b. Community Ecology and Conservation Group, Center for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla 666303, China
Trait-based plant ecology, which focuses on plant functional traits rather than taxonomic identities, has been shown to be a feasible approach to explore a wide range of research problems in ecology (Wright et al., 2004; McGill et al., 2006; Kunstler et al., 2016). Functional traits, by definition, are any measurable feature at the individual level affecting fitness directly or indirectly (Violle et al., 2007; Albert et al., 2010, 2012). Therefore, functional trait variation of plants within a community consists of both inter- and intraspecific variation, i.e., variation of averaged trait value between species and trait variation of individuals within species, respectively. Ecologists have long used mean trait values of species to infer ecological processes (McGill et al., 2006). However, recent studies have shown that intraspecific variation is substantial and plays an important role in community assembly and ecosystem processes (Siefert et al., 2015; Hart et al., 2016; Turcotte and Levine, 2016; Pérez-Izquierdo et al., 2019).
Intraspecific variation in plants may help species adapt to diverse abiotic and biotic environments (Jung et al., 2010; Violle et al., 2012; Silva et al., 2019), affect species coexistence (Crutsinger et al., 2008; Clark, 2010; Turcotte and Levine, 2016), and alter ecosystem processes (Schweitzer et al., 2004; Crutsinger et al., 2006). For example, in Brazil, intraspecific variation in Eremanthus erythropappus promotes its ability to occupy different habitats (forests and savannas) (Silva et al., 2019). Similarly, intraspecific variation of seed biomass in Brachypodium hybridum, an annual grass in California, facilitates its invasions into water-limited environments (Liu et al., 2019). Intraspecific variation has also been shown to impact species coexistence, with stable coexistence predicted when stabilizing niche differences exceed average fitness differences, and competitive exclusion predicted when it does not (Turcotte and Levine, 2016). The importance of intraspecific variation to competitive exclusion has been demonstrated in Solidago altissima, a plant which reduces richness, cover, and biomass of new colonizing species within communities by increasing stem density (Crutsinger et al., 2008). Intraspecific variation can also affect various ecosystem processes, the condensed tannins in the leaves of cottonwood trees of different genotypes in both Populus fremontii and P. angustifolia have been found to significantly affect litter decomposition and soil N mineralization (Schweitzer et al., 2004). In Pinus pinaster, intraspecific variation has been shown to affect its rhizospheric microbiome and the hydrolysis of celluloses, hemicelluloses, and chitin (Pérez-Izquierdo et al., 2019).
Although many studies have shown that plant traits vary substantially within species, and recent syntheses have encouraged the inclusion of intraspecific trait variation into ecological studies (Hulshof and Swenson, 2010; Bolnick et al., 2011; Violle et al., 2012; Siefert et al., 2015; Yang et al., 2018), direct comparisons between inter- and intraspecific variation of tropical plant species are still lacking. One key factor that is generally agreed to affect plant intraspecific variation is elevation (Cordell et al., 1998; Fajardo and Piper, 2011; Read et al., 2014). Therefore, in this study we compared the intraspecific leaf trait variation (leaf area, specific leaf area, leaf thickness, leaf density, and leaf chlorophyll content) of Pittosporopsis kerrii Craib (Icacinaceae) along an elevation gradient with that of the interspecific variation of 462 tree species coexisting in the same community (Yang et al., 2014). P. kerrii is a highly abundant understory tree species within the Xishuangbanna tropical seasonal rainforest in southwestern China (Lan et al., 2008). This species is widespread in the tropical forests of Southeast Asia, such as those of Laos, Myanmar, Thailand, and North Vietnam (Peng and Howard, 2008; Hong and Stephen, 2015). We asked two questions: (1) How does intraspecific leaf trait variation of P. kerrii compare to the interspecific variation of mean trait values for most tree species in the same area? (2) Do P. kerrii leaf traits vary systematically with elevation?
