communities throughout succession (sup. 1).

|
| Fig. 1 Four successional stages of Himalayan marmot (Marmota himalayana) mounds: (a) early, (b) medium, (c) late, and (d) last stage. |
Phylogenetic trees with branch lengths (sup. 2) containing all the plant species in the sites were reconstructed using "V.PhyloMaker" in R software (Jin and Qian, 2019). The mega-tree in "V.PhyloMaker" was a combination of GBOTB for seed plants (Smith and Brown, 2018) and the clades in a previously published phylogeny for pteridophytes (Zanne et al., 2014). The tree was determined by family-level clades according to APG IV (Angiosperm Phylogeny Group 2016) for angiosperms. Although it is more accurate to use DNA sequences to construct phylogenetic trees, evidence obtained by using the APG tree is also credible (Pei, 2012; Qian and Jin, 2021).
2.3. Trait dataWe selected six functional traits that may mediate plant responses to disturbance and succession: life form, leaf texture, inflorescence type, fruit type, fruit appendages, and plant height (sup. 3). These traits include taxonomic and quantitative characters. Taxonomic traits (life form, leaf texture, inflorescence type, fruit type, fruit appendages) were determined according toFlora of China (Wu et al., 1994-2013) and field observations. Plant height, a quantitative trait, was measured at least three times (in September) in the wild using a meter ruler. These traits may be related to successful local establishment and may change along environmental gradients (McIntyre et al., 1995, 1999; Diaz et al., 2001; Lake and Leishman, 2004; Wang and Ni, 2005; Prusinkiewicz et al., 2007).
Trait conservatism, or phylogenetic signal, of the traits was determined according to the method proposed by Maddison and Slatkin (1991) for categorical traits and using K statistic, as proposed by Blomberg et al. (2003), for the height of the plants. For categorical traits, phylogenetic signal was determined by testing whether the minimum number of character state changes was lower than expected by chance on a phylogenetic tree in which data were reshuffled 999 times at the tips (Maddison and Slatkin, 1991). Trait conservatism is indicated when the number of character state changes is lower than expected by chance, i.e., when closely related taxa are more similar than expected for the trait (Maddison and Slatkin, 1991). For qualitative traits, we tested phylogenetic signal in R v. 3.4.2 (R core Team, 2017), using the function 'phylo.signal.disc', which was made to correspond to Maddison and Slatkin (1991) as proposed by Enrico Rezende. We used Bloomberg's K to test for phylogenetic signal in plant height, which is a continuous variable. K values greater than 1 imply that closely related species are more similar than expected, which is a strong phylogenetic signal indicating niche conservatism (Blomberg et al., 2003). Significance was determined using randomization tests with 999 permutations.
2.4. Diversity indicesWe estimated phylogenetic and functional structures along successional gradients with a net related index (NRI) using the function 'ses.mpd' in Picante package (Kembel et al., 2010). We calculated NRI as follows:

|
NRI is based on mean phylogenetic or functional distance (MPD) (Webb et al., 2008, 2011), which is the average phylogenetic or functional relatedness between all possible taxon pairs at one site. The phylogenetic distance between species pairs was computed with the function 'cophenetic' in the Picante package (Kembel et al., 2010). Functional dissimilarity between species was computed with the Gower distance (Leps et al., 2006). NRI was calculated based on phylogeny (phylogenetic NRI, NRI-P) and on the functional traits (functional NRI, NRI-F). We compared succession on marmot mounds with succession on anthills common in alpine regions. For this purpose, we used data from Meng et al. (2011) to calculate phylogenetic and functional diversities of anthills across successional stages with the methods described herein.
2.5. AnalysesTo identify the phylogenetic and functional structures of plants along marmot mound successional series, phylogenetic and functional alpha diversities (NRI-P, and NRI-F) were calculated at each stage of the successional series for the plots on marmot mounds and the neighboring area. Phylogenetic and functional structures were compared between the mounds and neighboring sites along a succession series to detect whether they showed different patterns. The species pool used in the analyses included all species in the sites. All analyses were performed with R software (R core Team, 2017).
