b. CAS Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla 666303, China
Large-scale distribution patterns of species richness and their underlying causes are still subject of debate in ecology and bioge-ography (Gaston, 2000). Numerous factors have been proposed to explain large-scale patterns of species richness, including climate (O'Brien, 1993, 1998; O'Brien et al., 2000; Hawkins et al., 2003; Dufour et al., 2006; Whittaker et al., 2007; Wang et al., 2011), historical biogeography (Wiens and Donoghue, 2004), environ-mental heterogeneity (Ricklefs and Robert, 1977; Stein et al., 2014; Stein and Kreft, 2015), and human activity (Balmford, 2001; Gaston, 2005; Tang et al., 2006; White and Kerr, 2007).
Although the relative importance of various factors in shaping species richness patterns remains in dispute, researchers agree that climatic factors are among the most important (O'Brien, 1993, 2006; O'Brien et al., 1998; Francis and Currie, 2003; Field et al., 2005; Wang et al., 2011). For instance, previous studies have sug-gested that climate is closely correlated with species richness on regional, continental, or even global scales (Currie et al., 2004; Buckley and Jetz, 2007; Kreft and Jetz, 2007; Wang et al., 2011; Zhang et al., 2017). Among climatic parameters, environmental energy (Rahbek and Graves, 2001; Wang et al., 2011), water avail-ability (O'Brien, 1993; Kreft and Jetz, 2007; Whittaker et al., 2007), and rainfall seasonality (O'Brien, 1993; Aguilar-Santelises et al., 2013) may influence the geographical patterns of species richness.
The richest plant diversity and the most diverse array of vege-tation types in the world are located in China (Wu, 1980b; Wu, 2004), where the climate ranges from tropical to temperate. Thus, China is an ideal region to characterize geographical distributions of species richness and test whether these species richness patterns are correlated with specific climate types. Indeed, plant richness patterns in China have long been widely studied at various taxo-nomic and regional scales; however, these studies have yet to consistently identify which climatic parameters are most important in determining species richness patterns (Chen et al., 2011; Li et al., 2015; Lü et al., 2018; Wang et al., 2009, 2011; Zhang et al., 2017).
Recent studies have suggested that the monsoon climate plays an important role in plant biodiversity in China (Spicer, 2017; Spicer et al., 2017; Mosbrugger et al., 2018). The Asian monsoon climate, which was established in China as early as the Paleogene (Spicer et al., 2017), is characterized by wet summers and dry winters (Zhang, 1991). The development of the Asian monsoon might have been a major factor driving the evolution of the East Asian floras (Chen et al., 2018). Increased precipitation seasonality could lead to the migration or extinction of some plant taxa (Su et al., 2013; Wang et al., 2019), consequently shaping the vegetation of China (Li et al., 2015b). For example, the intensification, by the late Plio-cene, of the Asian monsoon and its pronounced dry winters may have hampered the survival of Cedrus seedlings, leading to the disappearance of Cedrus in western Yunnan (Su et al., 2013). Simi-larly, the genus Metasequoia did not survive the intensification of the Asian monsoon, which, during the Neogene, brought aridity in winter and spring, and may have been the main factor that contributed to its disappearance in southwestern China (Wang et al., 2019). Accordingly, the Asian monsoon climate, together with a complex topography, has made southern China a biodiver-sity hotspot (Spicer, 2017; Spicer et al., 2017; Mosbrugger et al., 2018). However, no previous research has examined how this monsoonal climate affects large-scale species richness patterns in China.
In this study, we first analyzed the distribution patterns of woody dicotyledons in humid regions of China. Second, we inves-tigated which geographical and climatic factors are correlated with species richness patterns in these same regions.
2. Materials and methods 2.1. Study regionsThis study focuses on humid regions of China, where the mean annual precipitation (MAP) is higher than 400 mm, which is a threshold for humid and arid regions in China (Ren and Bao, 1992); the eastern area of this isoline is mainly exposed to the Asian monsoon (Wang and Ho, 2002). These humid regions contain 2082 counties, representing 88% of the total 2380 counties in China.
2.2. Data set on the species distribution of Chinese woody dicotyledonsThe distribution of woody dicotyledons was obtained from the database of native woody dicotyledons in China, which includes 3166 native woody species belonging to 536 genera and 111 fam-ilies (see the electronic Supplementary Data 1 in Chen et al., 2014). This data set was derived from Seed Plant of China (Wu and Ding, 1999), specimen records at the Herbarium of the Kunming Insti-tute of Botany (KUN), and Flora of China (Wu, 1979; Wu, 1980a; Wu, 1982; Wu, 1984; Wu, 1988; Wu, 1995; Wu, 1996; Wu, 1998). The native Chinese woody dicotyledons in this data set include trees and large shrubs (defined as woody plants > 3 m in height), which contain 2272 trees and 894 large shrubs (see Supplementary Data 1); trees in the current study represent 77.3% of the total 2939 trees in China (Fang et al., 2011; Li et al., 2016). This sampling strategy has been useful in our recent studies on the relationship between the climate and spatial distribution patterns of leaf char-acters (Chen et al., 2014, 2019). Maps of the present-day distribu-tion of 3166 species were compiled and digitized using a Geographical Information System (GIS) (ArcView GIS 3.2, ESRI, New York, USA) at county level. The calibration data set used for analysis consisted of 732 sites at the county level, where each county contained at least 20 species (see electronic Supplementary Data 2 in Chen et al., 2014).
