b. Yunnan Forestry Technological College, 1 Jindian, Kunming, 650224, Yunnan, PR China;
c. Forest Inventory and Planning Institute of Yunnan Province, Kunming, 650051, Yunnan, China
Sexual reproduction is one of the most important life-cycle stages in determining plant distribution, abundance, and population dynamics (Stieha et al., 2014). Plants growing at high elevations are challenged by hostile environmental conditions, including reduced atmospheric pressure, low temperatures, short growth seasons and low soil nutrient availability, increased ultraviolet radiation, as well as abundant and prolonged snow cover (Körner, 2003). For flowering plants, especially those that depend on pollinators to reproduce, high elevations pose an additional challenge: at high elevations, pollinator diversity, abundance, and activity decrease (Arroyo et al., 1985; Bingham and Orthner, 1998). Compared with the disadvantages of sexual reproduction in stressful environments, clonal reproduction (i.e., production of potentially independent offspring by vegetative growth) has been demonstrated to be advantageous. Clonal growth enables plants to uptake nutrition from multiple sites through horizontal organs (Evans and Cain, 1995), share limited resources among ramets (Alpert, 1996), store resources in below-ground clonal structures to persist through cold seasons (Venn and Morgan, 2009), and increase floral displays, thus attracting more pollinators (Zhang and Zhang, 2006). Importantly, clonal reproduction provides a safe alternative to risky seed production and recruitment (Callaghan and Emanualson, 1985). Consequently, clonal reproduction is thought to play an important role in population establishment and maintenance in stressful environments (e.g., Grace, 1993; Körner, 2003; Evette et al., 2009), and higher proportions of clonal reproduction are predicted to occur in cold environments (e.g., alpine regions) than in temperate environments (e.g., lowlands) (Klimeš et al., 1997; Stamati et al., 2007). Despite recent progress, an improved understanding of the evolutionary adaptations of clonal reproduction requires the use of standardized methodologies to conduct comparative studies of different floras (Klimešová et al., 2011; Klimešová and Dolezal, 2011).
One natural laboratory for studying plant evolution is the subnival belt of the Hengduan Mountains (Sun et al., 2014). The Hengduan Mountains are located at the southeastern region of the Qinghai-Tibet Plateau; because of its high levels of endemism and species diversity, this region has been designated one of the 36 most important global biodiversity hotpots (Myers et al., 2000; Hrdina and Romportl, 2017). The alpine subnival belt (i.e., highest vertical zone of vegetation) is characterized by extreme environments, such as low temperatures, strong wind, frequent precipitation, intense ultraviolet radiation, and low insect abundance and activity (He et al., 2006; Wang, 2006). Although the subnival belt of the Hengduan Mountains experiences the same environmental characteristics as other subnival belts around the world, it is home to probably the most abundant subnival flora, with two or three times the number of plant species than any other known subnival belt (Körner, 2003; Xu et al., 2014). Furthermore, plants growing in these areas frequently exhibit specialized adaptations to the extreme environments, represented by 'cushion', 'glasshouse' or 'downy' plants (Tsukaya and Tsuge, 2001; Sun et al., 2014). However, most previous studies on reproductive adaptation of plants in this region have mainly focused on sexual reproduction (Peng et al., 2012; Sun et al., 2014), and information on clonal reproduction is strikingly scarce. Furthermore, the few studies available have mostly been conducted at the intra-specific level (e.g. Lu et al., 2007; Fan and Yang, 2009), whereas no study has been conducted at the community level or at relatively large scales, which is particularly important for understanding the adaptive strategies of plants (Hegland and Totland, 2005; Xu et al., 2020).
In this study, we aimed to characterize the clonal plant species in the subnival belt of the Hengduan Mountains. Previous studies have reported that plants with different sex systems may experience different levels of pollination limitation. For instance, because unisexual flowers rely on pollen vectors for pollination, they are more likely than bisexual flowers to receive insufficient pollen (Vamosi and Queenborough, 2010). In response to stronger pollination limitation, plants with unisexual flowers may evolve higher level of clonal reproduction for reproductive assurance. In addition, prolonged longevity in woody plants may compensate for reduced mate assurance (Arroyo and Squeo, 1990), thereby potentially mitigating the selective pressure for clonal reproduction. Thus, we determined whether clonality evolved at randomly or differently in various sex systems and growth forms. In general, species native to high-stress environments tend to have more adaptations or tolerance than species native to lower-stress habitats (Caldwell et al., 1982). Thus, we also determined whether the incidence of clonality is correlated with the elevational distribution of plants.
