b. Research Associate of Singapore Botanic Gardens Herbarium, Republic of Singapore;
c. Herbarium, Singapore Botanic Gardens, National Parks Board, 1 Cluny Road, 259569, Republic of Singapore;
d. CAS Key Laboratory of Biodiversity and Biogeography, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201, China
Senegalia Raf. (Leguminosae: Mimosoideae) is a genus of 217 species (233 taxa) that occurs in the tropics and subtropics of both the Old and New Worlds (Fig. 1). It is represented by 97 species in the Americas (with Brazil being the centre of species-richness), 62 species in Africa (plus 11 species in Madagascar and Mascarene Islands), 56 species in Asia (i.e. Arabian Peninsula to China and Southeast Asia), and 2 species in Australia. The above species numbers are derived from the WorldWideWattle website (web ref. 1), with the addition of those newly recognised in the present work. China is a centre of species-richness for Senegalia within the Asian region, with 21 indigenous species recognised. In recent decades, and prior to the fragmentation of Acacia sens. lat. (see below), species of Senegalia had been assigned to Acacia subg. Aculeiferum Vassal. Among other characters, species of this genus are recognised by having bipinnate foliage and cauline prickles, with many possessing a liana habit. As currently defined, Senegalia has moderate genetic support and comprises two sections, sect. Senegalia and sect. Monacanthea (Vassal) Maslin comb. nov. (see below under Taxonomy).
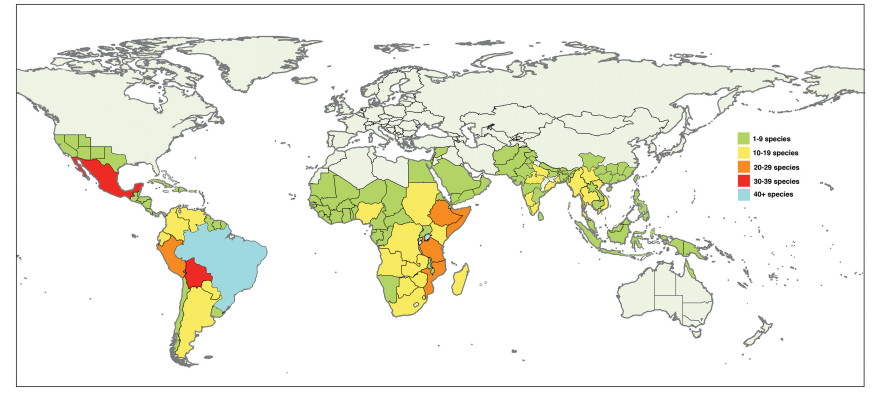
|
| Fig. 1 Senegalia global distribution showing patterns of species-richness. |
In recent years there have been substantial changes to both the classification and nomenclature of the group formerly known as Acacia Mill. in China (Senegalia is nested within this group). These changes have occurred for two main reasons. Firstly, molecular and other evidence have shown that the former broadly circumscribed, pantropical genus Acacia is polyphyletic and should be treated as comprising at least seven genera, namely, Acacia (1067 species), Acaciella Britton & Rose (15 species), Mariosousa Seigler & Ebinger (13 species), Parasenegalia Seigler & Ebinger (7 species), Pseudosenegalia Seigler & Ebinger (2 species), Senegalia (217 species) and Vachellia Wight & Arn. (164 species); species numbers derived from WorldWideWattle website (web ref. 1). This group of genera is referred to herein as Acacia sens. lat. and all indigenous species of the group in China belong to the genus Senegalia. Secondly, the name Acacia is now conserved with a new type, namely, the Australian species Acacia penninervis Sieber ex DC. which replaces the Afro-Asian species, Acacia nilotica (L.) Willd. ex Delile. This has meant that species which might otherwise have been called Acacia are now Vachellia. These two factors have had global repercussions as summarised below. However, the second matter scarcely affects China because it involves only two introduced species, namely, Vachellia farnesiana (L.) Wight & Arn. and Vachellia nilotica (L.) P.J.H.Hurter & Mabb.
The present study further impacts upon the Chinese Acacia sens. lat. flora because six new species are described, and five nomen-clatural novelties established. These changes involve half the indigenous species of Senegalia that occur within the country.
The 36 species of Acacia sens. lat. that are treated in detail here for China, represent a significant increase over previous numbers. These include 21 indigenous species and 15 species that are considered to be the most significant introductions. Acacia confusa Merr., which is both introduced and indigenous in Taiwan, is included with the introductions as a matter of convenience. The indigenous species belong to Senegalia sect. Monacanthea (Vassal) Maslin and are arranged in five informal species-groups (see under Classification below). These species do not form a conspicuous element of the Chinese flora; they occur in tropical and subtropical forest areas in the southern part of the country, south of latitude 30°N. Almost half of the indigenous Senegalia species are endemic or near-endemic to China, with the remainder ranging to Southeast Asia, and a few extending to the Indian subcontinent. The introduced species of Acacia sens. lat. in China belong to Acacia, Acaciella, Senegalia sect. Senegalia and Vachellia, with the Australian species of Acacia being the most numerous.
Although this is the first comprehensive, critical revision of Senegalia for China, there have been several previous taxonomic treatments of the group (as Acacia). The more important of the earlier publications which focus on China as a whole are those of Huang (1985), Wu (1988), Sun and Chen (1990), Sun (2006) and Wu and Nielsen (2010). These studies, as well as our own, benefitted greatly from the excellent work involving Acacia sens. lat. taxa for Southeast Asia by Nielsen(1980, 1981, 1985a, 1985b, 1992).
The taxonomic foundation for Senegalia in China today was essentially established by Huang (1985) and Wu (1988) who recognised (under Acacia) five and eight indigenous species respectively. Sun and Chen (1990) substantially increased species numbers by recognizing (under Acacia) 11 indigenous species (representing 14 taxa); the Flora of China treatment by Wu and Nielsen (2010) followed Sun (2006) to a large extent. Details concerning these treatments are discussed below under Historical perspective. The earliest enumerations of Chinese species under the name Senegalia were by Maslin (2015), who recognised 10 species (representing 13 taxa), followed by Zhu et al. (2015), who recognised 12 species (representing 15 taxa). Building on these previous studies, and from scrutiny of specimens in relevant herbaria in China, Southeast Asia and India, we here recognise 21 indigenous species (representing 22 taxa) of Senegalia for China.
The aim of this paper is to critically revise the indigenous species of Senegalia in China, and to provide a synopsis of the principal species of Acacia sens. lat. that are introduced to the country. Before presenting these taxonomic treatments (and a key to species identification) we provide background and general information to contextualise Senegalia within China.
1.1. Acacia sens. lat. classification and phylogeny: a synopsisTraditionally, Acacia sens. lat. and sometimes the monotypic genus Faidherbia Del. (native to Africa and West Asia) were regarded as comprising tribe Acacieae within subfamily Mimosoideae of family Leguminosae. However, this classification at present is under review. Recent molecular evidence shows the Mimosoideae is monophyletic, but nested within subfamily Caesalpinioideae (Legume Phylogeny Working Group, 2017), but tribal relationships within Mimosoideae are unresolved (Miller and Seigler, 2012).
As already noted, the former broadly circumscribed Acacia sens. lat. has now been split into seven genera (listed in Table 1). This new classification is based on results from many morphological, genetic and other studies that have occurred over the past almost 50 years. Maslin (2015); Maslin et al. (2003); Miller and Seigler (2012) provide synoptic overviews of this body of work, and discussion of relevant issues. Molecular sequence data involving both chloroplast and nuclear genes have been particularly helpful in understanding phylogenetic relationships within Acacia sens. lat. Over the past 15 years there have been seven large, comparative molecular studies involving species of this group which have also included members of the Mimosoid tribes Ingeae and Mimoseae, namely, Kyalangalilwa et al. (2013), Bouchenak-Khelladi et al. (2010), Luckow et al. (2003), Miller and Bayer(2000, 2001), Miller et al. (2003) and Miller and Seigler (2012). However, it is relevant to the present work to note that scarcely any species from China, Southeast Asia or the Indian subcontinent were included in these studies; a deficiency that should be redressed.
| Current namea | Previous nameb | No. sp.c | Generalised Distributiond [and occurrence in China] |
| Acacia (sens. str.) | Acacia subg. Phyllodineae | 1067 | Australia, Pacific, SE Asia [12 spp. introduced in China, but A. confusa is both introduced and indigenous in Taiwan] |
| Vachellia | Acacia subg. Acacia | 164 | Americas, Africa, Asia, N. Australia [2 spp. introduced in China] |
| Senegalia | Acacia subg. Aculeiferum | 217 | Americas, Africa, Asia, NE Australia [21 native & 2 introduced spp. in China] |
| Acaciella | Acacia subg. Aculeiferum sect. Filicineae | 15 | Americas [1 species introduced in China] |
| Mariosousa | Acacia subg. Aculeiferum ('oulteri' group) | 13 | Americas [Not represented in China] |
| Parasenegalia | Acacia subg. Aculeiferum ('kleroxyla' group) | 7 | Americas [Not represented in China] |
| Pseudosenegalia | Acacia subg. Aculeiferum (pro parte) | 2 | South America [Not represented in China] |
| a Currently accepted genus name following split of Acacia sens. lat. and conservation of the name Acacia with a new type. b The name that applied before the split of Acacia sens. lat. and conservation of the name Acacia. c Current species numbers obtained from WorldWideWattle website (web ref. 1). d Distribution maps for genera are provided on WorldWideWattle website (web ref. 2). | |||
All seven of the above-mentioned comparative molecular studies consistently showed that Acacia sens. lat. is clearly poly-phyletic. The congruence between the results of these studies provided strong evidence that Acacia sens. lat. could not be retained as a single genus. Furthermore, congruence between clades recognised in the studies and from prior morphological groupings, complemented by the work of Miller et al. (2017) and Terra et al. (2017), show that Acacia sens. lat. comprises at least seven monophyletic groups, representing seven genera. Acacia (sens. str.), Acaciella, Mariosousa, Parasenegalia, Pseudosenegalia and Senegalia are nested within a paraphyletic grade that also includes genera of tribe Ingeae, while Vachellia is nested within, or sister to, a clade containing the paraphyletic tribe Mimoseae. This phylogeny and classification for Acacia sens. lat. is shown in the schematic diagram (Fig. 1) in both Miller et al. (2017) and Terra et al. (2017), and is widely accepted by the international legume community.
1.2. Acacia sens. lat. nomenclature: a synopsisThe name Acacia is now conserved with a new type, A. penninervis, which replaces the former type, A. scorpioides (L.) W.Wight (=A. nilotica) (McNeill and Turland, 2011). This matter, which was prompted by a formal conservation proposal by Orchard and Maslin (2003), had been the subject of robust debates in scientific and other literature for many years. The decision to retypify Acacia was initially made at the 2005 International Botanical Congress in Vienna, and was ratified at the 2011 IBC in Melbourne. This resolution meant that nomenclatural stability was achieved concerning the applications of the names Acacia and Vachellia (Senegalia and the other genera of Acacia sens. lat. were not affected by the conservation proposal). The WorldWideWattle website (web ref. 3) provides further information regarding these nomenclatural issues, including references to relevant publications on both sides of the debate. A brief overview of these matters, with particular reference to East and Southeast Asia, is provided in Maslin (2015).
Following the taxonomic fragmentation of Acacia sens. lat. and the conservation of the name Acacia with a new type, the names of many "Acacia" species worldwide have changed in recent years. The currently accepted names for all taxa in the seven genera of Acacia sens. lat. can be obtained from using the Advanced Search feature of the WorldWideWattle website (web ref. 4); where appropriate, these names are accompanied by the names (now synonyms) that had been commonly used prior to fragmentation and retypification of Acacia. Most of these names are also available on the Catalogue of Life website (web ref. 5).
2. Materials and methodsWork for this project was conducted between 2013 and 2019, primarily at the Kunming Institute of Botany in China. The taxonomic conclusions presented here are based largely on analyses of herbarium specimens, but previous taxonomic studies involving Chinese Acacia sens. lat. taxa were also taken into account. Some targeted field work in Yunnan was conducted during the study and a little opportunistic collecting was undertaken in Guangdong and Guangxi Provinces. The third author has extensive field knowledge of particularly the Yunnan species on account of a long-established research interest in acacias of China, e.g. Sun and Chen (1990) and Sun (2006).
Scope of study. This study includes all species of the former broadly circumscribed genus Acacia that occur in China, either naturally or as major introductions. All Acacia sens. lat. names that apply to Senegalia, as either accepted names or synonyms, that appear in taxonomic literature involving China, are included.
The geographic coverage is China, including species from Taiwan where information could be sourced from literature, online databases or from herbarium records that we consulted.
Arrangement of text. The 21 indigenous species (all Senegalia) are treated first, followed by the 15 introduced species that belong to Acacia, Acaciella, Senegalia and Vachellia. In both cases the species are arranged alphabetically under their respective genus, and are numbered sequentially (i.e. 1-36).
Types and typification. Type terminology follows the International Code of Nomenclature (Turland et al., 2018) except that "emaining syntype" (not in the Code) is used to denote specimens (syntypes) remaining following selection of a lectotype. We have followed the Code and also recommendations by McNeill (2014) regarding the definition of a holotype. Type information from the protologue is presented under the heading "Type citation". This is done to contextualise type information that is derived from specimens and presented under the "Type" heading. Many type specimens were viewed as digital images obtained from the worldwide web, and these are designated by "digital image!" in the text. The one exception is Acacia hainanensis Hayata where this designation means that photographs of types from herb. TI and TAIF were supplied for our inspection (see Acknowledgements).
Synonymy. In most cases only synonyms pertinent to China are included, i.e. those names based on material collected from China and/or which have been listed in publications involving taxa that occur in China. Where additional synonyms exist, reference(s) to the relevant publication(s) are given at the end of the synonymy list under the heading 'Additional synonymy'. In synonymy lists the following conventions have been used: - denotes a nom. invalid. (which includes nom. nud.); = denotes a heterotypic (i.e. taxonomic) synonym; ≡ denotes a homotypic (i.e. nomenclatural) synonym. The symbol! indicates that the cited specimen (or digital image of the specimen) has been seen by us.
Descriptions. For the indigenous species of Senegalia, the descriptions are based on herbarium material of plants from China, unless otherwise indicated. The citation of specimens from countries other than China indicates that the description was not based exclusively on Chinese material. Information derived from relevant previous publications has also been used, and where presumed errors exist in these sources, they are noted in the text. For introduced species, the descriptions are based on sources that are given under the Taxonomy heading in each botanical treatment (those sources often include a reference to specimens seen in Chinese herbaria).
Distinctive features. The short paragraph preceding the description highlights some of the main morphological features that characterise the taxon. When taken in combination, these features are normally diagnostic for the taxon.
Specimen citations. Specimens are cited for all indigenous taxa, but in most cases only a selection of specimens examined has been listed. The citations for China are arranged alphabetically by Province and reflect the distribution of the taxon within the country (see Distribution citations below). Within Provinces, specimens are listed alphabetically by City or Autonomous Prefecture, which are the primary geo-administrative sub-divisions, then by County. Specific collection localities are normally not cited (except where possible, for type specimens) because these are infrequently given on specimen labels.
The inclusion of non-Chinese specimen citations indicates that at least some material from the countries listed was used to prepare the taxon description, but these citations are not intended to reflect the geographic distribution of taxa within those countries. A selection of voucher specimens (from Chinese herbaria) are given for introduced species, together with data sources used to prepare these treatments. Collector names, localities and other information given in Chinese on specimen labels were translated into English by the fourth author. Herbarium acronyms follow Index Herbariorum (web ref. 6). Both institution accession numbers and barcode numbers are given where these appear on the sheets. For barcodes, the institution acronym is repeated where this forms an integral part of the barcode.
Habitat and phenological information. These data were derived from herbarium specimens, complemented by information provided in relevant literature, especially Sun (2006) and Wu and Nielsen (2010).
Distribution citations. For each taxon its distribution within China is given, and where relevant, also its global distribution. Distribution statements for Chinese taxa are based on specimen records that we have confirmed as correct. Data sources validating global distribution records for species are given at the WorldWideWattle website (web ref. 4). Suspect or otherwise uncertain records are noted as requiring further investigation. The term nearendemic as used here refers to distributions that extend beyond the border of China by no more than about 100 km.
Distribution maps. Chinese counties represent the basic units used for generating distribution maps. The location-identifiers on these maps are the centre-points of each county from where the taxon is recorded as occurring. These counties are listed in the specimen citations (unless otherwise noted in the map caption). The maps were generated using GIS Diva and show taxon distri-butions within China only.
Vernacular names. These names are presented in both Chinese and English for each species. Where possible, we adopted names that had been used previously under Acacia. In cases where vernacular names did not exist, or were considered inappropriate, new names have been proposed. Where multiple common names had been applied to a species, we designate one as our preferred name for use in China.
The word "Wattle" that appears in some vernacular names is a common name that is often applied to many species of Acacia in Australia.
Abbreviations. Taxon author and literature abbreviations used in the taxonomic section of the paper follow those given in IPNI (web ref. 7), except for the following. For A.P. de Candolle's 1825 publication Prodromus Systematis Naturalis Regni Vegetabilis IPNI gives the abbreviation as "Prodr. (A.P. de Candolle)"; however, we adopt the shorter and more conventional abbreviation, "Prodr." Also, Willdenow's 4th edition of Species Plantarum is cited here as "Sp. Pl., ed. 4", not "Sp. Pl., ed. 4 [Willdenow]" as is given in IPNI, and the Philippine Journal of Science. Section C, Botany is cited here as "Philip. J. Sci. (Botany)", not "Philip. J. Sci., C" as given in IPNI.
Herbaria consulted. Specimens at the following Chinese herbaria were inspected in connection with this study: GXMG, GXMI, HITBC, HIB, IBK, IBSC, KUN, SWFC, SYS and YUKU. In order to contextualise and better-understand Chinese taxa, the first author examined relevant specimens at the following (mostly Asian) herbaria: BK, BKF, BO, CAL, CMU, DD, HN, HNU, KEP, PERTH, SING, VNM. Digital images of type and other specimens were obtained from the following herbaria: A, AAU, BM, C, E, K, L, P, PE, TAIF, TCD, TI and US.
When we commenced this study, all Senegalia specimens in Chinese herbaria were determined as Acacia. During the course of the work we comprehensively determined specimens with their respective Senegalia names at only KUN and SING. For other herbaria that were visited, the specimens were normally sorted according to taxa recognised herein (and names written on the outer specimen folder), but individual sheets were normally not determined as to their Senegalia name.
2.1. Application of rankAs noted by Cowan and Maslin (1995) the assignment of rank is largely a subjective exercise that is dependent on one's knowledge of the taxa under study. There are no universally accepted objective criteria that can be used for assigning rank. Furthermore, as noted by Johnson (1976), it does not matter too much what rank is applied to taxa, provided that they are named and that they represent meaningful biological entities.
We have been guided by these view-points when assigning rank to taxa of Chinese Senegalia. Our choice of rank has been made largely on a subjective judgement of the amount and significance of morphological separation between taxa. Consequently, several taxa that were formerly recognised as varieties or subspecies under Acacia are here treated as species, namely, Acacia delavayi var. kunmingensis C.Chen & H.Sun is now Senegalia kunmingensis (C.Chen & H.Sun) Maslin, B.C.Ho, H.Sun & L.Bai, Acacia megaladena var. garrettii I.C.Nielsen is now Senegalia garrettii (I.C.Nielsen) Maslin, B.C.Ho, H.Sun & L Bai and Acacia pennata subsp. kerrii I.C.Nielsen is now Senegalia kerrii (I.C.Nielsen) Maslin, B.C.Ho, H.Sun & L.Bai. Also, Acacia macrocephala Lace that was formerly a synonym of A. pennata subsp. hainanensis (Hayata) I.C.Nielsen is now resurrected as Senegalia macrocephala (Lace) Maslin, B.C.Ho, H.Sun & L.Bai. Although the morphological boundaries between some of our species may not seem especially large, this does not negate their recognition as species. This is exemplified especially by following species-pairs: Senegalia hainanensis - Senegalia stipitata, S. kerrii - S. garrettii and Senegalia teniana - Senegalia prominens. In these cases, the species within each pair are morphologically rather close and it could possibly be argued that each pair constitutes a single species with two subspecies, but for reasons discussed in the text we have chosen species rank. A relevant factor here is that we consider that the recognition of species rather than infraspecies obliges users to rigorously test our classification hypotheses, because the option of simply calling specimens by a collective species name does not exist.
We therefore consider that the ranks applied in this study provide a practical and workable framework that facilitates the understanding and discussion of variation, relationships, etc. within Chinese Senegalia. However, it is recognised that future studies may demonstrate the need to choose different ranks to ours in some cases.
3. Senegalia in China: an overview 3.1. Historical perspectiveMany of the matters discussed below are summarised in Table 2 which provides the names of indigenous species of Senegalia that have been recognised in publications encompassing the whole of China. As will be seen from the following discussion, the number of indigenous species of Senegalia (which were previously called Acacia) recognised for China has increased from one to 21 over the past 125 years.
| Publication | No. sp. (Senegalia/Acacia sens. lat.)a | Senegalia taxa included in publicationb | Notes |
| Forbes and Hemsley (1887) | 1/3 | S. rugata [A. concinna] | |
| Huang (1985) | 5/15 | S. delavayi [A. delavayi], A. intsia, A. pennata, S. rugata & S. prominens [A. sinuata], S. yunnanensis [A. yunnanensis]. | It is not known what species were included by Huang (1985) under the names Acacia intsia & A. pennata. The entity described as A. sinuata probably included elements of S. prominens and S. rugata. |
| Wu (1988) | 8/18 | S. caesia [A. caesia], S. delavayi [A. delavayi], S. megaladena [A. megaladena], A. pennata, S. pruinescens [A. pruinescens], S. rugata & S. prominens [A. sinuata], S. teniana [A. teniana], S. yunnanensis [A. yunnanensis]. | Re A. pennata & A. sinuata: see notes above. |
| Sun and Chen (1990) | 11/14 | S. caesia [A. caesia], S. rugata [A. concinna], S. delavayi [A. delavayi var. delavayi], S. kunmingensis [A. delavayi var. kunmingensis], S. hainanensis & S. stipitata [A. hainanensis], S. garrettii [A. megaladena var. garrettii], S. megaladena var. megaladena [A. megaladena var. megaladena], S. clandestina, S. pennata subsp. insuavis & S. kerrii [A. pennata], S. pruinescens [A. pruinescens, two vars.], S. teniana [A. teniana], S. tonkinensis [A. tonkinensis], S. andamanica & S. prominens [A. vietnamensis], S. yunnanensis [A. yunnanensis]. | Acacia hainanensis included elements of S. hainanensis & S. stipitata. Acacia pennata included elements of S. clandestina S. kerrii& S. pennata subsp. insuavis. Acacia vietnamensis (does not occur in China) included elements of S. andamanica, S. prominens & S. vietnamensis, |
| Wu and Nielsen (2010) | 11/20 | S. caesia [A. caesia], S. rugata [A. concinna], S. delavayi [A. delavayi var. delavayi], S. kunmingensis [A. delavayi var. kunmingensis], S. garrettii [A. megaladena var. garrettii], S. megaladena var. megaladena [A. megaladena var. megaladena], S. hainanensis [A. pennata subsp. hainanensis], S. kerrii [A. pennata subsp. kerrii], S. pruinescens [A. pruinescens], S. teniana [A. teniana], S. tonkinensis [A. tonkinensis], A. vietnamensis, S. yunnanensis [A. yunnanensis]. | The description of A. vietnamensis (= S. vietnamensis) was largely based on the protologue; this species does not occur in China. Wu and Nielsen (2010) regarded A. catechu (= S. catechu) as native in Yunnan, but we consider it introduced. |
| Maslin (2015) | 10/15 | S. caesia, S. delavayi, S. garrettiii [S. megaladena var. garrettii], S. hainanensis [S. pennata subsp. hainanensis], S. kerrii [S. pennata subsp. kerrii], S. kunmingensis [S. delavayi var. kunmingensis], S. megaladena var. megaladena, S. pruinescens, S. rugata, S. teniana, S. tonkinensis, S. yunnanensis. | In this publication S. catechu was regarded as native to China, following Wu and Nielsen (2010); see note above. |
| Zhu et al. (2015) | 11/25 | S. caesia, S. delavayi [S. delavayi var. delavayi], S. garrettiii [S. megaladena var. garrettii], S. hainanensis, S. kunmingensis [S. delavayi var. kunmingensis], S. megaladena var. megaladena, S. pennata, S. pruinescens [S. pruinescens var. pruinescens & var. luchunensis], S. rugata, S. teniana, S. tonkinensis, S. vietnamensis, S. yunnanensis. | Re S. pennata & S. vietnamensis: see notes above. Two varieties were recognised for S. pruinescens, but var. luchunensis is now not recognised. |
| Maslin et al. (the current paper) | 21/36 | S. andamanica, S. caesia, S. clandestina, S. delavayi, S. garrettii, | |
| S. guangdongensis, S. hainanensis, S. kerrii, S. kunmingensis, S. macrocephala, S. megaladena var. megaladena & var. indochinensis, S. obliqua, S. orientalis, S. pennata subsp. insuavis, S. prominens, S. pruinescens, S. rugata, S. stipitata, S. teniana, S. tonkinensis, S. yunnanensis. | |||
| a Number of Senegalia species and (after the/) number of Acacia sens. lat. species in publication listed in column 1. b Names in bold font = currently accepted Senegalia names; names in plain font are those used in the publication listed in column 1. (Where appropriate, taxa in column 3 are arranged alphabetically by the Acacia species name (mostly given in square brackets) under which they were described in the publication listed in column 1.) Abbre-viations: A. = Acacia, S. = Senegalia. | |||
The earliest enumeration of Acacia sens. lat. for all of China ap-pears to be that of Forbes and Hemsley (1887). In that work just three species were recognised, including one that is now referred to Senegalia, namely, Acacia concinna (Willd.) DC. (=Senegalia rugata (Lam.) Britton & Rose). The other two species in that paper wereAcacia farnesiana (L.) Willd. (=V. farnesiana) and Acacia richii A.Gray (which was a misapplication for A. confusa Merr.). About one hundred years later, Huang (1985) followed by Wu (1988), recognised 15 and 18 species of Acacia sens. lat. respectively for the country. About one third of the species included by Huang (1985) and about half of those included by Wu (1988) are now referred to Senegalia. Both Huang (1985) and Wu (1988) included A. pennata (L.) Willd. and Acacia sinuata (Lour.) Merr., but it is not known with certainty what taxa were encompassed by these names: Senegalia pennata (L.) Maslin is more narrowly defined than previously and is represented in China by only S. pennata subsp. insuavis; although A. sinuata is here regarded as a synonym of S. rugata, the entity described under this name by both Huang (1985) and Wu (1988) probably included elements of S. prominens Maslin, B.C.Ho, H.Sun & L.Bai and S. rugata. For further discussion of these matters see botanical treatments under S. prominens and S. rugata.
Subsequent to the work of Nielsen(1980, 1981, 1985a, 1985b) in Southeast Asia, Sun and Chen (1990) made significant advances for China by recognising 11 species that are now referred to Senegalia. Sun and Chen (1990) included most of the taxa that had been recognised by Wu (1988) but they also described two new taxa, A. delavayi var. kunmingensis C.Chen & H.Sun (=Senegalia kunmingensis) and Acacia pruinescens var. luchunensis C.Chen & H.Sun (this variety is not recognised in the present work), resurrected A. hainanensis Hayata (=S. hainanensis (Hayata) H.Sun) which had previously been recognised by Merrill (1927) but omitted by Huang (1985) and Wu (1988), and recorded for the first time in China A. megaladena var. garrettii I.C.Nielsen (=S. garrettii) and Acacia tonkinensis I.C.Nielsen (=Senegalia tonkinensis (I.C.Nielsen) Maslin, Seigler & Ebinger). Sun and Chen (1990) also recognised Acacia vietnamensis I.C.Nielsen (=Senegalia vietnamensis (I.C.Nielsen) Maslin, Seigler & Ebinger) as a new record for China, based on specimens that we now assign to Senegalia andamanica (I.C.Nielsen) Maslin, Seigler & Ebinger and S. prominens; S. vietnamensis does not occur in China. Sun and Chen (1990) did not include the confusing A. sinuata but they did include A. pennata (their treatment being based on specimens that are here assigned to Senegalia clandestina Maslin, B.C.Ho, H.Sun & L.Bai, S. pennata subsp. insuavis (Lace) Pedley and S. kerrii. Although Sun and Chen (1990) did not provide descriptions, they did provide synonymy, references to relevant literature and, importantly, voucher specimens upon which their taxon concepts were based.
The Flora of China treatment of Acacia by Wu and Nielsen (2010) contained essentially the same taxa as Sun and Chen (1990) except that the broad, four-subspecies concept of Nielsen (1980) for A. pennata was adopted, with subsp. kerrii I.C.Nielsen (=Senegalia kerrii) and subsp. hainanensis (Hayata) I.C.Nielsen (=S. hainanensis) being recorded for China. The Wu and Nielsen (2010) description of A. vietnamensis was based closely on the protologue, but as already noted, Senegalia vietnamensis does not occur in China.
The first listings of taxa for China under the name Senegalia was given by Maslin (2015) followed by Zhu et al. (2015) who recognised 10 species (13 taxa) and 12 species (15 taxa) respectively. The present work now recognises 21 species (22 taxa) of Senegalia for China. These species are listed in Tables 4 and 5 and are detailed in the Taxonomy section of this paper below.
| Bentham (1842) | Vassal (1972) | Pedley (1978) | Pedley (1986) | Current classification |
| ACACIA | ACACIA | ACACIA | SENEGALIA | SENEGALIA [217 spp.] |
| Series Vulgares | Subg. Aculeiferum | Subg. Aculeiferum | Sect. Senegalia | Sect. Senegalia |
| [52 spp.]a (Africa & Asia) | ||||
| Sect. Aculeiferum | Sect. Spiciflorae | |||
| Sect. Monacanthea | Sect. Monacanthea | |||
| [168 spp.]b (Pantropical) | ||||
| MARIOSOUSA | ||||
| [13 spp.] (Americas) | ||||
| PARASENEGALIA | ||||
| [7 spp.] (Americas) | ||||
| PSEUDOSENEGALIA | ||||
| [2 spp.] (Americas) | ||||
| Series Filicinae | Sect. Filicinae | Sect. Filicinae | Sect. Filicinae | ACACIELLA |
| [15 spp.] (Americas) | ||||
| Note: only infra-generic groups that have direct relevance to the discussions in the present paper are included in this table. Currently accepted names are given in bold font. Column 5: Current classification with global species numbers and generalised natural distribution in parentheses a Species assigned here to Senegalia sect. Senegalia have cauline prickles at the nodes, and flowers normally arranged in cylindrical spikes. b Species assigned here to Senegalia sect. Monacanthea have cauline prickles scattered between nodes, and flowers normally arranged in globose heads (less frequently cylindrical spikes). Abbreviations: Sect. = Section, Subg. = Subgenus. | ||||
| Taxaa of Senegalia | Groupb | China occurrencec | Non-Chinad | ||||||||||||||
| YN | SC | GZ | GX | HN | JX | ZJ | FJ | GD | HK | MO | HI | TW | SEA | IND | |||
| S. andamanica | TEN | × | × | × | × | ||||||||||||
| S. caesia | CAE | × | × | ? | × | × | |||||||||||
| S. clandestina (e) | PEN | × | |||||||||||||||
| S. delavayi (e) | TEN | × | |||||||||||||||
| S. garrettii | PEN | × | × | × | × | × | |||||||||||
| S. guangdongensis (e) | HAI | × | |||||||||||||||
| S. hainanensis | HAI | × | × | ||||||||||||||
| S. kerrii | PEN | × | × | × | × | ||||||||||||
| S. kunmingensis (ne) | TEN | × | × | × | × | ||||||||||||
| S. macrocephala (ne) | HAI | × | × | ||||||||||||||
| S. megaladena var. megaladena | PEN | × | ? | × | × | ||||||||||||
| S. megaladena var. indochinensis | PEN | × | × | ||||||||||||||
| S. obliqua (ne) | PEN | × | × | ||||||||||||||
| S. orientalis (ne) | PEN | × | × | × | × | × | × | ||||||||||
| S. pennata subsp. insuavis | PEN | × | × | × | × | ||||||||||||
| S. prominens (e) | TEN | × | × | × | × | ? | ? | × | × | ? | |||||||
| S. pruinescens | TEN | × | × | × | × | ||||||||||||
| S. rugata | RUG | × | × | × | ? | × | × | ||||||||||
| S. stipitata | HAI | × | × | × | |||||||||||||
| S. teniana (e) | TEN | × | × | ||||||||||||||
| S. tonkinensis | CAE | × | × | ||||||||||||||
| S. yunnanensis (e) | TEN | × | × | ||||||||||||||
| TOTAL TAXAf | 16 | 2 | 4 | 7 | 2 | 1 | 0 | 1 | 5 | 1 | 1 | 4 | 2 | 15 | 7 | ||
| Abbreviations used: aSpecies endemism: e - endemic to China; ne = near-endemic to China. bTaxonomic group (see introduction under Classification): CAE - Caesia, HAI - Hainanensis, PEN - Pennata, RUG - Rugata, TEN - Teniana. cChina occurrences (see species treatments under Taxonomy below): FJ - Fujian Province, GD - Guangdong Province, GX - Guangxi Province, GZ - Guizhou Province, HI - Hainan Province, HK - Hong Kong Territory, HN - Hunan Province, JX - Jiangxi Province, MO - Macau Territory, SC - Sichuan Province, TW - Taiwan, YN - Yunnan Province, ZJ - Zhejiang Province. dNon-Chinese distributions (see Table 5 for details): IND - Indian subcontinent, SEA - Southeast Asia. fTotals: uncertain occurrences (designated in table by?) not included in totals. | |||||||||||||||||
| Taxa of Senegalia | Group1 | Southeast Asia & Australia2 | Indian subcontinentc | ||||||||||||||||
| VN | LA | KH | TH | MY | ID | PH | SG | OT | MM | BD | IN | BT | NP | LK | |||||
| S. andamanica | TEN | ×n | × | × | |||||||||||||||
| S. caesia | CAE | × | × | × | × | × | × | × | × | × | |||||||||
| S. garrettii | PEN | ×ns | × | ×ne | |||||||||||||||
| S. hainanensis | HAI | ×c | |||||||||||||||||
| S. kerrii | PEN | × | × | × | × | × | × | × | × | ×ne | ? | ? | ? | ||||||
| S. kunmingensis | TEN | ×n | |||||||||||||||||
| S. macrocephala | HAI | × | |||||||||||||||||
| S. megaladena var. megaladena | PEN | × | × | × | × | × | × | × | × | × | |||||||||
| S. megaladena var. indochinensis | PEN | × | × | × | × | ? | ? | ||||||||||||
| S. obliqua | PEN | ×n | |||||||||||||||||
| S. orientalis | PEN | ×n | |||||||||||||||||
| S. pennata subsp. insuavis | PEN | ? | × | ? | |||||||||||||||
| S. pruinescens | TEN | ×n | × | × | ×ne | ||||||||||||||
| S. rugata | RUG | × | × | × | × | × | × | × | × | × | × | × | × | × | |||||
| S. stipitata | HAI | ×n | |||||||||||||||||
| S. tonkinensis | CAE | ×nc | ×n | × | × | ||||||||||||||
| TOTALTAXAd | 13 | 6 | 4 | 9 | 2 | 3 | 1 | 0 | 2 | 9 | 3 | 7 | 3 | 2 | 1 | ||||
| Abbreviations used: aTaxonomic group (see Introduction above under Classification): CAE - Caesia, HAI - Hainanensis, PEN - Pennata, RUG - Rugata, TEN - Teniana. bSoutheast Asia & Australia occurrences (distribution records derived from Maslin (2015) and the WorldWideWattle website, web ref. 4): ID - Indonesia, KH - Cambodia, LA - Laos, MM - Myanmar, MY - Malaysia, OT - Australia or East Timor or Papua New Guinea, PH - Philippines, SG - Singapore, TH - Thailand, VN - Vietnam. cIndian sub-continent occurrences (distribution records are derived from Deshpande et al. (2019) and the WorldWideWattle website, web ref. 4): BD - Bangladesh, BT - Bhutan, IN - India, LK - Sri Lanka, NP - Nepal. dTotals: uncertain occurrences (designated in table by?) not included in totals. Other abbreviations: c = central, - = eastern, n = northern. | |||||||||||||||||||
| Taxa of Senegalia | Southeast Asia occurrencesa | China occurrencesb | |||||||||||||||
| VN | ID | TH | MM | MY | KH | LA | PH | TL | PG | YN | GZ | GX | GD | HI | TW | ||
| S. bomeensis | × | × | |||||||||||||||
| S. clandestina | × | ||||||||||||||||
| S. donnaiensis | × | × | × | × | |||||||||||||
| S. garrettii | × | × | × | × | × | ||||||||||||
| S. guangdongensis | × | ||||||||||||||||
| S. hainanensis | × | × | |||||||||||||||
| S. kerrii | × | × | × | × | × | × | × | × | × | × | |||||||
| S. macrocephala | × | × | |||||||||||||||
| S. megaladena var. megaladena | × | × | × | × | × | × | ? | ||||||||||
| S. megaladena var. indochinensis | × | ? | × | ? | ? | × | × | × | |||||||||
| S. obliqua | × | × | |||||||||||||||
| S. orientalisc | × | × | × | × | |||||||||||||
| S. palawanensis | × | ||||||||||||||||
| S. pennata subsp. insuavis | × | ? | × | × | × | ||||||||||||
| S. pluricapitata | × | × | × | × | |||||||||||||
| S. pluriglandulosa | × | × | |||||||||||||||
| S. stipitata | × | × | × | ||||||||||||||
| S. sulitii | × | × | |||||||||||||||
| S. tawitawiensis | × | ||||||||||||||||
| S. thailandica | × | × | |||||||||||||||
| S. vietnamensis | × | × | |||||||||||||||
| TOTAL TAXAd | 10 | 7 | 6 | 5 | 4 | 4 | 4 | 3 | 1 | 1 | 8 | 1 | 3 | 3 | 3 | 2 | |
| Abbreviations used: aSoutheast Asian occurrences (distribution records derived from Maslin (2015) and the WorldWideWattle website, web ref. 4): ID - Indonesia, KH - Cambodia, LA - Laos, MM - Myanmar, MY - Malaysia, PG ePapua New Guinea, PH - Philippines, TH - Thailand, TL - East Timor, VN - Vietnam. bChina occurrences (see species treatments under Taxonomy below): GD - Guangdong Province, GX - Guangxi Province, GZ - Guizhou Province, HI - Hainan, TW - Taiwan, YN - Yunnan Province. cSenegalia orientalis: this species also occurs in Hunan & Fujian Provinces. dTotals: uncertain occurrences (designated in table by?) not included in totals. | |||||||||||||||||
It is of historical interest to note that Acacia concinna (=Senegalia rugata) appears to be the first species of Senegalia recognised for China, having been recorded for Hong Kong by Bentham (1861). The first species of Senegalia described for China, based on specimens collected from within the country, were Senegalia delavayi (Franch.) Maslin, Seigler & Ebinger and Senegalia yunnanensis (Franch.) Maslin, Seigler & Ebinger. These two species were described under Acacia by Franchet (1890), based on specimens collected in Yunnan by the French missionary, explorer and botanist, Père Jean-Marie Delavay (1834-1895). The first taxa of Senegalia described by resident Chinese botanists, based on material collected from within the country, were A. delavayi var. kunmingensis C.Chen & H.Sun (=S. kunmingensis) and A. pruinescens var. luchunensis C.Chen & H.Sun (this latter variety is not recognised here), that were described in Sun and Chen (1990).
3.2. ClassificationThe genus Senegalia was originally described by Rafinesque (1838) as one of seven new genera that he segregated from what was then called Acacia. None of Rafinesque's genera, except Senegalia, survives today. Senegalia was subsequently ignored until Britton and Rose (1928) recognised the genus for the Americas, assigning 66 species to it. However, after Rose's death in 1934 the somewhat radical Britton and Rose (1928) classification was ignored (Pedley, 1987) and most of their genera (including Senegalia) were rejected, with species being transferred back to Acacia where necessary. In 1986 Pedley re-assessed the classification of Acacia and proposed that the genus be divided into three genera, one being the resurrected Senegalia in which two infrageneric groups were recognised, namely, section Senegalia and section Filicinae (Benth.) Pedley. The classification of Pedley (1986) was not generally adopted at the time because it became associated with a robust debate concerning the application of the name Acacia (see A. sens. lat. nomenclature: a synopsis above), even though Senegalia was not directly involved in that matter. Following the decision concerning Acacia at the International Botanical Congress in Vienna in 2005 the name Senegalia began to appear in publications globally, including those in which new combinations from Acacia and also new species were described, e.g. Seigler et al. (2006a), Seigler and Ebinger(2009, 2010), Maslin (2012), Kyalangalilwa et al. (2013), Maslin et al. (2013), Seigler et al. (2012), Seigler et al. (2013), Ragupathy et al. (2014), Seigler (2014) and Boatwright et al. (2015). Over the past decade the definition of Senegalia has been refined, mainly as a result of taxonomic work involving the American species. Firstly, the genus Acaciella was resurrected by Rico Arce and Bachman (2006): species of this small genus have long been recognised as distinctive, and had been assigned to section or series Filicinae Benth. under both Acacia and Senegalia. Subsequently, three new genera were segregated from the "rump" of Senegalia: Mariosousa (Seigler et al., 2006b), Parasenegalia (Seigler et al., 2017) and Pseudosenegalia (Seigler et al., 2017). These changes, involving both the classification and nomenclature of Senegalia, are summarised in Table 3.
Apart from the original description of Senegalia, the concept of the genus as understood at present had its origins in the infrageneric classifications of Acacia by Bentham(1842, 1875). Among the six series created by Bentham in these works to accommodate the 340 and 432 species of Acacia respectively that he recognised, were two that contained species which, in the recent past or at present, belong to Senegalia, namely, ser. Filicinae and ser. Vulgares Benth. Species assigned by Bentham to ser. Filicinae are now called Acaciella; this genus is endemic to the Americas and contains 15 species of which one, Acaciella glauca (L.) L.Rico, is introduced in China. Species assigned by Bentham to ser. Vulgares now belong to the pantropical genus Senegalia sens. str. (containing 217 species) and to the American endemic genera Mariosousa (13 species) and Parasenegalia (7 species); the two species now included in Pseudosenegalia were not known to Bentham. Apart from Senegalia sens. str., there are no representatives of these four genera in China. Within series Vulgares Bentham(1842, 1875) recognised five and six subseries respectively, defining them primarily by prickle disposition along the stems and inflorescence shape characters. Unlike Bentham's series, his subseries appear not to have been adopted in subsequent literature.
Vassal (1972) proposed a new classification of Acacia that was based chiefly on information derived from his study of seed, seedling and stipule characters, supplemented by pollen data from Guinet (1969). Vassal's scheme recognised three subgenera within Acacia, subgenus Acacia (now Vachellia), subgenus Aculeiferum Vassal (now Senegalia) and subgenus Heterophyllum Vassal (= subg. Phyllodineae (DC.) Ser., now Acacia). These subgenera broadly corresponded to groupings of the six series of Bentham (1875) which, according to Ross (1981) was fortunate, because most of the characters on which Vassal's classification was based are not obvious from the gross morphology of conventional herbarium specimens. Ross (1979) provides a good discussion of Bentham's and Vassal's classifications, especially insofar as they apply to African species. Within Acacia subgenus Aculeiferum Vassal recognised two sections, sect. Aculeiferum (= Senegalia sect. Senegalia) and sect. Monacanthea (= Senegalia sect Monacanthea). In many publications subsequent to Vassal (1972), including those of Nielsen(1980, 1981, 1985a, 1985b, 1992) for Southeast Asia, species that are now assigned to Senegalia were presented under Acacia subgenus Aculeiferum.
Notwithstanding the recognition of five and six subseries within sect. Vulgares by Bentham (1842) and Bentham (1875) respectively, the only formal infrageneric categories that are often recognised within Senegalia today are the two sections that Vassal (1972) recognised within Acacia subg. Aculeiferum, namely:
Senegalia sect. Senegalia (syn. Acacia sect. Aculeiferum); characterised principally by cauline prickles located at the nodes, and inflorescences arranged in cylindrical spikes; 52 species distributed in Africa, Madagascar and Asia.
Senegalia sect. Monacanthea (syn. Acacia sect. Monacanthea Vassal); characterised principally by cauline prickles scattered between the nodes, and inflorescences mostly arranged in globose heads (but sometimes cylindrical spikes); 168 species distributed pantropically in the Americas, Africa, Madagascar, Asia and Australia.
Sections Aculeiferum and Monacanthea were shown to be sistergroups on a moderately supported clade containing all species of Senegalia included in a recent genetic study by Miller and Seigler (2012); the four species labelled Senegalia on an adjacent clade in that study are now referred to the genus Parasenegalia. However, relationships within Senegalia remain relatively poorly understood (Miller and Seigler, 2012; Terra et al., 2017), and scarcely any Asian species were included in previous broad-based analyses of Acacia sens. lat. These matters need to be addressed in the future by broad-based phylogenetic studies that include an appropriate representation of Asian Senegalia taxa. It is noted, however, that for China Ou Yang et al. (2013) conducted a DNA barcoding study involving 28 Acacia sens. lat. species (including 12 indigenous Senegalia, together with species of Vachellia and Acacia). However, this work contained scarcely any phylogenetic discussion, but it did include a tree (unrooted).
For the purpose of the present work, the 21 indigenous species of Senegalia in China are accommodated in six informal species-groups within sect. Monacanthea (the two introduced species of Senegalia in China belong to sect. Senegalia). These informal groups have been established primarily from our subjective assessment of species relationships based on comparative morphology. This arrangement is provided primarily to establish a framework for the discussions below under Biogeography, where further notes concerning these groups are provided.
Groups of Chinese Senegalia:
1. Prickles at nodes; inflorescences in spikes. Introduced species…………………………………………………Section Senegalia
• Senegalia catechu, S. senegal
1: Prickles internodal; inflorescences in heads, rarely spikes. Indigenous species (Section Monacanthea)………………………2
2. Pods thickly coriaceous & smooth (fresh), hard-textured & wrinkled (dry)………………………………Rugata species-group
• Senegalia rugata, S. rugata (Bukit Brown)
2: Pods chartaceous to thinly coriaceous……………………………3
3. Petiolule centric (with main vein starting from near centre of leaflet)…………………………………………Caesia species-group
• Senegalia caesia, S. tonkinensis
3: Petiolule excentric (with main vein starting from near edge of leaflet)…………………………………………………………………4
4. Leaflets narrow (normally 0.5-1 mm wide) (Pennata complex)…………………………………………………………………5
4: Leaflets normally more than 1 mm wide…Teniana species-group
• Senegalia delavayi, S. prominens, S. teniana, S. yunnanensis
• Senegalia kunmingensis
• Senegalia pruinescens
• Senegalia andamanica
5. Inflorescence axes & peduncles with conspicuous, dark-coloured resin hairs…………………Hainanensis species-group
• Senegalia guangdongensis, S. hainanensis, S. macrocephala, S. stipitata
5: Inflorescence axes & peduncles without (or with very few) glandular hairs……………………………Pennata species-group
• Senegalia orientalis, S. pennata subsp. insuavis
• Senegalia clandestina, S. garrettii, S. kerrii
• Senegalia megaladena
• Senegalia obliqua
3.3. BiogeographyAs already noted in the Introduction above, Senegalia is a pantropical genus containing 217 species. Apart from Australia, the Asian region has the fewest number of species, and within this region the geo-political areas having highest species numbers are Yunnan Province in China (16 species) and Vietnam (17 species). While the elevated species numbers for Yunnan is probably due in part to the present taxonomic scrutiny of Chinese Senegalia, the Province is nevertheless naturally species-rich. China represents the northeast limit of distribution of Senegalia in East Asia, with no indigenous species extending to Korea or Japan. As already noted, there are two introduced species of Senegalia in China, but these species are not included in the discussions below.
In the discussions that follow, the distributions of Chinese species are given in maps presented below in the Taxonomy section of this paper. The distributions of species from Southeast Asia and the Indian subcontinent are documented in Maslin (2015) and Deshpande et al. (2019) respectively, and on the WorldWideWattle website (web ref. 4).
Within China, Senegalia occurs in tropical and subtropical areas in the more southerly Provinces of the country and does not extend above latitude 30°N (Fig. 2). It is likely that low winter temperature is a significant factor in precluding species from more northerly regions. The most northerly distributed species are S. teniana (Harms) Maslin, Seigler & Ebinger and S. yunnanensis in Yunnan and Sichuan Provinces (in the valley of the Jinsha River and its branches, fide Sun and Chen (1990)), and S. prominens that extends to Hunan Province. There is no taxon that ranges across the entire east-west breadth of China (except possibly Senegalia pennata subsp. insuavis, see note below). However, there are several widespread species in both southwest and southeast China whose distributions end in the central Province of Guangxi. For example, S. garrettii, S. kunmingensis and S. pruinescens (Kurz) Maslin, Seigler & Ebinger are all widespread in the southwest Province of Yunnan, but do not extend eastwards beyond central Guangxi, whereas S. prominens and S. orientalis Maslin, B.C.Ho, H.Sun & L.Bai are widespread in a number of south eastern Provinces, but do not extend westward beyond Guangxi. Senegalia pennata subsp. insuavis is the only taxon recorded as having a trans-China distribution (Yunnan, Guangxi and Guangdong Provinces), but it is not known if these records all represent natural occurrences because the subspecies is commonly cultivated for culinary purposes.
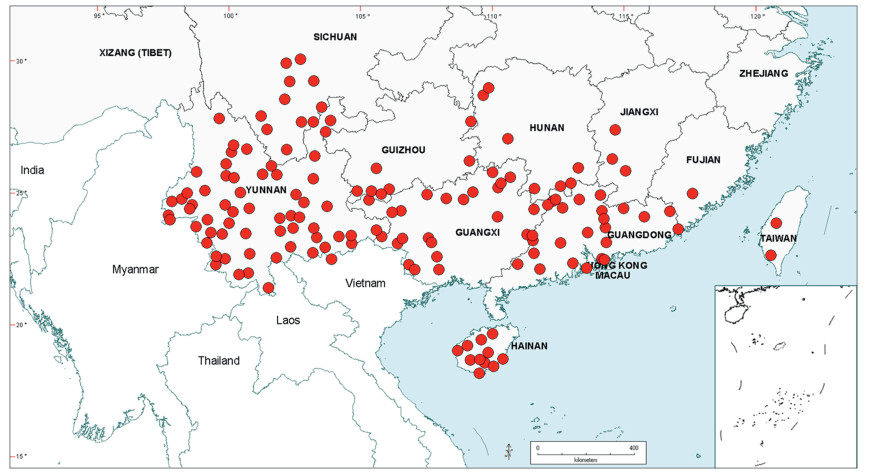
|
| Fig. 2 Distribution of the genus Senegalia in China based on location data cited for the 21 indigenous species in the text. The genus has been recorded for Zhejiang Province, but we have not verified this record (see text under Senegalia prominens). We have seen only a a few sterile specimens from Xizang Autonomous region (Tibet), but their identity is uncertain (see text under S. megaladena). |
The centre of species-richness for Senegalia in China is Yunnan Province which has 16 species (Table 4), representing c. 75% of the total taxa for the country. Senegalia in Yunnan includes representatives from all five species-groups that are recognised for the genus (Table 4), making this Province also the most species-diverse within China. As noted below, Yunnan also contains the highest number of endemics. Species numbers decrease markedly to the north and east of Yunnan, with Sichuan having just two species (Senegalia teniana and S. yunnanensis) and Guangxi, Guizhou, Guangdong and Hainan having between four and seven species each. The remaining Provinces are species-poor, having less than three species each.
The Chinese Senegalia flora comprises a mixture of both geographically restricted and widespread species, with the former being the more numerous (Tables 4 and 5). Fifteen species (16 taxa, representing 75% of the total for China) occur in just one or two Provinces (see Table 4), most notably Yunnan. However, almost half of the taxa that are geographically restricted have extensive distributions outside of China (e.g. Senegalia kerrii, S. megaladena var. indochinensis (I.C.Nielsen) Maslin, Seigler & Ebinger, S. tonkinensis; see Table 5 and discussions below). Species having the narrowest geographic ranges and which are restricted, or almost restricted, to China are: Senegalia guangdongensis Maslin, B.C.Ho, H.Sun & L.Bai in Guangdong Province; S. clandestina, S. delavayi, S. macrocephala and S. obliqua Maslin, B.C.Ho, H.Sun & L.Bai in Yunnan Province; S. teniana and S. yunnanensis in both Yunnan and Sichuan Provinces (Table 4). The two most widespread species (occurring in at least six Provinces) are the endemic Senegalia prominens and the near-endemic S. orientalis. Both these species predominate in south eastern regions of mainland China, but the latter extends to Taiwan and northeast Vietnam.
There are six endemic and four near-endemic species of Senegalia in China, which together represent almost half the total number of species for the genus in the country. The endemics are Senegalia guangdongensis in Guangdong Province, S. clandestina and S. delavayi in Yunnan Province, S. teniana and S. yunnanensis in both Yunnan and Sichuan Provinces, and the widespread S. prominens which occurs in Guangxi, Guizhou, Hunan, Guangdong and Jiangxi Provinces, Hong Kong Territory, and possibly also Fujian, Hainan and Zhejiang Provinces. The near-endemics are Senegalia macrocephala, which has a restricted distribution in western Yunnan and adjacent areas of Myanmar, S. kunmingensis which occurs in Yunnan, Guizhou and Guangxi but extends south to the Sapa region of northern Vietnam, S. obliqua which occurs in Yunnan and the western extremity of Guangxi but also extends south for a short distance into northern Vietnam, and S. orientalis which occurs in a number of Provinces of southeast China (including Taiwan), but extends to northeast Vietnam, not far south of the Chinese border. From the above it is seen that four of the six endemics and three of the near-endemics occur in Yunnan. About half of the endemic and near-endemic species occur in the 'Teniana species-group' (namely, Senegalia delavayi, S. kunmingensis, S. prominens, S. teniana, S. yunnanensis) with the other half in the "Pennata complex", three in the 'Pennata species-group' (S. clandestina, S. obliqua, S. orientalis) and two in the 'Hainanensis species-group' (S. guangdongensis, S. macrocephala).
Fourteen species (representing 15 taxa) have distributions that extend beyond China (Tables 4 and 5). These 14 species represent c. 70% of the Chinese Senegalia flora and, as shown in Table 5, all range to Southeast Asia with six (c. 30%) extending more widely to the Indian subcontinent (principally India). Southeast Asian countries that share highest species numbers with China are the neighbouring Vietnam (12 species, representing c. 60% of the Chinese Senegalia flora) and Myanmar (eight species, c. 40%); Laos and China, which also share a border, have only five species (23%) in common. Apart from Thailand which shares eight species with China, the other countries of Southeast Asia have less than four species in common (see Table 5) with China.
Species from all five of the informal species-groups recognised for Chinese Senegalia are included in those that range beyond the country. The most wide-spread of these species are S. caesia, S. rugata, and S. kerrii plus S. megaladena (Desv.) Maslin, Seigler & Ebinger, from the 'Caesia', 'Rugata' and 'Kerrii' species-groups respectively (this last-mentioned group is a member of the "Pennata complex").
The following discussion of biogeographic patterns for the five informal species-groups provides a better understanding and context for the Chinese Senegalia flora.
Hainanensis and Pennata species-groups. These two groups comprise the "Pennata complex" and will therefore be discussed together. The "Pennata complex" is the largest natural group of Senegalia in China, containing 11 species (representing 12 taxa; see Tables 4-6) which represent half the Senegalia flora of the country. The complex is named for Senegalia pennata, a species that until now has been broadly circumscribed with four subspecies recognised (Nielsen, 1980) and widely distributed in Asia (including China), but here we assign species rank to three of the four sub-species (with only subsp. insuavis occurring in China, as discussed under Senegalia pennata below). Most taxa of the "Pennata complex" in China occur in Yunnan Province, namely Senegalia clandestina, S. garrettii, S. kerrii, S. macrocephala, S. megaladena var. megaladena, S. obliqua, S. pennata subsp. insuavis and S. stipitata. The Chinese members of the "Pennata complex" include two endemics (Senegalia clandestina, S. guangdongensis) and three near-endemics (S. macrocephala, S. obliqua, S. orientalis), most of which have restricted geographic distributions within China (S. orientalis is the only exception, being widespread in southeast China and adjacent parts of northeast Vietnam). Outside of China the "Pennata complex" is especially speciose in Southeast Asia (18 species representing 19 taxa; see Table 6) and to a much lesser extent, the Indian subcontinent (represented there by Senegalia donaldii (Haines) Ragup., S. pennata (but it is not known if S. pennata subsp. insuavis is native or introduced in India), S. kerrii and S. megaladena var. megaladena. As seen from Table 6 the most species-rich countries in Southeast Asia for the "Pennata group" are Vietnam (nine species, 10 taxa), Indonesia (seven species), Thailand (five species, six taxa) and Myanmar (five species); the remaining countries have less than five species each. Senegalia tawitawiensis (I.C.Nielsen) Maslin, Seigler & Ebinger from the Philippines is the only species of the group in Southeast Asia that occurs in just one country; the remaining 17 species occur in two or more countries. Senegalia kerrii and S. megaladena are the most widely distributed members of the "Pennata complex" ranging beyond China to much of Southeast Asia and the Indian subcontinent (see Table 5). From the above it is evident that the "Pennata complex" has undergone active speciation in both Southeast Asia and China, with China having the most species (Vietnam coming a close second) and most endemics. Within China, Yunnan Province is the most species-rich region, having more species (eight) than most countries of South-east Asia.
Teniana species-group. This group is represented in China by seven species (Tables 4 and 5), making it the second-largest group in the country. Within China this group is represented by four endemics (Senegalia delavayi, S. prominens, S. teniana, S. yunnanensis) and one near-endemic (S. kunmingensis which extends just south of the border to the Sapa region of Vietnam). The remaining two species, S. andamanica and S. pruinescens, range more widely, to Vietnam, Thailand and India. Within China most species of the 'Teniana species-group' have relatively narrow distributions that are confined to Yunnan and/or the adjacent Provinces of Sichuan, Guangxi and Guizhou (these Provinces are located in the south-west and south-central regions of the country). Senegalia prominens is the exception, being widely distributed in southeast China and extending west to the eastern periphery of Guizhou and Guangxi; this species is most closely related to S. teniana which occurs in Yunnan and Sichuan. Outside of China the 'Teniana species-group' is represented in Southeast Asia by six species (S. andamanica, S. kekapur (I.C.Nielsen) Maslin, Seigler & Ebinger, S. kostermansii (I.C.Nielsen) Maslin, Seigler & Ebinger, Senegalia merrillii (I.C.Nielsen) Maslin, Seigler & Ebinger, S. pseudointsia (Miq.) Maslin, Seigler & Ebinger, S. verheijenii (I.C.Nielsen) Maslin, Seigler & Ebinger) and the Indian subcontinent by half this number (S. andamanica, S. hohenacker (Craib) Ragup. et al., S. pruinescens). The centre of species-richness for the 'Teniana species-group' in Southeast Asia is Indonesia (five species) and Thailand (four species), both of which have fewer species than China. It is noteworthy that apart from S. andamanica, S. kunmingensis and S. pruinescens, there are no representatives of the 'Teniana species-group' in countries of Southeast Asia that share a border with China, namely, in Laos, Myanmar and Vietnam. From the above it is seen that the 'Teniana species-group' has three, disjunct, centres of speciesrichness which are centred on China, Thailand and Indonesia. Furthermore, a majority of the Chinese species of this group are more or less endemic with only S. andamanica being shared with Thailand, Vietnam and India, and none with Indonesia. From this it could be speculated that the 'Teniana species-group' is possibly a relatively old assemblage which has had time enough to undergo independent speciation in China.
Caesia species-group. This group is represented in China by just two species, Senegalia caesia and S. tonkinensis. These species are relatively uncommon in China, having restricted distributions confined to the periphery of Yunnan and Hainan provinces. Outside of China, however, both species are widespread in northern regions of Southeast Asia, with S. caesia extending to the Indian subcontinent (Tables 4 and 5). The 'Caesia species-group' as a whole is represented in northern Southeast Asia by S. comosa (Gagnep.) Maslin, Seigler & Ebinger, S. caesia, S. gageana (Craib) Maslin, Seigler & Ebinger, S. meebii (Craib) Maslin, Seigler & Ebinger and S. tonkinensis which occur in Cambodia, Laos, Myanmar, Thailand and Vietnam. On the Indian subcontinent the group is represented by S. caesia, S. diadenia (R.Parker) Ragup. et al., S. gageana and S. torta (Roxb.) Maslin, Seigler & Ebinger. The 'Caesia species-group' has clearly speciated in both Southeast Asia and the Indian subcontinent but it has not diversified or spread within China, where it is possibly a relatively recent arrival.
Rugata species-group. This group is represented in China by Senegalia rugata and a poorly known entity that is informally called S. rugata (Bukit Brown) in this paper. Within China S. rugata has a disjunct distribution, being recorded from the southwest periphery of Yunnan Province (close to the Myanmar border) and from Macau and far eastern Guangdong. The species was also recorded (as Acacia concinna) for Hainan by Merrill (1927), but we have not verified this record. Senegalia rugata (Bukit Brown) is an entity of uncertain taxonomic status and is known from just two collections in far western Yunnan (close to the Myanmar border). It is not known if these China plants are native or introduced; the natural distribution of Senegalia rugata (Bukit Brown) outside of China is unknown. As currently defined, S. rugata is very variable morphologically and has an extensive geographic range in both Southeast Asia and the Indian subcontinent (Table 5). The only other species currently assigned to the 'Rugata species-group' is S. albizioides (Pedley) Pedley from northeast Australia. Within China the 'Rugata species-group' shows a similar geographic pattern to that of the 'Caesia species-group' and it may be speculated that it too is a relatively recent arrival in the country, perhaps having spread from Southeast Asia through Vietnam to Guangdong and Macau, and from the Indian subcontinent through Myanmar to Yunnan.
In summary it can be said that China is here shown to be a principal area of species-richness for Senegalia in the whole of Asia (21 species, representing 22 taxa), and represents the northeast limit of distribution of the genus for the region (with species not extending north of 30°N within China). China contains representatives of all Senegalia species-groups that are currently recognised for both Southeast Asia and the Indian subcontinent, and has strongest affinities with Southeast Asia. Most species are contained in the "Pennata complex" (comprising the 'Hainanensis and Pennata species-groups' and the 'Teniana species-group' which together contain 18 species (representing 86% of the Chinese Senegalia flora). These groups, which are perhaps the earliest arrivals in China, also have centres of species-richness in Southeast Asia, but the highest concentration of species occurs in China. Senegalia displays a high degree of endemism within China with almost half the species being either endemic (six species) or near-endemic (four species): these species occur in the "Pennata complex" and 'Teniana species-group' Similarly, there is a strong tendency for species of Senegalia in China to have short geographic ranges: the two most widely distributed species are S. prominens and S. orientalis, which predominate in the southeast of the country and which are endemic and near-endemic respectively. Of the 15 species that range beyond China, all occur in Southeast Asia, with the highest numbers shared with the neighbouring countries of Vietnam (12 species, 13 taxa) and Myanmar (nine species), and six species extending to the Indian subcontinent: the most widespread of these species are S. caesia, S. megaladena and S. rugata. The 'Rugata' and 'Caesia speciesgroups' which are widespread in both Southeast Asia and the Indian subcontinent, are poorly represented in China and possibly represent relatively recent arrivals to the country. Within China, Yunnan Province is the major centre of both species-richness (16 species) and species-diversity and contains most Chinese en-demics or near-endemics (seven species).
In conclusion it is noted that Sun and Chen (1990) discussed historical biogeography of the Chinese Acacia sens. lat. flora (including Senegalia, which was referred to in that paper as Acacia subg. Aculeiferum). However, such a broad-based phylogeographic analysis is not possible here because Asian Senegalia is very poorly represented in existing genetic datasets.
4. Taxonomy 4.1. Morphological charactersThe morphological characters used in both the taxon descriptions and the key to species below are based on dried herbarium material, unless otherwise indicated. The following notes are provided to assist readers to better-understand the structure, variation and taxonomic utility of some of these characters. Morphological terminology mostly follows that of Beentje (2016).
Habit. The indigenous species of Senegalia in China are commonly lianas, but some are shrubs or trees. While the third author has considerable field knowledge of especially the Yunnan species, much of the habit information presented in the taxon descriptions was derived from herbarium label information. These data sometimes appear to be misleading and therefore indepen-dent verification, and/or a more precise characterisation of the habit would be desirable for at least some species. For example, collectors may apply the terms tree and shrub differently and when plants are described as shrubs, collectors often do not explicitly state whether or not liana development exists for the terminal branches. Shrubs where it is known that some liana development occurs are termed lianescent shrubs herein. Another complication regarding the determination of habit from herbari-um label information is that, as discussed by Nielsen (1992), plants of Senegalia (which Nielsen treated under Acacia subg. Aculeiferum) in the Malesiana region of Southeast Asia display plasticity for habit, being lianas in dense vegetation and erect shrubs (or trees) with scandent branches in open vegetation, and a similar potential for change in habit had been observed in the African species. While it is likely that this same sort of variation also occurs in China, this needs to be confirmed by more targeted field studies. Similarly, very little information is known as to whether Senegalia species in China are evergreen or deciduous. Sun and Chen (1990) report that plants of S. yunnanensis growing close to watercourses show an evergreen habit, while those growing on drier sites tend to be deciduous. Also, one label for both Senegalia prominens and S. garrettii record the foliage as being deciduous, while in Thailand, herbarium labels occasionally record S. garrettii and S. rugata as being deciduous. From our field observations of Senegalia clandestina it is suspected that this species is deciduous.
Notwithstanding the above, it seems that most indigenous species of Senegalia in China are lianas or lianescent shrubs. In some species at least tendrils (i.e. naked, very prickly short branchlets that are used in climbing) occur in leaf axils, but more field knowledge is needed to quantify this character. Species that are seemingly shrubs (or sometimes trees) with no, or scarcely any, liana development are: S. delavayi, S. teniana and S. yunnanensis. The liana habit does not occur in either of the two introduced species of Senegalia (i.e. S. catechu and Senegalia senegal); they are always shrubs or trees.
Prickles and spines. All indigenous species of Senegalia in China are armed with rather small prickles that are scattered along the branchlet internodes and often the underside of the leaf petiole and rachis (but prickles are sometimes wanting on herbarium material). The two introduced species of Senegalia, S. catechu and S. senegal, have two or three prickles respectively that are located at or near the nodes. The disposition of cauline prickles (i.e. internodal vs nodal) help define the two major sub-groups within Senegalia, namely, sect. Senegalia (prickles at nodes) and sect. Monacanthea (prickles scattered between nodes). Apart from that, prickles have relatively little taxonomic value within Chinese Senegalia. Our prickle descriptions are based mostly on observations of herbarium material which normally comprises only the upper branches. It is known, however, that prickle morphology (especially size) and/or frequency on older stems of living plants is often different to that of the upper branches; where we have knowledge of prickles on older stems it is explicitly stated in the descriptions.
The only other species of Acacia sens. lat. in China that are armed are the introduced Vachellia farnesiana and V. nilotica, which have a pair of spines (i.e. modified stipules) at the nodes. In places such as the Indian subcontinent, Africa and the Americas where numerous species of both Senegalia and Vachellia occur, it is important to recognise the differences between spines and prickles (see Ross (1979) for discussion). In China this distinction is obvious because in V. farnesiana and V. nilotica the spiny stipules are perfectly straight whereas in Senegalia catechu and S. senegal the prickles are recurved to some degree.
Stipules. In Senegalia the stipules are not spinescent and are normally caducous. They occur as small appendages at the base of leaves on vegetative shoots or at the base of peduncles or peduncle-clusters on inflorescences (a primordial leaf is normally situated between the stipules in the latter case). Stipule characters, especially their shape and size, can sometimes be useful aids to identification but their utility is limited because they are commonly few or absent on herbarium material. Stipules are best observed when inflorescences are in young bud. In the species treatments below, the descriptions of stipules are based on those that occur at the base of the peduncles, unless otherwise indicated. In the Figures accompanying these treatments, we normally include an illustration of the stipule.
Leaves and leaflets. The mature foliage of all species of Senegalia (and of Acaciella and Vachellia) is bipinnately compound and consists of a primary axis (comprising the petiole and rachis) with a variable number of pinnae which support the leaflets. The petiole is the primary leaf axis lying between the leaf base and the first pair of pinnae; at its base is a relatively small (and often ill-defined) region of differentiated tissue called the pulvinus that facilitate leaf movement. The rachis is the primary leaf axis lying between the lowermost and uppermost pairs of pinnae. The pinnae (singular: pinna) are the secondary leaf axes that are inserted on the rachis and which support the leaflets; the axis of the pinna is termed the rachilla. The tiny stalk of the leaflets is termed the petiolule.
In most introduced Australian species of Acacia (and in A. confusa from Taiwan), the foliage is reduced to phyllodes (e.g. Acacia mangium Willd.), but in that genus bipinnate-leaved species also occur (e.g. Acacia mearnsii De Wild.). For indigenous Chinese species of Senegalia, the most informative leaf characters are the following: pinnae number (which range from 3 to 30 pairs), rachis indumentum (hair orientation and curvature), leaf glands (see below) and leaflet size (broadly speaking, species can be grouped by leaflet width, i.e. to c. 1.5 mm wide vs more than 1.5 mm wide), venation (see below) and apex shape (symmetric vs asymmetric and acute vs obtuse). The leaflet dimensions given in the descriptions below do not include the atypically small leaflets that are commonly found at the base of the pinnae.
The leaves of all Chinese species of Senegalia arise singularly at the nodes. Only the introduced species of Vachellia have leaves that (on mature branches) are grouped at the nodes where they form nodose clusters (sometimes called "short shoots". On the new shoots of Vachellia species the leaves occur singularly at the nodes.
Although leaflet venation is a somewhat subtle character it can often be useful for identification purposes. Most species have a single main vein that starts near the upper margin at the base of the leaflet and extends obliquely to the apex, e.g. Senegalia delavayi (Fig. 10B). However, in Senegalia caesia and S. tonkinensis the main vein starts more or less at the centre of the leaflet base, and this provides a very useful and easy character for recognizing these species (see Figs. 5B and 53B respectively). The lateral veins that diverge from the main vein in many species provide informative characters such as their orientation at the point of divergence (patent vs ascending) and more importantly, whether or not they anastomose to form a reticulum. The pres-ence or absence of a reticulum is useful for identification pur-poses, but care is needed when assessing this character. In a few species (most notably Senegalia rugata) the reticulum is well-developed with numerous, small vein-islands (Fig. 44B and C). In many species, however, the reticulum comprises larger and fewer vein-islands that are often imperfectly developed, for example, in Senegalia teniana (Fig. 50B). The best way to observe the leaflet veins on herbarium material is to carefully inspect the lower surface of the leaflet at ×10 magnification with an oblique light source; if an over-head light source is used it often "flattens" the veins, making them somewhat difficult to see. When leaflets are very narrow (i.e. 0.5-1 mm wide), lateral veins are normally not visible.
Leaf glands. All indigenous species of Senegalia in China possess glands (extra-floral nectaries) on their petiole, rachis (at or near insertion of pinnae), and often rachilla (at base of uppermost few pairs of leaflets). However, as discussed below, glands are occasionally wanting on some specimens. Typically, petiole glands are larger than rachis glands, with rachilla glands being the smallest. Although these glands are morphologically very variable, the petiole glands in particular often provide useful characters for identification, especially their shape (when viewed laterally), prominence (i.e. the degree to which the gland tissue is raised above the surface of the petiole) and size. As to size: the length is the measurement of the gland axis that lies parallel with the leaf axis upon which the gland is inserted, width is the measurement that is transverse to the leaf axis, and height is the measurement of the distance that the gland is elevated above the leaf axis.
Barros and Morim (2014) noted the importance of leaf glands in helping define and distinguish many American species of Senegalia, and the utility of these structures in the present study will become apparent from the identification key and taxon treatments below. However, further study of these glands, especially in living plants, would help to a better-understand their morphology and variation within Chinese Senegalia. Also, there is a need to develop a more effective terminology for describing these glands, not only for the Chinese species but also for the genus as a whole.
A single gland is present on the petioles of most species of Senegalia in China, but there are exceptions. In Senegalia yunnanensis the petiole gland is often absent and in S. teniana it is sometimes absent (but rachis glands are normally present in those species) while S. tonkinensis has two glands on its petiole (Figs. 53F and 55H); two petiole glands are also occasionally found on some leaves of S. kunmingensis, S. pruinescens, S. stipitata and S. rugata.
While gland size is helpful in characterising species, the amount of intra- and inter-specific variation generally precludes its use for discriminating species. In most species the petiole glands fall within the range of about 2-5 mm long, with a few (e.g. Senegalia guangdongensis, S. pennata subsp. insuavis) sometimes reaching 6-7 mm. Species having the shortest glands (mostly 1-2 mm long) are S. delavayi, S. macrocephala, S. teniana and S. yunnanensis.
Petiole gland position is variable (even on a single specimen) but most commonly it is situated on the lower half of the petiole. In some species, however, especially Senegalia garrettii, S. megaladena, S. obliqua and S. pruinescens, the gland is often situated near (or sometimes above) the middle of the petiole, and when this occurs it is a useful aid to identification. The discussion in the S. garrettii treatment below provides additional details concerning variation in petiole gland position for that species and for S. megaladena. It is extremely rare in Chinese Senegalia to find a petiole gland situated at the extreme distal end of the petiole near the base of the lowermost pair of pinnae. Although this is a common petiole gland position for species of Vachellia, we have observed it only once in Chinese Senegalia, in the rare variant of S. rugata that has two petiole glands (with the distal one located at the base of the proximal pair of pinnae).
The degree to which sessile petiole glands are thickened, and their shape, are useful characters for identification purposes. Species regarded as having prominent petiole glands are those where the nectiferous tissue is clearly raised (to c. 1-2 mm high), e.g. Senegalia megaladena (Fig. 27J), S. prominens (Fig. 38F and G), S. rugata (Fig. 44E). In these cases, the gland shape (when viewed laterally) is often conical, hemispheric or oblate, but sometimes it is difficult to describe the shape using conventional terminology on account of gland asymmetry. This asymmetry is normally caused by the nectiferous tissue being slightly or prominently more elevated at the distal end of the gland, as is often seen in, for example, S. kunmingensis (Figs. 22F and 24J) and S. prominens (Fig. 38G). In a number of species, the petiole gland is not or scarcely thickened (elevated to only c. 0.5 mm above the petiole), e.g. S. delavayi (Fig. 10D), S. orientalis (Fig. 33E) and S. teniana (Fig. 52H). These glands may also sometimes display asymmetry on account of being slightly elevated at their distal end, but in these cases the gland is raised from its base, not because of a thickening of nectiferous tissue, e.g. S. andamanica (Fig. 3E and F) and S. hainanensis (Fig. 17I). In a few species the petiole gland is cupular or elongate-cupular, e.g. sometimes in S. kerrii (Fig. 19H), or has a distinct "pore" on its upper surface, e.g. S. macrocephala (Fig. 25E and F). Most other sessile petiole glands appear to lack pores, but they are sometimes present (although commonly ill-defined) in S. caesia and S. megaladena. In a few species that have been inspected in the living state, the petiole gland has displayed a considerable degree of polymorphism; Senegalia kunmingensis is a good example of this (see Fig. 24J-N).
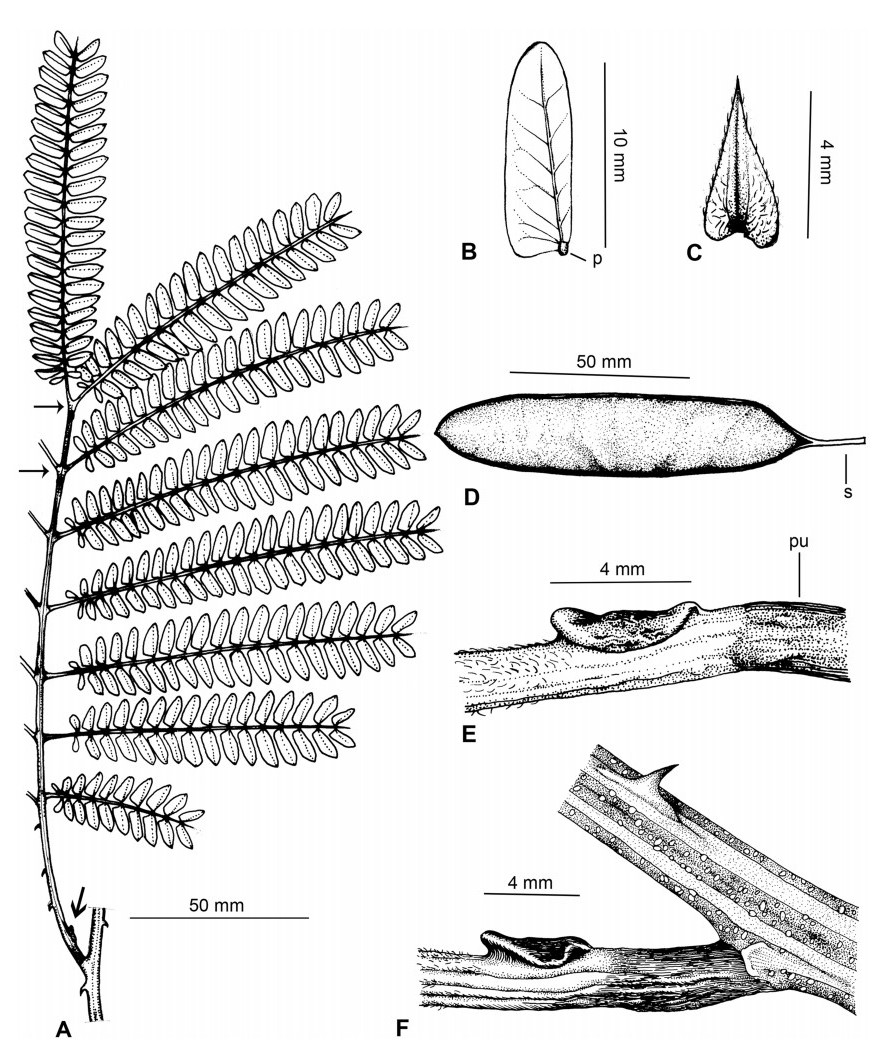
|
| Fig. 3 Senegalia andamanica. A - Leaf showing position of petiole gland (thick arrow) and rachis glands (thin arrows). B - Leaflet (lower surfac-) relatively large with apex rounded and lacking apiculum, and petiolule (p) extended below leaflet base. C - Stipule relatively large and lanceolate. D - Pod with long, slender stipe (s). E -Petiole gland quite large and close to pulvinus (pu), flattened but slightly up-turned at distal end. F - Node showing branchlet glabrous and petiole gland close to leaf base. Vouchers: P.A. Petelot 2178 (A, C & F); P.A. Petelot 2163 (B & E); S.K. Lee 200449 (D). Drawn by Joshua Yang. |
The term peripterous has been introduced here to help describe a particular type of gland that is found on the petiole and sometimes the rachis of a few species. This term refers to the relatively narrow rim ("wing") that comprises the outer margin of the gland lamina. This rim is variously orientated, commonly horizontally splayed but sometimes (at least in dried specimens) it ascends or descends in various ways to produce rather oddlooking glands (which are often difficult to describe in words). Peripterous glands commonly look to be sessile, especially when the gland lamina is horizontally splayed and flat-topped. However, these glands are fractionally elevated by a broad, microscopic stipe (which normally cannot be seen) and as a consequence the "wing" of the gland is not closely appressed to the surface of the petiole or rachis. When the entire rim of a peripterous gland is upturned, the form of gland becomes cupular or elongate-cupular. Glands that do not form a marginal "wing" are termed apterous, and in these cases the entire base of the gland is closely appressed to the petiole or rachis; most species of Senegalia possess apterous glands. Peripterous glands are best-developed in S. garrettii (Fig. 14E and F) but they commonly also occur in S. kerrii (Fig. 21F and G), and sometimes in S. andamanica, S. hainanensis and S. pennata subsp. insuavis. However, in the latter three cases the peripterous nature of the gland is often only partially, or rather poorly, developed (e.g. Fig. 3F).
Apart from Senegalia stipitata all species of Chinese Senegalia have sessile (or occasionally sub-stipitate) petiole and/or rachis glands. These stipitate glands display a remarkable range of variation in the shape (even on a single specimen) which, as illustrated in Fig. 47H, varies from cylindrical to obconic or calicioid (this latter term describes a gland possessing a slender stipe and a dilated apex, see Fig. 47Hd). Stipitate petiole glands are not especially common in Senegalia but they have been reported for some species in Southeast Asia, e.g. Senegalia pluricapitata (Steud. ex Benth.) Maslin, Seigler & Ebinger (fide Nielsen (1992), as Acacia pluricapitata Steud. ex Benth.) and South America, e.g. S. martiusiana (Steud.) Seigler & Ebinger (fide Barros and Morim (2014)).
A single gland normally occurs at or near the base of some, but not all, pairs of pinnae on the rachis of most species of Chinese Senegalia (only in S. delavayi (Franch.) Maslin, Seigler & Ebinger are rachis glands occasionally absent). Rachis glands are normally morphologically similar to the petiole glands, except a little smaller. They are commonly associated with the uppermost 1-4 pairs of pinnae, but in few a species they are more numerous, with S. stipitata having the most (6-17). By way of contrast, the introduced bipinnate-leaved species of Acacia normally have a rachis gland associated with all pairs of pinnae (including the lowermost pair) and this is a useful way to distinguish sterile specimens of these species from indigenous Senegalia species. In one species, A. mearnsii, there is more than one rachis gland between adjacent pairs of pinnae on at least some leaves.
Inflorescences and flowers. The inflorescence is the flower-bearing axis (called the receptacle) situated at the distal end of the peduncle, and in Senegalia it is either capitate (i.e. in the form of a globose or obloid head) or spicate (i.e. cylindrical). Senegalia yunnanensis is the only indigenous Chinese species of Senegalia with spicate inflorescences (Fig. 56B) but these also occur in the two introduced species, S. catechu and S. senegal, both of which belong to Senegalia sect. Senegalia. Spicate inflorescences are rare in Asian species of Senegalia sect. Monacanthea (the group to which S. yunnanensis belongs), being otherwise known in only S. donnaiensis (Gagnep.) Maslin, Seigler & Ebinger from Southeast Asia. When plants are in pod the shape of the inflorescence can normally be inferred from inspecting the disposition of flower scars on the receptacle.
The pedunculate inflorescences are commonly arranged in terminal panicles and/or racemes, but they can also be solitary (or in pairs or fascicled) within the leaf axils, in which case they are termed axillary. When axillary, the inflorescences in at least some species are initiated simultaneously with immature leaves on expanding new shoots, and the fertile region can assume the appearance of a "false raceme"; this is especially evident in Senegalia yunnanensis but is seen in other species such as S. kunmingensis and S. teniana. The synchronous development of inflorescences and foliage also occurs in some species of Acacia in Australia, e.g. A. synchronicia Maslin, (1992). While inflorescence arrangement is helpful in recognizing a few species, it is generally treated with caution because further study of living plants is needed to better understand variation within this character.
An inflorescence attribute that is useful in characterising the 'Hainanensis species-group' is the dense layer of dark-coloured (red-brown to black) glandular hairs that occur on the raceme and panicle axes, and often also on the peduncles, of Senegalia guangdongensis, S. hainanensis, S. macrocephala and S. stipitata. This same indumentum may also occur on the young branchlets and leaf axes of these species. While glandular hairs are commonly found in other species of Chinese Senegalia, they are very few and scattered. On old herbarium specimens it is often difficult to properly char-acterise the dark-coloured glandular indumentum, but in recently-preserved specimens it can be clearly recognised. Regrettably we have not inspected any of the above-mentioned species in the field, and therefore do not know how this indumentum manifests in the fresh state. Further study of this distinctive indumentum would be instructive.
The young inflorescences of Senegalia tonkinensis are distinctive in having bracteoles that are exserted beyond the flowers (Fig. 53D). This character is unique to this species in China, but has been reported by Parker (1929) (as Acacia diadenia R. Parker) for S. diadenia (R.Parker) Ragu et al. from India and in Senegalia comosa (Gagnep.) Maslin, Seigler & Ebinger and an apparently undescribed species from Thailand by Nielsen (1985a), as A. comosa Gagnep. (p. 166) and Acacia sp. in obs. (p. 181).
Individual flowers of Chinese Senegalia offer relatively few characters that are useful for identification purposes, but calyx and corolla colouring does warrant comment. Commonly the perianth of Senegalia species are pale yellow or green when fresh. However, we have observed in living plants of S. garrettii, S. megaladena var. megaladena and S. pruinescens in China that the upper portion of the calyx is very commonly a red-coloured. This colour is also seen in the inflorescence buds of these species, because at that stage of flower development, much of the unopened calyx is fully exposed. As noted below, it is likely that living plants of many indigenous species of Senegalia in China normally possess some sort of reddish-coloured perianth. It is therefore perhaps surprising that these colours are seldom recorded on herbarium labels or reported in literature. These fresh-state colours are rarely retained on herbarium specimens, but their presence can normally be inferred from a darker-than-normal brownish/greyish colouring of the inflorescence buds (and the upper portion of the calyx, and sometimes the petals). However, these dark colours can sometimes be rather subtle, making it difficult to speculate with confidence whether or not the fresh colour might have been reddish. The range of colour recorded for the perianth (and inflorescence buds) on living plants of Senegalia from China and elsewhere in Asia, varies from pink to dark red, brown-red or purple, and sometimes more than one colour is recorded for a single species. For example, Nielsen (1992) described the buds of Acacia concinna (=S. rugata) from Malaysia as dark red while Verdcourt (1979) described them as purple or dark red on plants from New Guinea; our images of that species from Thailand and Macau show the buds as varying from rich pink to pale pink respectively (see Fig. 46A and B). Judging from our field observations of a few populations, and those of others, from pub-lished information and from at least some specimen labels, at least sometimes the living plants of the following species of Chinese Senegalia possess reddish or purple perianth parts (thus inflorescence buds): S. caesia, S. garrettii, S. guangdongensis, S. hainanensis, S. kerrii, S. megaladena, S. orientalis, S. pruinescens, S. rugata and S. tonkinensis. Also, judging from the dark colouring of inflorescence buds and perianth parts on herbarium specimens, it is speculated that these same (or similar) colours will often be found in living plants of the following species: S. andamanica, S. delavayi, S. macrocephala, S. obliqua and S. stipitata. Chinese Senegalia that do not possess red/purple inflorescence buds when fresh, based on our field observations of a few populations, include the following: S. clandestina, S. prominens, S. teniana and S. yunnanensis; S. kunmingensis and S. pennata subsp. insuavis also possess green buds when fresh, but herbarium material suggests that sometimes red buds may also occur in these two species.
Further discussion of Senegalia garrettii and S. pruinescens provides a better understanding of variation for red perianth colouring within at least these two species. In the single population of these species that we examined in China, most inflorescence buds were of a dull red colour (see Figs. 14A and B and 43C) that was produced by the upper portion of the calyx (see Fig. 43E); the petals in both species were pale green. However, the two species showed variation for both the intensity of colour (pale to dark red) and its distribution within and between the heads (some buds, especially those not receiving direct sunlight, being completely or partially green, see Fig. 43D). Label information on some specimens of S. garrettii collected by J.F. Maxwell from Chiang Mai, Thailand, provides further insights into perianth colour variation for this species: J.F. Maxwell 01-288 (BKF 139619) records: "calyx pale light greencream, developing some dull reddish in places; corolla cream and often with dull red" while J.F. Maxwell 05-303 records: "calyx tube whitish, lobes and entire corolla pale light green".
From the above it is apparent that more study of perianth colour in Senegalia is warranted in order to better-assess the range of colours that occur in nature, to better-understand the variation within taxa, and to reassess the utility of using dark-coloured perianth parts on herbarium specimens to infer the colour of inflorescence buds and perianth parts on living plants.
Pods. With the exception of Senegalia rugata (see below) the pods of indigenous Senegalia in China are morphologically quite similar, but some species have characters that are useful for identification purposes. Typically, the pods are dehiscent, oblong to broadly linear, ±straight, flattened (but commonly slightly raised over seeds along the midline), thintextured (firmly chartaceous to thinly coriaceous-crustaceous), normally glabrous (but see below) and veinless or indistinctly veined; they possess a somewhat thickened margin that is infrequently constricted between the seeds and a basal stipe of variable length. Pod length varies considerably (50-220 mm long) and is determined by the number of seeds produced; it has scarcely any value in identifying species. Although pod width is also variable (normally 15-40 mm wide), it can sometimes be helpful in distinguishing species. The presence of an indumentum on the pods of Senegalia in China is rare, but microscopic hairs do occur in both Senegalia obliqua (indumentum velutinous) and S. tonkinensis (a downy indumentum sometimes present). Brown or red-brown, circular, sessile glands occur on the pods of many species; these glands are very small (scarcely visible to the unaided eye) and normally few and scattered. However, in S. guangdongensis, S. macrocephala, S. stipitata and S. tonkinensis the glands are often numerous and frequently embedded within a resin matrix; as the pods mature the glands often partially wear-off and those remaining are seen as discontinuous, irregular, dark-coloured patches. The presence of these glands in the above four species is a useful character that aids their identification.
Senegalia rugata differs significantly from all other species of Senegalia in Asia in having pods that are fleshy, smooth and thickly coriaceous when fresh but upon drying are very hard-textured (thickly crustaceous to ±woody) and possess a distinctively rugose surface. These pods are also often discernibly constricted between the seeds.
Seeds. It is difficult to meaningfully assess the variation and potential taxonomic value of seeds for Chinese Senegalia because very few mature seeds have been seen for most species. Never-theless, current knowledge shows the seeds to be uniseriate, transverse in the pods (except oblique in S. obliqua), oblong to elliptic (rarely ovate), brown to black and exarillate. Also, apart from those of Senegalia rugata, the seeds are clearly flattened and seated in poorly defined, very shallow depressions within the pods. In S. rugata the seeds are somewhat turgid and seated in discrete chambers within the pods. Seed size normally varies from about 8 to 12 mm long and 5-8 mm wide, but in a few species the seeds are slightly larger, e.g. S. obliqua (15 mm long, 11 mm wide), S. guangdongensis (10-15 mm long, 9-12 mm wide) and S. kunmingensis (10-15 mm long, 8-11 mm wide).
The opposing faces of most Mimosoideae seed are marked by a fine line (the pleurogram) that defines an area called the areole. The shape and size of the areole can be taxonomically important (Nielsen, 1985b), but in Chinese Senegalia it is relatively uniform, normally being quite large (relative to the seed size) and open at the hilar end. In two species, however, S. guangdongensis and S. yunnanensis, we were not able to observe a pleurogram; this matter requires further investigation because the absence of an areole would be atypical for Senegalia.
4.2. Key to species of Acacia sens. lat. in ChinaThis key is based on dried, flowering material unless otherwise stated. It includes both the native and introduced species of Acacia sens. lat. that are dealt with in this work. Numerals preceding taxon names refer to taxon numbers in the text. The three unnumbered species (Acacia elata, Senegalia senegal and Vachellia nilotica) are noted in the introductory text under Introduced species.
Notes on identification. Species of Senegalia are often difficult to identify, especially because they commonly look superficially similar, and distinguishing characters can be cryptic. Therefore, it is normally not a good idea to try and "spot identify" specimens. Reliable identifications normally require careful observation of characters to ensure that they are correctly interpreted.
Superscripts used in key.1 On fruiting specimens the shape of the inflorescence can normally be inferred by inspecting the disposition of flower scars on receptacle.2 Reticulum and other venation (e.g. lateral veins) are best observed by careful observation at ×10 magnification using oblique lighting.
1. Leaves bipinnate…………………………………………………2
1: Leaves reduced to phyllodes (Acacia; all introduced species except A. confusa in Taiwan)…45
2. Stipules spinescent, paired at the nodes, straight & mostly 5-20 mm long, prickles absent; leaves (at mature nodes) two or more in brachyblastic clusters (single at nodes on new growth); flowers in globose heads1. Introduced tree or shrub. (Vachellia)…………………………………………………………3
2: Stipules not spinescent; prickles (0.5-5 (-7) mm long, straight or recurved) often present between nodes or sometimes at nodes; leaves single at all nodes; flowers in globose or obloid heads or sometimes cylindrical spikes1. Tree, shrub or liana………………………………………………4
3. Involucre situated near apex of peduncle (obscured by anthers at anthesis); pods turgid (terete to compressed), blackish to dark brown, glabrous, finely longitudinally striate; seeds embedded in pith…………36. Vachellia farnesiana
3: Involucre on lower ½ of peduncle; pods & seeds not as above……………………………………………Vachellia nilotica
4. Branchlet prickles AND leaf glands absent; flowers with a distinct, slender pedicel 1-2 mm long; calyx very short (¼-⅕ length of corolla); leaflets 1.5-3 mm wide. Introduced shrub…………………………………………34. Acaciella glauca
4: Branchlet prickles AND/OR leaf glands (on petiole and/or rachis) present; flowers ±sessile OR if stipitate (rare) then leaflets < 1.5 mm wide; calyx > ½ length of corolla…………5
5. Flowers in cylindrical spikes1; prickles (when present) at nodes or inter-nodal. Tree or shrub……………………………6
5: Flowers in globose or obloid heads1; prickles (when present) inter-nodal. Tree, shrub or liana…8
6. Leaflets obviously appressed-hairy (especially on lower surface); prickles (when present) situated between the nodes, straight; petiole gland often absent. Indigenous species………………………………21. Senegalia yunnanensis
6: Leaflets glabrous or sub-glabrous (excluding margins); prickles (when present) situated at or near nodes, curved. Introduced species………………………………………………7
7. Pinnae > 8 pairs; prickles 2 per node; petiole gland 2-3.5 mm long…………………………………………35. Senegalia catechu
7: Pinnae < 8 pairs; prickles 1 or 3 per node; petiole gland < 1 mm long……………………………………Senegalia senegal
8. Leaflets 0.4-1.5 mm wide AND lateral veins not forming a reticulum2 on lower surface……………………………………9
8: Leaflets some or all > 1.5 mm wide AND/OR lateral veins forming a (sometimes imperfect) reticulum2 on lower surface; pinnae (3-) 4-12 (-14) pairs…………………………34
9. Rachis gland present at (or near) base of all pairs of pinnae, sessile; prickles absent from branchlets; peduncles 2-8 mm long; seed funicle dilated into an aril. Tree or shrub (never liana). Introduced species. (Acacia)……………………………10
9: Rachis gland absent from base of some (rarely all) pairs of pinnae (occasionally at base of all pinnae in Senegalia stipitata, in which case the glands are stipitate); prickles present & scattered on branchlets (but sometimes absent from herbarium material); peduncles normally 10-40 mm long; seed funicle not dilated into an aril. Commonly liana but sometimes tree or shrub. Mostly indigenous species………………………………………………12
10. Leaflets 5-10 (-15) mm long, narrowly linear; branchlets angled by winged ridges 0.5-2 mm high………………………………………27. Acacia decurrens
10: Leaflets < 5 mm long, mostly oblong to narrowly oblong; branchlets terete (or slightly angled at extremities)………11
11. Rachis with more than one gland between at least some pairs of pinnae; leaflets dark green and ±shiny above (best observed when fresh); pods appressed-hairy, narrow (4-8 mm wide)………………………………31. Acacia mearnsii
11: Rachis with one gland between each pair of pinnae; leaflets bluish grey with dull lustre; pods glabrous, broad (6-14 mm wide)……………………………………………26. Acacia dealbata
12. Leaves with up to 13 pairs of pinnae……………………………13
12: Leaves some or all with more than 13 pairs of pinnae………23
13. Raceme and/or panicle axes (and often young peduncles and young branchlets) dark-coloured by numerous glandular hairs, sometimes glandular-scurfy; leaflet main vein normally rela-tively close to and parallel with upper margin at least in lower 
13: Raceme axes etc. not as above (i.e. glandular hairs absent or very few & scattered); leaflet main vein generally not as above; mature pods without sessile glands, or glands very few and scattered; ………………………………………………17
14. Rachis gland at base of uppermost 1 or 2 pairs of pinnae; petiole gland 3-6 mm long, sessile, situated 9-17 mm above pulvinus; leaflet apices rounded to obtuse. Guangdong………………………………6. Senegalia guangdongensis
14: Rachis glands more numerous than above; petiole gland rarely exceeding 3 mm long/diam., sessile or stipitate, normally closer to pulvinus than above……………………………15
15. Heads large (15-22 mm diam. when dry) on long peduncles (20-45 mm); calyx obviously hairy; rachis hairs longish, generally straight and patent; pods 30-40 mm wide; petiole gland sessile, 1-2 mm long, situated 0-3 mm above illdefined pulvinus; leaflet apices clearly acute. Far western Yunnan……………………………………10. Senegalia macrocephala
15: Heads smaller (7-10 mm diam. when dry) on commonly shorter peduncles (normally 7-18 mm); calyx glabrous or sub-glabrous; rachis hairs short, curved forward and normally ±appressed (rarely spreading); pods < 30 mm wide; leaflet apices obtuse or sometimes broadly acute………………16
16. Petiole gland sessile, 1-3 (-4) mm long, asymmetric (raised from base at distal end: Fig. 17I), situated 0-3 (-5) mm above pulvinus; rachis glands ±sessile. Hainan……………………………………7. Senegalia hainanensis
16: Petiole gland stipitate or sub-stipitate, symmetrically shortcylindric to narrowly obconic or caliciod and 0.4-1 mm diam. at apex (Fig. 47H), situated 1-10 (-15) mm above pulvinus; rachis glands clearly stipitate or sub-stipitate. Southern Yunnan……………………………18. Senegalia stipitata
17. Leaflets closely sessile (i.e. petiolule not extended below base of leaflet: Figs. 22B and 24F and G)……………………………18
17: Leaflets petiolulate (the petiolule shortly but discernibly extended below base of leaflet: Fig. 52E)………………………………19
18. Branchlets not pruinose; leaflets some or all with lower margin ±shallowly concave immediately above the basal angle (Fig. 24G); peduncles normally ±glabrous; stipules narrowly oblong to triangular, 0.5-1.2 mm wide; inflorescences axillary (i.e. 1-3 within axils of often young leaves) or sometimes arranged in racemes……………………………9. Senegalia kunmingensis
18: Branchlets normally pruinose (pruinosity faint to distinct, occasionally absent; best observed on living plants); leaflets lower margin straight (not concave above the basal angle); peduncles densely hairy; stipules ovate, 1-4 mm wide; inflorescences arranged in panicles or racemes……………………………………16. Senegalia pruinescens
19. Petiole gland peripterous, not thickened, often situated near or above middle of petiole; leaflet apices ±symmetrically rounded to obtuse AND without an apiculum; indumentum on upper branchlets, rachis and peduncles comprising dense, spreading, straight hairs………………………5. Senegalia garrettii
19: Petiole gland (when present) apterous, thickened and ±slightly to prominently raised; leaflet apices asymmetric AND/OR with a centric or excentric apiculum; indumentum characters normally not combined as above………………………20
20. Petiole gland prominently raised, often convex on upper surface and situated near middle of petiole (Fig. 43I); peduncle indumentum often orange-brown; inflorescences arranged in panicles or racemes. Normally liana………………21
20: Petiole gland (when present) ±not prominently raised, mostly flat-topped and situated on lower ½ of petiole (Fig. 52H); peduncle indumentum absent or white; inflorescences not arranged in panicles (i.e. inflorescences 2-6 within axils of often young leaves or arranged in racemes). Shrub…………………………………………………………………22
21. Pods glabrous, 30-50 mm wide; branchlets normally glabrous and pruinose3 (pruinosity faint to distinct, but sometimes not visible on herbarium specimens) ………………………………………………16. Senegalia pruinescens
21: Pods microscopically velutinous, to 30 mm wide; branchlets densely puberulous, not pruinose………12. Senegalia obliqua
22. Peduncles with ±dense and straight, patent hairs; upper branchlets sparsely to densely hairy; leaflets (lower surface) with lateral veins rather evident; petiole gland sometimes absent………………………………………19. Senegalia teniana
22: Peduncles glabrous or with sparse, strongly curved, ±appressed hairs; upper branchlets glabrous; leaflets (lower surface) with lateral veins not visible or few and obscure; petiole gland always present………………4. Senegalia delavayi
23. Raceme and/or panicle axes (and often young peduncles and young branchlets) dark-coloured by numerous glandular hairs, sometimes glandular-scurfy; leaflet main vein nor-mally parallel with upper margin at least in lower 
23: Raceme axes etc. not as above (i.e. glandular hairs absent or very few & scattered); leaflet main vein not parallel with upper margin (except S. pennata subsp. insuavis); mature pods without sessile glands, or glands very few and scattered…24
24. Leaflet apices symmetrically rounded to obtuse AND without an apiculum…………………………………………………………25
24: Leaflet apices otherwise (i.e. asymmetric or acute or with an apiculum)…………………………………………………………………29
25. Petiole gland peripterous, not thickened, often situated near or above middle of petiole; indumentum on upper branchlets, rachis and peduncles comprising dense, spreading, sometimes orange-brown hairs; leaflets (lower surface) with relatively few, patent lateral veins2………5. Senegalia garrettii
25: Petiole gland apterous, thickened to some extent; indumentum characters not combined as above (hairs absent or if present then appressed and not orange-brown); leaflets (lower surface) with lateral veins ascending (rarely patent in S. clandestina) or not visible………………………………………26
26. Petiole gland rather prominently raised (Figs. 29I and J and 30A), position variable but commonly near middle of petiole, 1.5-5 mm long with l:w =0.7-2.5, orange to orange-brown when fresh (S. megaladena)………………………………………27
26: Petiole gland not prominently raised (Fig. 9G and H), normally situated on lower ½ of petiole and 3-6 mm long with l:w = 3-5, green when fresh (but colour unknown for S. orientalis)…………………………………………………………28
27. Leaflets 4-8 mm long, normally 0.8-1.5 mm wide, the lateral veins1 visible (obscure to somewhat evident). Yunnan………………………………11a. Senegalia megaladena var. megaladena
27: Leaflets (2-) 3-4 (-5) mm long, 0.4-0.6 mm wide, the lateral veins1 not visible or occasionally extremely faint. Hainan………………11b. Senegalia megaladena var. indochinensis
28. Branchlets dark-coloured (when dry); leaflets 4-6 mm long, often with a few hairs on under-surface at apex, marginal hairs often long & spreading; heads c. 45-60-flowered; pods 25-42 mm wide. SE China………………13. Senegalia orientalis
28: Branchlets pale-coloured (when dry); leaflets 3-4 mm long, without hairs on under-surface at apex; heads c. 25-30-flowered; pods 18-21 mm wide. Yunnan ………………………………………………3. Senegalia clandestina
29. Pods microscopically velutinous; petiole gland prominently raised, normally situated near middle of petiole; inflores-cence axes & peduncles very densely (often orange-brown) hairy………………………………………………12. Senegalia obliqua
29: Pods glabrous; petiole gland (when present) not prominently raised, normally situated on lower ½ of petiole (often near pulvinus); inflorescence axes & peduncles not orange-brown hairy (except sometimes in S. kerrii)……………………………30
30. Leaflets (lower surface) with lateral veins rather evident; pinnae to 14 pairs; inflorescences not arranged in panicles (i.e. inflorescences 2-6 within axils of young leaves or ar-ranged in racemes); petiole gland sometimes absent. Shrub………………………………………………19. Senegalia teniana
30: Leaflets (lower surface) with lateral veins not visible or few and obscure; pinnae 14-28 pairs; inflorescences arranged in panicles or racemes; petiole gland always present. Commonly liana………………………………………………31
31. Plants emitting a disagreeable odour when fresh (when branches cut or leaves crushed; odour lost upon drying); flowers shortly but discernibly pedicellate (pedicel 0.5-1 mm long); petiole 30-65 (-80) mm long; leaflets sometimes strongly folded lengthwise and/or strongly curved forward upon drying (Fig. 35D), apex finely acute by a normally excentric and slender point, main vein parallel to upper margin near base of leaflet……………………………14. Senegalia pennata subsp. insuavis
31: Plants not emitting disagreeable odour when fresh; flowers ±sessile; petiole normally 15-35 mm long; leaflets characters not combined as above………………………………………32
32. Petiole gland not thickened, flat to ±patelliform or shallowly cupular, sometimes narrowly peripterous, oblong to circular in plane view with l:w = 0.7-2 (Fig. 21E-G)………………………………………………8. Senegalia kerrii
32: Petiole gland thickened to some degree (±longitudinally wrinkled when dry: Fig. 9G and H), never patelliform, cupular or peripterous, mostly narrowly oblong to narrowly elliptic in plane view with l:w = 3-5………………………………33
33. Branchlets dark-coloured (when dry); leaflets 4-6 mm long, normally with a few hairs on under-surface at apex (Fig. 33B), marginal hairs often long & spreading; heads c. 45-60-flowered; pods 25-40 mm wide. SE China………………………………………………13. Senegalia orientalis
33: Branchlets pale-coloured (when dry); leaflets 3-4 mm long, without hairs on under-surface at apex, marginal hairs appressed and often short; heads c. 25-30-flowered; pods 18-21 mm wide. Yunnan………………3. Senegalia clandestina
34. Leaflets very large (20-60 mm long, 5-13 mm wide) and lanceolate; prickles absent from branchlets. Introduced tree…………………………………………………………………A. elata
34: Leaflets smaller (< 20 mm long, normally < 5 mm wide), never lanceolate………………………………………………35
35. Petiolule (= stalk of leaflet) centric or sub-centric, the midvein starting at or near middle of leaflet base (Fig. 53B)…36
35: Petiolule (= stalk of leaflet) clearly excentric, the midvein starting near upper margin of leaflet base (Fig. 10B)………37
36. Petiole glands 2; bracteoles exserted beyond flowers in young heads (Fig. 53D); branchlets with dense indumentum of patent, straight, brownish, tolerably long hairs………………………………………………20. Senegalia tonkinensis
36: Petiole gland 1; bracteoles not exserted beyond flowers in young heads; branchlets glabrous or occasionally with short, curved, ±appressed hairs………………………………2. Senegalia caesia
37. Leaflets closely sessile (i.e. petiolule not extended below base of leaflet: Figs. 22B and 24F and G)……………………………38
37: Leaflets petiolulate (the petiolule shortly but discernibly extended below base of leaflet: Fig. 52E)………………………………39
38. Branchlets not pruinose; leaflets normally not reticulately-veined on lower surface, some or all with lower margin ±shallowly concave immediately above the basal angle; inflorescences axillary (i.e. 1-3 within axils of young leaves) or sometimes arranged in racemes; peduncles normally ±glabrous; stipules narrowly oblong to triangular, 0.5-1.2 mm wide………………………………9. Senegalia kunmingensis
38: Branchlets normally pruinose (pruinosity faint to distinct, occasionally absent; best observed on living plants); leaflet often reticulately-veined on lower surface, the lower margin straight (not concave above the basal angle); inflorescences arranged in panicles or racemes; peduncles densely hairy; stipules ovate, 1-4 mm wide………16. Senegalia pruinescens
39. Leaflets (lower surface) reticulately-veined AND/OR petiole gland ±prominently raised (often ±rounded on top: Fig. 43I)……………………………………………………………………………40
39: Leaflets (lower surface) not reticulately-veined AND petiole gland (when present) not prominently raised (often ±flattopped: Fig. 52H………………………………………………43
40. Pods hard-textured (thickly crustaceous to ±woody) and wrinkled when dry; leaflets glabrous on lower surface (ignore margins); peduncles mostly 1-3 (-5) within axils of leaves, rarely arranged in short racemes; flower buds often reddish when fresh (dark-coloured when dry)………17. Senegalia rugata
40: Pods thin-textured (chartaceous to thinly coriaceous) and smooth when dry; leaflets appressed-hairy or glabrous on lower surface; peduncles arranged in panicles or racemes; flower buds reddish or green when fresh………………………………41
41. Branchlets normally glabrous and pruinose (pruinosity faint to distinct, sometimes not visible on herbarium specimens; best observed on living plants); flower buds mostly reddish when fresh (darkish coloured at apex when dry); petiole gland sometimes near middle of petiole. Often liana………………………………………………16. Senegalia pruinescens
41: Branchlets normally ±densely hairy towards apices, not pruinose; flower buds green when fresh (pale-coloured when dry); petiole gland on lower half of petiole or absent. Often shrub…………………………………………………………42
42. Peduncle hairs ±appressed; leaflets normally appressed-hairy on lower surface (rarely glabrous); petiole gland 2-4 mm long, 1-2 mm high, often conical-shaped, always present………………………………………15. Senegalia prominens
42: Peduncle hairs ±patent; leaflets normally glabrous on lower surface (rarely appressed-hairy); petiole gland 1.5-2 (-3) mm long, 0.5-1 mm high, never conical-shaped, sometimes absent………………………………………19. Senegalia teniana
43. Leaflets 2-4.5 mm wide, apices without an apiculum; petiole gland 2-5 mm long, often slightly upturned or raised from base at distal end (Fig. 3E and F); inflorescences often arranged in panicles; peduncles normally densely hairy. Liana or shrub……………………………………1. Senegalia andamanica
43: Leaflets 1-2 mm wide, apices often apiculate; petiole gland (when present) 0.8-2 (-3) mm long, not upturned or raised at distal end; inflorescences axillary (i.e. 1-6 within axils of often young leaves) or arranged in racemes. Shrub………44
44. Peduncles ±densely hairy; leaflets (lower surface) with veins rather evident and often forming an imperfect reticulum2, the apices with a poorly developed, blunt apiculum, or apiculum absent; petiole gland sometimes absent………………………………………………19. Senegalia teniana
44: Peduncles glabrous or sparsely hairy; leaflets (lower surface) with veins absent or very obscure (not forming reticulum), the apices with a finely acute apiculum; petiole gland always present………………………………………4. Senegalia delavayi
45. Phyllodes with a single longitudinal vein on each face; flowers in globose heads arranged in racemes………………………………………………33. Acacia podalyriifolia
45: Phyllodes with more than one longitudinal vein on each face; flowers in cylindrical spikes or globose heads………………………46
46. Flowers in cylindrical spikes1; phyllode main longitudinal veins (at base of phyllodes) often confluent with one another and/or with lower margin………………………………………47
46: Flowers in globose or obloid heads1; phyllode main longitudinal veins (at base of phyllodes) remaining separate from one another and from lower margin……………………………51
47. Phyllodes straight, with 3-5 prominent longitudinal veins, the minor veins forming a net-like reticulum2 with clearly defined vein-islands; branchlets often stout and acutely angled; pods coiled; aril yellow or orange, not encircling seed……………………………………………………………………48
47: Phyllodes normally falcately recurved, with numerous longitudinal veins (some more pronounced than others), minor veins often anastomosing but not forming a net-like reticulum; branchlets often slender and ±terete………………………49
48. Phyllodes with 4 (or 5) longitudinal veins; branchlets and phyllodes glabrous; spikes white to cream; seed aril orange; tree to 30 m tall……………………………30. Acacia mangium
48: Phyllodes with 3 longitudinal veins; branchlets and phyllodes appressed-hairy; spikes golden; seed aril yellow. Shrub or tree 3-8 m tall……………………………28. Acacia holosericea
49. Phyllodes with minor veins not anastomosing; pods ±straight, 20-35 (-45) mm wide; funicle/aril white, not encircling seed………………………………25. Acacia crassicarpa
49: Phyllodes with minor veins some or many anastomosing; pods < 20 mm wide; funicle/aril yellow or orange and encircling the seed………………………………………………50
50. Branchlets and phyllodes (at least when young) appressedhairy; new shoots densely appressed-hairy, hairs golden (tinged brown); spikes cream to pale yellow; pods tightly spirally coiled, valves 4-7 mm wide………23. Acacia cincinnata
50: Branchlets and phyllodes glabrous; new shoots not as above; spikes golden; pods strongly curved to openly coiled, valves 8-18 mm wide……………………………22. Acacia auriculiformis
51. Phyllodes with minor veins forming an obvious, net-like reticulum2 between the main longitudinal veins; inflorescences 3-5-headed racemes; seed aril red or pink and encircling the seed; pods strongly curved to openly coiled and often twisted………………………………32. Acacia melanoxylon
51: Phyllodes with minor veins not forming a net-like reticulum between the main longitudinal veins or reticulum2 poorly developed; seed aril absent or white and not encircling seed……………………………………………………………………52
52. Racemes 4-8-headed racemes and 10-45 mm long; heads cream to pale yellow; phyllodes 70-200 mm long, with numerous longitudinally anastomosing minor veins between main veins; pods coiled and twisted, thick-textured; seed aril white………………………………………………29. Acacia implexa
52: Racemes 1- or 2-headed and rudimentary (c. 1 mm long); heads golden to bright yellow; phyllodes 50-110 mm long, with few or no anastomosing minor veins between main veins; pods straight to shallowly curved, thin-textured; seeds exarillate………………………………………24. Acacia confusa
4.3. Indigenous species (all Senegalia) 4.3.1. SENEGALIA Raf., Sylva Tell. 119 (1838).金合欢属【jīn hé huān shǔ】(新拟)≡ Acacia subg. Aculeiferum Vassal, Bull. Soc. Nat. Hist. Toulouse 108: 138 (1972). [Also published in: Trav. Lab. Forest. Toulouse Tome 1, Vol. 8, Art. 17: 15 (1972).]. LECTOTYPE: Mimosa senegal L. (vide Britton & Rose, N. Amer. Fl. 23: 106. 1928) (Senegalia senegal (L.) Britton [Senegalia triacantha Raf., nom. illeg.]).
Two sections are recognised:
Senegalia sect. Senegalia
Distinctive features. Trees. Prickles 2 or 3 at or near nodes. Inflorescences comprising pedunculate spikes. [Two introduced species in China: see Introduced species below.]
Senegalia sect. Monacanthea (Vassal) Maslin, comb. nov.
≡ Acacia subg. Aculeiferum sect. Monacanthea Vassal, Bull. Soc. Nat. Hist. Toulouse 108: 139 (1972). [Also published in: Trav. Lab. Forest. Toulouse Tome 1, Vol. 8, Art. 17: 15 (1972).] TYPE: Acacia ataxacantha DC. [21 indigenous species recognised for China.]
Distinctive features. Lianas or sometimes shrubs or trees. Prickles scattered on the internodes. Stipules not spinescent and mostly caducous. Leaves bipinnate, single at nodes. Leaf glands (extra-floral nectaries) usually present; petiole gland 1 (rarely 2 or absent), normally situated near or below middle of petiole; rachis glands normally present at base of uppermost few pairs of pinnae. Inflorescences comprising pedunculate heads (globose or obloid) or rarely spikes, arranged in racemes or panicles or sometimes single or clustered in leaf axils; peduncles without involucre but sometimes a single bract on upper half. Pods flat, broad, thintextured and smooth, rarely hard-textured and wrinkled (Senegalia rugata).
Description. Lianas with stems to c. 15 m long or sometimes shrubs (often straggling or lianescent) normally 3-5 m tall or less frequently trees to c. 11 m tall, presumably normally evergreen, rarely (S. pennata subsp. insuavis) emitting a disagreeable odour when fresh (when leaves or branchlets crushed). Branchlets terete, glabrous or variably hairy, sometimes glandular-hairy, not pruinose (except S. pruinescens); lenticels present except some-times not visible on upper branchlets. Prickles scattered on the internodes, often also a few on under surface of petiole and rachis, sometimes absent from herbarium specimens, normally not prominent on upper branchlets (0.5-2 mm long) but longer (3-7 mm) on more mature branchlets, patent to slightly reflexed, straight to shallowly recurved, brown, splayed at base. Stipules caducous, not spinescent, 2-7 (-9) mm long, 0.5-3 (-4) mm wide. Leaves bipinnately compound, single at the nodes; pinnae (3-) 4-28 (-30) pairs, 20-110 (-145) mm long; petiole (10-) 15-60 mm long; rachis 30-190 (-300) mm long, normally hairy on upper surface (hairs straight and patent to curved and appressed), the axis terminated by a short, persistent, triangular point. Leaflets 7-80 (-100) pairs, (2-) 3-15 (-20) mm long, (0.3-) 0.4-6 (-9) mm wide, opposite, mostly narrowly oblong, thin-textured (often membranous in S. rugata), discolorous (sometimes strongly so, darkest abov-), rarely concolorous, straight or sometimes slightly curved laterally, flat (but often variously concave and/or ± shallowly curved forward upon drying, sometimes strongly concave and/or obviously curved forward in S. pennata subsp. insuavis), glabrous or sometimes appressed-hairy below, margins normally appressed-ciliate or appressed-ciliolate; apex symmetric or asymmetric, finely acute to rounded, with or without an apiculum or mucro that varies from centric to excentric; base normally unequal with an obvious rounded angle on lower edge of leaflet only and the clearly excentric petiolule situated near upper edge, occasionally (in S. caesia and S. tonkinensis) with an obvious rounded angle on both upper and lower edges and petiolule ±centric, sometimes sessile due to very short petiolule; main vein normally single (infrequently 2-4, e.g. S. caesia), more evident on lower surface of leaflet than upper surface (where it is commonly not or scarcely visibl-), starting near upper margin at base of leaflet or occasionally near middle; lateral veins (on lower surface of leaflets) visible or not visible, sometimes anastomosing to form a reticulum. Leaf glands (extra-floral nectaries) normally present; petiole glands e 1 (rarely 2, e.g. S. tonkinensis) or rarely absent (S. yunnanensis), sessile or rarely stipitate (S. stipitata) or substipitate (S. garrettii); rachis glands - normally present at or near base of some (but not all) pairs of pinnae and similar to petiole gland except smaller, sessile or rarely stipitate or substipitate (S. hainanensis, S. stipitata); rachilla glands - situated at base of uppermost 1-3 pairs of leaflets, sometimes absent, normally minute. Inflorescences comprising pedunculate heads (globose or sometimes obloid) or rarely spikes (i.e. S. yunnanensis) arranged in panicles or racemes, or sometimes single or clustered in leaf axils; peduncles (5-) 10-40 (-50) mm long, hairy or occasionally glabrous, sometimes glandular-hairy, single bract sometimes present on upper half of peduncle; heads and spikes white or pale yellow at anthesis; inflorescence buds often dark-coloured when dry (indicating red or purple when fresh). Bracteoles small, 0.5-1.5 (-2) mm long, not exserted beyond flowers in young buds (except S. tonkinensis). Flowers small, bisexual or sometimes male only, 5-merous, sessile or subsessile, the pedicel 0.1-0.5 (-1) mm long; calyx valvate, commonly c. ¾ length of corolla (sometimes shorter or longer), gamosepalous, normally shortly dissected (to c. ¼ its length), infrequently dissected to near base (S. tonkinensis); corolla valvate, gamopetalous, the petals 2-4 (-5) mm long and 1-veined or veins not visible; stamens numerous, filaments free or sometimes very shortly united at base, anthers sometimes with a small, caducous, stipitate gland at apex (Wu and Nielsen, 2010); ovary sessile or short-stipitate, normally appressed-hairy (rarely glabrous). Pods dehiscent, oblong to broadly linear, (50-) 70-230 mm long, (10-) 15-40 (-50) mm wide, normally firmly chartaceous to thinly coriaceous-crustaceous, flat and smooth, rarely (in S. rugata) coriaceous/fleshy and thickened when fresh but drying coarsely wrinkled and hard-textured (crustaceous to ±woody), straight or slightly curved, glabrous or sub-glabrous, rarely (S. obliqua) microscopically densely velutinous, sometimes with small, circular, sessile, dark-coloured glands, veins transverse and obscure or not visible, margins slightly thickened and (except S. rugata) not commonly con-stricted between the seeds, apex obtuse to acute, basal stipe indistinct to evident (2-15 mm long). Seeds (not seen for some species) uniseriate, transverse in the pods (oblique in S. obliqua, but only a single seed seen), normally oblong to elliptic, 7-15 mm long, 4-11 mm wide, flattened (except somewhat turgid in S. rugata), brown to black; pleurogram not prominent, not visible in S. guangdongensis and S. yunnanensis (but seeds sub-mature); areole open narrowly at the hilar end, 4-9 mm long, 2-5 mm wide, sometimes occupying most of seed surface; funicle thickly filiform, without an aril.
Etymology. The generic name is in reference to the northwest African country of Senegal. It was a specimen collected from Senegal by Michel Adanson between 1749 and 1753 that Linnaeus (1753) used to prepare his diagnostic phrase-name for M. senegal, see Ross (1975) for discussion.
1. Senegalia andamanica (I.C.Nielsen) Maslin, Ebinger & Seigler, Blumea 58: 40 (2013) (Fig. 3)安达曼金合欢【ān dá màn jīn hé huān】(新拟)
≡ Acacia andamanica I.C.Nielsen, Adansonia, ser 2, 19(3): 354 (1980). ≡ Acacia pseudointsia var. ambigua Prain, J. Asiat. Soc. Bengal, Pt. 2, Nat. Hist. 66: 249 & 511 (1897) [1898 publ. 1897]. Type citation: "Andamans; very common, King's Collectors!". Type: INDIA, Tytler Ghat near Port Mouat, Andamans, 12 July 1890, Dr. King's Collector s.n. (lectotype: K barcode K000791195, ex CAL [digital image!], designated by Nielsen (1980); isolectotype: CAL 140529 barcode 0000012918!). Remaining syntypes: South Andamans, Dr. King's Collector s.n., 30 July 1892 (CAL 140521 barcode 0000012920!), 22 July 1893 (CAL barcode 0000012929! & CAL 140527 barcode 0000012924!), 2 Sep. 1893 (CAL barcode 0000012928! & CAL 140524 barcode 0000012917!), 21 July 1894 (CAL 140514 barcode 0000012919!) and 28 July 1894 (CAL 140518 barcode 0000012923!).
[Acacia pseudointsia auct. non Miq.: Craib, Fl. Siam. 1: 551 (1928), fide Nielsen(1980, 1985a).]
[Acacia vietnamensis auct. non I.C.Nielsen: H.Sun & C.Chen, Acta Bot. Yunnan. 12: 261 (1990), pro parte, as to S.K. Lee 200449.]
Distinctive features. Branchlets normally glabrous. Stipules relatively large, 3-6 (-8) mm long, 1-4 mm wide, evident in young inflorescence buds but soon caducous. Pinnae mostly 4-8 pairs. Rachis hairs very short and antrorsely curved, rarely absent. Leaflets rather large, normally 7-15 mm long and 2-4.5 mm wide; apex symmetric, rounded to obtuse and without an apiculum; petiolule extending below base of leaflet; lateral veins (on lower surface of leaflet) not forming a reticulum except on plants from Andamans. Petiole gland quite large, 2-4 (-5) mm long and 1-1.5 mm wide, normally situated close to leaf base, very flattened except often elevated slightly from base or curved upwards at distal end. Inflorescences terminal open panicles or sometimes elongated racemes; peduncles densely hairy. Pods 15-26 mm wide, stipe slender.
Description. Lianas or shrubs. Branchlets darkish coloured (grey to brown), glabrous or occasionally (at extremities) puberulous, lenticels absent from upper branchlets but present on mature branchlets. Prickles few to sub-numerous on internodes and few or absent on under-surface of leaf axes, occasionally absent from herbarium specimens, often not prominent (0.5-3 mm long) but reasonably stout, patent to shallowly reflexed, straight to shallowly recurved. Stipules evident in young inflorescence buds but soon caducous, ovate (Andaman specimens) to lanceolate or narrowly lanceolate, relatively large, 3-6 (-8) mm long, 1-4 mm wide, brown or red-brown, glabrous (except appressed-ciliolate) or sparsely appressed-hairy and striate abaxially, base equally or slightly unequally rounded and sometimes ±auriculate, apex acuminate. Leaves bipinnate; pinnae (3-) 4-8 (-10) pairs, (30-) 40-130 mm long; petiole 17-50 mm long; rachis (35-) 60-110 mm long, with very short, antrorsely curved, spreading to ±appressed hairs on upper surface, rarely glabrous. Leaflets (9-) 15-33 pairs, oblong to narrowly oblong, mostly 7-15 mm long and 2-4.5 mm wide, occasionally smaller in Thailand (see note below under Leaflets), flat, moderately to strongly discolorous (darkest on upper surface), glabrous except margins often appressed-ciliolate; apex symmetric or sometimes asymmetric, rounded to obtuse, occasionally retuse, apiculum absent; base unequal with an obvious rounded angle on lower edge, petiolule distinct (extended below base of leaflet) and clearly excentric; main vein starting near upper margin at leaflet base and extending obliquely to apex, ±straight, with 2 or 3 additional, short veins diverging from petiolule; lateral veins (on lower surface of leaflets) ±few and not prominent, often bifurcating near margin, normally not forming a reticulum except on Andaman specimens (see discussion below under Taxonomy). Glands: petiole gland-quite prominent, normally situated 4-10 mm above leaf base and 0-5 (-7) mm above pulvinus, rarely to 16 mm above leaf base and 10 mm above pulvinus in Thailand (viz. Haniff & Hd Nur 4012), oblong to oblong-elliptic, 2-4 (-5) mm long, 1-1.5 mm wide, sessile, depressed and not thickened, flattopped or shallowly concave above, occasionally very shallowly elongate-cupular, often discernibly elevated slightly from base or upwardly curved at distal end, commonly peripterous but varying to apterous or both peripterous and apterous; rachis glandsd-situated at or near base of uppermost 1-3 (-5) pairs of pinnae, circular to oblong-elliptic, 1-2 mm long, depressed, flat-topped (and at least sometimes peripterous) or shallowly cupular, sessile or occasionally sub-stipitate with a broadish stipe; rachilla glands-situated at base of uppermost 1 or 2 pairs of leaflets, oblong to oblong-elliptic, 0.5-1 mm long, depressed, sessile. In-florescences comprising pedunculate heads arranged in terminal open panicles and/or sometimes elongated racemes, the panicle/ raceme axes glabrous to densely pubescent (hairs spreading to appressed and less dense than on peduncles), glandular hairs ab-sent or very few; peduncles (5-) 10-20 mm long, rarely to 30 mm when in fruit, mostly 2-4 together in clusters, densely pubescent when in flower, occasionally sparsely pubescent or glabrous in pod, the hairs appressed or patent, straight or slightly curved and brownish yellow to creamy white, glandular hairs absent or very few; heads globose to slightly obloid, 8-13 mm diam. at anthesis when dry, yellow or pale yellow (?or white, see notes below under Head colour), densely 40-45-flowered; inflorescence buds rather dark-coloured (greyish or brown) when dry (suggesting that they were red when fresh; see discussion under Morphological characters: Inflorescences and flowers above). Bracteoles linear-spathulate, 1-1.5 mm long, not exserted beyond flower in buds, claw narrowly linear, lamina small. Flowers 5-merous, sessile or sub-sessile; calyx ¾ or more length of petals, gamosepalous, dissected for ¼-½ its length into triangular or oblong lobes, calyx tube glabrous or sub-glabrous and veins not visible; petals 2-3 mm long, glabrous or occasionally sub-glabrous, veins not visible; ovary glabrous or appressed-hairy, sessile. Pods (slightly immature) oblong, 80-160 mm long, 15-26 mm wide, thinly coriaceous to firmly chartaceous, straight or sometimes shallowly curved, normally not constricted between the seeds but a few moderately deep constrictions sometimes occur, flat, not or scarcely raised over seeds along midline, light brown to mid-brown, glabrous, sessile glands absent, veins not or scarcely visible or (on Andamans) rather numerous, transverse and fine, marginal vein thickened, apex obtuse to broadly acute; basal stipe 4-11 mm long, slender, terete. Seeds not seen mature but described by Nielsen (1985a) for Thailand as broadly elliptic, 9-9.5 mm long, 7-7.5 mm wide, flat; areole narrowly elliptic, c. 5-6 mm long, 2.5-3 mm wide.
Selected specimens examined. CHINA: Guangxi. Chongzuo City, Longzhou County, 16 Sep. 1936, H.Y. Liang 68426 (IBK 91527 [barcode 00067644], IBSC 294534 [barcode 0158783]); Chongzuo City, Ningming County, 13 Aug. 1954, S.K. Lee 200449 (IBK 29198 [barcode 00067646], IBSC 226592 [barcode 0158768]); Guilin City, Lingui County, 5 Oct. 1956, H. Liang 100087 (IBSC 216591 [barcode 0158769], IBSC 216591 [barcode 0158796]); Hechi City, Huanjiang Maonan Autonomous County, 16 Aug. 1977, Huanjiang Expedition 43-326 (GXMI 25740 [barcode 015767]); Guizhou. Qianxinan Buyi & Miao Autonomous Prefecture, Wangmo County, 10 June 1929, P.C. Chung 1435 (KUN 0400049 [barcode 1206968]-sphalm. 29 July 1927, PE 01856723 barcode 00321387-viewed on Chinese Virtual Herbarium, IBSC 680560 [barcode 0158860]). INDIA: South Andaman, 12 Dec. 1900, R.L. Heinig s.n. (CAL 140522); Port Mouat, South Andamans, 15 Aug. 1891, Dr King s.n. (CAL 140528 barcode 0000012921), undoubtedly original material, but not a type. THAILAND: North-eastern. Bungkhlaa, Phutoknoi, Nongkhlai [Nong Khai], 21 June 1997, C. Niyomdham 5077 (AAU [digital image!], BKF, n.v.). Peninsular. Pulau Panji [= Ko Panyi in Thai language], Pungah [= Phangnga Province], 11 Dec. 1928, Haniff & Nur 4012 (BO, SING 063127 [barcode 0206815]). South-western. Sam Roi Yot [National Park], Prachuap Khiri Khan Province, 12-19 July 1926, A.F.G. Kerr 10947 (BK 213378), flowers white; Wang Khanai, Kanchanaburi [sphalm. 'Kanburi' ] Province, 15 May 1927, A.F.G. Kerr 12864 (BK 213379 [barcode 213379], TCD 0016528 [digital image]), flowers yellow; Sam Roy Yot [National Park], Prachuap Khiri Khan Province, K. & S. Larsen 33684 (BKF). VIETNAM: Northern. Entre Kep and Pho Vi, Bac Giang Province [= Lang Son Province], 5 June 1936, [P.A.] Petelot 5246 (VNM); same locality as in preceding, 8 June 1939, [P.A.] Petelot 2163 (HNU 1363, VNM 00012190); Entre Dong Mo et Van Linh, Lang Son Province, 12 May 1938, [P.A.] Petelot 2178 (HNU 1362, VNM barcode VNM00020832); Dong Mo, Lang Son Province, Chi Lang District, 24 May 1981, Tran Dinh Nghai T-881 (HNU 12103).
Distribution (Fig. 4). Nielsen (1985a) recognised A. andamanica as occurring in southern Thailand and the Andaman Islands (a protectorate of India), but the distribution is extended here to include northeast Thailand, northern Vietnam and southern China. In China it has a scattered distribution, being known from a single gathering in southern Guizhou and a few collections in northern and south western Guangxi. In Vietnam it is recorded only from Lang Son Province which is located on the border with Guangxi. In Thailand it occurs in the north eastern region on the border with Vietnam (from Nong Khai in Bueng Kan Provinc-), the South-western region (centred on Sam Roi Yot and Kui Buri National Parks in Prachuap Khiri Khan Province, and Kanchanaburi Provinc-) and the Peninsular region (from near Phuket in Phangnga Provinc-).
|
| Fig. 4 Distribution of Senegalia andamanica in China, based on specimen records at GXMI, IBK, IBSC, KUN and PE, as cited in text. |
Habitat. Grows on low limestone mountains.
Phenology. Judging from herbarium collections, in China Senegalia andamanica commences flowering between late April and early June (but it is not known when it ends) and pods with slightly immature seeds have been collected between mid-August and early October (perhaps maturing between about late September and mid-November).
Typification. The basionym of Senegalia andamanica, A. pseudointsia var. ambigua, was based on material collected from the Andaman Islands by one of "King's collectors.", but no date of collection or herbarium of specimen lodgement was given in the protologue. At CAL there are several specimens from the Andamans that were collected between 1892 and 1894 by "King's collector" and which are regarded here as types of var. ambigua. There is a duplicate of one of these gatherings at K. These specimens all represent the one species, and most are labelled (?by Prain) as "Acacia pseudo-intsia var. ambigua Prain" Nielsen (1980) lectoty-pified A. pseudointsia var. ambigua with the Kew specimen that is dated 12 July 1890. This specimen was annotated by Nielsen in 1978 as "Acacia prainii I.C.Nielsen ined. Type! (A. pseudo-intsia Miq. var. ambigua Prain)", but he subsequently published the species as A. andamanica, undoubtedly becoming aware that Maiden (1917) had published A. prainii. Chakrabarty and Gangopadhyay (1996) overlooked Nielsen's lectotypification and superfluously relectotypified var. ambigua, based on a specimen collected on 2 September 1893, also from the Andaman Islands by one of "King's collectors" (i.e. CAL barcode 0000012928). The "King" referred above was George King, who at that time was the Superintendent of Calcutta Botanic Garden and who commonly engaged people to collect plants on his behalf. David Prain was the then Curator of Herbarium CAL, that is located within the Garden (fide Burkill, 1954).
Taxonomy. Until now, most specimens of this species had commonly been labelled in Chinese herbaria as Acacia concinna or A. sinuata (these two names are now regarded as synonyms of Senegalia rugata) and in Vietnamese herbaria as Acacia intsia (L.) Willd. (this name is now regarded as a nomen confusum, fide Deshpande et al. (2019)). Also, as listed under misapplied names above and discussed below under Senegalia prominens, Sun and Chen (1990) included a specimen of S. andamanica within their concept of A. vietnamensis.
Senegalia andamanica is a distinctive species, especially in having a rather large, flattened, basal and often peripterous petiole gland that is often curved upwards or raised from the base at its distal end, large and obtuse leaflets that are not apiculate, and relatively few pinnae. A slightly broad concept of this species is adopted here by including within its circum-scription plants from both the type locality (the Andaman Islands) and from mainland Southeast Asia and China. While the differences between plants from these two regions (discussed below) do not warrant the recognition of two species, it is possible that future studies may show the need to recognise infraspecific taxa.
The morphological differences between plants of Senegalia andamanica from the Andaman Islands and those from mainland Southeast Asia and China require further investigation. The most significant of these differences relate to the venation of leaflets and pods. In plants from the Andamans the leaflets possess an open, but not especially prominent, reticulum on their lower surface. Elsewhere the leaflets are mostly not reticulately-veined, except occasionally a few develop an indistinct and/or imperfect reticulum. Similarly, the only fruiting collection that we examined from the Andamans possessed pods with numerous, fine transverse veins, whereas the many fruiting collections we examined from elsewhere were veinless (or occasionally possessed a very few, indistinct transverse veins). In all cases the pods examined were not quite mature. Further collections of mature pods, especially from the Andamans, are needed to check these apparent differences. The Andaman plants also show other slight differences from plants that occur elsewhere, but these are unlikely to be of much, if any, taxonomic significance: the stip-ules are slightly wider (mostly 3-4 mm compared with 1-2.5 mm), pinnae slightly more numerous (7-10 pairs compared with normally 4-8 pairs) and rachis glands 3-5 (compared with 1-3).
Affinities. In China, Senegalia andamanica may superficially resemble S. kunmingensis, S. prominens and S. pruinescens on account of having relatively few pinnae and rather large leaflets. These three species can be distinguished from S. andamanica by their petiole gland being more prominently raised and/or further removed from the leaf base, and their pods often wider. Senegalia kunmingensis is further recognised by its sessile leaflets and by other characters discussed under that species; S. pruinescens by its pruinose branchlets (but pruinosity sometimes not visible on herbarium material) and by leaflets normally reticulately-veined on their lower surface; and S. prominens by its ±prominently 5-veined calyx tube and by leaflets normally reticulately-veined and appressed-hairy on their lower surface.
Leaflets. The leaflets of Senegalia andamanica are normally quite large, 7-15 mm long and 2-4.5 mm wide. However, the specimen C. Niyomdham 5077 from northeast Thailand has slightly smaller leaflets, 5-7 mm long and 1.5-1.8 mm wide. This specimen appears otherwise to be typical of the species, as best can be judged from inspection of a digital image.
Head colour. There is some uncertainty concerning the colour of the heads at anthesis in living plants of Senegalia andamanica. Very few herbarium specimens record head colour but in China they are described as pale yellow whereas in Thailand they are described simply as yellow (e.g. Kerr 12864 and Niyomdham 5077) or occasionally white (only Kerr 10947). It is not known if head colour in this species is variable or if there has been a labelling error (particularly on Kerr 10947). It is not possible to accurately determine head colour of living plants from herbarium material.
Etymology. This species name refers to the Andaman Islands from where the types were gathered.
Vernacular name. Andaman Senegalia (following the Chinese common name that is proposed abov-).
2. Senegalia caesia (L.) Maslin, Seigler & Ebinger, Blumea 58: 40 (2013) (Fig. 5).双脉金合欢【shuāng mài jīn hé huān】(新拟)
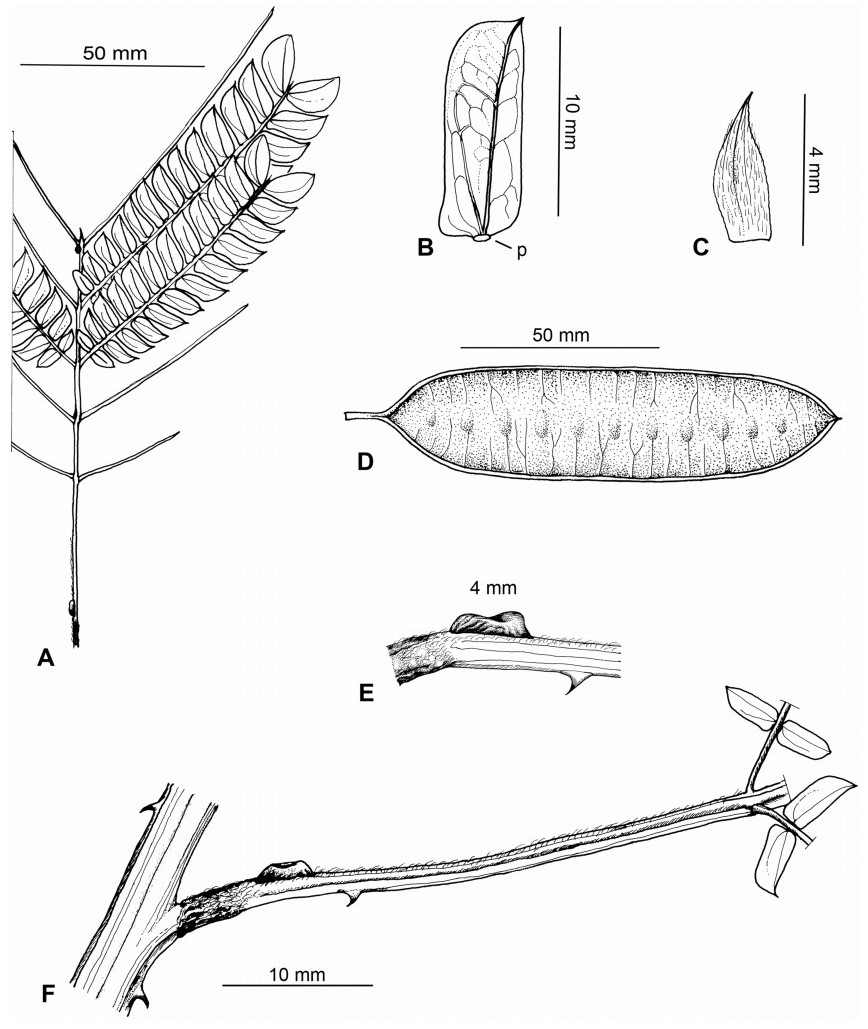
|
| Fig. 5 Senegalia caesia. A - Leaf showing few pinnae and position of petiole gland (thick arrow) and rachis gland (thin arrow). B - Leaflet (lower surface) showing ±indistinct, open reticulum, petiolule (p) in central position at base and apex obliquely truncate with a short but distinct mucro. C - Stipule. D - Pod. E Petiole gland prominent. F - Node showing branchlet glabrous and petiole gland close to leaf base. Vouchers: Sino-Vietnam Expedition 1309 (A & D); C. Wang 34134 (B, E & F); E. Poilane 16037 (C). Drawn by Joshua Yang. |
≡ Mimosa caesia L., Sp. Pl. ed.1, 1: 522 (1753). ≡ Acacia caesia (L.) Willd., Sp. Pl. ed. 4, 4(2): 1090 (1806). ≡ Acacia intsia var. caesia (L.) Wight & Arn. ex Baker, Fl. Brit. India [J.D. Hooker] 2: 297 (1878). ≡ Acacia caesia var. caesia, Contr. Gray Herb. 57: 7 (1919), established by publication of A. caesia var. oxyphylla (Graham ex Benth.) J.F.Macbr., see below. Type citation: No type cited but provenance given as "Habitat in India". Type: [SRI LANKA], Herb. Hermann 2: 50, No. 217 (lectotype: BM barcode BM000621675 and BM000621676 [digital images!], 2nd step designated by Rico Arce in Turland and Jarvis (1997), see discussion below under Typification; isolectotype: L n.v.-cited by Kostermans (1980)).
= Acacia oxyphylla Graham ex Benth., London J. Bot. 1: 514 (1842). ≡ Acacia intsia var. oxyphylla (Graham ex Benth.) Baker, Fl. Brit. India [J.D. Hooker] 2: 297 (1878). ≡ Acacia caesia var. oxyphylla (Graham ex Benth.) J.F.Macbr., Contr. Gray Herb. 57: 7 (1919). Type citation: "Silhet, Wallich". Type: BANGLADESH, Sillet, F. De S & W.G. [Sylhet, Bangladesh, F. De Silva & W. Gomez], in Wallich Numer. List no. 5252A (lectotype: K barcode K000791196 [digital image!], sheet stamped 'Herbarium Hookerianum' designated by Nielsen (1980) who incorrectly used the term holotype, see discussion under below Typification; isolectotypes: BM barcode BM000946892 [digital image!], E barcode E00318068 [digital image!], as '5252' K-W barcode K001120288 [digital image!]).
- "A. oxyphylla" Graham in N. Wallich, Numer. List no. 5252 (1831-32), nom. nud.
= Acacia oxyphylla var. subnuda Craib, F1. Siam. 1: 550 (1928). ≡ Acacia caesia var. subnuda (Craib) I.C.Nielsen, Adansonia, sér. 2, 19: 348 (1980). Type citation: "MAHARAT: Lampang, Mê Salop, 200 m, secondary growth forest, Winit, 1463". Type: THAILAND, Lampang, Mê Salop, 25 Oct. 1925, [K.] Winit 1463 (holotype: K barcode K000392266 [digital image!]; isotypes: ABD n.v.-cited in Nielsen (1980), BKF barcode SN036606!, C n.v.-cited in Nielsen (1980)).
Additional synonymy (not relevant to China): Deshpande et al. (2019).
Distinctive features. Pinnae few, 4-6 (-8) pairs. Leaflets large (mostly 10-15 mm long, 3-6 mm wid-), normally imperfectly 2-veined and openly reticulately-veined on lower surface; apex obliquely truncate or rounded-truncate with a short but distinct, clearly excentric, acute mucro; base ±sessile with petiolule ±centric. Petiole gland situated near base of leaf, large (mostly 3-7 mm long) and prominently raised (1-1.5 mm high). Bracteoles not exserted beyond flowers in buds.
Description. Lianas or straggling shrubs. Branchlets glabrous or infrequently with short, curved, ±appressed hairs. Prickles few to rather numerous on internodes and normally few on underside of leaf axes, often absent from herbarium specimens, 0.5-2 mm long, straight and patent or shallowly recurved. Stipules caducous, ±triangular to ovate-lanceolate, 2-4 mm long, 0.5-1 mm wide, (yellowish) light brown, very obscurely striate and (sometimes sparsely) appressed-hairy abaxially, acute to acuminate. Leaves bipinnate; pinnae 4-6 (-8) pairs, rarely 3 pairs (see note below under Variation), 35-100 mm long; petiole 30-60 mm long; rachis 30-115 mm long, with very short, ±appressed hairs (?or some-times glabrous). Leaflets 7-18 (-23) pairs, oblong to narrowly oblong but the apical pair often obovate, (7-) 10-15 (-20) mm long and (2-) 3-6 (-9) mm wide (lowermost pair often smaller), moderately to strongly discolorous, shiny and dark green above and light green below when fresh, glabrous or sometimes with very fine, appressed, white hairs over lower surface (indumentum sparse to moderately dense, difficult to see and easily overlooked); apex obliquely truncate or rounded-truncate, mucronate by a short (to c. 0.2 mm long) but distinct, excentric, acute point; base trun-cate, with rounded angles on both edges, the centric or sometimes sub-centric petiolule squat and very short so that leaflets appear sessile; main vein starting ±centrally at leaflet base and extending obliquely to mucro, a second less prominent vein diverging from base and extending c. ½ length of leaflet (rendering leaflets imperfectly 2-veined, or rarely imperfectly 3- or 4-veined if veins more numerous); lateral veins (on lower surface of leaflets) few and openly anastomosing to form an evident or obscure reticulum (in Thailand reticulum occasionally not visible). Glands: petiole gland-situated close to base of petiole 5-10 mm above leaf base and 0-5 mm above the pulvinus, ±oblong to slightly elliptic, (2-) 3-7 mm long, sessile, prominent, clearly raised (1-1.5 mm high, often slightly highest at distal end), shape (viewed laterally) somewhat variable; rachis glands-situated at base of uppermost 1-3 pairs of pinnae, commonly similar to petiole glands but smaller (1-1.5 mm long); rachilla gland-situated at base of uppermost pair of leaflets, small, depressed. Inflorescences comprising pedunculate heads arranged in terminal open panicles or elongated racemes, the axes of panicles and racemes appressed-puberulous (hairs white); peduncles single or clustered in groups of up to six, (7-) 8-25 mm long, puberulous; heads globose to slightly obloid, not especially large (c. 8 mm diam. at anthesis when dry), white or very pale yellow, scented, densely flowered; inflorescence buds often darkish coloured when dry (suggesting often reddish when fresh; see note below under Inflorescence bud colour). Bracteoles spathulate, 1.2-1.5 mm long, not exserted beyond flowers in buds. Flowers 5-merous, sessile or sub-sessile; calyx c. ¾ length of petals, gamo-sepalous, shortly dissected into acute lobes that are commonly dark-coloured at apices when dry (suggesting reddish when fresh), calyx tube glabrous or rarely slightly puberulous and veins not visible; petals 2-3 mm long, commonly dark-coloured at apices when dry (indicating presumably pink or red when fresh), glabrous, veins not visible; ovary hairy. Pods oblong, 70-180 mm long, 20-30 (-36) mm wide, firmly chartaceous to thinly coriaceous-crustaceous, straight or sometimes slightly curved, flat but shallowly convex over seeds along the midline, straight-edged or occasionally slightly constricted between seeds, light-to dark-brown or reddish brown, glabrous, sessile glands absent, finely transversely veined (some veins bifurcating), marginal vein narrow but thickened, apex acute, base narrowed to a short or distinct stipe 3-10 mm long. Seeds transverse in pods, oblong, 12 mm long, 7 mm wide, flattened, very dark brown, slightly shiny; areole 8 mm long, 4 mm wide, open at hilar end; funicle thickly filiform, exarillate.
Selected specimens examined. CHINA: Hainan. Sanya City, 22 Oct. 1987, Z.X. Li 2818 (IBSC 601601 [barcode 0158663]); Sanya City, 21 Sep. 1933, C. Wang 34134 (IBK 40325 [barcode 00067661], IBSC 63468 [barcode 0158659]. Yunnan. Dehong Dai and Jingpo Autonomous Prefecture, Yingjiang County, 18 Jan. 1989, H. Sun 1498 (KUN 0159827 [barcode 0598027]); Lincang City, Gengma Dai & Wa Autonomous County, H.C. Wang et al. 20110222 (KUN). Uncertain locality. Yunnan Expedition [without specific locality, unsure if China or Myanmar], 1868, D.J. Anderson 1175 (CAL 140509 & 140510). INDIA: Assam. Without precise locality, R.N. Parker 2138 (DD); East India, without date, W. Roxburgh s.n. (K barcode K000791176, digital image). LAOS: entre Ban Cha Tau et Kuang Si, Bassac Province [now Champassak Province], 19 Oct. 1928, [E.] Poilane 16037 (VNM 00012189). MYANMAR: Flora of Tenasserim [now Tanintharyi Region], 1905, S. Ahmuduli s.n. (CAL); Mawhau, Katha district, 16 Nov. 1928, C.E. Parkinson 1743 (DD). THAILAND: North-eastern. Ban Na Luang, Loei Province, 16 Jan. 1970, C.F. van Beusekom & C. Phengklai 3040 (BKF 66714 [barcode 036602]). Peninsular. Ranong, Kapor, 22 Nov. 1965, B. Sangkhachand 1151 (BKF [barcode 036603]). South-western. Ban Krang Camp, Kaeng Krachan National Park, Phetch-aburi Province, 25 Aug. 2004, I.C. Nielsen et al. 1936 (BKF 147171 [barcode 154178]). VIETNAM: Northern. Cuc Phuong, Ninh Binh Province, 26 Oct. 1963, Nguyen Dang Khoi 1470 (HN 0000042572); Thanh Pho, Lang Son Province, 14 Jan. 196, Sino-Vietnam Expedition 1309 (KUN 0400377 [barcode 0598601]); Cho Ganh, Ninh Binh Province, Sep. 1923, [P.A.] Petelot 1137 (HNU 1361).
Distribution (Fig. 6). Widespread on the Indian subcontinent (Bangladesh, Bhutan, India and Sri Lanka, see Deshpande et al. (2019)) eastwards through Myanmar to China and south into Laos, Thailand, Cambodia and Vietnam. Senegalia caesia was also recorded (as Acacia caesia) for Indonesia (Java) and Philippines by Nielsen (1981), but was not listed for those countries by Nielsen(1985a, 1992) and is unlikely to occur there (the earlier records most likely were based on specimens now known to be S. merrillii (I.C.Nielsen) Maslin, Seigler & Ebinger). In China, S. caesia is uncommon, being represented by a few gatherings in far western Yunnan (adjacent to the border with Myanmar) and the southern extremity of Hainan Island. Wu and Nielsen (2010) cited A. caesia also for Guangdong and Sichuan, but no specimens were found from either Province to verify these occurrences; these records are regarded as doubtful. caesiaAcacia was recorded for Taiwan by Huang and Ohashi (1993), based on C.C. Chuang 3266 (TAI); the digital image of this specimen we examined confirms that it is not S. caesia, but its real identity cannot be determined.