Each county covers an area from 231.6 to 41, 931.5 km2, with an average area of 2714.5 km2. To eliminate the effects of variation of area size on our estimations of species richness, we used maps based on equal-area counties (Rosenzweig, 1995; Wang et al., 2011). Thus, we changed each county area to an equal grid of 50×50 km; the refined species richness of each grid was as follows:
Species richness = sum of species in each county / county area×2500
The species richness of each calibration grid is shown in Supplementary Data 2.
2.3. Climate variablesTo test the effects of climatic variables on species richness, the climatic parameters used in this study were grouped into three categories: environmental energy, water availability, and season-ality rainfall. The energy-related variables included mean annual temperature (MAT), warmest month mean temperature (WMMT), coldest month mean temperature (CMMT), and growing season length (GSL, months when the mean monthly minimum temper-ature exceeds 10℃). For water-related variables, we chose MAP and growing season precipitation (GSP). In addition, we used pre-cipitation during the three wettest consecutive months (P3WET), precipitation during the three driest consecutive months (P3DRY), wettest month precipitation (Pmax), and driest month precipita-tion (Pmin) to represent the seasonality rainfall variables.
Asian monsoonal climates have significant characteristics, especially in terms of precipitation pattern; namely, these climates have abundant rainfall during the summer and drought during the winter (Lau and Chan, 1983). Previous studies have proposed that the difference between P3WET and P3DRY can serve as a proxy for the plant and leaf physiognomy response to the monsoon climate (Wang et al., 2006; Jacques et al., 2011; Xing et al., 2012; Khan et al., 2014; Chen et al., 2019). In this study, we followed this idea, using P3WET and P3DRY as signals of the Asian monsoon.
Climate data with 30-year records on average (1951-1980) were obtained from weather stations in each individual county from the Yunnan Provincial Meteorological Bureau (YPMB) and the China Meteorological Data Sharing Service System (available online: http://cdc.cma.gov.cn/). The climatic parameters of each county are shown in the Supplementary Data 2.
2.4. Data analysisCounty-level maps of the distribution of woody dicotyledon richness were compiled with transformed 50×50-km grids and digitized for application in a Geographical Information System (GIS) (ArcView GIS 3.2, ESRI, New York, USA).
Species richness was log-transformed for analyses because of the highly right-skewed distribution and the large standard devi-ation (SD) in relation to the mean (Kerkhoff and Enquist, 2009). Strong standard deviations for climate variables (MAP, GSP, P3WET, P3DRY, Pmax, and Pmin) were also log-transformed, whereas longitude, latitude, MAT, WMMT, CMMT, and GSL were not trans-formed because of the low standard variation. The relationships between log-transformed woody dicotyledons richness and geog-raphy, temperature, and log-transformed precipitation were quantified using single linear regressions (SLR). All statistical analyses were conducted using SPSS version 19 (SPSS Science, Chicago, IL, USA).
3. Results 3.1. Geographical patterns of species richness, precipitation during the three wettest consecutive months (P3WET), and precipitation during the three driest consecutive months (P3DRY)Species richness varied from 1 to 808 per grid, with an average of 142 species per grid (Table 1). In general, species richness was high in southern China, but significantly lower in northern China. Moreover, species richness was relatively high in mountainous areas and comparatively low in plains and on plateaus (Fig. 1).
| Range | Minimum | Maximum | Mean | SD | |
| Species richness | 807 | 1 | 808 | 141.6 | 149.90 |
| Log (Species richness) | 2.8 | 0.1 | 2.9 | 0.9 | 0.45 |
| Geographic variables | |||||
| Longitude (°E) | 42.9 | 91.1 | 134.0 | 110.6 | 6.74 |
| Latitude (°N) | 33.5 | 18.2 | 51.7 | 28.1 | 5.03 |
| Environmental energy | |||||
| Mean Annual Temperature (MAT, in℃) | 27.8 | 2.4 | 25.4 | 16.1 | 4.48 |
| Warmest Month Mean Temperature (WMMT, in℃) | 22.3 | 8.7 | 31.0 | 25.7 | 3.56 |
| Coldest Month Mean Temperature (CMMT, in℃) | 48.8 | 28.0 | 20.8 | 5.2 | 6.94 |
| Growing Season Length (GSL, in month) | 12.0 | 0.0 | 12.0 | 9.0 | 2.12 |
| Water availability | |||||
| Mean Annual Precipitation (MAP, in mm) | 2057.5 | 418.1 | 2475.6 | 1244.9 | 402.70 |
| Log (MAP) | 0.8 | 2.6 | 3.4 | 3.1 | 0.16 |
| Growing Season Precipitation (GSP, in mm) | 2475.6 | 0.0 | 2475.6 | 1145.1 | 401.98 |
| Log (GSP) | 1.1 | 2.4 | 3.4 | 3.0 | 0.18 |
| Seasonality rainfall | |||||
| Precipitation 3 Wettest Cons. Months (P3WET, in mm) | 1264.5 | 214.4 | 1478.9 | 618.2 | 189.27 |
| Log (P3WET) | 0.8 | 2.3 | 3.2 | 2.8 | 0.14 |
| Precipitation 3 Driest Cons. Months (P3DRY, in mm) | 202.9 | 1.2 | 204.1 | 87.4 | 57.73 |
| Log (P3DRY) | 2.2 | 0.1 | 2.3 | 0.8 | 0.42 |
| Wettest Month Precipitation (Pmax, in mm) | 430.0 | 86.1 | 516.1 | 233.0 | 69.95 |
| Log (Pmax) | 0.8 | 1.9 | 2.7 | 2.4 | 0.13 |
| Driest Month Precipitation (Pmin, in mm) | 58.3 | 0.1 | 58.4 | 23.7 | 16.06 |
| Log (Pmin) | 2.8 | 1.0 | 1.8 | 1.2 | 0.47 |
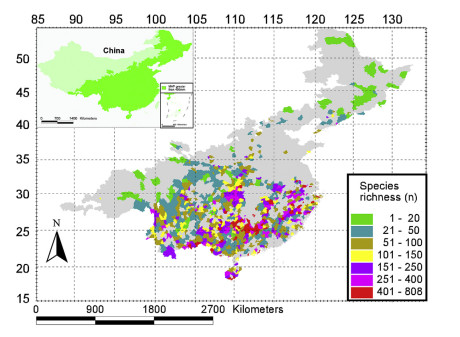
|
| Fig. 1 Distribution pattern of woody dicotyledon richness in humid regions of China with a mean annual precipitation (MAP) above 400 mm. |
The patterns of P3WET and P3DRY generally showed a decreasing trend from southeastern to northwestern China (Fig. 2a and b). For P3WET, some anomalous areas of high precipitation were observed, e.g., the western part of Yunnan Province, the southwest of Sichuan Province, and the northeast of Liaoning Province. In addition, based on the P3DRY, areas with extremely high precipitation were the Gaolingong Mountains west of Yunnan, while north of Hainan Island was characterized by an extremely low precipitation (Fig. 2, b).
