b. Yunnan Key Laboratory of Dai and Yi Medicines, Yunnan University of Chinese Medicine, Kunming, China;
c. College of Chinese Material Medica, Yunnan University of Chinese Medicine, Kunming, China;
d. Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, China
Temperature variation is one of the most important environmental factors that influences the geographical distribution of plant species, and exposure of plants to temperatures beyond optimum limits their growth and yield. The ability of plants to tolerate extreme temperatures may be enhanced by pre-treating plants with a period of moderately low or high temperature (Sung et al., 2003). However, different plant species show different abilities to tolerate moderate temperature stresses, even temperatures close to the environmental conditions of their grandparent or parent (Groot et al., 2017). The duration of these temperature treatments also influences the growth of plants, and long-term moderately low or high temperature may inhibit growth or even cause death of some plant species (Tang et al., 2016; Zheng et al., 2016). Understanding the tolerance of a plant to long-term moderate temperature stresses is critical for the introduction of economically important plant species, such as crops, flowers, and medicinal plants.
Membranes are sensitive to environmental changes. Therefore, to tolerate low or high temperature stress, plants must maintain the integrity and fluidity of membranes (Moellering et al., 2010; Murakami et al., 2000). Membrane fluidity and integrity are largely determined by glycerolipids, which also influence membrane function. Previous studies have shown that short-term extreme temperature stresses, such as freezing and heat shock, cause considerable glycerolipid degradation and increased production of phosphatidic acid (PA) and lysophospholipids (LPLs) (Li et al., 2004; Welti et al., 2002; Zheng et al., 2016; Zheng et al., 2011). Studies have also shown that chloroplastidic lipids are more sensitive to extreme temperature stresses than are extraplastidic lipids, which are largely degraded after freezing stress (Li et al., 2004; Zheng et al., 2016), and this may further cause the decline of photosynthesis.
Some studies have investigated the response of the glycerolipidome to short-term moderate temperature stresses. For example, after long-term moderately low temperature treatments, Arabidopsis accumulate massive long-chain unsaturated triacylglycerides (TAG), while the level of total membrane lipids is unaffected (Degenkolbe et al., 2012). However, exposure of Arabidopsis to short-term (6 h) moderately low (4℃) and high (32℃) temperatures has little effect on the composition of glycerolipids (Burgos et al., 2011). Furthermore, after three days of cold acclimation (4℃), Arabidopsis tend to synthesize more monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG), while phospholipids are unaffected; lipid species with more polyunsaturated acyl chains alsoincrease, while saturated lipid species decline significantly (Welti et al., 2002). We have also investigated plant lipidomic responses to long-term heat(35℃, 22days) and found thatthere are two phases of lipidome change during long-term heat treatment (Tang et al., 2016). We found that plants cope with long-term high (45℃) and low (-7℃) temperature treatments by adjusting the composition of glycerolipidsand maintaining saturation ofglycerolipids(Zheng et al., 2011). Research on tomato has shown that short-term (6 days) treatmentat high (38℃)temperature remodelthylakoid membranes (Spicher et al., 2016). However, little is known about the different responses of plants lipid profiles to long-term moderately high and low temperatures. Chloroplastidic lipids, such as MGDG, DGDG, SQDG, and PG are the main components of chloroplast membranes and are indispensable for the efficiency of photosynthesis. They play important roles in the stabilization of photosynthetic complexes, membrane architecture, and thylakoid stack formation (Dörmann, 2013). Temperature changes affect the synthesis and degradation of chloroplastidic lipids and thereafter the process of photosynthesis (Tang et al., 2016; Zheng et al., 2016). Heat-tolerant plants tend to synthesize more DGDG after long-term heat treatment than do heatsensitive plants, which may contribute to their heat-tolerant ability (Su et al., 2009). Temperature-stress-induced decline in photosynthesis may be partly attributed to the degradation of plastidic lipids (Tang et al., 2016; Zheng et al., 2016). The lipid-to-chlorophyll ratio is an effective way to estimate the protein-packing density in thylakoids-a high lipid-to-chlorophyll ratio reflects a low protein-packing density (Kirchhoff et al., 2013). However, little is known about the differences in chloroplastidic lipid remodeling between long-term treatments with high and low temperatures, and the relationships of these differences with variation in photosynthesis.
Panax notoginseng is a perennial herb belonging to the family Araliaceae and is a well-known traditional Chinese medicine rich in saponins. The average annual growth temperature in its habitat, Wenshan, Yunnan Province, China, is about 16-22℃ (Wang, 2006). P. notoginseng seeds have been shown to germinate better at 10℃ than at 20 or 30℃ (Zhou et al., 2012). However, little is known about the ability of this traditional Chinese medicine to tolerate long-term moderately high and low temperatures. In the present study, we treated P. notoginseng with temperatures of 10, 20, and 30℃ for 30 days and investigated the response of the glycerolipidome through an ESI-MS/MS based lipidomics method. In this study, our aim was three-fold. First, we asked how the P. notoginseng glycerolipidome is remodeled by long-term exposure to high and low temperatures. Second, we investigated whether changes in photosynthesis after long-term exposure to cold temperature are mediated by changes in the glycerolipidome. Finally, we determined which membrane lipid saturation levels were most sensitive to long-term moderate temperature stresses.
2. Materials and methods 2.1. Plant material and treatmentsSeeds of P. notoginseng were purchased from Dali Junfeng Biological Technology Co., Ltd. China. Seeds were sown in October at the Kunming Botanical Garden greenhouse (25°8'27"N, 102°44'18"E). Nine months after sowing, P. notoginseng plants were transferred to growth incubators under 12-h light/12-h dark (120 μmol m-2 s-1 light), 60% relative air humidity, and 20℃. After incubation for two weeks, the temperature of the incubators was set to 10, 20, and 30℃, respectively. Thirty days after initiation of temperature treatments, the plants were harvested for photosynthesis and lipidomic tests.
2.2. Determination of maximum photochemical efficiency of photosystem IIChlorophyll fluorescence was measured using an IMAGING-PAM chlorophyll fluorometer and the ImagingWin software application (Walz, Effeltrich, Germany). Five leaves of each treatment were used for measuring chlorophyll fluorescence. After dark adaptation for 15 min, minimum chlorophyll fluorescence yield in the dark-adapted state (F0) was recorded. Plants were then exposed to a saturating pulse of light (>1800 μmol photons m-2 s-1), and maximum chlorophyll fluorescence yield in the dark-adapted state (Fm) was recorded. Maximum photochemical efficiency of photosystem II (Fv/Fm) was calculated as (Fm-F0)/Fm. False-color images were produced using Imaging Win software according to the degree of the Fv/Fm parameter.
