b. Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201, China;
c. Kunming College of Life Science, University of Chinese Academy of Sciences, Kunming, Yunnan, 650201, China
Bamboos are important members of forest ecosystems from sea level to alpine mountains (Ohrnberger, 1999; Clark et al., 2015). The bamboo subfamily, Bambusoideae, is one of the 12 subfamilies of the grass family Poaceae, and it is a member of the BOP clade (including Bambusoideae, Oryzoideae and Pooideae) (GPWG, 2001; GPWG II, 2012; Soreng et al., 2015, 2017; Saarela et al., 2018). Nearly 1700 bamboo species in 127 genera are found around the world (BPG, 2012; Clark et al., 2015; Clark and Oliveira, 2018).
The classification of bamboos at the tribal level has entered a new phase since the first molecular phylogenetic study of bamboos on the basis of broad sampling (Clark et al., 1995) and subsequent phylogenetic and phylogenomic studies (e.g., Bouchenak-Khelladi et al., 2008; Sungkaew et al., 2009; Kelchner and BPG, 2013; Triplett et al., 2014; Ma et al., 2014; Saarela et al., 2018; Guo et al., 2019). The subfamily Bambusoideae is divided into three tribes: the Arundinarieae (the temperate woody bamboos), the Bambuseae (the tropical woody bamboos), and the Olyreae (the herbaceous bamboos) (BPG, 2012; Kelchner and BPG, 2013). Phylogenetic studies based on plastid regions suggested that the woody bamboos (tribes Arundinarieae and Bambuseae) were paraphyletic with the herbaceous bamboos sister to the tropical woody bamboos (Bouchenak-Khelladi et al., 2008; Sungkaew et al., 2009; Kelchner and BPG, 2013). However, phylogenies reconstructed from nuclear loci (Triplett et al., 2014), and more recently, nuclear genome sequences, indicated that the woody bamboos were monophyletic, and that during their evolutionary history reticulate evolution and allopolyploidization occurred (Guo et al., 2019).
The subtribal classification of tribes Bambuseae and Olyreae is much clearer than that of Arundinarieae. Molecular and morphological data support the division of the tribe Bambuseae into 11 subtribes and the division of tribe Olyreae into three subtribes (BPG, 2012; Kelchner and BPG, 2013; Soreng et al., 2017). However, plastid phylogenies indicated that few Arundinarieae genera were monophyletic and recovered 12 lineages (Triplett and Clark, 2010; Zeng et al., 2010; Yang et al., 2013; Attigala et al., 2014; Zhang et al., 2016). Strikingly, none of these lineages corresponded to the subtribes based on morphology (Table 1). In addition, Arundinarieae phylogenies based on several nuclear loci have failed to provide high resolution subtribal delimitation (Peng et al., 2008; Zhang et al., 2012; Yang et al., 2013). The low resolution of the phylogenetic relationships between members of the tribe Arundinarieae has hindered the proposal of subtribal classifications, and consequently, some researchers have instead adopted 12 plastid lineages (Kellogg, 2015). Thus, a formal subtribal classification is needed.
| Clayton and Renvoize (1986) | Soderstrom and Ellis (1987) | Li (1998) | Ohrnberger (1999) | The 12 plastid lineagesa |
| Arundinariinae (20)b | Arundinariinae (12) | Arundinariinae (14) | Arundinariinae (16) | Ⅰ. Bergbambos (1) |
| Bergbambos | ||||
| Acidosasa | Acidosasa | Acidosasa | Acidosasa | Ⅱ. African alpine bamboos (1) |
| Arundinaria (Bashania, | Ampelocalamus | Ampelocalamus | Arundinaria | Oldeania |
| Oligostachyum, Pleioblastus) | Arundinaria (Bashania, | Arundinaria | Bashania | Ⅲ.Chimonocalamus (2) |
| #Aulonemia Goudot | Clavinodum, Omeiocalamus, | Chimonocalamus | Ferrocalamus | Chimonocalamus |
| Chimonobambusa | Pleioblastus, Pseudosasa) | Drepanostachyum | Gaoligongshania | Ampelocalamus |
| #Chusquea Kunth | Chimonobambusa | (Himalayacalamus) | Gelidocalamus | Ⅳ. Shibataea clade (6) |
| #Colanthelia McClure & | (Oreocalamus, Qiongzhuea, | Fargesia | Indocalamus | Ferrocalamus |
| E. W. Smith | Tetragonocalamus) | (Borinda, Yushania) | Menstruocalamus | Gelidocalamus |
| #Glaziophyton Franchet | Chimonocalamus | Ferrocalamus | Metasasa | Indocalamus |
| #Guaduella Franchet | Drepanostachyum | Gaoligongshania | Oligostachyum | Pseudosasa |
| #Hitchcockella A. Camus | (Himalayacalamus) | Gelidocalamus | Pleioblastus | Sasa |
| Indocalamus | Fargesia (Burmabambus, | Indocalamus | Polyanthus | Shibataea |
| Indosasa | Butania, Sinarundinaria, Yushania) | Oligostachyum | Pseudosasa | Ⅴ. Phyllostachys clade (17) |
| #Myriocladus Swallen | Gelidocalamus | Pseudosasa | Sasa | Acidosasa, Ampelocalamus, |
| #Neurolepis Meisner | Indocalamus (Ferrocalamus) | Sasa | Sasaella | Bashania (Sarocalamus), Brachystachyum, |
| #Olmeca Soderstrom | #Perrierbambus | Thamnocalamus | Vietnamocalamus | Chimonobambusa (Menstruocalamus, Qiongzhuea), |
