b. College of Horticulture, Shanxi Agricultural University, Taigu, 030801, China;
c. College of Agriculture, Shanxi Agricultural University, Taigu, 030801, China;
d. Faculty of Tropical Crops, Yunnan Agricultural University, Puer, 665000, China
Crofton weed (Eupatorium adenophorum), a plant native to central Mexico and Costa Rica, is a perennial, shrubby, evergreen, semi-shrub plant (Yang et al., 2017). It is a worldwide malignant weed. Crofton weed contains a variety of natural active substances and has strong allelopathic effects on other plants (Zhang et al., 2012; Chuah et al., 2016). The aqueous leaf extracts from crofton weed markedly inhibit root growth and development of alligator weed (Mishra, 2017). Liu et al. (2016) found that the extract of crofton weed showed high antibacterial and antifungal properties. Zhang et al. (2012) found that treatment with ρ-cymene, a bioactive compound in crofton weed, increases reactive oxygen species (ROS) accumulation and malondialdehyde (MDA) levels in rice seedlings, thereby resulting in oxidative damage. The accumulation of bioactive compounds from crofton weed in the soil has potential to adversely affect seed germination and seedling growth of field crops and weeds (Yang et al., 2006, 2013, 2016). However, the detailed physiological and molecular mechanisms underlying the effect of crofton weed extract on the modulation of plant growth and root system development remain largely unclear.
Root border cells (RBCs) are a group of special cells gathered around the apex of roots to protect root tips (Hawes et al., 2000, 2016a, 2016b; Miyasaka, 2001; G. Yang et al., 2016; J. Yang et al., 2016). RBC production and separation from root cap meristematic cells is an energy-consuming process. Environmental stresses stimulate a marked increase in RBC number (Watson et al., 2015; Hawes et al., 2016a, 2016b; Yang et al., 2016), and the process is correlated with pectin methyl esterase (PME) activity in root tips (Hawes et al., 2000; Micheli et al., 2001). Up to 98% of root exudates are made up of RBCs and associated extracellular material (Griffin et al., 1976; Hawes et al., 2016a). RBCs can also secrete the mucilage layer and regulate the rhizosphere environment and micronutrient uptake in roots (Hawes et al., 2016a, 2016b). Many studies have demonstrated that RBCs function not only as a biological layer between root surface and soil but also in environmental sensing and root secretion, thereby playing important roles in root responses to abiotic and biotic stresses (Gunawardena et al., 2005; Gunawardena and Hawes, 2002; Hawes et al., 2016; Li et al., 2017; Sun et al., 2015). RBCs prevent pathogens from attacking the root and resist toxic compounds by rapidly increasing the thickness of the RBC mucilage layer in response to pathogenic bacteria and heavy metal toxicities (Bengough et al., 2005; Hawes et al., 2000; Breusegem and Dat, 2006; Sun et al., 2015; Yang et al., 2016; Li et al., 2017; Zhang et al., 2017). The importance of RBCs in the rhizosphere environment and soil health has been recognized (Rovira et al., 1956; Hawes et al., 2016a).
Programmed cell death (PCD) plays a role in both normal growth and development and in stress responses in plants (Li and Xing, 2010; Sun et al., 2015). Heavy metal toxicity-induced ROS accumulation and subsequent PCD in roots mediate heavy metal stress tolerance in plants (Yakimova et al., 2007; Xu et al., 2010). As a crucial second messenger in plants, ROS also act as an important signaling molecule in modulating PCD (Xu et al., 2010; Li and Xing, 2010; Sun et al., 2015; Petrov et al., 2015).
A previous study demonstrated that extracts from crofton weed have potential toxicity on crop growth (Zhang et al., 2012); however, how bioactive compounds in crofton weed affect root system growth and development remains largely unclear. In this study, we aimed to elucidate the effects of the leaf extract of crofton weed (LECW) on root system growth and the changes in RBCs in maize seedlings.