2.3. Determination of photosynthetic pigmentsPhotosynthetic pigments were determined, with minor modifications, according to the method of Minocha et al. (2009). Briefly, about 0.1 g of fresh leaves were cut and immediately put into 3 mL N, N-dimethylformamide and stored at 4℃ overnight. The absorbance (480, 647, and 664 nm) of the extract was determined using a SpectraMax Plus 384 Microplate Reader (Molecular Devices, Wokingham, UK). After extraction, the leaves were dried at 80℃ and weighed to determine dry weight. The concentration of photosynthetic pigments was calculated using the following equations:Chl a (μg/mL)=12.00 × A664-3.11 × A647; Chl b (μg/ mL)=20.78 × A647-4.88 × A664; [Carotenoid]=(1000 × A480-1.12 ×[Chla]-34.07 ×[Chlb])/245. These values were then converted to mg/g dry weight leaf.
2.4. Lipidomic analysisLipidomic analysis was performed according to our previous studies (Zheng et al., 2016) (Kansas Lipidomics Research Center, http://www.k-state.edu/lipid/lipidomics). To inhibit lipolytic activity, fresh leaves of P. notoginseng were cut and added to 3 mL of 75℃ isopropanol (with 0.01% butylated hydroxytoluene, m/v) for 15 min. After cooling, 0.6 mL deionized water and 1.5 mL chloroform were added to the isopropanol and vortexed for 1 h. The leaves were then extracted with chloroform/methanol (2:1, v/v) until they turned white. After extraction, the leaves were dried at 105℃ and the dry mass was recorded. The lipid extract was dried under N2 gas for analysis. There were five replicates for each treatment.
2.5. Statistical proceduresOne-way ANOVA and principal component analysis (PCA) were performed using SPSS 13.0. Significance was calculated using Fisher's least significant difference (LSD). The double-bond index (DBI) was calculated as follows:DBI=(∑[N × mol% lipid])/100, where N is the number of double bonds and acyl carbons in each lipid molecule, respectively (Bakht et al., 2006; Zheng et al., 2016).
3. Results 3.1. Photosynthesis of P. notoginseng was more sensitive to longterm heat treatmentTo investigate different effects of long-term chilling (LTC) and long-term heat (LTH) on P. notoginseng, we treated the plants with temperatures of 10, 20, and 30℃ for 30 days and examined the Fv/ Fm of leaves. We found that both LTC and LTH treatment induced a decline in Fv/Fm in P. notoginseng, and this decline was more severe after LTH treatment (Fig. 1). Some leaflets withered after LTH treatment. These results indicate that P. notoginseng is more sensitive to LTH than to LTC, and LTH treatment may accelerate the senescence of P. notoginseng.
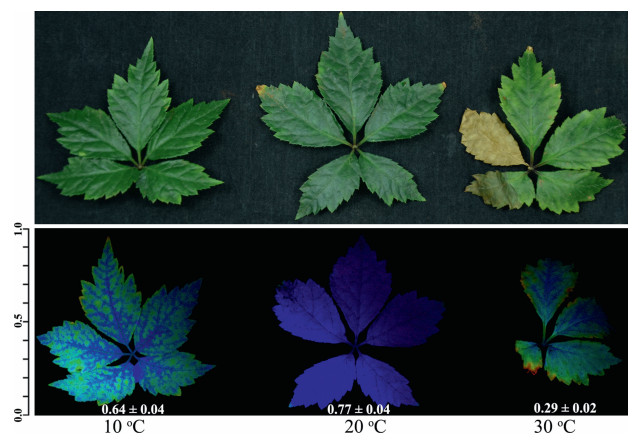
|
| Fig. 1 Effects of 30-day temperature treatments on phenotype (upper panel) and Fv/Fm fluorescence (lower panel) of Panax notoginseng leaves. The color bar indicates the Fv/Fm values. Inserted data is Fv/Fm value. Values are means ± SD (n = 5). |
Total photosynthetic pigments from P. notoginseng leaves were extracted and measured to determine the different effects of LTC and LTH on the content of photosynthetic pigments (Table 1). A significant decrease in chlorophyll a (Chl a), chlorophyll b (Chl b) and carotenoid was observed for P. notoginseng after both LTC and LTH treatment (Table 1). The decrease in Chl a observed in LTH-treated plants was more significant than that in LTC-treated plants. However, the ratio of Chl a/Chl b was maintained. These results indicate that long-term temperature stresses induced synchronic degradation of Chl a and Chl b, and that photosynthesis declined because photosynthetic pigments were degraded.
| Photosynthetic pigments (mg/g DW) |
Treatment | ||
| 10 ℃ | 20 ℃ | 30 ℃ | |
| Chl a | 7.23 ± 0.77b | 9.27 ± 1.31a | 5.99 ± 0.54c |
| Chl b | 3.45 ± 0.31b | 4.97 ± 0.92a | 3.12 ± 0.84b |
| Carotenoid | 1.88 ± 0.16b | 2.25 ± 0.28a | 1.72 ± 0.21b |
| Chl a + Chl b | 10.67 ± 1.05b | 14.24 ± 2.00a | 9.11 ± 1.09c |
| Chl a/Chl b | 2.10 ± 0.12a | 1.90 ± 0.28a | 1.99 ± 0.32a |
| DW, dry weight. Values in the same line with different superscript letters are statistically significant (p < 0.05). Values are means ± SD (n = 9 or 10). | |||
The effects of different long-term temperature stress treatments on the absolute levels of glycerolipids were clarified using PCA; the two principal components explained 61.46% and 15.09% of the overall variance (Fig. 2). The loadings of PC1 identified MGDG, SQDG, phos-phatidylinositol (PI), and phosphatidylserine (PS) as being most important for the separation, whereas for PC2, lysophosphatidyle-thanolamine (LPE) was identified (Table S1). According to the PC1, plants treated at 30℃ were separated from those treated at 10 and 20℃. PC2 represented the lysophospholipids (LPLs), products of glycerolipid hydrolysis through phospholipase A (PLA). Along this axis, some of the plants treated at20℃ were separated from the other samples, which may indicate that lipid degradation may occur after long-term moderately low and high temperature stresses.
