b. Aomori Prefectural Asamushi Aquarium, 1-25 Babayama, Asamushi, Aomori, Aomori, 039-3501, Japan;
c. Faculty of Agriculture, Niigata University, 8050 Ikarashi-ninocho, Nishi-ku, Niigata, 950-2181, Japan
Plants adapt to various ecological niches (e.g., diverse habitats and pollination systems) through morphological diversification and speciation (Futuyma and Slatkin, 1983). For example, leaf morphology in the silversword alliance (Asteraceae), which consists of 25 species that all evolved from a single colonizing ancestor in Hawaii, has been shown to diverge in dissimilar habitats (Barrier et al., 1999). Adaptations to a particular pollination syndrome promote speciation by attracting new pollinators through traits such as flower color and scent. Research has shown that changes in flower morphology lead to speciation by shifting pollinator preferences from bees to hummingbirds or bats (Grant and Grant, 1965; Castellanos et al., 2003; Wilson et al., 2007). Plants also hybridize, but because they are sessile, hybridization is less common than speciation, emphasizing the importance of pre-mating rather than post-mating isolation (Ando et al., 2001; Ramsey et al., 2003; Yasumoto and Yahara, 2006). Thus, identifying the factors that play a role in pre-mating isolation are critical to understanding plant speciation.
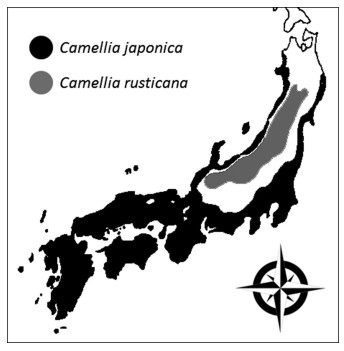
"Japan Sea elements" offer an opportunity to examine how ecological niches drive morphological divergence and speciation. Plant species unique to the region near the Sea of Japan, which are known as Japan Sea elements, are distributed according to levels of snowfall (Maekawa, 1977). Observations that species closely related to Japan Sea elements are distributed in the warm temperate zones of the Pacific regions have suggested that Japan Sea elements adapted to a past climate change and speciated from the temperate environments in Japan (Hotta, 1974; Sakai, 1982). In total, 212 taxa of Japan Sea elements and their relative species have been reported, including Camellia japonica L., and Camellia rusticana Honda (Sato, 2005; Honda, 1950) (Fig. 1, Fig. 2). C. japonica is distributed along the coast of the Natsudomari peninsula in the Aomori Prefecture of eastern Japan (Horikawa, 1972). It is also found in southern Korea and in the coastal regions of mainland China (Nagamasu, 2006). C. rusticana ranges from the Shiga prefecture to the snowy regions on the Japan Sea side of the Akita prefecture (Ishizawa, 1988) (Fig. 2). Therefore, it is often considered that C. rusticana is adapted to snowy regions and has speciated from C. japonica (Tsuyama, 1988).

|
| Fig. 1 Photos of Camellia japonica and C. rusticana. |
|
| Fig. 2 Distribution of Camellia sect. Camellia in Japan. This figure was transcribed from Ishizawa (1988). |
The taxonomic positions of Camellia japonica and C. rusticana remain controversial. Various researchers have classified them as either variety, subspecies, or species (Tsuyama, 1956; Kume et al., 1998; Ishizawa, 2005; Tateishi et al., 2007). According to Ishizawa (2003), C. rusticana was identified by Sugimoto (1936) as a variant, and initially named C. japonica var. decumbenus Sugimoto. C. rusticana was recognized by Honda (1950) as an independent species, but Kitamura (1950) later recognized it as a subspecies, C. japonica subsp. rusticana Kitamura. Individuals with an intermediate form called 'yuki-bata-tsubaki' can be seen in areas where the ranges of the two species are adjacent (Hagiya and Ishizawa, 1961; Orikawa et al., 1998), and hybridization occurs naturally (Hagiya and Ishizawa, 1961; Ueno, 2009). FTIR spectroscopy and microsatellite data have indicated that C. japonica and C. rusticana cluster together in the same clade (Shen et al., 2008; Caser et al., 2010; Eguchi et al., 1991; Ueno, 2009; Vijayan et al., 2009). Furthermore, anthocyanin pigment distribution in the petals indicates that C. rusticana possibly speciated from a common ancestor earlier than C. japonica (Sakata et al., 1987). The phylogeny of the genus Camellia is similarly inconsistent (Min and Bartholomew 2007; Vijayan et al., 2009; Yang et al., 2013; Huang et al., 2014; Kim et al., 2017; Li et al., 2019); therefore, estimating the evolution of Camellia requires integrating morphological, geographical, and genetic data.
Camellia japonica and C. rusticana differ morphologically, ecologically, and physiologically. C. japonica occurs in places where the average temperature in January is higher than ˗0.6 ℃ and the minimum warmth index proposed by Kira (1945) is 85 (Ishizuka, 1947). Although areas within this temperature range occur extensively in the inland snowy regions of the Japan Sea side, to the north of Fukui Prefecture, C. rusticana rather than C. japonica grows in places where snow accumulation exceeds 150 cm (Ishizawa, 1978) (Fig. 2). Morphological differences between C. japonica and C. rusticana include the presence or absence of petiole hair, presence or absence of a layer of hypodermis tissue under the epidermis, shape of the corolla, size of the seeds, and ratio of the filament coalescence of flowers (Tsuyama, 1956; Ishizawa, 2005). C. japonica flowers between December and April and is mainly pollinated by birds, as insects are scarce in winter (Yumoto, 1988; Kunitake et al., 2004; Abe and Hasegawa, 2008; Abe et al., 2011). C. rusticana flowers in early spring, from April to June, and is pollinated by insects such as flies, bees, and small beetles (Ishizawa, 1988). C. japonica and C. rusticana flowers have different traits, which may play a role in attracting different pollinator types (Ishizawa, 2005). The photosynthetic characteristics of C. japonica and C. rusticana also differ (Kume et al., 1998). The observable differences between these species suggest that speciation may have occurred through pre-mating isolation following morphological divergence of both flowers and leaves. However, little quantitative data is available on leaf and flower morphology or petal and filament color of these species.
