b. Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, People's Republic of China;
c. Yunnan Key Laboratory of Dai and Yi Medicines, Yunnan University of Chinese Medicine, Kunming, Yunnan, People's Republic of China
Atmospheric carbon dioxide concentrations ([CO2]) are predicted to rise to at least 730 and possibly up to 1000 μmol mol-1 by the end of this century (IPCC, 2007). CO2 is the main source of carbon for photosynthesis. Elevated [CO2] (E[CO2]) has a strong influence on the growth, development, seed yield and biochemical metabolism of plants (Ekman et al., 2007; Locke et al., 2013; Pal et al., 2014; Ward et al., 2012; Zhang et al., 2012). Moreover, crops are very important for human life, providing food and materials for clothing, biofuel, medicine and many other things. The trend in terms of crop responses to E[CO2] is expected to have a marked influence on the global food supply and may threaten the nutrition of human beings (Ainsworth et al., 2008; Myers et al., 2014). The effects of E[CO2] vary among different types of plants. E[CO2] directly enhances the photosynthesis of C3 plants but not of C4 plants (Leakey, 2009; Wang et al., 2012). In some C3 plant species, the enhancing effects of E[CO2] on photosynthesis occur only after short-term treatment and decrease or even disappear after longterm treatment, a phenomenon called "CO2 acclimation" (Bloom et al., 2002). In addition, Bloom et al. (2010) found that E[CO2] inhibits NO3- assimilation and decreases organic N content of plants, after which photosynthesis declines, which could be an important factor in "CO2 acclimation". Legumes are C3 plants and can use N2 from the air as a source of N to synthesize ammonia and organic N compounds. It has been reported that legumes maintain high rates of photosynthesis under E[CO2], even after long-term treatment (Arenque et al., 2014; Bernacchi et al., 2006), and E[CO2] has been shown to result in increased biomass, which is positively correlated with the ability to form nodules (Cernusak et al., 2011).
Soybean (Glycine max) is an economically important legume crop species worldwide and is rich in high-quality proteins, polyunsaturated fatty acids, and vitamins and minerals (Liu, 1997). It is also beneficial to human health due to its natural antioxidants such as tocopherols and isoflavones (Carrera et al., 2011). E[CO2] has been shown to promote photosynthesis and decrease stomatal conductance of soybean leaves (Ainsworth et al., 2002; Bishop et al., 2015; Sanz-Saez et al., 2017), and the average yields of soybean during 2002-2006 were shown to be higher than those in 1980 due to elevated CO2 (Sakurai et al., 2014). Photosynthesis in soybeans may be promoted by E[CO2] throughout the lifespan of the plants due to their ability to fix nitrogen. Because the ability of soybeans to fix nitrogen varies among different growth stages (Zapata et al., 1987), the positive effect of E[CO2] on soybean photosynthesis may be correlated with soybean growth stage. However, little is known about the effects of E[CO2] on photosynthesis at different growth stages of soybean (Sun et al., 2014).
The stimulatory effect of E[CO2] on soybean growth may dilute the nutritional quality of soybean (Högy et al., 2009; Taub et al., 2008; Uprety et al., 2010; Wieser et al., 2008). In contrast, the isoflavone content in dwarf soybean plants increases significantly in response to CO2 elevation (Caldwell et al., 2005), whereas seed protein, total oil, and fatty acid composition are not affected by E [CO2] in soybean (Thomas et al., 2003). The influence of E[CO2] on seed mineral elements varies in different studies. For example, Thomas et al. (2003) found that E[CO2] had no significant effect on either N concentration or P concentration in soybean seeds, while Rosenthal et al. (2014) found that it caused a decline in N. In these studies, the CO2 concentrations were lower than 800 μmol mol-1 (predicted at the end of this century). Furthermore, there is only limited information on the effects of E[CO2] on soil nutrients following the growth of soybean.
In this study, we propose two hypotheses: (1) under E[CO2], soybeans maintain relatively high levels of photosynthesis throughout their development; and (2) E[CO2] reduces the concentrations of both mineral elements in the leaves and chemical nutrients in the seeds due to the dilution effect. To test these hypotheses, we measured soybean photosynthetic physiology at three growth stages for plants grown at ambient (400 μmol mol-1) and elevated (800 μmol mol-1) CO2 concentrations, which simulate predicted end-of-the-century CO2 levels. We then measured the nutrient compositions of seeds, including mineral elements, natural antioxidants, and fatty acids after harvest. Finally, we quantified soil nutrient levels after soybean cultivation.
2. Materials and methods 2.1. Plant material and chemicalsSoybean (G. max) seeds (Liaoxin) (Yu et al., 2015) were purchased from the Jingdian Seed Company (Kunming, China). Standards of isoflavone components, including daidzin, glycitin, and genistin, were purchased from Chengdu Must Bio-Technology Co., Ltd., China (MUST-14031414, 14070311 and 14060511, respectively). Standards of tocopherols, including α-, δ- and γ-tocopherol, were purchased from Sigma-Aldrich (47783, 47784 and 47785, respectively). 5, 7-Dimethyltocol was purchased from Matreya, LLC. (1074), and the C17:0 standard was purchased from Sigma-Aldrich (T2151).
2.2. Plant growth and treatmentsSeeds were sown in pots (30 cm in diameter, 35 cm in height) with a humus soil consisting of 10% clay, and there was ultimately one seedling per pot. The seeds were allowed to germinate and grow under a 12-h light/12-h dark photoperiod at 23 ℃ (day) and 18 ℃ (night). Growth chamber light intensity was 350 μmol m-2 s-1, and humidity was 65%. Control plants were grown in growth chambers with ambient [CO2] (~400 μmol mol-1) and treated plants were grown in growth chambers under elevated [CO2] (~800 (±50) μmol mol-1). Fifteen plants were placed in each growth chamber, and the treatments were rotated every week. Plants were not fertilized in our experiment. The growth stages of soybeans were determined according to the guidelines of the University of Minnesota Extension service (https://extension.umn.edu/growingsoybean/soybean-growth-stages). Tests of seed composition were conducted on mature seeds (stage R8).
