b. School of Life Science, Yunnan Normal University, Kunming, 650500, Yunnan, China;
c. Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201, Yunnan, China;
d. National Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201, Yunnan, China
Seedling establishment is one of the most vulnerable stages in the plant life cycle. It is also critical in determining the success of population regeneration, especially in harsh habitats (Baskin and Baskin, 2014). Plants that live in alpine regions are exposed to extreme environmental conditions, including short growing seasons, low temperatures, and frequent frosts (Körner, 2003). In these environments, small seedlings do not have sufficient biomass to resist freezing cold in winter. To maximize the probability of seedling survival, and thus seedling establishment, many plants control the timing of germination (Jaganathan et al., 2015). Seed dormancy is a main mechanism controlling the timing of germination in alpine plants (Schwienbacher et al., 2011), which prevents germination in autumn and allows seeds to delay germination until the seasons are optimal for growth and survival of the seedlings (Baskin and Baskin, 2014; Porceddu et al., 2013).
Over 70% of alpine plants exhibit seed dormancy, the most common form of which is physiological dormancy (PD) (Baskin and Baskin, 2014; Schwienbacher et al., 2011). Seed dormancy can be released by both physiological and environmental factors. For example, GA3 and KNO3 are known to release seeds from dormancy (Baskin and Baskin, 2004), and are commonly applied to dormant seeds to promote germination. Several environmental factors are known to regulate seed dormancy-break and control germination under suitable conditions for seedling establishment (Batlla and Benech-Arnold, 2015). For example, if seeds require low temperatures in winter (cold stratification) for dormancy break, they usually become nondormant during winter and can germinate in spring or early summer (Mondoni et al., 2009; Schwienbacher et al., 2011; Wang et al., 2017). Daily fluctuating temperature as a seed germination cue is also a mechanism to control the timing of germination, especially for plants in wet habitats (Liu et al., 2013; Peng et al., 2017). Light also plays a pivotal role in controlling the timing of seed germination from the soil seed bank (Baskin and Baskin, 2014; Jaganathan et al., 2015; Peng et al., 2018; Wang et al., 2017). The requirement for light and temperature fluctuations to break dormancy in some alpine species not only prevents deep soil germination in late autumn, but also ensures germination occurs only at or near the soil surface in spring (Jaganathan et al., 2015).
Primula beesiana Forr. (Primulaceae) is a perennial herb endemic to the eastern Himalaya and Hengduan Mountains. It grows in wet mountain meadows and on the borders of streams at altitudes between 2700 and 3700 m (Hu and Kelso, 1996). P. beesiana is a wild ornamental plant, having large, rose-carmine umbels. For this reason, it has been indiscriminately collected in its natural habitats. The pollination biology and mating systems of this species have been well studied (Huang et al., 2015; Ma et al., 2014; Wu and Zhang, 2010); however, little is known about seed dormancy and germination in P. beesiana.
In this study, we investigated seed dormancy and germination in P. beesiana. We specifically addressed the following questions: (1) What is the dormancy type? How do GA3 and KNO3 release dormancy and promote germination? (2) How do temperatures and cold stratification affect dormancy and germination? What are the cardinal temperatures and thermal time for germination in different lengths of cold stratification? and (3) How do light conditions affect germination in different dormancy status? On the basis of the seed dormancy and germination responses to temperature, GA3, KNO3 and light measured in the laboratory, we would provide some valuable data for cultivation and conservation management of P. beesiana.
2. Materials and methods 2.1. Seed collectionFreshly-mature P. beesiana fruits were collected from at least 30 individuals in early October 2016 at the Lijiang Alpine Botanical Garden (27°00'55.42", 100°10'15.12", 3664 m a.s.l.), a natural botanical garden in the Yulong Shan Mountain range, Yunnan Province, China. Non-seed structures were removed by hand in the laboratory at the National Germplasm Bank of Wild Species (Kunming, Yunnan Province). Full seeds were air-dried (dry afterripening, DAR) in a paper bag at room temperature for six months until the onset of the experiments. After three months, we measured seed weight; the 100-seed weights were 21.2 ± 0.6 mg (n = 6; mean ± SE).
