b. Department of Ecology and Conservation Biology, Texas A&M University, College Station 77843-2138, TX, USA;
c. University of Chinese Academy of Sciences, Beijing 100049, PR China
Biodiversity is essential for the sustainability of global ecosystems (e.g., Rands et al., 2010), but it is consistently threatened by both climate change and human activity (Thomas et al., 2004; Feehan et al., 2009 and references therein; Brandt et al., 2019). An important step in conserving biodiversity is to identify areas with highly concentrated diversity of endemic and rare species (Zhang et al., 2015; Xu et al., 2019). Since Myers (1988), "biodiversity hotspots" has become a fundamental concept in conserving global biodiversity (e.g. Myers, 1988, Myers et al., 2000; Brooks et al., 2006; Yu et al., 2019b), and many nature reserves have been established in pursuit of this goal. However, large disconnects remain between centers of conservation efforts and the distribution of biodiversity, especially in China (Wang and Xie, 2004; Zhang and Ma, 2008; Zhang et al., 2015; Xu et al., 2019), highlighting that endangered plant conservation in China faces great challenges.
Historically, biodiversity hotspots have been defined based on taxon diversity (i.e., species richness, SR) but not on genetic and evolutionary attributes (e.g., Myers et al., 2000; Brooks et al., 2006; Zhang et al., 2016), resulting in incomplete depictions of the multiple dimensions of diversity (Thornhill et al., 2017). However, these missing dimensions may highlight the lineages and geographic regions most in need of conservation (e.g., Thornhill et al., 2016; Sosa et al., 2018). Rather than simply identifying areas of species richness and endemism, measures of phylogenetic diversity (PD) and endemism (PE) add an evolutionary dimension to understanding spatial patterns of diversity and endemism by measuring the geographic rarity of a phylogenetic tree in a given area (e.g., Forest et al., 2007; Thornhill et al., 2016, 2017; Sosa et al., 2018). To analyze the spatial patterns of phylogenetic endemism, Mishler et al. (2014) developed a randomization test for endemism called categorical analysis of neo- and paleo-endemism (CANAPE). CANAPE identifies regions that show high conservation values. In recent years, CANAPE analysis has been widely applied in local protection assessments (Thornhill et al., 2017; Sosa et al., 2018; Spalink et al., 2018; Dagallier et al., 2020; Xu et al., 2019). Indeed, phylogenetic approaches have been widely employed to identify evolutionarily important regions of diversity and to reevaluate biodiversity centers, resulting in strongly effective proposals/programs for biodiversity conservation (e.g., Thornhill et al., 2016; Sosa et al., 2018; Spalink et al., 2018; Xu et al., 2019).
Spatial distribution of biodiversity is largely driven by climate-related (e.g., climatic gradient, climatic stability, temperature and precipitation) and/or topography-related attributes (e.g., topographic heterogeneity/diversity) (e.g., Hortal et al., 2009; Fine, 2015; Yu et al., 2019a). Moreover, habitat diversity, which refers to the environmental variability within an area, including topography, habitat type and climate (e.g., Tews et al., 2004; Hortal et al., 2008), is positively correlated with diversity (e.g., Triantis et al., 2003; Hortal et al., 2009; Xu et al., 2014). However, few studies have compared the responses of different flora/ecosystem types in the same region to climatic and topographic factors. To effectively conserve biodiversity, then, we should also take various ecosystem types into consideration and focus on the protection of habitat diversity, not just species diversity.
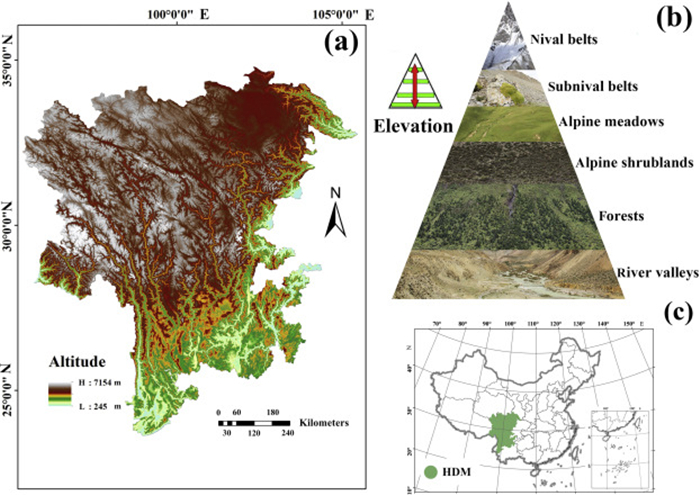
The Hengduan Mountains region (hereafter HDM) in southwestern China is located within a global biodiversity hotspot and is a conservation priority because of its high diversity of both species and ecological regions (Myers et al., 2000; Brooks et al., 2006; Zhang et al., 2017; Xu et al., 2019). Due to its climatic and geographical characteristics, the HDM supports a high diversity of vegetation types (Fig. 1) (Zhang et al., 1997). The mountain top areas (i.e., the subnival ecosystem) and the mountain bottom areas (i.e., the river valley ecosystem) are the two topographic and climatic extremes of the HDM with very different habitats. Both of these ecosystems harbor high plant diversity (Xu et al., 2014; Zhu, 2014). According to Xu et al. (2014), 942 plant species, belonging to 168 genera and 48 families, are found in the subnival belts. Of these, 295 species (31.32%) are endemic to the Sino-Himalayan alpine subnival belt and 151 (16.03%) are strictly endemic to the alpine subnival belt of the HDM. The river valleys harbor 2420 species, belonging to 805 genera and 147 families, also with a high proportion of endemism (Zhu, 2014). The two floras account for about 30% of the total plants in the HDM (Sun et al., 2017). In addition, the HDM region has also been identified as one of the distribution centers of many highly endangered plant species of China (Xu et al., 2019).
|
| Fig. 1 The geographical range of our study area. (a) Elevation map and main rivers. (b) Vertical landscape structure of the Hengduan Mountains (HDM). (c) Location of the research area in China. |
Conserving all species in all areas simultaneously is not feasible; thus, particularly biodiverse regions, vulnerable ecosystem types and/or threatened centers must be identified (e.g., Myers et al., 2000; Zhang et al., 2015; Xu et al., 2019). Many studies have attempted to provide guidance to biodiversity conservation by identifying spatial patterns of diversity in the HDM or the Qinghai-Tibet Plateau (e.g., Zhang et al., 2016; Xu et al., 2019). However, most of these studies have focused on species diversity, overlooking the importance of the evolutionary history of species (Thornhill et al., 2017; Yu et al., 2019b). As of 2017, 43 national nature reserves have been established in the HDM area. Most of these nature reserves have been primarily established in forest ecosystems and are intended to protect particular species (e.g., giant panda, snub-nosed monkey) (Fig. S1 in Appendix S1). However, in recent decades researchers have questioned whether these reserves are effective at protecting species (Zhang et al., 2015; Xu et al., 2019). Furthermore, distribution of these reserves in the HDM ignores the conservation value of the subnival and river valley ecosystems. This neglect may be partly due to a lack of data and limited understanding of these ecosystems, but the plant diversity and high proportion of endemism in these ecosystems suggests that special efforts be directed for their protection. Unfortunately, we are unaware of any studies that have compared the spatial patterns of plant diversity and endemism of these two ecosystems. Thus, it is important for conservation planning and prioritization to identify both spatial patterns of plant diversity and endemism in the subnival and river valley ecosystems and the factors (e.g., human, climatic, and topographical) that affect these ecosystems.