2.3. Measurement of physiological parametersGas exchange measurements were conducted in situ on new fully developed trifoliate leaves (the third trifoliate leaf from the top) of six randomly selected plants from each treatment using a LI-COR 6400 portable gas analysis system (LI-COR, USA) equipped with a 6 cm2 LED chamber (LI-6400-02 B). Measurements were performed after 20 min of stabilization at a light-saturating photosynthetic photon flux density (PPFD) of 1600 μmol m-2 s-1, at ambient humidity (~60%) and at a flow rate of 500 μmol s-1 through the gas exchange chamber. CO2 concentration was controlled using an LI-6400 CO2 injector system (LI-6400-01) and was set to match the growth conditions of the plants (400 or 800 μmol mol-1). Water-use efficiency (WUE) (μmol CO2 mol-1 H2O) was calculated as the net photosynthesis rate divided by the transpiration rate (Pn/Tr). We measured photosynthetic parameters at three soybean leaf growth stages: the first fully developed trifoliate leaves (V1), full flowering (R2), and full pods (R4).
Specific leaf area (SLA) was calculated as leaf dry weight divided by leaf area. After removing the petioles, the area and dry weight of the entire leaf were determined. The leaf area was determined using ImageJ. Dry weight was obtained by first desiccating leaves at 105 ℃ for 30 min and then drying them at 80 ℃ to a constant mass before weighing.
2.4. Sugar and proline measurementsLeaves were harvested at the V1, R2, and R4 growth stages. Sugar and proline levels were measured using a standard curve, according to the methods of Li et al. (2004). Sugar content was determined by measuring A625 of the sugareanthrone reaction. Proline content was determined by measuring A520 of the proline ninhydrin reaction.
2.5. Fatty acid determinationThe seed fatty acid proportion was determined by gas chromatography (GC) using the methyl ester method (Miquel and Browse, 1992). Briefly, after being methylated with 5% methanolic H2SO4, the fatty acids were extracted twice with hexane and dried under nitrogen. A total of 100 μl of C17:0 fatty acid standard (2 mg/ml in chloroform) was added to each sample as an internal standard prior to extraction. After drying under nitrogen, the samples were dissolved in hexane and separated by GC on an Agilent DB-225 MS column (30 m × 0.25 mm × 0.25 mm) and quantified using a flame ionization detector. The area normalization method was used to calculate the proportions of five fatty acid components: palmitic acid, stearic acid, oleic acid, linoleic acid, and linolenic acid. The levels of these fatty acids were determined using an internal standard. The double bond index (DBI), a measure of the degree of fatty acid desaturation, was calculated according to the formula DBI = (∑[N × mol% fatty acid])/100, where N is the number of double bonds in each fatty acid molecule.
2.6. Lipid peroxidationLipid peroxidation levels were estimated by determining malondialdehyde (MDA) levels (Duan et al., 2005). Approximately 0.5 g of seed powder was homogenized in 4 μl of chilled reagent containing 10% (w/v) trichloroacetic acid (TCA) and centrifuged at 12, 000 g for 10 min. Two milliliters of the supernatant were then reacted with 2 μl pf 0.6% (w/v) thiobarbituric acid (TBA) for 30 min in boiling water. After the mixture cooled and was centrifuged, absorption of the supernatant at 450, 532 and 600 nm was measured. MDA levels were subsequently calculated using the following formula: C (nmol ml-1) = 6.45 × (A532 - A600) - 0.56 × A450.
2.7. Isoflavone extraction and high-performance liquid chromatography assaysThe isoflavone concentration was analyzed by highperformance liquid chromatography (HPLC) (Ma et al., 2015). Briefly, 0.1 g of seed powder was extracted with 5 μl of extraction solution at room temperature overnight. After centrifugation, the supernatant was subjected to HPLC on an Agilent 1260 system equipped with a Waters XSelect CSH C18 column (4.6 × 75 mm in length, 2.5 μm × 75 mm, 2.5 μm). By comparing the retention time and the maximum UV absorbance for the three standards with our samples, we accurately determined the levels of isoflavone components based on the UV absorption value at 260 nm.
2.8. Tocopherol extraction and high-performance liquid chromatography assayTocopherol concentration was analyzed in accordance with the methods of Yang et al. (2011), with minor modifications. Totals of 0.1 g of seed powder and 1 μl of internal standard (50 mg/ml 5, 7- dimethyltocol) were added to 3 μl of methanol:chloroform (2:1 v/v) containing 0.01% (m/v) butylated hydroxytoluene. After 20 min of incubation, tocopherols were extracted using chloroform, dried under nitrogen and re-suspended in dichloromethane:methanol (1:5 v/v) for HPLC analysis, using an Agilent 1260 HPLC. Quantitative analysis was performed on an Agilent Zorbax SB-C18 column (4.6 × 150 mm in length, 5 μm particle size). By comparing the retention time and the maximum UV absorbance for the three standards with those of our samples, we accurately determined the levels of tocopherols based on the UV absorption value at 292 nm.
2.9. Determination of element levelsAfter photosynthesis was measured, the same leaves at the full flowering (R2) stage were measured for their levels of various elements. Leaves were dried at 80 ℃ and then ground to a powder after harvest. The leaf, seed, and soil powders were pulverized to pass through a 60-mesh sieve (0.25 mm in diameter). The levels of carbon (C) and nitrogen (N) were determined by Vario MAX CN instrument (Elementar Analysensysteme GmbH, Germany). For total element testing, the powders of the plant and soil samples were digested with concentrated HNO3-HClO4, and the levels of total phosphorus (P), potassium (K), calcium (Ca), magnesium (Me), iron (Fe), manganese (Mn), copper (Cu), zinc (Zn), aluminum (Al), sodium (Na), sulfur (S) and boron (B) were determined with an inductively coupled plasma atomic-emission spectrometer (IRIS Advantage-ER; Thermo Jarrell Ash Corporation, Franklin, MA, USA).
2.10. Statistical analysisTwenty-four plants were grown in each chamber. Seven samplings (20 seeds per sampling) were taken from each chamber for chemical analyses. The experiment was replicated three times. First, a Q-test was performed on all data, and data from discordant samples were removed. The data were then subjected to one-way analysis of variance (ANOVA) using SPSS 13.0. Statistical significance was tested by an independent-sample T test using SPSS 13.0. Differences at P < 0.05 were considered significant.