2.2. Seed germination experimentsTo determine the level of PD (if present) after DAR, seeds were incubated, on 1% water agar substrate with media containing 0 (control), or 100 mg L-1 of GA3, 10 mM KNO3 in plastic Petri dishes of 90 mm diameter in the light (12 h light/12 h darkness, hereafter) under two fluctuating temperature regimes (15/5 and 25/15 ℃). The two fluctuating temperatures were selected to approximate daily temperature regimes in the germination season (late April to late August). Plants were grown under 22.2 μmol·m-2·s-1 illumination from cool white fluorescent light and a 12-h photoperiod per day. Higher temperatures corresponded to light periods.
We further tested the effects of temperatures on germination after DAR by incubating seeds in light for 1-3 months under a range of constant temperatures (5, 10, 15, 20, 25, 28, 30 and 32 ℃). At the same time, three different cold stratification periods were started (1 ℃ in the light in 1% agar water in 90 mm diameter plastic Petri dishes for 4, 8 and 16 weeks) and, at the end of each pre-treatment, seeds were incubated, as detailed above (except for 28 and 32 ℃).
To determine the effect of cold stratification on light requirements, Petri dishes with untreated (0) and cold-stratified seeds (4, 8, and 16 weeks) were wrapped in two layers of aluminum foil, and then incubated under two constant temperatures (10 and 20 ℃) and two temperature fluctuation regimes (15/5 and 25/15 ℃). To avoid exposure to light, germination of darkincubated seeds was checked only once at the end of the dark incubation, and then the remaining ungerminated seeds were transferred to the light condition, and final germination was assessed when finishing the germination test.
Three replicates of 20 seeds each were used in each treatment. The Petri dishes were put into transparent plastic bags to prevent desiccation. Seeds incubated in the light were counted daily and germinated seeds were discarded. The criterion for germination was visible radicle protrusion. At the end of the germination tests, when no additional germination had occurred for two weeks. The viability of ungerminated seeds was checked by a cuttest. Seeds with a plump, firm, and white embryo were considered viable.
2.3. Data analysisThe final germination percentage was expressed as the mean ± standard error (SE) of the three replicates according to the total number of filled seeds. A one-way ANOVA was employed to determine whether GA3 and KNO3 significantly increased the final germination percentage or germination rate (1/t50) relative to control seeds at 15/5 and 25/15 ℃. The generalized linear model (GLM) was used to compare final germination percentages under control conditions (temperature and cold stratification). Tukey's HSD test was performed for multiple comparisons to determine significant (P < 0.05) differences between treatments. After ungerminated seeds were transferred from dark to light, one-way ANOVA was employed to determine whether germination percentage of coldstratified seeds increased significantly relative to untreated seeds at 10, 15/5, 20 and 25/15 ℃. When data did not follow a normal distribution, the non-parametric Kruskale-Wallis test was carried out. To determine differences between fluctuating and constant temperatures on germination (10 vs. 15/5, 20 vs. 25/15 ℃), an independent-sample t-test was employed. All data were analyzed using the procedures in PASW Statistics 18 (SPSS, Inc., 2009; Chicago, IL, www.spss.com), and all figures were drawn with OriginPro 9.5.
Cardinal temperatures were estimated with a thermal-time model (Hardegree, 2006). Germination time courses for all three replicates at a given temperature were combined and fitted using the Weibull Function (Brown and Mayer, 1988) in OriginPro 9.5. Estimates of time (tg, days) taken for cumulative germination to reach different percentiles (g) for successive increments of 5% germination were interpolated from the germination progress curves (Covell et al., 1986). Germination rate (1/t25) was plotted as a function of temperature and regressed using a linear model (Udo et al., 2017), to estimate the base temperature (Tb) below which germination rate was equal to zero. The slope of the linear regression line corresponded to the reciprocal of the thermal-time requirement at suboptimal temperatures (θ25).
3. Results 3.1. Effect of GA3 and KNO3 on seed germinationAt low fluctuating temperatures (15/5 ℃), in which the germination percentage of dried after-ripening seeds was low (5.0 ± 5.0%), GA3 and KNO3 treatment increased the germination percentage (Fig. 1). At higher fluctuating temperatures (25/15 ℃), in which the germination percentage of untreated seeds was high (80.4 ± 4.8%), GA3 and KNO3 did not significantly increase germination percentage, but increased germination rate (1/t50).
