b. Tobacco Breeding and Biotechnology Research Center, Yunnan Academy of Tobacco Agricultural Sciences, Key Laboratory of Tobacco Biotechnological Breeding, National Tobacco Genetic Engineering Research Center, Kunming, 650021, China;
c. School of Life Science, Yunnan University, Kunming, 650091, China
For 350 million years, the war between plants and insects has been raging; half of the nearly one million insect species on earth feed on plants (Gatehouse, 2002). To survive, plants have evolved complicated strategies to defend themselves against insects (Felton and Tumlinson, 2008). Coevolution of plants and insects has led to the evolution of a large number of defense-related genes and secondary metabolites that plants use in both direct and indirect defense against insect attack. Direct defenses include physical barriers and secondary metabolites such as toxins and antifeedants. Indirect defenses include volatile substances released by plants to attract natural predators of herbivorous insects.
The phytohormone jasmonic acid (JA), its precursors, and derivatives are collectively referred to as jasmonates. Jasmonates play important roles in many physiological processes, including root growth, fertility, and defense (Wasternack and Hause, 2013). Jasmonic acid (JA) is synthetized from linolenic acid, which is converted to 13-hydroperoxyliolenic acid (13-HPOT) by 13- lipoxygenase (13-LOX) in the chloroplast. 13-HPOT is further converted to oxophytodienoic acid (OPDA) by allene oxide synthase (AOS) and then allene oxide cyclase (AOC). Next, OPDA is transported into peroxisomes, where OPDA is converted to JA via 12- OPDA-reductase (OPR3) and three steps of β-oxidation (Wasternack and Hause, 2013; Wasternack and Song, 2017). JA can further conjugate with isoleucine by jasmonate-resistant1 (JAR1) to form jasmonoyl-isoleucine (JA-Ile), which is generally considered to be the only bioactive form of JA (Staswick and Tiryaki, 2004). When plants contain low levels of JA-Ile, jasmonate-zim-domain (JAZ) proteins recruit the co-repressor topless (TPL) and TPL-related proteins to form a repression complex, thereby inhibiting MYC2 from activating the expression of JA-related genes; while plants are subjected to stimuli, JA-Ile level increases, and JA-Ile associates with an F-box protein coronatine-insensitive1 (COI1), which is a part of SCFCOI, resulting in JAZ repressor complex degradation and release of MYC2 to activate the transcription of JA responsive genes (Chini et al., 2007; Thines et al., 2007; Pauwels et al., 2010).
Jasmonates play vital roles in plant resistance to herbivore attack. Herbivore attack induces JA and JA-Ile accumulation in plants (Howe and Jander, 2008). Elevated JA and JA-Ile activate transcription of defense-related genes and elevation of chemical defenses (Mithofer and Boland, 2012). For example, in Arabidopsis, insect feeding induces JA and JA-Ile, leading to increased levels of defensive metabolite glucosinolates (Mikkelsen et al., 2003). In the wild tobacco Nicotiana attenuata, feeding of the insect Manduca sexta leads to accumulation of nicotine, trypsin proteinase inhibitors, caffeoylputrescine, and malonylation of 17- hydroxygeranyllinalool diterpenoid glycosides (HGL-DTGs) (Woldemariam et al., 2013). Silencing LOX3 or JAR4/6 (JA and JA-Ile biosynthesis gene, respectively) in N. attenuata impairs resistance to insect herbivory, as the levels of anti-herbivore secondary metabolites including TPI and nicotine decrease in LOX3-or JAR4/6- silenced plants (Wang et al., 2008). Similarity, M. sexta herbivores fed the JA receptor COI1-silenced plants gain much more mass than those fed on wild-type (WT) plants (Paschold et al., 2007). Conversely, silencing two calcium-dependent protein kinases CDPK4 and CDPK5 strongly increases the level of JA/JA-Ile in N. attenuata, and these plants exhibit highly elevated levels of defensive metabolites and strong resistance to insects (Yang et al., 2012).
However, JA over-accumulation has a negative effect on plant growth and development (Wasternack and Kombrink, 2010). Thus, JA is also metabolized rapidly. JA can be catalyzed to methyl jasmonate (MeJA) by JA methyl transferase (JMT). MeJA was previously considered be a bioactive form, because its level alters during plant growth, development, and defense against herbivores (Seo et al., 2001). However, overexpression of JMT in N. attenuata increases plant susceptibility to insects, showing that MeJA is not a signal to activate insect defense system (Stitz et al., 2011). JA can also be decarboxylated to form cis-jasmone (Birkett et al., 2000). In addition, JA can be hydroxylated to 12-OH-JA, which can be further converted to 12-O-Glc-JA (TAG) or 12-OH-HSO4-JA. TAG was reported to be associate with leaf-closing movement in Samanea saman (Gidda et al., 2003; Nakamura et al., 2011). Furthermore, metabolism pathways of JA-Ile have been identified. The CYP94 subfamily, a subgroup of cytochrome P450 family, regulates JA-Ile hydroxylation. In Arabidopsis, CYP94B3 and CYP94B1 hydroxylates JA-Ile to 12-OH-JA-Ile, and CYP94C1 can further convert 12-OH-JA-Ile to 12-COOH-JA-Ile (Kitaoka et al., 2011; Koo et al., 2011; Heitz et al., 2012).
12-OH-JA occurs in various plant species. 12-OH-JA was considered an inactive form of JA, because 12-OH-JA neither induces the degradation of JAZ inhibitor protein and JA related genes expression, nor inhibits plant growth and seed germination (Miersch et al., 2008). However, 12-OH-JA induces formation of potato tubers, demonstrating that 12-OH-JA might function in some biological process (Yoshihara et al., 1989). Recent studies indicate that 12-OH-JA might be formed via two pathways. One pathway involves hydrolyzation from 12-OH-JA-Ile by amidohydrolases (AH) IAR3 and ILL6. The second pathway relies on direct oxidized from JA. In Arabidopsis, four genes from this second pathway have been identified:jasmonate-induced oxygenases 1-4 (JOX) (Caarls et al., 2017). JOXs have been shown to hydroxylate JA and repress resistance to herbivores. For example, joxQ quadruple mutant plants had enhanced resistance to the generalist caterpillar Mamestra brassicae (Caarls et al., 2017). Similarly, Smirnova et al. (2017) found that the same four hydroxylases of JA repress plant defense against the fungal infection. When plants are infected by pathogens, 12-OH-JA is mainly derived from the hydrolysis of 12- OH-JA-Ile; in contrast, when plants are subjected to wounding, 12-OH-JA is mainly produced from hydroxylation of JA (Smirnova et al., 2017).
