b. Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming, Yunnan, 650201, China;
c. Center of Economic Botany, Core Botanical Gardens, Chinese Academy of Sciences, Menglun, Mengla, Yunnan, 666303, China;
d. University of Chinese Academy of Sciences, Beijing, 100049, China
Nervonic acid (NA, cis-15-tetracosenoic acid) is a very long-chain monounsaturated fatty acid. It has been shown to be a core component of nerve fibers and nerve cells, and an essential fatty acid for brain growth and development (Sargent et al., 1994). Nervonic acid can be used to treat and prevent brain diseases, such as psychiatric disorders, cognitive impairment and Zellweger syndrome (Tanaka et al., 2007; Amminger et al., 2012). In addition, studies have shown that NA inhibits HIV-1 RT activity in a dose-dependent manner (Nobuyuki et al., 2008) and can be used to treat demyelinating disorders (Sargent et al., 1994).
Nervonic acid can be extracted from animal and plant tissues. Animal NA is mainly obtained from marine organisms, but in very small amounts. Consequently, commercially available NA is mainly derived from plants. To date, NA has been detected in the seeds of many plant species, although in most species NA represents, less than 5% of total seed oil content (http://sofa.mri.bund.de/). High levels of NA have been detected in relatively few plant species, such as Cardamine graeca L. (46%), Macaranga adenantha Gagnep (56%) and Malania oleifera Chun et S. Lee ex S. Lee (40%-67%) (Ou, 1981; Zhou et al., 2001; Ma et al., 2004; David et al., 2009). However, due to yield and oil quality issues, commercially available sources of NA mainly come from the seed oil of Acer truncatum Bunge.
Total seed oil content in A. truncatum, a maple widely distributed throughout northern and western China (Wang and Wang, 2005; Liang et al., 2019; Qiao et al., 2019), is about 24%-55%; NA content in the seed oil is only about 4%-7%. In 2011, the National Health Commission of the People's Republic of China approved the use of A. truncatum seed oil as a new food raw material (No. 9 Announcement, issued in 2011) (http://www.nhc.gov.cn/). However, the low NA content of A. truncatum limits the development of new and current applications. Some research has shown that the production unusual fatty acids is of taxonomic significance at the family and genus levels (Ghada et al., 2018). For example, six members of the genus Cardamine, which contains about 200 species worldwide, have been shown to contain less than 6% NA, although higher levels of NA were observed in one species, C. graeca (http://sofa.mri.bund.de/). Furthermore, three members of the genus Macaranga, which contains about 260 species worldwide, have been shown to have around 8% NA (unpublished results), which suggests that species containing higher NA contents remain to be discovered in large plant genera that contain NA-producing species.
The genus Acer, commonly known as maples, contains approximately 200 species worldwide and is widely distributed in northern temperate regions (Xu, 1998). Previous studies indicated that the NA content in the seed oils of different Acer species vary greatly, ranging from 2.50% (Acer carpinifolium) to 8.60% (Acer oliverianum) (Sun et al., 2018). Furthermore, γ-linolenic acid (GLA, (6Z, 9Z, 12Z)-octadeca-6, 9, 12-trienoic acid, 18:3, n-6), another unusual fatty acid with pharmaceutical applications, was produced at moderate levels in some tested species, but these levels were also highly variable. Additional Acer species are predicted to contain significantly higher levels of NA and may therefore serve as more suitable NA resource plants than A. truncatum.
To determine whether different Acer plants can serve as NA yield resources, we collected seeds from 46 Acer species and measured their fatty acid profiles, oil contents, and 100-seed weights, and then calculated the comprehensive evaluation value (W). We also examined correlations between fatty acid and oil contents of these species. Identifying Acer species that can be used as NA resources will provide a basis for the future development and breeding of Acer species.
2. Materials and methods 2.1. Seed collectionSeed materials were provided by the Germplasm Bank of Wild Species in Southwest China (GBWS, http://www.genobank.org) and collected from Kunming Botanical Garden (KBG), Chinese Academy of Sciences on October 22, 2019. The seed collection source and the corresponding serial number of the species are shown in Table S1.
2.2. Seed weight determinationAfter removal of wings and pericarps from samaras, seeds were dried to a constant weight in desiccators. Then the 100-seed weight (HW) was measured, and the oil content and fatty acid profiles of the seeds were determined.
2.3. Oil content analysisThe oil contents of the seeds were analyzed by the time-domain nuclear magnetic resonance (TD-NMR) technique. The TD-NMR determination was carried out using a minispec mq-one Seed Olive Analyzer (Bruker Optik GmbH, Germany), equipped with a sample tube 40 mm in diameter. A calibration curve was obtained from a reference oil sample extracted from A. truncatum seeds. Lipid extraction and separation were performed as previously described (Tian et al., 2020).
2.4. Fatty acid profile analysisFor each species, seeds (~50 mg) were sampled for fatty acid profile analysis. Each analysis was replicated three times. Seed samples were homogenized with a Superfine Homogenizer (FLUKO, Germany) and methylated with 2 mL of 3 N methanolic HCl. Then the samples were placed in a water bath at 85 ℃ for 2 h. After cooling, 2 mL of aqueous 0.9% NaCl was added, and fatty acid methyl esters were recovered by two sequential extractions with 4 mL of hexane. The fatty acid methyl esters were then analyzed by gas chromatography (PerkinElmer Clarus 680, Singapore) with flame ionization detection by a 30 m × 0.25 μm × 0.32 mm (inner diameter) Elite-225 column (PerkinElmer, Singapore). The following temperature program was applied: 150 ℃, held for 3 min; 10 ℃/min to 180 ℃, held for 9 min; and 5 ℃/min to 210 ℃, held for 8 min. The injector temperature was set to 250 ℃, the injection volume was 1 μL, and a split injection mode with a split ratio of 30:1 was used. The carrier gas was nitrogen at a flow rate of 1.5 mL/min. The fatty acids were qualified using fatty acid methyl ester standards (Sigma-Aldrich, USA), and the peaks were identified by comparing them to the patterns obtained using pure fatty acid standards analyzed in the same apparatus.
2.5. Comprehensive evaluation and screening analysisWe evaluated oil content, HW, erucic acid (EA) content, and NA content and subsequently calculated weight these values using the analytic hierarchy process (AHP) (Fig. S1). First, the fundamental scale (Table S2) was used to obtain a pairwise comparison matrix (Table S3), and then their weights were calculated, which are 0.2902, 0.1189, 0.0411, and 0.5499, respectively (Saaty, 1990). The value was normalized before the evaluation to obtain the relative membership degree (Wang et al., 2018) and to eliminate the incommensurability caused by the difference value and unit of the evaluation index. Candidate NA resource plant species will have high HW, high oil content, high NA content, and low EA content.