2. Materials and methods 2.1. Preparation of the leaf extract of crofton weedDry leaves of crofton weed were ground and then soaked in 95% ethanol for 72 h. The extract was filtered using a microporous filter membrane (0.45 μm) and then concentrated using a vacuum rotatory evaporator. The concentrated extract was dissolved in ddH2O and then extracted by petroleum ether twice. The petroleum ether extract was loaded onto a column and eluted with an eluent of petroleum ether:ethyl acetate (50:1). The eluent was loaded on the column again and eluted with n-hexane:ethyl acetate (5:1). The elution rate was 6-8 BV/h. The leaf extract of crofton weed (LECW) was then obtained and stored at 4 ℃. The LECW was dissolved in acetone (0.2 g/mL) as a stock solution, and then different concentrations (0, 50, 100, 150 and 200 mg/L) of aqueous solutions of the LECW were prepared for experiments. The same amount of acetone was added to the culture solution as the blank control.
2.2. Plant materials and growth conditionsThe maize cultivar Xianyu 335 was used throughout the experiments. The seeds were surface-sterilized with 0.2% HgCl2 for 5 min, washed 5 times with aseptic water, and then sown on an autoclaved sand bed with 1/2 Hoagland solution with or without the LECW at 20-25 ℃ under 12 h/12 h day/night periods. The germination was counted according to germination rate (G = Ga/ Gn × 100%, where Ga is the no. of germinated seeds and Gn is the no. of seeds). Three-day-old 1/2 Hoagland-grown maize seedlings were transferred to fresh culture solution with different concentrations (0, 50, 100, 150 and 200 mg/L) of the LECW for 6 d, and the primary root (PR) growth, root fresh weight (FW) and shoot FW were determined. The experiment was repeated four times with 30 seeds or seedlings in each replicate.
2.3. Detection of the accumulation of reactive oxygen species in rootsThree-day-old maize seedlings were grown in 1/2 Hoagland solution with 0, 50, 100, 150 or 200 mg/L LECW for 60 h H2O2- specific DAB and ROS-specific DCFH-DA fluorescent probe staining were performed as described by Xu et al. (2010) and Wan et al. (2019). The experiment was repeated three times with 15 seedlings in each treatment. The contents of O2- and H2O2 were measured as described by Xu et al. (2010). The experiment was repeated three times.
2.4. Observation of root border cellsApproximately 20 root tips 5 mm in length were soaked in 200 μL ddH2O, oscillated for 30 s, and washed 2 times with 50 μL ddH2O. The root tip cells were extruded in a syringe to disperse cells, and then the cell suspension was centrifuged for 3 min at 4 ℃ and 500 r/min. Part of the supernatant was discarded to obtain the root border cell (RBC) suspension with uniform cell dispersion and the appropriate concentration. A total of 20 μL acridine orange/ ethidium bromide AO-EB dye (AO/EB = 1/1) were added to 50 μL RBC suspension and stained for 1 min. Fluorescence was observed using a fluorescence microscope. Living cells showed green fluorescence, and dead cells showed orange or red fluorescence. The number of RBCs was counted using a blood cell counting chamber. The cell viability of RBCs was determined according to the cell viability rate (V= Cg/Ct × 100%, where Cg is the no. of RBCs with green fluorescence and Cg is the no. of RBCs). The experiment was repeated five times with 10 root tips in each replicate.
2.5. Hoechst 33258 stainingNext, 75 μL Hoechst 33258 dye was added to 50 μL RBC suspension and stained for 5 min in the dark, followed by washes in PBS buffer. The image was viewed microscopically with a UV light filter. The cell death of RBCs was calculated according to the cell death rate (D = Cb/Ct × 100%, where Cb is the no. of RBCs with strong blue fluorescence in the nuclei and Cg is the no. of RBCs). The experiment was repeated five times with 10 root tips in each replicate.
2.6. Scanning electron microscopy analysisApproximately 1 cm root tips were excised and used for scanning electron microscopy (SEM) analysis to image the surface of root tips as described by Ahmed et al. (2017). Briefly, the root tips were fixed in 0.1 M sodium phosphate buffer (pH 7.2) with 2% paraformaldehyde and 2.5% glutaraldehyde for 2 d at 0-4 ℃. The root tips were then dehydrated through a gradient series of ethanol (30%-50%-70%-80%-90%-95%, once for 15 min at each step). The root tips were then freeze-dried using a freeze-dryer (JEOL JFD-320, Tokyo, Japan). Dehydrated roots were then put on two-sided carbon tape fixed on an SEM stub and visualized under a JEM-6490 LV scanning electron microscope (JEOL, Tokyo, Japan). The experiment was repeated three times with 4 root tips in each replicate.
