b. Natural Resources Bureau of Guidong County, Chenzhou 423000, Hunan, China;
c. University of Chinese Academy of Sciences, Beijing 100049, China
Isodon (Schrad. ex Benth.) Spach is one of the largest genera in Lamiaceae with approximately 100 species distributed mainly in tropical and subtropical Asia (Harley et al., 2004; Li, 1988; Li and Hedge, 1994; Mabberley, 2008; Wu and Li, 1977). The Himalaya-Hengduan Mountains (HHM) global biodiversity hotspot, which accommodates ca. 70% of the species of Isodon, is considered the distribution and biodiversity center of the genus (Yu et al., 2014; Zhong et al., 2010). Isodon is recognized as the only genus in subtribe Isodoninae (Zhong et al., 2010), and it differs from other genera of Ocimeae by its pedunculate and bracteolate cymes, slightly or strongly 2-lipped (3/2) calyces, strongly 2-lipped (4/1) corollas, and free filaments inserted at the base of the corolla tube (Harley et al., 2004; Li, 1988; Paton and Ryding, 1998). Some species of Isodon have long been used as traditional folk medicine in China and Japan, and contemporary phytochemical studies of Isodon species have so far isolated and identified more than 1200 diterpenoids, some of which have important pharmaceutical functions (Liu et al., 2017; Sun et al., 2006).
Several new species of Isodon have been reported from China during the last decade (Chen et al., 2014, 2016b, 2017, 2019; Xiang and Liu, 2012). Recently, we collected a distinct species of Isodon from Hunan and Guangdong Provinces in southern China. Critical studies based on specimen and literature examination, as well as molecular phylogenetic analyses revealed it to be an undescribed species. Herein, we describe and illustrate the new species.
2. Material and methods 2.1. Morphological and taxonomic studiesComparison of morphological features between the new species and other species of Isodon were carried out based on our previous field observations, specimen examination, and unpublished mericarp data (Chen, 2017). Specimens of Isodon from 29 herbaria (A, AU, BM, CDBI, CSFI, E, G, GXMI, HHBG, HIB, IBK, IBSC, K, KUN, KYO, L, LBG, LE, MW, NAS, P, PE, S, SYS, SZ, TAI, TI, W, and WUK; abbreviations follow Thiers, 2020) and our field collections were examined. Meanwhile, protologues of all published names and all other taxonomic literature for Isodon were reviewed. The terminology used by Li (1988) and Li and Hedge (1994) was adopted for the morphological description of the new species.
2.2. Taxon sampling and DNA amplificationThe systematic placement of the new species was explored based on an ingroup sampling comprising 90 accessions of 84 species of Isodon from Asia, including two individuals of the new species from the type locality in Guangdong Province and from Hunan Province, respectively (Appendix A). Six genera representing all subtribes of Ocimeae except Isodoninae (Harley et al., 2004; Zhong et al., 2010) were selected as outgroups (Appendix A). Previous studies have shown that Isodon chloroplast DNA sequences have significantly lower numbers of variable sites than nuclear DNA sequences, and consequently generate poorly resolved phylogenies (Chen et al., 2019; Yu et al., 2014; Zhong et al., 2010). Thus, for phylogenetic analyses, we used two nuclear ribosomal DNA markers: the nuclear ribosomal internal and external transcribed spacers (ITS and ETS). A total of 172 sequences were downloaded from GenBank to complement our dataset, of which 168 sequences were generated from our previous study (Chen et al., 2019). Voucher information and GenBank accession numbers for all sequences are listed in Appendix A.
The modified CTAB method (Doyle and Doyle, 1987) was used to extract genomic DNA from the silica-gel-dried leaf material. For polymerase chain reaction (PCR) amplification, ITS was amplified using the primer pairs 17SE/26SE (Sun et al., 1994), ETS using ETS-B (Beardsley and Olmstead, 2002) and 18S-IGS (Baldwin and Markos, 1998). The PCR and sequencing protocols for the two markers followed those of Chen et al. (2016a).
2.3. Sequence alignment and phylogenetic analysesSequences were assembled and edited using Sequencher 4.1.4 (Gene Codes, Ann Arbor, Michigan, USA), and then aligned using MUSCLE (Edgar, 2004) and manually adjusted in MEGA v.6.0 (Tamura et al., 2013). Gaps were treated as missing data. Bayesian Inference (BI) and Maximum Likelihood (ML) analyses were conducted to reconstruct the phylogeny of Asian Isodon, using MrBayes v.3.2.6 (Ronquist et al., 2012) and RAxML-HPC2 (Stamatakis, 2014) on the Cyberinfrastructure for Phylogenetic Research Science (CIPRES) Gateway (http://www.phylo.org/; Miller et al., 2010), respectively. Parameters of each category of analysis followed that of Chen et al. (2019). TreeGraph 2 (Stover and Müller, 2010) was used to visualize the topology of phylogenetic trees with posterior probabilities (PP) and Bootstrap support (BS) values.
3. Results and discussionConsistent with previous molecular phylogenetic studies (Chen et al., 2019; Yu et al., 2014; Zhong et al., 2010), three well-supported clades (Fig. 1; Clades Ⅲ) are recognized for Asian Isodon. Clade Ⅲ contains ca. 80% of the species, but relationships within the clade are poorly resolved. Within Clade Ⅲ, a moderately supported subclade Clade Ⅲa (Fig. 1; BI-PP = 0.99/ML-BS = 72%) can be recognized, with most of the species having a Sino-Japanese distribution, as opposed to the remaining species of Clade Ⅲ that are predominantly distributed in the HHM region. The two individuals of the new species group together but with moderate support (Fig. 1; BI-PP = 0.94/ML-BS = 73%), which may partially result from our failure in obtaining the ITS sequence of the individual from Guangdong. The new species is further recovered in Clade Ⅲa. All species of this clade are perennial herbs, most of which are characterized with glandular or glandular and puberulent mericarps (Chen, 2017). One species that has glandular and puberulent mericarps but is not recovered in Clade Ⅲa is Isodon trichocarpus (Maxim.) Kudô, a species endemic to Japan.
