Being sessile does not pose a curse to the plants, but opens the gate of opportunity for plants to utilize each aspect of every single entity available to it. The entity either becomes the part of its system or acts as a signal to specify function for maintaining the living state of the plant. Reactive oxygen species (ROS) qualify as an example to justify this viewpoint. ROS are the reactive products of oxygen that have the potential to damage the essential components of a living cell. Existence of ROS in the living system provides direct evidence to their origin (Czarnocka and Karpiński, 2018). Thus to acquire functional complexity, ROS gets enough time to deep down their rooting in origin and presence in all events of an organism life cycle. Shifting from its negative role to positive one has provided foundation to refresh our insight and to broaden it in its efficient and effective routes. Reactive oxygen species (ROS) are the reactive products of oxygen that damage essential components of living cells. More recently, ROS have been shown to play important roles in plant growth and development. For instance, ROS actively participate in germination and flowering, as well as the development of root apical meristem and shoot apical meristem, root hair cells and pollen tubes, leaves, and lateral roots (Noctor et al., 2018; Mittler, 2017).
2. Sites of ROS production and ROS-scavengingROS production is important in plant cells because of its direct link with basic metabolic processes. ROS production is interlinked with ROS scavenging and cell survival depends on this link. The cell undergoes three states starting from ROS production, oxidative stress (ROS accumulation), and detoxification (Fig. 1) (Czarnocka and Karpiński, 2018). Whatever may be the reason of ROS production, the metabolic processes of life and natural selection does not eliminate the ROS production and detoxification. One important question is why ROS production is not naturally excluded? One of the more significant reasons has received much attention recently. Disruptions in the ROS production-scavenging cycle lead to ROS-mediated oxidative stress-induced damage, which may be lethal. ROS production sites in plant cells include chloroplasts, mitochondria, peroxisomes, and apoplasts (Table 1) (Corpas et al., 2015).
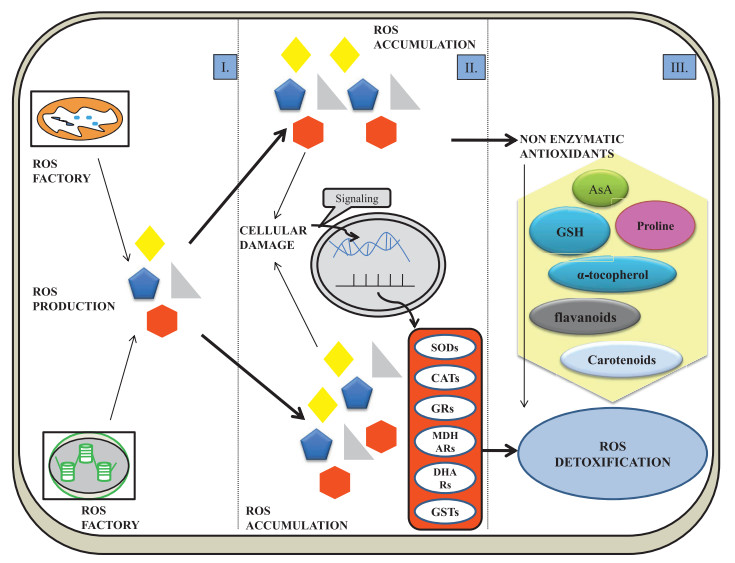
|
| Fig. 1 Fate of ROS in cell. Ⅰ. ROS production Ⅱ. Oxidative stress: ROS accumulation-induced cellular damage causes the production of antioxidant components Ⅲ. Detoxification: Enzymatic and non-enzymatic antioxidants (Proline, Carotenoids, Alpha-tocopherol, Glutathione, Ascorbic acid, Flavanoids and Carotenoids) act as ROS detoxicants. Superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), guaiacol peroxidase (POX), monodehydroascorbate reductase (MDHAR), glutathione-S-transferase (GSTs), dehydroascorbate reductase (DHAR), glutathione reductase (GR), ascorbate (AsA) and glutathione (GSH) |
| S. no. | Site of production | Cause of ROS | ROS | Factors favoring ROS production | Functional status | References |
| 1. | Cell wall | Class Ⅲ peroxidases (PRXs), germin-like oxalate oxidases, amine oxidases, lipoxygenases, and quinone reductase | O2- and H2O2 | Ozone, high light, salinity, heavy metal, cold, heat, wounding and pathogen | Apoplastic polyamine-dependent programmed cell death induced by Ca2+ influx across the plasma membrane | Pottosin et al. (2014) |
| 2. | Plasma membrane | NADPH oxidases | O2- and H2O2 | Ozone, high light, salinity, heavy metal, cold, heat, wounding and pathogen | Root hairs cell expansion, pollen tube growth, seed after-ripening, defense against pathogens, innate immunity and responses to abiotic stress | Wang et al.(2017) |
| 3. | Chloroplast | Reaction centers | 1O2, O2- and H2O2 | High light, UV radiation, low CO2, heat, cold, drought and pathogen | Local and systemic signaling, communication for proper non-photochemical quenching and develop systemic acquired acclimation or resistance | Wituszyńska and Karpiński (2013) |
| 4. | Mitochondria | Mitochondrial electron transport chain | O2- and H2O2 | Heat, cold, drought, salinity, high light, UV radiation, heavy metal and hypoxia | Release of cytochrome c from mitochondria for programmed cell death | Aken and Van Breusegem (2015) |
| 5. | Peroxisomes | Photorespiration and the fatty acid b-oxidation pathway | O2- and H2O2 | High light, low CO2, heat, salinity, drought and pathogen | Seed and pollen germination, and for stomatal movement senescence and fruit ripening | Corpas et al. (2017) |
The chloroplast is a major source of ROS production in plants. Within the chloroplast, ROS production is largely caused by incomplete oxidation of water, plastosemi-hydroquinone H2O2, and over-excitation of PSI (Fig. 2) (Pospíšil, 2016). H2O2 is produced via the action of chloroplastic superoxide dismutase on O2- and subsequently is converted to H2O by ascorbate and glutathione (Chang et al., 2009). Singlet oxygen (1O2) is produced in the chloroplast under stress conditions (Wituszyńska and Karpiński, 2013). Excess light, for example, affects electron distribution stabilization, which leads to the formation of triplet chlorophyll, generating 1O2 (Tripathy and Oelmüller, 2012). 1O2 provokes the loss of PSII via dysfunction of D1 polypeptide and pigment destruction. Incomplete oxidation of water, plastosemi-hydroquinone H2O2 and overexcited PSI are the main causes involved in ROS production (Fig. 2) (Pospíšil, 2016). H2O2 results from the action of chloroplastic superoxide dismutase on O2- and subsequently convert to H2O by ascorbate and glutathione (Chang et al., 2009).

|
| Fig. 2 Diagrammatic representation showing ROS production and cellular damage. A. Mitochondria (major site for ROS production via electron transport chain). B. Chloroplast (photosystems on thylakoids membrane generate ROS in the form of singlet oxygen and superoxide anions). C. Plasma membrane (NADPH oxidase and peroxidases at the membrane level produce ROS). D. Biomolecular damage (ROS accumulation causes protein degradation, lipid peroxidation and DNA damage) |
Mitochondria are also a site of ROS such as O2-. The mitochondrial electron transport chain (ETC) has a sufficient supply of electrons to reduce O2 to form ROS. Two main components of themtETC that act as electron donor agents in the production of ROS are Complex Ⅰ and Complex Ⅱ (Fig. 2). Mitochondria generally produce ROS during respiration, but ROS production increases under stress conditions, which may lead to programmed cell death. To counteract oxidative stress, ALTERNATIVE OXIDASE 1 (AOX1) and Mitochondrial SOD (Mn-SOD) are critical. AOX1 maintains a reduced state and decreases ROS production. The importance of AOX1 is clear from studies that show when AOX1 gene expression isdecreased, cell deathincreases (Robson and Vanlerberghe, 2002). Mn-SOD acts in the matrix to detoxify O2- into O2 and H2O2 (Navrot et al., 2007; Cvetkovska et al., 2013). Although ROS play significantly different roles in the mitochondria of animals and plants, ROS-mediated cell death in both requires cytochrome c (Mittler, 2017).