2.7. Determination of mucigel thicknessA total of 50 μL India ink was added to 50 μL RBC suspension and stained for 5 min. Fluorescence was observed using a fluorescence microscope (Olympus BX 53), and the thickness was measured. The experiment was repeated three times with 10 RBCs in each replicate.
2.8. Determination of pectin methyl esterase activityApproximately 20 root tips 5 mm long were ground in cold 800 μL pectin methyl esterase (PME) extract (0.2 mol/L Na2HPO4, 0.1 mol/L citric acid, and 1 mol/L NaCl, pH = 5.8), oscillated for 1 h, and then centrifuged for 10 min at 4 ℃ for 15000 r/min. Next, 4 mL substrate (0.5% w/v pectin, 0.05% w/v methyl red, and 0.2 mol/L NaCl, pH = 6.8) was added to 300 μL enzyme extract, and the mixture was incubated for 2 h at 37 ℃. The absorbance was recorded at 525 nm. The experiment was repeated three times.
3. Results 3.1. The leaf extract of crofton weed markedly inhibited seed germination and seedling growthThe surface-sterilized maize seeds were sown on an autoclaved sand bed supplemented with or without different concentrations of the LECW to estimate toxicity (Fig. 1A). After 24 h, 36 h, 48 h and 60 h of treatment with 0, 50, 150 and 200 mg/L LECW, the germination rate (Fig. 1B) was reduced in a dose-dependent manner.

|
| Fig. 1 The leaf extract of crofton weed (LECW) inhibited seed germination and seedling growth. (A, B) Nine-day-old maize seedlings grown in 1/2 Hoagland solution with 0 (Control), 50, 100, 150, or 200 mg/L LECW (A), the germination rate were counted at the pointed time (B). Bar = 5 cm. (C-E) Three-day-old 1/2 Hoagland-grown maize seedlings were transferred to fresh culture solution with different concentrations (0, 50, 100, 150 and 200 mg/L) of the LECW for 6 d, (C) the primary root (PR) growth, (D) shoot fresh weight (FW) and (E) root FW were determined. The error bars represent the SE. Different letters indicate significantly different values (P < 0.05 by Tukey's test). |
To elucidate the inhibitory effect of the LECW on seedling growth, three-day-old seedlings were transferred to fresh culture solution with different concentrations (0, 50, 100, 150 and 200 mg/L) of the LECW for 6 d. The primary root (PR) growth, root fresh weight (FW) and shoot FW were reduced by 62%, 42% and 60%, respectively, in 50 mg/L LECW-treated seedlings (Fig. 1C-E). These data indicated that the LECW markedly inhibited seed germination and early seedling growth in maize.
3.2. The leaf extract of crofton weed induced reactive oxygen species accumulation in root tips and cell death in root border cellsA previous study indicated that the extract of crofton weed induces reactive oxygen species (ROS) accumulation in rice seedlings (Zhang et al., 2012). To elucidate the physiological mechanisms of LECW-inhibited seedling growth, we examined whether the LECW also induces ROS accumulation in maize roots. As shown in Fig. 2A, DAB staining indicated that treatment with the LECW markedly induced H2O2 accumulation in roots. In support of these results, the DCFH-DA fluorescent probe also showed high ROS accumulation in LECW-treated roots (Fig. 2B). To further confirm the results, we also examined O2- and H2O2 levels in roots. In agreement with the results of DAB and DCFH-DA staining, treatment with the LECW elevated the levels of both O2- and H2O2 in roots (Fig. 2C and D). Taken together, these data indicated that the LECW induced ROS accumulation in maize roots.

