b. University of Chinese Academy of Sciences, Beijing, 100049, China;
c. Department of Biology & Environment, Faculty of Natural Sciences, University of Haifa-Oranim, Tivon, 36006, Israel
Terrestrial biodiversity is dominated by plants and the herbivores that consume them. Herbivory is the most important channel for energy and material flow from autotrophic plants to higher trophic levels (Futuyma and Agrawal, 2009). For example, on average, more than 10% of the annual plant biomass produced in natural communities is consumed by herbivores (Coley et al., 1985). In response to herbivory, plants have evolved defenses that operate in a variety of ways. Plant defense can be divided into physical defenses (Lucas et al., 2000; Hanley et al., 2007), chemical defenses (Mithofer and Boland, 2012), visual defenses (e.g., aposematism, camouflage, and masquerade; Lev-Yadun, 2016; Niu et al., 2017; Quicke, 2017), biological defenses (Kessler and Baldwin, 2001; Halpern et al., 2007), and statistical defenses (mast fruiting, flowering once in 100 years or so, being rare) (Janzen, 1976; Kelly and Sork, 2002). Herbivores tend to consume plants with low defense levels (Charles-Dominique et al., 2016), or develop a variety of antidefense traits (e.g., exploiting orally secreted bacteria to suppress plant defenses; Chung et al., 2013), eventually promoting species diversification (Futuyma and Agrawal, 2009). Therefore, interactions between plants and herbivores have profound effects on community structure and ecological functions. A better understanding of plante-herbivore interactions is particularly important for studying the form and maintenance of biodiversity. The role of chemical defenses in plante-herbivore interactions has been better studied and is more understood than that of physical defenses. Yet, most land plants rely at least in part on physical anti-herbivory defenses, including external structures that deter herbivores such as trichomes, thorns, prickles, and spines (Hanley et al., 2007).
Spinescence is a plant trait that refers to the presence of any sharp appendages or pointed tip on any part of the plant, including spines (a stiff, slender, sharp-pointed structure arising in a leaf from below the epidermis), thorns (a stiff woody modified branch), and prickles (a sharp outgrowth of the epidermis or bark) (Harris and Harris, 2001). Spinescence can wound mouths, digestive systems (Daniel and Paul, 1982) and other herbivore body parts, and can infect large herbivores with pathogenic bacteria (Halpern et al., 2007). Many spiny plants are visually aposematic (express warning coloration) through their spine colors and associated coloration (Lev-Yadun, 2001, 2016; Lev-Yadun and Ne'eman, 2006; Lev-Yadun et al., 2018). Spinescence has been thought to reduce herbivory by deterring consumption (Lev-Yadun, 2001, 2016; Midgley et al., 2016), and by reducing bite size (Cooper and Owen-Smith, 1986; Milewski et al., 1991; Ford et al., 2014). The size of spines, as well as the fact that invertebrates are infrequently damaged by spines (e.g., Yamazaki et al., 2014), suggests that spinescence evolved in response to vertebrates, especially large mammals (Young and Okello, 1998; Barton, 2016). Various studies have also found that spinescence sometimes performs additional functions. For example, in some plants, spines play important roles in thermal regulation and cooling (Nobel, 1983), the reduction of both water loss (Burns, 2016) and radiation flux (Nobel, 1988), and propagule dispersal (Gibson and Nobel, 1986), while in other plants spines function as climbing implements (Putz, 1990). Some authors have found that spinescence can structure grazed short grass steppe plant communities by providing refuge to less defended plant species (Rebollo et al., 2002). Nevertheless, most spinescence has been thought to have evolved as a defense against vertebrate herbivores (Hanley et al., 2007).
Several hypotheses have been proposed to explain the presence of spiny defenses in plants. For example, plants are known to make trade-offs between growth, reproduction and defense (Zust et al., 2015; Zust and Agrawal, 2017). These trade-offs may explain why some plant life-forms allocate limited resources to defense by spinescence (Raunkiaer, 1934; Ronel and Lev-Yadun, 2012). Alternatively, Lev-Yadun (e.g., 2001, 2009a, b, c, 2016, 2019) has recently proposed that spines may function not only to mechanically defend plants, but also may act as aposematic (warning) signals to potential vertebrate herbivores. Other studies have indicated that the presence of spines, thorns, and prickles may be partly related to phylogeny (Ronel and Lev-Yadun, 2012; Bagella et al., 2018; Zhang and Yao, 2018). However, determining which of these potential factors best explains the presence of spines in plants is difficult. Although previous studies on spines have included quantitative studies (Young et al., 2003; Ronel et al., 2007; Midgley et al., 2016), as well as investigations into ontogenetic (Lev-Yadun, 2003a; Burns, 2016) and geographical distribution patterns (Grubb, 1992; Tindall et al., 2017; Song et al., 2019), studies on spinescence in local and specific geographical areas are very rare, and relevant data are scarce (Ronel and Lev-Yadun, 2012; Bagella et al., 2018; Zhang and Yao, 2018).
To address these issues, we studied spinescence in the flora of the Jiaozi Snow Mountain, a nature reserve in southwestern China characterized by rich plant biodiversity. In this study, we asked three questions:(1) Did spinescence evolve in specific plant life forms and plant organs? (2) Is spinescence coloration aposematic in the flora of Jiaozi Snow Mountain? (3) Have phylogenetic position and phytogeographical origin affected the expression of spinescence?
Materials and methods Survey of research areaThe Jiaozi Snow Mountain is located in north-central Yunnan, China. Jiaozi Snow Mountain is the highest mountain in China east of the Qinghai-Tibet and the Yunnan-Guizhou Plateaus. In addition, it is one of the highest mountains at this latitude in the northern hemisphere. Its peak, which rises from a dry-hot valley (about 1100 m a.s.l.), is 4344 m a.s.l. (Peng and Liu, 2015). Because of its huge elevational range (more than 3000 m) and complex topography, Jiaozi Snow Mountain is exposed to a wide range of climates, from valley south subtropics, valley middle subtropics, mountain south subtropics in the foothills, mountain warm temperate zone, mountain middle temperate zone in the middle height ridges, to mountain cold temperate zone, mountain cold temperate zone in the subalpine belt, and a sub-frigid zone in the alpine belt. Along the increasing elevation, evergreen broad-leaved forest, temperate coniferous forest, shrub and alpine meadow, and alpine scree are replacing each other successively. Jiaozi Snow Mountain hosts a huge natural species gene pool with relatively rich biodiversity, consisting of 157 families, 563 genera and 1613 species of vascular plants (Peng and Liu, 2015).