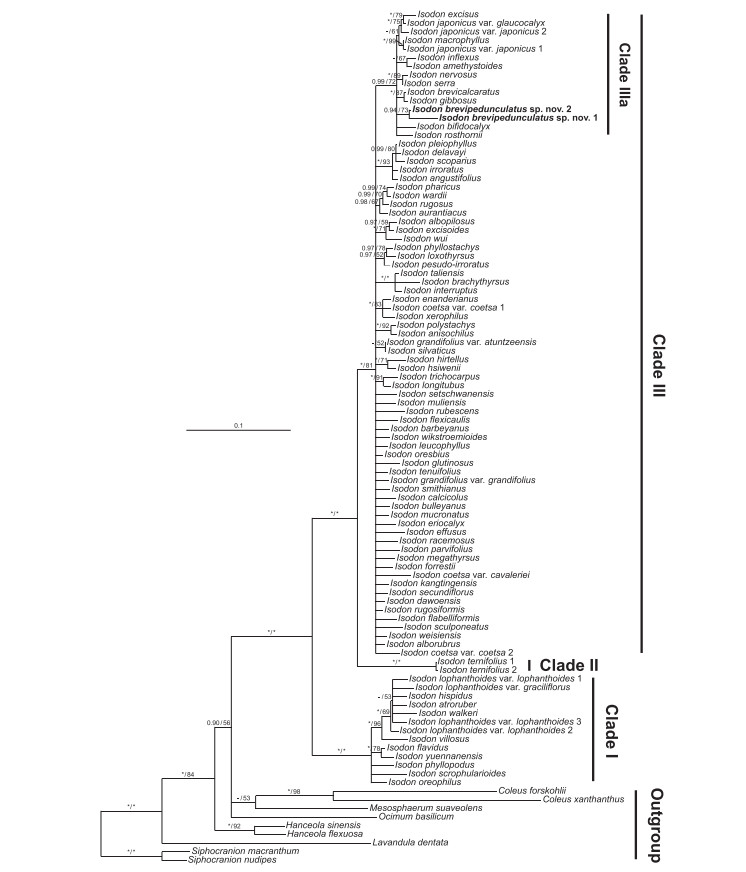
|
| Fig. 1 Phylogenetic placement of Isodon brevipedunculatus based on Maximum Likelihood analysis of the combined nuclear (ITS and ETS) dataset. Support values ≥ 0.90 PP or 50% BS are displayed on the branches following the order BI-PP/ML-BS ("*" indicates a support value of 100% and "-" indicates a PP < 0.90). Multiple accessions of the same species are numbered according to Appendix A. |
Although the relationships between Isodon brevipedunculatus and other species of Clade Ⅲa are not resolved, I. brevipedunculatus is united morphologically with Isodon amethystoides (Benth.) H. Hara, Isodon excisus (Maxim.) Kudô, Isodon inflexus (Thunb.) Kudô, and Isodon bifidocalyx (Dunn) H. Hara by having densely glandular mericarps. Morphologically and geographically, the new species is most closely related to I. amethystoides and I. bifidocalyx (Fig. 2, Fig. 3, Fig. 4; Appendix B).
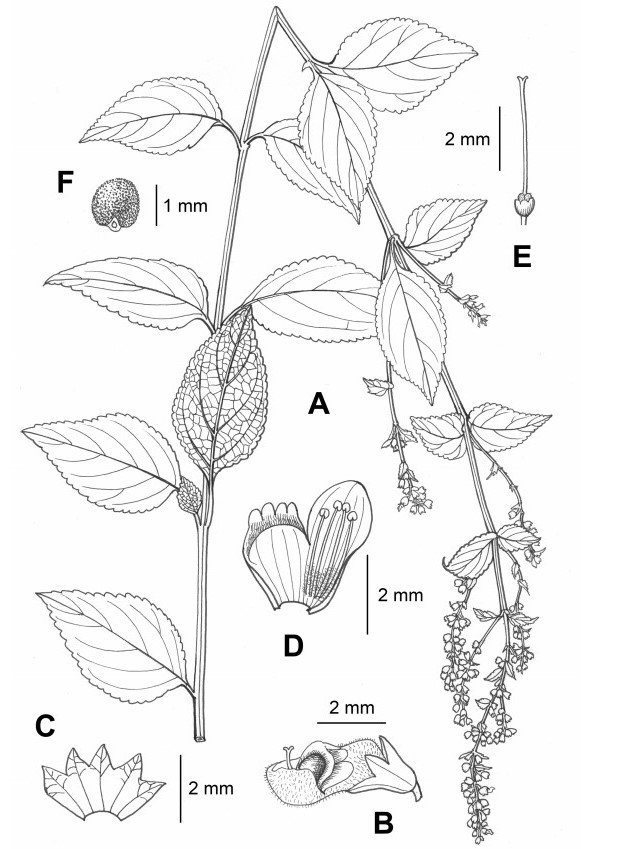
|
| Fig. 2 Isodon brevipedunculatus. (A) habit; (B) flower; (C) dissected calyx; (D) dissected corolla; (E) pistil; (F) mericarp. Drawn by L. Wang. |
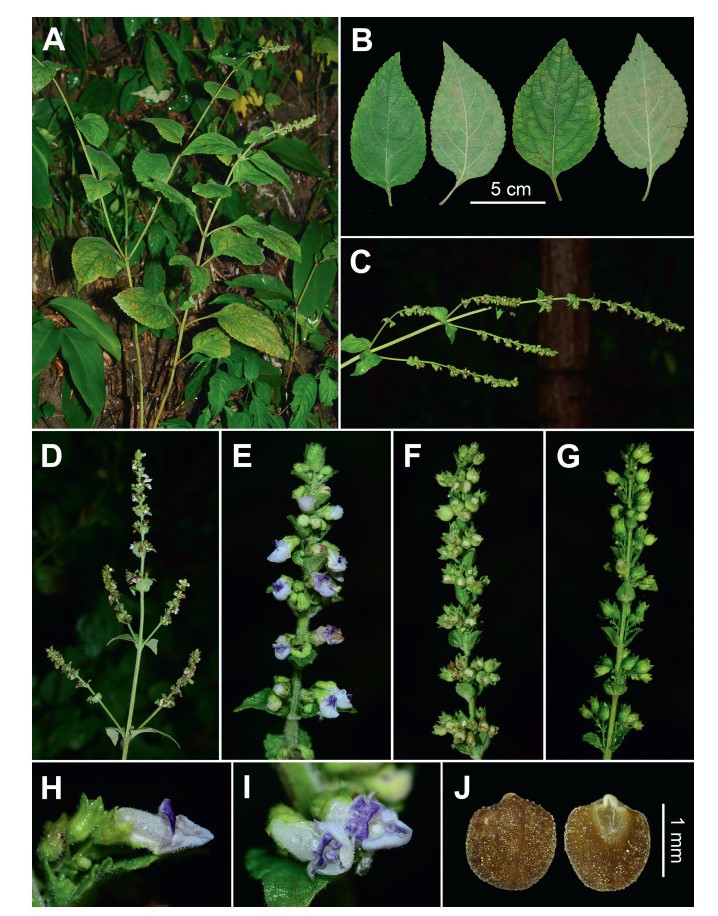
|
| Fig. 3 Isodon brevipedunculatus (from the type locality). (A) habit; (B) leaves; (C, D) panicles; (E) inflorescence; (F, G) infructescences; (H, I) flowers; (J) mericarps. Photographed by Y.P. Chen. |
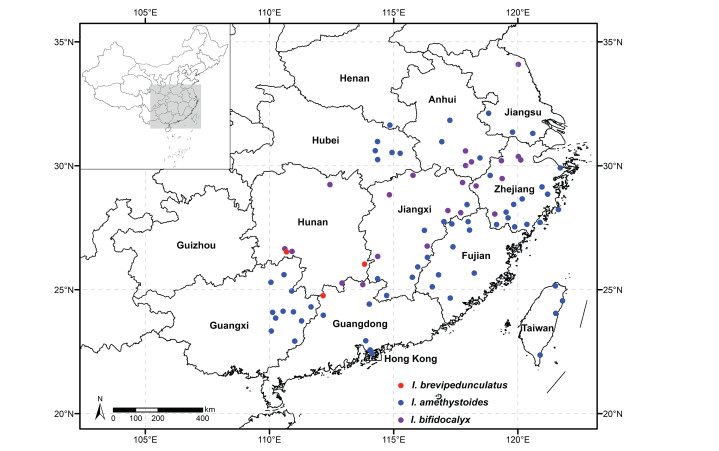
|
| Fig. 4 Distribution of Isodon amethystoides, I. bifidocalyx, and I. brevipedunculatus. |
Both Isodon brevipedunculatus and I. amethystoides have slightly 2-lipped flowering calyces with subequal teeth and erect fruiting calyces. The most noteworthy difference between the two species is the length of peduncles, which are 1–2 mm long in I. brevipedunculatus, but 1–4 cm long in I. amethystoides (Table 1). Meanwhile, laminae of I. amethystoides are usually lanceolate, whereas those of I. brevipedunculatus are ovate to broadly ovate; cymes are 3–7-flowered in the new species, but generally have more flowers per cyme (7–15-flowered) in I. amethystoides (Table 1).
| Character | I. amethystoides | I. bifidocalyx | I. brevipedunculatus |
| Lamina | lanceolate to ovate, densely pubescent to subglabrous | ovate to broadly ovate, subglabrous | ovate to broadly ovate, densely pubescent |
| Cyme | 7–15-flowered | 3–5-flowered | 3–7-flowered |
| Peduncle | 1–4 cm long | 2–4 mm long | 1–2 mm long |
| Flowering calyx | pubescent to subglabrous, and densely glandular outside, slightly 2-lipped to 1/3 its length, teeth subequal | densely glandular puberulent and glandular outside, strongly 2-lipped to 1/2 its length, teeth unequal | densely pubescent and glandular outside, slightly 2-lipped to 1/3 its length, teeth subequal |
| Fruiting calyx | erect | declinate | erect |
| Corolla | ca. 6 mm long | ca. 8 mm long | ca. 4 mm long |
