b. College of Forestry, Central South University of Forestry and Technology, Changsha, 410004, Hunan, China
Paraphlomis (Prain) Prain is a genus of the mint family (Lamiaceae) native to southern China, the Himalayas, and Southeast Asia (Wu and Li, 1977; Li and Hedge, 1994; Harley et al., 2004). Originally treated as a section of Phlomis L. by Prain (Phlomis sect. Paraphlomis Prain; see Prain, 1901), Paraphlomis, with only three recorded species, was later raised to the rank of genus (Prain, 1908). With the discovery and publication of more Chinese species (e.g., Handel-Mazzetti, 1936; Sun, 1955; Wu, 1959; Li, 1965), a total of 28 species and nine varieties are currently recognized within Paraphlomis, most of which are distributed in tropical and subtropical evergreen and mixed forests (Wu and Li, 1977; Li and Hedge, 1994). A new species of Paraphlomis discovered during our field investigations has raised questions about the phylogenetic relationships within the genus.
Previous molecular phylogenetic analysis assigned Paraphlomis to a newly established tribe, Paraphlomideae, within subfamily Lamioideae, along with two East Asian (southern China and Japan) genera, the oligotypic Matsumurella Makino (5 spp.) and the monotypic Ajugoides Makino (Bendiksby et al., 2011). Paraphlomis can be distinguished from these two genera and other taxa of Lamioideae by its herbaceous habit with simple hairs, actinomorphic calyx with five lobes less than half as long as the tube, corolla 2-lipped (1/3) with hairy upper lip but hardly bearded along the margin, included stamens, and an apically truncate ovary (Wu and Li, 1977; Li and Hedge, 1994; Harley et al., 2004; Bendiksby et al., 2011). Since only three species of Paraphlomis were sampled in the analysis of Bendiksby et al. (2011), phylogenetic relationships within the genus are still enigmatic.
Except for two species [Paraphlomis brevidens Merr. and P. oblongifolia (Blume) Prain] endemic to Indonesia, all species and varieties of Paraphlomis are recorded in China. The Chinese Paraphlomis was divided into two sections and five series based on the shape of calyx (tubular or obconical) and calyx teeth (e.g., conspicuous or inconspicuous, broadly triangular or subulate), namely, P. sect. Paraphlomis (including P. ser. Javanicae C.Y. Wu & H.W. Li and P. ser. Membranaceae C.Y. Wu & H.W. Li) and P. sect. Obconicocalyx C.Y. Wu & H.W. Li (including P. ser. Lancidentatae C.Y. Wu & H.W. Li, P. ser. Graciles C.Y. Wu & H.W. Li, and P. ser. Subcoriaceae C.Y. Wu & H.W. Li) (Li, 1965; Wu and Li, 1977). However, this classification has not been verified by molecular phylogenetic analysis.
Our study aims to resolve the phylogenetic relationships within Paraphlomis and assess the infrageneric classification of the genus by using molecular and morphological evidence. For this purpose, we reconstructed the phylogeny of Paraphlomis based on sequences of two nuclear ribosomal spacer regions and four plastid DNA markers of 16 species and five varieties, representing both sections and all five series. We also assigned a phylogenetic placement for a new species of Paraphlomis from China.
2. Material and methods 2.1. Taxon sampling and choice of markersThe ingroup sampling consisted of 29 accessions representing 16 species and five varieties of Paraphlomis from China, while the outgroup comprised two accessions of Matsumurella of tribe Paraphlomideae and two species representing the two genera (Phlomis L. and Phlomoides Moench) of tribe Phlomideae, which is one of the most closely related taxa of Paraphlomideae (Bendiksby et al., 2011; Appendix A). Nuclear ribosomal internal and external transcribed spacers (ITS and ETS) and four plastid DNA markers (rpl16, rpl32-trnL, rps16, and trnL-trnF) were selected for the phylogenetic reconstruction of Paraphlomis, based on previous phylogenetic studies in Lamioideae (e.g., Pan et al., 2009; Bendiksby et al., 2011; Salmaki et al., 2012).
2.2. DNA extraction and amplificationTotal genomic DNA was extracted either from silica-gel-dried leaf material using the modified CTAB method (Doyle and Doyle, 1987) or from herbarium specimens using the DNeasy Plant Pro Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Polymerase chain reaction (PCR) procedures followed those described inChen et al. (2016). The primer pairs 17SE and 26SE (Sun et al., 1994) were used for the amplification of ITS, and ETS-B (Beardsley and Olmstead, 2002) and 18S-IGS (Baldwin and Markos, 1998) for ETS. Plastid markers were amplified using the primers R1516 and F71 (Jordan et al., 1996) for rpl16, rpsR2 and rpsF for rps16 (Oxelman et al., 1997), trnL(UAG) and rpL32-F (Shaw et al., 2007) for rpl32-trnL, and "c" and "f" for trnL-trnF (Taberlet et al., 1991). We extracted and sequenced DNA from all taxa for this study. Voucher information and GenBank accession numbers for all sequences are listed in Appendix A.
2.3. Sequence alignment and phylogenetic analysesRaw sequences were assembled and edited using Sequencher 4.1.4 (Gene Codes, Ann Arbor, Michigan, USA), then aligned using MUSCLE (Edgar, 2004) and manually adjusted in MEGA 6.0 (Tamura et al., 2013). Partitioned Bayesian Inference (BI) and partitioned Maximum Likelihood (ML) analyses were employed to generate the phylogeny of Paraphlomis, using MrBayes (Ronquist et al., 2012) and RAxML-HPC2 (Stamatakis, 2014), respectively, on XSEDE on the Cyberinfrastructure for Phylogenetic Research Science (CIPRES) Gateway (http://www.phylo.org/; Miller et al., 2010). The combined nuclear data set and the combined plastid data set were initially analysed separately. Gaps were not coded for these markers. Other detailed parameters for BI and ML analyses followed those described in Chen et al. (2019). We used TreeGraph 2 (Stover and Müller, 2010) to annotate the phylogenetic trees with posterior probabilities (PP) and Bootstrap support (BS) values.
