Although most flowering plant species are hermaphrodite, during a reproductive season individual plants may serially adjust maternal investment, temporally regulating sexual resources allo-cated to female and male function (Lloyd, 1980a; Brunet and Charlesworth, 1995; Bishop et al., 2010). In diverse lineages of an-giosperms, some perfect flowers abort sex organs to produce pistillate (female) or staminate (male) flowers, resulting in gyno-monoecy and andromonoecy respectively (Darwin, 1877). The production of flowers is dependent on plants attaining a certain size threshold. Female function involves more resource cost than male function; consequently, small plants are expected to be either non-reproductive or functionally male (Lloyd, 1980a; Zhang and Jiang, 2002). For example, in the monoecious, insect-pollinated clonal herb Sagittaria trifolia (Alismataceae), plants developed from high-density clones are small and tend to be male, expressing staminate flowers in the upper racemes and aborted female flowers in the basal racemes (Han et al., 2011). One might expect that to maximize fitness, larger plants, which have more available re-sources to produce seeds, will tend to be female. Numerous theo-retical and empirical studies suggest that relative allocation to female function increases with plant size (see Lloyd and Bawa, 1984; Klinkhamer et al., 1997).
In hermaphrodites, early-opening flowers are usually perfect, but in late-opening flowers pistils may be aborted or undeveloped. The later occurrence of staminate flowers, usually near the tip of an inflorescence with perfect basal flowers, is a typical expression of andromonoecy. Because female function involves more resource cost than male function, loss of female function in late-opening flowers has been thought to be beneficial for optimal resource allocation (Stephenson, 1981; Bertin, 1982). Researchers have pro-posed that under variable environmental conditions plants can modify sex expression of individuals through the formation of hermaphrodite and unisexual flowers depending on plant size (Klinkhamer et al., 1997; Zhang et al., 2014). This concept is called size-dependent sex allocation, or size-dependent gender modifi-cation. Size-dependent sex allocation predicts that when plant size increases plants allocate more sexual resources to female function. However, this prediction does not appear to be accurate for the serial regulation of sex allocation in hermaphrodites (Lloyd, 1980a; Delph, 2003). For example, in the andromonoecious perennial herb Veratrum nigrum (Liliaceae) more staminate flowers have been observed in large plants than in small plants (Liao and Zhang, 2008). The underlying assumption of size-dependent sex alloca-tion is that as size increases fitness returns on female investment decelerate more rapidly than returns on male investment (Charnov, 1982; Lloyd and Bawa, 1984). However, late-opening staminate flowers in andromonoecious plants may enjoy enhanced male function. For instance, terminal flowers, which are in elevated po-sition, are likely to donate more pollen and experience less intra-plant self-pollination than basal perfect flowers (Harder and Barrett, 1996). A male-biased allocation of resources to staminate flowers may promote pollen donation and decrease geitonogamy in plants with large floral displays (Bertin, 1982; Solomon, 1986; Brunet, 1996; Harder and Barrett, 1996; Elle and Meagher, 2000). Furthermore, the production of staminate flowers does not inter-fere with the deposition of pollen on perfect flowers in the same plant (Vallejo-Marín and Rausher, 2007b). This discrepancy be-tween the theoretical predictions of size-dependent sex allocation and empirical observations of male-biased production of staminate flowers in some large plants remains largely unresolved (Vallejo-Marín and Rausher, 2007a).
To test the effect of plant size on sex expression, we investigated plant size and flower sex of individuals of the lily Lilium lankon-gense. Liliaceae species are a model system for investigating the effects of plant size on the plasticity of sex expression. Previous research suggests that sex expression in lily flowers may be related to plant size (Peruzzi, 2012; Zhang et al., 2014). In addition, male flowers frequently appear in Liliaceae species. In fact, three sexual types have been observed in L. lankongense, including male plants usually producing only one staminate flower, hermaphrodites producing only perfect flowers, and andromonoecious plants pro-ducing both perfect and staminate flowers. L. lankongense is an especially tractable model for testing predictions that arise from size-dependent sex allocation. The flowers are large, and perfect flowers have much longer styles than staminate flowers. Cross-pollination treatments on staminate flowers do not set any fruits, indicating that they are infertile, functional males. Also, this species is self-incompatible, without pleiotropic effects promoting out-crossing by staminate flowers. Furthermore, L. lankongense flowers are effectively pollinated by large butterflies (Sun and Yao, 2013) rather than pollen-collecting bees, which eliminates the con-founding effect of staminate flowers in andromonoecious species acting as a reward attractant for pollinators.