|
| Fig. 2 The leaf extract of crofton weed (LECW) induced reactive oxygen species (ROS) accumulation in root tips. (A, B) DAB staining (A) and DCFH-DA fluorescent probe staining (B) show ROS accumulation in maize root tips. (C, D) Determination of the contents of O2- (C) and H2O2 (D) in the roots of three-day-old maize seedlings grown in 1/2 Hoagland solution with 0 (Control), 50, 100, 150, or 200 mg/L LECW treatments for 60 h. The error bars represent the SD. Different letters indicate significantly different values (P < 0.05 by Tukey's test). |
Increased ROS accumulation triggered programmed cell death in plant cells (Xu et al., 2010; Li and Xing, 2010; Sun et al., 2015; Petrov et al., 2015). We thus wondered whether LECW-mediated ROS accumulation induces cell death in roots. To address this question, we first examined cell survival in root tips using AO-EB staining. With increasing LECW concentration, the green fluorescence gradually decreased, whereas the orange or red fluorescence gradually increased in root tips, especially in root border cells (RBCs) (Fig. 3A), indicating that the LECW induced cell death of the RBCs. To further confirm LECW-induced cell death in the RBCs, we examined the cell number and viability of them. With increasing concentrations of the LECW, the RBC number decreased by 17.4%, 31.7%, 41.5% and 50.5% (Fig. 3B), and the viability of RBCs decreased by 10.7%, 21.3%, 29.0% and 42.8% (Fig. 3C and D) with 50, 100, 150 and 200 mg/L LECW treatment, respectively. LECW-induced cell death was also further confirmed by Hoechst 33258 staining. Consistent with the AO-EB staining results, with increasing concentrations of the LECW, the cell death rate of RBCs increased by 67.1%, 77.7%, 81.3% and 83.1% in the 50, 100, 150 and 200 mg/L LECW groups, respectively (Fig. 3E and F). Taken together, these data indicated that exposure to LECW induced ROS accumulation in maize roots, thereby leading to cell death in the RBCs.

|
| Fig. 3 The leaf extract of crofton weed (LECW) induced cell death in RBCs. Three-day-old maize seedlings grown in 1/2 Hoagland solution with 0 (Control), 50, 100, 150 or 200 mg/L LECW for 60 h. (A) Detection of cell death in root tips using AO-EB staining. Bar = 500 μm. (B) Effects of different concentrations of the LECW on the cell number of RBCs. (C) Presentative image shows cell death of RBCs in LECW-treated seedlings using AO-EB staining. Living cells showed green fluorescence, and dead cells showed orange or red fluorescence. Bar = 20 μm. (D) Effects of different concentrations of the LECW on the cell viability of RBCs. (E) Presentative image shows cell death of RBCs in LECW-treated seedlings using Hoechest 33258 staining. 1 represents living cells, 2 represents apoptotic cells. Bar = 20 μm. (F) Effects of different concentrations of the LECW on cell death of RBCs. The error bars represent the SD. Different letters indicate significantly different values (P < 0.05 by Tukey's test). |
The above results indicated that the LECW induced ROS accumulation and PCD in RBCs, thereby inhibiting PR growth. To examine LECW-mediated PR growth inhibition in detail, we also analyzed the cell ultrastructure of root tips by scanning electron microscopy (SEM). As shown in Fig. 4, treatment with LECW markedly disrupted cell structure and led to cell swelling and deformation both in the root cap and elongation zone of the root tips. These results further confirmed that the LECW inhibited PR growth by disrupting the root cell structure.
