b. CAS Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650204, China;
c. Gongju National University of Education, 27, Ungjin-ro, Gongju-si, Chungcheongnam-do, 32553, Republic of Korea;
d. Graduate School of Horticulture, Chiba University, 648 Matsudo, Chiba, 271-8510, Japan;
e. College of Life Science, Northeast Agricultural University, Harbin, 150030, Heilongjiang, PR China
Species diversity of most plant groups tends to be concentrated in one or several geographic centers rather than having an even distribution within its geographic range (Jetz et al., 2012; Kiera et al., 2009; Myers et al., 2000; Pimm et al., 2014; Wu et al., 2006). Of primary interest is when and how the species diversity of plant groups accumulated in their geographic centers. For temperate woody plants of the northern hemisphere, East Asia is a major diversity center (Adair and Li, 1994; Latham and Ricklefs, 1993; Qian, 2002; Wang et al., 2011). The theoretical explanation for this phenomenon is that East Asia is an evolutionary cradle of species diversity, or a museum that accumulates lineages of both old and recent origins (Chen et al., 2018; Lu et al., 2018; Tang et al., 2018; Thome, 1999; Tiffney, 1985). However, understanding how temperate woody plant groups has accumulated diversity requires specific tests: time-calibrated molecular phylogenetic study and paleobotanical study. In recent years, many molecular phylogenetic studies on temperate woody plants have suggested that most of them originated in the Paleogene and diversified at different times throughout the Paleogene or Neogene (Chin et al., 2014; Forest et al., 2005; Grimm and Renner, 2013; Hinsinger et al., 2013; Li et al., 2018; Naciri et al., 2019; Renner et al., 2008; Yang et al., 2019a, 2019b). Compared to molecular phylogenetic studies, paleobotanical studies provide more direct evidence of the evolutionary history of plants in East Asia; however, few paleobotanical studies have been conducted on the temperate woody plants.
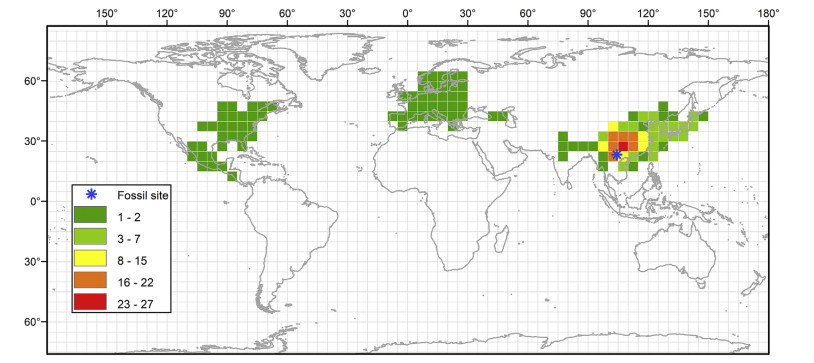
Carpinus Linnaeus in the birch family (Betulaceae Gray) is an important element of northern temperate forests (Chen, 1994a, b; Wu et al., 2006). The genus comprises about 50 species, of which 46 species are distributed in East Asia, two species in North America, and the remaining two species in Europe (Li and Skvortsov, 1999). East Asia represents a major species diversity center for the genus (Fig. 1). Earlier studies based on single or multiple gene fragment(s) show that Carpinus is not monophyletic due to Ostrya Scop. being nested within it (Chen et al., 1999; Grimm and Renner, 2013; Yang et al., 2019a; Yoo and Wen, 2002). However, a recent study based on the whole chloroplast genome indicates that the genus is monophyletic and sister to Ostrya (Yang et al., 2019b). Fossils of Carpinus are abundant in the Cenozoic of the northern hemisphere (Kim and Nam, 2017; Pigg et al., 2003). They have been extensively documented in the form of involucres, sometimes with an attached nut, and leaves, ranging from the Paleogene to the Pleistocene (Dai et al., 2013; Kim and Nam, 2017; Pigg et al., 2003; Stults et al., 2002). Despite extensive studies on Carpinus, few have been carried out to reveal how the diversity of the genus accumulated in East Asia.
Recently, abundant Carpinus involucre fossils were discovered from the early Miocene of southwestern China. In this study, we aim to (1) identify and describe these involucre fossils; (2) sum-marize fossil records of Carpinus in East Asia; and then (3) discuss the accumulation of species diversity within the genus in East Asia.
2. Material and methods 2.1. Geological settingThe involucre fossils were collected from the Maguan Basin in southeastern Yunnan province, southwestern China (23°1'N, 104°23'E, 1320 m a.s.l.; Fig. 1). The fossil-bearing layers, charac-terized by cyclic deposits of light-yellow or light-grey laminated mudstones and siltstones, belong to the Huazhige formation (Zhang, 1976). The Huazhige formation is also distributed in Wen-shan Basin, which is 45 km from the Magaun Basin. The formation in these two basins has uncovered diverse floras, i.e. the Maguan flora and the Wenshan flora (Huang, 2017; Jia, 2018). Paleobotanical studies show that the two flora are floristically similar except that the Maguan flora contains many extinct elements such as Cedre-lospermum Sapota (Jia et al., 2015), Diviacer Manchester, and Podocarpium A. Braun ex Stizenberger (Jia, 2018). Therefore, the age of the Maguan assemblage is likely older than the Wenshan assemblage. Based on magnetostratigraphic study, the age of Wenshan assemblage has been assigned to the earliest Middle Miocene (15.2-16.5 Ma) (Lebreton-Anberrée et al., 2016). Beneath the Maguan assemblage, a mammal fossil from the Huangzhige formation called Gigantamynodon was uncovered (Qi, 1992). Gigantamynodon is suggested as have lived from the middle to late Oligocene (Lucas and Emry, 1996; Qi, 1992). Therefore, previous researchers have suggested that the age of the Maguan flora is likely the early Miocene (Huang, 2017; Jia, 2018; Jia et al., 2019b).
|
| Fig. 1 Extant distribution of Carpinus and the position of the fossil locality in Maguan Basin, Yunnan province, China. Different colors in the map indicate the number of species in each grid. Extant occurrence data of Carpinus were from the Chinese Virtual Herbarium (CVH) and Global Biodiversity Information Facility (GBIF). |
Based on preliminary study, the Maguan flora represents a subtropical evergreen broad-leafed forest that includes floristic elements such as Burretiodendron Rehder (Lebreton-Anberrée et al., 2015), Pterolobium R. Br. ex Wight et Arn. (Jia et al., 2017), Sequoia Endlicher. (Zhang et al., 2015), Sladenia Kurz (Jia et al., 2019a), and Ulmus L. (Zhang et al., 2018).