In this study, we asked whether andromonoecious individuals of L. lankongense exhibit size-dependent sex allocation. To answer this question, we determined whether the numbers of perfect and staminate flowers are related to the size of individual L. lankongense plants. We also estimated male and female resource allocation by comparing the flower size and dry weight of floral organs of both perfect and staminate flowers in individual plants. In addition, we asked whether gender expression varies with environmental con-ditions. To answer this question, we calculated the proportion of the sexual types and the standardized phenotypic gender of L. lankongense at five field sites in 2017 and 2018. We predicted that small plants with limited resources for seed production were more likely to be functionally male, producing staminate flowers. We also predicted that production of more perfect flowers would be favored in larger individuals with more resources.
2. Material and methods 2.1. Study speciesLilium lankongense Franch. (Liliaceae) is a perennial that grows on forest margins along valleys and in rocky meadows at 2300-3500 m above sea level in subalpine areas of the Hengduan Mountains region, southwest China. Like many lilies, the plants are bulbous and produce an annual shoot. Flowering individuals are from 0.5 to 1.2 m tall, each with a terminal raceme composed of 1-10 or more large, pink nodding flowers (Sun and Yao, 2013). The flowers are protandrous, with six long stamens and six petals bearing many deep purple dots, with a green linear nectary at the base of each petal. Preliminary investigations showed that indi-vidual plants were either hermaphrodites that only produced perfect flowers or andromonoecious, producing later-opening sta-minate flowers at the top of racemes. These staminate flowers had rudimentary pistils with much smaller ovaries and shorter styles than perfect flowers (Fig. 1). Although bumblebees, honeybees and small bees were seen collecting nectar or pollen, potential polli-nators for these lily flowers are large butterflies (see also Sun and Yao, 2013).

|
| Fig. 1 Non-flowered plant (A) with an aborted floral bud and staminate- and perfect-flowered plant (B) of Lilium lankongense at Shangri-La Alpine Botanical Garden, southwestern China. Note the larger style in perfect flower. |
We sampled hundreds of wild plants within a field station of the university at Shangri-La Alpine Botanic Garden and on nearby slopes, at Shangri-La, Yunnan Province, southwestern China (27°53'53.70" N, 99°38'58.18"E-27°54'14.68' N, 99°38'14.02"E; elevation 3288-3364 m above sea level).
We recorded the sex of all flowers on each sampled individual by repeatedly identifying the status of the pistils throughout the flowering period from early July to late August 2017 and 2018.
2.2. Effect of size on sex expressionTo examine the effect of plant size on sex expression, we randomly sampled a total of 364 flowering individuals of L. lankongense from five sites (Site 1: 27°54'10"N, 99°38'18"E, 3346 m; Site 2: 27°54'8"N, 99°38'21"E, 3327 m; Site 3: 27°54'7"N, 99°38'22"E, 3317 m; Site 4: 27°54'6"N, 99°38'21"E, 3322 m; and Site 5: 27°54'9"N, 99°38'26"E, 3294 m) in summer 2017. Inflores-cence height was measured with a 2-m rule (to 1 mm) late in the flowering season (Zhang et al., 2014). Thirty non-flowered plants were also measured. These plants had no flowers, or smaller aborted terminal floral buds. For each plant, the numbers of perfect and staminate flowers and their position on racemes were recorded.
To test the pattern of size-dependent sex allocation (SDS), we examined whether large plants produced more perfect flowers. We calculated Pearson correlation coefficients between plant height and the number of hermaphrodite flowers, the number of stami-nate flowers, as well as the percentage of staminate flowers within individuals. To compare plant height of non-flowered and one-flowered individuals, a general linear model (GLM) with distribu-tion and identity-link function was performed.