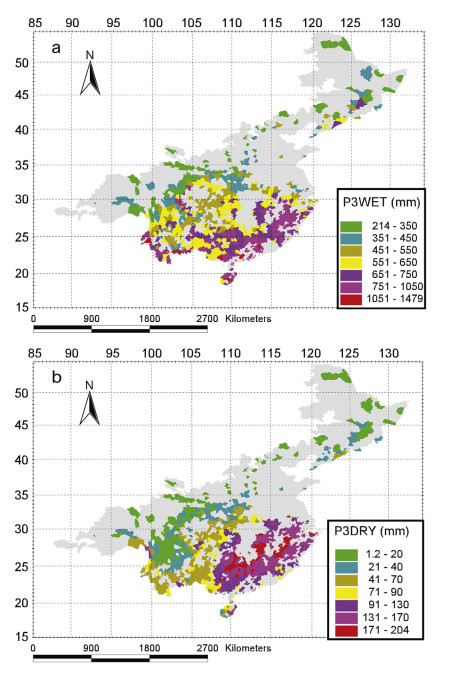
|
| Fig. 2 Distribution patterns of the change of the precipitation during the three wettest consecutive months (P3WET) and precipitation during the three driest consecutive months (P3DRY). |
Species richness of woody dicotyledons in humid regions of China was significantly negatively correlated with latitude variation (r2 = 0.18, P < 0.0001), but not with longitude variation (P > 0.05) (Fig. 3).
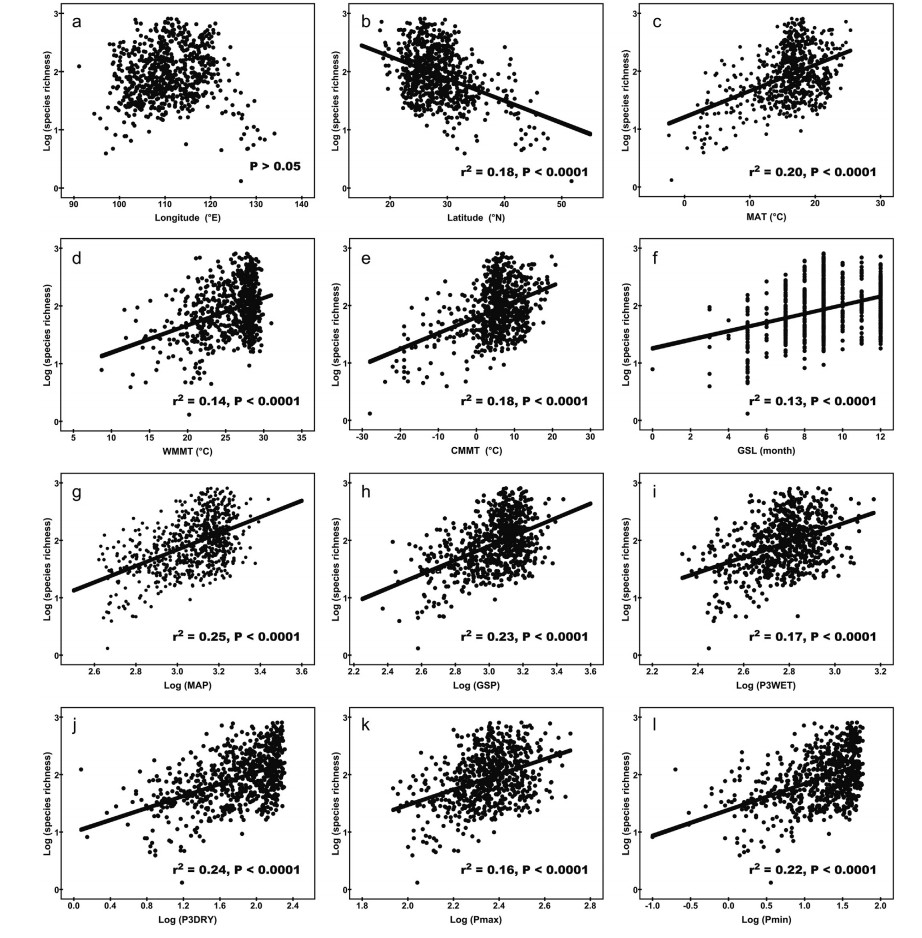
|
| Fig. 3 Relationships among geographic and climatic variables and log-transformed woody dicotyledon richness in humid regions of China. Abbreviations for climate parameters: MAT, mean annual temperature (℃); WMMT, warmest month mean temperature (℃); CMMT, coldest month mean temperature (℃); GSL, growing season length (month); MAP, mean annual precipitation (mm); GSP, growing season precipitation (mm); P3WET, precipitation during the three wettest consecutive months (mm); P3DRY, precipitation during the three driest consecutive months (mm); Pmax, wettest month precipitation (mm); Pmin, driest month precipitation (mm). |
Species richness was significantly correlated with all temperature-related climatic parameters in humid regions of China, including MAT, CMMT, WMMT, and GSL. The temperature-related parameter with the highest correlation to species richness was MAT (r2 = 0.20, P < 0.0001), while the temperature-related parameter with the lowest correlation to species richness was GSL (r2 = 0.13, P < 0.0001) (Fig. 3).