In this study, we asked first how plant diversity and endemism are spatially distributed in the river valleys and subnival ecosystems of the HDM. To answer this question, we used both taxonomic and phylogenetic measures of the biodiversity of the river valleys (thermophilic species) and the subnival belt (cold-adapted species) in the whole HDM. To understand which factors affect these patterns of diversity and endemism, we analyzed the potential drivers of plant diversity and endemism in this region, including habitat heterogeneity, climate (past and current) and human activity. Finally, to determine whether existing nature reserves protect centers of biodiversity in the HDM, we identified the distribution of diversity, endemism, threatened and high-priority species in these two ecosystems, and, in cases where there was little overlap between these important regions and nature reserves. As a result of these analyses, we compared the influence factors of different floristic distribution patterns and provide conservation significance for establishing new nature reserves and policies for ecosystem management in future.
2. Materials and methods 2.1. Study areaThe HDM, a well-known biodiversity hotspot in southwest China (Myers et al., 2000), includes northwestern Yunnan, eastern and southeastern Tibet, western Sichuan, southern Gansu, and southeastern Qinghai (Li, 1987). The HDM supports diverse vegetation types at different elevations. From low to high elevations, these include dry hot river valley scrub, broadleaved forest, coniferous broadleaved mixed forests, coniferous forest, shrub, meadow, cold subnival belts, and nival belts (glaciers) (Fig. 1; Zhang et al., 1997).
This study focuses on the river valley and the subnival flora as described by Xu et al. (2014) and Zhu (2014). Specifically, our study area extends between the boundaries of Chayu County, Basu County, Leiwuqi County, and Nangqian County in the west; Zhenkang County, Yongde County, and Wuding County in the south; Dari County, Shiqu County, Luqu County, and Diebu County in the north; and Yongshan County, Huize County, western Sichuan Basin, Mao County, and Wen County in the east.
The location of the river valley flora in the HDM region follows Zhu (2014), spanning the river valley region of Yunnan, Sichuan, and Tibet provinces. The region is dominated by tropical and subtropical climates. Zhu (2014) extracted records from Flora of China (Wu et al., 2013), Flora Reipublicae Popularis Sinicae (Flora Reipublicae Popularis Sinicae Editorial Board, 1961–2002), Vegetation of China (Wu, 1980), Vegetation of Yunnan (Wu et al., 1987), Vegetation of Sichuan (Vegetation of Sichuan Editorial Board, 1980) and Vegetation of Tibet (The Comprehensive Scientific Expedition to the Qinghai-Tibet Plateau, 1988) and identified valleys with xerophytic vegetation in the longitudinal ridge valley area of the Hengduan Mountains in southwest China, including the Salween/Nu, Lancang and Jinsha rivers and their main tributaries (main rivers see Fig. 1a).
The location of the subnival flora in the HDM region follows Xu et al. (2014), and includes western and northwestern Yunnan, eastern and southeastern Tibet, northern and western Sichuan, southern and southeastern Qinghai and southwestern Gansu. Xu et al. (2014) mainly based their work on Vascular plants of the Hengduan Mountains (The Comprehensive Scientific Expedition to the Qinghai-Tibet Plateau, 1993), Flora of China, Flora Reipublicae Popularis Sinicae, Flora of Yunnan (Kunming Institute of Botany Chinese Academy of Sciences, 2006), and Vegetation of Yunnan (Li, 1987).
2.2. Spatial indicesWe compiled a list of records of HDM river valley plant species from Zhu (2014) and a list of records of HDM subnival plant species from Xu et al. (2014), which included national and local floras and vegetation, herbarium records, and related monographs (Appendix S2). Considering the inadequacies of gymnosperm data and its potential impact on spatial analyses (total 28 gymnosperms), only angiosperms were included. For each species on the river valley and subnival flora lists, we collected county-level spatial records and elevational range data from the Chinese Virtual Herbarium (http://www.cvh.ac.cn), GBIF (http://www.gbif.org), National Specimen Information Infrastructure (NSII: http://www.nsii.org.cn), national and regional flora records, Xu et al. (2014) and Zhang et al. (2016). We subsequently removed dubious records and added new records. The final spatial distribution data include 2381 river valley species and 934 subnival species Appendix S2). We converted the county-level spatial records for each species to a 0.25-degree × 0.25-degree (approximately 27.5 km × 27.5 km) resolution by comparing the elevational range of each species with that of a grid cell (Yu et al., 2019a). The river valley flora was divided into 608 grid cells and the subnival flora was divided into 799 grid cells.
We constructed phylogenetic trees for the subnival species and the river valley species using Scenario 3 of V. PhyloMaker (Jin and Qian, 2019). V. PhyloMaker is a free R package used to construct phylogenies for vascular plants, combining GBOTB for seed plants (Smith and Brown, 2018) and the clades for pteridophytes (Zanne et al., 2014). It is the largest dated tree of life for vascular plants and includes 74, 533 species and all families of extant vascular plants. It adds species to the mega-phylogeny based on three scenarios: Scenario 1 adds species as basal polytomies within their genera or families; Scenario 2 randomly adds species among existing taxa within their parental taxa; Scenario 3 adds species as polytomies within their parental clades and assigns branch lengths using BLADJ. Species scientific names were standardized using the "plantlist" package (Zhang, 2018) in R 3.5.1 (R Core Team, 2018).
We used five indices to quantify plant diversity and endemism using Biodiverse V2.0 (Laffan et al., 2010): species richness (SR), phylogenetic diversity (PD), weighted endemism (WE), phylogenetic endemism (PE), and relative phylogenetic endemism (RPE). In biodiversity assessments, four of these spatial indices (SR, PD, WE, and PE) are widely used to measure the spatial distribution of diversity (richness) and endemism (Thornhill et al., 2017). Species richness (SR) is a basic index, which was calculated as the number of all species in a grid cell. Phylogenetic diversity (PD) is defined as the sum of branch lengths (from the terminal to the base of the tree) of all species in a region (Faith, 1992). Weighted endemism (WE) is defined as "the sum of the reciprocal of the total number of cells in which each species is found" (Linder, 2001), which emphasizes cells with high numbers of restricted species. PE highlights cells containing taxa with relatively long branches and restricted ranges, and is the sum of the range-weighted branch lengths (Rosauer et al., 2009), which considers the application of PD to range-restricted organisms.
Based on the randomization test for PE and RPE, Mishler et al. (2014) developed the categorical analysis of neo- and paleo-endemism (CANAPE). CANAPE can identify five types of grid cells: not significant, paleo-endemic, neo-endemic, mixed- and super-endemic. "Not significant" indicates that these cells are not a center of significantly high endemism (one-tailed test, α = 0.05). "Paleo-endemic" indicates that a cell has a significantly high value with respect to the RPE ratio (two-tailed test, α ≥ 0.95), where there is a high percentage of restricted long branches. This pattern is commonly considered to indicate refugial areas where clades may have suffered high extinction and range contraction historically (Mishler et al., 2014); we previously called these grid cells "museums for species evolution" (Dagallier et al., 2020). "Neo-endemic" indicates that a cell has a significantly low RPE ratio (two-tailed test, α ≤ 0.05), where there is a high percentage of restricted short branches. This pattern is commonly considered to indicate speciation centers (Mishler et al., 2014); we previously call these "cradles for species evolution" (Dagallier et al., 2020). "Mixed-endemic" indicates that a grid is a center of endemism, having a mix of rare long and rare short branches. "Super-endemic" is a further subdivision of mixed endemism at the α = 0.01 level. Mixed-endemic centers commonly reflect grid cells that act as both "museums" and "cradles". We ran 999 trials of the randomization test for PE and RPE in Biodiverse V2.0 and then used SDMtoolbox 2.0 (Brown et al., 2017) to conduct categorical analysis of neo- and paleo-endemism (CANAPE) (Mishler et al., 2014).