Data set compilationThe plant list of Jiaozi Snow Mountain was organized and compiled on the basis of the following literature:Flora of Yunnan (Wu, 1977-2006), The catalogue of seed plants in Yunnan Province (Chen et al., 2017), Flora of China (Wu and Raven, 1994-2011), Flora Reipublicae Popularis Sinicae (Flora Reipublicae Popularis Sinicae Editorial Committee, 1959-2013), Yunnan Jiaozishan National Nature Reserve (Peng and Liu, 2015), and Floristic Geography of Seed Plants in the Jiaozi Snow Mountain and Surrounding Areas (Wang, 2009). These sources were complemented by data from published papers (Xiang and Liu, 2012; Yang et al., 2015; Wang et al., 2018), a herbarium (KUN), and the Chinese Virtual Herbarium (http://www.cvh.ac.cn/). In 2018, we complemented this literature search with field surveys in Jiaozi Snow Mountain, where we collected and identified plants. During the field survey, we have found three new record species (i.e., Ligularia hookeri, Sinocarum schizopetalum, Saxifraga substrigosa) (Xu, unpublished data). Our data set includes 1488 angiosperm species belonging to 519 genera and 118 families (Table S1). The names of families were updated according to APG IV (Angiosperm Phylogeny Group, 2016), and the phylogenetic positions of genera were determined by Duocet Wiki of Plants (Duocet Group, 2016 onwards).
Species were defined as spinescent if they had any sharp appendages or pointed tips on any part of the plant (Bell, 1991; Harris and Harris, 2001; Hickey and King, 2001; Beentje, 2014) (Fig. S1). Specifically, according to the species descriptions in the Flora of China (Wu and Raven, 1994-2011), combined with specimen consultation, species with the term/s 'pungent tip', 'pungent point', 'spine*', 'spini*', 'spino*', 'spinu*', 'spiny', 'spicul*', 'thorn', 'thorny', 'prickle', 'prickly', 'armor', 'armour', 'armature', 'aculei', 'aculeate', 'aculeolate', 'acantha', 'asperity', 'burr', 'echinate' and 'echinulate' were sorted as 'spinescent'. In addition, 'glochid' and 'barb' in Boraginaceae, 'hook' in Agrimonia, and 'beak' in Carex also were labelled as 'spinescent'. Species lacking any of these features (including species described as not possessing the above features, e.g., 'unarmed' and 'prickless') were scored as 'non-spinescent' (Tindall et al., 2017). In this study, we only examined the qualitative traits; the quantitative aspects of spinescence were not considered.
To test the evolutionary pattern of spinescence in the whole flora and its relationship with other traits, we recorded the following traits:spinescent organ (spinescent type), life form, and spinescent color. Spinescent organs were divided in two major categories:vegetative and reproductive organs. Each category was divided into several subclasses (Table 1). We designated life forms as annuals, herbaceous perennials, shrubs, shrubs or trees, trees, and vines (Table 1). Due to their short life span, biennial herbs (8 such species) were categorized as annuals. We also divided plants in parallel into woody and non-woody species following Turcotte et al. (2014). We further divided woody plants based on leaf habit into deciduous, semi-evergreen, and evergreen. We listed semievergreen separately, because of this unique adaptation (Li, 1985). Because the color of spinescence in dry plant specimens in herbaria can sometimes be lost or changed, we also referred to our own field observation records and the photographs of living plants (http://www.plantphoto.cn/, http://www.cfh.ac.cn/default-en.html). For the traits described above, when the descriptions of species were vague and ambiguous, we referred to the descriptions of higher taxonomic units. For example, Aconitum has been previously described as an herb (Wu and Raven, 1994-2011). Thus, although some species of Aconitum Ser. Volubilia had twined stems, they were considered herbs. When there was no description or an incomplete description, we examined specimens or consulted experts in related taxa. In addition, we determined the effects of the phylogenetic position and phytogeographic origin on the distribution pattern of spinescence. Based on the areal types of genera [i.e., 1. Wide spread; 2. Pantropic; 3. Tropical Asia & Tropical America Disjuncted; 4. Old World Tropics; 5. Tropical Asia & Tropical Australasia; 6. Tropical Asia to Tropical Africa; 7. Tropical Asia (lndo-Malesia); 8. North Temperate; 9. E. Asia & N. America Disjuncted; 10. Old World Temperate; 11. Temperate Asia; 12. Mediterranean, W. Asia to C. Asia; 13. C. Asia; 14. E. Asia; 14(SH). Sino-Himalaya; 14(SJ). Sino-Japan; 15. Endemic to China] (Wu, 1991; Wu et al., 2006, 2010), and on different temperature conditions, type 8, 9, 10, 11, 12, 13, 14, 14(SH), 14(SJ) and 15 were classified as temperate genera; type 2, 3, 4, 5, 6 and 7 as (pan) tropical genera (We did not consider subtypes here except the major parts 14(SH) and 14(SJ)) (Good, 1974). Based on the distribution types of species (as described above areal types of genera, specially, 15-2-d means "shared with tropical Yunnan") (Peng and Liu, 2015), and different temperature condition, type 8, 9, 10, 11, 12, 13, 14, 14(SH), 14(SJ) and 15 (except for 15-2-d) were classified as temperate species, type 2, 3, 4, 5, 6, 7 and 15-2-d as (pan) tropical species (Table S1; Table S4) (We did not consider subtypes here except the major parts 14(SH), 14(SJ) and 15-2-d).
| Life form | Annuals | Herbaceous Perennials | Shrub | Tree | Shrub or tree | Vine | Total | |
| Number of species | 145 (9.7%) | 848 (57.0%) | 260(17.5%) | 97 (6.5%) | 73 (4.9%) | 65 (4.4%) | 1488 | |
| Number of spinescent species | 5 (3.4%) | 53 (6.3%) | 44 (16.9%) | 15 (15.5%) | 8 (11.0%) | 12 (18.5%) | 137 (9.2%) | |
| Spinescence in vegetative organ | 3 (60.0%) | 21 (39.6%) | 41 (93.2%) | 12 (80.0%) | 8 (100.0%) | 11 (91.7%) | 96 (70.0%) | |
| Stems and branches | 2 (40.0%) | 9 (17.0%) | 30 (68.2%) | 5 (33.3%) | 2 (25.0%) | 9 (75.0%) | 57 (41.6%) | |
| Leaves | 3 (60.0%) | 19 (35.8%) | 28 (63.6%) | 8 (53.3%) | 6 (75.0%) | 7 (58.3%) | 71 (51.8%) | |
| Spinescence in reproductive organ | 4 (80.0%) | 40 (75.5%) | 7 (15.9%) | 4 (26.7%) | 0 | 5 (41.6%) | 60 (43.8%) | |
| Flowers and inflorescence | 2 (40.0%) | 29 (54.7%) | 6 (13.6%) | 0 | 0 | 4 (33.3%) | 41 (29.9%) | |
| Fruits | 2 (40.0%) | 12 (22.6%) | 1 (2.3%) | 4 (26.7%) | 0 | 1 (8.3%) | 20 (14.6%) | |
| Seeds | 1 (20.0%) | 0 | 0 | 0 | 0 | 0 | 1 (0.7%) |
Spinescent proportion was calculated as the number of spiny species/the total number of species. The G-test was used to analyze whether the proportion of spinescence differed between plant organs, life forms, and phytogeographical origin (Harremoes and Tusnady, 2012). In addition, principal components analysis (PCA) was used to describe and visualize the structure of the data across life forms, leaf habit, spinescent organs, and phytogeographical origin. To test whether the evolution of spinescence is constrained by phylogeny, we measured phylogenetic signal by calculating D value (Fritz and Purvis, 2010).