2. Materials and method 2.1. Study site and focal speciesOur study was established in a 20-ha (400 m × 500 m) Forest Dynamic Plot (FDP) (21º36'42"-85"N and 101º34'26"-47'00"E; Lan et al., 2008) and surrounding forest (approximately 4 km2) in the Xishuangbanna tropical seasonal rainforest in southwestern China. Rain forest canopy reached ca. 50 m (Zheng et al., 2006). The region is part of the Indo-Burma biodiversity hotspot (Myers et al., 2000). The annual rainfall within the Xishuangbanna tropical seasonal rainforest has an average of 1493 mm, of which ca. 1256 mm (84%) occurs between May to October; soil type is derived from siliceous rock (Cao et al., 2006).
P. kerrii is a small shade-tolerant tree species, 4-7 m tall, that inhabits dense valley forests with an elevation range of 300-1600 m and is mainly distributed in South Yunnan (China), Laos, Myanmar, Thailand, and North Vietnam. P. kerrii seeds are edible and used medicinally (Peng and Howard, 2008). P. kerrii is the most abundant tree species in the Xishuangbanna tropical seasonal rainforest, accounting for over 20% of the total individuals within the community. Other dominant tree species at the site are Parashorea chinensis H. Wang (Dipterocarpaceae) and Garcinia cowa Roxburgh (Clusiaceae) (Lan et al., 2008).
2.2. Intraspecific trait variation of P. kerriiWe measured leaf area (LA), specific leaf area (SLA), leaf thickness (LT), leaf density (LD), and leaf chlorophyll content (Chl) of three mature leaves from 80 individuals of P. kerrii within an area of approximately 4 km2 in the Xishuangbanna tropical seasonal rainforest. Functional tradeoffs of these leaf traits between individuals and species have been described previously (Yang et al., 2014). Briefly, plants with high SLA usually have a fast resource acquisition and growth strategy, whereas plants with low SLA have a resource conservation and persistence strategy (Poorter et al., 2009). LT and LD, which are inversely related to SLA, can be calculated as SLA = 1/(LT × LD); therefore, increased LT or LD alone or simultaneously will lead to a decreased SLA (Poorter et al., 2009). Furthermore, leaf chlorophyll content is directly related to the photosynthetic rate of plants (Croft et al., 2017).
Because P. kerrii is a small shade-tolerant tree species, in most cases P. kerrii individuals do not receive direct light except for individuals in forest gaps. Therefore, to avoid the effects of sunlight, no P. kerrii individuals were selected within forest gaps. P. kerrii adults (generally with DBH > 3 cm and tree height > 4 m) were randomly sampled from valley to ridge habitat along elevation during the hot-dry season (April, 2015) to explore the elevation induced environment effects (e.g. water availability) on the leaf trait variation of P. kerrii. The elevation of each individual of P. kerrii sampled was determined by a hand-held GPS unit. Leaves were collected from the top of tree crowns without any obvious symptoms of pathogen attack, herbivore attack, or substantial epiphylls cover (Cornelissen et al., 2003). Both LT (mm) and leaf chlorophyll content (ChlSPAD) were measured in the field by an electronic digital micrometer (CANY Co., Shanghai, China) and a hand-held 'SPAD-502 Chl meter' (Minolta Camera Co., Osaka, Japan). Leaves were placed within a sealed plastic bag and taken to the laboratory immediately. LA (cm2) was measured using a leaf area meter (LI-COR 3100C Area Meter; LI-COR, USA). Each leaf was dried to a constant weight at 60 ºC and weighed to the nearest 0.01 g (Cornelissen et al., 2003). SLA (cm2 g-1) was calculated as the ratio of LA to oven-dry leaf mass (LM, g). The SPAD value was converted to chlorophyll concentration per unit leaf area (Chlarea, mmol m-2) by Chlarea = -112.9+(13.9 × ChlSPAD) (Anten and Hirose, 1999; Poorter and Bongers, 2006). The chlorophyll concentration per unit leaf mass (Chlmass, mmol/g) was calculated as Chlmass = Chlarea × (SLA/10, 000). LD (g cm-3) was calculated by LD = LM/(LA × LT/10) (Witkowski and Lamont, 1991). Trait values were averaged for the three leaves collected within an individual of P. kerrii.