To examine whether gender expression varies with environ-mental conditions, we calculated the proportion of the sexual types and the standardized phenotypic gender Gi (see Zhang et al., 2014 in Lilium apertum ) in each of five sites in 2018. Previous methods depicted this index as follows in cases where plant sex (either male or andromonoecious) could be temporally modified: Gi = Oi/ (Oi + piE), where Oi is the number of perfect flowers (i.e. ovule-bearing flowers), pi is the number of staminate flowers. E is the ratio of perfect to male flowers in each site:
Our hand pollination treatments indicated that this species was self-incompatible, and staminate flowers did not produce viable fruits and seeds under cross-pollination, confirming that the sta-minate flowers were functionally male (EiEi Shwi et al., unpub-lished data).
2.3. Floral allocation to perfect and staminate flowersTo compare relative resource allocation to female and male function, we randomly collected one perfect and one staminate flower from each of 15 andromonoecious plants in the early morning on the first day of flowering, before anther dehiscence. These fresh flowers were divided into three parts: (1) petals, (2) pistils and (3) stamens, and then oven-dried at 60°C for 5 d before weighing. The dry biomass of each organ was separately weighed on a Sartorius BSA224S electronic balance (0.1 mg, Sartorius Co., Goettingen, Germany).
The dry weight of perfect and staminate flowers was compared under GLM with distribution and identity-link function. All statis-tical analyses were performed in SPSS 21.0 (IBM Corporation, 2014).
3. Results 3.1. Plant size and sex expressionOf 364 sampled flowering individuals in 2017, 54 (14.8%) plants were male producing only staminate flowers, 277 (76.1%) were hermaphrodite having only perfect flowers and 33 (9.1%) were andromonoecious with both perfect and staminate flowers on one raceme. Male plants were significantly smaller than hermaphrodite and andromonoecious plants; both plant height and flower number were significantly lower.
Overall, flower number was positively correlated (r = 0.725; P < 0.001) with plant height (Fig. 2), indicating that large plants produced more flowers. Of 54 male plants examined, only one plant produced two flowers; the others were one-staminate-flowered, and plant height was not positively related to flower number (P = 0.662). Andromonoecious flower number per plant was positively correlated to plant height (r = 0.517; P < 0.001). Her-maphrodite individual flower numbers per plant were also posi-tively correlated to plant height (r = 0.694; P < 0.001). Of individuals having a single flower, hermaphrodite plants were taller (Wald χ2 = 5.37, df = 1, P = 0.02) (36.58 ± 1.05 cm, n = 144) than in males plants (31.86 ± 1.76, n = 53). Furthermore, we found plant height of 30 non-flowered individuals (13.22 ± 1.4) was significantly shorter than one-flowered male plants and her-maphrodite plants (P < 0.001).
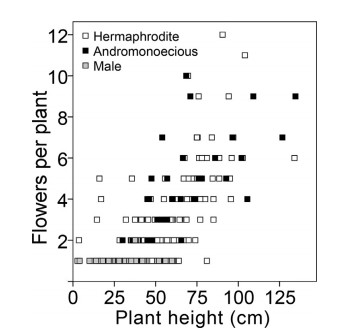
|
| Fig. 2 Effects of plant height on flower number in three types of individuals of Lilium lankongense: andromonoecious, hermaphrodite, and male plants. |
Individuals with more flowers tended to be andromonoecious (Fig. 3). Flower number per plant was highest in andromonoecious plants (4.76 ± 0.40); in contrast, flower number was significantly lower in male (1.02 ± 0.19) and in hermaphrodite plants (2.24 ± 0.11) (Wald 2 = 113.231, df = 2, P < 0.001).
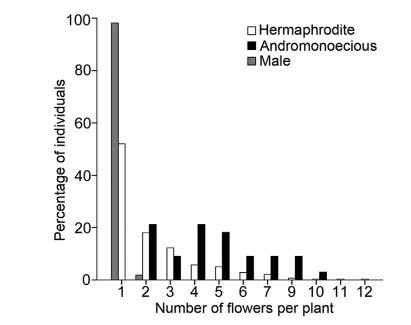
|
| Fig. 3 Percentage of individuals of the three sexual types with flower number per plant from all field sites of Lilium lankongense. |
Andromonoecious and male plants decreased between 2017 and 2018 (Fig. 4). Overall, 92.6% of individuals were hermaphrodite, whereas only 2.8% were andromonoecious, and 4.6% male. One site (Site 2) had a relatively higher proportion of individuals with sta-minate flowers (9%). Male plants were not observed at two sites(Site 1 and Site 4). Phenotypic gender analysis showed that all five L. lankongense sites surveyed were male-biased, but the proportion of phenotypic gender was approximately 0.5, suggesting an equal female and male function.