The wild tobacco N. attenuata has been intensively studied for its defense against insects (Wang and Wu, 2013). The direct and indirect defense traits with which JA signaling influences herbivore resistance are well-known (Heiling et al., 2010) (Steppuhn et al., 2004) (Halitschke et al., 2004). Many molecular tools, including transformation systems and virus-induced gene silencing (VIGS), have been well developed. Additionally, a set of transgenic N. attenuata lines, especially JA pathway genes silenced plants, are all available. These all make N. attenuata a very attractive system for studying plant resistance to insects. Here, we cloned four JOX homologs from N. attenuata, and named them NaJOX-like-1, -2, -3, and -4. We further investigated the functions of NaJOX-like genes in planteherbivore interactions by asking why silencing JA hydroxylases enhances plant resistance to herbivore attack. In addition, we evaluated the contribution of the two biosynthetic pathways of 12- OH-JA in herbivory-induced 12-OH-JA accumulation. Given that silencing NaJOX-like genes enhances plant resistance to herbivore attack, we highlight their potential applications in generating herbivore-resistant crops.
2. Materials and methods 2.1. Plant growth and treatmentsN. attenuata seeds were originally provided by Dr. Ian T. Baldwin (Max Planck Institute for Chemical Ecology). The irJAR4/6 transgenic line was created as described previously (Wang et al., 2008). Plants were growth at 26℃/16 h light, 24℃/8 h dark, and seedlings were transferred into pots 9-10 days after germination.
For simulated herbivore feeding treatment (W + OS), each leaf was wounded by rolling a pattern wheel six times along the midvein (three times each side) and 20 μL of diluted Spodoptera litura oral secretions (diluted in water 1:2 v/v) were immediately applied to the wounds. The W + OS-induced plant response is similar to that induced by herbivore attack (Machado et al., 2015, 2016).
2.2. Generation and characterization of NaJOX-like-silenced plantsWe used virus-induced gene silencing (VIGS) technology to create plants in which NaJOX-like-1, -2, -3, and -4 were independently and simultaneously silenced. cDNA fragments of NaJOX-like- 1, -2, -3 and -4 were amplified by PCR and fused into BamHI/ HindIII-linearized pTV00 vectors. To simultaneously silence the four NaJOX-like genes, all four cDNA fragments were ligated into pTV00 by the infusion cloning method based on homologous recombination using the ClonExpress MultiS kit (Vazyme, China). The primer pairs used for plasmid construction are listed in Table S1. The Agrobacterium tumefaciens (strain GV3101)-mediated transformation procedure for VIGS was described previously (Saedler and Baldwin, 2004). Plants for VIGS experiments were grown in a greenhouse at 23℃/8 h light. To monitor the progress of VIGS, we simultaneously silenced another set of plants with a construct specific for phytoene desaturase (NaPDS), resulting in visible bleaching of green tissue. When the leaves of NaPDSsilenced plants were sufficiently bleached (~6 weeks after germination), leaves of NaJOX-like-silenced (VIGS-NaJOXs), and empty vector-inoculated (VIGS-EV) plants were used for experiments.
2.3. Quantitative real-time PCR assay (qRT-PCR)Total RNA was isolated with Trizol reagent (Invitrogen) from leaf tissue according to the manufacturer's instructions. Each cDNA sample was synthesized from 500 ng of total RNA using RevertAid H Minus Reverse Transcriptase (Thermo Fisher Scientific). All qRT-PCR experiments were performed on a CFX connect Real-Time PCR Detection System using SYBR Green (Bio-Rad). Transcript abundance was normalized using N. attenuata NaActin as an internal control. qRT-PCR assays were used for each sample with five biological replicates. Primer pairs used in qRT-PCRs are listed in Table S1.
2.4. Insect bioassayS. litura eggs were ordered from the Genralpest Company (www.genralpest.com), and were hatched in a growth chamber at 26℃/ 16 h light and 24℃/8 h dark. The hatched larvae were first fed with an artificial diet for 5 days, then eight to ten freshly hatched larvae were allowed to feed on each individual plant. Larvae mass was measured on days 7, 10, and 15.
2.5. Quantification of jasmonates12-OH-JA and[2H2]12-OH-JA were purchased from Olchemim Ltd. (http://www.olchemim.cz/). Approximately 200 mg of leaf tissue was used for JA and JA-Ile quantification as previously described (Luo et al., 2016). For quantification of 12-OH-JA, approximately 200 mg of leaf material of each sample was pulverized in liquid nitrogen, then 1 mL of extraction buffer (70% methanol with 0.1% acetic acid spiked with 10 ng[2H2]12-OH-JA as internal standards) was added. After vortexing for 10 min, the suspensions were centrifuged at 16, 100 × g and 4℃ for 15 min. Samples were evaporated to ~500 μL in a vacuum concentrator (Eppendorf) under reduced pressure at 30℃. After centrifugation at 16, 100 × g for 15 min at 4℃, 400 μL of supernatants were used for HPLC-MS/MS as described previously (Smirnova et al., 2017).
2.6. Secondary metabolite analysisNicotine and 17-hydroxygeranyllinalool diterpene glycosides were analyzed by HPLC-MS/MS as described previously (Li et al., 2017). Approximately 100 mg of leaf material was mixed with 1 mL of extraction solution (40%[v/v] methanol with 0.1%[v/v] acetic acid). After vortexing, extracts were centrifuged at 12, 000 × g and 4℃ for 20 min, and 400 μL of supernatants were used for HPLC-MS/MS. For TPI activity, approximately 100 mg of tissue was used for total protein extraction with 300 μL of extraction buffer (0.1 M Tris-HCl, pH 7.6; 5% polyvinylpolypyrrolidone, 2 mg/mL phenylthiourea, 5 mg/mL diethyldithiocarbamate, 0.05 M Na2EDTA). TPI activity was analyzed with a radial diffusion assay as described previously (van Dam et al., 2001).