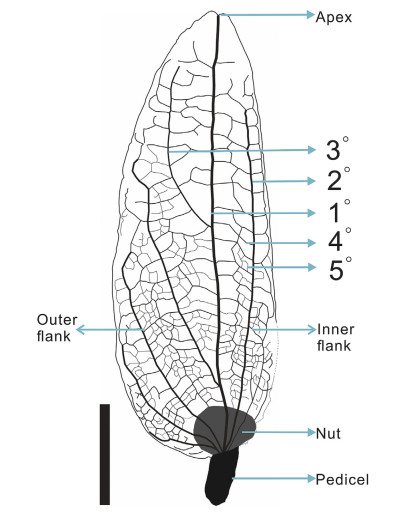
2.2. Examination and comparison of fossilsA total of 30 involucre fossils assigned to Carpinus were collected from the outcrop. These fossil specimens were first photographed using a digital camera (Nikon D700, Kanagawa, Japan), and then moved to a stereo microscope (Leica S8APO, Wetzlar, Germany) to observe fine details. Specimens from the Herbarium of the Kunm-ing Institute of Botany, Chinese Academy of Sciences (KUN) were studied for comparison purposes. Digitized specimens from the Chinese Virtual Herbarium (http://www.cvh.ac.cn/), the Global Plants on JSTOR (https://plants.jstor.org/), and the Royal Botanic Gardens, Kew (https://www.kew.org/) were also studied. Fossil records of Carpinus were compiled from the Cenozoic Angiosperm Database (http://www.fossil-cad.net/) (Xing et al., 2016), and published literature (Table 1). Because the leaves of Carpinus overlap morphologically with those of other genera in the Betula-ceae, we only include involucre fossils and leaf fossils with cuticular details in the discussion. Extant occurrences of Carpinus were obtained from the Chinese Virtual Herbarium and Global Biodiversity Information Facility (https://www.gbif.org/). Line drawings of the fossils were made by CorelDraw X7, and the distribution map of Carpinus was made by ArcGIS 10.0. As the involucre of Carpinus is leaf-like, the terminology used for description mainly follows Ellis et al. (2009) (Fig. 2).
| Species | Type | Age | Country | Locality | Reference | |
| 1 | C. sp. | Involucre | Early Oligocene | China | Lvhe, Yunnan | Linnemann et al. (2017) |
| 2 | C. sp. cf. C. longibracteata | Involucre | Oligocene | Japan | Ouchiyama-kami, | Uemura et al. (1999) |
| Hu & Chaney | Yamaguchi, Honshu | |||||
| 3 | C. sp. | Involucre | Oligocene | Japan | Ouchiyama-kami, | Uemura et al. (1999) |
| Yamaguchi, Honshu | ||||||
| 4 | C. subyedoensis Konno | Involucre | Early Miocene | Korea | Janggi flora | Huzioka (1972) |
| 5 | C. subcordata Nathorst | Involucre | Early Miocene | Korea | Janggi flora, | Huzioka (1972); Ablaev et al. (1993) |
| Yongdong flora | ||||||
| 6 | C. subyodoensis Kon'no | Involucre | Early Miocene | China | Weichang, Hebei | Bureau of Geology and Mineral |
| Resources of HebeiProvince (1989) | ||||||
| 7 | C. asymmetrica L. Xue & L.B. Jia | Involucre | Early Miocene | China | Maguan | This study |
| 8 | C. symmetrica L. Xue & L.B. Jia | Involucre | Early Miocene | China | Maguan | This study |
| 9 | C. cf. fangiana Hu | Involucre | Early Miocene | China | Maguan | This study |
| 10 | C. sp.1 | Involucre | Early Miocene | China | Maguan | This study |
| 11 | C. sp.2 | Involucre | Early Miocene | China | Maguan | This study |
| 12 | C. sp.3 | Involucre | Early Miocene | China | Maguan | This study |
| 13 | C. kyushinensis Endo | Involucre | Middle Miocene | Korea | Gungsim flora | Endo (1950) |
| 14 | C. endoi Huzioka | Involucre | Middle Miocene | Korea | Gungsim, Gogeonwon | Huzioka (1972); Lim et al. (1994) |
| floras | ||||||
| 15 | C. simplicibracteata Huzioka | Involucre | Middle Miocene | Korea | Janggi, Gogeonwon, | Huzioka (1943) |
| Hamjindong floras | ||||||
| 16 | C. stenophylla Nathorst | Involucre | Middle Miocene | Japan | Kaminokuni flora | Chaney (1963) |
| 17 | C. chaneyi Tanai & Suzuki | Involucre | Middle Miocene | China | Linju, Shandong | Sun (1999) |
| 18 | C. megabractcata Hu & Chaney | Involucre | Middle Miocene | China | Linju, Shandong | Sun (1999) |
| 19 | C. mioturczaninowii Hu & Chaney | Involucre | Middle Miocene | China | Linju, Shandong | Sun (1999) |
| 20 | C. oblongibracteata Hu & Chaney | Involucre | Middle Miocene | China | Linju, Shandong | Sun (1999) |
| 21 | C. shanwangensis Hu & Chaney | Involucre | Middle Miocene | China | Linju, Shandong | Sun (1999) |
| 22 | C. shimizui Tanai | Involucre | Middle Miocene | China | Linju, Shandong | Sun (1999) |
| 23 | C. subyedoensis Kon'no | Involucre | Middle Miocene | Japan | Tsuchikumazawa | Chaney (1963) |
| 24 | C. praejaponica Berger | Involucre | Middle Miocene | Japan | Yamakayakusa | Chaney (1963) |
| 25 | C. kodairae-bracteata Huzioka | Involucre | Middle Miocene | Korea | Yeonil flora | Kim and Nam (2017) |
| 26 | C. miofargesiana Tanai & Onoe | Involucre | Middle Miocene | Korea | Yeonil flora | Kim and Nam (2017) |
| 27 | C. oblongibracteata Hu & Chaney | Involucre | Middle Miocene | Korea | Yeonil flora | Kim and Nam (2017) |
| 28 | C. hokoensis Endo | Involucre | Middle Miocene | Korea | Yeonil flora | Endo (1950); Lim et al. (1994) |
| 29 | C. miofargesiana Tanai & Onoe | Involucre | Middle Miocene | Korea | Yeonil flora | Kim and Nam (2017) |
| 30 | C. oblongibracteata Hu & Chaney | Involucre | Middle Miocene | Korea | Yeonil flora | Lim et al. (1994) |
| 31 | C. sp. | Involucre | Middle Miocene | Korea | Yeonil flora | Chun (1982) |
| 32 | C. kodairae-bracteata Huzioka | Involucre | Middle Miocene | Korea | Yeonil, Gogeonwon | Huzioka (1943); Kim and Nam (2017) |
| floras | ||||||
| 33 | C. subcordata Nathorst | Involucre | Middle Miocene | Korea | Yeonil, Gogeonwon, | Huzioka (1972); Ablaev et al. (1993) |
| Hamjindong floras | ||||||
| 34 | C. stenophylla Nathorst | Involucre | Middle Miocene | Korea | Yeonil, Janggi, Gungsim, | Huzioka (1972) |
| Hamjindong floras | ||||||
| 35 | C. chaneyi Tanai & Suzuki | Involucre | Middle Miocene | Japan | Yoshioka flora | Chaney (1963) |
| and leaf | ||||||
| 36 | C. shimizui Tanai | Involucre | Middle Miocene | Japan | Yoshioka flora | Chaney (1963) |
| 37 | C. subcordata Nathorst | Involucre | Middle Miocene | Japan | Yoshioka, Kaminokuni, | Chaney (1963) |
| and leaf | Abura, Wakamatsu floras | |||||
| 38 | C. subyedoensis Kon'no | Involucre | Middle Miocene | Japan | Yoshioka, Kaminokuni, | Chaney (1963) |
| and leaf | Abura, Wakamatsu floras | |||||
| 39 | C. heigunensis Huzioka | Involucre | Middle/Late | Japan | Yagii flora | Ozaki (1991) |
| Miocene | ||||||
| 40 | C. heigunensis Huzioka | Involucre | Late Miocene | Japan | Seto, Itahanna, Ogawa | Ozaki (1991) |
| 41 | C. ovatiInvolucreeata Li & Sun | Involucre | Late Miocene | China | Shengxian, Zhejiang | Li (2010) |
| 42 | C. orbiInvolucreeata Li & Sun | Involucre | Late Miocene | China | Shengxian, Zhejiang | Li (2010) |
| 43 | C. miotschonoskii Li & Sun | Involucre | Late Miocene | China | Shengxian, Zhejiang | Li (2010) |
| 44 | C. nipponica Endo | Involucre | Miocene | Japan | Nishizawa | Endo (1950) |
| 45 | C. subcordata Nathorst | Leaf with | Miocene | China | Tengchong, Yunnan | Sun et al. (2003) |
| cuticle | ||||||
| 46 | C. honshuensis Endo | Involucre | Early Pliocene (?) | Japan | Maki, Kawanishi | Endo (1950) |
| 47 | C. cf. nipponica Endo | Involucre | Pliocene | Japan | Kabutoiwa Formation | Ozaki (1991) |
| 48 | C. tengchongensis | Leaf with | Pliocene | China | Tengchong, Yunnan | Dai et al. (2013) |
| Dai & B.N. Sun | cuticle | |||||
| 49 | C. japonica Blume | Involucre | Pleistocene | Japan | Siobara | Endo (1940) |
|
| Fig. 2 Morphology of Carpinus involucre and corresponding terms. Scale bar = 5 mm. |
Family: Betulaceae.
