b. Key Laboratory for Economic Plants and Biotechnology, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, China;
c. National Engineering Research Center for Ornamental Horticulture, Kunming, China
Plants of the genus Camellia (family Theaceae) are used throughout the world not only for beverages and oil, but also as important ornamental plants (Ming, 2000; Barman et al., 2008; Yang et al., 2015). As famous ornamental plants bearing large and bright flowers, camellias have been cultivated in China and elsewhere in Asia for thousands of years and are currently widely cultivated worldwide (Yu, 1985; Li et al., 2016a). In particular, the varieties that are crossed or selected from Camellia japonica L. and Camellia reticulata Lindl. are the most widely cultivated camellias. Although these two species belong to the genus Camellia section camellia, they exhibit significant differences in geographical distribution, environmental adaptability, and growth performance (Ming, 2000; Liu et al., 2003; Yang et al., 2011). For example, C. japonica is mostly a shrub, and grows in forests at altitudes of around 300-1100 m in eastern China, southern Korea and southern Japan. More than 10, 000 cultivars have been developed from C. japonica, with different flower colors and forms (Li et al., 2016a). These cultivars can be grown in most subtropical and warm temperate regions of the world, but should be planted in the shade. In contrast, C. reticulata is a small tree, and is native to southwestern China. It has a narrow distribution, with its wild populations restricted to mixed mountain forest at altitudes of 1600-2900 m in western and central Yunnan Province and southwestern Sichuan Province (Yu, 1985; Ming, 2000). This species is very susceptible to cold weather, and therefore has a much narrower cultivation range than C. japonica. The differing characteristics of C. japonica and C. reticulata suggest that they might have different environmental adaptations. But to date, no studies have compared the environmental adaptations of these two camellias, which limits their cultivation and utilization.
The growth and reproduction of plants depend on their physiological adaptabilities to the environments (Aleric and Kirkman, 2005; Wu and Campbell, 2006; Li et al., 2019). Empirical observations suggest that both C. japonica and C. reticulata produce more flowers under high light conditions, but may suffer from leaf burns under strong light, especially C. japonica. A previous study has also found that cultivars of C. japonica are susceptible to high light (Liu et al., 2003), whereas the photosynthetic adaptations of three cultivars of C. reticulata to light intensity differ (Yang et al., 2011). Furthermore, when C. reticulata plants produce new sprouts, even if the soil moisture conditions are good, the leaves and new shoots are prone to wilting; however, in C. japonica this phenomenon is not clearly observed. This indicates that these two species may differ in how they adapt to light and moisture.
Differences in plant photosynthesis and functional traits are widely used in explaining carbon gains, plant performance, and physiological tolerances to different environments (Niinemets and Kull, 1994; Hamerlynck and Knapp, 1996; Rozendaal et al., 2006; Niinemets, 2007; Li et al., 2016b). For example, acclimation to light environment is particularly important because light is critical for plant establishment, growth, and survival (Poorter, 1999; Goldstein et al., 2016). Insufficient light may reduce growth of plants by limiting photosynthetic gas exchange, while high light levels may lead to the increased formation of damaging reactive oxygen species as byproducts of photosynthesis, thereby damaging the photosynthetic apparatus and reducing photosynthetic carbon gain (Hjelm and Ögren, 2004; Aleric and Kirkman, 2005). Plants may optimize their photosynthesis under various light environments through regulating leaf nitrogen content, activation of Rubisco, nitrogen allocation among different pools within the photosynthetic apparatus, chlorophyll content, and diffusional conductance (Niinemets and Kull, 1994; Jensen, 2004; Rozendaal et al., 2006; Evans and Clarke, 2019). However, the photosynthetic adaptation to light intensity varies significantly across species. Sun plants often have higher light saturation points than shade plants (Pandey and Kushwaha, 2005; Zhang et al., 2006). But studies of the photosynthetic adaptation to differing light environment are rare for species within the genus Camellia, especially for C. reticulata and C. japonica (Liu et al., 2003; Barman et al., 2008; Yang et al., 2011).
Leaf morphological traits often adjust in response to different light environments and may affect the photosynthetic gas exchanges of plants (Murphy et al., 2012; Sack and Scoffoni, 2013; Scoffoni et al., 2015). For example, plants growing under shady conditions tend to develop lower leaf mass per unit area, vein density (Dv), and stomatal density (SD) than sun plants, but bigger stoma (Kim et al., 2011; Brodribb and Jordan, 2011; Scoffoni et al., 2015; Stewart et al., 2017). However, SD and Dv are not different in some species under different light intensities (Fetcher et al., 1983; Sack and Scoffoni, 2013). Stomatal density and size primarily dictate maximum stomatal conductance, and therefore potential transpiration demand (Franks et al., 2009; Brodribb and Jordan, 2011). Increased SD enhances the photosynthetic rate by modulating gas diffusion (Tanaka et al., 2013). Meanwhile, Dv is correlated with SD, maximum hydraulic conductance, and maximum photosynthetic rate (Sack and Frole, 2006; Zhang et al., 2012). However, in rice, an increase in SD does not enhance photosynthetic capacity (Schuler et al., 2018). Thus, the response pattern of leaf traits to the environment is highly species dependent (Xiong et al., 2018). But, differences in photosynthesis and functional traits are not well understood in species within the genus Camellia (Cai et al., 2017).
In the present study, the photosynthetic characteristics and leaf functional traits of four varieties of C. japonica and four varieties of C. reticulata were investigated under the same growing conditions. Our goals were to understand (1) the difference in the photosynthetic characteristics and leaf functional traits between C. japonica and C. reticulata; (2) the difference in the photosynthetic responses of C. japonica and C. reticulata to light intensity; and (3) how leaf morphological traits affect physiological traits and the adaptation of photosynthesis to light intensity. The results will be of great significance for guiding the cultivation of camellia plants.