2.4. Identification of incongruenceIncongruence between the phylogenies inferred from the combined nuclear data set and combined plastid data set was visually inspected for contrasting topologies. The thresholds for hard incongruence are defined here as: PP ≥ 0.90 and/or BS ≥ 75%. After excluding the taxa that exhibited strong incongruence, we combined the two data sets for phylogenetic analyses.
2.5. Taxonomic studiesSpecimen examination and morphological observation was carried out based on herbarium collections of Paraphlomis from 22 herbaria (BM, CDBI, CSFI, E, GNNU, GXMI, HAST, HIB, IBK, IBSC, JIU, JJF, K, KUN, KYO, MW, NAS, PE, SM, SZ, TI, and WUK; abbreviations follow Thiers, 2020). Taxonomic literature, especially protologues of all published names of Paraphlomis were reviewed. For the morphological description of Paraphlomis species, we followed the terminology used by Li and Hedge (1994).
3. ResultsA total of 190 new sequences were successfully amplified for the phylogenetic analysis of Paraphlomis. Eight sequencing reactions (three ITS, one trnL-trnF, and all four plastid sequences of P. brevifolia C.Y. Wu & H.W. Li) failed and these were treated as missing data. The final combined nuclear data set consists of 1255 positions (812 bp for ITS, 443 bp for ETS) and the combined plastid data set is 3317 bp (838 bp for rpl16, 855 bp for rpl32-trnL, 812 bp for rps16, 812 bp for trnL-trnF).
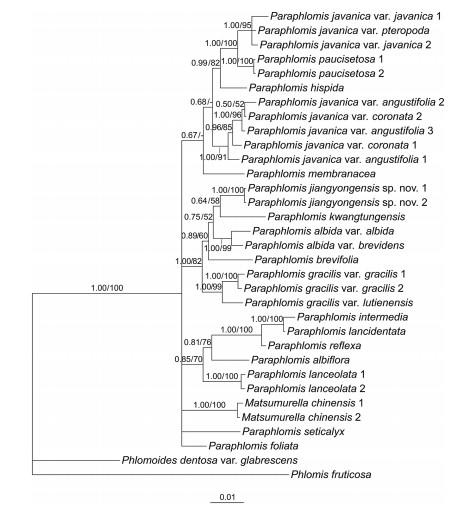
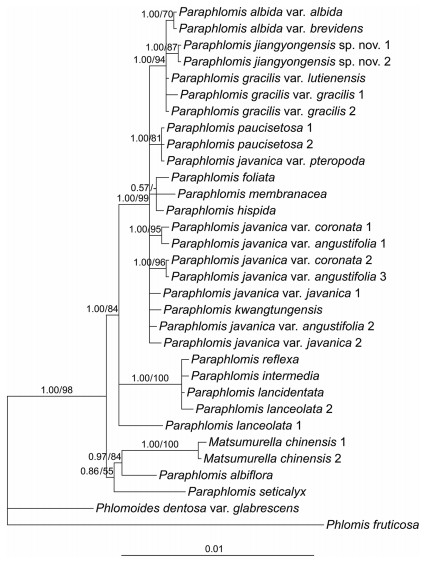
BI and ML analyses yield similar topologies. Only the Bayesian 50% majority-rule consensus trees of the combined nuclear data set (Fig. 1) and the combined plastid data set (Fig. 2) are presented here. Support values of ML trees are indicated.
|
| Fig. 1 Bayesian 50% majority-rule consensus tree of Paraphlomis based on combined nuclear (ITS and ETS) data set. Support values ≥ 0.50 PP or 50% BS are displayed above the branches ("-" indicates a support value < 50% BS). Scale bar denotes the expected number of substitutions per site in Bayesian analysis. Multiple accessions of the same species are numbered according to Appendix A. |
|
| Fig. 2 Bayesian 50% majority-rule consensus tree of Paraphlomis based on combined plastid (rpl16, rpl32-trnL, rps16, and trnL-trnF) data set. Support values ≥ 0.50 PP or 50% BS are displayed above the branches ("-" indicates a support value < 50% BS). Scale bar denotes the expected number of substitutions per site in Bayesian analysis. Multiple accessions of the same species are numbered according to Appendix A. |
Both the nuclear and plastid phylogenies place two accessions of Matsumurella chinensis (Benth.) Bendiksby deep within Paraphlomis, indicating that Paraphlomis is paraphyletic (Fig. 1, Fig. 2). Several moderately- to well-supported clades can be recognized in the nuclear tree (Fig. 1), although relationships among these clades are not resolved. In contrast, the combined plastid data set failed to resolve the interspecific relationships within each clade, but resulted in a relatively well supported backbone of Paraphlomis (Fig. 2). Hard incongruences can be recognized for the phylogenetic placements of two taxa, P. albiflora (Hemsl.) Hand.-Mazz. and P. javanica var. pteropoda D. Fang & K.J. Yan (Fig. 1, Fig. 2). The nuclear and plastid data sets were then combined for analyses after the exclusion of these two taxa.