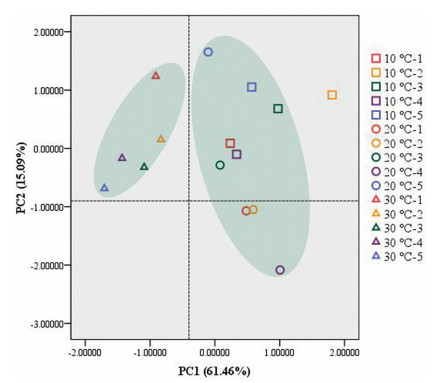
|
| Fig. 2 Principal component analysis (PCA) of lipid composition following different long-term temperature treatments of P. notoginseng. Squares represent 10 ℃ treatment, circles represent 20 ℃ treatment, and triangles represent 30 ℃ treatment. |
We employed a lipidomic analysis method based on ESI-MS/MS to investigate the glycerolipidome response of P. notoginseng to longterm (30 days) moderately high or low temperature treatment.
LTC treatment did not cause significant changes in the total amount of DGDG, MGDG, SQDG, PG, phosphatidylcholine (PC), phosphatidylethanolamine (PE), PI, or PS. Sub-zero low temperatures induced the synthesis of glycolipids with large head groups, such as trigalactosyldiacylglycerol (TrDG) and tetragalactosyldiacylglycerol (TeDG) (Moellering et al., 2010). However, cold did not induce the synthesis of DGDG, TrDG or TeDG; furthermore, the level of TrDG decreased after LTC treatment (data not shown). LTC induced an increase in PA (Fig. 3). This increase in PA levels contrasts with the changes in LPLs levels, which were unaltered by LTC. PA is a product of phospholipase D (PLD), whereas LPLsare products of phospholipase A (PLA).Thus, these findings suggest that PLD is more active than PLAat long-term moderately low temperature treatments.
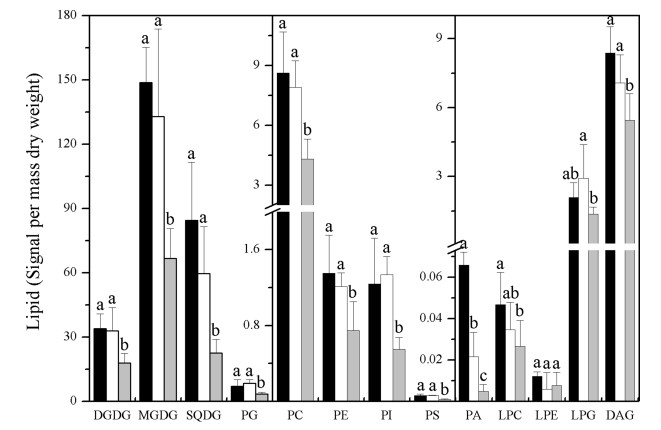
|
| Fig. 3 Total amount of lipid of each head-group class in P. notoginseng after treatment with long-term (30 days) low temperature (4 ℃, black bars), moderate temperature (20 ℃, blank bars), and high temperature (30 ℃, gray bars). Bars marked with different letters are significantly different from the others (p < 0.05). Values are means ± SD (n = 5). |
LTH treatment caused considerable degradation of glycerolipids in P. notoginseng, and the degradation of chloroplastidic lipids was similar to that of extraplastidic lipids (Fig. 3). This is different from results seen under short-term extreme temperature stresses, in which chloroplastidic lipids are degraded greatly (Zheng et al., 2016). During the LTH treatment, SQDG was the most degraded of the plastidic lipids, decreasing to 62.20% of the levels of the control. PC was the group of extraplastidic lipids that decreased the most, by more than 65% (Table S2). The levels of PA and lysophosphati-dylglycerol (LPG) declined after LTH treatment (Fig. 3), whereas under extreme temperature treatment, levels of these lipids greatly increased (Li et al., 2004). LTH also caused a considerable decrease in diacylglycerol (DAG) (Fig. 3), the most important precursor of glycerolipid synthesis. These results indicate that both an increase in degradation and a decrease in synthesis may contribute to the decrease in glycerolipids after LTH treatment. PLD and PLA may not participate in the process of long-term heat-induced glycerolipid decrease.
3.5. Influence of long-term temperature stresses on protein-packing density in thylakoids in P. notoginsengGlycerolipids, proteins, and photosynthetic pigments are the main components of photosynthetic organs, and the ratio of lipid to chlorophyll is a good indicator of protein-packing density. A high lipid-to-chlorophyll ratio reflects a low protein-packing density (Kirchhoff et al., 2013; Wang et al., 2014). The ratios of both DGDG to chlorophyll and SQDG to chlorophyll increased significantly after LTC treatment, whereas ratios of MGDG and PG to chlorophyll were unchanged. After LTH treatment, ratios of all plastidic lipids to chlorophyll were unchanged (Table 2). These results indicate that the decrease of Fv/Fm in LTC-treated plants may contribute to the low protein-packing density in thylakoids. LTH induced synchronic degradation of plastidic lipids and photosynthetic pigments.
| Lipid-to-chlorophyll ratio | Treatment | ||
| 10 ℃ | 20 ℃ | 30 ℃ | |
| MGDG-Chlorophyll | 3.20 ± 0.71a | 2.42 ± 0.87a | 2.16 ± 0.70a |
| DGDG-Chlorophyll | 14.11 ± 2.43a | 9.75 ± 3.17b | 7.97 ± 2.12b |
| SQDG-Chlorophyll | 7.96 ± 2.52a | 4.36 ± 1.64b | 2.72 ± 1.03b |
| PG-Chlorophyll | 0.65 ± 0.27a | 0.62 ± 0.16a | 0.42 ± 0.12a |
| The ratio (mmol/g) was calculated from the total MGDG and DGDG content (nmol signal per mass dry weight) and the chlorophyll content (mg/g dry weight). Values are means ± SD (n = 5). Values followed by the different letters within a line are significantly different (p < 0.05). | |||
To gain a detailed profile of glycerolipid molecules, we profiled more than 140 molecular species of membrane glycerolipids in P. notoginseng after long-term temperature stresses. P. notoginseng contains both 34:6 and 36:6 MGDG molecules (Fig. 4), which means that it is a 16:3 plant that harbors both prokaryotic and eukaryotic pathways of lipid synthesis.