Though Isodon brevipedunculatus resembles I. bifidocalyx in the ovate to broadly ovate laminae and narrow panicles (short peduncles), they can readily be distinguished by lamina indumentum, calyx morphology, and corolla length (Table 1). The new species is characterized by having densely pubescent laminae and inflorescences, while I. bifidocalyx has subglabrous laminae and densely glandular puberulent inflorescences. I. bifidocalyx also differs from I. brevipedunculatus in its strongly 2-lipped flowering calyces with unequal teeth and declinate fruiting calyces. Moreover, corollas of I. bifidocalyx are about 8 mm long, nearly twice the length of those of I. brevipedunculatus.
4. Taxonomic treatmentIsodon brevipedunculatus Y.P. Chen & C.L. Xiang, sp. nov. (Fig. 2, Fig. 3).
Type: CHINA. Guangdong, Lianshan County, Taibao Town, Dawu Mountain, in Chinese fir forest, 24°45′34″N, 112°9′40″E, alt. 690 m, 25 Oct. 2019, Y.P. Chen & Y. Zhao EM1381 (holotype: KUN!; isotypes: A!, K!, KUN!, P!, PE!, W!).
4.1. DiagnosisThe new species is morphologically similar to Isodon amethystoides and I. bifidocalyx, but differs from the former in its broadly ovate to ovate laminae (vs. ovate to lanceolate), 3–7-flowered cymes (vs. 7–15-flowered), and 1–2 mm long peduncles (vs. 1–4 cm long), and differs from the latter in having densely pubescent laminae (vs. subglabrous), flowering calyces slightly 2-lipped to 1/3 of their length with teeth subequal (vs. strongly 2-lipped to 1/2 their length with teeth unequal), erect fruiting calyces (vs. declinate), and corollas ca. 4 mm long (vs. ca. 8 mm long).
4.2. DescriptionPerennial herbs 50–150 cm tall. Rhizomes woody, tuberose. Stems erect, leafless at base, 4-angled, densely pubescent; internodes 5–9 cm long. Leaves opposite; lamina ovate to broadly ovate, papery, 5–12 × 3–6 cm, apex acute, margin crenate, base cuneate to rounded, adaxially dark green, pubescent, abaxially light green, densely pubescent, with colorless glands, lateral veins 4–5-paired; petioles 1–2 cm long. Panicles terminal and axillary, to 20 cm long; cymes 3–7-flowered, densely pubescent and glandular; peduncles and pedicels 1–2 mm long; floral leaves ovate, gradually reduced toward apex; bracts broadly ovate, sessile or subsessile, 5–10 mm long; bracteole linear, ca. 1 mm long. Calyx campanulate, ca. 2 mm long, 10-veined, densely pubescent and glandular outside, slightly 2-lipped to 1/3 as long as calyx; teeth 5, subequal, triangular, apex acute, fruiting calyx dilated to ca. 4 mm long, erect, slightly curved, broadly campanulate. Corolla white, ca. 4 mm long, pubescent and glandular outside; tube ca. 2 mm long, ca. 1.5 mm in diameter, saccate abaxially near base; apex 2-lipped, posterior lip 4-cleft, bluish purple at middle, lobes reflexed, ca. 1 mm long, anterior lip entire, suborbicular, concave, ca. 2 mm long. Stamens 4, included, inserted at base of corolla tube, filaments pubescent. Style included, apex slightly 2-cleft, ovaries glandular. Mericarps 4, yellowish brown, broadly ovoid, ca. 1.6 × 1.4 mm, densely glandular.