2. Materials and methods 2.1. Study regionThe Hengduan Mountains, a core region of the Sino-Himalayan Floristic Region, is located at the eastern edge of the Qinghai-Tibet Plateau, the highest and youngest plateau in the world (Wu, 1988; Zhang et al., 2009). This area stretches between 24°84′ to 34°80′N and 96°82′ to 104°83′E, and covers 364, 000 km2 that encompass northwestern Yunnan, western Sichuan, southwestern Tibet, southern Qinghai and southwestern Gansu (Li, 1987, Fig. 1). In contrast with other mountains and rivers in China, six mountain chains and rivers cross the region from north to south. The climate in this area is mainly affected by the southwest monsoon as well as plateau monsoon, with distinct dry (from October to mid-May) and wet (from mid-May to October) seasons (Zhang et al., 2009). Our study covered the subnival belt of the Hengduan Mountains, which is the transition zone between alpine meadows and the permanent snowline, with an average elevation usually above 4300 m. Representative climatic conditions of the subnival belt of the Hengduan Mountains, according to a meteorological station in the Baima Snow Mountains (28°23′N, 99°01′E, 4290 m a.s.l.), include an annual average air temperature of -1.0 ℃, and yearly precipitation between 680 and 790 mm, with means of 30 mm in May, 130 mm in June, and 500 mm between July and September (Wang, 2006).
|
| Fig. 1 The geographical location of the Hengduan Mountains, SW China. |
We compiled a list of all of the angiosperm species occurring in the study area, mainly based on Peng et al. (2012) and Xu et al. (2014). Varieties and subspecies were treated as independent species. In total, 793 angiosperm species were included in our dataset. The number of species analyzed in this study accounted for more than 80% of all angiosperms in the subnival belt of the Hengduan Mountains (Peng et al., 2012; Xu et al., 2014).
We categorized plant species into clonal plants and non-clonal plants based on morphological characters described in Flora of China (Wu et al., 1994–2012). Species were designated as clonal if they are capable of producing new and potentially independent ramets through vegetative reproduction (i.e., species were categorized as 'clonal' if they were described by the term/s 'rhizome', 'rhizocarp', 'sarment', 'tiller', 'stolon', 'runner', 'turion', 'leaf bud', 'caudex', 'perennating root', 'lateral stem', 'root stucker', 'rootstock', 'underground bud', 'bulb', 'pseudobulb', or 'creep'; species lacking these features were categorized as 'non-clonal'). For example, Aster flaccidus Bge., which is described as having a 'long, slender, sometimes stoloniferous' rhizome, was categorized as clonal. This is a commonly used method in identification of plant clonality (e.g., Ye et al., 2014, 2016; Zhang et al., 2018).
Information on the sex system and growth form for each species are available from a published data set (Peng et al., 2012). We classified each plant species into one of two growth-form categories: herbaceous plants or woody plants. Both dioecious and monoecious flowers depend on pollen vectors for pollination. In addition, there were a small number of species for each (dioecious plant: 46 species; monoecious plant: 48 species). Therefore, species were classified as bisexual (hermaphroditic) or unisexual (including dioecious and monoecious species). In addition, we collected data on elevational range for each species from Xu et al. (2014), complemented by data from the Chinese Virtual Herbarium, published papers, and personal communications with experts. We considered a species clonal at all elevations between its upper and lower limits if it was classified as clonal following Bhatta et al. (2018). We used distribution, elevation, and habitat to divide each species into one of two types: alpine-endemic species or non-alpine-endemic species. Alpine-endemic species only occur in zones above the timber line. Non-alpine-endemic species occur in zones above and below the timber line. The timber line in the Hengduan Mountains varies widely, from 3800 m in western Sichuan to 4600 m in southeastern Tibet (Wang et al., 2004).
2.3. Data analysesThe distribution of clonality according to sex systems (bisexual vs. unisexual), growth-forms (woody vs. herbaceous), and distribution ranges (alpine-endemic vs. non-alpine-endemic) was compared using G-test.
