Heavy metal pollution is a global environmental problem (Moreno-Caselles et al., 2000, Gao, 2016). In the European Union, approximately 3.5 million sites are contaminated by heavy metals, while half a million sites may be highly contaminated (Mahar et al., 2016). In China, 7.0% of soil sites that have been investigated are contaminated by Cd (http://www.gov.cn/xinwen/2014-04/17/content_2661765.htm). One of the largest harmful effects of heavy metals (especially high-toxicity Cd) on the environment is that they can enter the food chain through plant absorption, thereby threatening ecosystems and human health (Gao, 2016). Reducing the risks of heavy metal toxicity to humans relies on reducing heavy metal uptake in crop plants from soils. Hence, to cope with potential soil heavy metal pollution, scientists have focused on screening and breeding crop and vegetable cultivars with low heavy metal accumulation levels (Liu et al., 2010, Rizwan et al., 2016). However, several plant species that adsorb, absorb, transport, and/or transform heavy metals have been used to reduce heavy metal bioavailability in the environment; this process is called soil pollution phytoremediation (Pilon-Smits, 2005).
Phytoremediation is simple, environmentally friendly, and cost-effective (Pilon-Smits, 2005). Phytoremediation technologies can be divided into several categories based on remediation mechanisms, and include phytoextraction, phytostabilization, phytoevaporation, rizofiltration, and rhizodegradation (Mahar et al., 2016). Of these, phytoextraction and phytostabilization are the most widespread and effective soil remediation techniques (Mahar et al., 2016). Phytoextraction is the uptake of heavy metals from soils by plant roots and their translocation and accumulation in the aboveground parts (McGrath and Zhao, 2003). Phytoextraction directly depends on the absorption and transport capacity of the heavy metals in plants. Thus, heavy metal high-accumulating species (e.g., heavy metal hyperaccumulators) are effective tools for phytoextraction. Hyperaccumulators are used to define plant species that can accumulate more than the threshold values of heavy metal concentrations (e.g., 100 mg kg-1 for Cd; 1 000 mg kg-1 for Ni, Cu, and Pb; 10 000 mg kg-1 for Zn and Mn) in the dry aboveground parts (or leaves) without biomass reduction in heavy metal-polluted soils (Baker and Whiting, 2002, Li et al., 2016). In contrast, phytostabilization aims to decrease the mobility and bioavailability of the heavy metals in soils on the basis of their stabilization from off-site transport by the aid of plants (Pulford and Watson, 2003). Heavy metal fixation is primarily based on their absorption and accumulation in roots and the precipitation in the root zone through organic compound binding (Cunningham and Ow, 1996). Numerous studies have screened plant species for phytoextraction and/or phytostabilization by characterizing heavy metal accumulation and distribution in these candidate phytoremediators (Lorestani et al., 2013, Courchesne et al., 2017, Nikolic et al., 2017, Eisazadeh et al., 2019). Ornamental plants are strong potential phytoremediation materials that affect landscapes greatly.
Sansevieria trifasciata Prain, which now belongs to the Liliaceae family, is an evergreen, succulent, perennial plant that is native to tropical Western Africa. At present, S. trifasciata Prain, which has a number of cultivars, is commonly grown as an ornamental in tropical and subtropical regions and as an indoor potted plant in many areas worldwide. In China, S. trifasciata is known as an air purifier that absorbs noxious gases, such as formaldehyde, xylene, and total volatile organic compounds (Guo et al., 2007). However, few studies have examined the capacity of S. trifasciata to accumulate heavy metals. Yao et al. (2016) found that cultivar S. trifasciata 'Laurentii' accumulates a lower U concentration than other species, and that U is mainly accumulated in roots. No information about the accumulation characteristics of other heavy metals in S. trifasciata is available, although a patent (Alberto, 2017) declared that S. trifasciata can be used as a phytoremediator in an open dumpsite to decontaminate heavy metals, including Cu, Pb, iron, and Zn. In this study, we explored the Cd tolerance and accumulation characteristics of the three common S. trifasciata cultivars. The results will aid in evaluating the potential of S. trifasciata cultivars as phytoremediators to decontaminate Cd in soils.