Peroxisomes, which are the sites of photorespiration and β-oxidation of fatty acid, also produce ROS. O2- is generated in the peroxisome matrix by the electron transport chain and the action of xanthine oxidase. Other sources of ROS include the oxidation of glycolate and degradation of fatty acids. Glycolate, which is the final product of photorespiration in the chloroplast, enters the peroxisome where it is oxidized to glyoxylate with the aid of O2, leading to H2O2 production. The degradation of fatty acid by acyl-CoA oxidase also leads to H2O2 production. The ROS produced in the peroxisome are utilized in seed and pollen germination, fruit ripening, senescence, and stomatal movement (Corpas et al., 2017).
The apoplast is another source for ROS production, especially H2O2. In the apoplastic plasma membrane, NADPH oxidase in the main source of O2-, which is further metabolized to H2O2 by superoxide dismutase (Fig. 2) (Marino et al., 2012; Mittler, 2017). Enzymes localized in the cell wall that are responsible for apoplastic ROS production include class Ⅲ peroxidases, amine oxidases, germin-like oxalate oxidases, quinine reductase and lipoxygenases (Camejo et al., 2016).
The ROS scavenging system encompasses ROS homeostasis to ROS-dependent signaling under various environmental stresses (Noctor et al., 2018). ROS scavengers include ROS enzymatic as well as non-enzymatic systems that switch the cell from stressed to non-stressed phase. Enzymatic scavengers include Ascorbate Peroxidase (APX), Catalase (CAT), Glutathione Reductase (GRs), Superoxide Dismutase (SOD), Dehydroascorbate Reductase (DHAR), Glutathione-S-Transferase (GSTs) and Glutathione Peroxidase (GPX). The non-enzymatic scavenger system includes glutathione, α-tocopherol, flavonoids, carotenoids and proline. Both of these systems mitigate oxidative stress-induced damages (Wituszyńska and Karpiński, 2013).
3. ROS as a driver of cellular proliferation and differentiation during developmentIn multicellular organisms, the key events that determine growth and development are cell division and cellular differentiation. Disruption of the equilibrium between these two events leads to early termination of organogenesis, which may result in abnormal growth (Zhang et al., 2008). The transition from cellular proliferation to cell elongation during the earlier stages of differentiation are regulated by ROS homeostasis (Tsukagoshi et al., 2010). In Arabidopsis root, two ROS, oxygen radicals (O2-) and H2O2, are differentially distributed. H2O2 accumulates in the elongation region, whereas oxygen radicals are located in the meristematic region (Dunand et al., 2007). In the zone of transition, however, the distribution of O2- and H2O2 overlap. The equilibrium between these two ROS is under the control of UPBEAT1 (UPB1), a basic helix loop helix transcriptional factor that is upregulated in the transition region of roots (Tsukagoshi et al., 2010). Mutant plants that overexpress UPB1 have reduced root size due to smaller meristem size and fewer mature cells, while upb1 mutants in which the UPB1 transcription factor is absent are characterized by larger meristem size with elongated root cells. UPB1 negatively regulates the genes that encode for a set of peroxidases. Interestingly, positional information in the transition region is provided by a gradient of oxygen radicals and H2O2, which is required for cellular proliferation and differentiation. Hence, by mediating the gene expression of peroxidases, the requisite balance between oxygen radicals and H2O2 is maintained.
For the differentiation of root hairs PFT1/MED25 (mediator complex PHYTOCHROME AND FLOWERING TIME 1) and MED8 are essential. PFT1/MED25 are preferentially confined for cell growth whereas MED8 independently regulates the organ growth and ROS homeostasis in roots (Xu and Li, 2011). During the differentiation of root hairs, PFT1 causes the production of ROS by promoting class Ⅲ peroxidases to maintain balance elucidated by transcriptional profiling.
4. The function of ROS in diverse development stages 4.1. Seed dormancy and seed germinationROS play a key role seed dormancy and germination in Arabidopsis thaliana (Leymarie et al., 2012), wheat (Ishibashi et al., 2008), barley (Bahin et al., 2011), sunflower (Oracz et al., 2007) and cress (Müller et al., 2009). In dry seeds, enzymatic activity is low and lipid peroxidation serves as a source of ROS. In hydrated seeds, increased metabolism is correlated with ROS production in chloroplasts, mitochondria, glyoxysomes, peroxisomes, and the plasma membrane (McDonald, 1999; Bailly, 2004). During seed imbibition, subcellular compartmentalization of ROS and their target molecule regulates the expression of various genes. Unlike dry seeds in which ROS production sites must be near targets (Bailly et al., 2008), during seed imbibition, water allows the translocation of ROS (e.g., H2O2) over greater distances. Therefore, in addition to altering the transcriptional activity of genes, ROS facilitate the oxidation of several components, stimulating transcription factors for signaling (Laloi et al., 2004). For instance, most labile H2O2 messenger depends upon the redox state of active proteins to accelerate the redox sensitive transcriptional factor for activating downstream cascades, triggering the MAPKs and oxidation of precise peptides (Petrov and Van Breusegem, 2012; Foyer and Noctor, 2013). Such oxidized proteins inhibit translation by oxidizing mRNA and also drive germination.
During seed germination, ROS are known to play roles in endosperm deterioration, seed reserve mobilization, pathogen defense, and programmed cell death (Fig. 6) (El-Maarouf-Bouteau and Bailly, 2008; El-Maarouf-Bouteau et al., 2013). ROS action during seed germination relies on interactions with abscisic acid (ABA), gibberellic acid (GA), and ethylene (ET), phytohormones that regulate seed dormancy and germination. In cereals, ROS-mediated effects on germination are inhibited by ABA via the promotion of ROS-scavenging enzyme activity. However, these effects are counteracted by GA, which downregulates ROS-scavenging enzymes and induce ROS-mediated programmed cell death. ROS mediate cell wall polysaccharide deterioration and the activation of calcium (Ca2+) channels and MAPKs, which allows radicle enlargement (Diaz-Vivancos et al., 2013). The weakening of cell wall polysaccharide chains is regulated by apoplastic OH radicals, which mediate the breakdown of chitosan, pullulan-like polysaccharides, and hyaluronate (Stern et al., 2007). When GA interacts with the aleurone layer, it triggers alpha-amylase synthesis and releases other hydrolytic enzymes. In contrast, enzymes of the antioxidant system repress the production of hydrolytic enzymes. ABA acts as a negative regulator in seed germination via suppression of ROS production, although it also acts as a positive regulator that induces dormancy (Ishibashi et al., 2012; Finkelstein et al., 2008). Another phytohormone that plays a positive regulatory role in seed germination is ethylene. For instance, in soybean seeds ethylene production becomes elevated as a result of ROS production during imbibition (Ishibashi et al., 2013). During germination, ethylene and ABA antagonistically regulate seed germination (Fig. 4) (Arc et al., 2013).