2. Materials and methods 2.1. Plant materialsFour varieties of C. japonica and four varieties of C. reticulata were selected to compare the photosynthetic characteristics and leaf functional traits of these two camellia species. The four varieties of C. reticulata were Shizitou, Zaotaohong, Juban, and Songzilin. They are traditional varieties of C. reticulata and have a long cultivation history in Yunnan Province, China. The four varieties of C. japonica were Royal Velvet, Kramer's Supreme, Nuccio's Bella Rossa, and Flowerwood. These are introduced varieties, bred by growers in the United States of America. All materials used in the experiment were four-year old seedlings grown via cutting propagation.
2.2. Experimental site and designThe experiments were conducted in the Dachunhe Experimental Station of Flower Research Institute of Yunnan Academy of Agricultural Sciences in southwestern China. The study site is at an altitude of 2050 m a.s.l. It has a mean annual temperature of 14.8 ℃, a mean annual sunshine duration of 2291.2 h, and a mean annual precipitation of 900 mm, with 80% of this rainfall occurring between May and October. All seedlings were planted in plastic pots (30 cm in diameter) filled with peat moss. Before the emergence of new leaves, 30 seedlings of each variety were exposed to about 70% sunlight provided by shade nets applied on March 15, 2017. During the experiment, the seedlings were given slow-release fertilizer (N: P: K, 15:15:15) every two weeks, and watered every 1-3 days as needed. From July 3 to July 25 in 2017, the seedling's mature leaves that had formed in the current year were used for the observations on photosynthesis and leaf traits.
2.3. Measurements of photosynthesis and functional traitsThe photosynthetic responses to light intensity were measured on the fully expanded leaves using a Li-Cor 6400 portable photosynthesis system with a 6400-40 fluorescence chamber (Lincoln, NE, USA). After leaves were illuminated by an actinic light of 1200 μmol m-2s-1 (10% blue light, 90% red light) for 15 min to induce the maximum stomatal aperture, the recordings of photosynthetic rate in response to incident photosynthetic photon flux density (PPFD) were made between 1800 and 0 μmol m-2s-1 using an automated protocol built into the Li-Cor 6400 system. The program was configured to advance to the next step if the sum of three coefficients of variation (i.e. CO2, water vapor, and flow rate) was less than 0.3%, with a minimum waiting time of 3 min. Each leaf was equilibrated to initial conditions by waiting at least 15 min before executing the automated protocol. Photosynthetic light response curves of 3 individual leaves were measured at 11 light intensities under controlled levels of CO2 (400 μmol mol-1), flow rate (500 mmol s-1), leaf temperature (25 ℃), and leaf-to-air vapor pressure deficit (1.0-1.5 kPa). The values for light saturated photosynthetic rate (Pmax), apparent quantum efficiency (AQE), respiration rate (Rd), light compensation point (LCP), and light saturation point (LSP) were fitted for each leaf by a non-rectangular hyperbola (Prioul and Chartier, 1977).
Following the photosynthetic measurements, leaves were harvested from the sampled plants. Chlorophyll of fresh leaves was extracted in N, N-dimethylformamide for 48 h in the dark. Then, the absorbance of the extract was measured at 647.0 and 664.5 nm with a spectrophotometer (UV-2550, Shimadzu, Japan). Chlorophyll a content (Chla), chlorophyll b content (Chlb), and total chlorophyll content (Chl) were calculated following the method of Inskeep and Bloom (1985).
Leaf area per leaf (LA) was measured using a LI-3000A leaf area meter (Lincoln, NE, USA). Dry mass was determined after drying for 48 h at 70 ℃. Leaf dry mass per unit area (LMA) was calculated as the ratio of leaf dry mass to area. Subsequently, the leaves were ground into powder, and leaf nitrogen content was analyzed using a Vario MAX CN Elemental Analyzer (Elementar Analysensysteme GmbH, Germany). The leaf samples were then digested with HNO3-HClO4 and dissolved in HCl, and the phosphorus content was determined by an iCAP7400 inductively coupled plasma atomic-emission spectrometer (Thermo Fisher Scientific U.S.A, USA).
The section excised from the leaf was stained with 1% safranin and mounted in glycerol to obtain vein density (Dv). The sample was photographed at 10 × magnification with a digital camera mounted on a Leica DM2500 microscope (Leica Microsystems Vertrieb GmbH, Wetzlar, Germany). Vein length was determined from digital images via the IMAGEJ program (http://rsb.info.nih.gov/ij/). The value for Dv was expressed as vein length per unit leaf area. For stomatal observations, the lower and upper epidermises were peeled from the middle portions of fresh leaves, and the images were captured under the Leica DM2500 microscope. Stomata areas (SA) were observed in 30 randomly selected fields. Stomatal density (SD) was calculated as the number per unit leaf area, and stomatal length (SL) was represented as the length of the guard cell.
2.4. Data analysisAll statistical analyses were conducted using SPSS 16.0 for windows (SPSS Inc., Chicago, Illinois, USA). The differences in leaf functional traits between C. reticulata and C. japonica were tested by the Independent Samples t Test, while the differences among varieties were examined by one-way ANOVA with means discriminated by LSD multiple comparison tests. Relationships among variables were evaluated by a pair-wise Pearson correlation.