3. ResultsThe number of plant species on marmot mounds ranged from four to eight, and increased from the early stage to the late stage of succession. All six traits examined displayed significant phylogenetic signal (Table 1). For the categorical traits (life form, leaf texture, inflorescence type, fruit type, fruit appendages), the minimum number of character state changes was lower than expected by chance (P < 0.05). The K-value for plant height was greater than 1 (1.79). These results provide strong evidence for niche conservatism.
| Traits | K Value | Changes observed | Changes random | P-value |
| Life form | 3 | 3 | 0.041 | |
| Leaf texture | 3 | 5 | 0.026 | |
| Inflorescence type | 5 | 7 | 0.018 | |
| Fruit type | 4 | 4 | 0.041 | |
| Fruit appendages | 3 | 5 | 0.001 | |
| Plant height | 1.79346 | 0.011 |
Phylogenetic and functional structures (NRI-P and NRI-F) of plant communities showed a unimodal pattern along the succession gradients on marmot mounds (Fig. 2). During the early stage of succession, NRI values were negative, indicating that taxa growing on marmot mounds were phylogenetically overdispersed; at the medium and late stages, NRI values were positive, indicating that taxa were phylogenetically clustered. During the last stage, the values of NRI-P and NRI-F were negative, indicating phylogenetic overdispersion again (Fig. 2). The phylogenetic structure (NRI-P) showed no significant differences between the medium and late stage, whereas the functional structure (NRI-F) showed significant differences between the medium and late stage. Phylogenetic and functional structures (NRI-P and NRI-F) showed no significant differences between early and last stages; in contrast, phylogenetic and functional structures of the medium and late stages showed significant differences from early and last stages (Fig. 2).
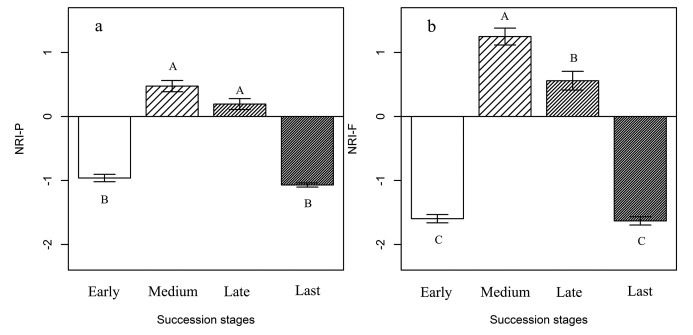
|
| Fig. 2 (a) Phylogenetic and (b) functional community structure (mean NRI-P, NRI-F, y-axis) on marmot mounds along the successional series. Specifically, NRI < 0 indicates phylogenetic overdispersion, whereas NRI > 0 indicates phylogenetic clustering. Letters indicate significant differences (α = 0.01) along succession. |
Sites neighboring marmot mounds were phylogenetically overdispersed (NRI-P and NRI-F), and showed no significant differences in community structure during the successional series, except during the medium stage (Fig. 3).
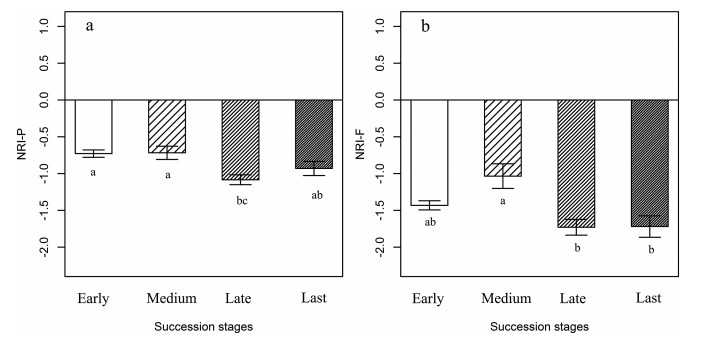
|
| Fig. 3 (a) Phylogenetic and (b) functional community structure (mean NRI-P, NRI-F, y-axis) surrounding marmot mounds (neighboring sites) along the succession series. Specifically, NRI < 0 indicates phylogenetic overdispersion, whereas NRI > 0 indicates phylogenetic clustering. |
Although plant community structures (NRI-P and NRI-F) on marmot mounds did not differ significantly from those in neighboring sites during the early and last stages of succession, community structures on marmot mounds were significantly different than those of neighboring sites at the medium and late stages (Table 2). In addition, plant species diversity was significantly changed by marmot disturbance during the early and last stages of community succession. Taken together, our results show that the phylogenetic and functional structures (NRI-P and NRI-F) of plant communities growing in a successional series on marmot mounds undergo a circular change.