There was a strong correlation between species richness and log-transformed MAP (r2 = 0.25, P < 0.0001) and GSP (r2 = 0.23, P < 0.0001). In particular, among all climatic variables, MAP showed the highest correlation with species richness (Fig. 3). Additionally, species richness was significantly correlated with seasonality rainfall variables, such as P3WET, P3DRY, Pmax, and Pmin (Fig. 3). In general, species richness was more affected by P3DRY (r2 = 0.24, P < 0.0001) than by P3WET (r2 = 0.17, P < 0.0001) (Fig. 3).
4. Discussion 4.1. The distribution pattern of species richness and its relationship with climateOur study shows that species richness is distributed along a latitudinal gradient in humid regions of China (Figs. 1 and 3b). This finding is consistent with previous results (Ying, 2001; Chen et al., 2011; Wang et al., 2011) and with the well-noted observation that species richness decreases with increasing latitude at a continental or even global scale (Green, 1994; Kaufman, 1995; Webb et al., 1997; Rohde, 1998; Qian, 1999; Gaston, 2000; Qian et al., 2003; Mutke and Barthlott, 2005; Barthlott et al., 2014).
All climatic variables showed significant and positive correla-tions with species richness in humid regions of China (Fig. 3). The climatic variables with the highest correlation with species rich-ness were MAP (r2 = 0.25, P < 0.0001) and P3DRY (r2 = 0.24, P < 0.0001). Thus, our study indicates that water availability (pre-cipitation-related climatic parameters), and not environmental energy (temperature-related climatic parameters), is the major climate factor in determining large-scale distribution patterns of species richness in humid regions of China, which is evidenced by previous studies at large scales (Meserve and Glanz, 1978; Richerson and Lum, 1980; Gentry, 1982, 1988; Wright, 1983; Glaser, 1992; O'Brien, 1993; Mourelle and Ezcurra, 1996; Schulze et al., 1996; Ganzhorn et al., 1997; Kay et al., 1997; Kessler, 2001; Li et al., 2015a, b; Lü et al., 2018).
However, our results disagree with several previous studies in China. For instance, previous studies suggested that the warmth index (an index of the growing season heat sum) was most significantly correlated with tree richness in northeast China (Wang et al., 2009), and that winter low temperature (mean tem-perature of the coldest quarter, MTCM) was the major factor that contributed to patterns of woody plant richness in China (Wang et al., 2011). Although energy, water, and climatic variability are the most important climatic factors that shape patterns of species richness on a large scale, the relative importance of particular cli-matic factors on these patterns may vary geographically (O'Brien, 1993; Hawkins et al., 2003; Kreft and Jetz, 2007; Whittaker et al., 2007; Wang et al., 2009). For instance, an analysis of 21 studies that examined the relationship between climatic factors and large-scale plant richness gradients showed that in the tropics, subtro-pics, and warm temperate zones, water variables usually represent the strongest predictors of plant richness, whereas in high latitude zones, water-energy (temperature) variables dominate (Hawkins et al., 2003). The proposed explanation for these findings is that in warm areas where energy is abundant, water availability might be a key constraint on plant richness. In cold regions, however, where energy inputs are lower and thus more likely to be limiting, energy interacts with water to contribute to plant richness. In our study, the humid regions of China are located in warm areas with abundant energy. Thus, our results show that water variables are the most important climatic parameters in determining large-scale patterns of plant richness in humid regions of China.
4.2. Species richness under the Asian winter monsoon climateIn this study, species richness correlated more significantly with P3DRY (r2 = 0.24, P < 0.0001) than with P3WET (r2 = 0.17, P < 0.0001). In China, the P3DRY occurs in winter and is related to the Asian winter monsoon. Thus, this finding demonstrates that the species richness in humid regions of China is largely affected by the Asian monsoon, especially the Asian winter monsoon, which may be explained by several factors.