The priority species list and threatened species list were evaluated based on Qin et al. (2017), Sun et al. (2019) and Information System of Chinese Rare and Endangered Plants (http://www.iplant.cn). The priority species list emphasizes species that are seriously disturbed by human economic activity (e.g., medicine, trade, gardening, etc.), and the threatened species list was classified according to the International Union for Conservation of Nature (IUCN) criteria (i.e., CR, critically endangered; EN, endangered; NT, near threatened; VU, vulnerable) (Appendix S3). From these data we determined richness hotspots for priority and threatened species. Moreover, we examined human population (HP) and gross domestic product (GDP) data for 107 counties in recent years from the National Bureau of Statistics as proxies for human activity. HP and GDP were interpolated into raster files based on a 0.05-degree × 0.05-degree grid, and the mean value for each grid cell was calculated (Wang et al., 2011b). To identify the priority regions in the HDM, we considered both the distribution of priority species and human activity. We overlaid the grids and identified the cells with both with the top 25% priority species richness and the top 25% GDP and HP. The boundary data of protected areas were extracted from Zhang et al. (2015).
2.3. Statistical analysesTo examine the mechanisms that drive diversity and endemism in the subnival and river valley flora, we calculated four types of predictors: topographic heterogeneity (Alt_SD), climatic gradient (Cvar_pre), modern climate (MAT_pre and MAP_pre), and climatic fluctuation velocity (Vel_MAT). To calculate Alt_SD, we extracted the SD of elevation for each grid cell using ArcGis 10.4 (http://www.esri.com). We used Yu et al. (2019a) method to calculate the climatic gradient (Cvar_pre). Bioclimatic variables were downloaded from the WorldClim database Version 1.4 (Hijmans et al., 2005). First, we removed collinearity with pairwise correlation r > 0.95 and retained nine bioclimatic variables (bio1, bio2, bio3, bio4, bio5, bio7, bio12, bio14, bio15), which were used to calculate the principal components analysis based on the "RStoolbox" package (Benjamin et al., 2019) in R 3.5.1. Then, we calculated the SD layers of the first three climate principal components (PC, explaining 90.1% of total variation) and summed them into the climatic gradient layer, weighted by the proportion explained by each PC. Modern climatic predictors include two bioclimatic variables: annual mean temperature (MAT_pre) and annual mean precipitation (MAP_pre) in each grid cell. Climatic fluctuation (Vel_MAT) is represented by the climate change velocity since the LGM in terms of mean annual temperature (Loarie et al., 2009; Sandel et al., 2011). Velocity is the regional displacement rate of climate, explained as the rate a species must migrate to maintain the same climatic conditions over time (Sandel et al., 2011). We calculated the velocity layer using the method described by Hamann et al. (2015).
We used ordinary least squares (OLS) linear regressions to explore the bivariate relationships between SR, PD, WE, PE and each predictor. We constructed spatial error simultaneous autoregressive (SAR) models for single and multiple variables to account for spatial autocorrelation. The model selection process was based on Akaike's information criterion (AIC). Averaged coefficients for the predictors in each model were calculated based on AIC weights, whilst the sum of AIC weights in all models for each predictor was calculated to reflect the statistical support. Because of the strong collinearity between topographic heterogeneity (Alt_SD) and climatic gradient (Cvar_pre) (subnival: r = 0.91, river valley: r = 0.86), we built two groups of models (group A: topographic heterogeneity + modern climate + climatic fluctuation; and group B: climatic gradient + modern climate + climatic fluctuation), each of which contained only one of the two predictors. These analyses were conducted using the packages "MuMIn" (Bartoń, 2019) and "spdep" (Bivand and Wong, 2018) in R 3.5.1.
3. Results 3.1. Observed plant diversity and endemismMaps with observed values of SR, WE, PD and PE for the subnival and river valley floras are presented in Fig. 2 (for main counties see Fig. S2 in Appendix S1). For both floras, the highest levels of SR and PD are found in northwestern Yunnan Province (Dêqên, Shangri-La, Weixi, Lijiang and Heqing counties) and southwestern Sichuan Province (Xiangcheng, Muli and south Litang counties) (26.5°–29°N, 98.5°–101°E). WE and PE for both floras are highest in the limited range that overlaps with that of phylogenetic diversity and species richness. Specifically, for the river valley flora, WE and PE are highest in Lijiang, Heqing, S Dêqên, southwestern Muli and northern Weixi counties. For the subnival flora, WE and PE are highest in Dêqên, Shangri-La, and Derong counties.

|
| Fig. 2 Spatial distribution of species richness (SR), weighted endemism (WE), phylogenetic diversity (PD), and phylogenetic endemism (PE) for the river valley flora (a, c, e, g) and the subnival flora (b, d, f, h) in the Hengduan Mountains region. |
Areas with significant phylogenetic endemism identified by CANAPE are shown in Fig. 3 (a, b). Flora of the river valley have centers of paleo-endemism in small regions of northwestern Yunnan and eastern Tibet, centers of neo-endemism in northern and eastern HDM, and centers of mixed- and super-endemism mostly in the south (i.e., the lower parts of the main streams and tributaries of Salween River, Lancang River, and Jinsha River).

|
| Fig. 3 Map of significant centers of phylogenetic endemism revealed by CANAPE analysis. Yellow indicates centers of neo-endemism, blue indicates centers of paleo-endemism, purple indicates mixed-endemism (α = 0.05) and dark purple indicates mixed super-endemism (α = 0.01) (a, c, d) The river valley flora (b. e, f) The subnival flora (c–f) Significant centers of human activity plotted against climatic fluctuation velocity for the area in which they occur. |
We identified only one area of paleo-endemism in the subnival flora of the Hengduan Mountains: eastern Tibet. Centers of neo-endemism in the subnival flora are found mostly in northwestern Yunnan and southwestern Qinghai. We identified centers of mixed- and super-endemism in the subnival flora in southeastern Qinghai, eastern Tibet, northwestern Yunnan, and northern Sichuan. Most centers of mixed-endemism of the river valley flora occur in areas with low velocity and high human activity (Fig. 3c-f). In contrast, centers of mixed-endemism of subnival flora occur in areas with high velocity and low human activity, although some subnival mixed-endemism centers also occur in the areas with higher human activity.
3.3. Protected statusAnalysis of human population and GDP indicates that human activity is highest in the southern and southeastern Hengduan Mountains (Fig. 4a, b). For both the subnival and river valley ecosystems, most centers of endemism identified by CANAPE were not protected (subnival: 73%, river valley: 86%; Fig. 3). Subnival centers of endemism in these southern unprotected areas of the HDM are exposed to strong human interference, whereas centers in the northern parts of the HDM, despite being unprotected, face less human interference (Figs. 3 and 4 a, b). Centers of endemism in the river valley ecosystem occur in the southern parts of the HDM, but are also unprotected and face severe human interference.