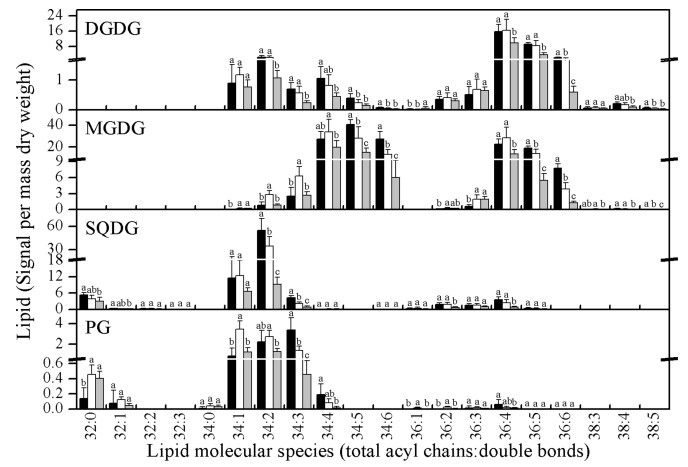
|
| Fig. 4 Changes in the molecular species of chloroplast lipids in P. notoginseng after treatment with long-term (30 days) low temperature (4 ℃, black bars), moderate temperature (20 ℃, blank bars), and high temperature (30 ℃, gray bars). Bars marked with different letters are significantly different from the others (p < 0.05). Values are means ± SD (n = 5). |
Overall (Figs. 4 and 5), the levels of most DGDG, SQDG, PI, and PS molecular species were maintained after LTC treatment.However, the glycerolipid molecular species MGDG, PG, PC, andPEwereremodeled after LTC treatment. Among these changes, glycerolipids with more double bonds showed increased abundance, e.g., 34:5 MGDG, 34:6 MGDG, 34:3 PG, 36:4 PC, 36:6 PC, and 36:4 PE, whereas glycerolipids with fewer double bonds decreased, e.g., 34:2 MGDG, 34:3 MGDG, 32:0PG, 34:1PG, 34:1PC, 36:1PC, 36:3PC, 34:1PE, and 36:1PE.These changes may be an important way to increase the fluidity of membranes under low temperatures. These results indicate that through adjustments of MGDG, PG, PC, and PE, P. notoginseng is able to respond to long-term chilling stresses.
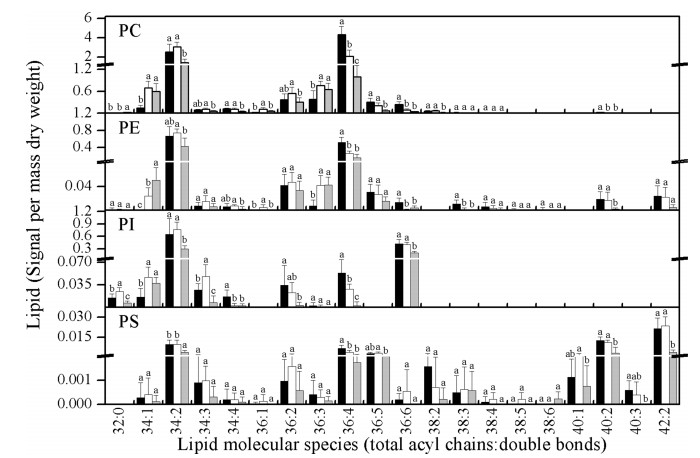
|
| Fig. 5 Changes in the molecular species of endoplasmic reticulum lipids in P. notoginseng after treatment with long-term (30 days) low temperature (4 ℃, black bars), moderate temperature (20 ℃, blank bars), and high temperature (30 ℃, gray bars). Bars marked with different letters are significantly different from the others (p < 0.05). Values are means ± SD (n = 5). |
The changes in glycerolipid molecular species levels under LTH treatment were different from those under LTC. Most glycerolipids decreased after LTH treatment, whereas some remained unchanged (Figs. 4 and 5). Lipid amounts for 36:6 DGDG, 34:6 MGDG, 36:6 MGDG, 34:2 SQDG, 34:3 PG, 36:4 PC, 36:4 PE, and 36:6 PI, which were the most abundant glycerolipids, decreased. These decreases may contribute most importantly to the decrease in the total content of these lipid classes. Meanwhile, levels of 34:1 MGDG, 36:3 MGDG, 32:0 PG, 34:0 PG, 34:1 PC, and 34:1 PI remained unchanged. These results may indicate that LTH-induced degradation of highly unsaturated glycerolipids may decrease the fluidity of membranes and make the membranes more rigid; this may contribute to LTH-accelerated senescence of P. notoginseng.
Levels of most LPL molecules were maintained after both LTC and LTH treatments, except for 18:2 lysophosphatidylcholine (LPC) and 16:1 LPG. 18:2 LPC increased after LTC treatment. 16:1 LPG decreased after LTH treatment, and this decrease contributed to the decrease in total LPG (Figs. 3 and 6). Surprisingly, the level of LPG in P. notoginseng was higher than in other species, being more than 100 times that seen in Arabidopsis, rice, Crucihimalaya himalaica, and Saussurea medusa, while the level of LPE was five times lower than that observed in these species (Zheng et al., 2011, 2016). LPGs that contain 16 carbons are the most abundant LPG molecules. We suspect that the PLA that hydrolyzes PG in P. notoginseng may be more effective than that found in other species.