| Pseudosasa | Sasa (Neosasamorpha, | Chimonocalamus, Drepanostchyum, Fargesia, | ||
| Sasa | Sasaella, Sasamorpha) | Gelidocalamus, Himalayacalamus, Indocalamus, | ||
| Sinobambusa | Thamnocalamus | Oligostachyum, Phyllostachys, Pleioblastus, | ||
| Sinarundinaria | Pseudosasa, Sasa, Yushania | |||
| Thamnocalamus | ||||
| #Perrierbambus A. Camus | ||||
| Bambusinae (3) | Shibataeinae (7) | Shibataeinae (7) | Shibataeinae (8) | |
| Phyllostachys | Brachystachyum | Chimonobambusa | Brachystachyum | Ⅵ. Arundinaria clade (12) |
| Semiarundinaria | Hibanobambusa | Indosasa | Chimonobambusa | Acidosasa (Metasasa), |
| Shibataea | Indosasa | Phyllostachys | Hibanobambusa | Arundinaria, Hibanobambusa, Indosasa, |
| Phyllostachys | Qiongzhuea | Indosasa | Oligostachyum, Pleioblastus, Pseudosasa, | |
| Semiarundinaria | Semiarundinaria | Phyllostachys | Sasa, Sasaella, | |
| Shibataea | (Brachystachyum) | Semiarundinaria | Sasamorpha, Semiarundinaria, Sinobambusa | |
| Sinobambusa (Neobambus) | Shibataea | Shibataea | ||
| Sinobambusa | Sinobambusa | |||
| Thamnocalaminae (8) | Ⅶ. Thamnocalamus (1) | |||
| Ampelocalamus | Thamnocalamus | |||
| Borinda | Ⅷ. Indocalamus wilsonii (1) | |||
| Chimonocalamus | Ⅸ. Gaoligongshania (1) | |||
| Drepanostachyum | Ⅹ. Indocalamus (1) | |||
| Fargesia | Ⅺ. Hsuehochloa (1) | |||
| Himalayacalamus | (Ampelocalamus calcareus = H.calcarea) | |||
| Thamnocalamus | Ⅻ. Kuruna (1) | |||
| Yushania | ||||
| Genera with # are not members of the tribe Arundinarieae; Guaduella belongs to the subfamily Puelioideae and the others belong to the tribe Bambuseae (Soreng et al., 2015, 2017). a Refers to Ma et al. (2014), Attigala et al. (2016) and Ma et al. (2017). b Denotes the number of genera included in the subtribes or lineages. | ||||
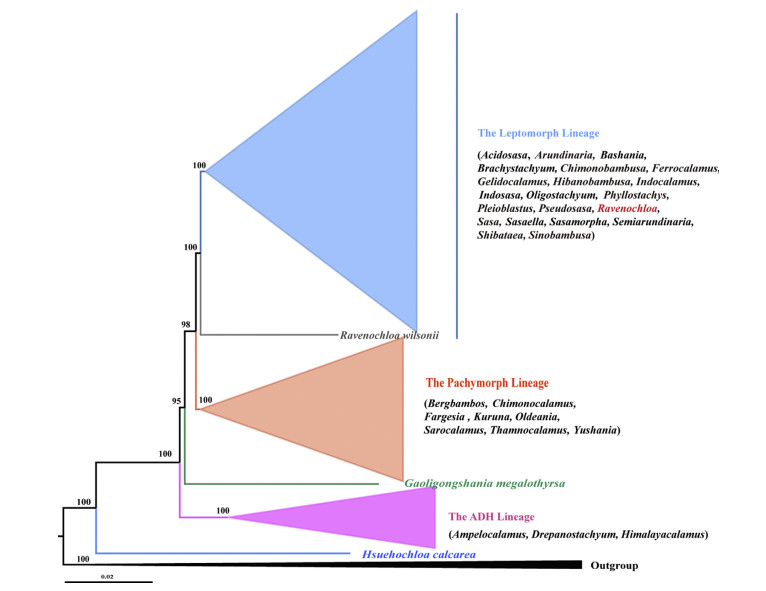
With the development of next generation sequencing technologies, genomic data can be obtained more easily and cheaply, such as with reduced-representation methods. As a pilot study, Wang et al. (2017) exploited the restriction-site associated DNA sequencing (RAD-seq) method to gain tens of thousands of single nucleotide polymorphisms (SNPs) and reconstructed a preliminary phylogeny of the temperate woody bamboos. The results demonstrated that the RAD-seq phylogeny largely agreed with the morphology-based taxonomy at the generic level with unprecedented resolution. Subsequently, Guo (2019) sampled more than 200 taxa of temperate woody bamboos and used a modified ddRADseq (double digest RAD-seq) method to reconstruct the phylogenetic backbone of this tribe. Using a dataset of all 12 plastid clades, five major lineages were recovered, i.e. the leptomorph lineage, the pachymorph lineage, Gaoligongshania megalothyrsa (Handel-Mazzetti) D. Z. Li, Hsueh & N. H. Xia, the ADH lineage, and Hsuehochloa calcarea (C. D. Chu & C. S. Chao) D. Z. Li & Y. X. Zhang. The relationships among those lineages and genera were well resolved.
In this paper, we propose a new subtribal classification for the tribe Arundinarieae based on these molecular phylogenies, with an attempt to obtain morphological synapomorphies in support of the classification. In addition, because Indocalamus wilsonii (Rendle) C. S. Chao & C. D. Chu had a unique phylogenetic position in plastid, plastomic, and nuclear genomic trees (Zeng et al., 2010; Ma et al., 2014, 2017; Guo, 2019), a new genus, Ravenochloa, is proposed to accommodate this isolated species.
2. Materials and methods 2.1. Specimen examinationWe examined specimens of representative clades of Arundinarieae deposited at herbaria from the 1990s to the present (E, HIB, HNNU, ISC, KUN, K, N, NF, P, PE, SIFS, SWFC, SZ, W, WU and US; herbarium acronyms following Thiers, 2019). Our examination emphasized type specimens of some noteworthy genera.
2.2. Molecular phylogeniesTo date the most comprehensive molecular phylogenies that have classified subtribal relationships in the tribe Arundinarieae are based on ddRAD-seq data (Guo, 2019). For the species Indocalamus wilsonii, phylogenies reconstructed from plastid regions, plastomes, and ddRAD-seq data were used as references (Zeng et al., 2010; Ma et al., 2014, 2017; Guo, 2019).
2.3. Morphological comparisonBecause of the high morphological heterogeneity of the five lineages recovered by Guo (2019), three reproductive characters and two vegetative characters were chosen for comparison. Four reproductive characters and six vegetative characters were selected and compared between I. wilsonii and the morphologically related genera Ferrocalamus Hsueh & P. C. Keng, Indocalamus Nakai and Sasa Makino & Shibata. All the morphological data were gathered based on specimens, field observation, and literature (Keng and Wang, 1996; Li et al., 2006).
