b. Departamento de Biología, Centro de Ciencias Básicas, Universidad Autónoma de Aguascalientes, Avenida Universidad 940, CP 20131, Aguascalientes, Mexico
Bouteloua aristidoides (Kunth) Griseb. (needle grama) is a grass species in subfamily Chloridoideae (Poaceae). The annual species is widely distributed in the deserts and other arid areas of southwestern USA, Mexico, and South America from sea level to ca. 2800 m in elevation. Its inflorescence is composed of 4–20 pendulous primary branches, each branch abscising at maturity with its 2–10 spikelets, the branch axis (rachis) extending as a point beyond the terminal spikelet node (Grisebach, 1864; Griffiths, 1912; Jones, 1912; Kearney and Peebles, 1960; Gould, 1980; Columbus, 1998; Herrera Arrieta et al., 2004).
In 1912, Jones described Bouteloua aristidoides var. arizonica M.E. Jones, which has a more restricted distribution (Arizona and New Mexico, USA; Sonora and possibly Durango, Mexico) than var. aristidoides. Gould (1980) claimed the presence of intermediates of these two taxa in sympatric populations. The two varieties have been distinguished using inflorescence branch length, number of spikelets per branch, and length of the branch axis extension. Compared to var. aristidoides, var. arizonica has longer branches (1.5–3.5 cm vs. < 1.6 cm), more spikelets per branch (5–10 vs. 2–5), and shorter branch axis extensions (1.5–7 mm vs. 6–10 mm) (Jones, 1912; Kearney and Peebles, 1960; Gould, 1980; Columbus, 1998; Wipff, 2003; Herrera Arrieta et al., 2004).
Grasses such as Bouteloua aristidoides commonly feature cleistogamy (Campbell et al., 1983), which refers to the production of closed self-pollinated flowers (cleistogamous flowers) and open flowers with the possibility of cross-pollination (chasmogamous flowers) in a plant species (Lord, 1981). Cleistogamous spikelets in B. aristidoides are frequent (Hackel, 1906; Columbus, 1998). In the field, we have not observed spikelets with exerted anthers or stigmas, an absence that indicates cleistogamy. However, Columbus (1998) reported dimorphic anther lengths from herbarium specimens representing both var. arizonica and var. aristidoides; furthermore, some plants of B. aristidoides he cultivated at Rancho Santa Ana Botanic Garden (RSABG) displayed varying numbers of chasmogamous spikelets having large anthers and stigmas. Therefore, chasmogamous spikelets may occur at low frequencies in natural populations.
Based on phylogenetic analyses of DNA sequences from the nuclear ribosomal internal transcribed spacer region (nrITS) and three predominantly noncoding chloroplast loci, Bouteloua aristidoides is strongly supported as sister to the narrowly distributed Bouteloua annua Swallen (Baja California Sur, Mexico) (Columbus, 1998; Columbus et al., 1998, Columbus et al., 2000; Peterson et al., 2015). In turn, this clade of two annual species is strongly supported as sister to the perennial clade of Bouteloua eriopoda (Torr.) Torr. + Bouteloua eriostachya (Swallen) Reeder. B. aristidoides was resolved as monophyletic in Columbus et al. (1998) and Peterson et al. (2015), who sampled more than one individual. Further, Peterson et al. (2015) included three samples of var. aristidoides, which also form a clade. However, no study has sampled more than one individual of var. arizonica, which means monophyly of this taxon has yet to be tested.
The difference in inflorescence form between the two varieties of Bouteloua aristidoides, which is even greater than that observed between other closely related species of Bouteloua Lag., led us to ask whether B. aristidoides var. arizonica and var. aristidoides are distinct species. To answer this question, we analyzed the distinguishing inflorescence characters of these varieties in a statistical framework, including principal component analysis and a micromorphological survey of the leaf blade. We also generated molecular phylogenies to provide context for morphological evaluation, by sequencing nuclear ITS and chloroplast rpoB, trnT-L-F, and trnC regions from a greatly expanded sampling of B. aristidoides, including multiple samples of var. arizonica. Plants of B. aristidoides var. arizonica were cultivated in greenhouse conditions, which allowed us to look for the presence of chasmogamous spikelets that indicate the viability of cross pollination with var.aristidoides.
2. Materials and methods 2.1. SamplingA total of 63 collections were used for the study complex, representing Bouteloua aristidoides var. aristidoides (33 total: 32 morphometrics, 7 leaf micromorphology, 18 DNA sequencing), B. aristidoides var. arizonica (21: 21, 4, 5), and outgroup species B. annua (6: 6, 2, 4), B. eriopoda (2: 1, 1, 1) and B. eriostachya (1: 1, 1, 1) (). For all the study complex taxa except B. eriopoda and B. eriostachya, collections from across their respective geographic ranges were selected. We included three collections from a sympatric population of var. aristidoides and var. arizonica in southern Arizona (at Patagonia Lake State Park). These collections represent each variety of B. aristidoides (Cuellar 46 and Columbus 2273, respectively) and one plant that appeared to be morphologically intermediate between the two varieties (Cuellar 44).
2.2. MorphometricsTwelve inflorescence characters (Appendix B) were selected for analysis, including three characters that have been used to distinguish the two varieties of Bouteloua aristidoides: inflorescence branch length, number of spikelets per branch, and length of the branch axis extension. All measurements were taken from herbarium specimens. On each specimen three mature inflorescences were arbitrarily selected from one or (if present) multiple individuals for measurement using millimetric paper under a stereoscopic microscope (see for details on the criteria used to measure each inflorescence character). The means of measured values were calculated (X = (X1+X2+X3)/3) and registered in Excel. All mean values were normalized (x – minimum value/Range of values) and used to create a matrix of morphological character states (Cuellar-Garrido L.F., Morphometry Matrix, Mendeley Data repository, 2020, https://doi.org/10.17632/2fzrv5sw58.1). The matrix was imported to the program NTSYSpc v.2.11T (Rohlf, 2000) for posterior creation of principal components analysis (PCA). Based on the obtained Eigen vector values (EVv), the five most informative characters (MICs) were used for statistical tests at R v.3.4.1 (R Development Core Team, 2017).