2.3. Interspecific trait variationInterspecific trait variation of 462 tree species (taxa) was first published in Yang et al. (2014), which includes most tree species in the study area. More than five leaves were sampled from each of five individuals of each taxon. LA, SLA, LT and ChlSPAD were measured as described above and averaged for each species. For more details, see Yang et al. (2014).
2.4. Statistical analysisTwo indexes were calculated to show the intra- and interspecific trait variation. One is the range of trait variation and the other is the coefficient of variation (CV), which is calculated as CV = SD/M, where SD is standard deviation and M is mean (Fajardo and Piper, 2011). Shapiro-Wilk tests were used to evaluate the normality of variables investigated in this study (leaf functional traits and elevation) and all variables were found to be acceptably normal (P > 0.05). Pearson correlation analysis was used to test the correlations among the traits and linear regression analysis was used to test the effects of elevation on leaf traits. Significance was obtained at P < 0.05. These tests were implemented using SPSS 16.0 (SPSS Inc., Chicago, USA).
3. Results 3.1. Intraspecific trait variation of P. kerrii and interspecific trait variation of 462 tree speciesIntraspecific variation of P. kerrii was substantial (Table 1; Fig. 1). LA varied threefold, ranging from 40.45 to 120.80 cm2. SLA varied from 110.90 to 194.90 cm2 g-1. LT varied from 0.20 to 0.34 mm and leaf chlorophyll content, as shown by SPAD value (ChlSPAD), varied from 48.26 to 75.02. The coefficient of variation (CV) of P. kerrii leaf traits varied from 0.10 to 0.24.
| Taits | Intraspecific | Interspecific | Range covered (%) | |||||
| Mean | CV | Range | Mean | CV | Range | |||
| Leaf area (cm2) | 74.61 | 0.24 | 40.45-120.80 | 31.94 | 3.53 | 1.86-239.53 | 33.80% | |
| Specific leaf area (cm2/g) | 146.23 | 0.13 | 110.90-194.90 | 56.67 | 0.72 | 14.48-394.34 | 22.11% | |
| Leaf chlorophyll content (SPAD) | 59.30 | 0.10 | 48.26-75.02 | 50.02 | 0.15 | 24.98-67.12 | a44.76% | |
| Leaf thickness (mm) | 0.27 | 0.11 | 0.20-0.34 | 0.23 | 0.25 | 0.11-0.63 | 26.92% | |
| Mass based leaf chlorophyll content (umol/g) | 10.32 | 0.11 | 7.61-12.25 | - | - | - | - | |
| Leaf density (g/cm3) | 0.26 | 0.13 | 0.19-0.37 | - | - | - | - | |
| CV, coefficient of variation. a The part of the range of intraspecific variation that exceeds the interspecific range was ignored to allow comparison; -no interspecific data for comparison. |
||||||||

|
| Fig. 1 The intraspecific trait variation of P. kerrii in the Xishuangbanna tropical seasonal rainforest in southwestern China. SLA, specific leaf area; ChlSPAD, Leaf chlorophyll content, showed by SPAD value; LT, leaf thickness; LA, Leaf area; Chlmass, mass based chlorophyll content; LD, leaf density |
The interspecific trait variation of 462 tree species is shown in Table 1. LA varied from 1.86 to 239.53 cm2, SLA varied from 14.48 to 394.34 cm2 g-1, LT varied from 0.11 to 0.63 mm and ChlSPAD varied from 24.98 to 67.12. The CV of these leaf traits varied from 0.15 to 3.53. The ranges of intraspecific trait variation of P. kerrii were 22.11%e44.76% of the ranges of interspecific trait variation among 462 tree species (Table 1, Fig. 2).