3. ResultsWe compared 20 leaf physiological and morphological traits of C. japonica and C. reticulata and found that half of the traits differed between the two species (Table 1). The varieties of C. reticulata had higher values for LSP, LCP, Pmax, LMA, ratio of Chla to Chlb and SA than those of C. japonica, but lower values for AQE, SD and leaf nitrogen content per unit mass. However, the values for Rd, leaf size, LT, chlorophyll content, leaf phosphorus content, Na, Chl/Na ratio, and Dv were not significantly different between the two species. The value for LSP was 1149.0 ± 24.9 μmol m-2s-1 in C. reticulata and 972.9 ± 33.7 μmol m-2s-1 in C. japonica. This indicates the difference between C. japonica and C. reticulata was mainly reflected in the photosynthetic adaptation of light intensity and leaf morphological traits (Table 1; Fig. 1).
| Traits | C. japonica | C. reticulata | P-value |
| Light saturation point (LSP, μmol m-2s-1) | 972.9 ± 33.7 | 1149.0 ± 24.9 | < 0.001*** |
| Light compensation point (LCP, μmol m-2s-1) | 16.87 ± 1.08 | 22.50 ± 1.78 | 0.013* |
| Apparent quantum efficiency (AQE, mol CO2 mol-1 photon) | 0.078 ± 0.004 | 0.065 ± 0.002 | 0.009** |
| Dark respiration rate (Rd, μmol m-2s-1) | 1.26 ± 0.09 | 1.34 ± 0.10 | 0.432NS |
| Light-saturated photosynthetic rate (Amax, μmol m-2s-1) | 11.21 ± 0.21 | 12.09 ± 0.19 | 0.003** |
| Leaf area per leaf (LA, cm2) | 32.38 ± 1.63 | 30.07 ± 1.61 | 0.468NS |
| Leaf dry mass per unit area (LMA, g m-2) | 149.9 ± 3.6 | 166.9 ± 4.9 | 0.006** |
| Leaf thickness (LT, μm) | 438.1 ± 9.6 | 440.5 ± 14.9 | 0.892NS |
| Chlorophyll a content per unit area (Chla, μg cm-2) | 42.13 ± 1.22 | 45.58 ± 1.47 | 0.079NS |
| Chlorophyll b content per unit area (Chlb, μg cm-2) | 19.77 ± 0.60 | 19.43 ± 0.55 | 0.674NS |
| Total chlorophyll content per unit area (Chl, μg cm-2) | 61.90 ± 1.42 | 65.01 ± 1.98 | 0.215NS |
| Ratio of Chla to Chlb | 2.171 ± 0.071 | 2.345 ± 0.036 | 0.029* |
| Leaf nitrogen content per unit mass (Nm, g kg-1) | 14.28 ± 0.28 | 12.85 ± 0.35 | 0.003** |
| Leaf phosphorus content per unit area (Pm, g kg-1) | 0.912 ± 0.029 | 0.862 ± 0.058 | 0.429NS |
| Leaf nitrogen content per unit area (Na, g m-1) | 2.140 ± 0.074 | 2.135 ± 0.104 | 0.967NS |
| Leaf phosphorus content per unit area (Pa, g kg-1) | 0.141 ± 0.005 | 0.143 ± 0.009 | 0.818NS |
| Chl/Na ratio | 0.288 ± 0.010 | 0.306 ± 0.014 | 0.345NS |
| Stomatal density (SD, number mm-2) | 252.4 ± 18.4 | 158.3 ± 4.2 | < 0.001*** |
| Stomatal area (SA, μm2) | 745.9 ± 34.8 | 1012.1 ± 18.3 | < 0.001*** |
| Leaf vein density (Dv, mm mm-2) | 4.65 ± 0.14 | 4.95 ± 0.21 | 0.230NS |
| The data in the table are means ± 1 SE. ***, P < 0.001; ***, P < 0.01; *, P < 0.05; NS, P > 0.05. | |||
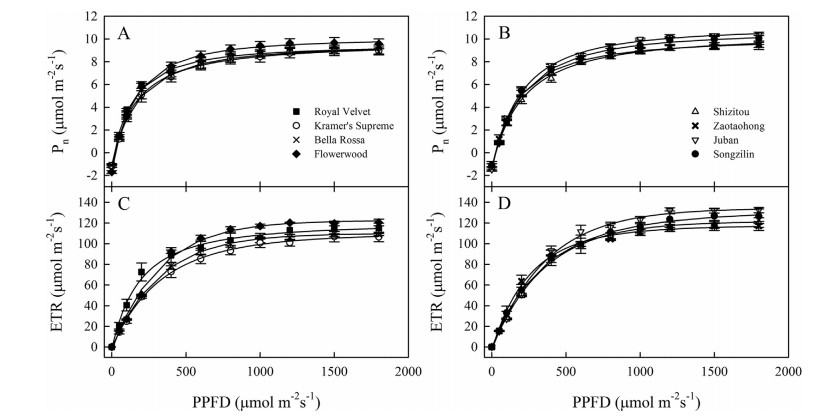
|
| Fig. 1 Responses of photosynthetic rates (Pn) and electron transport rates of photosystem Ⅱ (ETR) to photosynthetic photon flux density (PPFD) for different varieties of Camellia japonica (left) and C. reticulata (right). Each point represents the mean ± 1 SE of 3 measurements from different plants. |
There were differences in leaf functional traits among the varieties of both C. japonica and C. reticulata. For C. japonica, the values for Pmax, Chl, leaf size, SD, SA, and Dv differed among varieties. However, the differences in leaf morphological traits were more obvious than the other traits (Figs. 2-4). For C. reticulata, there were differences in Pmax, LMA, leaf size, SD, SA, and Dv among varieties, especially leaf size (Figs. 2-4). This indicates that leaf morphological traits were the main difference among the varieties of C. japonica or the varieties of C. reticulata.