|
| Fig. 6 Distribution of Senegalia caesia in China, based on specimen records at IBK, IBSC and KUN, as cited in text (and also Hengduan Mountains website, web ref. 36). Note. Wu and Nielsen (2010) recorded this species for Guangdong and Sichuan but we have not seen specimens from either of those Provinces; we regard those records as doubtful. |
Habitat. Grows in secondary, evergreen forests, at 200-300 m alt. Altitude recorded as 200-2500 m by Wu and Nielsen (2010), but is presumably an error. Some labels indicate that this species is common in the places where it grows.
Phenology. Specimen records show Senegalia caesia in China as flowering from late August to October, but because some specimens from Hainan have mature flower buds in mid-October, it is likely that flowering extends to about November, at least in that area. Wu and Nielsen (2010) record the fruiting period as November, but judging from the flowering period just given, it is likely that pods with mature seeds will occur for several months beyond November.
Typification. As noted by Jarvis (2007), both Kostermans (1980) and Nielsen (1980) indicated an element in Herb. Hermann (BM) as the lectotype of Mimosa caesia, but it was unclear as to which of the relevant specimens was meant. The lectotypification of this name by Rico Arce in Turland and Jarvis (1997) followed Kostermans (1980) and Nielsen (1980) but restricted their choice to the most suitable specimens. Rico Arce nominated a sheet of Herb. Hermann 217 as the lectotype; this sheet supports two specimens, the left-hand one being a branchlet with leaves and a terminal panicle, the right-hand one being a panicle only; no sheet number was provided by Rico Arce, but by Jarvis (2007) subsequently cited it as BM [barcode BM000594612]. However, that number no longer exists on the sheet and has been replaced by the following two barcode numbers: BM000621675 (referring to the upper left-hand branchlet with leaves and inflorescence) and BM000621676 (referring to the right-hand inflorescence). This material represents the same taxon and both the left-hand and right-hand pieces are labelled '217' by Linnaeus. Therefore, this material is here considered as constituting a single specimen (the lectotype), notwith-standing there being two barcodes on the sheet.
In the protologue of Acacia oxyphylla (which is treated above as a heterotypic synonym of Senegalia caesia), Bentham (1842) cited "Silhet, Wallich". There is one collection of this species from 'Silhet' in Wallich's Numerical List (Wallich, 1831-1832) under 5252A, collected by "F. DeS & W.G." [=Sylhet, Bangladesh, F. De Silva & W. Gomez, C. FrasereJenkins, pers. comm. 2016]. Nielsen (1980) correctly regarded 5252A as a type of A. oxyphylla. However, there are two relevant sheets at herb. K, and he treated a specimen in the general collection as the holotype and one in the 'Wallich Herbarium' as an isotype. Neither of these specimens is annotated by Bentham and it is not possible to determine with certainty if one or both were used to prepare the protologue. Accordingly, in conformity with ICN Art. 9.10 (Turland et al., 2018) and as discussed by McNeill (2014), Nielsen's holotype citation is corrected above to lectotype.
Taxonomy. For many years this species was treated as Acacia caesia var. subnuda in Southeast Asia, with A. oxyphylla given in synonymy Nielsen (1980), 1981, 1985a). In China Wu (1988), however, did not recognise the variety in his treatment of A. caesia; his taxonomy was subsequently adopted by Wu and Nielsen (2010) who placed var. subnuda in synonymy under A. caesia. Maslin (2015) and Deshpande et al. (2019) did the same thing under Senegalia.
Following Wu (1988) and Wu and Nielsen (2010), intraspecific rank is not recognised within Senegalia caesia here. Material from Sri Lanka and southern India (that corresponds to the type of the species), and also the entities from Bangladesh and Thailand (that were described as Acacia oxyphylla and A. caesia var. subnuda, respectively) are treated as a single species. However, on the Indian subcontinent Senegalia caesia is very variable and further study is needed to determine if it includes more than one taxon.
Senegalia caesia belongs to the taxonomically complex 'Caesia species-group'. On the Indian subcontinent, this group also includes S. diadenia (R.Parker) Ragup. et al., S. gageana (Craib) Maslin, Seigler & Ebinger and S. torta (Roxb.) Maslin, Seigler & Ebinger. In Southeast Asia, S. comosa (Gagnep.) Maslin, Seigler & Ebinger, S. meebii (Craib) Maslin, Seigler & Ebinger and S. tonkinensis are members of this same group. Only S. caesia and S. tonkinensis extend to China, where neither is common.
Affinities. Within China Senegalia caesia is most closely related to S. tonkinensis, which is readily distinguished in having two glands on its petioles and exserted bracteoles in the young heads (see S. tonkinensis for discussion).
Variation. The specimen H. Sun 1498 from Yunnan is atypical in having only 3 pairs of pinnae and an atypically small petiole gland (1-1.5 mm long) that is of a particularly odd shape (±flabelliform). This sterile specimen was taken from a young branch on the plant, which may account for these peculiarities.
Inflorescence bud colour. The often darkish colour of inflo-rescence buds on herbarium specimens of Senegalia caesia suggests that in living plants the buds were at least sometimes reddish (see discussion under Morphological characters: Inflorescences and flowers above). Although inflorescence buds on living plants of this species in China have not been observed in this study, Kostermans (1980) described them as brown-red in Sri Lanka, the label on I.C. Nielsen et al. 1936 (collected from Thailand) noted them as dark red, while A. Deshpande (pers. comm.) reports them as pinkish on living plants in southern India.
Uses. According to the label on C.E. Parkinson 1832 at DD, the bark of this species [in Myanmar] has been used for washing hair and clothes.
Etymology. The species name is derived from the Latin caesius (bluish grey, lavender blue). In the original description of Mimosa caesia, Linnaeus cited Plukenet's description of the leaves as being "subtus caesiis" [bluish grey below]. Plukenet may have been referring to colour seen on dry herbarium material because in the living state the leaflets are pale green or pale yellowish green below (A. Deshpande, pers. comm.).
Vernacular names. Double-veined Senegalia (following the Chinese common name that is proposed above). This name refers to the leaflets of the species which are typically 2-veined. In Myanmar, it has been called 'Kimunga' and 'Kinbunkta' (these names appear on labels of C.E. Parkinson 1743 and 1832 respectively at DD), and in northern Thailand 'Nam huang' (Nielsen, 1985a).
3. Senegalia clandestina Maslin, B.C.Ho, H.Sun & L.Bai, sp. nov (Figs. 7 and 9).玉溪金合欢【yù xī jīn hé huān】(新拟)
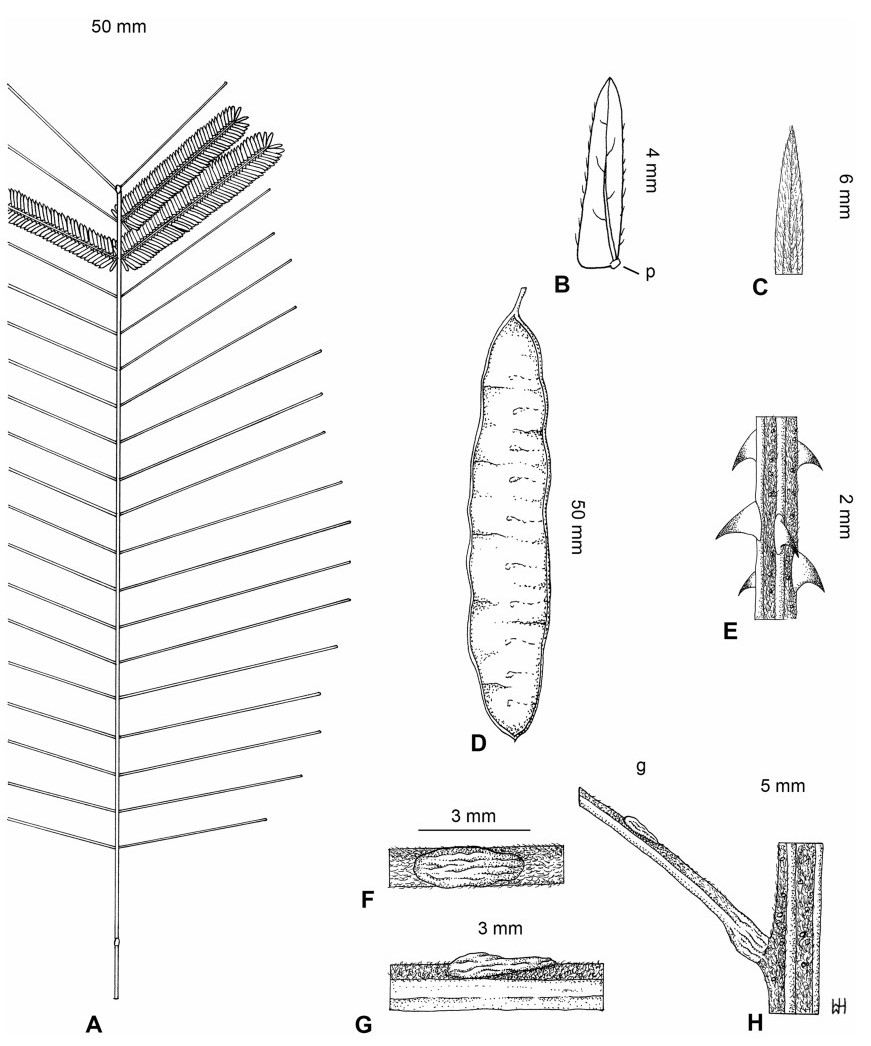
|
| Fig. 7 Senegalia clandestina. A - Leaf showing numerous pinnae and position of petiole gland (thick arrow) and rachis gland (thin arrow). B - Leaflet (lower surface) small. C - Stipule. D - Pod (sub-mature) pale-coloured. E - Prickles rather prominent on mature branch. F - Petiole gland (plane view) elongated and finely longitudinally wrinkled when dry. G - Petiole gland view) slightly elevated at distal end. H - Node showing low-profile, petiole gland (g) position. Vouchers: G. D. Tao 38697 (A, G & H); C.W. Wang 81633 (B, D & E); G. D. Tao 38697 (C); K.M. Feng 4479 (F). Drawn by Waiwai Hove. |
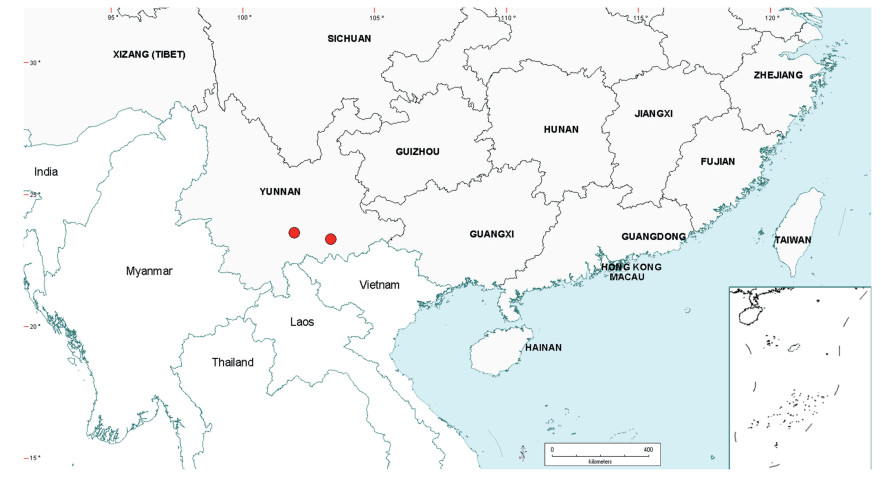
|
| Fig. 8 Distribution of Senegalia clandestina, based on specimen records at IBSC and KUN, as cited in text. |