In this study, we focused on the quantitative morphological differences between Camellia japonica and C. rusticana. First, we compared their leaf morphology to identify the differences in adaptation to the varied environments of their respective habitats. Second, we examined the degree of differentiation of the two species by quantitative comparison of flower form, petal color, and filament color.
2. Material and methods 2.1. Comparative analysis of leaf morphologyPrevious studies that examined five leaf morphology traits of Camellia japonica and C. rusticana (blade length, blade width, petiole length, distance of the widest point from base, distance of the widest point from base/blade length) found no significant differences (Orikawa et al., 1998). However, C. japonica has only two layers of palisade tissue beneath the upper epidermis, whereas C. rusticana has one layer of hypodermis next to the epidermis, which is made of slightly larger cells, followed by two layers of palisade tissue (Shimada and Hisada, 1966). Therefore, in this study, we used differences in leaf hypodermis to examine how ecological conditions drive morphological divergence.
We examined 80 individuals of Camellia japonica (Mean = 10 individuals; SD ± 0.0) from eight populations and 120 individuals of C. rusticana (Mean = 10 individuals; SD ± 0.0) from 12 populations for the presence of hypodermal tissue (Table 1). All specimens were collected from April to June 2014 and in July 2015. Specimens collected within previously reported ranges for respective species were considered to belong to those species (i.e., specimens collected in C. japonica range were considered C. japonica) (Ishizawa, 1988). At these sampling sites, we avoided areas adjacent to both species, where plants may have hybridized. Samples were taken from individuals more than 20 m apart. We also examined the leaves of 13 individuals from elevations between 20 and 220 m in Yoneyama, Niigata Prefecture, where hybridization is known to occur.
| Pop No. | Species | Pop | Flower samples | Flower petal color samples | Filament color samples | Leaf samples | Latitude | Longitude | Elevation (m) |
| 1 | C.rusticana | Senpoku | 0 | 0 | 0 | 10 | 39.6808 | 140.6654 | 423 |
| 2 | C.rusticana | Osyu | 5 | 4 | 3 | 10 | 39.1117 | 140.8546 | 472 |
| 3 | C.rusticana | Haguro | 0 | 0 | 0 | 10 | 38.7032 | 139.9831 | 379 |
| 4 | C.japonica | Murakami | 21 | 20 | 14 | 10 | 38.4921 | 139.5153 | 70 |
| 5 | C.rusticana | Nishikawa | 8 | 9 | 8 | 10 | 38.4553 | 140.0234 | 416 |
| 6 | C.japonica | Osado | 27 | 20 | 20 | 10 | 38.2534 | 138.4235 | 8 |
| 7 | C.rusticana | Yamagata | 0 | 0 | 0 | 10 | 38.2453 | 140.1967 | 565 |
| 8 | C.japonica | KunimiB | 14 | 0 | 0 | 10 | 38.0496 | 138.4706 | 41 |
| 9 | C.rusticana | KunimiA | 28 | 25 | 21 | 10 | 38.0023 | 138.5060 | 579 |
| 10 | C.japonica | Futami | 13 | 20 | 0 | 10 | 37.9785 | 138.2571 | 23 |
| 11 | C.rusticana | Shibata | 8 | 8 | 8 | 10 | 37.9010 | 139.4810 | 1137 |
| 12 | C.japonica | Niigata | 27 | 14 | 7 | 10 | 37.7293 | 138.7921 | 16 |
| 13 | C.japonica | Kashiwazaki | 12 | 0 | 0 | 10 | 37.4542 | 138.6116 | 71 |
| 14 | C.rusticana | Nagaoka | 7 | 8 | 7 | 10 | 37.4101 | 139.1231 | 1429 |
| 15 | C.rusticana | Tadami | 28 | 19 | 20 | 10 | 37.3154 | 139.2672 | 584 |
| 16 | C.rusticana | Tokamachi | 0 | 0 | 0 | 10 | 37.1000 | 138.6173 | 313 |
| 17 | C.rusticana | Joetsu | 1 | 2 | 0 | 10 | 36.8573 | 137.8276 | 985 |
| 18 | C.rusticana | Nagahama | 0 | 0 | 0 | 10 | 35.6165 | 136.1718 | 295 |
| 19 | C.japonica | Kitakyusyu | 2 | 0 | 0 | 10 | 33.7100 | 130.5814 | 109 |
| 20 | C.japonica | Kanoya | 8 | 0 | 0 | 10 | 31.4900 | 130.8346 | 781 |
| Total | 209 | 149 | 108 | 200 | |||||
| pop = population. Population names are listed starting from the North. | |||||||||
The hypodermal tissue was examined using the following procedure. Leaves were cut into 1-cm squares and were vertically sliced as thinly as possible using a razor blade. The thin slices were then observed under an optical microscope (50 ×) (CX41; Olympus, Tokyo, Japan) for the presence of hypodermal tissue.
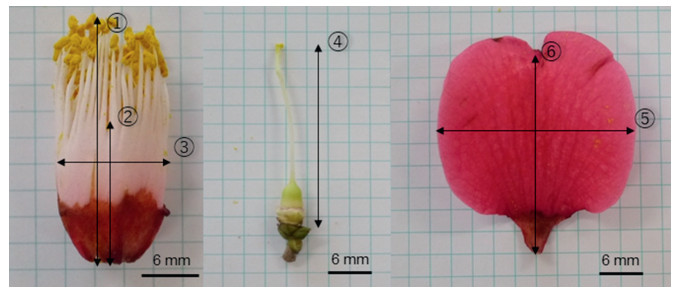
2.2. Comparative analysis of flower morphologyCamellia japonica and Camellia rusticana flowers differ in the shapes of the corolla and petal, rates of flower filament coalescence, lengths of stamen and pistil, and colors of flower filament (Ishizawa, 2005, 2010). In this study, we measured several quantifiable traits of C. japonica and C. rusticana flowers, including the length and breadth of the petal, aspect ratio of the petal, maximum length of the stamen, rate of flower filament coalescence (length of filament coalescence/length of filament), length of the pistil, and width of the stamen (Fig. 3). The morphological characters of flowers sometimes vary within a single individual plant, but these differences were not as large as the differences between respective individuals in the preliminary survey.