For HW, oil content, and NA content, the membership formula was
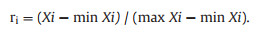
|
For EA content, the membership formula was
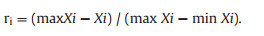
|
ri is the membership degree of index i; and max Xi and min Xi are the maximum and minimum values of the indicator, respectively. After the above normalization process, all index values were converted into membership degree values (Table 1).
| Species | HW | Oil content | Erucic acid C22:1 | Nervonic acid C24:1 | W | |||
| 0.1189 | 0.2902 | 0.0411 | 0.5499 | |||||
| A. amplum | 0.47 | 0.04 | 0.81 | 0.10 | 0.15 | |||
| A. barbinerve | 0.13 | 0.52 | 0.17 | 0.21 | 0.29 | |||
| A. buergerianum | 0.00 | 0.39 | 0.37 | 0.45 | 0.37 | |||
| A. caesium | 0.65 | 0.35 | 0.54 | 0.28 | 0.35 | |||
| A. campbellii var. serratifolium | 0.31 | 0.23 | 0.57 | 0.73 | 0.53 | |||
| A. cappadocicum | 0.52 | 0.38 | 0.68 | 0.00 | 0.20 | |||
| A. cappadocicum subsp. sinicum | 0.73 | 0.15 | 0.67 | 0.08 | 0.20 | |||
| A. caudatum | 0.04 | 0.24 | 0.40 | 0.28 | 0.25 | |||
| A. ceriferum | 0.21 | 0.67 | 0.34 | 0.33 | 0.42 | |||
| A. cordatum | 0.17 | 0.70 | 0.28 | 0.69 | 0.62 | |||
| A. coriaceifolium | 1.00 | 1.00 | 0.16 | 0.59 | 0.74 | |||
| A. crassum | 0.13 | 0.04 | 0.09 | 0.71 | 0.42 | |||
| A. davidiia | 0.38 | 0.34 | 0.42 | 0.24 | 0.29 | |||
| A. davidiib | 0.31 | 0.11 | 0.46 | 0.46 | 0.34 | |||
| A. davidii subsp. grosseri | 0.37 | 0.25 | 0.32 | 0.25 | 0.27 | |||
| A. elegantulum | 0.25 | 0.38 | 0.00 | 1.00 | 0.69 | |||
| A. fabri | 0.35 | 0.63 | 0.17 | 0.82 | 0.68 | |||
| A. flabellatum | 0.32 | 0.20 | 0.45 | 0.34 | 0.30 | |||
| A. forrestii | 0.27 | 0.26 | 0.46 | 0.31 | 0.30 | |||
| A. henryi | 0.08 | 0.64 | 0.28 | 0.47 | 0.47 | |||
| A. hookeri | 0.45 | 0.22 | 0.41 | 0.39 | 0.34 | |||
| A. laevigatum | 0.40 | 0.41 | 0.15 | 0.51 | 0.45 | |||
| A. laevigatum var. laevigatum | 0.16 | 0.55 | 0.40 | 0.31 | 0.37 | |||
| A. laxiflorum | 0.26 | 0.20 | 0.50 | 0.34 | 0.30 | |||
| A. maximowiczii | 0.37 | 0.34 | 0.44 | 0.17 | 0.25 | |||
| A. miaotaiense | 0.43 | 0.29 | 0.34 | 0.63 | 0.49 | |||
| A. negundo | 0.39 | 0.46 | 0.38 | 0.29 | 0.35 | |||
| A. oblongum | 0.38 | 0.51 | 0.27 | 0.32 | 0.38 | |||
| A. oblongum var. concolor | 0.04 | 0.56 | 0.41 | 0.90 | 0.68 | |||
| A. oblongum var. omeiense | 0.00 | 0.36 | 0.51 | 0.36 | 0.33 | |||
| A. oliverianum | 0.19 | 0.05 | 0.07 | 0.73 | 0.44 | |||
| A. palmatum | 0.20 | 0.47 | 0.63 | 0.10 | 0.24 | |||
| A. palmatum var. thunbergii | 0.01 | 0.42 | 0.13 | 0.54 | 0.43 | |||
| A. paxii | 0.19 | 0.59 | 0.61 | 0.23 | 0.35 | |||
| A. pectinatum | 0.07 | 0.36 | 0.37 | 0.23 | 0.26 | |||
| A. pectinatum subsp. taronense | 0.07 | 0.34 | 0.48 | 0.24 | 0.26 | |||
| A. pictum subsp. mono | 0.25 | 0.40 | 0.43 | 0.18 | 0.26 | |||
| A. sikkimense | 0.53 | 0.19 | 0.34 | 0.31 | 0.30 | |||
| A. sinense | 0.22 | 0.29 | 0.21 | 0.66 | 0.48 | |||
| A. stachyophyllum | 0.18 | 0.56 | 0.31 | 0.19 | 0.30 | |||
| A. stachyophyllum subsp. betulifolium | 0.05 | 0.39 | 0.49 | 0.16 | 0.22 | |||
| A. sterculiaceum subsp. franchetii | 0.18 | 0.00 | 1.00 | 0.22 | 0.18 | |||
| A. tataricum subsp. ginnala | 0.45 | 0.29 | 0.37 | 0.43 | 0.39 | |||
| A. tataricum subsp. semenovii | 0.29 | 0.29 | 0.32 | 0.39 | 0.35 | |||
| A. tataricum subsp. theiferum | 0.13 | 0.46 | 0.27 | 0.46 | 0.41 | |||
| A. wilsonii | 0.48 | 0.45 | 0.24 | 0.63 | 0.55 | |||
| a Collected from GBWS. b Collected from KBG. | ||||||||
IBM SPSS statistics 20 (IBM Corp, USA) was used to calculate the Spearman's correlation coefficients for oil and fatty acid content. Yaahp (MetaDecision Software, China) was used to calculate the weight value of the important indicators. Excel 2010 (Microsoft, USA) was used to calculate the means, and Original Pro 8.6 (OriginLab Corp, USA) was used to construct the graphics.
3. Results 3.1. Oil content and HW analysisOil content and HW are important agronomic traits of oil plants. In this study, the Acer species seed oil content ranged from values as low as 1.80% (Acer sterculiaceum subsp. franchetii) to as high as 44.84% (Acer coriaceifolium) in the 46 samples (Fig. 1). The oil content of Acer plants tested in present study were lower than 20% except for Acer ceriferum (30.68%), A. cordatum (32.05%), and A. coriaceifolium (44.84%). The HW ranged from 0.43 g (Acer buergerianum) to 4.65 g (A. coriaceifolium) (Fig. 1).