Genus: Carpinus L.
Repository: Fossil specimens are stored at the Herbarium of Kunming Institute of Botany, Chinese Academy of Sciences (KUN).
Locality: Maguan Basin, southeastern Yunnan, southwestern China.
Age: early Miocene.
Section Distegocarpus (Sieb. et Zucc.) Sarg.
Carpinus symmetrica L. Xue & L.B. Jia sp. nov. (Morphotype 1).
Holotype: MG0737 (Plate Ⅰ, 1a) (designated here).
|
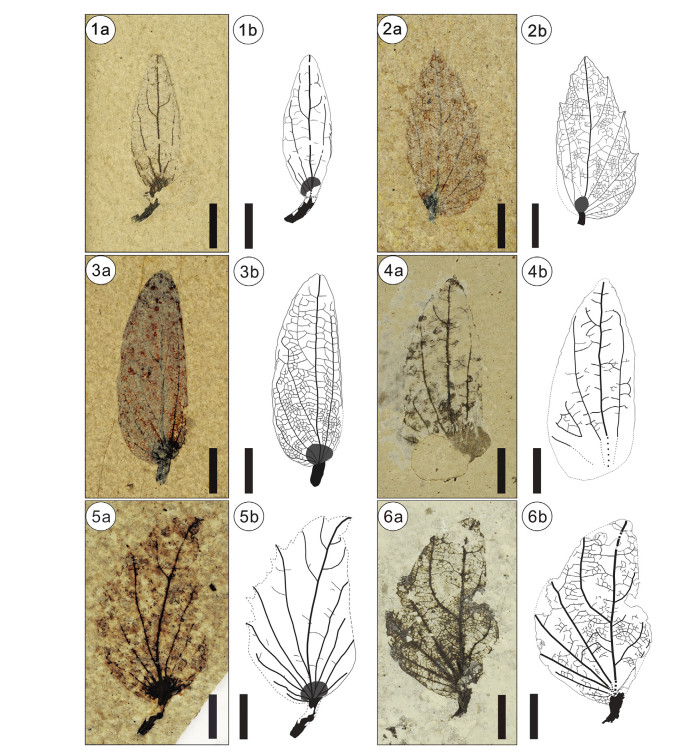
| Plate Ⅰ Fossil involucres of Carpinus and their line drawings. 1, C. symmetrica sp. nov. (morphotype 1); 2, C. cf. fangiana Hu (morphotype 2); 3, 4, C. asymmetrica sp. nov. (morphotype 4); 5, C. sp. 1 (morphotype 3); 6, C. sp. 2. (morphotype 5). 1, MG0737; 2, MG0743; 3, MG0735; 4, MG0742; 5, MG1154; 6, MG 2044. Scale bar = 5 mm. |
Etymology: The specific epithet "symmetrica" refers to the symmetry of the fossil involucre.
Specific diagnosis: Involucre oblong, symmetrical, without auricle, 19.8 mm long and 6.9 mm wide. Margin entire. Vein framework basal actinodromous with five veins.
Description: Pedicel stout, 4.9 mm long. Involucre oblong, almost symmetrical, without auricle, 19.8 mm long and 6.9 mm wide. Margin entire. Apex emarginate. Vein framework basal acti-nodromous with five veins. The middle vein extending straightly from the base to the apex of the bract. The left flank and the right flank populated by two to three secondary veins. Secondary and tertiary veins forming a series of loops, not reaching the margin. Quaternary vein convex.
Carpinus cf. fangiana Hu (Morphotype 2).
Specimen: MG0743 (Plate Ⅰ, 2a).
Description: Pedicel short, 0.9 mm long. Involucre ovate, sym-metrical, base of the left flank enfolded, without auricle, 18.0 mm long and 7.8 mm wide. Margin with sparse teeth. Apex acute. Vein framework basal actinodromous. The middle vein located at the center of the bract, extending straightly from the base to the apex. Secondary veins extending into the teeth. Left flank, two secondary veins visible after enfolding; right flank, four secondary veins. Tertiary vein originating from the midvein and extending into the teeth or forming a chevron. Quaternary vein irregular. Quinternary vein fabric irregularly reticulate.
Section Carpinus.
Carpinus sp. 1 (Morphotype 3).
Specimen: MG1154 (Plate Ⅰ, 5a).
Description: Pedicel stout, 5.0 mm long. Involucre ovate with upper part oblique to the inner (right) flank, the margin of inner flank not well preserved, 19.9 mm long, about 12.1 mm wide, asymmetrical. Outer (left) flank, 7.0 mm wide, inner flank, about 4.9 mm wide. Vein framework basal actinodromous. Inner and outer flank populating four and six secondary veins respectively. Secondary and tertiary veins terminating into teeth. The margin of the outer flank dentate. Quaternary vein not preserved. Nut, oval.
Carpinus asymmetrica L. Xue & L.B. Jia sp. nov. (Morphotype 4).
Holotype: MG0735; MG0742 (Plate Ⅰ, 3a, 4a). (designated here).
Etymology: The specific epithet "asymmetrica" means asym-metry which is an important character of the fossil involucres.
Specific diagnosis: Involucre long elliptical, asymmetrical, without auricle, 21.9-22.7 mm long and 7.7-8.8 mm wide. Margin entire. Vein framework basal actinodromous. Inner flank two veins; outer flank four veins. Nutlet oval, about 3 mm in diameter.
Description: Pedicel stout, 2.5 mm long. Involucre long ellip-tical, asymmetrical, without auricle, 21.9-22.7 mm long and 7.7-8.8 mm wide. Margin entire. Inner flank, 2.7-3.6 mm wide; outer flank, 5.0-5.2 mm wide. Vein framework basal actino-dromous. The middle vein situated close to the inner flank, extended from the base to the apex of the bract. Secondary veins forming a series loops with adjacent secondary veins or quaternary veins, not reaching the margin. The inner flank populated by two secondary veins and the outer flank populated by four secondary veins. Tertiary veins originating from the midvein, forming loops with secondary veins. Quaternary vein convex or forming a chevron, mixed percurrent. Quinternary vein irregularly reticulate.Nutlet small, about 3 mm in diameter.
Carpinus sp. 2 (Morphotype 5).
Specimens: MG0740; MG0748; DSC1543; MG1456; MG 2047; MG 2050; MG 2051 (Plate Ⅰ, 6a; Plate Ⅱ).