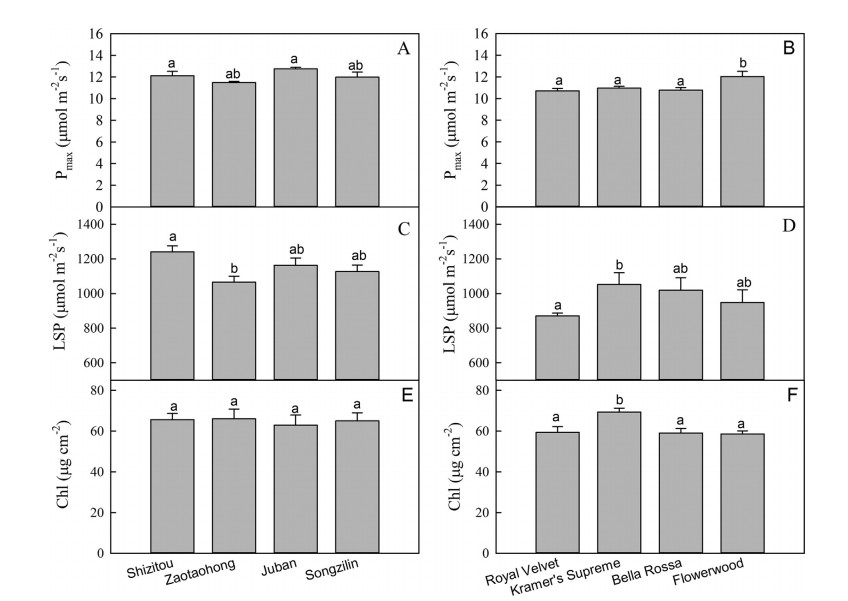
|
| Fig. 2 Values for light-saturated photosynthetic rate (Pmax), light-saturation point (LSP) and chlorophyll content per unit area (Chl) in different varieties of Camellia reticulata (left) and C. japonica (right). Each data point represents the average of three independent samples ± 1 SE. Different letters above bars in each graph indicate statistically different mean values (P < 0.05), as determined by LSD multiple comparison tests. |

|
| Fig. 3 Values for leaf area per leaf (LA), leaf thickness (LT) and leaf dry mass per unit area (LMA) in different varieties of Camellia reticulata (left) and C. japonica (right). Each data point represents the average of three independent samples ± 1 SE. Different letters above bars in each graph indicate statistically different mean values (P < 0.05), as determined by LSD multiple comparison tests. |
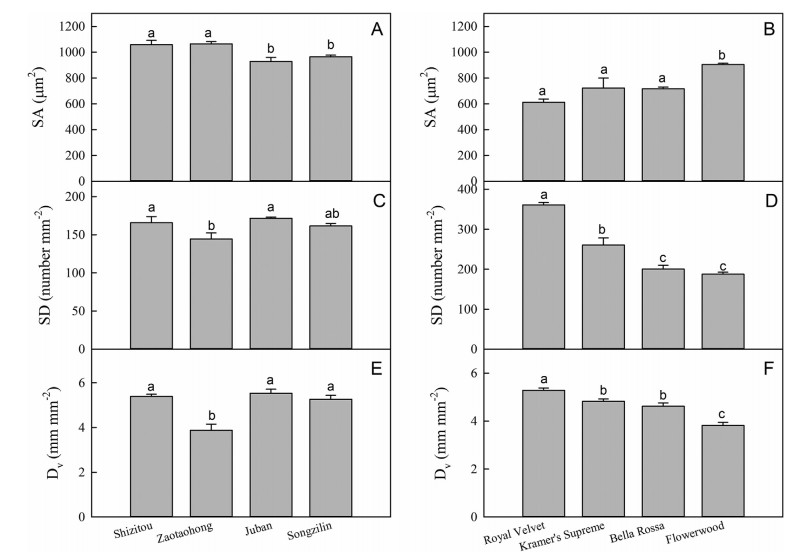
|
| Fig. 4 Values for stomatal area (SA), stomatal density (SD), and vein density (Dv) in different varieties of Camellia reticulata (left) and C. japonica (right). Each data point represents the average of three independent samples ± 1 SE. Different letters above bars in each graph indicate statistically different mean values (P < 0.05), as determined by LSD multiple comparison tests. |
Stomatal area (SA) was positively correlated with lightsaturated photosynthetic rate and light saturation point in the camellias used in the experiment but was negatively correlated with stomatal density. Leaf area per leaf was also positively correlated with vein density (Fig. 5). However, the correlations between Pmax and SD, Dv and LMA were very low.