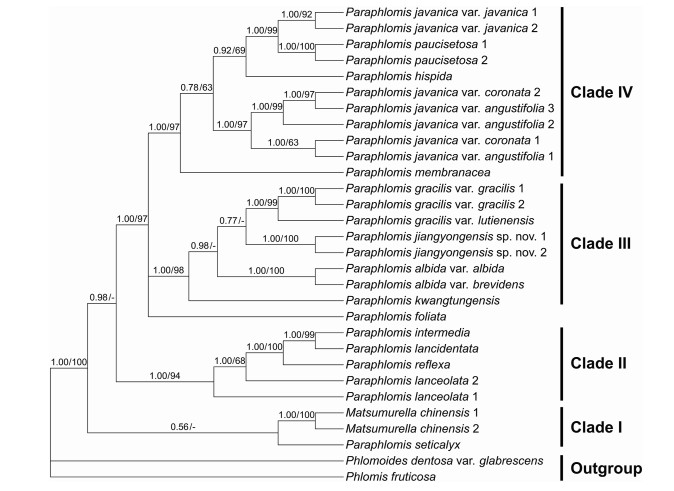
The combined nuclear and plastid data set comprises 4573 positions and the resulting phylogeny is well resolved (Fig. 3). Paraphlomis is not monophyletic as Matsumurella chinensis is recovered within the genus. Four clades can be recognized within the Paraphlomis-Matsumurella clade (Fig. 3; PP = 1.00/BS = 100%). Paraphlomis seticalyx C.Y. Wu is weakly supported (Fig. 3; PP = 0.56/BS < 50) as sister to the clade including the two accessions of M. chinensis, the two species forming the first diverging clade (Clade Ⅰ). Clade Ⅱ consists of four species, P. intermedia C.Y. Wu & H.W. Li, P. lancidentata Y.Z. Sun, P. reflexa C.Y. Wu & H.W. Li, and P. lanceolata Hand.-Mazz. (Fig. 3; PP = 1.00/BS = 94%). The excluded P. albiflora is recovered within this clade in the nuclear phylogeny (Fig. 1; PP = 0.85/BS = 70%), while in the plastid tree, P. albiflora groups with M. chinensis and P. seticalyx (Fig. 2; PP = 0.86/BS = 55%). Paraphlomis foliata (Dunn) C.Y. Wu & H.W. Li is recovered in a polytomy with Clade Ⅲ and Clade Ⅳ (Fig. 3; PP = 1.00/BS = 97%). Clade Ⅲ includes P. gracilis (Hemsl.) Kudô, P. albida Hand.-Mazz., P. kwangtungensis C.Y. Wu & H.W. Li, as well as the new species (here named as P. jiangyongensis X.L. Yu & A. Liu) (Fig. 3; PP = 1.00/BS = 98%). This clade is also recovered in the nuclear phylogeny (Fig. 1; PP = 1.00/BS = 81%), whereas in the plastid tree (Fig. 2), P. kwangtungensis does not group with the remaining species. Due to the failure of PCR reactions of the four plastid sequences, P. brevifolia was only included in the nuclear tree and it is also embedded within Clade Ⅲ (Fig. 1). Clade Ⅳ (Fig. 3; PP = 1.00/BS = 97%) consists of four species, P. javanica (Blume) Prain, P. paucisetosa C.Y. Wu, P. hispida C.Y. Wu, and P. membranacea C.Y. Wu & H.W. Li. Different accessions of the two varieties of P. javanica (P. javanica var. coronata (Vaniot) C.Y. Wu & H.W. Li and P. javanica var. angustifolia C.Y. Wu & H.W. Li ex C.L. Xiang, E.D. Liu & H. Peng) group together and form a strongly supported and distinct clade (Fig. 3; PP = 1.00/BS = 97%) within Clade Ⅳ; however, P. javanica var. javanica is shown to be sister to P. paucisetosa (Fig. 3; PP = 1.00/BS = 99%). The other variety, P. javanica var. pteropoda groups with the two accessions of P. javanica var. javanica in the nuclear tree (Fig. 1; PP = 1.00/BS = 95%), but forms a clade with the two accessions of P. paucisetosa in the plastid tree (Fig. 2; PP = 1.00/BS = 81%).
|
| Fig. 3 Bayesian 50% majority-rule consensus tree of Paraphlomis based on combined nuclear and plastid (ITS, ETS, rpl16, rpl32-trnL, rps16, and trnL-trnF) data set. Support values ≥ 0.50 PP or 50% BS are displayed above the branches ("-" indicates a support value < 50% BS). Multiple accessions of the same species are numbered according to Appendix A. |
Using three plastid DNA regions (matK, rps16, trnL-trnF), Bendiksby et al. (2011) carried out a comprehensive phylogenetic study of Lamioideae. In their updated classification of the subfamily, Ajugoides, Matsumurella, and Paraphlomis were assigned to a newly established tribe Paraphlomideae; however, the relationships among the three genera were not resolved. Matsumurella consists of five species distributed from Japan to southern China, while the monotypic Ajugoides is endemic to Japan. The morphological differences among the three genera are minute. For instance, Paraphlomis can only be distinguished from Matsumurella by its calyx lobes less than half as long as the tube (vs. over half as long as the tube) (Bendiksby et al., 2011). Bendiksby et al. (2011) also suggested that Matsumurella is morphologically less distinct from Ajugoides and the two genera may represent the same group.
In the present study, only one species of Matsumurella (M. chinensis) was sampled, and consistently embedded within Paraphlomis (Fig. 1, Fig. 2, Fig. 3), suggesting that the two genera may also be closely related. Moreover, the length of calyx teeth is quite variable within Paraphlomis. Some species (e.g., P. gracilis and P. reflexa) also have calyx teeth over half as long as the tube. Since the type species of Matsumurella [M. tuberifera (Makino) Makino] and the monotypic genus Ajugoides were not included in our study, we hesitate to make any taxonomic changes regarding the generic delimitations at this time.