We used logistic regression analysis to determine the relationship between clonality and elevation (see Pincheira-Donoso et al., 2017; Song et al., 2020). For this analysis, the elevation for each occurrence was extracted and the species' mean value was used (Chen et al., 2017). Clonality data was modeled as a binary response variable (0 = absence of clonality, 1 = presence of clonality) with logit link function (Pincheira-Donoso et al., 2017). First, to test for an overall association between elevation and incidence of clonal reproduction, we performed a simple logistic regression analysis using the full dataset where elevation predicted clonality. Second, to test whether elevational gradient of clonality depended on sex system or growth form, multiple logistic regressions were performed where clonality was predicted by elevation, sex system, growth form, and their interaction. If the interaction was significant, univariate logistic regressions with elevation as a predictor separately were performed to quantify the elevational pattern of clonality in each sex system or each growth form. Model selection was conducted based on the Akaike Information Criterion (AIC value). We also examined the relationship between incidence of clonal reproduction and elevation in the context of phylogeny following Chen et al. (2017). We constructed a 793-species phylogenetic tree following the approach of Qian and Jin (2016) and used phylogenetic logistic regression analysis to test for correlations.
All statistical analyses were conducted in the R computation platform (version 3.3.3) (R Development Core Team, 2017).
3. ResultsIn total, 793 species in 41 families of angiosperms in the subnival belt of the Hengduan Mountains were included in the data set we examined. Across the 41 families of angiosperms, 15 (36.59%) were exclusively clonal, 8 (19.51%) were exclusively non-clonal. The remaining 18 (43.90%) were families with clonal and non-clonal plants, i.e., 33 (80.49%) families had the ability to reproduce clonally. Of 131 genera, 57 (43.51%) were exclusively clonal, 47 (35.88%) were exclusively non-clonal, and the remaining 27 (20.61%) included both clonal and non-clonal plants, i.e., 84 (64.12%) genera had the ability to reproduce clonally. The overall proportion of species with clonal reproduction was 47.92% (380 clonal species).
Of all species, 699 produced bisexual flowers (88.15%) and 94 species produced unisexual flowers (11.85%). Clonality was significantly associated with sex systems (Table 1). Species with unisexual flowers had a significantly higher proportion of clonality than those with bisexual flowers.
| Group of traits | Percentage (%) | G | P |
| Growth form | |||
| Herbaceous | 51.04 | 33.87 | < 0.001 |
| Woody | 16.67 | ||
| Sex system | |||
| Bisexual | 43.63 | 45.67 | < 0.001 |
| Unisexual | 79.79 | ||
| Distribution range | |||
| Alpine-endemic | 58.33 | 11.03 | < 0.01 |
| Non-alpine-endemic | 44.60 | ||
There were 721 herbaceous species (90.92%) and 72 woody species (9.08%). The distribution of clonality differed significantly among growth forms (Table 1). Clonal reproduction was more represented among herbaceous plants and less represented among woody plants.
Based on their distributional range, elevation and habitat, 192 species (24.21%) were found to be endemic to the alpine zone and 601 species (75.79%) were also found in lower elevations. Although these species co-occurred in the subnival belt, alpine-endemic species had a significantly higher proportion of clonality than non-alpine-endemic species (Table 1). We further analyzed the relationship between clonality and elevational distribution range. A simple logistic regression showed a significantly positive association between incidence of clonality and elevation range (Fig. 2). Furthermore, such association did not change when the evolutionary relationships between species were considered (parameter α = 0.02; Appendix S1; Ives and Garland, 2010). When considering categorical factors (sex system, growth form) and their interactions with elevation in the multiple logistic regression analyzes, the best-fit model (AIC = 966, compared to AIC = 970 of the full model) detected a significant elevation × sex system and a significant elevation × growth form (Table 2), indicating that elevation gradients in clonal reproduction were contingent on sex system or growth form. In a separate regression analysis of clonality on elevation, a significantly positive relationship between clonality and elevation was detected in unisexual plants, but this relationship was marginally significant in bisexual plants; furthermore, a steeper elevational gradient in incidence of clonal reproduction was found in plants with unisexual flowers in comparison with their bisexual counterparts (slope: 0.002 vs. 0.0003; Fig. 3ab). In a separate regression analysis of clonality on elevation, the probability of clonal reproduction increased towards high elevations for herbaceous plants, but no significant gradient was found for woody plants (Fig. 3cd).