2. Materials and methods 2.1. Plant growth and treatmentsSeedlings of S. trifasciata cultivars (S. 'Trifasciata', S. trifasciata 'Laurentii', and S. trifasciata 'Silver Hahnii') were purchased from the flower market in Yunnan, China. The seedlings of each cultivar were neatly transplanted into flowerpots (d = 18.5 cm, h = 17.5 cm) with equal amounts of soils (one seedling in each pot). One control group and one treatment group were set for each cultivar. Control groups were grown in Cd-free soils (pH: 5.55, organic matter: 313 g kg-1, total P: 1.24 g kg-1, total N: 9.06 g kg-1, available P: 38.20 mg kg-1, hydrolytic N: 1.19 g kg-1, and total Cd: < 0.003 mg kg-1), and treatment groups were grown in soil with 50 mg Cd kg-1 dry weight (DW). This Cd concentration is commonly used in studies to estimate whether a plant is a potential Cd hyperaccumulator (Wang et al., 2012, Li et al., 2016). We first dissolved an accurate Cd content (CdCl2·2.5H2O) in an appropriate amount of deionized water and then fully mixed it with the weighed dry Cd-free soils to obtain 50 mg Cd kg-1 soil (DW). Pots were grown in greenhouse (light: 12–14 h, 20 ℃–25 ℃; darkness: 10–12 h, 18 ℃–20 ℃; humidity: 40%–60%) for 4 months (from mid-June to mid-October) with appropriate watering. Three biological replicates were prepared for each of the following measurements.
2.2. Plant harvesting and measurementThe fresh weight of the plants before and after growth was measured. When harvesting plants, roots were washed three times with deionized water, and root length (the maximum length of the root system) was measured. The shoot and root of each sample were dried under 80 ℃ for 48 h and subsequently weighed.
2.3. Detection of Cd concentrationDry samples of the treatment group were then used to measure Cd concentrations by inductively coupled plasma mass spectrometry (ICP-MS), as previously described (Li et al., 2017). Briefly, approximately 0.5–1.0 g samples were digested using 5 mL of HNO3 until reactions were finished. Polytetrafluoroethylene digestion tanks were then sealed and placed in a microwave digestion instrument to conduct the predefined digestion procedure (100 ℃, 3 min; 140 ℃, 3 min; 160 ℃, 3 min; 180 ℃, 3 min; and 190 ℃, 15 min). Cooled digestion solutions were entirely transferred to 50 mL volumetric flasks, and volumes were fixed to the measurement scale. Sample solutions were detected using ICP-MS, and Cd contents were calculated according to the standard curve.
2.4. Index calculationOn the basis of the Cd concentrations, we calculated the Cd bioconcentration factor (BCF) and translocation factor (TF) for each cultivar. We also estimated the Cd accumulation content in an individual plant and Cd transfer content in the shoot by combining tissue biomass and the corresponding Cd concentration, as follows:
BCF = shoot (root) Cd concentration/soil Cd concentration,
TF = shoot Cd concentration/root Cd concentration,
Cd content in the shoot of the individual plant = shoot Cd concentration × shoot biomass,
Cd content in the whole individual plant = shoot Cd concentration × shoot biomass + root Cd concentration × root biomass,
Cd transfer proportion in shoot = Cd content in the shoot of the individual plant/Cd content in the whole individual plant × 100%.
2.5. Statistical analysisSPSS version 18.0 was used for statistical analysis. One-way ANOVA was used to analyze the significant differences of the results among the three cultivars at 0.05 levels whereas an independent-samples t-test (2-tailed) was used between the control and treatment samples of the same cultivar.
3. Results and discussion 3.1. Cd tolerance characteristicsFor each phytoremediation technique, the primary requirement was that plants should have good tolerance to heavy metals. In this study, a high soil Cd concentration (50 mg kg-1) was used to identify Cd tolerance of the three S. trifasciata cultivars. The results showed that the plant growth of each S. trifasciata cultivar was not negatively affected by Cd treatment for 4 months. As shown in Table 1, the total fresh biomass of the individual plant of each S. trifasciata cultivar was similar between control and treatment groups before treatment and after treatment for 4 months. Thus, the increased biomass of each cultivar was unaffected by Cd treatment. The dry biomasses of the root and shoot of individual plants of three S. trifasciata cultivars were also similar under control and Cd treatment (Fig. 1a). The root lengths (Fig. 1b) of the three S. trifasciata cultivars were also not significantly affected by Cd treatment. These plant phenotypic results indicate that the three S. trifasciata cultivars have high tolerance to Cd stress.