First, China experiences a strong modern Asian monsoon climate, characterized by a wet summer and a dry winter (Lau and Chan, 1983; Zhang, 1991). The South Asian monsoon climate was established by the Paleogene, and the seasonality of rainfall increased progressively, achieving modern monsoon-like wet summers and dry winters, by the early Oligocene (Spicer et al., 2017). A recent study suggested that the formation and develop-ment of the Asian monsoon might be the main factor driving the evolution of the East Asian floras (Chen et al., 2018). Plant species must have adapted to the seasonality of rainfall in the geological past. Through natural selection, only plants that are able to tolerate seasonal drought can grow under monsoonal conditions, whereas those unable to adapt to this climate type become extinct (Su et al., 2013; Wang et al., 2019). Therefore, the seasonality of rainfall formed by monsoonal climate could influence species richness (O'Brien, 1998; Aguilar-Santelises et al., 2013; Spicer, 2017).
Second, under the Asian monsoon climate, the leaves of woody plants, which are the main functional trait, must adapt well to the extremely seasonal climatic conditions, and thus, leaf physiognomy is distinctive under a monsoon climate (Jacques et al., 2011; Spicer et al., 2016; Chen et al., 2019). It seems that plant leaves affected by the Asian monsoon climate, to a certain degree, may reflect the response of species distribution in relation to this climate.
Third, according to plant physiological studies, drought impedes normal growth, disturbs water relations, and reduces water use efficiency in plants (Farooq et al., 2012), even preventing seedling germination (Xia et al., 2016). Additionally, the low temperature during the winter can limit leaf net photosynthetic rate, damage enzyme function, disrupt membranes and cellular processes, and even cause irreparable tissue damage (Jones, 2014). Thus, seasonal drought or low temperatures may have affected plant physiology and shaped the distribution patterns of plants and species richness on a large scale (Woodward, 1987, 1990; Terradas and Save, 1992; O'Brien, 1993; Xu et al., 2016).
Finally, the distribution of the P3DRY and species richness largely overlap in areas where species number is higher than 150 (Fig. 4); thus, a relatively high species richness usually occurs in areas with high P3DRY in humid regions of China. Therefore, spe-cies richness in humid regions of China is influenced by the Asian monsoon climate with seasonal drought, accompanied by low winter temperatures.
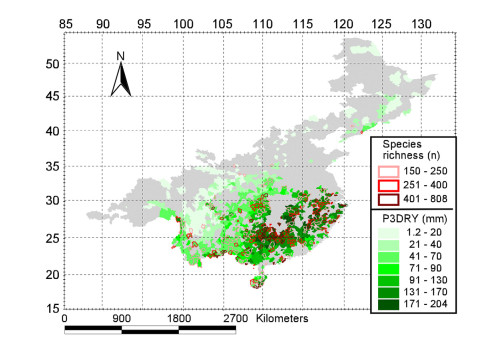
|
| Fig. 4 Distribution of precipitation during the three driest consecutive months (P3DRY) and woody dicotyledon richness where species number is above 150. |
In the present study, we investigated the distribution pattern of woody dicotyledons, based on a large-scale dataset, in humid re-gions of China. Our results suggest that the species richness shows a latitudinal trend, decreasing with increasing latitude. All climatic variables, especially MAP, significantly and positively correlate with species richness. Moreover, the seasonality of rainfall, controlled by the Asian monsoon climate, contributes to species richness, because species richness closely correlates with the winter monsoon, which is mainly characterized by P3DRY, in contrast to the summer monsoon, which is mainly characterized by P3WET. These results suggest that woody dicotyledon richness in humid regions of China is mainly affected by the Asian winter monsoon climate.
Author contributionsConceived and designed the research: Wen-Yun Chen; Wrote the paper: Wen-Yun Chen, Tao Su.
Declaration of Competing InterestNone.
AcknowledgementsThis work was supported by the National Natural Science Foundation of China (No. U1502231 and 41661134049), the Stra-tegic Priority Research Program of Chinese Academy of Sciences (No. XDA19050301).
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2020.03.003.
Aguilar-Santelises R., de los Remedios María Del Castillo, R. F., 2013. Factors affecting woody plant species diversity of fragmented seasonally dry oak forests in the Mixteca Alta, Oaxaca, Mexico. Rev. Mex. Biodivers, 84(2): 575-590. DOI:10.7550/rmb.30458 |
Balmford A., 2001. Conservation conflicts across Africa. Science, 291(5513): 2616-2619. DOI:10.1126/science.291.5513.2616 |
Barthlott, W., Erdelen, W.R., Rafiqpoor, M.D., 2014. Biodiversity and technical innovations: bionics. In: Lanzerath, D., Friele, M.B. (Eds.), Concepts and Values in Biodiversity. Routledge Studies in Biodiversity Politics and Management. Routledge, London, pp. 300-315.
|
Buckley L.B., Jetz W., 2007. Environmental and historical constraints on global patterns of amphibian richness. Proc. Biol. Sci, 274: 1167-1173. DOI:10.1098/rspb.2006.0436 |
Chen S.B., Jiang G.M., Ouyang Z.Y., et al, 2011. Relative importance of water, energy, and heterogeneity in determining regional pteridophyte and seed plant richness in China. J. Systemat. Evol, 49(2): 95-107. DOI:10.1111/j.1759-6831.2011.00120.x |
Chen W.Y., Su T., Adams J.M., et al, 2014. Large-scale dataset from China gives new insights into leaf margin-temperature relationships. Palaeogeogr. Palaeoclimatol. Palaeoecol, 402: 73-80. DOI:10.1016/j.palaeo.2014.03.016 |
Chen W.Y., Su T., Jia L.B., et al, 2019. The relationship between leaf physiognomy and climate based on a large modern dataset: implications for palaeoclimate reconstructions in China. Palaeogeogr. Palaeoclimatol. Palaeoecol, 527: 1-13. DOI:10.1016/j.palaeo.2019.04.022 |
Chen Y.S., Deng T., Zhou Z., et al, 2018. Is the East Asian flora ancient or not?. National Science Review, 5(6): 142-154. |
Currie D.J., Mittelbach G.G., Cornell H.V., et al, 2004. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett, 7(12): 1121-1134. DOI:10.1111/j.1461-0248.2004.00671.x |
Dufour A., Gadallah F., Wagner H.H., et al, 2006. Plant species richness and environmental heterogeneity in a mountain landscape: effects of variability and spatial configuration. Ecography, 29(4): 573-584. |
Fang, J.Y., Wang, Z.H., Tang, Z.Y., 2011. Atlas of Woody Plants in China: Distribution and Climate. Springer, Berlin.