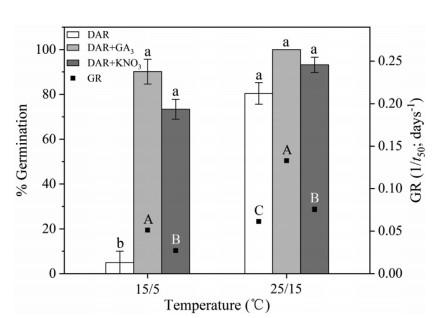
|
| Fig. 1 Final germination percentages (GP) and germination rate (GR, 1/t50) at two fluctuating temperatures for dry after-ripening (DAR), DAR + GA3, and DAR + KNO3 seeds of Primula beesiana in the light. Error bars indicate SE for three replicates of 20 seeds. Bars with the same lowercase (GP) and uppercase letters (GR) are not significantly different (P < 0.05). |
Cold stratification treatments increased germination percentage and germination rate, and widened the range of germination temperatures (Figs. 2 and 4). The germination percentage of control seeds (0) was high (> 70%) at higher temperature regimes (20, 25, 28, 30 and 25/15 ℃); but below 15 or above 30 ℃, germination percentage was approx. 10.0%, or even 3%. The fitted GLM showed that temperature (T), cold-stratified treatment (C) factors, and of their interaction (T × C) had a statistically significant effect on germination (P < 0.001; Fig. 2). Specifically, the effect of cold stratification was positive and statistically significant at lower temperatures (5, 10, 15 and 15/5 ℃). However, germination percentage did not increase in response to the length of stratification (i.e. from four-week to 16-weeks); moreover, cold-stratified seeds incubated at lower temperatures still had lower germination percentages than those at higher temperatures (20, 25, 30 and 25/ 15 ℃; Fig. 2).
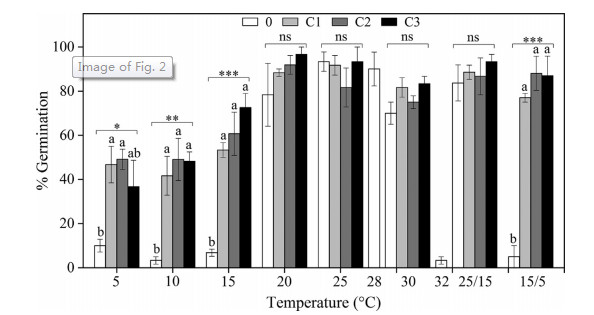
|
| Fig. 2 Effects of temperatures and cold treatments (0, control; C1, C2 and C3 cold stratification at 1 ℃ for 4, 8 and 16 weeks, respectively) on germination of Primula beesiana. Error bars indicate SE for three replicates of 20 seeds. Temperatures, treatments, and their interaction are statistically significant (P < 0.001 by GLM). Post hoc pairwise t-test comparisons (with Tukey's HSD) were carried out for each germination temperature. Asterisks indicate significant differences (*P < 0.05; **P < 0.01; ***P < 0.001) between germination percentage at the same temperature of the four treatments. Different letters indicate significant differences at P < 0.05 at each temperature. |

|
| Fig. 3 Effect of fluctuating and corresponding constant temperatures on germination percentages (GP) and germination rate (GR, 1/t50) of Primula beesiana under each cold treatment (0, control; C1, C2 and C3 cold stratification at 1 ℃ for 4, 8 and 16 weeks, respectively). Error bars indicate SE for three replicates of 20 seeds. * indicates significant difference of germination rate at P < 0.05; different letters indicate significant differences of germination percentages at P < 0.05, and the unmarked bars and boxes showed no difference between alternating and corresponding constant temperatures (10 vs. 15/5 ℃, 20 vs. 25/15 ℃). |
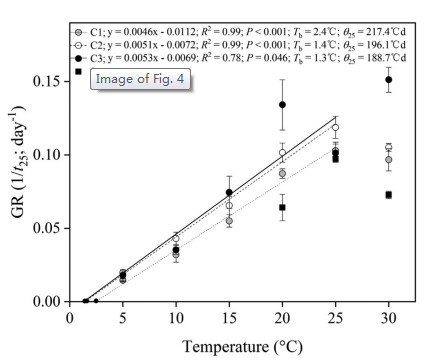
|
| Fig. 4 Base temperatures (Tb), calculated for 25% germination percentiles of Primula beesiana seeds, after each pre-treatment (0, control; C1, C2 and C3 cold stratifications at 1 ℃ for 4, 8 and 16 weeks, respectively) and incubation at the sub-optimal temperatures (5e25 ℃). Error bars indicate SE for three replicates of 20 seeds. Points correspond to the actual data and lines indicate the fitted line from the linear regressions. |
Low temperature fluctuations (15/5 ℃) did not significantly increase germination of untreated seeds; in contrast, after cold stratification, germination and germination rate (1/t50) increased significantly compared with the corresponding constant temperature (10 ℃; Fig. 3). Untreated and cold-stratified seeds all reached high germination when incubated at higher temperatures (Figs. 2 and 3). The effect of fluctuating temperature regime (25/15 ℃) on germination was not significant compared with the corresponding constant temperature (20 ℃; Fig. 3), except for that of the untreated seeds (Fig. 3).