|
| Fig. 1 The important agronomic traits and comprehensive evaluation value for 46 Acer species. a, collected from GBWS. b, collected from KBG. HW: 100-seed weight, EA: erucic acid, NA: nervonic acid, and W: comprehensive evaluation value. |
A total of 15 fatty acids were detected in the seed oils of the 46 species examined (Table 2), including linoleic acid, oleic acid (C18:1Δ9, C18:1Δ11), EA, palmitic acid, NA, linolenic acid (C18:3Δ6, 9, 12, C18:3Δ9, 12, 15), eicosenoic acid (C20:1Δ11, C20:1Δ13), stearic acid, behenic acid, tetracosanoic acid, arachidic acid, and eicosadienoic acid. In some samples, the contents of some fatty acids were zero. However, the contents of various specific fatty acids may have been too low to detect.
| Species | Fatty acid composition (%) | ||||||||||||||||
| Palmitic acid C16:0 | Stearic acid C18:0 | Oleic acid C18:1Δ9 | Oleic acid C18:1Δ11 | Linoleic acid C18:2 | Linolenic acid C18:3Δ6, 9, 12 | Linolenic acid C18:3Δ9, 12, 15 | Arachidic acid C20:0 | Eicosenoic acid C20:1Δ11 | Eicosenoic acid C20:1Δ13 | Dicosadienoic acid C20:2 | Behenic acid C22:0 | Eruic acid C22:1 | Tetracosanoic acid C24:0 | Nervonic acid C24:1 | ∑SFA | ∑UFA | |
| A. amplum | 20.52 ± 3.06 | 2.92 ± 0.87 | 22.58 ± 3.07 | 7.81 ± 1.34 | 25.27 ± 3.89 | - | 2.30 ± 0.55 | - | 4.11 ± 0.33 | - | - | 1.20 ± 0.05 | 7.29 ± 1.76 | 2.09 ± 0.71 | 3.90 ± 0.45 | 26.73 | 73.27 |
| A. barbinerve | 5.33 ± 0.22 | 2.11 ± 0.07 | 5.62 ± 0.58 | - | 42.91 ± 0.36 | 11.68 ± 0.95 | 0.73 ± 0.10 | 0.22 ± 0.01 | 7.74 ± 0.48 | 0.14 ± 0.12 | 0.50 ± 0.02 | 0.52 ± 0.04 | 17.00 ± 0.80 | 0.31 ± 0.02 | 5.19 ± 0.56 | 8.49 | 91.51 |
| A. buergerianum | 5.66 ± 1.66 | 2.38 ± 0.20 | 15.78 ± 0.43 | 8.11 ± 2.37 | 37.27 ± 2.43 | 1.00 ± 0.67 | 0.81 ± 0.41 | 0.24 ± 0.02 | 4.73 ± 0.38 | 0.40 ± 0.15 | 0.24 ± 0.03 | 1.13 ± 0.35 | 13.89 ± 2.91 | 0.58 ± 0.11 | 7.78 ± 3.29 | 10.00 | 90.00 |
| A. caesium | 7.68 ± 0.50 | 2.22 ± 0.17 | 20.50 ± 1.62 | - | 41.59 ± 2.07 | 2.67 ± 0.37 | 1.58 ± 0.10 | 0.12 ± 0.10 | 5.11 ± 0.16 | - | 0.38 ± 0.06 | 0.56 ± 0.05 | 11.32 ± 0.45 | 0.32 ± 0.03 | 5.94 ± 0.47 | 10.91 | 89.09 |
| A. campbellii var. serratifolium | 5.35 ± 0.49 | 2.41 ± 0.53 | 23.45 ± 2.96 | 7.92 ± 0.59 | 27.76 ± 3.61 | 3.25 ± 0.38 | 2.73 ± 0.89 | 0.19 ± 0.17 | 2.78 ± 0.21 | 0.39 ± 0.04 | - | 1.08 ± 0.24 | 10.96 ± 0.92 | 0.78 ± 0.09 | 10.95 ± 0.77 | 9.82 | 90.18 |
| A. cappadocicum | 11.02 ± 0.35 | 3.95 ± 0.47 | 15.81 ± 1.57 | 7.01 ± 0.27 | 40.56 ± 2.84 | 0.92 ± 0.14 | 1.60 ± 0.22 | 0.35 ± 0.05 | 5.10 ± 0.24 | 0.10 ± 0.09 | 0.20 ± 0.03 | 0.89 ± 0.07 | 9.31 ± 0.57 | 0.33 ± 0.04 | 2.84 ± 0.28 | 16.54 | 83.46 |
| A. cappadocicum subsp. sinicum | 10.89 ± 0.95 | 3.69 ± 0.23 | 11.95 ± 0.54 | 6.67 ± 1.29 | 43.58 ± 0.97 | 0.66 ± 0.18 | 2.93 ± 0.24 | 0.21 ± 0.18 | 4.30 ± 0.39 | 0.08 ± 0.14 | 0.19 ± 0.17 | 0.98 ± 0.08 | 9.50 ± 0.59 | 0.60 ± 0.06 | 3.76 ± 0.11 | 16.37 | 83.63 |
| A. caudatum | 7.62 ± 0.30 | 1.70 ± 0.05 | 7.24 ± 0.77 | 5.20 ± 0.51 | 41.15 ± 0.89 | 7.78 ± 0.38 | 3.66 ± 0.31 | 0.12 ± 0.11 | 4.43 ± 0.19 | 0.25 ± 0.01 | 0.44 ± 0.02 | 0.56 ± 0.02 | 13.53 ± 0.61 | 0.37 ± 0.02 | 5.95 ± 0.40 | 10.38 | 89.62 |
| A. ceriferum | 5.79 ± 0.22 | 2.50 ± 0.07 | 18.81 ± 0.84 | - | 40.06 ± 1.46 | 3.13 ± 0.03 | 0.85 ± 0.02 | 0.23 ± 0.03 | 6.26 ± 0.14 | - | 0.29 ± 0.02 | 0.82 ± 0.05 | 14.32 ± 0.39 | 0.41 ± 0.03 | 6.53 ± 0.22 | 9.75 | 90.25 |
| A. cordatum | 5.34 ± 0.26 | 2.36 ± 0.06 | 20.69 ± 2.77 | - | 35.09 ± 2.92 | 0.57 ± 0.09 | 3.25 ± 0.15 | 0.31 ± 0.02 | 4.10 ± 0.21 | 0.30 ± 0.15 | 0.23 ± 0.03 | 1.32 ± 0.10 | 15.27 ± 0.48 | 0.69 ± 0.12 | 10.48 ± 0.76 | 10.01 | 89.99 |
| A. coriaceifolium | 4.63 ± 0.19 | 3.93 ± 0.53 | 18.75 ± 1.28 | - | 36.37 ± 1.59 | 1.89 ± 0.04 | 0.41 ± 0.08 | 0.42 ± 0.07 | 4.09 ± 0.04 | 0.21 ± 0.11 | 0.26 ± 0.03 | 1.83 ± 0.31 | 17.09 ± 0.22 | 0.80 ± 0.04 | 9.33 ± 0.75 | 11.61 | 88.39 |