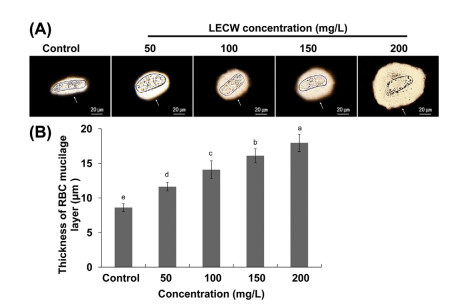
|
| Fig. 4 The leaf extract of crofton weed (LECW) affects RBC mucilage layer. Three-day-old maize seedlings grown in 1/2 Hoagland solution with 0 (Control), 50, 100, 150 or 200 mg/L LECW for 60 h. (A) Images of RBC mucilage layer subjected to different concentrations of LECW. Bar = 20 μm. (B) Effects of different concentrations of the LECW on the thickness of RBC mucilage layer. The error bars represent the SD. Different letters indicate significantly different values (P < 0.05 by Tukey's test). |
RBCs are synthesized and programmed to separate from the root tips by the action of pectolytic enzymes in the cell wall (Hawes et al., 2000). Several studies have demonstrated that RBC production is highly correlated with pectin methyl esterase (PME) activity in root tips (Hawes et al., 2000; Micheli et al., 2001). The above result indicated that the LECW decreased the RBC number; thus, we wondered whether exposure to the LECW affected the PME activity in root tips. As shown in Fig. 5, with increasing LECW concentration, the PME activity decrease by 4.8%, 5.5%, 6.9% and 10.3% with 50, 100, 150 and 200 mg/L LECW treatment, respectively. These data indicated that the LECW decreased the RBC number by reducing the PME activity in root tips.
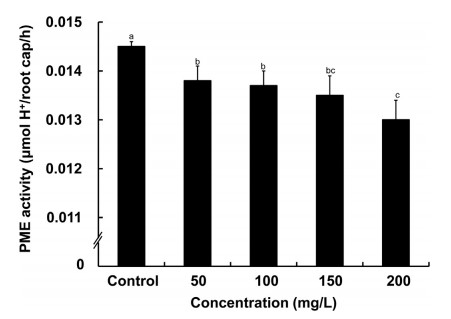
|
| Fig. 5 Effects of LECW on the PME activities of root caps. Three-day-old maize seedlings grown in 1/2 Hoagland solution with 0 (Control), 50, 100, 150 or 200 mg/L LECW. The error bars represent the SD. Different letters indicate significantly different values (P < 0.05 by Tukey's test). |
RBCs and their associated mucilage provide physical protection for the root meristem. An increase in the mucilage layer of RBCs is one of the adaptation mechanisms of roots to sense biotic and abiotic stress signaling, such as pathogen or heavy metal toxicity (Hawes et al., 2000). We thus examined whether the LECW modulated the mucilage layer of the RBCs. As shown in Fig. 6A and B, with increasing concentrations of the LECW, the thickness of the RBC mucilage layer increased by 35.0%, 63.8%, 87.1% and 108.4% with 50, 100, 150 and 200 mg/L LECW treatment, respectively.
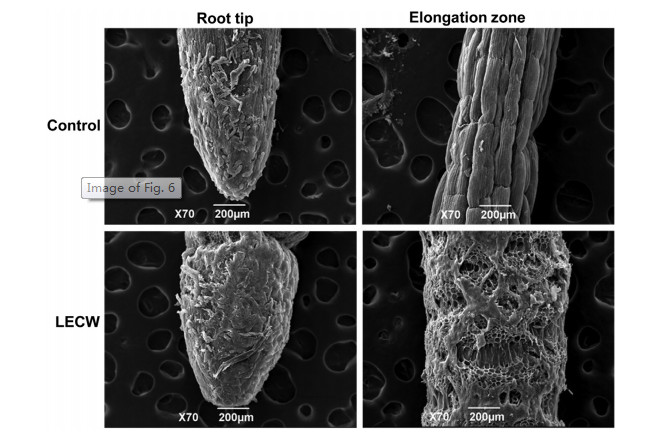
|
| Fig. 6 The leaf extract of crofton weed (LECW) disrupts cell structure in the root cap and elongation zones. Three-day-old maize seedlings grown in 1/2 Hoagland solution with or without 200 mg/L LECW for 4 d. |
Cytotoxicity is one of the characteristics of allelochemicals. Allelochemicals disrupt cell structure, mitosis, and gene expression in plant cells, thereby repressing plant growth and development (Cheng and Cheng, 2015). In this study, we found that treatment with the LECW repressed maize seed germination and seedling growth (Fig. 1A-G). Further study indicated that PR growth was inhibited in LECW-treated seedlings along with increased ROS accumulation (Fig. 2), subsequent emergence of PCD in RBCs (Fig. 1A and H, Figs. 2 and 3) and destruction of the root cell structure (Fig. 4). These results are consistent with those of previous studies by Li et al. (2017) and Zhang et al. (2017), who indicated that ROS are involved in heavy metal toxicity-mediated PCD in RBCs and supported the notion that RBCs serve an important purpose as environmental sensing cells (Gunawardena et al., 2005; Hawes et al., 2016; Sun et al., 2015; Zhang et al., 2017).