|
Farooq, M., Hussain, M., Wahid, A., et al., 2012. Drought Stress in Plants: an Overview. Plant Responses to Drought Stress. Springer Berlin Heidelberg.
|
Field R., O'Brien E.M., Whittaker R.J., 2005. Global models for predicting woody plant richness from climate: development and evaluation. Ecology, 86: 2263-2277. DOI:10.1890/04-1910 |
Francis A.P., Currie D.J., 2003. A globally consistent richness-climate relationship for angiosperms. Am. Nat, 161(4): 523-536. DOI:10.1086/368223 |
Ganzhorn J.U., Malcomber S., Andrianantoanina, et al, 1997. Habitat characteristics and lemur species richness in Madagascar. :-.. Biotropica, 29: 331-343. DOI:10.1111/j.1744-7429.1997.tb00434.x |
Gaston K.J., 2000. Global patterns in biodiversity. Nature, 405: 220-227. DOI:10.1038/35012228 |
Gaston K.J., 2005. Biodiversity and extinction: species and people. Prog. Phys. Geogr, 29(2): 239-247. |
Gentry A.H., 1982. Patterns of neotropical plant-species diversity. Evol. Biol, 15: 1-85. |
Gentry A.H., 1988. Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann. Mo. Bot. Gard, 75: 1-34. DOI:10.2307/2399464 |
Glaser P.H., 1992. Raised bogs in eastern North America: regional controls for species richness and floristic assemblages. J. Ecol, 80: 535-554. DOI:10.2307/2260697 |
Green J., 1994. The temperateetropical gradient of planktonic protozoa and rotifera. Hydrobiologia, 272: 13-26. DOI:10.1007/BF00006509 |
Hawkins B.A., Field R., Cornell H.V., et al, 2003. Energy, water, and broad-scale geographic patterns of species richness. :-.. Ecology, 84: 3105-3117. DOI:10.1890/03-8006 |
Jacques F.M.B., Su T., Spicer R.A., et al, 2011. Leaf physiognomy and climate: are monsoon systems different? Global Planet.. Change, 76: 56-62. |
Jones, H.G., 2014. Plants and Microclimate: a Quantitative Approach to Environmental Plant Physiology. Cambridge University press, Cambridge.
|
Kaufman D.M., 1995. Diversity of New World mammals: universality of the latitudinal gradients of species and bauplans. J. Mammal, 76: 322-334. DOI:10.2307/1382344 |
Kay, R.F., Madden, R.H., Van Schaik, C., et al., 1997. Primate species richness is determined by plant productivity: implications for conservation. Proc. Natl. Acad. Sci. U. S. A 94, 13023-13027.
|
Kerkhoff A.J., Enquist B.J., 2009. Multiplicative by nature: why logarithmic transformation is necessary in allometry. J. Theor. Biol, 257: 519-521. DOI:10.1016/j.jtbi.2008.12.026 |
Kessler M., 2001. Pteridophyte species richness in Andean forests in Bolivia. Biodivers. Conserv, 10: 1473-1495. DOI:10.1023/A:1011811224595 |
Khan, M.A., Spicer, R.A., Bera, S., et al., 2014. Miocene to pleistocene floras and climate of the eastern himalayan siwaliks, and new palaeoelevation estimates for the namling-oiyug basin, tibet. Global Planet. Change 113, 1-10.
|
Kreft H., Jetz W., 2007. Global patterns and determinants of vascular plant diversity. Proc. Natl. Acad. Sci. Unit. States Am, 104: 5925-5930. DOI:10.1073/pnas.0608361104 |
Lau K.M., Chan P.H., 1983. Short-term climate variability and Atmospheric teleconnections from satellite-observed outgoing longwave radiation. Part I:simultaneous relationships. J. Atmos. Sci, 40(12): 2751-2767. |
Li, L.P., Liu, Y.N., Wang, X.P., et al., 2015a. Different effects of regional species pool on plant diversity between forest and grassland biomes in arid northwest China. PloS One 10.
|
Li S.F., Mao L.M., Spicer R.A., et al, 2015b. Late Miocene vegetation dynamics under monsoonal climate in southwestern China. Palaeogeogr. Palaeoclimatol. Palaeoecol, 425: 14-40. DOI:10.1016/j.palaeo.2015.02.030 |
Li Y.Q., Wang Z.H., Xu X.T., et al, 2016. Leaf margin analysis of Chinese woody plants and the constraints on its application to palaeoclimatic reconstruction. Global Ecol. Biogeogr, 25(12): 1401-1415. DOI:10.1111/geb.12498 |
Lü L.S., Cai H.Y., Yang Y., et al, 2018. Geographic patterns and environmental determinants of gymnosperm species diversity in China. Biodivers. Sci, 26(11): 1133-1146. DOI:10.17520/biods.2018098 |
Meserve P.L., Glanz W.E., 1978. Geographical ecology of small mammals in the northern Chilean arid zone. J. Biogeogr, 5: 135-148. DOI:10.2307/3038168 |
Mosbrugger, V., Favre, A., Muellner-Riehl, A.N., et al., 2018. Cenozoic evolution of geobiodiversity in the Tibeto-Himalayan region. In: Hoorn, C., Perrigo, A., Antonelli, A. (Eds.), Mountains, Climate and Biodiversity. Wiley Blackwell, Oxford, pp. 429-448.