| Sample | Fresh weight before planting (g) | Fresh weight after planting (g) | Increased multiple times | |
| Species | Treatment | |||
| S. 'Trifasciata' | Control | 63.33 ± 15.28 a | 186.67 ± 56.86 a | 1.92 ± 0.18 a |
| Cd-50 | 73.33 ± 20.82 a | 220.00 ± 65.57 a | 2.00 ± 0.12 a | |
| S. trifasciata 'Laurentii' | Control | 73.33 ± 11.55 a | 166.67 ± 25.17 a | 1.28 ± 0.13 b |
| Cd-50 | 90.00 ± 10.00 a | 180.00 ± 17.32 a | 1.00 ± 0.11 b | |
| S. trifasciata 'Silver Hahnii' | Control | 70.00 ± 10.00 a | 163.33 ± 30.55 a | 1.32 ± 0.14 b |
| Cd-50 | 73.33 ± 15.28 a | 166.67 ± 47.26 a | 1.25 ± 0.17 b | |

|
| Fig. 1 Plant growth of three cultivars of Sansevieria trifasciata under control and 50 mg kg-1 Cd treatment (Cd-50) conditions. (a) Plant morphology after growing for 4 months. (b) Dry biomasses of roots and shoots. (c) Root length. For (b) and (c), data represent means ± standard deviation (n = 3); the difference between control and Cd treatment groups for the same cultivar is not significant according to independent-samples t-test (2-tailed). |
Cd tolerance is rare in the family Liliaceae. Chlorophytum comosum, the most studied member of Liliaceae, has been identified as a potential Cd hyperaccumulator (Wang et al., 2012). Researchers have proposed that chive (Allium schoenoprasum), which tolerates 60 mg kg-1 soil Cd, be designated a phytoextraction plant in Cd-contaminated soils (Eisazadeh et al., 2019). Thus, our results provide new insight into the response characteristics of the species under the Liliaceae family to Cd stress.
3.2. Cd accumulation and transfer characteristicsAlthough S. trifasciata cultivars tolerate Cd stress, their ability to accumulate high Cd levels in tissues had yet to be determined. After 4 months of growth in soil with Cd (50 mg kg-1), the mean Cd concentrations in the shoots of S. 'Trifasciata', S. trifasciata 'Laurentii', and S. trifasciata 'Silver Hahnii' were 63.0, 65.1, and 59.4 mg kg-1 DW, while those in the roots were 626.7, 571.7, and 272.3 mg kg-1 DW, respectively (Fig. 2a). The Cd concentrations in the shoots of the three cultivars were similar, whereas those in the roots of S. 'Trifasciata' and S. trifasciata 'Laurentii' were significantly higher than that in root of S. trifasciata 'Silver Hahnii' (P < 0.05, Fig. 2a). The reason for the difference in Cd accumulation among different cultivars should be explored in the future.
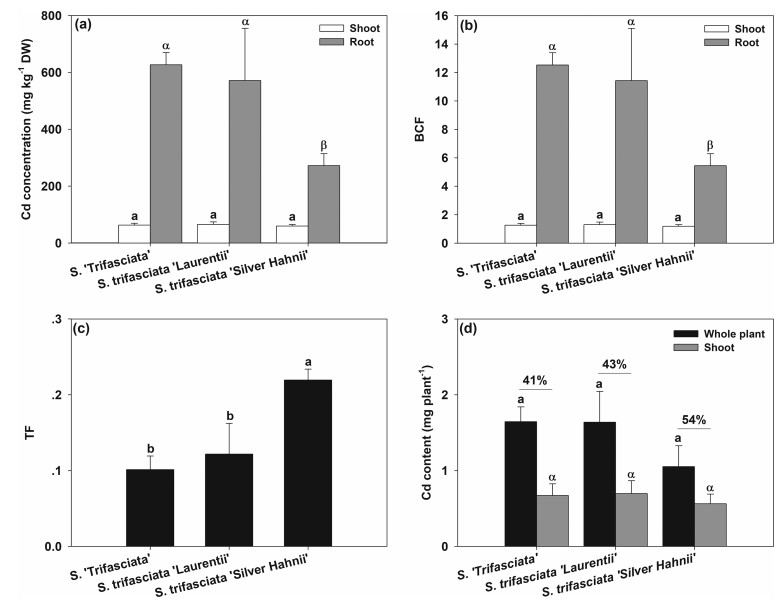
|
| Fig. 2 Cd accumulation characteristics of three cultivars of Sansevieria trifasciata under 50 mg kg-1 Cd treatment (Cd-50). (a) Cd concentration in the shoot and root. (b) The bioconcentration factor (BCF) of the shoot and root. (c) The translocation factor (TF) of the shoot. (d) Cd accumulation contents in single plant and its shoot. For (a)–(d), data represent means ± standard deviation (n = 3). Bars with the same color that are labeled with different letters (a, b or α, β) indicate significant differences among the three cultivars at P = 0.05. |
On the basis of the Cd concentrations in plant tissues and soils, we calculated BCFs and TFs for different cultivars. BCF is an important parameter that reflects the abilities of plants to absorb or ingest heavy metals from the soil environments (Liao et al., 2013). TF is another important index that reflects heavy metal transfer in plants (Rezapour et al., 2019). The mean Cd BCFs in the shoots of S. 'Trifasciata', S. trifasciata 'Laurentii', and S. trifasciata 'Silver Hahnii' were 1.26, 1.30, and 1.19, while those in the roots were 12.53, 11.43, and 5.45 respectively (Fig. 2b). Given that the plants were grown in the same soil, the significant differences between root and shoot Cd concentrations and Cd BCFs among various cultivars were similar (Fig. 2a and b). The mean Cd TFs of S. 'Trifasciata', S. trifasciata 'Laurentii', and S. trifasciata 'Silver Hahnii' were 0.10, 0.12, and 0.22, respectively (Fig. 2c). The Cd TF of S. trifasciata 'Silver Hahnii' was significantly higher than those of S. 