b. State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing, 100093, PR China;
c. University of Chinese Academy of Sciences, Beijing, 100049, PR China;
d. Sino-Africa Joint Research Center, Chinese Academy of Sciences, Wuhan, Hubei, 430074, PR China;
e. High School of Agricultural Sciences, University of Antananarivo, P.O. Box 175, Madagascar
Global climate change is a widely recognized problem that has serious impacts on local environments (IPCC, 2014). Accordingly, ecologists and conservation biologists have begun to study how global climate change affects particular regions and species (Fourcade, 2016). Madagascar is an important world conservation "hot spot" that supports a large number of endemic species. Located 400 km off the eastern coast of Africa, Madagascar is the fourth largest island in the world (Fig. 1) and its climate varies greatly, from a humid northeastern coast with tropical forests to a dry southwestern coast with dry deciduous and spiny forests (Tadross et al., 2008). In Madagascar, average temperatures increased rapidly between 1975 and 2000, and studies have predicted that climate changes will cause an increase in temperature, as well as an increase precipitation during the rainy season and a decrease in precipitation during the dry season (Tadross et al., 2008). The climate change-related increase in the frequency of the El Niño Southern Oscillation (ENSO) is predicted to cause drought events and associated wildfires in Madagascar, decreasing natural vegetation (Ingram and Dawson, 2005). Thus, understanding how native species respond to climate change in Madagascar is critical to conservation efforts.
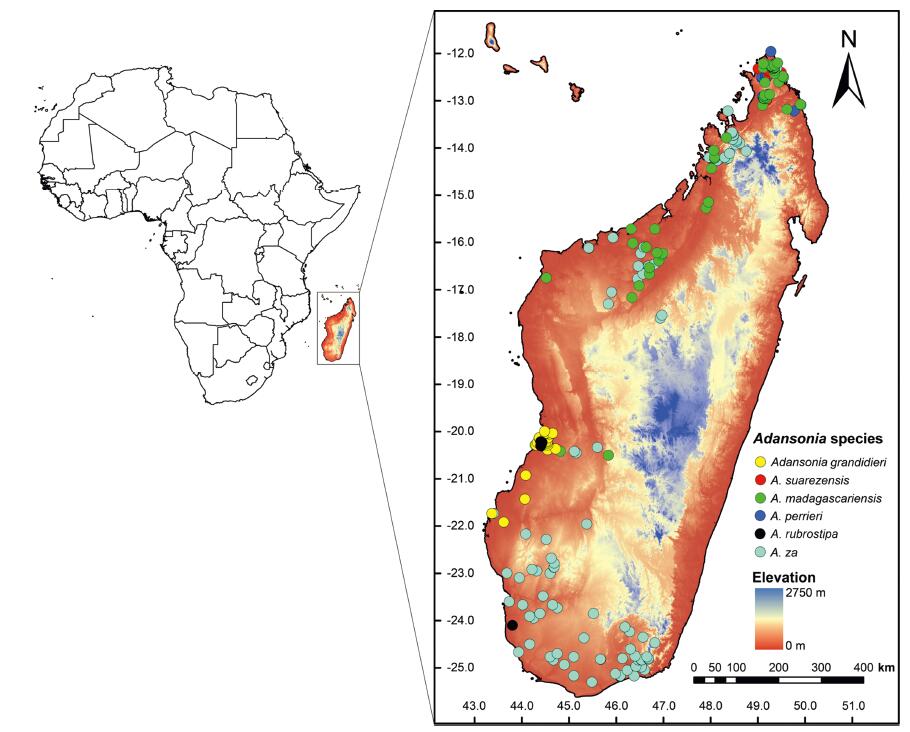
|
| Fig. 1 The 245 endemic baobab occurrence points used in modeling. |
Baobabs, one of the most remarkable trees in Africa, have great ecological and economic value. Belonging to subfamily Bombacoideae (Malvaceae), the baobab genus Adansonia L. consists of eight to nine species, six endemic to Madagascar: Adansonia grandidieri Baill., A. suarezensis H. Perrier, A. madagascariensis Baill., A. perrieri Capuron, A. rubrostipa Jum. & H. Perrier, A. za Baill (Bell et al., 2015; Cron et al., 2016; Douie et al., 2015; Jarnevich et al., 2015; Pettigrew et al., 2012). These endemic baobabs are currently distributed in western Madagascar, some within a narrow range while others are widely distributed along the western part of the island (Pettigrew et al., 2012; Vieilledent et al., 2013). Three of the six species (A. suarezensis, A. perrieri, A. grandidieri) are designated Endangered or Critically Endangered by the IUCN, and A. madagascariensis is Near Threatened (IUCN, 2020). Studies of Madagascar baobabs have focused on their uses, phylogeny, and pollination (Pettigrew et al., 2012; Sanchez et al., 2010). Few studies have examined baobab conservation (Lisao et al., 2018). Vieilledent et al. (2013) described the current distribution of three endangered baobab species in Madagascar (A. suarezensis, A. perrieri, A. grandidieri), and predicted the impact of climate change on suitable habitat in the future. However, information is lacking on suitable habitat for the three widespread baobab species (A. madagascariensis, A. rubrostipa, A. za), which are keystone species for ecosystems in Madagascar (Baum, 1996). Without comparatively complete knowledge of the whole species group, it is difficult to adopt appropriate conservation strategies (Carneiro et al., 2016; Ervin and Holly, 2011).