ResultsIn Jiaozi Snow Mountain, 9.2% (137 species) of plants are spinescent (Table 1). Of these spiny plants, 71.5% (98 taxa) of all spinescence is confined to one organ; 16.1% (22 taxa) of all spinescence occurs in two organs (Fig. 1). These findings are derived from a database which included 1488 angiosperm species, including 848 herbaceous perennials (57.0%), 260 shrubs (17.5%), 145 annuals (9.7%), with the remaining plants species composed of trees (6.5%), shrub or trees (4.9%), and vines (4.4%).
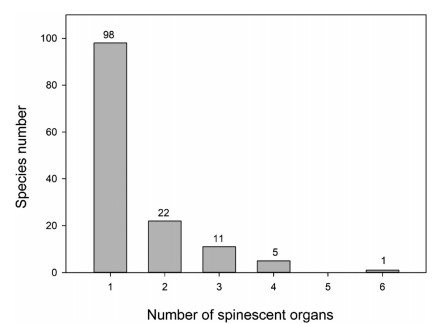
|
| Fig. 1 Frequency distribution of the number of organs with spinescence. |
Overall, spinescence in vegetative organs (70.0%) was significantly higher than in reproductive organs (43.8%; G = 8.84, P < 0.01) (Table 1). The plant organ with the highest incidence of spinescence was the leaf. In 51.8% of plants that had spines on a single organ, these spines were confined to the leaf. Spines were also common on stems and branches (41.6%). In the reproductive organs, spinescence was less common. For example, in flowers (29.9%), fruits (14.6%), and seeds (0.7%) had a lower probability of being spiny.
The prevalence of spinescence varied according to life form (Tables 1 and 2). The proportions of spinescent annual species (3.4%) and herbaceous perennials (6.3%) were significantly lower than those of shrubs (16.9%), trees (15.5%), and vines (18.5%), although no statistically significant differences were found between the former two life forms or between the latter three life forms. In addition, shrub or trees had a significantly higher proportion of spinescence (11.0%) than annuals, although they were not significantly different from all other life forms.
| Annuals | Herbaceous Perennials | Shrub | Tree | Shrub or tree | Vine | |
| Annuals | 1 | 2.01 | - | - | - | - |
| Herbaceous Perennials | 2.01 | 1 | - | - | - | - |
| Shrub | 18.88** | 24.82** | 1 | - | - | - |
| Tree | 10.98** | 8.83** | 0.11 | 1 | - | - |
| Shrub or tree | 4.55* | 2.07 | 1.64 | 0.74 | 1 | - |
| Vine | 12.38** | 10.07** | 0.09 | 0.25 | 1.56 | 1 |
| G-values are shown. * significant at P < 0.05; ** significant at P < 0.01. | ||||||
Life form had an effect on the organ of spinescence (Table 1). Three annual plant species had spinescence in vegetative organs and four in reproductive organs. Most spinescent herbaceous perennials had spinescence in one organ (75.5%); furthermore, a greater proportion of perennial species had spinescent reproductive organs (75.5%) than vegetative organs (39.6%; G = 14.30, P < 0.01). In shrubs, 59.1% of species had one spiny organ and 31.8% had two spiny organs. In contrast to herbaceous perennials, the proportion of spinescence in shrub vegetative organs (93.2%) was much higher than that in reproductive organs (15.9%). In trees, 93.3% of the species had one spiny organ, and only Corylus ferox had spines in three organs. Similar to shrubs, tree vegetative organs (80.0%) were more likely to be spiny than their reproductive organs (26.7%). In the group classified shrub or trees, all spinescence occurred in vegetative organs, with 6 in leaves and 2 in stems. For vines, spinescent proportion in vegetative organs (91.7%) was much higher than that in reproductive organs (41.6%).
When species were divided into woody and non-woody plants, life form also had a significant effect on spinescence (G = 34.42, P < 0.01). The proportion of spinescence in woody plants was about 190.6% greater than that in non-woody plants. For vegetative organs, the proportion of spinescence in woody plants was about 112.9% higher than that in non-woody plants (G = 41.21, P < 0.01). In contrast, the occurrence of spinescence in the reproductive organs of non-woody plants was 252.4% higher than that in woody plants (G = 39.52, P < 0.01).
The proportion of spinescence among evergreens was not significantly different from that in semi-evergreen and deciduous woody plants. However, the proportion of spinescent reproductive organs in deciduous plants (37.8%) was significantly higher than that in evergreen plants (5.6%; G = 7.78, P < 0.01); however, no significant difference was found for vegetative organs (G = 3.49, P = 0.06). For leaf blades, the proportion of spinescence was significantly higher in evergreen plants (55.6%) than in deciduous plants (27.0%; G = 6.53, P < 0.05). However, the proportion of spinescent petioles in deciduous plants (35.1%) was significantly higher than in evergreen plants (2.8%; G = 9.85, P < 0.01). Interestingly, semi-evergreen plants had a significantly higher proportion of stipular thorns (75.0%) than evergreen plants (2.8%; G = 13.90, P < 0.01) and deciduous plants (5.4%; G = 13.05, P < 0.01) (Table S3).
The colors of spinescence in the Jiaozi Snow Mountain flora varied, largely dependent on plant organ (Fig. 2). The most common color of spines was yellow (40.8%), followed by white (16.8%), red (15.8%), brown (14.3%), green (9.7%), black (1.5%) and purple (1.0%). All spines on seeds, and 83.3% of spines on stipules, were brown. The majority of leaf blade spines (72.7%) were yellow. The most common colors of spinescence on petioles were red and yellow. The most common colors for spines on flowers and inflorescences were yellow and green; for fruits, the most common color was white.