|
| Fig. 3 Measurement of flower morphology. 1, Maximum length of stamen; 2, Length of filament coalescence, 2/1; Rate of filament coalescence; 3, Stamen width; 4, Pistil length; 5, Petal width; 6, Petal length. |
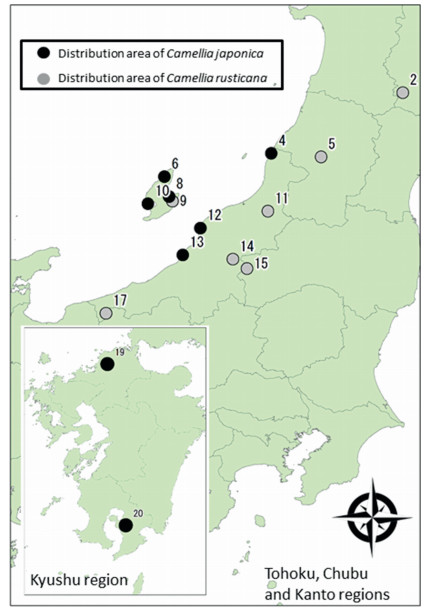
We compared the flower morphology of 124 individuals of Camellia japonica (Mean = 15.5 individuals; SD ± 8.9) from eight populations and 85 individuals of C. rusticana (Mean = 21.1 individuals; SD ± 11.1) from seven populations (Table 1; Fig. 4). We avoided sampling flowers in areas where both species occurred adjacent to each other. One flower per tree was collected from April 2014 to June 2014.
|
| Fig. 4 Collection sites of floral materials of Camellia sect. Camellia. Number shows sampling location (Table 1). |
Flowers were dissected into each part as much as possible. A t-test was used to compare the flower parts of Camellia japonica and C. rusticana. Principal Components Analysis (PCA) was performed using the top three items (maximum length of stamen, rate of flower filament coalescence, and length of pistil) with the highest scores on the PC 1 axis obtained from initial analysis. Statistical analyses were performed in R 3.1.1 (R Development Core Team, 2014).
2.3. Comparative analysis of petal and flower filament colorsPetal and flower-filament color were measured from 74 individuals of Camellia japonica (Mean = 18.5 individuals; SD ± 3.0) from four populations, and from 79 individuals of C. rusticana (Mean = 10.7 individuals; SD ± 8.3) from seven populations (Table 1). All specimens were collected from April to June 2014. Measurements were taken on the day of collection or the next day to measure the flower parts before discoloration. All discolored flowers were excluded from the analyses. The value and chroma in the Munsell color space were measured to estimate petal color using a spectrocolorimeter (CM- 2002; Konica Minolta, Tokyo, Japan). The colorimeter lights the petals, spectrally separates the light reflected from the petals, and converts the reflected light of each wavelength into numerical values. Wavelengths in the range of 400–700 nm were measured every 10 nm using the following conditions: 10° standard observer, illuminants DD65, and SCI setting, which means that the instrument's geometry and design allowed for the specular component to be included. The value and chroma in this system represent lightness and color purity, respectively. The value varies vertically along the color solid, from black (value 0) at the bottom, to white (value 10) at the top. Chroma, measured radially from the center of each color slice, represents the purity of a color related to saturation, with lower chroma being less pure and more washed out, as in pastels. Because the color of the flower filament could not be measured using the colorimeter due to its three-dimensional structure, we photographed flower filaments in a dark room (NIKON D100; Nikon, Tokyo, Japan) and processed the acquired images using ImageJ software (Abràmoff et al., 2004). During image processing, the anthers and petals attached to the flower filament were separated using ImageJ. The flower filament was extracted, and its color was transformed into the primary red (R), green (G), and blue (B) values (Motonaga et al., 1997, 2015).
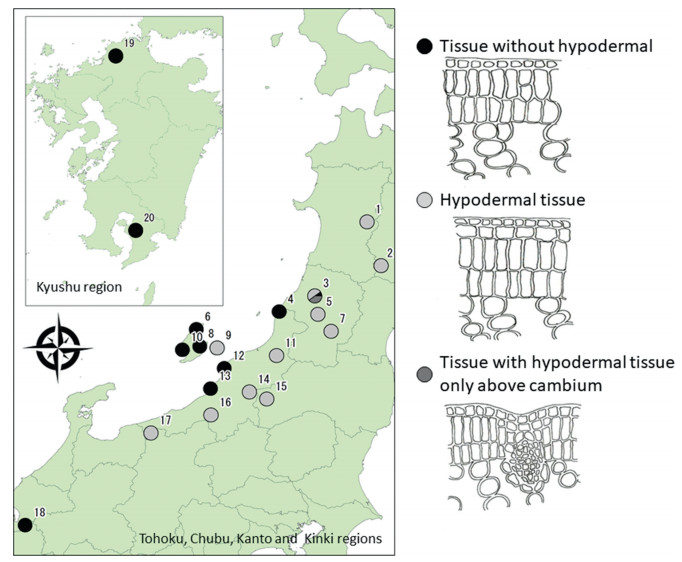
3. Results 3.1. Comparative analysis of leaf morphologyObservation of the cross-sectional morphology of the leaves revealed three types of individuals (Fig. 5): 105 with hypodermal tissue, 91 without hypodermal tissue, and four with hypodermal tissue only above the cambium. The populations that had hypodermal tissue were predominantly found in the Camellia rusticana distribution area, whereas the populations without hypodermal tissue were mainly found in the C. japonica distribution area (Fig. 5). However, the presence of hypodermal tissue was not observed in the population collected from Nagahama (Shiga Prefecture) in the distribution area of C. rusticana. In Mt. Haguro, Yamagata Prefecture, which is a distribution area of C. rusticana, five out of 10 individuals had hypodermal tissue, one did not have hypodermal tissue, and four had hypodermal tissue above the cambium (Table 1; population No. 3).