|
| Fig. 2 Radar chart showing (a) the intraspecific trait variation, and (b) the coefficient of trait variation (CV) for four traits in P. kerrii and of 462 tree species in the Xishuangbanna tropical seasonal rainforest. In Fig. 2a, green and blue dots show the trait limits of interspecific variation of the 462 tree species (n = 462), black and red dots show the trait limits of the intraspecific variation of P. kerrii (n = 80). The shallow blue area shows the intraspecific trait variation of the four leaf traits of P. kerrii. In Fig. 2b, black dots show the CV (coefficient of variation) of leaf traits of P. kerrii and red dots show the CV of leaf traits of 462 tree species in the same area. This figure was generated using the function "radarchart" in the R package "fmsb" (Nakazawa, 2018) |
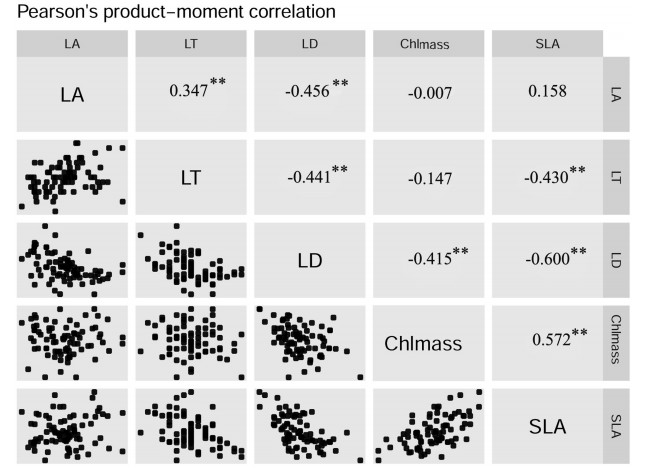
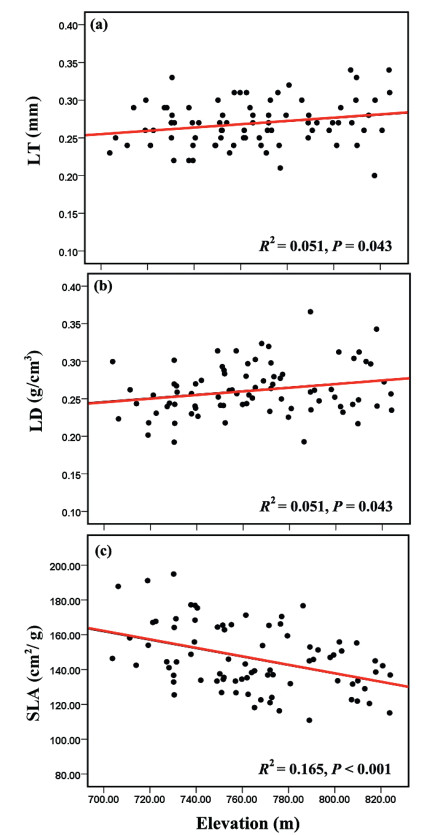
Most leaf traits of P. kerrii were significantly correlated (Fig. 3). For example, LT and LD were negatively correlated with one another (r = -0.441, P < 0.001) and also negatively correlated with SLA (r = -0.430, P < 0.001; r = -0.600, P < 0.001). Chlmass was positively correlated with SLA (r = 0.572, P < 0.001), while negatively correlated with LD (r = 0.415, P < 0.001). LA was positively correlated with LT but negatively correlated with LD (r = 0.347, P = 0.002; r = -0.456, P < 0.001). LT and LD increased, and SLA decreased significantly with increasing elevation (Fig. 4).
|
| Fig. 3 Correlations between leaf traits of P. kerrii within the Xishuangbanna tropical seasonal rainforest. The data shown in the figure are the Pearson correlation coefficient. **P < 0.01. This figure generated by function ggcorplot in the R package "Deducer" (Fellows, 2012; R Core Team, 2017). |
|
| Fig. 4 Variations in leaf thickness, density and specific leaf area of P. kerrii along an elevational gradient. R2 is the coefficient of determination in linear regression analysis. SLA, specific leaf area; LT, leaf thickness; LD, leaf density |
Our study indicates that intraspecific variation in leaf traits is substantial. This finding is consistent with previous studies (Fajardo and Piper, 2011; Laforest-Lapointe et al., 2014; Siefert et al., 2015; Umana et al., 2015; Luo et al., 2016). For example, previous work has shown that the size of within-species variation is comparable with that of species-level variation (Messier et al., 2010). Furthermore, intraspecific variation has been found to account for 25% of the total trait variation within communities and 32% of the total trait variation among communities on average (Siefert et al., 2015). Together, these findings show that using a simple mean value for a functional trait ignores substantial trait variation at the intraspecific level.