A one-way ANOVA (AV) test of each MIC variance was performed. Tukey's test plots were used to see the different associations of variance among taxa. To avoid creating type I or type II errors and the effects from violations of independence assumptions during the AV test (Scariano and Davenport, 1987), data sets were first tested for normal distribution and homoscedasticity with the Shapiro Wilk test (Shapiro and Wilk, 1965) and the Bartlett's homoscedasticity (Bartlett, 1937) test. For data that was not distributed normally or for which the homoscedasticity principle was not achieved (p < α), the Kruskall–Wallis (KW) test (Lantz, 2013) and the Welch (We) test (Welch, 1951) were used in addition to the AV. Atypical data were not included. The confidence level was set at α = 0.05 for all statistical tests.
2.3. Micromorphological analysisOf the 15 samples utilized for leaf micromorphology, 12 were fixed and preserved in FPA (1:1:18 37% formaldehyde: propionic acid: 70% ethanol) from living plants in the field or greenhouse, whereas the remaining 3 samples were removed from pressed and dried herbarium specimens. Middle sections of mature leaf blades were fixed for at least 72 h with a 50% ethanol-FPA. Posterior dehydration of the material was achieved by exposing the plant tissue through a series of increasing concentrations of ethanol (70%, 90%, 95% and 100%) and critical point dried with liquid CO2 at a high pressurized Pelco chamber. The dehydrated plant material was mounted on scanning electron microscope (SEM) stubs and sputter coated with gold at an argon based Pelco Sc-7 sputter coater. Imaging of the abaxial and adaxial leaf structures was made with a SEM (ISI brand, model WB-6).
2.4. Molecular analysisDNA was extracted from leaf material preserved in silica gel from 29 of the 63 study complex collections with a CTAB 2% protocol according to Doyle and Doyle (1987). Intergenic molecular regions from plastid (trnC-rpoB, trnT-L, and trnL-F) and nuclear (ITS) DNA was amplified on a Biometra TGradient thermocycler using a Phusion (BioLabs) polymerase kit. Because trnC-rpoB sequences have not previously been used in this genus, we only used molecular sequences (ITS/trnT-L-F) available at GenBank from 30 collections (Appendix A) representing 19 species (outside the study complex). For details on primer sequences and thermocycler conditions please refer to. Polymerase chain reaction (PCR) products were cleaned through a one-step precipitation of templates with polyethylene glycol (PEG) at 20% in 2.5M NaCl. A BigDye v.3.1 (Thermofisher) fluorescent marker was used during the double strand cycle sequencing of the clean PCR products. Cycle sequencing products were cleaned with hydrated Sephadex G-50 medium (SigmaAldrich) and sequenced at an ABI 3130xl sequencer. Second strand DNA sequences were reversed and aligned with their complementary first strand DNA at Geneious v10.2.2 (Kearse et al., 2012). Nucleotide consensus sequences from every sample were used to construct each separate and combined molecular marker matrix (trnT-L-F, trnC-rpoB, ITS, trnT-L-F/trnC-rpoB, trnT-L-F/ITS and trnT-L-F/trnC-rpoB/ITS). Maximum likelihood RAxML (Stamatakis, 2014) analyses of every matrix was performed at Geneious under the GTR Gamma nucleotide model and 1000 Bootstrap replicates. A 50% majority rule consensus tree was formed for each analysis.
The combined trnT-L-F/trnC-rpoB/ITS matrix was treated separately at jModeltest v.2.1.10 (Posada, 2008), for which the models TPM1uf and JC were respectively best scored for the plastid and nuclear data under the corrected Aikaike information criterion (AICc). Afterwards, the matrix was used for a Bayesian phylogenetic analysis with MrBayes v.3.1.1 (Huelsenbeck and Ronquist, 2001). Each data set was partitioned and customized to fit the best score models obtained with jModeltest. Parameters used at MrBayes were as follows: four Markov chains with 3, 000, 000 generations, a tree sample frequency of 500, and a 50% burn-in. A 50% majority rule consensus tree was formed for posterior probability calculations. Sequences of the three molecular markers for Bouteloua eriopoda (Columbus 2461) and B. eriostachya (Columbus 2843) were used as outgroups. An additional ITS Bayesian tree was constructed in the same manner as described above for the nuclear data set.
2.5. Ancestral state reconstruction analysisThe combined chloroplast-nuclear Bayesian 50% majority rule consensus molecular tree was used as a tree block in Mesquite v.3.61 (Maddison, 2008) to reconstruct the evolution of the MICs. The taxa and MIC- coded variable blocks were respectively constructed according to the tree and shared samples from the matrix of morphological character states. Characters with continuous data were organized into three categories according to the range of the characters ((maximum value – minimum value)/3). Maximum likelihood analyses of the ancestral reconstruction states of the characters were performed in the program under the Mk1 model with help of the "Trace Character History" command.
2.6. Cleistogamy testSeeds of Bouteloua aristidoides var. arizonica picked from five collections (Siqueiros 5216–5220) from Sonora (México) were cultivated in the greenhouse facilities at RSABG for a period of two years. Spikelets were visually monitored on a daily basis throughout their natural maturation cycle to look for the presence of exerted anthers.
3. Results 3.1. Taxonomic treatmentBouteloua arizonica (M.E.Jones) L.F.Cuellar & Columbus, stat. nov. BASIONYM: Bouteloua aristidoides var.arizonica M.E.Jones., Contrib.W.Bot.14, 13.1912.TYPE: U.S.A.Arizona: Pima Co., Tucson, 2400 ft, 2 September 1903, Thornber 177(holotype: RSA!; isotypes: MO!, NY!, US!).