Phenology
Flowering from August to November, fruiting from September to December.
Distribution and habitat
Isodon brevipedunculatus is currently known from Guangdong Province and Hunan Province in southern China (Fig. 4). It can be found in forests or on grassy slopes at altitudes of 600–1250 m.
Etymology
The specific epithet refers to the short peduncles of the new species, as compared to one of the most similar species, Isodon amethystoides.
Chinese name
Duan Geng Xiang Cha Cai (短梗香茶菜).
Additional specimens examined
CHINA. Guangdong: Lianshan County, Taibao Town, Dawu Mountain, 25 Oct. 1999, H.G. Ye et al. 2532 (IBSC). Hunan: Guidong County, Zhaiqian Town, Fangcun Village, Zhulongli, in the forest, alt. 1227 m, 1 Oct. 2019, C.Z. Huang GD0074 (CSFI); Xinning County, Ma-Ling-Tung, on the grassy slope, alt. 600 m, 9 Sept. 1935, C.S. Fan & Y.Y. Li 458 (BM, LE, NAS).
Author contributionsYPC, CZH, and YZ first discovered the new species in the field and collected the material. YPC carried out the molecular experiment and analyzed the data. YPC and CLX wrote the manuscript. All authors contributed to the revision of the manuscript.
Declaration of Competing InterestAll the authors declare that there is no conflict of interest.
AcknowledgementsWe would like to thank the staff of the following herbaria for their valuable help in research facilities: BM, CDBI, E, HIB, IBK, IBSC, K, KUN, KYO, LE, MW, NAS, PE, SZ, TI. Thanks are also given to Ms. Ling Wang from the Kunming Institute of Botany for her line drawing of the new species and Dr. Bryan T. Drew from the University of Nebraska for improving the English. Our manuscript also benefitted greatly from the constructive comments of two anonymous reviewers. This work was supported by the National Natural Science Foundation of China (Grant no. 31670197) and the CAS "Light of West China" Program, the Special Funds for the Young Scholars of Taxonomy of the Chinese Academy of Sciences (Grant no. ZSBR-006), the "Ten Thousand Talents Program of Yunnan" (YNWR-QNBJ-2018-279), and the "Yunnan Fundamental Research Projects" (2019FI009).
Appendix A. Sequence information for all samples used in the present study. Vouchers are only provided for samples newly collected here. Sequences newly generated in this study are marked in bold. "/" indicates missing data. All voucher specimens are deposited in the Herbarium of Kunming Institute of Botany (KUN).| Taxon | Voucher/Source | ITS | ETS |
| Isodon albopilosus (C.Y. Wu & H.W. Li) H. Hara | Chen et al. (2019) | MG232833 | MG232738 |
| Isodon alborubrus (C.Y. Wu) H. Hara | Chen et al. (2019) | MG232741 | MG232650 |
| Isodon amethystoides (Benth.) H. Hara | Chen et al. (2019) | MG232762 | MG232669 |
| Isodon angustifolius (Dunn) Kudô | Chen et al. (2019) | MG232803 | MG232709 |
| Isodon anisochilus (C.Y. Wu) H. Hara | Chen et al. (2019) | MG232789 | MG232695 |
| Isodon atroruber R.A. Clement | Chen et al. (2019) | MG232766 | MG232673 |
| Isodon aurantiacus Y.P. Chen & C.L. Xiang | Chen et al. (2019) | MG232764 | MG232671 |
| Isodon barbeyanus (H. Lév.) H.W. Li | Chen et al. (2019) | MG232790 | MG232696 |
| Isodon bifidocalyx (Dunn) H. Hara | Chen et al. (2019) | MG232744 | MG232653 |
| Isodon brachythyrsus (C.Y. Wu & H.W. Li) H. Hara | Chen et al. (2019) | MG232776 | MG232682 |
| Isodon brevicalcaratus (C.Y. Wu & H.W. Li) H. Hara | Y.P. Chen et al. EM1363 | MT603971 | MT614332 |
| Isodon brevipedunculatus sp. nov. 1 | Y.P. Chen et al. EM1381 | / | MT614331 |
| Isodon brevipedunculatus sp. nov. 2 | C.Z. Huang GD0074 | MT603978 | MT614339 |
| Isodon bulleyanus (Diels) Kudô | Chen et al. (2019) | MG232798 | MG232704 |
| Isodon calcicolus (Hand.-Mazz.) H. Hara | Chen et al. (2019) | MG232795 | MG232701 |
| Isodon coetsa (Buch.-Ham. ex D. Don) Kudô var. coetsa 1 | Chen et al. (2019) | MG232756 | MG232664 |
| Isodon coetsa (Buch.-Ham. ex D. Don) Kudô var. coetsa 2 | Chen et al. (2019) | MH557903 | MH557887 |
| Isodon coetsa var. cavaleriei (H. Lévl.) H.W. Li | Chen et al. (2019) | MG232759 | MG232666 |
| Isodon dawoensis (Hand.-Mazz.) H. Hara | Chen et al. (2019) | MG232804 | MG232710 |
| Isodon delavayi C.L. Xiang & Y.P. Chen | Chen et al. (2019) | MG232768 | MG232674 |