4.2. Intraspecific trait correlations of P. kerriiWe found that various P. kerrii leaf traits were significantly correlated (Fig. 3). For example, SLA was negatively correlated with LT and LD but positively correlated with Chlmass. SLA is a key trait that represents a functional tradeoff between growth and persistence for plant individuals or species (Sterck et al., 2006; Poorter et al., 2009). According to the resource availability hypothesis, plants in an environment with high resource availability invest more in growth than in persistence (Coley et al., 1986); therefore, the variation of SLA along with LT, LD and Chlmass can be taken as the consequences of the individuals of P. kerrii to adapt to the diverse environment.
We also found that LD was negatively correlated with Chlmass, indicating that denser leaves have a low mass-based content of chlorophyll, and chlorophyll content is directly related to the photosynthetic rate of plants (Croft et al., 2017). This is consistent with the discussions above that plants with denser leaves may invest more to persistence than to growth. Finally, we observed a significant positive correlation between LA and LT, which may indicate that LT plays a key role in determining the physical strength of leaves and thicker leaves are needed to support larger leaf area (Pérez-Harguindeguy et al., 2013).
4.3. Intraspecific trait variation along elevationPlant traits are generally correlated with elevation (Read et al., 2014). For example, SLA is observed to decrease when elevation increases (Cordell et al., 1998; Hovenden and Vander Schoor, 2004; Fajardo and Piper, 2011; Laforest-Lapointe et al., 2014). Elevation may affect air temperature and in turn affect plant functional traits that help plants cope with colder environments at higher elevations (Fajardo and Piper, 2011). However, elevation may also affect other environmental properties (e.g. soil water content, light and nutrient availability) that affect plant traits (Markesteijn et al., 2010). In this study, both LD and LT increased, and SLA decreased significantly as elevation increased (Fig. 4); however, SLA varied along elevation more significantly than LT and LD. According to Poorter et al. (2009), SLA can be calculated as SLA = 1/(LT × LD); therefore, SLA decreased when LT and LD increased alone or simultaneously. Thus, the more significant correlation between SLA and elevation could be a consequence of the simultaneous variation of LT and LD of P. kerrii in the Xishuangbanna tropical seasonal rainforest.
The elevational gradient within our study is relatively small (703-824 m); however, even within this elevational gradient, the intraspecific variation of P. kerrii leaf traits, is substantial and correlates with elevation (Figs. 1 and 4). A probable explanation for these patterns is that changes in environmental correlates are associated with topography. Within a relatively small elevational gradient, the effects of elevation on temperature could be small, whereas other factors, such as soil water and nutrient availability, could be significantly different. For instance, in the Xishuangbanna tropical seasonal rainforest, pH, phosphorous availability, and soil water availability are higher in valley habitats than in slopes or ridges at relatively higher elevations (Zheng et al., 2006; Xu et al., 2016). Water stress differences are especially pronounced in the hot-dry season (from March to April) (Cao et al., 2006). Again, in order to tolerate environmental stresses at higher elevations (e.g. soil water availability) in the Xishuangbanna tropical seasonal rainforest, it may be more beneficial for P. kerrii individuals to produce thicker and denser leaves rather than to increase growth rates (Fig. 4). Notably, we only investigated intraspecific variation of P. kerrii, the most abundant tree species; however, it has been shown that common species shown less intraspecific trait variation than rare species in the Xishuangbanna tropical seasonal rainforest because rare species are more likely struggling for success in a given environment (Umana et al., 2015).
5. Concluding remarksIs intraspecific variation negligible? Our study indicates that intraspecific variation is substantial and should not be ignored. Intraspecific variation may help species adapt to diverse environments and pass through biotic and abiotic filters. Our study provides support for a shift from species-based to individual-based community ecology (Violle et al., 2012).