PME catalyzes the hydrolysis of the pectin methyl ester group in the cell wall (Ren and Kermode, 2000) and is involved in modulating numerous biological processes, including fruit ripening, seed germination, pathogenesis, abscission and senescence, cell growth and cambial cell differentiation (Moustacas et al., 1991; Liners and van Cutsem, 1992; Gaffe et al., 1994; Steele et al., 1997; Baayen et al., 1997; Guglielmino et al., 1997; Ren and Kermode, 2000). PME activity is strongly correlated with RBC production and separation in root caps (Wen et al., 1999). PME activity results in reduced cell wall pH by releasing protons, thereby affecting the degrading enzyme activities in the cell wall (Micheli, 2001; Zhang et al., 2017). Several studies have demonstrated that PME activity regulates heavy metal tolerance and micronutrient uptake by the binding of these metal ions with the pectin stratum in the cell wall (Pan et al., 2001; Zhang et al., 2017). Our study found that the LECW repressed PME activity in root tips (Fig. 5) and decreased the RBC number (Fig. 3B), thereby inhibiting seedling growth. However, the detailed molecular mechanisms of LECW-repressed PME activity in plants need to be further investigated.
Previous studies indicated that Al-induced mucilage exudation in RBCs is a self-protection response in roots, and the thickness of the RBC mucilage layer is correlated with Al toxicity tolerance and Al accumulation in maize and snap bean seedlings. An increase in the mucilage layer was positively correlated with an inhibition of cell death in snap bean roots. In this study, we found that treatment with the LECW markedly increased the thickness of the RBC mucilage layer in a dose-dependent manner (Fig. 6), suggesting a positive feedback adaptation mechanism in regulating RBC activity.
In summary, our study reported that the LECW induced cell death in RBCs by increasing ROS accumulation in roots. Moreover, as a feedback adaptation regulation, the thickness of the RBC mucilage layer was markedly increased in LECW-treated maize roots. The present study provides insight into the evaluation of the toxicity of crofton weed extracts to crops and is helpful for future studies on the purification and identification of the compounds leading to these effects and, finally, weed control.
Author contributionsJ.H. M. conceived and designed the study, and supervised the experiments. J.H. M., X.X. F., X.H. Y., Y.H. C., W.F. Z., and L.L. S. performed the experiments. J.H. M., X.H. Y., Y.H. C., and L.L. S. analyzed the data. J.H. M., Y.H. C., and L.L. S. drafted and revised the manuscript. All authors read and approved the final manuscript.
Declaration of Competing InterestThe authors declare no conflicts of interest.
AcknowledgementsThis research was supported by the Key Project of Science and Technology of Shanxi Province (20150311016-5) and the Science and Technology innovation Foundation of Shanxi Agricultural University (2017ZZ09).
Ahmed B., Dwivedi S., Abdin M.Z., et al, 2017. Mitochondrial and chromosomal damage induced by oxidative stress in Zn 2+ ions, ZnO-Bulk and ZnO-NPs treated Allium cepa roots. Sci. Rep, 7: 1-14. |
Baayen R.P., Schoffelmeer E.A.M., Toet S., Elgersma D.M., 1997. Fungal polygalacturonase activity reflects susceptibility of carnation cultivars to fusarium wilt. Eur. J. Plant Pathol, 103: 15-23. DOI:10.1023/A:1008616008804 |
Bengough, A.G., Barlowa, P.W., Cooke, D.E.L., et al., 2005. Root border cells in plantsoil interactions. Environ. Times 160-161.