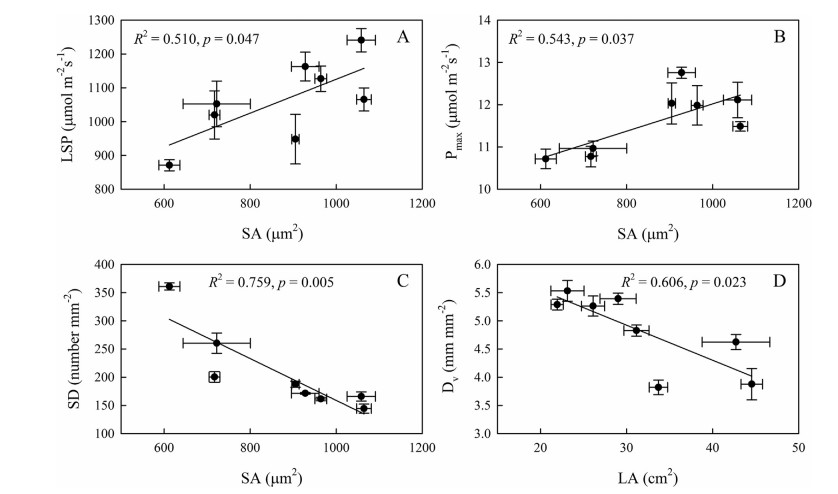
|
| Fig. 5 Relationships between stomatal area (SA) and light saturation point (LSP), light-saturated photosynthetic rate (Pmax), and stomatal density (SD), and between leaf area per leaf (LA) and vein density (Dv) in Camellia japonica and C. reticulata. |
The present study found that the light levels at which the varieties of C. reticulata achieved their maximal photosynthetic rates were higher than those of the C. japonica varieties. This was consistent with the empirical observation that C. reticulata had a higher light demand than C. japonica. The difference in photosynthetic adaptation is both genetically determined and environmentally induced (Hamerlynck and Knapp, 1996). In fact, C. reticulata is a small tree, while C. japonica is a shrub. This allows C. reticulata to grow higher into the forest canopy than C. japonica. Within forests, light intensity and humidity change significantly along vertical gradients. The upper canopy has a higher light intensity and is more xeromorphic than the lower canopy (Chazdon, 1988; Niinemets, 2007). Taller species make larger ontogenetic shifts in crown exposure than smaller species (Poorter et al., 2005). The most efficient way to acclimate for light is by adjusting leaf characteristics, such as leaf physiology, morphology, and anatomy (Mantovani, 1999; Rozendaal et al., 2006). A close association is often found between plasticity in leaf traits and maximum height of species (Thomas and Bazzaz, 1999; Cai et al., 2005). Shrub species are shade-adapted and can effectively utilize sun-flecks, while tree species are more likely to experience high light during their lifespans. After transferring to high light environment, the maximum photosynthetic rate of tree species increases considerably, but not in shrub species. Thus, tree species have a greater potential for light acclimation than shrub species (Cai et al., 2005). This indicates that species at different forest canopy layers have divergent light adaptation characteristics due to differences in the light environment experienced throughout their lifespans (Niinemets, 2007). Therefore, C. reticulata has a greater potential to adapt to high light environment than C. japonica.
Trees growing in gaps or in the upper canopy may experience higher light intensities and suffer more frequent photoinhibition, and rely strongly on biochemical mechanisms to dissipate excess energy and avoid damage to the light reaction centers (Niinemets, 2007; Goldstein et al., 2016). A high photochemical quenching coefficient is advantageous for the separation of electric charges in the reaction center and is beneficial to electron transport and photosystem Ⅱ yield (Mao et al., 2007). The plastic changes in hydraulic architecture may play an important role in the adaptation of trees to growth irradiance (Goldstein et al., 2016). When plants are exposed to high light, the temperatures of their leaves may increase (Zhang et al., 2005; Li et al., 2013). To avoid overheating and leaf burns, plants may increase their transpiration to lower leaf temperatures. Here, we found that the varieties of C. reticulata had higher LMA and SA than C. japonica, but a smaller leaf size. Meanwhile, SA was positively correlated with LSP. A previous study has suggested that leaf weight per area increases with relative light availability and species' light demand, while leaf size decreases with species' light demand and increasing plant height (Niinemets and Kull, 1994). A large leaf can increase the capture of light energy, but it also increases the heat load, water vapor interface layer, and water demand. In contrast, a small leaf has a thin interface layer which is beneficial for heat dissipation, ensuring the leaf is not easily damaged due to overheating (Rozendaal et al., 2006; Vogel, 2009; Li et al., 2013). Plasticity in leaf size may provide an efficient way for plants to acclimate hydraulic conductance and stomatal conductance to the contrasting evaporative conditions of sun and shade (Murphy et al., 2012). Plants exposed to high light may decrease their leaf sizes and increase transpiration rates (Dai et al., 2009; Sack and Scoffoni, 2013). Thus, leaf morphology is an important factor causing the difference in the light adaptation between C. reticulata and C. japonica.
In this study, whether between the two camellia species, or among the different varieties of the same species, the variation in leaf morphological traits were greater than the physiological traits. For example, although the light-saturated photosynthetic rate of C. reticulata was slightly higher than that of C. japonica, the lightsaturated photosynthetic rate was not significantly different in many cases. However, the leaf morphological traits such as leaf size and LMA varied significantly in many cases. A previous study has also found there are differential responses between ecotypes of Arabidopsis thaliana (Stewart et al., 2017). This seems to imply that morphological traits may be first noticed and selected during the breeding process of camellia.
Plants may enhance their adaptations to the environments or optimize their growth performance via shifts in trait-combinations (Mantovani, 1999; Fonseca et al., 2000; Zhang et al., 2012). Here, we found close associations among traits in camellias. For example, SA was positively correlated with light-saturated photosynthetic rate, but was negatively correlated with SD. Stomata are an important mechanism for regulating the entry of CO2 into the leaves and the loss of water from the leaves. The potential transpiration demand of a leaf is mainly determined by stomatal aperture and density (Franks et al., 2009; Brodribb and Jordan, 2011). The efficiency with which CO2 is absorbed and water loss is restricted appears to be partially a function of stomatal size (Aasamaa et al., 2001; Drake et al., 2012). Thus, stomatal aperture is linked to leaf conductance and photosynthetic rate (Büssis et al., 2006). The negative correlation between SD and SL has been previously observed (Sack et al., 2003; Beaulieu et al., 2008). This negative correlation may increase plasticity in maximum stomatal conductance to water vapor and CO2, with minimal alterations in the balance of water loss and epidermal allocations to the stomata (Sack et al., 2003; Franks et al., 2009). Thus, SA might affect the photosynthetic rate through regulating stomatal conductance in camellias.