Declaration of Competing InterestThe authors declare no conflict of interests.
AcknowledgmentsThis study was supported by the National Natural Science Foundation of China (Grant no. 31770569) and CAS 135 program (Grant no. XTBG-T01). The authors would like to thank the Xishuangbanna Station for Tropical Rain Forest Ecosystem Studies for assistance during the fieldwork and Prof. Richard Corlett for the insightful comments and revisions during the 2nd XTBG writing course 2017 at Kunming branch, XTBG.
Albert C., de Bello F., Boulangeat I., Pellet G., Lavorel S., Thuiller W., 2012. On the importance of intraspecific variability for the quantification of functional diversity. Oikos, 121: 116-126. DOI:10.1111/j.1600-0706.2011.19672.x |
Albert C.H., Thuiller W., Yoccoz N.G., Soudant A., Boucher F., Saccone P., et al, 2010. Intraspecific functional variability:extent, structure and sources of variation. J. Ecol, 98: 604-613. DOI:10.1111/j.1365-2745.2010.01651.x |
Anten N.P.R., Hirose T., 1999. Interspecific differences in above-ground growth patterns result in spatial and temporal partitioning of light among species in a tall-grass meadow. J. Ecol, 87: 583-597. DOI:10.1046/j.1365-2745.1999.00365.x |
Bolnick D.I., Amarasekare P., Araújo M.S., Bürger R., Levine J.M., Novak M., et al, 2011. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol, 26: 183-192. DOI:10.1016/j.tree.2011.01.009 |
Cao M., Zou X.M., Warren M., Zhu H., 2006. Tropical forests of xishuangbanna, China. Biotropica, 38: 306-309. DOI:10.1111/j.1744-7429.2006.00146.x |
Clark J.S., 2010. Individuals and the variation needed for high species diversity in forest trees. Science, 327: 1129-1132. DOI:10.1126/science.1183506 |
Cordell S., Goldstein G., Mueller-Dombois D., Webb D., Vitousek P.M., 1998. Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along an altitudinal gradient:the role of phenotypic plasticity. Oecologia, 113: 188-196. DOI:10.1007/s004420050367 |
Cornelissen J.H.C., Lavorel S., Garnier E., D#237;az S., Buchmann N., Gurvich D.E., et al, 2003. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot, 51: 335-380. DOI:10.1071/BT02124 |
Coley P.D., Bryant J.P., Chapin III F.S., 1986. Resource availability and plant antiherbivore defense. Science, 230: 895-899. |
Croft H., Chen J.M., Luo X., Bartlett P., Chen B., Staebler R.M., 2017. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Chang. Biol, 23: 3513-3524. DOI:10.1111/gcb.13599 |
Crutsinger G.M., Collins M.D., Fordyce J.A., Gompert Z., Nice C.C., Sanders N.J., 2006. Plant genotypic diversity predicts community structure and governs an ecosystem process. Science, 313: 966-968. DOI:10.1126/science.1128326 |
Crutsinger G.M., Souza L., Sanders N.J., 2008. Intraspecific diversity and dominant genotypes resist plant invasions. Ecol. Lett, 11: 16-23. |
Fajardo A., Piper F.I., 2011. Intraspecific trait variation and covariation in a widespread tree species (Nothofagus pumilio) in southern Chile. New Phytol, 189: 259-271. DOI:10.1111/j.1469-8137.2010.03468.x |
Fellows I., 2012. Deducer:A data analysis GUI for R. J. Stat. Softw, 49: 1-15. |
Hart S.P., Schreiber S.J., Levine J.M., 2016. How variation between individuals affects species coexistence. Ecol. Lett, 19: 825-838. DOI:10.1111/ele.12618 |
Hong, D.Y., Stephen, B., 2015. Plants of China: A Companion to the Flora of China. Science Press and Cambridge University Press, Beijing and Cambridge, PRC and UK.