'Trifasciata' and S. trifasciata 'Laurentii' (P < 0.05, Fig. 2c). The latest definition of hyperaccumulators requires the shoot BCF and TF to be greater than 1 (McGrath and Zhao, 2003, Li et al., 2016). Thus, the low TFs of the three S. trifasciata cultivars indicate that these cultivars are not potential Cd hyperaccumulators. However, Cd concentrations as high as those in the roots of S. 'Trifasciata' and S. trifasciata 'Laurentii' have rarely been reported in previously. Therefore, the mechanisms by which these roots tolerate such high Cd concentrations deserve further exploration. The mechanism underlying low Cd TF of S. trifasciata cultivars would be useful for agricultural production. Most plant foods are consumed for their aboveground tissues. Therefore, breeding crop cultivars with low Cd translocation may decrease Cd intake risk in human bodies.
In summary, the Cd accumulation characteristics of S. trifasciata cultivars suggest that the roots of these plants may include unique mechanisms of Cd distribution and detoxification. However, the change patterns in BCFs and TFs when soil Cd concentrations or plant growth periods vary still require studies.
3.3. Phytoremediation potential for Cd-contaminated soilsThe total Cd content in plants, which is dependent on biomasses and Cd concentrations, is the key factor in evaluating the potential of plants for phytoremediation. In this study, the mean Cd contents in the whole individual plant of S. 'Trifasciata', S. trifasciata 'Laurentii', and S. trifasciata 'Silver Hahnii' were 1.64, 1.64, and 1.05 mg, respectively (Fig. 2d). Approximately half of the total accumulated Cd (41%–54%) was allocated in the shoots of S. 'Trifasciata', S. trifasciata 'Laurentii', and S. trifasciata 'Silver Hahnii', which accounted for 0.67, 0.69, and 0.56 mg, respectively (Fig. 2d). Cd contents showed significant differences among the three cultivars in neither the whole individual plant nor the shoot of individual plant (Fig. 2d).
Previous studies suggested that heavy metal accumulation level and tissue distribution determines what phytoremediation technique (e.g., phytoextraction or phytostabilization) the plant is suitable for. Plants that accumulate high heavy metal concentrations in roots could be designed as phytostabilizers, whereas several species are still recommended as phytoextractors (De la Torre et al., 2016, Asensio et al., 2018, Manzoor et al., 2018, Eisazadeh et al., 2019). For example, chive is designated as a potential species for reducing Cd from contaminated soils despite the low Cd reallocation from roots to shoots (Eisazadeh et al., 2019). By contrast, Pteris melanocaulon, which exhibits a high Cu BCF of 4.04 and a low Cu TF of 0.01, has potential as a metallophyte for Cu phytostabilization (De la Torre et al., 2016). In the present study, the significantly high Cd concentrations in the roots and low TFs of the three S. trifasciata cultivars indicate that these plants are more suitable for phytostabilization, although approximately 1/2 of the total Cd was allocated in the shoots. Our results also showed that S. trifasciata cultivars had relatively slow growth rates (Table 1) for a perennial plant. Thus, these cultivars are unsuitable for Cd phytoextraction, which requires high Cd removal efficiency. S. trifasciata also contains glycosides and saponins and is extremely toxic (Mimaki et al., 1996, Mimaki et al., 1997). This result indicates that S. trifasciata has few predators, and is less likely to transfer Cd into the food chain. Overall, S. trifasciata can be designed as a potential Cd phytostabilizer in several regions worldwide. However, the plants should be uprooted to remove Cd when the phytostabilization processes are finished due to high Cd concentration in plants, especially in the roots.