|
| Plate Ⅱ Fossil involucres of 1 sp. 2 (morphotype 5) and their line drawings. 1, MG0748; 2, MG1456; 3, DSC1543; 4, MG 2047; 5, MG 2050; 6, MG0740. Scale bar = 5 mm. |
Description: Pedicel stout, 1.2-4.7 mm long. Bract broad sem-iovate, asymmetrical, without auricle, 12.0-14.9 mm long, 5.8-9.9 mm wide. Inner flank, 1.9-3.9 mm wide; outer flank, 3.9-7.2 mm wide. Margin of inner flank entire or occasionally with an inconspicuous tooth. Margin of outer flank serrate. Vein framework basal actinodromous. The distal end of the midvein slightly oblique to the inner flank. The inner flank populated by one or two secondary veins; the outer flank populated by three to five secondary veins. Secondary vein losing their gauge in the course extending to the apex. Tertiary vein concave, terminating at teeth in the outer flank, forming loops or gradually lose their gauge in the inner flank. Quaternary vein convex, mixed percurrent. Quinter-nary vein irregular reticulate. Nut oval, 3-4 mm in diameter.
Carpinus sp. 3 (Morphotype 6).
Specimens: MG0666; MG0735; MG0739; MG0746; MG1594; MG 2044 (Plate Ⅲ).

|
| Plate Ⅲ Fossil involucres of Carpinus sp. 3 (morphotype 6) and their line drawings. 1, MG0735; 2, MG0739; 3, MG0666; 4, MG1594; 5, MG0746; 6, MG2044. Scale bar = 5 mm. |
Description: Pedicel stout, 1.2-4.1 mm long. Bract semiovate to triangular, asymmetrical, 20.0-24.7 mm long, 7.8-12.5 mm wide. Inner flank, 2.9-4.1 mm wide; outer flank, 4.8-8.4 mm wide. The margin of inner flank entire, straight or sinuous. The margin of outer flank dentate; teeth spaced irregularly, sparse; tooth promi-nent, lobe-like in appearance. Vein framework basal actino-dromous. The middle vein extending straightly from the base to the apex or oblique to the inner flank. The inner and outer flank populated by two to three and three to four secondary veins respectively. Secondary vein terminating at teeth in the outer flank, or forming loops with adjacent tertiary vein and Quaternary vein. Tertiary vein originating from the midvein, extending into teeth or forming loops with adjacent veins. Quaternary vein straight, convex, mixed percurrent. Quinternary vein reticulate. Nutlet oval, 3.0-4.0 mm in diameter.
4. Discussion 4.1. Morphological comparisonsThe studied fossils are characterized by short and stout pedicels, ovate leaf-like laminae with basal actinodromous venation, and an oval nut positioned at the base of the lamina (Plate Ⅰ-Ⅲ). This combination of characters is identical to involucres of Carpinus. Five other genera in the Betulaceae, i.e., Corylus L., Cranea Manchester & ZD Chen, Ostrya Scop., Ostroyopsis Decne., and Paleocarpinus Crane produce more or less similar involucres (Crane, 1981; Li and Skvortsov, 1999; Manchester and Chen, 1998). However, the in-volucres of Ostrya are saccate, and those of Cranea, Corylus, Ostrya, and Ostroyopsis are campanulate or form a tubular sheath (Li and Skvortsov, 1999; Manchester and Chen, 1998), and therefore differ from the new fossils (Plate Ⅰ-Ⅲ). The involucres of Paleocarpinus are five-lobed, enclosing two nutlets (Crane, 1981), distinguishable from those of Carpinus, which are one to three lobed and encloseone nut (Plate Ⅰ-Ⅲ). Therefore, the new fossils can be unequivocally assigned to Carpinus.
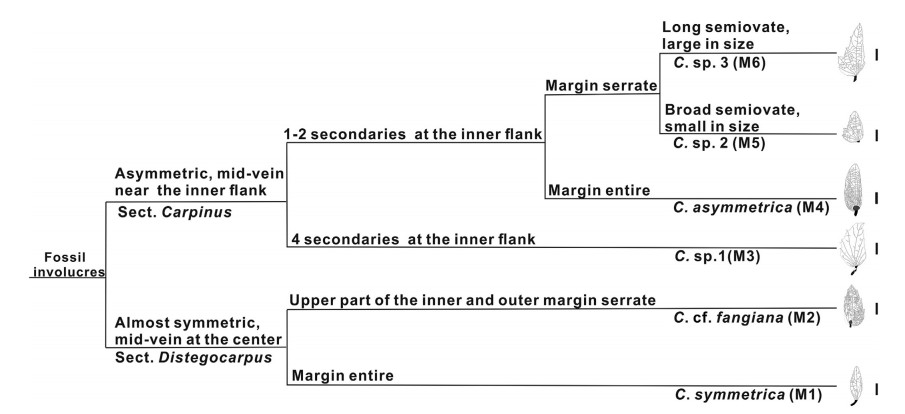
Carpinus is divided into two sections, namely, section Dis-tegocarpus (Sieb. et Zucc.) Sarg. and section Carpinus (Li and Cheng, 1979). Because the characters of the involucre are the basis for the infrageneric classification of Carpinus (Li and Cheng, 1979), it allows the new involucre fossils to be placed to section and even species level. In this study, we classified the new fossils by using both involucre characters of modern Carpinus classification systems, including the position of the midvein, presence or absence of lobes, teeth of inner and outer margins; and additional characters we regard as significant, including the numbers of veins in the inner flank and the overall shape. Based on all these characters, we recognized six morphotypes which may represent six species (Fig. 3).
|
| Fig. 3 Key for classifying involucre fossils of Carpinus from the Maguan Basin, Yunnan province, China. "M" is short for morphotype. Scale bars = 5 mm |
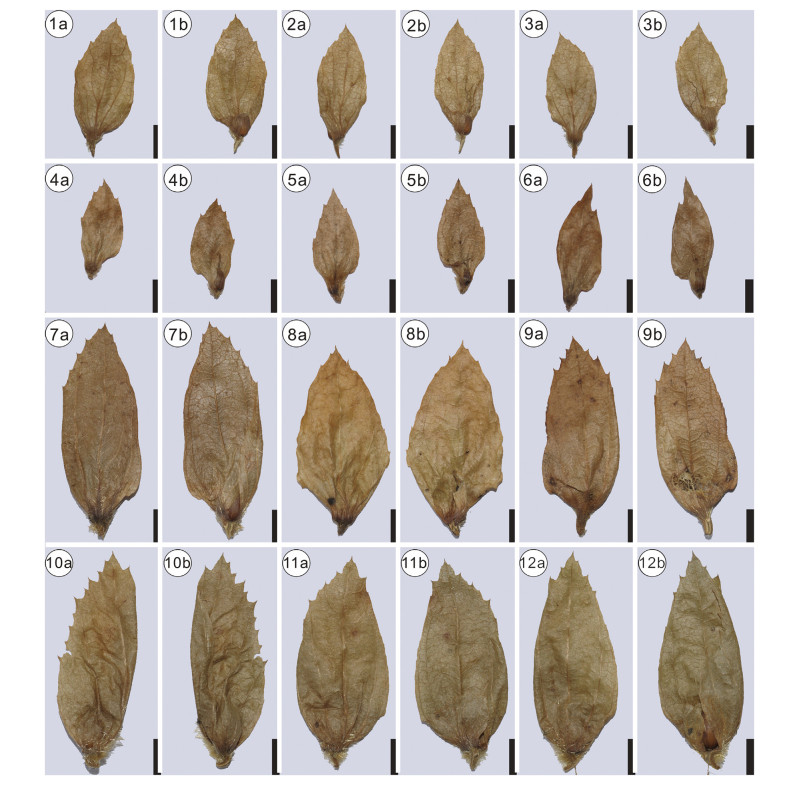
Morphotypes 1 and 2 are characterized by the midvein being situated nearly in the middle of the involucre. Although the base of morphotype 2 seems asymmetric at first glance, the obvious enfolded left side indicates that it was symmetric (Plate Ⅰ, 2a). This phenomenon is also observed in the extant involucres of Carpinus. Therefore, morphotype 1 and 2 should be assigned to the section Distegocarpus. Morphotype 1 has an entire margin (Plate Ⅰ, 1a), apparently different from the three extant species in section Dis-tegocarpus that possess sparse teeth at the apex of the involucre, i.e., Carpinus cordata Bl., Carpinus fangiana Hu, and Carpinus ran-kanensis Hayata. Thus morphotype 1 represents an extinct species within the genus. To the best of our knowledge, no fossil species have similar involucre morphology as our fossil. Therefore, we assign morphotype 1 to a new species, i.e., C. symmetrica. Mor-photype 2 is close to C. fangiana Hu in size and gross morphology (Plate Ⅰ, 2a; Plate Ⅳ,1-4 ).