3. Results and discussion 3.1. CO2 elevation stimulates photosynthesis and water-use efficiency at all growth stages of soybeanExposing soybean plants to elevated CO2 (E[CO2]) significantly increased net photosynthesis at all three growth stages examined (V1, "trifoliate leaves"; R2, "full flowering leaves"; and R4, "full pod leaves") (Table 1). Net photosynthesis increased the most in full flowering leaves (76.8%), while in first fully developed trifoliate leaves and full pod leaves, net photosynthesis increased by 28.6% and 46.5%, respectively.
| Growth stage | CO2 treatment | Pn (μmol CO2/m2/s) | Gs (mol H2O/m2/s) | Tr (mmol H2O/m2/s) | WUE (mmol/mol) | Ci/Ca |
| V1 | Ambient | 14.03 ± 1.08d | 0.26 ± 0.02abc | 4.34 ± 0.24a | 3.23 ± 0.21c | 0.73 ± 0.02c |
| Elevated | 18.04 ± 1.46c | 0.23±0.11bc | 3.44 ± 1.10b | 5.58 ± 1.32ab | 0.77 ± 0.07abc | |
| R2 | Ambient | 14.95 ± 0.61d | 0.22 ± 0.02c | 3.95 ± 0.31ab | 3.80 ± 0.32c | 0.67 ± 0.03d |
| Elevated | 26.43 ±0.81a | 0.31 ± 0.07ab | 4.35 ± 0.75a | 6.24 ± 1.10a | 0.78 ± 0.04ab | |
| R4 | Ambient | 14.59 ± 0.74d | 0.28 ± 0.03abc | 4.17 ± 0.35ab | 3.52 ± 0.35c | 0.74 ± 0.03bc |
| Elevated | 21.37 ± 0.70b | 0.30 ± 0.06ab | 4.32 ± 0.57a | 5.01 ± 0.68b | 0.81 ± 0.03a |
In some C3 plants, elevated CO2-induced increases in photosynthesis diminish over time (Bloom et al., 2002). This CO2 acclimation may attribute to the inhibition of nitrate assimilation under higher concentrations of CO2 (Bloom et al., 2010). However, the nitrogen-fixing ability of legumes suggests that they may not acclimate to high concentrations of CO2 after longterm treatment (Arenque et al., 2014; Bernacchi et al., 2006). In addition, nitrogen-fixing ability of soybean plants has been correlated with their growth stages, and compare to full flowering and full pods leaves, first fully developed trifoliate leaves have lower nitrogen-fixing ability (Zapata et al., 1987). This growth stage related nitrogen-fixing ability in soybean may explain why the promotion of net photosynthesis by E[CO2] was relatively low at first fully developed trifoliate leaves. We speculate that the nitrogen-fixing ability of soybeans may counteract the inhibitory effect of CO2 acclimation on nitrate assimilation and is capable of stimulating photosynthesis throughout the lifespan of soybean plants. Thus, examining the effects of E[CO2] on the nodule activity of soybeans may be an important part of future studies.
In previous studies, E[CO2] was found to decrease stomatal conductance of soybeans by approximately 40% (Ainsworth et al., 2002); similarly, E[CO2] decreased stomatal conductance of the N2-fixing species Lotus corniculatus (Ainsworth et al., 2003). Decreases in stomatal conductance together with increases in net photosynthesis have also been found to lead to higher water use efficiency (WUE) in soybeans (Prior et al., 2010). However, in our study, stomatal conductance in plants grown under E[CO2] did not decrease; in fact, at the full flowering stage, stomatal conductance of these plants was 40.9% higher than that of control plants (Table 1). The change in transpiration rate was also similar to that of stomatal conductance. This E[CO2]-induced increase in stomatal conductance at the full flowering stage may increase [CO2] at sites of Rubisco carboxylation and further promote photosynthesis. The relatively strong nitrogen-fixing ability and fast growth speed of soybeans at the full flowering stage (Zapata et al., 1987) lead us to speculate that the E[CO2]-induced increases in stomatal conductance at the full flowering stage may be caused by increased photosynthesis-promoted plant growth.
E[CO2] promoted water use efficiency of soybeans at all growth stages, which is the factor that contributed most to the increase in photosynthesis (Table 1). At all three growth stages, the ratio of intercellular CO2 to atmospheric CO2 (Ci/Ca) remained unchanged under E[CO2] treatment, and the soybean plants retained a high level of intracellular [CO2] (Table 1). Previous studies have shown that plants can maintain their level of 1-Ci/Ca via adjustments of stomatal anatomy and chloroplast biochemistry (Franks et al., 2013). We suspect that, after long-term treatment, plants may acclimate to E[CO2] via adjustments to stomatal conductance and chloroplasts. Taken together, these results suggest that soybean plants may have increased drought tolerance under high-CO2 conditions.
3.2. Soybean grown under CO2 elevation tended to have increased numbers of leaves and podsBecause sugar is the main product of photosynthesis, E[CO2]- induced promotion of photosynthesis is expected to increase sugar levels in leaves. However, in this study, E[CO2] had little effect on the sugar content of first full developed trifoliate leaves or full flowering leaves, while in full pod leaves, sugar content decreased by approximately 12% (from 11.53 to 10.18 mg/g Fresh Weight) (Table 2). In addition, at all growth stages, plants grown under E [CO2] had relatively low levels of proline (Table 2). In contrast, E [CO2] did not produce a discernible effect on the specific leaf area (SLA) at any growth stage (Table 2). Although the leaf area of soybean plants grown under ambient and elevated [CO2] conditions did not show any difference, the numbers of leaves (21%) and pods (15%) increased significantly in response to E[CO2] treatment (Table 2). After maturity, the total mass of seeds per plants grown under E[CO2] conditions were approximately 1 g heavier per plant (7.45 ± 0.07 g in ambient CO2 and 8.44 ± 0.11 g in E[CO2]).