| A. crassum | 11.43 ± 0.46 | 4.41 ± 0.31 | 29.87 ± 0.51 | - | 15.08 ± 3.92 | 0.30 ± 0.11 | 0.12 ± 0.11 | 0.46 ± 0.02 | 6.01 ± 0.77 | 0.31 ± 0.03 | 0.19 ± 0.04 | 1.88 ± 0.08 | 18.14 ± 2.01 | 1.04 ± 0.02 | 10.75 ± 0.99 | 19.24 | 80.76 |
| A. davidiia | 4.68 ± 0.47 | 3.38 ± 0.10 | 35.43 ± 0.72 | - | 23.88 ± 1.98 | 3.97 ± 0.83 | 1.81 ± 0.29 | 0.21 ± 0.18 | 6.62 ± 0.49 | - | 0.05 ± 0.09 | 0.88 ± 0.16 | 13.13 ± 1.51 | 0.42 ± 0.04 | 5.54 ± 0.60 | 9.58 | 90.42 |
| A. davidiib | 9.26 ± 0.70 | 3.24 ± 0.05 | 10.14 ± 1.93 | - | 39.52 ± 0.00 | 6.67 ± 0.16 | 4.84 ± 0.11 | - | 3.66 ± 0.14 | - | - | 1.19 ± 0.11 | 12.59 ± 0.54 | 1.01 ± 0.11 | 7.88 ± 0.63 | 14.70 | 85.30 |
| A. davidii subsp. grosseri | 5.44 ± 0.32 | 3.00 ± 0.17 | 30.17 ± 1.42 | - | 25.91 ± 1.12 | 4.67 ± 0.52 | 2.03 ± 0.23 | 0.29 ± 0.02 | 6.66 ± 0.28 | - | 0.17 ± 0.03 | 0.92 ± 0.01 | 14.71 ± 0.15 | 0.41 ± 0.02 | 5.61 ± 0.14 | 10.07 | 89.93 |
| A. elegantulum | 5.67 ± 0.45 | 1.79 ± 0.09 | 12.18 ± 1.54 | 1.35 ± 2.34 | 36.29 ± 3.30 | - | 2.79 ± 0.53 | 0.18 ± 0.17 | 3.81 ± 0.71 | 0.05 ± 0.08 | 0.44 ± 0.31 | 1.32 ± 0.19 | 19.48 ± 2.37 | 0.76 ± 0.03 | 13.9 ± 0.79 | 9.71 | 90.29 |
| A. fabri | 4.93 ± 0.35 | 2.05 ± 0.11 | 28.48 ± 1.23 | - | 28.11 ± 0.71 | 0.09 ± 0.16 | 0.31 ± 0.03 | 0.22 ± 0.02 | 4.55 ± 0.36 | 0.18 ± 0.05 | 0.18 ± 0.01 | 1.37 ± 0.03 | 16.9 ± 0.44 | 0.76 ± 0.03 | 11.86 ± 0.20 | 9.34 | 90.66 |
| A. flabellatum | 5.74 ± 0.31 | 2.56 ± 0.11 | 15.54 ± 0.45 | 6.15 ± 0.83 | 35.98 ± 1.07 | 6.10 ± 0.14 | 1.64 ± 0.10 | 0.25 ± 0.01 | 5.06 ± 0.14 | 0.12 ± 0.21 | 0.25 ± 0.03 | 0.86 ± 0.05 | 12.79 ± 0.49 | 0.40 ± 0.02 | 6.56 ± 0.19 | 9.81 | 90.19 |
| A. forrestii | 6.12 ± 0.05 | 2.82 ± 0.22 | 13.13 ± 0.57 | 6.32 ± 0.21 | 36.03 ± 0.40 | 7.61 ± 0.07 | 1.67 ± 0.09 | 0.25 ± 0.01 | 5.42 ± 0.16 | 0.25 ± 0.22 | 0.33 ± 0.01 | 0.84 ± 0.05 | 12.54 ± 0.34 | 0.41 ± 0.01 | 6.27 ± 0.54 | 10.43 | 89.57 |
| A. henryi | 8.05 ± 0.19 | 3.61 ± 0.12 | 16.33 ± 0.39 | - | 33.72 ± 0.16 | 5.44 ± 0.38 | 3.38 ± 0.09 | 0.35 ± 0.01 | 3.56 ± 0.36 | - | 0.15 ± 0.13 | 1.31 ± 0.07 | 15.30 ± 0.09 | 0.71 ± 0.05 | 8.08 ± 0.39 | 14.03 | 85.97 |
| A. hookeri | 9.20 ± 0.52 | 3.42 ± 0.22 | 12.67 ± 1.22 | - | 39.34 ± 0.69 | 6.04 ± 0.84 | 3.66 ± 0.41 | 0.20 ± 0.18 | 3.52 ± 0.26 | - | 0.20 ± 0.17 | 0.85 ± 0.75 | 13.34 ± 1.33 | 0.46 ± 0.40 | 7.10 ± 0.60 | 14.13 | 85.87 |
| A. laevigatum | 3.89 ± 0.26 | 3.89 ± 1.03 | 29.49 ± 5.06 | - | 28.56 ± 6.06 | - | 0.48 ± 0.06 | 0.41 ± 0.12 | 4.89 ± 0.26 | 0.09 ± 0.15 | 0.07 ± 0.12 | 1.89 ± 0.45 | 17.2 ± 0.42 | 0.68 ± 0.11 | 8.45 ± 0.13 | 10.76 | 89.24 |
| A. laevigatum var. laevigatum | 6.41 ± 1.22 | 4.18 ± 0.48 | 19.00 ± 9.67 | - | 40.93 ± 5.52 | 0.32 ± 0.28 | 1.01 ± 0.16 | 0.39 ± 0.05 | 5.56 ± 1.06 | 0.10 ± 0.10 | 0.25 ± 0.06 | 1.33 ± 0.17 | 13.55 ± 1.37 | 0.65 ± 0.05 | 6.32 ± 0.56 | 12.96 | 87.04 |
| A. laxiflorum | 6.50 ± 0.58 | 1.98 ± 0.08 | 17.98 ± 5.62 | 1.86 ± 3.22 | 38.23 ± 1.83 | 6.27 ± 0.53 | 1.73 ± 0.21 | 0.14 ± 0.12 | 5.47 ± 0.53 | 0.10 ± 0.18 | 0.24 ± 0.03 | 0.61 ± 0.07 | 11.91 ± 1.19 | 0.35 ± 0.06 | 6.63 ± 0.88 | 9.58 | 90.42 |
| A. maximowiczii | 6.59 ± 1.03 | 3.57 ± 0.50 | 34.97 ± 1.84 | - | 22.66 ± 1.29 | 5.72 ± 0.21 | 1.59 ± 0.10 | 0.36 ± 0.04 | 5.66 ± 0.24 | - | - | 0.85 ± 0.10 | 12.85 ± 0.83 | 0.44 ± 0.09 | 4.72 ± 0.10 | 11.82 | 88.18 |
| A. miaotaiense | 8.13 ± 1.28 | 2.01 ± 0.05 | 17.02 ± 4.67 | - | 39.90 ± 3.62 | 1.40 ± 0.18 | 0.78 ± 0.17 | 0.17 ± 0.15 | 4.07 ± 0.33 | 0.14 ± 0.13 | 0.29 ± 0.06 | 1.18 ± 0.05 | 14.38 ± 0.58 | 0.74 ± 0.06 | 9.79 ± 0.50 | 12.23 | 87.77 |
| A. negundo | 4.76 ± 0.42 | 1.50 ± 0.08 | 19.31 ± 1.03 | - | 36.65 ± 0.58 | 9.17 ± 0.36 | 1.02 ± 0.07 | 0.15 ± 0.02 | 6.43 ± 0.19 | - | 0.30 ± 0.03 | 0.54 ± 0.02 | 13.85 ± 0.34 | 0.32 ± 0.01 | 6.00 ± 0.11 | 7.27 | 92.73 |