|
| Fig. 2 The relationship between transition in clonal mode (0 = absence of clonality, 1 = presence of clonality) and elevation in all angiosperms in the subnival belt of the Hengduan Mountains. The fitted line displays the predicted probability of clonality as fit by logistic regression mode. |
| Variable | Z-value | P |
| Elevation | 2.36 | < 0.05 |
| Sex system | 2.11 | < 0.05 |
| Growth form | 2.03 | < 0.05 |
| Elevation × sex system | -2.31 | < 0.05 |
| Elevation × growth form | -2.27 | < 0.05 |
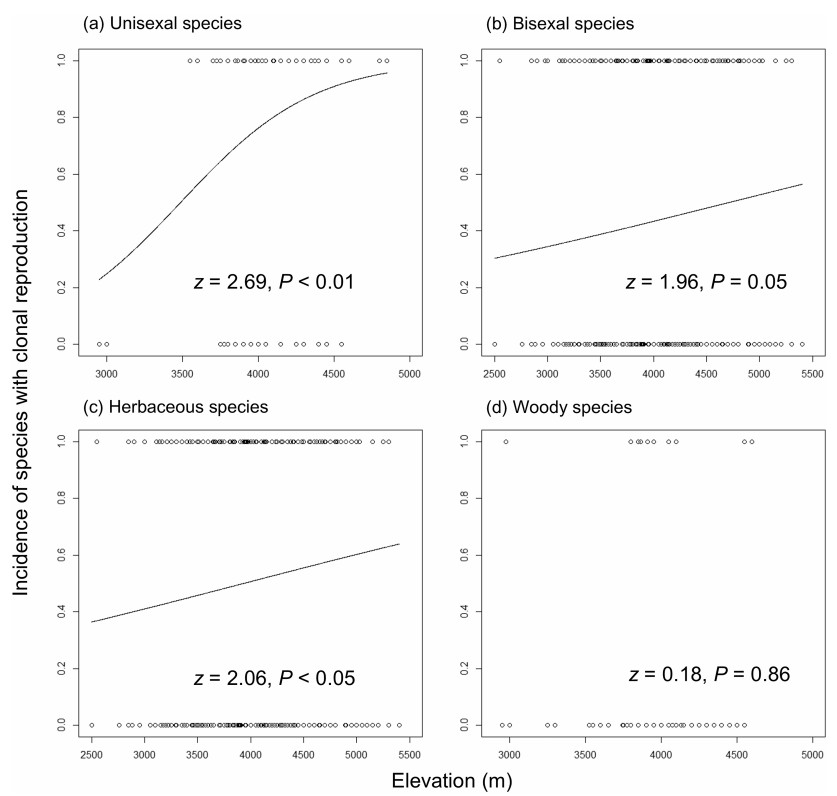
|
| Fig. 3 The relationships between transition in clonal mode (0 = absence of clonality, 1 = presence of clonality) and elevation in unisexual (a), bisexual (b), herbaceous (c), and woody species (d) in the subnival belt of the Hengduan Mountains. The fitted lines display the predicted probability of clonality as fit by logistic regression modes. |
The proportion of angiosperm species with clonal reproduction in the subnival belt of the Hengduan Mountains is 47.92%, which is significantly higher than that in Ladakh (26.7%; Klimeš, 2003), an alpine region adjacent to our study region, and also higher than that in Australia (9.4%; Zhang et al., 2018). These differences may to a large extent be attributed to environmental differences. For example, although Ladakh has a similar latitude (33°N) and elevation (4180–5970 m a.s.l.) to our study region, its mean annual precipitation (115 mm) is significantly lower, as it is rarely affected by the warm and wet monsoon (Miehe et al., 2001). Based on large-scaled horizontal analyses that spanned all of China from the tropics to the temperate zones, Ye et al. (2014) found that clonality was positively associated with precipitation. Similarly, Klimešová and Dolezal (2011) stated that aquatic habitats were more favorable for clonal reproduction, because plants can become fragmented and transported via water current and easily re-root. Thus, the arid environment in Ladakh may, to some extent, limit the evolution of clonality. In addition to lower precipitation, Zhang et al. (2018) proposed that the relatively low proportion of clonal species in Australian flora might be attributed to their unusual taxonomic composition, such as the preponderance of fire-prone vegetation (e.g. sclerophyll woodland), as it is difficult for these plant species to reproduce clonally (Bond and Midgley, 2001). Compared to subnival floras in the Alps and Caucasus (c. 70–90%; reviewed by Klimešová and Dolezal, 2011), the subnival flora in our study region had a much lower proportion of species with clonal reproduction. The reason for such a pattern is not yet clear. One possible explanation for this pattern is that the higher-latitude subnival belts of the Alps and Caucasus have harsher environments, which might result in stronger selection for clonal reproduction. Another possible reason for the higher proportion of clonal plants in the subnival belt of Caucasus might be attributed to the higher proportion of plants that easily reproduce clonally, such as Allium L. plants (Stearn, 1978). Thus, further studies comparing environmental condition and species composition between these floras should be conducted to improve our understanding of the selective agents on clonal reproduction.