|
| Fig. 9 Senegalia clandestina. A - Liana habit with insert showing cauline, inter-nodal prickles. B - Leaf showing numerous pinnae. C - Inflorescences forming an open terminal panicle (and elongated racemes at base of conflorescence). D - Panicle showing pale-coloured heads at anthesis and green inflorescence buds. E - Inflorescence node showing peduncle cluster flanked by narrowly oblong stipule. F - Fruiting panicle showing light brown pods. G & H - Petiole glands green, situated near base of petiole and slightly but discernibly thickened. I - Seeds dark brown with a sub-thickly filiform, exarillate funicle. Vouchers: B.R. Maslin & L. Bai BRM 11032 (A, B, D, E & H eupper & middle); B.R. Maslin & L. Bai BRM 11034 (C, G eupper & H elower); B.R. Maslin & L. Bai BRM 11033 (I). Photos: L. Bai. Scale bar = 5 mm (unless otherwise indicated). |
Type: CHINA, Yunnan Province, Yuxi City, Yuanjiang Hani, Yi & Dai Autonomous County, on Yuanmo Road, 23°38'01"N, 101°55'18"E, 4 June 2019, B.R. Maslin & L. Bai BRM 11032 (holotype: KUN barcode 1347978!; isotypes: BKF!, GXMI!, IBK!, IBSC!, K!, KUN barcode 1347977!, PE!, SING!).
Distinctive features. Branchlets pale coloured (greyish when fresh, commonly drying yellowish brown), inconspicuously hairy at extremities. Stipules narrowly oblong to narrowly triangular, 4-6 mm long, 1 mm wide. Pinnae 14-21 pairs. Rachis hairs short, antrorsely curved and appressed to sub-appressed. Leaflets small, (2-) 3-4 mm long, 0.5-0.8 (-1) mm wide; apex broadly acute to obtuse; lateral veins (on lower surface of leaflets) not or scarcely visible. Petiole gland elongated, normally 3-5 mm long, c. 1 mm wide, l:w = (2-) 3-5 (-6), solid, depressed, slightly thickened, normally finely but discernibly longitudinally wrinkled when dry, apterous. Inflorescences mostly arranged in terminal open panicles. Inflorescence buds pale green or yellowish green when fresh, drying yellowish brown. Peduncles densely hairy (hairs very short, curved and ±appressed). Pods 18-21 mm wide, light yellowish brown.
Description. Lianas or lianescent shrubs, probably deciduous. Branchlets (mature) pale-coloured (greyish when fresh but commonly yellowish brown when dry) and glabrous or subglabrous, young branchlets green (when fresh) and microscopically hairy (indumentum sparse to moderately dense and easily overlooked, the hairs c. 0.1-0.2 mm long, mostly curved and ±appressed). Prickles scattered on internodes and usually on inflorescence axes and under surface of leaf axes, numerous and prominent on living plants but sometimes sparse on herbarium specimens, 1-4 mm long, straight to shallowly recurved. Stipules at base of peduncles caducous (present at base of young inflorescence buds), narrowly oblong to narrowly triangular, 4-6 mm long, 1 mm wide, pale yellow or brownish yellow when fresh (drying light brown), glabrous to moderately appressed-hairy and obscurely striate abaxially, not lobed at base; stipules on vegetative shoots narrowly oblong to narrowly triangular, 6-10 mm long, 1-1.4 mm wide, light brown (dry), glabrous and finely striate abaxially, acute to acuminate. Leaves bipinnate, folding quickly after collection or when under water-stress on living plants; pinnae 14-21 pairs, infrequently few leaves with 11 pairs, 30-60 mm long, lowermost pair sometimes c. 15 mm long; petiole (10-) 15-35 (-40) mm long, indumentum as on rachis (except sparser) or absent; rachis 80-150 mm long, indumentum rather dense (especially on upper surface), the hairs uniformly ±short, antrorsely curved and appressed to sub-appressed. Leaflets 45-55 pairs, narrowly oblong except terminal pair sometimes slightly obovate, (2-) 3-4 mm long, 0.5-0.8 (-1) mm wide, moderately discolorous (mid-green above, pale green below), with dull lustre on both surfaces, straight and flat or sometimes very shallowly curved forward or very shallowly concave, glabrous except appressed ciliate or ciliolate; apex symmetric or asymmetric, broadly acute to obtuse; base unequal with an obvious rounded angle on lower edge, petiolule clearly excentric; main vein starting near upper margin at leaflet base and normally extending ±obliquely to apex, sometimes ±close to and parallel with lower margin, straight to very shallowly curved; lateral veins (on lower surface of leaflets) not visible or very few, obscure and patent, not forming a reticulum. Glands green when fresh: petiole gland-situated near base of petiole (rarely near middle of petiole on a few leaves) 6-15 mm above leaf base and 2-7 (-11) mm above the pulvinus, narrowly elliptic to oblong or narrowly oblong, (2-) 3-5 (-6) mm long (longest glands often adjacent to fertile region), 0.8-1 mm wide, l:w = (2-) 3-5 (-6), sessile, solid, depressed, slightly but discernibly thickened (raised to c. 0.5 mm high), normally flat-topped or shallowly concave on upper surface, occasionally asymmetric being slightly domed at distal end, smooth when fresh but normally very finely but discernibly longitudinally wrinkled when dry, apterous; rachis glands-situated at base of uppermost 1-3 pairs of pinnae, morphologically similar to petiole glands except smaller, oblong-elliptic, 0.8-1 mm long, 0.6-0.8 mm wide, sessile, depressed, a little thickened; rachilla gland-situated at base of uppermost pair of leaflets, minute, sessile. Inflorescences comprising pedunculate heads arranged in terminal open panicles and often a few elongated racemes, young leaves develop at base of secondary branches of panicles and sometimes peduncles during anthesis, occasionally (G.D. Tao 38697) 1-3 peduncles arising from within the axil of the developing leaves on new shoots; peduncles 10-20 mm long, densely hairy (hairs very short, curved, ±appressed and persisting to pod stage), with 1 or 2, short, caducous, scarious bracts on upper half; heads globose or (when in bud) shortly obloid, 10-13 mm diam. at anthesis when fresh, very pale lemon yellow; slightly fragrant, densely 25-30-flowered (flowers not especially densely arranged in the heads); inflorescence buds pale green or yellowish green when fresh, drying yellowish brown. Bracteoles minute (c. 1 mm long), not exserted beyond flowers in buds. Flowers 5-merous, sessile; calyx c. ¾ length of petals, gamosepalous, dissected for c. ¼ its length into triangular lobes, the calyx tube without veins or obscurely 5-veined and glabrous or sparsely hairy; petals 2.5-3 mm long, glabrous; ovary appressed-hairy at apex. Pods oblong to broadly linear, 100-155 mm long, 18-21 mm wide, firmly chartaceous, straight to slightly curved, flat, straight-edged or very slightly constricted between seeds, light brown or light yellowish brown, glabrous, sessile glands absent, without veins or very obscurely transversely veined, marginal vein thickened, apex obtuse to sub-acute and sometimes mucronate, basal stipe short (2-5 mm long). Seeds transverse in pods, oblong to elliptic, 10-13 mm long, 7-8.5 mm wide, flattened, dark brown, ±shiny or with a satin lustre; pleurogram obscure; areole elongate U-shaped, open at hilar end, 4-7 mm long, 2-3 mm wide; funicle sub-thickly filiform, exarillate.
Other specimens examined. CHINA: Yunnan. Honghe Hani & Yi Autonomous Prefecture, Mengzi City, 10 Sep. 1954, K.M. Feng 4479 (IBSC 312977 [barcode 0159290], KUN 0400186 [barcode 0598407] & KUN 0400195 [barcode 0598408]); Yuxi City, Yuanjiang Hani, Yi & Dai Autonomous County, 2 Apr. 1941, T.N. Liou 18212 (IBSC 221473 [barcode 0159277] & IBSC 680585 [barcode 0159288]); Yuxi City, Yuanjiang Hani, Yi & Dai Autonomous County, about 300 m E of Ega hamlet on Yuanmo Road, 23°38'59"N, 101°53'39"E, 4 June 2019, B.R. Maslin & L. Bai BRM 11033 (KUN [barcode 1347979], PE) & 11033A (KUN [barcode 1347982]); Yuxi City, Yuanjiang Hani, Yi & Dai Autonomous County, abandoned Xiaohedi Electricity Station (third level), 23°26'08"N, 101°19'42 "E, 5 June 2019, B.R. Maslin & L. Bai BRM 11034 (GXMG, KUN [barcode 1347983], PE, SING); Honghe Hani & Yi Autonomous Prefecture, Mengzi City, 7 Sep. 1939, C.W. Wang 81633 (KUN 0400181 [0598402] & KUN 0400190 [0598403]; IBSC 680591 [barcode 0159274]); Yuxi City, Yuanjiang Hani, Yi & Dai Autonomous County, 23 Oct. 1965, W.C. Yin 1731 (KUN 0400104 [barcode 0598313], KUN 0400204 [barcode 0598431]); Yuxi City, Yuanjiang Hani, Yi & Dai Autonomous County, 31 May 1984, G.D. Tao 38697 (HITBC 017853 [barcode 0010477]; KUN 0686022 [barcode 0598439] & KUN 0686023 [barcode 0598438]); Yunnan, 1939-1940, Z.S. Chao 1621 (KUN 0400176 [barcode 0598397], KUN 0400184 [barcode 0598399], KUN 0400185 [barcode 0598398]), see note under Distribution below regarding this collection.
Distribution (Fig. 8). Endemic to southern China. A relatively poorly collected species confined to Yunnan, c.150 km south of Kunming. Extant collections show Senegalia clandestina as having a narrow geographic range that extends for about 100 km east-west. Note: The Z.S. Chao 1621 specimen (listed above) was collected from Yunnan but without specific collection details. However, according to the following two references, Chao collected during 1939 and 1940 from western Yunnan (Bao et al., 1998) and central Yunnan (Wang, 2004). It is regrettable that a precise locality cannot be established for this gathering because Senegalia clandestina is currently known from relatively few localities.
Habitat. Grows at the base of sandstone mountains, or on adjacent flats, at alt. 300-500 m. In Yuxi City we observed plants as occurring singularly or in groups of a few individuals.
Phenology. Flowering commences in May, but it is not known when it finishes. Mature seed appear around early November, but sometimes old pods with few seeds persist on plants until the following flowering period.
Taxonomy. Specimens of this hitherto unrecognised species had previously been labelled in Chinese herbaria as Acacia pennata (=Senegalia pennata). However, it has a distinctive petiole gland and a combination of other characters that preclude its inclusion in any known species of Senegalia. It is therefore described here as S. clandestina.
Affinities. The general appearance of Senegalia clandestina is often very similar to that of S. kerrii, particularly when its leaflets are acute. Other characters shared by these species include pale-coloured branchlets, numerous pinnae, small leaflets, scarcely raised petiole glands normally situated near the leaf base and terminal and openly paniculate inflorescences. Although both species are found in Yunnan, current evidence shows them to be allopatric, with S. kerrii occurring in the far south of the Province in Xishuangbanna Dai Autonomous Prefecture, while S. clandestina occurs about 200 km to the northeast in Yuxi City and Honghe Hani & Yi Autonomous Prefecture. Morphologically, S. kerrii can be distin-guished from S. clandestina in the following ways: petiole gland often peripterous, generally wider (1-2 mm) with a lower l:w ratio (mostly 0.7-2), not thickened and brown or reddish on its upper surface (see under S. kerrii for discussion of petiole gland variation in that species); upper branchlets possessing a denser and more obvious indumentum of straight and patent hairs; stipules broader (normally 2-3 mm wide) and lanceolate; fresh inflorescence buds probably at least sometimes red; pods darker coloured (dark brown or dark purplish brown) and wider.
The basic petiole gland morphology of Senegalia clandestina is similar to that of the more easterly distributed S. orientalis and S. guangdongensis. Senegalia orientalis further resembles S. clandestina in having a similar number of pinnae, very narrow leaflets that are often acute and openly paniculate and elongated-racemose inflorescences. Senegalia orientalis, however, is distinguished from S. clandestina in the following ways: branchlets, inflorescence buds and pods dark-coloured when dry; heads 45-60-flowered; pods 25-42 mm wide; and leaflets often slightly but discernibly longer (4-6 mm) and normally possess longish marginal hairs that often extend to the lower surface of lamina at leaflet apices (these hairs are absent from S. clandestina). The relationship between S. clandestina and S. guangdongensis is less close, with the latter species being readily recognised by pinnae fewer (7-10 pairs), panicle and raceme axes dark-coloured by resinous hairs and pods broader (25-33 mm wide) with a dense, but often patchy, layer of dark-coloured, sessile glands.
Senegalia pennata subsp. insuavis is sometimes similar to S. clandestina, especially in having numerous pinnae, sometimes a ±similar petiole gland and openly paniculate and elongate-racemose inflorescences. However, Senegalia pennata subsp. insuavis can most readily be distinguished in the following ways: living plants emit a distinctive foetid odour when branchlets cut or leaves crushed (odour not present in S. clandestina); leaflets generally longer (normally 4-6 mm), finely acute and commonly some obviously folded lengthwise and/or strongly curved forward when dry; flowers with a short, but discernible pedicel 0.5-1 mm long; petiole often longer (30-65 (-80) mm); and peduncles generally longer (mostly 15-50 mm).
Variant. See note under Senegalia megaladena regarding an atypical specimen from Yunnan that has a petiole gland similar to S. clandestina but which otherwise has characters of S. megaladena.
Etymology. The species name is derived from the Latin clandestinus (secret, hidden) in reference to the long period that this entity has gone unrecognised as a distinct taxon.
Vernacular name. Yuxi Senegalia (following the Chinese common name that is proposed above). The name refers to Yuxi City, Yunnan, from where the type was collected.
4. Senegalia delavayi (Franch.) Maslin, Seigler & Ebinger, Blumea 58: 40 (2013) (Fig. 10).大理金合欢【dà lǐ jīn hé huān】(新拟)
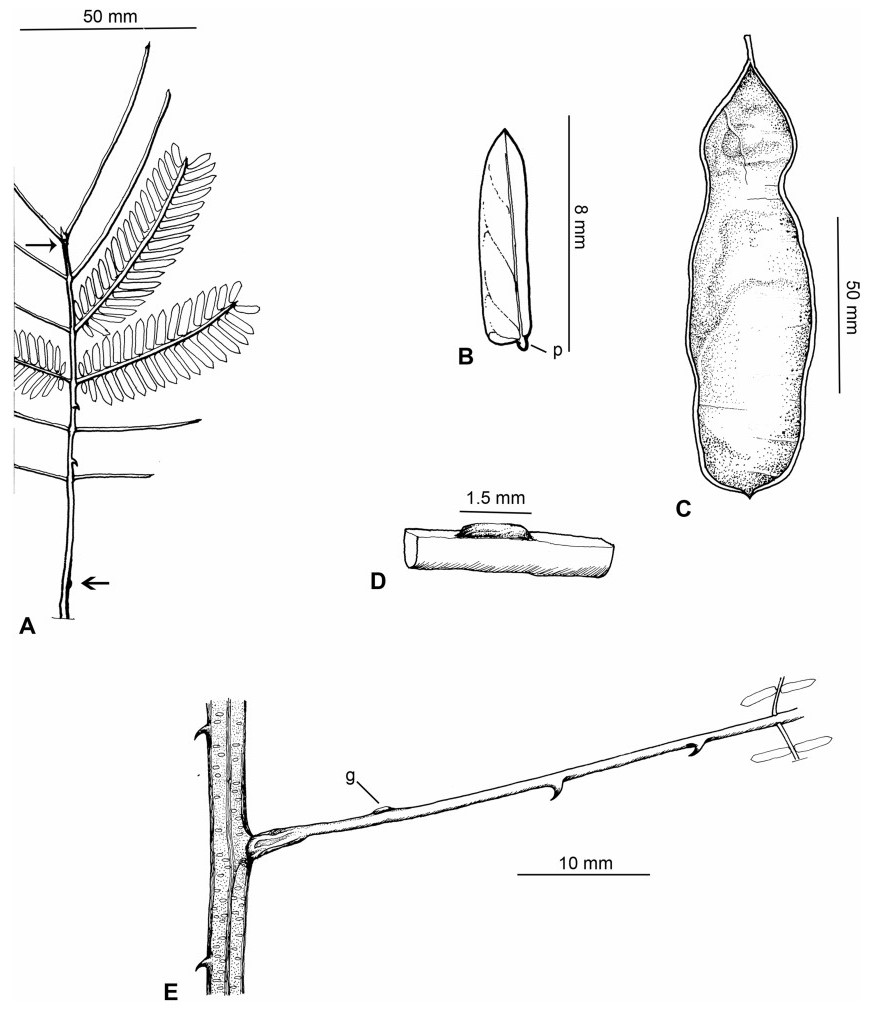
|
| Fig. 10 Senegalia delavayi. A - Leaf showing few pinnae and position of petiole gland (thick arrow) and rachis gland (thin arrow). B - Leaflet (lower surface) showing apex obtuse with a finely acute mucro, and excentric petiolule (p) clearly extended below base of leaflet. C - Pod. D - Petiole gland short and not prominently raised. E - Node showing glabrous branchlet and petiole gland (g) position. Vouchers: All from R.C. Ching 24699. Drawn by Joshua Yang. |
≡ Acacia delavayi Franch., Pl. Delavay. 194 (1890), as 'Delavayi'. Type citation: "Yun-nan, in collibus incultis ad Kiang-yn; fruct. 5 sept.1888 (Delavay)." Type: CHINA, Yunnan Province, 'Coteaux incultes, Kiang yn' [Uncultivated hill, Kiang-yu (sphalm. 'yn' fide Bretschneider (1898b), page 878); this locality is today known as Heqing County, located in Dali Bai Autonomous Prefecture, northwest Yunnan between Dali Lake and Lijiang], 5 Sep. 1888, J.M. Delavay s.n. (lectotype: P barcode P02436157 [digital image!], 2nd step lectotypification here designated, see discussion below under Typification; isolectotypes:?K n.v.-a note on P barcode P02992388 sheet states that a duplicate of that specimen is at herb. K, but we did not locate it there, KUN 0952825 barcode 1206520-specimen ex P!, P barcode P02436158 [digital image!], P barcode P02436159 [digital image!], P barcode 02992388 [digital image!]).
= Acacia delavayi Franch. var. delavayi, Acta Bot. Yunnan. 12: 261 (1990), autonym established by publication of A. delavayi var. kunmingensis C.Chen & H.Sun, Acta Bot. Yunnan. 12: 262 (1990). ≡ Senegalia delavayi (Franch.) Maslin, Seigler & Ebinger var. delavayi, Blumea 58: 40 (2013).
Distinctive features. Shrubs. Branchlets glabrous. Pinnae relatively few, 4-8 (-11) pairs. Petiole and rachis glabrous (or rachis sometimes sub-glabrous on upper surface). Leaflets (4-) 5-10 mm long, 1-2 mm wide, strongly discolorous; apex obtuse with a finely acute, centric apiculum; petiolule extended beyond base of leaflet; lateral veins (on lower surface of leaflets) not visible or few, obscure and not forming a reticulum. Petiole gland relatively short (0.8-1.5 (-2) mm long), not prominent (only slightly raised). Inflorescences arranged in racemes or 2-4 within leaf axils; peduncles long (18-40 mm), glabrous or with sparse, shortish, strongly curved and ±appressed hairs.
Description. Shrubs. Branchlets glabrous; lenticels not visible on upper branchlets. Prickles few or rather numerous on internodes with some also on under surface of leaf axes, sometimes absent from herbarium specimens, 1-4 mm long, straight or shallowly recurved, stout and quite vicious-looking when long. Stipules (few seen) caducous or sub-persistent, narrowly oblong, 4-5 mm long, 1 mm wide, light brown, glabrous or sub-glabrous and obscurely striate abaxially. Leaves bipinnate; pinnae 4-8 (-11) pairs, 25-55 mm long; petiole 15-40 mm long, glabrous; rachis (35-) 40-100 mm long, glabrous or with very few, short, curved, subappressed hairs on upper surface. Leaflets 16-27 pairs, to 12 pairs on lowermost pinnae, narrowly oblong, (4-) 5-10 mm long, 1-2 mm wide, strongly discolorous (darkest above), glabrous except sometimes appressed-ciliolate; apex symmetric or slightly asymmetric, obtuse-apiculate, the apiculum centric, short but distinct and finely acute; base unequal with a prominent rounded angle on lower edge, petiolule excentric, shortly but discernibly extended beyond base of leaflets; main vein starting near upper margin at leaflet base and extending obliquely to mucro, straight; lateral veins (on lower surface of leaflets) not visible or few, obscure, ±patent and sometimes bifurcating near margin, not forming a reticulum. Glands: petiole gland-situated on lower 
Other specimens examined. CHINA: Yunnan. Dali Bai Autonomous Prefecture, Eryuan County, 17 Sep. 1940, R.C. Ching 24699 (KUN 0159906 [barcode 0598122] & KUN 0159915 [barcode 0598121]; Dali Bai Autonomous Prefecture, Heqing County, 27 Apr. 1939, K.M. Feng 844 (KUN 0159907 [barcode 0598123] & KUN 0159908 [barcode 0598124]); without specific locality or date, J.M. Delavay s.n. (P barcode 02436160, digital image).
Distribution (Fig. 11). Endemic to southern China (northwest Yunnan). Senegalia delavayi is known from only a few collections in the general vicinity of the type locality, namely, the Jinsha River Valley area of Dali Bai Autonomous Prefecture. This species was erroneously recorded for Hainan by Merrill and Chun (1940), based on the specimen H.Y. Liang 62064, which is Senegalia hainanensis.

|
| Fig. 11 Distribution of Senegalia delavayi, based on specimen records at KUN and P, as cited in text. This species was erroneously recorded for Hainan by Merrill and Chun (1940), see text for notes. |
Habitat. Occurs in mountainous areas at 1700-2200 m alt (Wu and Nielsen, 2010), principally near watercourses on the sunny sides of valleys or hill slopes (Sun and Chen, 1990), in dry scrub or forests.
Phenology. Judging from the few herbarium specimens seen, Senegalia delavayi flowers in April and May, but Sun (2006) records flowering as extending to July. It produces pods with mature seeds in September (and possibly also October).
Typification. In the protologue of Acacia delavayi only a single, unnumbered Delavay collection (dated 5 September 1888 and collected from Kiang-yn) was cited by Franchet (1890), and no herbarium of lodgement was indicated (but it is noted that Franchet worked at herb. P at that time). The original description included both flowers and fruits. Sun and Chen (1990) cited Delavay s.n. at herb. P as the holotype of A. delavayi. However, at P there are five sheets of Delavay s.n., four supporting fruiting specimens and labelled as having been collected from "Kiang-yn" on 5 Sep. 1888. The fifth sheet supports a flowering specimen and is labelled as having been collected from Yunnan, but without a precise locality and is undated. While the flowering specimen is almost certainly original material (and the source of the flower description in the protologue) it is not regarded as a syntype because it does not bear the Delavay collection details that were given in the protologue. This specimen must have been collected at a different time from the fruiting ones because the flowering and fruiting periods for Senegalia delavayi do not overlap (see Phenology above). The four fruiting specimens are syntypes but there is no evidence that only one was used to prepare the original description. Therefore, in conformity with ICN Art. 9.10 (Turland et al., 2018) and as discussed by McNeill (2014), the Sun and Chen (1990) holotype citation is corrected to that of lectotype. Furthermore, a second step lectotypification is undertaken here to precisely fix the application of this name (cf. ICN Art. 9.17). Accordingly, P [barcode P02436157] has been chosen as the lectotype because it is a well-preserved specimen and is accompanied by a Delavay collection label that gives the collection details that appear in the protologue. In 1978 this specimen was labelled as holotype of A. delavayi by the late Ivan Nielsen (who worked extensively on Asian species of Acacia), but Nielsen never published that typification.
Taxonomy. Sun and Chen (1990) and Wu and Nielsen (2010) treated Acacia delavayi as comprising two varieties, var. delavayi and var. kunmingensis, but these taxa are treated here as separate species, namely, Senegalia delavayi and S. kunmingensis respectively. The character used to distinguish the varieties was the shape of the leaflet base, which was described as obtuse in var. delavayi and auriculate in var. kunmingensis (see S. kunmingensis for discussion of these two terms), but as discussed below there are additional characters that distinguish them.
Acacia cavaleriei, which was cited by Wu and Nielsen (2010) as a synonym of var. delavayi, is regarded here as a nomen nudum under Senegalia kunmingensis.
Affinities. Senegalia delavayi and the more widely distributed S. kunmingensis share the following characters that indicate their relationship: pinnae relatively few (normally 4-9 pairs), leaflets normally not reticulately-veined; inflorescences commonly axillary (i.e. peduncles arising from within axil of leaves); and branchlets often glabrous. Additional to the subtly different shape of the leaflet base (discussed under S. kunmingensis), S. kunmingensis can most readily be distinguished from S. delavayi in the following ways: leaflets clearly sessile (i.e. the petiolule does not extend below base of leaflet); petiole gland longer (2-6 mm) and often asymmetric in being slightly to obviously raised at its distal end (not raised in S. delavayi); rachis moderately to densely hairy on its upper surface; and pods generally wider (27-41 mm). In the field S. kunmingensis is often seen as a robust liana with whitish heads, whereas S. delavayi is a shrub with pale-yellow heads.
Senegalia delavayi is seemingly also related to S. teniana (see that species for differences), but the relationship between these two Chinese endemics requires further study. Both species have restricted distributions and occur in close proximity to one another in northern and north-western Yunnan (see Figs. 11 and 51 respectively).
Etymology. This species is named for Pere Jean-Marie Delavay (1834-1895), a French missionary, explorer and botanist who collected in northwest Yunnan and elsewhere in China between 1867 and 1895; his specimens are in herb. P (Bretschneider, 1898b).
Vernacular name. Dali Senegalia (following the Chinese common name that is proposed above). The name refers to Dali Bai Autonomous Prefecture in Yunnan, from where the type was collected and where the species is seemingly endemic.
5. Senegalia garrettii (I.C.Nielsen) Maslin, B.C.Ho, H.Sun & L.Bai, comb. & stat. nov. (Figs. 12 and 14).盘腺金合欢【pán xiàn jīn hé huān】
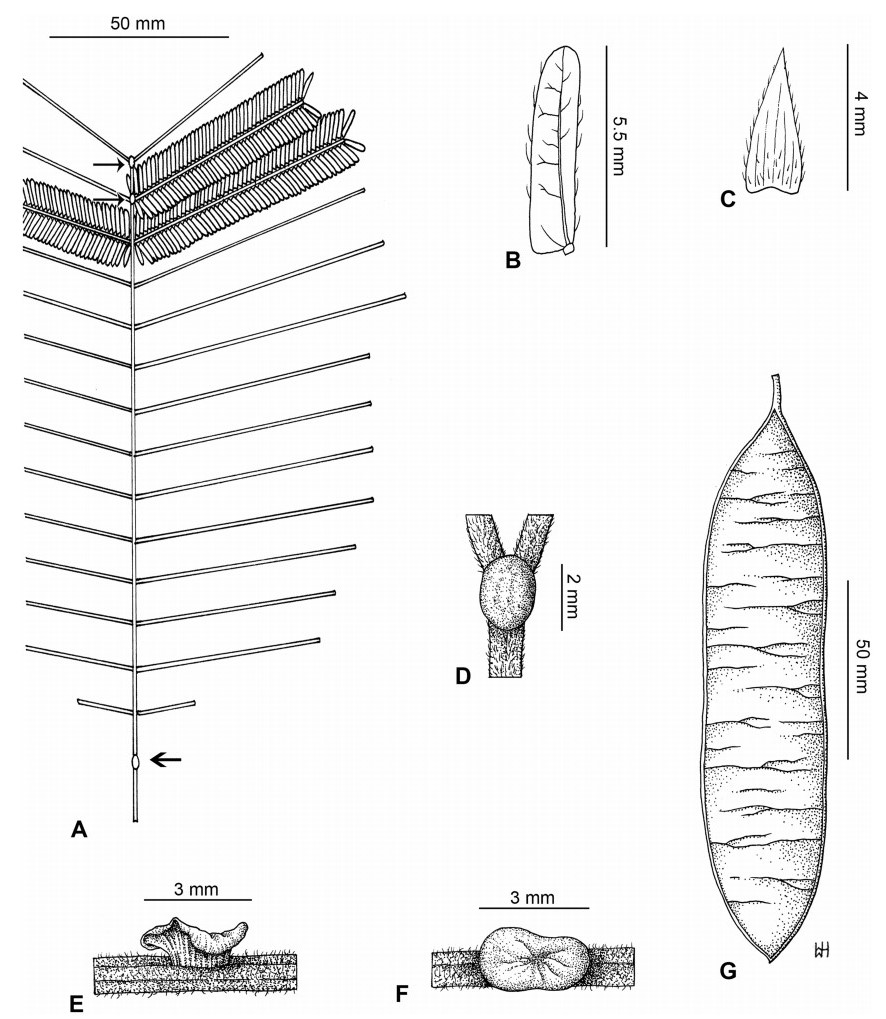
|
| Fig. 12 Senegalia garrettii. A - Leaf showing petiole gland (thick arrow) situated near middle of petiole and position of rachis glands (thin arrows). B - Leaflet (lower surface) showing symmetrically rounded apex and mostly patent lateral veins. C - Stipule. D - Rachis gland quite large. E - Petiole gland (lateral view) peripterous showing splayed upper surface curving downwards. F - Petiole gland (plane view) peripterous showing splayed upper surface extended beyond width of petiole. G - Pod. Vouchers: H.T. Tsai 56336 (A & G); H.T. Tsai 53403 (B, C & D); B.R. Maslin & L. Bai BRM 11022 (E); Q. Li 3-0466 (F). Drawn by Waiwai Hove. |
|
| Fig. 13 Distribution of Senegalia garrettii in China, based on specimen records at IBK, IBSC and KUN, as cited in text. |

|
Fig. 14 Senegalia garrettii. A - Inflorescences in terminal raceme showing some peduncle clusters subtended by developing leaves, flower heads white and most flower buds dull red. B - Young inflorescence showing flower buds dull red. C - Pinna (portion) showing obtuse leaflets. D - Rachis (portion) showing dense, straight, ±patent yellowish hairs, and prickle on lower surface. E - Peripterous petiole gland (lateral view). F - Peripterous petiole gland (oblique view). G - Petiole showing gland on lower half. H - Petiole showing gland on upper half (further removed from leaf base than normal). I - Inflorescence bud showing calyx red on upper  |
≡ Acacia megaladena var. garrettii I.C.Nielsen, Adansonia sér. 2, 19: 351 (1980). ≡ Senegalia megaladena var. garrettii (I.C.Nielsen) Maslin, Seigler & Ebinger, Blumea 58: 41 (2013). Type citation: "Garrett 1239, Thailand, Chiang Mai, Doi Chawm Hot, ca. 1420 m (holo-K; iso-, ABD, E)". Type: THAILAND, Doi Chawm Hot [=Doi Chom Hot, Phrao District, Chiang Mai Province], 13 June 1941, H.B.G. Garrett 1239 (holotype: K barcode K000791220 [digital image!]; isotypes: ABD n.v.-cited by Nielsen (1980), E n.v.-cited by Nielsen (1980), P barcode P02436184 [digital image!], P barcode P02436185 [digital image!]).
Distinctive features. Upper branchlets normally with dense indumentum of short, straight, patent hairs. Pinnae 10-15 (-18) pairs. Rachis sometimes orange-brown coloured, normally with dense indumentum of ±straight, patent hairs. Leaflets 4-6 (-7) mm long and mostly 0.8-1.3 mm wide; apex ±symmetrically rounded to obtuse and without a mucro; lateral veins (on lower surface of leaflets) ±patent, relatively few but normally reasonably evident (slightly raised) when dry. Petiole gland position variable but often near or above middle of petiole, l:w = 0.7-2, depressed, peripterous by a rather thin, spreading lamina that is variously curved upon drying, sometimes saddle-shaped; rachis glands 1-2 mm long, 1-2 mm wide. Panicle and raceme axes and flowering peduncles with a dense indumentum of short, patent, straight, often yellowish to pale orange-brown hairs (normally white when in pod); inflorescence buds at least sometimes dull red when fresh. Calyx glabrous or sparsely appressed-hairy. Pods clearly stipitate.
Description. Normally lianas, sometimes lianescent or straggly shrubs or trees. Branchlets normally densely hairy towards apices (hairs short, patent, straight and white or yellow-brown), mature branchlets glabrous or with indumentum persisting in longitudinal bands. Prickles scattered on internodes and infrequently on under surface of leaf axes, occasionally absent from herbarium specimens, not prominent (0.5-2 mm long), straight to shallowly recurved, patent to slightly reflexed. Stipules caducous (present only at ends of racemes where inflorescences first initiated), narrowly triangular to narrowly lanceolate, (3-) 4-7 mm long, 1-2 mm wide, pale-to mid-brown, glabrous (except ciliolate) or sparsely to densely puberulous and obscurely to ±obviously striate abaxially, base slightly lobed on one side or not lobed, apex acuminate. Leaves bipinnate; pinnae 10-15 (-18) pairs, 7-14 mm apart, (28-) 35-90 (-100) mm long; petiole (14-) 20-50 mm long, indumentum as on rachis; rachis 75-170 (-250) mm long, sometimes orange-brown coloured, densely hirsute to short-pilose by patent and ±straight hairs (but see discussion below under Variants). Leaflets (25-) 30-70 pairs, narrowly oblong, 4-6 (-7) mm long and (0.7-) 0.8-1.3 (-1.5) mm wide (sometimes the lowermost pair smaller), occasionally larger in Thailand (8-10 mm long and 2 mm wide on J.F. Maxwell 05-482, but this is presumably a cultivated specimen), flat and straight or sometimes very shallowly concave or shallowly curved forward, concolorous or discolorous (dark green above and paler green below), dull lustre on both surfaces, glabrous except normally appressed ciliate or ciliolate; apex symmetric or slightly asymmetric, rounded to obtuse, without an apiculum; base unequal with an obvious angle on lower edge, the petiolule excentric and short but clearly extended below base of leaflet; main vein starting near upper margin at leaflet base and extending obliquely to apex, straight or shallowly curved; lateral veins (on lower surface of leaflets) ±patent and normally reasonably evident (slightly raised when dry), relatively few, not forming a reticulum. Glands: petiole gland-variable (see discussion below under Petiole gland), situated near, above or below middle of petiole 7-20 (-36) mm above leaf base and 4-12 (-30) mm above pulvinus, oblong to oblongelliptic or sometimes circular, 2-4 mm long, 1.5-2.5 mm wide, l:w = 0.7-2, superficially sessile (but fractionally elevated from beneath) or sometimes sub-stipitate by broad stipe, depressed, peripterous (i.e. with a rather thin, spreading lamina that forms a rim which is not closely appressed to the petiole), ±flat-topped or the rim slightly upturned at proximal and/or distal end of gland and downturned along its sides (forming a ±saddle-shape), rarely entire rim upturned to form a concavity on upper surface, normally dark brown; rachis glands-situated at base of uppermost 1-3 pairs of pinnae, oblong to elliptic or circular, 1-2 mm long, 1-1.5 (-2) mm wide, appearing sessile but fractionally raised from beneath so that rim of gland is not closely appressed to the rachis, flat-topped or shallowly concave above, dark-coloured; rachilla gland-situated at base of uppermost pair of leaflets, sometimes absent, oblong, smaller than rachis glands, sessile, flat-topped. Inflorescences comprising pedunculate heads arranged in elongated, terminal racemes or panicles, sometimes juvenile or mature leaves present at base of peduncles during anthesis rendering inflorescences axillary, indumentum on raceme and panicle axes similar to peduncles; peduncles 10-30 mm long, single or clustered in groups of two or three, densely hirsute to short-pilose with short, patent, straight hairs that are often yellowish to pale orange-brown when in flower (normally ±white in pod), glandular hairs absent; heads globose, 7-10 (-13) mm diam. at anthesis when dry, white but becoming pale yellow as anthers wither, slightly fragrant, ±sub-densely 30-40-flowered; inflorescence buds at least partially dark brown or dark purplish brown when dry (commonly dull red when fresh; see notes below under Inflorescence bud colour). Flowers 5-merous, sessile or subsessile; calyx 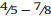
Selected specimens examined. CHINA: Guangxi. Baise City, Longlin Multinational Autonomous County, 19 May 1977, Q. Li 3-0466 (GXMI 21113 [barcode 015771]); Baise City, Longlin Multinational Autonomous County, 4 Oct. 1956, E.Y. Zhang & Y.K. Li P00678 (IBK 61819 [barcode 00067601], IBSC 224512 [barcode 0159299]). Guizhou. Qianxinan Buyi & Miao Autonomous Prefecture, Xingyi City, 12 July 1960, Guizhou Expedition 6120 (IBSC 680587 [barcode 0159153]). Yunnan. Dali Bai Autonomous Prefecture, Yangbi Yi Autonomous County, 7/8 Nov. 1946, T.N. Liou 22667 [IBSC 221875 [barcode 0159155], IBSC 391357 [barcode 0159151], KUN 0400139 [barcode 598352]); Dehong Dai & Jingpo Autonomous Prefecture, Lianghe County, 12 Dec. 1978, 780-Expedition 1013 (KUN 674615 [barcode 0598098]); Honghe Hani & Yi Autonomous Prefecture, Lühun County, 7 May 1974, Lühun Expedition 452 (KUN 0400157 [barcode 0598373]); Honghe Hani & Yi Autonomous Prefecture, Luxi County, 21 Feb. 1933, H.T. Tsai 56336 (KUN 0400133 [barcode 598348], KUN 0400134 [barcode 0598347]); Honghe Hani & Yi Autonomous Prefecture, Luxi County, 21 May 1933, H.T. Tsai 53403 (IBSC 105679 [barcode 0159287], KUN 0400135 [barcode 0598346]); Honghe Hani & Yi Autonomous Prefecture, Shiping County, 21 Feb. 1933, H.T. Tsai 56336 (IBSC 105080 [barcode 0159136]); Lincang City, Cangyuan Wa Autonomous County, 4 Sep. 1980, S.T. Li 1017 (KUN 0400164 [barcode 598376], KUN 0400165 [barcode 0598375]); Lincang City, Fengqing County, Feb. 1936, C.W. Wang 72091 (IBSC, 187544 [barcode 0159150], KUN 0400132 [barcode 598349]); Lincang City, Gengma Dai & Wa Autonomous County, 14 Jan. 2013, T. Zhang et al. 13CS5971 (KUN barcode 1370908); Lincang City, Linxiang District, 1 Sep. 1957, G.S. Sin 609 (KUN 0400150 [barcode 0598360], KUN 0400141 [barcode 598359]); Pu'er City, Jingdong Yi Autonomous County, 1934, Y. Tsiang 12265 (IBSC 73289 [barcode 0159157], KUN 0400127 [barcode 598344], also Kd see Nielsen (1980)); Pu'er City, Simao District, Caiyanghe Nature Reserve, 12 Jan. 2019, K.W. Jiang et al. 19 (CSH) [barcode CSH0160943 & CSH0160944). Wenshan Zhuang & Miao Autonomous Prefecture, Malipo County, 3 Oct. 1955, P.I. Mao 6457 (KUN 0400142 [barcode 598358], KUN 0400143 [barcode 598357]); Wenshan Zhuang & Miao Autonomous Prefecture, Xichou County, 24 Oct. 1947, K.M. Feng 12599 (KUN 0400138 [barcode 598353], KUN 0400124 [barcode 598332]); Xishuangbanna Dai Autonomous Prefecture, Jinghong City, 15 Oct. 1978, G.D. Tao et al. 19694 (IBSC 736088 [barcode 0738642], KUN 0400219 [barcode 0598423]); Xishuangbanna Dai Autonomous Prefecture, Menghai County, May 1936, C.W. Wang 73838 (IBSC, 187548 [barcode 0159152], IBSC 680588 [barcode 0159148], KUN 0400131 [barcode 0598350]); Yuxi City, Eshan Yi Autonomous County, 15 Oct. 1989, H. Sun 1663 (KUN 0400162 [barcode 0598378]); without specific locality, 1933, Y. Tsiang 12778 (HIB 25323 [barcode 0038698], IBSC 374980 [barcode 0158811]); Yuxi City, Xinping Yi & Dai Autonomous County, 23°56'31.1"N, 101°56'03.3"E, 19 May 2018, B.R. Maslin & L. Bai BRM11022 (GXMG, GXMI, IBSC, KUN barcode 1345215 & 1345216, PE); Yuxi City, Yuanjiang Hani, Yi & Dai Autonomous County, 7 Nov. 1958, S.K. Wu 726 (KUN 0400146 [barcode 598364], KUN 0400147 [barcode 0598363], KUN 0400148 [barcode 598362]). INDIA: Sikkim. Pawlabaree, June 1877, G. King 4852 (CAL 140643). MYANMAR: Shan hills, 1892, Abdul Huk 1056 (CAL 140702). THAILAND: Northern. Payap [= former name for Chiang Mai], 6 Dec. 1965, E. Hennipman 3241 (BKF 39705 barcode SN036638, also C & K but n.v.- see Nielsen (1980)); Chiang Mai, 26 May 2001, J.F. Maxwell 01-288 (BKF 139619 barcode SN143728); Doi Tung, Chiang Rai, 28 Apr. 2005, J.F. Maxwell 05-303 (BKF 161321 [SN172337]). South-western. Mahidol University, Kanchanaburi Campus, Doi Bin, c. 5 km N of Sai Yok, Kanchanaburi Province, Sai Yok District, 12 Sep. 2005, J.F. Maxwell 05-482 (BKF 159232 [barcode SN 169728]), specimen presumably cultivated. Peninsular. Ton Nga Chang Reserve, Songhala Province, 18 Apr. 1985, J .F. Maxwell 85-411 (BKF 090209 [SN 036644]).
Distribution (Fig. 13). Occurs in southwest China, Thailand, Myanmar and northeast India (Mizoram, Sikkim and perhaps West Bengal States, fide Deshpande et al. (2019). In China Senegalia garrettii is widespread in Yunnan (mostly south of the latitude of Kunming), extending to far western Guangxi (Longlin County) and far south western Guizhou (Xingyi City). Judging from this Chinese distribution, it is likely that the species will eventually be found also in Vietnam and Laos.
Habitat. In China, Senegalia garrettii grows in thickets and open forests in mountain regions at about 750-2200 m alt.; it is recorded from both dry and wet habitats.
Phenology. Flowers from May to July, and pods with mature seeds have been collected in January and February.
Taxonomy. Nielsen(1980, 1985a) and Wu and Nielsen (2010) treated this taxon as Acacia megaladena var. garrettii, assigning it to that species because the leaflets are obtuse, and the petiole gland is commonly located near the middle of the petiole. It was distinguished from the other two varieties of A. megaladena by "Calyx velutinous; corolla 4.2 mm long" which is curious because all specimens known to us (including the holotype of var. garrettii at Kew that was examined on our behalf by Yee Wen Low) show the calyx tube as glabrous or sub-glabrous, and the petals c. 3 mm long.
Following examination of many specimens in Chinese, Southeast Asian and Indian herbaria, this taxon should be appropriately treated as a distinct species, Senegalia garrettii. Its distinctive, peripterous, flattened petiole gland mandates its removal from S. megaladena, but as noted under Variants below there are a few specimens that may possibly be hybrids between the two species.
Affinities. Judging especially from its peripterous petiole gland, Senegalia garrettii is most closely related to S. kerrii. Additional characters shared by these two species include the following: upper branchlets densely hairy (hairs normally short, straight and patent, but sometimes otherwise on specimens of S. kerrii from outside of China); stipules rather large; inflorescences elongate-racemose or openly paniculate with the axes and peduncles having a dense indumentum similar to that of the branchlets; calyces glabrous or sparsely hairy; pods clearly stipitate. Senegalia kerrii can be most readily distinguished from S. garrettii in the following ways: leaflets finely to broadly acute and generally smaller (mostly 3-4 mm long, 0.4-0.8 mm wide); and pinnae more numerous (17-27 pairs) and more closely-spaced (5-8 mm apart). Also, in S. kerrii the petiole gland is always situated on the lower half of the petiole (often close to the pulvinus), never near or above its middle as often occurs in S. garrettii and its stipules are more persistent in young inflorescences. While it might be argued that S. garrettii and S. kerrii could be treated as infraspecific taxa of a single species, their morphological differences warrant recognition as separate species. Globally, S. kerrii has a much wider geographic range than that of S. garrettii, but within China the situation is reversed (compare Fig. 13, S. garrettii and Fig. 20, S. kerrii).
As already implied, Senegalia garrettii is not far removed taxo-nomically from S. megaladena. Both species have obtuse leaflets, a petiole gland that is commonly situated near the middle of the petiole and similar inflorescences. However, in addition to having a very different petiole gland (see Petiole gland below), S. megaladena can be distinguished from S. garrettii in the following ways: indumentum on upper branchlets and raceme/panicle axes much less conspicuous (the hairs commonly appressed and never yellow-brown); rachis hairs ±appressed and shallowly curved; lateral veins on lower surface of leaflets (when present) ascending.
Senegalia garrettii may also superficially resemble S. obliqua, but the two species appear not to be especially closely related (see S. obliqua for discussion).
Variants. The following collections, which seem to represent intermediates between Senegalia garrettii and S. megaladena, are not included in the above description. Further material is needed so that their taxonomic status can be properly assessed.
The specimen S.P. Ko 55808 (IBSC 88736 [barcode 0159298]), labelled Acacia pennata and collected from Guangxi (without specific locality) has atypically short (2-4 mm long) leaflets that are clearly curved forward and which lack lateral veins. These leaflets are very similar to those of Senegalia megaladena var. indochinensis (which is not recorded for Guangxi) but the specimen has a peripterous petiole gland as in S. garrettii (which occurs in far western Guangxi).
The leaf rachis of Senegalia garrettii normally possesses distinctively patent and ±straight hairs. However, scattered throughout the range of the species in China and elsewhere are a few unusual specimens with rachis hairs curved forward and ±appressed as in S. megaladena (this same range of variation is also found in S. kerrii). It is possible that these unusual specimens represent intermediates between S. garrettii and S. megaladena var. megaladena (see under Senegalia megaladena for other possible intermediates between these two species). Examples of S. garrettii specimens possessing curved, ±appressed rachis hairs include C.W. Wang 86117 from Malipo County, Yunnan, China (KUN 0400140 [barcode 0598351]) and J.F. Maxwell 94-594 from Lampoon Province, Thailand (BKF 172977).
Petiole gland. Senegalia garrettii has a distinctive petiole gland that is flattened and narrowly peripterous. The term peripterous means that the lamina of the gland forms a rather thin and relatively narrow marginal rim ('wing') that is not appressed to the petiole (because the gland is fractionally raised from its base) and which normally extends beyond the edges of the petiole. Often the gland is ±flat-topped, but upon drying at least the rim commonly curves in various ways as described above to produce somewhat different-looking glands (Fig. 14E and F show peripterous glands when fresh, and Fig. 12E shows one manifestation of this gland type when dry). By way of contrast, S. megaladena has a petiole gland that is thickened and clearly raised above the petiole: this gland is apterous (i.e. it does not possess a splayed rim) and the entire base of the gland is appressed to the petiole (see Fig. 29I and J, gland when fresh, and Fig. 27J, gland when dry). Peripterous glands are discussed in more detail under Morphological characters: Leaf glands above.
The position of the petiole gland in Senegalia garrettii is variable, being located below, near or above the middle of the petiole; this variation can be seen even on a single specimen. At herb. KUN (where there is a good representation of the species), about half the specimens show all leaves as having all glands located near or above the middle of the petiole. The remaining specimens show either all glands situated below the middle of the petiole or more commonly, show a mixture with glands located both below and near or above the middle of the petiole. A similar range of variation in gland position occurs in S. megaladena.
Inflorescence bud colour. In the one flowering population of Senegalia garrettii in China that we examined (represented by B.R. Maslin & L. Bai BRM11022), the predominant colour of most inflorescence buds was dull red (Fig. 14A, B & I). This colouring in living plants can normally be inferred from a dark-colouring of perianth parts in herbarium material (see discussion under Morphological characters: Inflorescences and flowers above above).
Etymology. The species name commemorates Henry Burton Guest Garrett (c. 1871-1959), who served as a forest officer under the Royal Thai Government and collected in northern Thailand between 1899 and 1959. His collection consisted of 1500 numbers, mainly kept at BM, K and BKF, with a few duplicates in other European herbaria (web ref. 8).
Vernacular name. Disk-gland Senegalia. This is a translation of the Chinese common name (see above) that had been used previously under A. megaladena var. garrettii by Sun (2006) and Wu and Nielsen (2010).
6. Senegalia guangdongensis Maslin, B.C.Ho, H.Sun & L.Bai, sp. nov. (Fig. 15).广东金合欢【guǎng dōng jīn hé huān】(新拟)
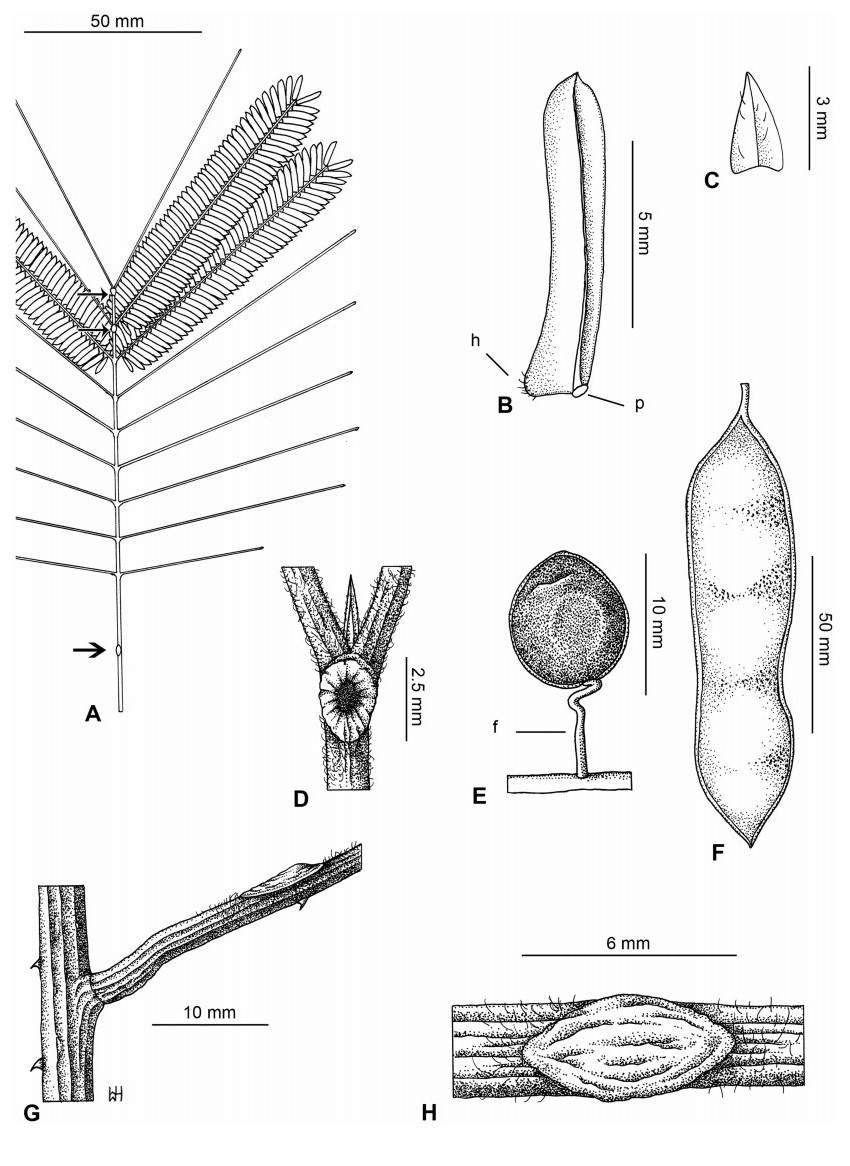
|
Fig. 15 Senegalia guangdongensis. A - Leaf showing relatively few pinnae and position of petiole gland (thick arrow) and rachis glands (thin arrows). B - Leaflet (lower surface) quite long, with rounded/obtuse and apiculate apex, poorly developed petiolule (p) and few hairs (h) on basal angle, also main vein close to and parallel with upper margin in lower  |
Type: CHINA, Guangdong Province, Yangjiang City, Yangxi County, Tangkou Township, Tongyou Village, 300 m alt., 18 June 1956, C. Wang 41872 (holotype: IBSC 233088 barcode 0159203!; isotypes: IBK 51376 barcode IBK0067606!, KUN 0400048 barcode 1206965!, SYS 119071 barcode SYS00079571!).
Distinctive features. Pinnae relatively few (7-10 pairs). Leaflets mostly 6-10 mm long and 1-1.5 mm wide, strongly discolorous, with few, short, often spreading hairs on basal angle otherwise glabrous; apex symmetric, mostly rounded to obtuse and apiculate; base sessile (the clearly excentric, squat petiolule not or scarcely extended below base of leaflet); main vein situated near upper margin in at least lower 
Description. Lianas or lianescent shrubs. Branchlets dark-coloured and microscopically hairy at extremities when young (non-glandular hairs straight and patent or clearly curved and ±appressed, easily overlooked) with sparse to ±dense glandular hairs intermixed, the mature branchlets pale brown, glabrous and without glandular hairs. Prickles scattered on internodes and under surface of leaf axes, sometimes absent from herbarium specimens, not prominent (0.5-1 mm long), straight to shallowly recurved. Stipules caducous, triangular, 2-3 mm long, 1-1.2 mm wide, sparsely puberulous with some glandular hairs intermixed (at least towards base of stipules) and veins obscure or not visible abaxially, apex acute. Leaves bipinnate; pinnae 7-10 pairs, 35-100 mm long; petiole 20-55 mm long; rachis 75-115 mm long, indumentum on upper surface comprising short, antrorsely curved and ±appressed hairs. Leaflets 35-65 pairs, narrowly oblong to almost linear, 6-10 mm long but lowermost few 4-5 mm long, (0.8-) 1-1.5 mm wide, strongly discolorous, shiny to sub-shiny and dark green above when fresh (drying dark greyish), dull and pale green below when fresh (drying brownish or light greyish), very close together (laminas touching or slightly imbricate) and wide-spreading, straight to slightly recurved laterally, flat, glabrous except a few, short, often spreading hairs on basal angle; apex mostly symmetric, rounded to obtuse and apiculate (apiculum minute but distinct); base truncate, unequal with a prominent rounded angle on lower edge, sessile (the clearly excentric, squat petiolule not or scarcely extended below base of leaflet); main vein starting near upper margin at leaflet base, excentric being situated relatively close to the upper margin and parallel with it in at least the lower 


Other specimens examined. CHINA: Guangdong. Maoming City, Xinyi City, 29 Apr. 1932, C. Wang 32260 (IBSC 32019 & [barcode 0159204] & PE [barcode 00321214]), this same collection also given at IBK as: H.Y. Liang 32260 (IBK 10455 [barcode 00067610]); Maoming City, Xinyi City, 6 Nov. 1951, Z.S. Zhu 1243 (IBSC 164694 [barcode 0159206]); Maoming City, Yangchun City, 8 May 2001, H.G. Ye 5428 (IBSC 00677813 [barcode 0159205]); Maoming City, Yangchun City, 11 May 1991, H.G. Ye & N. Liu 1076 (IBSC 604643 [barcode 0159208].
Distribution (Fig. 16). Endemic to southeast China (Guangdong). A poorly collected species known from only a small area c. 200 km due southwest of Guangzhou.
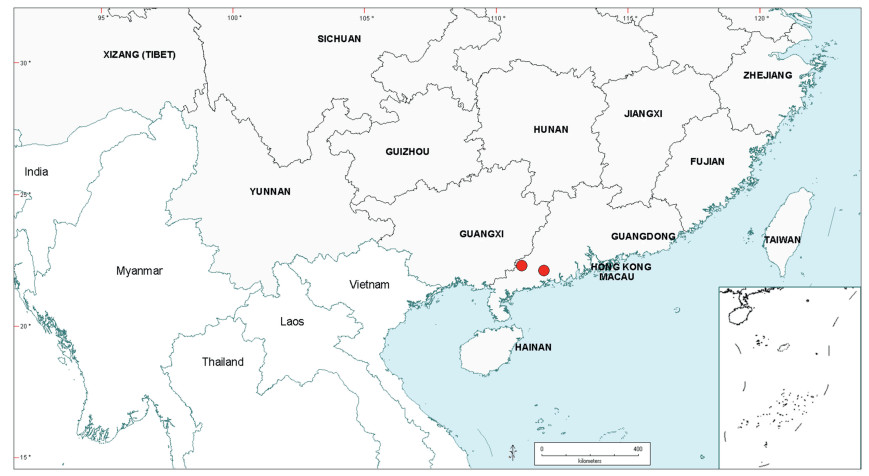
|
| Fig. 16 Distribution of Senegalia guangdongensis, based on specimen records at IBK, IBSC, KUN and SYS, as cited in text. |
Habitat. Grows in hilly areas.
Phenology. As best can be judged from the few extant specimens seen, this species commences flowering around late May or early June, but it is not known when flowering ends. Pods with near-mature seeds have been collected in early December.
Taxonomy. In the past, most herbarium specimens of this distinctive species had been determined as either Acacia pennata (which in China is now represented by only Senegalia pennata subsp. insuavis) or A. sinuata (=S. rugata). This distinctive species was first recognised as new, but not described, several years ago by the third author (H. Sun), based on a specimen of C. Wang 41872 at KUN.
Senegalia guangdongensis belongs to the 'Hainanensis species-group' which also includes S. hainanensis, S. macrocephala and S. stipitata. See discussion under S. hainanensis.
Affinities. Senegalia guangdongensis is seemingly related to both S. orientalis (which has a similar, elongated and depressed petiole gland) and S. hainanensis (which has similar dark-coloured, glandular-hairy inflorescence axes).
Senegalia orientalis further resembles S. guangdongensis by its broad pods and many-flowered heads (with buds dark-coloured when dry) which are mostly arranged in terminal panicles; both species occur in Guangdong but S. orientalis extends well beyond that Province. Senegalia orientalis is most readily distinguished from S. guangdongensis by its pinnae being more numerous (14-20 pairs), leaflets generally shorter (4-6 mm long) and normally with more obvious marginal hairs, inflorescence axes with very few glandular hairs and pods lacking sessile glands (or these glands very few and scattered).
Senegalia hainanensis further resembles S. guangdongensis in the following ways: pinnae number relatively few (8-14 pairs); leaflets strongly discolorous with the main vein situated rather close to the upper margin and ±parallel with it (at least towards base of leaflets); flower buds dark-coloured when dry; and inflorescences mostly arranged in open panicles. Within China, S. hainanensis occurs on Hainan Island, about 300 km south of where S. guangdongensis is found. Senegalia hainanensis is most readily distinguished from S. guangdongensis by its petiole gland which is shorter (mostly 1-3 mm long), normally situated closer to leaf base (mostly 4-6 mm) and slightly but discernibly raised at its distal end (not at all raised in S. guangdongensis); also, in S. hainanensis the rachis glands are more numerous (mostly 4-6) and never obovate, and the pods either lack sessile glands or these glands are very few and scattered.
The petiole gland in Senegalia guangdongensis is somewhat similar to that of the more westerly distributed S. clandestina, but the two species appear not to be especially closely related (see S. clandestina for discussion).
Inflorescence buds. The specimen label on C. Wang 32260 records the flower buds on living plants as being green but marked with purplish red. Discussion of inflorescence bud colour is given under Morphological characters: Inflorescences and flowers above.
Etymology. This species is named for the Chinese Province of Guangdong where it is endemic.
Vernacular name. Guangdong Senegalia (following the Chinese common name that is proposed above).
7. Senegalia hainanensis (Hayata) H.Sun, in X.Y.Zhu, Biodivers. Sci. 23(2): 249 (2015) (Fig. 17).海南金合欢【hǎi nán jīn hé huān】
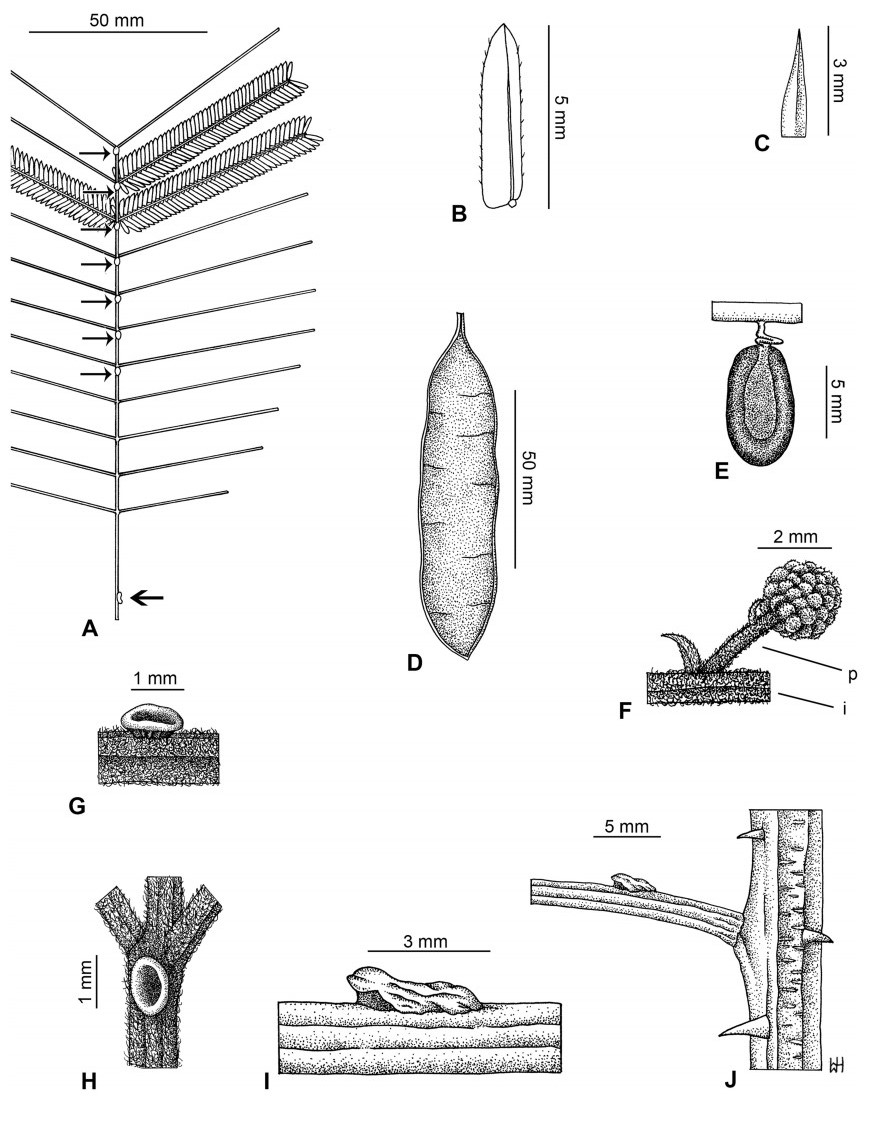
|
| Fig. 17 Senegalia hainanensis. A - Leaf showing position of petiole gland (thick arrow) and the numerous rachis glands (thin arrows). B - Leaflet (lower surface) showing main vein situated towards upper margin near base of leaflet. C - Stipule. D - Pod. E - Seed. F - Inflorescence (young) showing dark-colouring of inflorescence axis (i) and peduncle (p) due to dense layer of glandular hairs. G - Rachis gland (lateral view), sub-stipitate. H - Rachis gland (plane view). I - Petiole gland asymmetric being elevated from base at distal end. J - Node showing glabrous branchlet and petiole gland relatively close to leaf base. Vouchers: F.W. Xing (A, F & H); S.K. Lau 27182 (B); H.Y. Liang 65144 (C, G, I & J); W.T. Tsang 649 (D & E). Drawn by Waiwai Hove. |
≡ Acacia hainanensis Hayata, Icon. Pl. Formosan. 3: 86 (1913). ≡ Acacia pennata subsp. hainanensis (Hayata) I.C.Nielsen, Adansonia sér. 2, 19: 352 (1980). ≡ Senegalia pennata subsp. hainanensis (Hayata) Maslin, Seigler & Ebinger, Blumea 58: 41 (2013). Type citation: "Hainan, leg. Z. KATSUMADA, 1910". Type: CHINA, Hainan, 1910, Z. Katsumada s.n. (lectotype: TI barcode TI00002962 [digital image!], designated by Nielsen (1980), who incorrectly used the term holotype, see discussion under Typification below; isolectotypes (dated Oct. 1910, see discussion below under Phenology): IBSC 151878 barcode 0159249!-fragment probably ex TAIF, TAIF 12598 [digital image!], TAIF 12599 [digital image!]).
[Acacia delavayi auct. non Franch.: E.D. Merrill & W.Y. Chun, Sunyatsenia 5: 74 (1940), as to H.Y. Liang 62064.]
Distinctive features. Pinnae 8-14 pairs. Leaflets normally 5-7 mm long, 0.7-1.3 mm wide and obviously discolorous (darkest above); apex rounded to obtuse or broadly acute; main vein rather close to and ±parallel with upper margin at least in lower part of leaflet; lateral veins (on lower surface of leaflets) not or scarcely visible. Petiole gland situated close to base of petiole 4-6 (-10) mm above leaf base, 1-3 (-4) mm long, sessile, slightly but discernibly raised from base at distal end with the upper surface sloping downwards towards proximal end of gland; rachis glands at base of uppermost 4-6 (-9) pairs of pinnae, 0.7-1.5 long, 0.8-1 mm wide, sessile or sub-sessile by a minute stipe, rim of gland not closely appressed to rachis. Panicle and raceme axes and ±young peduncles densely covered by dark-coloured glandular hairs; inflorescence buds dark-coloured when dry (red when fresh). Pods 15-25 (-28) mm wide, mostly lacking small, sessile, dark-coloured, sessile glands.
Description. Lianas, or infrequently shrubs or trees 3-10 m tall. Branchlets dark grey or sometimes dark brown, glabrous or (at extremities) minutely and obscurely non-glandular hairy but sometimes glandular hairs intermixed. Prickles few to relatively numerous on internodes and (? sometimes) on under surface of leaf axes, not prominent, 0.5-2 (-3) mm long, patent and straight or slightly recurved. Stipules caducous, narrowly triangular, sometimes narrowly oblong-triangular, (1-) 2-4 mm long, 0.7-0.8 mm wide, glabrous or sparsely hairy and not or obscurely striate abaxially, acute to acuminate. Leaves bipinnate; pinnae 8-14 pairs, 35-60 (-80) mm long, sometimes slightly shorter on lowermost pair; petiole 20-40 mm long; rachis (40-) 60-120 mm long, often with a dense indumentum of short, curved and appressed to subappressed (occasionally spreading) hairs. Leaflets (20-) 30-50 (-60) pairs, narrowly oblong to ±linear, (4-) 5-7 (-8) mm long, 0.7-1.3 mm wide, straight or slightly curved forward, close together, obviously discolorous (dark-coloured and ±shiny above, paler-coloured and dull below), glabrous except margins often appressed-ciliolate; apex symmetric or asymmetric, rounded to obtuse or broadly acute, with or without a distinct, blunt apiculum; base truncate, unequal with an obvious angle on lower edge, the clearly excentric petiolule very short; main vein starting near upper margin at leaflet base, excentric being situated relatively close to the upper margin and parallel with it at least in lower 

Selected specimens examined. CHINA: Hainan. Baoting Li & Miao Autonomous County, 26 May 1935, K.Z. Hou 72597 (IBK 52735 [barcode 00067619], IBSC 87814 [barcode 0159251]); Changjiang Li Autonomous County, 11 May 1984, G.Y. Fu 3914 (IBSC 534828 [barcode 0159260]); Chengmai County, 11 June 1928, W.T. Tsang 649 (IBSC 65483 [barcode 0159217]); Danzhou City, Apr. 1928, W.T. Tsang 55 (IBSC 65095 [barcode 0159216]); Dongfang City, 22 Feb. 1934, H.Y. Liang 65144 (IBK 12010 [barcode 00067628], IBSC 67283 [barcode 0159255]); Ledong Li Autonomous County, 8 June 1936, S.K. Lau 27182 (IBSC 104971 [barcode 0159215]), KUN 0400061 [barcode 0598276]; Lingshui Li Autonomous County, 3-20 May 1932, H. Fung 20185 (CAL 140960, SING [barcode 0260863], SYS 21326 [barcode 00079577]); Qiongzhong Li & Miao Autonomous County, 17 May 1922, F.A. McClure 9659 (VNM 00022807); Sanya City, 11 July 1933, H.Y. Liang 62064 (IBK 30619 [barcode 00067614], IBSC 66118 [barcode 0159253]); Wanning City, 7 Mar. 1995, F.W. Xing (IBSC 633992 [barcode 0159263]); Wuzhishan City, 24 Apr. 1988, Z.X. Li & F.W. Xing 3654 (IBSC 624815 [barcode 0159270]). VIETNAM: Central. Hue, cultivated, 13 Mar. 1978, Tran Minh Quynh 34 (HN 000042595); Quang Binh Province, 24 Aug. 1981, T.K. Lien 406 (HN); in vicinities of Tra Ve forest protection station, Huong Nguyen Municipality, A Loui district, Thua ThieneHue Province, 4 May 2005, L. Averyanov, P.K. Loc, T.V. Thao & N.T. Vinh 7872 (HN); Dong Hoi [town], Quang Binh Province, 18 Feb. 1979, Thai Thuan 298 (HN 0000042596, 0000042597 and 0000042588).
Distribution (Fig. 18). Senegalia hainanensis has a rather restricted distribution in southeast China (Hainan, where it is widespread) and central Vietnam. In Vietnam it occurs in Quang Binh Province and Thua ThieneHue Province which are located on the northeast coast, opposite Hainan Island. As discussed below under Taxonomy Nielsen(1980, 1981) and Wu and Nielsen (2010) broadly circumscribed this species (as A. pennata subsp. hainanensis) and consequently recorded it as having a wide distribution in China (Fujian, Guangdong, Guangxi, Hainan and Yunnan Provinces) and Vietnam (northern and southern), extending westward to Myanmar and northeast India. This distribution is largely a result of those authors having included within their circumscription of subsp. hainanensis the species described here as Senegalia macrocephala, S. orientalis and S. stipitata. However, this does not explain the listing of subsp. hainanensis for India, which Nielsen (1980) recorded as occurring in Khasia (presumably referring to Khasia Hills in Meghalaya State). Although the specimen upon which that record was based has not been examined, we consider it unlikely that Senegalia hainanensis would occur in India. The record of Acacia subsp. hainanensis for northern Vietnam by Nielsen (1980) is most likely based on specimens of S. stipitata (see that species for discussion), while the southern Vietnam record in that work requires further investigation (it may possibly be S. pluricapitata).
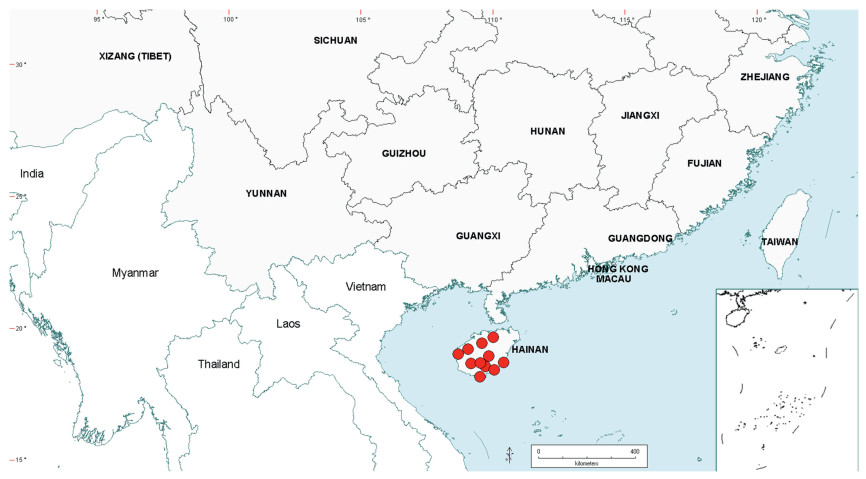
|
| Fig. 18 Distribution of Senegalia hainanensis in China, based on specimen records at CAL, IBK, IBSC, KUN, SYS and VNM, as cited in text. This species was formerly broadly circumscribed by Nielsen(1980, 1981) and Wu and Nielsen (2010), and as a consequence was regarded as having a much wider distribution than is shown here (see text for discussion). |
Habitat. In China, this species grows on gentle or steep slopes in hilly country, often near streams in dense or open forests or in thickets. A number of collectors note that it is fairly common in places where it grows.
Phenology. As best can be judged from herbarium specimens, Senegalia hainanensis in China flowers from March to June or July (but in the MarcheMay period often only mature buds are present on plants). In Vietnam, specimen records show it as flowering a little later, from about August to September. Mature seeds have been collected in China in mid-June and judging from immature pods on herbarium specimens, it seems likely that seeds would be present on some Chinese plants until about August. The fruiting period is unknown for Vietnam.
The type collection of Acacia hainanensis from Hainan comprises specimens with both mature inflorescence buds and a few heads at anthesis. The month of collection of this gathering is not given in the protologue or shown on the lectotype sheet at TI. However, labels on the two isolectotypes at TAIF give the month of collection as October (this same date appears on the type fragment at IBSC). How this date came to be recorded on the isolectotypes is not known, but judging from the phenology given above, it is most likely an error.
Typification. In the protologue of Acacia hainanensis only a single, un-numbered Katsumada collection was cited by Hayata (1913), no herbarium of lodgement was indicated and there was no indication that only a single specimen was used to prepare the original description. Stafleu and Cowan (1979) give TI as the location for most of Hayata's herbarium and types, and Nielsen (1980) subsequently cited Katsumada s.n. at TI as the holotype of A. hainanensis. However, it is now known that there are two additional sheets of Katsumada s.n at TAIF. Notwithstanding the October date mentioned above, none of the aforementioned specimens is at variance with the protologue and they are regarded here as original material. Accordingly, in conformity with ICN Art. 9.10 (Turland et al., 2018), and as discussed by McNeill (2014), the holotype citation by Nielsen (1980) is corrected above to lectotype.
Taxonomy. Over the past 90 years Senegalia hainanensis (as Acacia) has been variously treated in literature. Chun and How (1958) and Wu (1988) regarded it as a conspecific with Acacia pennata, Merrill (1927), Sun and Chen (1990) and Sun (2006) regarded it a distinct species of Acacia, while Nielsen(1980, 1981) and Wu and Nielsen (2010) treated it as a subspecies of A. pennata. The ILDIS database (Roskov et al., 2005) listed A. hainanensis as a synonym of both A. pennata and (surprisingly and erroneously) A. brevispica Harms subsp. brevispica. Under Senegalia, Maslin et al. (2013) followed the subspecies classification of Nielsen (1980) while Zhu et al. (2015) followed Sun and Chen (1990) and Sun (2006) in treating it as a distinct species. This entity is re-assessed here as a distinct species, but one that is more narrowly defined than by most previous authors because it excludes the three species that are noted in the following paragraph.
Nielsen(1980, 1981) adopted a broad concept for Acacia pennata subsp. hainanensis, including within its circumscription not only Senegalia hainanensis, but also other entities that are recognised here as distinct species, namely, S. macrocephala (resurrected below), S. orientalis and S. stipitata (the last two newly described below). Not all specimens cited by Nielsen(1980, 1981) have been examined and therefore it is not known if additional species were also encompassed by those treatments of subsp. hainanensis. The broad concept of A. pennata subsp. hainanensis by Nielsen(1980, 1981) was subsequently adopted by Wu and Nielsen (2010), and in that work the petiole gland description clearly refers to S. stipitata, while the distribution citations for China most likely refer to S. hainanensis (Hainan citation), S. stipitata (Yunnan and Guangxi citations) and probably S. orientalis (Fujian and Guangdong citations); the distribution citation for Myanmar was undoubtedly a reference to the type locality of S. macrocephala. Sun and Chen (1990) similarly included elements of not only S. hainanensis but also S. macrocephala and S. stipitata within a broadly defined A. hainanensis (see under S. stipitata for discussion).
Senegalia hainanensis, together with S. guangdongensis (which was unknown to any of the above-mentioned authors), S. macrocephala and S. stipitata constitute the informal 'Haina-nensis species-group' that is noted under both Classification and Biogeography in introductory chapters above. These four allopatric species are most obviously united by having darkcoloured glandular hairs on their inflorescence axes and often other organs. They also share the rather subtle character of the leaflet main vein being at least partially parallel with the upper margin; among Chinese Senegalia this character is otherwise known only in S. pennata subsp. insuavis. Species of the 'Hai-nanensis species-group' can be distinguished from one another by the morphology of their leaf glands and also by other characters given under their respective descriptions and in the Key to species above.
Affinities. Of the species formerly included within Senegalia (Acacia) hainanensis the one most closely related is S. stipitata. These two species can be easily distinguished by their leaf glands. In Senegalia stipitata the petiole glands are stipitate to sub-stipitate, small (0.4-1 mm diam. at apex) and situated 5-15 (-20) mm above the leaf base; their shape is normally symmetric and varies from short-cylindric to narrowly obconic or caliciod (i.e. having a slender stipe and a dilated apex) with a shallow or deep pore on the upper surface. As described above, the petiole glands in S. hainanensis are sessile, 1-3 (-4) mm long and asymmetric in being slightly but discernibly elevated from the base at their distal end; these glands are generally situated close to the leaf base (normally removed just 4-6 mm) and do not have a pore on their upper surface. These differences are illustrated in Fig. 17I (S. hainanensis) and Fig. 47H (S. stipitata). The two species also differ in their rachis glands. In Senegalia stipitata the rachis glands are situated at the base of the uppermost (5-) 6-11 pairs of pinnae and they are very similar to the petiole glands except they are 0.5-0.7 mm diam. at the apex. In S. hainanensis the rachis glands are generally fewer (situated at base of uppermost 4-6 (-9) pairs of pinnae), sessile or sub-stipitate (with stipe broader than that of S. stipitata) and have a larger lamina (0.7-1.5 mm long). These rachis gland differences are illustrated in Fig. 47A and G (S. stipitata) and Fig. 17A, G & H (S. hainanensis). Apart from leaf glands there appear to be no other strong Morphological characters that reliably distinguish these two allopatric species (S. stipitata occurs in Yunnan and Guangxi Provinces of China and northern Vietnam, whereas S. hainanensis occurs in Hainan Province of China and in central Vietnam). While it could be argued that these two entities might be better regarded as subspecies of S. hainanensis, such an action is considered inappropriate because it would create a species with an unacceptably wide range of variation for leaf gland morphology (see also discussion under Application of rank above).
Inflorescences. For specimen labels that record inflorescence colour, Senegalia hainanensis is most commonly described as having simply 'yellow' but sometimes white. What is precisely meant by yellow is not known but is possibly pale yellow. The specimen label on H.Y. Liang 65144 records the inflorescence buds of living plants as being dark red.
Etymology. The species name refers to Hainan Island, China, from where the type was collected.
Vernacular name. Hainan Senegalia. This name was used by Sun (2006), under A. hainanensis.
8. Senegalia kerrii (I.C.Nielsen) Maslin, B.C.Ho, H.Sun & L.Bai, comb. & stat. nov. (Figs. 19 and 21).柯氏金合欢【kē shì jīn hé huān】(新拟)
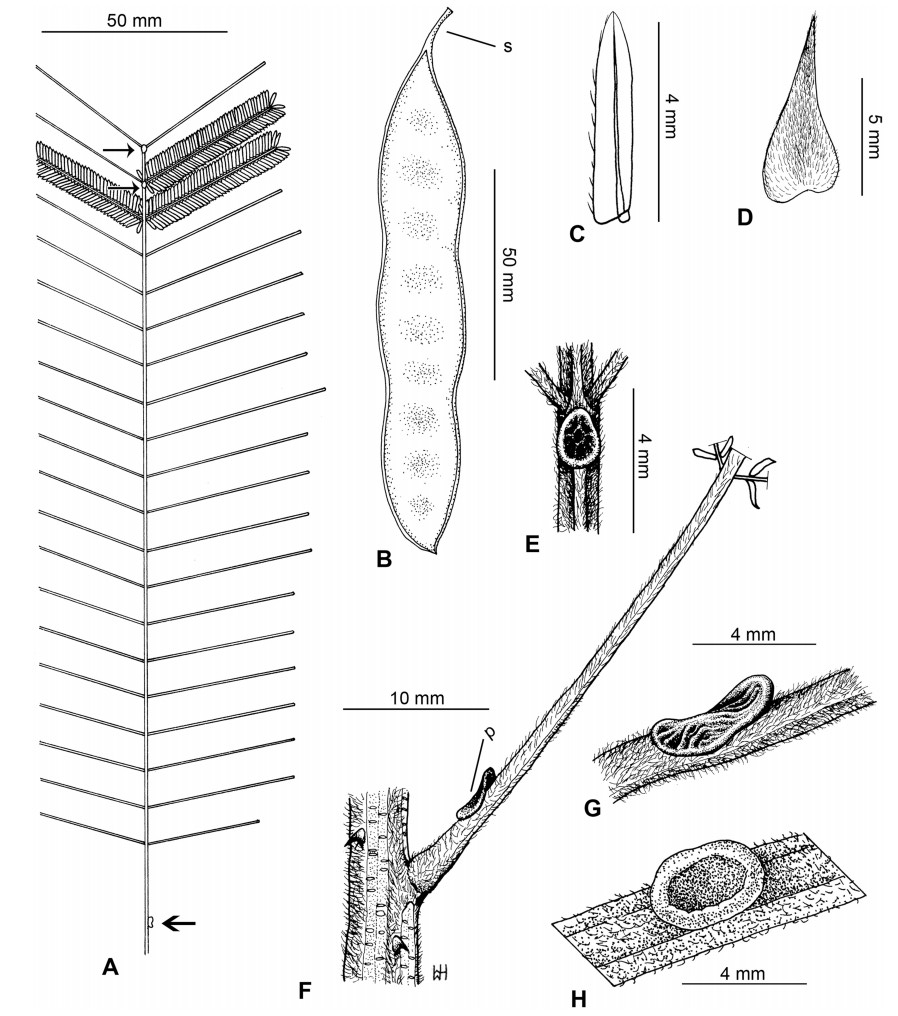
|
| Fig. 19 Senegalia kerrii. A - Leaf showing numerous pinnae and position of petiole gland (thick arrow) and rachis gland (thin arrows). B - Pod (sub-mature) showing distinct stipe (s). C - Leaflet (lower surface) small, showing broadly acute apex and rather evident main vein. D - Stipule. E - Rachis gland. F - Node showing branchlet with short, straight, patent hairs and petiole gland (p) close to leaf base. G - Petiole gland (oblique view, peripterous). H - Petiole gland (plane view, cupular). Vouchers: Y.H. Li 5191 (A & C); Sino-Vietnam Expedition s.n. (B & H); C.W. Wang s.n. (D, E, F & G). Drawn by Waiwai Hove (A, B, C & F) and Joshua Yang (D, E, G & H). |
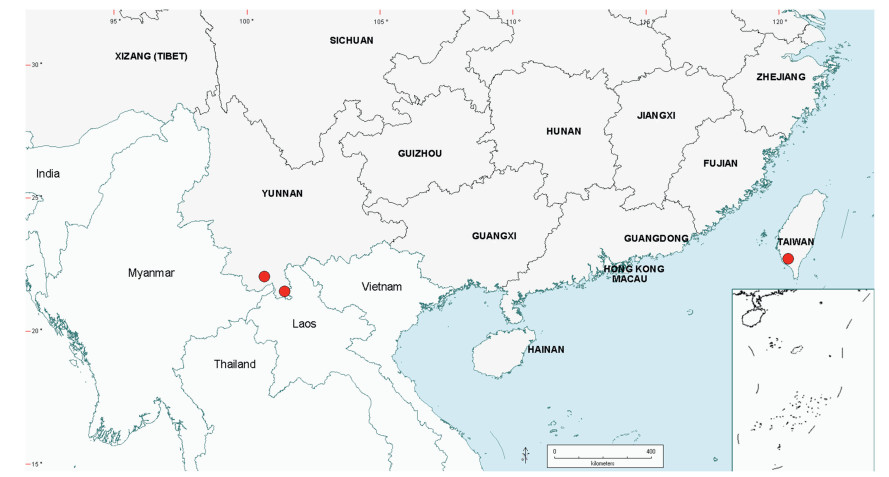
|
| Fig. 20 Distribution of Senegalia kerrii in China, based on specimen records at IBSC and KUN, as cited in text. |