3. Results 3.1. Herbivore feeding increases 12-OH-JA levelsTo clarify whether 12-OH-JA is induced in plant defense against herbivore attack, we quantified jasmonates (JA, JA-Ile, and 12-OH-JA) at several time points after simulated herbivore feeding (wounding plus S. litura oral secretions, W + OS). Consistent with previous reports (Luo et al., 2016), JA and JA-Ile levels increased rapidly after W + OS treatment, reaching peaks 1.5 h and 1 h, respectively, before quickly decreasing to control levels 6 h post treatment (Fig. 1A and B). The accumulation pattern of 12-OH-JA differed from those of JA and JA-Ile:12-OH-JA levels reached a peak at 3 h after treatment and declined slowly thereafter (Fig. 1C). Even 12 h after treatment, 12-OH-JA levels were at half the peak, indicating that 12-OH-JA is a relatively stable metabolite of JA. Herbivore attack induced the accumulation of 12-OH-JA, suggesting that it might be involved in N. attenuata resistance to herbivory.
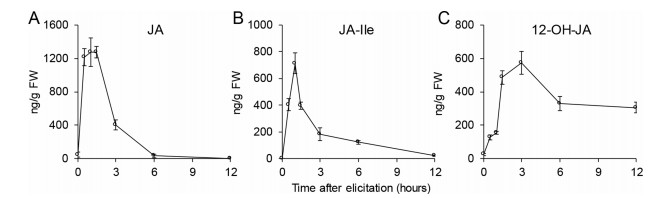
|
| Fig. 1 Herbivore feeding elevates levels of JA, JA-Ile, and 12-OH-JA. The concentrations of JA (A), JA-Ile (B), and 12-OH-JA (C) after simulated herbivore feeding (W + OS) treatment. The treated leaf tissues were harvested at 0, 0.5, 1, 1.5, 3, 6, and 12 h after treatment, the levels of jasmonic acid (JA), jasmonoyl-isoleucine (JA-Ile), and 12-hydroxy-jasmonic acid (12-OH-JA) were determined by HPLC-MS/MS. Error bars represent ± SE from five biological replicates. |
Previous research on Arabidopsis thaliana has identified four 2- oxoglutarate/Fe (II)-dependent oxygenases called jasmonateinduced oxygenases (JOX) 1-4 that hydroxylate JA to 12-OH-JA (Caarls et al., 2017). Additionally, the joxQ quadruple mutant has been shown to exhibit enhanced resistance to the cabbage moth M. brassicae. To further investigate the functions of JOX genes in plant defense against herbivores, we identified four homologs of JOX in N. attenuata using BLASTX, and named them NaJOX-like-1 to -4 (Fig. S1). We then examined their transcriptional patterns in response to simulated herbivore feeding. Transcription of NaJOXlike-1, -2, -3, and -4 increased following simulated herbivore feeding (Fig. 2). NaJOX-like-1 and NaJOX-like-2 transcript levels reached a maximum 3 h after W + OS treatment, and were about 1- and 3-fold higher than those in the untreated control plants, respectively (Fig. 2A and B). Meanwhile, the transcript levels of NaJOX-like-3 and NaJOX-like-4 reached peaks 1.5 h and 1 h after W + OS treatment, respectively, and both were about 1-fold higher than those in the untreated plants (Fig. 2C and D).

|
| Fig. 2 Transcription of NaJOX-like genes is induced by simulated herbivory. The transcriptional patterns of NaJOX-like-1 (A), NaJOX-like-2 (B), NaJOX-like-3 (C), and NaJOX-like-4 (D) in response to simulated herbivory. Fifty-day-old N. attenuata rosette-stage plants were treated with simulated herbivory. The treated leaf tissues were harvested at 0, 0.5, 1, 1.5, 3, 6, and 12 h after treatment for RNA extraction and reverse transcription. Relative expression of NaJOX-like genes was determined by qRT-PCR. Error bars represent ± SE from five biological replicates. |
To explore whether NaJOX-like-1, -2, -3, and -4 hydrolyze JA to 12-OH-JA, we used virus-induced gene silencing (VIGS) to generate plants silenced for each NaJOX-like gene. Sequence similarity between NaJOX-like-1, -2, -3, and -4 is relatively low; this enabled us to individually silence these genes by selecting gene-specific cDNA fragments (300-bp) from each NaJOX-like gene for VIGS vector construction (Fig. S2). Agrobacterium carrying either an empty vector or the constructed vectors were inoculated into N. attenuata to generate VIGS-EV and VIGS-NaJOX-like-1, -2, -3, and -4 plants. Compared with VIGS-EV plants, NaJOX-like-1, -2, -3, and -4 transcripts were 71, 82, 40, and 90% silenced in VIGS-NaJOX-like plants, respectively. At the same time, we confirmed that the four genes were independently silenced (Fig. S3). Notably, independently silencing NaJOX-like-1, -2, -3, or -4 did not affect plant growth at the rosette-stage (Fig. S4).
To determine whether NaJOX-like genes are JA hydroxylases, we measured 12-OH-JA level in VIGS-NaJOX-like-1, -2, -3, and -4 plants. Compared with VIGS-EV plants, 12-OH-JA accumulation decreased by 47, 23, 24, and 24% in VIGS-NaJOX-like-1, -2, -3, and -4 plants, respectively (Fig. 3). In addition, we found that compared with VIGS-EV plants, JA increased in VIGS-NaJOX-like-1 and VIGSNaJOX-like-3 plants (Fig. S5). These data confirm that NaJOX-like- 1, -2, -3, and -4 are JA hydroxylases. The large decrease in 12-OHJA levels in the VIGS-NaJOX-like-1 plants suggests that NaJOX-like- 1 plays a major role in hydroxylating JA.