Previous studies have used species distribution modeling (SDM) to elucidate the distribution of forest and their species (Boria et al., 2014; Chaturvedi et al., 2011; Kumar, 2012) and model the potential effects of climate change on biodiversity (Sinclair et al., 2010; Root et al., 2003). MaxEnt is widely used to predict the distribution of a species using only occurrence point records (Mesgaran et al., 2014; Baumgartner et al., 2018). Numbers of users contributed to the development of this method (Kumar and Stohlgren, 2009; Petitpierre et al., 2017; Radosavljevic and Anderson, 2014; Zeng et al., 2016; Zurell et al., 2016), making it one of the strongest tools to identify suitable habitats and predict future distributions for species.
In this study, we used MaxEnt to model the current distribution of suitable habitat for all baobab species endemic to Madagascar and the impact of climate change on suitable baobab habitat in the future (the years 2050 and 2070). Our modeling highlights the climate change-related loss of original baobab habitat over time. These findings may help policymakers identify new protected areas for baobab species.
2. Materials and methods 2.1. Distribution and environmental dataThe current distribution data (the longitude and latitude) of six baobab species in Madagascar were obtained from several sources, including Global Biodiversity Information Facility (https://www.gbif.org/en/), iNaturalist (https://www.inaturalist.org/), as well as our own field surveys in 2018 (Fig. 1, Fig. 2). Duplicated and erroneous records were removed from the combined distribution data set, and 245 occurrence points of six baobab species (31 for A. grandidieri, 21 for A. suarezensis, 57 for A. madagascariensis, 19 for A. perrieri, 9 for A. rubrostipa, 108 for A. za) were kept for the analyses after filtering. Current (1970-2000) and future(2050, 2070) climate data at 2.5 arc-min resolution were retrieved from the WorldClim database (http://www.worldclim.org). Nineteen bioclimatic variables for Madagascar were extracted through ArcGIS. To avoid the effect of highly correlated variables, Pearson correlation analysis (Pearson r < 0.8) (Pearson, 1920) for all nineteen bioclimatic variables was performed, and then seven bioclimatic variables with low correlation were selected for subsequent analysis: Annual Mean Temperature (BIO1), Mean Diurnal Range (BIO2), Isothermality (BIO3), Precipitation of Wettest Month (BIO13), Precipitation of Driest Month (BIO14), Precipitation of Warmest Quarter (BIO18), and Precipitation of Coldest Quarter (BIO19). To model how climate change will affect suitable habitat for baobab species in 2050 and 2070, we used the Representative Concentration Pathway climate scenario with a high radiative forcing of 8.5 W/m2 (RCP 8.5), which is a pessimistic emissions scenario for climate simulations (IPCC, 2014). Prediction were made using the Community Climate System Model (CCSM4).