| Isodon effusus (Maxim.) H. Hara | Chen et al. (2019) | MH557911 | MH557896 |
| Isodon enanderianus (Hand.-Mazz.) H.W. Li | Chen et al. (2019) | MG232740 | MG232649 |
| Isodon eriocalyx (Dunn) Kudô | Chen et al. (2019) | MG232805 | MG232711 |
| Isodon excisoides (Y.Z. Sun) H. Hara | Chen et al. (2019) | MG232806 | MG232712 |
| Isodon excisus (Maxim.) Kudô | Y.P. Chen et al. EM218 | MT603972 | MT614333 |
| Isodon flabelliformis (C.Y. Wu) H. Hara | Chen et al. (2019) | MG232771 | MG232677 |
| Isodon flavidus (Hand.-Mazz.) H. Hara | Chen et al. (2019) | MG232780 | MG232686 |
| Isodon flexicaulis (C.Y. Wu & H.W. Li) H. Hara | Y.P. Chen et al. EM673 | MT603977 | MT614338 |
| Isodon forrestii (Diels) Kudô | Chen et al. (2019) | MG232810 | MG232716 |
| Isodon gibbosus (C.Y. Wu & H.W. Li) H. Hara | Chen et al. (2019) | MH557901 | MH557885 |
| Isodon glutinosus (C.Y. Wu & H.W. Li) H. Hara | Chen et al. (2019) | MG232828 | MG232733 |
| Isodon grandifolius (Hand.-Mazz.) H. Hara var. grandifolius | Chen et al. (2019) | MG232801 | MG232707 |
| Isodon grandifolius var. atuntzeensis (C.Y. Wu) H.W. Li | Chen et al. (2019) | MG232812 | MG232718 |
| Isodon hirtellus (Hand.-Mazz.) H. Hara | Chen et al. (2019) | MG232813 | MG232719 |
| Isodon hispidus (Benth.) Murata | Chen et al. (2019) | MG232750 | MG232658 |
| Isodon hsiwenii Y.P. Chen & C.L. Xiang | Chen et al. (2019) | MG232770 | MG232676 |
| Isodon inflexus (Thunb.) Kudô | Chen et al. (2019) | MG232814 | MG232720 |
| Isodon interruptus (C.Y. Wu & H.W. Li) H. Hara | Chen et al. (2019) | MG232781 | MG232687 |
| Isodon irroratus (Forrest ex Diels) Kudô | Chen et al. (2019) | MG232779 | MG232685 |
| Isodon japonicus (Burm. f.) H. Hara var. japonicus 1 | Chen et al. (2019) | MG232783 | MG232688 |
| Isodon japonicus (Burm. f.) H. Hara var. japonicus 2 | Chen et al. (2019) | MH557910 | MH557895 |
| Isodon japonicus var. glaucocalyx (Maxim.) H.W. Li | Chen et al. (2019) | MG232818 | MG232723 |
| Isodon kangtingensis (C.Y. Wu & H.W. Li) H. Hara | Chen et al. (2019) | MG232819 | MG232724 |
| Isodon leucophyllus (Dunn) Kudô | Chen et al. (2019) | MG232821 | MG232726 |
| Isodon longitubus (Miq.) Kudô | Chen et al. (2019) | MH557912 | MH557898 |
| Isodon lophanthoides (Buch.-Ham. ex D.Don) H. Hara var. lophanthoides 1 | Chen et al. (2019) | MG232758 | MG232665 |
| Isodon lophanthoides (Buch.-Ham. ex D.Don) H. Hara var. lophanthoides 2 | Chen et al. (2019) | MH557907 | MH557892 |
| Isodon lophanthoides (Buch.-Ham. ex D.Don) H. Hara var. lophanthoides 3 | Chen et al. (2019) | MH557906 | MH557890 |
| Isodon lophanthoides var. graciliflorus (Benth.) H. Hara | Chen et al. (2019) | MG232761 | MG232668 |
| Isodon loxothyrsus (Hand.-Mazz.) H. Hara | Chen et al. (2019) | MG232823 | MG232728 |
| Isodon macrophyllus (Migo) H. Hara | Chen et al. (2019) | MG232742 | MG232651 |
| Isodon megathyrsus (Diels) H. Hara | Chen et al. (2019) | MG232800 | MG232706 |
| Isodon mucronatus (C.Y. Wu & H.W. Li) H. Hara | Y.P. Chen et al. EM676 | MT603968 | MT614328 |
| Isodon muliensis (W.W. Sm.) Kudô | Y.P. Chen et al. EM701 | MT603969 | MT614329 |
| Isodon nervosus (Hemsl.) Kudô | Y.P. Chen et al. EM444 | MT603973 | MT614334 |
| Isodon oreophilus (Diels) A.J. Paton & Ryding | Chen et al. (2019) | MG232825 | MG232730 |
| Isodon oresbius (W.W. Sm.) Kudô | Chen et al. (2019) | MG232827 | MG232732 |
| Isodon parvifolius (Batalin) H. Hara | Chen et al. (2019) | MG232777 | MG232683 |
| Isodon pharicus (Prain) Murata | Chen et al. (2019) | MG232817 | MG232722 |
| Isodon phyllopodus (Diels) Kudô | Chen et al. (2019) | MG232799 | MG232705 |
| Isodon phyllostachys (Diels) Kudô | Chen et al. (2019) | MG232785 | MG232690 |
| Isodon pleiophyllus (Diels) Kudô | Chen et al. (2019) | MG232830 | MG232735 |
| Isodon polystachys (Y.Z. Sun) H. Hara | Chen et al. (2019) | MG232802 | MG232708 |
| Isodon pseudo-irroratus (C.Y. Wu) H. Hara | Chen et al. (2019) | MG232775 | MG232681 |
| Isodon racemosus (Hemsl.) Murata | Chen et al. (2019) | MG232745 | MG232654 |
| Isodon rosthornii (Diels) Kudô | Chen et al. (2019) | MG232749 | MG232657 |
| Isodon rubescens (Hemsl.) H. Hara | Chen et al. (2019) | MG232760 | MG232667 |