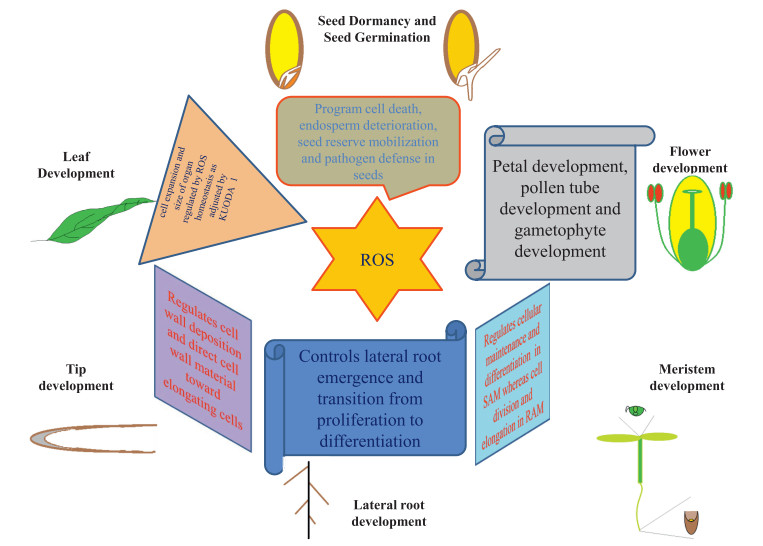
|
| Fig. 6 Schematic overview of the ROS functional aspects at different phases of plant development |
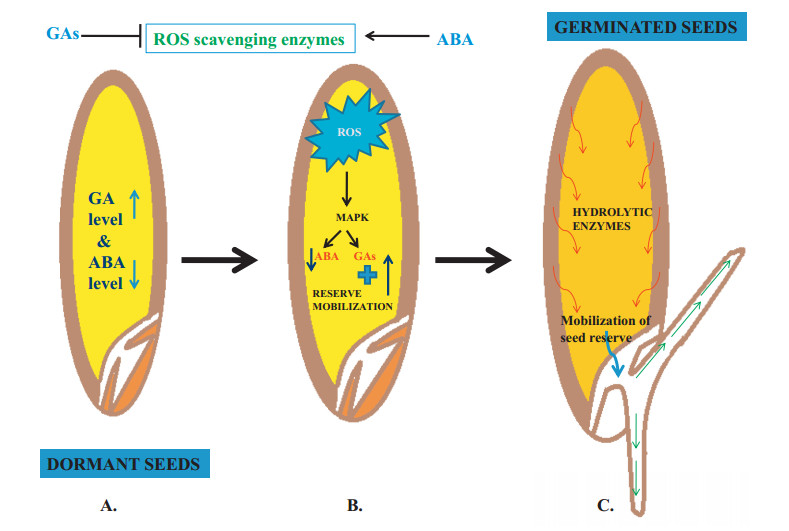
|
| Fig. 4 Model depicting the role of ROS in seed dormancy and germination. The levels of phytohormones ABA and GAs regulate ROS scavenging. ROS signaling mediates transcription factor MAPKs to activate the hydrolytic enzymes responsible for reserve degradation and its mobilization. MAPK (mitogen activating protein kinase) |
The ratio of ABA and GA regulates seed dormancy. Disturbance to this balance directly affects seed dormancy. For instance, high ABA/GA ratios favour dormancy, whereas low ABA/GA ratios result in a break in dormancy. The exogenous application of H2O2 decreases ABA levels and increases GA concentrations, which triggers the release of dormancy (https://www.frontiersin.org/articles/10.3389/fpls.2016.00864/full, Graeber et al., 2010; Oracz and Karpinski, 2016). Studies on the crosstalk between ROS-ABA and Ethylene-GA explain the role of ROS in the alleviation of seed dormancy in barley (Bahin et al., 2011). The establishment of crosstalk between ROS and hormonal signaling in seeds also triggers dormancy release, after-ripening, and germination (https://www.frontiersin.org/articles/10.3389/fpls.2016.00864/full, Bahin et al., 2011). Accumulation of ROS takes place apoplastically in the radicle and endosperm region of seeds under the regulation of hormones. In Lepidium sativum, ABA inhibits endosperm rupture, whereas GA counteracts the effect of ABA in the radicle to stimulate seed germination (https://www.frontiersin.org/articles/10.3389/fpls.2016.00864/full, Graeber et al., 2010).
4.2. Meristem developmentIn both the shoot apical meristem (SAM) and the root apical meristem (RAM), stem cells are organized in a central zone (CZ) surrounding an organizing center called the organizing zone (OZ), or quiescent center (QC). The maintenance of the meristem relies on exchange of information between the OZ/QC and central zone as well as feedback from differentiated tissues. The chief differences between root and shoot meristem development are the gene networks that regulate their activity and their response to growth hormones. Notably, the activity of SAM and RAM has been shown to be affected by the interplay between ROS, redox components and phytohormones (Schippers et al., 2016).
RAM activity is responsive to changes in cellular redox status. For instance, application of exogenous H2O2 reduces the number of meristem cells in the RAM (Tsukagoshi et al., 2010). In addition, DNA damage leads to H2O2 accumulation through FLAVINCONTAINING MONOOXYGENASE 1, and decreases root meristem size, indicating that H2O2 acts as a negative regulator of the RAM. Gradients in ROS have been reported in the different zones of roots, with H2O2 peaks in the zone of elongation, and O2- peaks in the zone of cell division (Fig. 5) (Rubio-Diaz et al., 2012; Tsukagoshi, 2016). Such distributions suggest that O2- and H2O2 act antagonistically (Zeng et al., 2017). Both O2- and H2O2 are associated with the maintenance and differentiation in the SAM by regulating the expression of the transcription factor WUS (Figs. 5 and 6; Table 2). O2- up regulates WUS expression, whereas H2O2 accumulates in the peripheral zone and is associated with cell differentiation (see Fig. 5).
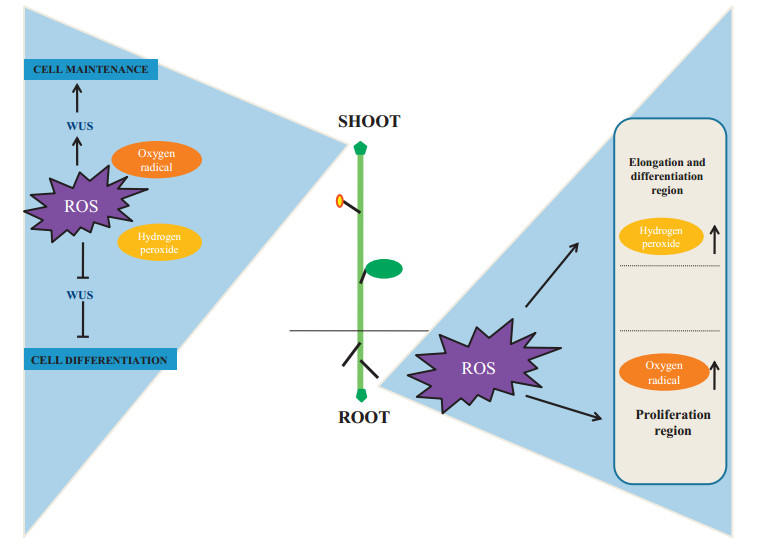
|
| Fig. 5 Differential response of ROS components in meristem development. In the SAM, H2O2 and O2- regulate the expression of the WUSCHEL (WUS) gene during cellular differentiation and maintenance respectively. In the root tip, increase in H2O2 induces elongation and differentiation whereas O2- induces proliferation SAM (Shoot apical meristem), WUS (WUSCHEL). |
| S. no. | Developmental event | Site of action | ROS action | ROS type | Plant | References |
| 1. | Release of dormancy and germination | Seed | Oxidation of specific peptides and MAPKs activation | H2O2 | Zinnia elegans, maize, wheat and soybean | Singh et al., (2016); Basbouss-Serhal et al., (2017) |
| 2. | Seed germination | Aleurone cells | PCD of aleurone layer | — | Cereal seeds | Yadegari and Drews (2004) |
| 3. | Leaf development | Leaves meristematic cells | Cell wall loosening and rigid cross-linking of cell wall components | H2O2 and O-2 | A. thaliana | Lu et al. (2014) |
| 4. | Leaf senescence | Senescent leaves | Crucial in regulation of senescence signaling | 1O2, H2O2 | — | Bhattacharjee (2012) |
| 5. | Senescence | Foral meristems | Protect the initial growth of the ovule, sepals, and petals after accomplishing the death of petal cells | H2O2 | Daylily plant | Halliwell and Gutteridge (1989); Tripathi and Tuteja (2007) |
| 6. | Development of trichome | Trichome initials | Switched the mitosis to endoreduplication, branching of cells, expansion, and cell death | H2O2 burst | - | Hulskamp (2004) |
| 7. | Development of male sex organs | Tapetal cells | Dictate the correct timing of tapetal PCD | H2O2 | A. thaliana | Durme and Nowack (2016) |
| 8. | Development of pollen tube on pistil | Pistil | Attract and guide the pollen tube growth by deteriorating the cells of pistil in a programmed manner & activating Ca2+ permeable channels to alter the cell wall extensibility | - | A. thaliana, | Duan et al., (2014); Lassig et al., (2014) |
| 9. | Self-incompatibility during pollination | Stigma | Induced PCD in incompatible pollen | H2O2 | Papaver | Wilkins et al. (2011) |
| 10. | Development of xylem tracheary elements | Vascular bundle cells | secondary wall differentiation or trigger xylem differentiation by PCD | H2O2 | - | Ros Barceló (2005) |
| 11. | Development of aerenchyma | Internode of stem | Induced PCD | H2O2 | rice | Steffens et al. (2011) |
| 12. | Rhizogenesis | Root | — | O2- (oxygen radical) | — | Libik-Konieczny et al. (2015) |
| 13. | Lateral root development | Root | Promoting transition from cellular proliferation to differentiation | H2O2 and O2- | A. thaliana | Manzano et al. (2014) |
| 14. | Root hair development | Epidermal root cells | Activation of MAPKs cascade | — | A. thaliana | Mangano et al. (2016) |
QC cells remain in a highly oxidized environment. The oxidized forms of glutathione and ascorbate are present and NADPH is barely detectable. In adjacent cells, however, higher antioxidant capacities and a more reducing environment is detected. Moreover, ROS-associated genes are differentially expressed in specific SAM and RAM tissues (Tognetti et al., 2017).