3.2. DiagnosisBouteloua arizonica differs from B. aristidoides in having inflorescence branches with five or more spikelets, each branch measuring 1.6–2.7 cm long, a branch extension equal or less than 6 mm long and having papillae on the adaxial surface of the leaf blades. B. arizonica differs from B. annua in having branches without terminal spikelet (non-truncated), a bigger branch extension (4–6 mm long) and having adaxial papillae on the leaf blades.
3.3. DescriptionAnnuals, culms 10–30 cm long, tufted at base, erect or geniculate. Sheaths shorter than the internodes, glabrous. Leaves short, scarce. Ligules 0.5 mm or less, ciliate. Inflorescence 5–20 cm long with 5–10 branches. Branches 1.6–2.7 cm long, extending 3.9–6.2 mm beyond the insertion of the terminal spikelet. Curved at maturity. Spikelets 0.5–0.8 cm long, 5–10 per branch. Lemmas 4–6 mm long. Paleas 3–4.5 mm long. 1st glume 2–4 mm long. 2nd glume 4–6 mm long. Caryopses 2–3 mm long.
Distribution and habitat - Arizona, southern Nevada, northern Sonora and possibly Durango (Mexico) (Fig. 1). Grows on dry mesas, plains and washes, at elevations from 1000 to 1250 m. Flowers in summer.
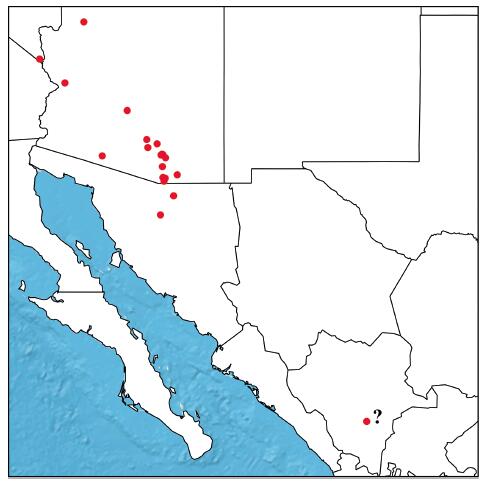
|
| Fig. 1 Distribution map of Bouteloua arizonica. Question mark = only known collection of Bouteloua arizonica in Durango, México. This collection was made by E. Palmer (717) in 1896 and it is unknown if the population is still extant. |
The sum of the Eigen values for the first three components (Co) explain 73.6% of the matrix variance (Co1 = 37.1%, Co2 = 21.7%, Co3 = 14.8%). The MICs include the number of spikelets per branch (EVv of 0.695 at the Co1), length of caryopsis (EVv of 0.672 at the Co2), number of branches per inflorescence (EVv of 0.657 at the Co2), branch length (EVv of 0.867 at the Co3), and branch extension (EVv of 0.446 at Co2 and 0.532 at Co3) (Fig. 2). The plot of the Co1 vs the Co2 (Fig. 3) clearly forms four different groups, one delimited by members of B. aristidoides var. arizonica (hereafter called AZ), two delimited by members of B. aristidoides var. aristidoides (hereafter AR), and one disperse group composed by B. annua (hereafter AN) members. The AZ group is located between the AR and AN groups, while the small group of AR is composed in its totality by specimens collected in Baja California Sur (hereafter BCS) (Columbus 4648; Siqueiros 5280, 5281, 5283).
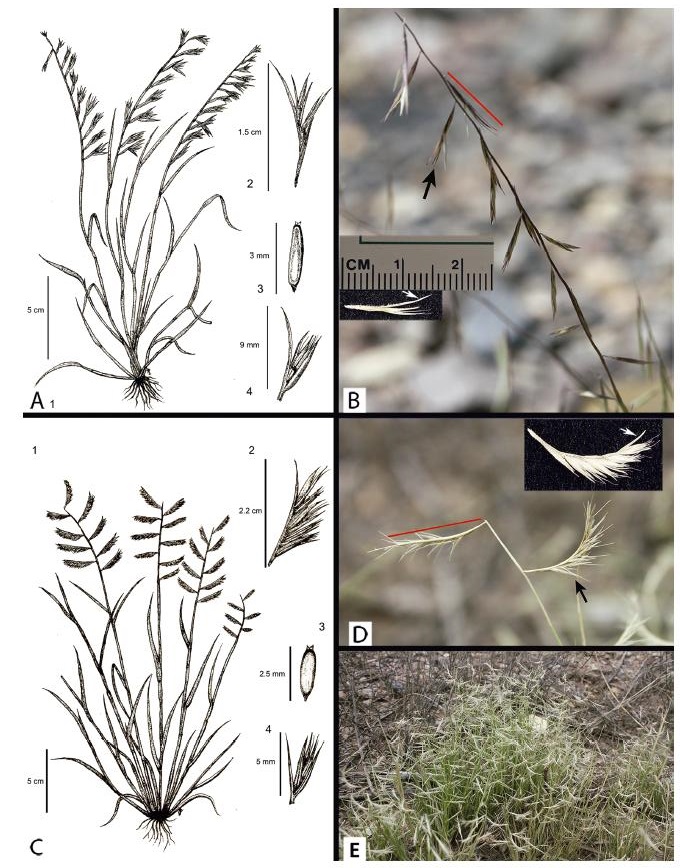
|