4. ConclusionsIn this study, we identified the Cd tolerance and accumulation characteristics of the three S. trifasciata cultivars. The results showed that these S. trifasciata cultivars can tolerate 50 mg kg-1 soil Cd concentration. The results of tissue Cd concentrations showed that all three S. trifasciata cultivars accumulated high Cd levels in roots and had low Cd translocation capacities. In combination with total Cd accumulation and distribution and its growth characteristics, S. trifasciata can be designed as a potential phytostabilizer in Cd-contaminated soils in tropical and subtropical regions. Further studies should be conducted to explore the mechanisms of the high-Cd-tolerance and accumulation characteristics in the roots of S. trifasciata.
Author contributionsY.P. Yang and X. Li conceived and designed the experiments. X. Li performed the experiments. X. Li analyzed the data. X. Li wrote the manuscript. Y.P. Yang revised the manuscript.
Declaration of Competing InterestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was financially supported by the Youth Innovation Promotion Association CAS (2020387).
Alberto, A.M.P., 2017. Decontaminating heavy metals e.g. copper in open dumpsite, involves using tropical plants e.g. Achyranthes aspera, Portulaca oleracea, and Sansevieria trifasciata as phytoremediators, and absorbing heavy metals using phytoremediators. PH1201600161-A1.
|
Asensio V., Florido F.G., Ruiz F., Perlatti F., Otero X.L., Ferreira T.O., 2018. Screening of native tropical trees for phytoremediation in copper-polluted soils. Int. J.Phytoremediation, 20: 1456-1463. DOI:10.1080/15226514.2018.1501341 |
Baker A.J.M., Whiting S.N., 2002. In search of the Holy Grail - a further step in understanding metal hyperaccumulation?. New Phytol., 155: 1-4. DOI:10.1046/j.1469-8137.2002.00449_1.x |
Courchesne F., Turmel M.C., Cloutier-Hurteau B., Constantineau S., Munro L., Labrecque M., 2017. Phytoextraction of soil trace elements by willow during a phytoremediation trial in Southern Quebec, Canada. Int. J. Phytoremediation, 19: 545-554. DOI:10.1080/15226514.2016.1267700 |
Cunningham S.D., Ow D.W., 1996. Promises and prospects of phytoremediation. Plant Physiol, 110: 715-719. DOI:10.1104/pp.110.3.715 |
De la Torre J.B., Claveria R.J., Perez R.E., Perez T.R., Doronila A.I., 2016. Copper uptake by Pteris melanocaulon Fee from a Copper-Gold mine in Surigao del Norte, Philippines. Int. J. Phytoremediation, 18: 435-441. DOI:10.1080/15226514.2015.1109603 |
Eisazadeh S., Kapourchal S.A., Homaee M., Noorhosseini S.A., Damalas C.A., 2019. Chive (Allium schoenoprasum L.) response as a phytoextraction plant in cadmium-contaminated soils. Environ. Sci. Pollut. R, 26: 152-160. DOI:10.1007/s11356-018-3545-2 |
Gao B., 2016. Cd isotopic signatures: a potential source tracer of metal pollution in the environment. Environ. Sci. Pollut. R, 23: 941-942. DOI:10.1007/s11356-015-4925-5 |
Guo X.Z., Huang P.C., Wang Y.Y., Wang J.G., Zeng A.P., 2007. Effects of plants on the absorption of indoor pollutant. Chin. J. Environ. Engi, 1: 104-106. |
Li X., Zhang X.M., Li B.Q., Wu Y.S., Sun H., Yang Y.P., 2017. Cadmium phytoremediation potential of turnip compared with three common high Cdaccumulating plants. Environ. Sci. Pollut. R, 24: 21660-21670. DOI:10.1007/s11356-017-9781-z |
Li X., Zhang X.M., Yang Y., Li B.Q., Wu Y.S., Sun H., Yang Y.P., 2016. Cadmium accumulation characteristics in turnip landraces from China and assessment of their phytoremediation potential for contaminated soils. Front. Plant Sci, 7: 1862. |