|
| Fig. 2 Malagasy baobabs. (a) & (b) Adansonia grandidieri; (c) A. suarezensis; (d), (e) & (f) A. madagascariensis; (g) & (h) A. perrieri; (i) & (j) A. rubrostipa; (k) A. za. |
Current climate background was used to model the current distribution of suitable baobab habitat using the MaxEnt 3.4.1 program (http://biodiversityinformatics.amnh.org/open_source/maxent/; Philips et al., 2006). Auto features function was used for the modeling, and the random test percentage was 25% with 10 replicates. Crossvalidate was used as the replicated run type, and other parameters were set as default. We computed the Area Under the Curve (AUC) to validate our model and specificity and sensitivity to the test data (Liu et al., 2011). We also computed the True Skill Statistic (TSS = sensitivity + specificity - 1) (Allouche et al., 2006; Liu et al., 2011) using the R code MaxEnt_TSS_calculations (https://github.com/KarlssonCatharina/MaxEnt_TSS_calculations). Then, to predict suitable habitat in the years 2050 and 2070, these models were projected using data from climate change scenarios. Based on the modeling results, we divided the potential habitat of each species into four classes using the reclassification function of ArcGIS: the highly suitable area (probability: 0.75-1), the medium suitable area (probability: 0.5-0.75), the low suitable area (probability: 0.25-0.5) and the unsuitable area (probability: < 0.25). Then the changes of the predicted potential habitats were calculated by overlap analysis in ArcGIS. This allowed us to quantify the range changes to illustrate the dynamicity of the range extensions, contractions, persistence, and shifts.
3. Results 3.1. The main bioclimatic variables affecting the distribution of AdansoniaThe AUC score for each of the six species modeling was above 0.8, indicating that models of these data sets performed better than a randomly chosen absence site. The score of TSS was equivalent to the AUC score in terms of performance.
For the current distribution modeling, the bioclimatic variable that contributed the most to the model of each species varied (Table 1). For four of six species (A. suarezensis, A. madagascariensis, A. perrieri, A. rubrostipa), the two variables that contributed the most to the model were both temperature-related (BIO1, BIO2 and BIO3); for the other two species (A. grandidieri, A. za), temperature-related variables also contributed largely. Precipitation (BIO13, BIO14, BIO18, BIO19) had a great impact on the distribution of A. grandidieri, A. rubrostipa and A. za, the three species that occur mainly in the southwest part of the island; however, for the three northern-distributed baobab species (A. suarezensis, A. madagascariensis, A. perrieri), precipitation had a limited impact.
| Species | Percent contribution (%) | ||||||
| BIO1 | BIO2 | BIO3 | BIO13 | BIO14 | BIO18 | BIO19 | |
| Adansonia grandidieri | 3.3 | 0.3 | 30.1 | 9.1 | 0.3 | 0 | 56.9 |
| A.suarezensis | 0.1 | 52.4 | 24.2 | 3.4 | 14.4 | 5.5 | 0 |
| A.madagascariensis | 29.0 | 33.0 | 27.2 | 0.2 | 7.4 | 2.2 | 1.0 |
| A.perrieri | 0.9 | 39.9 | 27.6 | 3.4 | 8.9 | 3.2 | 16.1 |
| A.rubrostipa | 27.5 | 0 | 46.3 | 0 | 26.2 | 0 | 0 |
| A.za | 24.5 | 2.3 | 0.4 | 42.7 | 22.4 | 5.5 | 2.2 |
Our modeling indicates that current potential suitable habitats for the six baobab species are all located at low elevations (mostly below 500 m) in western Madagascar (Fig. 1). Potential suitable habitats for A. madagascariensis, A. rubrostipa and A. za are relatively widely distributed, and that for A. madagascariensis is located in northern and northwestern Madagascar (Fig. 3g), while that for A. rubrostipa is mainly located along the west and southwest coasts of the island (Fig. 3m), with little overlapping distribution. Potential suitable habitat for A. za is the most widely distributed of Madagascar's baobab species, with two highly suitable habitats for this species in the northwest and south (Fig. 3p). The distribution of current potential suitable habitat for three endangered baobab species, A. grandidieri, A. suarezensis and A. perrieri, is limited. According to our modeling, suitable habitat for A. grandidieri is restricted to the southwest coast near the Morondava region (Fig. 3a), while A. suarezensis and A. perrieri habitat is restricted to a small region in northern Madagascar (Fig. 3d, j).