|
| Plate Ⅳ Involucre morphology of Carpinus section Distegocapus. 1-3, C. fangiana Hu; 4-6, C. rankanensis Hayata; 7-9, C. cordata Bl.; 10-12, C. cordata Bl. var. chinensis Franch. "a" indicates the reverse side of the involucre; "b" indicates the front side of the involucre. Scale bars = 5 mm. |
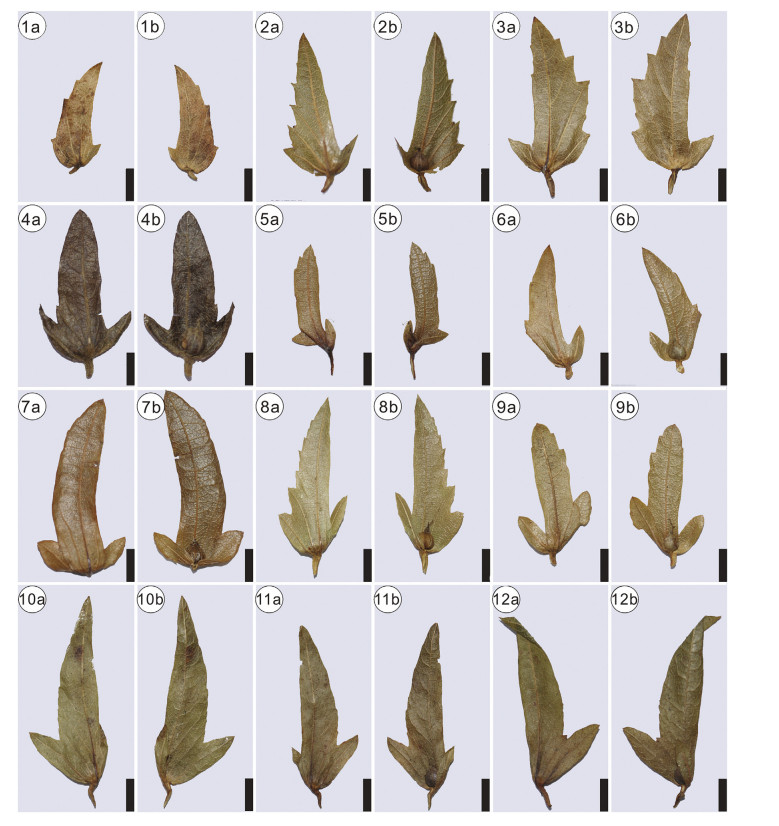
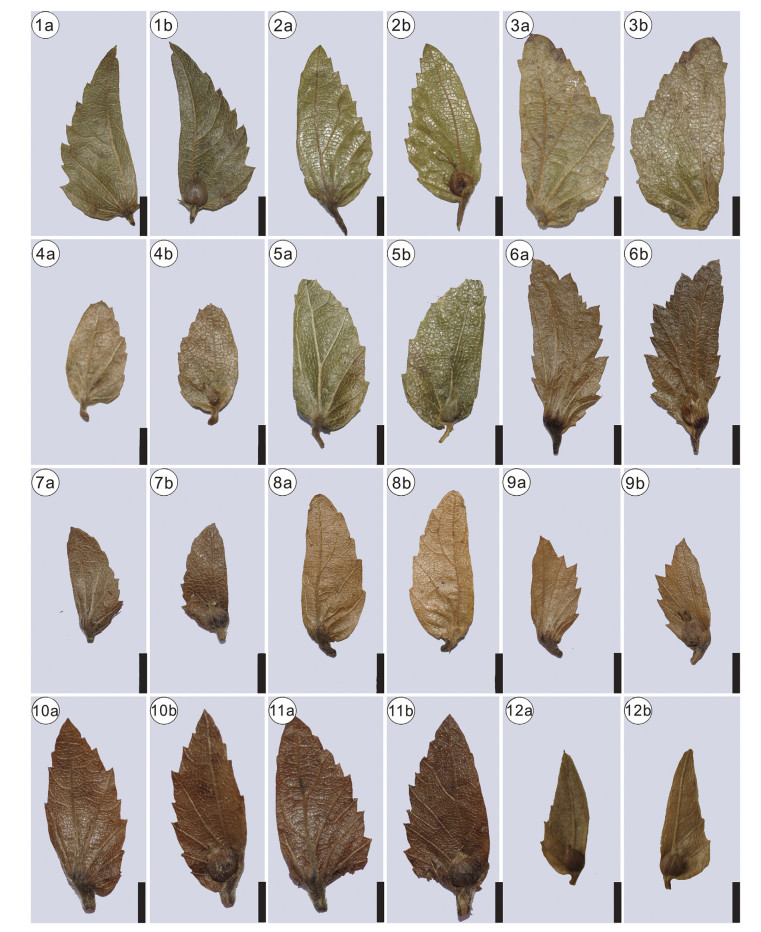
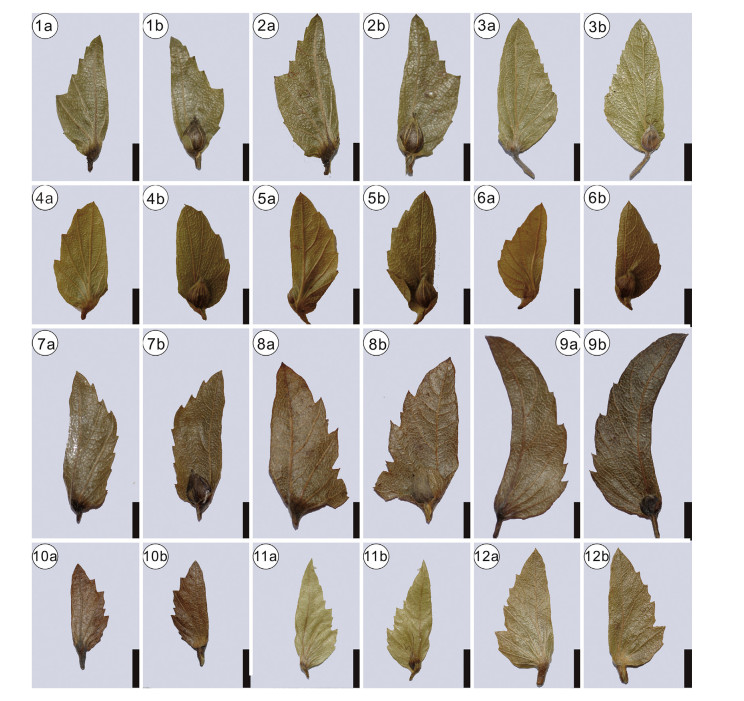
Morphotypes 3-6 are distinguished by the midvein being sit-uated at the inner flank, and thus should be assigned to section Carpinus. This section is divided into subsections Carpinus, Mon-beigianae (Hu). P. C. Li, and Polyneurae (Hu) P. C. Li (Li and Cheng, 1979). The inner and outer flanks of morphotypes 3-6 have no lobes, and thus differ from subsection Carpinus. Therefore, they represent members of subsection Monbeigianae or subsection Pol-yneurae. However, it is difficult to precisely assign them to either of the two subsections. Morphotype 3 has an almost equal number of veins at the inner and outer flank of involucre (Plate Ⅰ, 5a), which makes it to some extent similar to section Distegocarpus. However, morphotype 3 has five veins at the outer flank and an asymmetrical shape (Plate Ⅰ, 5a), which justifies our placement within section Carpinus. Compared with other extant species in section Carpinus, morphotype 3 has more secondary veins originating from the inner flank of involucre. Therefore, it may represent an extinct species in the genus. However, as the inner flank of the specimen is not well preserved, we here leave the nomenclature of morphotype 3 open for discussion. Morphotype 4 is to some extent similar to Carpinus pubescens Burk. in having an elliptical shape and round apex (Plate Ⅰ, 3a, 4a). However, the former is different from the latter in having an entire margin. To our best knowledge, no fossil species resemble morphotype 4. Therefore, morphotype 4 likely represents another extinct species. Here we assign morphotype 4 to a new species, C. asymmetrica . Morphotype 5 is small and broadly semiovate (Plate Ⅰ, 6a; Plate Ⅱ), and thus is morphologically similar to four extant species, Carpinus kweichowensis Hu, Carpinus monbeigiana Hand.-Mazz., Carpinus rupestris A. Camus, and Carpinus turczaniowii var. stiputale (H. Winkl.) H. Winkl. (Plate Ⅳ-Ⅶ). Morphotype 6 is elongated semiovate (Plate Ⅲ), closely resembling Carpinus molli-coma Hu and Carpinus omeiensis Hu & Fang (Plate Ⅳ-Ⅶ).