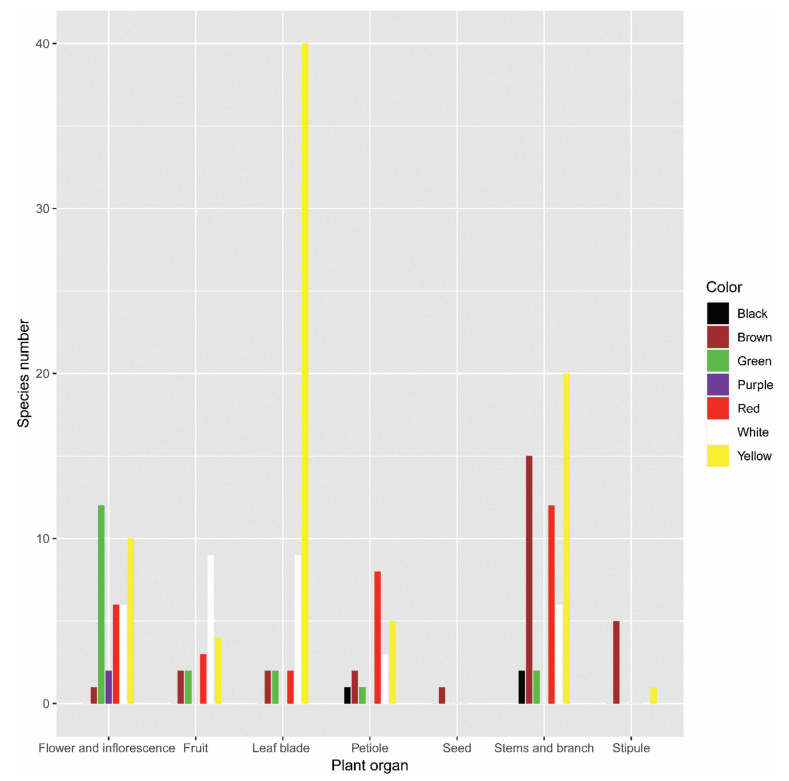
|
| Fig. 2 The color of spinescence on various plant organs in the Jiaozi Snow Mountain. |
Across the 118 families of angiosperms, 36 families were spinescent (30.5%). Berberiaceae, Phrymaceae, Capparaceae and Iteaceae had the highest proportion of spinescence (100%), followed by Elaeagnaceae, Rubiaceae, Fagaceae, Cucurbitaceae, Rhamnaceae (>50%). The families with the highest proportion of spinescence in vegetative organs were the Berberiaceae, Capparaceae and Iteaceae (100%). Fewer families had a high proportion of species with spinescent reproductive organs. For example, spinescent reproductive organs were present in over a third of species in only three families (i.e., Phrymaceae, Solanaceae and Boraginaceae) (Fig. 3). In total, 12.7% of genera and 9.2% of species were spinescent (Table S4). The phylogenetic signals were weak to moderate (0 < D ≤ 1) (Fritz and Purvis, 2010) (Table 3).
| Stems and branches | Leaf blade | Petiole | Stipule | Flower and inflorescence | Fruit | Seed |
| 0.888 | 0.281 | 0.775 | 0.825 | 0.892 | 0.643 | 0.972 |
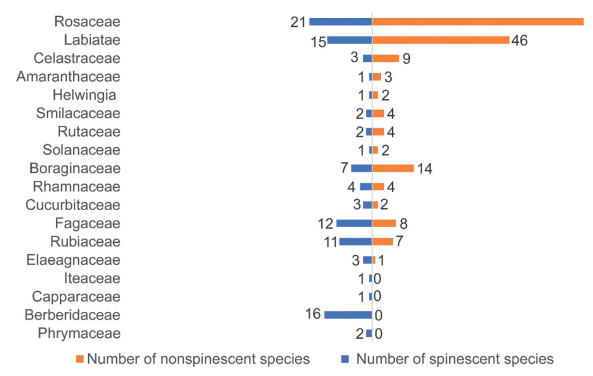
|
| Fig. 3 The number of species with or without spinescence in spinescent families in the Jiaozi Snow Mountain. |
We found that the proportions of spinescence in all areal types of genera were less than 1/3. In addition, type 12, 10, 2, 11, 8, and 14(SH) had a higher proportion of spinescence than average (12.7%). The proportion of spinescence of (pan) tropical genera (9.8%) was lower than that of temperate genera (13.2%), but the difference was not significant (G = 1.00, P = 0.32). At the species levels, the difference between (pan) tropical (7.6%) and temperate species (9.5%) was still not significant (G = 0.67, P = 0.41) (Table S4).
PCA analysis showed that Dim1 contributed 93.9% of the variation. We found that spinescence is highly correlated with plant organs and that the intra-organ correlation is higher than the interorgan correlation, regardless of whether examining vegetative or reproductive organs (Fig. 4, Figs. S2 and S3).
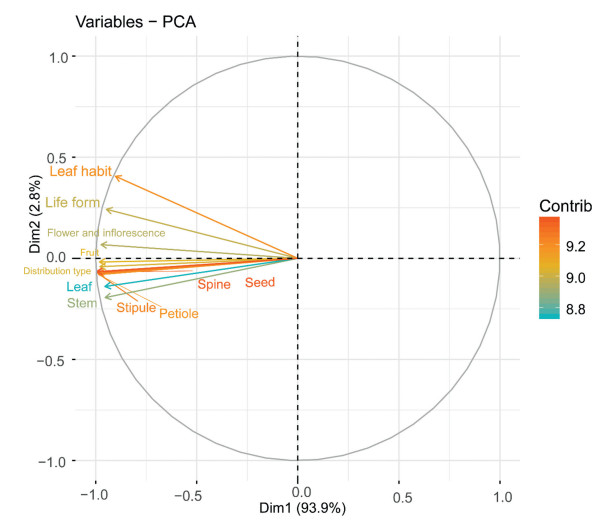
|
| Fig. 4 Principal Component Analysis of various traits. |
The proportion of angiosperm species with spinescence in Jiaozi Snow Mountain is 9.2%. This is somewhat lower than that reported in a previous study on Israel (11%; Ronel and Lev-Yadun, 2012). This may be attributed to the following two reasons. First, the Near East, including Israel, experienced a long history of intensive grazing from wild large herbivorous mammals, which thrived there during the Miocene and Pleistocene, and were replaced over the last several millennia by domesticated herbivores (Zohary, 1983; Perevolotsky and Seligman, 1998; Steiner, 2005; Tsahar et al., 2009), which increased grazing pressure. In addition, Israel and neighboring countries are characterized by arid conditions, including considerable deserts (Zohary, 1973). Thus, plants in the Near East are likely under selection pressure to reduce water loss (e.g., Burns, 2016) and radiation flux (e.g., Nobel, 1988), which may be indirectly responsible for the evolution of spinescence. However, when compared with the level of spinescence in Italy (5%; Bagella et al., 2018), Australia (4.2%) and New Zealand (3.9%; Tindall et al., 2017), the proportion of angiosperm plants with spinescence in Jiaozi Snow Mountain is much higher. The lower proportion of spinescent species in the floras of Italy, Australia, and New Zealand may be explained by either environmental and/or geographical factors or by the absence of predators. For example, in northern Italy, where the high Alps occupy large areas, the Italian alpine flora, like other alpine floras, is almost entirely spineless. Australia and New Zealand have historically lacked large mammalian herbivores (New Zealand) or had only marsupials (Australia) (e.g., Burns, 2016; Tindall et al., 2017). It should be noted that there are many similarities in component elements and climate between floras of North America (e.g., California) and East Asia (Wu et al., 2010). Thus, to further understand the evolution of spinescence, future studies should compare the patterns of spinescence between these two floras.