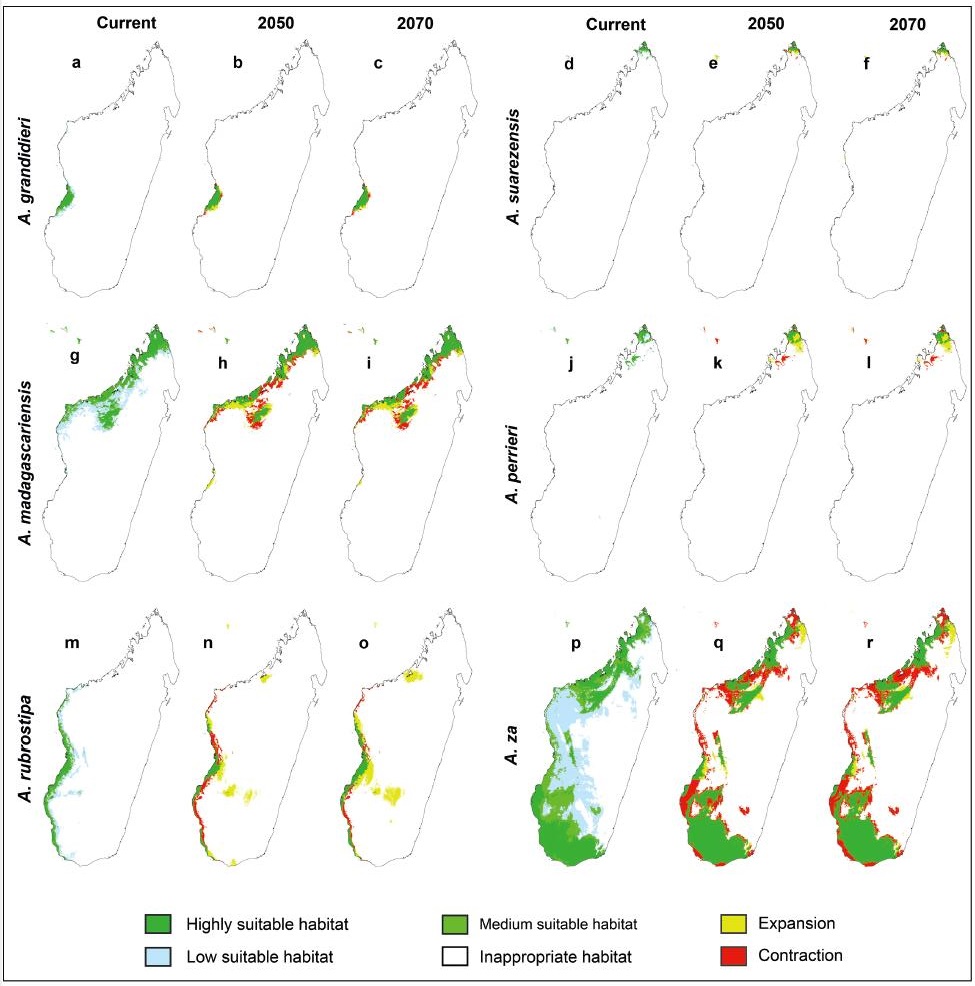
|
| Fig. 3 The distribution of potential suitable habitat for six baobab species in Madagascar at the present and the years 2050 and 2070. For the current distribution modeling, regions with different occurrence probability were filled with different colors. For the future distribution modeling, expansion and contraction of the highly suitable and medium suitable habitat were highlighted. |
The potential effects of climate change on suitable baobab habitat differed for each species. For A. suarezensis, A. perrieri and A. rubrostipa, the total area of suitable habitat in 2050 and 2070 is predicted to increase, whereas the total area of suitable habitat for A. grandidieri is likely to remain static. However, the area of the suitable habitat for A. madagascariensis and A. za may decrease significantly (Fig. 4).

|
| Fig. 4 The changes of potential suitable habitat area of the six baobab species from the current to the years 2050 and 2070. The area of original habitat predicted to be lost by the years 2050 and 2070 is also illustrated. |
Despite the predicted increase in habitat area for three baobab species, the current distribution of suitable habitat of all six species is predicted to undergo a contraction (Fig. 5). Notably, our modeling predicts that climate change will reduce the original habitats of A. rubrostipa and A. za by 40% (Fig. 4). According to our predicted distribution map, although most habitat contraction will be located along the margin of the original habitat, the narrow and long distribution pattern of A. madagascariensis, A. rubrostipa and A. za will be fragmented due to the loss of the original habitat (Fig. 3).
