b. University of Chinese Academy of Sciences, Beijing, 100049, China;
c. Fujian Agriculture and Forestry University, Fuzhou, 350002, China;
d. School of Life Science and Biopharmaceutics, Shenyang Pharmaceutical University, Shenyang, 110016, China;
e. Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201, China
Panax notoginseng (Burk.) F. H. Chen (Araliaceae) is an important plant that is used in traditional Chinese medicine to promote blood circulation, treat bruises, and slow internal and external bleeding (Gu et al., 2015, Gu et al., 2017, Ng, 2010, Wang et al., 2006). This economically important herb has been cultivated for over 400 years, traditionally in southwestern China (c. 23.5° north latitude) between elevations of 1500–1800 m, particularly in Wenshan district and the junction areas of Yunnan and Guangxi Provinces (Qiao et al., 2018, Wang et al., 2016). The herb is normally planted three years before harvest, and is very sensitive to environmental factors (e.g., light, humidity and temperature), which leave it susceptible to root rot, a disease caused mainly by microbial infections (Chen et al., 2002) that rots the root, withers the aerial part of plant, and ultimately leads to the death of the whole plant (Wang et al., 2003, Xun et al., 2013).
Continuous-cropping obstacles have become a serious problem for the cultivation of P. notoginseng. Because of the rapid expansion and intensive plantation in recent decades, the planting interval is at least 15–20 years (Zhu et al., 2015). The exudates of the plant or the secondary metabolites (allelochemicals) of microorganisms in soil might also be toxic (Inderjit, 2006). Previous studies on Panax species have found that secretions and rhizosphere soil extracts of P. ginseng have inhibitory effects on the growth of the herb itself (Li et al., 2009). Moreover, P. ginseng root exudates (e.g., benzoic acid, 1, 2-benzenedicarboxylicacid, bis(2-methyl- propyl) ester, hexadecanoic acid and 2, 2-bis(4-hydroxyphenyl) propane) have shown significant variation in both the allelopathic effect and the degree of that effect on colony growth and conidia germination rates of pathogens and probiotics of ginseng (Li et al., 2009). Several compounds have been isolated and identified from the continuously cultivated soil of P. notoginseng, including β-sitosterol, ferulic acid, ginsenoside Rh1, Rg1, Rh4, carotenoid and notoginsenoside R1 (Zhou et al., 2012). Aqueous extracts of notoginseng and high concentrations of P. notoginseng total saponins (PNS) have also shown significant inhibitory effects, while lower concentration of PNS have been found to increase seed germination of notoginseng trivially (Sun et al., 2008b). Moreover, PNS may promote the growth of two main pathogens of P. notoginseng, Cylindrocarpon destructans and Fusarium solani (Sun et al., 2008a).
To explore the chemical basis of continuous cropping obstacles of P. notoginseng, we used GC-MS analysis and column chromatography to identify and purify compounds from soil cultivated continuously with 3-year-old P. notoginseng. These analyses identified 13 volatile components, isolated another six triterpenes (1–4, 6–7) and one anthraquinone (5). We then tested the effects of compounds 1–7 on seed germination and root elongation in P. notoginseng, several crop plants, and Arabidopsis thaliana. Finally, we investigated the effects of compounds 1–7 on the rhizosphere microorganisms of P. notoginseng.
2. Materials and methods 2.1. General1D NMR spectra were recorded on Bruker AM-400 spectrometer, in CD3OD with TMS as an internal standard. Chemical shifts were reported in units of δ (ppm) and coupling constants (J) were expressed in Hz. ESI-MS were run on a Bruker HCT/Esquire spectrometer. Column chromatography was carried out over silica gel (200–300 mesh, Qingdao Marine Chemical and Industrial Factory, China), Sephadex LH-20 (Pharmacia Fine Chemical Co., Ltd. Uppsala, Sweden), MCI-gel CHP20P (75–100 μm, Mitsubishi Chemical Co., Ltd., Japan). Thin layer chromatography (TLC) analysis was carried out on GF254 silica gel (Qingdao Marine Chemical and Industrial Factory, China), using 10% sulfuric acid-alcohol solution as the chromogenic reagent.
2.2. Soil sample collectionNotoginseng soil samples (hereafter, notoginseng soil) were collected at harvest after three years of cultivation (November, 2012) from a P. notoginseng planting base in Kou-Zi-Shang Village (103.63°E, 24.73°N, 2098 m elev.), Shilin County, Yunnan province, China. At the same time a control soil sample (hereafter, control soil) was collected from an adjacent field in which notoginseng had not been previously cultivated. Before cultivation with P. notoginseng, both the notoginseng and control soils had been planted with corn. Before further examination, the notoginseng and control soil samples were comminuted and sieved through a 40-mesh screen to remove impurities, such as gravel, plastics, and plant debris, as well as any remaining fibrous roots of P. notoginseng.
2.3. Extraction and isolationNotoginseng and control soils (1000 kg) were soaked separately under room temperature with MeOH (600 L for each) three times (each 24 h), and then leached with ultrapure water. Vacuum removal of MeOH and H2O yielded several extracts of notoginseng soil (S3) and control soil (S0): S3–MeOH (435 g), S0–MeOH (337 g), S3–H2O (6.5 g), and S0–H2O (6.9 g). The MeOH extracts (S3: 430 g, S0: 335 g) were separately partitioned between n-hexane, EtOAc, n-BuOH and H2O successively to yield S3-n-hexane (143.0 g), S0-n-hexane (116.5 g), S3-EtOAc (54.1 g), S0-EtOAc (61.0 g), S3-n-BuOH (52.5 g), S0-n-BuOH (17.6 g), S3–H2O (126.0 g) and S0–H2O (107.8 g). The n-hexane fraction was used for subsequent GC-MS analysis.
The EtOAc fraction of S3 (54 g) was added to a chromatography column of silica gel (80 × 9 cm), and eluted with CHCl3–MeOH (50:1 → 5:1) to give five fractions (Fr. A-E). Fraction B (7.0 g) and fraction C (9.0 g) were chromatographed separately over silica gel (CHCl3–MeOH, 30:1 → 10:1), and then MCI-gel CHP20P (MeOH–H2O, 60:40, and 55:45 → 50:50) to yield compound 7 (40 mg) from fraction B, and compounds 1 (5 mg), 2 (3 mg), 3 (35 mg), 4 (4 mg) and 6 (6 mg) from fraction C, respectively. Fraction D (6 g) was applied successively to silica gel (CH3Cl–MeOH, 30:1 → 8:1) and a Sephadex LH-20 (MeOH–H2O, 50:50) chromatography column to yield compound 5 (40 mg).
2.4. GC-MS analysisThe n-hexane extracts of notoginseng and control soil samples were analyzed on a GC-MS system. GC-MS was performed using an Agilent HP 6890 GC equipped with an Agilent 5973 MS (Agilent Technologies) operated at 70 eV. A 2 μL aliquot of each sample was injected into an HP-5MS capillary column (0.25 mm × 30 m, 0.25 μm) with helium carrier gas at 1.0 mL min-1 in the splitless mode. After injection, the oven temperature was programmed to increase from 40 ℃ to 80 ℃ at 3 ℃/min, and from 80 ℃ to 280 ℃ at 5 ℃/min, and then held for 30 min. The injector temperature was maintained at 250 ℃, the ion source temperature at 230 ℃, and the quadrupole temperature at 150 ℃. The recorded mass range was 35 m/z to 500 m/z.