3. ResultsThe phylogenetic relationships within the Arundinarieae based on ddRAD data are discussed in Cen Guo's PhD dissertation (Guo, 2019). A simplified phylogeny of the tribe Arundinarieae is presented in Fig. 1. Taxa of the leptomorph lineage have the synapomorphy of leptomorph rhizomes, while the other major diagnostic features listed in Table 2 are quite heterogeneous among different genera. Taxa of the pachymorph lineage, Gaoligongshania D. Z. Li, Hsueh & N. H. Xia, the ADH lineage and, Hsuehochloa D. Z. Li & Y. X. Zhang all possess pachymorph rhizomes (except Sarocalamus Stapleton), semelauctant inflorescence, and three stamens. In addition, branch complements and number of stigmas differ among these three lineages.
|
| Fig. 1 The phylogenetic tree of Arundinarieae based on ddRAD data (adapted from Guo, 2019). |
| The leptomorph lineage | The pachymorph lineage | Gaoligongshania | The ADH lineage | Hsuehochloa | |
| Rhizome | Leptomorph | Pachymorph | Pachymorph | Pachymorph | Pachymorph |
| Branch complement | 1, 3, 5e7, >7 (20) | 1, 3, 5e8, >8 | 1 | Many, subequal or with one prominent branch | 1, 3-7 |
| Inflorescence | Semelauctant, iterauctant | Semelauctant | Semelauctant | Semelauctant | Semelauctant |
| Number of stamens | 3, 6 (occasionally 4 or 5) | 3 | 3 | 3 | 3 |
| Number of stigmas | 2, 3 | 2, 3 | 3 | 2 | 2 |
Indocalamus wilsonii was sister to the rest of the leptomorph lineage (Fig. 1). Morphological comparison between I. wilsonii and related genera is presented in Table 3. I. wilsonii, Indocalamus, and Sasa usually have shorter and thinner culms, while Ferrocalamus has taller and thicker culms. I. wilsonii and Sasa possess a solitary branch at each node, while Indocalamus and Ferrocalamus sometimes have more than one branch apically. Species of Sasa have six stamens, whereas the others possess three stamens. The leaves of I. wilsonii usually become wavy when dry (Fig. 2), and the leaves of Indocalamus (with the exception of a few species), Ferrocalamus and Sasa (except several taxa) are flat. In addition, I. wilsonii is the only species in the leptomorph lineage distributed at higher elevations (1500e3000 m) in Central China, while the other species of Indocalamus, species of Ferrocalamus, and Sasa are distributed usually on lower hills at 500-1000 m, and all below 2000 m.

|
| Fig. 2 Ravenochloa wilsonii. (A) Habitat; (B) Fresh foliage leaves; (C) Young culms; (D) Culm leaf sheath; (E) Branches; (F) A specimen showing the wavy leaves (Y. X. Zhang & Z. J. Wang 07088, KUN) |
| I. wilsonii | Indocalamus | Ferrocalamus | Sasa | |
| Culm height | 0.3-0.9 m | Usually less than 2 m | 2-7 m | < 2 m |
| Culm diameter | 0.2-0.4 cm | 0.3-2 cm | 0.8-4 cm | 0.5 cm |
| Internode | Glabrous or white pubescent, | Hairy, usually with a brown tomentose | Culm-wall thick, nearly solid at | Culm-wall thin, 10-25 cm long |
| 4-12 cm long | ring below nodes, 5-55 cm long, | the base, 50-70 cm long | ||
| usually 15-25 cm long | ||||
| Branch complement | Solitary | Solitary, sometimes 2-3 apically | Solitary at the base, 3-5 apically | Solitary |
| Culm sheath | Persistent, pale red-brown or | Persistent, longer or shorter than | Tardily deciduous or persistent, | Persistent, longer or shorter than |
| straw-colored, 1/2 as long as the | the internode, usually hairy | yellow-green, 1/2 as long as the | the internode, hairy or glabrous | |
| internode, densely deciduously | internode, densely brown or | |||
| white pubescent | dark brown setose | |||
| Leaf | Wavy when dry | Wavy or flat when dry | Flat when dry | Flat when dry (occasionally wavy) |
| Inflorescence | Panicles | Racemes or panicles | Panicles | Panicles |
| Number of florets | 3-7 | 3-15 | 3-10 | 4-8 |
| per spikelet | ||||
| Stamen | 3 | 3 | 3 | 6 |
| Stigma | 2, occasionally 3 | 2 | 2 | 3 |
The tribe Arundinarieae was established in 1902, making it the most recent tribe in Bambusoideae ( BPG, 2012 ). This is essentiallyan Old World tribe, with one genus, Arundinaria Michaux, native to the New World (southeastern North America) (Ohrnberger, 1999; Zhang et al., 2016). More than 500 species in 31 genera belong to the tribe Arundinarieae (Clark et al., 2015). They are mainly distributed in temperate woodlands, forest edges, and mountains of subtropical or tropical areas.
Previous subtribal divisions of this tribe have been mainly based on inflorescence type (iterauctant vs semelauctant), number of stamens, rhizome type (leptomorph vs pachymorph), culm leaves, and branch complements. The type of inflorescence and rhizome was treated as the most important diagnostic character at the subtribal level. For example, in the three widely used classification systems proposed by Soderstrom and Ellis (1987), Li (1998), and Ohrnberger (1999) (Table 1), the inflorescence type was the key feature that distinguished the subtribe Arundinariinae (semelauctant) from the subtribe Shibataeinae (iterauctant), while the rhizome type discriminated subtribes Arundinariinae and Shibataeinae (leptomorph) from the subtribe Thamnocalaminae (pachymorph) in Ohrnberger's system. Li (1997) proposed that the subtribe Arundinariinae could be divided into two groups on the differentiation of rhizome type and leaf anatomy, i.e. the Arundinaria group (leptomorph rhizome) and the Thamnocalamus Munro group and allies (pachymorph rhizome). The delimitation of the subtribe Arundinariinae has been the most controversial and complex. Genera with different types of rhizomes and branch complements, and different numbers of stamens were previously classified into this subtribe (Clayton and Renvoize, 1986; Soderstrom and Ellis, 1987; Li, 1998) (Table 1).