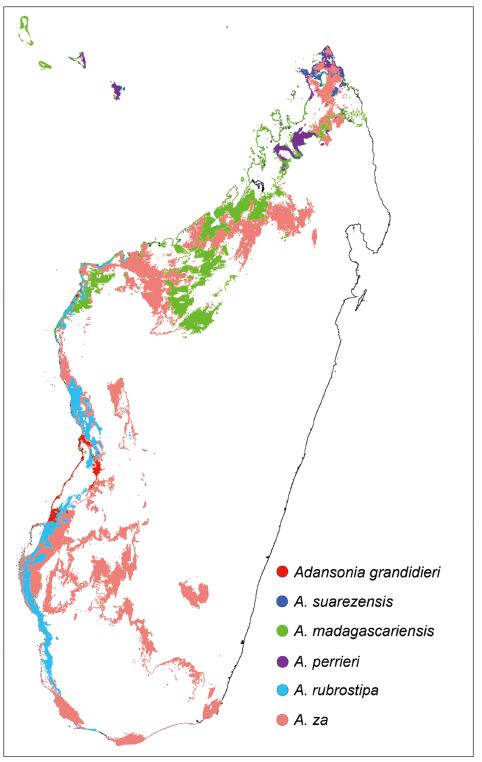
|
| Fig. 5 The geographical distribution showing the loss original habitat of six baobab species under climate change. |
In this study, we modeled the current distribution of suitable habitat for six species of baobabs endemic to Madagascar. Our modeling indicates that the distribution pattern of baobabs is temperature sensitive, and that these patterns are affected by both the average temperature and temperature fluctuation. These findings are consistent with previous studies that regard temperature as the most important factor for plant distribution patterns (Feng, 2008; Fišer et al., 2019; Yu et al., 2017; Huang et al., 2017). In our modeling, the distribution of three baobab species endemic to the dry forest in southern Madagascar (A. grandidieri, A. rubrostipa, A. za) is affected by precipitation, even though baobab trees are believed to exhibit a high tolerance to changes in rainfall as their massive trunks contain water (Archer et al., 2017). The mean rainfall in northern Madagascar is significantly higher than that in the southern part of the island, especially during the rainy season (Mitchell et al., 2004), indicating that baobab species in an arid environment may be sensitive to precipitation.
We also modeled the effects of climate change on the future distribution of suitable baobab habitat. Our modeling predicts that the distribution of suitable baobab habitat will undergo both expansions and contractions (Fig. 3). For baobab conservation, we wish to emphasize that more attention should be paid to the contraction of the original habitat than to habitat expansion. The contraction of suitable baobab habitat should be given great attention for three reasons. First, large baobab trees are generally old (Patrut et al., 2016) and are viewed as keystone species in dryland forests (Ebenman and Jonsson, 2005; Ellison et al., 2005). If climate change leads to the death of old baobab trees in the original habitat, it may take hundreds of years for young baobab trees in new habitats to restore the economic value of baobabs for Malagasy people and ecological value of related biocoenosis (Schäffler and Kappeler, 2014). Second, expansion of suitable habitat predicted by our modeling is based solely on climate data; however, other factors may limit this habitat expansion, including geological conditions, biological interactions, and human activities (Berger et al., 2019). Third, all baobab species in Madagascar occur in a narrow region along the west coast of the island; thus, baobab habitats are prone to fragmentation due to loss of their original habitat. Our modeling indicates that climate change during this century may lead to the loss of a large area of current baobab habitat (Fig. 5), highlighting the need to pay attention to all baobab conservation in Madagascar, regardless of species distribution or population size at present.
One limitation of our study is that our modeling is based on the RCP 8.5 scenario for climate change prediction, which is the most extreme scenario. Unfortunately, it is also believed that the RCP 8.5 scenario may be the most probable (IPCC, 2014). Two less extreme climate change scenarios include the RCP 2.6 and 4.5. However, we used the RCP 8.5 because we believe that, for species conservation, understanding the effects of the extreme scenario is important. Another limitation is that our modeling of baobab species distribution dynamic under climate change was based on climate factors only. Other environmental factors can also influence plant species distribution, including soil type, distance from water source, pollinator number, and human activity (Bio et al., 2002; Giannini et al., 2010; Blach-Overgaard et al., 2010). However, it is hard to predict how these factors will respond to global climate change. Thus, to support conservation strategies, in this study, we aimed to provide background information on a potential climate change-related shift in the distribution of Madagascar's baobabs.