We found that spinescence evolved differently in various life forms. For example, woody plants had a much higher proportion of spinescent species than non-woody plants. In addition, herbaceous perennials had a higher proportion of spinescent species than annuals. This is consistent with previous studies from the Mediterranean (Ronel and Lev-Yadun, 2012) and in the aquatic flora of the Yangtze Delta (Zhang and Yao, 2018). This difference in spinescent evolution among life forms can be partly explained by the plant apparency theory (Turcotte et al., 2014). Woody plants or herbaceous perennials generally experience higher levels of herbivore pressure than non-woody plants or annuals, and thus invest more structural defenses. This is because these plants are often more apparent to herbivorous mammalians within a community compared to non-woody plants or annuals (Turcotte et al., 2014). Additionally, herbaceous perennials that are not geophytes or hemicryptophytes are exposed to herbivory all year round. In contrast, most annual species grow during the best season, when the vegetation is lush, and thus enjoy group protection. Consequently, most annual species require less defense because they are exposed to lower herbivory pressure than herbaceous perennials. Another explanation for the pattern we observed is that resource allocation varies in plants. Specifically, plants must make trade-offs between either growth and reproduction or defense; strategies underlying these tradeoffs differ between life forms (Cates and Orians, 1975). For example, compared with herbaceous perennials or woody plants, annuals and many non-woody plants have much shorter life spans, thus investing more resources in rapid vegetative growth and seed production is favoured (Cates and Orians, 1975; Herms and Mattson, 1992). In addition, we found that the organs defended in non-woody plants differed from those in woody plants. In non-woody plants, the reproductive organs were more frequently spinescent, whereas in woody plants, the vegetative organs were more frequently spinescent. This is another example of a trade-off. Non-woody plant species, especially annuals, must not only complete their life cycle in a short time, but have only a single opportunity for sexual reproduction; thus, for the continuation of their genotype, defending reproductive organs is more important than defending vegetative organs. In contrast, woody plants reproduce repeatedly, and their reproductive organs, especially those of trees, because of their height, are fully or partly free from attack by large mammals; thus, their vegetative organs are more vulnerable to mammalian herbivores (Ronel and Lev-Yadun, 2012). This may also be the case for vines, because most of them climb in order to reach light, which simultaneously provides a height refuge from herbivorous large mammals.
Previous studies have found that leaves with longer lifespans often have higher levels of defense, because they are costly to produce (Coley, 1987; Wright et al., 2004). However, in our study, no significant difference in proportion of species with spinescent leaves was found between evergreen and deciduous woody plants. During our field survey, we found that most Rubus and Rosa (Rosaceae) plants, which are deciduous, had spinescence on their petioles but not on their leaf blades. In late autumn, the leaf blades fall off when an abscission layer is formed, but petioles remain vigorous and attached to the stems (Xu, personal observation). Thus, the spines of petioles continue to play a defensive role in winter and express leaf growth in the coming year, with a lower resource investment. Consequently, the lack of a significant difference in proportion of spiny leaves between evergreen and deciduous plants may result from the fact that petioles were included when we considered the spinescence on leaves. This interpretation is supported by our findings that the proportion of spinescence on leaf blades in evergreen plants was significantly higher than in deciduous plants. However, compared with evergreen plants, the proportion of reproductive organs with spinescence was significantly higher in deciduous plants. This can also be explained by plant apparency theory to some extent, because reproductive organs of deciduous plants are more apparent to herbivorous mammalian after the leaves have fallen off, and before the leaves emerge in spring (Turcotte et al., 2014).
Many studies have proposed that plants probably signal their spiny defense to potential mammalian herbivores by aposematic coloration (e.g., Lev-Yadun, 2001, 2003b, 2006, 2009a, 2009b, 2009c; Lev-Yadun et al., 2018; Midgley et al., 2016). In our study, the color of most spinescence is yellow, followed by white and red, suggesting the possible aposematic role of spinescent colors as advertisement of physical defense in the Jiaozi Snow Mountain flora (Ronel and Lev-Yadun, 2012). Furthermore, among these spinescence with aposematic color, 74.4% of spinescence showed different color relative to their growing organs (G = 5.38, P = 0.02) (Table S2), which can potentially enhance the visual signals themselves, thus making associative learning by herbivores quicker and stronger, eventually enhancing their protective effects against herbivores.
A study conducted on the flora of Israel showed that the evolution of spinescence is constrained by phylogenetic relatedness (Ronel and Lev-Yadun, 2012). In the Jiaozi Snow Mountain flora, however, most families contained both spiny and spineless plant species. Furthermore, the phylogenetic signals were weak or moderate. One possible explanation for these differences may be that phylogenetic analysis of the Israeli plants did not include sequence data (Ronel and Lev-Yadun, 2012). Our results indicate that the evolution of spinescence is relatively random and that phylogeny has weakly constrained spinescence in the Jiaozi Snow Mountain flora. Consequently, to fully understand the evolution of spinescence in the Jiaozi Snow Mountain flora, further studies should focus on ecological and environmental factors.
It is generally hypothesized that plants from low latitude tropical areas are better structurally defended than plants from higher latitudes (MacArthur, 1972; Coley and Aide, 1991). However, in our study, the proportion of spinescence in the tropical elements of the Jiaozi Snow Mountain flora is lower than that in temperate elements, although the difference is not significant. The reasons why the proportion of spinescent species in temperate and tropical elements were not significantly different remain unclear. However, one possible explanation is that the majority of defenses in tropical flora are aimed not at large herbivores but at insects (Zhang et al., 2016). A possible alternative explanation for this pattern is that ancient Mediterranean elements, which subsequently evolved into temperate elements, comprise a relatively high proportion of spinescent genera (33%; Table S4). With the retreat of the PaleoMediterranean, some of the original ancient members of the Mediterranean flora may have further evolved into the new types of the Sino-Himalayan elements (Zhou et al., 2016).