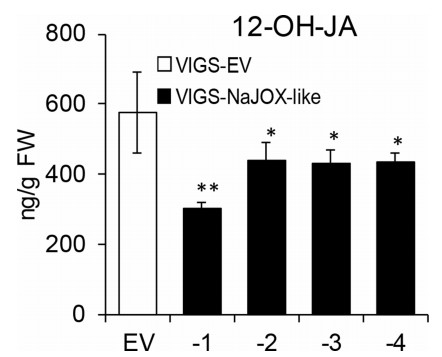
|
| Fig. 3 Independently silencing NaJOX-like genes reduces 12-OH-JA accumulation. VIGS-EV (white bars) and VIGS-NaJOX-like-1, -2, -3, and -4 (black bars) plants were treated with simulated herbivory. The treated leaf tissues were harvested 3 h after treatment and the 12-OH-JA levels were analyzed with HPLC-MS/MS. Error bars represent ± SE from five biological replicates. Asterisks indicate significant differences between VIGS-EV plants and VIGS-NaJOX-1, -2, -3, or -4 plants (Student's t -test: *, P < 0.05; **, P < 0.01). |
To investigate the function of JA hydroxylation in plant resistance to herbivores, we used VIGS to generate VIGS-NaJOXs plants by simultaneously silencing NaJOX-like-1, -2, -3, and -4. The rosette diameters of VIGS-EV and VIGS-NaJOXs plants were similar (Fig. S4). We treated VIGS-NaJOXs with W + OS, and determined the silencing efficiency of NaJOX-like-1, -2, -3, and -4 in the VIGSNaJOXs plants. Compared with VIGS-EV plants, NaJOX-like-1, -2, -3, and -4 in the VIGS-NaJOXs plants were silenced 77, 77, 67, and 70%, respectively (Fig. 4A).
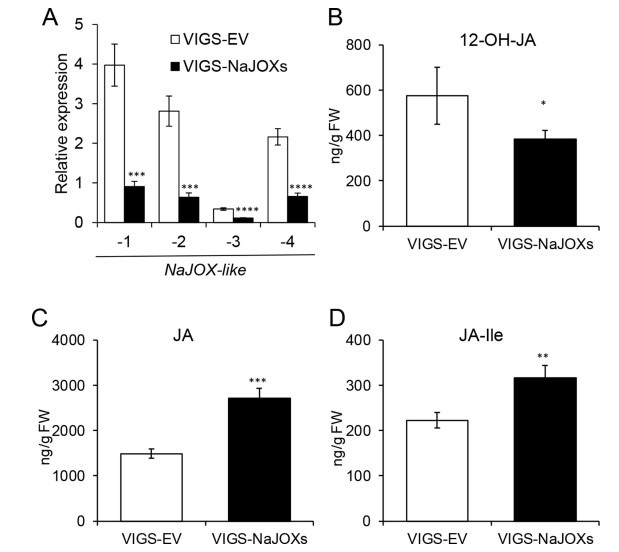
|
| Fig. 4 Simultaneously silencing NaJOX-like genes by VIGS reduces 12-OH-JA accumulation, but increases JA and JA-Ile levels. Transcripts of NaJOX-like genes were silenced in VIGS-NaJOXs plants (A). Levels of 12-OH-JA (B), JA (C), and JA-Ile (D) in VIGS-NaJOXs plants. For JA and JA-Ile measurements, leaves were harvested 1.5 h after W + OS treatment; for 12- OH-JA analysis, leaves were harvested 3 h after W + OS treatment. Error bars represent ± SE from eight biological replicates. The asterisks indicate significant differences between VIGS-EV plants and VIGS-NaJOX-1, -2, -3, or -4 plants (A) or between VIGS-EV and VIGS-NaJOXs plants (B to D) (Student's t-test: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). |
Next, the 12-OH-JA levels in VIGS-NaJOXs plants were measured after simulated herbivore feeding treatment. We found that the 12- OH-JA levels in the VIGS-NaJOXs plants decreased 33% compared with that in the VIGS-EV plants (Fig. 4B). In contrast, compared with VIGS-EV plants, JA and JA-Ile levels in VIGS-NaJOXs plants increased by 45 and 30%, respectively (Fig. 4C and D). These findings demonstrate that after insect feeding JA hydroxylation is a major source for 12-OH-JA accumulation.
12-OH-JA can be converted from 12-OH-JA-Ile by hydrolyzation (Widemann et al., 2013). To investigate whether hydrolyzation of 12-OH-JA-Ile contributes to 12-OH-JA production after herbivore attack, we measured 12-OH-JA level in irJAR4/6 plants, which are silenced for JAR4 and JAR6 genes and therefore have compromised JA-Ile and 12-OH-JA-Ile (Wang et al., 2008). The JA-Ile levels of irJAR4/6 plants were 90% lower than those of wild type plants (Fig. S6A), which is consistent with previous reports (Wang et al., 2008). In addition, the 12-OH-JA levels in irJAR4/6 plants were 34% lower than in wild type plants (Fig. S6B), suggesting that 12- OH-JA-Ile hydrolyzation and JA hydroxylation are both important pathways for 12-OH-JA production. Importantly, our finding that silencing JA hydroxylation increased JA levels by 45% (Fig. 4C) indicates that direct hydroxylation of JA by JOX is a crucial step in the herbivore-induced JA metabolic pathway.
Next, we allowed the herbivore S. litura to feed on the VIGS-EV and VIGS-NaJOXs plants, and recorded their larval mass. After 10 and 15 days of feeding, larvae that fed on VIGS-NaJOXs plants weighed much less than those fed on the VIGS-EV plants (both weighed about 36% less than controls on days 10 and 15) (Fig. 5). These results show that silencing NaJOX-like genes enhances plant resistance to herbivores, and 12-OH-JA does not induce plant defense against herbivore.