The balance between leaf vein and stomatal investments is important in supplying water to evaporative sites so that stomatal openings are maintained (Zhang et al., 2012; Brodribb et al., 2013). However, we did not find significant correlations between the Pmax and SD, or Dv and LMA in camellias. A previous study has also found that the various structural parameters in camellia leaves undergo modifications that differ in both their extent and degree of elasticity (Krüger et al., 1997). Such differences in plasticity of different traits may change the correlation among traits. Thus, the lack of correlation among different traits is a common phenomenon (Zhang et al., 2012). For example, a previous study found that leaf mechanical resistance and photosynthetic capacity are often unrelated (He et al., 2019). The increase in SD does not enhance photosynthetic capacity in rice because the increased numbers can be offset by the decreased functional stomatal aperture (Schuler et al., 2018). Meanwhile, locally stored water can buffer the transpiration stream, decreasing the dependence upon water uptake from the soil (Ogburn and Edwards, 2013). The lack of correlation among traits may also be due to the difference in selection pressure on different traits, and adaptative strategies to stress (Zhang et al., 2012; He et al., 2019). Thus, C. reticulata and C. japonica might have different strategies to adapt to their habitats.
Plants may improve their adaptations to light availability through physiological and biochemical regulation (Niinemets, 2007; Zhang et al., 2017). For example, shade plants often enhance their light capture by increasing chlorophyll content, whereas sun plants have higher leaf nitrogen content than shade plants (Niinemets, 2007). Nitrogen is preferentially allocated to chlorophyll related to light harvesting in the acclimation to low light and to CO2 fixation in the acclimation to high light (Hikosaka and Terashima, 1995; Niinemets, 2007). A higher Chl/N ratio indicates that a greater proportion of leaf N is invested in chlorophyll for light capture at the expense of investment in Rubisco in shade leaves (Hikosaka and Terashima, 1995; Zhang et al., 2017). Here, leaf nitrogen content per unit mass (Nm) in C. japonica was higher than C. reticulata, but chlorophyll content (Chl), leaf nitrogen content per unit area (Na), and Chl/Na ratio were not different between the two species. This indicates that leaf nitrogen investment in light harvesting is not different between the two species. Thus, the difference in physiology between C. japonica and C. reticulata is relatively small. Leaf morphological traits in these two species should have a greater adaptive significance than physiological traits.
In conclusion, C. reticulata can adapt to higher light intensity than C. japonica. This difference in light demand is related to two species' differing life forms. The variations in leaf morphological traits between C. reticulata and C. japonica were larger than physiological traits. Leaf morphological traits is an important factor responsible for differences in the light adaptation between C. reticulata and C. japonica, and might be first noticed and selected during the breeding process of camellia plants. Although further research is needed to focus on more intrinsic plant functional traits (such as flower trait and shoot trait) of C. reticulata and C. japonica and how these traits change under different environmental stresses, our findings will contribute to the cultivation of camellia plants.
Author contributionsJHW and SBZ conceived and designed the experiments. YFC and SFL performed the experiments. JHW and YFC analyzed and interpreted the data. JHW and SBZ wrote the manuscript. All authors reviewed and approved the manuscript.
Declaration of Competing InterestThe authors declare that there are no conflicts of interest.
AcknowledgmentsThis work is financially supported by the National Natural Science Foundation of China, China (31670342, 31760229), the Scientific and Technological Leading Talent Project of Yunnan Province (2016HA005); project for Construction of International Flower Technology Innovation Center and Achievement Industrialization. Thanks to Dr. John Meadows from the Tropical Forests and People Research Centre, University of the Sunshine Coast for English editing.
Aasamaa K., Sõber A., Rahi M., 2001. Leaf anatomical characteristics associated with shoot hydraulic conductance, stomatal conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Aust. J. Plant Physiol, 28: 765-774. |
Aleric K.M., Kirkman L.K., 2005. Growth and photosynthetic responses of the federally endangered shrub, Lindera melissifolia (Lauraceae), to varied light environments. Am. J. Bot, 92: 682-689. DOI:10.3732/ajb.92.4.682 |
Barman T.S., Baruah U., Saikia J.K., 2008. Irradiance influences tea leaf (Camellia sinensis L.) photosynthesis and transpiration. Photosynthetica, 46: 618-621. DOI:10.1007/s11099-008-0104-y |
Beaulieu J.M., Leitch I.J., Patel S., et al, 2008. Genome size is a stronger predictor of cell size and stomatal density in angiosperms. New Phytol, 179: 975-986. DOI:10.1111/j.1469-8137.2008.02528.x |
Brodribb T.J., Jordan G.J., 2011. Water supply and demand remain balanced during leaf acclimation of Nothofagus cunninghamii trees. New Phytol, 192: 437-448. DOI:10.1111/j.1469-8137.2011.03795.x |