Among the five lineages recovered by Guo (2019), the leptomorph lineage was the most complicated. There were representatives of 20 genera nested in this lineage. Some taxa were members of the subtribe Shibataeinae, such as Chimonobambusa Makino, Hibanobambusa Maruyama & Okamura, Indosasa McClure, Phyllostachys Siebold & Zuccarini, Semiarundinaria Makino ex Nakai, Shibataea Makino ex Nakai, and Sinobambusa Makino ex Nakai, whereas the others belonged to the subtribe Arundinariinae. All these taxa have leptomorph rhizomes. However, the branch complements and reproductive characters are quite diverse (Table 2). Acidosasa C. D. Chu & C. S. Chao ex P. C. Keng, Chimonobambusa, Indosasa, Pseudosasa Makino ex Nakai, and Sinobambusa usually have three branches per node, whereas Indocalamus and Sasa usually have a solitary branch at each node, and Phyllostachys possesses two branches at each node. Iterauctant inflorescences can be found in the genera Chimonobambusa, Indosasa, Phyllostachys, and Shibataea. Semelauctant inflorescences can be found in the genera Arundinaria, Acidosasa, Indocalamus and Pseudosasa. The stamen number of Acidosasa, Indosasa and Sasa is six, and the other genera have three stamens. Therefore, nearly all the diagnostic character states except the rhizome type in the tribe Arundinarieae can be found in this lineage.
The second large lineage recovered by Guo (2019) was the pachymorph lineage, which included eight genera. Chimonocalamus Hsueh & T. P. Yi, Fargesia Franchet, Thamnocalamus and Yushania P. C. Keng were assigned to Arundinariinae or Thamnocalaminae in different classification systems (Table 1). The recently established genera Bergbambos Stapleton (2013), Kuruna Attigala, Kaththriarachchi & L. G. Clark (Attigala et al., 2014), Oldeania Stapleton (Stapleton, 2013; Zhang et al., 2017), and Sarocalamus (Stapleton et al., 2004) were also members of Arundinariinae or Thamnocalaminae. The third large lineage, the ADH lineage, consisted of Ampelocalamus S. L. Chen, T. H. Wen & G. Y. Sheng, Drepanostachyum P. C. Keng and Himalayacalamus P. C. Keng, which were placed in Thamnocalaminae by Ohrnberger (1999) but in Arundinariinae by Soderstrom and Ellis (1987) and Li (1998). Gaoligongshania belonged to the subtribe Arundinariinae and the type species of Hsuehochloa was a member of Arundinariinae or Thamnocalaminae (Table 1). These four lineages all have pachymorph rhizomes (except Sarocalamus, which has leptomorph rhizomes), semelauctant inflorescences, and three stamens. There are some other fine distinctions among these lineages, such as the branch complements. Taxa of the pachymorph lineage have the most complex branch complements among the four lineages except the leptomorph lineage. The branch number at each node of the pachymorph lineage is one (some species of Yushania), three to eight (e.g. Chimonocalamus and Thamnocalamus), or many (Fargesia and Yushania) (Table 2).
Attigala et al. (2016) evaluated the evolution of several key morphological characters in the tribe Arundinarieae, including rhizomes and reproductive structures, based on the plastome phylogenetic tree. Their findings demonstrated that pachymorph rhizomes were possibly the ancestral state, leptomorph rhizomes evolved with reversions to the pachymorph condition once or multiple times, and pseudospikelets evolved at least twice in Arundinarieae. Recently, Guo (2019) estimated the evolution of three key diagnostic characters of Arundinarieae (i.e., rhizome type, inflorescence type, and branch complement) based on comprehensive sampling and well resolved phylogenetic trees retrieved from ddRAD data. It was inferred that inflorescence type and branch complement type experienced multiple transformations among different states, while rhizomes had some phylogenetic signal for distinguishing lineages. Those results suggested that some important diagnostic characters probably experienced convergent evolution in the Arundinarieae, similar to the situation in Arthrostylidiinae of the neotropical bamboo lineage (Bambuseae, Bambusoideae) (Tyrrell et al., 2012).
As Christenhusz et al. (2015) emphasized in their paper recommending that plant classification follow the Angiosperm Phylogeny Group (APG), the more we know about relationships, the more difficult it becomes to construct a reasonable classification. Classifications in the bamboos have had the same problem, especially in the tribe Arundinarieae. In this paper, we tried to follow the rules of monophyly and exploited the phylogenetic relationships as the main reference for the subtribal division of Arundinarieae. The five lineages recovered by Guo (2019) are proposed as five different subtribes. The leptomorph lineage is named subtribe Arundinariinae, as it includes the type genus, Arundinaria; the pachymorph lineage is named subtribe Thamnocalaminae; the ADH lineage is named subtribe Ampelocalaminae; and Gaoligongshania and Hsuehochloa are named subtribes Gaoligongshaniinae and Hsuehochloinae, respectively. These five newly delimited subtribes will have great value for teaching and for in-depth research on this group.
In the leptomorph lineage of Guo (2019), I. wilsonii was strongly supported as sister to the rest of the lineage, rather than grouping with species of Indocalamus (Fig. 1). The phylogenetic position of this species in the plastid trees was unique as well and it was treated as an independent lineage (Table 1) (Zeng et al., 2010; Ma et al., 2014, 2017; Guo, 2019). Morphologically, the vegetative features of this species are more similar to the species of Sasa. They all have short and thin culms and a solitary branch per node, although they have a different number of stamens. Species of Indocalamus usually possess a solitary branch at the basal and mid-culm nodes and 2e3 branches at the upper nodes. There is usually a brown tomentose ring below the nodes of most species of Indocalamus, but this is absent in I. wilsonii. The stigma number of I. wilsonii is usually 2 but occasionally 3, while species of Indocalamus have 2 stigmas. The vegetative features of Ferrocalamus are quite distinct from those of I. wilsonii, such as culm height and diameter, and internode length, although they have a solitary branch per node in common. Although the morphological differences between Indocalamus and I. wilsonii are not prominent, the unique phylogenetic position and geographic distribution of I. wilsonii motivated us to establish a new genus to accommodate its peculiarity.
5. Taxonomic treatment 5.1. Description of a new genusRavenochloa D. Z. Li & Y. X. Zhang, gen. nov.
雷文竹属【léi wén zhú shǔ】(新拟).
Diagnosis. Ravenochloa resembles Ferrocalamus, Indocalamus, and Sasa, but differs from those genera by its short and thin culms, pale red-brown or straw-colored culm leaves with deciduous white pubescence, wavy dry leaves, and distribution at higher elevations (usually above 2000 m). Moreover, Ravenochloa has yellow-villous rachilla making it distinct from Ferrocalamus, pulvinate flowering branches and 2 or 3 styles, which is different than Indocalamus, and three stamens, distinguishing it from Sasa (with six stamens).