ConclusionIn this study, we analyzed spinescence of wild plants in the flora of the Jiaozi Snow Mountain, an ecologically and taxonomically diverse nature reserve in southwestern China. We found that 137 out of 1488 angiosperm species in the Jiaozi Snow Mountain flora are spinescent. The life form of plants significantly influences whether they are spinescent and if so, which organs are spinescent. For example, the vegetative organs of woody plants have spines, whereas the reproductive organs of non-woody plants have spines. Our results also support the hypothesis that spinescence is visually aposematic to potential vertebrate herbivores. Weak phylogenetic signals suggest that spinescence is influenced more by environmental factors than by phylogenetic constraints.
Author contributionH Sun and B Song conceived and designated the experiments. Q Xu, L Sun and Z Chen collected and analyzed the data. Q Xu and B Song wrote the initial manuscript. S Lev-Yadun consulted about data collection and revised the manuscript.
Declaration of Competing InterestThe authors declare that they have no potential conflict of interest.
AcknowledgmentsWe thank Yunfei Deng (Acanthaceae expert), Limin Lu (Vitaceae expert), Fang Wen (Gesneriaceae expert), Bo Li (Polygonaceae expert), Bo Liu (Symplocaceae expert), Long Wang (Asteraceae expert), Yi Yang (Rhamnaceae expert) and Yaping Chen (Lamiaceae expert) for their assistance with species identification and information supplement in related taxa. We thank Buyun Zhang and Lu Gan for his sketch of spinescence, and Lishen Qian for his help in figure editing. This work was supported by the National Key Research and Development Program of China (2017YFC0505200), the Major Program of the National Natural Science Foundation of China (31590820), the National Natural Science Foundation of China (31570228, 31770249), the Young Academic and Technical Leader Raising Foundation of Yunnan Province (2016HB062), the Ten-thousand Talents Program of Yunnan Province (YNWR-QNBJ-2018-208), the Youth Innovation Promotion Association, CAS (2017437), and the CAS "Light of West China" Program to B.S. We thank three anonymous reviewers for their constructive suggestions.
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2019.12.002.
Angiosperm Phylogeny Group, 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot.J.Linn.Soc, 181: 1-20. DOI:10.1111/boj.12385 |
Bagella S., Filigheddu R., Benesperi R., Giordani P., Minuto L., Viciani D., Caria M.C., Pisanu S., Casazza G., 2018. Thorn, spine and prickle patterns in the Italian flora. Plant Biosyst, 153: 118-133. |
Barton K.E., 2016. Tougher and thornier: general patterns in the induction of physical defence traits. Funct.Ecol, 30: 181-187. DOI:10.1111/1365-2435.12495 |
Beentje, H., 2014. The Kew Plant Glossary: an Illustrated Dictionary of Plant Terms.Royal Botanic Gardens, Kew.
|
Bell, A.D., 1991. Plant Form: an Illustrated Guide to Flowering Plant Morphology.Oxhalpen University Press, New York.
|
Burns K.C., 2016. Spinescence in the New Zealand flora: parallels with Australia. New Z.J.Bot, 54: 273-289. DOI:10.1080/0028825X.2015.1130727 |
Cates R.G., Orians G.H., 1975. Successional status and the palatability of plants to generalized herbivores.. Ecology, 56: 410-418. DOI:10.2307/1934971 |
Charles-Dominique T., Davies T.J., Hempson G.P., Bezeng B.S., Daru B.H., Kabongo R.M., Maurin O., Muasya A.M., van der Bank M., Bond W.J., 2016. Spiny plants, mammal browsers, and the origin of African savannas. Proc.Natl.Acad.Sci.U.S.A, 113: E5572-E5579. DOI:10.1073/pnas.1607493113 |
Chen, J.H., Deng, T., Zhang, D.C., Yue, J.P., Zhou, Z., Sun, L., Li, Y.B., Li, W.Q., Shi, M.M., Sun, H., 2017. The Catalogue of Seed Plants in Yunnan Province. Science Data Bank. https://doi.org/10.11922/sciencedb.489.
|
Chung S.H., Rosa C., Scully E.D., Peiffer M., Tooker J.F., Hoover K., Luthe D.S., Felton G.W., 2013. Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc.Natl.Acad.Sci.U.S.A, 110: 15728-15733. DOI:10.1073/pnas.1308867110 |
Coley P.D., 1987. Interspecific variation in plant anti-herbivore properties: the role of habitat quality and rate of disturbance. New Phytol, 106(S1): 251-263. |
Coley, P.D., Aide, T.M., 1991. Comparison of herbivory and plant defences in temperate and tropical broad-leaved forests. In: Price, P.W., Lewinsohn, T.M., Fernandes, G.W., et al. (Eds.), Plant-animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. John Wiley, New York, p. 549.
|
Coley P.D., Bryant J.P., Chapin F.S., 1985. Resource availability and plant antiherbivore defense.. Science, 230: 895-899. DOI:10.1126/science.230.4728.895 |
Cooper S.M., Owen-Smith N., 1986. Effects of plant spinescence on large mammalian herbivores.. Oecologia, 68: 446-455. DOI:10.1007/BF01036753 |
Daniel H.J., Paul S.M., 1982. Neotropical anachronisms: the fruits the gomphotheres ate.. Science, 215: 19-27. DOI:10.1126/science.215.4528.19 |
Duocet Group, 2016. Duocet Wiki of Plants. onwards.Flora Reipublicae Popularis Sinicae Editorial Committee, 1959-2013. Flora Reipublicae Popularis Sinicae. Science Press, Beijing.
|
Ford A.T., Goheen J.R., Otieno T.O., Bidner L., Isbell L.A., Palmer T.M., Ward D., Woodroffe R., Pringle R.M., 2014. Large carnivores make savanna tree communities less thorny.. Science, 346: 346-349. DOI:10.1126/science.1252753 |
Fritz S.A., Purvis A., 2010. Selectivity in mammalian extinction risk and threat types: a new measure of phylogenetic signal strength in binary traits. Conserv.Biol, 24: 1042-1051. DOI:10.1111/j.1523-1739.2010.01455.x |
Futuyma D.J., Agrawal A.A., 2009. Macroevolution and the biological diversity of plants and herbivores. Proc.Natl.Acad.Sci.U.S.A, 106: 18054-18061. DOI:10.1073/pnas.0904106106 |
Gibson, A.C., Nobel, P.S., 1986. The Cactus Primer. Harvard University Press, Cambridge.