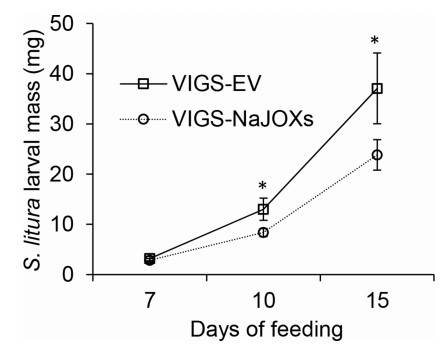
|
| Fig. 5 Simultaneously silencing NaJOX-like genes enhances N. attenuata resistance to S. litura. Freshly hatched larvae of the S. litura were placed on leaves of VIGS-EV and VIGS-NaJOXs plants. S. litura mass was measured at day 7, 10, and 15 after infestation. Error bars represent ± SE. The asterisks indicate significant differences between VIGS-EV and VIGS-NaJOXs plants (Student's t-test: *, P < 0.05; n = 20 to 30). |
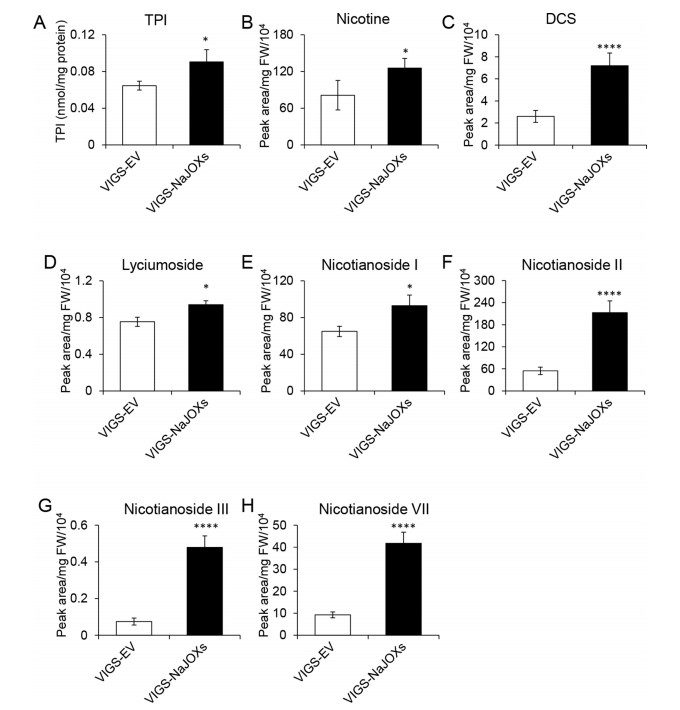
We speculated that anti-herbivore secondary metabolites were responsible for the reinforced defense in the VIGS-NaJOXs plants. To test this speculation, we determined the levels of the main defense metabolites in the VIGS-EV and VIGS-NaJOXs plants after W + OS induction. As expected, secondary metabolite accumulations were higher in VIGS-NaJOXs plants than in control plants (Fig. 6A-C). For example, dicaffepylspermidine (DCS) concentration was 64% higher in VIGS-NaJOXs plants than in control plants. Similarly, TPI activity and nicotine levels were 29% and 35% higher in VIGS-NaJOXs plants than in controls. In addition, we also examined HGL-DTGs levels, and found that lyciumoside, nicotianoside I, II, III, and VII levels in the VIGS-NaJOXs plants were 20, 30, 74, 84, and 78% higher than those in the VIGS-EV plants, respectively (Fig. 6D-H). These results indicate that enhanced herbivore resistance in VIGS-NaJOXs plants is correlated with elevated defenses.
|
| Fig. 6 Simultaneously silencing NaJOX-like genes increases direct defense. Trypsin proteinase inhibitor activity (TPI) (A), nicotine (B), dicaffepylspermidine (DCS) (C), and individual 17-hydroxygeranyllinalool diterpene glycosides (HGL-DTGs) (D-H) levels in VIGS-EV and VIGS-NaJOXs plants. VIGS-EV and VIGS- NaJOXs plants were treated with W + OS and leaf tissues were harvested after 72 h for analysis of direct defenses (n = 6 to 8; error bars represent ± SE). Asterisks represent significant differences between different plants (Student's t-test: *, P < 0.05; ****, < 0.0001). |
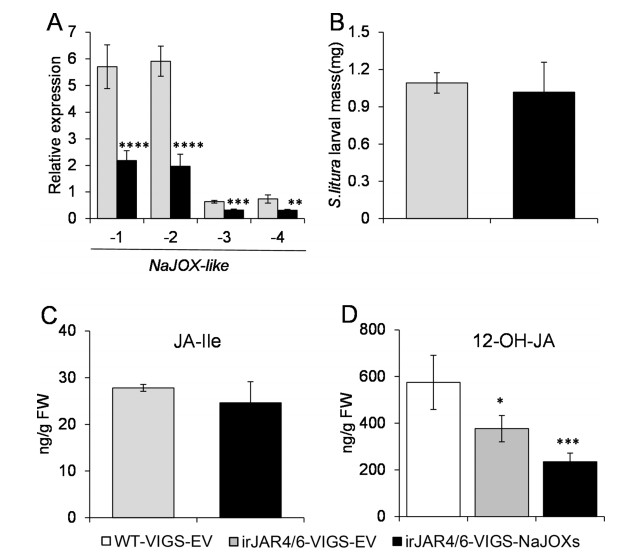
JA-Ile is generally considered the active jasmonate form that induces defense responses. Given that silencing NaJOXs increased levels of W + OS-induced JA and JA-Ile (Fig. 4), it is possible that enhanced plant resistant to S. litura resulted from the signaling effect of elevated JA-Ile. To examine this hypothesis, NaJOX-like genes were silenced by VIGS in JA-Ile deficient irJAR4/6 plants; VIGS-EV plants were also created using the irJAR4/6 plants (these plants are named irJAR4/6-VIGS-NaJOXs and irJAR4/6-VIGS-EV, respectively). NaJOX-like-1, -2, -3, and -4 transcript levels were 62, 67, 50, and 58% silenced in the irJAR4/6-VIGS-NaJOXs plants, respectively (Fig. 7A), indicating that the four NaJOX-like genes were well silenced in the irJAR4/6 background. Next, S. litura larvae were infested on the irJAR4/6-VIGS-EV and irJAR4/6-VIGS-NaJOXs plants. The larvae grown on the irJAR4/6-VIGS-EV did not gain more weight than those on the irJAR4/6-VIGS-NaJOXs plants (Fig. 7B). We also confirmed that the irJAR4/6-VIGS-EV and irJAR4/ 6-VIGS-NaJOXs plants had low JA-Ile levels (Fig. 7C). Notably, in irJAR4/6-VIGS-NaJOXs in which JA hydroxylation and the formation of JA-Ile are knocked down, the levels of 12-OH-JA decreased to 1/3, 2/3 compared to irJAR4/6-VIGS-EV and WT-VIGS-EV plants, respectively (Fig. 7D). Taken together, these results indicate that silencing NaJOXs increases herbivory-induced plant JA and JA-Ile level, and the JA-Ile is the signal that activates herbivore resistance in plants. Furthermore, both 12-OH-JA-Ile hydrolyzation and direct hydroxylation of JA are the major routes for herbivoreinduced production of 12-OH-JA.