|
| Fig. 21 Senegalia kerrii. A - Leaves. B - Leaf showing numerous, ±closely-spaced pinnae. C - Part of leaf showing acute leaflets and the rachis with dense, spreading hairs. D - Branchlet with short, straight, patent hairs and small, straight, patent prickles. E - Leaf base showing sub-circular petiole gland (with dark-coloured upper surface) situated near pulvinus. F - Petiole gland (narrowly peripterous) lateral view. G - Petiole gland (very narrowly peripterous) oblique view. Vouchers: All from L. Bai BLK-121. Photos: L. Bai. Scale bar = 5 mm (unless otherwise indicated). |
≡ Acacia pennata subsp. kerrii I.C.Nielsen, Adansonia, sér. 2, 19: 353 (1980). ≡ Senegalia pennata subsp. kerrii (I.C.Nielsen) Maslin, Nuytsia 22: 467 (2012). Type citation: "K. Bunchuai & B. Nimanong 1430, Thailand, Chiang Rai, Mae Suai, 25.7.1967 (holo-, K; iso-, BKF, C, P)" Type: THAILAND, Chiang Rai, Mae Suai, 25 July 1967, K. Bunchuai & B. Nimanong 1430 (holotype: K barcode K000791221 [digital image!]; isotypes: BKF 46294 barcode SN080094!, C barcode C10011409 [digital image!], E barcode E00318283 [digital image!], P n.v.-cited by Nielsen (1980)).
Additional synonymy (not relevant to China): Nielsen (1985b).
Distinctive features. Branchlets, raceme/panicle axes and peduncles densely hairy (the hairs normally short, ±straight and patent). Stipules evident in young inflorescences, normally 4-6 mm long, 2-3 mm wide and densely hairy abaxially, acuminate. Pinnae mostly 17-27 pairs. Leaflets small, 3-4 (-5) mm long and 0.4-0.8 (-1) mm wide; apices finely to broadly acute; the lateral veins not or scarcely visible. Petiole gland situated close to leaf base, l:w = 0.7-2 (-2.5), depressed, either flat-topped or shallowly concave above with lamina horizontally spreading (forming a very narrow wing) and ±patelliform or apterous and shallowly cupular to elongate-cupular. Heads rather small (7-9 mm diam. when dry). Flowers ±sessile; calyx tube ± glabrous. Pods rather dark-coloured when dry, stipitate (stipe normally 4-10 mm long).
Description. Lianas or shrubs to 5 m tall. Branchlets commonly rather pale-coloured (yellowish brown, light brown or light grey), upper branchlets with dense indumentum of short, straight and patent hairs (hairs sometimes curved, sub-appressed and sparse or absent on specimens outside of China), glandular hairs absent or few; lenticels (on upper branchlets) not visible or few and obscure. Prickles few to sub-numerous on internodes and sometimes on under surface of leaf axes, often absent from herbarium specimens, small (0.5-2 mm long), normally straight and patent to slightly reflexed, rarely recurved. Stipules evident in young inflorescences, normally lanceolate or narrowly lanceolate, (3-) 4-6 (-9) mm long, (1-) 2-3 mm wide, light brown to yellow-brown, densely hairy and obscurely striate or veins not visible abaxially, acuminate to short-acuminate. Leaves bipinnate; pinnae 17-27 pairs (occasionally 15 pairs on a few leaves), 5-8 mm apart, 25-60 (-70) mm long; petiole 20-35 mm long; rachis 80-180 mm long, hairs on upper surface either patent, ±straight and longish or appressed to sub-appressed, antrorsely curved and very short, sometimes admixture of both types (see notes below under Rachis indumentum), lower surface similarly hairy or glabrous. Leaflets 30-65 pairs, c. 20 pairs on lowermost pinnae, narrowly oblong, 3-4 (-5) mm long, 0.4-0.8 (-1) mm wide, straight to shallowly curved forward and flat to shallowly concave, weakly to moderately discolorous (darker green above than below), glabrous or sparsely ±appressed ciliolate or occasionally ciliate; apex obliquely or symmetrically narrowed to a finely or broadly acute point; base unequal with a rounded angle on lower edge, petiolule excentric; main vein starting near upper margin at leaflet base and extending obliquely to apex, straight or shallowly curved; lateral veins (on lower surface of leaflets) not visible or sometimes very few, obscure and patent, not forming a reticulum.Glands: petiole gland- variable (see discussion below under Petiole gland), situated close to base of petiole normally 5-11 mm above leaf base and 0-5 mm above pulvinus, oblong to circular, 1-4 (-5) mm long, 1-2 mm wide, l:w = 0.7-2, sessile (although often fractionally raised from beneath), depressed, flat-topped or shallowly concave on upper surface, peripterous and sometimes ±patelliform or apterous and shallowly cupular to elongate-cupular, upper surface brown or reddish and ±smooth; rachis glands - situated at base of uppermost 1-5 (-6) pairs of pinnae, oblong to elliptic or circular, 0.7-2 mm long, 0.5-1 (-1.3) mm wide, sessile, flat-topped or shallowly concave and occasionally fractionally elevated from beneath so that the rim of the gland is not closely appressed to the rachis, some-times shallowly cupular; rachilla glands - situated at base of uppermost 1-3 pairs of leaflets, smaller than rachis glands, sessile, flat-topped. Inflorescences comprising pedunculate heads arranged in terminal, open panicles or elongated racemes, often appearing axillary when in fruit, indumentum on raceme and panicle axes as on peduncles; peduncles (5-) 10-20 mm long, 2-4 together in clusters, the peduncle groups sometimes subtended by a prophyll, densely hairy (the hairs generally straight and patent, very rarely shallowly curved and ±appressed, and white or sometimes pale brown or yellowish brown), indumentum persisting to pod stage, glandular hairs absent; heads globose, rather small, 7-9 mm diam. (when dry), white to cream or pale yellow, slightly fragrant, densely or sub-loosely 35-40-flowered; inflorescence buds probably at least sometimes red when fresh (see notes below under Inflorescence bud colour). Bracteoles linearspathulate, 0.5-1 mm long; claw narrowly oblong and c. equal in length to the narrowly ovate, slightly hairy, acute lamina that is not exserted beyond flowers in buds. Flowers 5-merous, sessile to subsessile (pedicel minute, 0.1-0.2 mm long); calyx ¾-
Selected specimens examined. CHINA: Yunnan. Xishuang-banna Dai Autonomous Prefecture, Jinghong City, 15 Jan. 1964, Y.H. Li 5191 (IBSC 680596 [barcode 0159279], KUN 0400203 [barcode 0598432]); Xishuangbanna Dai Autonomous Prefecture, Mengla County, 26 Jan. 2019, L. Bai BLK-121 (BKF, HITBC, KUN [barcode 1347929 & 1347930], SING) & L. Bai BLK-123 (BKF, HITBC, IBSC, KUN [barcode 1347940], PE, SING); Xishuangbanna Dai Autonomous Prefecture, Mengla County, 7 Nov. 1952, K.M. Feng 14403 (KUN 0400196 [barcode 0598417], KUN 0400197 [barcode 0598416], KUN 0400205 [barcode 0598418]); Xishuangbanna Dai Autonomous Prefecture, Mengla County, Nov., C.W. Wang 80338 (IBSC, 187582 [barcode 0159281], IBSC 680594 [barcode 0159282], KUN 0400171 [barcode 0598395]); Yunnan, without specific lo-cality or date of collection, C.W. Wang s.n. (KUN 0400177 [barcode 0598392]); Yunnan (without specific locality), Sino-Vietnam Expedition s.n. (KUN 0400216 [barcode 0598445]). Taiwan. Kengting Park, Hengchun township, Pingtung County, 4 Sep. 1998, C.M. Wang 03453 (IBSC 00675307 [barcode 0159077]). LAOS: East bank of Mekong River, 30 Nov. 2013, H. Sun et al. 16932 (KUN 1067221 [barcoded 1296732]); Pahsan Road, 1 Aug. 1955, Talbot de Malahide 29 (SING 0206933 & 0206936). THAILAND: Northern. San Ban Dan Wildlife Sanctuary, Pang Mapha District, Mae Hong Son Province, 8 Aug. 1999, J.F. Maxwell 99-92 (BKF, 181710 [bar-code 193399]); Lampang, Me Pa, 31 Oct. 1925, [K.] Winit 1503 (BKF 036658). South-western. Brangkasi, c. 100 km S of Wangka, 22 June 1946, G. den Hoed & A. Kostermans 979 (BK 30600 [barcode 213363]); Kui Buri NP, [on] trail from park headquarters, Amphoe Pran Buri, Prachuap Khiri Khan, 20 Aug. 2002, D.J. Middleton, S. Suddee & C. Hemrat 1225 (BKF 140040 barcode SN144228). VIETNAM: Northern. Lang Nga [village], Cuc Phuong [commune], Ninh Binh Province, 17 Nov. 1965, Anonymous 4858 (HN 0000042603, 0000042604, 0000042605). Central. Moray [commune], Sa Thay District, Kontum Province, 17 Nov. 1978, Tran Dinh Dai 228 (HN 0000042607, 0000042608, 0000042609, 0000042610).
Distribution (Fig. 20). Senegalia kerrii is widespread in southeast Asia (Cambodia, East Timor, Indonesia, Laos, Peninsular Malaysia, Myanmar, Thailand and Vietnam) extending south to Australia (Queensland), north to China (including Taiwan) and west to Andaman Islands, and possibly northeast India, Bhutan and Nepal (Deshpande et al., 2019). Although Wu and Nielsen (2010) listed the species for Sri Lanka (as Acacia pennata subsp. kerrii), this record is not confirmed here. On mainland China, S. kerrii has a restricted distribution in southern Yunnan in Xishuangbanna Dai Autonomous Prefecture, close to the borders with Laos and Myanmar.
Habitat. In China this species occurs in secondary forests in hilly or mountainous country at 300-1200 m alt.
Phenology. No specimens from mainland China have been seen that enable the flowering period to be determined, but one specimen from Taiwan possessed flowers at anthesis and in bud in early September. In Thailand and Laos, herbarium specimens show flowers at anthesis (together with many buds) from early May to early August. Therefore, in these areas flowering extends from May to September. On mainland China, immature pods have been collected from November to February, suggesting that mature seeds will begin to appear in about March or April. However, the treatment of Acacia pennata subsp. kerrii in Malesia by Nielsen (1992) recorded quite a different phenology, namely, flowering from November to March and fruiting from April to August. In Australia, herbarium specimens show flowering as occurring in February and March, which is similar to that recorded by Nielsen (1992).
Taxonomy. Nielsen(1980, 1981, 1985a, 1985b) included Acacia pennata subsp. kerrii within a broadly defined A. pennata that comprised four subspecies. However, as discussed under Senegalia pennata, it is considered more appropriate to treat most of those subspecies as distinct species. Despite A. pennata subsp. kerrii having been included in the treatment for China by Wu and Nielsen (2010), the subspecies name did not appear on any specimens examined in Chinese herbaria (most were labelled simply as A. pennata).
Senegalia kerrii is a somewhat polymorphic species, especially with respect to its rachis indumentum and petiole gland morphology (see below). Although Nielsen (1985b) discussed variation within A. pennata subsp. kerrii for Indochina and Malesiana, further study of variation within Senegalia kerrii over its extensive geographic range would be worthwhile.
Affinities. Senegalia kerrii seems most closely related to S. garrettii and it often has a superficial resemblance to S. clandestina (see under these two species for discussion and differences). Senegalia kerrii often also resembles S. pennata subsp. insuavis, especially as both species have numerous pinnae, acute leaflets, depressed petiole glands situated close to the leaf base and clearly stipitate pods. Fresh specimens of S. pennata subsp. insuavis are easily recognised by the distinctively disagreeable odour they emit, especially when the branchlets are cut or the leaves crushed (this smell is lost upon drying; it is completely absent from S. kerrii). However, herbarium specimens can sometimes be somewhat difficult to distinguish, but Senegalia insuavis is recognised in the following ways: petioles and peduncles commonly longer (normally 30-65 mm and 15-40 mm long respectively), flowers shortly but discernibly pedicellate (pedicel 0.5-1 mm long), petiole gland often more elongated (l:w = 1.5-4). Also, in S. pennata subsp. insuavis at least some leaflets are commonly strongly folded lengthwise and/or ±strongly curved forward upon drying, whereas in S. kerrii the dry leaflets are ±flat and not or scarcely curved forward.
Senegalia kerrii sometimes superficially resembles S. megaladena on account of having numerous pinnae, small leaflets and panicu-late/racemose inflorescences. However, S. megaladena is most readily distinguished by its thickened and raised petiole gland that is commonly located near the middle of the petiole, and its obtuse to rounded leaflets; also, S. megaladena has less conspicuously hairy branchlets and raceme/panicle axes and a generally longer petiole (35-60 mm).
Rachis indumentum. The length, curvature and orientation of hairs on the upper surface of the rachis is often helpful in identifying species of Senegalia in China. Although species sometimes display variation for these attributes, the variation within S. kerrii is particularly noticeable where the rachis hairs can be either straight and patent or clearly curved and appressed. Specimens having one or other of these hair types have been observed on material from China, Myanmar and Thailand, and sometimes both hair types are found on a single specimen. More study is needed to better understand this variation and also to determine if there is a correlation between rachis hair type and other characters, particularly petiole gland morphology (see below).
Petiole gland. The petiole glands in Senegalia kerrii are variable in shape, size and form, and can therefore appear rather dissimilarlooking. At one extreme the gland is flat-topped or shallowly concave above, with the lamina horizontally spreading to form a very narrow rim that is not closely appressed to the petiole surface. This type of gland is termed peripterous, and it often has the general appearance of being ±patelliform. At the other extreme the lamina does not form a horizontal rim but instead the outer edge of the gland is slightly thickened and/or up-turned to surround a shallow depression that occupies most of the upper surface of the gland. This gland is termed apterous and it may have the appearance of being shallowly cupular or elongate-cupular. These two extremes in petiole gland morphology appear to be connected by intermediate forms. Both peripterous and apterous glands are found on specimens of S. kerrii in China (the former being the most common) and in several other countries, and they occasionally occur together on a single specimen. The shortest glands (1-2 mm long) are commonly ±circular, shallowly cupular and apterous; these glands look superficially rather different from those found on the type of A. pennata subsp. kerrii which has a partially peripterous, oblong, ±flat-topped gland c. 3 mm long. When specimens combine short, shallowly cupular petiole glands with curved, appressed, short rachis hairs they can assume a somewhat distinctive appearance, but at present it is not considered appropriate to afford formal (infraspecific) recognition to these specimens. Further study of petiole gland variation in S. kerrii is needed. Peripterous petiole glands also occur in S. garrettii where the peripterous nature of the gland is normally better-expressed than in S. kerrii. Senegalia hainanensis has a poorly-developed, partially peripterous petiole gland.
Inflorescence bud colour. The inflorescence buds and calyx lobes on herbarium specimens of this species are very often dark brown or purplish brown. As discussed under Morphological characters: Inflorescences and flowers above, this colouring suggests that in living plants the colour was most likely reddish. Although living plants of Senegalia kerrii have not been examined in China, herbarium label information on two Thailand specimens at BKF (namely, Maxwell 99-92 and Middleton et al. 1225) record the calyx as being dull red and red respectively. While it is likely that most living plants of this species will be shown to possess red inflorescence buds and calyx lobes, this needs to be confirmed.
Etymology. This species honours Arthur Francis George Kerr (1877-1942), originally a medical doctor in the service of the Royal Thai Government who later became the Government botanist. Kerr collected extensively (over 20, 000 numbers) in Thailand between 1902 and 1932. A complete set of his collections is at herb. K and BM, with duplicates distributed to many main herbaria of the world (fide web ref. 8). Several of Kerr's well-preserved Senegalia speci-mens have been found at herb. BK.
Vernacular name. Kerr's Senegalia (following Chinese common name that is proposed above).
9. Senegalia kunmingensis (C.Chen & H.Sun) Maslin, B.C.Ho, H.Sun & L.Bai, comb. & stat. nov. (Figs. 22 and 24).昆明金合欢【kūn míng jīn hé huān】
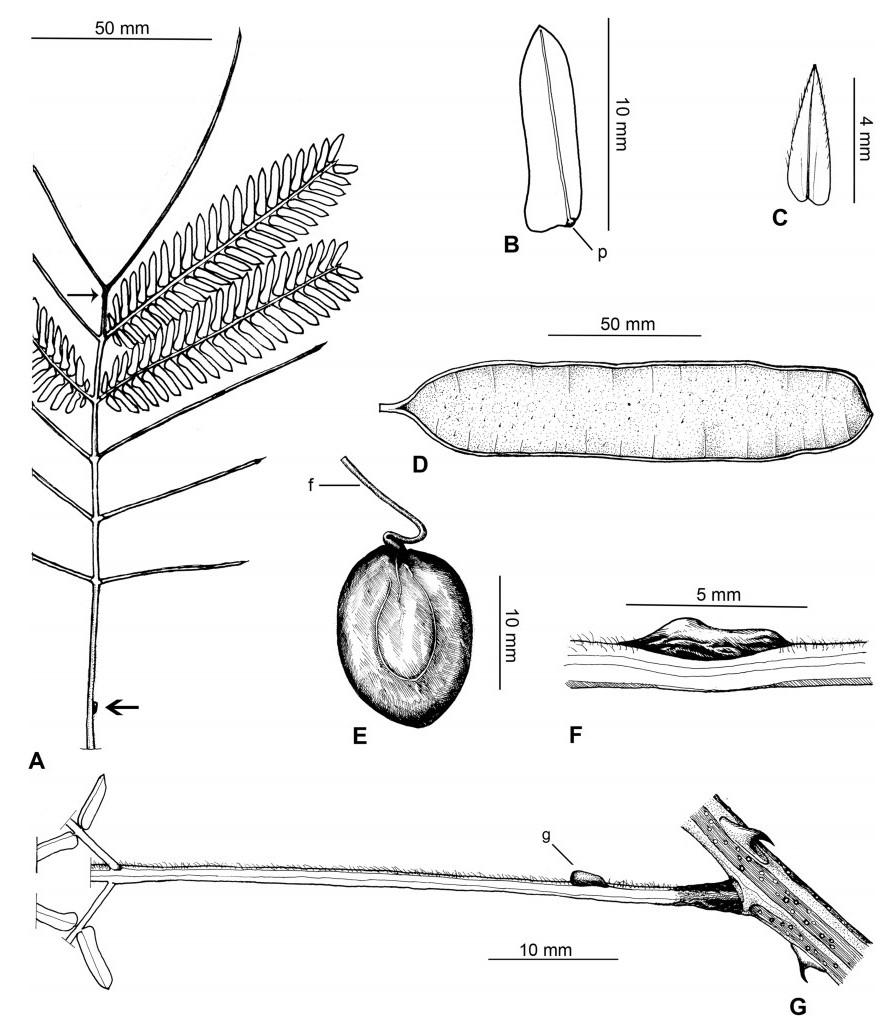
|
| Fig. 22 Senegalia kunmingensis. A - Leaf showing relatively few pinnae and position of petiole gland (thick arrow) and rachis gland (thin arrow). B - Leaflet (lower surface) large and sessile with petiolule (p) not extended below base. C - Stipule. D - Pod. E - Seed showing thickly filiform, exarillate funicle (f). F - Petiole gland elevated at distal end. G - Node showing glabrous branchlet and asymmetric petiole gland (g) relatively close to base of leaf. Vouchers: H. Sun 1662 (A); C.A. Wu 9438 (B, C & G); S.K. Wu 209 (D); M.K. Li 557 (E & F). Drawn by Joshua Yang. |
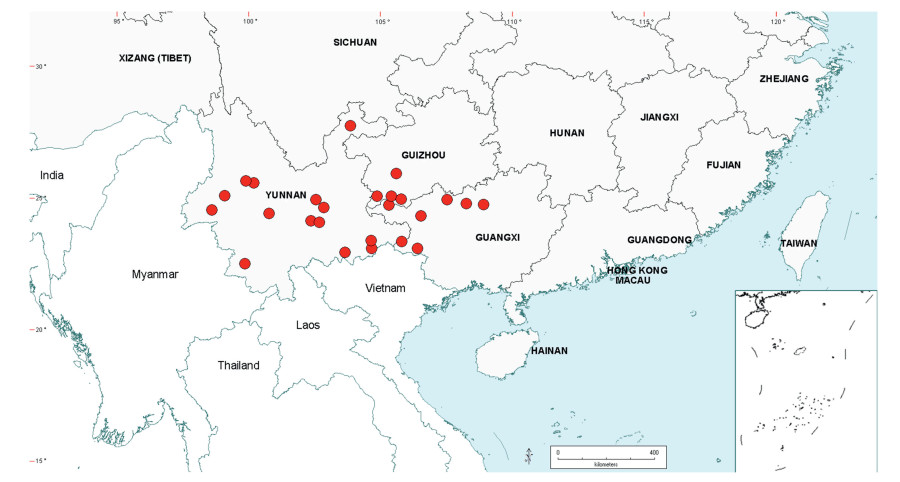
|
| Fig. 23 Distribution of Senegalia kunmingensis in China, based on specimen records at GXMG, GXMI, IBK, IBSC, KUN and SING, as cited in text. |
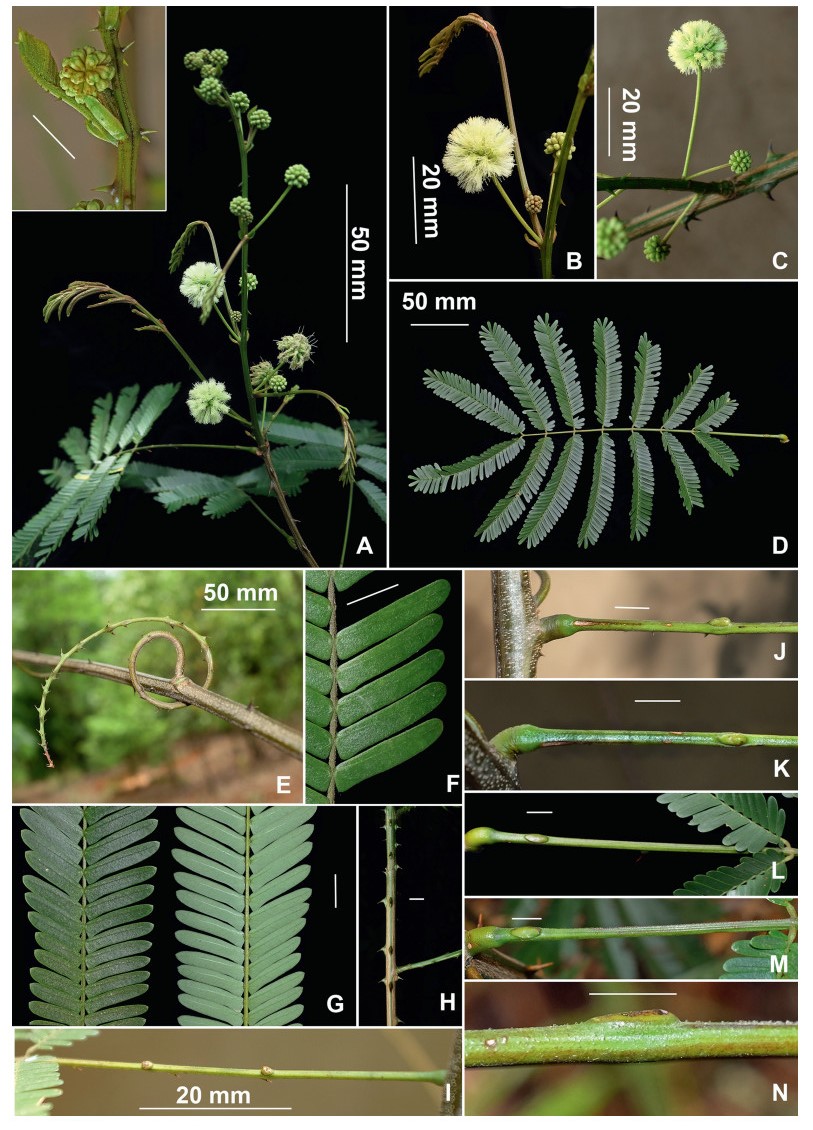
|
| Fig. 24 Senegalia kunmingensis. A - Inflorescences in terminal raceme with very pale yellow heads at anthesis and green flower buds, insert showing narrowly oblong stipule at base of peduncle. B - Inflorescence node showing young leaf subtending peduncles. C - Inflorescences showing pale-coloured head at anthesis and green flower buds. D - Leaf showing few pairs of pinnae. E - Tendril. F - Leaflets showing lower margin very shallowly concave above basal angle, except uppermost leaflet which is straight-edged. G - Leaflets discolourous (left: dark green with satin lustre on upper surface; right: paler green with dull lustre on lower surface). H - Prickles on stem. I - Leaf base showing two petiole glands (atypical). J &. K - Petiole gland associated with fertile region short and asymmetric being higher at distal end. L, M & N - Petiole gland on older leaves longer and more flattened (and often not elevated at distal end: not illustrated). Voucher: All from L. Bai & B.R. Maslin BLK-125. Photos: L. Bai. Scale bar =5 mm (unless otherwise indicated). |
≡ Acacia delavayi var. kunmingensis C.Chen & H.Sun, Acta Bot. Yunnan. 12: 262 (1990). ≡ Senegalia delavayi var. kunmingensis (C.Chen & H.Sun) Maslin, Seigler & Ebinger, Blumea 58: 40 (2013). Type citation: "Kunming, H.K. Tenn [Teng]" Type: CHINA, without date or locality [but according to Bao et al. (1998) Teng's collections 554-840 were gathered from Xishan Mountain, Xishan District, Kunming City, Yunnan Province in 1939], H.K. Teng 673 (holotype: KUN 0159923 barcode 1206964!; isotypes: KUN 0159924 barcode 1206966!, KUN 0159925 barcode 1206963!).
- "A. cavaleriei" H.Lév., Fl. Kouy-Tcheou 224 (1914), nom. nud.; see discussion below under Synonymy.
Distinctive features. Branchlets normally glabrous. Pinnae relatively few, (3-) 5-9 pairs. Leaflets rather large (6-12 mm long, 1.5-3 mm wide), wide-spreading, normally glabrous, sessile (i.e. the broad petiolule not extended beyond base of leaflet), lower edge often shallowly concave above basal angle; apex rounded to obtuse; lateral veins (on lower surface of leaflets) normally not prominent or forming a reticulum. Petiole gland 2-6 mm long, normally asymmetric being slightly to obviously thickened and raised at distal end, sometimes flattened and not raised at distal end. Inflorescences mostly comprising 1-3 pedunculate heads within leaf axils (initiated on new shoots); peduncles 15-35 (-45) mm long; heads large (10-18 mm diam. at anthesis when dry). Pods wide (27-41 mm), light brown (often tinged orange).
Description. Robust lianas or lianescent or scandent shrubs to c. 3 m tall; tendrils at least sometimes present in leaf axils. Branchlets glabrous or occasionally (e.g. Type collection) ±densely puberulous with hairs very short, patent and straight to shallowly curved (see notes below under Indumentum variation). Prickles normally rather few on internodes and extending to under surface of leaf axes, sometimes lacking on herbarium specimens, size variable, 1-5 mm long, longest prickles rather vicious-looking, recurved or straight and reflexed. Stipules at base of peduncles caducous, narrowly oblong to triangular, 2-5 mm long, 0.5-1.2 mm wide, glabrous or sparsely to densely puberulous and obscurely few-veined or veins not visible abaxially, base symmetric or asymmetric but not lobed, apex acute; stipules on vegetative shoots not prominent, narrowly triangular to narrowly lanceolate, 3-7 mm long, 1-2.5 mm wide, scarious, striate, glabrous. Leaves bipinnate; pinnae (3-) 5-9 pairs, 40-115 mm long (except to 30 mm long on lowermost pinnae); petiole (30-) 40-80 mm long, glabrous or sparsely to densely hairy; rachis (25-) 40-120 (-150) mm long, the upper surface moderately to densely hairy (hairs short, curved and ±appressed, or straight and patent) or occasionally sparsely hairy. Leaflets 21-33 (-45) pairs, to 14 pairs on lowermost pinnae, narrowly oblong, 6-12 mm long and 1.5-3 mm wide (but basal few sometimes smaller), close together, strongly to moderately discolorous (rather dark green with a satin lustre above and paler green with dull lustre below), normally glabrous except margins often sparsely appressed-ciliolate, occasionally (including the Type) finely appressed to sub-appressed villous on lower surface (hairs fine, white, ±sparse to moderately dense and extending over entire surface, indumentum on upper surface similar except sparser) (see notes below under Indumentum variation); apex symmetric or asymmetric, rounded to obtuse, normally with an indistinct, very short, blunt or acute apiculum; base unequal with a distinct rounded lobe on lower edge, petiolule clearly excentric, broad (c. 0.7 mm wide) and very short (not extended below base of leaflet, so that leaflets appear sessile), sometimes appearing ±auriculate (see notes below under Leaflet base); main vein starting near upper margin at leaflet base and extending obliquely to apex, straight; lateral veins (on lower surface of leaflets) normally not prominent or forming a reticulum, occasionally reticulum indistinct and imperfectly developed. Glands: petiole glanddsituated on lower 
Selected specimens examined. CHINA: Guangxi. Baise City, Jingxi County, 20 Dec. 1958, C.C. Chang 14844 (IBSC 298007 [bar-code 0158776]); Baise City, Lingyun County, 6 July 1937, S.K. Lau 28496 (IBSC 105012 [barcode 0158781]); Baise City, Longlin Multinational Autonomous County, 10 Sep. 1987, S.Q. Tang et al. 0079 (IBK, 204489 [barcode 00199492], IBK, 204490 [barcode 00199493]); Baise City, Napo County, 25 May 2015, D.X. Nong, Y.D. Peng & J.H. Li 451026150525011LY (GXMG 163267 [barcode 0109150]; Hechi City, Huanjiang Maonan Autonomous County, 21 Oct. 1991, Yunnan Guangxi & Guizhou Expedition 70030 (IBK, 200825 [barcode 00198744]); Hechi City, Luocheng Melao Autonomous County, 29 May 1977, Luocheng Expedition 4-1-1576 (GXMI 17000 [barcode 015768]); Hechi City, Nandan County, 28 Sep. 1977, Nandan Expedition 4-5-821 (GXMI 26979 [barcode 015765]). Guizhou. Qianxinan Buyi & Miao Autonomous Prefecture, Anlong County, 10 June 1960, Guizhou Expedition 5017 (IBSC 680563 [barcode 0158856]; Qianxinan Buyi & Miao Autonomous Prefecture, Ceheng County, 13 May 1960, Guizhou Expedition 2410 (IBSC 680564 [barcode 0158855], KUN 0159909 [barcode 0598153]; Qianxinan Buyi & Miao Autonomous Prefecture, Xingyi City, 8 July 1960, Guizhou Expedition 6710 (IBSC 680567 [barcode 0158852]. Yunnan. Baoshan City, Longling County, 7 Aug. 1941, C.W. Wang 89949 (KUN 0159922 [barcode 0598134]; Baoshan City, Longyang District, 26 Oct. 1978, 780-Expedition 22 (KUN 674617 [barcode 0598120]); Baoshan City, Longyang District, 17 Feb. 2013, W.L. Zhao BSGLGSly3049 (KUN 1013892 [barcode 1318451]); Dali Bai Autonomous Prefecture, Yangbi Yi Autonomous County, 6 Nov. 1958, W.T. Wang 604 [KUN 0159933 Dali Bai Autonomous Prefecture, Dali City, 21 June 1946, T.N. Liou 20833 (IBSC 215922 [barcode 0158801], IBSC 680553 [barcode 0158817], KUN 0159911 [barcode 0598125]) [barcode 0598144]); Honghe Hani & Yi Autonomous Prefecture, Pingbian Miao Autonomous County, 19 July 1934, H.T. Tsai 61015 (IBSC 105738 [barcode 0158828], KUN 0159928 [barcode 0598137], KUN 0159929 [barcode 0598138]); Kunming City, Xishan District, 24 Aug. 1953, P.Y. Chiu 50141 (KUN 0159936 [barcode 0598148], KUN 0159937 [barcode 0598149], KUN 0159938 [barcode 0598150]), branchlets hairy; Kunming City, Panlong District, Kunming Institute of Botany, Cultivated, Rare & Endangered Garden, 25°08'03"N, 102°44'29"E, 2 June 2019, L. Bai & B.R. Maslin BLK-125 (KUN [barcode 134796̶ 1347967]); Pu'er City, Jingdong Yi Autonomous County, 14 Oct. 1939, M.K. Li 557 (IBSC 390073 [barcode 0158802], KUN 0159921 [barcode 0598136]); Pu'er City, Jingdong Yi Autonomous County, 5 June 1963, C.A. Wu 9438 (KUN 0159939 [barcode 0598140], KUN 0159940 [barcode 0598141]); Pu'er City, Lancang Lahu Autonomous County, 7 Dec. 1994, Y.Y. Qian 3344 (KUN 455638 [barcode 1216624]; Wenshan Zhuang & Miao Autonomous Prefecture, Malipo County, 10 May 1993, Y.M. Shui 2591 (IBSC 714434 [barcode 0705086]); Wenshan Zhuang & Miao Autonomous Prefecture, Xichou County, 28 Sep. 1947, K.M. Feng 12089 (IBSC 312994 [barcode 0158799], KUN 0159913 [barcode 0598131], KUN 0159914 [barcode 0598132]; without specific locality, 1934, H.T. Tsai 57535 (BO, IBSC 105701 [barcode 0158829], KUN 0159927 [barcode 0598139]); Yuxi City, Chengjiang County, May 1939, Y. Tsiang & H. Wang 16137 (IBSC 440835 [barcode 0158809]); Yuxi City, Eshan Yi Autonomous County, 3 Oct. 1958, S.K. Wu 209 (KUN 0159931 [barcode 0598142]; Yuxi City, Eshan Yi Autonomous County, 15 Oct. 1988, H. Sun 1662 (KUN 0159945 [barcode 0598152]; Yuxi City, Tonghai County, 10 Aug. 1989, Yuxi Expedition 675 (KUN 0148343 [barcode 0598590], KUN 0148344 [barcode 0598589]), branchlets hairy; Zhaotong City, Daguan County, 21 June 1973, B.S. Sun et al. 718 (IBSC 641930 [barcode 0159419], KUN 0159926 [barcode 0598147]. VIETNAM: Northern. Chapa [ =Sapa], Tonkin, 1 Sep. 1941, A. Petelot 7023 (VNM 00022808).
Distribution (Fig. 23). Occurs in southwest China and northern Vietnam. In China, Senegalia kunmingensis is widespread in Yunnan and extends eastwards to Guangxi and southwest Guizhou. Judging from this distribution it is likely that the species will be found in Myanmar and perhaps northern Laos in the future. In Vietnam, Senegalia kunmingensis occurs in the far north of the country near Sapa, which is just south of the Chinese border.
Habitat. Grows in valleys and on mountain slopes at 700-1900 m alt., in open thickets or open secondary forests. According to Sun and Chen (1990) and Sun (2006) this species occurs mainly in limestone areas.
Phenology. Herbarium specimens show Senegalia kunmingensis as flowering from early May to late July (but it could extend to August) and having pods with near-mature seeds from early October to early December.
Synonymy. The name Acacia cavaleriei first appeared in Léveillé (1914) under the following terse entry: "A. cavaleriei Lévl. Mou-youse [=Huajiang township in Guanling Buyi & Miao Autonomous County, Guizhou Province] (Cavalerie, 2041) Juin 1904. La tige ligneuse court sur les murs. [The woody stem rambles over walls.]" Rehder (1932) and Lauener (1970) who conducted a thorough revision on Chinese plants described by Léveillé both regarded A. cavaleriei as a nomen nudum, placing it in synonymy under A. delavayi. Although Wu and Nielsen (2010) included A. cavaleriei as a synonym of A. delavayi var. delavayi, they did not declare it as a nomen nudum. While it is recognised that some workers may consider A. cavaleriei as being validly published, no publication that treated it as such has been found so far. Agreeing with Rehder (1932) and Lauener (1970), A. cavaleriei is treated here as not validly published, because the descriptive information provided by Léveillé is neither diagnostic for the species, nor is it considered adequate to qualify as a description. Furthermore, it is likely that Léveillé (1914) did not intend to describe A. cavaleriei as a new taxon because his practice was to insert 'nova sp.' after the names of entities he regarded as new, but this annotation does not accompany his A. cavaleriei entry. As suggested by Rehder (1932), it is suspected that Léveillé may have thought that the species had already been described earlier. However, despite extensive searches for Léveillé's publications, no other description of A. cavaleriei has been located, and it is unlikely that one exists; Rehder (1932) came to a similar conclusion. Web images of Cavalerie (2041) at E as well as K reveal that these specimens are clearly Senegalia kunmingensis, not S. delavayi.
Taxonomy. Until now this entity had been regarded as a variety of Acacia (Senegalia) delavayi by Sun and Chen (1990), Wu and Nielsen (2010) and Maslin et al. (2013), but as discussed under S. delavayi above, species rank is here considered more appropriate for it. The characters that unite S. kunmingensis and S. delavayi, and those that distinguish them, are discussed under the latter species. Senegalia kunmingensis can be readily recognised by characters given under Distinctive features above. Specimens of S. kunmingensis at IBSC were previously labelled A. concinna (=Senegalia rugata), but these two species are not closely related.
Affinities. Apart from its relationship with Senegalia delavayi (see that species for discussion), S. kunmingensis is often superficially similar to S. andamanica, S. pruinescens and S. prominens in having relatively few pinnae and often rather large leaflets. These three species can be distinguished from S. kunmingensis by their inflorescences arranged in panicles or racemes, and by their leaflets that are normally petiolulate (except occasionally sessile in S. pruinescens) and not basally auriculate (see discussion below under Leaflet base). Senegalia andamanica is further recognised by its distinctly depressed petiole gland and narrower pods (15-25 mm); S. pruinescens by its normally pruinose branchlets (although pruinosity is sometimes absent from herbarium specimens), wider stipules (1-3 (-4) mm), consistently densely hairy peduncles and by its leaflets that are often reticulately-veined on their lower surface; and S. prominens by its normally more obviously hairy upper branchlets, wider stipules (1.5-3 mm) and ±prominently 5-veined calyx tube.
The inflorescence structure in particular suggests that Senegalia kunmingensis has affinities with S. teniana which can be distinguished by its generally smaller leaflets (normally 5-7 mm long and 1-2 mm wide) that possess a short but distinct petiolule and which are often imperfectly reticulate on their lower surface, and by its generally smaller petiole gland (normally 1.5-2 mm long) that is never raised at its distal end.
Indumentum variation. The branchlets and leaflets of Senegalia kunmingensis are normally glabrous (except leaflet margins are often sparsely appressed-ciliolate) but there are a few collections (including the Type) that have rather densely hairy branchlets. In these cases, the leaflets are often, but not always, finely appressedhairy. This indumentum variation does not appear to have any taxonomic significance.
Leaflet base. Sun and Chen (1990) and Wu and Nielsen (2010) described the leaflet base of Acacia delavayi var. kunmingensis (=Senegalia kunmingensis) as auriculate and that of A. delavayi var. delavayi (=S. delavayi) as obtuse. In auriculate leaflets the lower margin in the region immediately above the basal angle descends in a curve to some extent (so that the margin is concave above the angle), whereas in obtuse leaflets this margin is straight (compare Figs. 24G and 52E). In S. kunmingensis the degree to which the margin descends varies from slight to pronounced, and the two extremes can be found on a single leaf. When the curve is only slightly pronounced the auriculate shape is not well-expressed, but when it is pronounced the auriculate shape is clearly seen. While most leaflets possess descending lower margins, they very often cooccur with a low proportion of leaflets where the margin is straight (see Fig. 24F). Although this is a subtle character it is useful in helping distinguish S. kunmingensis from its relatives, all of which have obtuse leaflet bases.
Petiole gland variation. There is some variation in the morphology of petiole glands in Senegalia kunmingensis. Most herbarium specimens show these glands as relatively short, 2-3 (-4) mm long, and asymmetric (being slightly to obviously more thickened, thus raised, at their distal end) (Fig. 24J). However, sometimes the petiole glands are longer (4-6 mm), flattened and not or scarcely raised at the distal end (Fig. 24M and N). This flattened form of the petiole gland has been observed on a few specimens from both Guangxi (e.g. R.C. Ching 6592 (IBSC 43151 [barcode 0158968]) and Yunnan (e.g. H. Sun 1662, KUN 0159944 [barcode 0 59815]). Observation of plants cultivated in Kunming (see L. Bai & B.R. Maslin BLK-125 cited above) show the longer, flattened glands as occurring on leaves at the base of the branches; higher up the branches, on leaves associated with the fertile region, the petiole glands are of the thickened, shorter, asymmetric type. It is not known if these two types of petiole gland are normally found on living plants of S. kunmingensis (with the shorter, asymmetrically thickened type having been disproportionally sampled by collectors).
Etymology. This species is named for the Chinese city of Kunming in Yunnan Province.
Vernacular name. Kunming Senegalia. This name was used by Sun (2006) and Wu and Nielsen (2010), under A. delavayi var. kunmingensis.
10. Senegalia macrocephala (Lace) Maslin, B.C.Ho, H.Sun & L.Bai, comb. nov. (Fig. 25).大头金合欢【dà tóu jīn hé huān】(新拟)
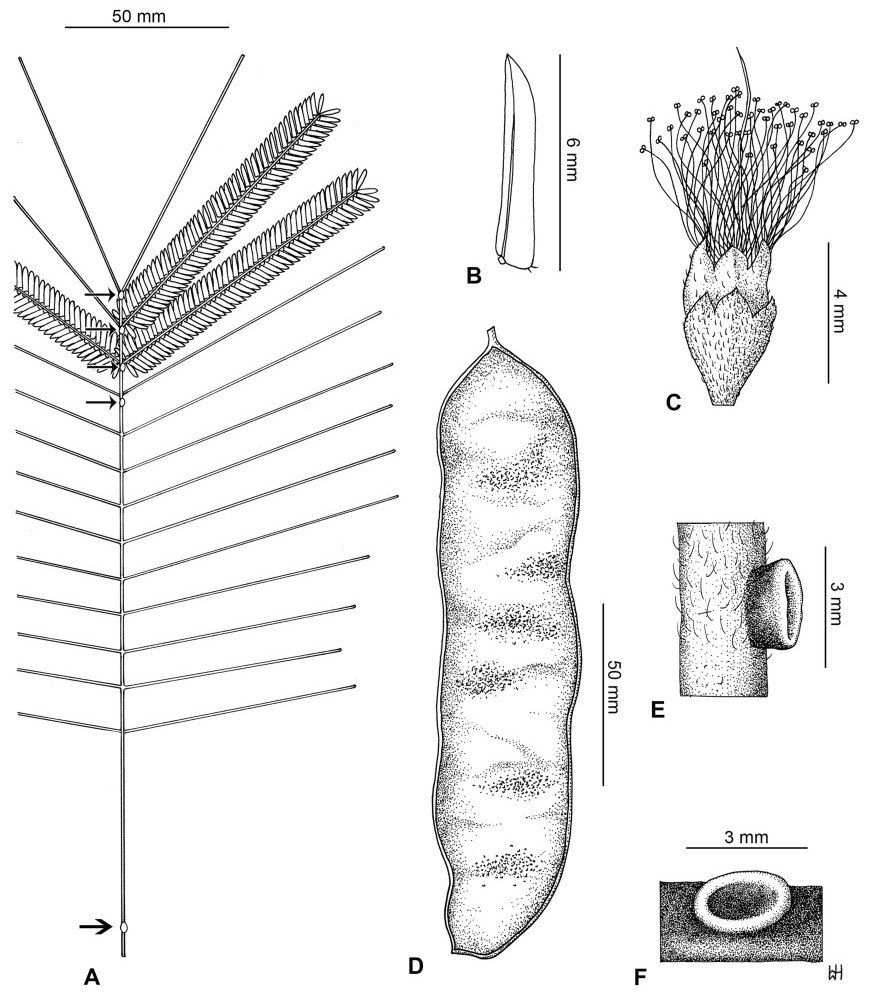
|
| Fig. 25 Senegalia macrocephala. A - Leaf showing position of petiole gland (thick arrow) close to leaf base and rachis glands (thin arrows). B - Leaflet (lower surface) showing main vein close to and parallel with upper margin in lower half of leaflet. C - Flower showing hairy calyx. D - Pod large, showing patches of dark-coloured, sessile glands. E - Petiole gland (lateral view) cupular. F - Petiole gland (plane view) cupular, showing yellow periphery and dark-coloured centre. Vouchers: J.H. Lace 5787 (A - lectotype); T.T. Yu 17292 (B - KUN 0400050); E.D. Liu 666 (C); T.T. Yu 17292 (D); T.T. Yu 17292 (E & F). Drawn by Waiwai Hove. |
≡ Acacia macrocephala Lace, Bull. Misc. Inform. Kew 1915: 401 (1915). Type citation: "INDO-CHINA. Burma: Bhamo District. Sinlum Kaba, 1700 m, Lace 5787" Type: MYANMAR, Bhamo District, Sinlum Kaba, Kachin Hills, [northeast Myanmar, not far S of Myitkyina, adjacent to border with Yunnan Province, China], 13 Apr. 1912, J.H. Lace 5787 (lectotype: E barcode E00318282 [digital image!], designated by Nielsen (1980) who incorrectly used the term holotype, see discussion below under Typification; isolectotypes: CAL 140711!, K barcode K000791194 [digital image!]).
[A. hainanensis auct. non Hayata: H.Sun & C.Chen, Acta Bot. Yunnan. 12(3): 258 (1990), pro parte, as to C.W. Wang 90201.]
Distinctive features. Young branchlets invested with dark reddish brown glandular hairs. Lenticels prominent on penultimate and mature branchlets. Pinnae 9-16 pairs. Rachis densely short-pilose to ±hirsute with ±straight, patent hairs and often also some reddish brown glandular hairs. Leaflets 4-8 mm long, 0.8-1 mm wide, discolorous (darkest above); apex obliquely narrowed to an acute point; main vein rather close to and ±parallel with upper margin at least in lower ¼ of leaflet; lateral veins (on lower surface of leaflets) not or scarcely visible. Petiole gland situated at extreme base of petiole 4-8 mm above leaf base, 1-2 mm long, not prominent but raised to c. 0.5 mm high, ±flat-topped or shallowly ±cupular; rachis glands at base of uppermost 4-6 pairs of pinnae, periphery yellow, centre often dark-coloured. Panicle and raceme axes reddish brown glandular-scurfy or glandular-hairy intermixed with some non-glandular white hairs; peduncles long (20-45 mm), with a mixture of dark red-brown glandular and white non-glandular hairs (glandular hairs very sparse on fruiting specimens); heads large (15-22 mm diam. at anthesis when dry). Calyx ±densely hairy. Pods wide (30-40 mm), with patchy clusters of circular, sessile brown glands.
Description. Trees to 8-10 m tall or scandent shrubs. Branchlets when young have dark reddish brown glandular hairs with often a few non-glandular hairs (white, straight and patent) intermixed, penultimate and mature branchlets glabrous or sub-glabrous with prominent lenticels. Prickles few on internodes and sometimes on under surface of leaf axes, often absent from herbarium specimens, normally not prominent and 0.5-1 mm long, ±straight and reflexed. Stipules (very few seen) caducous, oblong, c. 2 mm long. Leaves bipinnate; pinnae 9-16 pairs, 30-80 mm long; petiole 25-60 mm long; rachis 95-140 mm long, densely short-pilose to ±hirsute with hairs patent and ±straight, often intermixed with some dark reddish brown glandular hairs. Leaflets 35-65 pairs, narrowly oblong, 4-8 mm long, 0.8-1 mm wide, straight or sometimes slightly recurved at apex, flat or slightly concave, discolorous (darkest above), glabrous except for a few hairs on basal angle or margins; apex asymmetric, obliquely narrowed to an acute point; base truncate, unequal with an obvious angle on lower edge, the squat petiolule clearly excentric and only slightly extended below base of leaflet; main vein starting near upper margin at leaflet base, excentric being situated towards the upper margin and parallel with it at least in lower ¼ of leaflet; lateral veins (on lower surface of leaflets) not visible or very few, obscure and ±patent (except lowermost few which are ascending), not forming a reticulum. Glands: petiole gland-situated at extreme base of petiole 4-8 mm above leaf base and 0-3 mm above illdefined pulvinus, oblong to elliptic, 1-2 mm long, c. 1 mm wide, sessile or slightly stipitate, not prominent but raised to c. 0.5 mm high, ±flat-topped or more commonly shallowly ±cupular, often with a thickish, yellow rim surrounding a dark-coloured, slightly depressed centre, apterous; rachis glands - situated at base of uppermost 4-6 pairs of pinnae, similar to petiole gland except slightly smaller and circular to oblong-elliptic; SS glands-situated at base of uppermost 1-3 pairs of leaflets, circular, sessile, with a yellow periphery and dark-coloured centre. Inflorescences comprising pedunculate heads arranged in terminal open panicles or racemes, leaves often developing at base of peduncles during anthesis so that in-florescences may appear axillary, racemes sometimes growing out as a new shoot during anthesis, the panicle/raceme axes reddish brown glandular-scurfy or glandular-hairy intermixed with some non-glandular white hairs; peduncles 20-35 (-45) mm long, single or clustered in groups of up to five, with a mixture of dark red-brown glandular hairs and white non-glandular hairs (glandular hairs very sparse on fruiting specimens); heads globose, large, 15-22 mm diam. at anthesis when dry, c. 40-flowered; inflorescence buds darkish coloured at apices when dry (suggesting that they were red when fresh; see discussion under Morphological characters: Inflorescences and flowers above). Flowers 5-merous, sub-sessile (pedicel c. 0.5 mm long); calyx c. ¾ length of petals, gamosepalous, shortly dissected into triangular, dark brown lobes (?red when fresh), calyx tube moderately densely hirtellous or puberulous (hairs short, fine patent or appressed) and veins not visible; petals 4-5 mm long, ±glabrous, dark brown at apex (?red when fresh); ovary shortly hirsute. Pods oblong, 70-180 mm long, 30-40 mm wide, firmly chartaceous to thinly coriaceous, straight to slightly curved, flat, not constricted between seeds, with a ±moderately dense layer of brown, circular, sessile glands that sometimes partially wear off as pods mature (persisting in irregular patches but not embedded in resin matrix), marginal vein thickened, basal stipe short (c. 5 mm long). Seeds (very immature) transverse in pods.
Other specimens examined. CHINA: Yunnan. Baoshan City, Longling County, 28 Aug. 1941, C.W. Wang 90201 (KUN 0400054 [barcode 0598264]); Baoshan City, Tengchong County, 29 Jan. 2013, Baoshan Gaoligong Expedition BSGLGStc195 (KUN 1013810 [barcode 1318479]); Lincang City, Gengma Dai & Wa Autonomous County, 8 Aug. 1938, T.T. Yu 17292 (KUN 0400050 [barcode 0598262] & KUN 0400055 [barcode 0598263]); Lincang City, Yongde County, 8 May 2003, E.D. Liu 666 (KUN 0849155 [barcode 0555259], 0849156 [barcode 0555258]).
Distribution (Fig. 26). This poorly known species appears to have a restricted distribution in northeast Myanmar (the Type) and adjacent areas of far western Yunnan, China.
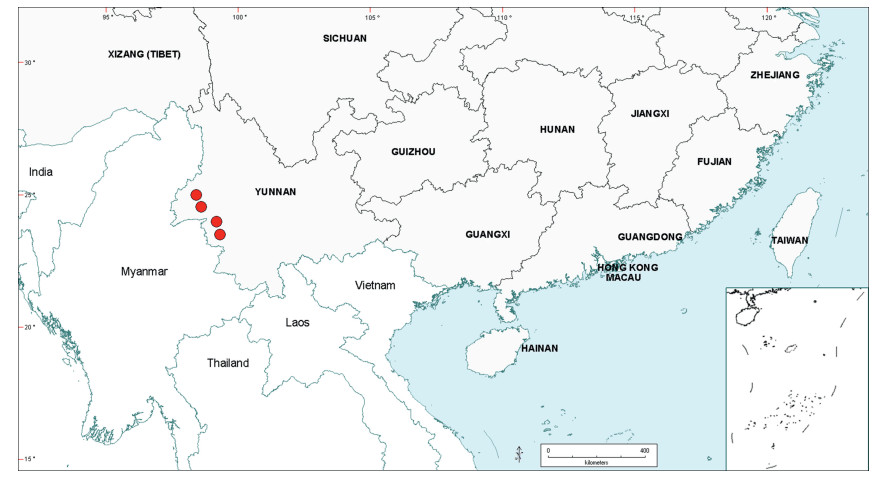
|
| Fig. 26 Distribution of Senegalia macrocephala in China, based on specimen records at KUN, as cited in text. |
Habitat. Grows in mountainous areas (alt. 1000-2450 m) in forests and on forest margins.
Phenology. As best can be judged from the relatively few specimens seen, flowering occurs from about mid-April to the end of May. Pods with very immature seed have been collected in mid-October, therefore mature seed will probably be present around NovembereDecember.
Typification. In the protologue of Acacia macrocephala only a single collection, Lace 5787, was cited by Lace; no herbarium of lodgement was indicated (although at that time Lace did spend time at Kew working up his Burmese collections, fide Lace (1915)) and there was no indication that only a single specimen was used to prepare the original description. Nielsen (1980) subsequently cited Lace 5787 at herb. E as the holotype (and annotated the specimen accordingly); this specimen is stamped as having originated from the J.H. Lace Herbarium and was purchased (by herb. E) in 1918. Nielsen (1980) noted that an isotype existed at herb. K, the specimen having been received there on 12 Nov. 1912, most likely from Lace himself; however, the origin of this isotype is not explicitly indicated on the sheet. There is a third sheet of this same collection at herb. CAL which Chakrabarty and Gangopadhyay (1996) cited as an isotype. These specimens all represent the same entity, none is at variance with the protologue and they should be treated as syntypes. Accordingly, in conformity with ICN Art. 9.10 (Turland et al., 2018) and as discussed by McNeill (2014), Nielsen's holotype citation is corrected above to lectotype, and the two other specimens corrected to isolectotype.
Taxonomy. Following its original publication in 1915 Acacia macrocephala appeared in two checklists of Myanmar plants, namely, Hundly and Ko (1961) and Kress et al. (2003). Nielsen(1980, 1981) treated this name as a synonym within a broadly defined A. pennata subsp. hainanensis (=Senegalia hainanensis from Hainan Island and central Vietnam) while Chakrabarty and Gangopadhyay (1996) treated it as a synonym of a broadly defined A. pennata. However, the entity described as A. macrocephala is morphologically distinctive, especially by its red-brown glandular hairy/scurfy young branchlets, raceme/panicle axes and peduncles, its large heads on long peduncles and its hairy calyces, and warrants recognition as a distinct species: the new combination in Senegalia is therefore provided above.
This species has not previously been recognised for China, but the specimen C.W. Wang 90201 (cited above) was included for the country by Sun and Chen (1990) within a broadly circumscribed Acacia hainanensis (see under Senegalia stipitata for further discussion of this publication).
Although the Wu and Nielsen (2010) treatment of Acacia pennata subsp. hainanensis for China did not give A. macrocephala as a synonym, the species was listed for Myanmar in that work, a record which was almost certainly based on the type of A. macrocephala.
Senegalia macrocephala belongs to the 'Hainanensis species-group' which also includes S. guangdongensis, S. hainanensis and S. stipitata. See discussion under Senegalia hainanensis.
Affinities. The dark-coloured glandular hairy/scurfy young branches, inflorescence axes and peduncles, and the leaflet main vein being located close to and partially parallel with the upper margin, indicate that Senegalia macrocephala has affinities with the more easterly distributed S. guangdongensis, S. hainanensis and S. stipitata. These four species constitute the 'Hainanensis species-group' that is noted under both Classification and Biogeography in introductory chapters above. The aforementioned three species are readily distinguished from Senegalia macrocephala by their leaf gland morphology and other characters such as calyx tubes glabrous to sub-glabrous, rachis hairs short, curved and normally ±appressed, leaflets commonly rounded to obtuse, heads smaller (to c. 10 mm diam. at anthesis when dry) and pods narrower (15-33 mm wide).
Etymology. The species name is derived from the Greek macros (large) and -cephalus (eheaded) in allusion to the large flower heads which are one of the distinctive features of the species.
Vernacular name. Large-headed Senegalia (following the Chinese common name that is proposed above).
11. Senegalia megaladena (Desv.) Maslin, Seigler & Ebinger, Blumea 58: 41 (2013) (Figs. 27, 29 & 30).钝叶金合欢【dùn yè jīn hé huān】
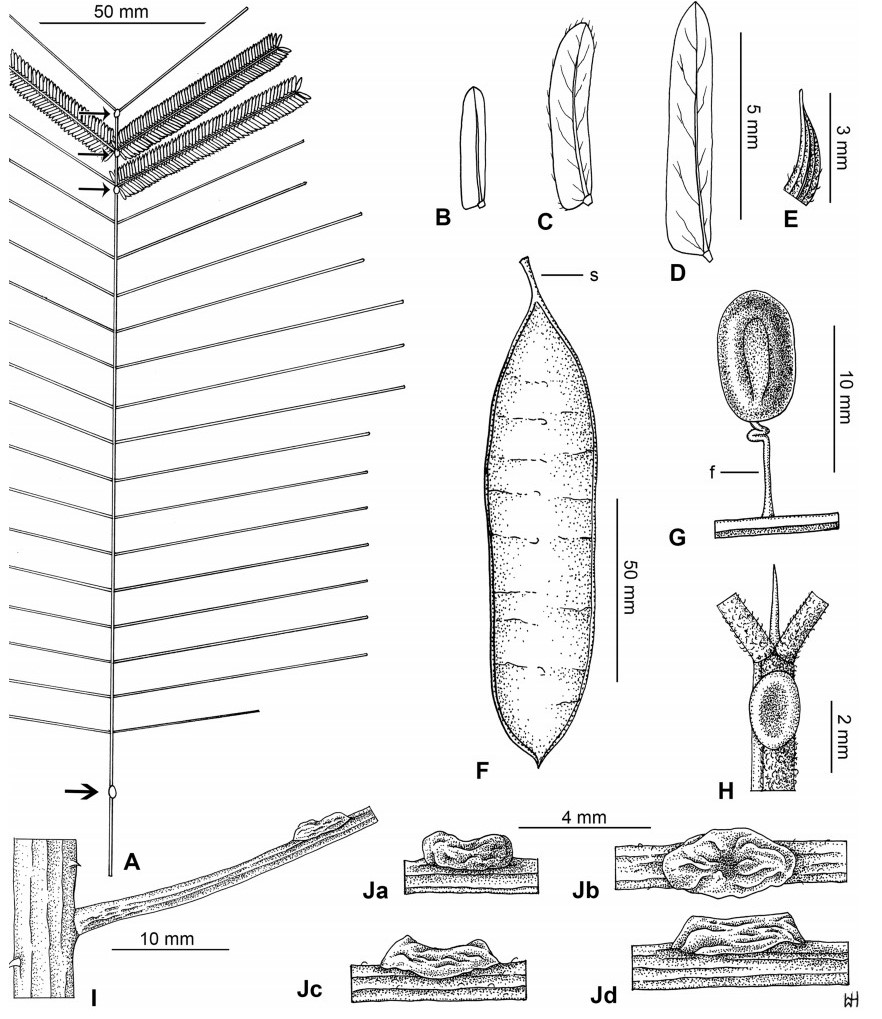
|
| Fig. 27 Senegalia megaladena. A - Leaf showing petiole gland (thick arrow) near centre of petiole and position of rachis glands (thin arrows). B - D -Leaflets (lower surface) showing size variation and rounded/obtuse apices (B = var. indochinensis; C & D - var. megaladena showing ascending, fine lateral veins). E - Stipule sparsely hairy abaxially. F - Pod with distinct stipe (s). G - Seed not especially large, showing thickly filiform, exarillate funicle (f). H - Rachis gland reasonably large. I - Node showing glabrous branchlet and petiole gland well-removed from leaf base. J - Petiole glands (dry) variation, showing characteristic thickening of gland and often upper surface concave. Vouchers: Sino-Soviet Joint Expedition 336 (A & C); Hainan Expedition 473 (B); D.D. Tao 562 (D); W.S. Liou 309 (E); Sino-German Expedition 1876 (F & H); Z.X. Li & F.W. Xing 1330 (G); Q. Lin 7742 (I); G.D. Tao 13471 (Ja); C.W. Wang 75618 (Jb); G.S. Sin 928 (Jc); C.W. Wang 74671 (Jd). Varieties: var. megaladena (A, C, D, E, I & J) and var. indochinensis (B, F, G & H). Drawn by Waiwai Hove. |
|
| Fig. 28 Distribution of Senegalia megaladena in China, based on specimen records at IBK, IBSC and KUN, as cited in text. Red dots =var. megaladena, blue dots =var. indochinensis. Wu and Nielsen (2010) recorded var; megaladena from Guangxi but we have not seen any specimens from that Province. Also, see text under var. megaladena (Variant) for discussion of specimens from Xizang Autonomous region (Tibet). |

|
| Fig. 29 Senegalia megaladena var. megaladena. A - Liana habit with insert showing basal portion of stem. B - Tendril. C - Leaf showing numerous pinnae. D - Prickles on branchlet. E - Leaflets (lower surface) showing lateral veins ascending and apex rounded to obtuse without apiculum. F - Portion of panicle showing dark red flower buds. G & H - Petiole gland prominent, brown to orange and situated near or above middle of petiole. I - Petiole gland (oblique view) prominent. J - Petiole gland (lateral view) prominent. Vouchers: All B.R. Maslin & L. Bai BRM 11040 except F - unvouchered and I - L. Bai & H. Wang, BLK-072. Photos: L. Bai except insert on Fig. A (B.R. Maslin). Scale bar = 5 mm (unless otherwise indicated). |

|
| Fig. 30 Senegalia megaladena var. indochinensis. A - Petiole gland prominent and orange, situated near middle of petiole. B - Leaflets short and with rounded apex (without apiculum). C - Pods purplish brown. D - Seed (sub-mature) showing areole closed or with a narrow opening at hilar end. Photos: B.C. Ho (from plants in Thailand). Scale bar = 5 mm (unless otherwise indicated). |
≡ Acacia megaladena Desv., J. Bot. Agric. 3: 69 (1814), as 'megalodena' Type citation: No type cited, but provenance given as "Habitat in India". Type: Habitatu in India orientalis, ex herb. A.N. Desvaux (holotype: P barcode P02436186 [digital image!]-see notes below under Typification.).
- "Mimosa megaladena" (Desv.) Poir., Encycl. [J. Lamarck et al.] Suppl.5, 530 (1817), as 'megalodena', nom. invalid.. (combination not actually made).
= Acacia arrophula D.Don, Prodr. Fl. Nepal. 247 (Dec. 1824, '1825'); Acacia pennata var. arrophula (D.Don) Baker, Fl. Brit. India [J.D. Hooker] 2(5): 298 (1878). Type citation: "Hab. in Sirinagur [now Srinagar located in Uttarakhand State, India: C.R. Fraser-Jenkins, pers. comm. April 2016]. Kamroop." Type: NEPAL, 1821, Wallich Numer. List no. 5257 (neotype: K-W barcode K001120304 [digital image!], here designated, see discussion below under Typification); isoneotypes: BM n.v.-cited by Nielsen (1980), CAL 140775!, K barcode K000791180 [digital image!], K barcode K000791181 [digital image!]).
- "A. arrophula" D.Don ex N.Wallich, Numer. List no. 5257 (1831), nom. nud.
[Acacia pennata auct. pl. non (L.) Willd.: Gagnep., Fl. Indo-Chine [P.H. Lecomte et al.] 2: 83 (1913), pro parte; Craib, Fl. Siam. 1: 550 (1928), pro parte; fide Nielsen (1981).]
Additional synonymy (not relevant to China): See Nielsen(1980, 1981, 1985a, 1985b, 1992).
Distinctive features. Upper branchlets glabrous or minutely hairy. Stipules (few seen) 0.6-1.5 mm wide, dark reddish brown, glabrous or sparsely appressed-hairy abaxially. Pinnae 12-24 pairs. Rachis hairs short, shallowly curved and ±appressed. Leaflets (2-) 3-8 mm long, 0.4-1.5 mm wide, lateral veins (when present) ascending, apex symmetrically rounded or obtuse. Petiole gland prominent, thickened (raised 0.5-1.5 mm high), often situated near middle of petiole, flat-topped or concave above. Inflorescence buds reddish when fresh. Pods 20-37 mm wide, stipe 5-12 mm long.
Description. Robust lianas (stems to c. 50 mm DBH) or straggly shrubs or trees 4-10 m tall; tendrils at least sometimes present in leaf axils. Branchlets glabrous to sub-glabrous (when in flower) or (when in pod) sparsely to densely minutely hairy towards apices (hairs white, spreading to appressed and straight to shallowly curved). Prickles few or relatively numerous on internodes and sometimes on under surface of leaf axes, occasionally absent from herbarium specimens, not especially prominent (0.5-2 mm long), straight and patent to reflexed or very shallowly recurved. Stipules (few seen) caducous or sometimes (in var. indochinensis) conspicuous in young inflorescence buds, triangular to narrowly triangular to narrowly lanceolate, 3-8 mm long, 0.6-1.5 mm wide, glabrous or sparsely appressed-hairy and obscurely to visibly striate abax-ially, dark reddish brown, acuminate. Leaves bipinnate; pinnae 12-24 pairs (but see note below under Variants), (15-) 20-110 mm long; petiole 35-60 mm long; rachis 130-160 (-250) mm long, ±appressed-hairy on upper surface (hairs short to longish and shallowly curved) (but see note below under Variants). Leaflets 56-64 pairs, (2-) 3-8 mm long, 0.4-1.5 mm wide, flat and straight when fresh, sometimes shallowly concave and (in var. indochinensis) obviously curved forward when dry, ±moderately dis-colorous (darkest above), green or slightly sub-glaucous with dull lustre on both surfaces (young growth light green), glabrous except normally ciliolate; apex rounded to obtuse, without an apiculum, glabrous or microscopically ciliolate; base unequal with an obvious rounded angle on lower edge, the petiolule clearly excentric, very short but discernible extended below base of leaflet; main vein starting near upper margin at leaflet base and extending obliquely to apex, straight to shallowly curved; lateral veins (on lower surface of leaflets) obscure to somewhat evident or (in var. indochinensis) not visible, ascending, not forming a reticulum. Glands: petiole gland-position variable (see note below under Petiole gland), often situated near middle of petiole (sometimes above or below middle) and 9-33 mm above leaf base and 3-28 mm above the pulvinus, oblong to oblong-elliptic or broadly elliptic, 1.5-4 (-5) mm long, 1-2 (-3) mm wide, l:w = 0.7-2.5, sessile, depressedoblate, rather prominent and thickened (raised 0.5-1 mm above petiole), upper surface flat-topped or concave when dry, commonly with a shallow or deep pore on upper surface, orange to orange-brown, smooth or coarsely wrinkled when dry, apterous; rachis glands - situated at base of terminal 1-4 (-5) pairs of pinnae, circular or oblong, 1.5-2 mm long, sessile; rachilla glands-present or absent, situated between uppermost 1 or 2 pairs of leaflets. Inflorescences comprising pedunculate heads arranged in terminal open panicles or occasionally elongated racemes, peduncles often subtended by a bract-like prophyll, the panicle/rachis axes sparsely to moderately hairy (hairs white, very short, appressed to spreading and straight to shallowly curved), resin hairs absent; peduncles (5-) 8-22 mm long, to 35 mm long in fruit, single or clustered in groups of up to four, densely pubescent but indu-mentum often sparser when in pod (the hairs white, short or ±long, normally shallowly curved and appressed to sub-appressed, occasionally straight and patent); heads globose, 8-10 (-13) mm diam. when dry, white to creamy white or pale yellow, slightly fragrant, densely 35-50-flowered; inflorescence buds dark brown to dark greyish when dry (red when fresh; see note below under Inflorescence bud colour). Bracteoles (var. indochinensis) not exserted beyond flowers in buds, spathulate, 1-1.5 mm long, glabrous or sometimes with very few marginal hairs on lamina; claw narrowly oblong, c. same length as the narrowly ovate, acute to short acuminate lamina. Flowers 5-merous, sessile or sub-sessile; calyx ¾-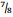