|
Cheng F., Cheng Z., 2015. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci, 6: 1020. |
Chuah T.S., Norhafizah M.Z., Naimah A.H., Ismail B.S., 2016. Phytotoxic activity of the allelochemical, 2, 4-Di-Tert-Butylphenol on two selected weed species. Sains Malays, 45: 963-967. |
Gaffe J., Tieman D.M., Handa A.K., 1994. Pectin methylesterase isoforms in tomato (Lycopersicon esculentum) tissues: effects of expression of pectin methylesterase antisense gene. Plant Physiol, 105: 199-203. DOI:10.1104/pp.105.1.199 |
Griffin G.J., Hale M.G., Shay F.J., 1976. Nature and quantity of sloughed organic matter produced by roots of axenic peanut plants. Soil Biol, 8: 29-32. DOI:10.1016/0038-0717(76)90017-1 |
Guglielmino N., Liberman M., Catesson A.M., et al, 1997. Pectin methylesterases from poplar cambium and inner bark: localization, properties, and seasonal changes. Planta, 202: 70-75. DOI:10.1007/s004250050104 |
Gunawardena U., Hawes M.C., 2002. Tissue specific localization of root infection by fungal pathogens. Mol. Plant Microbe Interact, 15: 1128-1136. DOI:10.1094/MPMI.2002.15.11.1128 |
Gunawardena U., Rodriguez M., Straney D., et al, 2005. Tissue specific localization of pea root infection by Nectria haematococca: mechanisms and consequences. Plant Physiol, 137: 1363-1374. DOI:10.1104/pp.104.056366 |
Hawes M., Allen C., Turgeon B.G., et al, 2016a. Root border cells and their role in plant defense. Annu. Rev. Phytopathol, 54: 143-161. DOI:10.1146/annurev-phyto-080615-100140 |
Hawes, M., McLain, J., Ramirez-Andreotta, M., et al., 2016b. Extracellular trapping of soil contaminants by root border cells: new insights into plant defense. Agronomy 6, 5.
|
Hawes M.C., Gunawardena U., Miyasaka S., Zhao X., 2000. The role of root border cells in plant defense. Trends Plant Sci, 5: 128-133. DOI:10.1016/S1360-1385(00)01556-9 |
Liners F., van Cutsem P., 1992. Distribution of pectic polysaccharides throughout walls of suspension-cultured carrot cells. Protoplasma, 170: 10-21. DOI:10.1007/BF01384453 |
Liu X., Ouyang C., Wang Q., et al, 2016. Evaluation of antibacterial and antifungal properties of 9-oxo-10, 11-dehydroageraphorone extracted from Eupatorium adenophorum. J. Plant Dis. Prot, 123: 93-99. DOI:10.1007/s41348-016-0006-3 |
Li X.W., Liu J.Y., Fang J., et al, 2017. Boron supply enhances aluminum tolerance in root border cells of pea (Pisum sativum) by interacting with cell wall pectins. Front. Plant Sci, 8: 742. DOI:10.3389/fpls.2017.00742 |
Li Z., Xing D., 2010. Mitochondrial pathway leading to programmed cell death induced by aluminum phytotoxicity in Arabidopsis. Plant Signal. Behav, 5: 1660-1662. DOI:10.4161/psb.5.12.14014 |
Micheli F., 2001. Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci, 6: 414-419. DOI:10.1016/S1360-1385(01)02045-3 |
Mishra S.K., 2017. Allelopathic potential of Phyllanthus niruri Linn. On seed germination and seedling growth of rice (Oryza sativa). Res. J. Pharmacogn. Phytochem, 9: 77-82. DOI:10.5958/0975-4385.2017.00014.0 |
Miyasaka S.C., 2001. Possible role of root border cells in detection and avoidance of aluminum toxicity. Plant Physiol, 125: 1978-1987. DOI:10.1104/pp.125.4.1978 |
Moustacas A.M., Nari J., Borel M., Noat G., Ricard J., 1991. Pectin methylesterase: metal ions and plant cell wall extension. The role of metal ions in plant cell wall extension. J. Biochem, 279: 351-354. DOI:10.1042/bj2790351 |
Pan J.W., Zhu M.Y., Chen H., 2001. Aluminum-induced cell death in root-tip cells of barley. Environ. Exp. Bot, 46: 71-79. DOI:10.1016/S0098-8472(01)00083-1 |