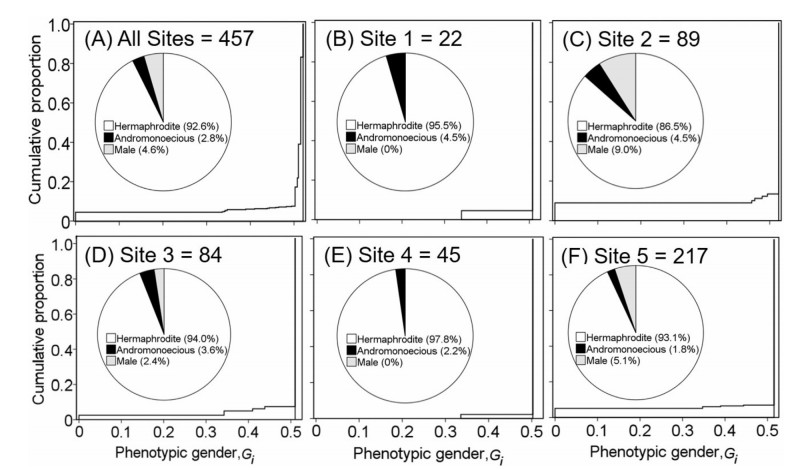
|
| Fig. 4 Variation in phenotypic gender of Lilium lankongense at five sites (S1-S5) in 2018. Lines represent the cumulative frequency distribution of standardized phenotypic fe-maleness (Gi) for plants sampled from each site (Gi = 0 for pure males, and Gi = 1 for pure females). Percentages of individuals of the three sexual types are shown in each insert pie. Data from all sites (A) and from Site 1 to Site 5 (B-F). |
Dry weight of petals and pistils was significantly greater in perfect than in staminate flowers, but that of stamens did not differ (Table 1). These comparisons indicate that resource allocation to perfect flowers was strongly female-biased, and male allocation did not decrease in staminate flowers.
| Floral organ | Perfect flowers | Staminate flowers | Waldχ2 | P |
| Petal (mg) | 0.14 ± 0.007 | 0.12 ± 0.006 | 6.813 | 0.009 |
| Pistil (mg) | 0.013± 0.0009 | 0.003± 0.0007 | 81.371 | < 0.0001 |
| Stamen (mg) | 0.046± 0.004 | 0.038± 0.003 | 2.608 | 0.106 |
| Total weight (mg) | 0.199± 0.0096 | 0.157± 0.0079 | 11.436 | 0.001 |
Our investigations confirmed predictions that in L. lankongense a size threshold must be reached to produce (perfect) flowers. We observed that small plants were functionally male, usually pro-ducing a single staminate flower. In andromonoecious individuals, staminate flowers had aborted pistils with smaller ovaries and shorter styles than perfect flowers, but resource allocation to male function (stamen and pollen production) did not decrease. The smallest plants were likely to be male, middle-sized plants were hermaphrodite and the largest plants were andromonoecious.
The majority of flowering individuals of L. lankongense produced significantly more hermaphrodite flowers than staminate flowers in the five sites we surveyed during two flowering seasons. Both small plants with a single flower and large plants with multiple flowers were likely to produce staminate flowers terminally on the inflorescences, suggesting that plant size affects sex expression (Fig. 3). Size-related sex expression has been observed in several herbs in the Liliaceae (reviewed by Zhang et al., 2014; Wang et al., 2018). For example, in Lilium apertum , which grows in similar mountainous areas as L. lankongense, male plants are the most common sexual type (mean frequency 57%) with male and her-maphrodite (28%) phenotypes usually having one flower and andromonoecious plants (15%) 2-3 flowers (Zhang et al., 2014). In Israel, individuals of Gagea chlorantha (Liliaceae) that produce be-tween 1 and 6 flowers were observed to be hermaphrodite, male and andromonoecious in five Mediterranean populations but pri-marily hermaphrodite in three desert populations; the overall proportions of the three sexual types were 70.6%, 12.5% and 16.9%, respectively (Wolfe, 1998). Single-flowered plants of G. chlorantha account for 54.5% of Mediterranean populations and 28% of desert populations, and the perfect flower of hermaphrodite plants are larger than the staminate flowers of male plants (and the bulb is heavier in hermaphrodites). These surveys of sex expression in various populations indicate that plasticity of sex expression is governed by the size of individual plants (see Zhang et al., 2014). Other studies have shown that the percentage of hermaphrodite flowers clearly increases with increasing nutrient supplementation (Primack and Lloyd, 1980; Emms, 1993). We were unable to label the individuals and identify the sex of the same plant over two years. Whether the lily flower we studied can change sex between seasons-as observed in L. apertum (Zhang et al., 2014) - needs further study.