| Stage | Mean NRI-P | Mean NRI-F | ||||
| Value difference | P-value | Value difference | P-value | |||
| Early stage | 0.2325 | 0.3847 | 0.1645 | 0.9706 | ||
| Medium stage | 1.1930 | 0.0000001 | 2.2837 | 0 | ||
| Late stage | 1.2783 | 0 | 2.2891 | 0 | ||
| Last stage | 0.1393 | 0.8720 | 0.0890 | 0.9992 | ||
| Significantly differences are shown in bold. | ||||||
The phylogenetic and functional structure of plant community successions on anthills showed a unimodal pattern (Fig. 4). During the first phase of succession, the phylogenetic structure was clustered. In mid-sized and large anthills, the phylogenetic and functional structures were clustered. The phylogenetic and functional structures of sites neighboring anthills were overdispersed.
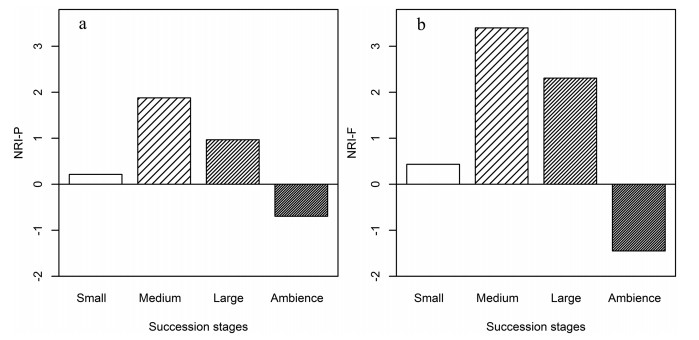
|
| Fig. 4 (a) Phylogenetic and (b) functional community structure (mean NRI-P, NRI-F, y-axis) on anthills along succession series. Specifically, NRI < 0 indicates phylogenetic or functional overdispersion, whereas NRI indicates phylogenetic or functional clustering. |
Analyzing the patterns of diversity along community succession is essential for understanding and predicting how plant communities respond to environmental changes (Prach and Walker, 2011). Our results show that phylogenetic and functional structures alpine plant communities growing on marmot mounds have significant successional patterns. This trend is probably caused by environmental changes on mounds related to marmot disturbance.
In plant communities growing on marmot mounds, traits were phylogenetically conserved. The phylogenetic and functional structures of these communities suggest that environmental filtering structures these alpine plant communities by selecting for traits capable of surviving harsh environmental conditions, thereby promoting the coexistence of closely related species with similar traits at the medium and late stages of the successional gradient. In these two stages, the environment (e.g., temperature and humidity) on the mound changes greatly (Meng et al., 2011). Species that cannot adapt to this environment are excluded, while species more closely associated with these environments remain, resulting in phylogenetic clustering. Under less stressful conditions, distantly related alpine plants tend to co-exist on the mounds at the last stage and away from the mounds, suggesting that these communities are probably structured by the effects of biotic interactions, such as competition between alpine plants. In the last stages and away from the mounds, the environment becomes consistent with the surrounding area. In addition, Himalayan marmots sometimes abandon their burrows, which reduces disturbance. Consequently, competition among alpine plants may become dominant again, leading to phylogenetic overdispersion.