|
Hovenden M.J., Vander Schoor J.K., 2004. Nature vs nurture in the leaf morphology of Southern beech, Nothofagus cunninghamii (Nothofagaceae). New Phytol, 161: 585-594. DOI:10.1046/j.1469-8137.2003.00931.x |
Hulshof C.M., Swenson N.G., 2010. Variation in leaf functional trait values within and across individuals and species:an example from a Costa Rican dry forest. Funct. Ecol, 24: 217-223. DOI:10.1111/j.1365-2435.2009.01614.x |
Jung V., Violle C., Mondy C., Hoffmann L., Muller S., 2010. Intraspecific variability and trait-based community assembly. J. Ecol, 98: 1134-1140. DOI:10.1111/j.1365-2745.2010.01687.x |
Kunstler G., Falster D., Coomes D.A., Hui F., Kooyman R.M., Laughlin D.C., et al, 2016. Plant functional traits have globally consistent effects on competition. Nature, 529: 204-207. DOI:10.1038/nature16476 |
Laforest-Lapointe I., Martinez-Vilalta J., Retana J., 2014. Intraspecific variability in functional traits matters:case study of Scots pine. Oecologia, 175: 1337-1348. DOI:10.1007/s00442-014-2967-x |
Lan G.Y., Hu Y.H., Cao M., Zhu H., Wang H., Zhou S.S., 2008. Establishment of Xishuangbanna tropical forest dynamics plot:species compositions and spatial distribution patterns. J. Plant Ecol, 32: 287-298. |
Liu S., Streich J., Borevitz J.O., Rice K.J., Li T., Li B., Bradford K.J., 2019. Environmental resource deficit may drive the evolution of intraspecific trait variation in invasive plant populations. Oikos, 128: 171-184. DOI:10.1111/oik.05548 |
Luo Y.H., Liu J., Tan S.L., Cadotte M.W., Xue K., Gao L.M., et al, 2016. Trait variation and functional diversity maintenance of understory herbaceous species coexisting along an elevational gradient in Yulong Mountain, southwest China. Plant Divers, 38: 303-311. DOI:10.1016/j.pld.2016.11.002 |
Markesteijn L., Iraipi J., Bongers F., Poorter L., 2010. Seasonal variation in soil and plant water potentials in a Bolivian tropical moist and dry forest. J. Trop. Ecol, 26: 497-508. DOI:10.1017/S0266467410000271 |
McGill B.J., Enquist B.J., Weiher E., Westoby M., 2006. Rebuilding community ecology from functional traits. Trends Ecol. Evol, 21: 178-185. DOI:10.1016/j.tree.2006.02.002 |
Messier J., McGill B.J., Lechowicz M.J., 2010. How do traits vary across ecological scales? A case for trait-based ecology. Ecol. Lett, 13: 838-848. DOI:10.1111/j.1461-0248.2010.01476.x |
Myers N., Mittermeier R.A., Mittermeier C.G., da Fonseca G.A.B., Kent J., 2000. Biodiversity hotspots for conservation priorities. Nature, 403: 853-858. DOI:10.1038/35002501 |
Nakazawa, M., 2018. Fmsb: Functions for Medical Statistics Book with Some Demographic Data. R package version 0.6.3. https: //CRAN.R-project.org/package=fmsb.
|
Peng, H., Howard, R.A., 2008. Icacinaceae (Flora of China), vol. 11. Science Press and Missouri Botanical Garden Press, Beijing and Saint Louis, PRC and USA, pp. 508-509.
|
Pérez-Izquierdo L., Zabal-Aguirre M., Gonzalez-Martinez S.C., Buee M., Verdu M., Rincon A., et al, 2019. Plant intraspecific variation modulates nutrient cycling through its below ground rhizospheric microbiome. J. Ecol, 107: 1594-1605. DOI:10.1111/1365-2745.13202 |
Pérez-Harguindeguy N., Díaz S., Garnier E., Lavorel S., Poorter H., Jaureguiberry P., et al, 2013. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot, 61: 167-234. |
Poorter L., Bongers F., 2006. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology, 87: 1733-1743. DOI:10.1890/0012-9658(2006)87[1733:LTAGPO]2.0.CO;2 |
Poorter H., Niinemets ülo, Poorter L., Villar W.R., 2009. Causes and consequences of variation in leaf mass per area (LMA):a meta-analysis. New Phytol, 182: 565-588. DOI:10.1111/j.1469-8137.2009.02830.x |
R Core Team, 2017. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. URL. https://www.Rproject.org/.