We observed that in L. lankongense perfect flowers were significantly larger than staminate flowers. Although stamen size did not differ between perfect and staminate flowers, perfect flowers had larger petals and pistils. This finding is consistent with previous findings that staminate flowers were usually smaller in andromonoecious plants (Solomon, 1986; Emms, 1993). One exception occurs in an andromonoecious annual herb Sagittaria guyanensis, in which staminate flowers have larger petals and more anthers than perfect flowers (Huang, 2003). Sex expression in S. guyanensis may be derived from a monoecious ancestor that had larger male than female flowers. In S. guyanensis, early hermaph-rodite flowers on the basal spikes are generally larger than later flowers, producing more ovules, but pollen production per flower does not decrease with sequentially opening flowers (Thomson, 1989). Although later-produced staminate flowers with undevel-oped pistils have been shown to be smaller, have lower biomass, and produce less pollen (Solomon, 1986; Emms, 1993; Zhang et al., 2014), the relative allocation of male to female investment may not decrease (see Huang et al., 2004; Liu and Huang, 2012).
Here we observed functional male flowers were borne on both small and large plants. Pollen-producing (infertile) flowers should be favored under conditions with limited resources. Female func-tion, which includes the production of fruit and seeds, is costlier than male function. In andromonoecious Zigadenus paniculatus (Liliaceae), individuals with large inflorescences have a higher proportion of male flowers (Emms, 1993). In one natural population of andromonoecious V. nigrum, Liao and Zhang (2008) observed that larger plants (measured as plant height) produce more sta-minate flowers. Furthermore, in this andromonoecious herb, large displays have relatively low female reproductive success (Liao et al., 2009). Large individuals of Solanum carolinense (Solanaceae) have numerous perfect (fruiting) and staminate (non-fruiting) flowers, and flowers in the terminal racemes are more likely to be func-tionally male (Solomon, 1986). Later-produced, less-costly flowers may enhance male reproductive success (Brunet, 1996; Elle and Meagher, 2000; Dai and Galloway, 2012). Alternatively, the main-tenance of staminate flowers may promote the female fitness of perfect flowers, enhancing seed production (Vallejo-Marín and Rausher, 2007b).
It is not surprising to observe that female and male resource allocation increased in parallel with plant size in a hermaphrodite plant (Xiong et al., 2016). An increase in both female and male in-vestment with plant size has been observed in hermaphrodites and in species with variable sex expression, e.g., in several andromo-noecious (Solomon, 1986; Emms, 1993; Liao and Zhang, 2008; Zhang et al., 2014), gynomonoecious (Mamut et al., 2017), and monoecious species (Han et al., 2011). If the fitness returns from female and male function are equal, resource investments to both sexes should be equal in hermaphrodites (Charnov, 1982; de Jong and Klinkhamer, 2005). Our calculation showed the proportion of phenotypic gender of sampled individuals was approximately 0.5 in all five sites surveyed, suggesting that female and male function contribute equally at the population level. Consistent with the null model of resource allocation variation with plant size, we observed that both perfect and staminate flowers increased with plant size in the lily.
Author contributionsSQH and EES designed the research; EES and BW performed the research; EES and SQH analyzed the data and wrote the paper. All authors discussed and commented on the manuscript.
Declaration of Competing InterestThe author declares no conflict of interest.