|
| Fig. 6 Changes in the molecular species of lysophospholipids in P. notoginseng after treatment with long-term (30 days) low temperature (4 ℃, black bars), moderate temperature (20 ℃, blank bars), and high temperature (30 ℃, gray bars). Bars marked with different letters are significantly different from the others (p < 0.05). Values are means ± SD (n = 5). |
After LTC treatment, the double bond index (DBI) of most glycerolipids increased, except for those of PI and PS, which did not show any statistically significant difference (Table 3). The DBI increase in extraplastidic lipids was more than that seen in plastidic lipids. Among plastidic lipids, the DBI of PG increased the most, by about 47.95%; in extraplastidic lipids, the DBI of PC increased by about 22.92%, which was the greatest increase among these lipids (Table 3). The DBI of most glycerolipids was unchanged after LTH treatment, except for DGDG and SQDG, which decreased by 2.83 and 11.28%, respectively. These results indicate that P. notoginseng may be able to adjust the degree of saturation of the most abundant glycerolipids to maintain the fluidity of the membrane under LTC treatment. During moderately high temperature-induced glycerolipid degradation, oxidative stress may not be the main injury to membranes.
| Lipid class | Treatment | RC (%) | DBI change | ||||||
| 10 ℃ | 20 ℃ | 30 ℃ | 10 ℃ | 30 ℃ | 10 ℃ | 30 ℃ | |||
| DBI | |||||||||
| DGDG | 4.16 ±0.03a | 4.06 ±0.05b | 3.94 ±0.06c | 2.53 | -2.83 | 0.10 | -0.12 | ||
| MGDG | 4.84 ±0.12a | 4.45 ±0.06b | 4.41 ±0.14b | 8.80 | -0.81 | 0.39 | -0.04 | ||
| SQDG | 1.91 ±0.06a | 1.81 ±0.07b | 1.60 ±0.05c | 5.54 | -11.28 | 0.10 | -0.21 | ||
| PG | 2.42 ±0.19a | 1.64 ±0.08b | 1.52 ±0.11b | 47.95 | -7.29 | 0.78 | -0.12 | ||
| CPL | 3.81 ±0.05a | 3.67 ±0.10b | 3.62 ±0.11b | 3.81 | -1.38 | 0.14 | -0.05 | ||
| PC | 3.29 ±0.09a | 2.68 ±0.11b | 2.58 ±0.11b | 22.92 | -3.86 | 0.61 | -0.1 | ||
| PE | 2.92 ±0.08a | 2.53 ±0.09b | 2.49 ±0.07b | 15.57 | -1.43 | 0.39 | -0.04 | ||
| PI | 3.55 ±0.41a | 3.22 ±0.29a | 3.37 ±0.20a | 10.31 | 4.82 | 0.33 | 0.15 | ||
| PS | 2.42 ±0.09a | 2.34 ±0.07a | 2.51 ±0.19a | 3.43 | 7.47 | 0.08 | 0.17 | ||
| EPL | 3.27 ±0.11a | 2.73 ±0.07b | 2.64 ±0.11b | 19.78 | -3.30 | 0.54 | -0.09 | ||
| PA | 3.16 ±0.22a | 2.43 ±0.69b | 2.17 ±0.55b | 29.88 | -10.54 | 0.73 | -0.26 | ||
| DAG | 2.99 ±0.07a | 2.61 ±0.07b | 2.52 ±0.04c | 14.77 | -3.26 | 0.38 | -0.09 | ||
| DBI =(∑[N × mol% lipid])/100, N is the total number of double bonds in the two fatty acid chains of each glycerolipid molecules. CPL represents chloroplastidic lipids which contains MGDG, DGDG, SQDG and PG. EPL represents extraplastidic lipids that contain PC, PE, PI and PS. The relative change (RC) in DBI at 10 and 30 ℃ is the percentage value for the difference between the DBI at 10 and 30 ℃ over the DBI at 20 ℃. Values in the same row marked with different superscript letters are significantly different (p < 0.05). Values are means ± SD (n =5). | |||||||||
The response of plants to long-term moderate temperature stresses is very important for the introduction of economically important plants, for example, medicinal plants. Adjustment of glycerolipid composition and saturation to maintain the integrity and fluidity of membranes is one of the most important ways plants cope with temperature stresses. However, little is known about glycerolipidome remodeling after long-term moderately low and high temperature treatment, and the relationship between glycerolipidome changes and changes in photosynthesis. In the present study, we used P. notoginseng, a traditional Chinese medicine, to investigate the responses of membrane lipids to long-term (30 days) chilling (10℃) and heat (30℃) temperature stresses.
The environmental temperature prevailing during growth of their parent or grandparent may influence the ability of plants to tolerate temperature stresses (Groot et al., 2017). The P. notoginseng plants used in this study were cultivated in Wenshan, Yunnan, China. The average temperature across the growth season is about 20℃, and the annual average temperature is about 16℃. Zhou et al. found that the germination of P. notoginseng seeds is accelerated at temperatures of 10℃ and inhibited at 30℃ (Zhou et al., 2012). In our experiment, treatment with both 10 and 30℃ induced a decline in photosynthesis of P. notoginseng, whereas the 30℃ treatment accelerated the senescence of leaves. P. notoginseng preferred the relatively low temperature rather than the high temperature during cultivation.
Photosynthesis is one of the most sensitive cellular processes and is immediately impaired under temperature stresses (Wahid et al., 2007). It is well known that extreme temperatures can induce the serious degradation of glycerolipids and a decline in photosynthesis (Ploschuk et al., 2014; Tang et al., 2016; Welti et al., 2002). Among the glycerolipids we tested, MGDG, DGDG, SQDG, and PG are the main components of chloroplast membranes, which were defined as plastidic lipids; and PC, PE, PI, PS are the main components of other membranes, which were defined as extraplastidic lipids. The content and composition of plastidic lipids are of great importance for the integrity and function of photosystems (Krumova et al., 2010). Deficiency in the synthesis of DGDG, MGDG, and PG could induce a decline in chlorophyll and thereafter the ability of photosynthesis (Jarvis et al., 2000; Xu et al., 2002). The degradation of plastidic lipids is one of the most prominent changes during extreme temperature treatment and other abiotic stresses, and this degradation may disturb the structure of photosystems and lead to a decline in photosynthesis (Li et al., 2008; Tang et al., 2016; Zheng and Li, 2017). In our study, both LTC and LTH caused a decrease in photosynthetic activity and photo pigments in P. notoginseng. LTH also induced serious degradation of both plastidic and extraplastidic lipids, suggesting that degradation of glycerolipids and photo pigments may have contributed to the decline of photosynthesis and senescence of leaves. In contrast, LTC had little effect on levels of glycerolipids. Notably, degradation of plastidic lipids did not occur following LTC. Long-term moderately low temperature stresses and short-term extreme temperature stresses affect photosynthesis differently. Short-term extreme temperature stresses cause chaotic degradation of lipids, proteins, and genetic materials, and thereafter the impairment of photosystems and decline in photosynthesis. Long-term moderately low temperature stresses decrease photosynthesis not by remodeling plastidic lipids, but instead by decreasing photo pigments and the protein-packing density in thylakoids.