| Isodon rugosiformis (Hand.-Mazz.) H. Hara | Chen et al. (2019) | MG232778 | MG232684 |
| Isodon rugosus (Wall. ex Benth.) Codd | C. Liu et al. 18CS17466 | MT603975 | MT614336 |
| Isodon scoparius (C.Y. Wu & H.W. Li) H. Hara | Chen et al. (2019) | MG232835 | MG232739 |
| Isodon scrophularioides (Wall. ex Benth.) Murata | Y.P. Chen et al. EM1224 | MT603970 | MT614330 |
| Isodon sculponeatus (Vaniot) Kudô | Chen et al. (2019) | MG232791 | MG232697 |
| Isodon secundiflorus (C.Y. Wu) H. Hara | Y.P. Chen et al. EM667 | MT603976 | MT614337 |
| Isodon serra (Maxim.) Kudô | Chen et al. (2019) | MG232743 | MG232652 |
| Isodon setschwanensis (Hand.-Mazz.) H. Hara | Chen et al. (2019) | MG232824 | MG232729 |
| Isodon silvaticus (C.Y. Wu & H.W. Li) H.W. Li | C.L. Xiang et al.1601 | MT603974 | MT614335 |
| Isodon smithianus (Hand.-Mazz.) H. Hara | Chen et al. (2019) | MG232816 | MG232721 |
| Isodon taliensis (C.Y. Wu) H. Hara | Chen et al. (2019) | MG232787 | MG232692 |
| Isodon tenuifolius (W.W. Sm.) Kudô | Chen et al. (2019) | MG232773 | MG232679 |
| Isodon ternifolius (D.Don) Kudô 1 | Chen et al. (2019) | MG232786 | MG232691 |
| Isodon ternifolius (D.Don) Kudô 2 | Chen et al. (2019) | MH557905 | MH557889 |
| Isodon trichocarpus (Maxim.) Kudô | Chen et al. (2019) | MH557909 | MH557894 |
| Isodon villosus Y.P. Chen & H. Peng | Chen et al. (2019) | MG232754 | MG232662 |
| Isodon walkeri (Arn.) H. Hara | Chen et al. (2019) | MG232755 | MG232663 |
| Isodon wardii (Marq. & Airy Shaw) H. Hara | Chen et al. (2019) | MG232763 | MG232670 |
| Isodon weisiensis (C.Y. Wu) H. Hara | Chen et al. (2019) | MG232807 | MG232713 |
| Isodon wikstroemioides (Hand.-Mazz.) H. Hara | Chen et al. (2019) | MG232811 | MG232717 |
| Isodon wui C.L. Xiang & E.D. Liu | Chen et al. (2019) | MG232769 | MG232675 |
| Isodon xerophilus (C.Y. Wu & H.W. Li) H. Hara | Chen et al. (2019) | MG232747 | MG232655 |
| Isodon yuennanensis (Hand.-Mazz.) H. Hara | Chen et al. (2019) | MG232808 | MG232714 |
| Coleus forskohlii (Willd.) Briq. | Chen et al. (2019) | MG232788 | MG232693 |
| Coleus xanthanthus C.Y. Wu & Y.C. Huang | Chen et al. (2019) | MN116780 | MN116786 |
| Hanceola exserta Y.Z. Sun | Chen et al. (2019) | MN116776 | MN116783 |
| Hanceola sinensis (Hemsl.) Kudô | Zhong et al. (2010); Chen et al., (2016) | FJ593353 | KT210227 |
| Lavandula dentata L. | Y.P. Chen s.n. | / | MT614340 |
| Mesosphaerum suaveolens (L.) Kuntze | Chen et al. (2019) | MN116777 | MN116784 |
| Ocimum basilicum L. | Walker and Sytsma (2007); Chen et al. (2016) | DQ667240 | KT210226 |
| Siphocranion macranthum (Hook. f.) C.Y. Wu | Chen et al. (2019) | MN116781 | MN116787 |
| Siphocranion nudipes (Hemsl.) Kudô | Chen et al. (2019) | MN116782 | MN116788 |
Specimens of I. amethystoides examined:
CHINA. Anhui: Hefei, Courtois 6058 (NAS), E China Workstation 3771 (NAS, PE); Jingde, Courtois 12529 (NAS); Tongcheng, Courtois 4071 (NAS); Precise locality not known, Courtois 6221 (NAS), R.C. Ching 8968 (NAS). Fujian: Dehua, P.C. Tsoong 114 (PE); Guangze, Xiamen Univ. Exped. to Wuyishan 800874 (AU); Jianyang, Wuyishan Exped. 820081 (NAS); Jiangle, Longxishan Exped. 1847, 1909, 3089, 3135 (PE); Liancheng, R. Lin 3257 (PE), 3747 (PE); Nanjing, C.J. Zeng 80 (AU), P. Lin 917, 960 (AU), Xiamen Univ. Exped. 951 (AU); Shanghang, Xiamen Univ. Exped. to Meihuashan 547, 626 (AU); Wuyishan, Wuyishan Exped. 469, 470 (KUN); Precise locality not known, R. Lin 3589 (PE), X.Q. Wang 82216 (NAS). Guangdong: Dongguan, S.Y. Lau 346 (SYS); Guangzhou, S.B. Guo W.217 (IBSC); Huaiji, W.T. Tsang 22761 (IBK, IBSC, SYS); Shenzhen, Shenzhen Exped. 356, 405, 1591, 1651 (PE); Wengyuan, X.Q. Liu 24353 (IBK, IBSC); Precise locality not known, W.T. Tsang 31360 (IBSC, SYS). Guangxi: Cangwu, Cangwu Exped. 7-098 (GXMI), X.F. Wu 12016 (GXMI, KUN); Cenxi, Cenxi Exped. 7-110, 7-402 (GXMI); Gongcheng, Y.X. Feng 6-5276 (GXMI); Guiping, N.K. Liang 15945 (GXMI); Hezhou, Hexian Exped. 7-185, 7-998 (GXMI), Anonymous 401375 (IBK); Jinxiu, D. Fang et al. 378 (GXMI); Lingui, G.Z. Li 15945 (PE); Mengshan, S.Z. Huang 15611 (GXMI); Pingnan, Y.P. Chen & L. Jiang EM283 (KUN); Xing'an, G.Z. Li & Z.X. Liao 10889 (IBK); Zhaoping, Zhaoping Exped. 