4.2. Infrageneric classification and phylogenetic relationships within ParaphlomisLi (1965) and Wu and Li (1977) subdivided the Chinese Paraphlomis into two sections and five series based mainly on the morphology of calyx teeth. In our phylogenetic analyses, both sections and all five series are represented (Appendix A), which enables a preliminary evaluation of the infrageneric classification of Paraphlomis. Most species of P. sect. Paraphlomis are grouped in Clade Ⅳ, the exceptions being P. albiflora, P. foliata, and P. seticalyx, which did not group with the rest of the section. Paraphlomis sect. Paraphlomis was separated from P. sect. Obconicocalyx by the tubular or tubular-campanulate (vs. obconical) calyces (Li, 1965; Wu and Li, 1977). However, our observations reveal that species of Clade Ⅳ share glabrous nutlets that are obviously inflated and exserted from the calyces. As a distinct feature within the genus, the significantly inflated nutlets might represent a synapomorphy of Clade Ⅳ. The two Paraphlomis species endemic to Indonesia (P. brevidens and P. oblongifolia) are morphologically very similar to P. javanica and might also be members of Clade Ⅳ.
Paraphlomis ser. Lancidentatae and P. ser. Graciles are also shown to be non-monophyletic, since taxa from both series are recovered in two separate clades (Clade Ⅱ and Clade Ⅲ). The two series were defined by the morphology of calyx teeth, which are lanceolate-triangular in P. ser. Lancidentatae, but broadly triangular in P. ser. Graciles (Li, 1965; Wu and Li, 1977). Our study, however, reveals that Clade Ⅱ and Clade Ⅲ can be distinguished by the morphology of nutlets. Species of Clade Ⅱ have glabrous nutlets included in the fruiting calyces, whereas hairy nutlets are presented in all members of Clade Ⅲ.
Paraphlomis ser. Subcoriaceae was defined by the presence of calyx teeth in which the apices elongate into winged extensions from veins, and it comprises P. brevifolia and P. subcoriacea C.Y. Wu (Li, 1965; Wu and Li, 1977). However, the two species also have hairy nutlets. Paraphlomis brevifolia is only included in our nuclear data set and recovered in Clade Ⅲ (Fig. 1). Thus, we can infer that the distinct hairy nutlets within the genus might be a synapomorphy of Clade Ⅲ.
Paraphlomis seticalyx was originally treated as a member of P. sect. Paraphlomis, but forms a clade (Clade Ⅰ) with M. chinensis. The relationship between the two species is weakly supported. Paraphlomis albiflora also groups with the two species in the plastid phylogeny (Fig. 2). As a heterogeneous clade within Paraphlomis, we fail to find any shared feature for these three species and their relationships still need to be further clarified.
Paraphlomis foliata forms a separate branch in the combined nuclear and plastid phylogeny. It is also morphologically distinct in the genus. Most species of Paraphlomis are characterized with axillary verticillasters, whereas leaves of P. foliata are usually rosette-like with stem leaves strongly reduced to bracts, resulting in the verticillasters forming a single and spike-like inflorescence.
Based on above analyses and discussion, the two sections and most of the series put forward by Li (1965) and Wu and Li (1977) are non-monophyletic. Moreover, nutlet morphology might be of more phylogenetic significance for the infrageneric classification of Paraphlomis than calyx morphology. A more comprehensive sampling and larger data sets are needed to make further advances in our understanding of the infrageneric relationships within Paraphlomis.
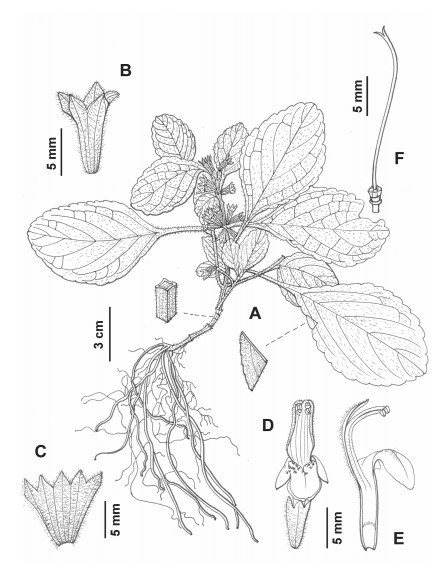
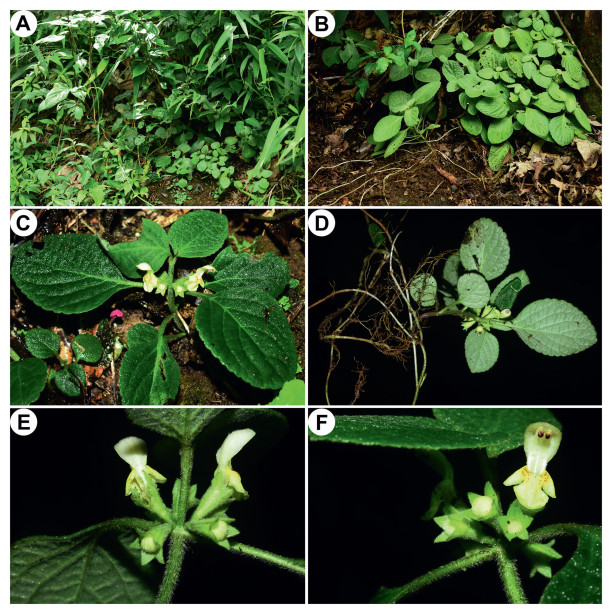
4.3. Phylogenetic placement and morphological comparison of Paraphlomis jiangyongensis sp. nov.Our molecular phylogenetic analyses reveal that the two individuals of the new species collected from Hunan Province, southern China, form a distinct clade within Clade Ⅲ and separate from existing species. It is either sister to P. kwangtungensis in the nuclear tree (Fig. 1; PP = 0.64/BS = 58%), or sister to P. gracilis in the combined nuclear and plastid tree (Fig. 3; PP = 0.77/BS < 50%), both with weak support values. Paraphlomis jiangyongensis have nutlets that are hairy at the apex, like the other species of Clade Ⅲ, but can be distinguished by several morphological traits. Specifically, P. jiangyongensis has ascending stems and is less than 30 cm tall (vs. erect and over 30 cm tall in other species), the laminae are ovate to subcircular (vs. lanceolate in P. gracilis) and densely strigose (vs. sparsely strigose in P. kwangtungensis), the cymes are sessile (vs. pedicels 1–2 mm long in P. albida and P. gracilis, ca. 1.5 mm long in P. kwangtungensis), the calyx teeth are triangular (vs. lanceolate in P. brevifolia, subulate in P. gracilis, broadly triangular in P. kwangtungensis), and the corollas are yellow (vs. white in P. albida and P. brevifolia) (Fig. 4, Fig. 5).