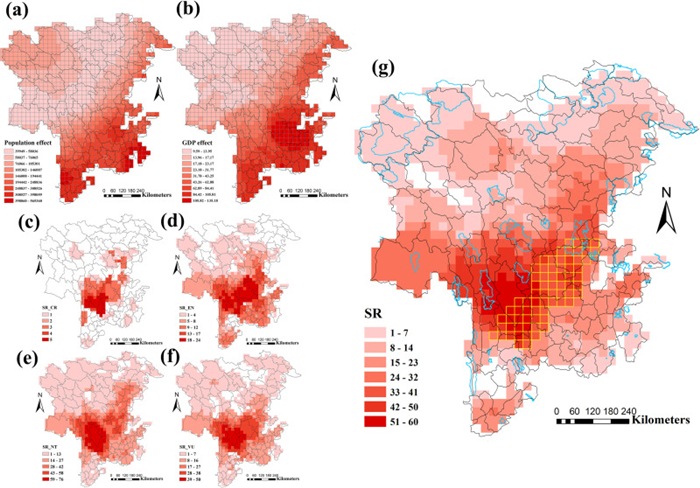
|
| Fig. 4 Map of human activity, threatened species richness, priority species richness and priority regions. Intensity of human activity based on (a) human population and (b) GDP (100 million). Categories were based on IUCN classification. (c) CR, critically endangered; (d) EN, endangered; (e) NT, near threatened; (f) VU, vulnerable. (g) Yellow grid cells indicate priority regions combined the results of both human population (HP) and GDP vs. priority species diversity. Blue polygons indicate protected areas (national nature reserves). |
The highest priority SR was also found in northwestern Yunnan and southwestern Sichuan, distributed in areas similar to those with the highest biodiversity (Fig. 3). These areas generally correspond to the distribution of threatened species (Fig. 4c-f). Specifically, these priority regions are located in southwestern Sichuan (Shimian, Jiulong, Mianning, Muli, Yanyuan counties) and northwestern Yunnan (Ninglang, Lijiang, S Shangri-La, Heqing, and Yongsheng counties). Furthermore, most priority regions are not designated as protected areas (Fig. 4g).
3.4. Environmental drivers of diversity and endemismThe river valley ecosystem. Both OLS and SAR indicated that SR, PD, WE and PE were negatively associated with climatic fluctuation, and positively associated with topographic heterogeneity, climatic gradient and modern temperature (Table 1). For SR and PD, SAR showed that climatic fluctuation, topographic heterogeneity and climatic gradient were significant, with climatic gradient having the highest coefficient (Table 1). For WE and PE, SAR showed that topographic heterogeneity, climatic gradient and modern precipitation were significant, with climatic gradient having the highest coefficient, followed by modern precipitation (negative coefficient) (Table 1). Multi-predictor regression models of the river valley flora indicated that for SR and PD, the models with the highest AIC weights both included climatic fluctuation; topographic heterogeneity and climatic gradient each had the highest AIC weights and significance; modern temperature and precipitation had lower AIC weights and were not significant (Table S1 in Appendix S1). For WE and PE, the models with the highest AIC weights both included modern precipitation; modern precipitation, topographic heterogeneity and climatic gradient all had the highest AIC weights; climatic fluctuation and modern temperature had lower AIC weights and were not significant (Table S1.
| OLS | SAR | OLS | SAR | ||||||||
| Coef | R2 | Coef | R2 | Coef | R2 | Coef | R2 | ||||
| River valley: single variable of OLS and SAR | |||||||||||
| SR | PD | ||||||||||
| Alt_SD | 0.48*** | 0.23 | 0.07** | 0.82 | 0.48*** | 0.23 | 0.06** | 0.84 | |||
| Cvar_pre | 0.59*** | 0.34 | 0.11*** | 0.82 | 0.60*** | 0.36 | 0.10*** | 0.84 | |||
| MAT_pre | 0.42*** | 0.18 | 0.02 | 0.82 | 0.46*** | 0.21 | 0.02 | 0.84 | |||
| MAP_pre | 0.38*** | 0.15 | −0.08 | 0.82 | 0.44*** | 0.19 | −0.06 | 0.84 | |||
| Vel_MAT | −0.26*** | 0.07 | −0.06* | 0.82 | −0.27*** | 0.07 | −0.05* | 0.84 | |||
| WE | PE | ||||||||||
| Alt_SD | 0.50*** | 0.25 | 0.09*** | 0.90 | 0.49*** | 0.25 | 0.08*** | 0.90 | |||
| Cvar_pre | 0.63*** | 0.39 | 0.15*** | 0.90 | 0.64*** | 0.40 | 0.14*** | 0.90 | |||
| MAT_pre | 0.57*** | 0.32 | 0.06* | 0.89 | 0.57*** | 0.33 | 0.05 | 0.90 | |||
| MAP_pre | 0.52*** | 0.26 | −0.11* | 0.89 | 0.54*** | 0.32 | −0.12* | 0.90 | |||
| Vel_MAT | −0.26*** | 0.06 | −0.03 | 0.89 | −0.26*** | 0.06 | −0.03 | 0.90 | |||
| Subnival: single variable of OLS and SAR | |||||||||||
| SR | PD | ||||||||||
| Alt_SD | 0.41*** | 0.17 | 0.08* | 0.73 | 0.41*** | 0.16 | 0.08* | 0.69 | |||
| Cvar_pre | 0.46*** | 0.21 | 0.16*** | 0.73 | 0.45*** | 0.20 | 0.16*** | 0.69 | |||
| MAT_pre | 0.33*** | 0.11 | 0.01 | 0.73 | 0.35*** | 0.12 | 0.01 | 0.69 | |||
| MAP_pre | 0.29*** | 0.09 | 0.06 | 0.73 | 0.29*** | 0.09 | 0.09 | 0.69 | |||
| Vel_MAT | −0.11** | 0.01 | −0.03 | 0.73 | −0.1** | 0.01 | −0.03 | 0.69 | |||
| WE | PE | ||||||||||
| Alt_SD | 0.412*** | 0.17 | 0.08** | 0.86 | 0.42*** | 0.17 | 0.08** | 0.84 | |||
| Cvar_pre | 0.46*** | 0.21 | 0.16*** | 0.86 | 0.45*** | 0.21 | 0.14** | 0.85 | |||
| MAT_pre | 0.25*** | 0.06 | −0.08** | 0.86 | 0.28*** | 0.08 | −0.08** | 0.84 | |||
| MAP_pre | 0.22*** | 0.05 | −0.04 | 0.85 | 0.21*** | 0.04 | −0.05 | 0.84 | |||
| Vel_MAT | −0.05 | 0.01 | −0.05** | 0.86 | −0.04 | 0.01 | −0.05** | 0.84 | |||
The subnival ecosystem. Both OLS and SAR indicated that SR, PD, WE and PE were negatively associated with climatic fluctuation, and positively associated with topographic heterogeneity and climatic gradient (Table 1). For SR and PD, SAR revealed that topographic heterogeneity and climatic gradient were significant, with climatic gradient having the highest coefficient (Table 1). For WE and PE, SAR showed that climatic fluctuation, topographic heterogeneity, modern temperature (negative coefficient) and climatic gradient were significant, with climatic gradient having the highest coefficient (Table 1). Multi-predictor regression models of the subnival flora indicate that for SR and PD, the models with the highest AIC weights in group A (topographic heterogeneity + modern climate + climatic fluctuation), only topographic heterogeneity was included; while group B (climatic gradient + modern climate + climatic fluctuation) included climatic gradient and modern temperature (only significant for SR), and climatic gradient had the highest coefficient (Table S2 in Appendix S1). For WE and PE, the models with the highest AIC weights both included climatic fluctuation and modern temperature; topographic heterogeneity and climatic gradient had high AIC weights; modern precipitation had lower AIC weights and was not significant (Table S2).