7-566 (GXMI). Henan: Xinxian, Anonymous 99 (KUN, PE). Hubei: Huangpi, Liu 857 (HIB); Huanggang, Medicine Inspecting Institute s.n. (HIB); Wuchang, C.H. Qian 1675 (WUK), Medicine Inspecting Institute s.n. (HIB), J.W. Wang 2 (PE); Wuhan, Z.E. Zhao 701 (HIB); Xishui, Anonymous s.n. (HIB). Jiangsu: Nanjing, J.S. Yue 600 (NAS); Suzhou, H. Migo s.n. (NAS); Yixing, Courtois 33045 (NAS), Z.L. Ding 39 (NAS), J. Shen 663 (NAS), G.J. Song s.n. (NAS). Jiangxi: Dayu, Anonymous 1741 (LBG); Guangchang, J.S. Yue et al. 2546 (IBSC, KUN); Huichang, K.M. Hu 3254 (IBSC, KUN, LBG, PE); Longnan, D.C. Wu 781198 (PE); Yanshan, M.X. Nie 4274 (IBSC, KUN, LBG); Ruijin, K.M. Hu 3905 (IBSC, KUN, LBG, PE); Shangrao, M.X. Nie 4781 (KUN, LBG, PE); Shicheng, M.X. Nie 4658 (KUN, LBG, PE); Wuyuan, Courtois 27597, 31475 (NAS); Yihuang, Q.H. Li & C. Chen 1772 (LBG, PE); Zixi, M.X. Nie 3242 (KUN, LBG). Taiwan: Hualian, Keng & Kao 2574 (TAI), S. Suzuki 8733 (TAI), M.T. Kao 6972 (TAI), T.C. Huang 4245, 4275, 4301, 4321 (TAI); Taibei, M. Taizo 4730 (TAI), N. Fukuyama 4471 (TAI), S. Tokio 18445 (NAS, PE, TAI), M.T. Kao 2113, 6298 (TAI), C.M. Kuo 8993, 9000, 9007 (TAI), C.C. Hsu 13342, 5564, 5565 (TAI); Taidong, S. Sasaki 380480 (TAI); Yilan, N. Taizo 4730 (TAI), C.I. Peng 1643 (TAI). Hong Kong: S.Y. Lau 3241 (HK), W.J. Tutcher 8394 (HK), W.Y. Chun 7847 (SYS), N.Q. Chen 41905 (IBK, IBSC), L. Jiang JL00444 (HK). Zhejiang: Chun'an, S. Chen 2394 (NAS, PE); Hangzhou, H. Migo s.n. (NAS), Oliver 90, 812 (NAS), J.L. Chu 162 (NAS), S.Y. Zhang 1342 (HHBG, NAS, PE), 1551 (HHBG, NAS, PE), 2720 (HHBG, PE), P. L. Zhu 105 (PE), Anonymous 1032 (HHBG); Jiande, S. Chen 2094 (PE); Jinyun, K.K. Tsoong 833 (PE); Jingning, Anonymous 3641 (NAS); Lishui, S.Y. Zhang 6398 (PE), Anonymous 6015 (NAS); Lin'an, H. Migo s.n. (NAS), Y.P. Chen & Q.R. Zhao EM039 (KUN), Y.Y. Ho 260 (IBSC, WUK), 716 (NAS), 849 (HHBG, IBSC, WUK), 869 (HHBG, IBSC), 25217 (HHBG), 25299 (NAS, PE), 25585 (HHBG, NAS), T.N. Liou 274 (PE), Y.H. Liu 1329 (NAS), Zhejiang Plant Resour. Exped. 29544 (NAS), Anonymous 770 (IBSC, KUN, PE); Linhai, H. Migo s.n. (NAS); Longquan, R.H. Shan 5677 (NAS, PE), Y.Y. Ho 3110 (NAS), 3114 (PE), T.S. Wang et al. 5566 (NAS), S.Y. Zhang 22829 (HHBG, NAS), Zhejiang Exped. 8449 (NAS), D.X. Zuo 21657 (HHBG, NAS), 22121 (NAS), 22904 (HHBG, NAS), Anonymous 5066 (NAS); Ningbo, S. Chen 4301 (PE), Y.Y. Ho 643 (PE), P.C. Tsoong 1081 (PE); Pingyang, Anonymous 4444, 24884 (NAS); Qingyuan, K.K. Tsoong 833 (PE); Rui'an, Y.Y. Ho 1504 (PE); Taishun, Zhejiang Exped. 8340 (NAS), D.X. Zuo et al. 23574 (NAS); Tiantai, S. Chen 3802 (PE), Y.L. Keng 1080 (NAS, PE), Hangzhou Bot. Garden Herb. 2697 (HHBG), Y.Y. Ho 27863 (NAS), S.Y. Zhang 28327 (NAS), Anonymous 1038 (NAS); Wenling, Hangzhou Bot. Garden Herb. 4083 (PE); Yunhe, S. Chen 672 (PE), Y.Y. Ho 3623 (NAS, PE), Anonymous 4001 (NAS).
Specimens of I. bifidocalyx examined:
CHINA. Anhui: Huangshan, Anonymous 4065 (NAS); Qingyang, E China Workstation 5914 (NAS, PE); Yixian, M. Liu et al. A130122 (PE). Guangdong: Renhua, L. Deng 7208 (IBSC, KUN, PE, SZ). Hunan: Nanxian, Y. Liu 334 (NAS, PE); Wugang, C.L. Xiang et al. 856, 866, 869 (KUN); Xinning, C.S. Fan & Y.Y. Li 458 (NAS); Yizhang, S.Q. Chen 2600 (IBK). Jiangxi: Binhai, S.R. Zhang 467 (NAS). Jiangxi: Guangchang, Q.M. Hu 5383 (IBSC, KUN); Guixi, M.X. Nie & S.S. Lai 3802 (PE); Jiujiang, H. Migo s.n. (NAS), H.C. Cheo 269 (NAS), K.J. Guan 74562 (PE), Y. Tsiang 10728 (NAS), M.X. Nie & S.R. Chen 7631 (LBG), C.M. Tan 95694 (HIB, IBSC, NAS, PE), M.J. Wang 978 (LBG, NAS, PE), 1076 (NAS, PE), 2190 (NAS), W.C. Cheng 5730 (PE), Y. Zhou 913 (LBG); Yanshan, M.X. Nie 4560 (PE); Suichuan, J.S. Yue et al. 4294 (IBSC, KUN); Wuyuan, Y. Tchong 27435 (NAS), R.C. Ching 8993 (PE); Xiushui, Y.Q. Miao & L.X. Li TanCM1257 (KUN). Zhejiang: Hangzhou, Y.Y. Ho 26289 (HHBG, IBSC, NAS), 26738 (NAS); Jiande, S. Chen 1965 (PE); Kaihua, M.L. Yu 26229 (NAS); Lin'an, Y.P. Chen & Q.R. Zhao EM040 (KUN), Y.Y. Ho 438 (HHBG, NAS), 726 (HHBG), 1868 (NAS), 25622 (HHBG, NAS), L.S. Liu et al. 79305 (NAS), T.S. Wang et al. 7771 (NAS), K.K. Tsoong D399 (PE), Anonymous 25683 (HHBG, PE, SZ); Longquan, Y.Y. Ho 3270 (PE), Anonymous 22516 (HHBG, NAS); Yuhang, S. Chen 2459 (PE).