|
| Fig. 4 Line drawing of Paraphlomis jiangyongensis. (A) habit; (B) calyx; (C) dissected calyx; (D) flower; (E) dissected corolla; (F) pistil. Drawn by L. Wang. |
|
| Fig. 5 Paraphlomis jiangyongensis from the type locality. (A) habitat; (B–D) habits; (E, F) verticillasters. Photographed by A. Liu. |
As a distinct species within Paraphlomis, the new species is described and illustrated below (see Taxonomic treatment).
4.4. Specific status of Paraphlomis javanica var. coronataParaphlomis javanica is the most widely distributed and variable species in Paraphlomis. A total of five varieties were previously established within P. javanica, including P. javanica var. javanica, P. javanica var. coronata, P. javanica var. angustifolia, P. javanica var. henryi (Yamam.) C.Y. Wu & H.W. Li, and P. javanica var. pteropoda. The latter two varieties were later reduced to synonyms of P. javanica var. javanica by Huang et al. (1998) and Xiang et al. (2016), respectively. Here we sampled four of the five varieties (P. javanica var. henryi was not sampled), and our results show that P. javanica is not a monophyletic group (Fig. 3). Paraphomis javanica var. javanica is shown to be sister to P. paucisetosa, with P. hispida further sister to the P. javanica var. javanica-P. paucisetosa clade. The three accessions of P. javanica var. angustifolia and two accessions of P. javanica var. coronata group together as sister to the clade of P. javanica var. javanica-P. paucisetosa-P. hispida. Strong cytonuclear discrepancy can be recognized for the placement of P. javanica var. pteropoda, which groups with P. javanica var. javanica in the nuclear tree (Fig. 1), but with P. paucisetosa in the plastid tree (Fig. 2), indicating that P. javanica var. pteropoda might be a hybrid of P. javanica var. javanica and P. paucisetosa. The variety was established based on the morphology of the base of its lamina, which is decurrent on the petiole and wing-like (Fig. 6) (Yan and Fang, 2009); however, further evidence is needed to confirm its status.

|
| Fig. 6 Type specimens of Paraphlomis javanica and its varieties. (A) holotype of Leonurus javanicus (≡ P. javanica var. javanica) (L0003739); (B) holotype of Lamium coronatum (≡ P. javanica var. coronata) (E00284215); (C) lectotype of P. rugosa var. angustifolia (≡ P. javanica var. angustifolia) (PE00834892); (D) holotype of P. javanica var. pteropoda (GXMI003993). |
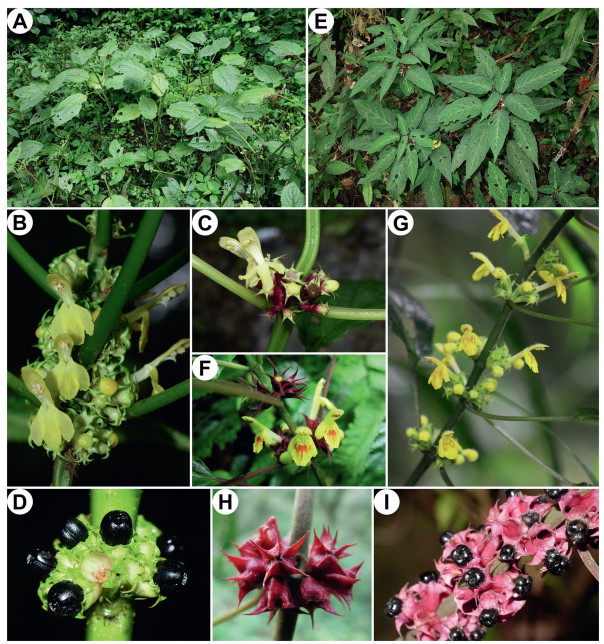
Paraphlomis javanica var. coronata differs from P. javanica var. javanica by having a smaller and succulent lamina, and a shallow and remotely serrate to crenate margin; P. javanica var. angustifolia differs from P. javanica var. javanica by having ovate-lanceolate to narrowly lanceolate lamina, an inconspicuously crenulate margin, and calyx teeth with an acicular and hispidulous apex (Fig. 6) (Wu and Li, 1977; Li and Hedge, 1994). Based on specimen examination and field observation, we find it difficult to differentiate the two varieties (P. javanica var. coronata and P. javanica var. angustifolia) from each other. The lamina morphology shows a continuous variation among different populations and even within the same population. The distribution of the two varieties also overlaps. Nevertheless, compared with P. javanica var. javanica, both P. javanica var. coronata and P. javanica var. angustifolia are slenderer plants with smaller leaves, wine-red rarely green (vs. green rarely red) calyces with apex of teeth subulate to acicular (vs. triangular to triangular-subulate), corolla bright yellow and villous outside (vs. light yellow or white, minutely puberulent outside), and lower corolla lip marked with orange-red spots (vs. without spots) (Fig. 7). Geographically, P. javanica var. javanica usually inhabits tropical forests, spreading from the eastern Himalayas and southern China to Southeast Asia, whereas P. javanica var. coronata and P. javanica var. angustifolia are mainly found in subtropical forests in southern China and northern Vietnam (Appendix B; Fig. 8).