Distribution (Fig. 28). Senegalia megaladena is widespread on the Indian Subcontinent (Bangladesh, Bhutan, Indiadincluding South Andaman Is., Nepal) extending to Southeast Asia (Cambodia, IndonesiadJava, Laos, ?Peninsular Malaysiadrecorded for northern Malaysia by Nielsen(1985b, 1992) but not listed for that region by Turner (1997), Myanmar, Thailand & Vietnam) and southern China (Yunnan and Hainan; see under var. megaladena below for note regarding Xizang Autonomous region (Tibet) records.
Typification. There is only a single specimen at herb. P that qualifies as a type of Acacia megaladena. It originated from Desvaux's herbarium and is not at variance with the protologue. The specimen is therefore here regarded as the holotype, and was determined as such by Ivan Nielsen in 1977, a decision that he subsequently published, see Nielsen(1980, 1981, 1985b).
In the protologue of Acacia arrophula, a single collection was cited by Don (1824 (Dec.) '1825'), namely, "Sirinagur. Kamroop" Today Sirinagur is called Srinagar and is located in Uttarakhand State of northern India, bordering Nepal (C.R. Fraser-Jenkins, pers. comm.); Kamroop (Kamrup) was a botanical collector employed by Nathaniel Wallich (Fraser-Jenkins, 2006). According to Stafleu and Cowan (1976), the types of taxa described by David Don in Prodromus florae nepalensis are at BM and LINN; however, there is no Sirinagur, Kamroop specimen of A. arrophula in either of those herbaria (Jacek Wajer and Mark Spencer, pers. comm. April 2016), and we located none elsewhere. The type is therefore regarded as lost and a neotype is needed. Accordingly, Wallich Numer. List no. 5257 is chosen above as neotype. This is an appropriate choice because hitherto this collection had been treated as the holotype of A. arrophula by Nielsen(1980, 1981) and Chakrabarty and Gangopadhyay (1996). This appears to have had its origins with Baker (1878) who listed only this collection when reducing Don's species to a variety of A. pennata.
Taxonomy. As noted by Nielsen (1980), Acacia megaladena had in the past commonly been combined with A. pennata, but Brenan and Exell (1957) clarified the situation by treating them as two separate species confined to the Asian region. Nielsen (1980) recognised three varieties within Acacia (Senegalia) megaladena, namely, var. megaladena, var. indochinensis I.C.Nielsen and var. garrettii I.C.Nielsen. In the present work, var. garrettii is regarded as a distinct species (see S. garrettii for discussion) but we recognise the other two varieties, both of which occur in China (see below under Varieties for discussion). In the treatment of Chinese Acacia by Wu and Nielsen (2010), only var. megaladena and var. garrettii were recognised.
With Senegalia garrettii now removed from S. megaladena, the latter is a more cohesive species than previously defined. Never-theless, Senegalia megaladena remains rather variable and a better understanding of this variation over its wide geographic range is needed. Plants from the Indian subcontinent are especially important to any such study, but until very recently an investigation of these has been constrained because Chakrabarty and Gangopadhyay (1996) included Acacia megaladena as a synonym within a broadly defined A. pennata. Although Deshpande et al. (2019) have now clarified the situation with respect to distribu-tion of these two species on the subcontinent, a detailed study of their morphological variation is still required. It is parenthetically noted here that during the course of the present study, the first author (BRM) inspected the herb. DD type of A. brunnescens C.E.Parkinson that was collected from Myanmar, and confirmed its placement within S. megaladena as currently defined; in the absence of having seen this type Nielsen (1980) provisionally treated A. brunnescens as a synonym of A. megaladena.
Because of variation within Senegalia megaladena, it is considered prudent to restrict our treatment of the species to just those plants that occur in China.
Varieties. The main characters used by Nielsen(1980, 1981, 1985a, 1992) to distinguish var. megaladena from var. indochinensis related to leaflet size and lateral vein prominence: var. megaladena was described as having leaflets 4-8 mm long and 0.8-1.5 mm wide with prominent lateral veins, whereas those of var. indochinensis were described as 1.5-4.5 mm long and 0.3-0.8 mm wide with hardly visible lateral veins. As to distribution Nielsen(1980, 1981, 1985a, 1992) recorded var. megaladena as ranging from the Indian subcontinent to Southeast Asia and China, whereas var. indochinensis was confined to Southeast Asia (not recorded for China). The other relatively few characters used by Nielsen (loc. cit.) to separate these two varieties are overlapping and/or not especially convincing.
While it is recognised that the leaflet characters used by Nielsen to distinguish varieties within Acacia (Senegalia) megaladena are rather slight, an examination of specimens from China revealed that leaflets on plants from Yunnan fell within the range of var. megaladena, whereas those from Hainan corresponded to var. indochinensis. While Nielsen's taxonomy is recognised in the present work, it is acknowledged that a more detailed study of S. megaladena over its entire geographic range may necessitate a change to this decision.
Affinities. Despite differences in petiole gland morphology Senegalia megaladena seems not far removed taxonomically from S. garrettii, and is sometimes superficially similar to S. kerrii (see these two species for discussion).
Variants. The following unusual collections are close to Senegalia megaladena but are not included within its circumscription here for reasons discussed below.
It appears that C.W. Wang made two independent collections from Yunnan Province to which he applied the collecting number 74671; specimens of these collections are at both IBSC and KUN. The specimens IBSC 187553 [barcode 0159145] and KUN 0400082 [barcode 0598295] cited above were collected in June 1936 from Menghai County, are Senegalia megaladena var. megaladena. However, the collections IBSC 680586 [barcode 159138] and IBSC 680589 [barcode 0159147] that were collected in 1935 without specific locality or exact date of collection are unusual. In all characters except petiole gland morphology they agree with S. megaladena var. megaladena; however, the petiole gland is less raised than normal for this variety and its overall morphology is not dissimilar to that of S. clandestina, i.e. depressed and finely longitudinally wrinkled. Further collections of this atypical petiole gland morphotype are needed in order to better assess its taxonomic status.
A second unusual collection from Yunnan (Pu'er City, Simao District) is P.I. Mao 6092 (KUN 0400086 [barcode 0598301] & KUN 0400095 [barcode 0598302]), which in some ways seems inter-mediate between Senegalia megaladena var. megaladena and S. garrettii. It is similar to S. garrettii in having patent rachis hairs (appressed in S. megaladena) and it has 6-9 pairs of pinnae, which is slightly closer to those of S. garrettii (pinnae 10-15 pairs) than to S. megaladena (pinnae 12-24 pairs). However, the leaflet and petiole gland morphology of this collection are clearly those of S. megaladena. There are specimens of typical S. garrettii and S. megaladena recorded from Pu'er City. See under Senegalia garrettii treatment above for other possible intermediates between these two species.
Petiole gland. The petiole gland in Senegalia megaladena is commonly located near the middle of the petiole and is often a useful character for identifying the species. However, there is variation for this character, and the variation in gland position is similar to that which has been described for Senegalia garrettii above.
Inflorescence bud colour. The inflorescence buds on both varieties of Senegalia megaladena are dark brown to dark greyish when dry. As discussed above under Morphological characters: Inflorescences and flowers this suggests that at least the sepals on living plants were red. As can be see from Fig. 29F the buds on plants of var. megaladena from Xishuangbanna Tropical Botanical Garden, China, are dark red, however, we have not confirmed the bud colour for var. indochinensis. In southern India the buds of var. megaladena are reputed to be always red (A. Deshpande, pers. comm.).
Etymology. From the Greek megalos (large) and adenos (gland) in allusion to the relatively large petiole gland that occurs on this species.
Vernacular name. Obtuse-leaflet Senegalia. This name was used by Sun (2006) and Wu and Nielsen (2010) under A. megaladena.
Key to varieties
Leaflets 4-8 mm long, normally 0.8-1.5 mm wide, lateral veins visible (obscure to somewhat evident). Yunnan. …var. megaladena. Leaflets (2-) 3-4 (-5) mm long, 0.4-0.6 mm wide, lateral veins not visible or occasionally extremely faint Hainan………………………………………………………… var. indochinensis.
11a. var. megaladena (Figs. 27A, C, D, E, I & J and 29)
=Acacia megaladena var. megaladena, Adansonia sér. 2, 19: 351 (1980), effected by the publication of A. megaladena var. garrettii I.C.Nielsen and var. indochinensis I.C.Nielsen.
Description. Stipules (few seen) triangular to narrowly triangular, 3 mm long, 0.6-1 mm wide. Pinnae 40-110 mm long. Leaflets 4-8 mm long, (0.7-) 0.8-1.5 mm wide, not or scarcely curved forward when dry; lateral veins visible and obscure to somewhat evident. Petiole gland 2.5-4 (-5) mm long.
Selected specimens examined. CHINA: Yunnan. Baoshan City, Longling County, 1956, Sino-Soviet Joint Expedition 336 (IBSC 641934 [barcode 0159414], KUN 0400094 [barcode 0598303]); Dali Bai Autonomous Prefecture, Nanjian Yi Autonomous County, 1 Jan. 2010, J.S. Li & Y.C. Guan NJWLS845 (KUN 0943518 [barcode 1318449]); Dehong Dai & Jingpo Autonomous Prefecture, Long-chuan County, 2 Dec. 1974, G.D. Tao 13471 (KUN 0400101 [barcode 0598316], KUN 0400110 [barcode 0598317]); Dehong Dai & Jingpo Autonomous Prefecture, Longchuan County, Mengyue Village, L. Bai & H. Wang, BLK-072 (KUN); Dehong Dai & Jingpo Autonomous Prefecture, Mang City, 14 Dec. 1997, F. Konta & R.S. Wang 3645 (KUN 0764123 [barcode 0579412]; Dehong Dai & Jingpo Autonomous Prefecture, Ruili City, 3 Dec. 1983, Q. Lin 770832 (KUN 0400112 [barcode 0598324]); Dehong Dai & Jingpo Autonomous Prefecture, Yingjiang County, Nov. 1952, R.C. Ching 50055 (KUN 0400089 [barcode 0598298]); Honghe Hani & Yi Autonomous Prefecture, Hekou Yao Autonomous County, 27 July 1953, W.S. Liou 309 (KUN 0400087 [barcode 0598300], KUN 0400088 [barcode 0598299]); Honghe Hani & Yi Autonomous Prefecture, Jinping Miao, Yao & Dai Autonomous County, near Manpeng village, 22°37'41"N, 103°08'38"E, 6 June 2019, B.R. Maslin & L. Bai BRM 11040 (IBSC, KUN [barcode 1347996]); Honghe Hani & Yi Autonomous Prefecture, Lühun County, 4 Oc. 1973, D.D. Tao 562 (KUN 0400102 [barcode 0598315]); Lincang City, Cangyuan Wa Autonomous County, 12 Sep. 1980, S.T. Li 1135 (KUN 0400111 [barcode 0598325], KUN 0400120 [barcode 0598326]); Lincang City, Fengqing County, 21 Nov. 1977, Q. Lin 7742 (KUN 0400114 [barcode 0598323]); Lincang City, Linxiang District, 23 Aug. 1957, G.S. Sin 246 (KUN 0400092 [barcode 0598305], KUN 0400093 [barcode 0598304]); Lincang City, Shuangjiang Lahu, Wa, Blang & Dai Autonomous County, 19570915, G.S. Sin 928 (IBSC 313189 [barcode 0159137], KUN 0400091 [barcode 0598306], KUN 0400100 [barcode 0598307]); Lincang City, Yongde County, 5 Sep. 2002, E.D. Liu 6395 (KUN 0798083 [barcode 0598419]); Lincang City, Yun County, 20 Sep. 1988, Anonymous 229 (KUN [barcode 0598440]); Pu'er City, Jingdong Yi Autonomous County, 3 July 1993, H. Peng & B. Bai 965 (KUN 0400130 [barcode 0598336]); Pu'er City, Jinggu Dai & Yi Autonomous County, 28 Nov. 1986, 1986 Expedition 1209 (KUN 0400118 [barcode 0598328], KUN 0400119 [barcode 0598327]); Pu'er City, Menglian Dai, Lahu and Wa Autonomous County, 18 Aug. 1973, Menglian Expedition 10220 (IBSC 474106 [barcode 0159286], KUN 0400103 [barcode 0598314]); Xishuangbanna Dai Autonomous Prefecture, Jinghong City, Aug. 1936, C.W. Wang 75618 (KUN 0400081 [barcode 0598296]) & 10292 (KUN 0400096 [barcode 0598311]; Xishuangbanna Dai Autonomous Prefecture, Menghai County, June 1936, C.W. Wang 74671 (IBSC 187553 [barcode 0159145]), KUN 0400082 [barcode 0598295]); Xishuangbanna Dai Autonomous Prefecture, Mengla County, 6 Jan. 1961, Z.W. Lin 3396 (KUN 0758883 [barcode 0598444]); Yuxi City, Xinping Yi & Dai Autonomous County, H. Sun 15776 (KUN); Yuxi City, Yuanjiang Hani, Yi & Dai Autonomous County, 6 Nov. 1958, S.K. Wu 681 (KUN 0400098 [barcode 0598309], KUN 0400099 [barcode 0598308]).
Distribution (Fig. 28). Variety megaladena is widespread on the Indian Subcontinent (Bangladesh, Bhutan, Indiadincluding South Andaman Is., Nepal) extending to Southeast Asia (IndonesiadJava, Laos, Myanmar, Thailand & Vietnam) and southern China (Yunnan), fide Nielsen(1980, 1981, 1985a, 1992). In China var. megaladena is common in southwest Yunnan (south of latitude 25°N). Although Wu and Nielsen (2010) recorded the variety from Guangxi, no specimens of it from that Province have not been found. (See below under Variant for an entity from Xizang Autonomous region (Tibet) that had been recorded as A. megaladena var. megaladena.)
Habitat. Grows in open or dense forest or thickets, in association with sandstone mountains; recorded on specimen labels as occurring between 230 and 1400 m alt. In Jinping County where we observed living plants they were quite common in the general area but occurred singularly or in groups of a few individuals.
Phenology. Flowers from July to September. Pods with mature seeds have been collected from December to February, but in December the pods are normally not quite mature.
Variant. Sun (2006) recorded Acacia megaladena var. megaladena from Xizang Autonomous region (Tibet), based on the following two collections (represented by four specimens) at KUN: H. Sun, Z.Q. Zhou & H.Y. Yu 192 and 1497. The specimen labels show these plants as lianas occurring in forest areas between 600 and 950 m alt. Although the specimens are sterile, they appear to represent a Senegalia because the branchlets possess scattered, internodal prickles and the leaves possess sessile glands on both the petiole and rachis. These specimens may superficially resemble S. megaladena var. megaladena (e.g. pinnae to 13 pairs, leaflets 7-10 mm long and 1-1.5 mm wide, petiole gland 2-4 mm long) but are considered unlikely to be that variety, principally because the leaflet apices are either clearly acute or obtuse-apiculate and the petiole gland is clearly flattened. In var. megaladena the leaflet apices are rounded to obtuse without an apiculum, while the petiole gland is clearly thickened and elevated. There is also a subtle (but perhaps significant) difference in the leaflet venation. In the Tibet plants the main vein on the lower surface of the leaflet is situated towards the upper margin with which it is parallel for some distance above the petiolule; this same arrangement is normally also seen in species of the 'Hainanensis species-group' and in S. pennata subsp. insuavis. In var. megaladena the main vein of the leaflets is not parallel with the upper margin. Flowering and fruiting collections of the Tibet entity are needed so that its identity can be further assessed.
11b. var. indochinensis (I.C.Nielsen) Maslin, Seigler & Ebinger, Blumea 58: 41 (2013) (Figs. 27B, F, G & H and 30)
≡ Acacia megaladena var. indochinensis I.C.Nielsen, Adansonia sér. 2, 19: 351 (1980), as 'indo-chinensis' Type citation: Larsen, Smitinand & Warncke 375, Thailand, S.E., Prachin Buri; Khao Yai National Park, alt. 750 m (holo-, AAU)". Type: Thailand, Prachinburi [Thai: Prachin Buri], Khao Yai National Park, alt. 750 m, 13 July 1966, K. Larsen, T. Smitinand & E. Warncke 375 (holotype: AAU [digital image!]; isotype: BKF 54811 barcode SN036634!).
[Acacia pennata var. arrophula auct. non (D.Don) Baker: Craib, Fl. Siam. 1: 550 (1928), pro parte; fide Nielsen (1980, 1981, 1985a).] [A. pennata (L.) Willd. var.: Craib, Fl. Siam. 1: 551 (1928); fide Nielsen(1981, 1985a).]
Description. Stipules (few seen) conspicuous in young inflorescence buds, narrowly triangular to narrowly lanceolate, 4-8 mm long, 1-1.5 mm wide. Pinnae (15-) 20-55 mm long. Leaflets (2-) 3-4 (-5) mm long, 0.4-0.6 mm wide, often shallowly to moderately curved forward when dry; lateral veins not visible or occasionally extremely faint. Petiole gland 1.5-2.5 mm long (to 4 mm in Thailand).
Distribution (Fig. 28). Variety indochinensis is widespread in Southeast Asia: Cambodia, Indonesia e Java where it is probably not native, Laos, ?Peninsular Malaysiadsee above, Thailand and Vietnam, see Nielsen(1980, 1981, 1985a, 1992). Although not previously recorded for China, var. indochinensis occurs in the south west of Hainan Island.
Selected specimens examined. CHINA: Hainan. Changjiang Li Autonomous County, 15 Dec. 1933, H.Y. Liang 66263 (IBSC 67554 [barcode 0159226]); Dongfang City, 28 Oct. 1933, H.Y. Liang 63863 (IBK 11925 [barcode 00067612], IBK 11925 [barcode 00067617], IBSC 66962 [barcode 0159227]); Ledong Li Autonomous County, 29 Nov. 1978, K.S. Chow 78241 (IBSC 451109 [barcode 0159240]); Lingshui Li Autonomous County, 18 May 1956, H.D. Zhang A205 (IBSC 224711 [barcode 0159211]); Sanya City, 21 Apr. 1984, Z.X. Li & F.W. Xing 1330 (IBSC 522465 [barcode 0159221]); Wuzhishan City, 7 Nov. 1959, Sino-German Expedition 1876 (IBSC 680590 [barcode 0159213]); without specific locality, 1955, Hainan Expedition 473 (KUN 0400083 barcode 0598339).
Habitat. Grows in open forest; 100-400 m alt.
Phenology. Flowers July to September. Pods with mature seeds have been collected in April.
Variant. See note under Senegalia garrettii regarding S.P. Ko 55808 collected from Guangxi.
12. Senegalia obliqua Maslin, B.C.Ho, H.Sun & L.Bai, sp. nov. (Fig. 31独眼龙【dú yǎn lóng(新拟)
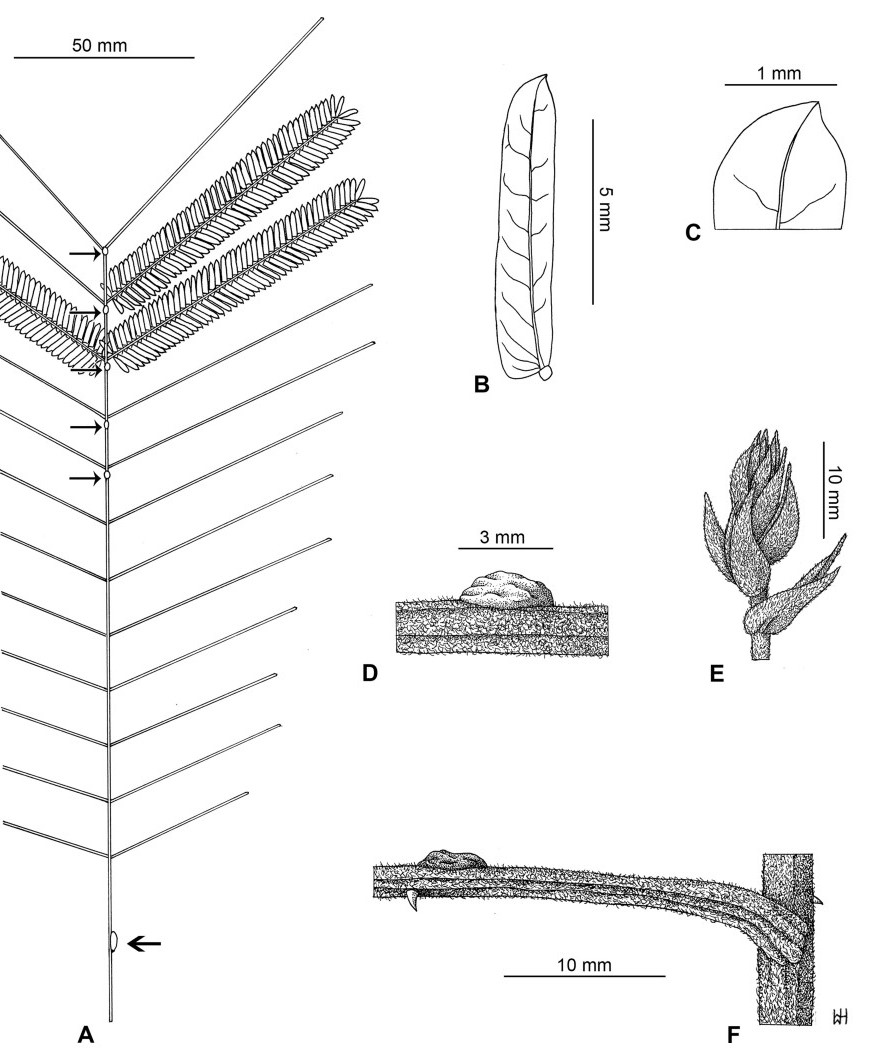
|
| Fig. 31 Senegalia obliqua. A - Leaf showing position of petiole gland (thick arrow) and rachis glands (thin arrows). B - Leaflet (lower surface) with rather evident patent lateral veins and apex showing excentric, bluntly acute apiculum. C - Leaflet apex. D - Petiole gland prominent. E - Young vegetative shoot (densely orange-brown hairy). F - Node showing densely (minutely) puberulous branchlet and petiole gland well-removed from leaf base. Vouchers: K.M. Feng 22449 - isotype (A & F); K.M. Feng 13873 (B & E); Z.G. Wang 3-1749 (C); P.I. Mao 453 (D). Drawn by Waiwai Hove. |
Type: CHINA: Yunnan Province, Wenshan Zhuang & Miao Autonomous Prefecture, Wenshan City, Kaihua [township], Laojun Mountain, 5 May 1962, K.M. Feng 22449 (holotype: KUN 0400300 barcode 0598512!; isotypes: IBSC 680612 barcode 0159167!; KUN 0400299 barcode 0598513!).
Distinctive features. Branchlets densely minutely puberulous. New shoots densely, appressed, orange-brown hairy when first initiated. Pinnae 10-15 pairs. Leaflets 6-9 mm long, 1-1.5 mm wide; apex normally obliquely rounded with a distinct, excentric, bluntly acute apiculum; lateral veins (on lower surface) ±patent and somewhat evident. Petiole gland prominently raised, normally situated near middle of petiole; rachis glands 2-6, prominently raised. Peduncles and inflorescence axes with very dense, spreading, orange-brown hairs. Pods microscopically velutinous, glandularscurfy when young.
Description. Lianas. Branchlets densely minutely puberulous (hairs straight to curved, light brown or white and patent to ±appressed); lenticels (upper branchlets) not evident. New shoots densely appressed orange-brown hairy when first initiated, indumentum less prominent as shoots expand. Prickles few to rather numerous on internodes and few on under surface of leaf axes, not prominent (0.5-3 mm long), straight to shallowly recurved, dark brown, normally glabrous towards apices and hairy (as on branchlets) towards base. Stipules at base of peduncles caducous, narrowly triangular-lanceolate, 4 mm long, 1.5-2 mm wide, densely hairy with veins not visible abaxially, often lobed (slightly to evidently) on one side at base, apex acute to short-acuminate; stipules on vegetative shoots conspicuous when first initiated, lanceolate, large (8-11 mm long and 3-5 mm wide), veins not visible or obscurely striate abaxially, base rounded on one or both edges and sometimes auriculate on one side, apex acuminate. Leaves bipinnate; pinnae 10-15 pairs, 40-90 mm long; petiole 35-45 mm long, hairy as on rachis; rachis 115-170 mm long, ±orange-brown, densely short-pubescent with hairs patent to appressed and straight to curved. Leaflets 30-50 (-65) pairs, narrowly oblong, 6-9 mm long, 1-1.5 mm wide, discolorous (often prominently so), glabrous or sparsely ±appressed-ciliate (hairs not prominent); apex asymmetric, obliquely rounded or slanting and ending in a distinctly excentric and bluntly acute apiculum, occa-sionally (especially on the Isotype) a few leaflets with symmetrical apices and ±centric apiculum; base unequal with a rounded angle on lower edge, the petiolule clearly excentric and shortly but discernible extended below base of leaflet; main vein starting near upper margin at leaflet base and extending obliquely to apex, ±straight or shallowly curved but normally obviously curved near leaflet apex; lateral veins (on lower surface of leaflets) somewhat evident and ±patent, not forming a reticulum. Glands: petiole gland-normally situated near middle of petiole (or occasionally on upper or lower 
Other specimens examined. CHINA: Guangxi. Baise City, Napo County, 10 Apr. 1978, Z.G. Wang 3-1749 (GXMI 21729 [barcode 052141]). Yunnan. Honghe Hani & Yi Autonomous Prefecture, Jinping Miao, Yao & Dai Autonomous County, 21 Aug. 1951, P.I. Mao 453 (IBSC 680593 [barcode 0159285], KUN 0400281 [barcode 0598503], KUN 0400282 [barcode 0598502]); Wenshan Zhuang & Miao Autonomous Prefecture, Funing County, 6 May 1940, C.W. Wang, 87066 (IBSC 680592 [barcode 0159273], KUN 0400182 [barcode 0598401] & KUN 0400183 [barcode 0598400]); Wenshan Zhuang & Miao Autonomous Prefecture, Malipo County, 15 Dec. 1947, K.M. Feng 13873 (KUN 0400289 [barcode 0598485] & KUN 0400290 [barcode 0598484]). VIETNAM: Northern. vicinity of Yen Lac Village, Bao Lac District, Yen Lac Municipality, Cao Bang Province, 22°44'N, 105°50'E, 16 Apr. 1999, P.K. Loc, P.H. Hoang & L. Averyanov CBL [sphalm. CLB] 1434 (HN, MO).
Distribution (Fig. 32). Southern China and northern Vietnam. In China, Senegalia obliqua occurs in southeast Yunnan near the border with Vietnam, and extends to the western extremity of Guangxi (Napo County), over about 250 km. In Vietnam, it is known from only a single gathering near the Chinese border, about 150 km southeast of Wenshan.
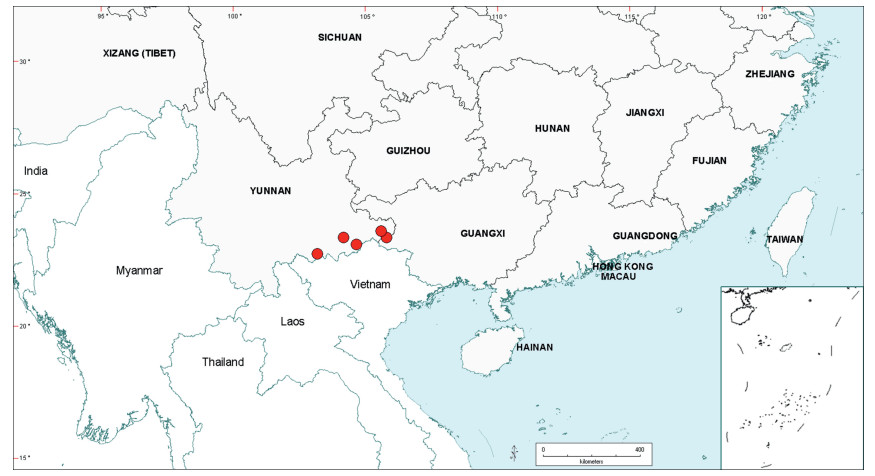
|
| Fig. 32 Distribution of Senegalia obliqua in China, based on specimen records at IBSC, KUN and GXMI, as cited in text. |
Habitat. Grows in limestone mountainous areas where it is recorded as both occasional and common. It occurs from 900 to 1500 m alt. in thickets or secondary wet broadleaved evergreen forest, in valleys and often along rivers or streams.
Phenology. The relatively few collections prevent an accurate phenological assessment. However, specimens with mature buds and some flowers at anthesis have been collected in early May; immature pods occur in late August and the one collection with mature seed (Loc et al. 1434) was collected in mid-April.
Taxonomy. Specimens of this hitherto unrecognised distinctive species had been labelled as Acacia rugata (=Senegalia rugata) at KUN, but the two species are not closely related. Among other characters, S. rugata is distinguished by its lack of the above-mentioned distinctive orange-brown indumentum, its reticulately-veined leaflets and its hard-textured pods that are coarsely wrinkled when dry. Senegalia obliqua differs significantly from all other Senegalia in China by its minutely velutinous pods.
Affinities. The closest relatives of Senegalia obliqua are uncertain, but it has some similarities with both S. garrettii and S. pruinescens, e.g. pinnae relatively few (normally 8-15 pairs) and petiole gland sometimes located near middle of the petiole; all three species occur in Malipo County, Yunnan. It further resembles S. garrettii in having densely hairy branchlets, narrow leaflets with patent lateral veins, and normally orange-brown, densely hairy flowering peduncles and inflorescence axes. Senegalia garrettii differs significantly from S. obliqua in its depressed, peripterous petiole gland, ±symmetrically rounded to obtuse leaflet apices that lack an apiculum and glabrous pods. While S. pruinescens has a prominently raised petiole gland and often asymmetric leaflet apices like those of S. obliqua, it can be distinguished in the following ways: branchlets normally pruinose and glabrous, leaflets often wider (normally 1.5-3 mm) and normally reticulatelyveined on their lower surface, pods wider (35-50 mm) and glabrous.
Note. When the new shoots of Senegalia obliqua are first initiated they are densely and distinctively orange-brown hairy, but as the shoots elongate much of the indumentum is less obvious. The significance of this indumentum character is unknown because new shoots at such and early stage of development have not been examined for most other species of Chinese Senegalia.
Uses. According to label information on Z.G. Wang 3-1749, this species can be used to cure high blood pressure, but no other details were provided.
Etymology. The species name is derived from the Latin obliquus (oblique, slanting), in reference to the leaflet apices that are obliquely rounded or slanting (and terminated by a distinct, bluntly acute apiculum). Although this character is not diagnostic for the species, it is characteristic of it.
Vernacular name. One-eye Dragon (following the Chinese common name that is proposed above). This name appeared on the label of Z.G. Wang 3-1749, but its meaning is unknown (but possibly it is in reference to the prominent petiole gland).
13. Senegalia orientalis Maslin, B.C.Ho, H.Sun & L.Bai, sp. nov. (Fig. 33).东方金合欢【dōng fang jīn hé huān】(新拟)
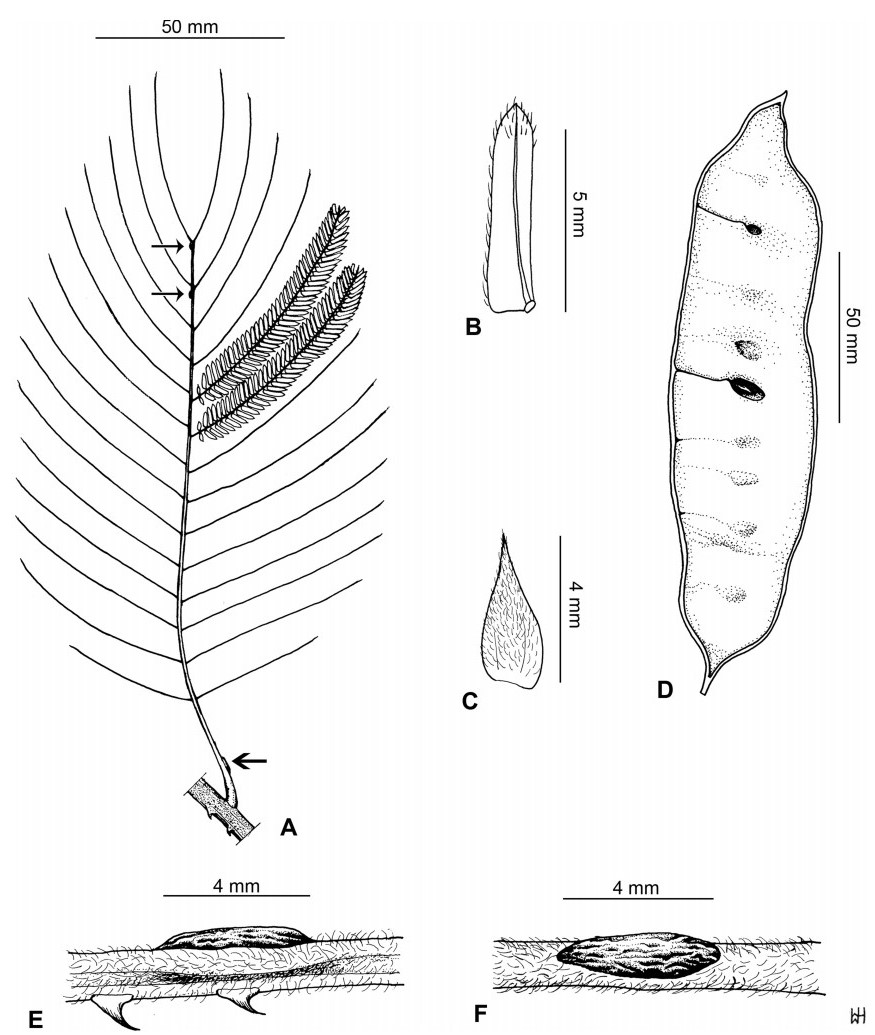
|
| Fig. 33 Senegalia orientalis. A - Leaf showing position of petiole gland (thick arrow) and rachis glands (thin arrows). B - Leaflet showing few hairs on lower surface of lamina at apex. C - Stipule. D - Pod (inner surface showing some immature seeds) rather large. E - Petiole gland (lateral view) depressed and flat-topped, with prickles on under surface of petiole. F - Petiole gland (plane view) elongated and finely longitudinally wrinkled when dry. Vouchers: C.C. Chang 10895 (A, D, E & F); W.T. Tsang 30328 (B); W.T. Tsang 29826 (C). Drawn by Joshua Yang (A, C - F) and Waiwai Hove (B). |
Type: VIETNAM, Dong Mo [township], Chi Lang District, Lang Son Province, 20 Apr. 1981, Tran Dinh Nghai & Pham Ke Loc T749 (holotype: HNU 12102!; isotypes!: HN, HNU, IBSC, KUN barcode 1345223, PE, VNM).
[Acacia pennata auct. non (L.) Willd.: E.D. Merrill, Lingnan Sci. J. 5: 89 (1927), pro parte, as to F.A. McClure 9369 (other specimens cited by Merrill under A. pennata not seen).]
[Acacia pennata subsp. hainanensis auct. non (Hayata) I.C.Nielsen: I.C.Nielsen, Fl. Cambodge, Laos & Vietnam 19: 67 (1981), pro parte, as to W.T. Tsang 29826 & 30328.]
Distinctive features. Branchlets dark-coloured (very dark grey to blackish) when dry, with lenticels absent or obscure. Prickles often evident. Pinnae numerous (14-20 pairs). Leaflets 4-6 mm long, 0.4-1 mm wide, strongly discolorous; apex acute (infrequently obtuse), margins normally ciliate by rather long hairs (to c. 0.5 mm) with some often extending to lower surface of lamina at leaflet apex; lateral veins (on lower surface of leaflet) not visible. Petiole gland elongated (3-6 mm long), depressed (a little thickened but not prominently raised), apterous, normally finely or coarsely longitudinally wrinkled when dry. Inflorescence buds darkcoloured when dry (dark greyish), purple when fresh. Pods broad (25-42 mm wide), dark brown, glabrous, reddish sessile glands absent or very few.
Description. Lianas or lianescent shrubs. Branchlets dark-coloured (very dark grey to blackish) when dry, sparsely to densely and often patent-hairy, the hairs short or long and mostly straight; lenticels absent from upper branchlets, obscure and scattered or absent on mature branchlets. New shoots with young leaflets membranous, undulate and with conspicuous long hairs on margins at least. Prickles normally evident and numerous on internodes and sometimes scattered on under surface of leaf axes, occasionally absent from herbarium specimens, on upper branchlets mostly straight and reflexed or shallowly recurved and 0.5-2 mm long, on mature branchlets (of one specimen, P.A. Petelot 6245) to 5 mm long, clearly recurved and quite vicious-looking. Stipules ovate to lanceolate or triangular-lanceolate, 3-5 mm long, 1.5-2.5 mm wide, sparsely to densely hairy with longitudinal veins obscure to evident (but not strongly pronounced) abaxially, rounded at base (but not auriculate), apex acute to acuminate. Leaves bipinnate; pinnae 14-20 pairs, 20-45 (-55) mm long; petiole 15-35 mm long; rachis 80-185 mm long, indumentum dense on upper surface, the hairs variable (either all longish, patent and straight or curved, or all short, curved and ±sub-appressed, or a mixture of both types). Leaflets 35-60 pairs, narrowly oblong to almost linear, 4-6 mm long, 0.4-1 mm wide, strongly discolorous, dark greyish above and pale greenish or brown below when dry, straight to shallowly or sometimes ±moderately curved forward, flat to shallowly or ±obviously concave, margins normally ciliate by rather long hairs (to c. 0.5 mm) with some often extending to lower surface of lamina at leaflet apex (see notes below under Leaflet indumentum); apex mostly symmetric, narrowed to a centric, acute or broadly acute point, infrequently obtuse (not rounded); base unequal with an obvious lobe or rounded angle on lower edge, petiolule very reduced and clearly excentric; main vein starting near upper margin at leaflet base and extending obliquely to apex, straight or very shallowly curved; lateral veins (on lower surface of leaflets) not visible. Glands: petiole gland-situated on lower ¼ of petiole normally 5-12 mm above leaf base and 0-6 mm above pulvinus, narrowly oblong to narrowly elliptic, elongated, 3-6 mm long, 1-1.5 (-2) mm wide, l:w = (2-) 3-4, sessile, solid, depressed, a little thickened but not prominently raised (0.5-1 mm high), flattopped or slightly convex or concave on upper surface, apterous, often finely or coarsely longitudinally wrinkled when dry; rachis glands - situated at base of uppermost 1-3 (-5) pairs of pinnae, oblong to oblong-elliptic, 1-1.5 mm long, 0.8-1 mm wide, depressed, sessile; rachilla gland-situated at base of uppermost pair of leaflets, similar to rachis glands but smaller (c. 0.3 mm long). Inflorescences comprising pedunculate heads arranged in terminal open panicles or racemes, the panicle/raceme axes dark-coloured when dry, puberulous, glandular hairs very few; peduncles (10-) 15-25 (-30) mm long, densely puberulous (hairs short, straight or curved) with indumentum persisting to pod stage; heads globose or slightly obloid, 9-13 mm diam. when dry, white to pale yellow, fragrant, densely 45-60-flowered; inflorescence buds dark-coloured (greyish) when dry, purple when fresh (see note below under Inflorescence bud colour). Bracteoles spathulate, 1-1.3 mm long, not exserted beyond flowers in buds; claw linear or narrowly oblong and about same length as the narrowly ovate, acute lamina which are appressed-hairy abaxially. Flowers 5-merous, sessile or sub-sessile (pedicel 0.2-0.3 mm long); calyx c. ¾-
Selected specimens examined. CHINA: Fujian. Zhangzhou City, Hua'an County, 9 June 1959, S.M. Hwang 190525 (IBSC 301772 [barcode 0159301]). Guangdong. Huizhou City, Longmen County, 6 Apr. 1981, Nankun Mountain Expedition 71347 (IBSC 475440 [barcode 0158747]); Qingyuan City, Yangshan County, Oct. 1984, Anonymous s.n. (IBSC 655596 [barcode 0159374]); Yunfu City, Yunnan County, 2 Apr. 1955, C. Wang & L. Teng 4118 (IBSC 206719 [barcode 0159202]); Zhaoqing City, Dinghu District, 28 Feb. 2010, H.G. Ye et al. 18437 (IBSC 755564 [barcode 0758091]). Guangxi. Baise City, Longlin Multinational Autonomous County, 14 Nov. 1957, C.C. Chang 10895 (IBK 76576 [barcode 00067608], IBSC 245556 [barcode 0159294]); Fangchenggang City, Shangsi County, 29 Mar. 1991, C.K. Huang 21456 (GXMI 58592 [barcode 058182] & GXMI 58593 [barcode 058183]), slightly atypical in leaflets glabrous; Laibin City, Jinxiu Yao Autonomous County, 27 May 1928, S.S. Sin 136 (IBK 190716 [barcode 00067611], IBSC 136933 [barcode 0159292]); Wuzhou City, Wanxiu District, 1 Nov. 1958, S.Q. Zhong 302050 (IBK 87990 [barcode 00067608], KUN 0400065 [barcode 0598272]). Hainan. Lingshui Li Autonomous County, 26 Apr. 1932, S.P. Ko 52208 (IBK 50414 [barcode 00067613], IBSC 31641 [barcode 159229]). Wuzhishan City, 29 Apr. 1922, F.A. McClure 9369 (IBSC 53550 [barcode 0159241]). Hunan. Yongzhou City, Jianghua Yao Autonomous County, 11 Apr. 1963, C.J. Qi 30030 (IBSC 318466 [barcode 0159306]). Taiwan. Nantou County, 16 July 1998, M. Wang 3413 (IBSC 675175 [barcode 0159078]). VIETNAM: Northern. Environs du Phan Me, Thai Nguyen Province, 17 May 1936, [P.A.] Petelot 6245 (HNU 1364); vicinity of Long Ngong Village, Dam-ha, Quang Ninh Province, 18-31 May 1940, W.T. Tsang 29826 (BKF 27925 [barcode SN 036632] & SING barcode 0206823dspecimens labelled "Sai Wong Mo Shan [Sai Vong Mo Leng]), Lung Wan Village, Dam-ha, Tonkin, 18 Maye5 July, 1940" IBSC 145045 [barcode 0159310]); Sai Wong Mo Shan [Sai Vong Mo Leng]), Long Ngong Village, Dam-ha, 13 Aug. 1940, W.T. Tsang 30328 (IBSC 145074 [barcode 0159311]dwithout specific locality, SING barcode 0206822ddated 18 Julye9 Sep. 1940).
Distribution (Fig. 34). Occurs in southeast China (including Taiwan) and northeast Vietnam. Within China, Senegalia orientalis has a scattered but wide distribution that extends from Fujian (Zhangzhou City) west-southwest from Hunan to Guangxi (west to Baise City) and Guangdong to southern Hainan and Taiwan. In Vietnam, the species occurs in Quang Ninh and Thai Nguyen Provinces which are not far south of the Chinese border.
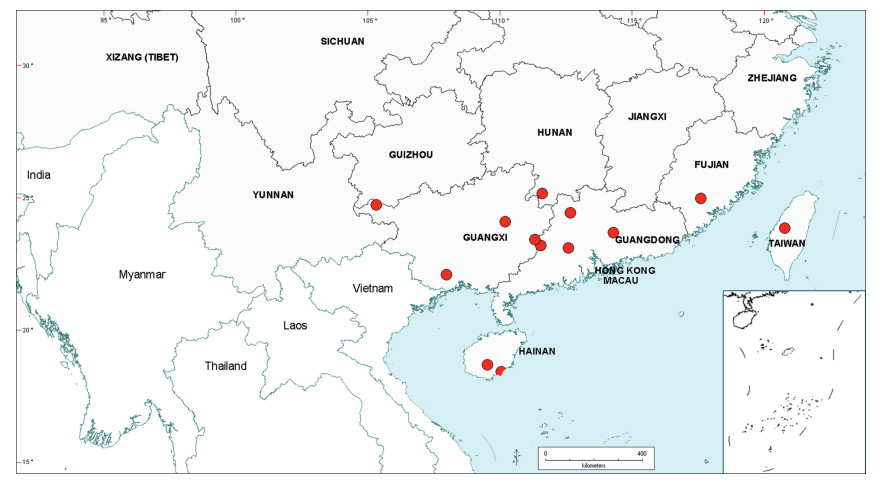
|
| Fig. 34 Distribution of Senegalia orientalis in China, based on specimen records at IBK, IBSC, GXMI and KUN, as cited in text. |
Habitat. Grows in hilly country (200-800 m alt.) in open or dense forest.
Phenology. Flowers at anthesis (often accompanied by flowers in bud) have been collected from late April to late June, therefore the flowering period extends to at least July. Pods with mature seeds have been collected only in mid-November.
Taxonomy. Specimens of this hitherto unrecognised species had in the past been variously named in herbaria, but most commonly as Acacia pennata (=Senegalia pennata). The specimens W.T. Tsang 29826 & 30328 from Vietnam cited under A. pennata subsp. hainanensis (=S. hainanensis) by Nielsen (1981) are S. orientalis, as is the specimen F.A. McClure 9369 from Hainan Island cited by Merrill (1927) as A. pennata
Affinities. The closest relative of Senegalia orientalis is uncertain, but its distinctive petiole gland suggests affinities with S. clandestina, S. guangdongensis and especially S. pennata subsp. insuavis (see these taxa for discussion). Although the leaflets of S. stipitata sometimes resemble those of S. orientalis, and both species have dark-coloured branchlets and raceme/panicle axes, they appear not to be especially closely related. In S. stipitata, the dark colouring of the raceme/panicle axes is due to the presence of a dense layer of glandular hairs (which are absent from S. orientalis). Senegalia stipitata also has distinctively stipitate or sub-stipitate petiole and rachis glands (with the latter normally being more numerous than in S. orientalis). In China Senegalia stipitata is recorded only from Yunnan where S. orientalis is not known to occur; however, both species occur in northern Vietnam.
Leaflet indumentum. The leaflet indumentum in Senegalia orientalis is often rather distinctive and can be a useful, albeit cryptic, aid to identification. Normally the hairs on leaflet margins are rather long, often somewhat spreading and quite obvious, especially towards the leaflet apex where a few hairs often extend to the lower surface of the leaflets. It is the presence of hairs on the lower surface of the lamina near the leaflet apex that is unusual. However, this character is variable, because occasionally the hairs are very short and/or closely appressed, and may be absent from the lower surface of the leaflets. The specimen C.K. Huang 21456 from Guangxi is atypical in having glabrous leaflets but is otherwise typical of the species.
Inflorescence bud colour. The label on one specimen of Senegalia orientalis (i.e. C.K. Huang 21456) records the inflorescence bud colour as purple. Because inflorescence buds on herbarium specimens of this species are always dark greyish, it is likely that they are always purple (perhaps ranging to red) in living plants (see discussion above under Morphological characters: Inflorescences and flowers).
Etymology. The species name is derived from the Latin orientalis (eastern) in reference to the species' generally easterly distribution within China.
Vernacular names. Oriental Senegalia. Both this name and the Chinese common name that is proposed above are derived from the scientific name.
14. Senegalia pennata subsp. insuavis (Lace) Maslin, Seigler & Ebinger, Blumea 58: 42 (2013) (Figs. 35 and 37).臭菜藤【chòu cài téng】
|
| Fig. 35 Senegalia pennata subsp. insuavis. A - Leaf showing numerous pinnae and position of petiole gland (thick arrow) and rachis glands (thin arrows). B - Leaflet (flattened, lower surface) showing finely acute point. C - Stipule. D - Leaflets (folded longitudinally upon drying) showing fine, acute points. E - Pod long with distinct stipe (s). F - Node showing flattened petiole gland (g) close to leaf base. G - Petiole gland flattened. Vouchers: B.R. Maslin et al. 11016 (A); Lühun Expedition 976 (B); S.X. Zhao 86 (C); K.M. Feng 20857 (D & F); S.J. Pei 9772 (E); H.K. Lua & A.T.K. Yee 2014-334 (G). Drawn by Joshua Yang. |
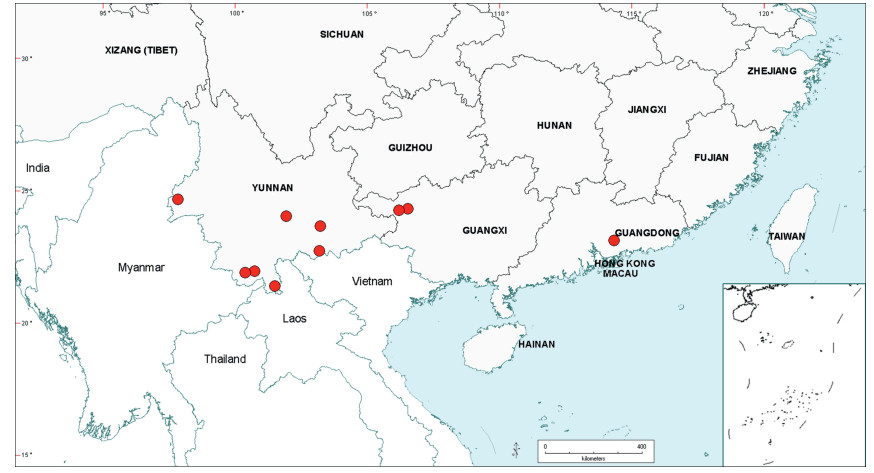
|
| Fig. 36 Distribution of Senegalia pennata subsp. insuavis in China, based on specimen records at GXMI, IBK, IBSC, KUN and SING, as cited in text. In China this subspecies has a discontinuous distribution. However, as it is commonly cultivated for culinary purposes, it is often difficult to know with certainty if specimen records given here represent natural or introduced occurrences. |
|
| Fig. 37 Senegalia pennata subsp. insuavis. A - Liana habit showing large green leaves (Singapore plant). B - Shrub habit showing sub-glaucous leaves (cultivated plant in China). C - Vegetative shoot (left) showing lanceolate stipule subtending developing leaf, and very young inflorescence (right) with stipules subtending youngest heads. D - 'Smelly Senegalia' on sale in Chiang Mai, Thailand, street market. E - Mature stem (light grey) showing robust prickles. F - Leaf showing 18 pairs of pinnae (which is at lower end of number range). G - Leaflets (apiculate) with dull lustre and pale green on lower surface (left) and darker green on upper surface (right); these leaflets are atypical in that apiculum is often centric and main vein is not parallel to upper margin. H - Petiole gland slightly thickened, situated very near pulvinus (with insert showing flattened profile). I - Petiole gland very shallowly elongate-cupular, situated at distal end of pulvinus. Vouchers: B.R. Maslin et al. BRM 11016 (A); B.R. Maslin & L. Bai BRM 11025 (B & I); B.R. Maslin & L. Bai BRM 11043 (C, E, F, G & H). Photos: B.R. Maslin (A, B, D) and L. Bai (C, E - I). Scale bar = 5 mm (unless otherwise indicated). |
≡ Acacia insuavis Lace, Bull. Misc. Inform. Kew 1915: 401 (1915). ≡ Acacia pennata subsp. insuavis (Lace) I.C.Nielsen, Adansonia, sér. 2, 19: 353 (1980). ≡ Senegalia insuavis (Lace) Pedley, Austro-baileya 9(2): 314 (2014). Type citation: "BURMA, Ani Sakan, near Maymyo, 900 m., Lace 6173." Type: MYANMAR, Maymyo Plateau, near Ani Sakan, alt. 3000 ft., 18 May 1913, J.H. Lace 6173 (lectotype: E barcode E00318280 [digital image!], 2nd step here designated, see discussion below under Typification; iso-lectotypes: CAL n.v.-cited by Chakrabarty and Gangopadhyay (1996), E barcode E00318279 [digital image!], E barcode E00318281 [digital image!], K barcode K000791207 [digital image!]).
Distinctive features. Plants emitting a disagreeable odour when fresh, especially when branches are cut, or leaves crushed. Branchlets normally rather pale-coloured. Pinnae numerous (normally 17-28 pairs). Petiole 30-65 (-80) mm long; Rachis hairs antrorsely curved. Leaflets normally 4-6 mm long, 0.5-1 mm wide and often at least some obviously curved forward and/or folded lengthwise when dry; main vein ±close to and parallel with upper margin for at least a short distance above base of leaflet; apex apiculate by a finely acute, short, slender point; lateral veins (on lower surface of leaflets) not visible. Petiole gland situated close to leaf base, mostly 3-6 mm long, flattened but normally slightly thickened and finely longitudinally wrinkled when dry, apterous or occasionally narrowly peripterous. Peduncles normally 15-40 mm long, densely hairy. Flowers shortly but discernibly pedicellate at anthesis (pedicel 0.5-1 mm long). Pods glabrous (no resin gland present), clearly stipitate (stipe 5-10 mm long).
Description. Lianas or shrubs to c. 5 m tall, emitting a disagreeable odour when fresh (especially when branches are cut, or the leaves crushed: see discussion below under The living plant smell). Branchlets light grey (mature) except pale green at extremities when fresh, normally somewhat pale-coloured when dry, indumentum variable (see note below under Branchlet indumentum), upper branchlets sparsely to densely hairy or sometimes glabrous with the indumentum persisting or not persisting on mature branchlets, the hairs normally short, antrorsely curved and spreading to appressed, resin hairs absent. Young growth bright green. Prickles few to numerous on internodes and normally some on under surface of leaf axes, occasionally absent from herbarium specimens, length very variable (1-7 mm long), straight to recurved. Stipules caducous, narrowly lanceolate to narrowly triangular or sometimes narrowly oblong, (2-) 3-5 mm long, 1-2 mm wide, densely appressed-hairy abaxially (indumentum obscuring venation). Leaves bipinnate; pinnae normally 17-28 (-30) pairs, rarely 12-16 pairs (e.g. K.M. Feng 20857), 30-100 mm long, lowermost pinnae 15-25 mm long; petiole 30-65 (-80) mm long; rachis 110-240 (-300) mm long, upper surface with appressed to somewhat spreading hairs that are always antrorsely curved. Leaflets 30-80 (-100) pairs, mostly 4-6 mm long, sometimes to 8 mm long or interspersed with a few 3 mm long, 0.5-1 mm wide at base but when folded lengthwise can be narrower near and above the middle, straight and flat (when fresh) but when dry commonly at least some clearly folded lengthwise (i.e. concave to conduplicate) and/or moderately to strongly curved forward or sometimes shallowly twisted or irregularly and shallowly undulate (see notes below under Leaflet curvature), concolorous to moderately discolorous (darkest above), green or sub-glaucous, dull lustre on both surfaces, ±appressed ciliate/ciliolate otherwise glabrous; apex normally asymmetric being obliquely rounded or slanting and terminated by distinct, finely acute, often slender excentric point (infrequently symmetric with the point centric); base unequal with an obviously rounded angle on lower edge, the petiolule short but distinct and clearly excentric; main vein starting near upper margin and normally remaining ±close to it and parallel with it for at least a short distance above base of leaflet; lateral veins (on lower surface of leaflets) not visible, not forming a reticulum. Glands: petiole gland-situated near base of petiole 5-10 (-14) mm above leaf base and 0-3 (-10) mm above pulvinus, oblong to narrowly oblong or narrowly elliptic to obovate in plane view, rarely a few leaves with circular glands, (2-) 3-6 (-7) mm long, 1-2 mm wide, l:w = 1.5-4, sessile, flattened but commonly slightly thickened and finely longitudinally wrinkled when dry, apterous and shallowly elongate-cupular (with a narrow rim surrounding a very shallow central depression) or sometimes very narrowly peripterous; rachis glands - situated at base of upper-most 1-3 (-5) pairs of pinnae, circular to oblong, 0.8-1.5 mm long, c. 1 mm wide, depressed, fractionally raised from the base so that the outer rim of gland not closely appressed to rachis; rachilla glands - situated at base of uppermost 2 or 3 pairs of leaflets, oblong, 0.3-0.5 mm long, sessile. Inflorescences comprising pedunculate heads arranged in terminal, elongated racemes or open panicles, sometimes young leaves subtend peduncle clusters, panicle and raceme axes densely puberulous, the hairs short, soft, curved and ±appressed; peduncles (10-) 15-40 (-50) mm long, single or clustered in groups of up to five, indumentum as on panicle/raceme axes; heads globose, 10-15 mm diam. at anthesis when dry, white or creamy white to pale yellow, densely (30-) 35-55-flowered; inflorescence buds green when fresh (based on a single observation, BRM 11043) but pale yellowish brown or dark brown to darkish purple when dry (suggesting that at least sometimes buds will also be reddish when fresh: see discussion under Morphological characters: Inflorescences and flowers above). Bracteoles linear-spathulate, c. 1 mm long, not visible in mature buds; claw linear, glabrous and abruptly expanded to form a narrowly elliptic, acute, obviously appressed-hairy lamina that is about as long as the claw. Flowers 5-merous, with a short, but discernible pedicel 0.5-1 mm long; calyx ¾-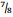

Selected specimens examined. AUSTRALIA: Northern Territory. Lambells Lagoon, outer Darwin, 55 Ewart Road (Jolly's fruit & vegetable farm), 16 Apr. 2013, J.O. Westaway 4148 (BRI, CANB, DNA, PERTH, MEL, SING). CHINA: Guangdong. Huizhou City, Boluo County, 6 May 1993, Y.Q. Wang 636 (IBSC 619759 [barcode 0159207]). Guangxi. Baise City, 24 May 1936, H.H. Soo 67533 (IBK 33813 [barcode 00067627], IBSC 92134 [barcode 0159291]); Baise City, Lingyun County, 12 Oct. 1977, Lingyun Expedition 3-26080 (GXMI 21391 [barcode 015762]); Baise City, Tianlin County, 11 June 1964, D. Fang 26584 (GXMI 04263 [barcode 015761], GXMI 58597 [barcode 058187]). Yunnan. Dehong Dai & Jingpo Autonomous Prefecture, Yingjiang County, 14 Jan. 1989, H. Sun 1479 (KUN 0400220 [barcode 0598422]); Honghe Hani & Yi Autonomous Prefecture, Jinping Miao, Yao & Dai Autonomous County, 20 May 1974, Lühun Expedition 976 (KUN 0400218 [barcode 0598424]); Honghe Hani & Yi Autonomous Prefecture, Jinping Miao, Yao & Dai Autonomous County, Jinshuihe village, 22°37'49"N, 103°07'04"E, 6 June 2019, B.R. Maslin & L. Bai BRM 11043 (BKF, IBSC, KUN [barcode 1348003̶ 1348005], PE); Honghe Hani & Yi Autonomous Prefecture, Kaiyuan City, s.d., Y.C. Xu s.n. (SWFC 0021136 & 0009674); Xishuangbanna Dai Autonomous Prefecture, Jinghong City, 15 May 1955, K.M. Feng 20857 (IBSC 314313 [barcode 0159284], KUN 0400189 [barcode 0598404]); Xishuangbanna Dai Autonomous Prefecture, Jinghong City, Sep. 1936, C.W. Wang 79607 (IBSC 179456 [barcode 0159283] & KUN 0400172 [barcode 0598394]); Xish-uangbanna Dai Autonomous Prefecture, Jinghong City, 28 May 1958, S.X. Zhao 86 (KUN 0400214 [barcode 0598436]); Xishuang-banna Dai Autonomous Prefecture, Menghai County, July 1936, C.W. Wang 76089 (IBSC 187562 [barcode 0159289], IBSC 680595 [barcode 0159280] & KUN 0400173 [barcode 0598393]); Xishuang-banna Dai Autonomous Prefecture, Mengla County, cultivated in Xishuangbanna Tropical Botanical Garden, 9 Apr. 2015, B.C. Ho & B.R. Maslin BRM 11009 (KUN, SING barcode 0216280); Xishuangbanna Dai Autonomous Prefecture, 3 Sep. 1959, Mengla County, S.J. Pei 9772 (KUN 0400209 [barcode 598429]); Yuxi City, Xinping Yi & Dai Autonomous County, 19 May 2018, B.R. Maslin & L. Bai BRM 11025 (KUN barcode 1345219 & 1345222). INDIA: Andhra Pradesh. Warangal District, Pakhal Lake, 9 Aug. 1961, K.M. Sebastine 13149 (CAL). MYANMAR: Upper Burma, Fort Stedman, 1894, Abdul Khail s.n. (CAL 140717). SINGAPORE: 'Lentor Forest', near Florissa Park estate, 0.5 km N of Yo Chu Kang Road, 5 Feb. 2016, B.R. Maslin, B.C. Ho, H.K. Lua & A.T.K. Yee BRM 11016 (SING barcodes SING0261281-261283); Lentor Drive, Lentor Forest, between Teachers Estate and Florissa Park, 20 Oct. 2014, H.K. Lua & A.T.K. Yee SING 2014-334 (SING barcode SING0261306). THAILAND: Northern. Lampang, Feb. 1921, A.F.G. Kerr 4805 (BK 8132 barcode BK213353); Chiang Mai Province, Doi Chiang Dao, SE foothills at Ban Yang Pong Luang, 29 Apr. 1989. J.F. Maxwell 89-526 (BKF 94309).
Distribution (Fig. 36). Because this subspecies has long been cultivated for culinary purposes it is often difficult to know with certainty if specimen records represent native or introduced occurrences. However, as best can be determined, Senegalia pennata subsp. insuavis occurs naturally (and is also sometimes cultivated) in Myanmar and southern China. Its status in Laos is uncertain since Nielsen (1981) regarded it as introduced while Newman et al. (2007) regarded it as native; similarly, in India it is not known if it is native or introduced (Deshpande et al., 2019). It is introduced in Australia (Northern Territory and Queensland), Cambodia (Nielsen, 1981), Singapore, Thailand (Nielsen, 1985a) and U.S.A. (Florida). In China, S. pennata subsp. insuavis has a discontinuous distribution that extends from far western and southern Yunnan (adjacent to borders with Myanmar and Laos) to western Guangxi (Baise City) and also Guangdong; a few possibly cultivated specimens occur throughout this range. The single gathering from Guangdong was collected from a valley within a mountain range and is provisionally considered as native.
Habitat. Recorded in China as growing in hilly or mountainous sandstone areas (alt. 330-1300 m), in open or mixed forests.
Phenology. Flowers from late April to June. Immature pods have been collected from China in September and October, and pods with one slightly immature seeds have been collected (in Thailand) in February.
Typification. In the protologue of Acacia insuavis Lace (1915) cited only a single collection, Lace 6173, but no herbarium of lodgement was indicated. Gamble (1918) mentioned that Lace specimens are at both herb. E and K; there are three specimens of Lace 6173 at E and one at K. Because there was no indication in the protologue of A. insuavis that the original description was based on a single specimen, a lectotype is needed. Nielsen (1980) cited Lace 6173 at herb. E as the holotype (but did not specify which of the three specimens this applied to), with an isotype at herb. K. Accordingly, in conformity with ICN Art.9.10 (Turland et al., 2018) and as discussed by McNeill (2014), Nielsen's holotype and isotype type citations are corrected above to lectotype and isolectotype respectively, and a second step lectotypification is undertaken here to more precisely typify this name (cf. ICN Art. 9.17). All three specimens at E are well-preserved, their label information is basically identical and none is at variance with the original description. The specimen E barcode E00318280 has been chosen as the lectotype; this sheet is stamped "Herb. J.H. Lace. Purchased 1918".
Pedley (2014) considered the herb. K specimen of Lace 6173 as the holotype of Acacia insuavis, arguing that around the time the species was described, Lace was a frequent visitor to Kew and published in its journal. Nevertheless, this is rejected here because as stated above there is no indication that only one specimen was used to prepare the original description, and because Nielsen (1980) had already typified the name based on Lace 6173 at herb. E.
Subspecies. As discussed below under Notes on Senegalia pennata, S. pennata is here more narrowly defined than previously and includes just two subspecies, subsp. pennata (which occurs naturally in Sri Lanka and India) and subsp. insuavis. These two sub-species are united principally by the following characters: flowers shortly but distinctly pedicellate; leaflet apices normally obliquely rounded or slanting and terminated by a finely acute, often slender excentric point; leaflet main vein relatively close to and parallel with the upper margin, at least near base of leaflet; petiole gland situated near base or petiole, clearly flattened and sometimes peripterous; and inflorescence axes lacking dark-coloured glan-dular hairs. Subspecies pennata is most readily distinguished from subsp. insuavis in the following ways: pinnae normally fewer (11-18 pairs); petiole gland and stipules slightly shorter (1.5-2 mm and 2-3 mm long respectively); leaflets never strongly curved forward or conduplicate when dry; petioles, rachides and peduncles generally shorter (15-40 mm, 60-150 mm and 7-18 mm long respectively). These characters are either contiguous with, or slightly overlapping, those of subsp. insuavis. While living plants of subsp. insuavis probably always possess an offensive smell when the branchlets are crushed, there are uncertainties regarding this character for subsp. pennata (see discussion below under The living plant smell). Further study, especially of material from the Indian subcontinent, is needed to better-understand the relationship between these two subspecies.
Taxonomy. Senegalia pennata subsp. insuavis was originally described by Lace (1915) as Acacia insuavis, based on material collected from Myanmar. Since then it has been maintained as a distinct species of Acacia for Myanmar by Craib (1928), treated as a subspecies of A. pennata (Nielsen 1980, 1981, 1985a) and of Senegalia pennata by Maslin et al. (2013) and Maslin (2015), regarded as a synonym of A. pennata by Chakrabarty and Gangopadhyay (1996), and treated as a distinct species of Senegalia by Pedley (2014). As discussed below under Notes on Senegalia pennata, we consider that subspecies rank is appropriate for this entity. It can be easily recognised by the characters given under Distinctive features above, especially the disagreeable odour that occurs in living plants. Senegalia pennata subsp. insuavis appears to have been first recognised for China by Sun (2006), who treated it as A. pennata (assigning it the common name, 'Smelly Acacia'), however, it was not included in the treatment of Acacia by Wu and Nielsen (2010).
Affinities. Within China Senegalia pennata subsp. insuavis often, at least superficially, resembles S. orientalis in having numerous pinnae, acute leaflets and a flattened, elongated, often longitudinally wrinkled petiole gland that is situated close to the leaf base. Senegalia orientalis can most readily be distinguished from subsp. insuavis in the following ways: living plants not reported as emitting a foetid odour; branchlets, inflorescence buds and pods darker-coloured, at least when dry; petiole generally shorter (15-35 mm); petiole gland a little thickened; flowers sessile or sub-sessile. Although both taxa have acute leaflets, those of S. orientalis are strongly discolorous, often have promi-nent marginal hairs, are not terminated by a slender point as in S. pennata subsp. insuavis, and are never strongly conduplicate and strongly curved forward as sometimes occurs in that subspecies.
Senegalia pennata subsp. insuavis is sometimes superficially similar to S. clandestina and especially S. kerrii (see these species for discussion).
The living plant smell. In the protologue of Acacia insuavis Lace (1915) referred to a disagreeable (foetid) odour that was emitted when its branches were crushed. We have observed this same smell in living plants from China and Singapore when the branchlets are cut for collection and/or the leaves are crushed, and it has been recorded on herbarium labels of plants introduced in Australia and Florida. In our experience this smell is lost once specimens are dried. The smell is also present, but less strong, on fresh young shoots of Senegalia pennata subsp. insuavis that are often sold as a vegetable in markets in China, Singapore and Thailand. In view of the above, it is perhaps curious that only a few herbarium labels of plants collected in China record this unmistakable odour.
The only other record known to us of an Asian Senegalia possessing a possibly similar odour to that of subsp. insuavis is that of Kostermans (1980) who reported a strong smell of hydrogen sulphide from plants of Acacia (Senegalia) pennata in Sri Lanka, "when collected in alcohol (wet method)". However, It is not known if Kostermans' comments referred to the indigenous S. pennata subsp. pennata, or if that author was referring to an introduced plant of S. pennata subsp. insuavis. We are informed by A. Deshpande (pers. comm.) that in southern India where subsp. pennata is indigenous, the smell is not present on living plants when their young branchlets are crushed; however, the wood does have a 'strong smell', which is lost upon drying. This matter concerning the presence or absence of an unpleasant smell in living plants of subsp. pennata requires further study.
Branchlet indumentum. Anecdotally, it appears that the branchlet indumentum on cultivated plants is sparser (or absent) compared with plants presumed to be of wild origin (which have densely hairy branchlets). However, this apparent correlation requires further investigation.
Leaflet curvature. The flat, straight leaflets that occur on living plants of this subspecies often quickly change their form following collection, folding lengthwise and/or curving forward (sometimes very obviously so) or become twisted or undulate. The degree to which these changes occur is related to the length of time it takes to press freshly-cut specimens. If specimens are pressed quickly upon collection the leaflets remain flat, but if there is a delay in pressing the leaflets fold/curve as described above. Most herbarium specimens of Senegalia pennata subsp. insuavis show at least some leaflets that are clearly folded lengthwise (concave to conduplicate) and/or are moderately to strongly curved forward, and these attributes are useful aids to identification when present. However, there are some herbarium specimens collected from China where all the leaflets are perfectly flat and straight.
Uses. The soft new shoots of Senegalia pennata subsp. insuavis are commonly used in Asian cooking. Plants of this subspecies are sometimes used as a hedge-row shrub in Thailand, fide Nielsen (1985a) and Australia (recorded on specimen labels). According to Nielsen (1981), the roots of this subspecies in Laos are used in local medicine to help combat anaemia.
Etymology. The subspecies name is derived from the Latin insuavis (unpleasant, disagreeable) in allusion to the rather offensive smell that is emitted from living plants, at least when their leaves or branches are first crushed or cut.
Vernacular names. Smelly Vegetable. This is the name commonly applied to this subspecies in markets in China and was used for it (under A. pennata) by Sun (2006). The name 'Smelly Vegetable' is also applied to Caesalpinia mimosoides Lam. in western Yunnan (Liu and Zhao, 2009), but the smell is different to that of Senegalia pennata subsp. insuavis. Various other common names have also been used for this taxon (under either A. insuavis or A. pennata subsp. insuavis) in Southeast Asia, e.g. 'Subok' in Myanmar (Lace, 1915), 'Laotien' in Laos (Nielsen, 1981), and 'Cha om' in central Thailand by Nielsen (1985a), who also provided several other Thai common names.
Notes on Senegalia pennata
A brief discussion of Acacia (Senegalia) pennata is required here because for the past four decades this name has often appeared in literature and on herbarium specimens, but the circumscription of the species has been greatly changed as a result of the present study.
Originally Brenan and Exell (1957) demonstrated that Acacia pennata is a species that is restricted to Asia and did not occur in Africa as previously thought. Subsequently, Nielsen (1980) recognised four subspecies within the species, namely: subsp. pennata, subsp. insuavis, subsp. hainanensis (with A. macrocephala given in synonymy) and subsp. kerrii. It is this classification that became widely adopted, not only in China but also in South East Asia and elsewhere. However, the broad concept of A. pennata by Nielsen (1980) brought together disparate taxa (united primarily by having narrow, acute leaflets), many of which we consider warrant recognition as separate species. Therefore, following a reassessment of Nielsen's classification we consider it more appropriate that Senegalia pennata be more narrowly defined, containing just subsp. pennata and subsp. insuavis. Subspecies hainanensis and subsp. kerrii are treated as distinct species, S. hainanensis and S. kerrii respectively, and A. macrocephala has been resurrected as S. macrocephala. Furthermore, a new species, Senegalia stipitata, is recognised; this species incorporates the specimens from Vietnam with stipitate petiole glands that Nielsen (1980) noted under A. pennata subsp. hainanensis. These five species can be most readily distinguished from one another by their leaf gland morphology, position and orientation of the main vein of the leaflets, and the presence or absence of dark-coloured resin hairs on their inflorescence.
We regard the above as a more workable classification than that of Nielsen (1980), and one that facilitates discussion of relationship between the species and their relatives.
As noted above, in the present work we recognise an informal group called the 'Pennata complex' which includes the species of Senegalia sect. Monacanthea that possess very narrow leaflets (normally 0.5-1 mm wide). This complex includes the 'Hainanensis species-group' (four species, characterized by having numerous dark-coloured glandular hairs on inflorescence axes) and the 'Pennata species-group' (seven species with darkcoloured glandular hairs absent or very few on inflorescence axes). Of the species discussed above, Senegalia hainanensis, S. macrocephala and S. stipitata belong to the 'Hainanensis species-group' while S. kerrii and S. pennata belong to the 'Pennata species-group'
Most of the numerous specimens examined in Chinese herbaria that had been identified as A. pennata (=Senegalia pennata), are now referred to other species of Senegalia, as discussed under the relevant species in this work.
15. Senegalia prominens Maslin, B.C.Ho, H.Sun & L.Bai, sp. nov. (Figs. 38 and 40).老虎刺金合欢【lǎo hǔ cì jīn hé huān】(新拟)
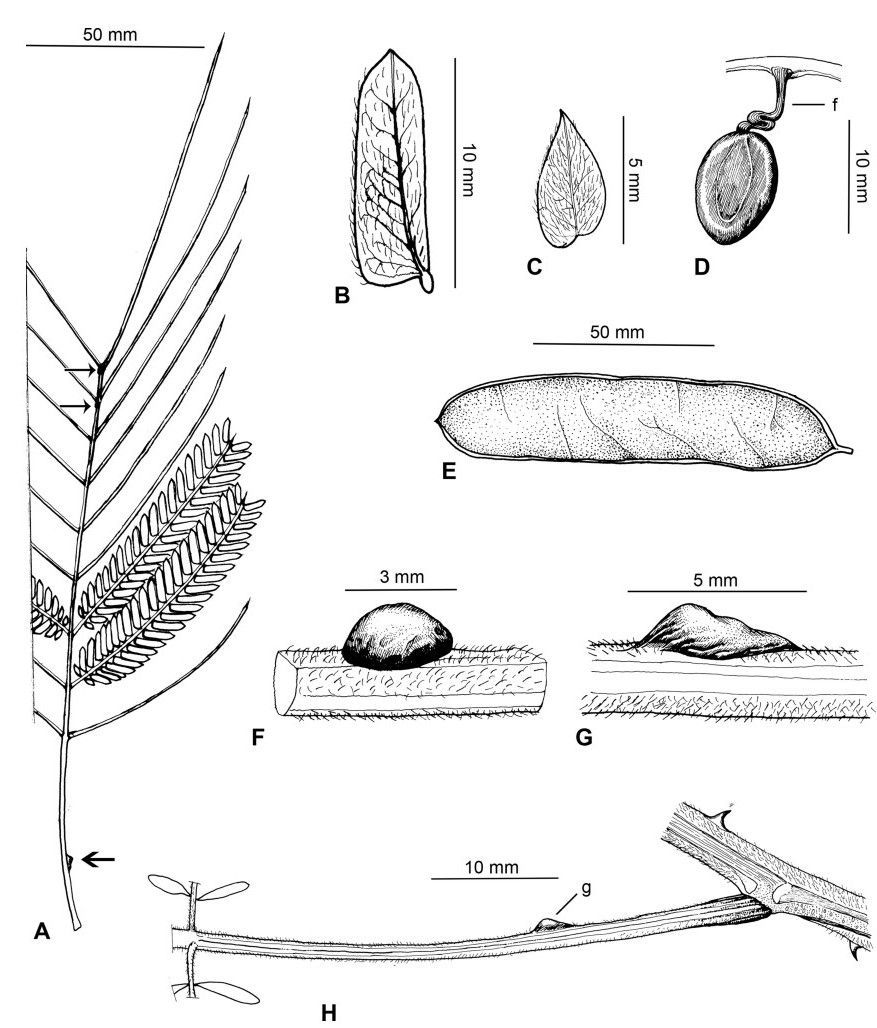
|
| Fig. 38 Senegalia prominens. A - Leaf showing relatively few pinnae and position of petiole gland (thick arrow) and rachis glands (thin arrows). B - Leaflet (lower surface) showing appressed indumentum. C - Stipule large, showing unequal base. D - Seed showing thickly filiform, exarillate funicle (f). E - Pod. F - Petiole gland (lateral view) hemispheric and prominently raised. G - Petiole gland (lateral view) conical and prominently raised. H - Node showing puberulous branchlet and petiole gland well-removed from leaf base. Vouchers: C.L. Tso 21029 (A, B, G & H); T.M. Tsui 252 (C); S.H. Lai 5470 (D & E); N. Liu et al. 2403 (F). Drawn by Joshua Yang. |
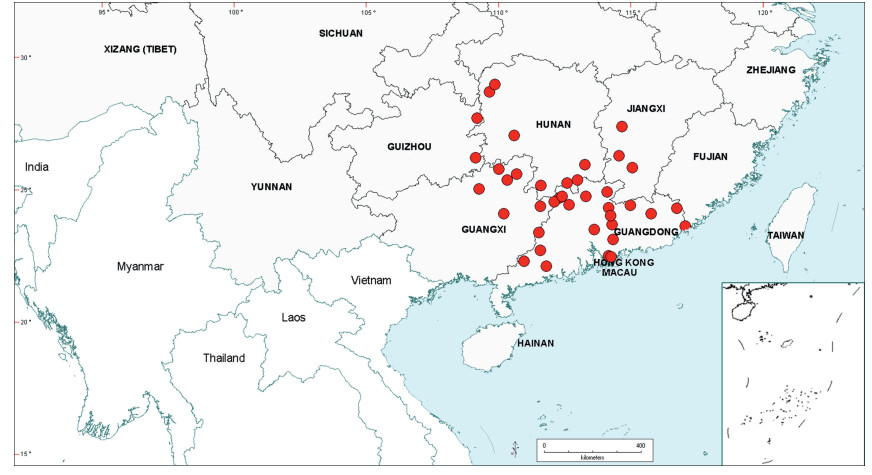
|
| Fig. 39 Distribution of Senegalia prominens, based on specimen records at GXMI, IBK, IBSC and KUN, as cited in text. |

|
| Fig. 40 Senegalia prominens. A - Shrub habit. B - Inflorescences arranged in open panicles. C to G - Petiole glands (prominent) showing variation (all from the one plant, BRM 11019, except G). H - Inflorescence apex showing white heads at anthesis and green flower buds. I - Leaf showing relatively few pinnae. J - Leaflets (lower surface) wide, appressed-hairy (hairs difficult to see) and apex rounded to obtuse. K - Young inflorescence subtended by a primordial leaf, showing broad, lanceolate stipule at base of peduncle and green flower buds. Vouchers: All from B.R. Maslin & L. Bai BRM 11019 (the type plant) except G & H (from unvouchered Bai & Maslin collection from near Guilin). Photos: B.R. Maslin. Scale bar = 5 mm (unless otherwise indicated). |
Type: CHINA, Guangxi Province, Guilin City, Xing'an County, Mao'er Mountain Nature Reserve, Guangxi, 25° 51'28"N, 110° 29'14"E, 16 June 2017, B.R. Maslin & L. Bai BRM11019 (holotype: KUN barcode 1345210!; isotypes: GXMI!, IBSC!, KUN barcode 1345212!, PE!).
[Acacia concinna auct. non (Willd.) DC.: T.L.Wu, Fl. Hong Kong 2: 42 (2008) and T.L.Wu & I.C.Nielsen, Fl. China 10: 58 (2010), pro parte, see notes below under Taxonomy.]
[Acacia sinuata auct. pl. non (Lour.) Merr.: P.C.Huang, Sylva Sinica 2: 1263 (1985) and T.L.Wu, Fl. Reipubl. Popularis Sin. 39: 34 (1988), pro parte, see notes below under Taxonomy and discussion under Senegalia rugata; H. Ye & S. Peng, Pl. Diversity Invent. Guangdong 223 (2006) and H. Ye et al., Med. Pl. S. China 162 (2013)djudging from the distribution cited for China in these two publications, the species discussed is probably Senegalia prominens.]
[Acacia vietnamensis auct. non Nielsen: H.Sun & C.Chen, Acta Bot. Yunnan. 12: 261 (1990) and Wu & I.C. Nielsen, Fl. China 10: 57-58 (2010), pro parte, see notes below under Taxonomy.]
Distinctive features. Branchlets moderately to densely puberulous towards apices, not pruinose. Stipules large (3-6 (-8) mm long and 1.5-3 mm wide), appressed-hairy abaxially, unequally lobed at base. Pinnae (5-) 6-10 (-13) pairs. Leaflets normally 6-10 mm long and 1.5-2.5 mm wide, the lower surface appressedhairy or sometimes glabrous; lateral veins (on lower surface of leaflets) normally forming an imperfect or indistinct reticulum. Petiole gland 5-20 (-25) mm above leaf base, 2-4 mm long, prominent (raised 1-2 mm high), often conical (symmetrically or asymmetrically) but sometimes otherwise. Inflorescences arranged in racemes and panicles; flower buds pale green when fresh; peduncles densely puberulous by ±appressed and curved, white-cream hairs. Calyx ±strongly 5-veined (so that flower buds often appear somewhat angular).
Description. Commonly much-branched or lianescent shrubs 2-6 m tall (infrequently trees to 11 m tall), sometimes (according to some herbarium labels) lianas. New shoots bright green. Branchlets moderately to densely puberulous (hairs uniformly short, curved and appressed to sub-appressed, infrequently ±straight and patent) towards apices, the indumentum persisting to penultimate and mature branchlets (but often sparser) or sometimes absent. Prickles normally relatively few on internodes and sometimes on under surface of leaf axes, sometimes lacking on herbarium specimens, commonly 0.5-2 mm long, straight to slightly recurved and patent to slightly reflexed on upper branchlets, on mature branches (seldom seen on herbarium material) may reach 4-6 mm long and be rather vicious-looking. Stipules caducous, lanceolate or sometimes ±triangular, large (3-6 (-8) mm long and 1.5-3 mm wide), pale green or yellowish green when fresh, normally densely appressed-hairy abaxially (veins obscured by indumentum), slightly to obviously unequally lobed at base with one side more rounded-convex than the other, rarely shortly auriculate on the more prominently lobed side, apex acute to acuminate. Leaves bipinnate; pinnae (5-) 6-10 (-13) pairs, 70-110 mm long when in flower but often 40-50 mm long when in pod; petiole 25-65 (-75) mm long when in flower, sometimes 12-20 mm long when in pod; rachis 105-145 (-180) mm long when in flower, sometimes 70-100 mm long in pod, with short, spreading to appressed, antrorsely curved hairs on upper surface. Leaflets (14-) 18-34 pairs, narrowly oblong, (5-) 6-10 (-12) mm long, 1.5-2.5 (-3) mm wide, moderately discolorous, mid-green to slightly sub-glaucous above and pale green below, dull lustre on both surfaces except shiny above on young leaflets, normally finely appressed-hairy on lower surface (indumentum dense to moderately dense), glabrous to ±sparsely appressed-hairy on upper surface, sometimes glabrous on both surfaces (except margins appressed ciliate or ciliolate); apex symmetric or asymmetric, rounded to obtuse, rarely broadly acute, with a very short and often ill-defined, bluntly acute apiculum or apiculum lacking; base unequal with a distinct rounded angle on lower edge, petiolule distinct (extended below base of leaflet) and clearly excentric; main vein starting near upper margin at leaflet base and extending obliquely to apex, ±straight; lateral veins (on lower surface of leaflets) rather evident, patent to ascending, sometimes bifurcating near margin and coalescing (at least along lower edge of leaflet) to form an intra-marginal vein, anastomosing to form a normally imperfect and/or indistinct reticulum (which could be misinterpreted as absent). Glands: petiole gland-situated on lower 

Selected specimens examined. CHINA: Guangdong. Chaozhou City, Raoping County, 15 Apr. 1931, N.K. Chen 42647 (IBSC 21452 [barcode 0158722]); Guangzhou City, Conghua District, 3 Dec. 1958, L. Teng 8821 (IBSC 680549 [barcode 0158749], KUN 0400348 [barcode 0598570]); Heyuan City, Heping County, 12 June 1958, C.F. Wei 120489 (IBSC 305760 [barcode 0158734]); Huizhou City, Boluo County, 19 Dec. 1928, Y. Tsiang 1623 (IBSC 3944 [barcode 0158731]); Huizhou City, Longmen County, 9 June 1935, W.T. Tsang 25417 (IBSC 169019 [barcode 0158733]); Maoming City, Xinyi City, Oct. 1969, C.L. Lo 1054 (IBSC 490964 [barcode 0158625]); Meizhou City, Dabu County, 19 Aug. 1958, S.C. Lee 202601 (IBK 115748 [barcode 00067645], IBSC 376636 [barcode 0158744]); Meizhou City, Xingning City, 12 Oct. 1959, Nanzhidi Expedition 7679 (IBSC 337239 [barcode 0158756]); Qingyuan City, Liannan Yao Autonomous County, 11 Sep. 1958, P.C. Tuam 59371 (KUN 0400350 [barcode 0598568]); Qingyuan City, Lianshan Zhuang & Yao Autonomous County, 25 Oct. 1999, H.G. Ye 2539 (IBSC 714271 [barcode 0705083]); Qingyuan City, Lianzhou City, 7 Oct. 1958, P.C. Tam 50579 (IBSC 641649 [barcode 0159373]); Qingyuan City, Yangshan County, Nanling Nature Reserve. Shrubs 3 m tall with spreading shoots, flowers creamy white. Alt. 1200 m, 9 July 2016, L. Bai & J. Wang BL 001 (KUN barcode 1345211, SING barcodes SING0261304 & SING0261305); Qingyuan City, Yangshan County, 12 June 1956, L. Teng 1442 (IBSC 230031 [barcode 0158729], KUN 0400343 [barcode 0598565]); Shaoguan City, Lechang City, 9 June 1929, C.L. Tso 21029 (IBSC 8324 [barcode 0158736]); Shaoguan City, Ruyuan Yao Autonomous County, 27 Oct. 1956, C. Wang 42399 (IBSC 231576 [barcode 0158751]); Shaoguan City, Shixing County, 12 July 1958, L. Teng 6788 (IBSC 301163 [barcode 0158724]); Shaoguan City, Wengyuan County, 4 Oct. 1924, S.K. Lau 24736 (IBSC 89039 [barcode 0158732]); Shaoguan City, Xinfeng County, 15 July 1985, H.G. Ye 967 (IBSC 587655 [barcode 0158725]); Shenzhen City, Luohu District, Apr. 1932, T.M. Tsui 252 (IBSC 117706 [barcode 0158730]); Yangjiang City, Yangchun City, 24 Nov. 1991, N. Liu et al. 3069 (IBSC 606553 [barcode 0158748]); Yunfu City, Luoding City, 22 Oct. 1991, N. Liu et al. 2403 (IBSC 605904 [barcode 0158757]); Zhaoqing City, Fengkai County, 15 June 1958, S. Wang 164316 (IBSC 267684 [barcode 0158740], KUN 0400257 [barcode 0598529]). Guangxi. Baise City, Debao County, s.d., Sangzhi County Institute of Forestry 1702 (KUN 0401717 [barcode 0579461]); Guilin [City], 8 Aug. 1963, X.C. Huang 1947 (GXMI 04287 [barcode 015766]); Guilin City, Lingchuan County, 23 Aug. 1937, W.T. Tsang 27981 (IBSC 170192 [barcode 0158784]); Guilin City, Longsheng Multinational Autonomous County, 31 Aug. 1957, H.F. Tan & C.T. Li 71192 (IBK 64168 [barcode 00067641], IBSC 274019 [barcode 0158792]); Guilin City, Xing'an County, 12 June 1936, J.X. Zhong 81774 (IBK 58955 [barcode 00067637], IBSC 91972 [barcode 0158791]); Hezhou City, Babu District, 19580912, Y.K. Li 401542 (IBK 71857 [barcode 00067642], IBSC 310315 [barcode 0158786]); Laibin City, Jinxiu Yao Autonomous County, 2 July 1958, Y.K. Li 400487 (IBK 70808 [barcode 00067636], IBSC 310306 [barcode 0158767]); Liuzhou City, Rongshui Miao Autonomous County, 17 Nov. 1958, S.H. Chen 17303 (IBSC 298646 [barcode 0158789], KUN 0400064 [barcode 0598273]). Guizhou. Qiandongnan Miao & Dong Autonomous City, Liping County, 21 June 1981, Yong Kang Li 8697 (IBSC 540158 [barcode 0158858]); Tongren City, Bijiang District, 4 July 1988, Wuling Mountain Expedition 1415 (KUN 0571008 [barcode 0579369]). Hong Kong. 8 Jan. 1941, Y. Tsiang 16589 (IBK 91397 [barcode 00067658], IBSC 145126 [barcode 0158726]); 3 June 1933, C. Wang 32437 (IBK 40168 [barcode 00067656], IBSC 38383 [barcode 0158758]. Hunan. Chenzhou City, Linwu County, 16 Sep. 1942, P.H. Liang 83456 (IBK 56639 [barcode 00067635], IBSC 177777 [barcode 0158843]); Chenzhou City, Yizhang County, 11 Oct. 1964, M.X. Huang 112967 (IBSC 374508 [barcode 0158832]); Chenzhou City, Zixing City, 22 Aug. 1951, P.H. Liang 176047 (IBSC 176047 [barcode 0158842]); Shaoyang City, Dongkou County, 30 Oct. 1957, C.J. Qi 3123 (IBSC 310519 [barcode 0158837]); Xiangxi Tujia & Miao Autonomous City, Baojing County, 16 Sep. 1958, L.H. Liou 9809 (IBSC 311622 [barcode 0158835]); Xiangxi Tujia & Miao Autonomous City, Yongshun County, 4 Aug. 1953, Western Hunan Expedition 485 (IBSC 303181 [barcode 0159305]); Yongzhou City, Jianghua Yao Autonomous County, 4 Aug. 1953, C.J. Qi 3742 (IBSC 267317 [barcode 0158830]). Jiangxi. Ganzhou City, Gan County, 16 July 19640, C.B. Yang 1107 (IBSC 404731 [barcode 0158846]); Ji'an City, Anfu County, 3 Sep. 1963, C.S. Yok 3802 (IBSC 680558 [barcode 0158847]); Ji'an City, Suichuan County, 4 Nov. 1965, S.H. Lai 5470 (IBSC 405962 [barcode 0158845]).
Distribution (Fig. 39). Endemic and widespread in southeast China, south of latitude 29°N. It extends from Hunan, Jiangxi and far eastern Guizhou, south to northeast Guangxi, Guangdong (where it is widespread southwards to Maoming City) and Hong Kong. It is possible that the species also occurs in Fujian, Hainan and Zhejiang, but no specimens have been seen to verify these occurrences; these three Provinces were among those listed by Wu and Nielsen (2010) for Acacia concinna and A. vietnamensis (see discussions under Taxonomy below).
Habitat. Grows in hilly or mountainous country, often in valleys near watercourses, in open or closed forests or thickets.
Phenology. Judging from herbarium specimens, flowering commences from mid-June to mid-July (depending on location) and appears to extend to around mid-October (but we have not seen flowering specimens for August and September). Pods with mature seeds occur from early October to early December (again, depending on location). This rather large range of variation for flowering and fruiting is probably related to the wide geographic range of the species.
Taxonomy. Senegalia prominens has until now had a somewhat confusing taxonomic and nomenclatural history. Although the species has been extensively collected in China, it was commonly included in S. rugata, and together these two species were reported as either Acacia concinna or A. sinuata (both these names are regarded here as synonyms of S. rugata); most specimens of S. prominens at herb. IBK and IBSC were determined as one or other of these Acacia names. More recently, the name A. vietnamensis had been applied to S. prominens and most specimens at herb. KUN were determined with this name.
Deciphering and interpreting past Chinese literature accounts of Acacia concinna and A. sinuata is somewhat challenging, especially because no voucher specimens were cited in most relevant works. Nevertheless, it appears most likely that elements of both Senegalia prominens and S. rugata were included in the descriptions of A. sinuata by Institute of Botany (1972), Huang (1985) and Wu (1988), and in the descriptions of A. concinna by Xing (2005), Wu (2008) and Wu and Nielsen (2010). However, the illustrations in Huang (1985), Wu (1988) and Wu (2008) are clearly of S. prominens, while the photo in Xing (2005) is most likely of S. rugata. Although the description of A. concinna by Wu and Nielsen (2010) applies to S. rugata, most Chinese provincial occurrences they cited for that species probably refer to S. prominens.
Under Acaci vietnamensis, Sun and Chen (1990) listed many specimens but did not provide a description of the species. Apart from the specimen S.K. Lee 200449 (IBK, IBSC), which is Senegalia andamanica, and the type of A. vietnamensis (E. Poilane 19678), the other specimens cited by Sun and Chen (1990) that have been examined belong to S. prominens (H.Y. Liang 68476 & P.C. Tam 59391 could not be located). The name A. vietnamensis was subsequently adopted in the Chinese flora by Wu and Nielsen (2010). In that work, the description was based largely on the protologue, but the Chinese Provinces cited correspond very closely to those in which S. prominens is found. Senegalia vietnamensis, as delimited here, occurs in southern Vietnam and Laos, and can be distinguished from S. prominens by: stipules smaller (c. 3 mm long and 1 mm wide) and not lobed at their base; pinnae (when in flower) shorter (20-60 mm long); leaflets generally narrower (mostly 1-1.5 mm wide) and glabrous or sub-glabrous on their lower surface; and petiole gland shorter (0.5-2 mm long), less prominently raised and flat-topped or shallowly concave. Also, according to the protologue, the calyx and corolla of Acacia (Senegalia) vietnamensis are glandular puberulous (glabrous or non-glandular hairy in S. prominens).
As discussed below, Senegalia prominens is allied to S. teniana. Although the individual Morphological characters separating these two allopatric taxa may seem not especially strong, when taken in combination they are considered sufficient to warrant the recognition of two species, rather than two subspecies within a more broadly defined S. teniana. Furthermore, species rank provides a more convenient framework for discussing variation and relationships involving these taxa within Senegalia. Additional comments regarding rank as applied in this work are provided above under Application of rank.
Affinities. Senegalia prominens is most closely related to the more westerly distributed S. teniana. These two endemic Chinese species share a similar number of pinnae, their leaflets are of a similar shape and size and are commonly reticulately-veined on their lower surface, their peduncles are densely hairy, and their flower buds are uniformly pale green when fresh (not stained reddish as often occurs in many other Chinese Senegalia). Furthermore, both species are shrubs or small trees (herbarium labels record S. prominens as also being a liana, but this habit needs to be confirmed by field observations). Senegalia teniana is most readily distinguished from S. prominens in the following ways: leaflets normally glabrous (apart from hairs on margins); petiole gland less prominent (raised to c. 0.5-1 mm high) and never conical-shaped; peduncle hairs ±patent; ovary densely appressed-tomentulose with hairs covering entire surface; stipules smaller (2-3×0.5-2 mm) (but regrettably few stipules have been seen for S. teniana). Additionally, in S. teniana the inflorescences are seemingly never paniculate, the calyx is often less prominently veined, and the leaves are generally slightly smaller. Although both species have hairy upper branchlets, the indumentum on S. teniana tends to be less evident and less persistent than that of S. prominens.
At least superficially, Senegalia pruinescens can resemble S. prominens because of often having reticulately-veined leaflets more than 1 mm wide, a prominent petiole gland and densely hairy peduncles that are arranged in panicles and racemes. Senegalia pruinescens is a robust liana or sometimes a lianescent shrub that can be distinguished from S. prominens in the following ways: branchlets pruinose (but pruinosity is sometimes not visible on herbarium specimens) and commonly glabrous; leaflets normally glabrous (rarely sparsely hairy on lower surface); petiole glands rarely conical; inflorescence buds dull red when fresh; and pods (in China) 35-50 mm wide. Senegalia pruinescens is distributed in northern Vietnam and Thailand, westwards through Myanmar to northeast India; in China the species is known only from Yunnan and far western Guangxi (to the west of where S. prominens occurs, compare Fig. 42, S. pruinescens and Fig. 39, S. prominens).
Senegalia prominens may also superficially resemble S. andamanica, S. kunmingensis or S. rugata on account of having relatively few pinnae and relatively large leaflets (see these species for discussion of differences).
Etymology. The species name is taken from the Latin prominens (prominent), in allusion to the prominent petiole gland which is one of the distinctive features of the species.
Vernacular name. Tiger Prickle Senegalia (following the Chinese common name that is proposed above). This name appears on the label of S.H. Lai 5470 and refers to the rather prominent, recurved prickles found on the mature branchlets of this species.
16. Senegalia pruinescens (Kurz) Maslin, Seigler & Ebinger, Blumea 58: 41 (2013) (Figs. 41 and 43).粉被金合欢【fěn bèi jīn hé huān】
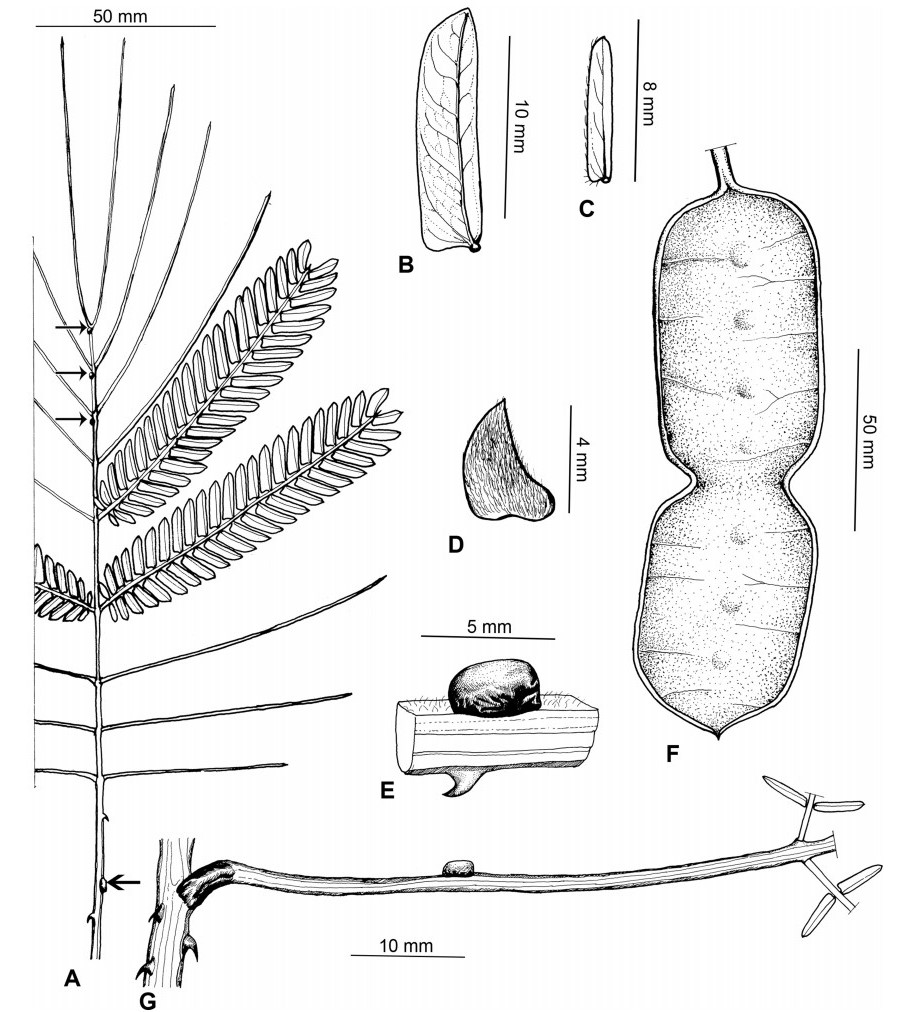
|
| Fig. 41 Senegalia pruinescens. A - Leaf showing relatively few pinnae and position of petiole gland (thick arrow) and rachis glands (thin arrows). B & C - Leaflets (lower surface) showing size variation. D - Stipule. E - Petiole gland prominent. F - Pod wide. G - Node showing pruinose, glabrous branchlet and petiole gland well-removed from leaf base. Vouchers: Y.C. Du 580179 (A, D & E); M.K. Li 2849 (B & F); South-to-North Water Diversion Project 8003 (C & G). Drawn by Joshua Yang. |
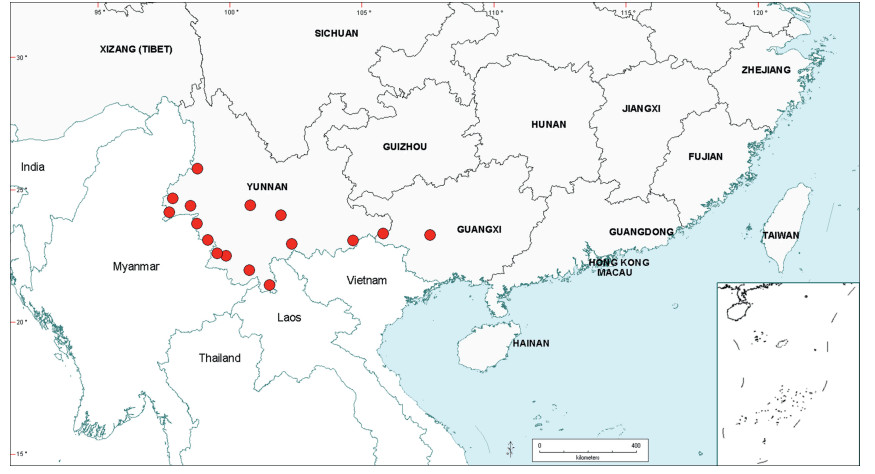
|
| Fig. 42 Distribution of Senegalia pruinescens in China, based on specimen records at GXMI, HITBC, IBK, IBSC and KUN, as cited in text. |