|
| Fig. 5 Cross section of the morphology of leaves in the Camellia sect. Camellia. Number shows sampling location (Table 1). |
Of the thirteen individuals sampled from the hybridization zone (20–220 m), plants with hypodermal tissue were not observed below 50 m. Of the thirteen individuals sampled from the hybridization zone (20–220 m), plants with hypodermal tissue were not observed below 50 m. An intermediate form appeared in the two individuals at an elevation of approximately 50 m, and the presence of hypodermal tissue was observed in all individuals found at elevations higher than 50 m
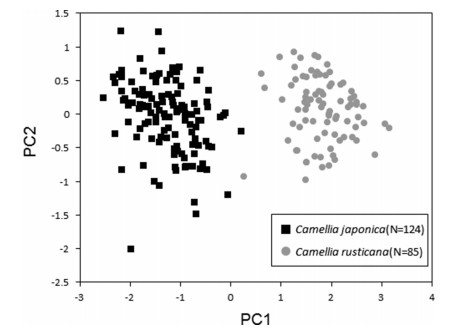
3.2. Comparative analysis of flower morphologyFlower morphological traits of Camellia japonica and C. rusticana differed significantly (Table 2; Fig. 3). The length of stamen (P < 0.01), length of pistil (P < 0.01), width of stamen (P < 0.01), length of petal (P < 0.01), and width of petal (P < 0.01) were significantly larger in C. japonica than in C. rusticana. The rate of flower filament coalescence was significantly higher in C. japonica than in C. rusticana (P < 0.01). With respect to the petal aspect ratio, C. rusticana had more elongated petals (P < 0.01). PCA divided the traits into two groups (Fig. 6). The cumulative contribution rate was about 87% on the PC 1 axis and about 96% on the PC 2 axis (Table 3). On the PC 1 axis, eigenvalues reached close to an upper ˗0.5, with the maximum length of the stamen, coalescence rate, and length of the pistil. Only one individual collected at Mt. Kunimi, Sado City, Niigata Prefecture, which is a distribution area of C. rusticana, showed values on the C. japonica side of the PC 1 axis (Table 1; population No. 9, Fig. 6). Other than this individual, most of the individuals were largely divided into two distributional areas, that of C. japonica or C. rusticana, by the PC 1 axis around 0.5 (Fig. 6).
| Species | No. | Stamen length | Stamen width | Rate of filament coalescence | Pistil length | Petal width | Petal length | Aspect ratio of petals |
| Camellia japonica | 124 | 34.59 (±4.53) | 15.64 (±2.45) | 0.62 (±0.07) | 32.00 (±3.47) | 35.8 (±5.92) | 43.97 (±6.97) | 1.24 (±0.17) |
| C.rusticana | 85 | 15.64 (±4.56) | 11.95 (±2.94) | 0.34 (±0.07) | 15.43 (±5.10) | 23.95 (±4.25) | 33.02 (±5.41) | 1.40 (±0.21) |
| Collection sites are shown in Table 1. Significant differences were observed in all traits (P < 0.01; t-test). | ||||||||
|
| Fig. 6 Principal component analysis (PCA) on flower morphology of Camellia japonica and C. rusticana. PCA analysis was repeated using the top three items (stamen maximum length, rate of flower filament coalescence, and length of pistil) that had the highest score on the PC1 axis obtained from initial analysis. |
| PC1 | PC2 | |
| Stamen length | ˗0.58 | 0.43 |
| Pistil length | ˗0.59 | 0.37 |
| Connate range | ˗0.56 | ˗0.83 |
| Cumulative proportion | 0.87 | 0.96 |
| Scatterplot of this PCA is shown in Fig. 6. | ||
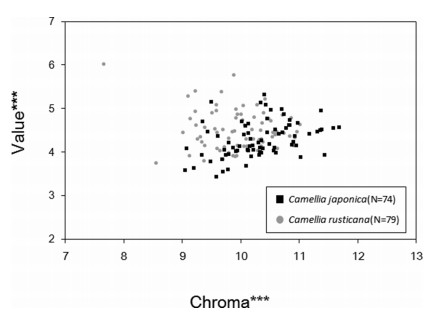
Petal color analysis showed that the brightness and saturation of Camellia japonica and C. rusticana flowers differed significantly (P < 0.01;Fig. 7). C. japonica had darker and purer red petals than C. rusticana. The average Munsell value of C. japonica was 4.28, whereas that of C. rusticana was 4.50. The average Munsell chroma of C. japonica was 10.34, whereas that of C. rusticana was 9.96.
|
| Fig. 7 Comparison of petal color of Camellia japonica and C. rusticana. Brightness and saturation were determined for the petal color using a colorimeter (CM- 2002; Konica Minolta, Tokyo, Japan). The measurement wavelength is from 400 to 700 nm at 10 nm pitch. "Chroma" indicates saturation and "Value" indicates lightness. ***; P < 0.01 (t-test). |
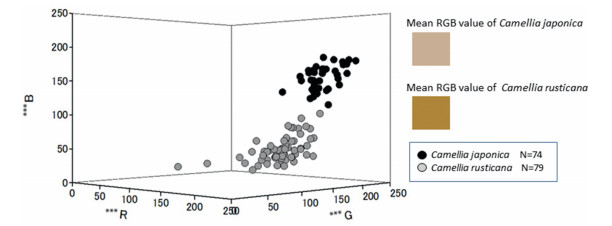
Average combined RGB values showed that Camellia japonica flower filaments were closer to white, whereas C. rusticana flower filaments were closer to yellow (Fig. 8). The average R, G, and B values for C. japonica flower filaments were 184, 141, and 51, respectively; for C. rusticana, R, G, and B values were 210, 184, and 157, respectively. The largest difference in RGB values was observed in the B values (P < 0.01).
|
| Fig. 8 Distribution filament color of Camellia japonica and C. rusticana in the RGB color space. RGB values of filament color were analyzed using camera (NIKON D100; Nikon, Tokyo, Japan) and Image J (Abràmoff et al., 2004). ***; P < 0.01 (y-test). i: average R, G, B values of C. japonica (184, 141 and 51, respectively); ii: average R, G, B values of C. rusticana (210, 184, and 157, respectively). |
In this study, we observed hypodermal tissue in leaves of plants sampled from Camellia rusticana distribution areas but not in leaves of those sampled from C. japonica distribution areas (Fig. 5). Our findings support those from previous studies that distinguish these species based on their distribution areas (Ishizawa, 1988) and leaf morphology (Shimada and Hisada, 1966; Lu et al., 2012). However, leaf morphology of plants in Mt. Haguro, Yamagata Prefecture (Table 1; population No. 3) and Nagahama City, Shiga Prefecture (Table 1; population No. 18), which are distribution areas of C. rusticana, were inconsistent with findings from a previous study (Ishizawa, 1988).