|
Mourelle C., Ezcurra E., 1996. Species richness of Argentine cacti: a test of biogeographic hypotheses. J. Veg. Sci, 7: 667-680. DOI:10.2307/3236378 |
Mutke J., Barthlott W., 2005. Patterns of vascular plant diversity at continental to global scales. Biol. Skr, 55: 521-531. |
O'Brien E.M., 1993. Climatic gradients in woody plant species richness: towards an explanation based on an analysis of southern Africa's woody flora. J. Biogeogr, 20: 181-198. DOI:10.2307/2845670 |
O'Brien E.M., 1998. Water-energy dynamics, climate, and prediction of woody plant species richness: an interim general model. J. Biogeogr, 25: 379-398. DOI:10.1046/j.1365-2699.1998.252166.x |
O'Brien E.M., 2006. Biological relativity to watereenergy dynamics. J. Biogeogr, 33: 1868-1888. DOI:10.1111/j.1365-2699.2006.01534.x |
O'Brien E.M., Field R., Whittaker R.J., 2000. Climatic gradients in woody plant (tree and shrub) diversity: watereenergy dynamics, residual variation, and topography. Oikos, 89: 588-600. DOI:10.1034/j.1600-0706.2000.890319.x |
O'Brien E.M., Whittaker R.J., Field R., 1998. Climate and woody plant diversity in southern Africa: relationships at species, genus and family levels. Ecography, 21: 495-509. DOI:10.1111/j.1600-0587.1998.tb00441.x |
Qian H., 1999. Spatial pattern of vascular plant diversity in North America north of Mexico and its floristic relationship with Eurasia. Ann. Bot, 83: 271-283. DOI:10.1006/anbo.1998.0816 |
Qian H., Song J.S., Krestov P., et al, 2003. Large-scale phytogeographical patterns in East Asia in relation to latitudinal and climatic gradients. J. Biogeogr, 30: 129-141. DOI:10.1046/j.1365-2699.2003.00807.x |
Rahbek C., Graves G.R., 2001. Multiscale assessment of patterns of avian species richness. Proc. Natl. Acad. Sci. Unit. States Am, 98: 4534-4539. DOI:10.1073/pnas.071034898 |
Ren, M.E., Bao, H.S., 1992. China's Natural Regions and Their Development and Renovation. Science Press, Beijing (In Chinese).
|
Richerson P.J., Lum K.L., 1980. Patterns of plant species diversity in California:relation to weather and topography. Am. Nat, 116: 504-536. DOI:10.1086/283645 |
Ricklefs Robert, E ., 1977. Environmental heterogeneity and plant species diversity: a hypothesis. Am. Nat, 111(978): 376-381. DOI:10.1086/283169 |
Rohde K., 1998. Latitudinal gradients in species diversity. Area matters, but how much? :-.. Oikos, 82: 184-190. DOI:10.2307/3546928 |
Rosenzweig, M.L., 1995. Species Diversity in Space and Time. Cambridge University Press, Cambridge, UK.
|
Schulze E.D., Ellis R., Schulze W., et al, 1996. Diversity, metabolic types and delta C-13 carbon isotope ratios in the grass flora of Namibia in relation to growth form, precipitation and habitat conditions. Oecologia, 106: 352-369. DOI:10.1007/BF00334563 |
Spicer R.A., 2017. Tibet, the Himalaya, Asian monsoons and biodiversityeIn what ways are they related?. Plant Diversity, 39: 233-244. DOI:10.1016/j.pld.2017.09.001 |
Spicer R.A., Yang J., Herman A.B., et al, 2016. Asian Eocene monsoons as revealed by leaf architectural signatures. Earth Planet Sci. Lett, 449: 61-68. DOI:10.1016/j.epsl.2016.05.036 |
Spicer R.A., Yang J., Herman A.B., et al, 2017. Paleogene monsoons across India and South China: drivers of biotic change. Gondwana Res, 49: 350-363. DOI:10.1016/j.gr.2017.06.006 |
Su T., Liu Y.S., Jacques F.M.B., et al, 2013. The intensification of the East Asian winter monsoon contributed to the disappearance of Cedrus (Pinaceae) in southwestern China. Quat. Res, 80: 316-325. DOI:10.1016/j.yqres.2013.07.001 |
Stein A., Gerstner K., Kreft H., 2014. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett, 17: 866-880. DOI:10.1111/ele.12277 |
Stein A., Kreft H., 2015. Terminology and quantification of environmental heterogeneity in species-richness research. Biol. Rev, 90: 815-836. DOI:10.1111/brv.12135 |
Tang Z.Y., Wang Z.H., Zheng C.Y., et al, 2006. Biodiversity in China's Mountains. Front. Ecol. Environ, 4: 347-352. DOI:10.1890/1540-9295(2006)004[0347:BICM]2.0.CO;2 |
Terradas, J., Savé, R., 1992. The in fluence of summer and winter stress and water relationships on the distribution of Quercus ilex L. Vegetatio 99-100, 137-145.