In summary, the results of multi-predictor regression models reflect similar results compared with single-predictor regression models.
4. DiscussionThis study is the first to simultaneously examine and compare patterns of plant diversity, endemism and threatened species in the two extreme environments of the HDM, using both traditional taxonomic measurements (i.e., SR, WE) and phylogenetic approaches (i.e., PD, PE and CANAPE analyses). In addition, we overlaid spatial phylogenetic patterns onto maps of current protected areas and human activity levels. Based on this information, we identified conservation gaps in this region and thus were able to present practical conservation proposals.
4.1. Spatial patterns of diversity and endemismIn general, patterns of diversity and endemism in the river valley and subnival ecosystems are similar, with the middle-southern HDM harboring the highest levels of both diversity and endemism. These patterns are consistent with previous studies (Zhang et al., 2009, 2015; Zhu, 2014). Specifically, northwestern Yunnan supports the highest biodiversity (also see Guo and Long, 1998; Zhang et al., 2015).
Biodiversity in the HDM has been driven by tectonic activities (e.g., Xing and Ree, 2017), climate effects (e.g., Yu et al., 2019a) and topographic heterogeneity (e.g., Zhang et al., 2009; Yu et al., 2019a). Our results show that SR, PD, WE and PE are negatively associated with climatic fluctuation but positively associated with topographic heterogeneity and climatic gradient (Table 1). These results are consistent with studies that have attributed the plant diversity of the Qinghai-Tibet Plateau to both the Neogene plateau uplift and subsequent Quaternary climate fluctuations (e.g. Wen et al., 2014; Yu et al., 2019a).
The effect of topographic heterogeneity and climatic gradients on diversity and endemism are likely related to the role that these factors play in creating habitat diversity (Table 1). Habitat diversity in a region can support various ecological niches for diversification and increase rates of speciation (Wang et al., 2017; Shrestha et al., 2018). Habitat diversity increases the likelihood that habitats will meet the specialized requirements of endemic species (Crisp et al., 2001). Therefore, we argue that habitat diversity in the HDM is one of the main drivers of the current plant diversity and endemism of river valley and subnival ecosystems.
Our finding that climatic fluctuation is negatively correlated with diversity and endemism indicates that regions with lower levels of climatic fluctuations may act as refugia during glaciations, thus preserving abundant biodiversity (Wallis et al., 2016). Higher levels of diversity and endemism may also be attributed to increased extinction and reduced speciation (e.g., Sandel et al., 2011; Feng et al., 2016). Interestingly, in our study, regions of the subnival ecosystem with higher MAT had lower levels of endemism; however, in the river valley ecosystem, regions with higher MAP had lower levels of endemism. These findings suggest that plants in these ecosystems use different adaptive strategies. For example, river valley plants are more drought- and heat-adapted (Jin et al., 1995), whereas subnival plants are more cold-adapted (Xu et al., 2014). We argue that different floras respond differently to environmental factors even in the same region, suggesting that the drivers of biodiversity in mountainous areas are complex. It is, therefore, inappropriate to explore the drivers of biodiversity in mountainous areas without taking various ecosystem types into consideration.
Although the patterns of diversity (SR, PD) and endemism (WE, PE) are similar in river valley and subnival ecosystems (Fig. 2), the patterns of neo- and paleo-endemism differ (Fig. 3). The majority of areas that constitute the river valley and subnival ecosystems are not centers of neo- or paleo-endemism. We identified a small region of paleo-endemism for both subnival and river valley floras in northwestern Yunnan and eastern Tibet. Centers for neo-endemism for the river valley flora were identified in small areas of the eastern and northeastern Hengduan Mountains. We also identified a relatively large center for neo-endemism of subnival flora that ranges across northwestern Yunnan and southeastern Qinghai (Fig. 3). Centers of mixed- and super-endemism for river valley flora occur in the southern and southeastern parts of the Hengduan Mountains (Fig. 3). Centers of neo-endemism in subnival ecosystems suggest that the subnival ecosystems in the southern and northern HDM served as centers for species differentiation and speciation after the Quaternary glaciation (Luo et al., 2016, 2017); conversely, the absence of centers of neo-endemism in the river valleys suggests that these ecosystems played a more limited role in this process. In addition, these results suggest that the warmer southern region in the HDM may have simultaneously served as a "museum" and a "cradle of diversification" over the last glacial–interglacial cycles, especially the river valley ecosystem where thermophilic plants could migrate to and take refuge and diversify (e.g., Yang et al., 2008). However, cold-adapted plants may have survived in some central or northern areas (Shimono et al., 2010; Wang et al., 2011a; Luo et al., 2016), which may explain the northern distribution pattern of neo-endemism and mixed-endemism in the subnival ecosystem. In general, CANAPE results identify the different spatial phylogenetic patterns of river valley and subnival ecosystems, which confirms the necessity to study individual floras separately.
It should be noted that the CANAPE approach was first used to analyze phylogenetic diversity and endemism in Australian Acacia species (Mishler et al., 2014). Although CANAPE analysis has been widely applied in local protection assessments in Australia (Thornhill et al., 2017), Mexico (Sosa et al., 2018), Wisconsin (Spalink et al., 2018), tropical Africa (Dagallier et al., 2020) and the karst flora in China (Xu et al., 2020), some researchers question whether the CANAPE approach can be used to identify centers of neo- and paleo-endemism in the complete flora of a region (not just endemic flora). In fact, when we are taking a phylogenetic approach, larger-scale lineages will occur outside our study area even if some species are endemic, so it is not really possible to eliminate all lineages that occur outside our area. Furthermore, PE is relative to a given study region, as is indeed true of all biodiversity metrics. Until data exist to analyze spatial phylogenetics at a global scale, this will continue to limit interpretations. Even though relative endemism only reflects local conditions, it is important for understanding the biogeographic processes in a region. Moreover, understanding relative endemism is quite important for conservation, especially because countries and decision-makers often target organisms for conservation that are rare within their boundaries, regardless of whether these organisms are present elsewhere.
4.2. Implications for conservationPhylogenetic measurements can be used to determine whether highly important areas or taxonomic groups are protected (Sosa et al., 2018). Currently, nature reserves in the HDM are scattered and mainly protect forest ecosystems (Fig. S1).
Global biodiversity is threatened by climate change and human activity (Thomas et al., 2004; Feehan et al., 2009 and references therein; Brandt et al., 2019). Our results show that the intensity of human activity is highest in the south and southeast HDM (Fig. 4), indicating biodiversity in this area confronts higher threats than in other areas. Biodiversity-rich areas often overlap with regions of high human population density (Cincotta et al., 2000; Luck, 2007) and species extinction risks tend to be high in such areas (Cardillo et al., 2004). Unfortunately, the centers of highest plant diversity, endemism, and threatened species overlap significantly with the highest intensity of human activity (Fig. 2, Fig. 3, Fig. 4). Most of these centers are not currently protected by nature reserves (Fig. 3, Fig. 4). This suggests that concerted efforts to conserve biodiversity and appropriate protection policies in these areas are urgently required.