|
| late Ⅴ Involucre morphology of Carpinus subsection Carpinus. 1-3, C. viminea Wall.; 4, C. tientaiensis Cheng; 5-6, C. londoniana H. Winkl. var. Lanceolata (Hand. -Mazz.) P. C. Li; 7-9, C. londonniana H. Winkl.; 10-12, C. londonniana H. Winkl. var. xiphocteata P. C. Li. "a" indicates the reverse side of the involucre; "b" indicates the front side of the involucre. Scale bars = 5 mm. |
|
| Plate Ⅵ Involucre morphology of Carpinus subsection Monbeigianae (Hu). P. C. Li. 1-2, C. fargesiana H. Winkl.; 3, C. kweichowensis Hu; 4-5, C. monbeigiana Hand.-Mazz.; 6, C. turczaniowii Hance; 7-8, C. pubescens Burk.; 9, C. turczaninowii Hance var. stipulata (H. Winkl.) H. Winkl.; 10-11, C. tsaiana Hu; 12, C. chuniana Hu. "a" indicates the reverse side of the involucre; "b" indicates the front side of the involucre. Scale bars = 5 mm. |
|
| Plate Ⅶ Involucre morphology of Carpinus subsection Polyneurae. 1-2, C. mollicoma Hu; 3, C. omeiensis Hu; 4-6, C. rupestris A. Camus; 7-8, C. tschonoskii Maxima.; 9, C. tschonoskii Maxim. var. falcatibracteata (Hu) P. C. Li; 10-12, C. polyneura Franch. "a" indicates the reverse side of the involucre; "b" indicates the front side of the involucre. Scale bars = 5 mm. |
Reliable fossil records of Carpinus from the East Asian Paleogene are relatively scarce (Table 1). Based on our knowledge, the invo-lucre fossil record from the early Oligocene of the Lvhe flora in southwestern China represents the earliest record of the genus in East Asia (Linnemann et al., 2017). The other Paleogene record is from the Oligocene Ouchiyama-kami flora in Japan, where two species were recognized (Uemura et al., 1999) (Table 1). For the early Miocene, only three species have been reported from north-ern China and Korea (Ablaev et al., 1993; Bureau of Geology and Mineral Resources of HebeiProvince, 1989; Huzioka, 1972) (Table 1). Our discovery of six Carpinus species from the Maguan flora constitutes the southernmost fossil occurrence, and also the earliest occurrence that shows a high diversity for the genus. This find demonstrates that Carpinus had already reached southern East Asia and achieved a relatively high species diversity there at least by the early Miocene. In total, nine Carpinus species are known from the early Miocene of East Asia (Table 1), indicating that the species diversity of the genus has increased since then. Middle Miocene Carpinus fossils has been extensively described from northern and southern East Asia (Table 1). In all, 18 species have been reported, showing that the genus had achieved a high di-versity by the middle Miocene (Table 1). Few studies have reported Carpinus species from the late Miocene and Pliocene. This gap in fossil may be due to under-investigation, taphonomic bias, or possible extinction event (Table 1).
A distinctive distributional feature of Carpinus in East Asia is that a small region commonly harbors a high diversity of Carpinus species. For example, in the Wenshan Nature Reserve and Mount Jinggangshan regions, 12 Carpinus species have been documented(Liao, 2014; Yang et al., 2008); and in Mount Emei, five Carpinus species were discovered (Li and Shi, 2007). Thus, understanding when this pattern was formed is of great interest. Because mega-fossils usually represent floristic elements of regions proximal to the site of deposition, the diversity of plant groups in paleofloras can directly reflect the level of species diversity of taxa in a region in the geological past (Huang et al., 2015). Six Carpinus species were discovered from the early Miocene of southeastern China, southern East Asia and, five species have been reported from the middle Miocene of Shanwang flora, China, central East Asia (Sun, 1999), while six species have been documented from the middle Miocene of Yeonil flora, Korea, northern East Asia (Kim and Nam, 2017). This pattern is indicative that the phenomenon which a small region harbors a high diversity of Carpinus species can at least be traced back to the middle Miocene across East Asia.
4.3. Extinction and persistence in southern East AsiaExtinction events are an essential aspect of the evolution of diversity. In this study, we identified three extinct Carpinus species, namely C. symmetrica, C. asymmetrica, and C. sp. 1. The discovery of these species shows that extinction events have taken place in the evolution of Carpinus in East Asia. Such extinction events have been also found in other taxa such as Cedrelospermum Saporta, Cedrus Trew, and Sequoia Endlicher. in southwestern China (Jia et al., 2015, 2019b; Su et al., 2013; Zhang et al., 2015). These extinctions have been ascribed to the southeastern extrusion of the Tibetan Plateau and the intensification of the East Asian monsoon (Jia et al., 2015; Su et al., 2013). The three Carpinus species described in this study might have experienced a similar fate, although the exact reason for the extinction could not yet be determined.
Although C. cf. fangiana, C. 2, and C. 3 closely resemble several extant Carpinus species in morphology, they could not be unequivocally assigned to a specific extant species. These extant species now have a wide a distribution range in southwestern China. The resemblance of these fossils to extant species raises the possibility that the wide climatic tolerance species of these species has allowed their sustained presence in southwestern China since the early Miocene.
To conclude, our findings indicate that the accumulation of Carpinus species diversity in East Asia was a complex process, involving extinction, persistence, and possible subsequent specia-tion because the species diversity of the genus known in fossil re-cords is much lower than its modern diversity in East Asia.
Author contributionsLJ and SZ conceived and designed the research. LX, LJ, GN, YH, SZ, YW, ZZ and YC performed research and analyzed data. LX and LJ wrote the paper.
Declaration of Competing InterestNone.