ChemStation software (Agilent Technologies) was used for data acquisition. The peak areas in a GC-MS chromatogram were automatically integrated and corrected through the ChemStation software. Peaks with area lower than 100, 000 were rejected. Peak width was set at 0.1 s, and threshold was set at 14.0. The compounds were identified by searching wiley7n.l library and NIST14 library. Compounds with more than 80% matching value were selected. In addition, three masses per compound were selected as qualifier ions to assist in identification (Zhang et al., 2018a). Peak alignment was performed by comparing retention time among all samples manually. In the end, the relative percentage of each compound in a sample was normalized based on total ion current (TIC) (Cho et al., 2012).
2.5. Seedling germinationTo test the phytotoxic effects of the soil samples on the germination of P. notoginseng seeds, we used a sand culture assay. Seeds of P. notoginseng (provided by Yunnan Academy of Agricultural Sciences) were sterilized with 0.1% mercuric chloride for 1 h after the epidermis was peeled off, and then washed three times with sterile water. For test fraction derived from P. notoginseng soil (S3) and control soil (S0), 100 mg of S0–MeOH and S3–MeOH, and 5 mg of different solvent fractions (n-hexane, EtOAc, n-BuOH and H2O) were dissolved in 5 mL of MeOH and added to a 6-cm sterile glass Petri dish filled with sterilized sand (60 g) separately. An additional Petri dish treated with only MeOH (5 mL) was used as a control (CK). After evaporating the MeOH at 45 ℃ for approximately 10 h, 10 mL sterilized water was sprayed on the sand, and 30 surface-sterilized seeds were placed in each Petri dish, sealed and shaded to germinate in growth chambers. Three repetitions were conducted, the germination levels were recorded after 30 days of incubation (Hoagland and Williams, 2004).
2.6. Root cell death detection and seedling growth testTo determine whether compounds 1–7 inhibited seed germination and induced apoptosis in root tips cells of P. notoginseng and several crop plants, we first sterilized the seeds of P. notoginseng, corn, wheat, turnip and water spinach successively with ethanol (70% v/v) and sodium hypochlorite (5% v/v), each 2 min, and then rinsed with sterile distilled water (three times). The sterilized seeds of P. notoginseng and 20 mL aliquot of testing compounds (1–7) (1.0 μg mL-1) were added to sterilized 50 mL glass bottle. Control seeds were treated the same except for the addition of testing compounds. For each treatment, three repetitions (bottles) were used and each repetition included ten seedlings. The seedlings were incubated in a growth chamber for 24 h; fibrous roots from the seeds of P. notoginseng longer than 2 cm were excised with a sterile razor blade. Seeds of corn, wheat, turnip and water spinach were treated the same as P. notoginseng (Yang et al., 2015a).
We also examined the effects of compounds 1–7 on seeds Columbia (Col) ecotype A. thaliana, which were provided by Wei-Qi Li's research group in the Germplasm Bank of Wild Species. Surface sterilized A. thaliana seeds were cold stratified for 2 days at 4 ℃, and subsequently sown in Petri dishes that contained MS agar (Yang et al., 2015b), 0.2% gellan gum (G1910; Sigma–Aldrich) and 1% sucrose with different concentrations of compounds 1–7. Petri dishes with seeds were placed vertically in growth chambers (23/18 ℃ with 150 μmol m-2 s-1 photosynthetic photon flux density under a day/night cycle of 12/12 h) for 5 days (Yang et al., 2015a).
To detect root death, roots of P. notoginseng, corn, wheat, turnip and water spinach and A. thaliana were counterstained with propidium iodide (PI, 10 μg mL-1) (Sigma Aldrich) for 5–15 min (depending on their size) separately, washed once in distilled water, and mounted in water for confocal microscopy. The roots under all above treatments were observed with an Olympus confocal laser scanning microscope (Olympus Fluoview 1000, Japan) at 535 nm. The experiment was repeated three times, and 10 roots were tested for each treatment. Approximately 10–15 images were examined, and at least two independent experiments were performed. Images were processed with Adobe Photoshop CC (Shaw and Dolan, 2008).
Seedling growth of A. thaliana was used to assess the inhibitory effects of different extract fractions. After incubation for 5 days, the root lengths of ten seedlings per plate were recorded. These tests were conducted separately on three replicates per treatment (Chen et al., 2017, Hu et al., 2016, Romagni, 2004, Bogatek et al., 2006).
2.7. Antimicrobial assayAll the bacteria and fungi were isolated and identified from the rhizosphere soil of P. notoginseng by Yi-Xuan Zhang's lab and were deposited at the School of Life Science and Biopharmaceutics, Shenyang Pharmaceutical University. Prior to these experiments, bacteria Achromobacter marplatensis, Bacillus siamensis and Nocardia rhamnosiphila were reactivated by cultivation at 37 ℃ for 24 h in LB media in glass tubes, while fungi Bionectria ochroleuca, Chaetomium globosum, Cladosporium gossypicola, Cl. uredinicola, Cl. tenuiussimum, Fusarium oxysporium and Penicillium janthinellum were reactivated by cultivation at 28 ℃ for 7 days in PDA media in culture dishes. The LB/PDB bacterial/spore suspension was diluted to the predetermined starting concentration (optical density at 600 nm; OD600 = 0.2), and 1 mL of bacterial/spore solution was mixed with 24 mL media. The prepared solutions of compounds 1–7 (0.1 mg mL-1 per well) and the bacterial/spore solutions of different strains (198 μL per well) were inoculated into 96-well plates, using untreated bacterial solution served as the negative control group, and bacterial solutions treated with ampicillin (Amp, 0.02 mg mL-1), kanamycin (Kan, 0.02 mg mL-1), geneticin (G418, 0.02 mg mL-1) and nystatin (Nys, 0.02 mg L-1) served as positive controls, respectively. The 96-well plates were placed in the incubator at 37/28 ℃ for 24 h, and the growth was measured using a multiplate photometric reader. Every experiment was performed in triplicate to validate the screening method (Liu et al., 2010, Liu et al., 2017, Xie et al., 2017).
3. Results 3.1. Effects of different soil extracts and fractions on the seed germination of P. notoginsengThe seeds of P. notoginseng began to germinate about one month after being buried in sterilized sand, and germination rates were calculated after two months. The solvent extracts and fractions of notoginseng and control soil samples, except for the n-hexane extract fraction from control soil, had a phytotoxic effect on the germination of P. notoginseng seeds (Fig. 1). The strongest inhibitory effects were observed from n-hexane and EtOAc fractions of notoginseng soil (at a concentration of 83 ppm). Thus, we examined these fractions to identify their chemical constituents.