Brodribb T.J., Jordan G.J., Carpenter R.J., 2013. Unified changes in cell size permit coordinated leaf evolution. New Phytol, 199: 559-570. DOI:10.1111/nph.12300 |
Büssis D., von Groll U., Fisahn J., et al, 2006. Stomatal aperture can compensate altered stomatal density in Arabidopsis thaliana at growth light conditions. Funct. Plant Biol, 33: 1037-1043. DOI:10.1071/FP06078 |
Cai Z.Q., Rijkers T., Bongers F., 2005. Photosynthetic acclimation to light changes in tropical monsoon forest woody species differing in adult stature. Tree Physiol, 25: 1023-1031. DOI:10.1093/treephys/25.8.1023 |
Cai Y.-F., Yang Q.-Y., Li S.-F., et al, 2017. The water-water cycle is a major electron sink in Camellia species when CO2 assimilation is restricted. J. Photochem. Photobiol., B, 168: 59-66. DOI:10.1016/j.jphotobiol.2017.01.024 |
Chazdon R.L., 1988. Sunflecks and their importance to understorey plants. Adv. Ecol. Res, 18: 1-63. DOI:10.1016/S0065-2504(08)60179-8 |
Dai Y., Shen Z., Liu Y., et al, 2009. Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Environ. Exp. Bot, 65: 177-182. DOI:10.1016/j.envexpbot.2008.12.008 |
Drake P.L., Froend R.H., Franks P.J., 2012. Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot, 64: 495-505. |
Evans J.R., Clarke V.C., 2019. The nitrogen cost of photosynthesis. J. Exp. Bot, 70: 7-15. DOI:10.1093/jxb/ery366 |
Fetcher N., Strain B.R., Oberbauer S.F., 1983. Effects of light regime on the growth, leaf morphology, and water relations of seedlings of two species of tropical trees. Oecologia, 58: 314-319. DOI:10.1007/BF00385229 |
Fonseca C.R., Overton J.M., Collins B., et al, 2000. Shifts in trait-combinations along rainfall and phosphorus gradients. J. Ecol, 88: 964-977. DOI:10.1046/j.1365-2745.2000.00506.x |
Franks P.J., Drake P.L., Beerling D.J., 2009. Plasticity in maximum stomatal conductance constrained by negative correlation between stomatal size and density: an analysis using Eucalyptus globulus. Plant Cell Environ, 32: 1737-1748. DOI:10.1111/j.1365-3040.2009.002031.x |
Goldstein, G., Santiago, L.S., Campanello, P.I., et al., 2016. Facing shortage or excessive light: how tropical and subtropical trees adjust their photosynthetic behavior and life history traits to a dynamic forest environment. In: Goldstein, G., Santiago, L.S. (Eds.), Tropical Tree Physiology. Springer International Publishing, Switzerland, pp. 319-336.
|
Hamerlynck E., Knapp A.K., 1996. Photosynthetic and stomatal responses to high temperature and light in two oaks at the western limit of their range. Tree Physiol, 16: 557-565. DOI:10.1093/treephys/16.6.557 |
He P., Wright I.J., Zhu S., et al, 2019. Leaf mechanical strength and photosynthetic capacity vary independently across 57 subtropical forest species with contrasting light requirements. New Phytol, 223: 607-618. DOI:10.1111/nph.15803 |
Hikosaka K., Terashima I., 1995. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ, 18: 605-618. DOI:10.1111/j.1365-3040.1995.tb00562.x |
Hjelm U., Ögren E., 2004. Photosynthetic responses to short-term and long-term light variation in Pinus Sylvestris and Salix dasyclados. Trees, 18: 622-629. DOI:10.1007/s00468-004-0329-8 |
Inskeep W.P., Bloom P.R., 1985. Extinction coefficients of chlorophyll a and b in N, N-dimethylformamide and 80% acetone. Plant Physiol, 77: 483-485. DOI:10.1104/pp.77.2.483 |
Jensen R.G., 2004. Activation of Rubisco controls CO2 assimilation in light: a perspective on its discovery. Photosynth. Res, 82: 187-193. DOI:10.1007/s11120-004-1060-4 |
Kim S.J., Yu D.J., Kim T.-C., et al, 2011. Growth and photosynthetic characteristics of blueberry (Vaccinium corymbosum cv. Bluecrop) under various shade levels. Sci. Hortic, 129: 486-492. DOI:10.1016/j.scienta.2011.04.022 |
Krüger G.H.J., Tsimilli-Michael M., Strasser R.J., 1997. Light stress provokes plastic and elastic modifications in structure and function of photosystem Ⅱ in camellia leaves. Physiol. Plantarum, 101: 265-277. DOI:10.1111/j.1399-3054.1997.tb00996.x |
Li P.-R., Liu Y.-Z., Zhai M., et al, 2016a. Recent advances in Camellia japonica L. J. Plant Genet. Resour, 17: 1022-1030. |
Li S., Zhang Y.-J., Sack L., et al, 2013. The heterogeneity and spatial patterning of structure and physiology across the leaf surface in giant leaves of Alocasia macrorrhiza. PloS One, 8: e66016. DOI:10.1371/journal.pone.0066016 |
Li X., Liu X., Li C., et al, 2019. Growth and antioxidant physiology effects of Camellia azalea seedlings under different light conditions. Chin. J. Top. Crops, 40: 688-692. |
Li X., Yang Y., Yang S., et al, 2016b. Comparative proteomics analyses of intraspecific differences in the response of Stipa purpurea to drought. Plant Divers, 38: 101-117. DOI:10.1016/j.pld.2016.03.002 |
Liu D., Zhao S., Zhang Z., et al, 2003. Photosynthesis of different cultivars of Camellia japonica L. in greenhouse. Acta Hortic. Sin, 30: 65-68. |
Mantovani A., 1999. Leaf morpho-physiology and distribution of epiphytic aroids along a vertical gradient in a Brazilian rain forest. Selbyana, 20: 241-249. |
Mao L.Z., Lu H.F., Wang Q., et al, 2007. Comparative photosynthesis characteristics of Calycanthus chinensis and Chimonanthus praecox. Photosynthetica, 45: 601-605. DOI:10.1007/s11099-007-0103-4 |
Ming, T.-L., 2000. Monograph of the Genus Camellia. Yunnan Science and Technology Press, Kunming, China.