Liao Q.L., Liu C., Cai Y.M., Zhu B.W., Wang C., Hua M., Jin Y., 2013. A preliminary study of element bioconcentration factors within milled rice and wheatmeal in some typical areas of Jiangsu Province. Chin. Geol, 40: 331-340. |
Liu W.T., Zhou Q.X., Zhang Y.L., Wei S.H., 2010. Lead accumulation in different Chinese cabbage cultivars and screening for pollution-safe cultivars. J. Environ.Manag, 91: 781-788. DOI:10.1016/j.jenvman.2009.10.009 |
Lorestani B., Yousefi N., Cheraghi M., Farmany A., 2013. Phytoextraction and phytostabilization potential of plants grown in the vicinity of heavy metalcontaminated soils: a case study at an industrial town site. Environ. Monit.Assess, 185: 10217-10223. DOI:10.1007/s10661-013-3326-9 |
Mahar A., Wang P., Ali A., Awasthi M.K., Lahori A.H., Wang Q., Li R., Zhang Z., 2016. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: a review. Ecotoxicol. Environ. Saf, 126: 111-121. DOI:10.1016/j.ecoenv.2015.12.023 |
Manzoor M., Gul I., Silvestre J., Kallerhoff J., Arshad M., 2018. Screening of indigenous ornamental species from different plant families for Pb accumulation potential exposed to metal gradient in spiked soils. Soil Sediment Contam, 27: 439-453. DOI:10.1080/15320383.2018.1488238 |
McGrath S.P., Zhao F.J., 2003. Phytoextraction of metals and metalloids from contaminated soils. Curr. Opin. Biotechnol, 14: 277-282. DOI:10.1016/S0958-1669(03)00060-0 |
Mimaki Y., Inoue T., Kuroda M., Sashida Y., 1996. Steroidal saponins from Sansevieria trifasciata. Phytochemistry, 43: 1325-1331. DOI:10.1016/S0031-9422(96)00397-4 |
Mimaki Y., Inoue T., Kuroda M., Sashida Y., 1997. Pregnane glycosides from Sansevieria trifasciata. Phytochemistry, 44: 107-111. DOI:10.1016/S0031-9422(96)00477-3 |
Moreno-Caselles J., Moral R., Perez-Espinosa A., Perez-Murcia M.D., 2000. Cadmium accumulation and distribution in cucumber plant. J. Plant Nutr, 23: 243-250. DOI:10.1080/01904160009382011 |
Nikolic N., Zoric L., Cvetkovic I., Pajevic S., Borisev M., Orlovic S., Pilipovic A., 2017. Assessment of cadmium tolerance and phytoextraction ability in young Populus deltoides L. and Populus x euramericana plants through morphoanatomical and physiological responses to growth in cadmium enriched soil. Iforest, 10: 635-644. DOI:10.3832/ifor2165-010 |
Pilon-Smits E., 2005. Phytoremediation. Annu. Rev. Plant Biol, 56: 15-39. DOI:10.1146/annurev.arplant.56.032604.144214 |
Pulford I.D., Watson C., 2003. Phytoremediation of heavy metal-contaminated land by trees - a review. Environ. Int, 29: 529-540. DOI:10.1016/S0160-4120(02)00152-6 |
Rezapour S., Atashpaz B., Moghaddam S.S., Kalavrouziotis I.K., Damalas C.A., 2019. Cadmium accumulation, translocation factor, and health risk potential in a wastewater-irrigated soil-wheat (Triticum aestivum L.) system. Chemosphere, 231: 579-587. DOI:10.1016/j.chemosphere.2019.05.095 |
Rizwan M., Ali S., Abbas T., Zia-ur-Rehman M., Hannan F., Keller C., Al-Wabel M.I., Ok Y.S., 2016. Cadmium minimization in wheat: a critical review. Ecotoxicol.Environ. Saf, 130: 43-53. DOI:10.1016/j.ecoenv.2016.04.001 |
Wang Y.B., Yan A.L., Dai J., Wang N.N., Wu D., 2012. Accumulation and tolerance characteristics of cadmium in Chlorophytum comosum: a popular ornamental plant and potential Cd hyperaccumulator. Environ. Monit. Assess, 184: 929-937. DOI:10.1007/s10661-011-2010-1 |
Yao T.Y., Wang D., Li Z.H., Long C., Jiang W.J., Chen L., Xiang M.W., 2016. Enrichment characteristics of eight ornamental plants to uranium in soil. Environ. Sci. Technol, 39: 24-30. |