Different lipid classes have different head groups, and the composition of these lipids is closely correlated with the integrity and function of membranes. PC and PE are the main extraplastidic lipids, while MGDG and DGDG are the main plastidic lipids. PC and DGDG, harboring a large polar head group, tend to form bilayer membranes. In contrast, MGDG and PE, which have relatively small head groups, tend to form non-bilayer HII-type membranes (Hazel and Williams, 1990). The ratio of PC/PE and DGDG/MGDG has been found to increase in plants in response to freezing; this is very important for plants to maintain the integrity of membranes under sub-zero temperatures (Moellering et al., 2010; Welti et al., 2002). During our long-term moderately low temperature treatment of P. notoginseng, the ratio of PC/PE and DGDG/MGDG was maintained (Table S2). We suspect that the mechanism P. notoginseng has adopted to maintain the integrity of membranes after LTC treatment differs from that employed by other species during sub-zero temperature stresses (Moellering et al., 2010; Welti et al., 2002). In our experiment, after LTC treatment, the ratio of SQDG/PG increased significantly (Table S2), and this increase was attributed to an increase in SQDG and a decrease in PG. Among the four plastidic lipids, SQDG and PG are negatively charged lipids, in contrast to the non-charged MGDG and DGDG. PG can substitute for most SQDG functions, whereas SQDG cannot entirely compensate for PG functions (Shimojima, 2011). Furthermore, both SQDG and PG are sensitive to the availability of phosphate. Therefore, we suspect that P. notoginseng tends to synthesize more SQDG to maintain the anionic surface charge of thylakoid membranes due to the decrease in PG after LTC treatment. The decrease in PG and increase in SQDG may cause a decline in Fv/Fm, because, as stated previously, PG can substitute for most of the SQDG function, whereas SQDG cannot entirely compensate for PG function (Shimojima, 2011).
Phospholipases are the main enzymes that participate in the degradation of phospholipids. Phospholipases can be divided into four groups, PLA, phospholipase B (PLB), phospholipase C (PLC), and phospholipase D (PLD) (Aloulou et al., 2012). PLA, PLC, and PLD are the main phospholipases in higher plants, and the products of these phospholipases are LPL, DAG, and PA (Wang et al., 2012). These products are very important messengers in cells and participate in plant stress responses (Testerink and Munnik, 2005). PLB also has lysophospholipase activity, which is similar to PLA. PLB exists in some fungal species, bacteria, and in mammalian cells (Aloulou et al., 2012). In our previous study and other reports, the levels of these LPLs were not species specific and were detected at similar levels in Arabidopsis thaliana, C. himalaica, S. medusa, Solms-Lau-bachia linearifolia, and Oryza sativa (Zheng et al., 2011, 2016). In P. notoginseng, the contents of LPC and LPE were similar to those in other species, whereas the content of LPG was about 200 times that of other species, and the main LPG contained 16 C (16:0 and 16:1). These findings suggest that in P. notoginseng, the activity of PLA is very strong and specifically degrades PG, or that another phospholipase, such as PLB, may act.
Changing the degree of unsaturation of glycerolipids to maintain the fluidity of membranes is important for plant responses to long-term temperature stresses (Zheng et al., 2011, 2016). Photosynthesis is one of the most sensitive processes influenced by temperature stresses. The high degree of unsaturation of chloroplast lipids is critical for the fluidity of chloroplast membranes and the low temperature tolerance of photosynthesis (Webb and Green, 1991). Changing the content of trienoic fatty acid through transgenic methods may influence the fluidity and membrane organization of chloroplast membranes and further change the thermosensitivity of plants (Murakami et al., 2000). We suspect that under long-term moderate temperature stresses the fluidity of plastidic membrane is more sensitive than that of extraplastidic membranes. Surprisingly, we found that the DBI of all plastidic lipids was maintained after both LTC and LTH treatment, whereas the DBI of extraplastidic lipids increased after LTC treatment and decreased after LTH treatment (Table 3). These results indicate that fluidity of membranes other than the chloroplast membrane may be affected the most severely after longterm moderate temperature stresses. Because the plastidic lipids have a high DBI, chloroplast membranes can keep their fluidity under LTC or LTH treatments. Meanwhile, extraplastidic lipids have a relatively low DBI; therefore, the membrane fluidity is low and needs to adjust to maintain the biochemical reactions on these membranes.
PG is the major phospholipid in chloroplast membranes and an important constituent of photosystems (Nishihara et al., 1980). It plays important roles in the development of thylakoid membranes, biogenesis of the photosystem I (PS I) complex, and the electron transport in photosystem II (Droppa et al., 1995; Sato et al., 2004). The most abundant PG molecules in P. notoginseng leaves have acyl chains of 32 or 34 carbons (Fig. 4). Most of the sn2-positions of PG are 16:0 or 16:1 fatty acids (Nishihara et al., 1980). PG molecules with 32 acyl carbons, such as 16:0/16:0 (32:0) and 16:0/16:1 (3 t) (32:1), are high-melting-point molecules, and these lipids decrease under low-temperature stresses (Sakamoto et al., 2004). 34:1 and 34:2 PG are extraplastidic lipids, whereas 34:4 PG are plastidic lipids that form part of the plastidic membranes (Jia et al., 2013). The saturation of PG is closely correlated with the composition and contents of these lipid molecules, and further influences the activity of photosynthesis (Ivanov et al., 2012; Sakamoto et al., 2004). Increases in the proportion of saturated PG in transgenic tobacco have been shown to slow the rate of damage and repair of the D1 protein at low temperatures (Moon et al., 1995). Sakamoto et al. (2004) also found that decreases in cis-unsaturated PG caused chilling sensitivity in tobacco. Ivanov et al. (2012) found that a decrease in the unsaturation of PG in tobacco could accelerate the photoinhibition of PS I under low temperatures. Among the four chloroplastidic lipids in our study, the increase in the DBI of PG was the greatest after LTC treatment. This increase in the DBI of PG may mostly be attributed to the decrease in high-meltingpoint PG and the increase in PG species having three or four double bonds, which may be localized in the chloroplast membranes. This change in DBI was also attributed to a decrease in PG with fewer double bonds (32:0 and 34:1 PG) and an increase in PG with more double bonds (34:3 and 34:4 PG) (Fig. 4). We speculate that a decrease in high-melting-point and extraplastidic PG and an increase in plastidic PG, which further increases the unsaturation of PG in P. notoginseng under long-term low temperatures, may have important roles in maintaining the function of photosystems. This indicates that under long-term moderately low temperature stresses, saturation of PG molecules rather than other plastidic lipids may be more important for the function of photosynthesis of photosystems I and II of P. notoginseng.