| Fig. 2 Most informative characters of Bouteloua aristidoides varieties. A. Illustration of var. aristidoides. B. Inflorescence of var. aristidoides. C. Illustration of var. arizonica. D. Inflorescence of var. arizonica. E. var. arizonica in its natural habitat. 1: Number of branches per inflorescence. 2: Branch length and number of spikelets per branch. 3: Caryopsis length. 4: Branch extension. Black arrow: Branch spikelet. Red bar: Inflorescence Branch. White arrow: Branch extension. |
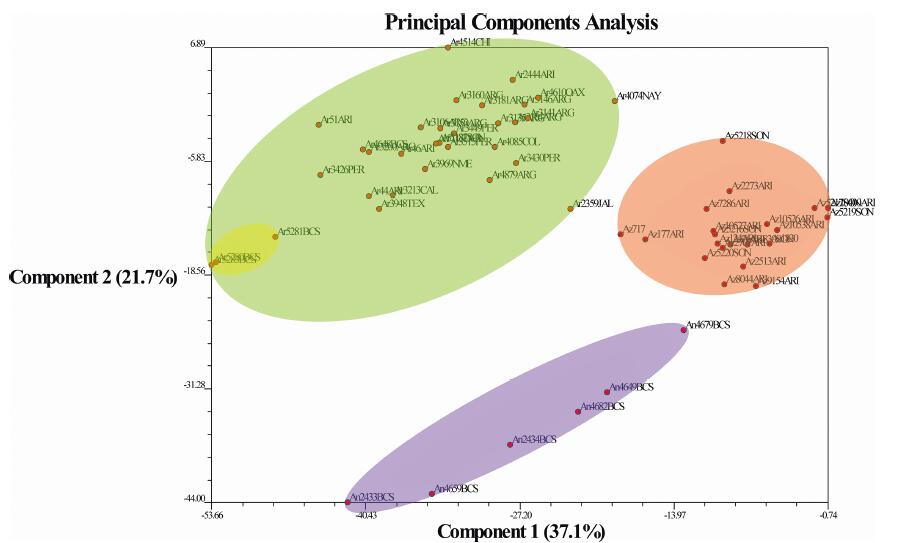
|
| Fig. 3 PCA of the 12 morphological characters measured for each specimen. X axis = Component 1 vs. Y axis = Component 2 (eigen values for each component is depicted in parenthesis). Blue oval = B. annua. Red oval = var. arizonica. Green oval = var. aristidoides. Yellow oval = Subgroup of var. aristidoides with specimens from Baja California Sur. Red dots = each individual population measured, followed of the taxa acronym, collection number, and place of collection acronym (ARG = Argentina; ARI = Arizona; BCS = Baja; California Sur; CAL = California; CHI = Chihuahua; COL = Colima; JAL = Jalisco; NAY = Nayarit; NME = New Mexico; OAX = Oaxaca; PER = Perú; SON = Sonora; TEX = Texas). |
AR is clearly differentiated from AZ and AN in having fewer spikelets per branch (AV p = 2.2e−16; KW p = 8.504e−11; We p = 1.23e−9), an increased number of branches per inflorescence (AV p = 4.799e−12; We p = 8.479e−8), a bigger caryopsis (AV p = 1.857e−8; KW p = 1.111e−6), a smaller branch length (AV p = 3.669e−2; KW p = 1.788e−4; We p = 2.025e−2), and an increased length of the branch extension (AV p = 1.086e−8; KW p = 9.667e−11; We p = 3.451e−7). In addition, AN is differentiated from AZ by having a smaller range of spikelets per branch and a smaller branch extension (Fig. 4).
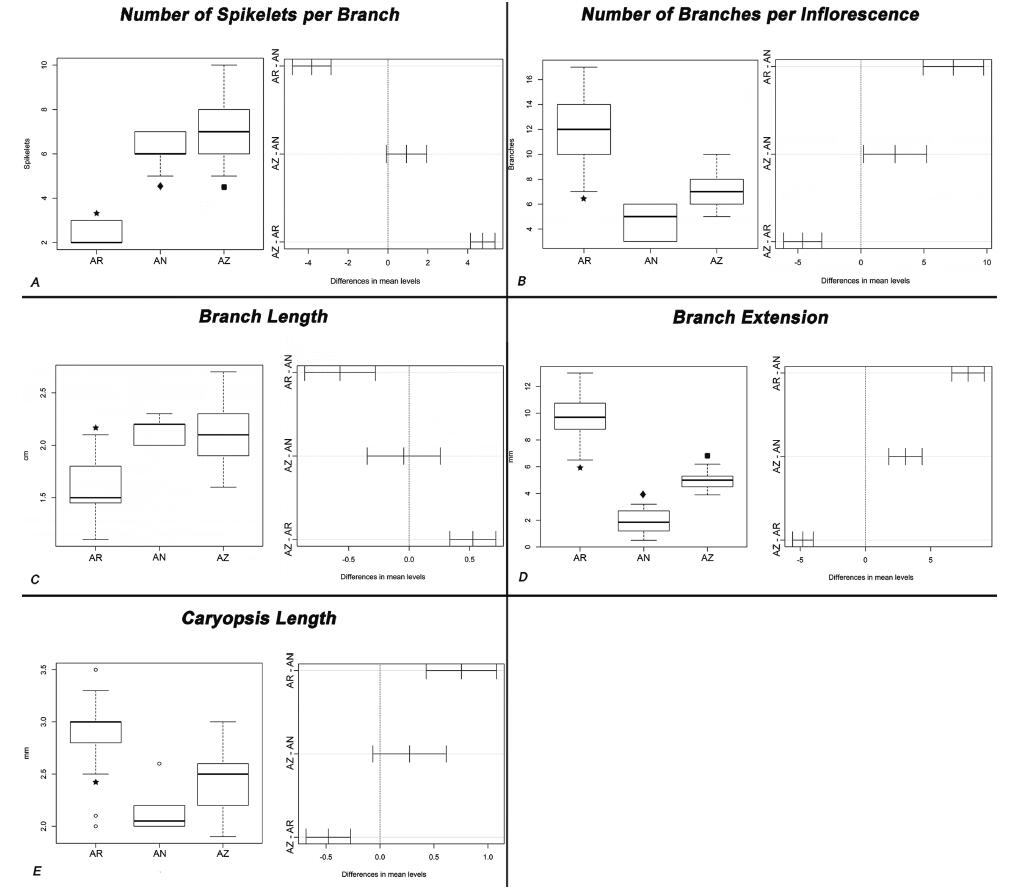
|
| Fig. 4 Box plot (left) and Tukey's test plot (right) for each most informative character statistical test of variance. A. Number of spikelets per branch. B. Number of branches per inflorescence. C. Branch length. D. Branch extension. E. Caryopsis length. AR = B. aristidoides. AZ = B. arizonica. AN = B. annua. Star = variance of AR is statistically different from variances of AZ and AN. Diamond = variance of AN is statistically different from AZ and AR variances. Square = variance of AZ is statistically different from variances of AR and AN. α = 0.05 for all the statistical tests. |
AZ was the only studied taxa to have papillae on cells of the adaxial side of the leaf. Specifically, this trait is found in bulliform cells at margins of the intercostal zone, and in interstomatal and long cells of the costal zone. Each of these cells bears a single papilla positioned at distal sides (Fig. 5).