| A. oblongum | 5.86 ± 0.13 | 2.89 ± 0.08 | 7.94 ± 0.01 | - | 44.01 ± 0.99 | 6.97 ± 0.50 | 1.50 ± 0.01 | 0.30 ± 0.02 | 6.73 ± 0.22 | 0.41 ± 0.03 | 0.42 ± 0.01 | 0.77 ± 0.01 | 15.38 ± 0.15 | 0.36 ± 0.01 | 6.43 ± 1.14 | 10.19 | 89.81 |
| A. oblongum var. concolor | 8.66 ± 0.83 | 1.47 ± 0.11 | 13.29 ± 1.13 | 4.56 ± 0.16 | 38.04 ± 1.52 | - | 2.88 ± 0.38 | - | 3.16 ± 0.14 | - | - | 0.86 ± 0.09 | 13.39 ± 0.88 | 0.90 ± 0.10 | 12.79 ± 0.50 | 11.89 | 88.11 |
| A. oblongum var. omeiense | 13.04 ± 0.89 | 4.77 ± 0.72 | 17.27 ± 1.22 | 4.95 ± 0.93 | 32.49 ± 2.86 | - | 0.48 ± 0.14 | 0.57 ± 0.23 | 4.12 ± 0.11 | 0.37 ± 0.06 | - | 1.91 ± 0.29 | 11.81 ± 1.60 | 1.34 ± 0.20 | 6.87 ± 0.75 | 21.64 | 78.36 |
| A. oliverianum | 6.11 ± 0.54 | 2.90 ± 0.30 | 8.15 ± 0.32 | - | 41.11 ± 3.08 | 3.50 ± 3.12 | 1.08 ± 0.22 | 0.31 ± 0.04 | 4.69 ± 1.69 | 0.43 ± 0.03 | 0.39 ± 0.04 | 1.35 ± 0.58 | 18.36 ± 2.13 | 0.67 ± 0.30 | 10.94 ± 4.44 | 11.34 | 88.66 |
| A. palmatum | 7.02 ± 0.31 | 3.55 ± 0.23 | 20.69 ± 3.70 | 4.32 ± 3.75 | 41.51 ± 0.29 | 0.40 ± 0.35 | 0.83 ± 0.08 | 0.25 ± 0.21 | 5.63 ± 0.11 | 0.11 ± 0.13 | 0.18 ± 0.15 | 1.00 ± 0.05 | 10.07 ± 0.04 | 0.44 ± 0.05 | 3.99 ± 0.31 | 12.26 | 74.34 |
| A. palmatum var. thunbergii | 5.02 ± 0.05 | 2.58 ± 0.13 | 28.80 ± 1.66 | - | 25.08 ± 1.49 | 1.71 ± 0.14 | 2.85 ± 0.40 | 0.34 ± 0.03 | 4.75 ± 0.14 | 0.23 ± 0.13 | 0.15 ± 0.02 | 1.41 ± 0.18 | 17.56 ± 0.57 | 0.66 ± 0.07 | 8.85 ± 0.20 | 10.01 | 89.99 |
| A. paxii | 7.65 ± 0.07 | 3.86 ± 0.04 | 13.02 ± 1.22 | 6.87 ± 0.14 | 42.64 ± 1.38 | 2.52 ± 0.14 | 0.74 ± 0.03 | 0.36 ± 0.02 | 4.00 ± 0.14 | 0.35 ± 0.04 | 0.25 ± 0.02 | 1.33 ± 0.05 | 10.35 ± 0.38 | 0.66 ± 0.07 | 5.40 ± 0.27 | 13.87 | 86.14 |
| A. pectinatum | 8.00 ± 0.58 | 2.33 ± 0.29 | 10.58 ± 0.98 | 4.55 ± 0.82 | 39.87 ± 0.14 | 5.57 ± 0.37 | 3.62 ± 0.29 | 0.22 ± 0.02 | 4.44 ± 0.25 | 0.09 ± 0.16 | 0.35 ± 0.01 | 0.68 ± 0.04 | 13.92 ± 0.54 | 0.35 ± 0.04 | 5.43 ± 0.43 | 11.59 | 88.41 |
| A. pectinatum subsp. taronense | 6.24 ± 0.24 | 1.88 ± 0.07 | 10.24 ± 0.36 | 5.05 ± 0.31 | 41.68 ± 0.52 | 8.43 ± 0.28 | 2.65 ± 0.06 | 0.18 ± 0.01 | 4.62 ± 0.11 | - | 0.37 ± 0.04 | 0.55 ± 0.04 | 12.28 ± 0.14 | 0.33 ± 0.03 | 5.49 ± 0.07 | 9.19 | 90.81 |
| A. pictum subsp. mono | 6.52 ± 2.05 | 2.21 ± 0.32 | 22.37 ± 1.24 | - | 38.88 ± 2.77 | 2.72 ± 0.53 | 0.82 ± 0.18 | 0.16 ± 0.15 | 6.95 ± 0.30 | - | 0.31 ± 0.03 | 0.73 ± 0.18 | 13.07 ± 0.94 | 0.43 ± 0.15 | 4.83 ± 0.44 | 10.05 | 89.95 |
| A. sikkimense | 6.23 ± 1.76 | 3.00 ± 0.25 | 23.84 ± 3.11 | - | 35.16 ± 2.89 | 1.23 ± 0.15 | 2.74 ± 0.34 | 0.20 ± 0.17 | 5.28 ± 0.02 | 0.25 ± 0.09 | 0.12 ± 0.11 | 0.98 ± 0.06 | 14.31 ± 0.98 | 0.40 ± 0.05 | 6.25 ± 1.06 | 10.80 | 89.20 |
| A. sinense | 5.34 ± 0.70 | 2.74 ± 0.33 | 21.80 ± 1.87 | - | 34.97 ± 1.69 | 0.13 ± 0.22 | 0.83 ± 0.19 | 0.30 ± 0.02 | 4.85 ± 0.22 | - | 0.25 ± 0.04 | 1.47 ± 0.18 | 16.39 ± 0.36 | 0.81 ± 0.09 | 10.14 ± 0.66 | 10.65 | 89.35 |
| A. stachyophyllum | 6.41 ± 0.33 | 2.33 ± 0.05 | 8.63 ± 0.31 | 5.00 ± 0.33 | 41.30 ± 0.37 | 7.30 ± 0.28 | 1.83 ± 0.11 | 0.24 ± 0.01 | 5.59 ± 0.11 | 0.26 ± 0.01 | 0.51 ± 0.00 | 0.53 ± 0.03 | 14.84 ± 0.16 | 0.28 ± 0.01 | 4.95 ± 0.25 | 9.79 | 90.21 |
| A. stachyophyllum subsp. betulifolium | 6.87 ± 0.38 | 1.84 ± 0.07 | 12.62 ± 0.24 | 7.40 ± 0.37 | 40.76 ± 0.38 | 4.08 ± 0.27 | 1.98 ± 0.07 | 0.22 ± 0.01 | 6.08 ± 0.13 | 0.34 ± 0.03 | 0.31 ± 0.00 | 0.53 ± 0.07 | 12.08 ± 0.43 | 0.35 ± 0.01 | 4.56 ± 0.19 | 9.80 | 90.20 |
| A. sterculiaceum subsp. franchetii | 21.04 ± 2.79 | 1.69 ± 0.36 | 6.52 ± 1.99 | 3.15 ± 1.17 | 39.58 ± 0.54 | 7.08 ± 1.09 | 6.80 ± 0.91 | - | 1.55 ± 0.31 | - | 0.14 ± 0.24 | 1.01 ± 0.25 | 4.48 ± 0.77 | 1.65 ± 0.48 | 5.30 ± 0.20 | 25.39 | 74.61 |