AcknowledgementsWe thank professor Yaowu Xing for generously providing Car-pinus fossil records in his database (the Cenozoic Angiosperm Database); the Chinese Virtual Herbarium (CVH) for providing the extant occurrence data of Carpinus; Miss Wenqing Li and Mr. Lu Sun for technical assistance with software; professor Torsten Utescher for giving valuable comments; and professor Bob Spicer and the editor Raymond Porter for polishing the manuscript. This work was supported by the National Natural Science Foundation of China (No. 31670216, No. 31900194), the Foundation of the State Key Labora-tory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences (No. 183112).
Ablaev, A.G., Sin, E.U., Vassiliev, I.G., et al., 1993. Miocene of the North Korea and the South Primorye (Beds with Engelhardia). Feb Ran, Vladivostok.
|
Adair K.T., Li S., 1994. Seed plant species pools: the development of species richness in eastern Asia and North America. Chin. Biodivers, 2: 18-29. |
Bureau of Geology and Mineral Resources of Hebei Province, 1989. Regional Geology of Hebei Province, Beijing Municipality and Tianjin Municipality. Geology Press, Beijing.
|
Chaney, R.W., 1963. Tertiary Floras of Japan: Miocene Floras. Geological Survey of Japan, Tokyo.
|
Chen Y.-S., Deng T., Zhou Z., et al, 2018. Is the East Asian flora ancient or not? Nat. Sci. Rev, 5: 920-932. |
Chen Z.-D., Manchester S.R., Sun H.-Y., 1999. Phylogeny and evolution of the Betulaceae as inferred from DNA sequences, morphology, and paleobotany. Am. J. Bot, 86: 1168-1181. DOI:10.2307/2656981 |
Chen Z., 1994a. Phylogeny and phytogeography of the Betulaceae. Acta Phytotaxon. Sin, 32: 1-32. |
Chen Z., 1994b. Phylogeny and phytogeography of the Betulaceae (Cont. ). Acta Phytotaxon. Sin, 32: 101-153. |
Chin S.W., Shaw J., Haberle R., et al, 2014. Diversification of almonds, peaches, plums and cherriesemolecular systematics and biogeographic history of Prunus(Rosaceae). Mol. Phylogenet. Evol, 76: 34-48. DOI:10.1016/j.ympev.2014.02.024 |
Chun H.Y., 1982. Plant fossils from the tertiary pohang sedimentary basin, Korea. Korea Inst. Energ. Res, 14: 7-23. |
Crane P.R., 1981. Betulaceous leaves and fruits from the British upper palaeocene. Bot. J. Linn. Soc, 83: 103-136. DOI:10.1111/j.1095-8339.1981.tb01224.x |
Dai J., Sun B., Xie S., et al, 2013. A new species of Carpinus (Betulaceae) from the pliocene of yunnan province, China. Plant Systemat. Evol, 299: 643-658. DOI:10.1007/s00606-012-0750-1 |
Ellis, B., Daly, D.C., Hickey, L.J., et al., 2009. Manual of Leaf Architecture. Cornell University, New York.
|
Endo, S., 1940. A Pleistocene Flora from Shiobara. Japan. Sci. Rep. Tohoku Imperial Univ., pp. 47-80
|
Endo S., 1950. On the fossil Carpinus from Japan and Korea. Short Papers IGPS, 2: 51-57. |
Forest F., Savolainen V., Chase M.W., et al, 2005. Teasing apart molecular-versus fossil-based error estimates when dating phylogenetic trees: a case study in the birch family (Betulaceae). Syst. Bot, 30: 118-133. DOI:10.1600/0363644053661850 |
Grimm G.W., Renner S.S., 2013. Harvesting Betulaceae sequences from GenBank to generate a new chronogram for the family. Bot. J. Linn. Soc, 172: 465-477. DOI:10.1111/boj.12065 |
Hinsinger, D.D., Basak, J., Gaudeul, M., et al., 2013. The phylogeny and biogeographic history of Ashes (Fraxinus, Oleaceae) highlight the roles of migration and vicariance in the diversification of temperate Trees. PloS One 8, e80431.
|
Huang J., 2017. The Middle Miocene Wenshan Flora, Yunnan, Southwestern China and its Palaeoenvironment Reconstruction. Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun, p, 320. |
Huang Y.-J., Jacques F.M.B., Liu Y.-S.C., et al, 2015. Rubus (rosaceae) diversity in the late pliocene of yunnan, southwestern China. Geobios, 48: 439-448. DOI:10.1016/j.geobios.2015.08.001 |
Huzioka K., 1943. On some fossil involucres of Ostrya and Carpinus from the Miocene deposits of hokkaido and tyosen. J. Geol. Soc. Jpn, 50: 285-291. DOI:10.5575/geosoc.50.285 |
Huzioka K., 1972. The tertiary floras of Korea. J. Min. Coll. Akita Univ. Series, A 5: 1-83. |
Jetz W., McPherson J.M., Guralnick R.P., 2012. Integrating biodiversity distribution knowledge: toward a global map of life. Trends Ecol. Evol, 27: 151-159. DOI:10.1016/j.tree.2011.09.007 |
Jia L.B., 2018. The Early Miocene Maguan Flora from Yunnan, Paleovegetation and Floristic Evolution. Kunming Institute of Botany, Kunming, p, 253. |
Jia L.B., Huang Y.J., Sun H., 2017. First fossil of Pterolobium (leguminosae)from the middle Miocene yunnan, south China. Rev. Palaeobot. Palynol, 242: 21-32. DOI:10.1016/j.revpalbo.2017.03.002 |
Jia, L.B., Manchester, S.R., Huang, J., et al., 2019a. First fossil record of an East Asian endemic genus Sladenia (Sladeniaceae) from its modern range: implications for floristic evolution and conservation biology. J. Systemat. Evol. https://doi.org/10.1111/jse.12518.
|
Jia L.B., Manchester S.R., Su T., et al, 2015. First occurrence of Cedrelospermum(ulmaceae) in Asia and its biogeographic implications. J. Plant Res, 128: 747-761. DOI:10.1007/s10265-015-0739-2 |
Jia L.B., Su T., Huang Y.J., et al, 2019b. First fossil record of Cedrelospermum(ulmaceae) from the qinghai-Tibetan plateau: implications for morphological evolution and biogeography. J. Systemat. Evol, 57: 94-104. DOI:10.1111/jse.12435 |
Kiera G., Kreft H., Leeb T.M., et al, 2009. A global assessment of endemism and species richness across island and mainland regions. Proc. Natl. Acad. Sci. U.S.A, 106: 9322-9327. DOI:10.1073/pnas.0810306106 |
Kim J.-H., Nam K.-S., 2017. Fossil involucres of Carpinus and their significances from the duho formation of Yeonil group, Korea. J. Geol. Soc. Korea, 53: 759-772. DOI:10.14770/jgsk.2017.53.6.759 |
Latham R.E., Ricklefs R.E., 1993. Continental comparisons of temperate-zone tree species diversity. Spec. Diver. Ecol. Comm.: Hist. Geogr. Perspect: 294-314. |
Lebreton-Anberrée Li, S. H., Li S.-F., et al, 2016. Lake geochemistry reveals marked environmental change in southwest China during the mid Miocene climatic optimum. Sci. Bull, 61: 897-910. DOI:10.1007/s11434-016-1095-x |
Lebreton-Anberrée J., Manchester S.R., Huang J., et al, 2015. First fossil fruits and leaves of Burretiodendron s. l. (Malvaceae s.l.) in Southeast Asia: implications for taxonomy, biogeography, and paleoclimate. Int. J. Plant Sci, 176: 682-696. |
Li, X.C., 2010. The Late Cenozoic Floras from Eastern Zhejiang Province and Their Paleoclimatic Reconstruction. Lanzhou University, Lanzou.