Plant community composition on marmot mounds changes during succession. In theory, phylogenetic clustering in a community is caused by an increase in related species (Norden et al., 2012), or a decrease in distantly related species. Phylogenetic overdispersion, in contrast, is caused by a decrease in related species (Norden et al., 2012) or an increase in distantly related species. During the early stages of succession on the marmot mounds, marmots directly decrease the number of plants of Kobresia pygmaea (C.B. Clarke) C.B. Clarke by burying the plants under the mound. However, K. pygmaea, which is a dominant species and a monocotyledonous plant, increased from 1.66 in the middle stage to 9.33 in the late stage. Thus, phylogenetic and functional structures showed a trend toward overdispersion. At the medium stage, K. pygmaea remained rare due to marmot disturbance on the mounds. Instead, at this stage Potentilla tatsienluensis Th. Wolf., Potentilla saundersiana Royle and other dicotyledonous species were dominant. The decrease in distantly related species and the increase in closely related species led to highly clustered phylogenetic and functional structures. At same time, the phylogenetic and functional structures differed between the mounds and the neighboring sites, likely because marmot activity was more concentrated on the mounds than on the neighboring sites. At the late stage, dicotyledonous species also dominated, but K. pygmaea returned to the mounds and became common. The phylogenetic and functional structures showed clustering, but they showed a tendency towards overdispersion. During this late stage, the phylogenetic and functional structures of plant communities on marmot mounds differed significantly from those on neighboring sites. In the last stage, K. pygmaea again became the dominant species andP. saundersiana and P. tatsienluensis, which are closely related, were less common than in the medium and late stages. Therefore, a decrease in related species and an increase in distantly related species resulted in overdispersion of phylogenetic and functional structures. Along the successional gradient, neighboring sites were disturbed little because Himalayan marmots eat few plants that grow around their burrow; therefore, the phylogenetic and functional structures of neighboring plant communities, which were overdispersed, showed almost no difference during the stages of succession.
In alpine regions, plant communities commonly undergo succession on anthills. On the marmot mounds, plants are basically buried during the first stage of succession; in contrast, plants that grow on anthills are not completely buried during the first stages of succession because anthills are built slowly (King, 1977). The patterns of phylogenetic and functional structure in plant communities growing on anthills are similar to those of plant communities growing on marmot mounds. Both show a unimodal pattern of community succession. In the first phase of plant succession on anthills, the phylogenetic structure is clustered, whereas the first stage of plant succession on marmot mounds is phylogenetically overdispersed. In mid-sized and large ant-hills, the plant phylogenetic and functional structures are clustered, which is consistent with our findings on the middle and late stages of plant succession on marmot mounds. Sites neighboring anthills are relatively stable in favorable environments. Phylogenetic and functional community structures show overdispersion at these neighboring sites, similar to the patterns of phylogenetic and functional structures away from marmot mounds. Studies on phylogenetic diversity consistently show a similar unimodal pattern along successional gradients: at the medium and late stages, when the environment is harsh, plant communities become clustered; during the last stage, when the environment is favorable, plant communities become overdispersed. Harsh environmental conditions are expected to filter numerous lineages that are not adapted to changing habitats, leaving those species that can tolerate abiotic conditions, thereby resulting in the coexistence of related species and leading to phylogenetic clustering. Favorable environments encourage the competition between related species, leaving distantly related species in the community, and leading to phylogenetic overdispersion.
5. ConclusionsThis study shows that different ecological drivers can change community assemblages during plant community succession on the mounds of Himalayan marmots (Marmota himalayana). Phylogenetic and functional community structures changed greatly on marmot mounds, but were relatively stable away from the mounds during succession and showed significance differences during the medium and late stages. Phylogenetic and functional clustering in the middle and late successional stages indicate that environmental filtering is an important force for structuring alpine plant communities in extreme habitats, whereas phylogenetic and functional overdispersion in the early and last successional stages suggest that biotic interactions result in the co-existence of distantly related species under more favorable conditions. Thus, based on a combination of traits that lead to community assemblages along an environmental gradient, plants can survive during temporal dynamic processes.