Disrupted glutaredoxin (GRX) activity is closely associated with meristem deficiencies. In Arabidopsis, GRXS17 controls the translocation and sensitivity of auxin (Cheng et al., 2011). In maize (Zea mays), GRX ABERRANT PHYLLOTAXY (ABPHYL2) regulates shoot meristem size and phyllotaxy through post translational modification the bZIP transcription factor FASCIATED EAR4 (Yang et al., 2015; Pautler et al., 2015). Moreover, Arabidopsis GRXs ROXY1 and ROXY2 reduce the disulfide bonds in the heteromeric TGA9/TGA10 transcription factor complex, a step that is required to activate gene expression during floral transition (Murmu et al., 2010). The auxinsynthesizing Flavin monooxygenase YUCCA6 has thiol reductase activity, suggesting that there is a link between redox and auxin pathways (Cha et al., 2015).
The plastid thioredoxin TRXm3 regulates ROS homeostasis adjacent to plasmodesmata, targeting callose deposition that ultimately regulates transport through the plasmodesmata (Benitez-Alfonso et al., 2009). ROS also interact with the plant defense hormone salicylic acid (SA). In both Arabidopsis and rice, ABNORMAL INFLORESCENCE MERISTEM (AIM1) plays key role in the SA biosynthetic pathway and is required for meristem development. At the transcriptional level, SA downregulated WRKY transcription factors and thus alleviates the repression of antioxidative enzymes, such as glutathiones and catalases (Bussell et al., 2014; Xu et al., 2017).
4.3. Leaf developmentThe development of terminal plant organs entails a complex harmonization of cell proliferation and cell expansion (Lu et al., 2014). Leaf development is characterized by the proliferation of meristematic cells followed by expansion without further division (Beemster et al., 2005). This cell expansion requires changes to the structure and content of the plant cell wall. These cell wall changes are mediated by peroxidase-associated ROS gradients. Apoplastic peroxidases directly regulate the rigidity of cell wall by either restricting or promoting cellular extension (Lee et al., 2013). Cell wall peroxidases operate as cell wall loosening agent by producing O2-, which break down cell wall polysaccharides (Müller et al., 2009). Cell wall stiffness is enhanced by the production of H2O2, which increases cross linking (Table 2). Studies in Arabidopsis have shown that a repressor of peroxidases called KUODA1 (KUA1) acts as a promoter of cell expansion during leaf development by changing ROS homeostasis (Fig. 6). Mutant plants that overexpress KUA1 are characterized by larger leaves with massive cells. In addition, kua 1 mutants have increased levels of H2O2 and increased class Ⅲ peroxidase activities. Disruption of KUA1 activity triggers peroxidase activity, which results in leaves with smaller cells. These findings indicate that KUA 1-mediated ROS homeostasis regulates cell expansion and organ size during leaf development (Lu et al., 2014).
4.4. Tip developmentRoot hair cells and pollen tubes show tip expansion growth mediated by membrane deposition and wall material synthesis in the direction of elongating cells. Cell expansion must be precisely regulated to produce the correct size and shape. Polar cell growth is maintained by oscillatory feedback loops that consist of three main components. One of the main components is ROS that, with pH and Ca2+ ions maintain polar cell growth (Mangano et al., 2016). During cell expansion, NADPH oxidase and class Ⅲ peroxidases regulate apoplastic ROS homeostasis to affect cell wall properties. Expansion in polar cells of root hair cells and pollen tubes are associated with higher cytoplasmic Ca2+ parallel with the apoplastic ROS production in the apical zone (Figs. 3 and 6) (Steinhorst and Kudla, 2013). Ca2+ is derived from vacuoles, ER, Golgi bodies, the cell wall and the exterior of the cell; the apoplastic pH is altered by plasma membrane-localized H+ pump activation or deactivation, which releases Ca2+ from the cell wall and exterior into the cytosol from cell wall and exterior. Fluctuating Ca2+ concentrations are regulated by auto-inhibitory P-type Ⅱ B Ca2+ ATPases, which facilitate the movement of Ca2+ into the apoplast, and H+/Ca2+ antiporters that supplement Ca2+ movement and H+ influx into the cytoplasm. Such elevated levels of cytosolic Ca2+ in tip zone are required to activate NADPH oxidase (NOXs). NADPH oxidase generates ROS, which trigger the release of Ca2+ into the cytosol (Duan et al., 2014).
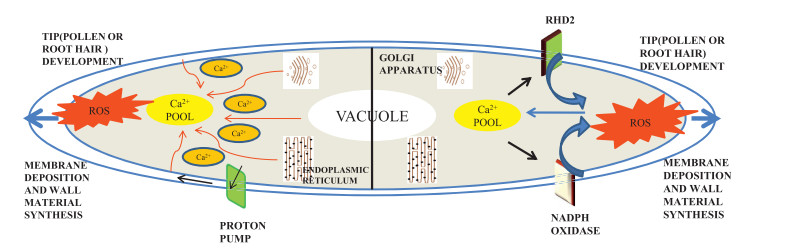
|
| Fig. 3 Illustration showing the role of ROS and interacting components in tip development. Peak in calcium concentration in the cytoplasm activates the proton pump to acidify the cell wall and activate RHD2 and NADPH oxidase to produce ROS, thereby allowing tip development. RHD2 (Root hair defective gene 2), Ca2+ (Calcium) |
The ROOT HAIR DEFECTIVE 2 (RHD2) gene, which transfers of electrons to its acceptor from NADPH, leads to ROS production. rhd2 (root hair defective 2) mutants properly initiate develop of protuberances on epidermal cells but do not show tip growth elongation (Foreman et al., 2003). oxi 1 mutants have demonstrated that OXIDATIVE BURST INDUCIBLE 1 (OXI1), an important Ser-Thr kinase, plays a role in root hair elongation (Rentel and Knight, 2004). During root hair growth, RHD2 triggers ROS resulting in the establishment of MAPKs cascade via OXI1. This ROS burst is essential for pollen tube rupture and sperm release (Duan et al., 2014). NADPH, Ca2+ and pH are the factors that oscillate in such a way that with their climax concentration, growth is favored (Lovy-Wheeler et al., 2006; Macpherson et al., 2008). For instance, in root hair, there is highest oscillatory fluctuation in cytoplasmic as well as apoplastic pH. Apoplastic ROS level increases the growth by 7-8s, whereas cytoplasmic Ca2+ fluctuation suspends the growth oscillation by approximately 5-6s (Monshausen et al., 2009). Pollen tube growth was found to be delayed by 11s with the oscillation in cytoplasmic Ca2+ concentration (Pierson et al., 1994). Application of H2O2 pauses the tip growth and its scavenging continues tip growth, thus suggesting the importance of ROS in cell wall rigidity (Monshausen et al., 2007).