Both sex system and growth form had significant influences on the incidence of clonal reproduction: unisexual species included higher proportions of clonal species than bisexual species; and herbaceous species included higher proportions of clonal species than woody species. Although various abiotic factors (e.g., low soil fertility, cold weather and wet habitat) can affect clonal reproduction (Zhang et al., 2018), the differences in incidence of clonality between sex system and growth form may mainly be attributed to different selective pressures for reproductive assurance. Plants with different sex systems have been widely thought to have different levels of pollination limitation (Larson and Barrett, 2000). For instance, because bisexual plants can self-pollinate, they have an advantage over unisexual plants when pollinators are absent (Crawford, 1989; Zhang and Li, 2008; Song et al., 2014). In contrast, unisexual flowers completely depend on vectors for cross-pollination. In addition, although clonal growth can increase floral display and thus enhance pollinator attraction, it can also bring a fitness cost due to geitonogamy, particularly for bisexual plants (Zhang and Zhang, 2006). Thus, the selection for clonal reproduction should be stronger in unisexual plants than in bisexual plants. The same causal pathway can be used to explain the difference in incidence of clonality between growth forms. Woody plants live longer than herbaceous plants. This increased longevity of woody plants may compensate for reduced pollination service (Pannell and Barrett, 1998), thereby potentially reducing selection for clonal reproduction as a mode of reproductive assurance.
We found a significantly higher proportion of clonality in alpine-endemic species than in co-occurring non-alpine-endemic species. This is consistent with the idea that the more often organisms are exposed to unfavorable environmental conditions, the more able they are to adapt to and/or tolerate these conditions (e.g., Barnes et al., 1987). Thus, it is not surprising that alpine-endemic species had higher levels of clonality than did species distributed from the alpine zone to lower elevations. The effect of elevational distribution on clonality was further supported by our logistic regression analyses, which showed a highly significant increase in the incidence of clonal reproduction with increasing elevation. There are obvious environmental gradients in Hengduan Mountains, and higher elevation ecosystems can experience harsher environments, including lower temperatures, stronger ultraviolet radiation, shorter growth season and lower pollinator abundance and activity (He et al., 2006; Wang, 2006). Thus, in contrast to some previous studies (e.g., de Kroon and Knops, 1990; Klimešová and Dolezal, 2011), our results support the idea that clonal reproduction is associated with high elevational habitats (Körner, 2003; Evette et al., 2009). The difference in incidence of clonality between sex systems or between growth forms should be especially pronounced in species distributed at high elevations, because selection for clonal reproduction to compensate for failure of sexual reproduction should be stronger in unisexual or herbaceous plants (see above). As expected, the elevational gradients in clonality were contingent on sex system or growth form. It is worth noting that the different pattern of clonality between growth forms, particularly the lack of a significant elevational gradient in clonality in woody plants, may result from their relatively small elevational range and/or relatively small number of species sampled. Therefore, future studies sampling a range of woody plant species along a wide elevational range are needed to fully understand the evolution of clonality. Nevertheless, our study further supports the idea that biological characteristics (e.g., plant growth form) should be considered in quantifying geographical gradients in clonality (and other traits) (Moeller et al., 2017; Zhang et al., 2018).
Note that, in our study, when a species was considered clonal, it just meant that this species had the potential to reproduce independent offspring by vegetative growth. Compared to clonal reproduction, sexual reproduction in most plant species has a distinct priority, particularly for maintaining population genetic diversity and adapting to changeable environments (Zhang and Zhang, 2006). For example, a study on Polygonum viviparum L. showed that more resources were allocated to sexual reproduction compared with vegetative reproduction in harsher environments (Fan and Yang, 2009). Consequently, further studies determining the relative importance of clonal reproduction vs. sexual reproduction between growth forms, sexual systems, and elevation ranges are particularly crucial for understanding the evolution of clonal reproduction (Dorken and Eckert, 2001).
5. ConclusionThe adaptive strategies of plants that live in the harsh environmental conditions of the subnival belt of the Hengduan Mountains have been well studied (Sun et al., 2014). For example, previous studies have shown that this region harbors a high proportion of plants with specialized phenotypes, bisexual flowers, and generalized flowers (e.g. Tsukaya and Tsuge, 2001; Peng et al., 2012; Song et al., 2013). To this list, our study adds information about asexual reproduction, a crucial part of plant adaptations. Previous studies have found clonality was associated with various unfavorable environmental conditions, including low or high temperatures, high soil moistures, and low nutrient availability (Klimeš et al., 1997; Klimešová et al., 2011; Ye et al., 2014). Further studies that explore the mechanisms that underlie patterns of clonal reproduction are particularly important to understanding the likely responses of alpine ecosystems to climate change in the subnival belt of the Hengduan Mountains.