Author contributionsLi Xinhui was responsible for data analysis and for writing the manuscript. Yang Tao was responsible for the field investigation and for collecting the data. Wang Dandan was responsible for the design of the paper.
Declaration of competing interestWe declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted. Li Xinhui, Yang Tao and Wang Dandan.
AcknowledgementsThis work was supported by the National Natural Science Foundation of China (NSFC) (Grant No. 31560063) and Applied Basic Research Program of Yunnan Province, China (2018FB067).
Bhaskar, R., Dawson, T. E., Balvanera, P., 2014. Community assembly and functional diversity along succession post-management. Funct. Ecol., 28: 1256-1265. DOI:10.1111/1365-2435.12257 |
Blomberg, S. P., Garland, T., Ives, A. R., 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution, 57: 717-745. DOI:10.1111/j.0014-3820.2003.tb00285.x |
Carson, W. P., Schnitzer, S. A., 2008. Tropical forest community ecology. Blackwell Scientific, Oxford.
|
Diaz, S., Noy-Meir, I., Cabido, M., 2001. Can grazing response of herbaceous plants be predicted from simple vegetative traits?. J. Appl. Ecol., 38: 497-508. DOI:10.1046/j.1365-2664.2001.00635.x |
Jin, Y., Qian, H., 2019. V.PhyloMaker: an R package that can generate very large phylogenies for vascular plants. Ecography, 42: 1353-1359. DOI:10.1111/ecog.04434 |
Kembel, S. W., Cowan, P. D., Helmus, M. R., et al., 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics, 26: 1463-1464. DOI:10.1093/bioinformatics/btq166 |
King, T. J., 1977. Plant ecology of ant-hills in calcareous grasslands: II. succession on mounds. J. Ecol., 65: 257-278. DOI:10.2307/2259078 |
Lake, J. C., Leishman, M. R., 2004. Invasion success of exotic in natural ecosystems: the role of disturbance, plant attributes and freedom from herbivores. Biol. Conserv., 117: 215-226. DOI:10.1016/S0006-3207(03)00294-5 |
Lebrija-Trejos, E., Perez-Garcia, E. A., Meave, J. A., et al., 2010. Functional traits and environmental filtering drive community assembly in a species-rich tropical system. Ecology, 91: 386-398. DOI:10.1890/08-1449.1 |
Leibold, M. A., Holyoak, M., Mouquet, N., et al., 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecol. Lett., 7: 601-613. DOI:10.1111/j.1461-0248.2004.00608.x |
Leps, J., de Bello, F., Lavorel, S., et al., 2006. Quantifying and interpreting functional diversity of natural communities: practical considerations matter. Preslia, 78: 481-501. |
Lohbeck, M., Poorter, L., Martinez-Ramos, M., et al., 2014. Changing drivers of species dominance during tropical forest succession. Funct. Ecol., 28: 1052-1058. DOI:10.1111/1365-2435.12240 |
Maddison, W. P., Slatkin, M., 1991. Null models for the number of evolutionary steps in a character on a phylogenetic tree. Evolution, 45: 1184-1197. DOI:10.1111/j.1558-5646.1991.tb04385.x |
McIntyre, S., Lavorel, S., Landsberg, J., et al., 1999. Disturbance response in vegetation towards a global perspective on functional traits. J. Veg. Sci., 10: 621-630. DOI:10.2307/3237077 |
McIntyre, S., Lavorel, S., Tremont, R. M., 1995. Plant life-history attributes: their relationship to disturbance responses in herbaceous vegetation. J. Ecol., 83: 31-44. DOI:10.2307/2261148 |
Meng, F. Q., Gao, X. M., Sun, S. C., 2011. Plant community succession on ant-hills of a sub-alpine meadow in northwestern Sichuan, China: species composition and diversity. Plant Divers. Resour., 33: 191-199. |