Although effective decision making to limit climate change is a world-wide challenge for all countries (Xu et al., 2017), specific suggestions should be made to reduce the risks of climate change on baobab species in Madagascar. This study predicted which currently suitable habitats for different baobab species will be lost over the next century due to climate change. Thus, local baobab trees in these areas should be protected with greater urgency (Guisan and Thuiller, 2005). In addition to climate change, other factors may threaten the original suitable habitat, such as fire, logging and shifting agriculture. These human activities should be limited to reduce the rate of habitat contraction in these areas. Another way to protect the threatened baobabs from climate change is to preserve and introduce these baobab trees into areas in which suitable habitat is predicted to expand. To avoid climate change-related extinction of the baobab trees endemic to Madagascar (Bell et al., 2015), conservationists must invest more time and resources.
In this study, we modeled the current distribution of suitable habitat for baobab species endemic to Madagascar and how climate change may alter this distribution. Our modeling predicts that current suitable habitat of all baobab species will undergo a contraction. In our modeling, baobab distribution is most affected by temperature. We support previous recommendations (Kreft et al., 2006; Wiens et al., 2011) to protect areas that currently contain suitable baobab habitat under threat of contraction.
Author contributionsYDZ and QFW conceived and designed the study. BL, BNM, JRER, HPX and YDZ conducted the field surveys and collected the distribution data. JNW, NJM and SWW performed the analysis. JNW and NJM wrote the manuscript. All authors reviewed and approved the manuscript.
Declaration of Competing InterestWe declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
AcknowledgmentsThis study was supported by the funds from Sino-Africa Joint Research Center, CAS, China (Y323771W07 and SAJC201322) and National Natural Science Foundation of China (31800176).
O.Allouche, A.Tsoar, R.Kadmon, 2006. Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol., 43: 1223-1232. DOI:10.2307/4123815 |
Archer, E.R.M., Landman, W.A., Tadross, M.A., et al, 2017. Understanding the evolution of the 2014–2016 summer rainfall seasons in southern Africa: key lessons. Clim. Risk Manag., 16: 22-28. DOI:10.1016/j.crm.2017.03.006 |
D.A. Baum, 1996. The ecology and conservation of the baobabs of Madagascar. Prim. Rep., 46: 311-327. |
J.B.Baumgartner, M.Esperón-Rodríguez, L.J.Beaumont, 2018. Identifying in situ climate refugia for plant species. Ecography, 41: 1850-1863. DOI:10.1111/ecog.03431 |
K.L. Bell, H. Rangan, C.A. Kull, 2015. The history of introduction of the African baobab (Adansonia digitata, Malvaceae: Bombacoideae) in the Indian subcontinent. R. Soc. Open Sci., 2: 150370. DOI:10.1098/rsos.150370 |
C. Berger, M. Bieri, K. Bradshaw, 2019. Linking scales and disciplines: an interdisciplinary cross-scale approach to supporting climate-relevant ecosystem management. Clim. Change, 156: 139-150. DOI:10.1007/s10584-019-02544-0 |
Bio, A.M.F., de Becker, P., de Bie, E., et al, 2002. Prediction of plant species distribution in lowland river valleys in Belgium: modelling species response to site conditions. Biodivers. Conserv., 11: 2189-2216. DOI:10.1023/A:1021346712677 |
Blach-Overgaard, A., Svenning, J.C., Dransfield, J., et al, 2010. Determinants of palm species distributions across africa: the relative roles of climate, non-climatic environmental factors, and spatial constraints. Ecography, 33: 380-391. DOI:10.1111/j.1600-0587.2010.06273.x |
Boria, R.A., Olson, L.E., Goodman, S.M., et al, 2014. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model., 275: 73-77. DOI:10.1016/j.ecolmodel.2013.12.012 |
Carneiro, L., Lima, A., Machado, R., et al, 2016. Limitations to the use of species-distribution models for environmental-impact assessments in the Amazon. PLoS One, 11: e0146543. DOI:10.1371/journal.pone.0146543 |
R.K. Chaturvedi, A.S. Raghubanshi, J.S. Singh, 2011. Plant functional traits with particular reference to tropical deciduous forests: a review. J. Biosci., 36: 963-981. DOI:10.1007/s12038-011-9159-1 |