4.5. Lateral root developmentThe interplay between auxin and ABA is crucial for the development of the lateral root. Auxin triggers the separation of the pericycle initials and cellular expansion, whereas ABA is necessary to balance the equilibrium between cellular proliferation and cell differentiation in both the meristem and lateral primordia of the root (Table 2) (Lavenus et al., 2013). Both phytohormones induce ROS production to promote cell expansion in growing roots. IAA is degraded by peroxidases, decreasing auxin pools (Cosio et al., 2008). As observed in tobacco plants, decreased levels of free available IAA in the roots elicits significant changes in auxin that inhibit lateral root formation (Moriwaki et al., 2011). Almost all gpx (glutathione peroxidase) mutants have enlarged lateral root primordia, indicating that the redox-mediated GPX family plays a role in regulating root architecture (Passaia et al., 2014). GPX1 and GPX7 are the major peroxidases that regulate root architecture, lateral root development, and auxin-mediated lateral root formation. Several factors contribute to the development of later roots, including oxygen radicals (O2-) and H2O2 as well as increased ROS production initiated by enzymes such as lipoxygenases, cytochrome P450 carrier proteins and AtrbohC (Manzano et al., 2014). Overall, lateral root emergence is determined by peroxidases through ROS signaling that promotes the transition from cellular proliferation to cell differentiation (Fig. 6).
5. Negative consequences of ROSOxidative stress occurs when the production of enhanced reactive oxygen species exceeds their degradation. Numerous factors are capable of disturbing ROS equilibrium in plants, including drought, high light, and salinity. When ROS target vital biomolecules (i.e., DNA, lipids, and proteins), they affect cell physiological pathways, signaling cascades, membrane properties, which ultimately cause cell death (Fig. 2; Table 3).
| S. no. | ROS type | Half life | Diffusion range | Target of ROS | Oxidative damage | References |
| 1. | 1O2 | 3 μs | 100 nm | Proteins, lipids, nucleic acids, and pigments | Lipid peroxidation, photosystem Ⅱ activity loss and PCD | Das and Roychoudhury (2014) |
| 2. | O2- | 2—4 μs | 30 nm. | Proteins | Oxidize enzymes containing the [4Fe—4S] clusters (aconitase or dehydratase) and reduce cytochrome C | Halliwell (2006) |
| 3. | OH- | 1 μs | 1 nm | Proteins, lipids and nucleic acids | Lipid peroxidation, production of cytotoxic lipid aldehydes, deoxyribose oxidation, removal of nucleotides, DNA-protein crosslinks, and strand breakage | Hossain et al. (2015) |
| 4. | H2O2 | 1 ms | 1 μm | Cysteine and methionine residues, and oxidize thiolates | Oxidation of Calvin cycle enzymes, transcription factors, signaling kinases, phosphatases, proteases, RNA-binding proteins and cell death | Waszczak et al. (2015) |
DNA is a potential target of ROS damage and numerous environmental stresses have the potential to cause DNA degradation in plants (Das and Roychoudhury, 2014). Regardless of the source of DNA, damage ultimately causes aberrations in the resulting protein, which affects various aspects of cell physiology. ROS attacks break DNA strands, remove and/or alter nucleotides, and oxidize deoxyribose.
ROS oxidize both deoxyribose and DNA base units. For instance, hydroxyl radicals can react with the deoxyribose backbone as well pyrimidine and purine bases. These oxidative attacks on DNA generally cause several mutagenic aberrations. For instance, hydrogen liberation from deoxyribose leads to sugar damage. However, when ROS removes hydrogen atoms from deoxyribose at C-4 position, additional radicals are produced, which leads to DNA single strand breaks (Evans et al., 2004). The addition of OH radicals also damages bases (Halliwell, 2006; Das and Roychoudhury, 2014). Specifically, ROS directly cause mutations by altering G:C sites and indirectly damage DNA by generating potential products of macromolecules (lipids).
The reactivity of ROS varies for different molecules. Fe2+ is the most reactive towards ROS damage as it functions in the Fenton reaction to form hydroxyl radical (Mignolet-Spruyt et al., 2016). Hydroxyl ions target DNA and DNA binding proteins, which leads to cross-linking between the protein and DNA and is lethal without repair before transcription and replication. Chloroplastic and mitochondrial DNA are more vulnerable to ROS damage than chromosomal DNA. This is because of the absence of histone incorporation and presence of ROS production sites in these organelles. Thus, excessive ROS production leads to permanent DNA damage and ultimately to lethality in the cell.
5.2. ProteinIn plants exposed to various stresses, protein modification increases. Generally, tissue rich in oxidative damage is recognized by the presence of high level of protein carbonylation, which acts as a marker for protein oxidation. ROS damage proteins in a variety of ways either directly or indirectly. Direct consequences of ROS attack on proteins include carbonylation, disulphide bond formation, glutathionylation and nitrosylation. Indirectly, ROS damage proteins via the products of fatty acid peroxidation, which bind with proteins to alter their activity (Yamauchi et al., 2008; Sharma et al., 2012). In addition, extreme ROS mediate the aggregation of products of cross-linked reactions, alter electric charge, fragment amino acid peptide chains, modify site-specific amino acids and increase the susceptibility of proteins to proteolysis.
The degree of ROS damage to proteins varies depending on amino acid composition. Sulfur- and thiol-containing amino acids are more vulnerable to ROS attack. For example, activated oxygen removes the H atom from cysteine amino acids to cross link with other equivalent cysteine amino acids via disulphide bonds. Furthermore, oxygen binds with methionine to generate methionine-sulphoxide derivative products. Oxygen radicals target iron-sulfur centers to permanently inactivate enzymes. Iron metal present in its binding form, located on the cation binding site and at this site metal undergoes Fenton reaction to generate hydroxyl radical in order to quickly oxidize the amino acids in the or nearby cation binding site of protein (Mittler, 2017).
5.3. LipidsLipid peroxidation increases ROS above threshold levels, which interfere with the functions of cellular and organelle membranes. Accordingly, lipid peroxidation is correlated with ROS production. Hence, lipid peroxidation levels during stress conditions are good indicators of ROS-induced damage to cell membranes. Such lipid peroxidation potentially damages DNA and proteins by generating the radicals of lipid derivatives, thus worsening oxidative stress. For instance, in the peroxidation of unsaturated fatty acids in phospholipids malondialdehyde damages the cell membrane.
ROS target double or unsaturated bonds and the ester bonds between fatty acid and glycerol resting on phospholipids (Sharma et al., 2012). Polyunsaturated fatty acids are more vulnerable to ROS attack. Membrane properties are altered due to polyunsaturated fatty acid peroxidation by ROS attack resulting in chain cleavage (Das and Roychoudhury, 2014). Furthermore, a single hydroxyl radical can mediate lipid peroxidation of a number of fatty acids.
The lipid peroxidation process is divided into three steps: initiation, progression, and termination. The initiation step is the rate limiting step that is activated by O2. Hydroxyl radicals and O2- generate conjugating diene hydroperoxides and lipid peroxy radicals. These radicals, which are extremely reactive and capable of continuing the chain reaction, react with the methylene group of polyunsaturated fatty acids. The lipid hydroperoxides generated in the reaction undergo cleavage by reduced metals. Lipid hydroperoxide decomposes to produce numerous reactive species such as aldehydes, alkanes, alcohols, lipid alkoxyl radicals and lipid epoxides.
6. Concluding remarks and perspectivesAlthough ROS are considered messengers that lead to oxidative signaling, they may play two roles in plant biology. First, ROS trigger biological activities in response to stress. Second, evidence from several decades indicates that ROS are involved in plant growth and developmental processes. ROS regulate the cell cycle, seed dormancy and germination, root growth, pollen tube and leaf development and more. Much progress has been made in understanding the roles and mechanisms by which ROS regulate the plant life cycle. Despite our understanding of ROS production and activities, one emerging question is how ROS function and communicate between cell compartments. Finally, the range of ROS function still needs much attention and provides an opportunity for researchers interested in this area of plant physiology.