Type. Ravenochloa wilsonii (Rendle) D. Z. Li & Y. X. Zhang, comb. nov.
雷文竹【léi wén zhú 】(新拟).
Basionym. Arundinaria wilsonii Rendle, Journal of the Linnean Society, Botany, 36: 437. 1904. Holotype: CHINA, Hubei, Fang County, 2310-2880 m ("scrub on top of highest mountains, 7700-9600 ft"), E. H. Wilson 1887 (K!, sheet No. K000912158); isotype: E. H. Wilson 1887 (K! "ex Herb. Hongkong.", sheet No. K000912157).
Homotypic synonyms. I. wilsonii (Rendle) C. S. Chao & C. D. Chu; Sinarundinaria wilsonii (Rendle) P. C. Keng.
Heterotypic synonyms. Indocalamus nubigenus (P. C. Keng) H. R. Zhao & Y. L. Yang; Indocalamus shimenensis B. M. Yang; Sasa nubigena P. C. Keng; Sasamorpha nubigena (P. C. Keng) P. C. Keng.
Description. Rhizomes leptomorph. Culms 30-90 cm tall, 0.2-0.4 cm in diameter; internodes 4-12 cm long, glabrous or white pubescent. Culm leaf sheaths pale red-brown or strawcolored, closely embracing the culm, ca. 1/2 as long as the internode, densely deciduously white pubescent, densely pubescent or glabrescent near the outer margins, veins conspicuous, transverse veins sometimes distinct; auricles and oral setae absent; ligule short, ca. 0.6 mm tall; blades erect, deciduous, ovate-lanceolate or narrowly triangular, base contracted, apex acute. Branch sheaths orange-red when dry, glabrous; inner ligule 1.5-4 mm tall; blades lanceolate or narrowly ovate-lanceolate, 2.5-4 cm long. Foliage leaves 3 (-5) per ultimate branch; sheaths yellow-green, tinged with red, glabrous or pubescent; auricles and oral setae absent; ligule 2.5e9 mm tall; blades oblong-lanceolate, 6-17 × 1.5-4.7 cm, wavy when dry, abaxially gray-green and pilose, adaxially yellowgreen and glabrous. Panicles 5e10 cm long, base encircled by a leaf sheath; branches ascending, slender, glabrous, pulvinate; spikelets usually purple green; florets 3-7; rachilla internodes ca. 4 mm, densely yellow-villous; glumes usually 2, glabrous; lemma puberulent, 7-9 veined, apex acuminate with a short mucro, callus densely white villous; palea puberulent; stamens 3, anthers yellow; styles 2 or 3.
Etymology. Ravenochloa is named in honor of Dr. Peter H. Raven, member of the National Academy of Sciences of the USA, and foreign member of the Chinese Academy of Sciences. As the coEditor-in-Chief of the Flora of China, Dr. Raven has made great contributions to the study of systematics and biogeography, and conservation of plants in China and the rest of the world in general, and inspired bamboo taxonomy and evolutionary study in particular. Raven refers to his family name and chloa means grass.
Distribution and habitat. Central China (Chongqing, Hubei and Hunan). Under forests at elevations of (1500-) 2000-3000 m.
Additional specimens examined. CHINA. Chongqing: Fengjie, 1800 m alt., 1 June 1964, H . F. Zhou & H. Y. Su 108411(SZ); Nanchuan, 1800 m, 6 September 1943, Keng & P. C. Keng 3882 (N), 1700 m, 24 May 1957, G . F. Li 61519 (SZ), 2100 m, 4 October 1957, J . H. Xiong & Z. L. Zhou 93794 (KUN, SZ), 2250 m, 29°02.060'N, 107°11.340'E, 5 July 2010, P . F. Ma MPF10146 (KUN), 2243 m, 29°2.100'N, 107°11.560'E, 4 September 2015, C . Guo & X. Y. Wang GC122 (KUN). Hubei: Changyang, 1530 m, 30°37.000'N, 110°42.940'E, 10 July 2007, Y . X. Zhang & Z. J. Wang 07088 (KUN), Shennongjia, 5 December 1976, T . P. Yi 76342 (HIB). Hunan: Shimen, 1 August 1982, B . Z. Wu s.n. (HNNU), 1818 m, 32°2.830'N, 110°31.500'E, 23 July 2015, X . Y. Ye & J. X. Liu YXY233 (KUN).
5.2. Re-circumscription of subtribes in ArundinarieaeⅠ. Subtribe Arundinariinae Bentham in J. Linn. Soc. Bot. 19: 31. 1881.
Type. Arundinaria Michaux, Flora of Boreali-Americana, 1: 73. 1803.
Description. Rhizomes leptomorph. Culms diffuse or pluricaespitose; internodes terete or quadrate, sulcate or not; sheath scars prominent or inconspicuous. Branch complements various, solitary, 2, 3, 3-7, or many branches per node. Culm leaves deciduous or persistent, longer or shorter than the internodes, sheaths glabrous or hairy, sometimes spotted; auricles conspicuous or absent; blades erect, reflexed or recurved, linear, subulate, lanceolate, or ovate-lanceolate. Foliage leaves several or many per ultimate branch; sheaths glabrous, pubescent or setose; blades lanceolate or broadly lanceolate, glabrous or pubescent abaxially. Inflorescences iterauctant or semelauctant, usually subtended by bracts or prophyll when iterauctant; stamens 3 or 6, occasionally 4 or 5, anthers yellow or purple; stigmas 2 or 3, plumose.
Included genera. Acidosasa (Metasasa W. T. Lin), Arundinaria, Bashania P. C. Keng & T. P. Yi, Brachystachyum Keng, Chimonobambusa (Menstruocalamus T. P. Yi, Oreocalamus Keng, Qiongzhuea Hsueh & T. P. Yi), Ferrocalamus, Gelidocalamus T. H. Wen, Hibanobambusa, Indocalamus, Indosasa, Oligostachyum Z. P. Wang & G. H. Ye (Clavinodum T. H. Wen), Phyllostachys, Pleioblastus Nakai (Nipponocalamus Nakai, Polyanthus C. H. Hu), Pseudosasa Makino ex Nakai, Ravenochloa, Sasa (Neosasamorpha Tatewaki), Sasaella Makino, Sasamorpha Nakai, Semiarundinaria, Shibataea, Sinobambusa (Neobambus Keng ex P. C. Keng).
Incertae Sedis. Vietnamocalamus T. Q. Nguyen.