|
| Fig. 43 Senegalia pruinescens. A - Habit showing large, terminal, open panicles. B - Liana habit (with insert showing prickles on stem). C - Inflorescences (side facing sun) showing flower buds dull red; this is same inflorescence as in Fig. D. D - Inflorescences (side not facing sun) showing flower buds green; this is same inflorescence as in Fig. C. E - Inflorescence at anthesis showing stamens white and upper portion of calyx red. F - Inflorescence bud showing upper part of calyx red and petals green. G - Branchlet pruinose (density of pruinosity variable in this species, see text for discussion). H -. Petiole gland (lateral and plane views) hemispheric and prominent. I - Leaf (lower portion) showing prominent gland situated near middle of petiole. J - Vegetative shoot showing prominent, lanceolate stipules. Vouchers: All from B.R. Maslin & L. Bai BRM 11023 except G - L. Bai & H. Wang BLK-073. Photos: L. Bai. Scale bar = 5 mm (unless otherwise indicated). |
≡ Acacia pruinescens Kurz, J. Asiat. Soc. Bengal, Pt. 2, Nat. Hist. 45 (4): 296, 298 (1877, '1876'). Type citation: "Not unfrequent in tropical forests of the southern Pegu Yomah; also Ava, Khakyen Hills, east of Bhamo (J. Anderson)". Type: CHINA, "Poneshee" [located in Yunnan on the border with Myanmar, c. 51 km E of Bhamo, in Dehong Dai & Jingpo Autonomous Prefecture, Yingjiang County, fide Pei et al. (1991)], 26 Apr. 1868, D.J. Anderson s.n. (lectotype: CAL 140803 barcode 0000012937!, 2nd step lectotypification designated here, see discussion below under Typification; isolectotype: CAL, without accession or barcode number!).
=Acacia pruinescens var. luchunensis C.Chen & H.Sun, Acta Bot. Yunnan. 12: 260 (1990); Senegalia pruinescens var. luchunensis (C.Chen & H.Sun) X.Y.Zhu, Biodivers. Sci. 23: 249 (2015). Type citation: "Yunnan Province, Lüchun, Lüchun Expedition 751." Type: CHINA, Lühun Xian [Lühun County is located in Honghe Hani and Yi Autonomous Prefecture, southeast-central Yunnan], suburbs, 1500-1600 m. alt., 13 May 1974, Lühun Expedition 751 (holotype: KUN 0400231 barcode 1206967!; isotype: KUN 0400236 barcode 1206970!).
Distinctive features. Branchlets pruinose (pruinosity faint to conspicuous, sometimes not visible on fruiting specimens) and normally glabrous. Pinnae relatively few (8-11 pairs). Leaflets normally 6-15 mm long, 1.5-3 mm wide and glabrous; lateral veins (on lower surface of leaflets) often forming an imperfect or welldeveloped reticulum; apex normally asymmetric & obtuse. Petiole gland normally well-removed from leaf base (13-40 mm), prominent, 2-4 (-5) mm long and raised 1-2 mm high, shape often irregular but often ±depressed-oblate to ±hemispheric (infrequently conical). Inflorescences arranged in open panicles or racemes, the axes densely short-hairy as on peduncles; inflores-ence buds dull red or pinkish red when fresh (often darkish purple to brown when dry). Pods wide (35-50 mm in China).
Description. Often robust lianas, sometimes lianescent shrubs 3-4 m tall. Branchlets finely striate-ribbed and without lenticels at apices, pruinose (pruinosity faint to conspicuous, sometimes not visible on fruiting specimens, see discussion below under Variation), penultimate and mature branchlets glabrous or infrequently hairy. Prickles few to somewhat numerous on internodes and on under surface of leaf axes, occasionally absent from herbarium material, commonly 1-3 mm long but sometimes to 5 mm, somewhat stout, straight and patent to shallowly or obviously recurved. Stipules often persistent in young inflorescence buds, ovate, 2-4 (-5) mm long, 1-3 (-4) mm wide, sparsely to densely minutely hairy with veins evident or not visible (obscured by indumentum) abaxially, normally at least some lobed (auriculate) on one or both side at base, apex acute. Leaves bipinnate; pinnae 8-11 pairs (to 13 (-15) pairs in India), (20-) 30-95 (-145) mm long; petiole 25-75 mm long; rachis 80-150 (-185) mm long, with short, curved, normally ±appressed, sparse to dense hairs on upper surface. Leaflets 20-37 (-50) pairs, to 12 pairs on lowermost pinnae, narrowly oblong, (5-) 6-15 (-20) mm long, (1-) 1.5-3 (-5) mm wide, moderately to strongly discolorous (darkest above), green, dull lustre on both surfaces or somewhat shiny above, glabrous or occasionally finely appressed-hairy on upper and/or lower surfaces; apex asymmetric or sometimes symmetric, obtuse, with or without an often inconspicuous, blunt apiculum; base truncate, unequal with a prominent rounded angle on lower edge, the petiolule excentric and normally shortly but discernibly extended below base of leaflet, rarely not or scarcely extended below base as in Senegalia kunmingensis; main vein starting near upper margin at leaflet base and extending obliquely to apex, straight except often curved towards apiculum at leaflet apex; lateral veins (on lower surface of leaflets) normally ±evident, patent or sometimes ascending and normally bifurcating near margin, often anastomosing to form an imperfect or well-developed reticulum, sometimes not reticulate. Glands: petiole gland (see notes below under Variation)don lower 
Selected specimens examined. CHINA: Guangxi. Baise City, Napo County, 19 Oct.1009, D. Fang et al. 0694 (GXMI 051841 [barcode 052142]); Baise City, Pingguo County, 2 June 1957, Y.K. Li 1357 (KUN 0400250 [barcode 0598477]). Yunnan. Dehong Dai & Jingpo Autonomous Prefecture, Longchuan County, 4 Sep. 2017, L. Bai & H. Wang BLK-073 (IBSC, KUN barcode 1345221 & 1345224); Dehong Dai & Jingpo Autonomous Prefecture, Mang City, 26 Jan. 1934, H.T. Tsai 56751 (IBSC 105137 [barcode 0159334], KUN 0400247 [barcode 0598469]), KUN 0400248 [barcode 0598470]); Dehong Dai & Jingpo Autonomous Prefecture, Yingjiang County, 17 Nov. 1989, H. Sun 1496 (KUN 0400237 [barcode 0598464]); Honghe Hani & Yi Autonomous Prefecture, Lühun County, 13 May 1974, Lühun Expedition 751 (KUN 0400231 [barcode 1206967], KUN 0400236 [barcode 1206970]); Lincang City, Cangyuan Wa Autonomous County, 19 Dec. 1958, T.P. Zhu 503 (KUN 0400228 [barcode 0598454], KUN 0400229 [barcode 0598453])datypical in having constricted pods; Lincang City, Zhenkang County, 11 Jan. 2013, T. Zhang et al. 13CS5893 (KUN barcode 1440965); Nujiang Lisu Autonomous Prefecture, Lushui County, 11 Apr. 1960, Southto-North Water Diversion Project (western-Yunnan) 8003 (KUN 0400242 [barcode 0598467]); Pu'er City, Jingdong Yi Autonomous County, 7 June 1940, M.K. Li 2849 (KUN 0400251 [barcode 0598476]); Pu'er City, Jingdong Yi Autonomous County, 7 Nov. 1963, Z.H. Yang et al. 101473 (KUN 0400232 [barcode 0598460]), atypical in having 2 glands on petiole; Pu'er City, Lancang Lahu Autonomous County, 25 July 1990, Y.Y. Qian 2617 (HITBC 111115); Pu'er City, Ximeng Wa Autonomous County, 10 Mar. 1958, Y.C. Du 580179 (KUN 0400230 [0598452]); Wenshan Zhuang & Miao Autonomous Prefecture, Malipo County, 3 Feb. 1940, C.W. Wang 86589 (IBSC 680550 [barcode 0158823], KUN 0400246 [barcode 0598471], KUN 0400255 [barcode 0598472])dpetiole gland closer to base than normal; Xishuangbanna Dai Autonomous Prefecture, Jinghong City, 31 Mar. 1957, H.T. Tsai 80126 (KUN 0400275 [barcode 0598488]); Xishuangbanna Dai Autonomous Prefecture, Mengla County, 26 Jan. 2019, L. Bai BLK-119 (BKF, HITBC, KUN [barcode 1347926])dbranchlets/stems pruinose, and L. Bai BLK-122 (BKF, HITBC, KUN [barcode 1347934], PE, SING)da seemingly non-pruinose morphotype; Xishuangbanna Dai Autonomous Prefecture, Mengla County, 23 Aug. 1959, S.J. Pei 9508 (KUN 0400227 [barcode 0598455]; Yuxi City, Xinping Yi & Dai Autonomous County, 23°55'55"N, 101°56'41"E, 19 May 2018, B.R. Maslin & L. Bai BRM 11023 (IBSC, KUN barcode 1345217 & 1345218, PE). INDIA: Assam. Talap-Lakhimpur, 24 Mar. 1984, G.A. Gammie 160 (CAL 140796). MYANMAR: flanks of the Taping valley, 1913, G. Forrest 9830 (IBSC 44554 [barcode 0159338]-specimen ex E, KUN 0400245 [barcode 0598450]-photo, SYS 80414 [barcode 00044311]-photo). THAILAND: South-western. Dong Yai, Srisawat [district], Kanburi [Kanchanaburi], 9 Apr. 1995, Somruay, Sakarm & Aditep 202 (BKF 60529, barcode SN036705).
Distribution (Fig. 42). Occurs in southwest China and northern Vietnam, eastwards through Thailand (a new record for that country) and Myanmar to northeast India. In China the species is scattered in western, southern and central Yunnan, extending to western Guangxi (Baise City). The northern Vietnam occurrence is based on Poilane 25200, cited by Nielsen (1981), but we have not seen this specimen. Judging from its known distribution (especially in China), it is likely that Senegalia pruinescens will be shown to occur in Laos in the future.
Habitat. In China, this species grows in open forests on hills or mountains at 700-1600 m alt.
Phenology. This species has a relatively long flowering period that extends from April to August. Specimens with immature seeds occur from late November to early February. Only a single specimen with near-mature seeds has been seen, and this was collected in early January. Wu and Nielsen (2010) record the fruiting period as June to October which seems rather unusual.
Typification. In the protologue of Acacia pruinescens, Kurz (1877 '1876') cited the species as occurring in "Southern Pegu Yomah; also Ava, Khakyen Hills, east of Bhamo (J. Anderson)". Although not explicitly stated in that work, the Yomah collection was very likely a reference to Kurz's own gathering of the species, see Kurz (1874). Acacia pruinescens is therefore regarded here as having been based on Anderson and Kurz specimens which are regarded here as original material. Nielsen (1980) designated a J. Anderson specimen at herb. CAL that was collected from 'Poneshee' as the lectotype of A. pruinescens, presuming that it was the same gathering as was cited in the protologue, despite having a seemingly different locality. Nielsen's interpretation is regarded as reasonable because Anderson (1878) clearly indicated that "Ponsee" was in the Kakhyen hills (which was cited in protologue, as "Khakyen Hills", and attributed to Anderson). This locality is in far western Yunnan, on the border with Myanmar (see lectotype citation above) and is shown on the map of Bretschneider (web ref. 9, given as "Pangsi"). However, because there are two specimens of the Anderson gathering at CAL, a second step lectotypification is done above to more precisely typify this name (cf. ICN Art. 9.17). These two specimens have identical labels, each annotated 'A. pruinescens' by Kurz and showing the collection information as given by Nielsen (1980), except the date of collection is 1868, not 1866 as cited by Nielsen. CAL 140803 [barcode 0000012937] has been selected as the lectotype because it is the better-preserved specimen.
Chakrabarty and Gangopadhyay (1996) were seemingly unaware of the lectotypification of Acacia pruinescens by Nielsen (1980), and consequently cited "Myanmar, Pegu, Yomah to Poungyee, Kurz 1744" at CAL as the holotype of the species. Despite this locality being very similar to one of those given in the protologue, Kurz 1744 was not explicitly cited there, but this collection is certainly original material. It is noted that Kurz 1744 appears to be atypical for Senegalia pruinescens in having glabrous peduncles, even though Kurz (1877 '1876') described A. pruinescens as having hairy or non-hairy peduncles.
Bibliography. Although the date 1876 appears on the cover of the bound volume 45 of the Journal of the Asiatic Society of Bengal, on page iv of the journal it clearly states that Part 4 (in which A. pruinescens was described) was issued on 19 April 1877.
Taxonomy. Sun and Chen (1990) described Acacia pruinescens var. luchunensis to accommodate Chinese plants with broad leaflets (2.5-4 mm wide), applying the name var. pruinescens to plants where leaflets did not exceed 1.5 mm wide. Sun (2006) maintained var. luchunensis but Wu and Nielsen (2010) subsequently treated it as a synonym under A. pruinescens. While the leaflets on Chinese plants are generally wider than those on specimens from further west (i.e. Myanmar and India), the differences are not especially large, and the variation appears to be continuous (see Variation below). Therefore, in the absence of other distinguishing characters var. luchunensis is treated here as a synonym under A. pruinescens.
Affinities. The closest relatives of Senegalia pruinescens are uncertain. As discussed under S. andamanica, S. kunmingensis, S. obliqua, S. prominens, S. teniana and S. rugata these species sometimes superficially resemble S. pruinescens, (especially as most have relatively few pinnae and rather large leaflets), but none seem especially closely related to S. pruinescens. Senegalia pruinescens can be distinguished from the above-mentioned species by its pruinose branchlets (but see notes below under Variation) as well as the combination of other characters listed under Distinctive features above.
Variation. Senegalia pruinescens is rather variable in a number of characters and this variation sometimes confounds the distinction between the species and those listed under Affinities above.
Senegalia pruinescens is the only species in China that has pruinose branchlets (the pruinosity varies from faint to conspicuous). While most herbarium specimens show this pruinosity, it is absent from a few (especially fruiting specimens) in which cases it is not known if the pruinosity was lost as a result of the drying process, was present on living plant-parts that were not sampled, or was totally absent from the plant sampled. In the protologue of Acacia pruinescens, Kurz (1877 '1876') described the branchlets, inflorescences and peduncles as being more or less pruinose, but it is not known if those were observations based on living plants or dry specimens (the original material of this species at herb. CAL does not show any obvious signs of being pruinose). This matter of pruinosity within S. pruinescens, its variation on plant organs and also between different individuals/populations, warrants further investigation.
The leaflets of Senegalia pruinescens vary in both width and venation. In India and Myanmar, they are consistently narrow (c. 1 mm wide) and although sometimes similar on plants in China, they co-occur with wider ones. The normal range of variation for leaflet width in China is 1.5-3 (-4) mm and can vary considerably, even on the same specimen. On specimens from India and Myanmar (including the type of A. pruinescens) and on a number of specimens from China, the minor veins on the lower surface of the leaflets do not form a reticulum, or if present it is imperfectly developed. However, many Chinese specimens do possess a discrete reticulum, with the anastomoses varying from relatively few to numerous. In the latter case the leaflet venation is not dis-similar to that which is often seen in S. prominens.
The petiole glands of Senegalia pruinescens are prominent but their form is variable and irregular, and it is rather difficult to describe using conventional terminology (see description above). Only infrequently are these glands conical as in S. prominens (e.g. D. Fang et al. 0694 and the lectotype). Normally the petiole gland is well-removed from the leaf base (13-40 mm), but one collection, C.W. Wang 86589, is very unusual in having a gland just 6-7 mm above the leaf base. As in most other Chinese species of Senegalia, S. pruinescens normally has only a single gland on the petiole. However, on Z.H. Yang et al. 101473 from Jingdong Yi Autonomous County, there are two petiole glands on some of the leaves; other specimens from this county possess a single gland on all leaves. Occasional specimens with two petiole glands are also seen in S. kunmingensis, S. rugata and S. stipitata, while S. tonkinensis is the only species in this paper that consistently has two glands on its petioles.
Although Wu and Nielsen (2010) described Acacia pruinescens as having linear leaflets and a pubescent calyx tube, this species is more correctly described as having narrowly oblong leaflets and a glabrous calyx tube. Wu and Nielsen (2010) also described the seeds as 7-10 mm long, which is a little shorter than we have observed (i.e. 12-13 mm long), but it is possible that the Wu and Nielsen (2010) measurement was taken from Nielsen (1981) where the seeds were said to be immature.
Variant. The following unusual specimen is not included in the above description: IBK 94365 [barcode 00067657] from Guangxi (no other collection details apart from a field tag attached to the specimen that is annotated '85161'. The specimen has pruinose branchlets but differs from Senegalia pruinescens by its stipules being glabrous and strongly veined, and its leaflets having a centric apiculum. These branchlet and stipule characters also distinguish IBK 94365 from S. prominens which it resembles in some ways (e.g. petals strongly 1-veined). It would be desirable to recollect this entity if possible, and to better-assess its taxonomic status.
Uses. The tough bark and fibre are used for poisoning fish in Myanmar (Kurz, 1877 '1876').
Etymology. The species name is taken from the Latin pruinosus (pruinose, a white powdery covering). See note above under Variation regarding this pruinosity.
Vernacular names. Powder-bark Senegalia. This name was used by Wu (1988), Sun (2006) and Wu and Nielsen (2010) under Acacia pruinescens. Various other names have been applied to this species in Southeast Asia, e.g. 'Thau duc ngu' (in Vietnam by the Tho ethnic group), fide Nielsen (1981) and 'Kinmum gyin' in Myanmar (Kress et al., 2003).
17. Senegalia rugata (Lam.) Britton & Rose, N. Amer. Fl. 23(2): 120 (1928) (Figs. 44 and 46).紫荚金合欢【zǐ jiá jīn hé huān】(新拟)
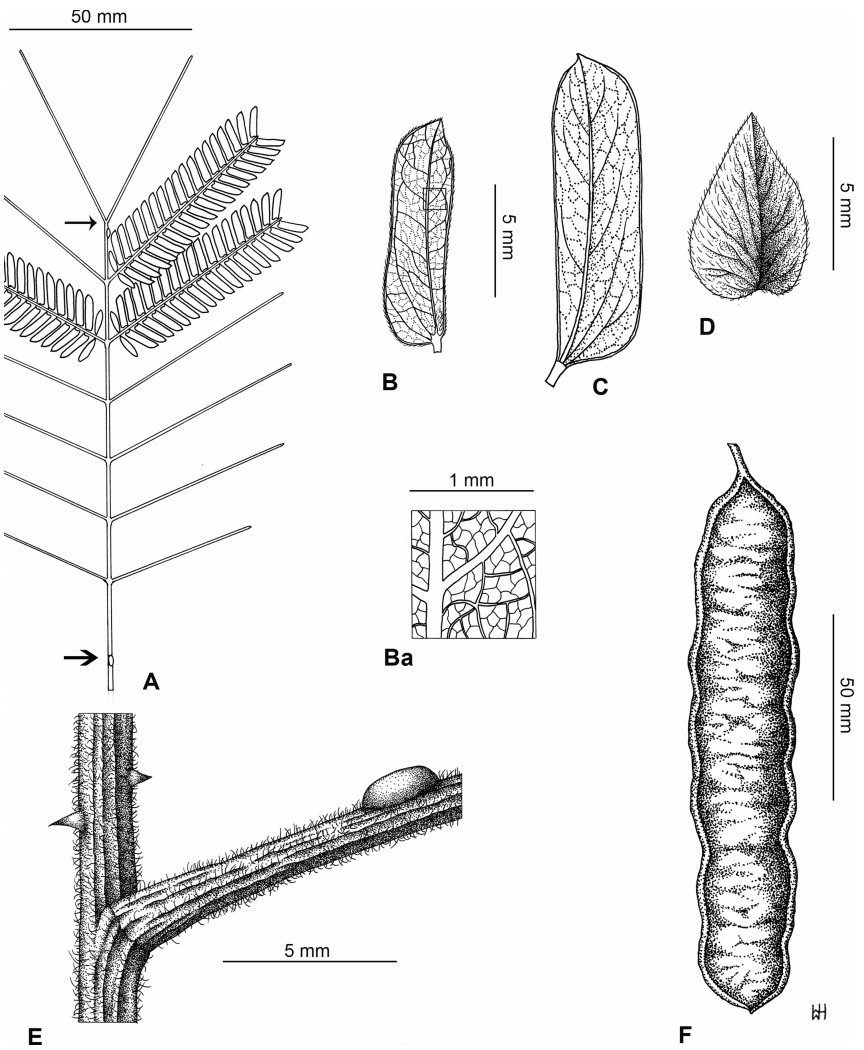
|
| Fig. 44 Senegalia rugata. A - Leaf showing relatively few pair of pinnae and position of petiole gland (thick arrow) and rachis gland (thin arrow). B & C - Leaflets (lower surface) showing size variation, reticulum and terminal apiculum (B = typical S. rugata; C =large leaflet morphotype of S. rugata, i.e. Bukit Brown variant). Ba - Leaflet reticulum showing detail. D - Stipule large, lobed at base. E Node showing prominent petiole gland. F - Pod dark-coloured and coarsely wrinkled when dry. Vouchers: P.I. Mao 7419 (A & E); S. Chow 604 (B & Ba); A.T. Gwee et al. (C - SING barcode 0096345); S. Chow 604 (D); J.F. Zhang 12 (F). Drawn by Waiwai Hove. |
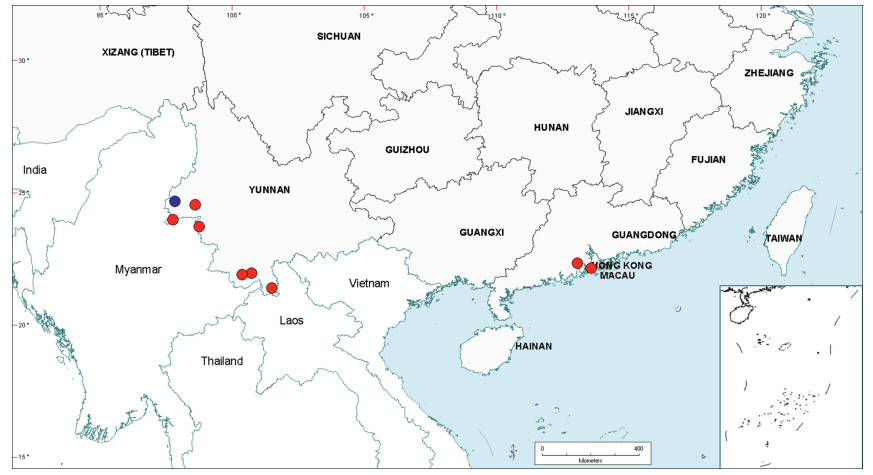
|
| Fig. 45 Distribution of Senegalia rugata in China, based on specimen records at IBSC and KUN, as cited in text. The wider distribution given for Senegalia rugata (as Acacia concinna) in China by Wu and Nielsen (2010) was based mostly on misidentifications of Senegalia prominens (see text for discussion). Also, both Forbes and Hemsley (1887) and Merrill (1927) recorded this species (as Acacia concinna) from Hainan, based on a B.C. Henry specimen, but we have not seen this or any other specimens to verify this occurrence and therefore regard Hainan as uncertain for Senegalia rugata. Also, Sun (2006) recorded Acacia concinna from southeast Yunnan, but again we have not seen specimens that verify the record. Red dots =typical Senegalia rugata, blue dot =S. rugata (Bukit Brown variant); both these entities are recorded for the Dehong Dai & Jingpo Autonomous Prefecture. |

|
| Fig. 46 Senegalia rugata. A - Inflorescence showing obviously pink flower buds (from plant in Chiang Mai, Thailand). B - Inflorescence showing flower buds (atypically) yellowish green and also slightly tinged pink (from Macau, China). C - Stem of liana. D - Pods obviously constricted between seeds. E - Petiole gland prominent. F - Prickles on branchlet. G - Leaf showing relatively few pinnae. Vouchers: B.C. Ho 15-007 (A - SING); Yi Qi-Fei 3124 (B); B.R. Maslin 11000 (C, E, F & G - SING); Yi Qi-Fei 2318 (D). Photos: B.C. Ho (A), B.R. Maslin (C, E, F & G, from plant in Singapore) and Yi Qi-Fei (B & D). Scale bar =5 mm (unless otherwise indicated). |
≡ Mimosa rugata Lam., Encycl. [J. Lamarck et al.]. 1: 20 (1783). ≡ Acacia rugata (Lam.) Buch.-Ham. ex Voigt, Hort. Suburb. Calcutt. 263 (1845), nom. illeg., non Buch.-Ham. ex Bentham (1842). ≡ Acacia rugata (Lam.) Buch.-Ham. ex Merr., Philip. J. Sci. (Botany) 5: 28 (1910), isonym. ≡ Acacia rugata (Lam.) Buch.-Ham. ex Fawc. & Rendle, Fl. Jamaica [Fawcett & Rendle] 4: 141 (1920), isonym. Type citation: "Cet arbre croît dans l'Inde, & m'a été communiqué par M. Sonnerat.". Type: INDIA, comm. P. Sonnerat s.n (holotype: P-LA barcode P00297138, specimen ex herb. Lamarck [digital image!]), fide Seigler et al. (2014). Note: Despite the above orthography which appears in the publications cited, there is no evidence that A. rugata Buch.-Ham. (nom. nud., see below) is based on M. rugata Lam. although Voigt and later authors made that assumption.
= Mimosa sinuata Lour., Fl. Cochinch. 2: 653 (1790). ≡ Acacia sinuata (Lour.) Merr., Trans. Amer. Philos. Soc. n.s. 24: 186 (1935), nom. illeg., non Jacques (1860). Type citation: No type cited but provenance given as "Habitat in sylvis Cochinchinae" Type: Unknown (see discussion below under Taxonomy).
= Mimosa concinna Willd., Sp. Pl. ed. 4, 4: 1039 (1806). ≡ Acacia concinna (Willd.) DC., Prod. 2: 464 (1825). ≡ Acacia rugata Buch.-Ham. ex Benth. var. concinna (Willd.) Kurz, J. Asiat. Soc. Bengal, Pt. 2, Nat. Hist. 45: 297 (1877, '1876'. Type citation: "Habitat in India orientalis … Klein". Type: D. Klein s.n., Ind. Or (holotype: BeW, fide Nielsen (1980)).
- "A. rugata" Buch.-Ham. in Wallich Numer. List no. 5251 (1831-32), nom. nud.
= Acacia. rugata Buch.-Ham. ex Benth., London J. Bot. 1: 514 (1842). ≡ Acacia concinna var. rugata (Buch.-Ham. ex Benth.) Baker, Fl. Brit. India [J.D. Hooker] 2: 297 (1878). ≡ Acacia rugata Buch.-Ham. ex Gamble, Fl. Madras 1: 429 (1919), isonym. ≡ Acacia gamblei Bahadur & R.C.Gaur, Acta Bot. Indica 4: 67 (1976), nom. illeg. (superfluous). Type citation: "E. Indies, Hamilton …. Ham. in Wall. Cat. no. 5251" Type: Wallich Cat. no. 5251 (holotype: KeW!, fide Nielsen (1985b)).
= Acacia philippinarum Benth., London J. Bot. 1: 514 (1842). Type citation: "Philippine Islands, Cuming n. 953 and 1166". Type: PHILLIPINES, 1841, H. Cuming 953 (lectotype: K barcode K000295861 & K000724530, designated by Nielsen (1985b) who incorrectly used the term holotype).
Additional synonymy (not relevant to China): See Nielsen (1985b).
Distinctive features. Stipules wide (3-4 mm), obviously lobed on one or both sides at base. Pinnae relatively few, 4-8 (-11) pairs. Leaflets normally 7-10 mm long, and 1-2.5 mm wide; apex obtuse with a short, acute, centric or excentric apiculum; lateral veins (on lower surface of leaflets) forming a net-like reticulum (comprising small vein-islands). Petiole gland somewhat prominent, normally situated on lower ¼ of petiole. Inflorescences 2-3 (-5) within leaf axils but sometimes racemosely arranged; inflorescence buds red or pink when fresh (dark-coloured when dry). Flowers not particularly densely arranged in heads. Pods flattened but thick, smooth and coriaceous/fleshy when fresh, coarsely wrinkled and very hard-textured when dry, margins often shallowly constricted between the seeds. Seeds seated within discrete chambers in pod.
Description. Lianas or scandent shrubs or small trees to c. 5 m tall. Branchlets glabrous or (towards apices) puberulous; lenticels evident on mature branchlets. Prickles few to rather numerous on internodes and normally a few on under surface of leaf axes, 1-3 mm long on upper branchlets but 4-5 mm on mature branches, rather stout, straight and patent or shallowly to obviously recurved on branchlets, recurved on leaf axes. Stipules caducous, ovate, 4-6 mm mm long, 3-4 mm wide, sub-glabrous (except ciliolate) or densely hairy abaxially, obviously lobed on one or both side at base, apex acute to short-acuminate. Leaves bipinnate; pinnae 4-8 (-11) pairs, 40-85 mm long; petiole 20-40 mm long; rachis 40-110 mm long. Leaflets 14-28 pairs, narrowly oblong, (5-) 7-10 (-12) mm long, 1-2.5 mm wide, thin-textured (often membranous), flat but often slightly undulate or folded lengthwise when dry, green, concolorous or weakly discolorous (darkest above), green, dull lustre on both surfaces, glabrous (except mar-gins often sparsely ±appressed-ciliate); apex symmetric to asymmetric, obtuse, apiculate by a short but distinct, acute, centric to excentric point; base unequal with a prominent rounded angle on lower edge, the petiolule excentric and evident (extended below base of leaflet); main vein starting near upper margin at leaflet base and extending obliquely to apex, straight to slightly curved; lateral veins (on lower surface of leaflets) anastomosing to form a rather dense, net-like reticulum comprising small vein-islands. Glands: petiole gland-normally situated on lower ¼ of petiole or occasionally near its middle 7-18 (-25) mm above leaf base, rarely a second gland situated at base of proximal pair of pinnae (see SinoSoviet Joint Expedition 346), oblong to elliptic, 1.5-2 (-3) mm long, 1.5 mm wide, sessile, solid, somewhat prominent (raised to c. 1 mm high), shape variable (but often hemispheric or depressed-oblate); rachis glands-normally situated at base of uppermost 1-3 (-4) pinnae, oblong to elliptic, 1-2 mm long, sessile; rachilla glands - situated at base of uppermost 1 or 2 pair of leaflets. Inflorescences comprising 2-3 (-5) pedunculate heads within leaf axils or sometimes arranged in short racemes (see notes below under Inflorescences); peduncles 15-25 (-43) mm long, normally with a rather dense indumentum of patent, ±straight, longish hairs, sometime hairs appressed or (when in pod) almost absent; heads globose, 8-10 mm diam. when dry, white or pale yellow, 25-35-flowered, the flowers not especially densely arranged (best observed in young buds); inflorescence buds all or most dark-coloured when dry (pink or red when fresh, see notes below under Inflorescences). Bracteoles not exserted beyond flowers in buds. Flowers 5-merous, sessile or sub-sessile; calyx about as long as petals, dark-coloured (as in buds) throughout or only near apex, gamosepalous, shortly dissected into broadly triangular lobes, calyx tube glabrous and obscurely 5-veined or veins not visible; petals 2.5-3 mm long, glabrous, veins not visible; ovary glabrous or sparsely appressed-hairy. Pods oblong, 35-100 mm long, (10-) 15-20 (-25) mm wide, smooth and coriaceous/fleshy when fresh, coarsely wrinkled and very hard-textured (thickly crustaceous to ±woody) when dry, normally ±straight, infrequently curved, shallowly or sometimes strongly constricted between seeds, sometimes straight-edged, flattened but thick, black or brown when dry, dark brown or purplish brown when fresh, glabrous or rarely obscurely appressed-hairy, stipe short (5-8 mm long) but distinct. Seeds (based on T. Smitinand et al. 444 from Thailand) transverse and seated within discrete chambers in pod, oblong, 10-11 mm long, 7-8 mm wide, somewhat turgid (c. 4 mm thick), black, somewhat shiny; areole open at hilar end, c. 7 mm long and 5 mm wide, occupying much of the seed surface; funicle thickly filiform, exarillate.
Selected specimens examined. CHINA: Guangdong. Jiangmen City, Xinhui City, Feb. 1933, S.L. Chang 129 (IBSC 53766 [barcode 0158754]). Macau. Macau, 6 Feb. 2015, Yi Qi-Fei 2318 and 21 Apr. 2016, Yi Qi-Fei 3124 (both IBSC).Yunnan. Baoshan City, Longling County, 26 June 1956, Sino-Soviet Joint Expedition 346 (KUN 0400294 [barcode 0598489]), atypical in having two petiole glands; Dehong Dai & Jingpo Autonomous Prefecture, Ruili City, 27 Apr. 1961, S. Chow 604 (KUN 0400297 [barcode 0598515], KUN 0400298 [barcode 0598514]); Lincang City, Zhenkang County, Mar. 1936, C.W. Wang 72735 (KUN 0400287 [barcode 0598483]); Xishuangbanna Dai Autonomous Prefecture, Jinghong City, 31 Oct. 19581, K.L. Le 1435 (KUN 0400262 [barcode 0598506]), atypical in having 11 pair of pinnae; Xishuangbanna Dai Autonomous Prefecture, Jinghong City, 19 Nov. 1955, P.I. Mao 7419 (IBSC 311347 [barcode 0158815], KUN 0400271 [barcode 0598501], KUN 0400272 [barcode 0598500]); Xishuangbanna Dai Autonomous Prefecture, Jinghong City, 3 Mar. 1953, J.F. Zhang 12 (KUN 0400285 [barcode 0598492]); Xishuangbanna Dai Autonomous Prefecture, Menghai County, May 1936, C.W. Wang 74014 (KUN 0400288 [barcode 0598482]); Xishuangbanna Dai Autonomous Prefecture, Mengla County, s.d., H.C. Mao s.n. (SWFC 0021086 & 0052951). THAILAND: South-western. Kanchanaburi Province, Thung Yai Naresuan, Ban Nong Daeng, 27 Feb. 1993, T. Smitinand, T. Santisuk et al. 444 (BKF, 191957 barcode SN208700).
Distribution (Fig. 45). Widespread on the Indian subcontinent (Bangladesh, Bhutan, India and Nepal) and in Southeast Asia (Cambodia, Indonesia, Laos, Peninsular Malaysia, Myanmar, Papua New Guinea, Philippines, Thailand and Vietnam), and of restricted occurrence in China. Introduced in Australia (Queensland), Brazil, Caribbean, Japan (Okinawa), Madagascar, Mauritius, Reunion Island and Singapore. In China, Senegalia rugata occurs in Guangdong (coastal areas adjacent to Macau), Macau and in far western and southern Yunnan (not far from the Myanmar border). Although both Forbes and Hemsley (1887) and Merrill (1927) cited a B.C. Henry specimen of A. concinna (=Senegalia rugata) for Hainan, neither this collection, nor any other of this species from that area, has been seen by us. Therefore, the occurrence of S. rugata on Hainan Island is regarded as uncertain. Sun (2006) recorded the species (as A. concinna) from southeast Yunnan, but again no specimen to verify this record has been seen. The wider distribution given for Senegalia rugata (as A. concinna) in China by Wu and Nielsen (2010) is based mostly on misidentifications of Senegalia prominens (see discussion below). Senegalia rugata (as A. concinna) was the first indigenous species of Senegalia recorded in western literature for China, see Bentham (1861).
Habitat. In China, this species grows in forests or thickets at 800-1500 m alt., commonly in valleys near watercourses.
Phenology. Judging from herbarium specimens, Senegalia rugata in China flowers in March and April (with plants in March often having only inflorescence buds). Wu and Nielsen (2010) recorded flowering (under A. concinna) as April to June, but as noted below, it is likely that that treatment included elements of both S. rugata and the later-flowering S. prominens. Mature seeds have not been seen for Chinese plants, but judging from immature pods on herbarium specimens, they are likely to be present from about late February to March/April. In Thailand, one fruiting specimen with mature seeds (together with inflorescence buds) has been seen, it was collected in late February.
Taxonomy. Senegalia rugata has a very complex nomenclatural history and many synonyms are currently recognised for it (see above). The synonyms of relevance to China are Acacia concinna and A. sinuata, and the relationship between these two names and that of S. rugata is briefly discussed here.
As detailed by Nielsen (1980), the entities described as Acacia concinna and A. rugata have at times been treated as separate species or as varieties of the one species; however, Nielsen (1980) considered that they were best regarded as conspecific (with the species being recognised primarily by its thick, fleshy pods that are characteristically hard-textured and wrinkled when dry). Under Acacia, Nielsen (1980) applied the name A. concinna to the combined entity, but under Senegalia, the correct name for it is S. rugata. It is this broadly defined S. rugata that has been adopted in the present work. However, it is evident from an examination of herbarium specimens in Southeast Asia, China and India that S. rugata as presently defined is very variable and is in need of further study over its extensive geographic range. Such a study is beyond the scope of this paper and therefore the treatment of S. rugata presented here is restricted to plants from China, except for the seeds which are based on a specimen from Thailand.
In past Chinese literature, Senegalia rugata had most often been called Acacia concinna but its circumscription was commonly confounded with that of A. sinuata. Although the name A. sinuata is treated here as synonymous with S. rugata, the entity described in China in the past under A. sinuata commonly included elements of both S. rugata and our new species, S. prominens (see discussion below and under S. prominens above).
The Wu and Nielsen (2010) treatment of Acacia concinna in China contains elements of both Senegalia rugata and S. prominens, and in part, seems to have been based on the descriptions given in Institute of Botany (1972), Huang (1985) and Wu (1988). For example, the heads 'arranged in a panicle' applies to S. prominens (not S. rugata) while the pods 'fleshy, with wrinkled surfaces' applies to S. rugata (not S. prominens). As to the Chinese Provinces cited by Wu and Nielsen (2010), Yunnan applies to S. rugata (not S. prominens) while the other more easterly Provinces that are cited apply to S. prominens (not S. rugata). As already noted above, the flowering phenology given in Wu and Nielsen (2010) for A. concinna is possibly a combination of that for S. rugata and S. prominens.
Like Nielsen (1980) we have been unable to locate a type for Mimosa sinuata. This is not surprising because, as noted by Merrill (1935), very few Loureiro types are extant. Although no type specimen was cited in the protologue, Loureiro (1790) did say that the species occurred in "Cochinchinae" This geographic name is not in current use, but in the past it most often referred to the southern one third of present-day Vietnam (although originally it referred to the southern half of Vietnam, extending southwards from just north of Hue). Loureiro's chief place of residence during the 36 years that he lived in Cochinchina was Hue (Merrill, 1935) so it is possible that the entity he described as M. sinuata was based on material collected from that general vicinity.
We agree with the interpretation by Merrill (1935) that Loureiro's description of Mimosa sinuata probably refers to the entity hitherto called Acacia concinna (=Senegalia rugata), especially as the inflorescences were described as axillary and solitary and the pods as sinuous (presumably a reference to the pod margins that are normally constricted between the seeds). These two characters are otherwise uncommon in Asian Senegalia. However, the description of the flowers as 4-merous and the corolla as absent by Loureiro (1790) are curious; these may be errors or perhaps there was some species additional to S. rugata that was included in Loureiro's concept of M. sinuata.
Notwithstanding the above, Acacia sinuata has been variously treated by authors in the past, and there has been much confusion concerning this name in Chinese literature. Nielsen (1980) regarded it as a dubious name, but later treated it as a provisional synonym of A. concinna Nielsen(1981, 1985b), as did Sun and Chen (1990). Nielsen (1992), Sun (2006) and Wu and Nielsen (2010) treated A. sinuata as conspecific with A. concinna, without qualification.
The name Acacia sinuata appears to have been first introduced into Chinese taxonomic literature by Institute of Botany (1972), followed by Huang (1985) and Wu (1988). These three publications all treated A. sinuata as a distinct species with the last two giving A. concinna as a synonym. The three descriptions are very similar to one another, and each is accompanied by an illustration, but no voucher specimens were cited. It appears most likely that all three descriptions contain elements that apply to Senegalia prominens (e.g. leaflets hairy and inflorescences paniculately arranged) while the first two describe pods that are clearly those of S. rugata (i.e. slightly succulent, wrinkled when dry: these attributes were not mentioned by Wu (1988)); the illustrations in all three works appear to be of S. prominens only. This confusion concerning the definition of A. sinuata in China is also seen on herbarium specimens. During the course of this study we located many specimens of S. prominens at herb. IBK and IBSC that were determined as either A. concinna or A. sinuata.
Affinities. When in pod, Senegalia rugata is very distinctive and would not be confused with any other species of Senegalia in Asia. The dry pods of this species are characteristically very hardtextured (thickly crustaceous to ±woody) and coarsely wrinkled whereas in other species of Senegalia the pods are thin-textured (firmly chartaceous to thinly coriaceous) and not at all wrinkled. When in flower though, S. rugata may at times superficially resemble S. prominens, S. pruinescens or S. teniana, all of which often possess ±reticulately-veined leaflets, relatively few pinnae and often a prominent petiole gland. However, compared with those three species, the leaflet reticulum in Senegalia rugata is normally better-developed with more numerous, small vein-islands. Flowering specimens of S. prominens can be further recognised by its leaflets normally being appressed-hairy on their lower surface, its inflorescences often paniculately arranged and its flower buds pale green when fresh; these two species occur near one another in the general vicinity of Macau. Flowering specimens of S. pruinescens are most readily distinguished from those of S. rugata by having pruinose young branchlets and often paniculately arranged inflorescences; these two species are broadly sympatric in western and southern Yunnan (compare Fig. 45, S. rugata and Fig. 42, S. pruinescens). Senegalia teniana also has pale green (not red) inflorescence buds and is further recognised by its normally shrubby habit, often shorter pinnae and a slightly less prominent petiole gland. These two species occur in Yunnan but they are allopatric, with S. teniana occurring about 400 km to the northeast of where S. rugata is found (compare Fig. 45, S. rugata and Fig. 51, S. teniana).
Inflorescences. The inflorescences of Senegalia rugata in China and elsewhere are normally clustered within the leaf axils, but sometimes they are also arranged in short racemes. Panicles are rare in this species but have been observed on a few collections, especially from northern Thailand (e.g. J.F. Maxwell 97-745, CMU 11374). Although Wu and Nielsen (2010) described the inflorescences of this species as being arranged in panicles, it is most likely that their description referred to plants of S. prominens (see under Taxonomy above); we have not seen any specimens of S. rugata from China with paniculately arranged inflorescences.
The inflorescence buds on herbarium specimens of Senegalia rugata specimens are dark-coloured (but sometimes only near the bud apex), suggesting that they were reddish in living plants (see discussion above under Morphological characters: Inflorescences and flowers). Indeed, Nielsen (1992) described the inflorescence buds of this species (as Acacia concinna) in Malaysia as being dark red, while Verdcourt (1979) described them as either dark red or purple on plants from New Guinea. Fig. 46 shows further variation, not only for bud colour (which is pinkish red) but also for how well the colour is expressed. In the plant from Chiang Mai, Thailand, the pinkish red colour is very well-developed and is more or less uniform within the heads (Fig. 46A), whereas the plant from Macau shows the red col-ouring as poorly developed, with many flowers in the heads being a greenish yellow (Fig. 46B).
Bukit Brown variant. A variant not included in the above description, referred to herein as Senegalia rugata (Bukit Brown), is known in China from only the two collections cited below. Although these specimens are without stipules, inflorescences or pods, their leaf characters show the diagnostic features of S. rugata, except that the leaflets are atypically large (i.e. 15-18 mm long, 5-5.5 mm wide, see Fig. 44C). The two collections are from far western Yunnan (in Dehong Dai & Jingpo Autonomous Prefecture; typical S. rugata also occurs in this Prefecture), c. 100 km east of the Myanmar border; H. Sun 1478 was gathered from the outskirts of a small village, but it is not known if it was native or introduced in that area (H. Sun, pers. obs.). This variant appears most likely to be the same entity that occurs in Singapore (including the Bukit Brown Cemetery) where it is thought to have been introduced, perhaps for culinary purposes; the Singapore plants are also sterile, but they do possess the characteristic stipules of S. rugata. Until flowering and fruiting material can be collected, and further detailed studies conducted, it is not possible to assess the taxonomic status of this entity within the currently broadly circumscribed S. rugata, or to know its natural distribution.
Selected specimens examined (Bukit Brown variant). CHINA:Yunnan. Dehong Dai & Jingpo Autonomous Prefecture, Yingjiang County, 14 Jan. 1989, H. Sun 1478 (KUN 0159825 [barcode 0598024]) and 1 Apr. 1979, Kunming Institute of Botany Plant Population Project s.n. (KUN 674608 [barcode 0598442]). SINGAPORE: Bukit Brown Cemetery, adjacent to Kheam Hock Road, 23 Oct. 2014, B.R. Maslin, H.K. Lua, B.C. Ho & A.T.K. Yee BRM 11002 (BKF & SING barcode 0208533) and BRM 11003 (KUN & SING barcode 0208534 & 0208535).
Etymology. The species name is derived from the Latin rugatus (wrinkled) in reference to the pods that are distinctively coarsely wrinkled when dry (smooth when fresh).
Vernacular names. Purple-pod Senegalia (following the Chinese common name that is proposed above). In Thailand this species has been called (under Acacia concinna) 'Som khon' or 'Som poi', fide Nielsen (1985a).
18. Senegalia stipitata Maslin, B.C.Ho, H.Sun & L.Bai, sp. nov. (Figs. 47 and 49).柄腺金合欢【bǐng xiàn jīn hé huān】(新拟)
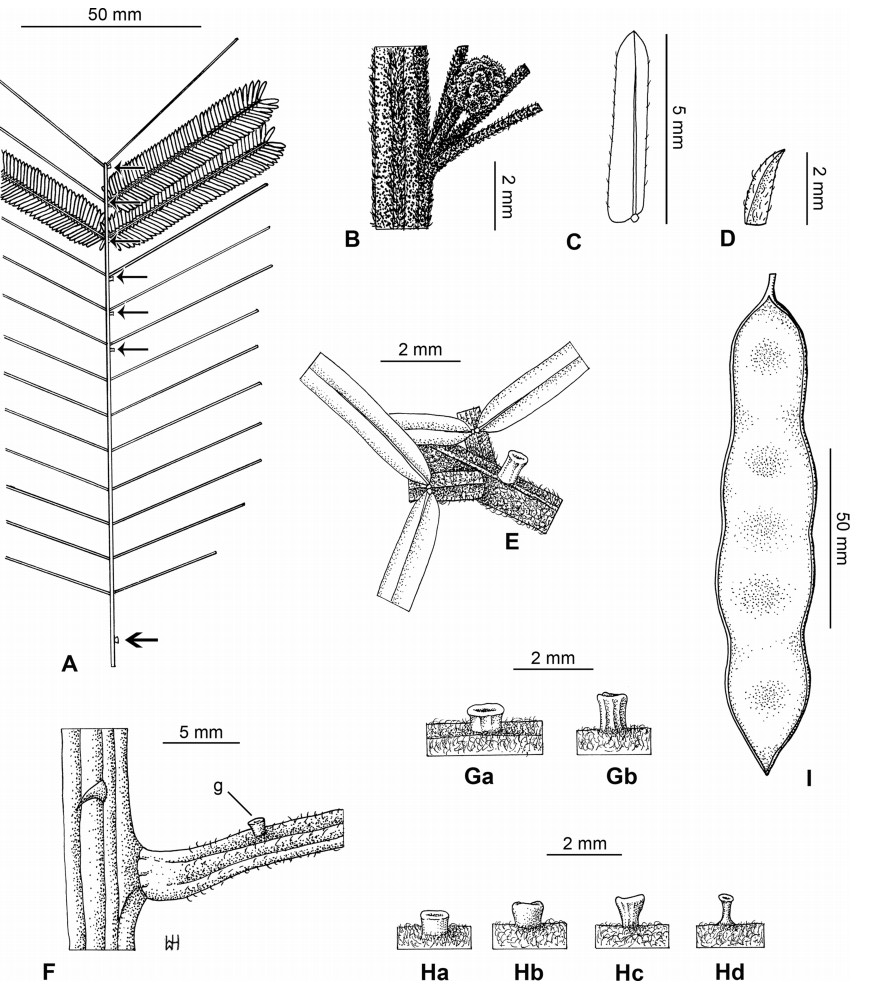
|
| Fig. 47 Senegalia stipitata. A - Leaf showing position of petiole gland (thick arrow) and numerous rachis glands (thin arrows). B - Inflorescence bud at node, showing dark-colouring due to dense indumentum of resin hairs. C - Leaflet (lower surface) showing main situated slightly towards and parallel with upper margin near base of leaflet. D - Stipule small. E - Rachis gland (stipitate) at base of pinnae. F - Node showing small, stipitate petiole gland position. G - Rachis glands (stipitate) showing variation. H - Petiole glands (stipitate) showing variation (Hd = calicioid; Hc & Hd - note variation from the one specimen). I - Pod. Vouchers: Sino-Vietnam Expedition 1603 (A, C, E, F, Gb & Ha); P.I. Mao 3922 (B & D); A.J.B. Chevalier 37410 (Ga); Ban, Phuong, Khoi, Binh & Bach 2067 (I); Anonymous (Hb eIBSC 680597); K.H. Cai 1265 (Hc & Hd). Drawn by Waiwai Hove. |

|
| Fig. 48 Distribution of Senegalia stipitata in China, based on specimen records at GXMI, HITBC, IBK IBSC and KUN, as cited in text. |

|
| Fig. 49 Senegalia stipitata. A - Leaf. B - Tendril with vegetative bud at apex. C - New shoot (young) showing reddish, sessile glands and narrowly oblong, acute stipules. D - Leaflets (upper surface) dark green and rather shiny. E - Leaflets (lower surface) paler green with dull lustre, the main vein close to and ± parallel with upper margin towards base of leaflet. F - Prickles on stem. G - Petiole gland stipitate (upper: gland single; lower: two glands atypical). H - Rachis glands stipitate. Voucher: All from B.R. Maslin & L. Bai BRM 11044. Photos: L. Bai. Scale bar =5 mm (unless otherwise indicated). |
Type: CHINA, Guangxi Province, Chongzuo City, Fusui County, Zhongdong District to Damingshan Mountain, 23 Apr. 1957, S.H. Chen 11949 (holotype: IBSC 298557 barcode 0159300!; isotypes: IBK 00067629 barcode IBK00067601!, KUN 0400056 barcode 0598271!).
[Acacia hainanensis auct. non Hayata: H.Sun & C.Chen, Acta Bot.Yunnan. 12: 258 (1990), pro parte, as to Sino-Soviet Joint Expedition 434, K.H. Cai 1265 and S.H. Chen 11949, see discussion under Senegalia hainanensis; Ou-Yang (2013), Pl. Diversity Resources 35: 549 (2013), as to P.I. Mao 3922! & H.C. Wang 20110182!]
[Acacia pennata subsp. hainanensis auct. non (Hayata) I.C.Nielsen: I.C.Nielsen, Fl. Camboge, Laos & Vietnam 19: 67 (1981), pro parte, as to Balansa 2171, Chevalier 29742, Eberhardt 3907 & 4806, see note under Senegalia hainanensis; T.L.Wu & I.C. Nielsen, Fl. China 10: 59 (2010), pro parte, as to citation of Yunnan and Guangxi under distribution, see discussion under Senegalia hainanensis.]
Distinctive features. Pinnae 11-18 pairs. Leaflets normally 4-6 mm long and 0.6-1 mm wide, strongly discolorous (darkest above); apex obtuse or sometimes rounded or broadly acute; main vein situated rather close to and ±parallel with upper margin at least towards base of leaflet; lateral veins (on lower surface of leaflets) not or scarcely visible. Leaf glands not prominent, stipitate to sub-stipitate, ±symmetrically short-cylindric to obconic or calicioid; petiole gland situated 7-15 (-20) mm above leaf base; rachis glands numerous (normally at base of uppermost 6-11 pairs of pinnae), 0.5-1.5 high, 0.5-0.7 mm diam. at apex. Panicle/raceme axes and ±young peduncles dark-coloured by a dense layer of glandular hairs; inflorescence buds dark-coloured when dry (?red when fresh). Pods (sub-mature) with scattered, small, obscure, reddish brown, sessile glands.
Description. Lianas or lianescent shrubs; tendrils sometimes (? often) present in axil of leaves, sometimes with vegetative bud at apex. Branchlets glabrous or (at extremities) minutely and obscurely non-glandular hairy but glandular hairs sometimes intermixed. Prickles scattered on internodes and on under surface of leaf axes, numerous or (on herbarium specimens at least) few, not prominent, 0.3-1.5 (-2) mm long, straight or shallowly recurved, patent or shallowly reflexed. Stipules at base of peduncles caducous, triangular to narrowly oblong or sometimes lanceolate, c. 2 mm long and 1 mm wide, faintly striate abaxially, base not lobed, apex acute; stipules on vegetative shoots present when leaves very young, triangular to narrowly oblong, 2-4 mm long, 0.5-0.7 mm wide, green when fresh, glabrous, veins not visible or obscurely striate. New shoots with a dense layer of red-brown hairs when first initiated, the subsequent expanding young foliage bright light green. Leaves bipinnate; pinnae 11-18 pairs, (20-) 25-65 (-80) mm long; petiole 15-40 mm long; rachis 90-180 mm long, with ±dense indumentum of short, antrorsely curved, appressed to somewhat spreading hairs. Leaflets (20-) 30-68 pairs, narrowly oblong, (3.5-) 4-6 (-7) mm long, 0.6-1 (-1.3) mm wide, straight or sometimes shallowly but discernibly curved forward, close together, strongly discolorous (mid-green to dark-green and rather shiny above, pale green with dull lustre below), glabrous except normally ±sparsely appressed-ciliolate; apex symmetric or asymmetric, normally obtuse but sometimes rounded or broadly acute, with or without a blunt apiculum; base truncate, unequal with an obvious rounded angle on lower edge, sub-sessile with the clearly excentric, squat petiolule very short (scarcely extended below base of leaflets); main vein starting near upper margin at leaflet base, excentric being situated towards the upper margin and parallel with it at least near base of leaflet; lateral veins (on lower surface of leaflets) not visible or obscure and patent, not forming a reticulum. Glands not prominent, green: petiole glands-single or rarely 2, when single normally situated on lower ¼ of petiole (rarely situated near or above middle of petiole) 5-15 (-20) mm above leaf base and 1-10 (-15) mm above pulvinus, the second gland situated 0-10 mm below the lowermost pair of pinnae, stipitate or sub-stipitate, mostly 0.5-1 mm high, shape variable (see notes below under Leaf glands), symmetrically (or occasionally obliquely) shortcylindric to narrowly obconic or calicioid, not or only slightly dilated at apex (0.4-1 mm diam.), with a shallow or deep orifice on the upper surface; rachis glands - situated at base of uppermost (5-) 6-11 pairs of pinnae (or occasionally to 17 being situated at base of all pairs of pinnae, including the lowermost pair), stipitate to sub-stipitate, short-cylindric to calicioid, 0.5-1.5 mm high, 0.5-0.7 mm diam. at apex; rachilla glands - situated at base of uppermost 1 or 2 pairs of leaflets, occasionally absent (e.g. Chevalier 37410), oblong-elliptic, minute (c. 0.3 mm long), sessile. Inflorescences comprising pedunculate heads arranged in terminal panicles and/or racemes (which sometimes grow out as a leafy shoot), the raceme/panicle axes dark-coloured by a dense layer of glandular hairs (with some white, non-glandular hairs intermixed); peduncles 7-15 (-25) mm long, young peduncles with glandular indumentum as on raceme/panicle axes but as peduncles mature the glandular hairs become sparser (or absent) exposing white, non-glandular hairs that are patent to appressed and straight to curved; heads globose to shortly obloid (shape best observed in mature bud), 8-10 mm diam. when dry, yellow (see note below under Inflorescence colour), densely 50-60-flowered; inflorescence buds dark-coloured when dry (suggesting that they were red when fresh; see discussion under Morphological characters: Inflorescences and flowers above). Bracteoles not exserted beyond flowers in buds. Flowers 5-merous, sessile or sub-sessile; calyx c. ¾ length of petals, gamosepalous, dissected for ¼-
Selected specimens examined. CHINA: Guangxi. Baise City, Napo County, 10 May 1989, Hongshui River Expedition 477 (IBSC 616182 [barcode 0159370]); Nanning City, Long'an County, 20 Apr. 1978, X.X. Chen & Y.P. Huang 2-243 (GXMI 31310 [barcode 015754]).Yunnan. Honghe Hani & Yi Autonomous Prefecture, Hekou Yao Autonomous County, 10 June 1953, K.H. Cai 1265 (KUN 0400052 [barcode 0598266] & KUN 0400053 [barcode 0598265]); Honghe Hani & Yi Autonomous Prefecture, Jinping Miao, Yao & Dai Autonomous County, Mengla village, 22°37'49"N, 103°07'04"E, 6 June 2019, B.R. Maslin & L. Bai BRM 11044 (BKF, KUN [barcode 1348009 & 1348010], PE, SING); Honghe Hani & Yi Autonomous Prefecture, Jinping Miao, Yao & Dai Autonomous County, 14 Apr. 1956, SinoSoviet Joint Expedition 434 (KUN 0400058 [barcode 0598270]); Honghe Hani & Yi Autonomous Prefecture, Pingbian Miao Autonomous County, 19 Apr. 1954, P.I. Mao 3922 (KUN 0400059 [barcode 0598269] & KUN 0400060 [barcode 0598268]); Xishuangbanna Dai Autonomous Prefecture, Mengla County, 3 Apr. 1982, A nonymous 31704 (HITBC 017874 [barcode 0010440]). VIETNAM: Northern. Tonkin, Tuyen Quang [Province], 17-19 Apr. 1918, A.J.B. Chevalier 37410 (VNM 00008366); Nui Bieu, Da Bac [District], Hoa Binh [Province], 21 June 1999, Ban, Phuong, Khoi, Binh & Bach 2067 (HN 0000042600, 0000042601 & 0000042602); Thu Phap [Ba Vi District, Hanoi Province], 22 Apr. 1986, Phuong 43 (HN 0000042611, 0000042612 & 0000042613); Ngoc Thanh [commune], Mi Linh [Phuc Yen, District], Vinh Phuc [Province], 13 Jan. 2007, Phuong 4095 (HN); Hoang Lien Son [Province, now Yen Bai Province], Mar. 1959, Pham Ke Loc s.n. (HNU 12104); 10 km SE of Tỉnh, Lạng Sơn Province, 18 Jan. 1965, Sino-Vietnam Expedition 1603 (IBSC 680605 [barcode 0159275], KUN 0735851 [barcode 0598280]); anonymous, without collection details, IBSC 680597 [barcode 01593090].
Distribution (Fig. 48). Occurs in southern China and northern Vietnam. In China it is found in southern Yunnan close to the borders with Vietnam and Laos, and western Guangxi. Judging from the distribution in China it is likely that the species will be recorded for Laos in the future. In Vietnam Senegalia stipitata has a scattered distribution from the vicinity of Hanoi northwards towards the Chinese border (adjacent to about where it occurs in China). It is recorded from Hanoi, Hoa Binh, Tuyen Quang, Vinh Phuc and Yen Bai Provinces in Vietnam.
Habitat. Grows in hilly or mountainous country (60-900 m alt.) in open forests or scrub, commonly near watercourses in valleys. In Jinping County where we observed living plants of this species, they occurred singularly or in groups of a few individuals.
Phenology. Flowers from April to June. The one fruiting specimen seen (Ban et al. 2067 from Vietnam, with sub-mature pods) suggests that mature seeds would be available in about August.
Taxonomy. This hitherto unrecognised new species had formerly been included within a broadly defined Acacia hainanensis (syn. A. pennata subsp. hainanensis). For example, the Sun and Chen (1990) treatment of A. hainanensis included specimens of both Senegalia hainanensis (i.e. F.C. How 70721 and S.C. Chen 1117) and S. stipitata (i.e. Sino-Soviet Expedition 434, K.H. Cai 1265 and S.C. Chen 11949), and also S. macrocephala (i.e. C.W. Wang 90201). The treatment of A. pennata subsp. hainanensis by Wu and Nielsen (2010) also included elements of S. hainanensis and S. stipitata (as noted under S. hainanensis above), and at least one of the two voucher specimens cited for A. hainanensis by Ou Yang et al. (2013) is S. stipitata (namely, P.I. Mao 3922; we have not seen the second voucher cited in that work). Also, under A. pennata subsp. hainanensis Nielsen (1980) cited four specimens from northern Vietnam (i.e. Balansa 2171, Chevalier 29742, Eberhardt 3907, 4806) that were characterised by having small, columnar petiole glands; although we have not seen these specimens they are undoubtedly S. stipitata. Most herbarium specimens of S. stipitata seen in China had previously been labelled as either A. hainanensis or A. pennata.
Senegalia stipitata belongs to the 'Hainanensis species-group' which also includes S. guangdongensis, S. hainanensis and S. macrocephala, and which is discussed under S. hainanensis.
Affinities. Within China, Senegalia stipitata is most closely related to S. hainanensis from Hainan and central Vietnam (see S. hainanensis for discussion and differences).
Senegalia stipitata is also related to S. pluricapitata (Steud. ex Benth.) Maslin, Seigler & Ebinger which occurs in Indonesia, (Java, Sumatra and Kalimantan), Malaysia (Peninsular Malaysia and Sabah), Thailand and possibly southern Vietnam; it doubtfully occurs in Myanmar or Philippines, despite having been recorded for those countries by Merrill (1923). Senegalia pluricapitata, like S. stipitata, has stipitate or sub-stipitate petiole and (numerous) rachis glands. However, S. pluricapitata is distinguished by its pinnae being more numerous (20-30 pairs) and generally shorter (20-30 mm long), leaflets smaller (2-4 mm long, 0.3-0.5 mm wide), petiole glands wider (1-2 mm), heads fewer-flowered (30-35) and glandular hairs few or absent on the raceme/panicle axes (which possess a rather dense indumentum of short, non-glandular hairs). A few species from the Philippines also have stipitate petiole and/or rachis glands (see below under Leaf glands), but these species do not possess dark-coloured, densely glandular-hairy inflores-cence axes.
Senegalia stipitata may sometimes superficially resemble S. orientalis from southeast China and Vietnam, but the two species appear not to be especially closely related (see that species for discussion)
Leaf glands. The distinctive, small, stipitate to sub-stipitate petiole and rachis glands of Senegalia stipitata are morphologically variable, as described above and illustrated in Fig. 47G and H. Senegalia stipitata is the only species of this genus in China with stipitate leaf glands, but as already noted they also occur in S. pluricapitata from Southeast Asia. They are also found on a few species from the Philippines (i.e. S. borneensis (I.C.Nielsen) Maslin, Seigler & Ebinger, S. sulitii (I.C.Nielsen) Maslin, Seigler & Ebinger and S. tawitawiensis (I.C.Nielsen) Maslin, Seigler & Ebinger) and on some species of Senegalia in Brazildsee Barros and Morim (2014). The specimen A.J.B. Chevalier 37410 from Vietnam is atypical in having two petiole glands; other species that occasionally have two petiole glands include S. kunmingensis, S. pruinescens and S. rugata.
Inflorescence colour. P.I. Mao 3922 is the only collection examined that records the colour of inflorescences at anthesis, namely, 'yellow'. What is precisely meant by yellow is unknown, but is possibly pale yellow; this same descriptor is most often used for the heads of Senegalia hainanensis.
Etymology. The species name is derived from the Latin stipitatus (stipitate, provided with a stalk), in allusion to the petiole and rachis glands that are distinctively stipitate or sub-stipitate.
Vernacular name. Stalked-gland Senegalia (following the Chinese common name that is proposed above).
19. Senegalia teniana (Harms) Maslin, Seigler & Ebinger, Blumea 58: 42 (2013) (Figs. 50 and 52).盐丰金合欢【yán fēng jīn hé huān】
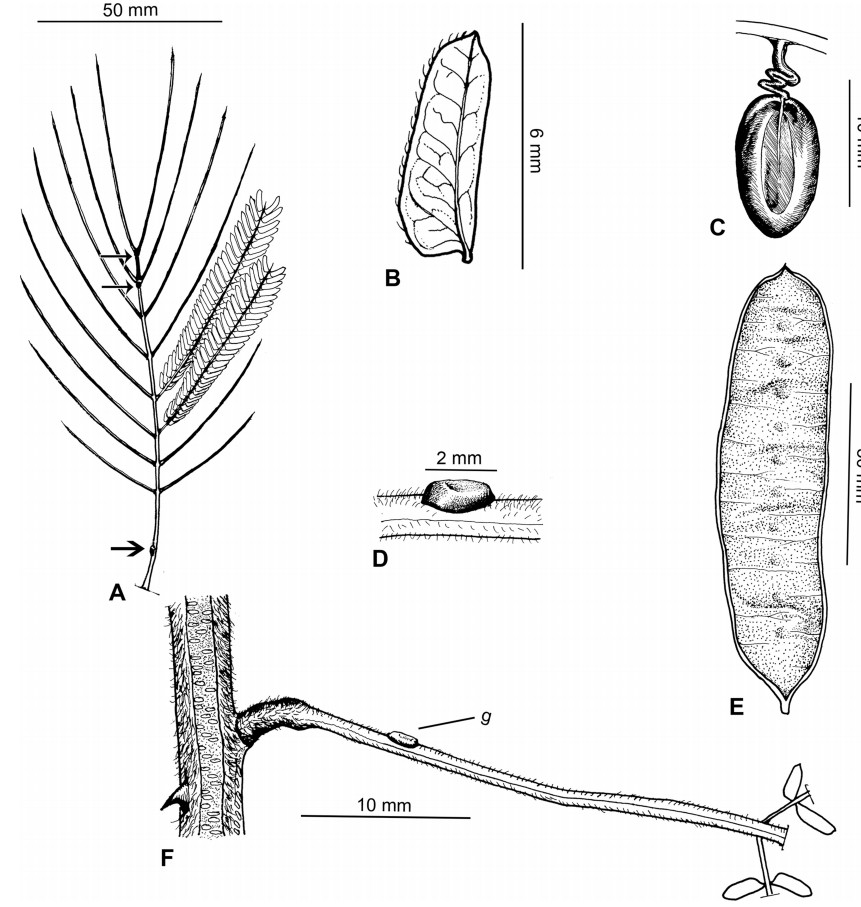
|
| Fig. 50 Senegalia teniana. A - Leaf showing relatively few pinnae and position of petiole gland (thick arrow) and rachis glands (thin arrows). B - Leaflet (lower surface) glabrous and showing lateral veins evident and forming an imperfect reticulum. C - Seed showing thickly filiform & exarillate funicle. D - Petiole gland short and not prominently raised. E Pod. F - Node showing hairy branchlet and low-profile petiole gland (g). Vouchers: S. Jiang 7552 (A, B, D, E & F); Heng Li 273 (C). Drawn by Joshua Yang. |
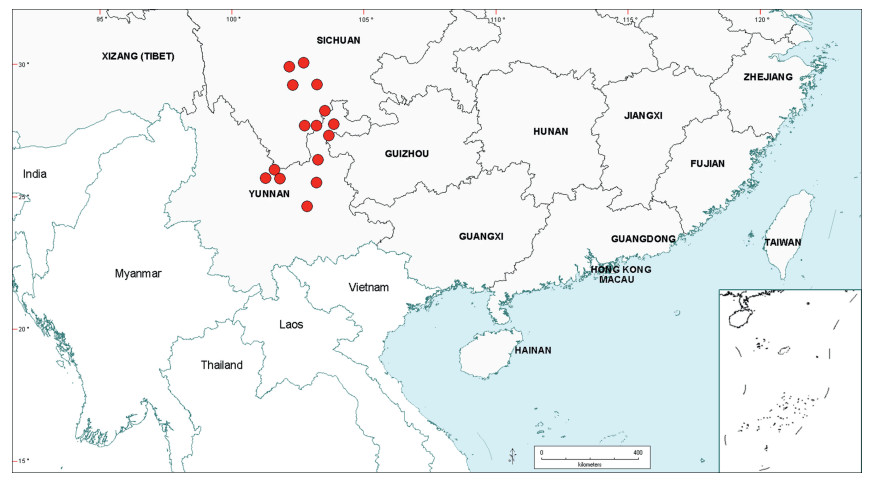
|
| Fig. 51 Distribution of Senegalia teniana, based on specimen records at A, IBSC and KUN, as cited in text. |
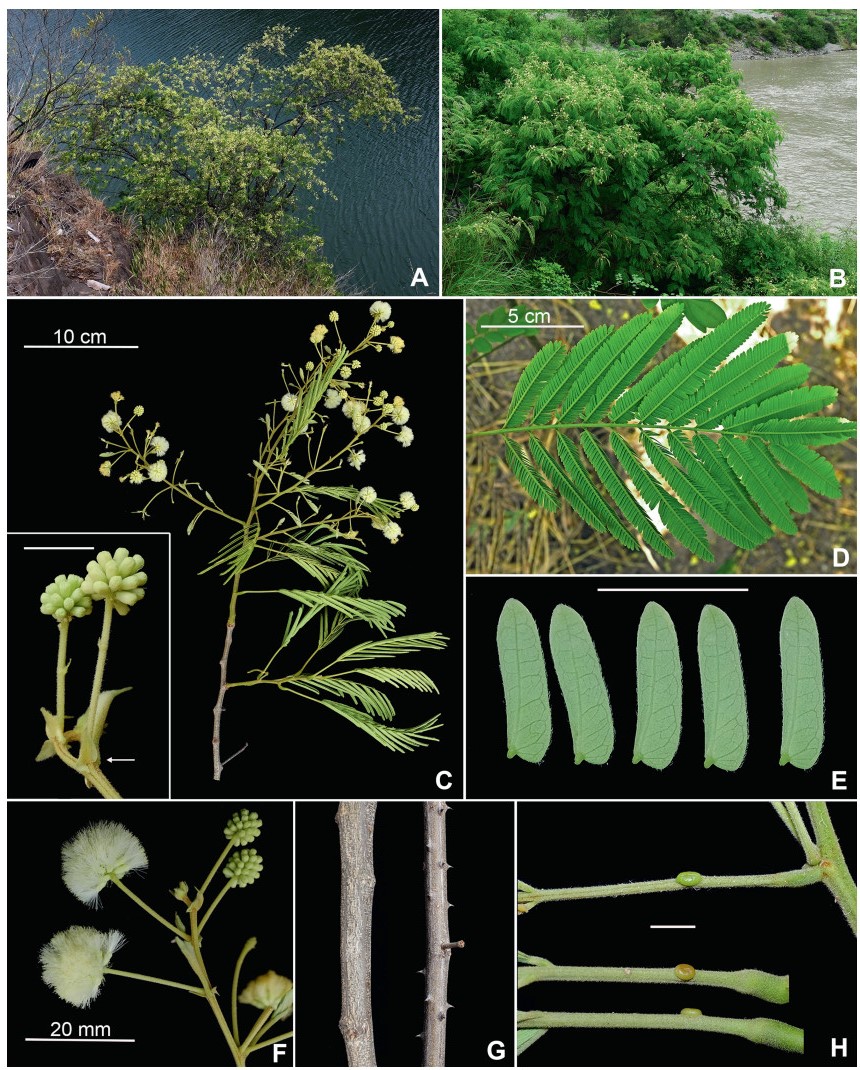
|
| Fig. 52 Senegalia teniana. A - Shrub habit (open growth form). B - Shrub habit (dense growth form). C - Branchlet showing terminal racemes (insert: inflorescence buds showing stipule (arrowed), yellowish green heads and bract on peduncles). D - Leaf. E-Leaflets (lower surface) showing imperfect reticulum. F - Raceme apex showing heads white at anthesis and the sub-loosely arranged flower buds green. G - Branchlets without prickles (left) and with prickles (right). H - Petiole gland thickened, not prominently raised and flat-topped, position normally near base of petiole. Voucher: All from L. Bai & F.C. Ning BLK-124 except B (unvouchered). Photos: All L. Bai except B (H. Sun). Scale bar = 5 mm (unless otherwise indicated). |
≡ Acacia teniana Harms, Repert. Spec. Nov. Regni Veg. 17: 133 (1921), as 'Teniana'. Type citation: "Yunnan: Pe yen tsin (Simeon Ten, no. 349, no. 113); San ly tsin Kouty (S. Tenin Herb. Haun.; 23.Ⅳ.1919)". Type: CHINA, northwest Yunnan, near Pe Yen Tsin [Pe Yen Tsin is today's Baiyanjin village in Shiyang township, Dayao County, Chuxiong Yi Autonomous Prefecture], 24 Apr. 1917, Pater Siméon Ten 349 (lectotype: A barcode 00058295 [digital image!], here designated, see notes below under Typification; isolectotype: US 1174864 barcode 00000624 [digital image!]). Remaining syntypes: CHINA, northwest Yunnan, near Pe Yen Tsin, 20 May 1916, Pater Siméon Ten 113 (A barcode 00058296 [digital image!]). CHINA, Yunnan, in silvis San ly tsin Kouty, 23 Apr. 1919, Siméon Ten s.n. (C barcode C10011410 [digital image!]dsheet stamped "Herbarium Botanicum Hauniense".
Distinctive features. Normally shrubs 3-5 m tall. Stipules relatively small (2-3 long and 0.5-2 mm wide). Pinnae (5-) 6-12 (-14) pairs. Leaflets normally 5-7 mm long, 1-2 mm wide and glabrous (except margins ciliate or ciliolate), rarely finely appressed-hairy; lateral veins (on lower surface of leaflets) rather evident and often forming an imperfect reticulum. Petiole gland 1.5-2 (-3) mm long, variably prominent and normally ±flat-topped (never conical). Inflorescences 2-6 pedunculate heads in leaf axils or arranged in elongated racemes; flower buds pale green when fresh; peduncle hairs dense and ±patent. Calyx tube sparsely to densely hirsutellous, 5-veined but veins not especially prominent; ovary densely appressed-tomentulose. Pods (10-) 15-30 mm wide.
Description. Normally shrubs or occasionally lianescent shrubs or trees 3-5 m tall (seemingly sometimes to 10 m tall, see note below under Variation). Branchlets sparsely to densely minutely puberulous to hirsutellous towards apices (indumentum not conspicuous, the hairs straight to shallowly curved and mostly patent), penultimate and mature branchlets glabrous or occasionally sparsely hairy and with narrowly oblong to linear, transverse lenticels. Prickles very few or absent from internodes on upper branches, normally absent from leaves, c. 1 mm long on upper branches, on mature branches (not often seen in herbarium material) to c. 4 mm long, stout, patent and straight or shallowly recurved. Stipules caducous, triangular-lanceolate, 2-3 mm long, 0.5-2 mm wide, densely hairy and obscurely veined or veins not visible abaxially, not lobed or prominently lobed on one side at base, apex acute to short-acuminate. Leaves bipinnate; pinnae (5-) 6-12 (-14) pairs, 30-50 (-80) mm long, sometimes to 20 mm long on lowermost pair; petiole 20-35 (-45) mm long; rachis 65-120 mm long, sub-glabrous to moderately (rarely ±densely) short-hairy, the hairs not especially conspicuous, ±patent and straight to shallowly curved. Leaflets 25-35 pairs, to 18 pairs on lowermost pinnae, narrowly oblong, normally 5-7 mm long, rarely 9-10 mm long (on specimen Z.S. Zheng 198), 1-2 mm wide, discolorous (darkest above), green, dull lustre on both surfaces, normally glabrous (except margins normally appressed ciliate or ciliolate with hairs often sparse), rarely finely appressed-hairy on both surfaces (see note below under Variation); apex symmetric or asymmetric, rounded to obtuse or sometimes broadly acute, with an often poorly developed and blunt apiculum or sometimes without apiculum; base unequal with a distinct rounded angle on lower edge, petiolule discrete (extended below base of leaflet) and clearly excentric; main vein starting near upper margin at leaflet base and extending obliquely to apex, straight or very shallowly curved; lateral veins (on lower surface of leaflets) rather evident, patent or sub-patent, often bifurcating near margin and sometimes coalescing to form an intra-marginal vein, often anastomosing to form an imperfect reticulum. Glands: petiole gland-situated on lower ½ or rarely near middle of petiole (4-) 8-15 (-20) mm above leaf base and 2-7 (-15) mm above pulvinus, sometimes absent from some (rarely all) leaves, oblong to elliptic or occasionally circular, 1.5-2 (-3) mm long, c. 1 mm wide, sessile, solid, discernibly thickened but variably prominent (raised to c. 0.5-1 mm high), normally ±flat-topped (rarely shallowly convex or concave on upper surface), green or sometimes light brown when fresh; rachis glands - situated at base of uppermost 1-3 pairs of pinnae, c. 1 mm long, sessile, depressed; rachilla glands - situated at base of uppermost 1-3 pairs of leaflets, tiny or to c. 1 mm long, sessile. Inflorescences comprising (1-) 2-6 pedunculate heads within leaf axils or arranged in elongated terminal or axillary racemes (see note below under Variation) with young leaves often developing at base of peduncles during anthesis, indumentum on raceme axes similar to peduncles; peduncles 10-25 (-30) mm long, with dense or sub-dense, short, straight to sub-straight or sometimes curved ±patent, white hairs; heads globose or (at least when in bud) obloid, 15-17 mm diam. at anthesis when fresh (c. 10 mm when dry), white to cream or pale yellow (darker yellow as anthers whither), slightly fragrant (apple-scented), c. 30-flowered, the flowers not especially densely arranged in the heads (best observed in mature buds); inflorescence buds pale green when fresh, pale yellowish or pale brownish when dry. Bracteoles inconspicuous, c. 0.5 mm long, not exserted beyond flowers in buds. Flowers 5-merous, sessile to sub-sessile; calyx ¾-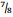
Selected specimens examined. CHINA: Sichuan. Ganzi Zang Autonomous Prefecture, Luding County, 9 Oct. 1976, Z.Y. Wu et al. 6268 (KUN 0400313 [barcode 0598540], KUN 0400314 [barcode 0598541]); Leshan City, Ebian Yi Autonomous County, Nov. 1938, Z.S. Zheng 198 (KUN 0400320 [barcode 0598536]); Liangshan Yi Autonomous Prefecture, Butuo County, 20 Aug. 1959, J.L. Chuan 5647 (KUN 0400318 [barcode 0598542]); Liangshan Yi Autonomous Prefecture, Jinyang County, 27 Oct. 1985, Heng Li 273 (KUN 0400325 [barcode 0598545]); Liangshan Yi Autonomous Prefecture, Leibo County, 7 July 1960, S. Jiang 7552 (KUN 0400316 [barcode 0598544]); Ya'an City, Shimian County, 1955, C.T. Hsieh 41598 (IBSC 252579 [barcode 0159380]); Ya'an City, Tianquan County, without date, H.L. Tsiang 35502 (IBSC, 194072 [barcode 0159384]).Yunnan. Chuxiong Yi Autonomous Prefecture, Dayao County, 26°00'58"N, 100°57'22" E, 3 May 2019, L. Bai & F.C. Ning BLK-124 (BKF, GXMG, GXMI, HITBC, IBK, IBSC, KUN [barcode 1347948 & 1347949], PE, SING); Chuxiong Yi Autonomous Prefecture, Yongren County, May 1965, Anonymous s.n. (SWFC 0021180 & 048371); Chuxiong Yi Autonomous Prefecture, Yuanmou County, 7 Aug. 1965, Tree Oil Plants Expedition 715 (KUN 0400361 [0598581], KUN 0400370 [barcode 0598582]; Kunming City, Xundian County, 19 Aug. 1974, Anonymous 1 (KUN 0400291 [0598524]); Qujing City, Huize County, 12 Dec. 1952, P.I. Mao 02014 (IBSC 680607 [barcode 0159386]), leaflets appressed-hairy; Yuxi City, Chengjiang County, 9 Aug. 1939, H. Wang 41558 (IBSC 141236 [barcode 0158816]); Zhaotong City, 18 May 1932, H.T. Tsai 50909 (IBSC 113926 [barcode 0159390], KUN 0400286 [barcode 0598486], KUN 0400295 [barcode 0598487]); Zhaotong City, Daguan County, 20 June 1973, B.S. Sun et al. 0705 (IBSC 680610 [barcode 0159382], KUN 0400324 [barcode 0598535], YUKU 0037516 [barcode 02022666]), leaflets appressed-hairy and heads obloid.
Distribution (Fig. 51). Endemic to southwest China where it has a somewhat restricted distribution in southern Sichuan and adjacent areas of northeast Yunnan. It occurs mainly in the drainage system of Jinsha River to the east of Dukou City, but also in the drainage system of the Yalong River to the north of Dukou City. This distribution is largely parapatric with that of Senegalia yunnanensis.
Habitat. Occurs in forests on mountain slopes and river valleys at 750-1500 alt.; this altitude was recorded by Sun (2006) and Wu and Nielsen (2010).
Phenology. Judging from specimens examined and from information provided in Wu and Nielsen (2010), Senegalia teniana flowers from late April to about July, and has pods with mature seeds from early October to January.
Typification. The name Acacia teniana was described based on three gatherings from Yunnan Province by Father Simeon Ten, namely, Ten 113, 349 and s.n. Sun and Chen (1990) nominated Ten 349 (P) as the type, inadvertently calling it holotype, instead of lectotype. Despite having searched at P (O. Poncy, herb. P, pers. comm. 2017), the Ten 349 specimen was not located, and is presumed lost. Therefore, in accordance with ICN Art. 9.11 (Turland et al., 2018) an alternative lectotype needs to be selected and accordingly, the duplicate of Ten 349 at herb. A has been selected above as the lectotype of A. teniana. This is a better-preserved specimen than the duplicate of Ten 349 at herb. US which becomes an isolectotype.
Taxonomy. Following its original publication in 1921, Acacia teniana was first included in Chinese taxonomic literature by Wu (1988) and subsequently by Sun (2006) and Wu and Nielsen (2010). While it could perhaps be argued that the delimitation of Senegalia teniana proposed here should be expanded to accommodate S. prominens as a subspecies, these taxa are considered more appropriately treated as separate species (see under S. prominens for discussion).
Affinities. Senegalia teniana appears to be most closely related to S. prominens and S. yunnanensis. These three species are endemic to China and are normally shrubs or sometimes trees (some specimen labels record S. prominens as also a liana, but these records need to be confirmed). Senegalia prominens and S. teniana have globose or slightly obloid heads with the flower buds pale green when fresh (not tinged reddish as often occurs in other species of Senegalia in China), and the leaflets are normally reticulately-veined on their lower surface. These two species are allopatric with S. prominens having a more easterly distribution than that of S. teniana (compare Fig. 51, S. teniana and Fig. 39, S. prominens). Senegalia prominens differs from S. teniana in having a more prominent, often conical petiole gland and ±appressed peduncle hairs (see under S. prominens for further discussion). Senegalia yunnanensis has flowers arranged in cylindrical spikes (colour of fresh flower buds is unknown) and its leaflets that are not reticulately-veined; its distribution is essentially parapatric with that of S. teniana (compare Fig. 51, S. teniana and Fig. 57, S. yunnanensis). See under Senegalia yunnanensis treatment below for further discussion.
Senegalia teniana seemingly is also related to the poorly-known S. delavayi, which also has a restricted distribution in northwest Yunnan (not far to the west of where S. teniana occurs, compare Fig. 51, S. teniana and Fig. 11, S. delavayi). However, the relationship between these two species requires further study. Senegalia delavayi differs most obviously from S. teniana in the following ways: branchlets glabrous; peduncles glabrous or sparsely hairy (hairs strongly curved and ±appressed); leaflets with a slender, distinctly acute apiculum and lateral veins (on lower surface of leaflets) not visible or few and obscure and which do not form a reticulum.
Senegalia teniana appears also to have some affinities with S. kunmingensis (see that species for discussion).
The following two species sometimes superficially resemble Senegalia teniana, but they are not as closely related to it as the preceding ones.
Senegalia pruinescens and S. teniana have a similar number of pinnae and both commonly possess reticulately-veined leaflets. Senegalia pruinescens is a robust liana or sometimes a lianescent shrub that can most readily be distinguished from S. teniana in the following ways: branchlets pruinose (although pruinosity some-times not visible on herbarium specimens); petiole gland more prominent and normally situated further from leaf base (usually 13-40 mm); inflorescences often paniculately arranged with the flower buds dull red when fresh; ovary glabrous or sparsely hairy; and pods broader (mostly 35-50 mm wide).
Senegalia rugata, like S. teniana has reticulately-veined leaflets and often axillary inflorescences, but it has very distinctive, hard-textured pods (see S. rugata for further differences).
Variation. Most specimens record the height of this species as about 3-5 m tall. However, the Siméon Ten 113 syntype gives the height as 8-10 m (the other two Siméon Ten syntypes record the height as 3 m); also, the specimen H.T. Tsai 50909 records plants as reaching 10 m tall.
The leaflets of Senegalia teniana are normally glabrous on both surfaces (except margins are appressed ciliate or ciliolate), but on a few Yunnan specimens they are finely appressed-hairy on both surfaces (e.g. P.I. Mao 02014 and B.S. Sun et al. 705). The latter specimen is further a little atypical in having flowers arranged in obloid heads.
In the original description of Acacia teniana (Harms, 1921), and in the descriptions of the species by Wu (1988), Sun (2006) and Wu and Nielsen (2010), inflorescences are described as paniculately arranged and (except Sun (2006)) the peduncles as reaching 40 mm long. However, from material we have examined (including photographs of the type collection) there is no clear evidence of panicle development in Senegalia teniana and its peduncles seem not to exceed 30 mm in length. Also, Wu and Nielsen (2010) recorded the pinnae as 70-120 mm long (seemingly an error for the rachis length) and the ovary as glabrous (which appears to be an error).
Etymology. This species is named for Father Siméon Ten who collected in northern Yunnan Province in the early part of the 20th century (Bao et al., 1998).
Vernacular name. Yanfeng Senegalia. This name was used by Sun (2006) under A. teniana. The name is derived from the old Chinese County name for Dayao County, from where type was collected (and which is where Siméon Ten lived, after whom the species is named).
20. Senegalia tonkinensis (I.C.Nielsen) Maslin, Seigler & Ebinger, Blumea 58: 42 (2013) (Figs. 53 and 55).老街金合欢【lǎo jiē jīn hé huān】(新拟)
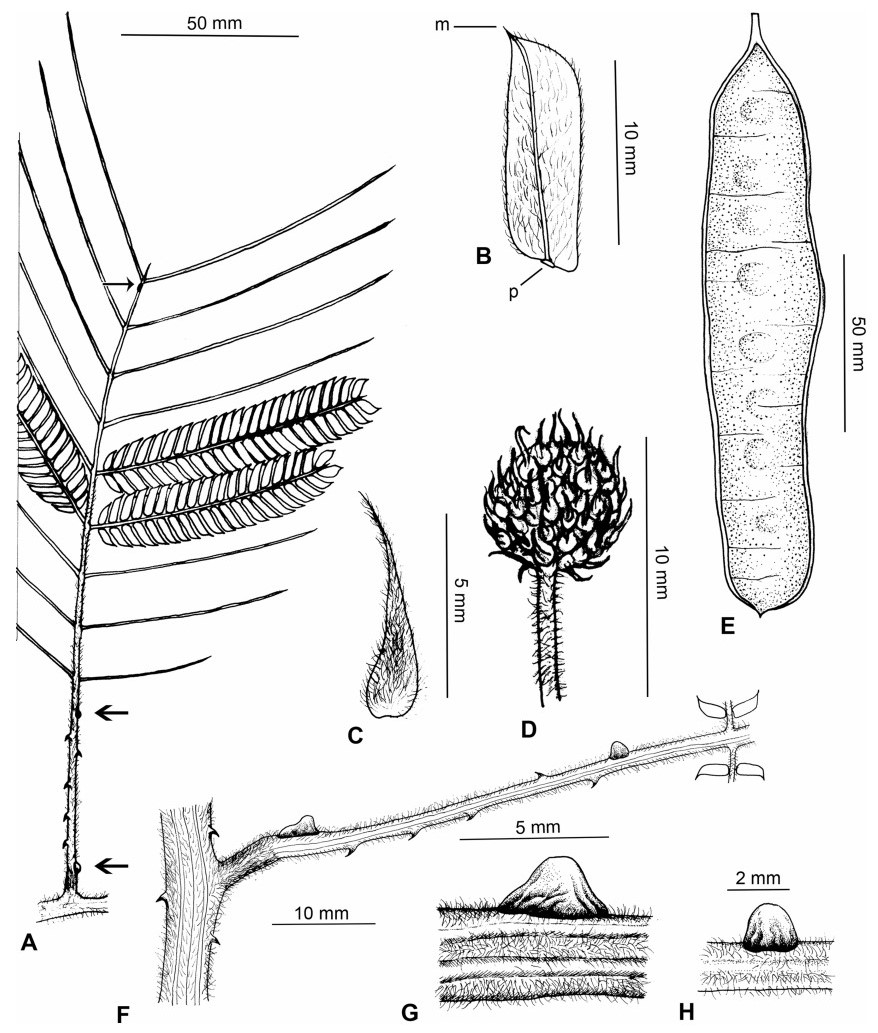
|
| Fig. 53 Senegalia tonkinensis. A - Leaf showing relatively few pinnae and position of petiole glands (thick arrow) and rachis gland (thin arrow). B - Leaflet (lower surface) appressed-hairy, sessile, with petiolule (p) centrally-situated and ±not extended below base of leaflet, and obliquely truncate at apex with distinct mucro (m). C - Stipule caudate-acuminate. D - Inflorescence bud showing acuminate bracteoles distinctively exserted. E - Pod. F - Node showing hairy branchlet and leaf with two petiole glands. G - Petiole gland (lowermost) prominent and conical. H - Petiole gland (uppermost). Vouchers: H. Sun 1356 (A, C, D & F); Y.Y. Qian 2085 (B, E, G & H). Drawn by Joshua Yang. |
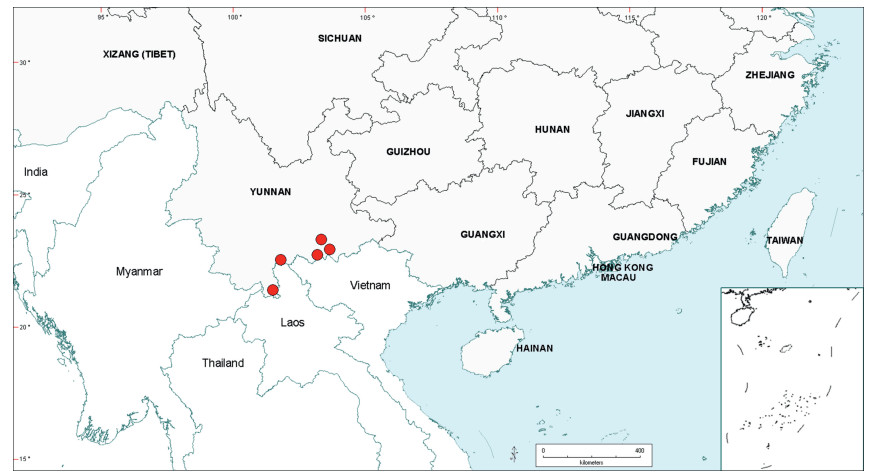
|
| Fig. 54 Distribution of Senegalia tonkinensis in China, based on specimen records at IBSC and KUN, as cited in text. |
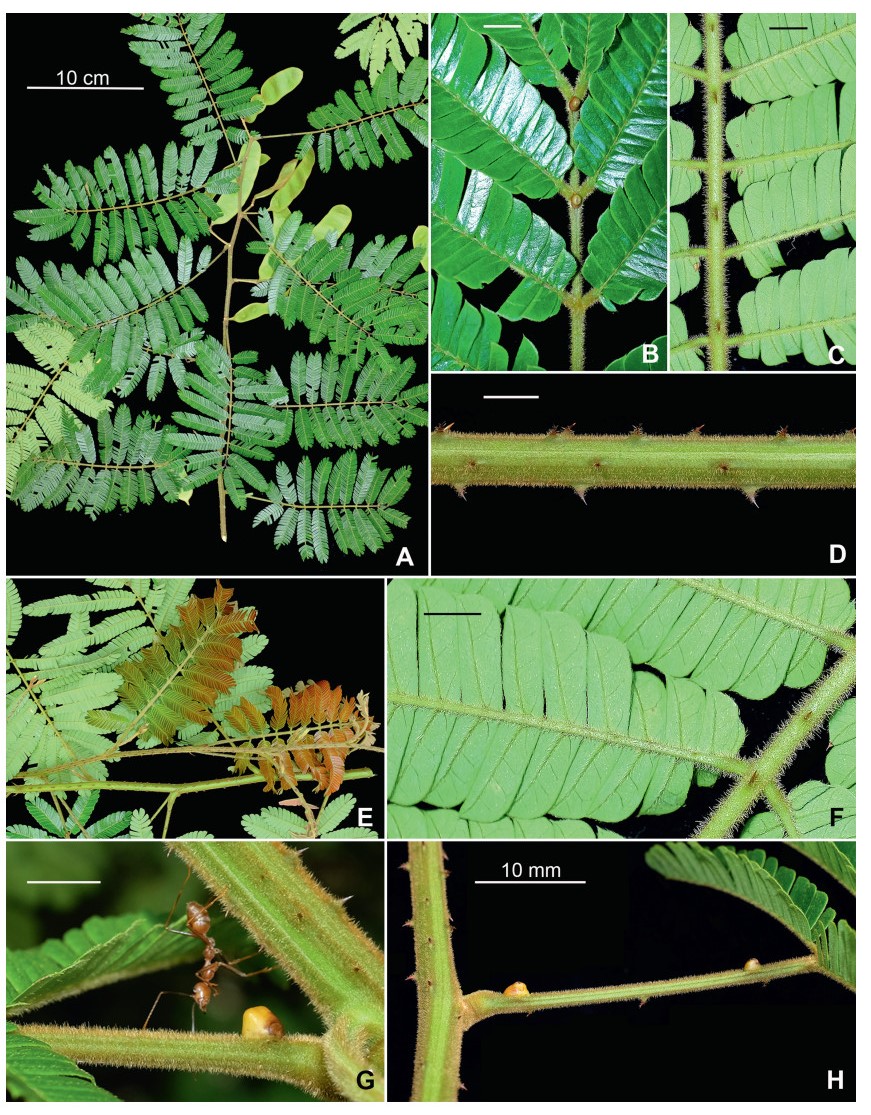
|
| Fig. 55 Senegalia tonkinensis. A - Branchlet showing leaves and immature pods. B - Rachis (portion) showing gland at base of pinnae and leaflets dark green and glossy on their upper surface. C - Rachis (portion) showing prickles on lower surface and leaflets pale green with dull lustre on their lower surface. D - Branchlet showing prickles and dense indumentum of short, straight, patent, light brown hairs. E New shoot showing reddish brown young leaflets. F - Leaflets (lower surface) showing main vein starting centrally at base and ±truncate apex with an excentric, fine point. G - Lowermost petiole gland (with ant visitation). H - Leaf base showing two prominent petiole glands. Voucher: All from L. Bai & F.C. Ning BLK-124 except B (unvouchered). Photos: L. Bai BKL-118 (A, B, C, E & F) and B.R. Maslin & L. Bai BRM 11041 (D, G & H). Scale bar = 5 mm (unless otherwise indicated). |
≡ Acacia tonkinensis I.C.Nielsen, Adansonia, sér. 2, 19: 358, pl. 2 (1980). Type citation: "Wilson 2715, N. Vietnam, Lao Cai, 8.1899 (holo-, K)". Type: VIETNAM, Laokai Tonking [Làao Cai, northern Vietnam], Aug. 1899, E.H. Wilson 2715 (holotype: K barcode K000724533 [digital image!]).
[Acacia torta auct. non (Roxb.) Craib: I.C.Nielsen, Adansonia sér. 2, 19: 360 (1980).]
Distinctive features. Branchlets, rachis and peduncles densely patent-hairy (hairs normally light brown or yellowish brown). Pinnae relatively few, 6-13 pairs. Leaflets wide (1.5-4 mm in China), (4-) 5-15 mm long, strongly discolorous, appressed shortpilose on lower surface; apex obliquely rounded-truncate and distinctly mucronate by a clearly excentric, slender point; base truncate and ±sessile, the much-reduced petiolule ±centrally located. Petiole glands 2, rather prominent, often conical-shaped. Bracteoles distinctly acuminate and exserted beyond flowers (at least in young inflorescence buds). Inflorescence buds, sepal and petal apices red when fresh (dark-coloured when dry). Calyx dissected to near base. Pods with scattered to moderately dense, dark-coloured, small, sessile glands (sometimes forming patchy clusters embedded within a resin matrix).
Description. Lianas or lianescent shrubs. Branchlets densely hirsute to short-pilose or infrequently (in Vietnam) short-puberulous, the hairs straight, patent and normally light brown or yellowish brown. New shoot foliage reddish brown or pale brown when young, ageing light green. Prickles reasonably numerous on internodes and extending to under surface of leaf axes, 0.5-2 mm long, obscured by indumentum when very short, ±patent, straight to slightly recurved. Stipules caducous, very narrowly triangular to narrowly lanceolate, 4-6 mm long, c. 1 mm wide, densely hirsute to short-pilose with veins not visible abaxially, caudate-acuminate. Leaves bipinnate; pinnae 6-12 (-13) pairs, 30-115 mm long; petiole 30-65 mm long; rachis 85-150 mm long, to 200 (-250) mm long in Thailand (Srisanga and Sasirat, 2000), indumentum as on branchlets. Leaflets (11-) 14-33 pairs, oblong to narrowly oblong but terminal pair obovate, (4-) 5-15 mm long, 2-4 mm wide, to 5 (-6) mm wide in Myanmar, strongly discolorous (dark green and glossy above, pale green with dull lustre below), moderately to densely appressed short-pilose on lower surface (hairs white to pale yellow and normally distributed over entire surface; sometimes confined to main vein in Thailand), glabrous or appressed short-pilose on upper surface; apex obliquely rounded-truncate, mucronate by a distinct, excentric, fine point 0.3-0.5 mm long; base truncate, with rounded angles on both edges but more acutely so on the lower edge, the centric or sub-centric petiolule very reduced so that leaflets appear sessile; main vein starting ±centrally at leaflet base and extending obliquely to mucro, with 1 (or 2) less evident veins ascending from the base; lateral veins (on lower surface of leaflets) not visible or very obscure, not forming a reticulum. Glands: petiole glands-2, occasionally 1 in Thailand (Srisanga and Sasirat, 2000), sessile, solid, rather prominent; proximal petiole gland situated on lower ¼ of petiole (3-) 5-12 mm above leaf base and normally 1-5 mm above pulvinus, 1.2-3 mm long, prominent, symmetric or asymmetric and normally conical, raised 1.5-2 mm high; distal petiole gland situated at or above middle of petiole 25-35 mm above leaf base, prominent, hemispheric to conical, a little smaller than proximal gland; rachis glands - situated at base of uppermost 1-2 (-3) pairs of pinnae, relatively prominent, hemispheric or conical, raised to c. 1 mm high, sessile; rachilla gland-situated at base of uppermost pair of leaflets, elliptic, 1 mm long, sessile. Inflorescences comprising pedunculate heads arranged in terminal panicles or elongated racemes (to c. 150 mm long) in upper axils, indumentum on panicle and raceme axes as on branchlets; peduncles 10-25 mm long, single or clustered in groups of up to three, indumentum as on branchlets; heads globose to slightly obloid, yellow or pale yellow, densely flowered; inflorescence buds dark-coloured when dry (dark red when fresh; see note below under Inflorescence bud colour). Bracteoles ±filiform to very narrowly elongate-triangular, c. 2 mm long; lamina distinctly acuminate and clearly exserted beyond flowers in young buds (sometimes not exserted in mature buds), short-pilose abaxially with hairs spreading. Flowers 5-merous, sessile; calyx dissected to near base, the sepals oblong, c. 2 mm long, acute and glabrous or with a few scattered hairs at apex, the apices dark-coloured when dry (dark-red when fresh); petals 2.5 (-3) mm long, glabrous or sub-glabrous, the apex dark-coloured when dry (dark-red when fresh); ovary puberulous. Pods broadly oblong, 125-170 mm long, 20-30 mm wide, firmly chartaceous, straight or sometimes slightly curved, flat but slightly raised over seeds along midline, reddish brown, sub-glabrous or with very fine, patent, scattered, very short hairs that impart a slight downy feel to the pods (hairs difficult to see with unaided eye), sessile glands (tiny, circular & dark-coloured) scattered to moderately dense and sometimes aggregated into patchy clusters embedded within a resin matrix, veins inconspicuous, basal stipe short (c. 5 mm long). Seeds transverse in pods, irregularly elliptic or oblong, 8-12 mm long, 5-7 mm wide, black; areole narrowly oblong, open at hilar end, pitted, c. 4-7 mm long, 2 mm wide; funicle thickly filiform, exarillate.
Selected specimens examined. CHINA:Yunnan. Honghe Hani & Yi Autonomous Prefecture, Jinping Miao, Yao & Dai Autonomous County, near Manpeng village, 22°38' 20"N, 103°08' 26"E, 6 June 2019, B.R. Maslin & L. Bai BRM 11041 (BKF, KUN [1347998]); Honghe Hani & Yi Autonomous Prefecture, Mengzi City, 12 Apr. 1941, T.N. Liou 18808 (IBSC barcode 0159423); Honghe Hani & Yi Autonomous Prefecture, Pingbian Miao Autonomous County, 29 Aug. 1953, P.I. Mao 3099 (KUN 0400327 [barcode 0598551], KUN 0400328 [barcode 0598550]); Pu'er City, Jiangcheng Hani & Yi Autonomous County, 5 Feb. 1990, Y.Y. Qian 2085 (IBSC 582269 [barcode 0159393]; Xishuangbanna Dai Autonomous Prefecture, Mengla County, 26 Jan. 2019, L. Bai BKL-118 (BKF, GXMI, HITBC, IBSC, KUN, PE, SING); Xishuangbanna Dai Autonomous Prefecture, Mengla County, 12 Aug. 1987, H. Sun 1356 (KUN 0400333 [barcode 0598554], KUN 0400334 [barcode 0598553]). MYANMAR: Martaban, without date, S. Kurz 1740 (CAL 140592). THAILAND: Northeastern. Nahaew, Hueng river, 1995, [P.] Srisanga 989 (QBG), n.v.- but cited by Srisanga and Sasirat (2000). Peninsular. [Ranong], Tasan, 22 Dec. 1928, A.F.G. Kerr 16287 (BK 213414, K, n.v.-but cited by Nielsen (1980) as A. torta). South-western. Kanchanaburi Province, Sangklaburi District, Toong Yai Naresaun Wildlife Reserve, Lai wo Subdistrict, Ban Saneh Pawng (Karen village), along Po Kee stream, J.F. Maxwell 93-1225 (BKF [barcode 182742], CMU). VIETNAM: Northern. Environs de Tu-Phap [Thu Phap, Hanoi City], 27 Mar. 1887, B. Balansa 2168 (P [barcode P02934832]); Lao Cai Province, 19 Dec. 1964, Sino-Vietnam Expedition 613 (KUN 0400332 [barcode 0598555]), the specimen HN 0000042552 with collection number 3113 is seemingly a duplicate of this gathering. Central. Pu Mat [National Park], Nghe An Province, 16 June 1998, Anonymous 499 (HNU 14088).
Distribution (Fig. 54). Scattered in southern China, northern Laos- but the Poilane 11668 specimen cited by Nielsen (1981) as the only record for Laos has not been seen, Myanmar, Thailand and Vietnam (northern & central). In China it occurs in southern Yunnan Province along the border with Laos and Vietnam.
Habitat. In China this species grows in evergreen forest in rugged sandstone terrain, mostly between 350 and 700 m alt., often near watercourses. In Jinping County where we observed one population of living plants, they occurred as a localised, small group of individuals.
Phenology. Sun (2006) recorded the flowering period in China as July to September, but Srisanga and Sasirat (2000) gave a slightly broader flowering period (June to October) for Thailand. Pods with mature seeds have been collected in March, but the range of the fruiting period is unknown.
Taxonomy. Senegalia tonkinensis belongs to the taxonomically complex S. Caesia species-group' that is distributed in Southeast Asia and the Indian subcontinent (see notes under S. caesia above). In the protologue of Acacia tonkinensis, Nielsen (1980) recorded A. torta (Roxb.) Craib for Thailand based on Kerr 16287. However, we have now re-identified this collection as S. tonkinensis; other occurrences of this species in Thailand are documented in Srisanga and Sasirat (2000). It is unlikely that S. torta occurs in Thailand; this species is widespread on the Indian subcontinent, and may possibly also occur in Myanmar, fide Deshpande et al. (2019).
Affinities. Senegalia tonkinensis is closely related S. diadenia (R.N.Parker) Ragupathy et al. which occurs in northeast India (Assam) and possibly Nepal. Apart from possessing the unusual characters of two petiole glands and exserted bracteoles in young heads, these two species have similar leaves (including relatively few pinnae and wide, ±sessile leaflets with a ±centrally located petiolule and an obliquely truncate apex that terminates in a distinct, fine, excentric mucro), inflorescences and pods (which often possess patches of small, sessile, circular glands within a resin matrix). Senegalia diadenia is most readily distinguished from S. tonkinensis by its branchlets, peduncles and rachides possessing an indumentum of short, ±appressed hairs, and its leaflets that are glabrous or very sparsely hairy on their lower surface (hairs confined to the main vein when they are present). Further study, especially of plants from the Indian subcontinent, is needed to reassess the relationship between these two species; it is possible that S. tonkinensis might be better treated as an infraspecific taxon of S. diadenia. The one specimen from Nepal that we have seen (G. Panigrahi 16917, CAL) was provisionally assigned to S. diadenia by Deshpande et al. (2019), but it possesses some characters very similar to those of S. tonkinensis.
Within China, Senegalia tonkinensis is most similar to S. caesia, especially on account of its broad, ±sessile leaflets that possess a centric petiolule and a clearly excentric mucro, and its red inflorescence buds (when observed on fresh material). However, S. caesia is most readily distinguished in the following ways: petiole gland single; bracteoles not exserted in inflorescence buds; branchlets glabrous or with short, ±appressed hairs; leaflets reticulately-veined, imperfectly 2 (3-4)-veined, mucro less pronounced (to c. 0.2 mm) and the lower surface normally glabrous; pods lacking dark-coloured, sessile glands.
Inflorescence bud colour. Srisanga and Sasirat (2000) describe the apex of the sepals and petals of living plants in Thailand as dark red. Although not explicitly stated by those authors, this dark red colour would also be clearly visible in inflorescence buds. Because inflorescence buds on herbarium specimens of this species are always dark-coloured (purplish grey), it is very likely that they are always dark red in living plants (see discussion above under Morphological characters: Inflorescences and flowers).
Etymology. The species name refers to the Tonkin region of northern Vietnam which today is centred on the Red River Delta. During the 18th and 19th centuries the term Tonkin had a wider meaning; it was used by Westerners to describe approximately the northern one third of Vietnam.
Vernacular names. Lao Cai Senegalia (following the Chinese common name that is proposed above). The name refers to Lao Cai in Vietnam from where the type was collected.
21. Senegalia yunnanensis (Franch.) Maslin, Seigler & Ebinger, Blumea 58: 42 (2013) (Figs. 56 and 58).云南金合欢【yún nán jīn hé huān】
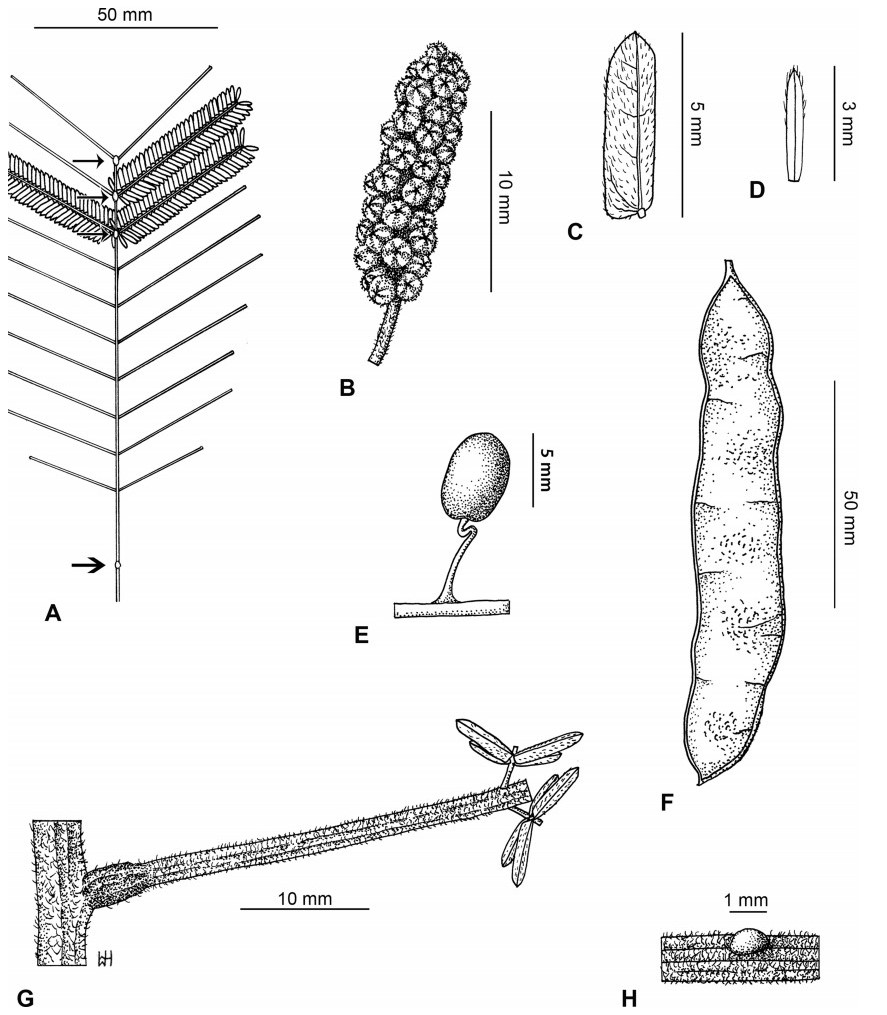
|
| Fig. 56 Senegalia yunnanensis. A - Leaf showing position of petiole gland (thick arrow) and rachis glands (thin arrows). B - Inflorescence cylindrical. C - Leaflet (lower surface) softly pubescent. D - Stipule. E Seed (sub-mature) relatively small, seemingly without pleurogram. F - Pod. G - Node showing branchlet lacking prickles and petiole gland absent. H - Petiole gland very small. Vouchers: S.W. Yu & Q.T. Zhang 251 (A & D); J.S. Ying 3318 (B); K.M. Feng 9231 (C & H); T.T. Yu 6358 (E, F & G). Drawn by Waiwai Hove. |
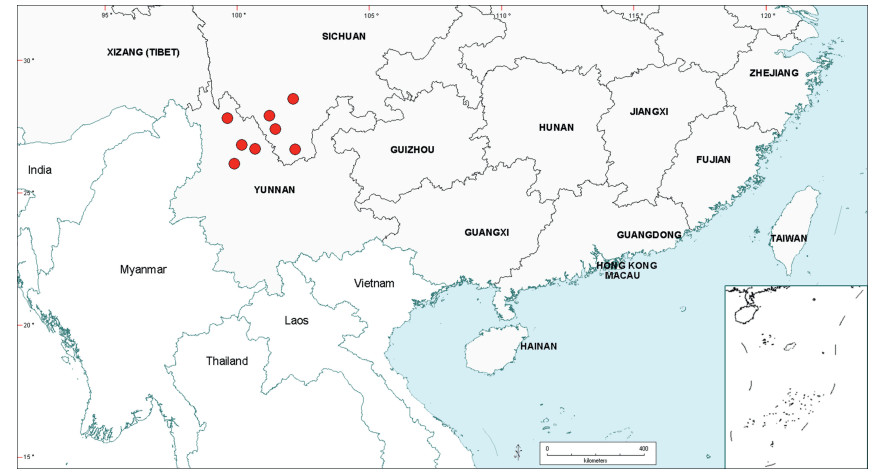
|
| Fig. 57 Distribution of Senegalia yunnanensis, based on specimen records A, P and KUN, as cited in text. |