Declaration of Competing InterestThe authors declares that there is no conflict of interest.
Aken O.V., Van Breusegem F., 2015. Licensed to kill:mitochondria, chloroplasts, and cell death. Trends Plant Sci, 20: 754-766. DOI:10.1016/j.tplants.2015.08.002 |
Arc E., Sechet J., Corbineau F., Rajjou L., Marion-Poll A., 2013. ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Front. Plant Sci, 4: 63. |
Bahin E., Bailly C., Sotta B., Kranner I., Corbineau F., Leymarie J., 2011. Crosstalk between reactive oxygen species and hormonal signaling pathways regulates grain dormancy in barley. Plant Cell Environ, 34: 980-993. DOI:10.1111/j.1365-3040.2011.02298.x |
Bailly C., 2004. Active oxygen species and antioxidants in seed biology. Seed Sci.Res, 14: 93-107. DOI:10.1079/SSR2004159 |
Bailly C., El-Maarouf-Bouteau H., Corbineau F., 2008. From intracellular signaling networks to cell death:the dual role of reactive oxygen species in seed physiology. C. R. Biol, 331: 806-814. DOI:10.1016/j.crvi.2008.07.022 |
Basbouss-Serhal I., Pateyron S., Cochet F., Leymarie J., Bailly C., 2017. 5' to 3' mRNA decay contributes to the regulation of Arabidopsis seed germination by dormancy. Plant Physiol, 173: 1709-1723. DOI:10.1104/pp.16.01933 |
Beemster G.T.S., Veylder L.D., Vercruysse S., West S., Rombaut D., Hummelen P.V., Galichet A., Gruissem W., Inzé D., Vuylsteke M., 2005. Genome-wide analysis of gene expression profiles associated with cell cycle transitions in growing organs of Arabidopsis. Plant Physiol, 138: 734-743. DOI:10.1104/pp.104.053884 |
Benitez-Alfonso Y., Cilia M., San Roman A., Thomas C., Maule A., Hearn S., Jackson D., 2009. Control of Arabidopsis meristem development by thioredoxin-dependent regulation of intercellular transport. Proc. Natl. Acad. Sci, 106: 3615-3620. DOI:10.1073/pnas.0808717106 |
Bhattacharjee S., 2012. The language of Reactive oxygen species signaling in plant. J. Bot., Le, 2012: 1-22. |
Bussell J.D., Reichelt M., Wiszniewski A.A., Gershenzon J., Smith S.M., 2014. Peroxisomal ATP-binding cassette transporter COMATOSE and the multifunctional protein abnormal INFLORESCENCE MERISTEM are required for the production of benzoylated metabolites in Arabidopsis seeds. Plant Physiol, 164: 48-54. DOI:10.1104/pp.113.229807 |
Camejo D., Guzmán-Cedeño A., Moreno A., 2016. Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol. Biochem, 103: 10-23. DOI:10.1016/j.plaphy.2016.02.035 |
Cha J.-Y., Kim W.-Y., Kang S.B., Kim J.I., Baek D., Jung I.J., Kim M.R., Li N., Kim H.-J., Nakajima M., 2015. A novel thiol-reductase activity of Arabidopsis YUC6 confers drought tolerance independently of auxin biosynthesis. Nat. Commun, 6: 8041. DOI:10.1038/ncomms9041 |
Chang C.C.C., Ślesak I., Jordá L., Sotnikov A., Melzer M., Miszalski Z., Mullineaux P.M., Parker J.E., Karpińska B., Karpiński S., 2009. Arabidopsis chloroplastic glutathione peroxidases play a role in cross talk between photooxidative stress and immune responses. Plant Physiol, 150: 670-683. DOI:10.1104/pp.109.135566 |
Cheng N.H., Liu J.Z., Liu X., Wu Q., Thompson S.M., Lin J., Chang J., Whitham S.A., Park S., Cohen J.D., Hirschi K.D., 2011. Arabidopsis monothiol-glutaredoxin, AtGRXS17, is critical for temperature-dependent postembryonic growth and development via modulating auxin response. J. Biol. Chem, 286: 20398-20406. DOI:10.1074/jbc.M110.201707 |
Corpas F.J., Barroso J.B., Palma J.M., Rodriguez-Ruiz M., 2017. Plant peroxisomes:a nitro-oxidative cocktail. Redox Biol, 11: 535-542. DOI:10.1016/j.redox.2016.12.033 |
Corpas, F.J., Gupta, D.K., Palma, J.M., 2015. Production sites of reactive oxygen species (ROS) in organelles from plant cells. In: React Oxyg Species Oxidative Damage W. Plants Stress Springer Cham, pp. 1-22.
|
Cosio C., Vuillemin L., DeMeyer M., Kevers C., Penel C., Dunand C., 2008. An anionic classIII peroxidase from zucchini may regulate hypocotyl elongation through its auxin oxidase activity. Planta, 229: 823-836. |
Cvetkovska M., Alber N.A., Vanlerberghe G.C., 2013. The signaling role of a mitochondrial superoxide burst during stress. Plant Signal. Behav, 8: e22749. DOI:10.4161/psb.22749 |
Czarnocka W., Karpiński S., 2018. Friend or foe? Reactive oxygen species production scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med, 122: 4-20. DOI:10.1016/j.freeradbiomed.2018.01.011 |
Das K., Roychoudhury A., 2014. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci, 2: 53. |
Diaz-Vivancos P., Barba-Espin G., Hernandez J.A., 2013. Elucidating hormonal/ROS networks during seed germination:insights and perspectives. Plant Cell Rep, 32: 1491-1502. DOI:10.1007/s00299-013-1473-7 |
Duan Q., Kita D., Johnson E.A., Aggarwal M., Gates L., Wu H.M., Cheung A.Y., 2014. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat. Commun, 5: 3129. DOI:10.1038/ncomms4129 |
Dunand C., Crevecoeur M., Penel C., 2007. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development:possible interaction with peroxidases. New Phytol, 174: 332-341. DOI:10.1111/j.1469-8137.2007.01995.x |
Durme M.V., Nowack M.K., 2016. Mechanisms of developmentally controlled cell death in plants. Curr. Opin. Plant Biol, 29: 29-37. DOI:10.1016/j.pbi.2015.10.013 |
El-Maarouf-Bouteau H., Bailly C., 2008. Oxidative signaling in seed germination and dormancy. Plant Signal. Behav, 3: 175-182. DOI:10.4161/psb.3.3.5539 |
El-Maarouf-Bouteau H., Meimoun P., Job C., Job D., Bailly C., 2013. Role of protein and mRNA oxidation in seed dormancy and germination. Front. Plant Sci, 4: 77. |
Evans M.D., Dizdaroglu M., Cooke M.S., 2004. Oxidative DNA damage and disease:induction, repair and significance. Mutat. Res. Rev. Mutat. Res, 567: 1-61. DOI:10.1016/j.mrrev.2003.11.001 |
Finkelstein R., Reeves W., Ariizumi T., Steber C., 2008. Molecular aspects of seeddormancy. Ann. Rev. Plant Biol, 59: 387-415. DOI:10.1146/annurev.arplant.59.032607.092740 |
Foreman J., Demidchik V., Bothwell J.H., Mylona P., Miedema H., Torres M.A., Linstead P., Costa S., Brownlee C., Jones J.D., Davies J.M., Dolan L., 2003. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature, 422: 442-446. DOI:10.1038/nature01485 |
Foyer C.H., Noctor G., 2013. Redox signaling in plants. Antioxidants Redox Signal, 18: 2087-2090. DOI:10.1089/ars.2013.5278 |
Graeber K., Linkies A., Müller K., Wunchova A., Rott A., Leubner-Metzger G., 2010. Cross-species approaches to seed dormancy and germination:conservation and biodiversity of ABA-regulated mechanisms and the Brassicaceae DOG1 genes. Plant Mol. Biol, 73: 67-87. DOI:10.1007/s11103-009-9583-x |
Halliwell B., 2006. Reactive species and antioxidants. redox biology is a fundamental theme of aerobic life. Plant Physiol, 141: 312-322. DOI:10.1104/pp.106.077073 |
Halliwell B., Gutteridge J.M.C., 1989. Free Radicals in Biology and Medicine. Oxford: Clarendon Press: pp :450-499.