Cron, G.V., Karimi, N., Glennon, K.L., et al, 2016. One African baobab species or two? A re-evaluation of Adansonia kilima. South Afr. J. Bot., 103: 312. DOI:10.1016/j.sajb.2016.02.036 |
C. Douie, J. Whitaker, I. Grundy, 2015. Verifying the presence of the newly discovered African baobab, Adansonia kilima, in Zimbabwe through morphological analysis. South Afr. J. Bot., 100: 164-168. DOI:10.1016/j.sajb.2015.05.025 |
B. Ebenman, T. Jonsson, 2005. Using community viability analysis to identify fragile systems and keystone species. Trends Ecol. Evol., 20: 568-575. DOI:10.1016/j.tree.2005.06.011 |
Ellison, A.M., Elliott, K., Kloeppel, B.D., et al, 2005. Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ., 3: 479-486. DOI:10.2307/3868635 |
G.N. Ervin, D.C. Holly, 2011. Examining local transferability of predictive species distribution models for invasive plants: an example with Cogongrass (Imperata cylindrica). Invas. Invasive Plant Sci. Manag., 4: 390-401. DOI:10.1614/IPSM-D-10-00077.1 |
J.M.Feng, 2008. Spatial patterns of species diversity of seed plants in China and their climatic explanation. Biodivers. Sci., 16: 470-476. DOI:10.3724/SP.J.1003.2008.08027 |
Fiser, C., Delic, T., Lustrik, R., et al, 2019. Niches within a niche: ecological differentiation of subterranean amphipods across Europe's interstitial waters. Ecography, 42: 1212-1223. DOI:10.1111/ecog.03983 |
Y. Fourcade, 2016. Comparing species distributions modeled from occurrence data and from expert-based range maps. The implication for predicting range shifts with climate change. Ecol. Inf., 36: 8-14. DOI:10.1016/j.ecoinf.2016.09.002 |
T.C. Giannini, A.M. Saraiva, I. Alves-Dos-Santos, 2010. Ecological niche modeling and geographical distribution of pollinator and plants: A case study of Peponapis fervens (Smith, 1879) (Eucerini: Apidae) and Cucurbita species (Cucurbitaceae). Ecol. Inf., 5: 59-66. DOI:10.1016/j.ecoinf.2009.09.003 |
A. Guisan, W. Thuiller, 2005. Predicting species distribution: offering more than simple habitat models. Ecol. Lett., 8: 993-1009. DOI:10.1111/j.1461-0248.2005.00792.x |
Huang, J., Yu, H., Dai, A., et al, 2017. Drylands face potential threat under 2 ℃ global warming target. Nat. Clim. Change, 7: 417-422. DOI:10.1038/nclimate3275 |
J.C. Ingram, T.P. Dawson, 2005. Climate change impacts and vegetation response on the island of Madagascar. Phil. Trans. R. Soc. A., 363: 55-59. DOI:10.1098/rsta.2004.1476 |
IPCC, 2014. Climate Change 2014: synthesis Report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change.
|
IUCN, 2020. IUCN Red List of Threatened Species. Version 2020.1. https://www.iucnredlist.org.
|
Jarnevich, C.S., Stohlgren, T.J., Kumar, S., et al, 2015. Caveats for correlative species distribution modeling. Ecol. Inf., 29: 6-15. DOI:10.1016/j.ecoinf.2015.06.007 |
H. Kreft, J.H. Sommer, W. Barthlott, 2006. The significance of geographic range size for spatial diversity patterns in Neotropical palms. Ecography, 29: 21-30. DOI:10.2307/3683492 |
P. Kumar, 2012. Assessment of the impact of climate change on Rhododendrons in Sikkim Himalayas using MaxEnt modeling: limitations and challenges. Biodivers. Conserv., 21: 1251-1266. DOI:10.1007/s10531-012-0279-1 |
S. Kumar, T.J. Stohlgren, 2009. MaxEnt Modeling for predicting suitable habitat for threatened and endangered tree Canacomyrica monticola in New Caledonia. J. Ecol. Nat. Environ., 1: 94-98. |
K.Lisao, C.J.Geldenhuys, P.W.Chirwa, 2018. Assessment of the African baobab (Adansonia digitata L.) populations in Namibia: Implications for conservation. Glob. Ecol. Conserv., 14: e00386. DOI:10.1016/j.gecco.2018.e00386 |
C.Liu, M.White, G.Newell, 2011. Measuring and comparing the accuracy of species distribution models with presence-absence data. Ecography, 34: 232-243. DOI:10.1080/j.1600-0587.2010.06354.x |
M.B.Mesgaran, R.D.Cousens, B.L.Webber, 2014. Here be dragons: a tool for quantifying novelty due to covariate range and correlation change when projecting species distribution models. Divers. Distrib., 20: 1147-1159. DOI:10.1111/ddi.12209 |
Mitchell, T.D., Carter, T.R., Jones, P.D., et al., 2004. A comprehensive set of high-resolution grids of monthly climate for Europe and the globe: the observed record (1901-2000) and 16 scenarios (2001-2100). Norwich, UK, Tyndall centre for climate change research.