| Growth stage | CO2 treatment | Sugars (mg/g FW) | Proline (mg/g FW) | SLA (mg DW/cm2) |
| V1 | Ambient | 7.66 ± 0.55c | 162.33 ± 8.72b | 2.12 ± 0.22d |
| Elevated | 7.95 ± 0.53c | 141.56 ± 9.12c | 2.39 ± 0.51cd | |
| R2 | Ambient | 9.99 ± 0.21b | 176.60 ± 9.29b | 3.02 ± 0.51ab |
| Elevated | 9.49 ± 0.42b | 132.23 ± 7.92c | 2.84 ± 0.43bc | |
| R4 | Ambient | 11.53 ±0.67a | 234.67 ± 14.31a | 3.27 ± 0.26ab |
| Elevated | 10.18 ± 0.64b | 224.84 ± 17.46a | 3.40 ± 0.28a | |
| Leaf area (cm2) | No. trifoliate leaves | No. pods | ||
| R4 | Ambient | 40.81 ±3.76a | 11.82 ± 1.78b | 13.10 ± 1.07b |
| Elevated | 39.98 ± 5.45a | 14.30 ± 1.33a | 15.00 ± 1.63a |
The increase in photosynthesis in response to CO2 enrichment may accelerate the growth and, ultimately, cause a dilution effect in plants (Höogy et al., 2009; Lenka et al., 2017; Taub et al., 2008; Uprety et al., 2010; Wieser et al., 2008). For example, under E [CO2] treatment, the level of proline decreased at all growth stages. E[CO2] showed a similar dilution effect on the sugar content only of full pod leaves. However, other leaf components, such as amino acids and fatty acids, may not be subjected to the dilution effect associated with E[CO2] because the SLA did not change during any of the growth stages. Compared with plants grown under ambient CO2 concentrations, soybean plants grown under E[CO2] grew faster and had more trifoliate leaves, which may be attributable to the promotive effect of E[CO2] on soybean growth at all growth stages. These results may indicate that the increase in pod number in soybean plants under E[CO2] is primarily attributable more to the rapid growth of plants rather than to the accumulation of functional components in the leaves.
3.3. Effects of CO2 elevation on C and N metabolism in soybean leaves and seedsE[CO2] induced minor decreases in C in soybean plants-1.54% and 0.46% in leaves and seeds, respectively (Table 3). Under E[CO2] treatment, leaf N levels decreased by 24.68%, while the N change in seeds was not statistically significant (Table 3). The carbon-to-nitrogen ratio (C/N) in leaves increased by approximately 33% under E[CO2] but remained unchanged in the soybean seeds (Table 3).
| Tissue | Ambient | Elevated | Relative change (%) | |
| C (g/kg) | leaves | 454.83 ± 0.41 | 447.83 ± 2.04* | -1.54 |
| seeds | 501.33 ± 1.86 | 499.00 ± 1.41* | -0.46 | |
| N (g/kg) | leaves | 35.13 ± 1.89 | 26.46 ± 4.00* | -24.68 |
| seeds | 68.23 ± 1.32 | 69.33 ± 0.85 | 1.61 | |
| C/N | leaves | 12.98 ± 0.73 | 17.26 ± 2.66* | 32.97 |
| seeds | 7.35 ± 01.6 | 7.20 ± 0.10 | -2.04 |
Because the leaf area and SLA were unchanged after E[CO2] treatment (Table 2), the decrease in soybean leaf N that we observed may not be related to the dilution of sugar and other structural components. Instead, the decrease may have been induced by relatively low N absorption under E[CO2] conditions due to the accelerated growth of soybean plants. Rogers et al. (2006) found that E[CO2] decreased N content in soybeans at the beginning of the season, but this inhibition diminished in the middle of the season. However, soybeans can overcome this N limitation, and N assimilation is promoted by E[CO2] (Rogers et al., 2006). The decrease in N content in soybean leaves at the full flowering stage may not affect the N assimilation process because of the relatively high nitrogen-fixing ability of soybeans at this stage. Because of this, the decrease in total N content in soybeans at the full flowering stage has little effect on the E[CO2]-promoted photosynthesis. C and N are the most abundant elements in plants, and CO2 enrichment increases the C/N ratio of plant tissues (Ainsworth et al., 2002) and affects the metabolism of both C and N (Loladze, 2002). In chickpea, E[CO2] increases grain C (higher C/N ratio) (Saha et al., 2015), while in our experiment, the C/N ratio of the seeds was not affected by E[CO2] (Table 3). Our results suggest that the E[CO2]-induced changes in C and N metabolism in the leaves may not affect C and N metabolism in seeds.
3.4. Effects of E[CO2] on the concentrations of mineral nutrients in the leaves and seeds of soybean and in the soilIn leaves grown under elevated CO2, the content of 13 out of 14 mineral elements decreased; Na, which did not show a statistically significant change in leaf content, was the exception (Fig. 1). The levels of Mn and Zn decreased the most-by as much as 30%. P and K, macroelements that are very important for soybean growth, decreased by 26.08% and 19.34%, respectively (Fig. 1). The increase in [CO2] may have adverse effects on plant macro- and microelements (Uprety et al., 2010) and thus may trigger micronutrient malnutrition (Loladze, 2002). These results indicate that E[CO2] induced a dilution effect for most mineral nutrients in soybean leaves, and under future conditions of high [CO2], increased amounts of P- and K-rich fertilizer will be required for soybean fields.
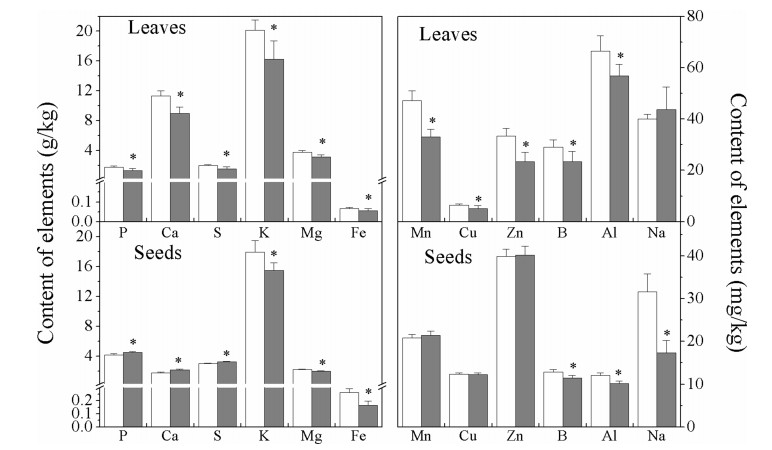
|
| Fig. 1 Effects of ambient [CO2] (400 μmol mol-1, white bars) and elevated [CO2] (800 μmol mol-1, gray bars) treatments on the levels of mineral nutrients in soybean leaves and seeds. The asterisk means that the value at 800 μmol mol-1 differs significantly from that at 400 μmol mol-1 (P < 0.05) |
In our experiment, E[CO2] caused overall increases (8.84%, 23.15% and 7.13%) in the levels of phosphorus (P), calcium (Ca) and sulfur (S) in soybean seeds, respectively (Fig. 1). However, the levels of Mn, Cu, and Zn remained unchanged (Fig. 1). Because E[CO2] promoted the biomass of soybean (Table 2), there were pronounced dilution effects for K, Mg, Fe, B, Al and Na caused by E[CO2], for which the overall reductions were 13.60%, 10.25%, 36.41%, 11.05%, 15.53% and 45.02%, respectively (Fig. 1). Depending on the magnitude of the E[CO2]-induced dilution effect of mineral nutrients in crops, humans that consume these crops may encounter health problems related to microelement deficiencies (Uprety et al., 2010).