|
Good, R., 1974. The Geography of the Flowering Plants, third ed. Longmans, Green and Co., London.
|
Grubb P.J., 1992. A positive distrust in simplicity - lessons from plant defences and from competition among plants and among animals. J.Ecol, 80: 585-610. DOI:10.2307/2260852 |
Halpern M., Raats D., Lev-Yadun S., 2007. Plant biological warfare: thorns inject pathogenic bacteria into herbivores. Environ.Microbiol, 9: 584-592. DOI:10.1111/j.1462-2920.2006.01174.x |
Hanley M.E., Lamontb B.B., Fairbanksb M.M., Rafferty C.M., 2007. Plant structural traits and their role in anti-herbivore defence. Perspect.Plant Ecol, 8: 157-178. DOI:10.1016/j.ppees.2007.01.001 |
Harremoes P., Tusnady G., 2012. Information divergence is more chi squared distributed than the chi squared statistic.. Mathematics, 538: 543. |
Harris, J.G., Harris, M.W., 2001. Plant Identification Terminology: an Illustrated Glossary. Spring Lake Publishing, Spring Lake.
|
Herms D.A., Mattson W.J., 1992. The dilemma of plants: to grow or defend. Q.Rev.Biol, 67: 283-335. DOI:10.1086/417659 |
Hickey, M., King, C., 2001. The Cambridge Illustrated Glossary of Botanical Terms.Cambridge University Press, Cambridge.
|
Janzen D.H., 1976. Why bamboos wait so long to flower. Annu.Rev.Ecol.Systemat, 7: 347-391. DOI:10.1146/annurev.es.07.110176.002023 |
Kelly D., Sork V.L., 2002. Mast seeding in perennial plants: why, how, where? Annu. Rev.Ecol.Evol.Syst, 33: 427-447. DOI:10.1146/annurev.ecolsys.33.020602.095433 |
Kessler A., Baldwin I.T., 2001. Defensive function of herbivore-induced plant volatile emissions in nature.. Science, 291: 2141-2144. DOI:10.1126/science.291.5511.2141 |
Lev-Yadun S., 2001. Aposematic (warning) coloration associated with thorns in higher plants. J.Theor.Biol, 210: 385-388. DOI:10.1006/jtbi.2001.2315 |
Lev-Yadun S., 2003a. Why do some thorny plants resemble green zebras? J. Theor.Biol, 244: 483-489. |
Lev-Yadun S., 2003b. Weapon (thorn) automimicry and mimicry of aposematic colorful thorns in plants. J.Theor.Biol, 224: 183-188. DOI:10.1016/S0022-5193(03)00156-5 |
Lev-Yadun, S., 2006. Defensive coloration in plants: a review of current ideas about anti-herbivore coloration strategies. In: Teixeira da Silva, J.A. (Ed.), Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues (IV). Global Science Books, London, pp. 292-299.
|
Lev-Yadun, S., 2009a. Aposematic (warning) coloration in plants. In: Baluska, F.(Ed.), Plant - Environment Interactions. From Sensory Plant Biology to Active Plant Behavior. Springer-Verlag, Berlin, pp. 167-202.
|
Lev-Yadun S., 2009b. Müllerian and Batesian mimicry rings of white-variegated aposematic spiny and thorny plants: a hypothesis. Isr.J.Plant Sci, 57: 107-116. DOI:10.1560/IJPS.57.1-2.107 |
Lev-Yadun S., 2009c. Müllerian mimicry in aposematic spiny plants. Plant Signal.Behav, 4: 482-483. DOI:10.4161/psb.4.6.8848 |
Lev-Yadun, S., 2016. Defensive (Anti-herbivory) Coloration in Land Plants: Antiherbivory Plant Coloration and Morphology. Springer, Zug.
|
Lev-Yadun S., 2019. Defensive (anti-herbivory) Batesian mimicry in plants. Isr.J.Plant Sci, 66: 34-51. DOI:10.1163/22238980-00001044 |
Lev-Yadun S., Ne'eman G., 2006. Color changes in old aposematic thorns, spines, and prickles. Isr.J.Plant Sci, 54: 327-333. |
Lev-Yadun S., Ne'eman G., Keasar T., 2018. Differences in flower colors between spiny and non-spiny Asteraceae species: a possible case of aposematism?. Flora, 239: 98-103. DOI:10.1016/j.flora.2017.12.002 |
Li B.S., 1985. A semi-evergreen broad-leaf forest on the south slope of the eastern Himalayas. Acta Bot.Sin, 27: 334-336. |
Lucas P.W., Turner I.M., Dominy N.J., Yamashita N., 2000. Mechanical defences to herbivory. Ann.Bot, 86: 913-920. DOI:10.1006/anbo.2000.1261 |
MacArthur, R.H., 1972. Geographical Ecology: Patterns in the Distribution of Species.Princeton University Press, Princeton.
|
Midgley J.J., Abbas H., Armel M.P., 2016. Further evidence that in African acacia white is a warning colour to herbivores; the white pseudo-galls of Vachellia seyal.. Afr.J.Range & Forage Sci., 33: 127-129. |
Milewski A.V., Young T.P., Madden D., 1991. Thorns as induced defenses: experimental evidence.. Oecologia, 86: 70-75. DOI:10.1007/BF00317391 |
Mithofer A., Boland W., 2012. Plant defense against herbivores: chemical aspects. Annu.Rev.Plant Biol, 63: 431-450. DOI:10.1146/annurev-arplant-042110-103854 |
Niu Y., Chen Z., Stevens M., Sun H., 2017. Divergence in cryptic leaf colour provides local camouflage in an alpine plant.. Proc.R.Soc.B, 284: 20171654. DOI:10.1098/rspb.2017.1654 |
Nobel P.S., 1983. Spine influences on PAR interception, stem temperature, and nocturnal acid accumulation by cacti. Plant Cell Environ, 6: 153-159. DOI:10.1111/j.1365-3040.1983.tb01888.x |
Nobel, P.S., 1988. Environmental Biology of Agaves and Cacti. Cambridge University Press, Cambridge.
|
Peng, H., Liu, E.D., 2015. Yunnan Jiaozishan National Nature Reserve. China Forestry Publishing House, Beijing.
|
Perevolotsky A., Seligman N., 1998. Role of grazing in Mediterranean rangeland ecosystems.Inversion of a paradigm.. Bioscience, 48: 1007-1017. DOI:10.2307/1313457 |
Putz F.E., 1990. Growth habits and trellis requirements of climbing palms (Calamus spp.) in North-Eastern Queensland.. Aust.J.Bot, 38: 603-608. DOI:10.1071/BT9900603 |
Quicke, D.L.J., 2017. Mimicry, Crypsis, Masquerade and Other Adaptive Resemblances. Wiley Blackwell, Oxford.