AcknowledgementsWe thank Li-Bing Jia, Kai Hao, Mo-Han Hu, and Ju Tang for their help in the field study; Director Zhen-Dong Fang and staff at Shangri-La Botanical Garden for logistical support; Dr. Ying-Ze Xiong for help with data analysis; and Sarah Corbet (University of Cambridge) and two anonymous referees for valuable comments on this manuscript. The first author, who is from Myanmar, thanks the Chinese government for an international scholarship for foreign students. This work was supported by the National Natural Science Foundation of China (No. U1402267, 31270281) to S.Q.H.
Bertin R.I., 1982. The evolution and maintenance of andromonoecy. Evol. Theor, 6: 25-32. |
Bishop E.J., Spigler R.B., Ashman T.-L., 2010. Sex-allocation plasticity in hermaphrodites of sexually dimorphic Fragaria virginiana (Rosaceae). Botany, 88: 231-240. DOI:10.1139/B10-005 |
Brunet J., 1996. Male reproductive success and variation in fruit and seed set in Aquilegia caerulea (Ranunculaceae). Ecology, 77: 2458-2471. DOI:10.2307/2265746 |
Brunet J., Charlesworth D., 1995. Floral sex allocation in sequentially blooming plants. Evolution, 49: 70-79. DOI:10.1111/j.1558-5646.1995.tb05959.x |
Charnov, E.L., 1982. The Theory of Sex Allocation. Princeton Univ. Press, Princeton.
|
Dai C., Galloway L.F., 2012. Male flowers are better fathers than hermaphroditic flowers in andromonoecious Passiflora incarnata. New Phytol, 193: 787-796. DOI:10.1111/j.1469-8137.2011.03966.x |
Darwin, C., 1877. The Different Forms of Flowers on Plants of the Same Species. John Murrary, London, UK.
|
Delph L.F., 2003. Sexual dimorphism in gender plasticity and its consequences for breeding system evolution. Evol. Dev, 5: 34-39. DOI:10.1046/j.1525-142X.2003.03006.x |
de Jong, T.J., Klinkhamer, P.G.L., 2005. Evolutionary Ecology of Plant Reproductive Strategies. Cambridge Univ. Press, Cambridge.
|
Elle E., Meagher T.R., 2000. Sex allocation and reproductive success in the andromonoecious perennial Solanum carolinense (Solanaceae). ò. Paternity and functional gender. Am. Nat, 156: 622-636. |
Emms S.K., 1993. Andromonoecy in Zigadenus paniculatus (Liliaceae): spatial and temporal patterns of sex allocation. Am. J. Bot, 80: 914-923. DOI:10.1002/j.1537-2197.1993.tb15312.x |
Han B., Wang X.F., Huang S.Q., 2011. The production of male flowers does not decrease with plant size in insect-pollinated Sagittaria trifolia, contrary to predictions of size-dependent sex allocation. J. Systemat. Evol, 49: 379-385. DOI:10.1111/j.1759-6831.2011.00141.x |
Harder, L.D., Barrett, S.C.H., 1996. Pollen dispersal and mating patterns in animalpollinated plants. In: Lloyd, D.G., Barrett, S.C.H. (Eds.), Floral Biology: Studies on Floral Evolution in Animal-Pollinated Plants. Chapman & Hall, New York, USA, pp. 140-190.