|
Murphy M.R.C., Jordan G.J., Brodribb T.J., 2012. Differential leaf expansion can enable hydraulic acclimation to sun and shade. Plant Cell Environ, 35: 1407-1418. DOI:10.1111/j.1365-3040.2012.02498.x |
Niinemets Ü., Kull K., 1994. Leaf weight per area and leaf size of 85 Estonian woody species in relation to shade tolerance and light availability. For. Ecol. Manag, 70: 1-10. DOI:10.1016/0378-1127(94)90070-1 |
Niinemets U., 2007. Photosynthesis and resource distribution through plant canopies. Plant Cell Environ, 30: 1052-1071. DOI:10.1111/j.1365-3040.2007.01683.x |
Ogburn R.M., Edwards E.J., 2013. Repeated origin of three-dimensional leaf venation releases constraints on the evolution of succulence in plants. Curr. Biol, 23: 722-726. DOI:10.1016/j.cub.2013.03.029 |
Pandey S., Kushwaha R., 2005. Leaf anatomy and photosynthetic acclimation in Valeriana jatamansi grown under high and low irradiance. Photosynthetica, 43: 85-90. DOI:10.1007/s11099-005-5090-8 |
Poorter L., 1999. Growth responses of 15 rain-forest tree species to a light gradient: the relative importance of morphological and physiological traits. Funct. Ecol, 13: 396-410. DOI:10.1046/j.1365-2435.1999.00332.x |
Poorter L., Bongers F., Sterck F.J., et al, 2005. Beyond the regeneration phase: differentiation of heightelight trajectories among tropical tree species. J. Ecol, 93: 256-267. DOI:10.1111/j.1365-2745.2004.00956.x |
Prioul J.L., Chartier P., 1977. Partitioning of transfer and carboxylation components of intracellular resistance to photosynthetic CO2 fixation: a critical analysis of the methods used. Ann. Bot, 41: 789-800. DOI:10.1093/oxfordjournals.aob.a085354 |
Rozendaal D.M.A., Hurtado V.H., Poorter L., 2006. Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature. Funct. Ecol, 20: 207-216. DOI:10.1111/j.1365-2435.2006.01105.x |
Sack L., Grubb P.J., Marañón T., 2003. The functional morphology of juvenile plants tolerant of strong summer drought in shaded forest understories in southern Spain. Plant Ecol, 168: 139-163. DOI:10.1023/A:1024423820136 |
Sack L., Frole K., 2006. Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology, 87: 483-491. DOI:10.1890/05-0710 |
Sack L., Scoffoni C., 2013. Leaf venation: structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol, 198: 983-1000. DOI:10.1111/nph.12253 |
Schuler M.L., Sedelnikova O.V., Walker B.J., et al, 2018. SHORTROOT-mediated increase in stomatal density has no impact on photosynthetic efficiency. Plant Physiol, 176: 757-772. DOI:10.1104/pp.17.01005 |
Scoffoni C., Kunkle J., Pasquet-Kok J., et al, 2015. Light-induced plasticity in leaf hydraulics, venation, anatomy, and gas exchange in ecologically diverse Hawaiian lobeliads. New Phytol, 207: 43-58. DOI:10.1111/nph.13346 |
Stewart J.J., Polutchko S.K., Adams III W.W., et al, 2017. Acclimation of Swedish and Italian ecotypes of Arabidopsis thaliana to light intensity. Photosynth. Res, 134: 215-229. DOI:10.1007/s11120-017-0436-1 |
Tanaka Y., Sugano S.S., Shimada T., et al, 2013. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytol, 198: 757-764. DOI:10.1111/nph.12186 |
Thomas S.C., Bazzaz F.A., 1999. Asymptotic height as a predictor of photosynthetic characteristics in Malaysian rain forest trees. Ecology, 80: 1607-1622. DOI:10.1890/0012-9658(1999)080[1607:AHAAPO]2.0.CO;2 |
Vogel S., 2009. Leaves in the lowest and highest winds: temperature, force and shape. New Phytol, 183: 13-26. DOI:10.1111/j.1469-8137.2009.02854.x |
Wu C.A., Campbell D.R., 2006. Environmental stressors differentially affect leaf ecophysiological responses in two Ipomopsis species and their hybrids. Oecologia, 148: 202-212. DOI:10.1007/s00442-006-0363-x |
Xiong D., Douthe C., Flexas J., 2018. Differential coordination of stomatal conductance, mesophyll conductance, and leaf hydraulic conductance in response to changing light across species. Plant Cell Environ, 41: 436-450. DOI:10.1111/pce.13111 |
Yang Y.-J., Chang W., Hu H., 2011. The photosynthetic characteristics of three horticultural cultivars of Camellia reticulate. North. Horti, 21: 65-69. |
Yang G.-Y., Wang B.-Y., He H., et al, 2015. Drought resistance potential of different ploidy of Camellia reticulata from leaf anatomic traits view. Southwest China J. Agric. Sci, 28: 2714-2719. |
Yu D., 1985. A historical review and future development of Camellia reticulata in Yunnan. Acta Hortic. Sin, 12: 131-136. |
Zhang S.-B., Zhou Z.-K., Hu H., et al, 2005. Photosynthetic performances of Quercus pannosa vary with altitude in the Hengduan Mountains, southwest China. For. Ecol. Manag, 212: 291-301. DOI:10.1016/j.foreco.2005.03.031 |
Zhang S.-B., Hu H., Xu K., et al, 2006. Photosynthetic performances of five Cypripedium species after transplanting. Photosynthetica, 44: 425-432. DOI:10.1007/s11099-006-0046-1 |
Zhang S.-B., Guan Z.-J., Sun M., et al, 2012. Evolutionary association of stomatal traits with leaf vein density in Paphiopedilum, Orchidaceae. PloS One, 7: e40080. DOI:10.1371/journal.pone.0040080 |
Zhang W., Huang W., Zhang S.-B., 2017. The study of a determinate growth orchid highlights the role of new leaf production in photosynthetic light acclimation. Plant Ecol, 218: 997-1008. DOI:10.1007/s11258-017-0747-5 |