|
Raunkiaer, C., 1934. The Life-form of Plants and Statistical Plant Geography. Oxford University Press, Oxford.
|
Rebollo S., Milchunas D.G., Noy-Meir I., Chapman P.L., 2002. The role of spiny plant refuge in structuring grazed shortgrass steppe plant communities.. Oikos, 98: 53-64. DOI:10.1034/j.1600-0706.2002.980106.x |
Ronel M., Malkiel H.M., Lev-Yadun S., 2007. Quantitative characterization of the thorn system of the common shrubs Sarcopoterium spinosum and Calicotome villosa. Isr.J.Plant Sci, 55: 63-72. DOI:10.1560/IJPS.55.1.63 |
Ronel M., Lev-Yadun S., 2012. The spiny, thorny and prickly plants in the flora of Israel. Bot.J.Linn.Soc, 168: 344-352. DOI:10.1111/j.1095-8339.2011.01211.x |
Song, B., Sun, L., Lev-Yadun, S., Moles, A.T., Zhang, S., Jiang, X.L., Gao, Y.Q., Xu, Q., Sun, H., 2019. Plants are more likely to be spiny at mid-elevations in the Qinghai-Tibetan Plateau, south-western China. J. Biogeogr. https://doi.org/10.1111/jbi.13724.
|
Steiner, M.C., 2005. The Faunas of Hayonim Cave (Israel): a 200, 000-year Record of Paleolithic Diet, Demography and Society. Peabody Museum of Archaeology and Ethnology, Cambridge.
|
Tindall M.L., Thomson F.J., Laffan S.W., Moles A.T., 2017. Is there a latitudinal gradient in the proportion of species with spinescence?. J.Plant Ecol, 10: 294-300. |
Tsahar E., Izhaki I., Lev-Yadun S., Bar-Oz G., 2009. Distribution and extinction of ungulates during the Holocene of the southern Levant.. PloS One, 4: e5316. DOI:10.1371/journal.pone.0005316 |
Turcotte M.M., Davies T.J., Thomsen C.J.M., Johnson M.T.J., 2014. Macroecological and macroevolutionary patterns of leaf herbivory across vascular plants.. Proc.R.Soc.B, 281: 20140555. DOI:10.1098/rspb.2014.0555 |
Wang, H.C., 2009. Floristic Geography of Seed Plants in the Jiaozi Snow Mountain and Surrounding Areas. Yunnan University.
|
Wang J.Y., He J., Xu Z.F., Meng J., Liu E.D., Wang H., 2018. Aconitum wumengense(Ranunculaceae), a new species from Yunnan, China.. Phytotaxa, 343: 60-66. DOI:10.11646/phytotaxa.343.1.5 |
Wright I.J., Reich P.B., Westoby M., et al, 2004. The worldwide leaf economics spectrum.. Nature, 428: 821. DOI:10.1038/nature02403 |
Wu, Z.Y., 1977-2006. Flora of Yunnan. Science Press, Beijing.
|
Wu, Z.Y., 1991. Areal-types of Chinese genera of seed plants. Acta Bot. Yunnanica 1-139.
|
Wu, Z.Y., Raven, P.H., 1994-2011. Flora of China. Science Press/Missouri Botanical Garden Press, Beijing/St. Louis.
|
Wu, Z.Y., Sun, H., Zhou, Z.K., Li, D.Z., Peng, H., 2010. Floristics of Seed Plants from China. Science Press, Beijing.
|
Wu, Z.Y., Zhou, Z.K., Sun, H., Li, D.Z., Peng, H., 2006. The Areal-Types of Seed Plants and Their Origin and Differentiation. Yunnan Science and Technology Press, Kunming.
|
Xiang C.L., Liu E.D., 2012. A new species of Isodon (Lamiaceae, Nepetoideae) from Yunnan province, southwest China. Syst.Bot, 37: 811-817. DOI:10.1600/036364412X648751 |
Yamazaki K., Nakatani K., Masumoto K., Kawamura K., 2014. Slug caterpillars of Parasa lepida (Cramer, 1799) (Lepidoptera: limacodidae) become stuck on rose prickles. Pan-Pacific Entomol, 90: 221-225. DOI:10.3956/2014-90.4.221 |
Yang Q.E., Wang L., Ren C., 2015. Cremanthodium wumengshanicum (asteraceae, senecioneae), a new species from yunnan, China.. Phytotaxa, 238(3): 265. DOI:10.11646/phytotaxa.238.3.5 |
Young T.P., Okello B.D., 1998. Relaxation of an induced defense after exclusion of herbivores: spines on Acacia drepanolobium.. Oecologia, 115: 508-513. DOI:10.1007/s004420050548 |
Young T.P., Stanton M.L., Christian C.E., 2003. Effects of natural and simulated herbivory on spine lengths of Acacia drepanolobium in Kenya.. Oikos, 101: 171-179. DOI:10.1034/j.1600-0706.2003.12067.x |
Zhang G., Yao R., 2018. The spinescent aquatic plants in the Yangtze Delta, East China. Isr.J.Plant Sci, 65: 9-16. |
Zhang S., Zhang Y.X., Ma K.M., 2016. Latitudinal variation in herbivory: hemispheric asymmetries and the role of climatic drivers. J.Ecol, 104: 1089-1095. DOI:10.1111/1365-2745.12588 |
Zhou Z.K., Zhou Z.H., Wang Y., 2016. Coevolution between terrestrial ecosystem and Earth environment. Adv.Earth Sci, 31: 682-688. |
Zohary, M., 1973. Geobotanical Foundations of the Middle East. Gustav Fischer, Stuttgart.
|
Zohary, M., 1983. Man and vegetation in the Middle East. In: Holzner, W., Werger, M.J.A., Ikusima, I. (Eds.), Man's Impact on Vegetation. Dr W. Junk Publishers, The Hague, pp. 287-295.
|
Zust T., Agrawal A.A., 2017. Trade-offs between plant growth and defense against insect herbivory: an emerging mechanistic synthesis. Annu.Rev.Plant Biol, 68: 513-534. DOI:10.1146/annurev-arplant-042916-040856 |
Zust T., Rasmann S., Agrawal A.A., 2015. Growth-defense tradeoffs for two major anti-herbivore traits of the common milkweed Asclepias syriaca.. Oikos, 124: 1404-1415. DOI:10.1111/oik.02075 |