Author contributionsY.Q.G, F.D, and X.Q.L. planned and designed the research; Y.Q.G. and J.X.Z. collected the data; Y.Q.G. and J.X.Z. analyzed data; Y.Q.G. wrote the manuscript.
Declaration of Competing InterestThe authors declare that they have no potential conflict of interests.
AcknowledgementsThis work was supported by the Science Research Foundation of Yunnan Provincial Department of Education (2019J0551 to Y. Q. Gao).
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2020.06.006.
Alpert P., 1996. Nutrient sharing in natural clonal fragments of Fragaria chiloensis. J. Ecol, 84: 395-406. DOI:10.2307/2261201 |
Arroyo M.T.K., Armesto J.J., Primack R.B., 1985. Community studies in pollination ecology in the high temperate Andes of central Chile Ⅱ. Effect of temperature on visitation rates and pollination possibilities. Plant Systemat. Evol, 149: 187-203. DOI:10.1007/BF00983305 |
Arroyo, M.T.K., Squeo, F.A., 1990. Relationship between plant breeding systems and pollination. In: Kawano, S. (Ed.), Biological Approaches and Evolutionary Trends in Plants. Academic Press, London, pp. 205-227.
|
Barnes P.W., Flint S.D., Caldwell M.M., 1987. Photosynthesis damage and protective pigments in plants from a latitudinal Arctic/Alpine gradient exposed to supplemental UV-B radiation in the field. Arct. Alp. Res, 19: 21-27. DOI:10.2307/1550996 |
Bhatta K.P., Grytnes J.A., Vetaas O.R., 2018. Scale sensitivity of the relationship between alpha and gamma diversity along an alpine elevation gradient in central Nepal. J. Biogeogr, 45: 804-814. DOI:10.1111/jbi.13188 |
Bingham R.A., Orthner A.R., 1998. Efficient pollination of alpine plants. Nature, 391: 238-239. DOI:10.1038/34564 |
Bond W.J., Midgley J.J., 2001. Ecology of sprouting in woody plants: the persistence niche. Trends Ecol. Evol, 16: 45-51. DOI:10.1016/S0169-5347(00)02033-4 |
Caldwell M.M., Robbercht R., Nowak R., 1982. Differential photosynthetic inhibition by ultraviolet radiation in species from the arctic-alpine life zone. Arct. Alp.Res, 14: 195-202. DOI:10.2307/1551152 |
Callaghan, T.V., Emanualson, U., 1985. Population structure and process of tundra plants and vegetation. In: White, J. (Ed.), The Population Structure of Vegetation. Junk Publishing, Dordrecht, pp. 399-439.
|
Chen S.C., Cornwell W.K., Zhang H.X., Moles, et al, 2017. Plants show more flesh in the tropics: variation in fruit type along latitudinal and climatic gradients. Ecography, 40: 531-538. DOI:10.1111/ecog.02010 |
Crawford, R.M.M., 1989. Study in Plant Survival: Ecological Case Histories of Plant Adaptation to Adversity. Blackwell Scientific Publications, Oxford, p. 296.
|
de Kroon H., Knops J., 1990. Habitat exploration through morphological plasticity in two chalk grassland perennials. Oikos, 59: 39-49. DOI:10.2307/3545120 |
Dorken M.E., Eckert C.G., 2001. Severely reduced sexual reproduction in northern populations of a clonal plant, Decodon verticillatus (Lythraceae). J. Ecol, 89: 339-350. DOI:10.1046/j.1365-2745.2001.00558.x |
Evans J.P., Cain M.L., 1995. A spatially explicit test of foraging behavior in a clonal plant. Ecology, 76: 1147-1155. DOI:10.2307/1940922 |
Evette A., Bedecarrats A., Bornette G., 2009. Environmental constraints influence clonal traits of herbaceous plant communities in an alpine Massif. Folia Geobot, 44: 95-108. DOI:10.1007/s12224-009-9039-8 |
Fan D.M., Yang Y.P., 2009. Altitudinal variations in flower and bulbil production of an alpine perennial, Polygonum viviparum L. (Polygonaceae). Plant Biol, 11: 493-497. DOI:10.1111/j.1438-8677.2008.00188.x |
Grace J.B., 1993. The adaptive significance of clonal reproduction in angiosperms:an aquatic perspective. Aquat. Bot, 44: 159-180. DOI:10.1016/0304-3770(93)90070-D |
He Y.P., Duan Y.W., Liu J.Q., et al, 2006. Floral closure in response to temperature and pollination in Gentiana straminea Maxim. (Gentianaceae), an alpine perennial in the Qinghai-Tibetan Plateau. Plant Systemat. Evol, 256: 17-33. |
Hegland S.J., Totland O., 2005. Relationships between species' floral traits and pollinator visitation in a temperate grassland. Oecologia, 145: 586-594. DOI:10.1007/s00442-005-0165-6 |
Hrdina A., Romportl D., 2017. Evaluating global biodiversity hotspots e very rich and even more endangered. J. Landscape Ecol, 10: 108-115. DOI:10.1515/jlecol-2017-0013 |
Ives A.R., Garland T., 2010. Phylogenetic logistic regression for binary dependent variables. Syst. Biol, 59: 9-26. DOI:10.1093/sysbio/syp074 |
Klimeš L., 2003. Life-forms and clonality of vascular plants along an altitudinal gradient in E Ladakh (NW Himalayas). Basic Appl. Ecol, 4: 317-328. DOI:10.1078/1439-1791-00163 |
Klimeš, L., Klimešová, J., Hendriks, R., et al., 1997. Clonal plant architecture: a comparative analysis of form and function. In: de Kroon, H., van Groenendael, J.(Eds.), The Ecology and Evolution of Clonal Plants. Backhuys, Kerkwerve, pp. 1-29.