3.4. Effect of light on seed germinationUntreated and cold-stratified seeds did not germinate, or rarely germinated, in the dark at any temperature (Table 1). After transfer to the light, fresh ungerminated seeds began germinating slowly to large numbers, except at 10 ℃, where germination was 0 or < 10%. Seeds that were cold stratified and incubated at fluctuating temperatures showed significantly higher germination, especially after cold stratification for four week and eight weeks.
| Cold stratification | 10 ℃ | 15/5 ℃ | 20 ℃ | 25/15 ℃ | ||||||||
| Dark | Dark to light | Dark | Dark to light | Dark | Dark to light | Dark | Dark to light | |||||
| 0 | 0 | 0b | 1.67 ± 1.67 | 11.67 ± 4.41b | 0 | 38.33 ± 6.01b | 0 | 21.81 ± 4.96b | ||||
| C1 | 0 | 0b | 0 | ***5.09 ± 0.09b | 0 | 35.00 ± 2.89b | 0 | ***91.67 ± 3.33a | ||||
| C2 | 3.33 ± 1.67 | 8.33 ± 3.33a | 0 | **60.00 ± 7.64a | 0 | 83.33 ± 4.41a | 0 | *96.67 ± 1.67a | ||||
| C3 | 0 | 0b | 0 | 4.76 ± 4.76b | 4.76 ± 4.76 | 85.71 ± 8.25a | 4.76 ± 4.76 | 95.24 ± 4.76a | ||||
Based on the germination rate (1/t25) for the cold-stratified seeds, goodness of fit (R2) for the linear regressions of 1/t25 against temperature showed that the best sub-optimal model included data only up to 25 ℃ (i.e. excluding 30 ℃; Fig. 4). It was possible to estimate the base temperature for germination (Tb) in the suboptimal temperature range for each treatment (Fig. 4). Tb values and thermal time (θ25) for four week of cold stratification were 2.4 ℃ and 217.4 ℃d; for eight weeks of cold stratification, 1.4 ℃ and 196.1℃d; and for 16 weeks of cold stratification, 1.3 ℃ and 188.7 ℃d. In addition, considering that germination percentage was higher at 20e28 ℃, but suddenly decreased by one-third at 30 ℃ to only 3% at 32 ℃ in untreated seeds (Fig. 2), the optimal temperature (To) for germination was estimated to range between 25 and 28 ℃, and the maximum temperature (Tm) was estimated to range between 30 and 32 ℃ (Fig. 4).
4. DiscussionIn this study, we provide evidence that P. beesiana seeds have type 3 non-deep physiological dormancy. The dormancy type of P. beesiana has been demonstrated in three ways. Untreated (0) seeds had high germination percentages at higher temperatures (25/15 ℃), but very low germination percentages at lower temperatures (15/5 ℃). In addition, GA3 and KNO3 significantly increased germination percentage at 15/5 ℃, but had no effect at 25/15 ℃. These results suggest that fresh P. beesiana seeds might have physiological dormancy (PD), whereas dry after-ripening was not sufficient to break dormancy. Second, after cold stratification, seeds germinated at significantly higher levels compared to untreated (0) seeds, especially at low temperatures (5-15 ℃). This finding confirms that P. beesiana seeds have PD and supports earlier observations of other Primula species (Baskin and Baskin, 2014; Hitchmough et al., 2011; Peng et al., 2019a; Wang et al., 2017). The response of P. beesiana seeds to both GA3 and cold stratification suggests that they have non-deep PD (Baskin and Baskin, 2004). Further, the widening of the temperature range at which seeds germinated - from medium to both high and low - indicates that P. beesiana seeds have type 3 non-deep PD (Baskin and Baskin, 2014).