|
Wang B., Ho L., 2002. Rainy season of the Asian-Pacific summer monsoon. J. Clim, 15(4): 386-398. |
Wang, B., Trenberth, K., Hurrell, J., et al., 2006. The Asian Monsoon. Springer Berlin Heidelberg.
|
Wang L., Kunzmann L., Su T., et al, 2019. The disappearance of Metasequoia(cupressaceae) after the middle miocene in yunnan, southwest China: evidences for evolutionary stasis and intensification of Asian monsoon. Rev. Palaeobot. Palynol, 264: 64-74. DOI:10.1016/j.revpalbo.2018.12.007 |
Wang X.P., Fang J.Y., Sanders N.J., et al, 2009. Relative importance of climate vs local factors in shaping the regional patterns of forest plant richness across northeast China. :-.. Ecography, 32: 133-142. DOI:10.1111/j.1600-0587.2008.05507.x |
Wang Z.H., Fang J.Y., Tang Z.Y., et al, 2011. Patterns, determinants and models of woody plant diversity in China. Proc. Biol. Sci, 278: 2122-2132. DOI:10.1098/rspb.2010.1897 |
Webb G.E., Sando W.J., Raymond A., 1997. Mississippian coral latitudinal diversity gradients (western interior United States): testing the limits of high resolution diversity data. J. Paleontol, 71: 780-791. DOI:10.1017/S0022336000035733 |
Wright D.H., 1983. Species-energy theory: an extension of speciesearea theory. :-.. Oikos, 41: 496-506. DOI:10.2307/3544109 |
White P.J.T., Kerr J.T., 2007. Human impacts on environmentediversity relationships: evidence for biotic homogenization from butterfly species richness patterns. Global Ecol. Biogeogr, 16: 290-299. DOI:10.1111/j.1466-8238.2007.00298.x |
Whittaker R.J., Nogues-Bravo D., Araujo M.B., 2007. Geographical gradients of species richness: a test of the water-energy conjecture of Hawkins et al. . 2003. using European data for five taxa. Global Ecol. Biogeogr, 16: 76-89. |
Wiens J.J., Donoghue M.J., 2004. Historical biogeography, ecology and species richness. Trends Ecol. Evol, 19: 639-644. DOI:10.1016/j.tree.2004.09.011 |
Woodward, F.I., 1987. Climate and Plant Distribution. Cambridge University Press, Cambridge.
|
Woodward F.I., 1990. The impact of low temperatures in controlling the geographical distribution of plants. Phil. Trans. Biol. Sci, 326: 585-593. |
Wu, Z.Y. (Ed.), 1979. Flora of China, vols. 20-72. Science Press, Beijing (In Chinese).
|
Wu, Z.Y. (Ed.), 1980a. Flora of China, vols. 20-72. Science Press, Beijing (In Chinese).
|
Wu, Z.Y. (Ed.), 1982. Flora of China, vols. 20-72. Science Press, Beijing (In Chinese).
|
Wu, Z.Y. (Ed.), 1984. Flora of China, vols. 20-72. Science Press, Beijing (In Chinese).
|
Wu, Z.Y. (Ed.), 1988. Flora of China, vols. 20-72. Science Press, Beijing (In Chinese).
|
Wu, Z.Y. (Ed.), 1995. Flora of China, vols. 20-72. Science Press, Beijing (In Chinese).
|
Wu, Z.Y. (Ed.), 1996. Flora of China, vols. 20-72. Science Press, Beijing (In Chinese).
|
Wu, Z.Y. (Ed.), 1998. Flora of China, vols. 20-72. Science Press, Beijing (In Chinese).
|
Wu, Z.Y. (Ed.), 1980b. Vegetation of China. Science Press, Beijing, pp. 363-372 (In Chinese).
|
Wu, Z.Y. (Ed.), 2004. Flora of China, vol. 1. Science Press, Beijing, p. 1044 (In Chinese).
|
Wu, Z.Y., Ding, T.Y., 1999. Seed Plants of China. Yunnan Science and Technology Press, Kunming (In Chinese).
|
Xia K., Fan L., Sun W.B., et al, 2016. Conservation and fruit biology of Sichou oak(Quercus sichourensis, Fagaceae)-a critically endangered species in China. Plant Diversity, 38: 233-237. DOI:10.1016/j.pld.2016.07.001 |
Xing, Y.W., Utescher, T., Jacques, F.M.B., et al., 2012. Paleoclimatic estimation reveals a weak winter monsoon in southwestern China during the late Miocene: evidence from plant macrofossils. Palaeogeogr. Palaeoclimatol. Palaeoecol. 358-360, 19-26.
|
Xu X., Wang Z., Rahbek C., et al, 2016. Geographical variation in the importance of water and energy for oak diversity. J. Biogeogr, 43: 279-288. DOI:10.1111/jbi.12620 |
Ying, T.S., 2001. Species diversity and distribution pattern of seed plant in China. Biodivers. Sci. 9 (4), 393-398 (In Chinese with English abstract).
|
Zhang, J.C., 1991. Climate in China: the Pandect. China Meteorological Press, Beijing, p. 477 (In Chinese).
|
Zhang P.P., Shao M.A., Zhang X.C., 2017. Spatial pattern of plant species diversity and the influencing factors in a gobi desert within the heihe river basin, northwest China. Journal of Arid Land 9 :379-393.. Journal of Arid Land, 9: 379-393. DOI:10.1007/s40333-017-0056-9 |