Previous research indicated that various forms of Camellia japonica, C. rusticana, and their hybrids are distributed at lower elevations; in contrast, at higher elevations, only populations of C. rusticana and its hybrids are found (Orikawa et al., 1998). Although C. rusticana is distributed at high elevations, the boundaries of its distribution are likely found at low to mid-elevations that comprise hybrid-dominant zones. In this study, differences in leaf morphology indicate that C. rusticana is distributed from 295 to 1429 m (average 631 m), Camellia japonica is distributed from 8 to 781 m (average 140 m), and C. rusticana grows in snowy areas at higher elevations (Table 1). Elevation and snow depth are not necessarily positively correlated; however, owing to atmospheric pressure, higher elevations are more likely to have greater levels of snow.
We discovered an intermediate leaf type for the first time. Specifically, of thirteen individuals sampled at an elevation of 20–220 m, two individuals sampled at approximately 50 m had an intermediate. These findings suggest that plants without hypodermal tissue are Camellia japonica, those with hypodermal tissue are C. rusticana, and those with the intermediate type are hybrids. There are some areas in Shiga Prefecture, where the distribution of both species overlaps. The sampling site in Tsubakizaka, Nagahama city (No. 18) was near the south boundary and located at a mid-elevation of 295 m, which is the lowest elevation for C. rusticana in this study. Therefore, hybridization appears to occur under natural conditions. We collected leaves from around a shrine in Mt. Haguro, Yamagata Prefecture, where leaf morphology could be predicted by the distribution area. Although hybridization may occur under natural conditions, there is a high probability that hybridization also occurs due to artificial planting near the shrine.
Previous studies have indicated that the presence or absence of hypodermal tissue reflects a difference in the physiological characteristics of plants living in snowy environments. For example, Camellia rusticana is distributed in areas that receive annual snowfall of 150 cm or more and are snow-covered for 90–200 days (Ishizawa, 1978). Photosynthetic characteristics of C. japonica and C. rusticana have suggested that C. rusticana must covered by snow in winter (Kume and Tanaka, 1996). In addition, Kume et al. (1998) also showed that under experimental conditions, C. rusticana maintains physiological activity under snow for over 360 days. These findings lead us to speculate that the presence of hypodermal tissue may be related to the maintenance of photosynthetic properties and physiological activity under snow, and that differences in environmental conditions have driven physiological adaptations that caused speciation. In our study, however, we have found only circumstantial evidence of the correspondence between the distribution and leaf morphology of C. japonica and C. rusticana. Additional work is required to confirm this relationship and elucidate the mechanisms that underlie this evolutionary process.
4.2. Comparison of flower morphologyAnalysis of flower morphology revealed that Camellia japonica has larger petals than C. rusticana (Table 2; P < 0.01). While the flower filament of C. japonica has a high coalescence rate and a tubular shape, that of C. rusticana was found to be spread (P < 0.01). This is consistent with the findings of Ishizawa (2005). PCA showed that flower morphology was divided into two groups at approximately 0.5 on the PC 1 axis (Fig. 6). This is consistent with the distribution area of C. japonica and C. rusticana reported in a previous study (Ishizawa, 1988). Hakoda and Akihama (1988) used 44 morphological traits of flowers and leaves from Camellia sasanqua (three native individuals and 61 individuals from the line varieties), Camellia oleifera (three individuals from three varieties), and C. japonica (three native individuals, and three individuals from three varieties) for PCA, and demonstrated that these three strains could be distinguished by these traits. This study also suggested that discrimination between species based on flower morphology is quantitatively possible.
Petals serve as landing guides for pollinators (Faegri and Van der Pijl, 1979). In some cases, altered flower morphology results in speciation by changing pollinator preference (Grant and Grant, 1965; Zung et al., 2015). As such, altered flower morphology in Camellia japonica and C. rusticana may have resulted in a pollinator shift (e.g., from birds to insects) that drove speciation.
Adaptations to different environments may have changed pollination systems in Camellia japonica and C. rusticana. The main pollinators of C. japonica are birds; the plant flowers in winter when the availability of fruits and insects in temperate forests is limited (Yumoto, 1988; Kunitake et al., 2004; Abe and Hasegawa, 2008; Abe et al., 2011). In contrast, C. rusticana is pollinated by small insects in the early spring, which marks the beginning of the life cycles of many insects (Ishizawa, 1988). Flower morphology may also have played a role in pollinator shifts. C. japonica has a larger petal than C. rusticana. Small birds, such as Japanese white-eyes (Zosterops japonicus), are known to grip the petals to drink the nectar. Thus, we speculate increases in petal size allowed birds to begin visiting the C. japonica flowers. Furthermore, pollination syndromes are associated with particular pollinator types (Faegri and Van der Pijl, 1979). As viewed from these categories, the coalescence of the flower filament of C. japonica is thought to be a structure that can hold a larger amount of nectar, and this phenomenon may be related to the bird pollination syndrome (Hainsworth and Wolf, 1976; Bolten and Feinsinger, 1978). In fact, in a preliminary study, it has also been shown that the amount of nectar is considerably higher in C. japonica than in C. rusticana. We therefore speculate that the flower morphology of C. japonica is more suitable for bird pollination than that of C. rusticana.
4.3. Comparison of petal and flower filament colorsThe petals of Camellia japonica are more red, while the petals of C. rusticana are slightly lighter (P < 0.01) (Fig. 7), although petal colors widely overlap between the two species (89.3% of the entire area). C. rusticana contains more of the petal pigment 3-galactoside than the other species of the genus Camellia (Sakata et al., 1986, 1987). This pigment may account for the difference between C. japonica and C. rusticana petal color. Red is commonly associated with bird pollination syndrome (Faegri and Van der Pijl, 1979; Rodríguez-Gironés and Santamaría, 2004), suggesting that C. japonica flowers are adapted for bird pollination.
Pollinators are known to be attracted not only by petal colors, but also by color contrasts within the entire flower (Hempel de Ibarra and Vorobyev, 2009; Sletvold et al., 2016). Flower filaments of Camellia japonica and C. rusticana showed a large difference in the RGB values, with combined RGB values indicating that C. japonica flower filaments are white and C. rusticana flower filaments are yellow (P < 0.01;Fig. 8). The contrast between the red petals and white filaments of C. japonica flowers may make it easier for birds to find larger flowers from a distance, and also helps birds, which drink nectar from an unstable platform, identify where nectar is present (Cronk and Ojeda, 2008). Contrasting colors within flowers are also an important signal for insects (Hempel de Ibarra and Vorobyev, 2009; Sletvold et al., 2016). However, for insects such as flies, bees, and small beetles, the contrast of flower colors of Camellia, whose flower is many times larger than their body sizes, may be less important.