Ⅱ. Subtribe Thamnocalaminae P. C. Keng in J. Bamboo Res. 11: 25. 1992.
Type. Thamnocalamus Munro, Transactions of the Linnean Society of London, 26: 33. 1868.
Description. Rhizomes pachymorph (except Sarocalamus with leptomorph), some taxa with elongated necks. Culms unicaespitose, pluricaespitose or diffuse; internodes terete, glabrous, white powdery or hairy; sheath scar prominent or not. Branch complements consisting of solitary to many branches per node. Culm leaves deciduous, tardily deciduous or persistent, sheaths glabrous or hairy; auricles absent or present; blades erect or reflexed, linear to ovatelanceolate. Foliage leaves a few per ultimate branch; sheaths glabrous or hairy; blades lanceolate, glabrous or pubescent abaxially. Inflorescences semelauctant, racemose or paniculate, bracteate or ebracteate; stamens 3, anthers yellow or purple; stigmas 2 or 3, plumose.
Included genera. Bergbambos, Chimonocalamus, Fargesia (Borinda Stapleton, Sinarundinaria Nakai), Kuruna, Oldeania, Sarocalamus, Thamnocalamus, Yushania (Burmabambus P. C. Keng, Butania P. C. Keng, Monospatha W. T. Lin).
This subtribe is referred to as the alpine bamboos, which diversified in the mountains of Southwest China in the late Pliocene (Ye et al., 2019).
Ⅲ. Subtribe Gaoligongshaniinae D. Z. Li & Y. X. Zhang, subtrib. nov.
贡山竹亚族【gòng shān zhú yà zú 】(新拟).
Diagnosis. The subtribe Gaoligongshaniinae resembles the subtribe Thamnocalaminae in pachymorph rhizomes, semelauctant and paniculate inflorescences and three stamens, but differs from Thamnocalaminae by epiphytic habit, solitary branch per node (as thick as the culm), large foliage leaves and open, large, ebracteate inflorescences.
Type. Gaoligongshania D. Z. Li, Hsueh & N. H. Xia, Acta Phytotaxonomica Sinica, 33: 597. 1995.
Description. Shrubby scrambling bamboo, sometimes epiphytic on trees. Rhizomes short necked, pachymorph. Culms unicaespitose; internodes terete, glabrous; nodes flat. Branches solitary per node. Culm leaves persistent, sheaths leathery, densely setose abaxially, shorter than the internodes; ligule short; auricles embracing culms, large; blades recurved, lanceolate. Foliage leaves 7e9 per ultimate branch; blades large, oblong-lanceolate, glabrous abaxially. Inflorescence an open, large, ebracteate panicle on leafy or leafless flowering branches; spikelets 4-9-flowered, followed by a sterile floret, long pedicellate; glumes 2; lemma many veined, sometimes long mucronate; palea subequal to lemma, 2-keeled, 2- cleft at apex; rachilla internodes ca. 1/2 as long as florets, disarticulating; lodicules 3, transparent; stamens 3, anthers yellow; ovary glabrous; style 1; stigmas 3, plumose.
Included genus. Gaoligongshania.
Ⅳ. Subtribe Ampelocalaminae D. Z. Li & Y. X. Zhang, subtrib. nov.
悬竹亚族【xuán zhú yà zú】(新拟).
Diagnosis. The subtribe Ampelocalaminae is similar to the subtribe Thamnocalaminae in pachymorph rhizomes, semelauctant inflorescences, and three stamens, but the new subtribe has pendulous or scrambling culms, many branches per node sometimes with one dominant, conspicuous sheath scars, and ebracteate inflorescences, which distinguish it from Thamnocalaminae.
Type. Ampelocalamus S. L. Chen, T. H. Wen & G. Y. Sheng, Acta Phytotaxonomica Sinica, 19: 332. 1981.
Description. Rhizomes pachymorph. Culms caespitose, apically pendulous or scrambling; internodes terete, finely ridged or not; nodal sheath scars prominent. Branch complements consisting of many branches per node, these subequal or the central one dominant. Culm leaves deciduous or persistent, longer or shorter than the internode; auricles absent or minute, occasionally prominent; oral setae usually absent; blades linear to ovate-lanceolate, erect or reflexed. Foliage leaves 3e11 per ultimate branch; sheaths glabrous or pubescent; blades papery, lanceolate, glabrous or pubescent abaxially. Inflorescences semelauctant, ebracteate, paniculate or racemose; florets 1e7 per spikelet; stamens 3, anthers yellow; stigmas 2, plumose.
Included genera. Ampelocalamus (Petrocalamus Z. P. Wang, N. X. Ma & W. Y. Zhang), Drepanostachyum, Himalayacalamus.
Ⅴ. Subtribe Hsuehochloinae D. Z. Li & Y. X. Zhang, subtrib. nov.
纪如竹亚族【jì rú zhú ya zú 】(新拟).
Diagnosis. The subtribe Hsuehochloinae resembles the subtribe Ampelocalaminae in scrambling habit, pachymorph rhizomes, semelauctant inflorescence and three stamens, but differs from it by having 3e7 subequal branches per node, inconspicuous sheath scars and purple stamens.
Type: Hsuehochloa D. Z. Li & Y. X. Zhang, PhytoKeys, 109: 67. 2018.
Description. Rhizomes pachymorph. Culms caespitose, apically drooping, procumbent or scrambling; internodes terete, densely white pubescent initially on the upper part, later subglabrous; nodes and sheath scars inconspicuous. Branch complements with one branch proximally and 3e7 branches apically, branches slender, subequal. Culm leaves persistent, 1/2 as long as internodes, sheaths densely white pubescent abaxially, glabrescent, margins densely white ciliate; auricles falcate, amplexicaul; oral setae many, radiate; ligule short, apex densely white fimbriate; blades reflexed, green, ovate-lanceolate. Foliage leaves 2e5 per ultimate branch; sheaths glabrous, glossy; blades thinly leathery, lanceolate, abaxially slightly glaucous, glabrous on both surfaces. Inflorescences imperfectly known, semelauctant, ebracteate, racemose possibly with 1 or few spikelets; glumes not seen; florets 5; lemma and palea purple green; lodicules not seen; stamens 3, anthers purple; ovary and style not seen; stigmas 2, plumose.
Included genus. Hsuehochloa.
Author contributionsYu-Xiao Zhang drafted this paper; Cen Guo conducted the phylogenomic work and revised the draft; De-Zhu Li conceived and wrote this paper.