|
| Fig. 58 Senegalia yunnanensis. A - Upper branchlet. B - Raceme apex showing cylindrical inflorescences subtended by young leaves, and flower buds green. Photos: H. Sun. |
≡ Acacia yunnanensis Franch., Pl. Delavay. 193 (1890). Type citation: "Yun-nan, in collibus calcareis et silvulis ad Che-tong, prope Tapin-tze; fl. 13 Maj. 1886 (Delav. n. 2555.)". Type: CHINA, northwest Yunnan Province, 'Arbuste de 4 â 5 m. Les bois de coteaux calaires a Che tong pres Tapin tze' [Shrub 4-5 m. Woods and limestone hills; Che tong is a cave at 1200 m alt., located south of Ta pin tze (a one-time French missionary station that was located and east of "Tali fu" which is now Eryuan County of Dali Bai Autonomous Prefecture in NW Yunnan, fide Bretschneider (1898b)], 13 May 1886, J.M. Delavay 2555 (lectotype: P barcode P02436191 [digital image!], 2nd step lectoty-pification designated here, see discussion below under Typification; isolectotypes: A barcode 00058297 [digital image!]dfragment ex P02436189, P barcode P02436189 [digital image!], P barcode P02436190 [digital image!], P barcode P02436192 [digital image!]).
Distinctive features. Normally shrubs to 4-5 m tall. New shoots canescent. Prickles often absent. Pinnae (8-) 10-15 pairs. Leaflets normally 3-5 mm long, 0.7-1.5 mm wide, strongly discolorous and rather densely appressed soft-pubescent on lower surface (upper surface the same but it varies to glabrous); lateral veins (on lower surface of leaflets) not or scarcely visible. Petiole gland very small (0.7-1 mm long), often absent. Inflorescences spicate and arising within axils of immature leaves on expanding, new shoots. Calyx densely hairy and often about as long as the petals. Seeds (sub-mature) relatively small (6-7 mm long, 4-5 mm wide).
Description. Normally shrubs to 4-5 m tall, sometimes trees to 8 m, sometimes deciduous (see comment under Note below). Branchlets pubescent towards extremities (hairs short, patent, straight or shallowly curved), mature branchlets glabrous or hairs confined to longitudinal bands. New shoots canescent. Prickles absent or occasionally few on under surface of leaf axes, normally absent from internodes on herbarium specimens but when present (usually on old branches, fide Wu and Nielsen (2010)) they are few to reasonably numerous, 2-5 mm long, stout, patent and straight or shallowly recurved. Stipules caducous, narrowly triangular or sometimes almost linear, 3-6 mm long, 0.5-1 mm wide, light brown. Leaves bipinnate, often not fully mature at anthesis; pinnae (8-) 10-15 pairs, 15-50 mm long; petiole 12-40 (-50) mm long; rachis 60-120 mm long. Leaflets 25-47 pairs, narrowly oblong, 3-5 (-6) mm long, 0.7-1.5 (-2) mm wide, strongly discolorous, green, rather densely appressed soft-pubescent on lower surface, upper surface indumentum similar but it varies to glabrous; apex symmetric or asymmetric, acute to sub-acute or obtuse, normally bluntly apiculate; base unequal with a prominent rounded angle on lower edge, petiolule clearly excentric; main vein starting near upper margin at leaflet base and extending ±obliquely to apiculum, straight to shallowly curved; lateral veins (on lower surface of leaflets) not visible or few and obscure (commonly obscured by indumentum), ±patent and sometimes bifurcating near margin, not forming a reticulum. Glands:petiole gland-often absent, when present situated near or below middle of petiole 8-12 mm above leaf base and 5-9 mm above pulvinus, very small (0.7-1 mm long), circular or oblong, sessile, depressed or raised to c. 0.5 mm high, flat-topped or convex above; rachis glands - situated at base of uppermost 1-3 pairs of pinnae, circular to shortly oblong-elliptic, 0.5-0.7 mm long, sessile; rachilla glands - situated at base of uppermost 1 or 2 pairs of leaflets, sessile. Inflorescences comprising pedunculate spikes arising within axils of immature leaves on new shoots, often appearing racemose or occasionally paniculate due to delayed development of subtending leaves; peduncles 10-25 mm long, densely hairy; spikes cylindrical, 16-30 mm long at anthesis when dry, white or pale yellow; inflorescence buds pale green. Flowers 5-merous, sessile to sub-sessile or shortly pedicellate (pedicel 0.5-1 mm long); calyx equaling or slightly shorter than petals, gamosepalous, dissected for ⅕-¼ its length into triangular lobes, calyx tube rather densely hirtellous (hairs white and ±patent) and obscurely 5-veined or veins not visible; petals (2.5-) 3 (-4) mm long, sub-glabrous to moderately appressed-hairy (hairs short and white), veins not visible; ovary densely hairy. Pods oblong, (65-) 80-150 mm long, 20-30 mm wide, firmly chartaceous to thinly coriaceous, generally straight, flat, scarcely raised over seeds, light brown, glabrous, sessile glands (minute, circular & dark brown) scattered, apex obtuse and often apiculate, base short-stipitate (stipe c. 5 mm long). Seeds (sub-mature), transverse in pods, oblong-elliptic, 6-7 mm long, 4-5 mm wide, flattened, brown; pleurogram not visible; funicle thickly filiform, exarillate.
Selected specimens examined. CHINA: Sichuan. Liangshan Yi Autonomous Prefecture, Huili County, 12 Dec. 1952, P.I. Mao 2014 (KUN 0400359 [barcode 0598595], KUN 0400360 [barcode 0598594]); Liangshan Yi Autonomous Prefecture, Mianning County, 12 July 1959, S.K. Wu 2211 (KUN 0400357 [barcode 0598597], KUN 0400358 [barcode 598596]); Liangshan Yi Autonomous Prefecture, Muli Zang Autonomous County, 17 June 1937, T.T. Yu 6358 (KUN 0400351 [barcode 0598593]); without specific locality, 5 May 1960, J.S. Ying 3318 (KUN 0400352 [barcode 598592]).Yunnan. Diqing Zang Autonomous Prefecture, Shangri'la County, 27 May 1939, K.M. Feng 1082 (KUN 0400371 [barcode 0598578], KUN 0400372 [barcode 0598577]); Lijiang City, Lijiang Naxi Autonomous County, 3 Aug. 1942, K.M. Feng 9231 (KUN 0400375 [barcode 0598576]); Lijiang City, Lijiang Naxi Autonomous County, 17 May 1981, S.W. Yu & Q.T. Zhang 251 (KUN 0751585 [barcode 0598576]); Lijiang City, Yongsheng County, Apr. 1940, R.C. Ching 31189 (KUN 0400366 [barcode 0598585], KUN 0400367 [barcode 0598588]).
Distribution (Fig. 57). Endemic to southwest China where it has a somewhat restricted distribution in southern Sichuan and adjacent areas of north-western Yunnan. It occurs mainly in the drainage system of the Jinsha River to the west of Dukou City but also in the drainage system of the Yalong River to the north of Dukou City. This distribution is largely parapatric with that of Senegalia teniana(see Fig. 51).
Habitat. Grows in thickets and open sites in mountain regions at 1200-2500 m alt.
Phenology. Based on information provided by Sun (2006) and from specimens at KUN, this species flowers from April to July and produces pods with mature seeds from about August to December.
Typification. In the protologue of Acacia yunnanensis, a single collection (Delavay 2555) was cited by Franchet (1890); no herbarium of lodgement was indicated (but it is noted that Franchet worked at herb. P at that time) and there was no indication that only a single specimen of the Delavay collection was used to prepare the original description. Sun and Chen (1990) subsequently cited Delavay 2555 at herb. P as the holotype of A. yunnanensis. However, in that herbarium there are four sheets of Delavay 2555; two sheets (barcodes P02436190 and P02436191) show handwriting by Delavay giving collection details and stating that the plant was a shrub 4-5 m [tall], while another (barcode P02436189) supports drawings of a dissected flower and flower parts (the protologue diagnosis specifically mentions the small flowers of this species). None of these sheets is labelled type by Sun or Chen, none of the specimens is at variance with the original description and they should all be treated as syntypes. Accordingly, in conformity with ICN Art. 9.10 (Turland et al., 2018) and as discussed by McNeill (2014), the Sun and Chen (1990) holotype citation is corrected above to lectotype. Furthermore, in order to more precisely typify this name a second step lectotypification (cf. ICN Art. 9.17) is undertaken. Accordingly, P [barcode P02436191] has been chosen above as the lectotype because it is a well-preserved specimen with label information providing a direct reference to the protologue. Although the equally well-preserved specimen P02436189 had been labelled (in 1978) as type of A. yunnanensis by the late Ivan Nielsen, this typification was never published, and the sheet does not include a handwritten Delavay label.
Taxonomy. Senegalia yunnanensis is unique among indigenous Chinese Senegalia in having its flowers arranged in cylindrical spikes. The only other species of Senegalia in China with spicate inflorescences are the introduced S. catechu and S. senegal which have prickles at their nodes, and which belong to Senegalia sect. Senegalia. Neither of these introductions is closely related to S. yunnanensis (which belongs to Senegalia sect. Monacanthea because its cauline prickles are internodal).
This species is described (as Acacia yunnanensis) in Chinese in Huang (1985) and also by Wu (1988), accompanied by illustrations. While the flowering branch illustrated in plate 10, Fig. 1 of Wu (1988) accurately depicts Senegalia yunnanensis, the flowers that are illustrated in Fig. 2 on that same plate do not appear to belong to this species (the calyx is too short).
The Wu and Nielsen (2010) treatment of Senegalia yunnanensis contains discordant elements, the most obvious of which are the following: leaflets to 10 mm long and sparsely pubescent on both surfaces, petals densely golden tomentose, and to 5 mm long. These characters were most likely taken from two speci-mens of an unknown Mimosoid that was collected in Yunnan and which had been misidentified as Acacia yunnanensis at herb. IBSC, namely, T.N. Liou 13028 & 15049. Also, Wu and Nielsen (2010) recorded A. yunnanensis as having as few as five pairs of pinnae, an error possibly caused by the inclusion of the specimen B.S. Sun et al. 0705 within their concept of the species. This specimen is regarded here as S. teniana, but it is slightly atypical for that species in having obloid inflorescences and finely appressed-hairy leaflets.
Affinities. Senegalia yunnanensis appears most closely related to the Chinese endemic, S. teniana. These two species are shrubs or sometimes trees and have rather restricted, broadly parapatric distributions in northwest Yunnan and southern Sichuan (see under Distribution above). Senegalia teniana is most readily distinguished from S. yunnanensis by its flowers arranged in globose or sometimes obloid heads, leaflets normally glabrous (but when hairs are present they are less obvious than those of S. yunnanensis) and imperfectly reticulately-veined on their lower surface, petiole gland more evident (1.5-3 mm long) and only sometimes absent from a few leaves. Senegalia teniana has seeds which are presum-ably larger than those of S. yunnanensis (however, fully-mature seeds have not been seen for this species).
Senegalia donnaiensis (Gagnep.) Maslin, Seigler & Ebinger, a liana from Indochina, is superficially similar to S. yunnanensis in having spicate inflorescences, small petiole glands and relatively small leaflets that are hairy on their lower surface. Apart from differences in growth form, S. donnaiensis can be readily distinguished from S. yunnanensis by branchlets being long-pilose (hairs yellow-brown), petioles longer (mostly 45-55 mm), petiole glands 2, calyx glabrous and pod stipes about twice as long.
Note. According to Sun and Chen (1990), plants of this species that grow close to watercourses show an evergreen habit, while those growing on drier sites far from watercourses tend to be deciduous.
Etymology. The species name refers to the Chinese Province of Yunnan from where the type was collected.
Vernacular name. Yunnan Senegalia. This name was used by Sun (2006) and Wu and Nielsen (2010) under A. yunnanensis.
4.4. Introduced species (of Acacia, Acaciella, Senegalia and Vachellia)Determining what introduced species of Acacia sens. lat. to include here often proved troublesome. Some difficulties were encountered in deciding which exotic species are found in China today, where and to what extent they are grown, and for what purpose they are cultivated. In reviewing this subject, we relied upon information derived from literature and especially from advice provided by knowledgeable colleagues (see below under Acknowledgements); although herbarium records were consulted, these often did not provide useful information as to the frequency or utilisation of species. About 30 taxa of Acacia sens. lat. have been reported in literature as cultivated or otherwise introduced to the country over the past 50 years or so (Table 7). Here we recognise 15 species (representing nearly half the Acacia sens. lat. of China) as being the most relevant introductions today. Acacia confusa is included with these introductions as a matter of convenience: this species is introduced in mainland China but is both introduced and indigenous in Taiwan. The introduced species have been used most commonly in landscape amelioration projects and in commercial plantations, but some have been used for other purposes such as medicines, amenity plantings, honey plants, etc. The 15 species are listed in Table 8, are included in the Key to species above and are provided with a short botanical profile below.
| Name used in literature [and validating references] | Currently accepted name | Notes | ||
| A. arabica (Lam.) Willd. [Huang (1985); Wang and Fang (1991).] | V. nilotica (L.) P.J.H.Hurter & Mabb. | Acacia arabica has long been considered conspecific with A. nilotica (=V. nilotica). see below. | ||
| A. arundelliana F.M.Bailey [Zhu et al. (2015).] | A. oshanesii F.Muell. & Maiden | Acacia oshanesii is an Australian species not known to be | ||
| introduced in China (Huoran Wang, pers. comm.); it is not | ||||
| known to what taxon the non-current name A. arundelliana | ||||
| that was listed by Zhu et al. (2015) refers to. | ||||
| A. aulacocarpa A.Cunn. ex Benth. [Wang et al. (1994); Bai et al. (1998); Pan and You (1994); Turnbull et al. (1998).] | A. aulacocarpa A.Cunn. ex Benth. (A. peregrinalis M.W.McDonald & Maslin) | Plants trialled as A. aulacocarpa in China were A. peregrinalis (M. McDonald, pers. comm.), a Papua New Guinea endemic; it showed potential for utilisation but has apparently not been domesticated. | ||
| A. auriculiformis A.Cunn. ex Benth. [Wu et al. (1983); Huang (1985), as 'auriculaeformis'; Wu (1988); Wang and Fang (1991); Pan and You (1994); Wang et al. (1994); Wu and Nielsen (2010); Zhu et al. (2015).] | A. auriculiformis A.Cunn. ex Benth. | Currently utilised in China (see Table 8). | ||
| A. baileyana F.Muell. [Wu et al. (1983)]. | A. baileyana F.Muell. | Few plants of this Australian species grown in Nanning City, | ||
| Guangxi, but not currently regarded as domesticated to any | ||||
| extent in China (Huoran Wang, pers. comm.) | ||||
| A. catechu (L.f.) Willd. [Wu et al. (1983); Huang (1985); Wu (1988); Pan and You (1994); Wu and Nielsen (2010)]. | S. catechu (L.f.) P.J.H.Hurter & Mabb. | Currently utilised in China (see Table 8). | ||
| A. catechu var. wallichiana (DC.) P.C.Huang [Huang (1985).] | S. catechu (L.f.) P.J.H.Hurter & Mabb. | This variety is no longer regarded as distinct from typical S. catechu. | ||
| A. cincinnata F.Muell. [Wang et al. (1994); Turnbull et al. (1998).] | A. cincinnata F.Muell. | Currently utilised in China (see Table 8). | ||
| A. confusa Merr. [Wu (1988); Zheng and Yang (1993); Zhu et al. (2015).] | A. confusa Merr. | Currently utilised in China (see Table 8). | ||
| A. crassicarpa A.Cunn. ex Benth. [Wang and Fang (1991); Pan and You (1994); Wang et al. (1994); Bai et al. (1998); Turnbull et al. (1998).] | A. crassicarpa A.Cunn. ex Benth. | Currently utilised in China (see Table 8). | ||
| A. cunninghamii Hook. [Wang and Fang (1991); Wang et al. (1994).] | A. concurrens Pedley | The illegitimate name, A. cunninghamii Hook. was widely misapplied in the past (Pedley, 1978); it is not known what species was grown in China (introduced in 1979) under this name. | ||
| A. dealbata Link [Wu et al. (1983); Huang (1985); Wu (1988); Wang and Fang (1991); Pan and You (1994); Wang et al. (1994); Wu and Nielsen (2010); Zhu et al. (2015).] | A. dealbata Link | Currently utilised in China (see Table 8). | ||
| A. deanei (R.T.Baker) M.B.Welch et al. [Wang et al. (1994).] | A. deanei (R.T.Baker) M.B.Welch et al. | Although formerly considered prospective for utilisation in China, this Australian species has not been domesticated. | ||
| A. decurrens Willd. [Wu et al. (1983); Huang (1985); Wu (1988); Wang and Fang (1991); Wu and Nielsen (2010); Zhu et al. (2015).] | A. decurrens Willd. | Currently utilised in China (see Table 8). | ||
| A. decurrens var. mollis Lindl. [Wu et al. (1983)] | A. dealbata Link (A. mearnsii De Wild.) | The plant grown in China as var. mollis was A. mearnsii, fide Huang (1985) and Wu (1988). However, nomenclaturally A. decurrens var. mollis is a synonym of A. dealbata. | ||
| A. decurrens var. normalis Benth. [Wu et al. (1983).] | A. decurrens Willd. | Variety normalis is the same taxon as A. decurrens (see above). | ||
| A. elata A.Cunn. ex Benth. [Wu et al. (1983); Wang and Fang (1991).] | A. elata A.Cunn. ex Benth. | An Australian species considered prospective for utilisation in China, but not yet extensively used (Huoran Wang, pers. comm.). It is included in our identification key but a detailed description is not presented. | ||
| A. farnesiana (L.) Willd. [Wu et al. (1983); Huang (1985); Wu (1988); Wang and Fang (1991); Wu and Nielsen (2010); Zhu et al. (2015).] | V. farnesiana (L.) Wight & Arn. | Currently utilised in China (see Table 8). | ||
| A. glauca (L.) Moench [Huang (1985); Wu (1988); Wang and Fang (1991); Wu and Nielsen (2010); Zhu et al. (2015).] | Acaciella glauca (L.) L.Rico | Currently utilised in China (see Table 8). | ||
| A. holosericea A.Cunn. ex G.Don [Wang and Fang (1991); Wang et al. (1994); Pan and You (1994); Turnbull et al. (1998); Zhu et al. (2015).] | A. holosericea A.Cunn. ex G.Don | Currently utilised in China (see Table 8). | ||
| A. implexa Benth. [Wang et al. (2017).] | A. implexa Benth. | Currently utilised in China (see Table 8). | ||
| A. leptocarpa A.Cunn. ex Benth. [Wang et al. (1994).] | A. leptocarpa A.Cunn. ex Benth. | An Australian species formerly considered prospective for utilisation in China, but apparently has not been domesticated. | ||
| A. mangium Willd. [Wang and Fang (1991); Pan and You (1994); Wang et al. (1994); Bai et al. (1998); Turnbull et al. (1998); Zhu et al. (2015).] | A. mangium Willd. | Currently utilised in China (see Table 8). | ||
| A. mearnsii De Wild. [Wu et al. (1983); Huang (1985); Wu (1988); Wang and Fang (1991); Pan and You (1994); Wang et al. (1994); Turnbull et al. (1998); Wu and Nielsen (2010); Zhu et al. (2015).] | A. mearnsii De Wild. | Currently utilised in China (see Table 8). | ||
| A. melanoxylon R.Br. [Wu et al. (1983); Wang and Fang (1991); Wang et al. (1994); Turnbull et al. (1998).] | A. melanoxylon R.Br. | Currently utilised in China (see Table 8). | ||
| A. mollissima Hort. ex Willd. [Wu et al. (1983).] | A. pubescens (Vent.) R.Br. (A. mearnsii De Wild.) | The plant grown in China under this name was A. mearnsii, fide Wu (1988). Nomenclaturally A. pubescens is a synonym of A. pubescens. | ||
| A. neriifolia A.Cunn. ex Benth. [Wang et al. (1994).] | A. neriifolia A.Cunn. ex Benth. | An Australian species was formerly considered prospective for utilisation in China, but apparently has not been domesticated. | ||
| A. nilotica (L.) Willd. ex Delile [Wu (1988); Wu and Nielsen (2010); Zhu et al. (2015).] | V. nilotica (L.) P.J.H.Hurter & Mabb. | An African/Asian species regarded by Wu and Nielsen (2010) as rarely cultivated in China. It is included in our identification key but a detailed description is not presented. | ||
| A. oswaldii F.Muell. [Wu et al. (1983).] | A. oswaldii F.Muell. | A very slow-growing Australian species that has not been domesticated in China (Huoran Wang, Wang, pers. comm.). | ||
| A. podalyriifolia A.Cunn. ex G.Don [Zhu et al. (2015).] | A. podalyriifolia A.Cunn. ex G.Don | Currently utilised in China (see Table 8). | ||
| A. richii A.Gray [Huang (1985).] | A. confusa Merr. (A. richii) | Acacia richii is endemic to Fiji and is not introduced in China; Huang (1985) misapplied this name to plants of A. confusa. | ||
| A. senegal (L.) Britton [Huang (1985); Wu (1988); Wang and Fang (1991); Wu and Nielsen (2010); Zhu et al. (2015).] | S. senegal (L.) Britton | African/Asian species regarded by Wu and Nielsen (2010) as rarely cultivated in China. It is included in our identification key but a detailed description is not presented. | ||
| Abbreviations: A. =Acacia, S. =Senegalia, V. =Vachellia. | ||||
| Name | Country of origin; introduction to China | Main uses in China |
| Acacia auriculiformis A.Cunn. ex Benth. | Native of Australia & southern SE Asia. Introduced to China in 1961 [Pan and Yang (1987)]. | Solid wood products & pulpwood (but volumes of timber & pulpwood available only from older remnant plantings, and are very limited in extent, Roger Arnold, pers. comm.), environmental (soil & water conservation plantings), amenity plantings and (at the local level) fuelwood, farm tools, biofertiliser, honey plant. [Wang and Fang (1991); Turnbull et al. (1998); Wang et al. (2017).] |
| Acacia cincinnata F.Muell. | Native of Australia. Introduced to China in latter decades of 20th century. | Prospective for solid wood products & pulpwood [Wang et al. (2017); Wang et al. (1994)]. In recent years considered desirable for veneer production but usage constrained by seed hard to acquire (Roger Arnold, pers. comm.). |
| Acacia confusa Merr. | Native of Philippines & presumably Taiwan. Not known when introduced to mainland China. | Amenity plantings, fuelwood, environmental (soil conservation plantings), solid wood products (farm tools, furniture & house construction), medicinal (treatment for skin ulcers). [Wang and Fang (1991); Zheng and Yang (1993); Ye et al. (2013).] Wood volume for solid wood products is very limited and mostly coming from isolated or small groups of trees (Roger Arnold, pers. comm.). In the past was used for charcoal production. |
| Acacia crassicarpa A.Cunn. ex Benth. | Native of Australia & New Guinea. Introduced to China in 1979 [Wang et al. (1994)]. | Considered suitable for solid wood products (e.g. building construction, flooring, etc.), pulpwood and for environmental purposes (e.g. coastal dune greening, forest windbreaks). [Wang et al. (2017); Wang et al. (1994).] However, limited areas remain under cultivation in China today (Roger Arnold, pers. comm.). |
| Acacia dealbata Link | Native of Australia. Introduced to China in the 1950s [Wang and Fang (1991)]. | Environmental (erosion control & water conservation plantings, ornamental, fuelwood, honey plant. [Wang and Fang (1991); Wang et al. (2017); Wang et al. (1994); Wu and Nielsen (2010).] |
| Acacia decurrens Willd. | Native of Australia. Introduced to China in 1950s [Wang and Fang (1991)]. | Environmental (soil & water conservation plantings, vegetation restoration, forest protection). [Wang et al. (2017).]. |
| Acacia holosericea A.Cunn. ex G.Don | Native of Australia. Introduced to China in 1979 [Wang and Fang (1991)]. | Environmental (soil & water conservation plantings), fuelwood. [Turnbull et al. (1998); Wang and Fang (1991); Wang et al. (1994).] |
| Acacia implexa Benth. | Native of Australia. Uncertain when introduced to China (perhaps late 20th century). | Environmental (soil & water conservation plantings, landscaping). [Wang et al. (2017).] |
| Acacia mangium Willd. | Native of Australia, New Guinea & Moluccas. Introduced to China in 1979 [Wang and Fang (1991)]. | Solid wood products, pulpwood. [Wang et al. (2017); Wang et al. (1994).]. Significant areas of commercial plantations established in China around beginning of 21st century but now plantations in decline and wood volumes very limited (Roger Arnold, pers. comm.) |
| Acacia mearnsii De Wild. | Native of Australia. Introduced to China in the early 1930s [Turnbull et al. (1998)]. | Environmental (especially soil & water conservation plantings). Tannin production important in the past; the species also produced solid wood products (e.g. furniture manufacture, mine props), fuelwood, honey plant. [Wang et al. (2017); Wang et al. (1994); Ho and Fang (1997); Midgley and Turnbull (2003); Wu and Nielsen (2010).] Today there are almost no commercial plantations remaining in China (Roger Arnold, pers. comm.) |
| Acacia melanoxylon R.Br. | Native of Australia. Unknown when first introduced to China but was trialled in 1987 [Zhang et al. (2004)]. | Solid wood products (especially furniture). [Wang et al. (2017); Wang et al. |
| (1994).]) Today there is almost no plantation resource of this species in China. However, as this species produces a valuable timber, it would be a good candidate for more extensive genetic and silvicultural research in China (Roger Arnold, pers. comm.). | ||
| Acacia podalyriifolia A.Cunn. ex G.Don | Native of Australia. Not known when introduced to China. | Ornamental plantings. (Huoran Wang, pers. comm.) |
| Acaciella glauca (L.) L.Rico | Native of Caribbean & Venezuela. Not known when first introduced to China but was included (as Acacia glauca) in Huang (1985). | Ornamental plantings, host plant of lac insect, fuelwood. [Huang (1985); Wu (1988); Wu and Nielsen (2010).] |
| Senegalia catechu (L.f.) P.J.H.Hurter & Mabb. | Native of the Indian subcontinent and Myanmar. Not know when first introduced to China. | Medicinal (for a range of ailments), tanning leather, dye manufacture, solid wood products (building construction and other purposes). [Huang (1985); Wu (1988).] |
| Vachellia farnesiana (L.) Wight | Native of the Americas; globally widespread. Introduced to Taiwan in 17th century; unknown when first introduced to mainland China but recorded for Hong Kong by Bentham (1861). | Hedge plant, medicinal, dye & perfume manufacture. [Huang (1985); Wu (1988); Ye et al. (2013).] |
Despite some of the 15 introduced species having been reported as invasive in some countries, the only documented account of invasiveness in China that we have found is for A. mearnsii at Kunming Changshui Airport (Liu et al., 2016). Nevertheless, we have observed A. dealbata as common and presumably spreading in some disturbed sites (especially road verges) in Yunnan, while A. confusa has become somewhat naturalised, but not regarded as invasive, in parts of southern Guangdong and Fujian (Roger Arnold, pers. comm.). Many species of Acacia sens. lat. possess biological characteristics that favour their establishment and spread, especially in open disturbed sites. These characteristics include the production of large quantities of easily-dispersed, hard-coated seeds which remain viable in the soil for many years; rapid growth rate; root nodulation that enables them to fix atmospheric nitrogen and survive and prosper in nutrient-deficient soils; and the ability of some species to produce root suckers. It therefore would be prudent to monitor, and manage if necessary, the introduced species in China to ensure that they do not become serious environmental weeds in the future. Griffin et al. (2011) have provided a useful summary of potential invasiveness of Australian species of Acacia within the context of species having been introduced to areas beyond their natural range for utilisation purposes.
The earliest introduced species of Acacia sens. lat. to China appears to be Vachellia (Acacia) farnesiana. According to Li et al. (2015) this species was introduced to Taiwan by the Dutch East Company in the 17th century. Although it is not known when it was first introduced to mainland China (where it is now widespread), it was recorded (as A. farnesiana) for Hong Kong by Bentham (1861). Vachellia farnesiana is a native of the Americas and has been introduced into numerous countries around the globe; the WorldWideWattle website (web ref. 33) documents this distribution. Another early introduction to China was A. confusa, a species that is now widely distributed not only within the country but also in the AsiaePacific region where it is commonly used as an ornamental. This species is a native of the Philippines and probably also Taiwan. It was recorded (as Acacia richii) for Taiwan by Forbes and Hemsley (1887), but it is not known when the species was first introduced to mainland China.
A major period of Acacia sens. lat. introductions into China occurred during the second half of the 20th century, and involved species originating mainly from the Australian region. Environmental enhancement and commercial considerations were the primary drivers for these introductions, with an emphasis on species that might help ameliorate the effects of environmental degradation and/or had potential for producing useful products (particularly solid wood and pulp wood). Overviews of the extent and utilisation of these and other Acacia species are provided by Turnbull et al. (1998), Midgley and Turnbull (2003), Midgley and Beadle (2007) and Griffin et al. (2011).
In China, many Australian species of Acacia were trialled (Zhang et al., 1998), but relatively few were assessed as potentially suitable for afforestation programs or commercial plantations. The comprehensive evaluation of Australian acacias for use in China commenced in 1985 (Wang and Fang, 1991) and in ten years about 100 species had been assessed (Wang et al., 1994). Of the 13 species that Wang et al. (1994) listed as most promising, not all subse-quently proved successful, but some did become incorporated in plantation forestry, soil and water conservation programs, and as sources of fuelwood in southern China (in areas south of about latitude 30°N). The use of these Acacia species, together with those of Australian Allocasuarina and Eucalyptus, and also tropical pines, greatly changed the traditional Chinese landscape in many rural areas (Wang et al., 1994). As noted by Bai et al. (1998), the successful Acacia species were especially suited to the acidic, infertile soils on hilly sites in southern China.
From a commercial perspective at an industrial scale, the most important Australian species that were established in plantations in China around the turn of the 21st century were A. auriculiformis, A. crassicarpa and A. mangium (Huoran Wang, pers. comm.). Acacia cincinnata and A. melanoxylon were also planted, but in limited numbers of pilot-scale plantations (Roger Arnold & Huoran Wang, pers. comm.). All these species were grown for timber and/or pulp wood in southern China (Hainan, Fujian, Guangdong, Guangxi and Yunnan Provinces, although not all species were grown in all Provinces). Acacia mearnsii which was introduced to China in the 1930s primarily as a source of tannin, was also established in plantations. However, except for A. crassicarpa in Fujian, Guangdong and Guangxi, A. mangium on Hainan Island and A. mearnsii in southern China (Li et al., 1994), mainly in Fujian and Jiangxi, the utilisation of these species was on a relatively small scale (Roger Arnold, pers. comm.).
Over the past decade or so, there has been a decline in the commercial use and importance of these acacias in China, in preference for species of Eucalyptus. There are many reasons for this decline, but the most significant factors include the following. Firstly, eucalypts proved more suitable (and profitable) than acacias for use in the recently developed wood veneer industry (with veneer mills preferring the straighter logs that are produced by Eucalyptus species). Secondly, the commonly available clonal eucalypts in China produce higher volumes of merchantable logs on short rotations than do the acacias. How-ever, the superior productivity of eucalypts is very dependent upon high rates of fertilisation, so if farmers/growers cannot afford fertiliser then perhaps acacias offer greater productivity on lower nutrient soils in warmer regions. Thirdly, and perhaps most importantly, the tropical species of Acacia are not well-adapted to low winter temperatures, and in particular to the intermittent but prolonged periods of extremely cold weather that is experienced across most of southern China (Hainan Island excepted). Many upper parts of crowns of these acacias have been observed to die back from prolonged exposure to temper-atures below 10 ℃, but above freezing (i.e. 0 ℃). Furthermore, southern China has a marked dry season that extends from late summer to early winter: A. mangium (previously the foremost commercial species of Acacia in China) is not well-suited to such a prolonged dry season, although A. auriculiformis and A. crassicarpa are better adapted to these conditions. Most of the above information concerning the current status of commercial Acacia plantations in China was kindly provided by Roger Arnold (pers. comm.).
Apart from being used as sources of solid timber and pulp wood derived from commercial plantations, Australian species of Acacia have been used in China in landscape amelioration projects, especially in the south of the country. For example, species such as A. dealbata, A. decurrens, A. holosericea, A. implexa and A. mearnsii have been planted for erosion control and/or to assist in water conservation. A few species are used in amenity plantings (e.g. A. auriculiformis) or as ornamentals (e.g. A. podalyriifolia). Wang and Fang (1991) provide a synopsis of some important Australian Acacia introductions in China. Wang et al. (2017) provide a comprehensive enumeration (in Chinese) of Australian species of Acacia (a majority of which are not yet introduced in China), suggesting where in the country each might be suited for cultivation, and in some cases, summarising their known uses.
Other than Australian species, those of Acacia sens. lat. that have been recorded in taxonomic literature as introduced in China and used for a range of purposes include Acacia confusa, Acaciella glauca (as Acacia glauca), Senegalia catechu (as Acacia catechu and A. catechu var. wallichiana) and Vachellia farnesiana (as A. farnesiana).
Information concerning specific usage of these above-mentioned species is given in the species profiles below and is summarised in Table 8.
Additional to the aforementioned, the following introduced species are also included in the identification key but are not detailed by species profiles for reasons noted below.
Vachellia nilotica (L.) P.J.H.Hurter & Mabb. was included (as Acacia arabica (Lam.) Willd.) in Huang (1985), who recorded it for Guangdong, Hainan and Taiwan, and (as A. nilotica) in Wu (1988), who recorded it for Hainan andYunnan. However, Wu and Nielsen (2010) regarded this species as being only rarely cultivated in China and therefore did not include it in their treatment. We have followed Wu and Nielsen (2010) because we did not locate any specimens of this species in any Chinese herbarium we visited. Vachellia nilotica includes seven subspecies and it is not possible to know with certainty which of these occurs in China without inspecting specimens. Vachellia nilotica is therefore included in the identification key at the species level. At HITBC and KUN a few specimens labelled A. nilotica were located but were shown to be misidentifications for Senegalia senegal. Taxonomic treatments of V. nilotica (as A. nilotica) and its subspecies are provided in Ali and Faruqi (1969) and Ross (1979). The natural distribution of this species, which extends from the Indian subcontinent through the Arabian Peninsula and West Asia to Africa, together with introduced occurrences, are documented on the WorldWideWattle website (web ref. 10).
Senegalia senegal (L.) Britton was included (as Acacia senegal (L.) Willd.) in both Huang (1985) and by Wu (1988), accompanied by an illustration, where it was recorded as cultivated in Yunnan and Taiwan. However, again Wu and Nielsen (2010) regarded the species as being only rarely cultivated in China and therefore excluded from their treatment; a decision that we have followed. Very few specimens of this species have been seen in Chinese herbaria (HITBC, KUN and IBSC). A detailed taxonomic treatment of Senegalia senegal (as A. senegal) is provided by Ross (1979), who recognised four varieties; the natural distribution of the species, which extends from the Indian subcontinent through the Arabian Peninsula to Africa, together with introduced occurrences, are documented on the WorldWideWattle website (web ref. 11).
Acacia elata A.Cunn. ex Benth. (Cedar wattle, Mountain cedar wattle, Pepper-tree wattle) is currently not commonly grown in China but it may have potential for more extensive cultivation in Hainan, Guangdong, Guangxi, Yunnan, Fujian and Zhejiang (Wang et al. (2017) and Huoran Wang, pers. comm.). This fast-growing bipinnate-leaved Australian species that attains heights of 7-20 (-30) m has been used as a windbreak and for ornamental purposes; it is a suitable fuelwood and has potential for pulpwood (fide Doran and Turnbull (1997) who provide a comprehensive summary for this species). A detailed botanical profile of A. elata, based on plants from Australia and accompanied by photographs, is provided at web ref. 12.
4.4.1. ACACIA Mill., Gard. Dict. Abr., ed. 4, [25] (1754), nom. cons.相思属【xiāng sī shǔ】≡ Racosperma (DC.) Mart., Hort. Reg. Monac. Semin. 4 (1835); ≡ Acacia subg. Phyllodineae (DC.) Ser., Fl. Jard. 3: 472 (1849). TYPE: Acacia penninervis Sieber ex DC. (typ. cons., vide Regnum Veg. 146: App. Ⅲ, 286. 2006).
= Acacia subg. Heterophyllum Vassal, Bull. Soc. Nat. Hist. Toulouse 108: 139 (1972). TYPE: Acacia stenophylla A.Cunn. ex Benth.
Trees or shrubs characterised by: prickles absent; stipules not spinescent; leaves bipinnate with numerous pairs of pinnae or modified to phyllodes; leaf glands (on bipinnate leaves) at or near base of all pairs of pinnae; inflorescences pedunculate heads or spikes, 1 or few within axil of phyllodes or leaves, or arranged in racemes or panicles; funicle expanded into an aril near attachment to seed.
A very large genus of 1067 species, the majority of which are confined to Australia (about 20 species occur naturally in Southeast and East Asia and the Pacific), see web ref. 2. The 15 species considered the most significant introductions to China are described below and are included in the Key to species above.
22. Acacia auriculiformisA.Cunn. ex Benth., London J. Bot. 1: 377 (1842), as auriculaeformis. 'auriculaeformis'.耳荚相思【ěr jiá xiāng sī】(大叶相思)
≡ Racosperma auriculiforme (A.Cunn. ex Benth.) Pedley, Bot. J. Linn. Soc. 92: 247 (1986).
Distinctive features. Unarmed glabrous (except ovary) trees with foliage phyllodinous. Phyllodes mostly falcately recurved, with numerous longitudinal veins (some more pronounced than the others); main veins remaining separate from one another and not confluent with lower margin at base of phyllode; minor veins fairly close together with some longitudinal anastomoses (but not forming a clear reticulum). Spikes interrupted, bright yellow to golden. Pods irregularly circinate to coiled and sometimes twisted, (7-) 10-18 wide, margin undulate. Aril yellow or orange, encircling seed.
Description. Unarmed, commonly heavily branched small trees 8-10 m tall but reaching 25-35 m tall with long, straight bole under favourable conditions, glabrous (except ovary). Branchlets slender, terete. Phyllodes narrowly elliptic, shallowly to obviously falcately recurved or sometimes (?when young) straight and dimidiate (i.e. lower margin ±straight, upper margin convex), 80-200 mm long, (10-) 15-40 (-60) mm wide; longitudinal veins parallel and numerous, 3-5 (-6) more pronounced that the rest, free from one another and not confluent with lower margin at base of phyllode; minor veins reasonably close together or clearly separated from one another (3-6/mm), anastomoses relatively few to sub-numerous and clearly longitudinally trending but not forming a net-like reticulum; base tapered with pulvinus 2-6 mm long; apex acute to acuminate or obtuse. Gland not prominent but slightly raised, 0-1 mm above pulvinus. Inflorescences comprising 1 to several spikes within axils of phyllodes; peduncles 5-20 mm long; spikes 50-80 (-100) mm long, interrupted (receptacle clearly visible between flowers or flower clustersdbest observed in mature buds), bright yellow to golden. Flowers 5-merous; calyx cupular, 
Distribution. Native of Australia (Northern Territory and Queensland), Papua New Guinea (Central and Western provinces) and Indonesia (Kai Islands in the Moluccas, and West Papua); map provided in Pinyopusarerk et al. (2018). Acacia auriculiformis has been introduced to many countries, especially those of tropical Asia (including China, Southeast Asia and the Indian subcontinent), but also the Pacific islands, Africa and South America; the WorldWideWattle website (web ref. 13) provides details of this distribution. It was introduced in China in 1961 according to Pan and Yang (1987), and has been recorded for Fujian, Guangdong, Guangxi, Hainan, Hong Kong, Macau and Zhejiang (Wang et al., 2017; Wu, 2008; Wu and Nielsen, 2010; Xing, 2005), and also Yunnan (KUN 0159814 [barcode 0598017]) and Taiwan (Huang et al., 1991). This species is reported by Nghiem et al. (2015) as invasive in Singapore, but there are no records of it being invasive in China.
Taxonomy. Acacia auriculiformis belongs to Acacia sect. Juliflorae (Benth.) Maiden & Betche and is related to A. cincinnata (see that species for differences). Hybrids between A. auriculiformis and A. mangium (= A. × mangiiiformis Maslin & L.A.J.Thomson) occur naturally in Papua New Guinea and are cultivated in various countries of Southeast Asia. It is doubtful that any significant cultivation of this hybrid exists in China today (see under A. mangium for discussion).
The above description is based on information provided in Wu and Nielsen (2010) and on specimens seen in Chinese herbaria GXMG, IBSC and KUN (e.g. Fujian: IBSC 457731 [barcode 0158643]; Guangxi: KUN 0159942 [barcode 0598156] & GXMG 13827 [barcode 0068742]; Hainan: IBSC 744264 [barcode 0738680]); and specimen cited under Distribution above). Acacia auriculiformis is described in Chinese and is illustrated in Huang (1985) and Wu (1988). A detailed botanical profile, based on plants from Australia and accompanied by photographs, is provided at web ref. 14.
Utilisation. Acacia auriculiformis is a member of a group of tropical Australian species (that also includes A. auriculiformis× mangium, A. crassicarpa and A. mangium).These species are now extensively used in commercial plantations, particularly in Asia, where they form a significant component of the plantation forestry industry (primarily as a source of pulpwood and solid timber). Overviews of the extent and utilisation of these and other Acacia species are provided in Midgley and Turnbull (2003), Midgley and Beadle (2007) and Griffin et al. (2011). Acacia auriculiformis is an adaptable, fast-growing species that has been used for a wide range of purposes including fuelwood, construction timber, paper and furniture manufacture, soil improvement and erosion control; it is a common street tree in many countries. According to Doran and Turnbull (1997), few other species can match the ability of this species to grow on harsh sites in the tropics. In China commercial plantations of A. auriculiformis were established in Fujian, Guangdong, Guangxi and Hainan Provinces around the turn of the present century, but these were limited in area and today few remain (Roger Arnold, pers. comm.), for reasons noted in the introduction to Introduced species above. Today A. auriculiformis is little-used in China as a source of wood products, with volumes of timber and pulpwood available only from older remnant plantings (Roger Arnold, pers. comm.). Nevertheless, this species has been incorporated in Chinese afforestation programs for soil and water conservation plantings in upland areas (Turnbull et al., 1998). It is also used in amenity plantings, and at a local level as fuelwood, farm tools, biofertiliser and as a source of pollen for honey bees. Wang and Fang (1991) provide discussion and references to the then utilisation of this species in China. Details of biology, ecology, silviculture, utilisation, etc. of A. auriculiformis are given in Turnbull (1986), Doran and Turnbull (1997) and Pinyopusarerk et al. (2018).
Etymology. The species name is derived from the Latin auricula (ear-like appendage) and forma (shape) in allusion to the shape of the pods.
Vernacular names. Ear-pod Wattle (preferred name), Northern Black Wattle (standard trade name) - see Standards Association of Australia (1983), Darwin Black Wattle, and more, see Doran and Turnbull (1997) and Pinyopusarerk et al. (2018).
23. Acacia cincinnata F.Muell., Fragm. (Mueller) 11: 35 (1878).螺旋荚相思【luó xuán jiá xiāng sī】(新拟)
≡ Racosperma cincinnatum (F.Muell.) Pedley, Austrobaileya 2: 347 (1987).
Distinctive features. Unarmed, large shrubs or trees with foliage phyllodinous. Branchlets with longish, appressed, white hairs at extremities. New shoots golden yellow by a dense layer of longish, appressed hairs. Phyllodes dimidiate to shallowly falcate, rather large (100-160 mm long, 14-30 mm wide), obviously narrowed at base, appressed white-hairy when young, ageing glabrous; with 3 prominent longitudinal veins that run together at base of phyllode (but normally remaining separate from lower margin); minor veins longitudinal, some anastomosing (like A. auriculiformis) but not forming a prominent reticulum. Spikes interrupted, cream to pale yellow. Pods tightly spirally coiled, the valves (4-) 5-7 mm wide, coriaceous, glabrous and often pruinose. Funicle-aril encircling seed in double-loop, orange or yellow.
Description. Unarmed, large shrubs or trees 5-25 m high. Branchlets slender, terete except angular at extremities, with white, appressed, longish, straight hairs at extremities. New shoots golden yellow by a dense layer of appressed, longish, straight hairs. Phyllodes narrowly elliptic, dimidiate to shallowly falcate, 100-160 mm long, (12-) 14-30 mm wide, thinly coriaceous, appressed white-hairy when young but ageing glabrous, dark green; longitudinal veins parallel and numerous, 3 veins more pronounced that the rest and running together at phyllode base but normally remain separate from lower margin, the lowermost of these veins not reaching the apex (normally extending ½-