Because our results suggest that habitat diversity can promote plant diversity and endemism, habitat diversity should be promoted not only to support current biodiversity, but also to preserve areas for potential future evolution. Although we examined diversities in the river valley and subnival ecosystems simultaneously, the human-induced impacts on these two ecosystems must be considered separately because of the different intensities of human activity (e.g., Paudel et al., 2018). The river valley ecosystem is suitable for human colonization. Residential settlement (e.g., urban expansion), infrastructure construction (e.g., road and hydropower construction), resource exploitation (e.g., mineral and wood extraction) and tourism development (e.g., hotels, restaurants and entertainment site construction) have already caused substantial damage to the local diversity (Fig. 5). For example, due to the increasing intensity of human activity, the ecosystem in the Jinsha river valley is extremely degraded, resulting in local diversity loss (Zhong, 2000). Due to the high overlap between diversity, endemism (especially mixed-endemism identified by CANAPE), threatened species and human activity, we recommend that special attention be paid to the river valleys in the southern and southeastern parts of the HDM.

|
| Fig. 5 Potential damage caused by human activity in the river valley (a–e) and the subnival (f–k) ecosystems. (a) Road construction; (b) Hydropower station; (c) Heavy-duty machinery; (d) Factory construction; (e) Agricultural reclamation; (f) Road construction (g, h, j) Collecting and selling medicinal plants; (i) Mechanical excavation (k) Over-grazing. (a, e, j, k) by YZ Zhang; (b) by R Tang; (c) by JG Chen; (d, i) by WG Sun; (f) by HL Chen; (g) by B Song; (h) by LS Qian. |
The subnival ecosystem is very fragile and sensitive to climate change (Körner, 2003). Many studies suggest that biodiversity shifts in the high mountains have been induced by climate change (e.g., Steinbauer et al., 2018; Salick et al., 2019). Besides climatic effects, human activity, such as livestock grazing and collection of medicinal plants/fungi, which have been sustained for millennia, have recently increased (Xu and Wilkes, 2004). For example, the subnival ecosystem is a traditional area for the collection of Tibetan medicinal herbs (e.g., Fritillaria delavayi, Rhodiola crenulata, Saussurea medusa, Polygonatum hookeri), but the intensity of collection has increased yearly, causing significant threats to local plants (Law and Salick, 2005; Liang et al., 2018; Peng et al., 2019). Some alpine plants are in danger of local or global extinction (Dullinger et al., 2012), especially those with extremely restricted distributions (Liang et al., 2018). In addition, due to its striking scenery, the tourism industry has developed greatly in mountain areas. Scientists, tourists, photographers, plant hunters and mountaineers are surging into the high alpine areas across the whole of the HDM, which may also threaten local plant diversity. More conservation efforts are urgently needed.
Availability of data and materialAll data generated and analyzed during this study are included in this published article and its supplementary information files. The software and codes used in this study were described in the methods section.
Declaration of competing interestThe authors declare that they have no conflict of interest.
Author contributionsY.Z.Z., J.G.C. and H.S. conceived the idea and designed the study. Y.Z.Z., L.S. and L.S.Q. collected original data; Y.Z.Z., S.L.Q and J.G.C. produced and analyzed data; Y.Z.Z. and J.G.C. wrote the manuscript; D.S. and H.S. revised the manuscript. All the authors read and approved the manuscript.
AcknowledgmentsThis study was supported equally by the National Key R & D Program of China (2017YFC0505200 to H Sun) and the Major Program of the National Natural Science Foundation of China (31590823 to H Sun), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA20050203 to H Sun), and USDA National Institute of Food and Agriculture (McIntire Stennis #101869 to DS). We thank XX Zhu, B Xu, XH Li, WG Sun and JH Chen for providing the original distribution data. We would like to thank R Tang and YC Yang for their help with data analysis. Thanks to XG Ma, D Luo and HL Chen for writing advice. We also thank S Christian for his useful suggestions about organizing this study and data analyses.
AcknowledgmentsAppendix A. Supplementary data Supplementary data to this article can be found online at https://CRAN.R-project.org/package=MuMIn.
Bartoń K., 2019. MuMIn: Multi-Model Inference. R package version 1, 43: 6. |
Benjamin L., Ned H., Jakob S.W., 2019. RStoolbox: Tools for Remote Sensing Data Analysis. R package version 0, 2: 6. |
Bivand R.S., Wong D.W.S., 2018. Comparing implementations of global and local indicators of spatial association.. Test, 27: 716-748. DOI:10.1007/s11749-018-0599-x |
Brandt J.S., Radeloff V., Allendorf T., et al, 2019. Effects of ecotourism on forest loss in the Himalayan biodiversity hotspot based on counterfactual analyses. Conserv. Biol, 33: 1318-1328. DOI:10.1111/cobi.13341 |
Brooks T.M., Mittermeier R.A., da Fonseca G.A.B., et al, 2006. Global biodiversity conservation priorities.. Science, 313: 58. DOI:10.1126/science.1127609 |
Brown J.L., Bennett J.R., French C.M., 2017. SDMtoolbox 2., 0:the next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ, 5: e4095. DOI:10.7717/peerj.4095 |
Cardillo M., Purvis A., Sechrest W., et al, 2004. Human population density and extinction risk in the world's carnivores. PLoS Biol, 2: E197-E197. DOI:10.1371/journal.pbio.0020197 |
Cincotta R.P., Wisnewski J., Engelman R., 2000. Human population in the biodiversity hotspots.. Nature, 404: 990-992. DOI:10.1038/35010105 |
Crisp M.D., Laffan S., Linder H.P., et al, 2001. Endemism in the Australian flora. J. Biogeogr, 28: 183-198. DOI:10.1046/j.1365-2699.2001.00524.x |
Dagallier L.-P.M.J., Janssens S.B., Dauby G., et al, 2020. Cradles and museums of generic plant diversity across tropical Africa. New Phytol, 225: 2196-2213. DOI:10.1111/nph.16293 |
Dullinger S., Gattringer A., Thuiller W., et al, 2012. Extinction debt of highmountain plants under twenty-first-century climate change.. Nat. Clim. Change, 2: 619-622. DOI:10.1038/nclimate1514 |
Faith D.P., 1992. Conservation evaluation and phylogenetic diversity. Biol. Conserv, 61: 1-10. DOI:10.1016/0006-3207(92)91201-3 |
Feehan J., Harley M., van Minnen J., 2009. Climate change in Europe. 1. Impact on terrestrial ecosystems and biodiversity. A review. Agron. Sustain. Dev, 29: 409-421. |
Feng G., Mao L., Sandel B., et al, 2016. High plant endemism in China is partially linked to reduced glacial-interglacial climate change. J. Biogeogr, 43: 145-154. DOI:10.1111/jbi.12613 |
Fine P.V.A., 2015. Ecological and evolutionary drivers of geographic variation in species diversity. Annu. Rev. Ecol. Evol. Syst, 46: 369-392. DOI:10.1146/annurev-ecolsys-112414-054102 |
Flora Reipublicae Popularis Sinicae Editorial Board, 1961-2002. Flora Reipublicae Popularis Sinicae. Science Press, Beijing.
|
Forest F., Grenyer R., Rouget M., et al, 2007. Preserving the evolutionary potential of floras in biodiversity hotspots.. Nature, 445: 757-760. DOI:10.1038/nature05587 |
Guo H., Long C., 1998. Yunnan's Biodiversity. Yunnan Science and Technology Press, Kunming, China.. Kunming, China.: Yunnan's Biodiversity. Yunnan Science and Technology Press.