| A. tataricum subsp. ginnala | 4.32 ± 0.10 | 2.55 ± 0.23 | 18.80 ± 0.42 | - | 40.50 ± 1.38 | 3.70 ± 0.86 | 1.19 ± 0.05 | 0.19 ± 0.01 | 5.41 ± 0.26 | - | 0.26 ± 0.01 | 0.97 ± 0.12 | 13.86 ± 0.66 | 0.65 ± 0.05 | 7.60 ± 0.63 | 7.69 | 81.03 |
| A. tataricum subsp. semenovii | 4.60 ± 0.25 | 1.99 ± 0.04 | 16.68 ± 0.86 | - | 40.97 ± 0.51 | 5.42 ± 0.25 | 1.60 ± 0.04 | 0.21 ± 0.01 | 4.88 ± 0.07 | - | 0.34 ± 0.01 | 0.77 ± 0.06 | 14.75 ± 0.20 | 0.60 ± 0.04 | 7.19 ± 0.16 | 8.17 | 94.17 |
| A. tataricum subsp. theiferum | 4.21 ± 0.58 | 2.58 ± 0.22 | 30.00 ± 2.95 | - | 29.69 ± 5.17 | 1.23 ± 0.24 | 0.92 ± 0.22 | 0.23 ± 0.02 | 5.88 ± 0.12 | - | 0.23 ± 0.12 | 1.11 ± 0.07 | 15.38 ± 0.90 | 0.64 ± 0.10 | 7.9 ± 0.72 | 8.77 | 91.23 |
| A. wilsonii | 4.58 ± 1.54 | 2.72 ± 0.35 | 14.46 ± 2.12 | 6.39 ± 2.42 | 37.20 ± 0.73 | 0.43 ± 0.57 | 0.88 ± 0.24 | 0.29 ± 0.02 | 4.50 ± 0.31 | 0.33 ± 0.18 | 0.30 ± 0.09 | 1.5 ± 0.39 | 15.86 ± 2.70 | 0.73 ± 0.15 | 9.85 ± 2.84 | 9.81 | 90.19 |
| Minimum | 3.89 ± 0.26 | 1.47 ± 0.11 | 5.62 ± 0.58 | 0.00 | 15.08 ± 3.92 | 0.00 | 0.12 ± 0.11 | 0.00 | 1.55 ± 0.31 | 0.00 | 0.00 | 0.52 ± 0.04 | 4.48 ± 0.77 | 0.28 ± 0.01 | 2.84 ± 0.28 | 7.27 | 73.27 |
| Maximum | 21.04 ± 2.79 | 4.77 ± 0.72 | 35.43 ± 0.72 | 8.11 ± 2.37 | 44.01 ± 0.99 | 11.68 ± 0.95 | 6.80 ± 0.91 | 0.57 ± 0.23 | 7.74 ± 0.48 | 0.43 ± 0.03 | 0.51 ± 0.00 | 1.91 ± 0.29 | 19.48 ± 2.37 | 2.09 ± 0.71 | 13.9 ± 0.79 | 26.73 | 94.17 |
| ∑SFA: Total saturated fatty acids = C16:0 + C18:0 + C20:0 + C22:0 + C24:0;
∑UFA: Total unsaturated fatty acids = C18:1 + C18:2 + C18:3 + C20:1 + C20:2 + C22:1 + C24:1.
a Collected from GBWS. b Collected from KBG. -: undetected. | |||||||||||||||||
Nervonic acid was detected in all samples and ranged from 2.84% (Acer cappadocicum) to 13.90% (A. elegantulum). NA content was greater than 9% in 11 Acer species, including A. oliverianum (10.94%), A. elegantulum (13.90%), A. fabri (11.86%), A. oblongum var. concolor (12.79%), A. campbellii var. serratifolium (10.95%), Acer wilsonii (9.85%), A. coriaceifolium (9.33%), A. miaotaiense (9.79%), A. sinense (10.14%), A. cordatum (10.48%), and A. crassum (10.75%).
Oleic acid and linoleic acid were the most abundant fatty acid component in the samples. They ranged from 5.62% (Acer barbinerve) to 35.43% (A. davidiia) and from 15.08% (A. crassum) to 44.01% (A. oblongum), respectively. Erucic acid was the third most abundant fatty acid and ranged from 4.48% in A. sterculiaceum subsp. franchetii oil to 19.48% in A. elegantulum oil. EA content was greater than 10% in most seed oil samples. Palmitic acid is also one of the major fatty acids in Acer derived oils and ranged from 3.89% (A. laevigatum) to 21.04% (A. sterculiaceum subsp. franchetii) in this study.
Some species contained two linolenic acids with different configurations, namely, γ-linolenic acid (C18:3Δ6, 9, 12, GLA) and α-linolenic acid (C18:3Δ9, 12, 15, ALA). ALA was a minor fatty acid that ranged from 0.12% (Acer crassum) to 6.80% (A. sterculiaceum subsp. franchetii). In contrast, GLA content in most species was significantly higher (up to 11.68% in A. barbinerve) than that of ALA. Seven species contained GLA levels > 7%: A. sterculiaceum subsp. franchetii (7.08%), A. stachyophyllum (7.30%), A. forrestii (7.61%), A. caudatum (7.78%), A. pectinatum subsp. taronense (8.43%), A. negundo (9.17%), and A. barbinerve (11.68%). The total saturated fatty acid content varied from 7.27% to 26.73%, and total unsaturated fatty acids varied from 73.27% to 94.17%. The unsaturated fatty acid content of the Acer species was usually much higher than the saturated fatty acid content, which is common in woody plant oils (http://sofa.mri.bund.de/).