|
| Fig. 7 Increased S. litura resistance in NaJOXs-silenced plants was caused by elevated JA-Ile levels. Silencing efficiency of NaJOX-like genes (A), S. litura larval mass (B), JA-Ile (C) and 12-OH-JA (D) levels in WT-VIGS-EV, irJAR4/6-VIGS-EV and irJAR4/6-VIGS-NaJOXs plants. In these experiment, irJAR4/6 or WT plants were used as the genetic background. irJAR4/6- VIGS-EV and irJAR4/6-VIGS-NaJOXs plants were treated with W + OS, and 1.5 h and 3 h after treatment, samples were collected for JA-Ile, 12-OH-JA quantification and NaJOX-like gene silencing efficiency test. For herbivore performance, freshly hatched S. litura larvae were infested on the leaves of irJAR4/6-VIGS-EV and irJAR4/6-VIGS-NaJOXs plants. For each group of plants, 20e30 larvae were used and S. litura mass was measured at day 7 post infestation. Error bars represent ± SE from eight biological replicates. The asterisks indicate significant differences between VIGS-EV plants and VIGS-NaJOX-1, -2, -3, or -4 plants (A) or between VIGS-EV and VIGS-NaJOXs plants (B to C) or between irJAR4/6-VIGS-NaJOXs and WT-VIGS-EV or irJAR4/6-VIGS-EV plants. (Student's t test: **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). |
12-OH-JA, which is an important metabolite of JA active in many plant species, occurs in high concentrations in some plant tissues such as unmatured soybean (Glycine max) seeds and maize (Zea mays) filaments (Miersch et al., 2008). Although previous studies have shown that 12-OH-JA may mediate the induction of potato tuber formation (Yoshihara et al., 1989), plant response to salt (Abdala et al., 2003), and interactions with AM-fungi (Pedranzani et al., 2016), the function of 12-OH-JA in plant-herbivore interactions remains largely unexplored.
Previous studies have found that 12-OH-JA is not capable of triggering the degradation of JAZ9 and does not induce expression of JAresponsive genes or inhibit root growth; thus, 12-OH-JA has been characterized as an inactive form of JA (Miersch et al., 2008). The identification of JOX genes, which encode JA hydroxylases, has facilitated the functional analysis of 12-OH-JA. Accordingly, previous studies found that JOX enzymes negatively affect many JA responses, including resistance to herbivores and necrotrophic pathogens (Caarls et al., 2017). However, it is not clear how JA hydroxylation negatively impacts plant defenses. However, it has been proposed that hydroxylation of JA can quickly inactivate JA. Our finding that simultaneously silencing four JA hydroxylases in N. attenuata increased plant resistance to the generalist herbivore S. litura is consistent with previous studies that found that Arabidopsis joxQ mutants show enhanced M. brassicae resistance. Importantly, we demonstrate that the enhanced herbivore resistance resulted from elevated JA-Ile levels. In our experiments, when we simultaneously silenced all four JA hydroxylases in the JA-Ile deficient irJAR4/6 background, the enhanced herbivore resistance vanished (Fig. 7B).
Simulated herbivory studies have shown that JA reaches a peak level around 1 h after elicitation (Howe and Jander, 2008; Luo et al., 2016). However, we found that the catabolism of JA is also rapid, the concentration of JA returning to control levels within 6 h after elicitation (Fig. 1A). Blocking JA hydroxylation greatly increases levels of JA and JA-Ile, indicating that hydroxylation is a major catabolic pathway of JA and plays a crucial role in maintaining the homeostasis of JA and JA-Ile in plants (Fig. 4C and D).
12-OH-JA formation has two putative ways:12-OH-JA-Ile hydrolyzation or direct hydroxylation of JA (Wasternack and Strnad, 2018). A previous study has shown that when plants are infected by the pathogen Botrytis cinerea, 12-OH-JA is mainly derived from the hydrolysis of 12-OH-JA-Ile. However, when plants are subjected to wounding, 12-OH-JA is mainly produced from hydroxylation of JA (Smirnova et al., 2017). Here, we found that in N. attenuata response to herbivore attack the two pathways are equally important for the accumulation of 12-OH-JA. 1) Simulta-neously silencing four JA hydroxylase genes led to about 1/3 decrease in 12-OH-JA peak levels (Fig. 4B); 2) in irJAR4/6 plants, in which JA-Ile synthesis is greatly impaired, and thus 12-OH-JA-Ile levels are highly decreased (Luo et al., 2016), simulated herbivoryinduced 12-OH-JA levels were also decreased by about 1/3 (Fig. S6); 3) furthermore, simultaneously knocking down JA hydroxylation and the formation of JA-Ile by silencing JA hydroxylase in irJAR4/6 plants led to a 66% decrease in 12-OH-JA level (Fig. 7D).
JOXs are common in land plants, including monocots (Kawai et al., 2014). The negative effect that JOX genes have on plant resistance to insects makes these genes prime targets for genome modification.
Declaration of Competing InterestThe authors declare no conflict of interest.
AcknowledgementsThis work was supported by the Key Project of Applied Basic Research Program of Yunnan (2017FA015), the Young Academic and Technical Leader Raising Foundation of Yunnan Province (no. 2017HB063), and the Yunnan Academy of Tobacco Agricultural Sciences (2018530000241002 and 2019530000241003). We also thank the Biotechnology Experimental Center at the Kunming Institute of Botany, CAS, for supporting plant cultivation.
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2019.11.005.