Declaration of Competing InterestThe authors have no conflicts of interest to declare.
AcknowledgementsThis study was supported by the National Natural Science Foundation of China (Grant 31430011). We are indebted to Dr. Chao-Nan Fu of the Kunming Institute of Botany, Chinese Academy of Sciences for assistance in literature search. Thanks also go to curators of herbaria mentioned in Materials and Methods for their help in specimen examination. We also would like to thank Prof. Lynn G. Clark of Iowa State University, U. S. A. and the anonymous reviewers for their constructive suggestions.
Attigala, L., Triplett, J.K., Kathriarachchi, H.S., Clark, L.G., 2014. A new genus and a major temperate bamboo lineage of the Arundinarieae (Poaceae: Bambusoideae) from Sri Lanka based on a multi-locus plastid phylogeny. Phytotaxa 174, 87-205.
|
Attigala L., Wysocki W.P., Duvall M.R., Clark L.G., 2016. Phylogenetic estimation and morphological evolution of Arundinarieae (Bambusoideae: Poaceae) based on plastome phylogenomic analysis. Mol. Phylogenet. Evol, 101: 111-121. DOI:10.1016/j.ympev.2016.05.008 |
Bamboo Phylogeny Group (BPG), 2012. An updated tribal and subtribal classification of the bamboos (Poaceae: Bambusoideae). In: Gielis, J., Potters, G. (Eds.), Proceedings of the 9th World Bamboo Congress, 10-15 April 2012. World Bamboo Organization, Antwerp, Belgium, pp. 3-27.
|
Bouchenak-Khelladi Y., Salamin N., Savolainen V., Forest F., van der Bank M., Chase M.W., Hodkinson T.R., 2008. Large multi-gene phylogenetic trees of the grasses (Poaceae): progress towards complete tribal and generic level sampling. Mol. Phylogenet. Evol, 47: 488-505. DOI:10.1016/j.ympev.2008.01.035 |
Christenhusz M.J.M., Vorontsova M.S., Fay M.F., Chase M.W., 2015. Results from an online survey of family delimitation in angiosperms and ferns: recommendations to the Angiosperm Phylogeny Group for thorny problems in plant classification. Bot. J. Linn. Soc, 178: 501-528. DOI:10.1111/boj.12285 |
Clark, L.G., Loñdono, X., Ruiz-Sanchez, E., 2015. Bamboo taxonomy and habitat. In: Liese, W., Köhl, M. (Eds.), Bamboo-the Plant and its Uses. Springer (eBook).
|
Clark, L.G., Oliveira, R.P., 2018. Diversity and Evolution of the New World Bamboos(Poaceae: Bambusoideae: Bambuseae, Olyreae). Keynote Lecture in the 11th World Bamboo Congress, 14-18 August 2018. Xalapa, Mexico.
|
Clark L.G., Zhang W.P., Wendel J.F., 1995. A phylogeny of the grass family (Poaceae) based on ndhF sequence data. Syst. Bot, 20: 436-460. DOI:10.2307/2419803 |
Clayton, W.D., Renvoize, S.A., 1986. Genera Graminum, Grasses of the World. Her Majesty's Stationery Office, London.
|
Grass Phylogeny Working Group (GPWG), 2001. Phylogeny and subfamilial classification of the grasses (Poaceae). Ann. Mo. Bot. Gard, 88: 373-457. DOI:10.2307/3298585 |
Grass Phylogeny Working Group ò (GPWG ò), 2012. New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytol, 193: 304-312. DOI:10.1111/j.1469-8137.2011.03972.x |
Guo, C., 2019. Phylogenomics of Arundinarieae (Poaceae: Bambusoideae). Kunming Institute of Botany, Chinese Academy of Sciences. PhD dissertation.
|
Guo, Z.H., Ma, P.F., Yang, G.Q., Hu, J.Y., Liu, Y.L., Xia, E.H., Zhong, M.C., Zhao, L., Sun, G.L., Xu, Y.X., Zhao, Y.J., Zhang, Y.C., Zhang, Y.X., Zhang, X.M., Zhou, M.Y., Guo, Y., Guo, C., Liu, J.X., Ye, X.Y., Chen, Y.M., Yang, Y., Han, B., Lin, C.S., Lu, Y., Li, D.Z., 2019. Genome sequences provide insights into the reticulate origin and unique traits of woody bamboos. Mol. Plant 12, 1353-1365.
|
Kelchner S.A., Bamboo Phylogeny Group (BPG), 2013. Higher level phylogenetic relationships within the bamboos (Poaceae: Bambusoideae) based on five plastid markers. Mol. Phylogenet. Evol, 67: 404-413. DOI:10.1016/j.ympev.2013.02.005 |
Kellogg, E.A., 2015. Flowering plants. Monocots: Poaceae. In: Kubitski, K. (Ed.), The Families and Genera of Vascular Plants, vol. XⅢ. Springer International, Cham, Switzerland.
|
Keng, P.C., Wang, Z.P., 1996. Flora Reipublicae Popularis Sinicae (1), vol. 9. Science Press, Beijing, China.
|
Li, D.Z., 1997. The Flora of China Bambusoideae project-problems and current understanding of bamboo taxonomy in China. In: Chapman, G.P. (Ed.), The Bamboos. Linnean Society Symposium Series 19. Academic Press, London, United Kingdom, pp. 61-81.
|
Li, D.Z., 1998. Taxonomy and biogeography of the Bambuseae (Gramineae: Bambusoideae). In: Rao, A.N., Rao, V.R. (Eds.), Proceedings of Training Course/workshop 10-17 May 1998. Kunming and Xishuangbanna, Yunnan, China, pp. 14-23.
|
Li D.Z., Wang Z.P., Zhu Z.D., Xia N.H., Jia L.Z., Guo Z.H., Yang G.Y., Stapleton C.M.A., 2006. Bambuseae (Poaceae). In: Wu Z.Y., Raven P.H., Hong, D.Y. (Eds.), Flora of China, vol, 22. |
Ma P.F., Vorontsova M.S., Nanjarisoa O.P., Razanatsoa J., Guo Z.H., Haevermans T., Li D.Z., 2017. Negative correlation between rates of molecular evolution and flowering cycles in temperate woody bamboos revealed by plastid phylogenomics. BMC Plant Biol, 17. |
Ma P.F., Zhang Y.X., Zeng C.X., Guo Z.H., Li D.Z., 2014. Chloroplast phylogenomic analyses resolve deep-level relationships of an intractable bamboo tribe Arundinarieae (Poaceae). Syst. Biol, 63: 933-950. DOI:10.1093/sysbio/syu054 |
Ohrnberger, D., 1999. The Bamboos of the World: Annotated Nomenclature and Literature of the Species and the Higher and Lower Taxa. Elsevier Science BV, Amsterdam.