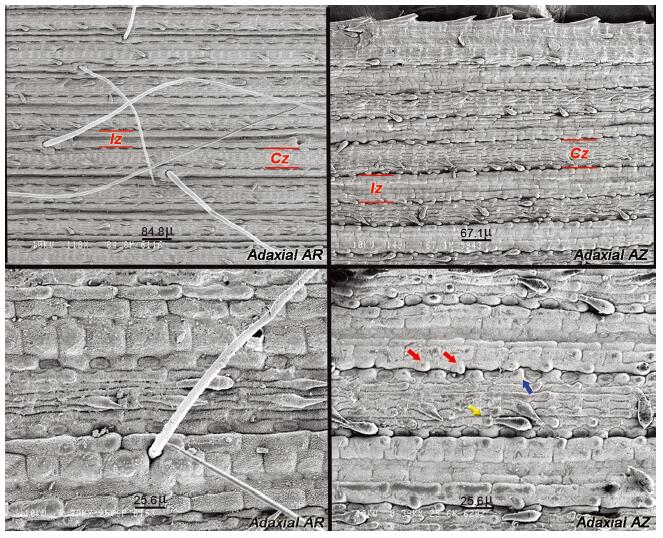
|
| Fig. 5 SEM pictures of the middle section of mature leaf blades of var. aristidoides and var. arizonica. Upper left, adaxial side of leaf var. aristidoides at 118x (Siqueiros 5283). Upper right, adaxial side of leaf of var. arizonica at 149x (Columbus 2273). Lower left, zoom at 390x of upper left image. Lower right, zoom at 390x of upper right image. Red bars = delimitation of the costal zone (Cz) and intercostal zone (Iz) of cells. AR = var. aristidoides; AZ = var. arizonica; Red arrows = papilla at each bulliform cell at the margin of the intercostal zone; Blue arrow = papilla at interstomatal cell at margin of the costal zone; yellow arrow = papilla at long cell of the costal zone. |
Except from the ITS tree, each study complex taxon (i.e., AN, AR, and AZ) is depicted as monophyletic in all the Maximum Likelihood chloroplast trees (trnT-L-F, trnC-rpoB, chloroplast combined trnT-L-F/trnC-rpoB), combined chloroplast-nuclear trees, (trnT-L-F/ITS and trnT-L-F/trnC-rpoB/ITS) (Figs. 6 and S1) and the Bayesian combined chloroplast-nuclear tree (Fig. 7). AZ and AR are sister, whereas AN is represented as an earlier divergent lineage of the other two. Good bootstrap values and posterior probabilities at each node of all the combined trees (Figs. 6 and 7) and trnC-rpoB tree (Fig. S1) support this topology. Each of the sympatric populations of AZ and AR from the Patagonia Lake State Park (Columbus 2273; Cuellar 46) fits within their respective species clade in these trees (Figs. 6 and 7 and S1). The phylogenetic tree-branch of the AZ group is conspicuously bigger than any other tree-branches formed by AR groups at the Bayesian ITS tree (Fig. 6).
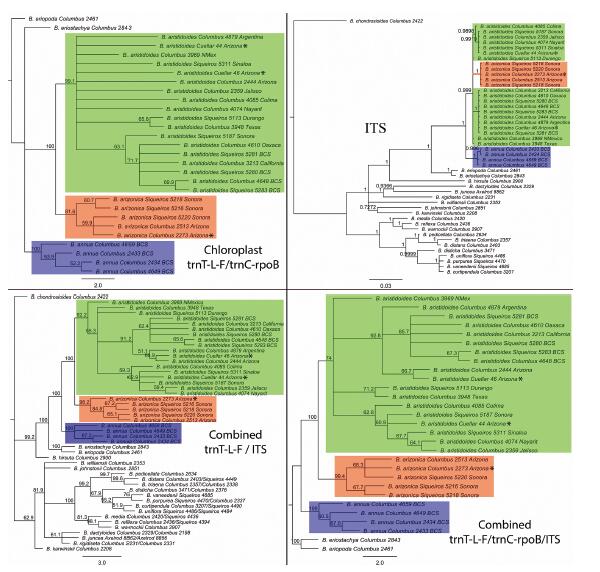
|
| Fig. 6 Phylogeny of the Bouteloua annua – B. aristidoides clade based on the 50% majority-rule consensus trees from the Maximum Likelihood phylogenetic analyses of the chloroplast (trnC-rpoB/trnT-L-F), combined trnT-L-F/ITS, combined trnT-L-F/trnC-rpoB/ITS and the Bayesian phylogenetic analysis of the nuclear ribosomal (ITS) molecular data. Bootstrap values and posterior probabilities are depicted at each node. Green square = group of var. aristidoides. Red square = group of var. arizonica. Blue square = group of B. annua. Asterisk = sympatric populations of var. aristidoides and var. arizonica at the Patagonia Lake State Park (Arizona). Information following taxon names refer to voucher information (Appendix A) and place of collection. Red bar at ITS tree denotes a conspicuously big tree-branch of the var. arizonica group. |
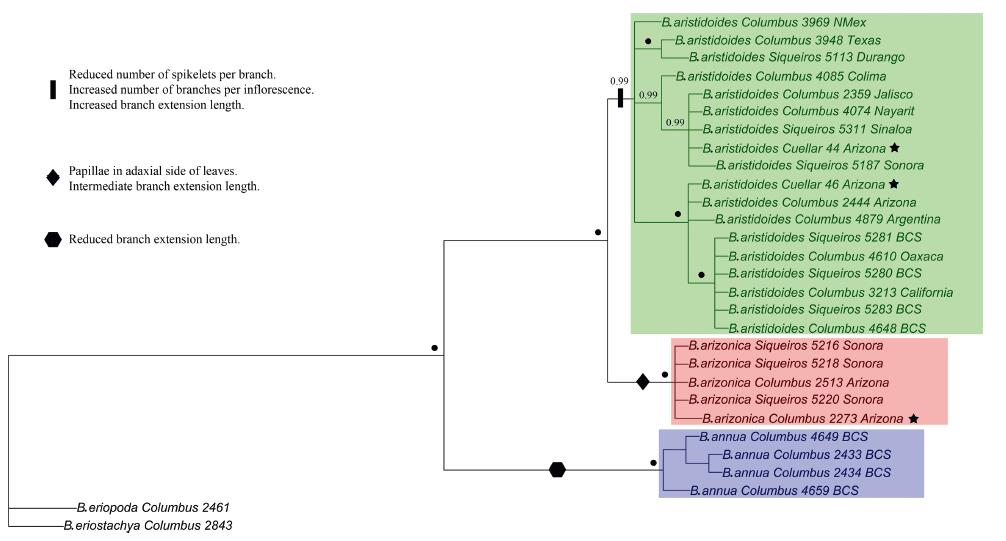
|
| Fig. 7 Mapping of the ancestral state reconstruction analyses for the most informative characters of the Bouteloua annua – B. aristidoides clade at the 50% majority-rule consensus tree from the Bayesian phylogenetic analysis of the concatenated chloroplast (trnC-rpoB, trnT-L-F) and nuclear ribosomal (ITS) molecular data. Posterior probabilities of 1 are depicted with a black circle at each node. Green square = group of var. aristidoides. Red square = group of var. arizonica. Blue square = group of B. annua. Black bar, diamond, and hexagon symbols denote the mapped characters for each group. Star = sympatric populations of var. aristidoides. and var. arizonica at the Patagonia Lake State Park (Arizona). Information following taxon names refer to voucher information and place of collection (Appendix A). |
Phylogenetic structure was found inside the monophyletic AR trees due to formation of different clades inside the group. Moreover, the alleged intermediate form from sympatric populations of AR and AZ (Cuellar 44) align inside the monophyletic AR clade trees, and in a separate subclade from its sympatric AR population (Cuellar 46) in the combined chloroplast-nuclear trees (Figs. 6 and 7).