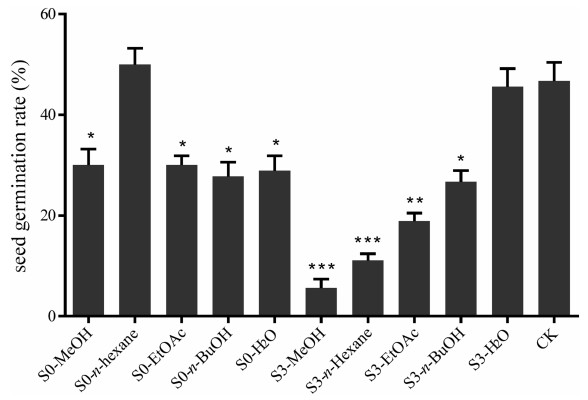
|
| Fig. 1 Inhibitory effect of different extracts and fractions of S0 and S3 soil samples on the seed germination of P. notoginseng. S0: soil sample from uncultivated field; S3: cultivated soil sample of 3-year-old P. notoginseng. All test solutions were prepared using MeOH as solvent. Data are mean ± SD of three independent experiments, *P < 0.05, **P < 0.01, ***P < 0.001. |
The volatile components in the n-hexane fraction of the soil (S3) cultivated continuously with 3-year-old P. notoginseng were analyzed by GC-MS. In total 13 volatile components of seven chemical structure types (alkene, ester, phenanthrene, parazole, benzoheterocycle, terpene and steroid) were identified. The same compounds appeared in the blank control (uncultivated soil, S0) were eliminated from the list (Table S2, Fig. S2).
3.3. Identification of compounds 1–7 from the soil of 3-year-cultivated P. notoginsengBy using column chromatography to purify the EtOAc fraction of notoginseng soil, we isolated six ursane-type triterpenoid acids (1–4, 6–7) and one anthraquinone glucoside (5) (Fig. 2). After comparison of their spectroscopic data with those reported in references, these compounds were identified as ursolic acid (1) (Ju et al., 2003), asiatic acid (2) (Jiang et al., 2013), corosolic acid (3) (Niu et al., 2013), pomolic acid (4) (Ju et al., 2003), chrysophanol-1-O-β-D-glucopyranoside (5) (Annamalai et al., 2013), euscaphic acid (6) (Wu et al., 2014) and tormentic acid (7) (Zhang et al., 2018b).
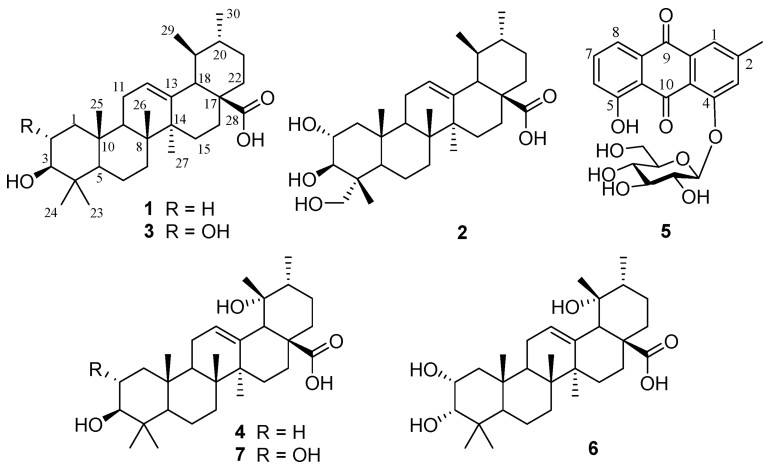
|
| Fig. 2 The chemical structures of compounds 1–7 isolated from the soil cultivated with P. notoginseng. |
Ursolic acid (1): white amorphous powder, C30H48O3, ESI-MS (positive ion mode): m/z 479 [M+Na]+. 1H NMR (pyridine-d5, 400 MHz): δH 1.24 (3H, s, Me-23), 0.96 (3H, s, Me-24), 0.99 (3H, d, J = 6.6 Hz, Me-30), 1.21 (3H, s, Me-26), 1.54 (3H, s, Me-27), 0.95 (3H, d, J = 6.6 Hz, Me-29), 0.93 (3H, s, Me-25). 13C NMR (pyridine-d5, 100 MHz): see Table S1.
Asiatic acid (2): white amorphous powder, C30H48O5, ESI-MS (positive ion mode): m/z 511 [M+Na]+. 1H NMR (pyridine-d5, 400 MHz): δH 0.96 (3H, s, Me-24), 0.98 (3H, d, J = 6.6 Hz, Me-30), 0.99 (3H, s, Me-26), 1.27 (3H, s, Me-27), 0.98 (3H, d, J = 6.6 Hz, Me-29), 1.09 (3H, s, Me-25). 13C NMR (pyridine-d5, 100 MHz): see Table S1.
Corosolic acid (3): white amorphous powder, C30H48O4, ESI-MS (positive ion mode): m/z 495 [M+Na]+. 1H NMR (pyridine-d5, 400 MHz): δH 1.16 (3H, s, Me-23), 0.96 (3H, s, Me-24), 0.98 (3H, d, J = 6.6 Hz, Me-30), 0.98 (3H, s, Me-26), 1.26 (3H, s, Me-27), 0.94 (3H, d, J = 6.6 Hz, Me-29), 1.07 (3H, s, Me-25). 13C NMR (pyridine-d5, 100 MHz): see Table S1.
Pomolic acid (4): white amorphous powder, C30H48O4, ESI-MS (positive ion mode): m/z 495 [M+Na]+. 1H NMR (pyridine-d5, 400 MHz): δH 1.25 (3H, s, Me-23), 0.98 (3H, s, Me-24), 1.23 (3H, s, Me-26), 1.55 (3H, s, Me-27), 0.94 (3H, s, Me-25), 1.43 (3H, s, Me-29), 1.12 (3H, d, J = 6.6 Hz, Me-30). 13C NMR (pyridine-d5, 100 MHz): see Table S1.
Chrysophanol-1-O-β-D-glucopyranoside (5): yellow needle crystal, C21H20O9, ESI-MS (positive ion mode): m/z 439 [M+Na]+. 1H NMR (DMSO-d6, 400 MHz): δH 12.82 (1H, s, OH-5), 7.69 (1H, d, J = 7.8 Hz, H-6), 7.83 (1H, d, J = 7.8 Hz, H-8), 7.87 (1H, t, J = 7.8 Hz, H-7), 7.48 (1H, s, H-1), 7.18 (1H, s, H-3), 4.78 (1H, d, J = 7.8 Hz, H-1'), 2.41 (3H, s, Me-11). 13C NMR (DMSO-d6, 100 MHz): 161.1 (C-1), 124.3 (C-2), 147.9 (C-3), 120.8 (C-4), 119.6 (C-5), 136.2 (C-6), 122.6 (C-7), 158.7 (C-8), 187.7 (C-9), 182.6 (C-10), 114.1 (C-1a), 132.2 (C-4a), 134.6 (C-5a), 117.3 (C-8a), 100.6 (C-1′), 77.5 (C-2′), 76.7 (C-3′), 69.7 (C-4′), 73.4 (C-5′), 60.8 (C-6′), 21.7 (Me-11).
Euscaphic acid (6): yellow needle crystal, C30H48O5, ESI-MS (positive ion mode): m/z 511 [M+Na]+. 1H NMR (pyridine-d5, 400 MH): δH 3.71 (1H, d, J = 1.8 Hz, H-3), 4.26 (1H, d, J = 10.2 Hz, H-3), δH 1.63 (3H, s, Me-27), 1.40 (3H, s, Me-29), 1.23 (3H, s, Me-23), 1.09 (3H, s, Me-26), 0.94 (3H, s, Me-25), 0.88 (3H, s, Me-24), 1.12 (3H, d, J = 6.6 Hz, Me-30). 13C NMR (pyridine-d5, 100 MHz): see Table S1.