We found that the germination temperature of cold-stratified seeds (4-16 weeks) widened from higher to lower temperatures compared with untreated seeds (0), which had a low germination percentage (< 10%) at lower temperatures (5, 10, 15 and 15/5 ℃). This finding suggests that cold-stratification is an effective agent for breaking seed dormancy in P. beesiana. We estimated that the optimal temperature (To) for P. beesiana germination is between 25 and 28 ℃, as the best fit of the germination rate data in the suboptimal temperature range excluded 30 ℃, which fell in the supra-optimal temperature range. The base temperatures for germination (Tb) in seeds of P. beesiana varied from approx. 15 ℃ for untreated seeds (0) to 1.3 ℃ for cold-stratified seeds (16 weeks). Seeds of P. beesiana had similar low Tb values (< 5 ℃) as other Primula species in the alpine region of Hengduan Mountains (Peng et al., 2019a). A lower Tb allows seeds to accumulate heat when ambient temperatures are low (below 5 ℃). The requirement of cold stratification for release from dormancy is a universal mechanism for preventing winter germination that is shared by most spring-germinating alpine plants (Peng et al., 2017, 2018, 2019b; Wang et al., 2017).
Final germination of P. beesiana responded positively to temperature fluctuations (15/5 ℃) in the light (Fig. 3), even after a period of cold stratification (4e16 weeks). These results are consistent with previous studies (Liu et al., 2013; Thompson and Grime, 1983) that suggested that seed germination of many species living in wet habitats require temperature fluctuations. P. beesiana occupy moderately wet habitats in spring, and must detect the water levels to avoid germinating in deep water. Because the temperature of shallow water fluctuates much more than that of deep water, a positive germination response to temperature fluctuations is a good mechanism to limit germination in shallow water. Moreover, temperature fluctuations occur frequently in alpine environments during the spring germination period when solar irradiance slowly increases. Thus, the response of P. beesiana seeds to fluctuating temperatures (especially at 15/5 ℃) also ensures germination in spring.
Our results show that light significantly increased P. beesiana germination. These results are in agreement with those of previous studies on other Primula species (Hitchmough et al., 2011; Peng et al., 2019a; Shimono and Washitani, 2004; Wang et al., 2017). In spring, P. beesiana habitats are generally wet; therefore, this requirement for light, which is a common germination requirement or germination stimulus (Grime et al., 1981), may be an adaptation to wet habitats. Aside from niche preference (e.g. wet habitat), functional traits (e.g. seed mass) also affect the light requirement of germination (Milberg et al., 2000). For instance, many species of Primula have relatively small seeds (http://www.kew.org/data/sid); for one gram of P. beesiana, there are approximately 4760 seeds. Small-seeded species are known to form buried soil seed banks were inhibited by darkness and in the absence of light do not respond to fluctuating temperatures (Thompson and Grime, 1983). In this study, seeds of P. beesiana were shown to have strict light requirements; moreover, these requirements were not rescued by fluctuating temperatures in darkness. Germinability in darkness was negligible even after experiencing cold stratification. However, after transfer to the light, germinability increased greatly, especially at fluctuating temperatures. Such positive responses to light and temperature fluctuations are likely mechanisms that enable seeds to detect water level and soil depth. These mechanisms ensure that germination occurs only in gaps and at/near the soil surface, thus avoiding seedling death by inundation or germination deep in the soil.