|
Hossain M.A., Bhattacharjee S., Armin S.M., Qian P., Xin W., Li H.Y., Burritt D.J., Fujita M., Tran L.S., 2015. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance:insights from ROS detoxification and scavenging. Front. Plant Sci, 6: 420. |
Hulskamp M., 2004. Plant trichomes:a model for cell differentiation. Nat. Rev. Mol. Cell Biol, 5: 471-480. |
Ishibashi Y., Koda Y., Zheng S.H., Yuasa T., Iwaya-Inoue M., 2013. Regulation of soybean seed germination through ethylene production in response to reactive oxygen species. Ann. Bot, 111: 95-102. DOI:10.1093/aob/mcs240 |
Ishibashi Y., Yamamoto K., Tawaratsumida T., Yuasa T., Iwaya-Inoue M., 2008. Hydrogen peroxide scavenging regulates germination ability during wheat(Triticum aestivum L.) seed maturation. Plant Signal. Behav, 3: 183-188. DOI:10.4161/psb.3.3.5540 |
Ishibashi Y., Tawaratsumida T., Kondo K., Kasa S., Sakamoto M., Aoki N., Zheng S.H., Yuasa T., Iwaya-Inoue M., 2012. Reactive oxygen species are involved in gibberellin/abscisic acid signaling in barley aleurone cells. Plant Physiol, 158: 1705-1714. DOI:10.1104/pp.111.192740 |
Laloi C., Apel K., Danon A., 2004. Reactive oxygen signalling:the latest news. Curr. Opin. Plant Biol, 7: 323-328. DOI:10.1016/j.pbi.2004.03.005 |
Lassig R., Gutermuth T., Bey T.D., Konrad K.R., Romeis T., 2014. Pollen tube NAD(P)H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. Plant J, 78: 94-106. DOI:10.1111/tpj.12452 |
Lavenus J., Goh T., Roberts I., Guyomarch S., Lucas M., De Smet I., Fukaki H., Beeckman T., Bennett M., Laplaze L., 2013. Lateral root development in Arabidopsis:fifty shades of auxin. Trends Plant Sci, 18: 450-458. DOI:10.1016/j.tplants.2013.04.006 |
Lee Y., Rubio M.C., Alassimone J., Geldner N., 2013. A mechanism for localized lignin deposition in the endodermis. Cell, 153: 402-412. DOI:10.1016/j.cell.2013.02.045 |
Leymarie J., Vitkauskaite G., Hoang H.H., Gendreau E., Chazoule V., Meimoun P., Corbineau F., El-Maarouf-Bouteau H., Bailly C., 2012. Role of reactive oxygen species in the regulation of Arabidopsis seed dormancy. Plant Cell Physiol, 53: 96-106. DOI:10.1093/pcp/pcr129 |
Libik-Konieczny M., Kozieradzka-Kiszkurno M., Desel C., Michalec-Warzecha Z., Miszalski Z., Konieczny R., 2015. The localization of NADPH oxidase and reactive oxygen species in in vitro-cultured Mesembryanthemum crystallinum L. hypocotyls discloses their differing roles in rhizogenesis. Protoplasma, 252: 477-487. |
Lovy-Wheeler A., Kunkel J.G., Allwood E.G., Hussey P.J., Hepler P.K., 2006. Oscillatory increases in alkalinity anticipate growth and may regulate actin dynamics in pollen tubes of lily. Plant Cell, 18: 2182-2193. DOI:10.1105/tpc.106.044867 |
Lu D., Wang T., Persson S., Mueller-Roeber B., Schippers J.H.M., 2014. Transcriptional control of ROS homeostasis by KUODA1 regulates cell expansion during leaf development. Nat. Commun, 5: 3767. DOI:10.1038/ncomms4767 |
Macpherson N., Takeda S., Shang Z., Dark A., Mortimer J.C., Brownlee C., 2008. NADPH oxidase involvement in cellular integrity. Planta, 227: 1415-1418. DOI:10.1007/s00425-008-0716-2 |
Mangano S., Denita Juárez S., Estevez J.M., 2016. ROS regulation of polar growth in plant cells. Plant Physiol, 171: 1593-1605. DOI:10.1104/pp.16.00191 |
Manzano C., Pallero-Baena M., Casimiro I., DeRybel B., Orman-Ligeza B., VanIsterdael G., Beeckman T., Draye X., Casero P., Del Pozo J.C., 2014. The emerging role of reactive oxygen species signaling during lateral root development. Plant Physiol, 165: 1105-1119. DOI:10.1104/pp.114.238873 |
Marino D., Dunand C., Puppo A., Pauly N., 2012. A burst of plant NADPH oxidases. Trends Plant Sci, 17: 9-15. DOI:10.1016/j.tplants.2011.10.001 |
McDonald M.B., 1999. Seed deterioration:physiology, repair and assessment. Seed Sci. Technol, 27: 177-237. |
Mignolet-Spruyt L., Xu E., Idänheimo N., Hoeberichts F.A., Mühlenbock P., Brosché M., Van Breusegem F., Kangasjärvi J., 2016. Spreading the news:subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot, 67: 3831-3844. DOI:10.1093/jxb/erw080 |
Mittler R., 2017. ROS are good. Trends Plant Sci, 22: 11-19. DOI:10.1016/j.tplants.2016.08.002 |
Monshausen G.B., Bibikova T.N., Messerli M.A., Shi C., Gilroy S., 2007. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc. Natl. Acad. Sci. U.S.A, 104: 20996-21001. DOI:10.1073/pnas.0708586104 |
Monshausen G.B., Bibikova T.N., Weisenseel M.H., Gilroy S., 2009. Ca2+ regulates reactive oxygen species production and pH during mechano-sensing in Arabidopsis roots. Plant Cell, 21: 2341-2356. DOI:10.1105/tpc.109.068395 |
Moriwaki T., Miyazawa Y., Kobayashi A., Uchida M., Watanabe C., Fujii N., Takahashi H., 2011. Hormonal regulation of lateral root development in Arabidopsis modulated by MIZ1 and requirement of GNOM activity for MIZ1 function. Plant Physiol, 157: 1209-1220. DOI:10.1104/pp.111.186270 |
Müller K., Linkies A., Vreeburg R.A.M., Fry S.C., Krieger-Liszkay A., LuebnerMetzger G., 2009. In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol, 150: 1855-1865. DOI:10.1104/pp.109.139204 |
Murmu J., Bush M.J., DeLong C., Li S., Xu M., Khan M., Malcolmson C., Fobert P.R., Zachgo S., Hepworth S.R., 2010. Arabidopsis basic leucine zipper transcription factors TGA9 and TGA10 interact with floral glutaredoxins ROXY1 and ROXY2 and are redundantly required for anther development. Plant Physiol, 154: 1492-1504. DOI:10.1104/pp.110.159111 |
Navrot N., Rouhier N., Gelhaye E., Jacquot J.-P., 2007. Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol. Plant, 129: 185-195. DOI:10.1111/j.1399-3054.2006.00777.x |
Noctor G., Reichheld J.-P., Foyer C.H., 2018. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol, 80: 3-12. DOI:10.1016/j.semcdb.2017.07.013 |
Oracz K., El-Maarouf-Bouteau H., Farrant J.M., Copper K., Belghazi M., Job C., Job D., Corbineau F., Bailly C., 2007. ROS production and protein oxidation as novel mechanism of seed dormancy alleviation. Plant J, 50: 452-465. DOI:10.1111/j.1365-313X.2007.03063.x |
Oracz K., Karpiński S., 2016. Phytohormones signaling pathways and ROS involvement in seed germination. Front. Plant Sci, 7: 864. |
Passaia G., Queval G., Bai J., Margis-Pinheiro M., Foyer C.H., 2014. The effects of redox controls mediated by glutathione peroxidases on root architecture in Arabidopsis thaliana. J. Exp. Bot, 65: 1403-1413. DOI:10.1093/jxb/ert486 |
Pautler M., Eveland A.L., LaRue T., Yang F., Weeks R., Lunde C., Je B.I., Meeley R., Komatsu M., Vollbrecht E., Sakai H., Jackson D., 2015. FASCIATED EAR4 encodes a bZIP transcription factor that regulates shoot meristem size in maize. Plant Cell, 27: 104-120. DOI:10.1105/tpc.114.132506 |
Petrov V.D., Van Breusegem F., 2012. Hydrogen peroxide-a central hub for information flow in plant cells. AoB Plants, 2012: ls014. |
Pierson E.S., Miller D.D., Callaham D.A., Shipley A.M., Rivers B.A., Cresti M., Hepler P.K., 1994. Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient:effect of BAPTA type buffers and hypertonic media. Plant Cell, 6: 1815-1828. |
Pospíšil, P., 2016. Production of reactive oxygen species by photosystem Ⅱ as a response to light and temperature stress. Front. Plant Sci. 7, 1950.