|
Huang S.Q., 2003. Flower dimorphism and the maintenance of andromonoecy in Sagittaria guyanensis ssp lappula (Alismataceae). New Phytol, 157: 357-364. DOI:10.1046/j.1469-8137.2003.00676.x |
Huang S.Q., Tang L.L., Yu Q., et al, 2004. Temporal floral sex allocation in protogynous Aquilegia yabeana contrasts with protandrous species: support for the mating environment hypothesis. Evolution, 58: 1131-1134. DOI:10.1111/j.0014-3820.2004.tb00446.x |
Klinkhamer P.G.L., de Jong T.J., Metz H., 1997. Sex and size in cosexual plants. Trends Ecol. Evol, 12: 260-265. DOI:10.1016/S0169-5347(97)01078-1 |
Liao W.J., Zhang D.Y., 2008. Increased maleness at flowering stage and femaleness at fruiting stage with size in an andromonoecious perennial. Veratrum nigrum. J. Integr. Plant Biol, 50: 1024-1030. DOI:10.1111/j.1744-7909.2008.00691.x |
Liao W.J., Hu Y., Zhu B.R., et al, 2009. Female reproductive success decreases with display size in monkshood, Aconitum kusnezoffii Ranunculaceae. Ann. Bot, 104: 1405-1412. DOI:10.1093/aob/mcp237 |
Liu C.Q., Huang S.Q., 2012. Does the relative importance of resource competition and architectural effect in floral variation vary with stages of floral ontogeny? J. Systemat. Evol, 50: 119-124. DOI:10.1111/j.1759-6831.2011.00175.x |
Lloyd D.G., 1980a. Sexual strategies in plants. I. An hypothesis of serial adjustment of maternal investment during one reproductive session. New Phytol, 86: 69-79. |
Lloyd D.G., 1980b. Sexual strategies in plants ó. A quantitative method for describing the gender of plants. N. Z. J. Bot, 18: 103-108. |
Lloyd D.G., Bawa K.S., 1984. Modification of the gender of seed plants in varying conditions. Evol. Biol, 17: 255-338. |
Mamut J., Xiong Y.Z., Tan D.Y., et al, 2017. Flexibility of resource allocation in a hermaphrodite-gynomonoecious herb through deployment of female and male resources in perfect flowers. Am. J. Bot, 104: 461-467. DOI:10.3732/ajb.1600397 |
Peruzzi L., 2012. Male flowers in Liliaceae are more frequent than previously thought. Bocconea, 24: 301-304. |
Primack R.B., Lloyd D.G., 1980. Andromonoecy in the New Zealand montane shrub manuka, Leptospermum scoparium (Myrtaceae). Am. J. Bot, 67: 361-368. DOI:10.1002/j.1537-2197.1980.tb07661.x |
Solomon B.P., 1986. Sexual allocation and andromonoecy, resource investment in male and hermaphrodite flowers of Solanum carolinense Solanaceae. Am. J. Bot, 73: 1215-1221. DOI:10.1002/j.1537-2197.1986.tb08568.x |
Stephenson A.G., 1981. Flower and fruit abortion, proximate causes and ultimate functions. Annu. Rev. Ecol. Systemat, 12: 253-279. DOI:10.1146/annurev.es.12.110181.001345 |
Sun S.G., Yao C.Y., 2013. Increased seed set in down slope-facing flowers of Lilium duchartrei. J. Systemat. Evol, 51: 405-412. DOI:10.1111/jse.12002 |
Thomson J.D., 1989. Deployment of ovules and pollen among flowers within inflorescences. Evol. Trends Plants, 3: 65-68. |
Vallejo-Mar#237;n M. Rausher M.D., 2007a. The role of male flowers in andromonoecious species, energetic costs and siring success in Solanum carolinense. Evolution, 61: 404-412. DOI:10.1111/j.1558-5646.2007.00031.x |
Vallejo-Mar#237;n M. Rausher M.D., 2007b. Selection through female fitness helps to explain the maintenance of male flowers. Am. Nat, 169: 563-568. DOI:10.1086/513112 |
Wang J., Zhai Y., Zhang A., 2018. Temporal variation of plant sexes in a wild population of Tulipa sinkiangensis over seven years. Biodivers. Sci, 26: 519-526. DOI:10.17520/biods.2018038 |
Wolfe L.M., 1998. Regulation of sex expression in desert and Mediterranean populations of an andromonoecious plant Gagea chlorantha, Liliaceae. Israel J. Plant Sci, 46: 17-25. DOI:10.1080/07929978.1998.10676703 |
Xiong Y.Z., Xie M., Huang S.Q., 2016. Influence of plant size on female-biased sex allocation in a single-flowered, nectarless herb. AoB PLANTS 8, 139. |
Zhang D.Y., Jiang X.H., 2002. Size-dependent resource allocation and sex allocation in herbaceous perennial plants. J. Evol. Biol, 15: 74-83. DOI:10.1046/j.1420-9101.2002.00369.x |
Zhang Z.Q., Zhu X.F., Sun H., et al, 2014. Size-dependent gender modification in Lilium apertum (Liliaceae): does this species exhibit gender diphasy? Ann. Bot, 114: 441-453. |