|
Hamann A., Roberts D.R., Barber Q.E., et al, 2015. Velocity of climate change algorithms for guiding conservation and management. Global Change Biol, 21: 997-1004. DOI:10.1111/gcb.12736 |
Hijmans R.J., Cameron S.E., Parra J.L., et al, 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol, 25: 1965-1978. DOI:10.1002/joc.1276 |
Hortal J., Rodr#237;guez J., Nieto-D#237;az M., et al, 2008. Regional and environmental effects on the species richness of mammal assemblages. J. Biogeogr, 35: 1202-1214. DOI:10.1111/j.1365-2699.2007.01850.x |
Hortal J., Triantis Kostas A., Meiri S., et al, 2009. Island species richness increases with habitat diversity. Am. Nat, 174: E205-E207. DOI:10.1086/645085 |
Jin Y., Qian H., 2019. V.PhyloMaker: an R package that can generate very large phylogenies for vascular plants.. Ecography, 42: 1-7. DOI:10.1111/ecog.03836 |
Jin N.Z., Yang Y.P., Tao G.D., 1995. The floristic characteristics, nature and origin of seed plants in the dry-hot river valley of SW China.. Acta Bot. Yunnanica, 17: 129-143. |
Körner C., 2003. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems, second ed.. Berlin: Springer.
|
Kunming Institute of Botany Chinese Academy of Sciences, 2006. Flora of Yunnan.. Beijing: Science Press.
|
Laffan S.W., Lubarsky E., Rosauer D.F., 2010. Biodiverse, a tool for the spatial analysis of biological and related diversity.. Ecography, 33: 643-647. DOI:10.1111/j.1600-0587.2010.06237.x |
Law W., Salick J., 2005. Human-induced dwarfing of himalayan snow lotus, Saussurea laniceps (asteraceae).. Proc. Natl. Acad. Sci. USA, 102: 10218-10220. DOI:10.1073/pnas.0502931102 |
Li B.Y., 1987. On the boundaries of the hengduan mountains. Mt. Res, 5: 74-82. |
Liang Q., Xu X., Mao K., et al, 2018. Shifts in plant distributions in response to climate warming in a biodiversity hotspot, the Hengduan Mountains. J. Biogeogr, 45: 1334-1344. DOI:10.1111/jbi.13229 |
Linder H.P., 2001. On areas of endemism, with an example from the african restionaceae. Syst. Biol, 50: 892-912. DOI:10.1080/106351501753462867 |
Loarie S.R., Duffy P.B., Healy H., et al, 2009. The velocity of climate change.. Nature, 462: 1052-1055. DOI:10.1038/nature08649 |
Luck G.W., 2007. A review of the relationships between human population density and biodiversity. Biol. Rev, 82: 607-645. DOI:10.1111/j.1469-185X.2007.00028.x |
Luo D., Yue J.P., Sun W.G., et al, 2016. Evolutionary history of the subnival flora of the Himalaya-Hengduan Mountains: first insights from comparative phylogeography of four perennial herbs. J. Biogeogr, 43: 31-43. DOI:10.1111/jbi.12610 |
Luo D., Xu B., Li Z.-M., et al, 2017. The nWard LineeMekongeSalween Divideo is an important floristic boundary between the eastern Himalaya and Hengduan Mountains: evidence from the phylogeographical structure of subnival herbs Marmoritis complanatum (Lamiaceae). Bot. J. Linn. Soc, 185: 482-496. DOI:10.1093/botlinnean/box067 |
Mishler B.D., Knerr N., Gonzalez-Orozco C.E., et al, 2014. Phylogenetic measures of biodiversity and neo- and paleo-endemism in Australian Acacia. Nat. Commun, 5: 4473. DOI:10.1038/ncomms5473 |
Myers N., 1988. Threatened biotas: "Hot spots" in tropical forests.. Environmentalist, 8: 187-208. DOI:10.1007/BF02240252 |
Myers N., Mittermeier R.A., Mittermeier C.G., et al, 2000. Biodiversity hotspots for conservation priorities.. Nature, 403: 853-858. DOI:10.1038/35002501 |
Paudel P.K., Sipos J., Brodie J.F., 2018. Threatened species richness along a Himalayan elevational gradient: quantifying the influences of human population density, range size, and geometric constraints. BMC Ecol, 18: 6. DOI:10.1186/s12898-018-0162-3 |
Peng D., Sun L., Pritchard H.W., et al, 2019. Species distribution modelling and seed germination of four threatened snow lotus (Saussurea), and their implication for conservation. Glob. Ecol. Conserv, 17: e00565. DOI:10.1016/j.gecco.2019.e00565 |
Qin H., Yang Y., Dong S., et al, 2017. Threatened species list of China's higher plants. Biodivers. Sci, 25: 696-744. DOI:10.17520/biods.2017144 |
R Core Team, 2018. R: A Language and Environment for Statistical Computing.. R Foundation for Statistical Computing, Vienna, Austria.. |
Rands M.R.W., Adams W.M., Bennun L., et al, 2010. Biodiversity conservation: challenges beyond 2010.. Science, 329: 1298-1303. DOI:10.1126/science.1189138 |
Rosauer D., Laffan S.W., Crisp M.D., et al, 2009. Phylogenetic endemism: a new approach for identifying geographical concentrations of evolutionary history. Mol. Ecol, 18: 4061-4072. DOI:10.1111/j.1365-294X.2009.04311.x |
Salick J., Fang Z., Hart R., 2019. Rapid changes in eastern Himalayan alpine flora with climate change. Am. J. Bot, 106: 520-530. DOI:10.1002/ajb2.1263 |
Sandel B., Arge L., Dalsgaard B., et al, 2011. The influence of Late Quaternary climate-change velocity on species endemism.. Science, 334: 660-664. DOI:10.1126/science.1210173 |
Shimono A., Ueno S., Gu S., et al, 2010. Range shifts of Potentilla fruticosa on the Qinghai-Tibetan Plateau during glacial and interglacial periods revealed by chloroplast DNA sequence variation.. Heredity, 104: 534. DOI:10.1038/hdy.2009.158 |
Shrestha N., Wang Z., Su X., et al, 2018. Global patterns of Rhododendron diversity: the role of evolutionary time and diversification rates. Global Ecol. Biogeogr, 27: 913-924. DOI:10.1111/geb.12750 |
Smith S., Brown J.W., 2018. Constructing a broadly inclusive seed plant phylogeny. Am. J. Bot, 105: 302-314. DOI:10.1002/ajb2.1019 |
Sosa V., De-Nova J.A., Vásquez-Cruz M., 2018. Evolutionary history of the flora of Mexico: dry forests cradles and museums of endemism. J. Syst. Evol, 56: 523-536. DOI:10.1111/jse.12416 |
Spalink D., Kriebel R., Li P., et al, 2018. Spatial phylogenetics reveals evolutionary constraints on the assembly of a large regional flora. Am. J. Bot, 105: 1938-1950. DOI:10.1002/ajb2.1191 |
Steinbauer M.J., Grytnes J.A., Jurasinski G., et al, 2018. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature, 556: 231-234. DOI:10.1038/s41586-018-0005-6 |
Sun H., Zhang J., Deng T., et al, 2017. Origins and evolution of plant diversity in the Hengduan Mountains, China. Plant Divers, 39: 161-166. DOI:10.1016/j.pld.2017.09.004 |
Sun W.B., Yang J., Dao Z.L., 2019. Study and Conservation of Plant Species with Extremely Small Population (PSESP) in Yunnan Province. Beijing: Science Press.