Tormentic acid (7): white amorphous powder, C30H48O5, ESI-MS (positive ion mode): m/z 511 [M+Na]+. 1H NMR (pyridine-d5, 400 MHz): δH 1.69 (3H, s, Me-27), 1.42 (3H, s, Me-29), 0.98 (3H, d, J = 6.6 Hz, Me-30), 1.08 (3H, s, Me-26), 1.03 (3H, s, Me-23), 1.24 (3H, s, Me-24), 0.98 (3H, s, Me-25). 13C NMR (pyridine-d5, 100 MHz): see Table S1.
3.4. Root cell death detection and seedling growth test on different plantsSeedlings of P. notoginseng, corn, wheat, turnip, water spinach, and A. thaliana were cultivated separately with compounds 1–7 (each 1.0 μg mL-1) for 5 days, counterstained with propidium iodide (PI, 10 μg mL-1) for 5–15 min, and then observed under 535 nm. As shown in Fig. 3, compared with the blank control (treated with MeOH), the root tip cell structures of all the experimental groups in P. notoginseng were damaged to varying degrees. The dead cell numbers in root tips were counted and statistically analyzed (Fig. 4). The numbers of dead root tip cells treated with compounds 3 and 5–7 were significantly higher than those of the control treatment, indicating that 3 and 5–7 can cause severe apoptosis of P. notoginseng root tip cells.
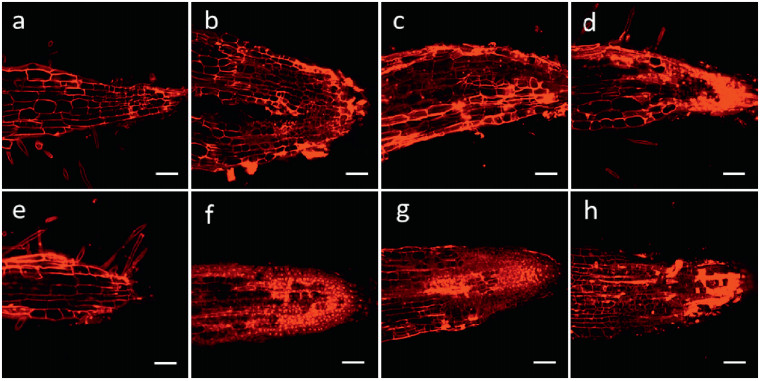
|
| Fig. 3 Representative confocal microscopy images of P. notoginseng root tips treated with compounds 1–7 (each 1.0 μg mL-1, b-h) for 24 h and stained with PI (10.0 μg mL-1). Cell nuclei of dead cells were dyed red by PI. Control group was treated with MeOH (a). Bars = 100 μm. |
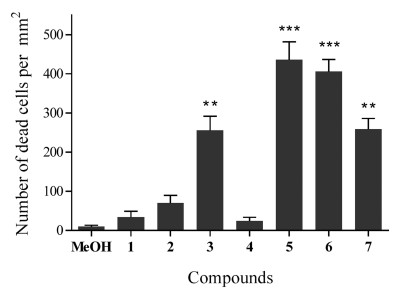
|
| Fig. 4 Quantity statistics of dead cells in the apical roots of P. notoginseng after treatment with compounds 1–7. Statistically significant differences compared with roots without treatments with compounds 1–7 are indicated; *P < 0.05, **P < 0.01, ***P < 0.001. |
The effects of compounds 1–7 on the roots of corn, wheat, turnip and water spinach are shown in Fig. 5 (A-F), compared with the root tips not exposed to these compounds (CK-1-CK-4). Compounds 1 and 6 caused high levels of apoptosis in the root tip cells of corn (Fig. 5A and B). Compound 2 caused similar levels of apoptosis in spinach (Fig. 5C), as did compound 7 in turnip (Fig. 5D), and compounds 3 and 4 in wheat (Fig. 5E and F).
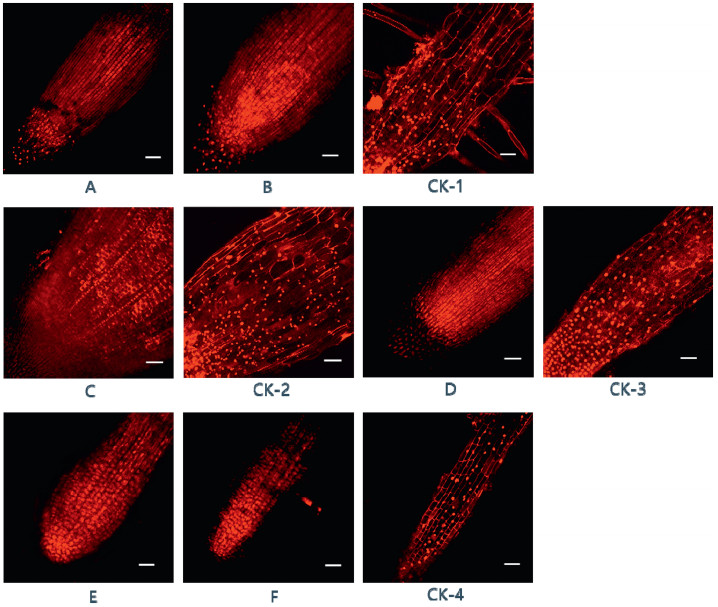
|
| Fig. 5 Representative confocal microscopy images of different crop root tips treated with compounds 1–7 (1.0 μg mL-1) and MeOH (0.1%) for 24 h and stained with PI (10.0 μg mL-1). Corn root with 1 (A), 6 (B), and MeOH (CK-1); water spinach root with 2 (C) and MeOH (CK-2); turnip root with 7 (D) and MeOH (CK-3); wheat root with 3 (E), 4 (F) and MeOH (CK-4). Cell nuclei in dead cells were dyed red by PI. Bars = 100 μm. |
Compounds 2, 6 and 7 induced apoptosis in A. thaliana root tip cells (Fig. 6). Compounds 6 and 7 had a particularly strong effect, inducing cell death in almost all cells in the central elongation zone (CEZ). In contrast, compounds 1–4 promoted the growth of cells and the division of root apical meristem.