5. ConclusionIn conclusion, we demonstrated that P. beesiana seeds undergo type 3 non-deep physiological dormancy, which is consistent with other Primula species (Baskin and Baskin, 2014; Hitchmough et al., 2011). We also found that cold stratification, GA3 and KNO3 are effective methods for dormancy release. Seed dormancy mechanisms and thermal characteristics (e.g. Tb and θ25) of germination suggest that seeds of P. beesiana may delay germination until the next spring when water levels and temperatures of habitat are suitable for germination, which may be advantageous in alpine habitats (Peng et al., 2017, 2018, 2019b). The requirement of light and fluctuating temperatures for germination allows buried seeds to sense depth and may initiate establishment from seeds within suitable gaps. These mechanisms are especially important for P. beesiana, which have small seeds that are often buried deep in the gravelly soils of alpine habitats. This study also demonstrates that propagation from seeds is a feasible and inexpensive method for cultivation of P. beesiana and that high temperature, temperature fluctuations, cold stratification, GA3, and KNO3 are effective methods to improve germination.
Author contributionsD-L.P., H.S. and Z-M.L. designed the study. L-E.Y., D-L.P., L.H. and J.Y. performed the experiments. D-L.P. analyzed the data. D-L.P. and L-E.Y. wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Declaration of Competing InterestWe declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled "Cold stratification, temperature, light, and plant growth regulators effects on seed germination of P. beesiana from Yunnan, China".
AcknowledgementsWe would like to thank two anonymous reviewers for their comments and suggestions. We thank Yun-Gang, Guo, Ya-Juan, Yang, Dr. Xiao-Jian, Hu, and Dr. Xiang-Yun, Yang of the National Germplasm Bank of Wild Species for help during the experiments. We are grateful to hang-Qiu, Liu and Min-Shu, Song for their assistance in seed collection and laboratory work. This study was supported by the National Key R & D Program of China (2017YF0505200 to H. Sun), the Strategic Priority Research Program of Chinese Academy of Sciences (XDA 20050203 to H. Sun), the Key Program of the National Natural Science Foundation of China (U1802232 to H. Sun), National Natural Science Foundation of China (grant 31700284 to D.L. Peng, 31670206 to Z.M. Li and 31900185 to L.E. Yang).
Baskin, C.C., Baskin, J.M., 2014. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, second ed. Elsevier Academic Press Inc., San Diego.
|
Baskin J.M., Baskin C.C., 2004. A classification system for seed dormancy. Seed Sci. Res, 14: 1-16. DOI:10.1079/SSR2003150 |
Batlla D., Benech-Arnold R., 2015. A framework for the interpretation of temperature effects on dormancy and germination in seed populations showing dormancy. Seed Sci. Res, 25: 1-12. DOI:10.1017/S0960258515000033 |
Brown R.F., Mayer D.G., 1988. Representing cumulative germination: 2. the use of the Weibull function and other empirically derived curves. Ann. Bot, 61: 127-138. DOI:10.1093/oxfordjournals.aob.a087535 |
Covell S., Ellis R.H., Roberts E.H., et al, 1986. The influence of temperature on seed germination rate in grain legumes: I. a comparison of chickpea, lentil, soyabean and cowpea at constant temperatures. J. Exp. Bot, 37: 705-715. DOI:10.1093/jxb/37.5.705 |
Grime J.P., Mason G., Curtis A.V., et al, 1981. A comparative study of germination characteristics in a local flora. J. Ecol, 69: 1017-1059. DOI:10.2307/2259651 |
Hardegree S.P., 2006. Predicting germination response to temperature. I. cardinaltemperature models and subpopulation-specific regression. Ann. Bot, 97: 1115-1125. DOI:10.1093/aob/mcl071 |
Hitchmough J., Innes S., Mitschunas N., 2011. The effect of seed treatment and depth of sowing on seedling emergence in Primula species. Seed Sci. Technol, 39: 539-551. DOI:10.15258/sst.2011.39.3.01 |
Hu, C.M., Kelso, S., 1996. In: Wu, Z.Y., Raven, P.H., Hong, D.Y. (Eds.), Primulaceae. Science Press, Missouri Botanical Garden Press, Beijing, China, St. Louis, MO, USA.
|
Huang Y., Li N.W., Ren Z.X., et al, 2015. Reproductive biology of Primula beesiana (Primulaceae), an alpine species endemic to Southwest China. Plant Ecol. Evol, 148: 289-296. DOI:10.5091/plecevo.2015.867 |
Jaganathan G.K., Dalrymple S.E., Liu B., 2015. Towards an understanding of factors controlling seed bank composition and longevity in the alpine environment. Bot. Rev, 81: 70-103. DOI:10.1007/s12229-014-9150-2 |
Körner, C. (Ed.), 2003. Alpine Plant LifedFunctional Plant Ecology of High Mountain Ecosystems. Springer, Berlin.