Because soybean plants generate nodules that provide nitrogen to the plants, we speculated that E[CO2] could affect soil nutrients following the growth of soybeans. However, E[CO2] conditions only decreased the levels of B and available K, while other parameters remained unchanged (Table 4). Organic matter and available nitrogen concentrations in the soil also remained unchanged after the growth of soybeans (Table 4). Soil pH at ambient and elevated CO2 concentrations was 5.51 and 5.49, respectively. E[CO2] promotes photosynthesis and, thus, soybean growth. This promotion of photosynthesis requires soybeans to absorb increased amounts of available K from the soil. The available K constitutes a relatively small portion of the K in the soil (Table 4), so the greater consumption of available K by soybean under E[CO2] may not affect the total K content in the soil. These results may indicate that in future climate scenarios, fertilizers containing increased amounts of K should be applied, and plants may need more fertilizer due to their accelerated growth.
| Mineral element | Content (g/kg) | Mineral element | Content (mg/kg) | ||
| Ambient | Elevated | Ambient | Elevated | ||
| Ca | 7.02 ± 0.32 | 6.94 ± 0.28 | Mn | 685.00 ± 18.71 | 703.33 ± 12.11 |
| K | 9.31 ± 0.24 | 9.24 ± 0.23 | Cu | 85.33 ± 3.26 | 87.08 ± 4.22 |
| Mg | 4.43 ±0.11 | 4.50 ± 0.08 | Zn | 97.52 ± 3.99 | 99.17 ± 2.28 |
| Fe | 53.59 ± 1.16 | 55.40 ± 3.60 | B | 54.23 ± 4.70 | 48.24 ± 2.37* |
| Al | 56.26 ± 2.04 | 57.65 ± 2.50 | Na | 1.32 ± 0.04 | 1.31 ± 0.04 |
| C | 155.25 ± 7.64 | 153.84 ± 6.90 | S | 491.67 ± 19.41 | 480.00 ± 17.89 |
| N | 8.52 ± 0.29 | 8.39 ± 0.53 | Hy.N | 720.50 ± 15.29 | 706.17 ± 6.43 |
| P | 1.00 ± 0.01 | 0.99 ± 0.02 | A.P | 12.48 ± 1.88 | 13.67 ± 1.20 |
| O.M. | 344.12 ± 9.29 | 341.97 ± 18.00 | A.K | 338.00 ± 74.47 | 254.8 ± 13.85* |
Isoflavones and tocopherols are important antioxidant compounds in soybean seeds. Using HPLC, we examined the levels of three isoflavones (daidzin, glycitin and genistin) and three tocopherols (α-, γ- and δ-tocopherol, the main tocopherols in soybean seeds). Compared with soybeans grown under ambient CO2 concentrations, soybeans grown under E[CO2] showed lower levels of all three isoflavones, and daidzin and glycitin decreased by 5.34% and 15.6%, respectively (Table 5). After E[CO2] treatment, the levels of α- and γ-tocopherol were maintained, but that of d-tocopherol greatly decreased (Table 5). Soybean plants grown under elevated CO2 had an average δ-tocopherol level of 170.76 μg/g (±14.92), representing a 15.95% decrease compared with that of plants grown under ambient CO2 conditions, which averaged 143.52 μg/g (±12.01) (Table 5). This E[CO2]-induced decrease in soybean seed antioxidant content may be due to decreased antioxidant capacity in leaves (Gillespie et al., 2011). The decrease in natural antioxidants in soybean seeds caused by E[CO2] may have a strong influence on human diet and on the sector of the food industry that produces isoflavone and tocopherol antioxidants from soybean seeds.
| Compound | Ambient CO2 | Elevated CO2 | Relative change (%) | |
| Isoflavone (mg/g) | Daidzin | 191.22 ±5.91 | 181.01 ±4.90* | -5.34% |
| Glycitin | 27.49 ± 3.21 | 23.20 ± 3.42* | -15.61% | |
| Genistin | 188.71 ± 11.93 | 185.41 ± 9.85 | -1.75% | |
| Tocopherol (mg/g) | α-tocopherol | 28.55 ± 3.05 | 30.12 ± 2.64 | 5.50% |
| γ-tocopherol | 216.41 ± 18.82 | 214.30 ± 17.18 | -0.98% | |
| δ-tocopherol | 170.76 ± 14.92 | 143.52 ± 12.01* | -15.95% |
Previous studies have suggested that CO2 enrichment may result in a decrease in saturated fatty acids due to a lower O2/CO2 ratio (Uprety et al., 2010). However, we did not find that CO2 elevation induced fatty acid peroxidation in the seeds, and the level of malondialdehyde (MDA) and degree of DBI did not show any difference between soybeans treated with ambient and elevated CO2 (Table 6). The concentration of most fatty acids was maintained after CO2 elevation, except for that of oleic acid, which decreased significantly from 55.85 to 45.70 mg/g (Table 6). The proportion of palmitic acid, stearic acid, and linoleic acid increased, while only the level of oleic acid decreased, and that of linolenic acid remained unchanged (Table 6). The increase in linoleic acid was approximately 2.5%, and the decrease in oleic acid was approximately 3.5% (Table 6).