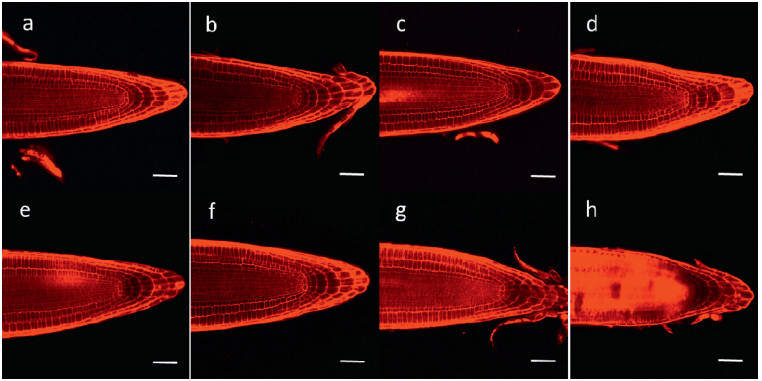
|
| Fig. 6 Representative confocal microscopy images of A. thaliana root tips treated with compounds 1–7 (each 1.0 μg mL-1, b-h) for 5 days and stained with PI (10.0 μg mL-1). Cell nuclei in dead cells were dyed red by PI. Control group was treated with DMSO (a). Bars = 50 μm. |
Compounds 1–7 inhibited A. thaliana root elongation to varying degrees (Fig. 7, Fig. 8, and S3-S9). After treatment with 0.5 μg mL-1 of compound 2, A. thaliana roots were less than 50% the length of those observed under control conditions. Root lengths were similarly reduced after treatment with compounds 2, 3 and 7 at 1 μg mL-1, compounds of 2, 3, 5 and 7 at 5 μg mL-1, and compounds 1–5 and 7 at 10 μg mL-1. In addition, compounds 2 and 4 inhibited root elongation almost completely when administered at 10 μg mL-1. However, these compounds affected root growth differently at high and low concentrations. For example, at high concentrations, compounds 1, 2, 5 and 6 significantly inhibited growth, whereas at lower concentrations they promoted growth. Furthermore, at concentrations between 5 and 10 μg mL-1, compounds 3 and 4 induced lateral root differentiation.
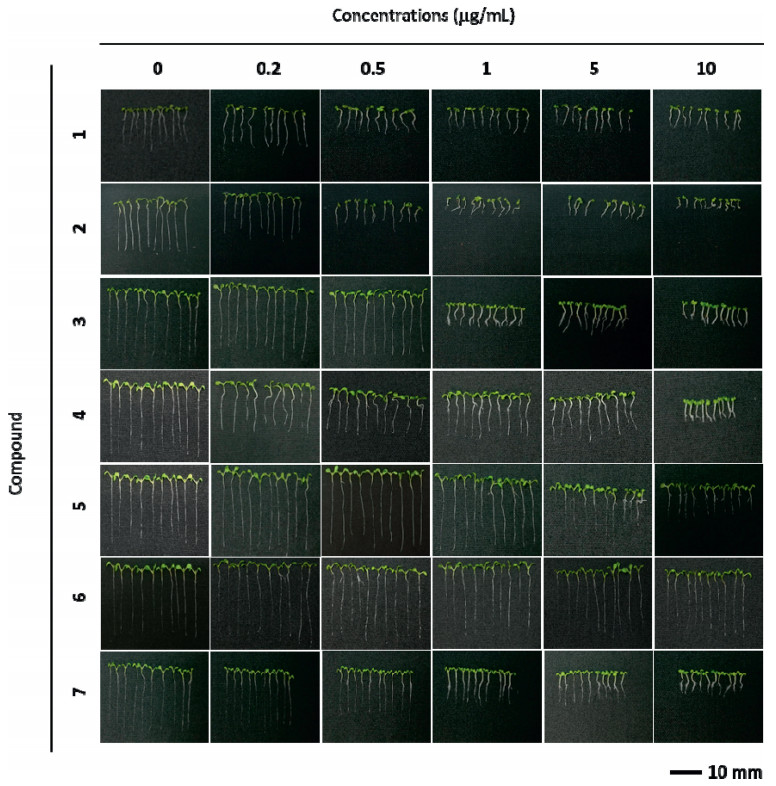
|
| Fig. 7 Five-day-old wild type (Col-0) A. thaliana seedlings grown with compounds 1–7 (0, 0.2, 0.5, 1, 5, 10 μg mL-1). |
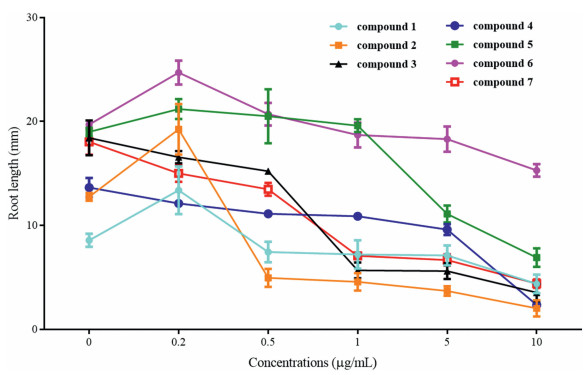
|
| Fig. 8 Quantification of the root length of seedlings shown in Fig. 7. Data represent mean ± SD (n = 10). |
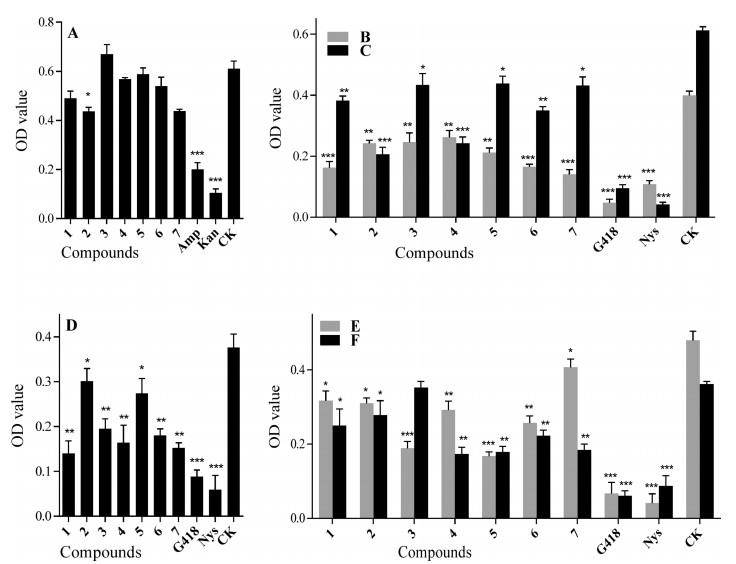
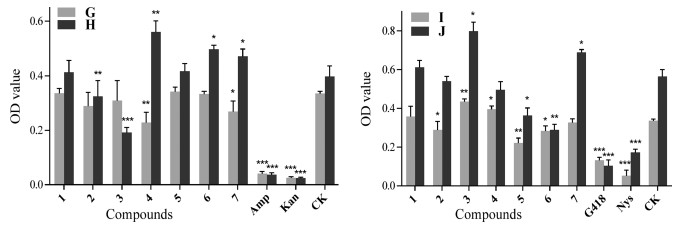
The antimicrobial activities of the compounds 1–7 against six probiotics (bacteria: Bacillus siamensis; fungi: Bionectria ochroleuca, Chaetomium globosum, Cladosporium uredinicola, Cl. tenuiussimum, P. janthinellum) and four pathogens (bacteria: A. marplatensis, N. rhamnosiphila; fungi: Cl. gossypicola, F. oxysporium) from the rhizosphere soil of P. notoginseng were tested according to the methods of previous studies (Xie et al., 2017, Liu et al., 2010, Zhang et al., 2004). At a concentration of 0.1 mg mL-1, compounds 2 and 5 showed substantial antibacterial activity on Ba. siamensis (Fig. 9A). Compounds 1–7 (0.1 mg mL-1) showed an inhibitory effect against the growth of all the fungal probiotics, especially Ch. globosum, Cl. uredinicola, Bi. ochroleuca and Cl. tenuiussimum (Fig. 9B–E). All compounds except 3 exhibited strong antagonistic activity against P. janthinellum (Fig. 9F). Additionally, compound 5 showed a slightly enhanced effect on the growth of bacterium N. rhamnosiphila at a concentration of 0.1 mg mL-1 (Fig. 10G), while 4–7 displayed remarkable promoting effects on pathogenic bacterium A. marplatensis (Fig. 10H). Meanwhile, compounds 1, 3, 4 and 7 displayed increasing effects from one level to another on two fungal pathogens (Cladosporium gossypicola and F. oxysporium) which can cause the root rot in P. notoginseng (Fig. 10I and J).