4.4. Speciation of Camellia based on morphological studyIn this study, Camellia japonica and C. rusticana were quantitatively distinguished by the presence or absence of a hypodermis, flower morphology, and petal and filament colors.
The differentiation of flower traits is one of the barriers to genetic exchange for plants that rely on pollinators for pollen dispersion. A previous study demonstrated that in Petunia pollinator type depends on flower color (Hoballah et al., 2007). Another study reported that in Campanula the size of the corolla depends on pollinator type (Inoue and Amano 1986). In this study, we showed that flower traits of Camellia japonica and C. rusticana are clearly distinct. These distinct flower traits suggest that differences in pollinator type let to reproductive isolation and speciation of C. japonica and C. rusticana. Differences in leaf morphology indicate that environmental conditions drove physiological adaptations that may also have caused speciation. Pre-mating mechanisms include physiological or ecological barriers to fertilization (Mayr, 1970). Taken together, the differences between these species mean pre-mating isolation, which could promote geographical and ecological reproductive isolation and consequently speciation.
Our findings in Camellia japonica and C. rusticana may represent speciation processes for other species as well. Sakata et al. (1987) reported that the distribution of anthocyanin pigments in the petals suggests that C. rusticana probably diverged from the common ancestor earlier than C. japonica. Isozyme and nrDNA ITS sequences of C. japonica and C. rusticana suggest something similar (Eguchi et al., 1991; Vijayan et al., 2009). There is a high probability that C. japonica and C. rusticana are not closely related enough to be considered sister species. However, as previously stated, the phylogeny within the genus Camellia is very complicated (Vijayan et al., 2009; Yang et al., 2013; Huang et al., 2014; Kim et al., 2017: Li et al., 2019). Thus, some of these specimens may represent hybrids. Accurate predictions of Camellia evolution require integration of molecular phylogenetic results with geographic morphological information. In addition, it is necessary to examine the evolutionary background that might have led to speciation by elucidating the phylogenetic relationships and physiological and ecological characteristics of all plants in the genus Camellia. Importantly, researchers should examine speciation based on pollination syndrome shifts.
Analysis of leaf morphology identified an intermediate type between Camellia japonica and C. rusticana in two regions (population No. 3 and population No. 18). Because both species grow adjacent to each other, introgressive hybridization may occur in which genetically differentiated taxa are secondarily contacted and crossed. However, since phenology does not overlap between the two plants, with C. japonica usually blooming in winter and C. rusticana usually blooming in early spring, we speculate that there are only limited opportunities for these two species to hybridize under natural conditions.
In the future, we need to examine the historical dynamics leading to speciation in the genus Camellia, by matching genetic classification and morphology, clarifying the influence of introgressive hybridization of the two species, and performing phylogenetic analysis of the entire Camellia genus.
5. ConclusionsIn this study, we compared leaf morphology of Camellia japonica and C. rusticana to identify diverse adaptive strategies to different environments. We also examined the degree of speciation of these species by quantitative comparative analysis of flower form, petal color, and filament color. We were able to distinguish species by the presence or absence of leaf hypodermis. PCA indicated that the flower morphologies of these species were significantly different. We also found that petal and filament colors were significantly different. These findings suggest that pre-mating isolation caused by differences in pollinator type and environmental conditions promote speciation in Camellia.
Author contributionsHarue Abe: Conception and design of the study. Drafting of the article. Critical revision of the article for important intellectual content. Final approval of the article. Hiroki Miura: Collection and assembly of data. Drafting of the article. All authors: Analysis and interpretation of data.
Declaration of competing interestThe authors declare no conflicts of interest associated with this manuscript. All the authors agreed to submit this manuscript.
AcknowledgmentThe authors thank Susumu Ishizawa, Kazuhiko Hoshizaki, Naoko Kan, Makoto Kobayashi, Taiga Kuhara, Yoshinari Moriguchi and Hiroshi Tomimatsu for cooperating during sample collection, and Saneyoshi Ueno, Kosuke Homma, and Hitoshi Sakio for providing helpful comments. We also thank the staff of the Field Center for Sustainable Agriculture and Forestry, Niigata University, for assistance in the field. We were given permission to access to its off-limits area by Ministry of Agriculture, Forestry and Fisheries, and had got the collection permission application by relevant prefectures and by Ministry of Agriculture, Forestry and Fisheries. This work was supported by Sado City Grant for Scientific Research on Biodiversity and Tadami-machi (2014–2016), and was supported in part by the JSPS KAKENHI (Grant Number JP15K07473).
Abe H., Hasegawa M., 2008. Impact of volcanic activity on a plant-pollinator module in an island ecosystem: the example of the association of Camellia japonica and Zosterops japonica. Ecol. Res, 23: 141-150. DOI:10.1007/s11284-007-0345-4 |
Abe, H., Ueno, S., Tsumura, Y., Hasegawa, M., 2011. Expanded home range of pollinator birds facilitates greater pollen flow of Camellia japonica in a forest heavily damaged by volcanic activity. In: Isagi, Y., Suyama, Y. (Eds. ), Single-Pollen Genotyping. Springer, Tokyo, pp. 47-62.