| CO2 treatment | Fatty acid content (mg/g) | MDA (nmol/g) | |||||
| Palmitic acid 16:0 | Stearic acid 18:0 | Oleic acid 18:1 | Linoleic acid 18:2 | Linolenic acid 18:3 | MDA | ||
| Ambient | 27.35 ± 2.28 | 5.72 ± 0.53 | 55.85 ± 4.32 | 114.91 ± 12.45 | 31.28 ± 2.48 | 10.45 ± 0.51 | |
| Elevated | 27.04 ± 3.87 | 6.02 ± 0.83 | 45.07 ± 6.80* | 113.96 ± 16.90 | 29.75 ± 5.15 | 10.54 ± 0.39 | |
| CO2 treatment | Fatty acid composition (mol%) | DBI | |||||
| 16:0 | 18:0 | 18:1 | 18:2 | 18:3 | |||
| Ambient | 11.63 ±0.19 | 2.43 ± 0.08 | 23.83 ±2.13 | 48.79 ± 1.93 | 13.32 ± 0.66 | 1.61 ± 0.03 | |
| Elevated | 11.97 ±0.16* | 2.67 ± 0.04* | 20.34 ± 1.43* | 51.36 ± 1.21* | 13.66 ± 0.17 | 1.63 ± 0.02 | |
Soybean oil is a good source of linoleic and linolenic fatty acids, which directly benefit human health. It has been reported that linoleic acid is very important for lowering blood cholesterol (Shenolikar, 1980; Uprety et al., 2010). The increase in linoleic acid caused by E[CO2] is thus a beneficial change in the quality of oil for human consumption. E[CO2] has a major influence on the composition of fatty acids in other plant species. For example, linoleic acid levels have been shown to increase in CO2-enriched seeds of Brassica plants and sunflower (Pal et al., 2014; Uprety et al., 2010). Oleate desaturase, which desaturates oleic acid to linoleic acid, is sensitive to environmental changes, and reductions in oleic acid are usually associated with an increase in linoleic acid and vice versa (Izquierdo et al., 2009; Zapata et al., 1987). Photosynthetic oxygen supply and concentration of O2 in different seed tissues can affect fatty acid metabolism (Borisjuk and Rolletschek, 2009; Rolletschek et al., 2005; Vigeolas et al., 2003), and an increase in O2 supply can increase the activity of oleate desaturase and promote the content of linoleic acid (Rolletschek et al., 2007). In our experiment, E[CO2] accelerated the photosynthesis of soybeans at all growth stages, which may increase the O2 supply, thereby increasing oleate desaturase activity and, ultimately, linoleic acid content. The change in the proportion of soybean seed oil caused by E[CO2] would be beneficial to human health.
4. ConclusionsIn this study, we found that elevated CO2 concentrations increased photosynthesis at all soybean growth stages. This increase in photosynthesis may be related to the nitrogen-fixing capacity of soybean. E[CO2] caused decreases in the levels of most mineral nutrients in the leaves, which may cause malnutrition in plants. However, in our experiments, E[CO2] had no effect on soil nutrients. E[CO2] induced decreases in natural antioxidants, including tocopherols and isoflavones in soybean seeds. Furthermore, E[CO2] may have a dilution effect on K, Mg, Fe, B, Al and Na in the seeds. Finally, E[CO2] caused a decrease in the proportion of oleic acid, but the proportion of linoleic acid increased, which is beneficial with respect to human consumption. Our experiment is a preliminary study of the response of soybeans to E[CO2] under growth chamber conditions. In field conditions, light, temperature, and humidity change greatly, therefore future studies should focus on the response of soybean to E[CO2] under field conditions.
Author contributionsG.Z and J.C. conducted the experiments. W.L. and G.Z. planned the research. G.Z. designed the experiments. G.Z. and W.L. wrote the manuscript.
Declaration of Competing InterestThe authors declare no competing financial interest.
AcknowledgmentsThis work was supported by the High Level Talents Project of Yunnan University of Chinese Medicine (2019YZG07), Yunnan Provincial Department of Education Science Research Funding (2018JS290), the Yunnan Applied Basic Research Project (2016FA042, 2017FB057 and 2015FB171), Discipline funding of School of Chinese Material Medica, Yunnan University of Chinese Medicine (2019ZY014), grants from the National Natural Science Foundation of China (31600215, 31401313), and by Joint Special Funds for Basic Research of Yunnan Local College. We thank Dr. Shihong Luo for help with the analysis of the isoflavones and tocopherols. We also thank Zhen Yu for assistance with the GCeMS analysis.
Ainsworth E.A., Beier C., Calfapietra C., Ceulemans R., Durand-Tardif M., Farquhar G.D., et al, 2008. Next generation of elevated[CO2] experiments with crops:a critical investment for feeding the future world. Plant Cell Environ, 31: 1317-1324. DOI:10.1111/j.1365-3040.2008.01841.x |
Ainsworth E.A., Davey P.A., Bernacchi C.J., Dermody O.C., Heaton E.A., Moore D.J., et al, 2002. A meta-analysis of elevated[CO2] effects on soybean (Glycine max) physiology, growth and yield. Glob. Chang. Biol, 8: 695-709. DOI:10.1046/j.1365-2486.2002.00498.x |