|
| Fig. 9 Antimicrobial activities of compounds 1–7 against one bacterial [Ba. siamensis (A)] and five fungal [Ch. globosum (B), Cl. uredinicola (C), Bi. ochroleuca (D), Cl. tenuiussimum (E), P. janthinellum (F)] probiotics from the rhizosphere soil of P. notoginseng. Data are mean ± SD of three independent experiments, *P < 0.05, **P < 0.01, ***P < 0.001. |
|
| Fig. 10 Antimicrobial activities of compounds 1–7 against two bacterial [N. rhamnosiphila (G) and A. marplatensis (H)] and two fungal [Cl. gossypicola (I) and F. oxysporium (J)] pathogens from the rhizosphere soil of P. notoginseng. Data are mean ± SD of three independent experiments, *P < 0.05, **P < 0.01, ***P < 0.001. |
Further investigation on the active fractions of cultivated soil (S3), which showed strong inhibitory effect on seed germination of P. notoginseng, resulted in the isolation of six ursane-type triterpenoid acids (1–4, 6–7) and one anthraquinone glucoside (5) from the EtOAc fraction, as well as detection of 13 volatile substances from the n-hexane fraction. The soil sample was comminuted and sieved to remove the impurities before study, in order to avoid the direct interference caused by the largely existing fibrous roots of P. notoginseng. All the components were reported from soil for the first time.
Although compounds 1–7 have been reported as secondary metabolites of various plants (e.g., Crataegus pinnatifida [Chen et al., 2008], Eriobotrya japonica [Ju et al., 2003], Psidium guajava [Diwakar et al., 2016], Rheum tanguticum [Gao et al., 2011]) and microorganisms (Passari et al., 2017), none to date has been identified as the exudate of P. notoginseng, from which only tetracyclic triterpenes have been reported (Qiao et al., 2018). Furthermore, P. notoginseng is not cultivated with any of the above-mentioned plants; therefore, these plants could not be the sources of 1–7. However, shelters used during P. notoginseng cultivation are commonly constructed of cedar branches and pine needles, both of which have been reported to produce pentacyclic triterpenes; thus, compounds 1–7 may be derived from the shelters through leaching rather than from P. notoginseng (Zhao et al., 2010). These compounds may also originate from the secondary metabolites of microbes or their transformation products. Besides, the volatile components detected in soils may be derived from a wide variety of other sources, such as leakage and spills of crude oil and its products, deposition from the air, discharge from industrial and domestic sources, dumping of solids, or degradation of naturally occurring organic compounds (Zhang et al., 2004, Zhu et al., 2014, Xie et al., 2007).
In addition, the content of chemicals in the soil is dynamic, because the soil environment is a complex ecosystem which could be easily affected by many factors, such as microorganisms, insects and the plant itself, as well as tillage, mulch and the effect of leaching. Also, during our extraction and separation processes, the amount of the compounds would be inevitably decreased to some extent. Taking these factors into consideration, it could be only supposed that the content of compounds in soil detected in experiment was close to the actual content under natural conditions, however it could not be construed to be the totally actual content.
4.2. Interactions between chemicals and plantsPrevious research showed that the germination rate and germination index of P. notoginseng seeds were lower in soil that has been cultivated with P. notoginseng (Zhang et al., 2010). The EtOAc extract of P. notoginseng soil has previously displayed the most obvious inhibition of notoginseng seed germination rate, germination index, and root elongation (You et al., 2009a). Allelochemicals have been shown to exist in rhizosphere soil of P. notoginseng and affect the growth of the herb. The extracts of P. notoginseng soil have also been shown to have allelopathic effects on other plants, such as cabbage, radish and lettuce (You et al., 2009b). The effects of compounds isolated in this study are consistent with these previous findings.
Previous research showed that ginsenosides exert autotoxic effects on P. notoginseng seed germination and root cell vigor at a concentration of 1.0 μg/mL (Yang et al., 2015a). Thus, we selected this concentration to test the allelopathic activities of compounds 1–7. Although this concentration may not be relevant to natural conditions, our experiments were designed to examine a cause-and-effect scenario.
When we exposed P. notoginseng, corn, wheat, turnip and water spinach to compounds 1–7, the root tip cell structures were damaged to varying degrees. These results imply that these compounds are not only harmful to P. notoginseng, but also to other crops. Moreover, in A. thaliana these compounds not only inhibited root elongation but also bifurcated and distorted the roots, and induced apoptosis in root tip cells. The results for many studies of developmental processes that have been performed in A. thaliana have subsequently been extended to other plants. Such evidence suggests that compounds 1–7 may have the same effects on seed germination and root elongation in numerous plant species.
4.3. Interactions between chemicals and rhizosphere microorganismsPrevious studies on rhizosphere microorganisms of P. notoginseng have mainly focused on the microbial community structure and the pathogenic effect of microorganisms on the plant (Miao et al., 2015, Guan et al., 2005, Guan et al., 2006, Guan et al., 2010). Only one study has reported the inhibitory effects of the secondary metabolites from endophytic fungi of P. notoginseng on other rhizosphere microorganisms (Xie et al., 2017). In the present study, compounds 3, 4, 6 and 7 significantly inhibited the growth of probiotics and promoted the growth of pathogens, at a concentration of 0.1 mg mL-1, indicating that they may be potential allelochemicals causing root-rot disease, and manifest as antagonists against probiotics in the rhizosphere soil of P. notoginseng.
Microorganisms and chemical constituents isolated from the cultivated soil were found to not only have great impact on the plant separately but also interact with each other. In this process, certain chemicals promoted the growth of pathogenic bacteria/fungi in the soil, or inhibited the growth of probiotics, or in both ways, thereby reducing the number of beneficial microorganisms in the soil and increasing the number of harmful microorganisms. Some pathogens infect or indirectly lead to a weakened ability of plants to resist external disturbances or infringement (Xiao et al., 2013, Zhu et al., 2015). Obviously, as some of the ecological factors in the continuous cropping obstacle of P. notoginseng, soil chemical components exert various effects on plants in different ways, and thus play a role in the whole soil micro-ecological system.
5. ConclusionsWe used column chromatography to isolate six ursane-type triterpenoid acids (1–4, 6–7) and one anthraquinone glucoside (5) and GC-MS analysis to identify 13 volatile components from the soil of 3-year-old P. notoginseng. Compounds 1–7 were reported from the soil and tested for their bioactivities on plant seed germination and the growth of rhizosphere microorganisms of P. notoginseng for the first time. The triterpenoid acids (1–4, 6–7) and quinone (5) were found to cause apoptosis in root tips of P. notoginseng, several crops and A. thaliana, as well as to inhibit seed germination and elongation, and to lead to some abnormal phenomena, such as root division, root apical meristem division, and morphological changes of root tip cells. These compounds also showed different effects on the growth of the rhizosphere microorganisms of P. notoginseng. For example, they inhibited probiotics, but promoted pathogens. These results suggest that these compounds may contribute to continuous cropping obstacle of P. notoginseng.