Petrov V., Hille J., Mueller-Roeber B., Gechev T.S., 2015. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci, 6: 69. |
Ren C., Kermode A.R., 2000. An increase in pectin methyl esterase activity accompanies dormancy breakage and germination of yellow cedar seeds. Plant Physiol, 124: 231-242. DOI:10.1104/pp.124.1.231 |
Rovira A.D., 1956. Plant root excretions in relation to the rhizosphere effect. Plant Soil, 3: 209-217. |
Steele N.M., McCann M.C., Roberts K., 1997. Pectin modification in cell walls of ripening tomatoes occurs in distinct domains. Plant Physiol, 114: 373-381. DOI:10.1104/pp.114.1.373 |
Sun L., Song J., Peng C., et al, 2015. Mechanistic study of programmed cell death of root border cells of cucumber (Cucumber sativus L.) induced by copper.. Plant Physiol. Biochem, 97: 412-419. DOI:10.1016/j.plaphy.2015.10.033 |
Van Breusegem F., Dat J.F., 2006. Reactive oxygen species in plant cell death. Plant Physiol, 141: 384-390. DOI:10.1104/pp.106.078295 |
Wan J., Wang R., Wang R., et al, 2019. Comparative physiological and transcriptomic analyses reveal the toxic effects of ZnO nanoparticles on plant growth. Environ. Sci. Technol, 53: 4235-4244. DOI:10.1021/acs.est.8b06641 |
Watson B.S., Bedair M.F., Urbanczyk-Wochniak E., et al, 2015. Integrated metabolomics and transcriptomics reveal enhanced specialized metabolism in Medicago truncatula root border cells. Plant Physiol, 167: 1699-1716. DOI:10.1104/pp.114.253054 |
Wen F., Zhu Y., Hawes M.C., 1999. Effect of pectin methylesterase gene expression on pea root development. Plant Cell, 11: 1129-1140. DOI:10.1105/tpc.11.6.1129 |
Xu J., Yin H., Li Y., Liu X., 2010. Nitric oxide is associated with long-term zinc tolerance in Solanum nigrum. Plant Physiol, 154: 1319-1334. DOI:10.1104/pp.110.162982 |
Yakimova E.T., Kapchina-Toteva V.M., Woltering E.J., 2007. Signal transduction events in aluminum-induced cell death in tomato suspension cells. J. Plant Physiol, 164: 702-708. DOI:10.1016/j.jplph.2006.03.018 |
Yang G.Q., Qiu W.R., Jin Y.N., Wan F.H., 2013. Potential allelochemicals from root exudates of invasive Ageratina adenophora. Allelopathy J, 32: 233-242. |
Yang G.Q., 2006. Physiological effects of allelochemicals from leachates of Ageratina adenophora (Spreng.) on rice seedlings. Allelopathy J, 18: 237-246. |
Yang, G., Gui, F., Liu, W., Wan, F., 2017. Crofton weed Ageratina adenophora (Sprengel). In: Biological Invasions and its Management in China, pp. 111-129.
|
Yang G., Guo J., Zhu X., et al, 2016a. Soil chemicals from croftonweed (Ageratina adenophora) are phytotoxic. Weed Sci, 64: 223-230. DOI:10.1614/WS-D-15-00115.1 |
Yang J., Qu M., Fang J., et al, 2016b. Alkali-soluble pectin is the primary target of aluminum immobilization in root border cells of pea (Pisum sativum). Front. Plant Sci, 7: 1297. |
Zhang F., Chen F., Liu W., et al, 2012a. r-Cymene inhibits growth and induces oxidative stress in rice seedling plants. Weed Sci, 60: 564-570. DOI:10.1614/WS-D-12-00029.1 |
Zhang F., Guo J., Chen F., et al, 2012b. Identification of volatile compounds released by leaves of the invasive plant crofton weed (Ageratina adenophora, Compositae), and their inhibition of rice seedling growth. Weed Sci, 60: 205-211. DOI:10.1614/WS-D-11-00156.1 |
Zhang Y., Wu Y., Xu G., et al, 2017. Effects of iron toxicity on the morphological and biological characteristics of rice root border cells. J. Plant Nutr, 40: 332-343. DOI:10.1080/01904167.2016.1240193 |