Ainsworth E.A., Rogers A., Blum H., Nosberger J., Long S.P., 2003. Variation in acclimation of photosynthesis in Trifolium repens after eight years of exposure to free air CO2 enrichment (FACE). J. Exp. Bot, 54: 2769-2774. DOI:10.1093/jxb/erg309 |
Arenque B.C., Grandis A., Pocius O., de Souza A.P., Buckeridge M.S., 2014. Responses of Senna reticulata, a legume tree from the Amazonian floodplains, to elevated atmospheric CO2 concentration and waterlogging. Trees Struct. Funct, 28: 1021-1034. DOI:10.1007/s00468-014-1015-0 |
Bernacchi C.J., Leakey A.D.B., Heady L.E., Morgan P.B., Dohleman F.G., McGrath J.M., et al, 2006. Hourly and seasonal variation in photosynthesis and stomatal conductance of soybean grown at future CO2 and ozone concentrations for 3 years under fully open-air field conditions. Plant Cell Environ, 29: 2077-2090. DOI:10.1111/j.1365-3040.2006.01581.x |
Bishop K.A., Betzelberger A.M., Long S.P., Ainsworth E.A., 2015. Is there potential to adapt soybean (Glycine max Merr.) to future[CO2]? An analysis of the yield response of 18 genotypes in free-air CO2 enrichment. Plant Cell Environ, 38: 1765-1774. DOI:10.1111/pce.12443 |
Bloom A.J., Burger M., Rubio-Asensio J.S., Cousins A.B., 2010. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science, 328: 899-903. DOI:10.1126/science.1186440 |
Bloom A.J., Smart D.R., Nguyen D.T., Searles P.S., 2002. Nitrogen assimilation and growth of wheat under elevated carbon dioxide. Proc. Natl. Acad. Sci. U. S. A, 99: 1730-1735. DOI:10.1073/pnas.022627299 |
Borisjuk L., Rolletschek H., 2009. The oxygen status of the developing seed. New Phytol, 182: 17-30. DOI:10.1111/j.1469-8137.2008.02752.x |
Caldwell C.R., Britz S.J., Mirecki R.M., 2005. Effect of temperature, elevated carbon dioxide, and drought during seed development on the isoflavone content of dwarf soybean[Glycine max (L.) Merrill] grown in controlled environments. J. Agric. Food Chem, 53: 1125-1129. DOI:10.1021/jf0355351 |
Carrera C., Martínez M.J., Dardanelli J., Balzarini M., 2011. Environmental variation and correlation of seed components in nontransgenic soybeans:protein, oil, unsaturated fatty acids, tocopherols, and isoflavones. Crop Sci, 51: 800-809. DOI:10.2135/cropsci2010.06.0314 |
Cernusak L.A., Winter K., Martinez C., Correa E., Aranda J., Garcia M., et al, 2011. Responses of legume versus nonlegume tropical tree seedlings to elevated CO2 concentration. Plant Physiol, 157: 372-385. DOI:10.1104/pp.111.182436 |
Duan B., Lu Y., Yin C., Junttila O., Li C., 2005. Physiological responses to drought and shade in two contrasting Picea asperata populations. Physiol. Plant, 124: 476-484. DOI:10.1111/j.1399-3054.2005.00535.x |
Ekman Å., Bülow L., Stymne S., 2007. Elevated atmospheric CO2 concentration and diurnal cycle induce changes in lipid composition in Arabidopsis thaliana. New Phytol, 174: 591-599. DOI:10.1111/j.1469-8137.2007.02027.x |
Franks P.J., Adams M.A., Amthor J.S., Barbour M.M., Berry J.A., Ellsworth D.S., et al, 2013. Sensitivity of plants to changing atmospheric CO2 concentration:from the geological past to the next century. New Phytol, 197: 1077-1094. DOI:10.1111/nph.12104 |
Gillespie K.M., Rogers A., Ainsworth E.A., 2011. Growth at elevated ozone or elevated carbon dioxide concentration alters antioxidant capacity and response to acute oxidative stress in soybean (Glycine max). J. Exp. Bot, 62: 2667-2678. DOI:10.1093/jxb/erq435 |
Högy P., Wieser H., Kohler P., Schwadorf K., Breuer J., Erbs M., et al, 2009. Does elevated atmospheric CO2 allow for sufficient wheat grain quality in the future? J. Appl. Bot. Food Qual, 82: 114-121. |
IPCC, 2007. Climate change 2007: the physical science basis. In: Solomon, S., Qin, D., Manning, M., Marquis, M., Averyt, K., Tignor, M.M.B., Miller, Henry LeRoy, Chen, Z. (Eds.), Contribution of Working Group Ⅰ to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp. 1-18.
|
Izquierdo N.G., Aguirrezábal L.A.N., Andrade F.H., Geroudet C., Valentinuz O., Pereyra Iraola M., 2009. Intercepted solar radiation affects oil fatty acid composition in crop species. Field Crop. Res, 114: 66-74. DOI:10.1016/j.fcr.2009.07.007 |
Leakey A.D.B., 2009. Rising atmospheric carbon dioxide concentration and the future of C4 crops for food and fuel. Proc. R. Soc. B Biol. Sci, 276: 2333-2343. DOI:10.1098/rspb.2008.1517 |
Lenka N.K., Lenka S., Thakur J.K., Elanchezhian R., Aher S.B., Simaiya V., et al, 2017. Interactive effect of elevated carbon dioxide and elevated temperature on growth and yield of soybean. Curr. Sci, 113: 2305-2310. DOI:10.18520/cs/v113/i12/2305-2310 |
Li W., Li M., Zhang W., Welti R., Wang X., 2004. The plasma membrane-bound phospholipase Ddelta enhances freezing tolerance in Arabidopsis thaliana. Nat. Biotechnol, 22: 427-433. DOI:10.1038/nbt949 |
Liu, K.S., 1997. Chemistry and nutritional value of soybean components. In: Liu, K.S.(Ed.), Soybeans: Chemistry, Technology and Utilization. Chapman & Hall, New York, pp. 15-113.