5. ConclusionsBy treating P. notoginseng with temperatures of 10, 20, and 30℃ for 30 days, we found that P. notoginseng is more sensitive to high temperatures than to low temperatures. LTH treatment induced synchronous degradation of both photo pigments and glycerolipids, and thereafter the decline of photosynthesis and senescence of leaves. LTC caused a decrease in photo pigments and protein-packing density in thylakoids and may contribute to the decline of Fv/Fm. P. notoginseng is a 16:3 plant that harbors both prokaryotic and eukaryotic pathways of lipid synthesis. It has an especially high level of LPG and can cope with LTC treatment through adjustment of saturation of extraplastidic membranes rather than plastidic membranes. Regulation of PG saturation is very important for the function of photosystems of P. notoginseng under LTC treatment.
Declaration of Competing InterestThe authors declare no competing financial interest.
AcknowledgmentsThis work was supported by grants from National Natural Science Foundation of China (31560085, 81460581 and 31600215), High Level Talents Project of Yunnan University of Chinese Medicine (2019YZG07), Natural Science Fund of Yunnan Province (2017FG001), Yunnan Applied Basic Research Project (2016FA042, 2017FB057 and 2015FB171), Innovation Guidance and Scientific and Technological Enterprise Cultivation Plan in Yunnan Province (2017RA001). We thank Kansas Lipidomics Research Center for performing the lipidomic analysis.
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2019.11.002.
Aloulou, A., Ali, Y.B., Bezzine, S., Gargouri, Y., Gelb, M.H., 2012. Phospholipases: an overview. In: Sandoval, G. (Ed.), Lipases and Phospholipases. Humana Press, New York, pp. 63-85.
|
Bakht J., Bano A., Dominy P., 2006. The role of abscisic acid and low temperature in chickpea (Cicer arietinum) cold tolerance.II.Effects on plasma membrane structure and function. J.Exp.Bot, 57: 3707-3715. DOI:10.1093/jxb/erl120 |
Burgos A., Szymanski J., Seiwert B., Degenkolbe T., Hannah M.A., Giavalisco P., et al, 2011. Analysis of short-term changes in the Arabidopsis thaliana glycerolipidome in response to temperature and light. Plant J, 66: 656-668. DOI:10.1111/j.1365-313X.2011.04531.x |
Dörmann, P., 2013. Galactolipids in plant membranes. In: eLS. John Wiley & Sons, Ltd, Chichester, pp. 1-6.
|
Degenkolbe T., Giavalisco P., Zuther E., Seiwert B., Hincha D.K., Willmitzer L., 2012. Differential remodeling of the lipidome during cold acclimation in natural accessions of Arabidopsis thaliana. Plant J, 72: 972-982. DOI:10.1111/tpj.12007 |
Droppa M., Horvath G., Hideg E., Farkas T., 1995. The role of phospholipids in regulating photosynthetic electron transport activities: treatment of thylakoids with phospholipase C. Photosynth.Res, 46: 287-293. DOI:10.1007/BF00020442 |
Groot M.P., Kubisch A., Ouborg N.J., Pagel J., Schmid K.J., Vergeer P., et al, 2017. Transgenerational effects of mild heat in Arabidopsis thaliana show strong genotype specificity that is explained by climate at origin. New Phytol, 215: 1221-1234. DOI:10.1111/nph.14642 |
Hazel J.R., Williams E.E., 1990. The role of alterations in membrane lipidcomposition in enabling physiological adaptation of organisms to their physical-environment. Prog.Lipid Res, 29: 167-227. DOI:10.1016/0163-7827(90)90002-3 |
Ivanov A.G., Allakhverdiev S.I., Huner N.P.A., Murata N., 2012. Genetic decrease in fatty acid unsaturation of phosphatidylglycerol increased photoinhibition of photosystem I at low temperature in tobacco leaves. Biochim.Biophys.Acta Bioenerg, 1817: 1374-1379. DOI:10.1016/j.bbabio.2012.03.010 |
Jarvis, P., Dormann, P., Peto, C.A., Lutes, J., Benning, C., Chory, J., 2000. Galactolipid deficiency and abnormal chloroplast development in the Arabidopsis MGD synthase 1 mutant. In: Proceedings of the National Academy of Sciences of the United States of America, 97, pp. 8175-8179.
|
Jia Y., Tao F., Li W., 2013. Lipid profiling demonstrates that suppressing Arabidopsis phospholipase Ddelta retards ABA-promoted leaf senescence by attenuating lipid degradation.. PLoS One, 8: e65687. DOI:10.1371/journal.pone.0065687 |
Kirchhoff H., Sharpe R.M., Herbstova M., Yarbrough R., Edwards G.E., 2013. Differential mobility of pigment-protein complexes in granal and agranal thylakoid membranes of C3 and C4 Plants.. Plant Physiology, 161: 497-07. DOI:10.1104/pp.112.207548 |
Krumova S.B., Laptenok S.P., Kovacs L., Toth T., van Hoek A., Garab G., et al, 2010. Digalactosyl-diacylglycerol-deficiency lowers the thermal stability of thylakoid membranes. Photosynth.Res, 105: 229-242. DOI:10.1007/s11120-010-9581-5 |
Li W., Li M., Zhang W., Welti R., Wang X., 2004. The plasma membrane-bound phospholipase Dδ enhances freezing tolerance in Arabidopsis thaliana. Nat.Biotechnol, 22: 427-433. DOI:10.1038/nbt949 |
Li W., Wang R., Li M., Li L., Wang C., Welti R., et al, 2008. Differential degradation of extraplastidic and plastidic lipids during freezing and post-freezing recovery in Arabidopsis thaliana. J.Biol.Chem, 283: 461-468. DOI:10.1074/jbc.M706692200 |
Minocha R., Martinez G., Lyons B., Long S., 2009. Development of a standardized methodology for quantifying total chlorophyll and carotenoids from foliage of hardwood and conifer tree species. Can.J.For.Res, 39: 849-861. DOI:10.1139/X09-015 |
Moellering E.R., Muthan B., Benning C., 2010. Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane.. Science, 330: 226-228. DOI:10.1126/science.1191803 |
Moon, B.Y., Higashi, S.I., Gombos, Z., Murata, N., 1995. Unsaturation of the membrane-lipids of chloroplasts stabilizes the photosynthetic machinery against low-temperature photoinhibition in transgenic tobacco plants. In: Proceedings of the National Academy of Sciences of the United States of America, 92, pp. 6219-6223.