|
Read Q.D., Moorhead L.C., Swenson N.G., Bailey J.K., Sanders N.J., 2014. Convergent effects of elevation on functional leaf traits within and among species. Funct. Ecol, 28: 37-45. DOI:10.1111/1365-2435.12162 |
Schweitzer J.A., Bailey J.K., Rehill B.J., Martinsen G.D., Hart S.C., Lindroth R.L., et al, 2004. Genetically based trait in a dominant tree affects ecosystem processes. Ecol. Lett, 7: 127-134. DOI:10.1111/j.1461-0248.2003.00562.x |
Silva M.C., Teodoro G.S., Bragion E.F.A., van den Berg E., 2019. The role of intraspecific trait variation in the occupation of sharp forest savanna ecotones. Flora, 253: 35-42. DOI:10.1016/j.flora.2019.03.003 |
Siefert A., Violle C., Chalmandrier L., Albert C.H., Taudiere A., Fajardo A., et al, 2015. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol. Lett, 18: 1406-1419. DOI:10.1111/ele.12508 |
Sterck F.J., Poorter L., Schieving F., 2006. Leaf traits determine the growth survival trade-off across rain forest tree species. Am. Nat, 167: 758-765. DOI:10.1086/503056 |
Turcotte M.M., Levine J.M., 2016. Phenotypic plasticity and species coexistence. Trends Ecol. Evol, 31: 803-813. DOI:10.1016/j.tree.2016.07.013 |
Umana M.N., Zhang C.C., Cao M., Lin L.X., Swenson N.G., 2015. Commonness, rarity, and intraspecific variation in traits and performance in tropical tree seedlings. Ecol. Lett, 18: 1329-1337. DOI:10.1111/ele.12527 |
Violle C., Enquist B.J., McGill B.J., Jiang L., Albert C.H., Hulshof C., et al, 2012. The return of the variance:intraspecific variability in community ecology. Trends Ecol. Evol, 27: 244-252. DOI:10.1016/j.tree.2011.11.014 |
Violle C., Navas M.L., Vile D., Kazakou E., Fortunel C., Hummel I., et al, 2007. Let the concept of trait be functional!. Oikos, 116: 882-892. DOI:10.1111/j.0030-1299.2007.15559.x |
Witkowski E.T.F., Lamont B.B., 1991. Leaf specific mass confounds leaf density and thickness. Oecologia, 88: 486-493. DOI:10.1007/BF00317710 |
Wright I.J., Reich P.B., Westoby M., Ackerly D.D., Baruch Z., Bongers F., et al, 2004. The worldwide leaf economics spectrum. Nature, 428: 821-827. |
Xu W.M., Liu L., He T., Cao M., Sha L., Hu Y., et al, 2016. Soil properties drive a negative correlation between species diversity and genetic diversity in a tropical seasonal rainforest. Sci. Rep, 6: 20652. DOI:10.1038/srep20652 |
Yang J., Zhang G.C., Ci X.Q., Swenson N.G., Cao M., Sha L., 2014. Functional and phylogenetic assembly in a Chinese tropical tree community across size classes, spatial scales and habitats. Funct. Ecol, 28: 520-529. DOI:10.1111/1365-2435.12176 |
Yang J., Cao M., Swenson N.G., 2018. Why functional traits do not predict tree demographic rates. Trends Ecol. Evol, 33: 326-336. DOI:10.1016/j.tree.2018.03.003 |
Zheng Z., Feng Z., Cao M., Li Z., Zhang J., 2006. Forest structure and biomass of a tropical seasonal rain forest in xishuangbanna, southwest China. Biotropica, 38: 318-327. |