3.8. Ancestral state reconstruction analysesAnalyses indicate that AR has the following synapomorphies: reduced number of spikelets per branch, an increased number of branches per inflorescence, and an increased branch extension length. Reduced branch extension length is synapomorphic for AN. For the monophyletic clade of AZ, the presence of papillae in the adaxial side of leaves is synapomorphic. Results for the character of branch length were inconclusive (Figs. 7 and S2).
3.9. Cleistogamy testNo exerted anthers were found in spikelets of AZ collections grown under greenhouse conditions during the span of two years.
4. DiscussionThe treatment on Bouteloua by Gould (1980) represents one of the most extensive and detailed work for taxonomic consultation on this genus. In terms of the circumscription of B. aristidoides, Gould (1980) acknowledged the existence of AZ and made the following statement based on personal observations: "in its extreme form, AZ differs strikingly from AR, but the two varieties intergrade freely". However, based on the results presented in this paper we differ from Gould (1980) and Jones (1912) on including AZ as part of the B. aristidoides circumscription. Moreover, we present the likely scenario of existing undescribed varieties in B. aristidoides (different from AZ), which Gould (1980) probably thought were intermediates of AR and AZ. We elaborate on these ideas in the following paragraphs.
PCA (Fig. 3) and molecular analyses (except the ITS tree) depict AR and AZ as separate groups, even in sympatric populations (Figs. 6 and 7 and S1). In addition, the micromorphology of leaves (Fig. 5), morphology statistical tests (Fig. 4) and ancestral state reconstruction analyses (Figs. 7 and S2) expand some of the specific characters that distinguish each group. AZ contains papillae on the adaxial side of leaves as a synapomorphy (Fig. 5, Fig. 7 and S2). Has from 5 to 10 branches per inflorescence. Each branch measures from 1.6 to 2.7 cm long, extending 3.9–6.2 mm beyond the insertion of the terminal spikelet, and bears 5 to 10 spikelets. In contrast, AR has 7 to 17 branches per inflorescence. Each branch in AR is 1.1–2.1 cm long, extending 6.5–13 mm beyond the insertion of the terminal spikelet, and bears 2 to 3 spikelets (Figs. 4 and S2). In very rare occasions, an atypical apical branch with 4 spikelets can be found in AR. Also, AR caryopsis tends to be bigger than in AZ.
Peterson et al. (2015) described how chloroplast molecular markers give more structure than ITS in Bouteloua and in general how chloroplast-nuclear combined data give phylogenies with better resolution and backbone structure for this genus. Our results are in accord to these findings. Moreover, although ITS did not show the monophyly of AZ in this study, the Bayesian tree of this marker (Fig. 6) shows a conspicuously big tree-branch for this group. The length of this phylogenetic tree-branch relates to the number of molecular differences between AZ and the other AR groups formed. Most likely, the inclusion of another nuclear molecular marker (like ETS) in addition to ITS will provide additional nucleotide synapomorphies that will show the monophyly for the nuclear evolutionary history of AZ (Baldwin and Markos, 1998).
With the exception of the ITS tree, the alleged intermediate forms from sympatric populations of AR and AZ at Patagonia Lake State park (Cuellar 44) align inside the monophyletic AR clades in all molecular trees (Figs. 6 and 7 and S1), and its morphological variance matches other AR populations that are not in sympatry with AZ (Fig. 3). Therefore, individuals of this population should be treated as AR and not as an intermediate between AR and AZ.