|
Patrut, A., Patrut, R.T., Danthu, P., et al, 2016. AMS radiocarbon dating of large za baobabs (Adansonia za) of Madagascar. PLoS One, 11: e0146977. DOI:10.1111/ddi.12209 |
K. Pearson, 1920. Notes on the history of correlation. Biometrika, 13: 25-45. DOI:10.2307/2331722 |
Pettigrew F.R.S, J.D., Bell, K.L., Bhagwandin, A., et al, 2012. Morphology, ploidy and molecular phylogenetics reveal a new diploid species from Africa in the baobab genus Adansonia (Malvaceae: Bombacoideae). Taxon, 61: 1240-1250. DOI:10.1002/tax.616006 |
Petitpierre, B., Broennimann, O., Kueffer, C., et al, 2017. Selecting predictors to maximize the transferability of species distribution models: lessons from cross-continental plant invasions. Global Ecol. Biogeogr., 26: 275-287. DOI:10.1111/geb.12530 |
S.J.Philips, R.P.Anderson, R.E.Schapire, 2006. Maximum entropy modelling of species geographic distributions. Ecol. Model., 190: 231-259. DOI:10.1016/j.ecolmodel.2005.03.026 |
A.Radosavljevic, R.P.Anderson, 2014. Making better MaxEnt models of species distributions: complexity, over fitting, and evaluation. J. Biogeogr., 41: 629-643. DOI:10.1111/jbi.12227 |
Root, T.L., Price, J.T., Hall, K.R., et al, 2003. Fingerprints of global warming on wild animals and plants. Nature, 421: 57-60. DOI:10.1038/nature01333 |
A.C.Sanchez, P.E.Osborne, N.Haq, 2010. Identifying the global potential for baobab tree cultivation using ecological niche modeling. Agrofor. Syst., 80: 191-201. DOI:10.1007/s10457-010-9282-2 |
L.Schäffler, P.M.Kappeler, 2014. Distribution and abundance of the world's smallest primate, Microcebus berthae, in central western Madagascar. Int. J. Primatol., 35: 557-572. DOI:10.1007/s10764-014-9768-2 |
S.J.Sinclair, M.D.White, G.R.Newell, 2010. How useful are species distribution models for managing biodiversity under future climates?. Ecol. Soc., 15: 299-305. DOI:10.5751/ES-03089-150108 |
Tadross, M., Randriamarolaza, L., Rabefitia, Z., et al., 2008. Climate change in Madagascar; recent past and future. Tech. Rep., World Bank.
|
Vieilledent, G., Cornu, C., Cuni Sanchez, A., et al, 2013. The vulnerability of baobab species to climate change and effectiveness of the protected area network in Madagascar: towards new conservation priorities. Biol. Conserv., 166: 11-22. DOI:10.1016/j.biocon.2013.06.007 |
J.A.Wiens, N.E.Seavy, D.Jongsomjit, 2011. Protected areas in climate space: what will the future bring?. Biol. Conserv., 144: 2119-2125. DOI:10.1016/j.biocon.2011.05.002 |
Xu, Y., Shen, Z., Ying, L., et al, 2017. Hotspot analyses indicate significant conservation gaps for evergreen broadleaved woody plants in China. Sci. Rep., 7: 1859. DOI:10.1038/s41598-017-02098-0 |
Yu, F., Skidmore, A.K., Wang, T., et al, 2017. Rhododendron diversity patterns and priority conservation areas in China. Divers. Distrib., 23: 1143-1156. DOI:10.1111/ddi.12607 |
Y.Zeng, B.W.Low, D.C.J.Yeo, 2016. Novel methods to select environmental variables in MaxEnt: a case study using invasive crayfish. Ecol. Model., 341. DOI:10.1016/j.ecolmodel.2016.09.019 |
Zurell, D., Thuiller, W., Pagel, J., et al, 2016. Benchmarking novel approaches for modeling species range dynamics. Global Change Biol., 22: 2651-2664. DOI:10.1111/gcb.13251 |