|
Locke A.M., Sack L., Bernacchi C.J., Ort D.R., 2013. Soybean leaf hydraulic conductance does not acclimate to growth at elevated[CO2] or temperature in growth chambers or in the field. Ann. Bot, 112: 911-918. DOI:10.1093/aob/mct143 |
Loladze I., 2002. Rising atmospheric CO2 and human nutrition:toward globally imbalanced plant stoichiometry? Trends Ecol. Evol, 17: 457-461. |
Ma L., Li B., Han F., Yan S., Wang L., Sun J., 2015. Evaluation of the chemical quality traits of soybean seeds, as related to sensory attributes of soymilk. Food Chem, 173: 694-701. DOI:10.1016/j.foodchem.2014.10.096 |
Miquel M., Browse J., 1992. Arabidopsis mutants deficient in polyunsaturated fatty acid synthesis. Biochemical and genetic characterization of a plant oleoylphosphatidylcholine desaturase. J. Biol. Chem, 267: 1502-1509. |
Myers S.S., Zanobetti A., Kloog I., Huybers P., Leakey A.D., Bloom A.J., et al, 2014. Increasing CO2 threatens human nutrition. Nature, 510: 139-142. DOI:10.1038/nature13179 |
Pal M., Chaturvedi A., Pandey S., Bahuguna R., Khetarpal S., Anand A., 2014. Rising atmospheric CO2 may affect oil quality and seed yield of sunflower(Helianthus annus L.). Acta Physiol. Plant, 36: 2853-2861. DOI:10.1007/s11738-014-1651-4 |
Prior S.A., Runion G.B., Rogers H.H., Arriaga F.J., 2010. Elevated atmospheric carbon dioxide effects on soybean and sorghum gas exchange in conventional and notillage systems. J. Environ. Qual, 39: 596-608. DOI:10.2134/jeq2009.0181 |
Rogers A., Gibon Y., Stitt M., Morgan P.B., Bernacchi C.J., Ort D.R., Long S.P., 2006. Increased C availability at elevated carbon dioxide oncentraiton improves N assimilation in a legume. Plant Cell Environ, 29: 1651-1658. DOI:10.1111/j.1365-3040.2006.01549.x |
Rolletschek H., Borisjuk L., Sánchez-García A., Gotor C., Romero L.C., Martínez-Rivas J.M., et al, 2007. Temperature-dependent endogenous oxygen concentration regulates microsomal oleate desaturase in developing sunflower seeds. J. Exp. Bot, 58: 3171-3181. DOI:10.1093/jxb/erm154 |
Rolletschek H., Radchuk R., Klukas C., Schreiber F., Wobus U., Borisjuk L., 2005. Evidence of a key role for photosynthetic oxygen release in oil storage in developing soybean seeds. New Phytol, 167: 777-786. DOI:10.1111/j.1469-8137.2005.01473.x |
Rosenthal D.M., Ruiz-Vera U.M., Siebers M.H., Gray S.B., Bernacchi C.J., Ort D.R., 2014. Biochemical acclimation, stomatal limitation and precipitation patterns underlie decreases in photosynthetic stimulation of soybean (Glycine max) at elevated[CO2] and temperatures underfully open air field conditions. Plant Sci, 226: 136-146. DOI:10.1016/j.plantsci.2014.06.013 |
Saha S., Chakraborty D., Sehgal V.K., Pal M., 2015. Potential impact of rising atmospheric CO2 on quality of grains in chickpea (Cicer arietinum L.). Food Chem, 187: 431-436. DOI:10.1016/j.foodchem.2015.04.116 |
Sakurai G., Iizumi T., Nishimori M., Yokozawa M., 2014. How much has the increase in atmospheric CO2 directly affected past soybean production?. Sci. Rep, 4: 4978. |
Sanz-Saez A., Koester R.P., Rosenthal D.M., Montes C.M., Ort D.R., Ainsworth E.A., 2017. Leaf and canopy scale drivers of genotypic variation in soybean response to elevated carbon dioxide concentration. Glob. Chang. Biol: 1-13. |
Shenolikar I., 1980. Fatty-acid profile of myocardial lipid in populations consuming different dietary fats. Lipids, 15: 980-982. DOI:10.1007/BF02534427 |
Sun J., Feng Z., Leakey A.D.B., Zhu X., Bernacchi C.J., Ort D.R., 2014. Inconsistency of mesophyll conductance estimate causes the inconsistency for the estimates of maximum rate of Rubisco carboxylation among the linear, rectangular and non-rectangular hyperbola biochemical models of leaf photosynthesisda case study of CO2 enrichment and leaf aging effects in soybean. Plant Sci, 226: 49-60. DOI:10.1016/j.plantsci.2014.06.015 |
Taub D.R., Miller B., Allen H., 2008. Effects of elevated CO2 on the protein concentration of food crops:a meta-analysis. Glob. Chang. Biol, 14: 565-575. DOI:10.1111/j.1365-2486.2007.01511.x |
Thomas J.M.G., Boote K.J., Allen L.H., Gallo-Meagher M., Davis J.M., 2003. Elevated temperature and carbon dioxide effects on soybean seed composition and transcript abundance. Crop Sci, 43: 1548-1557. DOI:10.2135/cropsci2003.1548 |
Uprety D.C., Sen S., Dwivedi N., 2010. Rising atmospheric carbon dioxide on grain quality in crop plants. Physiol. Mol. Biol. Plants, 16: 215-227. DOI:10.1007/s12298-010-0029-3 |
Vigeolas H., van Dongen J.T., Waldeck P., Hühn D., Geigenberger P., 2003. Lipid storage metabolism is limited by the prevailing low oxygen concentrations within developing seeds of oilseed rape. Plant Physiol, 133: 2048-2060. DOI:10.1104/pp.103.031963 |
Wang D., Heckathorn S.A., Wang X.Z., Philpott S.M., 2012. A meta-analysis of plant physiological and growth responses to temperature and elevated CO2. Oecologia, 169: 1-13. DOI:10.1007/s00442-011-2172-0 |
Ward J.K., Samanta Roy D., Chatterjee I., Bone C.R., Springer C.J., Kelly J.K., 2012. Identification of a major QTL that alters flowering time at elevated[CO2] in Arabidopsis thaliana. PLoS One, 7: e49028. DOI:10.1371/journal.pone.0049028 |
Wieser H., Manderscheid R., Erbs M., Weigel H.J., 2008. Effects of elevated atmospheric CO2 concentrations on the quantitative protein composition of wheat grain. J. Agric. Food Chem, 56: 6531-6535. DOI:10.1021/jf8008603 |
Yang W., Cahoon R.E., Hunter S.C., Zhang C., Han J., Borgschulte T., et al, 2011. Vitamin E biosynthesis:functional characterization of the monocot homogentisate geranylgeranyl transferase. Plant J, 65: 206-217. DOI:10.1111/j.1365-313X.2010.04417.x |
Yu X.M., Li A.H., Li W.Q., 2015. How membranes organize during seed germination:three patterns of dynamic lipid remodelling define chilling resistance and affect plastid biogenesis. Plant Cell Environ, 38: 1391-1403. DOI:10.1111/pce.12494 |
Zapata F., Danso S.K.A., Hardarson G., Fried M., 1987. Time course of nitrogen fixation in field-grown soybean using nitrogen-15 methodology. Agron. J, 79: 172-176. DOI:10.2134/agronj1987.00021962007900010035x |
Zhang F.-F., Wang Y.-L., Huang Z.-Z., Zhu X.-C., Zhang F.-J., Chen F.-D., et al, 2012. Effects of CO2 enrichment on growth and development of Impatiens hawkeri. Sci. World J, 2012: 601263. |