Author contributionsZhang Y.J., Yang C.R., and Wang D. conceived and designed the research; Qiao Y.J and Gu C.Z. performed experiments and analyzed data under direction of Zhang Y.J.; Zhu H.T. gave suggestion and direction of interaction experiments; Zhu H.T., Zhang M.Y. and Zhang Y.X. gave suggestion and direction of antimicrobial assay; Qiao Y.J. interpreted results of experiments, drafted manuscript and prepared figures; Zhang Y.J. and Wang D. edited and revised manuscript; Zhang Y.J. revised and approved final version of manuscript.
Declaration of Competing InterestThe authors declare no conflict of interest.
AcknowledgmentsThe authors are grateful to the members of the Analytical Group in State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, for measuring all the spectra. We also appreciate Prof. Wei-Qi Li, Dr. Yan-Xia Jia and Dr. Xing Huang for providing materials and technical support on plant bioassays. This work is supported by the Science and Technology Planning Project (2013FC008) and the Major Science and Technique Programs (2016ZF001-001) of Yunnan Province, China, and Yung-Chi Cheng academician workstation of Yunnan provincial academy of science and technology (2015IC017).
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2020.04.003.
Annamalai T., Venkateswara R.G., Mukhopadhyay T., 2013. Pulmatin from the roots of Rumex acetosa. Der Pharm. Lett, 5: 116-119. |
Bogatek R., Gniazdowska A., Zakrzewska W., Oracz K., Gawronski S.W., 2006. Allelopathic effects of sunflower extracts on mustard seed germination and seedling growth. Biol. Plantarum, 50: 156-158. DOI:10.1007/s10535-005-0094-6 |
Chen H.Y., Yu X.M., Zhang X.D., Yang L., Huang X., Zhang J., Pritchard H.W., Li W.Q., 2017. Phospholipase Dα1-mediated phosphatidic acid change is a key determinant of desiccation-induced viability loss in seeds. Plant Cell Environ, 41: 50-63. |
Chen L.S., Lv Y., Xu S.W., Yi Y., 2008. Study on the triterpene acids in fruit of Crataegus pinnatifida. Lishizhen Med. Mater. Med. Res, 19: 2909-2910. |
Chen Z.J., Wang Y., Sun Y.Q., Feng G.Q., 2002. Present situation investigation of Panax notoginseng planting industry. J. Chin. Med. Mater, 25: 387-389. |
Cho I.H., Lee H.J., Kim Y.S., 2012. Differences in the volatile compositions of ginseng species (Panax sp.). J. Agric. Food Chem, 60: 7616-7622. DOI:10.1021/jf301835v |
Diwakar R., Shaikh I., Dawda H., Mukundan U., 2016. Phytochemical evaluation and quantitative estimation of corosolic and ursolic acid from Psidium guajava. World J. Pharmaceut. Res, 5: 1318-1327. |
Gao L.L., Xu X.D., Nan H.J., Yang J.S., Chen S.L., 2011. Chemical constituents in Rheum tanguticum. Chin. Tradit. Herb. Drugs, 42: 443-446. |
Gu C.Z., Lv J.J., Zhang X.X., Qiao Y.J., Yan H., Li Y., Wang D., Zhu H.T., Luo H.R., Yang C.R., Xu M., Zhang Y.J., 2015. Triterpenoids with promoting effects on the differentiation of PC12 cells from the steamed roots of Panax notoginseng. J. Nat.Prod, 78: 1829-1840. DOI:10.1021/acs.jnatprod.5b00027 |
Gu C.Z., Qiao Y.J., Wang D., Zhu H.T., Yang C.R., Xu M., Zhang Y.J., 2017. New triterpenoid saponins from the steaming treated roots of Panax notoginseng. Nat. Prod. Res, 32: 294-301. |
Guan H.L., Chen Y.J., Liu S.Q., Zhang W.D., Xia C.F., 2006. Study on the relationship between root rot in Panax notoginseng and soil microbes. J. South China Normal Univ. (Soc. Sci. Ed.), 5: 706-709. |
Guan H.L., Chen Y.J., Zhu H.C., Zhang W.D., Yin F., Yang J.Z., Liu S.Q., 2005. Characteristic study of rhizome-microorganism in soil of diseased Panax notoginseng. Agric. Tec. (Santiago), 25: 56-70. |
Guan H.L., Yang J.Z., Chen Y.J., Cui X.M., Wang Y., Zhang Y.F., 2010. Change of rhizospheric microbe colony in cultivated soil and its correlation to root rot disease in Panax notoginseng. Soils, 3: 378-384. |
Hoagland R.E., Williams R.D., 2004. Bioassays-useful tools for the study of allelopathy. Libr. Work Study, 39: 1-10. |
Hu Z.B., Vanderhaeghen R., Cools T., Wang Y., Clercq I.D., Leroux O., Nguyen L., Belt K., Millar A.H., Audenaert D., Hilson P., Small I., Mouille G., Vernhettes S., Breusegem F.V., Whelan J., Höfte H., Veyldera L.D., 2016. Mitochondrial defects confer tolerance against cellulose deficiency. Plant Cell, 28: 2276-2290. DOI:10.1105/tpc.16.00540 |
Inderjit, 2006. Experimental complexities in evaluating the allelopathic activities in laboratory bioassays: a case study. Soil Biol. Biochem, 38: 256-262. DOI:10.1016/j.soilbio.2005.05.004 |
Jiang Y.P., Li H., Pan X.Z., Sun X.G., Tang Q.F., Shao M., 2013. Chemical constituents from the roots of Ilex pubescens. J. Chin. Med. Mater, 36: 1774-1778. |
Ju J.H., Zhou L., Lin G., Liu D., Wang L.W., Yang J.S., 2003. Studies on constituents of triterpene acids from Eriobotrya japonica and their anti-inflammatory and antitussive effects. Chin. Pharmaceut. J, 38: 752-757. |
Li Y., Liu S.L., Huang X.F., Ding W.L., 2009. Allelopathy of ginseng root exudates on pathogens of ginseng. Acta Ecol. Sin, 29: 161-168. |
Liu C.K., Deng H.P., Yin C., Lei S.Y., 2010. Allelopathic effects of Chenopodium ambrosioides L. On seed germination and seedling growth in five crops. J. South China Normal Univ. (Soc. Sci. Ed.), 350: 152-155. |
Liu M.L., Luo G.X., Wang Y.Z., Xu R., Wang Y., He W.F., Tan J.L., Xing M., Wu J., 2017. Nano-silver-decorated microfibrous eggshell membrane: processing, cytotoxicity assessment and optimization, antibacterial activity and wound healing. Sci. Rep, 7: 436. DOI:10.1038/s41598-017-00594-x |
Miao C.P., Mi Q.L., Qiao X.G., 2015. Rhizospheric fungi of Panax notoginseng: diversity and antagonism to host phytopathogens. J. Ginseng Res, 21: 127-134. |
Ng T.B., 2010. Pharmacological activity of sanchi ginseng (Panax notoginseng). J. Pharm. Pharmacol, 58: 1007-1019. |
Niu B.J., Ma Z.K., Liao Z.X., Ji L.J., Sun H.F., 2013. Chemical constituents from Dracocephalum heterophyllum. Chin. Tradit. Herb. Drugs, 44: 147-152. |
Passari A.K., Mishra V.K., Singh G., Singh P., Kumar B., Gupta V.K., Sarma R.K., Saikia R., Donovan A.O., Sing B.P., 2017. Author Correction: insights into the functionality of endophytic actinobacteria with a focus on their biosynthetic potential and secondary metabolites production. Sci. Rep, 7: 11809. DOI:10.1038/s41598-017-12235-4 |
Qiao Y.J., Shang J.H., Wang D., Zhu H.T., Yang C.R., Zhang Y.J., 2018. Research of Panax spp. in Kunming Institute of botany. Nat. Prod. Bioprospect, 8: 245-263. DOI:10.1007/s13659-018-0176-8 |
Romagni J.G., 2004. Allelopathic effect of volatile cineoles on two weedy plant species. J. Chem. Ecol, 26: 303-313. |
Shaw P., Dolan L., 2008. Chromatin and Arabidopsis root development. Semin. Cell Dev. Biol, 19: 580-585. DOI:10.1016/j.semcdb.2008.09.001 |
Sun Y.Q., Chen Z.J., Li G.C., Yang S.C., Wei M.L., Cui X.M., 2008a. Preliminary study of allelochemicals on propagation of Sanqi pathogen. Res. Pract. Chin. Med, 22: 19-21. |
Sun, Y.Q., Wei, M.L., Chen, Z.J., Ke, J.H., 2008b. Preliminary study of allelochemicals influence on the seed germination of Panax Notoginseng (Burk.) F.H. Chen. Spec.Wild Econ. Anim. Plant Res. 30, 44-46.