|
Abràmoff M.D., Magalhães P.J., Ram S.J., 2004. Image processing with ImageJ. Biophot. Int., 11: 36-42. |
Ando T., Nomura M., Tsukahara J., et al, 2001. Reproductive isolation in a native population of Petunia sensu Jussieu (Solanaceae). Ann. Bot, 88: 403-413. DOI:10.1006/anbo.2001.1485 |
Barrier M., Baldwin B., Robichaux R., et al, 1999. Interspecific hybrid ancestry of a plant adaptive radiation: allopolyploidy of the Hawaiian silversword alliance(Asteraceae) inferred from floral homeotic gene duplications. Mol. Biol. Evol, 16: 1105-1113. DOI:10.1093/oxfordjournals.molbev.a026200 |
Bolten A.B., Feinsinger P., 1978. Why do hummingbird flowers secrete dilute nectar?. Biotropica, 10: 307-309. DOI:10.2307/2387684 |
Caser M., Torello Marinoni D., Scariot V., 2010. Microsatellite-based genetic relationships in the genus Camellia: potential for improving cultivars. Genome, 53: 384-399. DOI:10.1139/G10-012 |
Castellanos M.C., Wilson P., Thomson J.D., 2003. Pollen transfer by hummingbirds and bumblebees, and the divergence of pollination modes in Penstemon. Evolution, 57: 2742-2752. DOI:10.1111/j.0014-3820.2003.tb01516.x |
Cronk Q., Ojeda I., 2008. Bird-pollinated flowers in an evolutionary and molecular context. J. Exp. Bot, 59: 715-727. DOI:10.1093/jxb/ern009 |
Eguchi T., Okubo H., Fujieda K., et al, 1991. Genetic divergence among intraspecific taxa of Camellia japonica L. J. Jpn. Soc. Hortic. Sci, 59: 803-814. DOI:10.2503/jjshs.59.803 |
Faegri K., Van der Pijl L., 1979. The Principles of Pollination Ecology. third ed. Oxford: Pergamon Press: p. 242.
|
Futuyma, D.J., Slatkin, M. (Eds. ), 1983. Coevolution. Sinauer Associates Inc., Massachusetts, p. 556.
|
Grant V., Grant K., 1965. Flower Pollination in the Phlox Family. New York: Family. Columbia University Press: p. 224.
|
Hagiya K., Ishizawa S., 1961. Studies on snow-camellia (Camellia rusticana). I. On variations and distribution of native and domesticated Camellias' in Niigata Prefecture. J. Jap. Soc. Hort. Sci, 30: 270-290. DOI:10.2503/jjshs.30.270 |
Hainsworth F.R., Wolf L.L., 1976. Nectar characteristics and food selection by hummingbirds. Oecologia, 25: 101-113. DOI:10.1007/BF00368847 |
Hakoda N., Akihama T., 1988. Morphological classification of cultivars in Camellia sasanqua Thunb. using principal component analysis and cluster analysis. J. Jpn.Soc. Hortic. Sci, 57: 233-242. DOI:10.2503/jjshs.57.233 |
Hempel de Ibarra N., Vorobyev M., 2009. Flower patterns are adapted for detection by bees. J. Comp. Physiol, 195: 319-323. DOI:10.1007/s00359-009-0412-0 |
Hoballah M.E., Gübitz T., Stuurman J., et al, 2007. Single gene-mediated shift in pollinator attraction in Petunia. Plant Cell, 19: 779-790. DOI:10.1105/tpc.106.048694 |
Honda M., 1950. Further notes on Camellia rusticana Honda. Acta Phytotax. Geobot, 12: 176-178. |
Horikawa, Y., 1972. Atlas of the Japanese Flora: an Introduction to Plant Sociology of East Asia. Gakken Co., Tokyo, p. 500.
|
Hotta, M., 1974. History and geography of plants. In: Evolutionary Biology in Plants Ⅲ. Sanseido Co. Ltd., Tokyo, p. 400 (In Japanese).
|
Huang H., Shi C., Liu Y., et al, 2014. Thirteen Camellia chloroplast genome sequences determined by high-throughput sequencing: genome structure and phylogenetic relationships. BMC Evol. Biol, 14: 151. DOI:10.1186/1471-2148-14-151 |
Inoue K., Amano M., 1986. Evolution of Campanula punctata Lam. in the Izu Islands: changes of pollinators and evolution of breeding systems. Plant Species Biol, 1: 89-97. DOI:10.1111/j.1442-1984.1986.tb00018.x |
Ishizawa, S., 1978. Climatic factors affecting the distribution of snow-camellia(Camellia rusticana Honda). In: Plant Ecology to the Memory of Dr Kuniji Yoshioka (ed. Society of Tohoku Plant Ecology), pp. 296-308 (In Japanese).
|
Ishizawa, S., 1988. Life history of snow Camellia. In: Newton Special Issue the World of Plant 1. Kyoikusya, pp. 28-55 (In Japanese).
|
Ishizawa S., 2003. The plants in snowy regions, Camellia rusticana 25. Niigata Plant Conservation Association, 9: 13-16. |
Ishizawa, S., 2005. Tree shape of Camellia rusticana, In: special issue Trees that bloom in spring (2). Puranta 99, 22-32 (In Japanese).
|
Ishizawa, S., 2010. Trees of Niigata, Camellia rusticana. In: A Tour of Camellia rusticana in Aga Town. Aga-machi Yukitsubaki Utilization Promotion Council, Niigata (In Japanese).
|
Ishizuka K., 1947. Distribution of evergreen forest in Tohoku rejoin and Korean Peninsula. Seitaigaku Kenkyu, 10: 98-100. |
Kim S.H., Cho C.H., Yang M., et al, 2017. The complete chloroplast genome sequence of the Japanese Camellia (Camellia japonica L.). Mitochondrial DNA Part B, 2: 583-584. DOI:10.1080/23802359.2017.1372719 |
Kira T., 1945. New Classification of Climate in Southeast Asia and the Western Pacific. Hort. Inst. Kyoto Univ., Kyoto: p. 23pp. |
Kitamura S., 1950. Tea and camellia. Acta Phytotax. Geobot, 14: 56-63. |
Kume A., Tanaka C., 1996. Adaptation of stomatal response of Camellia rusticana to a heavy snowfall environment: winter drought and net photosynthesis. Ecol.Res, 11: 207-216. DOI:10.1007/BF02347687 |
Kume A., Tanaka C., Matsumoto S., Ino Y., 1998. Physiological tolerance of Camellia rusticana leaves to heavy snowfall environments: the effects of prolonged snow cover on evergreen leaves. Ecol. Res, 13: 117-124. DOI:10.1046/j.1440-1703.1998.00251.x |
Kunitake Y.K., Hasegawa M., Miyashita T., et al, 2004. Role of a seasonally specialist bird Zosterops japonica on pollen transfer and reproductive success of Camellia japonica in a temperate area. Plant Species Biol, 19: 197-201. DOI:10.1111/j.1442-1984.2004.00115.x |
Li W., Zhang C.P., Guo X., et al, 2019. Complete chloroplast genome of Camellia japonica genome structures, comparative and phylogenetic analysis. PLoS One, 14: e0216645. DOI:10.1371/journal.pone.0216645 |
Lu H., Jiang W., Ghiassi M., et al, 2012. Classification of Camellia (Theaceae) species using leaf architecture variations and pattern recognition techniques. PLoS One, 7: e29704. DOI:10.1371/journal.pone.0029704 |
Maekawa F., 1977. Floristic Division of Japan. Tokyo: Tamagawa University Press.