|
Liu K., Baskin J.M., Baskin C.C., et al, 2013. Effect of diurnal fluctuating versus constant temperatures on germination of 445 species from the eastern Tibet Plateau. PloS One, 8: e69364. DOI:10.1371/journal.pone.0069364 |
Ma Y.P., Tian X.L., Zhang J.L., et al, 2014. Evidence for natural hybridization between Primula beesiana and P. bulleyana, two heterostylous primroses in NW Yunnan, China. J. Systemat. Evol, 52: 500-507. DOI:10.1111/jse.12077 |
Milberg P., Andersson L., Thompson K., 2000. Large-seeded species are less dependent on light for germination than small-seeded ones. Seed Sci. Res, 10: 99-104. DOI:10.1017/S0960258500000118 |
Mondoni A., Daws M.I., Belotti J., et al, 2009. Germination requirements of the alpine endemic Silene elisabethae Jan: effects of cold stratification, light and GA3. Seed Sci. Technol, 37: 79-87. DOI:10.15258/sst.2009.37.1.10 |
Peng D.L., Chen Z., Hu X.J., et al, 2017. Seed dormancy and germination characteristics of two Rheum species in the Himalaya-Hengduan Mountains. Plant Divers, 39: 180-186. DOI:10.1016/j.pld.2017.05.009 |
Peng D.L., Hu X.J., Sun H., et al, 2019a. Dry after-ripening, light, cold stratification, and temperature effects on seed germination of Primula poissonii from Yunnan, China. Seed Sci. Technol, 47: 1-6. DOI:10.15258/sst.2019.47.1.01 |
Peng D.L., Hu X.J., Yang J., et al, 2018. Seed dormancy, germination and soil seed bank of Lamiophlomis rotata and Marmoritis complanatum (Labiatae), two endemic species from Himalaya-Hengduan Mountains. Plant Biosyst, 152: 642-648. DOI:10.1080/11263504.2017.1311959 |
Peng D.L., Sun L., Pritchard H.W., et al, 2019b. Species distribution modelling and seed germination of four threatened snow lotus (Saussurea), and their implication for conservation. Global Ecol. Conserv, 17: e00565. DOI:10.1016/j.gecco.2019.e00565 |
Porceddu M., Mattana E., Pritchard H.W., et al, 2013. Thermal niche for in situ seed germination by Mediterranean mountain streams: model prediction and validation for Rhamnus persicifolia seeds. Ann. Bot, 112: 1887-1897. DOI:10.1093/aob/mct238 |
Schwienbacher E., Navarro-Cano J.A., Neuner G., et al, 2011. Seed dormancy in alpine species. Flora, 206: 845-856. DOI:10.1016/j.flora.2011.05.001 |
Shimono A., Washitani I., 2004. Seedling emergence patterns and dormancy/ germination physiology of Primula modesta in a subalpine region. Ecol. Res, 19: 541-551. DOI:10.1111/j.1440-1703.2004.00667.x |
Thompson K., Grime J.P., 1983. A comparative study of germination responses to diurnally-fluctuating temperatures. J. Appl. Ecol, 20: 141-156. DOI:10.2307/2403382 |
Udo N., Tarayre M., Atlan A., 2017. Evolution of germination strategy in the invasive species Ulex europaeus. J. Plant Ecol, 10: 375-385. |
Wang G.Y., Baskin C.C., Baskin J.M., et al, 2017. Timing of seed germination in two alpine herbs on the southeastern Tibetan plateau: the role of seed dormancy and annual dormancy cycling in soil. Plant Soil, 421: 465-476. DOI:10.1007/s11104-017-3400-0 |
Wu Z.K., Zhang C.Q., 2010. Comparative study of pollination biology of two closely related alpine Primula species, namely Primula beesiana and P. bulleyana (Primulaceae). J. Systemat. Evol, 48: 109-117. DOI:10.1111/j.1759-6831.2010.00069.x |