|
Wang, C.Z., McEntee, E., Wicks, S., Wu, J.A., Yuan, C.S., 2006. Phytochemical and analytical studies of Panax notoginseng (Burk.) F.H. Chen. J. Nat. Med. 60, 97-106.
|
Wang, T., Guo, R.X., Zhou, G.H., Zhou, X.D., Kou, Z.Z., Sui, F., Li, C., Tang, L.Y., Wang, Z.J., 2016. Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (Burk.) F.H. Chen: a review. J. Ethnopharmacol. 188, 234-258.
|
Wang Y., Fan C., Chen Y.J., Liu Y.Z., 2003. Main disease and prevention status of Panax notoginseng. Res. on Ginseng, 15: 43-45. |
Wu X.P., Huang X.Y., Zhang X.P., Ma G.X., Huang Z., Yuan J.Q., Xu X.D., Zhong X.M., 2014. Triterpenoid components from Rosa cymosa. Chin. Tradit.Herb. Drugs, 45: 626-630. |
Xiao Y.H., Li J., Liu Z.X., Li Y.J., Li G., Long H., 2013. Advances in studies on rhizospheric microorganism of medicinal plants. Chin. Tradit. Herb. Drugs, 44: 497-504. |
Xie G.X., Qiu Y.P., Qiu M.F., Gao X.F., Liu Y.M., Jia W., 2007. Analysis of dencichine in Panax notoginseng by gas chromatographyemass spectrometry with ethyl chloroformate derivatization. J. Pharmaceut. Biomed. Anal, 43: 920-925. DOI:10.1016/j.jpba.2006.09.009 |
Xie J., Wu Y.Y., Zhang T.Y., Zhang M.Y., Zhu W.W., Gullen E.A., Wang Z.J., Cheng Y.C., Zhang Y.X., 2017. New and bioactive natural products from an endophyte of Panax notoginseng. RSC Adv, 7: 38100-38109. DOI:10.1039/C7RA07060H |
Xun L.L., Zhao H.G., Liang Z.S., Wei M.T., Liu F.H., Han R.L., 2013. Study of microecology in root rot and healthy Panax notoginseng soil. Acta Agric. Borealioccidentalis Sin, 22: 146-151. |
Yang M., Zhang X.D., Xu Y.G., Mei X.Y., Jiang B.B., Liao J.J., Yin Z.B., Zheng J.F., Zhao Z., Fan L.M., He X.H., Zhu Y.Y., Zhu S.S., 2015a. Autotoxic ginsenosides in the rhizosphere contribute to the replant failure of Panax notoginseng. PloS One, 10: e0118555. DOI:10.1371/journal.pone.0118555 |
Yang S.G., Li C.L., Zhao L.M., Gao S.J., Lu J.X., Zhao M.L., Chen C.Y., Liu X.C., Luo M., Cui Y.H., Yang C.W., Wu K.Q., 2015b. The arabidopsis SWI2/SNF2 chromatin remodeling ATPase BRAHMA targets directly to PINs and is required for root stem cell niche maintenance. Plant Cell, 27: 1670-1680. DOI:10.1105/tpc.15.00091 |
You P.J., Wang W.Q., Zhang Y., 2009a. Allelopathic effects of extracts from root zone soil of Panax Notoginseng on notoginseng's seedlings. Southwest China J.Agric. Sci, 2: 308-310. |
You P.J., Zhang Y., Wang W.Q., 2009b. Allelopathic effects of continuous cropping soil of Panax Notoginseng on seed and seedling of some vegetables. Mod. Chin.Med, 5: 12-13. |
Zhang J.J., Li Y., Guo J.P., Du B., He G.C., Zhang Y.J., Chen R.Z., Li J.R., 2018a. Lipid profiles reveal different responses to brown planthopper infestation for pest susceptible and resistant rice plants. Metabolomics, 14: 120. DOI:10.1007/s11306-018-1422-0 |
Zhang Y.T., Wu W.H., Zhou F.J., Duan J.Y., Zhang C.P., Yin X.X., 2018b. Chemical constituents from Potentilla discolor Bge. Guangzhou Chem. Ind, 46: 72-74. |
Zhang Z.H., Tao S., Ye B.X., Peng Z.Q., Yuan J.P., 2004. Pollution sources and identification of hydrocarbons in soil and sediment using molecular markers. Chin. J. Soil Sci, 35: 793-798. |
Zhang Z.L., Wang W.Q., Yang J.Z., 2010. Effects of continuous Panax notoginseng cropping soil on P. notoginseng seed germination and seedling growth. Soils, 6: 1009-1014. |
Zhao H.L., Wang Q., Ruan X., Pan C.D., Jiang D.A., 2010. Phenolics and plant allelopathy. Molecules, 15: 8933-8952. DOI:10.3390/molecules15128933 |
Zhou, J.M., Zhang, W.B., Yang, J.Z., Cui, X.M., Zeng, H.C., Zhu, L., 2012. Preliminary isolation and identification of allelopathy from the rhizosphere soil of Panax notoginseng (Buck) F. H. Chen. Chin. Med. J. Res. Prac. 26, 14-16.
|
Zhu L., Ma N., Cui X.M., Zhu Y., Zhou J.M., 2014. Analysis of volatile components in planting soil and crop residues of Panax notoginseng. Chin. Med. J. Res. Prac, 28: 3-5. |
Zhu Y., Teng Y., Zhang M.Y., Ma W.T., Liu W.X., 2015. Pollutant accumulation and microbiological characteristics change in Notoginseng-planting soils. Soils, 47: 121-127. |