Baldwin B.G., Markos S., 1998. Phylogenetic utility of the external transcribed spacer (ETS) of 18S-26S rDNA: congruence of ETS and ITS trees of Calycadenia(Compositae). Mol. Phylogenet. Evol, 10: 449-463. DOI:10.1006/mpev.1998.0545 |
Beardsley P.M., Olmstead R.G., 2002. Redefining Phrymaceae: the placement of Mimulus, tribe Mimuleae, and Phryma. Am. J. Bot, 89: 1093-1102. DOI:10.3732/ajb.89.7.1093 |
Chen, Y.P., 2017. Taxonomic and Molecular Phylogenetic Studies on Isodon (Schrad. Ex Benth. ) Spach (Lamiaceae) in China. University of Chinese Academy of Sciences, Beijing. Dissertation.
|
Chen Y.P., Hu G.X., Xiang C.L., 2014. Isodon delavayi (Ocimeae, Nepetoideae, Lamiaceae): a new species from Yunnan Province, Southwest China. Phytotaxa, 156: 291-297. DOI:10.11646/phytotaxa.156.5.5 |
Chen Y.P., Drew B.T., Li B., et al, 2016a. Resolving the phylogenetic position of Ombrocharis (Lamiaceae), with reference to the molecular phylogeny of tribe Elsholtzieae. Taxon, 65: 123-136. DOI:10.12705/651.8 |
Chen Y.P., Xiang C.L., Sunojkumar P., et al, 2016b. Isodon villosus (Nepetoideae, Lamiaceae), a new species from Guangxi, China. Phytotaxa, 268: 271-278. DOI:10.11646/phytotaxa.268.4.5 |
Chen Y.P., Hu G.X., Zhao F., et al, 2017. Taxonomic notes on Isodon (Lamiaceae) in China, Ⅱ: I. aurantiacus, a new species from Tibet, China. Ann. Bot. Fenn, 54: 239-243. DOI:10.5735/085.054.0606 |
Chen Y.P., Wilson T.C., Zhou Y.D., et al, 2019. Isodon hsiwenii (Lamiaceae: Nepetoideae), a new species from Yunnan, China. Syst. Bot, 44: 913-922. DOI:10.1600/036364419X15710776741486 |
Doyle J.J., Doyle J.D., 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull, 19: 11-15. |
Edgar R.C., 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res, 32: 1792-1797. DOI:10.1093/nar/gkh340 |
Harley, R.M., Atkins, S., Budantsev, A.L., et al., 2004. Labiatae. In: Kubitzki, K., Kadereit, J.W. (Eds. ), The Families and Genera of Vascular Plants, vol. 7. Springer, Berlin & Heidelberg, pp. 167-275.
|
Li H.W., 1988. Taxonomic review of Isodon (Labiatae). J. Arnold Arbor, 69: 289-400. |
Li, H.W., Hedge, I.C., 1994. Lamiaceae. In: Wu, C.Y., Raven, P.H. (Eds. ), Flora of China, vol. 17. Science Press, Beijing & Missouri Botanical Garden Press, St. Louis, pp. 269-291.
|
Liu M., Wang W.G., Sun H.D., et al, 2017. Diterpenoids from Isodon species: an update. Nat. Prod. Rep, 34: 1090-1140. DOI:10.1039/C7NP00027H |
Mabberley, D.J., 2008. Mabberley's Plant-Book: a Portable Dictionary of the Plants, third ed. Cambridge University Press, Cambridge.
|
Miller, M.A., Pfeiffer, W., Schwartz, T., 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE). New Orleans, LA, pp. 1-8.
|
Paton A.J., Ryding O., 1998. Hanceola, Siphocranion and Isodon and their position in the Ocimeae (Labiatae). Kew Bull, 53: 723-731. DOI:10.2307/4110492 |
Ronquist F., Teslenko M., van der Mark P., et al, 2012. MrBayes 3. 2:efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol, 61: 539-542. DOI:10.1093/sysbio/sys029 |
Stamatakis A., 2014. RAxML version 8:a tool for phylogenetic analysis and postanalysis of large phylogenies. Bioinformatics, 30: 1312-1313. DOI:10.1093/bioinformatics/btu033 |
Stover B., Müller K., 2010. TreeGraph 2:Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinf, 11: 1-9. |
Sun Y., Skinner D.Z., Liang G.H., et al, 1994. Phylogenetic analysis of Sorghum and related taxa using internal transcribed spacers of nuclear ribosomal DNA. Theor.Appl. Genet, 89: 26-32. DOI:10.1007/BF00226978 |
Sun H.D., Huang S.X., Han D.B., 2006. Diterpenoids from Isodon species and their biological activities. Nat. Prod. Rep, 23: 673-698. DOI:10.1039/b604174d |
Tamura K., Stecher G., Peterson D., et al, 2013. MEGA6:molecular evolutionary genetics analysis version 6. 0. Mol. Biol. Evol, 30: 2725-2729. DOI:10.1093/molbev/mst197 |
Thiers, B., 2020. Index Herbariorum: a Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden's Virtual Herbarium. http://sweetgum.nybg.org/science/ih/. (Accessed 3 April 2020).
|
Walker J.B., Sytsma K.J., 2007. Staminal evolution in the genus Salvia (Lamiaceae): molecular phylogenetic evidence for multiple origins of the staminal lever. Ann.Bot, 100: 375-391. DOI:10.1093/aob/mcl176 |
Wu, C.Y., Li, H.W., 1977. Rabdosia (Bl. ) hassk. In: Wu, C.Y., Li, H.W. (Eds. ), Flora Reipublicae Popularis Sinicae, vol. 66. Science Press, Beijing, pp. 416-534.
|
Xiang C.L., Liu E.D., 2012. A new species of Isodon (Lamiaceae, Nepetoideae) from Yunnan Province, Southwest China. Syst. Bot, 37: 811-817. DOI:10.1600/036364412X648751 |
Yu X.Q., Maki M., Drew B.T., et al, 2014. Phylogeny and historical biogeography of Isodon (Lamiaceae): rapid radiation in south-west China and Miocene overland dispersal into Africa. Mol. Phylogenet. Evol, 77: 183-194. DOI:10.1016/j.ympev.2014.04.017 |
Zhong, J.S., Li, J., Li, L., et al., 2010. Phylogeny of Isodon (Schard. ex Benth. )Spach (Lamiaceae) and related genera inferred from nuclear ribosomal ITS, trnL-trnF region, and rps16 intron sequences and morphology. Syst. Bot. 35, 207-219.
|