3.3. Correlation relationship analysisCorrelation analysis was conducted to identify relationships between fatty acid content and oil contents of the 46 species tested in this study and 55 samples referenced from previous reports (Table 2, Table 3 and S4). Oil content was negatively correlated with palmitic acid content (r = - 0.406, p < 0.01), and positively correlated with eicosenoic acid content (r = 0.232, p < 0.05), eicosadienoic acid content (r = 0.384, p < 0.01), behenic acid content (r = 0.278, p < 0.01), and EA content (r = 0.299, p < 0.01) (Table 3). Oleic acid was negatively correlated with linoleic acid content (r = - 0.703, p < 0.01), linolenic acid content (r = - 0.312, p < 0.01), and eicosadienoic acid content (r = - 0.559, p < 0.01) were observed. Linoleic acid content was positively correlated with eicosadienoic acid content (r = 0.415, p < 0.01) and negatively correlated with behenic acid content (r = - 0.266, p < 0.01). Linolenic acid content was negatively correlated with arachidic acid content (r = - 0.486, p < 0.01), and had a positive correlation with eicosadienoic acid content (r = 0.400, p < 0.01). NA content was negatively correlated with palmitic acid content (r = - 0.375, p < 0.01), oleic acid content (r = - 0.381, p < 0.01) and eicosenoic acid content (r = - 0.380, p < 0.01). Conversely, NA content was positively correlated with eicosadienoic acid content (r = 0.250, p < 0.05), behenic acid content (r = 0.585, p < 0.01), EA (r = 0.649, p < 0.01) and tetracosanoic acid content (r = 0.576, p < 0.01).
| Oil content | C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C20:1 | C20:2 | C22:0 | C22:1 | C24:0 | C24:1 | |
| Oil content | 1.000 | ||||||||||||
| C16:0 | -0.406** | 1.000 | |||||||||||
| C18:0 | 0.037 | 0.168 | 1.000 | ||||||||||
| C18:1 | -0.134 | -0.008 | 0.347** | 1.000 | |||||||||
| C18:2 | 0.066 | 0.102 | -0.269** | -0.703** | 1.000 | ||||||||
| C18:3 | 0.013 | 0.095 | -0.137 | -0.312** | 0.045 | 1.000 | |||||||
| C20:0 | -0.112 | -0.082 | 0.273** | 0.204* | -0.086 | -0.486** | 1.000 | ||||||
| C20:1 | 0.232* | -0.315** | 0.037 | 0.226* | 0.035 | -0.133 | 0.197* | 1.000 | |||||
| C20:2 | 0.384** | -0.120 | -0.159 | -0.559** | 0.415** | 0.400** | -0.326** | 0.045 | 1.000 | ||||
| C22:0 | 0.278** | -0.108 | 0.375** | -0.075 | -0.266** | 0.046 | -0.107 | -0.368** | 0.206* | 1.000 | |||
| C22:1 | 0.299** | -0.600** | -0.165 | -0.318** | -0.058 | -0.189 | 0.252* | 0.123 | 0.181 | 0.217* | 1.000 | ||
| C24:0 | 0.142 | 0.018 | 0.253* | -0.128 | -0.213* | 0.125 | -0.339** | -0.462** | 0.294** | 0.847** | 0.083 | 1.000 | |
| C24:1 | 0.184 | -0.375** | -0.110 | -0.381** | -0.125 | 0.022 | -0.071 | -0.380** | 0.250* | 0.585** | 0.649** | 0.576** | 1.000 |
| *P < 0.05; **P < 0.01. | |||||||||||||
High seed oil and NA contents, large HWs, and low EA contents are desired agronomic traits for NA resource plant. The weight of each indicator was calculated according to the AHP (Tables S2 and S3, Fig. S1, and the relative membership degree was also calculated according to the membership formula (Table 1). The comprehensive evaluation value (W) was then calculated for each species (Table 1). The W ratings for the potentially most important species were as follows: Acer coriaceifolium (0.74), A. elegantulum (0.69), A. henryi (0.68), and A. fabri (0.68).
4. DiscussionNervonic acid is an unusual fatty acid with pharmaceutical applications (Sargent et al., 1994; Calder, 2015); however, these applications are limited by the scarcity of natural resources. The most promising resources for the production of NA include NA-enriched edible vegetable oils produced by members of the genus Acer (Sun et al., 2018; Bohannon and Kleiman, 1976). Accordingly, in 2011, the National Health Administration of China approved seed oil from A. truncatum as a new resource food. However, A. truncatum seed oil only contains small amounts of NA; therefore, plant resources with higher NA yields must be identified.
In this study, the seeds of 46 Acer species (Table S1) were collected and their HWs, oil contents and fatty acid profiles were determined. Oil content, HW, and fatty acid content varied among the 46 Acer species (Table 2, Fig. 1). Previous studies have reported the fatty acid profiles and oil contents for 10 species tested in our study (Table 2 and S4); however, the oil content and fatty acid contents reported in previous studies have not been consistent, even for the same species. The variation is probably due to the geographic environment, genetic factors, and individual differences (Sun et al., 2018).
Nervonic acid content was an important evaluation criterion for the Acer species screened in this study. It was detected in all samples, but the content varied greatly among species, with the highest NA content being recorded in A. elegantulum (13.90%). A total of 26 samples had NA contents were lower than 7%, while nine samples had NA contents in the 7%-9% range. Eleven samples had NA contents > 9% (Table 2). Previous studies have reported that NA content in A. truncatum ranges between 4% - 7% (Wang and Wang, 2005; Liang et al., 2019; Qiao et al., 2019). An NA level higher than 9% has also been reported in two other Acer species, Acer tataricum (10.30%) (Bohannon and Kleiman, 1976), and Acer palmatum (9.41%) (Wei and Liang, 2005) (Table S4). In this study, the NA contents for 20 of the samples analyzed were higher than those for A. truncatum (4%-7%), and our findings indicate that A. coriaceifolium is a potential NA resource plant. However, NA content in A. coriaceifolium is still lower than that in many plants, such as, M. adenantha (56%) and M. oleifera (40%-67%). Therefore, it is important to further expand the scope of future plant screening programs in order to discover and identify better NA resource plants.
This study demonstrated that samples with high NA contents usually have relatively high EA contents. For example, the samples with NA contents > 9% all contained more than 10% EA (Table 2 and S4). Furthermore, NA and EA content were positively correlated (r = 0.649, p < 0.01). Previous studies have reported that the long-term use of high concentrations of EA in animals cause myocardial lipid deposition, reduced contractility, and even damage to tissues (Kramer et al., 1992). Consequently, low EA contents are a requisite for high quality oil, and screening criteria for potential sources of NA should consider both NA and EA content.
Biosynthesis of NA is achieved through elongation of the precursor EA (Guo et al., 2009), a molecular that is obtained by two elongations of oleic acid (Sébastien, 2018). In this study, Spearman's correlation coefficients between the fatty acid and oil contents of species were determined reveal the possible role of fatty acids in NA biosynthesis (Table 3). We found that NA content was negatively correlated with palmitic acid and oleic acid content, but positively correlated with EA content. However, EA content was negatively correlated with palmitic acid and oleic acid content. These results suggest that, in the genus Acer, palmitic acid may play a role in EA and NA biosynthesis. Of course, this needs further research.
Future efforts to screen Acer species for potential NA sources should examine oil content and HW. In this study, the oil contents of most samples were less than 30%. There were only three samples with oil contents above 30%, of which the oil content in A. coriaceifolium was up to 44.84%. In addition, A. coriaceifolium had the greatest HW weights at up to 4.65 g (Fig. 1). The species with the highest W value was A. coriaceifolium (0.74) (Table 1). The four reference indicators and their W values are shown in Fig. 1. A total of ten samples contained more NA than A. coriaceifolium (9.33%) in this study (Table 2), but the A. coriaceifolium oil content and HW were much higher than the values of the other samples. Therefore, the overall W value for A. coriaceifolium was the highest (Fig. 1). This indicates that A. coriaceifolium should be considered as a potential new source of NA, and should be included in further elite breeding research programs.