We recognize that complex biological processes like lineage sorting can mask events of introgressive hybridization (Buckley et al., 2006), for which the inclusion of even more molecular markers and cloning of ITS to search for multiple molecular copies representing AR and AZ in this study would have been ideal. However, no single nucleotide shared-polymorphisms were detected in our current molecular data from sympatric populations, indicating that hybridization events have not occurred. In addition, even if Bouteloua aristidoides is previously known for having very small rates of chasmogamous spikelets (Columbus, 1998), AZ never presented exerted anthers in the field or in greenhouse conditions. The collections of AZ grown in the facilities of the RSABG for a period of two years produced only cleistogamous spikelets with very small anthers (0.5 mm) that contain a small number of mature pollen mother cells. Columbus (1998) never explicitly or implicitly states in which variety he observed chasmogamous spikelets; however, our results suggest he solely referred to AR specimens. We cannot assert that AZ is strictly cleistogamous, but the current observations propose a strong correlation with this condition. Hence, gene flow between AR and AZ seems unlikely.
Based on the morphological and molecular evidence in our study, coupled with the lack of convincing evidence for gene flow between AR and AZ, we conclude that Bouteloua aristidoides var. arizonica should be elevated to the rank of species. Therefore, we propose B. arizonica (M.E. Jones) L.F. Cuellar & Columbus, stat. nov.
In regard to the existence of undescribed varieties of Bouteloua aristidoides (not to confuse B. arizonica as a variety of B. aristidoides hereafter), our results show high molecular and morphological variability among individuals of B. aristidoides. Specifically, populations from BCS form a subgroup inside B. aristidoides in the PCA (Fig. 3). This subgroup has an exceedingly atypical branch length (2.4–3.6 cm long) and branch extension from the terminal spikelet (15–28 mm long), and forms a clade (Columbus 4648; Siqueiros 5280, 5281, 5283) along with Oaxaca (Columbus 4610) and California (Columbus 3213) populations in most of the molecular trees (Figs. 6 and 7 and S1). However, PCA does not group BCS individuals with the Oaxaca and California populations (Fig. 3). Moreover, although the present data are indicative of intraspecies variability, the presence of polytomies inside the monophyletic B. aristidoides in our phylogenetic trees makes it difficult to draw further conclusions about the circumscription of varieties in this group. Future phylogenetic works employing next generation sequencing techniques of a wider number of samples of B. aristidoides are encouraged to resolve these relationships.
As our data show, the great morphological diversity of members of Bouteloua aristidoides occurs in all populations across the taxon distribution (Fig. 3) and is not limited to places where it co-occurs with B. arizonica. One possible explanation for the molecular and morphological diversity in B. aristidoides is the increase of the spikelet branch extension morphology (hence the common name Needle grama), which has been previously reported to be involved in the successful wide-range distribution of the species, due to the role it plays in epizoochory events (Columbus, 1998). The extensive geographic distribution of B. aristidoides would aid in the creation of different isolated genotypes leading to novel morphological evolution.
5. ConclusionResults (except ITS tree) support elevation of Bouteloua aristidoides var. arizonica to species rank and depict papillae on the adaxial side of leaf blades as a synapomorphy. Thus, a new combination B. arizonica (M.E. Jones) L.F. Cuellar & Columbus is proposed.
The chloroplast-nuclear molecular trees describe the most accurate phylogenies of the study complex. These are strongly supported and show phylogenetic structure within Bouteloua aristidoides (different from B. arizonica). However, current polytomies make it difficult to draw further conclusions for intraspecies circumscription.
The morphology of inflorescence in Bouteloua aristidoides varies across and among its distribution. The phylogenetic trees (except the ITS tree) and PCA results support the inclusion of populations with different morphologies between sympatric populations of B. aristidoides and B. arizonica as members of B. aristidoides. Cultivated plants of B. arizonica with strict cleistogamous spikelets suggest lack of gene flow with B. aristidoides.
Author contributionsLuis Fernando Cuellar-Garrido wrote the paper, performed the morphometrics, cleistogamy test, and micromorphological/molecular analyses. María Elena Siqueiros-Delgado performed the ancestral state reconstruction analyses, revised this paper and supervised all other analyses.
Declaration of competing interestThe authors declare no conflict of interest.
AcknowledgmentsThe authors acknowledge to the Consejo Nacional de Ciencia y Tecnología (Mexico) for its support to Luis Fernando Cuellar-Gar- rido with the master's degree scholarship 615539.We thank the Howard and Phoebe Brown Endowed Fellowship, the RSABG/Claremont Graduate University (CGU), and the Universidad Autonoma de Aguascalientes (UAA) financial support.Thanks to J.Travis Columbus (RSABG/California Botanic Garden) for his contributions on the realization of this study, which include advise in the experimental design, teaching on some of the methods, expertise in the study group, fieldwork help, facilitation of resources (vouchers and samples), revision of an early draft of this manuscript and being the first one to verbally address the possibility of var.arizonica being a species and for which, he is acknowledged in the co-authorship of the name B.arizonica.Thanks to Juvenal Aragon Parada for making illustrations of Fig. 2.Special thanks to the anonymous editors and to Paul M.Peterson and additional anonymous reviewers for their constructive criticisms and useful suggestions which helped to greatly improve this paper.
Supplementary data: Appendix A and C, Fig. S1 and Fig. S2Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2020.09.009.
、AppendicesAppendix B. List and measurement description of characters used for PCA
1.Inflorescence length (cm): from tip of the inflorescence to point of emersion from the sheath.2.Number of branches per inflorescence.3.Branch length (cm): from the tip of the branch to point of attachment to the inflorescence axis.4.Number of spikelets per branch.5.Branch extension length (cm): from tip of the branch to point of attachment of the most distal spikelet.6.Spikelet length (cm): from the tip of the spikelet to point of attachment to the branch axis.7.First glume length (mm).8.Second glume length (mm).9. Proximal lemma length (mm).10.Palea length (mm).11.Caryopsis length (mm).12.Awn length: from the tip of the most distal point of extension of the awn to the point of attachment to the rachilla.