|
| Fig. 7 Morphology of Paraphlomis javanica and P. coronata. Paraphlomis javanica: (A) habit; (B–C) inflorescences; (D) infructescence. Paraphlomis coronata: (E) habit; (F–G) inflorescences; (H) post-flowering calyces; (I) infructescence. A, D, photographed by X.-X. Zhu; B, E, photographed by Y.-P. Chen; C, photographed by L.-B. Jia; F, H, photographed by C.-L. Xiang; G, photographed by R.-B. Zhu; I, photographed by Y.-C. Xu. |

|
| Fig. 8 Distribution of Paraphlomis jiangyongensis, P. javanica, and P. coronata. |
Based on molecular phylogenetic, morphological, and geographic evidence, we conclude that P. javanica var. coronata and P. javanica var. angustifolia represent the same species, which is distinct from P. javanica var. javanica. Considering that the basionym of P. javanica var. coronata was published much earlier than that of P. javanica var. angustifolia and thus has the priority, P. javanica var. coronata is here combined as P. coronata, with P. javanica var. angustifolia treated as its synonym (see Taxonomic treatment).
5. Taxonomic treatment 5.1. Paraphlomis jiangyongensis X.L. Yu & A. Liu, sp. nov. (Figs. 4 and 5). 江永假糙苏【jiāng yǒng jiǎ cāo sū】Type: CHINA, Hunan, Jiangyong County, Yuankou Natural Reserve, Chaleiping, in forest or at streamside, 24°57′5.43″N, 111°1′20.16″E, alt. 600 m, 8 Jun. 2020, A. Liu & C. Ding JY0001 (holotype: CSFI!; isotypes: CSFI!, K!, KUN!, PE!).
Diagnosis. Paraphlomis jiangyongensis is morphologically most similar to P. albida and P. brevifolia, but can be distinguished from the former by having ascending stems less than 30 cm tall (vs. erect and over 30 cm tall), apex of laminae obtuse to subrounded (vs. acute to acuminate), cymes sessile (vs. pedicels 1–2 mm long), and corollas yellow (vs. white), and from the latter by having ascending stems less than 30 cm tall (vs. erect and over 30 cm tall), laminae ovate to subcircular (vs. oblong), calyx teeth triangular (vs. lanceolate), and corollas yellow (vs. white).
Description. Perennial herbs 5–30 cm tall, stoloniferous. Stems simple, ascending, 4-angled, densely retrorse strigose. Leaves opposite; lamina ovate to subcircular, papery, 3–8 × 2–5 cm, apex obtuse to subrounded, margin shallowly crenate, base cuneate, broadly cuneate to subrounded, decurrent, adaxially green, densely appressed strigose, abaxially light green, densely strigose, lateral veins 3–5-paired; petioles 1–5 cm long, densely retrorse strigose. Verticillasters 2–8-flowered, flowers sessile; bracteoles minute, early deciduous, subulate, ca. 1 mm long, densely strigose. Calyx green, tubular-campanulate, 7.5–10 mm long, densely strigose on both sides; teeth 5, subequal, triangular, straight, ca. 2 mm long, apex acute. Corolla yellow, 1.5–2 cm long; tube 7.5–10 mm long, straight, slightly dilated toward throat, villous annulate inside at 1/3 toward base; 2-lipped, upper lip oblong, entire, erect, concave, 7.5–10 mm long, ca. 3 mm wide, sparsely villous outside, lower lip spreading, dotted with red spots, 3-lobed, medium lob largest, subcircular, apex slightly emarginate, 3–4 mm wide, lateral lobes ovate, ca. 2 mm wide. Stamens 4, straight, included, filaments flat, villous at base, anther cells 2, parallel. Style included, glabrous, apex subequally 2-lobed, lobes subulate. Ovary apex truncate, strigose.
Phenology. Flowering from June to August.
Distribution and habitat. Paraphlomis jiangyongensis is currently known from Jiangyong County of Hunan Province in southern China (Fig. 8). It occurs in shady and moist places in evergreen broad-leaved forests at altitudes of 600–650 m.
Etymology. The specific epithet refers to Jiangyong County of Hunan Province, China, from where the type was collected.
Additional specimens examined. CHINA. Hunan: Jiangyong County, Yuankou Town, Xuetangpo, alt. 610 m, 4 Aug. 2020, A. Liu et al. LK1104 (CSFI, KUN).
5.2. Paraphlomis coronata (Vaniot) Y.P. Chen & C.L. Xiang, comb. & stat. nov. (Fig. 7E–I). 小叶假糙苏【xiǎo yè jiǎ cāo sū】≡ Lamium coronatum Vaniot, Bull. Acad. Int. Géogr. Bot. 14: 174 (1904). ≡ Paraphlomis rugosa var. coronata (Vaniot) C.Y. Wu, Acta Phytotax. Sin. 8(1): 38 (1959). ≡ Paraphlomis javanica var. coronata (Vaniot) C.Y. Wu & H.W. Li, Acta Phytotax. Sin. 13(1): 72 (1975). Type: CHINA, Guizhou, Ganpin, 2 Aug. 1897, L. Martin & E. Bodinier 1777 (holotype: E00284215!).
= Loxocalyx vaniotiana H. Lév., Repert. Spec. Nov. Regni Veg. 9(208–210): 224 (1911). Type: CHINA, Guizhou, Ganpin, 2 Aug. 1897, L. Martin & E. Bodinier 1777 (syntype: E00284215!); Guizhou, 13 Nov. 1907, J. Cavalerie 3195 (syntypes: E00284216!, E00284217!).
= Paraphlomis rugosa var. angustifolia C.Y. Wu, Acta Phytotax. Sin. 8(1): 38 (1959). ≡ Paraphlomis javanica var. angustifolia C.Y. Wu & H.W. Li ex C.L. Xiang, E.D. Liu & H. Peng, Nordic J. Bot. 28: 668 (2010). Type: CHINA, Sichuan, Emei Shan, Hongchunping, 30 Aug. 1939, Z.W. Yao 4920 [lectotype: PE00834892!, designated by Xiang et al. (2010)].syn. nov.