Previous studies have shown that GLA has many nutritional and medicinal application (Horrobin, 1992; Reddy et al., 1998). In 1976, A. negundo and A. tataricum were found to have relatively high GLA contents at 8% and 6.80%, respectively (Bohannon and Kleiman, 1976). Another previous study also showed that A. tataricum was also a good GLA resource plant (Codreanu et al., 2007). In this study, there were three samples with GLA contents higher than 8%. These were A. pectinatum subsp. taronense (8.43%), A. negundo (9.17%), and A. barbinerve (11.68%) (Table 2), which indicated that Acer plants may potentially be exploited as GLA resource plants.
Author contributionsBT and DZL conceived research. XH performed the experiments and wrote the article with contributions of all the authors. All authors contributed to data analysis, and reviewed and approved the final manuscript.
Declaration of competing interestThe authors declares no potential conflict of interest. All the authors agreed to submit this manuscript.
AcknowledgementWe thank the Germplasm Bank of Wild Species in Southwest China and Kunming Botanical Garden, Chinese Academy of Sciences for their help in seed material collection. We thank Gong Xun, Shen Zongfang and Li Jiangying for their assistance in seed collection and the Tang Mingyong for his assistance in oil content detection. We also would like to thank the reviewers and editor for their comments and suggestions, and Editage (www.editage.com) for English language editing. This work was supported by the National Natural Science Foundation of China (grant number 31671732).
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2020.10.003.
Amminger G.P., SchaFer M.R., Klier C.M., et al, 2012. Decreased nervonic acid levels in erythrocyte membranes predict psychosis in help-seeking ultra-high-risk individuals. Mol. Psychiatr, 17: 1150-1152. DOI:10.1038/mp.2011.167 |
Bohannon R., Kleiman, 1976. γ-linolenic acid in Acer seed oils. Lipids, 11: 157-159. DOI:10.1007/BF02532667 |
Calder P.C., 2015. Functional roles of fatty acids and their effects on human health. J. Parenter. Enteral Nutr, 39: 18S-32S. DOI:10.1177/0148607115595980 |
Codreanu M.V., Istudor V., Dociu N., 2007. Acer tataricum L. seeds-a new convenient source of gamma-linolenic acid. Acta Chromatogr, 19: 238-245. |
David C.T., Tammy F., Guo Y.M., et al, 2009. Molecular cloning and characterization of a KCS gene from Cardamine graeca and its heterologous expression in Brassica oil seeds to engineer high nervonic acid oils for potential medical and industrial use. Plant Biotechnol. J, 7: 925-938. DOI:10.1111/j.1467-7652.2009.00454.x |
Ghada K., Mohamed H., Sabrine S., et al, 2018. A systematic comparison of 25 Tunisian plant species based on oil and phenolic contents, fatty acid composition and antioxidant activity. Ind. Crop. Prod, 123: 768-778. DOI:10.1016/j.indcrop.2018.07.008 |
Guo Y., Mietkiewska E., Francis T., et al, 2009. Increase in nervonic acid content in transformed yeast and transgenic plants by introduction of alunaria annual. 3-ketoacyl-CoA synthase (KCS) gene. Plant Mol. Biol, 69: 565-575. DOI:10.1007/s11103-008-9439-9 |
Horrobin D.F., 1992. Nutritional and medical importance of gamma-linolenic acid. Prog. Lipid Res, 31: 163-194. DOI:10.1016/0163-7827(92)90008-7 |
Kramer J.K.G., Sauer F.D., Wolynetz M.S., et al, 1992. Effects of dietary saturated fat on erucic acid induced myocardial lipidosis in rats. Lipids, 27: 619-623. DOI:10.1007/BF02536120 |
Liang Q., Wang W.W., Yuan F.L., et al, 2019. Characterization of yuanbaofeng (Acer truncatum Bunge) samaras: oil, fatty acid, and phytosterol content. Ind. Crop.Prod, 135: 344-351. DOI:10.1016/j.indcrop.2019.04.032 |
Ma B.L., Liang S.F., Zhao D.Y., et al, 2004. Study on plants containing nervonic acid. Acta Bot. Boreali Occident. Sin, 24: 2362-2365. |
Nobuyuki K., Yoshiyuki M., Fumio S., et al, 2008. Three-dimensional structural model analysis of the binding site of an inhibitor, nervonic acid, of both DNA polymerase β and HIV-1 reverse transcriptase. J. Biochem, 132: 819-828. |
Ou G.Z., 1981. A new presence of important fatty acid (cis-tetracos-15-enoic)-oil of Malania olefera chun et lee. Acta Bot. Yunnanica, 3: 181-184. |
Qiao Q., Wang X., Ren H.J., et al, 2019. Oil content and nervonic acid content of Acer truncatum seeds from 14 regions in China. Horti. Plant J, 5: 24-30. DOI:10.1016/j.hpj.2018.11.001 |
Reddy D.R., Prassad V.S.S.V., Das U.N., 1998. Intratumoral injection of gamma linolenic in malignant gliomas. J. Clin. Neurosci, 5: 36-39. DOI:10.1016/S0967-5868(98)90199-0 |
Saaty T.L., 1990. How to make a decision: the analytic hierarchy process. Eur. J. Oper.Res, 48: 9-26. DOI:10.1016/0377-2217(90)90057-I |
Sargent J.R., Coupland K., Wilson R., 1994. Nervonic acid and demyelinating disease. Med. Hypotheses, 42: 237-242. DOI:10.1016/0306-9877(94)90122-8 |
Sébastien B., 2018. Seeds as oil factories. Plant Reprod, 31: 213-235. DOI:10.1007/s00497-018-0325-6 |
Sun J.Y., Wang X.K., Smith M.A., 2018. Identification of n-6 monounsaturated fatty acids in Acer seed oils. J. Am. Oil Chem. Soc, 95: 21-27. DOI:10.1002/aocs.12020 |
Tanaka K., Shimizu T., Ohtsuka Y., et al, 2007. Early dietary treatments with lorenzo's oil and docosahexaenoic acid for neurological development in a case with Zellweger syndrome. Brain Dev, 29: 586-589. DOI:10.1016/j.braindev.2007.02.005 |
Tian B., Sun M., Jayawardana K., et al, 2020. Characterization of a pldz2 homology gene from developing castor bean endosperm. Lipids, 55: 537-548. DOI:10.1002/lipd.12231 |
Wang B.Q., Zhang J., Xu L., et al, 2018. Physicochemical property, evaluation and screening of seed oil from 88 species of non-food biodiesel plants in xiangxi region. Chem. Ind. For. Prod, 38: 94-104. |
Wang X.Y., Wang S.Q., 2005. A new resource of nervonic acid: purpleblow maple oil. China Oils Fats, 9: 60-62. |
Wei X.Y., Liang J., 2005. Potentially medicinal plants such as Yuanbao Feng and other maple plants. J. Chin. Med. Mater, 28: 14-15. |
Xu T.Z., 1998. The systematic evolution and distribution of the genus Acer. Acta Bot.Yunnanica, 20: 383-393. |
Zhou Y.H., Li W.G., Yi F.P., et al, 2001. Determination of fatty acids in Malania oleifera oil by gas chromatography-mass spectrometry. Chin. J. Chromatogr, 19: 147-148. |