Baldwin B.G., Markos S., 1998. Phylogenetic utility of the external transcribed spacer (ETS) of 18S-26S rDNA: Congruence of ETS and ITS trees of Calycadenia(Compositae). Mol. Phylogenet. Evol, 10: 449-463. DOI:10.1006/mpev.1998.0545 |
Bartlett M.S., 1937. Properties of sufficiency and statistical tests. Proc. R. Soc. London, Ser. A, 901: 268-282. |
Buckley T.R., Cordeiro M., Marshall D.C., et al, 2006. Differentiating between hypotheses of lineage sorting and introgression in New Zealand alpine cicadas(Maoricicada Dugdale). Syst. Biol, 55: 411-425. DOI:10.1080/10635150600697283 |
Campbell C.S., Quinn J.A., Cheplick G.P., et al, 1983. Cleistogamy in grasses. Annu.Rev. Ecol. Systemat, 14: 411-441. DOI:10.1146/annurev.es.14.110183.002211 |
Columbus J.T., 1998. Morphology and leaf blade anatomy suggest a close relationship between Bouteloua aristidoides and B. (Chondrosium) eriopoda (Gramineae: Chloridoideae). Syst. Bot, 24: 467-478. |
Columbus J.T., Kinney M.S., Pant R., et al, 1998. Cladistic parsimony analysis of internal transcribed spacer region (nrDNA) sequences of Bouteloua and relatives(Gramineae: Chloridoideae). Aliso, 17: 99-130. DOI:10.5642/aliso.19981702.03 |
Columbus, J.T., Kinney, M.S., Siqueiros-Delgado, M.E., et al., 2000. Phylogenetics of Bouteloua and relatives (Gramineae: Chloridoideae): cladistic parsimony analysis of internal transcribed spacer (nrDNA) and trnL-F (cpDNA) sequences. In: Jacobs, S.W., Everett, J. (Eds. ), Grasses: Systematics and Evolution. CSIRO PUBLISHING, Melbourne, pp. 189-194.
|
Doyle J.J., Doyle J.L., 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull, 19: 11-15. |
Gould F.W., 1980. The genus Bouteloua (Poaceae). Ann. Mo. Bot, 66: 348-416. |
Griffiths D., 1912. The grama grasses: Bouteloua and related genera. Contr. Nat.Herb, 14: 396-398. |
Grisebach, A., 1864. Flora of the British West Indian Islands. Reeve & Company, London.
|
Hackel E., 1906. Uber kleistagamie bei den grasern. Oesterr. Bot. Z, 56: 143-154. DOI:10.1007/BF01791796 |
Herrera Arrieta, Y., Peterson, P.M., Cerda Lemus, M., 2004. Revisión de Bouteloua Lag. (Poaceae), Politecnico Nacional, CⅡDIR y Comisión Nacional para el Con-ocimiento y Uso de la Biodiversidad, Durango.
|
Huelsenbeck J.P., Ronquist F., 2001. MRBAYES: bayesian inference of phylogenetic trees. Bioinformatics, 17: 754-755. DOI:10.1093/bioinformatics/17.8.754 |
Jones M.E., 1912. New species and notes. Contr. W. Bot, 14. |
Kearney, T.H., Peebles, R.H., 1960. Arizona Flora. Univ. of California Press, California.
|
Kearse M., Moir R., Wilson A., et al, 2012. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28: 1647-1649. DOI:10.1093/bioinformatics/bts199 |
Lantz B., 2013. The impact of sample non-normality on ANOVA and alternative methods. Br. J. Math. Stat. Psychol, 66: 224-244. DOI:10.1111/j.2044-8317.2012.02047.x |
Lord E.M., 1981. Cleistogamy: a tool for the study of floral morphogenesis, function and evolution. Bot. Rev, 47: 421-449. DOI:10.1007/BF02860538 |
Maddison W.P., 2008. Mesquite: a modular system for evolutionary analysis. Evolution, 62: 1103-1118. DOI:10.1111/j.1558-5646.2008.00349.x |
Peterson P.M., Romaschenko K., Herrera Arrieta Y., 2015. Phylogeny and subgeneric classification of Bouteloua with a new species, B. herrera-arrietae(Poaceae: Chloridoideae: Cynodonteae: Boutelouinae). J. Systemat. Evol, 53: 351-366. DOI:10.1111/jse.12159 |
Posada D., 2008. jModelTest: phylogenetic model averaging. Mol. Biol. Evol, 25: 1253-1256. DOI:10.1093/molbev/msn083 |
R Development Core Team, 2017. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
|
Rohlf, F.J., 2000. NTSYS-PC: Numerical Taxonomy and Multivariate Analysis System, Version 2.11T. Exeter Software, New York.
|
Scariano S.M., Davenport J.M., 1987. The effects of violations of independence assumptions in the one-way ANOVA. Am. Statistician, 41: 123-129. |
Shapiro S.S., Wilk M.B., 1965. An analysis of variance test for normality (complete samples). Biometrika, 65: 591-611. |
Stamatakis A., 2014. RAxML version 8:a tool for phylogenetic analysis and postanalysis of large phylogenies. Bioinformatics, 30: 1312-1313. DOI:10.1093/bioinformatics/btu033 |
Welch B.L., 1951. On the comparison of several mean values: an alternative approach. Biometrika, 38: 330-336. DOI:10.1093/biomet/38.3-4.330 |
Wipff, J.K., 2003.17.46 Bouteloua Lag. In: Barkworth, M.E., Capels, K.M., Long, S., et al., (Eds. ), Flora of North America North of Mexico Vol. 25, Magnoliophyta: Commelinidae (In Part): Poaceae, Part 2. Oxford University Press, New York, pp. 250-271.
|