|
Klimešová J., Dolezal J., 2011. Are clonal plants more frequent in cold environments than elsewhere?. Plant Ecol. Divers, 4: 373-378. DOI:10.1080/17550874.2011.586734 |
Klimešová J., Dolezal J., Dvorsky M., et al, 2011. Clonal growth forms in eastern Ladakh, western Himalayas: classification and habitat preferences. Folia Geobot, 46: 191-217. DOI:10.1007/s12224-010-9076-3 |
Körner, C.H., 2003. Alpine Plant Life. Springer Verlag, Berlin.
|
Larson B.M.H., Barrett S.C.H., 2000. A comparative analysis of pollen limitation in flowering plants. Biol. J. Linn. Soc, 69: 503-520. DOI:10.1111/j.1095-8312.2000.tb01221.x |
Li B.Y., 1987. On the boundaries of the Hengduan mountains. Mt. Res, 5: 74-82. |
Lu J.Y., Ma R.J., Sun K., 2007. Clonal diversity and structure in Polygonum viviparum. J. Plant Ecol, 31: 561-567. DOI:10.17521/cjpe.2007.0072 |
Miehe G., Winiger M., Bohner J., et al, 2001. The climatic diagram map of High Asia. Purpose and concepts. Erdkunde, 55: 94-97. DOI:10.3112/erdkunde.2001.01.06 |
Moeller D.A., Runquist R.D.B., Moe A.M., et al, 2017. Global biogeography of mating system variation in seed plants. Ecol. Lett, 20: 375-384. DOI:10.1111/ele.12738 |
Myers N., Mittermeier R.A., Mittermeier C.G., et al, 2000. Biodiversity hotspots for conservation priorities. Nature, 403: 853-858. DOI:10.1038/35002501 |
Pannell J.R., Barrett S.C.H., 1998. Baker's law revisited: reproductive assurance in a metapopulation. Evolution, 52: 657-668. DOI:10.1111/j.1558-5646.1998.tb03691.x |
Peng D.L., Zhang Z.Q., Xu B., et al, 2012. Patterns of flower morphology and sexual systems in the subnival belt of the Hengduan Mountains, SW China. Alpine Bot, 122: 65-73. DOI:10.1007/s00035-012-0104-1 |
Pincheira-Donoso D., Jara M., Reaney A., Garcia-Roa R., et al, 2017. Hypoxia and hypothermia as rival agents of selection driving the evolution of viviparity in lizards. Global Ecol. Biogeogr, 26: 1238-1246. DOI:10.1111/geb.12626 |
Qian H., Jin Y., 2016. An updated megaphylogeny of plants, a tool for generating plant phylogenies and an analysis of phylogenetic community structure. J. Plant Ecol, 9: 233-239. DOI:10.1093/jpe/rtv047 |
R Development Core Team, 2017. R: a Language and Environment for Statistical Computing. R Fundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. (Accessed September 2017).