– Paraphlomis javanica var. angustifolia (C.Y. Wu) C.Y. Wu & H.W. Li, Acta Phytotax. Sin. 13(1): 73 (1975), nom. inval.
Description. Perennial herbs 30–100 cm tall. Stems erect, obtusely 4-angled, densely hispid to minutely retrorse strigose, leafless toward base. Leaves opposite; lamina narrowly lanceolate, ovate-lanceolate, elliptic or ovate-oblong, 3–15 × 1.5–6 cm, membranous or papery, apex acute to acuminate, base rounded to cuneate, margin crenate-serrate to inconspicuously serrate or crenulate, adaxially green, sometimes white on midribs and lateral veins, subglabrous to sparsely hispid, abaxially light green, subglabrous to minutely strigose; petioles to 8 cm long, minutely retrorse strigose, green or purplish green. Verticillasters many-flowered, globose, flowers sessile, rarely pedicels ca. 1 mm long; bracteoles few, subulate, 2–4 mm long. Calyx wine red or purplish green, rarely green, tubular-campanulate, glabrescent to densely minutely strigose outside, inconspicuously veined, tube 6–8 mm long; teeth 5, subequal, 2–5 mm long, triangular, base plicate, apex acicular or subulate, hispidulous, strongly reflex, rarely straight; fruiting calyx enlarged. Corolla bright yellow, 1.6–2.2 cm long; tube 1–1.6 mm long, straight, villous on upper part of tube outside, villous annulate inside at 1/3 toward base; 2-lipped, upper lip oblong, entire, erect, concave, 5–7 mm long, densely villous outside, lower lip spreading, dotted with orange-red spots, 5–7 mm long, 3-lobed, medium lob largest, trapeziform, apex slightly emarginate, lateral lobes oblong. Stamens 4, straight, included, filaments flat, villous at base, anther cells 2, parallel. Style included, glabrous, apex subequally 2-lobed, lobes subulate. Nutlets 4, black, triquetrous-obovoid, ca. 4 mm long, apex rounded, glabrous, strongly enlarged and exserted.
Phenology. Flowering from May to October, fruiting from September to February the next year.
Distribution and habitat. Paraphlomis coronata is widely distributed in southern China and northern Vietnam (Fig. 8). It is most accustomed to shady and moist places in evergreen or mixed forests at altitudes of 200–2100 m. The species can also be found at roadsides, among shrubs, or in limestone areas.
Author contributionsYPC, XLY, CLX, and AL conceived this research. YPC, AL, and CLX collected the materials. YPC carried out the molecular experiment and analyzed the data. YPC and AL wrote the manuscript. All authors contributed to the revision of the manuscript.
Declaration of competing interestAll the authors declare that there is no conflict of interest.
AcknowledgementsWe would like to thank the staff of following herbaria for their warmly help in research facilities: BM, CDBI, E, GXMI, HIB, IBK, IBSC, K, KUN, KYO, MW, NAS, PE, SZ, TI. We are indebted to the Herbarium of Shanghai Chenshan Botanical Garden (CSH) and Dr. Xin Zhong for providing leaf material of Paraphlomis, to Mr. Xiong Li, Dr. Maxim Nuraliev, Dr. Zhu-Zhu Yang, Dr. Wei-Bin Xu, Dr. Lin-Bo Jia, Dr. Xin-Xin Zhu, Dr. Yi Yang, and Mr. Cun-Zhong Huang for their kind help with material collection, to the Big Data Platform for Ex Situ Plant Conservation, Dr. Ren-Bin Zhu, Mr. Ye-Chun Xu, Dr. Xin-Xin Zhu, and Dr. Lin-Bo Jia for providing the photographs of Paraphlomis. Thanks also to Ms. Ling Wang from the Kunming Institute of Botany for her line drawing of the new species. Our manuscript also benefitted greatly from the insightful comments of two anonymous reviewers. This work was supported by the National Natural Science Foundation of China (Grant No. 31872648), the Yunnan Fundamental Research Projects (Grant No. 2019FI009), and the "Ten Thousand Talents Program of Yunnan" (Grant No. YNWR-QNBJ-2018-279) to CLX, the CAS "Light of West China" program to CLX and YPC, and the "Special Investigation on Wild Orchidaceae Plant Resources in Hunan Province" program (Grant No. 2020070711) to XLY.
Appendix A. Supplementary dataSupplementary data to this article can be found online at http://sweetgum.nybg.org/science/ih/.
Baldwin B.G., Markos S., 1998. Phylogenetic utility of the external transcribed spacer (ETS) of 18S-26S rDNA: congruence of ETS and ITS trees of Calycadenia(Compositae). Mol. Phylogenet. Evol, 10: 449-463. DOI:10.1006/mpev.1998.0545 |
Beardsley P.M., Olmstead R.G., 2002. Redefining Phrymaceae: the placement of Mimulus, tribe Mimuleae, and Phryma. Am. J. Bot, 89: 1093-1102. DOI:10.3732/ajb.89.7.1093 |
Bendiksby M., Thorbek L., Scheen A.C., et al, 2011. An updated phylogeny and classification of Lamiaceae subfamily Lamioideae. Taxon, 60: 471-484. DOI:10.1002/tax.602015 |
Chen Y.P., Drew B.T., Li B., et al, 2016. Resolving the phylogenetic position of Ombrocharis (Lamiaceae), with reference to the molecular phylogeny of tribe Elsholtzieae. Taxon, 65: 123-136. DOI:10.12705/651.8 |
Chen Y.P., Wilson T.C., Zhou Y.D., et al, 2019. Isodon hsiwenii (Lamiaceae: Nepetoideae), a new species from Yunnan, China. Syst. Bot, 44: 913-922. DOI:10.1600/036364419X15710776741486 |
Doyle J.J., Doyle J.D., 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull, 19: 11-15. |
Edgar R.C., 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res, 32: 1792-1797. DOI:10.1093/nar/gkh340 |
Handel-Mazzetti, H., 1936. Paraphlomis Prain. In: Handel-Mazzetti, H. (Ed. ), Symbolae Sinicae, vol. 7(4). Springer, Vienna, pp. 921-922.