|
Li, P.C., Cheng, S.H., 1979. Betulaceae. In: Kuang, K.Z., Li, P.C. (Eds.), Flora Republicae Popularis Sinicae. Science Press, Beijing, pp. 44-137.
|
Li, P.C., Skvortsov, A.K., 1999. Betulaceae. In: Wu, Z.Y., Raven, P., Hong, D. (Eds.), Flora of China. Science Press/Missouri Botanical Garden Press, Beijing/St. Louis, pp. 286-313.
|
Li Y., Yang Y., Yu L., et al, 2018. Plastomes of nine hornbeams and phylogenetic implications. Ecol. Evol, 8: 8770-8778. DOI:10.1002/ece3.4414 |
Li, Z.Y., Shi, L., 2007. Plants of Mount Emei. Beijing science and technology press, Beijing.
|
Liao, W., 2014. Integrated Study on Biodiversity of Mount Jinggangshan Regions in China. Science Press, Beijing.
|
Lim, K.H., Jang, C.B., Kwon, J.R., et al., 1994. The Fossils of Chosen 3. Science Engineering Press, Pyongyang.
|
Linnemann, U., Su, T., Kunzmann, L., et al., 2017. New U-Pb dates show a Paleogene origin for the modern Asian biodiversity hot spots. Geology 41, 3-6.
|
Lu L.-M., Mao L.-F., Yang T., et al, 2018. Evolutionary history of the angiosperm flora of China. Nature, 554: 234-238. DOI:10.1038/nature25485 |
Lucas S.G., Emry R.J., 1996. Biochronological significance of amynodontidae(mammalia, perissodactyla) from the Paleogene of Kazakhstan. J. Paleontol, 70: 691-696. DOI:10.1017/S0022336000023647 |
Manchester S.R., Chen Z.-D., 1998. A new genus of Coryloideae (Betulaceae) from the paleocene of north America. Int. J. Plant Sci, 159: 522-532. DOI:10.1086/297569 |
Myers N., Mittermeier R.A., Mittermeier C.G., et al, 2000. Biodiversity hotspots for conservation priorities. Nature, 403: 853-858. DOI:10.1038/35002501 |
Naciri Y., Christe C., Betrisey S., et al, 2019. Species delimitation in the East Asian species of the relict tree genus Zelkova (Ulmaceae): a complex history of diversification and admixture among species. Mol. Phylogenet. Evol, 134: 172-185. DOI:10.1016/j.ympev.2019.02.010 |
Ozaki K., 1991. Late Miocene and pliocene floras in central honshu, Japan. Bull. Kanagawa Prefect. Mus: 1-188. |
Pigg K.B., Manchester S.R., Wehr W.C., 2003. Corylus, Carpinus, and palaeocarpinus(Betulaceae) from the middle eocene klondike mountain and allenby formations of northwestern north America. Int. J. Plant Sci, 164: 807-822. DOI:10.1086/376816 |
Pimm, S.L., Jenkins, C.N., Abell, R., et al., 2014. The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752.
|
Qi T., 1992. A new species of Gigantamynodon (amynodontidae) from yunnan, China. Vertebr. Palasiat, 30: 229-232. |
Qian H., 2002. A comparison of the taxonomic richness of temperate plants in East Asia and North America. Am. J. Bot, 89: 1818-1825. DOI:10.3732/ajb.89.11.1818 |
Renner S.S., Grimm G.W., Schneeweiss G.M., et al, 2008. Rooting and dating maples (Acer) with an uncorrelated-rates molecular clock: implications for north American/Asian disjunctions. Syst. Biol, 57: 795-808. DOI:10.1080/10635150802422282 |
Stults D.Z., Axsmith B.J., Haywick D., 2002. Evidence of Carpinus (Betulaceae) in the late tertiary (bliocene) of Alabama. Am. J. Bot, 89: 1547-1549. DOI:10.3732/ajb.89.9.1547 |
Su T., Liu Y.S., Jacques F., et al, 2013. The intensification of the East Asian winter monsoon contributed to the disappearance of Cedrus (Pinaceae) in southwestern China. Quat. Res, 80: 316-325. DOI:10.1016/j.yqres.2013.07.001 |
Sun B.-N., Cong P.-Y., Yan D.-F., et al, 2003. Cuticular structure of two angiosperm fossils in Neogene from Tengchong, Yunnan Province and its paleoenvironmental significance. Acta Palaeontol. Sin, 42: 216-222. |
Sun, B., 1999. Fossil Plants from Shanwang Flora. Shandong Science and Technology Press, Jinan.
|
Tang, C.Q., Matsui, T., Ohashi, H., et al., 2018. Identifying long-term stable refugia for relict plant species in East Asia. Nat. Commun. 9, 4488.
|
Thome R.F., 1999. Eastern Asia as a living museum for archaic angiosperms and other seed plants. 44 :413-422.. Taiwania, 44: 413-422. |
Tiffney B.H., 1985. Perspectives on the origin of the floristic similarity between eastern Asia and eastern North America. :-.. J. Arnold Arbor, 66: 73-94. DOI:10.5962/bhl.part.13179 |
Uemura K., Doi E., Takahashi F., 1999. Plant megafossil assemblage from the kiwado formation (Oligocene) from ouchiyama-kami in yamaguchi pref. , western honshu, Japan. Bull. Mine City Mus. Yamaguchi Prefect. JPN, 15: 1-59. |
Wang Z., Fang J., Tang Z., et al, 2011. Patterns, determinants and models of woody plant diversity in China. Proc. Biol. Sci, 278: 2122-2132. DOI:10.1098/rspb.2010.1897 |
Wu, Z.Y., Zhou, Z.K., Sun, H., et al., 2006. The Areal-Types of Seed Plants and Their Origin and Differentiation. Kunming.
|
Xing Y., Gandolfo M.A., Onstein R.E., et al, 2016. Testing the biases in the rich Cenozoic angiosperm macrofossil record. Int. J. Plant Sci, 177: 371-388. DOI:10.1086/685388 |
Yang, X.-Y., Wang, Z.-F., Luo, W.-C., et al., 2019a. Plastomes of Betulaceae and phylogenetic implications. J. Systemat. Evol. https: //doi.org/10.1111/jse.12479.
|
Yang, Y., Tian, K., He, S., 2008. Study on the Scientific Survey of Wenshan National Reserve in China. Science Press, Beijing.
|
Yang, Z., Wang, G., Ma, Q., et al., 2019b. The complete chloroplast genomes of three Betulaceae species: implications for molecular phylogeny and historical biogeography. PeerJ 7, e6320.
|
Yoo K.O., Wen J., 2002. Phylogeny and biogeography of Carpinus and subfamily Coryloideae (Betulaceae). Int. J. Plant Sci, 163: 641-650. DOI:10.1086/340446 |
Zhang J.W., D'Rozario A., Adams J.M., et al, 2015. Sequoia maguanensis, a new Miocene relative of the coast redwood, Sequoia sempervirens, from China: implications for paleogeography and paleoclimate. Am. J. Bot, 102: 103-118. DOI:10.3732/ajb.1400347 |
Zhang, C.-H., 1976. The Report to the Regional Geological Survey (1/200, 000) of Wenshan/Maguan Scope (F-48-3, F-48-9). Geological Bureau of Yunnan Province: Yuxi.
|
Zhang Q.Y., Huang J., Jia L.B., et al, 2018. Miocene Ulmus fossil fruits from Southwest China and their evolutionary and biogeographic implications. Rev. Palaeobot. Palynol, 259: 198-206. DOI:10.1016/j.revpalbo.2018.10.007 |