Abdala G., Miersch O., Kramell R., Vigliocco A., Agostini E., Forchetti G., Alemano S., 2003. Jasmonate and octadecanoid occurrence in tomato hairy roots.Endogenous level changes in response to NaCl. Plant Growth Regul, 40: 21-27. |
Birkett M.A., Campbell C.A., Chamberlain K., Guerrieri E., Hick A.J., Martin J.L., Matthes M., Napier J.A., Pettersson J., Pickett J.A., et al, 2000. New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc.Natl.Acad.Sci.U.S.A, 97(16): 9329-9334. DOI:10.1073/pnas.160241697 |
Caarls L., Elberse J., Awwanah M., Ludwig N.R., de Vries M., Zeilmaker T., Van Wees S.C.M., Schuurink R.C., Van den Ackerveken G., 2017. Arabidopsis jasmonate-induced oxygenases down-regulate plant immunity by hydroxylation and inactivation of the hormone jasmonic acid. Proc.Natl.Acad.Sci.U.S.A, 114(24): 6388-6393. DOI:10.1073/pnas.1701101114 |
Chini A., Fonseca S., Fernandez G., Adie B., Chico J.M., Lorenzo O., GarciaCasado G., Lopez-Vidriero I., Lozano F.M., Ponce M.R., et al, 2007. The JAZ family of repressors is the missing link in jasmonate signalling.. Nature, 448(7154): 666-671. DOI:10.1038/nature06006 |
Felton G.W., Tumlinson J.H., 2008. Plant-insect dialogs: complex interactions at the plant-insect interface. Curr.Opin.Plant Biol, 11(4): 457-463. DOI:10.1016/j.pbi.2008.07.001 |
Gatehouse J.A., 2002. Plant resistance towards insect herbivores: a dynamic interaction. New Phytol, 156(2): 145-169. DOI:10.1046/j.1469-8137.2002.00519.x |
Gidda S.K., Miersch O., Levitin A., Schmidt J., Wasternack C., Varin L., 2003. Biochemical and molecular characterization of a hydroxyjasmonate sulfotransferase from Arabidopsis thaliana. J.Biol.Chem, 278(20): 17895-17900. DOI:10.1074/jbc.M211943200 |
Halitschke R., Ziegler J., Keinanen M., Baldwin I.T., 2004. Silencing of hydroperoxide lyase and allene oxide synthase reveals substrate and defense signaling crosstalk in Nicotiana attenuata. Plant J, 40(1): 35-46. DOI:10.1111/j.1365-313X.2004.02185.x |
Heiling S., Schuman M.C., Schoettner M., Mukerjee P., Berger B., Schneider B., Jassbi A.R., Baldwin I.T., 2010. Jasmonate and ppHsystemin regulate key Malonylation steps in the biosynthesis of 17-Hydroxygeranyllinalool Diterpene Glycosides, an abundant and effective direct defense against herbivores in Nicotiana attenuata.. Plant Cell, 22(1): 273-292. DOI:10.1105/tpc.109.071449 |
Heitz T., Widemann E., Lugan R., Miesch L., Ullmann P., Desaubry L., Holder E., Grausem B., Kandel S., Miesch M., et al, 2012. Cytochromes P450 CYP94C1 and CYP94B3 catalyze two successive oxidation steps of plant hormone Jasmonoylisoleucine for catabolic turnover. J.Biol.Chem, 287(9): 6296-6306. DOI:10.1074/jbc.M111.316364 |
Howe G.A., Jander G., 2008. Plant immunity to insect herbivores. Annu.Rev.Plant Biol, 59(20): 41-66. |
Kawai Y., Ono E., Mizutani M., 2014. Evolution and diversity of the 2-oxoglutaratedependent dioxygenase superfamily in plants. Plant J, 78(2): 328-343. DOI:10.1111/tpj.12479 |
Kitaoka N., Matsubara T., Sato M., Takahashi K., Wakuta S., Kawaide H., Matsui H., Nabeta K., Matsuura H., 2011. Arabidopsis CYP94B3 encodes jasmonyl-L-isoleucine 12-hydroxylase, a key enzyme in the oxidative catabolism of jasmonate. Plant Cell Physiol, 52(10): 1757-1765. DOI:10.1093/pcp/pcr110 |
Koo A.J., Cooke T.F., Howe G.A., 2011. Cytochrome P450 CYP94B3 mediates catabolism and inactivation of the plant hormone jasmonoyl-L-isoleucine. Proc.Natl.Acad.Sci.U.S.A, 108(22): 9298-9303. DOI:10.1073/pnas.1103542108 |
Li R., Wang M., Wang Y., Schuman M.C., Weinhold A., Schafer M., JimenezAleman G.H., Barthel A., Baldwin I.T., 2017. Flower-specific jasmonate signaling regulates constitutive floral defenses in wild tobacco. Proc.Natl.Acad.Sci.U.S.A, 114(34): E7205-E7214. DOI:10.1073/pnas.1703463114 |
Luo J., Wei K., Wang S., Zhao W., Ma C., Hettenhausen C., Wu J., Cao G., Sun G., Baldwin I.T., et al, 2016. COI1-regulated hydroxylation of Jasmonoyl-Lisoleucine Impairs Nicotiana attenuata's resistance to the generalist herbivore Spodoptera litura. J.Agric.Food Chem, 64(14): 2822-2831. DOI:10.1021/acs.jafc.5b06056 |
Machado R.A., Arce C.C., Ferrieri A.P., Baldwin I.T., Erb M., 2015. Jasmonatedependent depletion of soluble sugars compromises plant resistance to Manduca sexta. New Phytol, 207(1): 91-105. DOI:10.1111/nph.13337 |
Machado R.A.R., Robert C.A.M., Arce C.C.M., Ferrieri A.P., Xu S.Q., JimenezAleman G.H., Baldwin I.T., Erb M., 2016. Auxin is rapidly induced by herbivore attack and regulates a subset of systemic, Jasmonate-dependent defenses. Plant Physiol, 172(1): 521-532. |
Miersch O., Neumerkel J., Dippe M., Stenzel I., Wasternack C., 2008. Hydroxylated jasmonates are commonly occurring metabolites of jasmonic acid and contribute to a partial switch-off in jasmonate signaling. New Phytol, 177(1): 114-127. |
Mikkelsen M.D., Petersen B.L., Glawischnig E., Jensen A.B., Andreasson E., Halkier B.A., 2003. Modulation of CYP79 genes and glucosinolate profiles in Arabidopsis by defense signaling pathways. Plant Physiol, 131(1): 298-308. |