|
Harley, R.M., Atkins, S., Budantsev, A.L., et al., 2004. Labiatae. In: Kubitzki, K., Kadereit, J.W. (Eds. ), The Families and Genera of Vascular Plants, vol. 7. Springer, Berlin & Heidelberg, pp. 167-275.
|
Huang, T.C., Hsieh, T.H., Cheng, W.T., 1998. Labiatae. In: Huang, T.C. (Ed. ), Flora of Taiwan,, second ed., vol. 4. Editorial Committee of the Flora of Taiwan, Second Edition, Taipei, pp. 432-548.
|
Jordan W.C., Courtney M.W., Neigel J.E., 1996. Low levels of intraspecific genetic variation at a rapidly evolving chloroplast DNA locus in North American duckweeds (Lemnaceae). Am. J. Bot, 83: 430-439. DOI:10.1002/j.1537-2197.1996.tb12724.x |
Li H.W., 1965. Revisio generis Paraphlomis Labiatarum Sinensium. Acta Phytotaxon.Sin, 10: 57-74. |
Li, H.W., Hedge, I.C., 1994. Lamiaceae. In: Wu, C.Y., Raven, P.H. (Eds. ), Flora of China, vol. 17. Science Press, Beijing & Missouri Botanical Garden Press, St. Louis, pp. 269-291.
|
Miller, M.A., Pfeiffer, W., Schwartz, T., 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE). New Orleans, LA, pp. 1-8.
|
Oxelman B., Lidén M., Berglund D., 1997. Chloroplast rps16 intron phylogeny of the tribe Sileneae (Caryophyllaceae). Plant Syst. Evol, 206: 393-410. DOI:10.1007/BF00987959 |
Pan Y.Z., Fang L.Q., Hao G., et al, 2009. Systematic positions of Lamiophlomis and Paraphlomis (Lamiaceae) based on nuclear and chloroplast sequences. J. Syst.Evol, 47: 535-542. DOI:10.1111/j.1759-6831.2009.00050.x |
Prain D., 1901. Phlomis L. Ann. Roy. Bot. Gard. (Calcutta), 9: 59-60. |
Prain D., 1908. Paraphlomis Prain. J. Asiat. Soc. Bengal, Pt. 2. Nat. Hist., 74: 721. |
Ronquist F., Teslenko M., van der Mark P., et al, 2012. MrBayes 3.2:efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol, 61: 539-542. DOI:10.1093/sysbio/sys029 |
Salmaki Y., Zarre S., Ryding O., et al, 2012. Phylogeny of the tribe Phlomideae(Lamioideae: Lamiaceae) with special focus on Eremostachys and Phlomoides: new insights from nuclear and chloroplast sequences. Taxon, 61: 161-179. DOI:10.1002/tax.611012 |
Shaw J., Lickey E.B., Schilling E.E., et al, 2007. Comparison of whole genome chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare Ⅲ. Am. J. Bot, 94: 275-288. DOI:10.3732/ajb.94.3.275 |
Stamatakis A., 2014. RAxML version 8:a tool for phylogenetic analysis and postanalysis of large phylogenies. Bioinformatics, 30: 1312-1313. DOI:10.1093/bioinformatics/btu033 |
Stover B., Müller K., 2010. TreeGraph 2:combining and visualizing evidence from different phylogenetic analyses. BMC Bioinf, 11: 1-9. |
Sun Y.Z., 1955. Notes on the Chinese species of Paraphlomis. Acta Phytotaxon. Sin, 4: 47-51. |
Sun Y., Skinner D.Z., Liang G.H., et al, 1994. Phylogenetic analysis of Sorghum and related taxa using internal transcribed spacers of nuclear ribosomal DNA. Theor.Appl. Genet, 89: 26-32. DOI:10.1007/BF00226978 |
Taberlet P., Gielly L., Pautou G., et al, 1991. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol, 17: 1105-1109. DOI:10.1007/BF00037152 |
Tamura K., Stecher G., Peterson D., et al, 2013. MEGA6:molecular evolutionary genetics analysis version 6. 0. Mol. Biol. Evol, 30: 2725-2729. DOI:10.1093/molbev/mst197 |
Thiers, B., 2020. Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium. _aaaaaa_paichu__. (Accessed 15 December 2020).
|
Wu C.Y., 1959. Revisio Labiatarum Sinensium. Acta Phytotaxon. Sin, 8: 1-66. |
Wu, C.Y., Li, H.W., 1977. Paraphlomis Prain. In: Wu, C.Y., Li, H.W. (Eds. ), Flora Reipublicae Popularis Sinicae, vol. 65(2). Science Press, Beijing, pp. 545-572.
|
Xiang C.L., Liu E.D., Peng H., 2010. Nomenclatural notes on the genus Paraphlomis(Lamiaceae: Lamioideae) from China. Nord. J. Bot, 28: 667-669. DOI:10.1111/j.1756-1051.2009.00691.x |
Xiang C.L., Hu G.X., Peng H., 2016. New combinations and new synonyms in Lamiaceae from China. Biodivers. Sci, 24: 719-722. DOI:10.17520/biods.2016018 |
Yan K.J., Fang D., 2009. A supplement to the Paraphlomis (Lamiaceae) from Guangxi, China. J. Trop. Subtropical Bot, 17: 91-92. |