|
Murakami Y., Tsuyama M., Kobayashi Y., Kodama H., Iba K., 2000. Trienoic fatty acids and plant tolerance of high temperature.. Science, 287: 476-479. DOI:10.1126/science.287.5452.476 |
Nishihara M., Yokota K., Kito M., 1980. Lipid molecular species composition of thylakoid membranes.. Biochim.Biophys.Acta, 617: 12-19. DOI:10.1016/0005-2760(80)90219-2 |
Ploschuk E.L., Bado L.A., Salinas M., Wassner D.F., Windauer L.B., Insausti P., 2014. Photosynthesis and fluorescence responses of Jatropha curcas to chilling and freezing stress during early vegetative stages. Environ.Exp.Bot, 102: 18-26. DOI:10.1016/j.envexpbot.2014.02.005 |
Sakamoto A., Sulpice R., Hou C.X., Kinoshita M., Higashi S.I., Kanaseki T., et al, 2004. Genetic modification of the fatty acid unsaturation of phosphatidylglycerol in chloroplasts alters the sensitivity of tobacco plants to cold stress. Plant Cell Environ, 27: 99-105. DOI:10.1046/j.0016-8025.2003.01131.x |
Sato N., Suda K., Tsuzuki M., 2004. Responsibility of phosphatidylglycerol for biogenesis of the PSI complex. Biochim.Biophys.Acta Bioenerg, 1658: 235-243. DOI:10.1016/j.bbabio.2004.06.008 |
Shimojima M., 2011. Biosynthesis and functions of the plant sulfolipid. Prog.Lipid Res, 50: 234-239. DOI:10.1016/j.plipres.2011.02.003 |
Spicher L., Glauser G., Kessler F., 2016. Lipid antioxidant and galactolipid remodeling under temperature stress in tomato plants. Front.Plant Sci, 7. |
Su K., Bremer D.J., Jeannotte R., Welti R., Yang C., 2009. Membrane lipid composition and heat tolerance in cool-season Turfgrasses, including a hybrid Bluegrass. J.Am.Soc.Hortic.Sci, 134: 511-520. DOI:10.21273/JASHS.134.5.511 |
Sung D.Y., Kaplan F., Lee K.J., Guy C.L., 2003. Acquired tolerance to temperature extremes. Trends Plant Sci, 8: 179-187. DOI:10.1016/S1360-1385(03)00047-5 |
Tang T., Liu P.L., Zheng G.W., Li W.Q., 2016. Two phases of response to long-term moderate heat: variation in thermotolerance between Arabidopsis thaliana and its relative Arabis paniculata.. Phytochemistry, 122: 81-90. DOI:10.1016/j.phytochem.2016.01.003 |
Testerink C., Munnik T., 2005. Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci, 10: 368-375. DOI:10.1016/j.tplants.2005.06.002 |
Wahid A., Gelani S., Ashraf M., Foolad M.R., 2007. Heat tolerance in plants: an overview. Environ.Exp.Bot, 61: 199-223. DOI:10.1016/j.envexpbot.2007.05.011 |
Wang, G., Ryu, S., Wang, X., 2012. Plant phospholipases: an overview. In: Sandoval, G. (Ed.), Lipases and Phospholipases. Humana Press, New York, pp. 123-137.
|
Wang S., Uddin M.I., Tanaka K., Yin L., Shi Z., Qi Y., et al, 2014. Maintenance of chloroplast structure and function by overexpression of the rice MONOGA-LACTOSYLDIACYLGLYCEROL SYNTHASE gene leads to enhanced salt tolerance in tobacco.. Plant Physiol, 165: 1144-1155. DOI:10.1104/pp.114.238899 |
Wang, Y., 2006. Yunnan Mountain Climate. Yunnan Science and Technology Press, Kunming, Yunnan.
|
Webb M.S., Green B.R., 1991. Biochemical and biophysical properties of thylakoid acyl lipids.. BBA- Bioenergetics, 1060: 133-158. DOI:10.1016/S0005-2728(09)91002-7 |
Welti R., Li W., Li M., Sang Y., Biesiada H., Zhou H.-E., et al, 2002. Profiling membrane lipids in plant stress responses: role of phospholipase Dα in freezing-induced lipid changes in Arabidopsis. J.Biol.Chem, 277: 31994-32002. DOI:10.1074/jbc.M205375200 |
Xu C., H#228;rtel H., Wada H., Hagio M., Yu B., Eakin C., et al, 2002. The pgp 1 mutant locus of Arabidopsis encodes a phosphatidylglycerol phosphate synthase with impaired activity.. Plant Physiology, 129: 594-604. DOI:10.1104/pp.002725 |
Zheng G., Li W., 2017. Profiling membrane glycerolipids during gamma-rayinduced membrane injury. BMC Plant Biol, 17: 203. DOI:10.1186/s12870-017-1153-9 |
Zheng G.W., Li L.X., Li W.Q., 2016. Glycerolipidome responses to freezing- and chilling-induced injuries: examples in Arabidopsis and rice. BMC Plant Biol, 16: 70. DOI:10.1186/s12870-016-0758-8 |
Zheng G.W., Tian B., Zhang F.J., Tao F.Q., Li W.Q., 2011. Plant adaptation to frequent alterations between high and low temperatures: remodelling of membrane lipids and maintenance of unsaturation levels. Plant Cell Environ, 34: 1431-1442. DOI:10.1111/j.1365-3040.2011.02341.x |
Zhou J., Kulkarni M.G., Huang L.Q., Guo L.P., Van Staden J., 2012. Effects of temperature, light, nutrients and smoke-water on seed germination and seedling growth of Astragalus membranaceus, Panax notoginseng and Magnolia officinalis - highly traded Chinese medicinal plants. South Afr.J.Bot, 79: 62-70. DOI:10.1016/j.sajb.2011.11.004 |