Mithofer A., Boland W., 2012. Plant defense against herbivores: chemical aspects. Annu.Rev.Plant Biol, 63: 431-450. DOI:10.1146/annurev-arplant-042110-103854 |
Nakamura Y., Mithofer A., Kombrink E., Boland W., Hamamoto S., Uozumi N., Tohma K., Ueda M., 2011. 12-hydroxyjasmonic acid glucoside is a COI1-JAZindependent activator of leaf-closing movement in Samanea saman. Plant Physiol, 155(3): 1226-1236. |
Paschold A., Halitschke R., Baldwin I.T., 2007. Co(i)-ordinating defenses: NaCOI1 mediates herbivore- induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. Plant J, 51(1): 79-91. DOI:10.1111/j.1365-313X.2007.03119.x |
Pauwels L., Barbero G.F., Geerinck J., Tilleman S., Grunewald W., Perez A.C., Chico J.M., Bossche R.V., Sewell J., Gil E., et al, 2010. NINJA connects the corepressor TOPLESS to jasmonate signalling.. Nature, 464(7289): 788-791. DOI:10.1038/nature08854 |
Pedranzani H., Rodr#237;guez-Rivera M., Gutiérrez M., Porcel R., Hause B., Ruiz-Lozano M., J ., 2016. Arbuscular mycorrhizal symbiosis regulates physiology and performance of Digitaria eriantha plants subjected to abiotic stresses by modulating antioxidant and jasmonate levels. Mycorrhiza, 26(2): 141-152. DOI:10.1007/s00572-015-0653-4 |
Saedler R., Baldwin I.T., 2004. Virus-induced gene silencing of jasmonate-induced direct defences, nicotine and trypsin proteinase-inhibitors in Nicotiana attenuata. J.Exp.Bot, 55(395): 151-157. |
Seo H.S., Song J.T., Cheong J.J., Lee Y.H., Lee Y.W., Hwang I., Lee J.S., Choi Y.D., 2001. Jasmonic acid carboxyl methyltransferase: a key enzyme for jasmonateregulated plant responses. Proc.Natl.Acad.Sci.U.S.A, 98(8): 4788-4793. DOI:10.1073/pnas.081557298 |
Smirnova E., Marquis V., Poirier L., Aubert Y., Zumsteg J., Menard R., Miesch L., Heitz T., 2017. Jasmonic acid oxidase 2 hydroxylates jasmonic acid and represses basal defense and resistance responses against Botrytis cinerea infection.. Mol.Plant, 10(9): 1159-1173. DOI:10.1016/j.molp.2017.07.010 |
Staswick P.E., Tiryaki I., 2004. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis.. Plant Cell, 16(8): 2117-2127. DOI:10.1105/tpc.104.023549 |
Steppuhn A., Gase K., Krock B., Halitschke R., Baldwin I.T., 2004. Nicotine's defensive function in nature. PLoS Biol, 2(8): E217. DOI:10.1371/journal.pbio.0020217 |
Stitz M., Gase K., Baldwin I.T., Gaquerel E., 2011. Ectopic expression of AtJMT in Nicotiana attenuata: creating a metabolic sink has tissue-specific consequences for the jasmonate metabolic network and silences downstream gene expression. Plant Physiol, 157(1): 341-354. |
Thines B., Katsir L., Melotto M., Niu Y., Mandaokar A., Liu G., Nomura K., He S.Y., Howe G.A., Browse J., 2007. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling.. Nature, 448(7154): 661-665. DOI:10.1038/nature05960 |
van Dam N.M., Horn M., Mares M., Baldwin I.T., 2001. Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. J.Chem.Ecol, 27(3): 547-568. |
Wang L., Allmann S., Wu J., Baldwin I.T., 2008. Comparisons of LIPOXYGENASE3-and JASMONATE-RESISTANT4/6-silenced plants reveal that jasmonic acid and jasmonic acid-amino acid conjugates play different roles in herbivore resistance of Nicotiana attenuata. Plant Physiol, 146(3): 904-915. |
Wang L., Wu J., 2013. The essential role of jasmonic acid in plant-herbivore interactions–using the wild tobacco Nicotiana attenuata as a model. J.Genet.Genom, 40(12): 597-606. DOI:10.1016/j.jgg.2013.10.001 |
Wasternack C., Hause B., 2013. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development.An update to the 2007 review in Ann. Bot, 111(6): 1021-1058. |
Wasternack C., Kombrink E., 2010. Jasmonates: structural requirements for lipidderived signals active in plant stress responses and development. ACS Chem.Biol, 5(1): 63-77. DOI:10.1021/cb900269u |
Wasternack C., Song S., 2017. Jasmonates: biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J.Exp.Bot, 68(6): 1303-1321. |
Wasternack C., Strnad M., 2018. Jasmonates: news on occurrence, biosynthesis, metabolism and action of an ancient group of signaling compounds. Int.J.Mol.Sci, 19(9). |
Widemann E., Miesch L., Lugan R., Holder E., Heinrich C., Aubert Y., Miesch M., Pinot F., Heitz T., 2013. The amidohydrolases IAR3 and ILL6 contribute to jasmonoyl-isoleucine hormone turnover and generate 12-hydroxyjasmonic acid upon wounding in Arabidopsis leaves. J.Biol.Chem, 288(44): 31701-31714. DOI:10.1074/jbc.M113.499228 |
Woldemariam M.G., Dinh S.T., Oh Y., Gaquerel E., Baldwin I.T., Galis I., 2013. NaMYC2 transcription factor regulates a subset of plant defense responses in Nicotiana attenuata. BMC Plant Biol, 13(5): 73. |
Yang D.H., Hettenhausen C., Baldwin I.T., Wu J., 2012. Silencing Nicotiana attenuata calcium-dependent protein kinases, CDPK4 and CDPK5, strongly upregulates wound- and herbivory-induced jasmonic acid accumulations. Plant Physiol, 159(4): 1591-1607. |
Yoshihara T., Omer E.A., Koshino H., Sakamura S., Kikuta Y., Koda Y., 1989. Structure of a tuber-inducing stimulus from potato leaves (Solanum-Tuberosum-L). Agric.Biol.Chem, 53(10): 2835-2837. |