Norden, N., Letcher, S. G., Boukili, V., et al., 2012. Demographic drivers of successional changes in phylogenetic structure across life-history stages in plant communities. Ecology, 93: S70-S82. DOI:10.1890/10-2179.1 |
Pei, N. C., 2012. Building a subtropical forest community phylogeny based on plant DNA barcodes from Dinghushan plot. Plant Divers. Resour., 34: 263-270. DOI:10.3724/SP.J.1143.2012.11173 |
Prach, K., Walker, L. R., 2011. Four opportunities for studies of ecological succession. Trends Ecol. Evol., 26: 119-123. DOI:10.1016/j.tree.2010.12.007 |
Prinzing, A., Durka, W., Klotz, S., et al., 2001. The niche of higher plants: evidence for phylogenetic conservatism. Proc. R. Soc. B-Biol. Sci., 268: 2383-2389. DOI:10.1098/rspb.2001.1801 |
Prusinkiewicz, P., Erasmus, Y., Lane, B., et al., 2007. Evolution and development of inflorescence architectures. Science, 316: 1452-1456. DOI:10.1126/science.1140429 |
Qian, H., 2001. A comparison of generic endemism of vascular plants between East Asia and North America. Int. J. Plant Sci., 162: 191-199. DOI:10.1086/317909 |
Hong, Q., Yi, J., 2021. Are phylogenies resolved at the genus level appropriate for studies on phylogenetic structure of species assemblages?. Plant Divers., 43: 255-263. DOI:10.1016/j.pld.2020.11.005 |
R core Team, 2017. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. URL https://www.R-project.org/.
|
Shooner, S., Chisholm, C., Davies, T. J., 2015. The phylogenetics of succession can guide restoration: an example from abandoned mine sites in the subarctic. J. Appl. Ecol., 52: 1509-1517. DOI:10.1111/1365-2664.12517 |
Smith, S. A., Brown, J. W., 2018. Constructing a broadly inclusive seed plant phylogeny. Am. J. Bot., 105: 302-314. DOI:10.1002/ajb2.1019 |
The Angiosperm Phylogeny Group, 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc., 181: 1-20. DOI:10.1111/boj.12385 |
Tucker, C. M., Cadotte, M. W., Carvalho, S. B., et al., 2016. A guide to phylogenetic metrics for conservation, community ecology and macroecology. Biol. Rev., 92: 698-715. |
Wang, G. H., Ni, J., 2005. Plant traits and environmental conditions along the Northeast China Transect. Ekologia-Bratisl., 24: 170-185. |
Webb, C. O., 2000. Exploring the phylogenetic structure of ecological communities: an example for rain forest trees. Am. Nat., 156: 145-155. DOI:10.1086/303378 |
Webb, C. O., Ackerley, D. D., Kembel, S. W., 2011. "Software for the analysis of phylogenetic community structure and character evolution (with phylomatic and ecovolve). " User´s Manual Version 4.2. from http://phylodiversity.net/phylocom/.
|
Webb, C. O., Ackerly, D. D., Kembel, S. W., 2008. Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics, 24: 2098-2100. DOI:10.1093/bioinformatics/btn358 |
Webb, C. O., Ackerly, D. D., McPeek, M. A., et al., 2002. Phylogenies and community ecology. Annu. Rev. Ecol. Systemat., 33: 475-505. DOI:10.1146/annurev.ecolsys.33.010802.150448 |
Wu, Z. Y., Raven, P. H., Hong, D. Y., 1994-2013. Flora of China. Science Press, Beijing; Missouri Botanical Garden Press, St. Louis.
|
Zanne, A. E., Tank, D. C., Cornwell, W. K., et al., 2014. Three keys to the radiation of angiosperms into freezing environments. Nature, 506: 89-92. DOI:10.1038/nature12872 |