|
Song B., Chen G., Stöcklin J., et al, 2014. A new pollinating seed-consuming mutualism between Rheum nobile and a fly fungus gnat Bradysia sp., involving pollinator attraction by a specific floral compound. New Phytol, 203: 1109-1118. DOI:10.1111/nph.12856 |
Song B., Sun L., Lev-Yadun S., et al, 2020. Plants are more likely to be spiny at midelevations in the Qinghai-Tibetan Plateau, south-western China. J. Biogeogr, 47: 250-260. DOI:10.1111/jbi.13724 |
Song B., Zhang Z.Q., Stöcklin J., et al, 2013. Multifunctional bracts enhance plant fitness during flowering and seed development in Rheum nobile (Polygonaceae), a giant herb endemic to the high Himalayas. Oecologia, 172: 359-370. DOI:10.1007/s00442-012-2518-2 |
Stamati K., Hollingsworth P.M., Russell J., 2007. Patterns of clonal diversity in three species of sub-arctic willow (Salix lanata, Salix lapponum and Salix herbacea). Plant Systemat. Evol, 269: 75-88. DOI:10.1007/s00606-007-0578-2 |
Stearn W.T., 1978. European species of Allium and allied genera of Alliaceae: a synonymic enumeration. Ann. Mus. Goulandris, 4: 83-198. |
Stieha C.R., Middleton A.R., Stieha J.K., et al, 2014. The dispersal process of asexual propagules and the contribution to population persistence in Marchantia(Marchantiaceae). Am. J. Bot, 101: 348-356. |
Sun H., Niu Y., Chen Y.S., et al, 2014. Survival and reproduction of plant species in the Qinghai-Tibet Plateau. J. Systemat. Evol, 52: 378-396. DOI:10.1111/jse.12092 |
Tsukaya H., Tsuge T., 2001. Morphological adaptation of inflorescences in plants that develop at low temperatures in early spring: the convergent evolution of "downy plants". Plant Biol, 3: 536-543. DOI:10.1055/s-2001-17727 |
Vamosi S.M., Queenborough S.A., 2010. Breeding systems and phylogenetic diversity of seed plants along a large-scale elevational gradient. J. Biogeogr, 37: 465-476. DOI:10.1111/j.1365-2699.2009.02214.x |
Venn S.E., Morgan J.W., 2009. Patterns in alpine seedling emergence and establishment across a stress gradient of mountain submits in south-eastern Australia. Plant Ecol. Divers, 2: 5-16. DOI:10.1080/17550870802691356 |
Wang X.P., Zhang L., Fang J.Y., 2004. Geographical differences in alpine timberline and its climatic interpretation in China. Acta Geograph. Sin, 59: 871-879. |
Wang, Y., 2006. Yunnan Mountain Climate. Science and Technology Press, Kunming.
|
Wu Z.Y., 1988. The Hengduan Moutain flora and her significance. J. Jpn. Bot, 63: 1-14. |
Wu, C.Y., Raven, P.H., Hong, D.Y., 1994-2012. Flora of China. Science Press, Beijing.
|
Xu B., Li Z.M., Sun H., 2014. Seed Plants of the Alpine Subnival Belt from the Hengduan Mountains, SW China. Beijing|: Science Press.
|
Xu Q., Lev-Yadun S., Sun L., et al, 2020. Spinescent patterns in the flora of Jiaozi snow mountain, southwestern China. Plant Divers, 42: 83-91. DOI:10.1016/j.pld.2019.12.002 |
Ye D., Hu Y., Song M., et al, 2014. Clonality-climate relationships along latitudinal gradient across China: adaptation of clonality to environments. PloS One, 9: e94009. DOI:10.1371/journal.pone.0094009 |
Ye D., Liu G.F., Song Y.B., et al, 2016. Strong but diverging clonality-climate relationships of different plant clades explain weak overall pattern across China. Sci. Rep, 6: 26850. DOI:10.1038/srep26850 |
Zhang D.C., Zhang Y.H., Boufford D.E., et al, 2009. Elevational patterns of species richness and endemism for some important taxa in the Hengduan Mountains, southwestern China. Biodivers. Conserv, 18: 699-716. DOI:10.1007/s10531-008-9534-x |
Zhang H.X., Bonser S.P., Chen S.C., et al, 2018. Is the proportion of clonal species higher at higher latitudes in Australia?. Austral Ecol, 43: 69-75. DOI:10.1111/aec.12536 |
Zhang Z.Q., Li Q.J., 2008. Autonomous selfing provides reproductive assurance in an alpine ginger Roscoea schneideriana (Zingiberaceae). Ann. Bot, 102: 531-538. DOI:10.1093/aob/mcn136 |
Zhang Y.F., Zhang D.Y., 2006. Asexual and sexual reproductive strategies in clonal plants. J. Plant Ecol, 30: 174-183. DOI:10.17521/cjpe.2006.0024 |