|
Peng, S., Yang, H.Q., Li, D.Z., 2008. Highly heterogeneous generic delimitation within the temperate bamboo clade (Poaceae: Bambusoideae): evidence from GBSSI and ITS sequences. Taxon 57, 799-810.
|
Saarela, J.M., Burke, S.V., Wysocki, W.P., Barrett, M.D., Clark, L.G., Craine, J.M., Peterson, P.M., Soreng, R.J., Vorontsova, M.S., Duvall, M.R., 2018. A 250 plastome phylogeny of the grass family (Poaceae): topological support under different data partitions. PeerJ 6, e4299.
|
Soderstrom, T.R., Ellis, R.P., 1987. The position of bamboo genera and allies in a system of grass classification. In: Soderstrom, T.R., Hilu, K.W., Campbell, C.S., Barkworth, M.E. (Eds.), Grass Systematics and Evolution. Smithsonian Institution Press, Washington, D.C., pp. 225-238
|
Soreng R.J., Peterson P.M., Romaschenko K., Davidse G., Zuloaga F.O., Judziewicz E.J., Filgueiras T.S., Davis J.I., Morrone O., 2015. A worldwide phylogenetic classification of the Poaceae (Gramineae). J. Systemat. Evol, 53: 117-137. DOI:10.1111/jse.12150 |
Soreng R.J., Peterson P.M., Romaschenko K., Davidse G., Teisher J.K., Clark L.G., Barber#225; P. Gillespie L.J., Zuloaga F.O., 2017. A worldwide phylogenetic classification of the Poaceae (Gramineae) ò: an update and comparison of two 2015 classifications. J. Systemat. Evol, 55: 259-290. DOI:10.1111/jse.12262 |
Stapleton, C.M.A., 2013. Bergbambos and Oldeania, new genera of African bamboos(Poaceae, Bambusoideae). PhytoKeys 25, 87-103.
|
Stapleton, C.M.A., Ní Chonghaile, G., Hodkinson, T.R., 2004. Sarocalamus, a new Sino-Himalayan bamboo genus (Poaceae: Bambusoideae). Novon 14, 345-349.
|
Sungkaew S., Stapleton C.M.A., Salamin N., Hodkinson T.R., 2009. Non-monophyly of the woody bamboos (Bambuseae; Poaceae): a multi-gene region phylogenetic analysis of Bambusoideae s. s. J. Plant Res, 122: 95-108. DOI:10.1007/s10265-008-0192-6 |
Thiers, B., 2019. Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium (continuously updated). http://sweetgum.nybg.org/science/ih/.
|
Triplett J.K., Clark L.G., 2010. Phylogeny of the temperate bamboos (Poaceae:Bambusoideae: Bambuseae) with an emphasis on Arundinaria and allies. Syst. Bot, 35: 102-120. DOI:10.1600/036364410790862678 |
Triplett J.K., Clark L.G., Fisher A.E., Wen J., 2014. Independent allopolyploidization events preceded speciation in the temperate and tropical woody bamboos. New Phytol, 204: 66-73. DOI:10.1111/nph.12988 |
Tyrrell C.D., Santos-Gon#231;alves A.P. Londoño X., Clark L.G., 2012. Molecular phylogeny of the arthrostylidioid bamboos (Poaceae: Bambusoideae: Bambuseae: Arthrostylidiinae) and new genus Didymogonyx. Mol. Phylogenet. Evol, 65: 136-148. DOI:10.1016/j.ympev.2012.05.033 |
Wang X.Q., Ye X.Y., Zhao L., Li D.Z., Guo Z.H., Zhuang H.F., 2017. Genome-wide RAD sequencing data provide unprecedented resolution of the phylogeny of temperate bamboos (Poaceae: Bambusoideae). Sci. Rep, 7. |
Yang H.M., Zhang Y.X., Yang J.B., Li D.Z., 2013. The monophyly of Chimonocalamus and conflicting gene trees in Arundinarieae (Poaceae: Bambusoideae) inferred from four plastid and two nuclear markers. Mol. Phylogenet. Evol, 68: 340-356. DOI:10.1016/j.ympev.2013.04.002 |
Ye X.Y., Ma P.F., Yang G.Q., Guo C., Zhang Y.X., Chen Y.M., Guo Z.H., Li D.Z., 2019. Rapid diversification of alpine bamboos associated with the uplift of the Hengduan Mountains. J. Biogeogr, 46: 2678-2689. DOI:10.1111/jbi.13723 |
Zeng C.X., Zhang Y.X., Triplett J.K., Yang J.B., Li D.Z., 2010. Large multi-locus plastid phylogeny of the tribe Arundinarieae (Poaceae: Bambusoideae) reveals ten major lineages and low rate of molecular divergence. Mol. Phylogenet. Evol, 56: 821-839. DOI:10.1016/j.ympev.2010.03.041 |
Zhang X.Z., Zeng C.X., Ma P.F., Haevermans T., Zhang Y.X., Zhang L.N., Guo Z.H., Li D.Z., 2016. Multi-locus plastid phylogenetic biogeography supports the Asian hypothesis of the temperate woody bamboos (Poaceae: Bambusoideae). Mol. Phylogenet. Evol, 96: 118-129. DOI:10.1016/j.ympev.2015.11.025 |
Zhang Y.X., Ma P.F., Haevermans T., Vorontsova M.S., Zhang T., Nanjarisoa O.P., Li D.Z., 2017. In search of the phylogenetic affinity of the temperate woody bamboos from Madagascar, with description of a new species (Bambusoideae, Poaceae). J. Systemat. Evol, 55: 453-465. DOI:10.1111/jse.12256 |
Zhang Y.X., Zeng C.X., Li D.Z., 2012. Complex evolution in Arundinarieae (Poaceae:Bambusoideae): incongruence be tween plastid and nuclear GBSSI gene phylogenies. Mol. Phylogenet. Evol, 63: 777-797. DOI:10.1016/j.ympev.2012.02.023 |