|
Mayr E., 1970. Populations, Species, and Evolution. Cambridge: Harvard Univ. Press: p. 453.
|
Min, T.L., Bartholomew, B., 2007. Theaceae. In: Flora of China Editorial Committee(ed. ) Flora of China 12. Science Press/Missouri Botanical Garden Press, Beijing, pp. 366-478.
|
Motonaga Y., Kameoka T., Hashimoto A., 1997. Color development of tomato during post-ripening. AIC Color, 97: 929-932. |
Motonaga Y., Nedu K., Suzuki T., et al, 2015. Color ripening chart for 'Shine Muscat' grape for in situ evaluation. Agr. Info. Res, 24: 1-14. |
Nagamasu, H., 2006. Theaceae. In: Iwatsuki, K., Boufford, D.E., Ohba, H. (Eds. ), Flora of Japan Volume Ⅱ a. Kodansya, Tokyo, pp. 394-411.
|
Orikawa T., Iwatsubo Y., Oht M., 1998. Morphological variation of diploid Camellia japonica L. var. intermedia. Tovama Science Museum Report, 21: 2-8. |
R Development Core Team, 2014. A language and environment for statistical computing. In: R Foundation for Statistical Computing. Vienna.
|
Ramsey J., Bradshaw H.D., Schemske D.W., 2003. Components of reproductive isolation between the monkey flowers Mimulus lewisii and M. cardinalis(Phrymaceae). Evolution, 57: 1520-1534. DOI:10.1111/j.0014-3820.2003.tb00360.x |
Rodríguez-Gironés M.A., Santamaría L., 2004. Why are so many bird flowers red?. PLoS Biol, 2: e350. DOI:10.1371/journal.pbio.0020350 |
Sakai, A., 1982. Freezing Tolerance and Cold Adaptation of Plants. Gakujyutsu Publication Center. -10: 4762253103 469 pp. (In Japanese).
|
Sakata Y., Arisumi K., Miyajima I., 1986. Cyanidin 3-Galactoside, a new anthocyanin from Camellia japonica subsp. rusticana (Honda) Kitamura and its occurrence in the garden forms of Camellia of Japanese origin. J. Jpn. Soc. Hortic. Sci, 55: 82-88. DOI:10.2503/jjshs.55.82 |
Sakata Y., Arisumi K., Miyajima I., 1987. Constitution of anthocyanins in flowers of the wild forms of section Camellia of Japanese and Formosan origin. J. Jpn. Soc. Hortic. Sci, 56: 208-214. DOI:10.2503/jjshs.56.208 |
Sato T., 2005. Plant called Japan sea elements. Sea of Japan Cultural Res. Inst., Toyama, 18: 13-21. |
Shen J.B., Lu H.F., Peng Q.F., et al, 2008. FTIR spectra of Camellia sect. Paracamellia, and sect. Camellia (Theaceae) with reference to their taxonomic significance. J. Syst. Evol, 46: 194-204. |
Shimada G., Hisada Y., 1966. Anatomical studies on the leaves of Camellia japonica and C. rusticana. J. Jpn. Bot, 41: 33-36. |
Sletvold N., Trunschke J., Smit M., et al, 2016. Strong pollinator-mediated selection for increased flower brightness and contrast in a deceptive orchid. Evolution, 70: 716-724. DOI:10.1111/evo.12881 |
Sugimoto, J., 1936. Japanese tree index. In: Inoue Bookstore (In Japanese).
|
Tateishi N., Oishi M., Ozaki Y., et al, 2007. Chloroplast DNA variation in the genus Camellia with reference to the origin of 'Tamanoura'. J. Hortic. Sci. Biotechnol, 82: 377-382. DOI:10.1080/14620316.2007.11512246 |
Tsuyama T., 1956. On some morphological features of Camellia japonica and C. rusticana. J. Jpn. Bot, 31: 225-228. |
Tsuyama, T., 1988. Distribution and evolutionary process of Japanese Camellia. In: Newton special issue Syokubutu no sekai. Kyoikusha, Tokyo, pp. 56-57 (In Japanese).
|
Ueno, S., 2009. Genetic structure in hybrid zones between Camellia japonica and C. rusticana. In: JSPS KAKENHI Grant Number NO. 18770024 (2006-2008) (In Japanese).
|
Vijayan K., Zhang W.J., Tsou C.H., 2009. Molecular taxonomy of Camellia (Theaceae) inferred from nrITS sequences. Am. J. Bot, 96: 1348-1360. DOI:10.3732/ajb.0800205 |
Wilson P., Wolfe A.D., Armbruster W.S., et al, 2007. Constrained lability in floral evolution: counting convergent origins of hummingbird pollination in Penstemon and Keckiella. New Phytol, 176: 883-890. DOI:10.1111/j.1469-8137.2007.02219.x |
Yang J.B., Yang S.X., Li H.T., et al, 2013. Comparative chloroplast genomes of Camellia species. PLoS One, 8: e73053. DOI:10.1371/journal.pone.0073053 |
Yasumoto A.A., Yahara T., 2006. Post-pollination reproductive isolation between diurnally and nocturnally flowering daylilies, Hemerocallis fulva and Hemerocallis citrina. J. Plant Res, 119: 617-623. DOI:10.1007/s10265-006-0028-1 |
Yumoto T., 1988. Pollination systems in the cool temperate mixed coniferous and broad-leaved forest zone of Yakushima Island. Ecol. Res, 3: 117-129. DOI:10.1007/BF02346934 |
Zung J.L., Forrest J.R.K., Castellanos M.C., et al, 2015. Bee- to bird-pollination shifts in Penstemon: effects of floral-lip removal and corolla constriction on the preferences of free-foraging bumble bees. Evol. Ecol, 29: 341-354. DOI:10.1007/s10682-014-9716-9 |