|
Tews J., Brose U., Grimm V., et al, 2004. Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J. Biogeogr, 31: 79-92. DOI:10.1046/j.0305-0270.2003.00994.x |
The Comprehensive Scientific Expedition to the Qinghai-Tibet Plateau, 1988. Vegetation of Tibet.. Beijing: Science Press.
|
The Comprehensive Scientific Expedition to the Qinghai-Tibet Plateau, 1993. Vascular Plants of the Hengduan Mountains.. Beijing: Science Press.
|
Thomas C.D., Cameron A., Green R.E., et al, 2004. Extinction risk from climate change.. Nature, 427: 145-148. DOI:10.1038/nature02121 |
Thornhill A.H., Mishler B.D., Knerr N.J., et al, 2016. Continental-scale spatial phylogenetics of Australian angiosperms provides insights into ecology, evolution and conservation. J. Biogeogr, 43: 2085-2098. DOI:10.1111/jbi.12797 |
Thornhill A.H., Baldwin B.G., Freyman W.A., et al, 2017. Spatial phylogenetics of the native California flora. BMC Biol, 15: 96. DOI:10.1186/s12915-017-0435-x |
Triantis K.A., Mylonas M., Lika K., et al, 2003. A model for the species-area-habitat relationship. J. Biogeogr, 30: 19-27. DOI:10.1046/j.1365-2699.2003.00805.x |
Vegetation of Sichuan Editorial Board, 1980. Vegetation of Sichuan.. Chengdu: Sichuan Renmin Press.
|
Wallis G.P., Waters J.M., Upton P., et al, 2016. Transverse alpine speciation driven by glaciation. Trends Ecol. Evol, 31: 916-926. DOI:10.1016/j.tree.2016.08.009 |
Wang S., Xie Y., 2004. China Species Red List.. Beijing: Higher Education Press.
|
Wang L., Wu Z.Q., Bystriakova N., et al, 2011a. Phylogeography of the SinoHimalayan fern Lepisorus clathratus on "the roof of the world.. PLoS One, 6: e25896. DOI:10.1371/journal.pone.0025896 |
Wang Z., Fang J., Tang Z., et al, 2011b. Patterns, determinants and models of woody plant diversity in China. Proc. Biol. Sci, 278: 2122-2132. |
Wang Q., Su X., Shrestha N., et al, 2017. Historical factors shaped species diversity and composition of Salix in eastern Asia. Sci. Rep, 7: 42038. DOI:10.1038/srep42038 |
Wen J., Zhang J.Q., Nie Z.L., et al, 2014. Evolutionary diversificatons of plants on the Qinghai-Tibetan plateau. Front. Genet, 5: 4. |
Wu C.Y., 1980. Vegetation of China.. Beijing: Science Press.
|
Wu C.Y., Zhu Y.C., Jiang H.Q., 1987. Vegetation of Yunnan.. Beijing: Science Press.
|
Wu C.Y., Raven P.H., Hong D.Y., 2013. Flora of China.. Beijing & St. Louis: Science Press & Missouri Botanical Garden Press.
|
Xing Y.W., Ree R.H., 2017. Uplift-driven diversification in the Hengduan Mountains, a temperate biodiversity hotspot. Proc.. Natl. Acad. Sci. USA, 114: E3444. DOI:10.1073/pnas.1616063114 |
Xu J., Wilkes A., 2004. Biodiversity impact analysis in northwest Yunnan, southwest China. Biodivers. Conserv, 13: 959-983. DOI:10.1023/B:BIOC.0000014464.80847.02 |
Xu B., Li Z.M., Sun H., 2014. Seed Plants of the Alpine Subnival Belt from the Hengduan Mountains, SW China. Beijing: Science Press.
|
Xu C., Huang Z.Y.X., Chi T., et al, 2014. Can local landscape attributes explain species richness patterns at macroecological scales? Global Ecol. Biogeogr, 23: 436-445. |
Xu Y., Huang J., Lu X., et al, 2019. Priorities and conservation gaps across three biodiversity dimensions of rare and endangered plant species in China. Biol. Conserv, 229: 30-37. DOI:10.1016/j.biocon.2018.11.010 |
Xu M.Z., Yang L.H., Kong H.H., et al, 2020. Congruent spatial patterns of species richness and phylogenetic diversity in karst flora: the case study of Primulina(Gesnariaceae). J. Syst. Evol.. DOI:10.1111/jse.12558 |
Yang F., Li Y., Ding X., et al, 2008. Extensive population expansion of Pedicularis longiflora (Orobanchaceae) on the Qinghai-Tibetan Plateau and its correlation with the Quaternary climate change. Mol. Ecol, 17: 5135-5145. DOI:10.1111/j.1365-294X.2008.03976.x |
Yu H., Deane D.C., Sui X., et al, 2019a. Testing multiple hypotheses for the high endemic plant diversity of the Tibetan Plateau. Global Ecol. Biogeogr, 28: 131-144. DOI:10.1111/geb.12827 |
Yu H., Favre A., Sui X., et al, 2019b. Mapping the genetic patterns of plants in the region of the Qinghai-Tibet Plateau: implications for conservation strategies. Divers. Distrib, 25: 310-324. DOI:10.1111/ddi.12847 |
Zanne A.E., Tank D.C., Cornwell W.K., et al, 2014. Three keys to the radiation of angiosperms into freezing environments.. Nature, 506: 89-92. DOI:10.1038/nature12872 |
Zhang J., 2018. Plantlist: Looking up the Status of Plant Scientific Names Based on the Plant List Database. R package version 0, 6.0.. |
Zhang Y.B., Ma K.P., 2008. Geographic distribution patterns and status assessment of threatened plants in China. Biodivers. Conserv, 17: 1783. DOI:10.1007/s10531-008-9384-6 |
Zhang R., Zheng D., Yang Q., et al, 1997. Physical Geography of Hengduan Mountains.. Beijing: Science Press.
|
Zhang D., Boufford D.E., Ree R.H., et al, 2009. The 29#176;N latitudinal line: an important division in the Hengduan Mountains a biodiversity hotspot in southwest China. Nord. J. Bot, 27: 405-412. DOI:10.1111/j.1756-1051.2008.00235.x |
Zhang Z., He J.S., Li J., et al, 2015. Distribution and conservation of threatened plants in China. Biol. Conserv, 192: 454-460. DOI:10.1016/j.biocon.2015.10.019 |
Zhang D.C., Ye J.X., Sun H., 2016. Quantitative approaches to identify floristic units and centres of species endemism in the Qinghai-Tibetan Plateau, south-western China. J. Biogeogr, 43: 2465-2476. DOI:10.1111/jbi.12819 |
Zhang M.G., Slik J.W.F., Ma K.P., 2017. Priority areas for the conservation of perennial plants in China. Biol. Conserv, 210: 56-63. |
Zhong X., 2000. Degradation of ecosystem and ways of its rehabilitation and reconstruction in dry and hot valley-Take representative area of Jinsha river, Yunan province as an example. Resour. Environ.. Yangtze Basin, 9: 376-383. |
Zhu X., 2014. The Flora of Seed Plants in Three Rivers Valley of SW China.. University of Chinese Academy of Sciences.. |