|
Pottosin I., Velarde-Buendía A.M., Bose J., Zepeda-Jazo I., Shabala S., Dobrovinskaya O., 2014. Cross-talk between reactive oxygen species and polyamines in regulation of ion transport across the plasma membrane:implications for plant adaptive responses. J. Exp. Bot., 65: 1271-1283. DOI:10.1093/jxb/ert423 |
Rentel M.C., Knight M.R., 2004. Oxidative stress-induced calcium signaling in Arabidopsis. Plant Physiol, 135: 1471-1479. DOI:10.1104/pp.104.042663 |
Robson C.A., Vanlerberghe G.C., 2002. Transgenic plant cells lacking mitochondrial alternative oxidase have increased susceptibility to mitochondria-dependent and independent pathways of programmed cell death. Plant Physiol, 129: 1908-1920. DOI:10.1104/pp.004853 |
Ros Barceló A., 2005. Xylem parenchyma cells deliver the H2O2 necessary for lignifications in differentiating xylem vessels. Planta, 220: 747-756. DOI:10.1007/s00425-004-1394-3 |
Rubio-Diaz S., Perez-Perez J.M., Gonzalez-Bayon R., Munoz-Viana R., Borrega N., Mouille G., Hernández-Romero D., Robles P., Höfte H., Ponce M.R., Micol J.L., 2012. Cell expansion-mediated organ growth is affected by mutations in three EXIGUA genes. PLoS One, 7: e36500. DOI:10.1371/journal.pone.0036500 |
Schippers J.H.M., Foyer C.H., Van Dongen J.T., 2016. Redox regulation in shoot growth, SAM maintenance and flowering. Curr. Opin. Plant Biol, 29: 121-128. DOI:10.1016/j.pbi.2015.11.009 |
Sharma P., Jha A.B., Dubey R.S., Pessarakli M., 2012. Reactive oxygen species oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot., Le, 2012: 1-26. |
Singh R., Singh S., Parihar P., Mishra R.K., Tripathi D.K., Singh V.P., Chauhan D., Prasad S.M., 2016. Reactive oxygen species (ROS):beneficial companions of plants' developmental processes. Front. Plant Sci, 7: 1299. |
Steffens B., Geske T., Sauter M., 2011. Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol, 190: 369-378. DOI:10.1111/j.1469-8137.2010.03496.x |
Steinhorst L., Kudla J., 2013. Calcium-a central regulator of pollen germination and tube growth. Biochim. Biophys. Acta, 1833: 1573-1581. DOI:10.1016/j.bbamcr.2012.10.009 |
Stern R., Kogan G., Jedrzejas M.J., Soltés L., 2007. The many ways to cleave hyaluronan. Biotechnol. Adv, 25: 537-557. DOI:10.1016/j.biotechadv.2007.07.001 |
Tognetti V.B., Bielach A., Hrtyan M., 2017. Redox regulation at the site of primary growth:auxin, cytokinin and ROS crosstalk. Plant Cell Environ, 40: 2586-2605. DOI:10.1111/pce.13021 |
Tripathi S.K., Tuteja N., 2007. Integrated signaling in flower senescence. Plant Signal. Behav, 2: 437-445. DOI:10.4161/psb.2.6.4991 |
Tripathy B.C., Oelmüller R., 2012. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav, 7: 1621-1633. DOI:10.4161/psb.22455 |
Tsukagoshi H., 2016. Control of root growth and development by reactive oxygen species. Curr. Opin. Plant Biol, 29: 57-63. DOI:10.1016/j.pbi.2015.10.012 |
Tsukagoshi H., Busch W., Benfey P.N., 2010. Transcriptional regulationof ROS controls transition from proliferation to differentiation in the root. Cell, 143: 606-616. DOI:10.1016/j.cell.2010.10.020 |
Wang F., Chen Z.-H., Liu X., Colmer T.D., Shabala L., Salih A., Zhou M., Shabala S., 2017. Revealing the roles of GORK channels and NADPH oxidase in acclimation to hypoxia in Arabidopsis. J. Exp. Bot, 68: 3191-3204. |
Waszczak C., Akter S., Jacques S., Huang J., Messens J., Van Breusegem F., 2015. Oxidative post-translational modifications of cysteine residues in plant signal transduction. J. Exp. Bot, 66: 2923-2934. DOI:10.1093/jxb/erv084 |
Wilkins K.A., Bancroft J., Bosch M., Ings J., Smirnoff N., Franklin-Tong V.E., 2011. Reactive oxygen species and nitric oxide mediate actin reorganization and programmed cell death in the self incompatibility response of Papaver. Plant Physiol, 156: 404-416. DOI:10.1104/pp.110.167510 |
Wituszyńska, W., Karpiński, S., 2013. Programmed Cell Death as a Response to High Light, UV and Drought Stress in Plants, pp. 207-245.
|
Xu L., Zhao H., Ruan W., Deng M., Wang F., Peng J., Luo J., Chen Z., Yi K., 2017. ABNORMAL INFLORESCENCE MERISTEM1 functions in salicylic acid biosynthesis to maintain proper reactive oxygen species levels for root meristem activity in rice. Plant Cell, 29: 560-574. DOI:10.1105/tpc.16.00665 |
Xu R., Li Y., 2011. Control of final organ size by mediator complex subunit 25 in Arabidopsis thaliana. Development, 138: 4545-4554. DOI:10.1242/dev.071423 |
Yadegari R., Drews G.N., 2004. Female gametophyte development. Plant Cell, 16: S133-S141. DOI:10.1105/tpc.018192 |
Yamauchi Y., Furutera A., Seki K., Toyoda Y., Tanaka K., Sugimoto Y., 2008. Malondialdehyde generated from peroxidized linolenic acid causes protein modification in heat-stressed plants. Plant Physiol. Biochem, 46: 786-793. DOI:10.1016/j.plaphy.2008.04.018 |
Yang F., Bui H.T., Pautler M., Llaca V., Johnston R., Lee B.-H., Kolbe A., Sakai H., Jackson D., 2015. A maize glutaredoxin gene, Abphyl2, regulates shoot meristem size and phyllotaxy. Plant Cell, 27: 121-131. DOI:10.1105/tpc.114.130393 |
Zeng J., Dong Z., Wu H., Tian Z., Zhao Z., 2017. Redox regulation of plant stem cell fate. EMBO J, 36: 2844-2855. DOI:10.15252/embj.201695955 |
Zhang L., Ren F., Zhang Q., Chen Y., Wang B., Jiang J., 2008. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev. Cell, 14: 377-387. DOI:10.1016/j.devcel.2008.01.006 |