Distribution. A native of Australia (north-eastern Queensland). Acacia cincinnata was one of many Australian acacias trialled in the later decades of the twentieth century for utilisation in China. Today there are small trials and pilot-scale plantations of this species established in Fujian, Guangdong, Guangxi, Hainan and Yunnan (Fangqiu Zhang and Roger Arnold, pers. comm.).
Taxonomy. Acacia cincinnata belongs to Acacia sect. Juliflorae and judging particularly from its phyllode venation and brightly coloured aril that encircles the seed, is related to A. auriculiformis. Acacia auriculiformis, however, is most readily distinguished by its glabrous branchlets and phyllodes, bright yellow to golden coloured spikes and curved to openly coiled pods that are wider (8-18 mm); furthermore, it does not possess the distinctively golden-hairy new shoots of A. cincinnata.
The above description is based mainly on specimens at PERTH. No specimens from Chinese herbaria have been seen. A detailed botanical profile, based on plants from Australia and accompanied by photographs, is provided at web ref. 15.
Utilisation. Acacia cincinnata has performed well in Chinese plantation trials with plants showing some cold tolerance and good stem form, and therefore regarded as promising for production of solid wood products and pulpwood, see Wang et al. (1994). In recent years A. cincinnata has become appealing for veneer production on account of it having a more suitable stem form for this purpose than many other species of Acacia (Roger Arnold, pers. comm.). Wang et al. (2017) described the beautiful, fine-textured, brown wood as being suitable for building construction, furniture manufacture and paper making. While there are pilot plantings of A. cincinnata in China today, the scale of plantings is constrained by difficulties in acquiring adequate seed supplies and various other factors (Roger Arnold, pers. comm.). Comprehensive information concerning this species, including details of its biology, ecology, silviculture, utilisation, etc. is provided by Turnbull (1986) and Doran and Turnbull (1997).
Etymology. The species name is derived from the Latin cincinnus, the classical meaning of which refers to a lock of curled hair or ringlet (Alex George, pers. comm.). Mueller (loc. cit.) used this term in reference to the spirally coiled pods of this species.
Vernacular name. Coil-pod Acacia (following the Chinese common name that is proposed above.).
24. Acacia confusa Merr., Phillip. J. Sci. (Bot.) 5: 27 (1910).台湾相思【tái wān xiāng sī】(相思树、台湾柳、相思仔)
≡ Racosperma confusum (Merr.) Pedley, Bot. J. Linn. Soc. 92: 248 (1968).
= A. confusa var. inamurai Hayata, Icon. Pl. Formosan. 4: 4 (1914).
[A. richii auct. pl., non A.Gray: Forbes & Hemsley, J. Linn. Soc., Bot. 23: 215 (1887); Perkins, Fragm. Fl. Philipp. 6 (1904); Matsumrua & Hayata, Enum. Pl. Formosa 117 (1906): fide Merrill, Enum. Philipp. Fl. Pl. 2(3): 248 (1923), Nielsen (1992), Huang, Silva Sinica 2: 1249 (1985) and Wu, Fl. Reipubl. Popularis Sin. 39: 24 (1988).]
Distinctive features. Unarmed trees with foliage phyllodinous. Branchlets rather slender, glabrous, lenticels evident. Phyllodes mostly 50-110 mm long, 4-10 mm wide and shallowly recurved with an up-turned apical point, with normally 4-7 widely spaced longitudinal veins and sometimes rather obscure anastomosing minor veins in between. Gland not prominent, 1-5 (-8) mm above pulvinus. Heads in rudimentary racemes (raceme axes c. 1 mm long), 1-4 within axil of phyllodes. Pods 7-10 (-12) mm wide. Funicle of seed exarillate.
Description. Unarmed trees 6-16 m. Branchlets terete except angled at extremities, rather slender, glabrous, lenticels evident. Phyllodes narrowly elliptic to linear-lanceolate, (45-) 50-110 mm long, (3-) 4-10 (-14) mm wide (width very variable), l:w = (5-) 7-20, thinly coriaceous, mostly shallowly recurved (sometimes with a few straight), rarely all phyllodes straight, glabrous; main longitudinal veins mostly 4-7, occasionally 3 (when phyllodes very narrow) or 8, 3 veins slightly more evident than the rest, widely spaced, not confluent with one another or with margin at phyllode base; minor veins (between main veins) not prominent or sometimes not visible, normally sparingly anastomosing with anastomoses longitudinally orientated; apex acute to acuminate or occasionally obtuse-mucronulate, the tip slightly to obviously upturned (occasionally absent from some phyllodes); pulvinus 1-2 mm long. Gland situated 1-5 (-8) mm above pulvinus, no additional glands along margin, not prominent. Inflorescences comprising 1 or 2 pedunculate heads arranged in 1 or 2 rudimentary racemes (raceme axis c. 1 mm long), resulting in 1-4 per axil; peduncles 7-13 mm long, slender, glabrous, the basal peduncular bract single, persistent, small and dark brown; heads globose, 4-7 mm diam. at anthesis when dry, c. 25-flowered, golden to bright lemon yellow, slightly fragrant. Flowers 5-merous, sepals free. Pods narrowly oblong, 4-9 (-12) cm long, 7-10 (-12) mm wide, firmly chartaceous to thinly crustaceous, straight to shallowly curved, flat but slightly convex over seeds, straight-edged or shallowly constricted between seeds, brown or blackish, glabrous, obscurely veined or veins not visible. Seed longitudinal to longitudinally oblique in the pods, oblong-elliptic to widely oblong, 5-6 mm long, dull brown; pleurogram continuous; areole quite large (c. 4 mm long and 2.5 mm wide); funicle linear, thick, not expanded into an aril.
Distribution. A native of Philippines and Taiwan. In the Philippines native stands of Acacia confusa are confined to Zambales Province (in dry forests on slopes at low altitudes) which is located on the north-western side of the northern-most island of Luzon; the species is planted in other parts of the country (Pelser, 2015; see web ref. 16). In Taiwan A. confusa is found throughout the island where it is very common in secondary forest and also wastelands, fide Huang and Ohashi (1993)); it is also cultivated in Taiwan. In the past it had been regarded as native in Taiwan by Merrill (1910), Merrill and Chun (1940), Pedley (1975), Nielsen (1992) and Pelser (loc. cit.), but Wu and Nielsen (2010) considered it introduced there. We have followed advice from Jer-Ming Hu (pers. comm., see Acknowledgements) in regarding A. confusa as both native and introduced in Taiwan. It was recorded (as A. richii) for that island by Forbes and Hemsley (1887) and Henry (1896); in both those publications the species was seemingly treated as a native, although this was not explicitly stated. It is not known when A. confusa was first introduced to mainland China, but Henry (1896) reported that it was believed to occur at 'Amoy' (= Xiamen in southeast Fujian) and Merrill and Chun (1940) recorded it for Hainan. The species today is widely cultivated in Fujian, Guangdong, Guangxi, Hainan, Jiangxi, Sichuan, Yunnan and Zhejiang according to Wu and Nielsen (2010), and is also known to occur in Guizhou (IBSC 430851 barcode 0158933l), Hubei (HIB 176776 barcode 0038687), Hong Kong (Wu, 2008) and Macau (Xing, 2005). In southern Guangdong and Fujian A. confusa has become somewhat naturalised in many places because it self-sows, but it is not regarded as invasive (Roger Arnold, pers. comm.). At a broader level A. confusa has been recorded as introduced (and sometimes adventive) in many places, including India, Southeast Asia (Indonesia, Malaysia, Singapore, Vietnam), Japan (Ryuku Islands), Indian Ocean islands (Mauritius, Seychelles) and Pacific Ocean islands (Fiji, Hawaii, Northern Marianas, Ogasawara-Shoto). The WorldWideWattle website (web ref. 17) provides reference details for these distributions.
Taxonomy. Acacia confusa belongs to Acacia sect. Plurinerves (Benth.) C.Moore & Betche. In the protologue Merrill (1910) compared the species with A. richii A.Gray (which is endemic to Fiji), under which name it had previously been misapplied (see above). While A. confusa does have affinities with A. richii (Pedley, 1975) its closest relative is Acacia simsii A.Cunn. ex Benth. from northern Australia and New Guinea. Acacia simsii is distinguished from A. confusa by having narrower pods (normally 4-5 mm wide) that are obviously raised over the smaller seeds, and straight to incurved phyllodes that have multiple small glands along their upper margin (the lowermost of which is located 0-2 mm above distal end of the pulvinus).
The above description is based on information from Pedley (1975) and Nielsen (1992) and on specimens in Chinese herbaria GXMG, HIB, IBSC and KUN (e.g. Guangdong: IBSC 732526 [barcode 0738705]; Guangxi: GXMG 54559 [barcode 0068858); Sichuan: KUN 0159883 [barcode 0598090]; and specimens cited under Distribution above). Acacia confusa is described in Chinese and is illustrated (as A. richii) in Huang (1985), and in Wu (1988).
Utilisation. In places where this fast-growing species is introduced it is commonly used in amenity plantings and sometimes (e.g. Ishigaki Island, Japan) in reforestation programs, fide Walker (1976). In China, Acacia confusa is widely planted along roadsides and on farms. It is used for fuelwood and soil conservation according to (Wang and Fang (1991) and also as a shade tree in tea gardens and for solid wood products such as farm tools, furniture and house-building according to Zheng and Yang (1993). The volume of wood used for solid wood products is very limited and mostly comes from isolated or small groups of trees (Roger Arnold, pers. comm.). In the past this species was used for charcoal production, but many kilns have now shut down for environmental reasons (Roger Arnold, pers. comm.). Ye et al. (2013) reported that fresh branch and leaves of this species can be boiled in water and the liquid used to cure skin ulcers; for external use only (its seeds are said to be poisonous when ingested). The fragrant flowers can be used to extract a fragrant oil according to Sun (2006).
Etymology. The species name is derived from the Latin confusus (confused) in allusion to the fact that prior to its recognition as a distinct species, A. confusa was confused with the Fijian species, A. richii.
Vernacular names. Taiwan Acacia (preferred name), also called Acacia Amarela in China, fide Xing (2005).
25. Acacia crassicarpa A.Cunn. ex Benth., London J. Bot. 1:厚荚相思【hòu jiá xiāng sī】
≡ Racosperma crassicarpum (A.Cunn. ex Benth.) Pedley, Austro-baileya 2: 347 (1987).
Distinctive features. Unarmed trees with foliage phyllodinous. Phyllodes large (80-270 mm long, 10-45 mm wide), normally lanceolate-falcate (i.e. broadest below the middle, tapered gradually towards apex and curved along both margins), with numerous, closely-spaced, non-anastomosing longitudinal veins, muchtapered at base, pulvinus long (normally 8-16 mm). Spikes often numerous (2-6) within phyllode axils, (20-) 30-70 mm long, light golden to pale yellow. Ovary densely hairy on upper half. Pods large (normally 40-120 mm long and 20-45 mm wide), flat, woody, dehiscing along ventral suture, with numerous transverse to transversely oblique, obvious veins. Seed aril much-folded, 5-20 mm long (unextended) and cream to white.
Description. Unarmed trees 6-25 (-30) m tall, boles to 60 cm dbh. Branchlets slender, somewhat angular at extremities but soon terete, glabrous. Phyllodes lanceolate or sometimes narrowly elliptic, falcate or sometimes sub-falcate, 80-270 mm long, 10-45 mm wide, coriaceous to thinly coriaceous, glabrous, pale green to grey-green; longitudinal veins numerous and parallel, none anastomosing, 3 prominent and 3 or 4 sub-prominent veins more pronounced than the closely spaced longitudinal minor veins, the veins free to phyllode base or more commonly the lowermost main veins confluent with lower margin for a short distance above the pulvinus; base narrowly tapered with pulvinus (5-) 8-16 mm long; apex gradually tapered. Gland normally not prominent, situated on upper margin of phyllode at distal end of pulvinus. Inflorescences comprising 2-6 pedunculate spikes within axil of phyllodes; peduncles 3-10 mm long, glabrous; spikes (20-) 30-70 mm long, ±interrupted (best observed in mature buds), light golden to pale yellow. Flowers 5-merous; calyx gamosepalous, shallowly dissected into broadly triangular lobes, calyx tube glabrous and truncate at base; petals 1-1.5 mm long, glabrous and 1-veined (vein thickened and most evident at petal apices); ovary densely white-hairy on upper half. Pods oblong to narrowly oblong, (30-) 40-120 mm long, (10-) 20-45 mm wide, flat, sometimes spirally once twisted, ±straight, dehiscing along ventral suture (i.e. splitting along the side opposite to where the funicle attaches seed to pod, thus, when the pod first opens the funicle and aril cannot be seen), woody, with numerous transverse to transversely oblique veins; stipe 2-6 mm long. Seeds transverse in pods, oblong to ovate, 5-6 mm long, 3-4 mm wide, glossy, black; areole narrowly oblong, open at hilar end; funicle/aril much-folded, 5-20 mm long (unextended), cream to white, ageing cream or pale yellow.
Distribution. A native of Australia (north-eastern Queensland and islands in the Torres Straits) and New Guinea (far southeast West Papua, Indonesia, eastwards through adjacent parts of Papua New Guinea to the Oriomo River region in Western Province); map provided in Midgley and Thomson (2018). In relatively recent years plantations of Acacia crassicarpa have been established in Southeast Asia, particularly Indonesia and Thailand (Harwood et al., 1993), and some areas of commercial plantations have been established in China (Huoran Wang and Roger Arnold, pers. comm.), including in Fujian, Guangdong, Guangxi, Hainan and Yunnan (Wang et al., 2017). This species was introduced into China in 1979 (Wang et al., 1994).
Taxonomy. Acacia crassicarpa belongs to Acacia sect. Juliflorae and is a member of the informal 'A. aulacocarpa Group'. A comprehensive taxonomic revision of A. crassicarpa, including an illustration, is presented in McDonald and Maslin (2000) and much of the information presented here is based on that treatment. No specimens from Chinese herbaria have been seen. A detailed botanical profile, based on plants from Australia and accompanied by photographs, is provided at web ref. 18.
Utilisation. Acacia crassicarpa is a member of a group of tropical Australian species (that also includes A. auriculiformis, A. auriculiformis × mangium and A. mangium). These species are now extensively used in commercial plantations, particularly in Asia, where they form a significant component of the plantation forestry industry (primarily as a source of pulpwood and solid timber). Overviews of the extent and utilisation of these and other Acacia species are provided by Turnbull (1986), Midgley and Turnbull (2003), Midgley and Beadle (2007) and Griffin et al. (2011). Doran and Turnbull (1997) described A. crassicarpa as one of the fastest-growing tropical acacias and suitable for a wide range of purposes, which are summarised by McDonald and Maslin (2000). For China, Wang et al. (1994) and Wang et al. (2017) described the hard, durable wood of this species as being suitable for building and ship construction, furniture manufacture, flooring etc, as well as for paper making, while the plants were considered suitable for greening coastal sand dunes and for forest windbreaks. Limited areas of commercial plantations of A. crassicarpa were established in Fujian, Guangdong and Guangxi in the 1990s (Fangqiu Zhang, pers. comm.), but according to Roger Arnold (pers. comm.) few of these exist today, although some other plantations have been established as recently as 2016. Details of biology, ecology, silviculture, utilisation, etc. of A. crassicarpa are given in Turnbull (1986), Doran and Turnbull (1997) and Midgley and Thomson (2018).
Etymology. The species name is derived from the Latin crassus (thick) and the Greek carpos (a fruit), in reference to the thick, woody pods.
Vernacular name. Thick-podded Salwood, the preferred name that was proposed by McDonald and Maslin (2000) who also discuss other common names that have been applied to this species. Brown Salwood is the standard trade name (Standards Association of Australia, 1983), a name that is also been applied to Acacia aulacocarpa (sens. lat.), A. crassicarpa and A. mangium.
26. Acacia dealbata Link, Enum. Hort. Berol. Alt. 2: 445 (1822).银荆【yín jīng】
≡ A. decurrens var. dealbata (Link) F.Muell. ex Maiden, Wattles & wattle-barks 3rd edn, 39 (1906); Racosperma dealbatum (Link) Pedley, Austrobaileya 2: 358 (1987).
= A. decurrens var. mollis Lindl., Bot. Reg. 5: t. 371 (1819).
Distinctive features. Unarmed trees or shrubs with bluish grey bipinnate foliage. Branchlets terete except slightly angled at extremities, densely soft-pubescent, pruinose (but pruinosity often not visible in herbarium specimens). Pinnae 10-25 pairs. Leaflets small (2-3.5 mm long and 0.4-0.8 mm wide), dull lustre. Petiole gland usually situated near base of lowermost pair of pinnae; rachis glands situated at base of all pinnae pairs, without additional interjugary glands. Inflorescences prolific, comprising globose heads arranged in racemes or short-panicles; peduncles 2-4 mm long. Pods 6-14 mm wide, flat but convex over seeds along midline, straight-edged or slightly constricted between seeds, glabrous, ±lightly pruinose. Seeds small (4-5 mm long, 2.5-3 mm wide); aril clavate.
Description. Unarmed trees or shrubs commonly 5-15 m tall and freely root-suckering. Branchlets terete except slightly angled at extremities, densely soft-pubescent by short, straight and patent hairs, pruinose (but pruinosity often not visible in her-barium specimens). Young foliage-tips white to cream or golden velvety-tomentose. Leaves bipinnate; pinnae 10-25 pairs, 15-30 mm long, lowermost pair sometimes 10 mm long; petiole 7-20 mm long, densely hairy; rachis 40-80 mm long, densely hairy. Leaflets 20-50 pairs, narrowly oblong, 2-3.5 mm long, 0.4-0.8 mm wide, opposite to sub-opposite, concolorous or weakly discolorous, dull on both surfaces, bluish grey, glabrous or appressed-hairy; apex rounded to obtuse; base unequal but lower edge not prominently lobed, petiolule poorly developed and normally central; main vein not visible or very poorly developed, central. Glands:petiole gland-usually present at base of proximal pair of pinnae; rachis glands - situated at base of all pinnae, no additional interjugary glands present, tomentellose. Inflorescences prolific, comprising pedunculate heads arranged in axillary racemes or mostly terminal or axillary false-panicles; peduncles 2-4 mm long, ±densely hairy; heads globose, 13-42-flowered, small (5-6 mm diam. when dry), bright yellow to golden. Flowers 5-merous; calyx c. ½ length of corolla, gamosepalous, very shortly dissected. Pods oblong, 3-8 cm long, 6-14 mm wide, firmly crustaceous to thinly coriaceous, flat, convex over seeds alternately on either side along midline of pod, straight-edged or slightly to moderately constricted between some or all seed, glabrous, ±lightly pruinose. Seeds longitudinal or obliquely longitudinal in pods, oblong-elliptic, 4-5 mm long, 2.5-3 mm wide, black; funicle ending in a clavate aril.
Distribution. A native of southeast Australia (New South Wales, Australian Capital Territory, Victoria and Tasmania), but is naturalised in southwest Western Australia and southern South Australia. This species has been introduced in many countries for utilisation purposes, and in some places such as South Africa and New Zealand it has become invasive (Maslin and McDonald, 2004); the global distribution of this species is documented at the WorldWideWattle website (web ref. 19). According to Wang and Fang (1991), A. dealbata and A. decurrens were introduced together into China, shortly after A. mearnsii [in the early 1950s]; A. dealbata was planted as a street tree in Kunming. This species is now established in Fujian, Guangdong, Guangxi, Guizhou, Hainan, Jiangsu, Jiangxi, Shanghai, Sichuan, Yunnan and Zhejiang (Wang et al., 2017), and is also found in Taiwan (Wu and Nielsen, 2010) and Hong Kong (specimen record: IBSC 419884 [barcode 0158957]). Although we have not seen any records of A. dealbata having become invasive in China, the possibility of this happening is likely to be relatively high in some places.
Taxonomy. Acacia dealbata belongs to Acacia sect. Botrycephalae (Benth.) Taub. The species has two subspecies, subsp. dealbata and subsp. subalpina Tindale & Kodela. Acacia dealbata may superficially resemble A. mearnsii (see that species for differences).
The above description is based primarily on the account by Wu and Nielsen (2010) and on specimens in Chinese herbaria GXMI, IBK, IBSC, KUN (e.g. Guangxi: GXMI 04255 [barcode 015759] and IBK 125039 [barcode 00067598]; Guizhou: IBSC 483347 [barcode 0159130]; Yunnan: KUN 0159897 [barcode 0598105]); and specimen cited under Distribution above). It is noted that in Australia some specimens show a greater range of character-variation than has been described for China: compare above description with that of Kodela and Tindale (2001a). Acacia dealbata is described in Chinese and is illustrated in Huang (1985) and Wu (1988). A detailed botanical profile, based on plants from Australia and accompanied by photographs, is provided at web ref. 20.
Utilisation. Acacia dealbata is a fast-growing, frost hardy species that (in its native environment in Australia at least) can form thickets by prolific root-suckering following disturbance. It is recognised as a source of good quality pulpwood suitable for a range of paper and paperboard products. In China this species is widely used in the landscape amelioration projects, e.g. erosion control and water conservation (Wang et al., 2017); it is well-adapted to nutrient-poor sites and is able to colonise bare land (Wang and Fang, 1991). Wang et al. (2017) noted that plants of A. dealbata from different origins can vary greatly in their growth form char-acteristics. On account of its prolific flowering the species is also used as an ornamental (Wang et al., 2017) and as a honey plant (Wu and Nielsen, 2010). Details of the biology, ecology, silviculture, utilisation, etc. of this species are given in Doran and Turnbull (1997); a comprehensive review of these matters is provided by Maslin and McDonald (2004).
Etymology. The species name is derived from the Latin dealbatus (whitened, covered with a white powder), in allusion to the pruinosity that is visible on various organs, e.g. branches, of the plants (especially evident when fresh).
Vernacular name. Silver Wattle. This is also the standard trade name for this species (Standards Association of Australia, 1983).
27. Acacia decurrens Willd., Sp. Pl. ed. 4, 4(2): 1072 (1806).绿荆【lǜ jīng】(线叶金合欢, 悉尼黑荆)
≡ Racosperma decurrens (Willd.) Pedley, Austrobaileya 2: 358 (1987).
- "Mimosa decurrens" Donn, Hort. Cantabrig.: 114 (1796), nom. nud.
= Mimosa decurrens J.C.Wendl., Bot. Beob. [Wendland] 57 (1798).
- Acacia decurrens var. normalis (Benth.) Maiden, Wattles & wattle-barks 2nd edn, 70 (1891), nom. illeg. (type variety).
Additional synonymy (not relevant to China): Tindale and Kodela (2001).
Distinctive features. Unarmed shrubs or trees with dark green, bipinnate foliage. Branchlets obviously angled by winged ridges, ±glabrous. Pinnae 7-15 pairs. Leaflets narrowly linear, 5-10 (- 15) mm long, 0.3-0.8 mm wide. Petiole gland situated near base of lowermost pair of pinnae; rachis gland situated at base of all pinnae pairs. Inflorescences comprising pedunculate heads arranged in axillary racemes or terminal false panicles; peduncles 2-7 mm long. Pods glabrous.
Description. Unarmed, shapely shrubs or trees 3-10 m tall, sometimes taller under favourable conditions, commonly with a single, ±straight trunk. Branchlets obviously angled by winged ridges 0.5-2 mm high, glabrous or (at extremities) appressed-hairy. Leaves bipinnate, dark green; pinnae normally 7-15 pairs, 30-70 mm long; petiole 20-30 mm long; rachis 60-120 mm long (can be shorter in Australia), glabrous to sub-glabrous. Leaflets widely spaced, 15-45 pairs, narrowly linear, 5-10 (-15) mm long, 0.3-0.8 mm wide, opposite to sub-opposite, straight, glabrous; apices obtuse or sub-acute; main vein obscure, sub-central; lateral veins not visible. Glands: petiole gland-situated at or near base of lowermost pair of pinnae, prominent; rachis glands - situated at base of all pinnae, hemispheric to bluntly conical, c. 1 mm long. Inflorescences comprising pedunculate heads arranged in axillary racemes (30-110 mm long) or terminal false panicles; peduncles 2-7 mm long, glabrous or sparsely appressed-hairy; heads globose, 20-32-flowered, rather small (4-6 mm diam. when dry), golden yellow. Flowers 5-merous; calyx c. ½ as long as corolla, gamosepalous, scarcely dissected, calyx tube dark-coloured; petals c. 2 mm long. Pods narrowly oblong, 2-10 cm long, 4-8.5 (-11) mm wide, thinly coriaceous, flat but convex over seeds alternately on either side, straight-edged or irregularly and mostly slightly constricted between the seeds, glabrous. Seeds longitu-dinal to oblique in the pods, oblong-elliptic, 5-6 mm long, c. 3 mm wide, ±turgid, very dark brown; funicle short and expanded into a ±clavate aril.
Distribution. A native of southeast Australia (New South Wales) but naturalised in other parts of Australia and is introduced into many countries around the world; this distribution is documented at the WorldWideWattle website (web ref. 21). According to Wang and Fang (1991), Acacia decurrens and A. dealbata were introduced together into China, shortly after A. mearnsii [in the early 1950s]. Acacia decurrens is now grown in Fujian, Guangdong, Guangxi, Hainan, Yunnan and Zhejiang according to Wu and Nielsen (2010) and Wang et al. (2017).
Taxonomy. This species belongs to Acacia sect. Botrycephalae. It is most readily distinguished from the other two Australian bipinnate-leaved acacias that are commonly grown in China (i.e. A. dealbata and A. mearnsii) by its branchlets acutely angled by obvious winged ridges and its longer, narrowly linear leaflets. Acacia decurrens and A. mearnsii are reported to hybridise, see Philp and Sherry (1949) and Sherry (1971).
The above description is based on specimens at KUN (e.g. Fujian: KUN 0400072 [barcode 0598291]) and PERTH, supplemented by information in Tindale and Kodela (2001) and Wu and Nielsen (2010). Acacia decurrens is described in Chinese by Huang (1985)d accompanied by illustration, and Wu (1988). A detailed botanical profile, based on plants from Australia and accompanied by photographs, is provided at web ref. 22.
Utilisation. This fast-growing but relatively short-lived species has a wide range of potential uses, including fuelwood, solid timber and pulp wood, landscape amelioration, and more. It is sometimes used as a source of natural tannin, but Acacia mearnsii is normally preferred for this purpose. A comprehensive review of the utilisation and also the biology, ecology and silviculture of this species is provided in Maslin and McDonald (2004). In China, A. decurrens is used for environmental purposes, including soil and water conservation, vegetation restoration and forest protection (Wang et al., 2017).
Etymology. The species name is derived from the Latin decurrens (prolonged below the point of insertion, as if running down-wards) in reference to the winged ridges that extend down the branchlets from below insertion of leaves.
Vernacular names. Green Wattle (preferred name), this is also the standard trade name for this species (Standards Association of Australia, 1983), Black Wattle, Sydney Black Wattle.
28. Acacia holosericea A. Cunn. ex G. Don, Gen. Hist. 2: 407 (1832).灯台相思【dēng tái xiāng sī】
≡ Acacia mangium var. holosericea (A.Cunn. ex G. Don) C.T.White, Contr. Arnold Arbor. 4: 42 (1933). ≡ Racosperma holosericeum (A.Cunn. ex G. Don) Pedley, Austrobaileya 2: 349 (1987).
Distinctive features. Unarmed shrubs or trees with foliage phyllodinous. Branchlets sericeous, acutely angled at extremities, penultimate branchlets normally ±terete. Young shoots sericeous. Phyllodes large (100-200 mm long, 20-50 mm wide), straight, sericeous, apical mucro distinct and often with a small gland near its base, with 3 prominent longitudinal veins (2 of which run together and ±contiguous with lower margin at base of phyllode), openly elongated-reticulate between main veins. Spikes golden. Petals hairy. Pods tightly and often somewhat irregularly coiled, valves 2.5-4 mm wide and thin-textured. Seed aril bright yellow, not encircling seed.
Description. Unarmed shrubs or trees 3-9 m high. Branchlets acutely angled at extremities but penultimate branchlets (c. 20-30 cm below apex) normally ±terete, sericeous by dense, silvery, appressed hairs. Young shoots sericeous, silvery or pale fawn-coloured. Phyllodes obliquely narrowly elliptic, 100-200 mm long, 20-50 mm wide, straight (not shallowly recurved at apex as in Acacia colei), silvery dull green or sub-glaucous, sericeous; with 3 prominent longitudinal veins (2 of which run together and ±confluent with lower margin at base of phyllode); minor veins forming a somewhat open, longitudinally orientated, evident reticulum; base unequal; apex with a distinct oblong to linear mucro 1-3 mm long. Gland situated on upper margin of phyllode near distal end of pulvinus, additional gland normally present at base of apical mucro on at least some phyllodes. Inflorescences comprising pedunculate spikes within axil of phyllodes; peduncles 3-7 mm long, sericeous; spikes normally 20-40 mm long, golden, flowers sub-loosely aggregated (best observed in mature buds). Flowers 5-merous; calyx gamosepalous, very shortly dissected, calyx tube sericeous or tomentulose; petals sericeous or tomentulose; ovary densely hairy. Pods tightly and often somewhat irregularly coiled, valves 2.5-4 mm wide, thinly crustaceous to coriaceous-crustaceous, dark brown to blackish and glabrous, remaining on plants as entangled clumps following dehiscence. Seeds longitudinal in pods, oblong-elliptic, 3.5 mm long, shiny, dark brown; aril bright yellow, not encircling seed.
Distribution. A native of northern Australia (Western Australia, Northern Territory & Queensland). In previous years the species was introduced to various countries (especially sub-Saharan Africa) for multi-purpose utilisation, but some of these introductions are now known to represent plants of A. colei Maslin & L.A.J.Thomson (see below), which is not known to occur in China. Acacia holosericea was introduced to China in 1979 (Wang and Fang, 1991). Wang et al. (2017) consider that it would be suitable for growing in Hainan, Guangdong, Guangxi, Yunnan, Fujian. Specimens that we have seen of this species at GXMI & IBSC show it as occurring in Guangdong and Guangxi; it is also recorded for Macau by Xing (2005).
Taxonomy. Acacia holosericea belongs to Acacia sect. Juliflorae. This species was reviewed by Maslin and Thomson (1992) and as a consequence was more narrowly defined than previously through the exclusion of A. neurocarpa A. Cunn. ex Hook. and A. colei Maslin & L.A.J.Thomson. Taxonomic summaries and illustrations of A. holosericea are given in Maslin and Thomson (1992) and Maslin (2001). A detailed botanical profile, based on plants from Australia and accompanied by photographs, is provided at web ref. 23.
The above description is based on information provided in Maslin and Thomson (1992) and Maslin (2001) and on specimens at the Chinese herbaria GXMG, HITBC and IBSC (e.g. Guangdong: IBSC 244175 [barcode 159066]; Guangxi: GXMG 11194 [barcode 0068801]; Yunnan: HITBC 057808 [no barcode number given]).
Acacia holosericea is related to A. mangium Willd. on account of having acutely angled branchlets (but normally more prominently angled in A. mangium), large, reticulately-veined phyllodes, longspicate inflorescences, coiled pods and brightly coloured seed arils. However, A. mangium differs most obviously in its more arborescent stature, glabrous branchlets, new shoots, phyllodes and petals, generally larger phyllodes with 4 (-5) longitudinal veins, paler-coloured, longer spikes, generally more thickly-textured pods and orange seed arils. Although some plants of A. holosericea in Australia have glabrous branchlets, young shoots, phyllodes and petals (Maslin and Thomson, 1992) these morphotypes are unknown in China.
Utilisation. An adaptable, fast-growing but short-lived species regarded as having potential as a source of fuelwood and charcoal as well as in soil conservation and as a windbreak (Doran and Turnbull, 1997). In China, small areas of Acacia holosericea have been planted, sometimes in mixed plantations (Turnbull et al., 1998), where it has proved capable of adapting to poor and dry soils (Wang and Fang, 1991). Comprehensive information, including details of biology, ecology, silviculture, utilisation, etc. is provided for this species by Turnbull (1986) and Doran and Turnbull (1997).
Etymology. The species name is derived from the Greek holo-(entire, complete, whole) and the Latin sericeus (sericeous, i.e. with silky appressed hairs), in reference to the indumentum found on the branchlets, phyllodes and flowers.
Vernacular names. Candelabra Wattle (preferred name), Soap Bush, Silver Wattle, Silver-leaved Wattle.
29. Acacia implexa Benth., London J. Bot. 1: 368 (1842).浅木相思【qiǎn mù xiāng sī】
≡ Racosperma implexum (Benth.) Pedley, Austrobaileya 2: 350 (1987).
Distinctive features. Unarmed trees with foliage phyllodinous, often root suckering. Phyllodes narrowly elliptic, 70-200 mm long, 6-25 mm wide, falcate, much narrowed at base; with 3-7 main longitudinal veins and numerous longitudinally anastomosing minor veins in between. Inflorescences 4-8-headed racemes; heads globose, cream to pale yellow. Pods linear, to 250 mm long, 4-7 mm wide, coiled and twisted, ±woody to thickly coriaceous. Funicle/aril fleshy, white, folded beneath the seed (not encircling seed).
Description. Unarmed trees 5-12 (-15) m high, single-stemmed or with 2 or 3 main stems from base, boles commonly straight and 15-30 cm dbh, often gregarious due to root suckering; bipinnate leaves may persist on young plants. Bark rough unevenly tessellated, becoming longitudinally fissured with age. Branchlets terete, commonly lightly pruinose, glabrous. Phyllodes narrowly elliptic, 70-200 mm long, 6-25 mm wide, falcate, thinly coriaceous, green, glabrous; with 3-7 main longitudinal veins and numerous longitudinally anastomosing minor veins in between (but not forming a close, net-like retic-ulum as in A. melanoxylon); base much narrowed; apex normally acute to acuminate. Inflorescences 4-8-headed racemes 10-45 mm long; peduncles 6-15 mm long, glabrous; heads globose, cream to pale yellow. Flowers 5-merous; sepals ¾ united. Pods linear, to 250 mm long, 4-7 mm wide, coiled and twisted, ±woody to thickly coriaceous, glabrous. Seeds longitudinal in pods, oblong-oval, 4-5 mm long, sub-glossy, dark brown; funicle/ aril fleshy, white, folded beneath the seed.
Distribution. A widespread, native of eastern Australia (Queensland southwards through New South Wales and Victoria to King Island in Tasmania). Acacia implexa was introduced to China around the turn of the present century for environmental and landscaping purposes in Fujian, Guangdong, Guangxi, Hainan, Sichuan and Yunnan (Wang et al., 2017).
Taxonomy. This species belongs to Acacia sect. Plurinerves. It is sometimes confused with A. melanoxylon but the two species are not closely related.
Acacia implexa is described and illustrated in Cowan and Maslin (2001a) and the above description is based on that treatment, supplemented by specimens at PERTH. No specimens from Chinese herbaria have been seen. A detailed botanical profile, based on plants from Australia and accompanied by photographs, is provided at web ref. 24.
Utilisation. Acacia implexa is a vigorous, long-lived, fast to moderately fast-growing species that coppices well and commonly root suckers (particularly if the main stems are cut back severely or if the roots are disturbed). It performed well in growth trials con-ducted in China in 1994 (Zhang et al., 1998) and is now used for soil and water conservation and landscaping purposes (Wang et al., 2017). A comprehensive summary of biological, ecological and silvicultural features, and the utilisation of A. implexa is provided by Maslin and McDonald (2004).
Etymology. The species name is derived from the Latin implexus (entangled, entwined), in reference to the coiled and twisted pods that often form a tangled mass.
Vernacular names. Lightwood (preferred name), this is also the standard trade name for this species (Standards Association of Australia, 1983), Hickory Wattle, and moredsee Cowan and Maslin (2001a).
30. Acacia mangium Willd., Sp. Pl., ed. 4, 4: 1053 (1806), as 'Mangium'.马占相思【mǎ zhàn xiāng sī】
≡ Racosperma mangium (Willd.) Pedley, Austrobaileya 2: 352 (1987).
= Mangium montanum Rumph., Herb. Amboin. (Rumphius) 3: 123, t. 81 (1743), a pre-Linnean name.
Distinctive features. Unarmed large trees with foliage phyllodinous. Branchlets glabrous, stout and acutely angled. Phyllodes large (mostly 110-270 mm long, 30-100 mm wide), glabrous, normally with 4 prominent longitudinal veins (some or all running together at base of phyllode, but not confluent with lower margin), openly reticulate between main veins. Spikes long (50-120 mm), white to cream. Petals glabrous. Pods ±irregularly coiled and twisted, thick-textured (coriaceous-crustaceous to sub-woody), valves 3-6 mm wide. Seed aril bright orange, not encircling seed.
Description. Unarmed trees 7-30 m high, normally with a straight bole that may occupy over half of the total tree height and which is sometimes fluted at the base. Branchlets acutely angled, stout, glabrous. New shoots glabrous, youngest phyllodes often scurfy towards their apices. Phyllodes obliquely narrowly elliptic to obovate, sometimes ±dimidiate with sub-straight lower edge and clearly convex upper edge, 110-270 (-370) mm long, 30-100 mm wide, straight, thinly coriaceous to ±chartaceous, glabrous; with 4 (-5) prominent longitudinal veins (with some or most veins running together at base of phyllode but not confluent with the lower margin, the veins close to but remaining free from lower margin); minor veins anastomosing to form a fine reticulum with elongated vein-islands; apex obtuse with a ±knob-like point (but normally missing, perhaps eaten by insects). Gland situated on upper margin of phyllode to 4.5 mm above pulvinus, additional gland sometimes present at or near base of apical point of phyllode. Inflorescences comprising pedunculate spikes within axil of phyllodes; peduncles 4-13 mm long, minutely hairy; spikes 50-120 mm long, white to cream, flowers loosely to sub-loosely aggregated (best observed in mature buds). Flowers 5-merous; calyx gamosepalous, shortly dissected into broadly triangular lobes, calyx tube tomentulose and veinless; petals 1.8-2 mm long, glabrous; ovary densely hairy. Pods linear, openly or tightly ±irregularly coiled and twisted, valves 3-6 mm wide, coriaceous-crustaceous to sub-woody, glabrous. Seeds longitudinal in pods, oblong-elliptic to slightly reniform, 3.5-5 mm long, black; aril bright orange, not encircling seeds.
Distribution. A native of Australia (northeast Queensland), New Guinea (Volgekop Peninsula, West Papua Province and southeast Papua Province, Indonesia, eastwards through adjacent parts of Papua New Guinea to the Oriomo River region in Western Province) and the Moluccas (Aru Islands, Seram and Sula), Indonesia; distribution map is provided in Arnold (2018). The species has been introduced into many countries as a plantation crop, especially in the humid tropical lowlands of Asia, and also in parts of Africa as well as Central and South America, see Midgley and Turnbull (2003) and Arnold (2018). Acacia mangium was introduced to China in 1979 (Wang and Fang, 1991) as a source of timber and high-quality pulpwood (Wang et al., 1994). It has been recorded for Guangdong, Guangxi, Hainan, Yunnan (Wang et al., 2017), Hong Kong (Wu, 2008) and Macau (Xing, 2005).
Taxonomy. This species belongs to Acacia sect. Juliflorae. In the past A. mangium had sometimes been confused with Acacia holosericea (see this species for discussion of differences). Hybrids between A. mangium and A. auriculiformis (=A. × mangiiiformis) occur in nature in Papua New Guinea and are also in cultivation in Southeast Asia; see note below concerning this hybrid species.
The above description is based on specimens in Chinese herbaria GXMG, KUN and IBSC (e.g. Guangdong: IBSC 739475 [barcode 0738689]; Guangxi: GXMG 84767 [0068654] and KUN 0400073 [barcode 0598285]) supplemented by some information extracted from literature. A detailed botanical profile, based on plants from Australia and accompanied by photographs, is provided at web ref. 25.
Utilisation. Acacia mangium is a member of a group of tropical Australian species that also includes A. auriculiformis, A. auriculiformis× mangium (=A. mangiiformis) and A. crassicarpa. These species are now extensively used in commercial plantations, particularly in Asia, where they form a significant component of the plantation forestry industry (primarily as a source of pulpwood and solid timber). Overviews of the extent and utilisation of these and other Acacia species are provided by Turnbull et al. (1998), Midgley and Turnbull (2003), Midgley and Beadle (2007) and Griffin et al. (2011). Around the beginning of the 21st century, significant areas of commercial plantations of A. mangium were established in warmer parts of China, especially in Hainan, Fujian, Guangdong and Guangxi Provinces (Fangqiu Zhang, pers. comm.). However, today the areas under plantation are in decline due to a multiplicity of factors (Roger Arnold, pers. comm.), as noted in the introduction to Introduced species above. Comprehensive information, including details of biology, ecology, silviculture, utilisation, etc. are provided for A. mangium by Turnbull (1986), Doran and Turnbull (1997) and Arnold (2018).
The hybrid Acacia auriculiformis × mangium (= A. × mangiiiformis)is important in plantation forestry in Vietnam and some other countries of Southeast Asia. In the past, small scale commercial plantations of this species in China had been established in Guangdong (Fangqiu Zhang, pers. comm.), but it is doubtful that any exist today (Roger Arnold, pers. comm.).
Etymology. The species name is derived from the vernacular (Moluccan) name "Mangi Mangi Goenong" that Rumphius used when he described this species as Mangium montanum.
Vernacular names. Mangium (preferred name), Brown Salwood is the standard trade name (Standards Association of Australia, 1983) who apply this name to Acacia aulacocarpa (sens. lat.), A. crassicarpa and A. mangium; however, these days the commonly used International Trade name for this species is Mangium, L. Thomson, pers. comm.), Hickory Wattle, Black Wattle and more-see Doran and Turnbull (1997).
31. Acacia mearnsii De Wild., Pl. Bequaert. 3: 62 (1925), as 'mearnsi'.黑荆【hēi jīng】(直干相思)
≡ Racosperma mearnsii (De Wild.) Pedley, Bot. J. Linn. Soc. 92: 249 (1986).
- 'A. decurrens var. mollissima Linkl.'; this name was treated as a synonym of A. mearnsii by Huang (1985) but without details of place of publication. It appears that the author "Linkl." is a typographical error for "Lindl." because the only place where we have found the name A. decurrens var. mollissima is in Bentham, London J. Bot. 1: 385 (1842); in that work Bentham mistakenly gives the epithet 'mollissima' for A. decurrens var. mollis Lindl.
[Acacia mollissima auct. non Hort. ex Willd.: Bentham, London J. Bot. 1: 342 (1842).]
[Acacia decurrens var. mollis auct. non Lindl.: Brenan & Melville, Kew Bull. 14: 37 (1960).]
Distinctive features. Unarmed trees with green bipinnate foliage. Branchlets densely velvety-tomentulose. New shoots golden to pale yellow or almost cream by velvety indumentum. Pinnae 6-31 pairs. Leaflets small (2-4 mm long, 0.5-1 mm wide), close together, ±shiny and glabrous above, often appressed-hairy below. Petiole gland situated up to c. 8 mm below lowermost pair of pinnae; rachis glands situated at base of all pinnae pairs, normally with 1 or 2 additional glands between some or all pinnae. Inflorescences comprising globose heads arranged in racemes or short-panicles; peduncles 2-10 mm long. Pods linear to ±sub-moniliform, 4-8 mm wide, black (at least when dry), ±densely and softly appressed grey-hairy. Seeds small (4 mm long, 2-2.5 mm wide); aril clavate.
Description. Unarmed trees or large shrubs 5-10 (-15) m tall. Branchlets densely velvety-tomentulose (hairs short, soft, patent and white), not pruinose. New shoots golden to pale yellow or almost cream by velvety indumentum. Leaves bipinnate; pinnae 6-31 pairs, (15-) 25-45 (-60) mm long; petiole 10-30 mm long, densely hairy; rachis 30-135 mm long, densely hairy. Leaflets (16-) 23-55 (-78) pairs, oblong to narrowly oblong except terminal pair normally narrowly obovate, 2-4 mm long, 0.4-0.8 (-1) mm wide, close together, discolorous or sometimes ±concolorous, dark green, shiny or sub-shiny (best seen when fresh) and ±glabrous above, often appressed-hairy below; apex rounded to obtuse; base unequal, petiolule central to excentric; main vein not or scarcely visible; lateral veins not visible. Glands: petiole gland-situated up to c. 8 mm below lowermost pair of pinnae; rachis glands - situated at or near base of each pair of pinnae, 1 or 2 additional glands normally present between some or all pinnae (these additional glands sometimes absent or occur at low frequency on some specimens); rachilla glands-absent. Inflorescences comprising pedunculate heads arranged in racemes or short panicles; heads globose, small (c. 5 mm when dry), 20-40-flowered, light yellow to cream; peduncles 2-10 mm long, white- or golden-hairy. Flowers 5-merous; calyx gamosepalous. Pods linear to ±sub-moniliform, convex over seeds and slightly but discernibly constricted between them, 50-150 mm long, 4-8 mm wide, coriaceous, black (at least when dry), ±densely and softly appressed grey-pubescent to velutinous. Seeds longitudinal in pods, elliptic, 4 mm long, 2-2.5 mm wide, black, shiny; aril clavate, c. ½ length of seed and pale yellowish brown when dry.
Distribution. A native of southeast Australia (New South Wales, Australian Capital Territory, Victoria, Tasmania and South Australia) but introduced into many countries for utilisation purposes; the global distribution of this species is documented at the WorldWideWattle website (web ref. 26). Within Australia A. mearnsii is naturalised in few areas; outside Australia it is often an invasive species (Luque et al., 2014) and has become invasive in many countries, including France, India, Israel, Italy, New Zealand, Portugal, Reunion, South Africa, Spain, Uganda and the United States (Liu et al., 2016). Acacia mearnsii was introduced to China in the early 1930s (Turnbull et al., 1998) for commercial tannin pro-duction. It has been recorded for Fujian, Guangdong, Guangxi, Guizhou, Hainan, Hunan, Jiangxi, Sichuan, Yunnan and Zhejiang (Wang et al., 2017), and also Hong Kong and Taiwan (Ye et al., 2013). Liu et al. (2016) reported this species as having become invasive at Kunming Changshui Airport in Yunnan but has not yet been recorded as invasive elsewhere in China.
Taxonomy. Acacia mearnsii belongs to Acacia sect. Botrycephalae. It may superficially resemble A. dealbata which is recognised by its pruinose branchlets (but pruinosity often not seen on herbarium specimens), bluish grey foliage with leaflets having a dull lustre on both surfaces (foliage green and leaflets shiny on their upper surface in A. mearnsii; these characters best observed in fresh material) and glabrous pods that are generally wider (i.e. 6-14 mm vs 4-8 mm). Furthermore, in A. dealbata there is only a single gland at the base of the pinnae whereas in A. mearnsii a proportion of the leaves normally have multiple glands between the pinnae. In the past, the name A. mollissima had often been erroneously applied to plants of A. mearnsii. This species is reported to hybridise with A. decurrens, see Philp and Sherry (1949) and Sherry (1971).
The above description is based on information in Boland et al. (1984), Tindale & Kodela (web ref. 27) and Wu and Nielsen (2010), and on specimens at Chinese herbaria GXMG, GXMI, HIB, IBK, IBSC and KUN (e.g. Guangdong: IBSC 605000 [barcode 0159112]; Guangxi: GXMG 5100 [barcode 0068819], GXMI 04257 [barcode 015756] and IBK 125039 [barcode 00067598]; Hunan: HIB 159691 [barcode 0038694]); Yunnan: KUN 0400076 [barcode 0598289]). Acacia mearnsii is described in Chinese by Huang (1985), accompanied by illustration, and by Wu (1988). A detailed botanical profile, based on plants from Australia and accompanied by photographs, is provided at web ref. 27.
Utilisation. Acacia mearnsii is a fast-growing species that has bark which is the world's most important source of vegetable tannin. Additionally, its wood has been used for a range of purposes including construction timber, cabinet making, flooring, paper making and more. Acacia mearnsii is planted in appropriate areas of China for environmental purposes, especially soil and water conservation (Ho and Fang, 1997) and in the past was an important source of natural tannin according to Wang and Fang (1991) and Midgley and Turnbull (2003). Wu and Nielsen (2010) report it as a honey plant. Today there are almost no commercial plantations of A. mearnsii remaining in China and there is no tannin production or significant use of wood from this species (Roger Arnold, pers. comm.). There is a large body of literature concerning A. mearnsii and some useful, comprehensive reviews involving aspects of utilisation, silviculture, biology, ecology etc. are provided in Turnbull (1986), Wiersum (1991), Brown and Ho (1997), Doran and Turnbull (1997), Searle (2000) and Midgley and Turnbull (2003); also Maslin and McDonald (2004).
Etymology. This species is named for Colonel Edgar Alexander Mearns (1856-1916), a doctor in the United States Army who became a naturalist after retiring in 1906. Mearns collected the type material for this Australian species that was described from trees cultivated in Africa, see Hall and Johnson (1993).
Vernacular name. Black Wattle. This is also the standard trade name for this species (Standards Association of Australia, 1983).
32. Acacia melanoxylon R.Br., Hort. Kew. ed. 2 [Aiton], 5: 462 (1813).黑木相思【hēi mù xiāng sī】 (黑木荆、澳洲乌木)
≡ Racosperma melanoxylon (R.Br.) Pedley, Bot. J. Linn. Soc. 92: 240 (1986).
Distinctive features. Unarmed trees with foliage phyllodinous, but bipinnate leaves often long-persistent on young plants and sometimes as reversion foliage on adult plants. Phyllodes narrowly elliptic to slightly oblanceolate, 40-160 mm long, 6-30 mm wide, straight to shallowly recurved, with 3-5 main longitudinal veins that are free to the base and with minor veins forming a prominent net-like reticulum in between. Inflorescences 3-5-headed racemes; heads globose, creamy pale yellow to white. Pods to 150 mm long, 3.5-8 mm wide, strongly curved to openly coiled and often twisted, coriaceous to sub-woody. Seeds twice-encircled by a fleshy, pink to deep red funicle/aril.
Description. Unarmed trees often 10-20 m high with dbh c. 0.5 m, sometimes to 40 m high with dbh to 1.5 m; may spread by root suckers; bipinnate leaves often long-persistent on young plants and sometimes as reversion foliage on adult plants. Branchlets terete, normally glabrous. Phyllodes narrowly elliptic to slightly oblanceolate, 40-160 mm long, 6-30 mm wide, straight to shallowly recurved, ±coriaceous, dark green, glabrous; main longitudinal veins 3-5, remaining free to the base and not confluent with lower margin; minor veins forming a prominent net-like reticulum between main veins; apex obtuse to acute. Inflorescences 3-5-headed racemes 10-40 mm long; peduncles 4-13 mm long, glabrous or puberulous; heads globose, creamy pale yellow to white. Flowers 5-merous; sepals ¾ or more united. Pods to 150 mm long, 3.5-8 mm wide, strongly curved to openly coiled and often twisted, coriaceous to sub-woody, glabrous. Seeds longitudinal in pods, broadly elliptic, 3-5 mm long, glossy, black; funicle/aril fleshy, pink to deep red, twice-encircling seed.
Distribution. A widespread native of eastern Australia (Queensland, New South Wales, Australian Capital Territory, Victoria, Tasmania and South Australia). It is not known when A. melanoxylon first arrived in China but it was included in Zhong Lun Wu et al. (1983) and was initially trialled for utilisation purposes in 1987 (see below); the species was subsequently included in formal and/or informal field trials in Fujian, Guangdong, Guangxi, Guizhou, Hainan, Sichuan, Yunnan and Zhejiang (Wang et al., 2017). In some countries (e.g. South Africa) this species has become an environmental weed which is difficult to control. This is attributed to the species fast growth rate, vigorous regrowth from root suckers and production of high numbers of seeds which are likely to be dispersed by birds on account of having brightly coloured arils (Stirton, 1978). There are no records of A. melanoxylon having become invasive in China.
Taxonomy. This species belongs to Acacia sect. Plurinerves. It is sometimes confused with A. implexa which is recognised by its commonly pruinose branchlets, its phyllode reticulum comprising clearly more elongated vein islands and its white funicle/aril that does not encircle the seed.
The above description of Acacia melanoxylon is based largely on the treatment by Cowan and Maslin (2001b) who also provide notes on variation within Australian native stands of the species, and an illustration. Boland et al. (1984) provided some additional descriptive information. A detailed botanical profile, based on plants from Australia and accompanied by photographs, is given at web ref. 28. Only a single specimen in a Chinese herbarium has been seen (Sichuan: KUN 0159884 [barcode 0598089]).
Utilisation. Acacia melanoxylon is renowned for its valuable timber that is used for veneer, panelling, furniture and stringed instruments; the species is also cultivated in some countries for lumber, fuelwood and amenity plantings. Although the initial performance trials of A. melanoxylon in China (on Hainan Island in 1987) showed poor results, trialling continued through the late 1980s and 1990s (Zhang et al., 2004); Wang et al. (1994) subsequently regarded the species as promising for cultivation in parts of China and limited areas of commercial plantations were established in Fujian and Guangdong in the late 1990s and 2000s (Fangqiu Zhang, pers. comm.). However, today there is almost no plantation resource of this species in China (Roger Arnold, pers. comm.). Acacia melanoxylon produces a valuable timber and would be a good candidate for more extensive genetic and silvicultural research in China (Roger Arnold, pers. comm.). In cultivation A. melanoxylon needs to be managed properly to ensure that it does not become invasive. A comprehensive summary of biological, ecological and silvicultural features, and the utilisation of A. melanoxylon is provided by Doran and Turnbull (1997) and Maslin and McDonald (2004). Much useful information concerning this species is also found in papers presented at various Blackwood Workshops sponsored by the Australian Rural Industries Research and Development Corporation (RIRDC), e.g. Brown (2004), Beadle and Brown (2007).
Etymology. The species name is derived from the Greek melas, melanos (black, dark) and xylon (wood, timber) in reference to the colour of the heart wood of this species. As noted by Hall and Johnson (1993) this heart wood varies from dark brown to almost golden.
Vernacular names. Blackwood (preferred name; this is also the standard trade name for this species (Standards Association of Australia, 1983), Australian Blackwood (China), Black Sally, Black Wattle, and more.
33. Acacia podalyriifolia A.Cunn. ex G.Don, Gen. Hist. 2: 405 (1832), as 'podalyriaefolia'.昆士兰银叶相思【kūn shì lán yín yè xiāng sī】
≡ Racosperma podalyriifolium (A.Cunn. ex G.Don) Pedley, Austro-baileya 2: 354 (1987).
Distinctive features. Unarmed spreading small trees with foliage phyllodinous. Branchlets densely velvety with short, straight, soft, patent hairs. Phyllodes rather short and wide (20-40 mm long, 10-30 mm wide, l:w normally 1.5-2.5), silvery grey to glaucous, indumentum as on branchlets but sparser, 1-veined, mucronate. Inflorescences showy, arranged in racemes (mostly 20-40 mm long); peduncles short (4-5 mm), indumentum similar to branchlets; heads globose, showy. Pods wide (15-23 mm), thinly coriaceous, velvety at least when young.
Description. Unarmed, spreading trees 3-7 m high. Branchlets pruinose (but pruinosity not always visible on herbarium specimens), densely ±velvety with short, straight, soft, white, patent hairs (rarely glabrous in Australia). Phyllodes elliptic to widely elliptic, sub-circular, oblong-elliptic or ovate, 20-40 mm long, 10-30 mm wide (to 50 mm in Australia), l:w normally 1.5-2.5, mucronate, thin-textured, silvery grey to glaucous (rarely green in Australia), with indumentum as on branchlets but sparser, midrib slightly excentric, finely penniveined. Gland normally inconspicuous, situated on upper margin of phyllode 7-18 mm above pulvinus. Inflorescences comprising numerous pedunculate heads arranged in racemes that exceed the phyllodes (mostly 20-40 mm long), axes with indumentum similar to branchlets; peduncles 4-5 mm long (to 10 mm in Australia), with indumentum similar to branchlets; heads showy, fragrant, globose, 15-30-flowered, 5-6 mm diam. at anthesis when dry, bright light golden. Flowers 5-merous; calyx gamosepalous. Pods to 120 mm long, 15-23 mm wide, thinly coriaceous, flat, velvety and pruinose at least when young, sometimes glabrous with age, dehiscing unilaterally, margins often undulate. Seeds longitudinal in pods, oblong, 6-7.5 mm long, dull, black; aril clavate, white.
Distribution. A native of eastern Australia (Queensland and New South Wales) and naturalised in other parts of the country (web ref. 30); it has also been introduced for ornamental purposes into many countries. The global distribution of this species is documented at the WorldWideWattle website (web ref. 29). At present A. podalyriifolia is seemingly not common in China but has been recorded for Guangdong (Ye and Peng, 2006) and Hong Kong (Zhu et al., 2015); Wang et al. (2017) consider this species would be suitable for growing in Hainan, Guangdong, Guangxi, Fujian, Zhejiang, Yunnan. It is not known when A. podalyriifolia was first introduced to China; it was not included in either Huang (1985) or Wu (1988) but is included in Zhu et al. (2015).
Taxonomy. Acacia podalyriifolia belongs to Acacia sect. Acacia.
The above description is based on information in Maslin (web ref. 30) and on specimens at Chinese herbaria GXMG and IBSC (e.g. Guangdong: IBSC 748868 [barcode 0751803]; Guangxi: GXMG 12302 [barcode 0068764]). Further information concerning the taxonomy (including full synonymy) of this species, and an illustration, is provided in Maslin (web ref. 30). A detailed botanical profile, based on plants from Australia and accompanied by photographs, is provided at web ref. 30.
Utilisation. Acacia podalyriifolia is a fast-growing species that is highly decorative on account of its masses of bright golden, perfumed heads and normally silvery grey to glaucous foliage. In China it is used in amenity plantings and for horticulture in some areas (see above) and has the potential for greater uses (Huoran Wang, pers. comm.). Comprehensive information, including details of biology, ecology, silviculture, utilisation, etc. is provided for this species by Turnbull (1986).
Etymology. The species name is derived from the name Podalyria (a genus of Leguminosae from southern Africa) and the Latin folium (a leaf). Presumably applied because of the resemblance of the phyllodes to the leaves of a species of Podalyria (Hall and Johnson, 1993).
Vernacular names. Queensland Silver Wattle (preferred name), Mt Morgan Wattle.
4.4.2. ACACIELLA Britton & Rose, N. Amer. Fl. 23: 96 (1928).美洲相思属【měi zhōu xiāng sī shǔ】(新拟)TYPE: Acaciella villosa (Swartz) Britton & Rose.
Shrubs characterised by: prickles and spinescent stipules absent; leaves bipinnate, glands absent from both petiole and rachis; flowers distinctly pedicellate; pods flat and thin-textured.
Fifteen species globally, all confined to the Americas (web ref. 2); one species is introduced in China and is described below and included in the Key to species above).
34. Acaciella glauca (L.) L.Rico in L. Rico Arce & S. Bachman, Anales Jard. Bot. Madrid 63(2): 210 (2006).灰美洲相思【huī měi zhōu xiāng sī】(新拟)
≡ Mimosa glauca L., Sp. Pl. 1: 520 (1753). ≡ Acacia glauca (L.) Moench, Meth. Pl.: 466 (1794).
Distinctive features. Unarmed shrubs with bipinnate foliage. Pinnae 5-11 pairs. Leaflets mostly 2.5-6 mm long and 1.5-3 mm wide. Leaf glands absent. Inflorescences comprising 2-6 pedunculate heads in axils of uppermost leaves, sometimes forming false racemes at ends of branchlets. Flower heads globose or obloid heads. Flowers distinctly pedicellate, the pedicel slender, 1-2 mm long and with an abscission joint at union with perianth so that often only pedicels seen on receptacle following anthesis. Pods 10-20 mm.
Description. Unarmed shrubs 2-8 m tall. Branchlets terete, obscurely ribbed, glabrous or sometimes sub-glabrous. Stipules caducous, ±narrowly triangular to lanceolate, c. 2 mm long, scarious. Leaves bipinnate; pinnae 5-11 pairs, 15-60 mm long; para-phyllidia linear-triangular, c. 0.5 mm long; petiole 15-45 mm long; rachis (20-) 40-75 mm long. Leaflets 10-30 pairs, oblong to narrowly oblong or slightly obovate, 2.5-6 (-8) mm long, 1.5-3 mm wide, opposite, discolorous (darkest above), flat, glabrous or occasionally with few appressed hairs on lower surface, margins often appressed-ciliolate; apex obtuse, apiculate; base unequal with a rounded angle on lower edge more pronounced that upper edge, petiolule excentric and distinct; main vein central or sub-central, visible but not pronounced; lateral veins obscure or not visible, bifurcating near margin and sometimes forming open reticulum. Glands absent from petiole, rachis and rachilla. Inflorescences comprising 2-6 pedunculate heads in axils of upper-most leaves, sometimes forming false racemes at ends of branchlets; peduncles 7-15 mm long, slender, glabrous or sparsely appressed-hairy; heads globose or obloid, large (10-15 mm long, 10-13 mm wide when dry), white to cream but becoming yellowish when dry, the flowers not densely arranged in the heads, slightly fragrant. Flowers 5-merous, distinctly pedicellate, the pedicel slender, 1-2 mm long and with abscission joint at union with perianth so often only pedicels remaining on receptacle following anthesis; calyx very short (¼-⅕ length of corolla), gamosepalous, cupulate, sinuate-toothed, glabrous, veinless; petals glabrous and veinless. Pods oblong, 30-80 long, 10-20 mm wide, chartaceous, straight, flat but obviously convex over seeds along midline, brown to reddish brown, glabrous, finely reticulate-veined, stipitate by a slender stipe 4-5 mm long. Seeds transverse to oblique in pods, black.
Distribution. A native of the eastern Caribbean and northern South America (Venezuela) while cultivated as an ornamental in parts of Asia and Australia; the WorldWideWattle website (web ref. 31) provides details for this distribution. In China Acaciella glauca is recorded for Fujian and Guangdong by Wu and Nielsen (2010) and we have also seen specimens from Guanxi, Hainan and Yunnan (cited below under Taxonomy). It is not known when it was first introduced to China, but it was included (as Acacia glauca) in Huang (1985).
Taxonomy. Although Acacia (Acaciella) villosa (Sw.) Willd. was listed as a synonym of Acacia (Acaciella) glauca by Wu (1988), Wu and Nielsen (2010) and Zhu et al. (2015), it is treated as a distinct species by Rico Arce and Bachman (2006). These authors remarked that Acaciella glauca has frequently been introduced to various countries for experimental agroforestry and ornamental purposes, but they did not nominate the countries of introduction. Acaciella villosa (Sw.) Britton & Rose is not known to occur in China. The treatment by Wu and Nielsen (2010) described the branchlets of Acacia glauca as pubescent, however, based on material we have seen the branchlets of this species are only occasionally hairy, and in these cases the indumentum is best described as sub-glabrous.
The above description is based on specimens in Chinese herbaria GXMG, HITBC, IBSC (e.g. Guangdong: IBSC 587923 [barcode 0159041]; Guangxi: GXMG 8035 [barcode 0068767]; Hainan: IBSC 309883 [barcode 0159043]. Yunnan: IBSC 680583 [barcode 0159046]) and at BKF, PERTH and SING, supplemented by infor-mation in Wu and Nielsen (2010). Acaciella glauca is described in Chinese (as Acacia glauca) by Huang (1985) and Wu (1988) which is accompanied by illustration.
Utilisation. As already noted this species is cultivated as an ornamental in various places. Both Huang (1985) and Wu (1988) considered it suitable as a source of fuelwood on account of its fast growth rate and sprouting ability. Acaciella glauca is a host plant of the lac insect (Wu and Nielsen, 2010). In Malaysia the species has been tried as a cover plant in tea plantations (Nielsen, 1992). In Australia it was planted during the 1970s and 1980s to assess its potential as cattle forage, but the trials concluded that it was of limited value for that purpose, and that its invasive potential probably outweighed any benefits likely to be gained (web ref. 32).
Etymology. The species name is derived from the Latin glaucus (glaucous, covered with a fine bloom of the colour of a Cabbage leaf). This term was used by Jacob Breyne whose work was cited by Linnaeus (1753) in his protologue of M. glauca; the term presumably refers to the under surface of the leaflets.
Vernacular name. Grey Acaciella (following the Chinese common name that is proposed above). Redwood is the common name applied to this species in Australia (web ref. 32).
4.4.3. SENEGALIA Raf.Two species introduced in China (both belonging to Senegalia sect. Senegalia) but S. senegal is rare (as already noted) and is therefore not described below (but is included in the Key to species above). Details of the 21 native species of Senegalia in China (all belonging to Senegalia sect. Monacanthea) are described under Indigenous species above.
35. Senegalia catechu (L.f.) P.J.H.Hurter & Mabb., Mabberley's Pl.-Book ed. 3, 1020 (2008).儿茶【ér chá】
= Mimosa catechu L. f., Suppl. Pl. 439 (1782 [dated '1781']). ≡ A. catechu (L.f.) Willd., Sp. Pl., ed. 4, 4(2): 1079 (1806).
=?Acacia wallichiana DC., Prodr. 2: 458 (1825). ≡ A. catechu var wallichiana (DC.) P.C.Huang in W. Cheng, Sylva Sinica 2: 1259, Fig. 579: 5-6 (1985).
= Mimosa catechuoides Roxb., Fl. Ind. (Roxburgh) 2: 562 (1832) ≡ Acacia catechuoides (Roxb.) Benth., London J. Bot. 1: 510 (1842).
Additional synonymy (not relevant to China): Deshpande et al. (2019).
Distinctive features. Spreading trees. Prickles in pairs at nodes, often absent from herbarium specimens, recurved. Pinnae 9-25 pairs, relatively short (15-40 mm long). Leaflets 3-5 mm long, 0.5-1 mm wide, apex obtuse. Petiole gland normally situated near or above middle of petiole. Inflorescences comprising 1-3 pedun-culate spikes initiated within axils of young leaves on new growth. Calyx ⅕-
Description. Spreading trees 6-10 m tall. Bark brown. Branchlets terete, obscurely veined, glabrous or (especially at extremities) sparsely hairy. Prickles in pairs at the nodes, often absent from herbarium specimens, (-1-) 2-6 mm long, c. 1.5 mm wide at the splayed base, rather robust, shallowly to obviously recurved, laterally flattened when young, turgid when mature. Stipules triangular, 1.5-2 mm long, c. 0.8 mm wide. Leaves bipinnate; pinnae 9-25 pairs, 15-40 mm long, the axes slender; petiole 10-45 mm long; rachis 50-140 mm long. Leaflets 30-50 pairs, narrowly oblong, 3-5 mm long, 0.5-1 mm wide, straight, discolorous, glabrous except appressed-ciliate; apex obtuse; base unequal, with a lobed angle on lower edge only, petiolule clearly excentric; main vein not prominent, centric; lateral veins absent or few and obscure. Glands:petiole gland-normally situated near or above middle of petiole (sometimes at or near insertion of lowermost pair of pinnae) (10-) 15-35 mm above leaf base, oblong to oblong-elliptic, 2-3.5 mm long, 1-1.5 mm wide, evident but not overly prominent, depressed (not strongly raised), oblong to elliptic, upper surface with a normally shallow depression surrounded by a low rim; rachis glands - situated at base of uppermost 1or 2 pairs of pinnae, 0.7-1 mm long; rachilla glands-absent. Inflorescences comprising 1-3 pedunculate spikes initiated within axils of young leaves on new growth; peduncles (8-) 10-20 mm long when in flower, to 30 mm long in pod, glabrous to sub-densely short-pilose; spikes (25-) 35-65 mm long and 10-12 mm wide at anthesis when dry, the flowers rather loosely arranged (receptacle clearly visible between adjacent flowers in mature buds), white or pale yellow. Flowers 5emerous, sessile; calyx ⅕-
Distribution. A native of the Indian subcontinent (where it is widespread in Bangladesh, Bhutan, India, Nepal and Pakistan) and Myanmar. Senegalia catechu is introduced in East Asia (China, including Taiwan; Ryuku Islands), Southeast Asia (Indonesia, Philippines and Vietnam) and the Indian Ocean island of Mauritius. The WorldWideWattle website (web ref. 33) provides data sources documenting this distribution. It is possible that some of the introduced records, including those in China, may ultimately prove to be Senegalia chundra (Roxb. ex Röttler) Maslin (see discussion below under Taxonomy). It is not known when Senegalia catechu was first introduced to China but Zhong Lun Wu et al. (1983) said that [at that time] it had been cultivated for more than 60 years in southern Yunnan; however, it was not listed for China in Handel-Mazzetti (1933). Today, Senegalia catechu is cultivated in Fujian, Guangdong, Guangxi, Hainan, Taiwan, Yunnan and Zhejiang (Wu and Nielsen, 2010). Although Wu (1988), followed by Wu and Nielsen (2010), regarded this species as native in Yunnan (Lincang, Xishuangbanna) we consider it more likely to be introduced in that Province.
Taxonomy. As discussed by Kshirsagar (2012), Acacia catechu has had a complex nomenclatural history involving (among other species) A. chundra Roxb. ex Röttler (syn. A. catechu var. sundra (Roxb.) Kurz). In that work (which was based on specimens from India) A. catechu and A. chundra were treated as separate species that were most reliably distinguished by certain flower characters: in A. catechu the calyx and corolla were described as densely hairy, with the corolla being twice as long as the calyx; in A. chundra the calyx and corolla were described as glabrous, with the corolla being 2 or 3 times as long as the calyx. Today both Senegalia catechu and S. chundra are recognised in India where they are native (Deshpande et al., 2019). However, plants introduced in China are very variable with respect to the above-mentioned flower characters, and many cannot be confidently assigned to one species or the other. Consequently, we have here adopted a conservative approach by recognising only Senegalia catechu for China, which is the name (as A. catechu) under which the species has long been known. Further study of Senegalia catechu and S. chundra in their native habitats would be desirable to re-assess their taxonomic status.
This species belongs to Senegalia sect. Senegalia (because prickles are located at the nodes). All indigenous species of Senegalia in China belong to Senegalia sect. Monacanthea (because prickles are internodal).
The description above is based on specimens in Chinese herbaria HITBC, IBK, IBSC, KUN and YUKU (e.g. Hainan: IBSC 449743 [barcode 0158681]; Guangxi: IBK 131862 [barcode 00067553]; Yunnan: KUN 0159839 [barcode 0598037]). Senegalia catechu is described in Chinese and is illustrated in Huang (1985), as both A. catechu and A. catechu var. wallichiana, and Wu (1988), as A. catechu.
Utilisation. Extracts from this plant are used medicinally for a range of ailments, its tannin extract for leather tanning and as a dye, as well as its wood for building construction and other purposes, fide Huang (1985) and Wu (1988).
Etymology. The species name is taken from the word 'Catechu' which appears in the protologue; the word refers to the extract that is derived from this species.
Vernacular names. Catechu (preferred name), Cutch Tree, and more.
4.4.4. VACHELLIA Wight & Arn., Prodr. 272 (1834).鸭皂树属【yā zào shù shǔ】(新拟)TYPE: Vachellia farnesiana (L.) Wight & Arn.
Shrubs or small trees characterised by: stipules spinescent; prickles absent; leaves bipinnate, fascicled in nodose clusters on mature branches (single at nodes on young growth); petiole glands normally present; inflorescences pedunculate heads or spikes, normally not in racemes or panicles; peduncles with involucre of bracts (hidden by heads at anthesis in V. farnesiana); pods variable.
There are 164 species (distributed pan-tropically: see web ref. 2) with two introduced in China, but Vachellia nilotica is rare (as already noted) and is therefore not described below (but is included in Key to species above). Prior to the retypification of Acacia (see discussion above under Introduction) the species of this genus were included within the former Acacia subg. Acacia.
36. Vachellia farnesiana (L.) Wight & Arn., Prodr. Fl. Ind. Orient. 1: 272 (1834).鸭皂树【yā zào shù】
≡ Mimosa farnesiana L., Sp. Pl. 1: 521 (1753). ≡ A. farnesiana (L.) Willd., Sp. Pl. 4: 1083 (1806). ≡ Poponax farnesiana (L.) Raf., Sylva Tellur. 118 (1838).
Additional synonymy (not relevant to China): Seigler and Ebinger (2005).
Distinctive features. Shrubs or small trees with bipinnate foliage. Branchlets with obvious lenticels. Prickles absent. Stipules spinose, paired at nodes, mostly 5-20 mm long, straight. Leaves bipinnate, 2-3 in brachyblastic clusters at mature nodes but single at nodes on young growth. Pinnae mostly 4-6 pairs, 10-35 mm long. Petiole gland situated near or above middle of petiole, not prominent. Inflorescences comprising 1-4 pedunculate heads within axil of leaves; involucre situated at or near apex of peduncle (obscured by heads, especially at anthesis); heads large (9-15 mm diam. when dry), golden yellow. Pods terete to compressed, ±not constricted between seed, scarcely dehiscent, very hard textured (±woody). Seed biseriate, seated in discrete chambers within pith; funicle rudimentary or absent, exarillate.
Description. Shrubs or small trees 2-4 m tall. Branchlets flexuose, terete, glabrous, marked with obvious lenticels. Prickles absent. Stipules spinose, paired at the nodes and about equal in length, (3-) 5-20 (-30) mm long, rigid, straight, subulate, light brown or ivory-coloured. Leaves bipinnate, 2-3 in brachyblastic clusters at mature nodes but single at nodes on young growth; pinnae (3-) 4-6 (-8) pairs, 10-35 mm long; petiole 5-20 mm long; rachis 15-50 (-60) mm long. Leaflets 10-20 pairs, narrowly oblong, (2-) 3-6 mm long, 1-1.5 mm wide, glabrous, dull green; apex obtuse; base ±asymmetric by a more obviously rounded angle on lower edge, petiolule sub-central; main vein sub-central; lateral veins obscure or not visible. Glands: petiole gland-situated near or above the middle of the petiole 4-12 mm above leaf base and 3-8 mm below the proximal pair of pinnae, circular to oblong, small (0.2-0.8 mm long), depressed cupular, sessile; rachis gland-sometimes with a small gland at base of distal pair of pinnae. Inflorescences comprising 1-4 pedunculate heads within axil of leaves; heads globose, 9-15 mm diam. when dry, golden yellow, sweetly fragrant; peduncles 8-40 (-50) mm long, glabrous to densely hairy; involucre situated at or near apex of peduncle, obscured by heads (especially at anthesis). Flowers 5-merous; calyx c. 
Distribution. A native of the Americas but widely distributed, and often naturalised, in many areas of the globe, including: Africa, Australia, East Asia, South Asia (Indian subcontinent), Southeast Asia, West Asia, southern Europe, Indian Ocean islands (e.g. Madagascar, Mascarenes and Seychelles) and Pacific Ocean Islands (e.g. Hawaii and Nauru). The WorldWideWattle website (web ref. 34 provides reference details for these distributions. Li et al. (2015) reported that this species was introduced to Taiwan by the Dutch East Company in the 17th century. We do not know when it was first introduced to mainland China, but Bentham (1861) recorded it (as Acacia farnesiana) for Hong Kong. Vachellia farnesiana is now widespread on mainland China where occurs in Fujian, Guangxi, Guangdong, Guizhou, Hainan, Hong Kong, Hunan, Sichuan, Yunnan and Zhejiang. In Yunnan it is widely cultivated (and has become naturalised in places) in tropical areas and dry, hot valleys (Sun, 2006).
Taxonomy. Seigler and Ebinger (2005), based on the work of Ebinger et al. (2002), recognised three varieties within this species, namely, var. farnesiana, var. minuta (M.E.Jones) Seigler & Ebinger and var. pinetorum (F.J.Herm.) Seigler & Ebinger. It is the typical variety that occurs in China.
The above description is based on information in Wu and Nielsen (2010) and on specimens in Chinese herbaria GXMG, HITBC, IBK, IBSC and KUN (e.g. Guangdong: IBSC 680578 [barcode 0158983]; Guangxi: GXMG, 19643 & 20146 [no barcodes given] and IBK 141838 [barcode 00067595]; Hainan: KUN 0159953 [barcode 0598247]; Yunnan: KUN 0159977 [barcode 0598223]). This species is described (as A. farnesiana) in Chinese and is illustrated in Huang (1985) and Wu (1988). A detailed botanical profile, based on plants from Australia and accompanied by photographs, is provided at web ref. 35.
Utilisation. Because of its spiny and often brambly nature, Vachellia farnesiana can be grown as a hedge plant. The tannin and gum produced by this species can also be used in the manufacture of black dye and for medicinal purposes; the fragrant flowers can be used to manufacture perfume, see Wu (1982), Huang (1985), Wu (1988), Mabberley (2008) and Ye et al. (2013). In Australia, the foliage and young, green pods of V. farnesiana are palatable to cattle and sheep, but the species is potentially invasive in grassland areas.
Etymology. The species name commemorates the illustrious Italian family Farnese at whose villa near Rome this species was cultivated and from which it was originally described (Hall and Johnson, 1993).
Vernacular names. Duck soap Tree (preferred name for China, origin unknown but used by Sun (2006) for A. farnesiana), Cassie Flower, Sweet Acacia, Farnesian Acacia, and many more (Kodela and Tindale, 2001b).
Conflict of interestThere is no known Conflict of Interest in this paper.
AcknowledgementsMuch of this study was conducted at KUN (Herbarium, Kunming Institute of Botany, Kunming) and the first author in particular expresses his thanks to Dr Ende Liu and Dr Tao Deng for facilitating visits to that herbarium, and to Mrs Jinghua Wang for day to day assistance with the collections. We also extend our thanks for the hospitality and assistance provided by management and staff at other Chinese herbaria that were visited, namely: GXMG (Guangxi Medicinal Botanic Garden, Nanning), GXMI (Guangxi Institute of Traditional Medical and Pharmaceutical Sciences, Nanning), HIB (Wuhan Institute of Botany, Wuhan), HITBC (Xishuangbanna Tropical Botanical Garden, Xishuangbanna), IBK (Guangxi Institute of Botany, Guilin), IBSC (South China Botanical Garden, Guangzhou), SWFC (South West Forestry College, Kunming), SYS (Zhongshan University, Guangzhou) and YUKU (Yunnan University, Kunming). Similarly, thanks are extended to management and staff at the following non-Chinese herbaria where work was also conducted, primarily by the first author: BK (Bangkok Herbarium, Bangkok, Thailand), BKF (Bangkok Forestry Herbarium, National Park, Wildlife and Plant Conservation Department, Bangkok, Thailand), BO (Bogor Herbarium, Research Centre for Biology, Cibinong, Indonesia), CAL (Calcutta Herbarium, Botanical Survey of India, Howrah, India), CMU (Chiang Mai University Herbarium, Chiang Mai, Thailand: the late James F. Maxwell is especially thanked), HN (Hanoi Herbarium, Vietnam Academy of Science and Technology, Hanoi, Vietnam), HNU (Hanoi University Herbarium, University of Science, Hanoi, Vietnam), KEP (Forest Research Institute Malaysia, Kuala Lumpur, Malaysia), PERTH (Western Australian Herbarium, Department of Biodiversity, Conservation and Attractions, Perth, Western Australia), SING (Herbarium, Singapore Botanic Gardens, Singapore), VNM (Institute of Tropical Biology, Ho Chi Minh City, Vietnam). We also thank the European and American herbaria (that are listed under Materials & Methods) for making available via the web specimen details and digital images that were consulted in connection with this study.
During this five year study many individuals generously provided specialised assistance that greatly facilitated our work; we therefore gratefully acknowledge and thank following (mostly arranged alphabetically by surname, and without titles): We are especially indebted to Roger Arnold (China Eucalypt Research Centre, Zhanjiang) for his comprehensive and incisive comments concerning the cultivation status of Australian Acacias in China today; Anup Deshpande (Goa University, India) for comments on various species of Senegalia in India; Christopher Fraser-Jenkins (Nepal) for information relevant to the typification Acacia arrophula and Acacia oxyphylla; Alex George (Perth, Australia) for his assistance with Latin translations, the formulation of new species epithets and interpreting etymology of species names herein; Phillip Kodela and Australian Biological Resources for permission to cite as web reference the comprehensive botanical profiles and photographs of Australian species of Acacia that are presented on the Flora of Australia Online website; Charles (Charlie) Jarvis and Steve Cafferty (Natural History Museum, London) for comments and assistance concerning Hermann types of Mimosa caesia and M. pennata at BM; Jer-Ming Hu (National Taiwan University) for comments on the occurrence of Acacia confusa in Taiwan; Hock Keong Lua (National Biodiversity Centre, Singapore) for field assistance in collecting Senegalia pennata subsp. insuavis and S. rugata in Singapore; Serena Lee (Singapore Botanic Gardens) and especially Kimberly Maslin (Australia) for assistance with Photoshop® Phan Ke Loc (Hanoi University Herbarium) for donation of isotype specimens of Senegalia orientalis to Chinese herbaria; Yee Wen Low (Singapore Botanic Gardens) for examining the type of Acacia megaladena var. garrettii on our behalf at K; David Mabberley (Australia), Nicholas Turland (Berlin) and Xiangyun Zhu (Herbarium PE, Beijing) for assistance with some nomenclatural matters, especially those relating to the name Acacia cavaleriei H. Lev.; Stephen (Steve) Midgley, Maurice McDonald, Suzette Searle and Lex Thomson (formerly Australian Tree Seed Centre, CSIRO, Canberra) for comments concerning Acacia species cultivated in China; Fachang Ning, Bin Yang and Xueyuan Yang (China) for their assistance during our field work; Odile Poncy (Muséum National d'istoire Naturalle, Paris) for undertaking a search at P for the type of Acacia teniana; V.P. Prasad (Herbarium CAL, Kolkata) and Lalji Singh (BSI Andaman & Nicobar Regional Centre, Port Blair) for assistance with interpreting the locality information on the lectotype of Acacia pseudointsia var. ambigua (basionym of Senegalia andamanica); Rachun Pooma (Forestry Herbarium, Bangkok) for assistance with interpretation of some Thailand collecting localities; Akiko Shimizu (Herbarium TI, Tokyo) and Yu-Hsin Tseng (Taiwan) for digital images of the types of Acacia hainanensis at TI and TAIF respectively; Mark Spencer and Jacek Wajer (Natural History Museum, London) for searching at LINN and BM respec-tively for the type of Acacia arrophula; Tim Utteridge (Royal Botanic Gardens, Kew) for information concerning specimens in Wallich Herbarium at Kew, especially Acacia oxyphylla; Robert Vogt (Botanischer Garten und Botanisches Museum Berlin-Dahlem, Berlin) for undertaking a search at B for the type of Acacia teniana and also for confirming H. Harms handwriting; the late Jan-Frits (JeF) Veldkamp (Naturalis, Leiden) for advice on several complex nomenclature matters, especially those relating to synonymy under Senegalia rugata; Huoran Wang (Chinese Academy of Forestry, Beijing) for considerable assistance and advice regarding Australian Acacia species introduced in China; Marie-Helene Weech (Digital Collections Unit, RBG Kew) for information relating to the Wallich Cata-logue and specimens at K; Annette Wilson (Perth, Australia) for editorial comments and critical review of an early draft of the manuscript and for assistance with some nomenclatural matters; Waiwai Hove and Joshua Yang (both Singapore) for undertaking the artwork included herein (funded by Singapore Herbarium); Fangqiu Zhang (Guangdong Forestry Academy, Guangzhou) for information concerning the location of commercial plantings of Australian Acacia species in China. Finally, we are very grateful for the excellent comments on our manuscript provided by three anonymous referees and also the editorial staff of Plant Diversity.
Ali S.I., Faruqi S.A., 1969. A taxonomic study of Acacia nilotica complex in W. Pakistan. Pakistan J. Bot, 1: 1-8. |
Anderson, J., 1878. Anatomical and Zoological Researches: Comprising an Account of the Zoological Results of the Two Expeditions to Western Yunnan in 1868 and 1875; and a Monograph of the Two Cetacean Genera. Platanista and Orcella.B. Quaritch, London.
|
Arnold, R., 2018. Acacia mangium. In: Thomson, L., et al. (Eds.), Trees for Life in Oceania: Conservation and Utilisation of Genetic Diversity. Australian Centre for International Agricultural Research (ACIAR), Canberra, pp. 42-46.
|
Bai, J.Y., Zhang, F.Q., Chen, Z.X., 1998. Progress in silviculture, improvement and market prospects for tropical acacias in southern China. In: Turnbull, J.W., et al.(Eds.), Recent Developments in Acacia Planting. Australian Centre for International Agricultural Research, Hanoi, Vietnam, pp. 332-334.
|
Baker, J.G., 1878. Acacia. In: Hooker, J.D. (Ed.), Flora of British India. L. Reeve, London, pp. 292-298.
|
Bao S.Y., Mao P.Y., Xiu Y.S., 1998. A Brief History of Plant Collection in Yunnan. Beijing: Science and Technology of China Press.
|
Barros M.J.F., Morim M.P., 2014. Senegalia (Leguminosae, Mimosoideae) from the Atlantic domain, Brazil. Syst. Bot, 39: 452-477. DOI:10.1600/036364413X680807 |
Beadle, C.L., Brown, A.G., 2007. Acacia utilisation and management-Adding value.In: Proceedings of a Blackwood Industry Group (BIG) Workshop, Victoria, 26-29 April 2006. Rural Industries Research and Development Corporation, Canberra.
|
Beentje H., 2016. The Kew Plant Glossary. second ed. London: Kew Publishing.
|
Bentham G., 1842. Notes on Mimoseae, with a synopsis of species. London J. Bot, 1: 318-528. |
Bentham G., 1861. Flora Hongkongensis. London: Lovell Reeve.
|
Bentham G., 1875. Revision of the suborder Mimoseae. Trans. Linn. Soc. London, 30: 335-664. DOI:10.1111/j.1096-3642.1875.tb00005.x |
Boatwright J.S., Maurin O., van der Bank M., 2015. Phylogenetic position of Madagascan species of Acacia s. l. And new combinations in Senegalia and Vachellia (Fabaceae, Mimosoideae, Acacieae). Bot. J. Linn. Soc, 179: 288-294. DOI:10.1111/boj.12320 |
Boland D.J., Brooker M.I.H., Chippendale G.M., Hall N., Hyland B.P.M., Johnston R.D., Kleinig D.A., Turner J.D., 1984. Forest Trees of Australia. 4th ed. Melbourne: Thomas Nelson Australia & CSIRO.
|
Bouchenak-Khelladi Y., Maurin O., Hurter J., van der Bank M., 2010. The evolutionary history and biogeography of Mimosoideae (Leguminosae):an emphasis on African acacias. Molec. Phylogen. Evol, 57: 495-508. DOI:10.1016/j.ympev.2010.07.019 |
Brenan J.P.M., Exell A.W., 1957. Acacia pennata (L.) Willd. and its relatives in tropical Africa. Bol. Soc. Brot, 31: 99-140. |
Bretschneider E., 1898a. History of European Botanical Discoveries in China, vol. 1. London: Sampson Low, Marston & Co. Ltd.
|
Bretschneider E., 1898b. History of European Botanical Discoveries in China, vol. 2. London: Sampson Low, Marston & Co. Ltd.
|
Britton, N.L., Rose, J.N., 1928. Mimosaceae. In: Britton, N.L., et al. (Eds.), North American Flora. New York Botanical Gardens, New York, pp. 1-194.
|
Brown, A.G., 2004. Blackwood management: Learning from New Zealand. In: Proceedings of an International Workshop Rotorua, New Zealand, 22 November 2002. Rural Industries Research and Development Corporation, Canberra.
|
Brown, A.G., Ho, C.K. (Eds.), 1997. Black Wattle and its Utilisation. Rural Industries Research and Development Corporation, Canberra.
|
Chakrabarty T., Gangopadhyay M., 1996. The genus Acacia P. Miller (Leguminosae:Mimosoideae) in India. J. Econ. Taxon. Bot, 20: 599-633. |
Chun W.Y., How F.C., 1958. Contributions to the flora of south China. Acta Phytotax.Sin, 7: 1-90. |
Cowan, R.S., Maslin, B.R., 1995. Acacia miscellany 10. New taxa and notes on previously described taxa of Acacia, mostly section Juliflorae (Leguminosae: Mimosoideae) in Western Australia. Nuytsia 10, 15-62. https://florabase.dpaw.wa.gov.au/nuytsia/article/223.
|
Cowan, R.S., Maslin, B.R., 2001a. Acacia implexa. In: Orchard, A.E., Wilson, A.J.G.(Eds.), Flora of Australia. Australian Biological Resources Study & CSIRO Publishing, Melbourne, p. 142.
|
Cowan, R.S., Maslin, B.R., 2001b. Acacia melanoxylon. In: Orchard, A.E., Wilson, A.J.G. (Eds.), Flora of Australia. Australian Biological Resources Study & CSIRO Publishing, Melbourne, pp. 141-142.
|
Craib W.G., 1928. Florae Siamensis Enumeratio. Bangkok: Siam Society.
|
Deshpande A., Krishnan S., Janarthanam M., Maslin B., 2019. Annotated checklist of Senegalia and Vachellia (Leguminosae:Mimosoideae) for the Indian subcontinent. Nordic J. Bot, 37: 1-20. DOI:10.1111/njb.02047 |
Don, D., 1824 Dec. '1825'. Prodromus Florae Nepalensis. J. Gale, London.
|
Doran, J.C., Turnbull, J.W., 1997. Australian Trees and Shrubs: Species for Land Rehabilitation and Farm Planting in the Tropics. ACIAR Monograph 24.Australian Centre for International Agricultural Research, Canberra.
|
Ebinger J.E., Seigler D.S., Clarke H.D., 2002. Notes on segregates of Acacia farnesiana(L.) Willd. (Fabaceae:Mimosoideae) and related species in north America. Southwest. Nat, 47: 86-147. DOI:10.2307/3672805 |
Forbes F.B., Hemsley W.B., 1887. An enumeration of all the plants known from China proper, Formosa, Hainan, Corea, the Luchu archipelago, and the island of Hongkong, together with their distribution and synonymy. J. Linn. Soc. Bot, 23: 1-489. |
Franchet A., 1890. Plantae Delavayanae. Paris: Paul Klincksieck.
|
Fraser-Jenkins, C.R., 2006. The First Botanical Collectors in Nepal. The Fern Collections of Hamilton, Gardner and Wallich. Bishen Singh Mahendra Pal Singh, Dehra Dun, India.
|
Gamble, J.S., 1918. XXXIII. Miscellaneous notes. J.H. Lace, C.I.E., F.L.S. Bull. Misc.Inform. Kew. 1918, 341. https://www.jstor.org/stable/4107427.
|
Griffin A.R., Midgley S.J., Bush D., Cunningham P.J., Rinaudo A.T., 2011. Global uses of Australian acacias-recent trends and future prospects. Divers. Distrib, 17: 837-847. DOI:10.1111/j.1472-4642.2011.00814.x |
Guinet, P., 1969. Les Mimosacées. Etude de palynologie fondamentale, correlations, evolution. Trav. Sect. Sci. Techn. Inst. Franç. Pondichéry 9, 1-293.
|
Hall, N., Johnson, L.A.S., 1993. The Names of Acacias of New South Wales with a Guide to Pronunciation of Botanical Names. Royal Botanic Gardens Sydney, Sydney.
|
Handel-Mazzetti, H., 1933. Mimosaceae. In: Brotherus, V.F., et al. (Eds.), Symbolae sinicae: botanische Ergebnisse der Expedition der Akademie der Wissenschaften in Wein nach Südwest-China, 1914-1918. Julius Springer, Vienna, pp. 538-539.
|
Harms H., 1921. Acacia teniana. Repert. Spec. Nov. Regni Veg, 17: 133. |
Harwood C.E., Haines M.W., Williams E.R., 1993. Early growth of Acacia crassicarpa in a seedling seed orchard at Melville Island. Forest Genet. Resources Inform, 21: 46-53. |
Hayata, B., 1913. Icones Plantarum Formosanarum. Bureau of Productive Industry, Government of Formosa, Taihoku.
|
Henry A., 1896. A list of plants from Formosa:with some preliminary remarks on the geography, nature of the flora, and economic botany of the island. Trans.Asiat. Soc. Japan, 24(Suppl. l): 1-118. |
Ho, C.K., Fang, Y.L., 1997. Development of black wattle Acacia mearnsii De Wild plantations in China. In: Brown, A.G., Ho, C.K. (Eds.), Black Wattle and its Utilisation. Rural Industries Research and Development Corporation, Canberra, pp. 83-88.
|
Huang, P.C., 1985. Acacieae. In: Zheng, W.J. (Ed.), Silva Sinica. China Forestry Publishing House, Beijing, pp. 1248-1263.
|
Huang, T.C., Hsieh, C.F., Huang, S.F., 1991. A catalogue of the TAI herbarium, National Taiwan University. Part 1. Leguminosae. Taiwania 36, 318-395. https://doi.org/10.6165/tai.1991.36.318.
|
Huang, T.C., Ohashi, H., 1993. Leguminosae. In: Editorial Committee of the Flora of Taiwan (Ed), Flora of Taiwan, 2nd ed. Editorial Committee of the Flora of Taiwan, Taipei, pp. 161-162.
|
Hundly, H.G., Ko, U.C.K., 1961. List of Trees, Shrubs, Herbs and Principal Climbers, Etc. Recorded from Burma with Vernacular Names, 3rd ed. Supdt. Govt. Printing and Staty, Rangoon.
|
Institute of Botany, 1972. Acacia. Science Press, Beijing, pp. 324-326. Figure 2381.
|
Jarvis C.E., 2007. Order Out of Chaos:Linnaean Plant Names and Their Types. London: Linnean Society of London & The Natural History Museum.
|
Johnson L.A.S., 1976. Problems of species and genera in Eucalyptus (Myrtaceae). Pl.Syst. Evol, 125: 144-167. DOI:10.1007/BF00986148 |
Kodela, P.G., Tindale, M.D., 2001a. Acacia dealbata. In: Orchard, A.E., Wilson, A.J.G.(Eds.), Flora of Australia. Australian Biological Resources Study & CSIRO Publishing, Melbourne, pp. 243-244.
|
Kodela, P.G., Tindale, M.D., 2001b. Acacia farnesiana. In: Orchard, A.E., Wilson, A.J.G.(Eds.), Flora of Australia. Australian Biological Resources Study & CSIRO Publishing, Melbourne, pp. 205-207.
|
Kostermans, A.J.G.H., 1980. Mimosaceae. In: Dassanayake, M.D. (Ed.), A Revised Handbook to the Flora of Ceylon. Amerind Publishing Co., New Delhi, pp. 459-508.
|
Kress, W.J., DeFilipps, R., Farr, E., Yin Yin Kyi, D., 2003. A checklist of the trees, shrubs, herbs and climbers of Myanmar Contr. U.S. Natl. Herb. 45, 1-590.https://www.jstor.org/stable/23493222.
|
Kshirsagar S.R., 2012. Observations and taxonomic assessment of Acacia catechu Willd. complex (Mimosaceae) in India. Life Sci. Leafl, 6: 68-77. |
Kurz, K., 1874. Contributions towards a knowledge of the Burmese flora. J. Asiat. Soc.Bengal Pt. 2 Nat. Hist. 43, 39-180.
|
Kurz, K., 1877. '1876'. Contributions towards a knowledge of the Burmese flora.J. Asiat. Soc. Bengal Pt. 2 Nat. Hist. 45, 204-310.
|
Kyalangalilwa B., Boatwright J.S., Daru B.H., Maurin O., van der Bank M., 2013. Phylogenetic position and revised classification of Acacia s. l. (Fabaceae:Mimosoideae) in Africa, including new combinations in Vachellia and Senegalia. Bot.J. Linn. Soc, 172: 500-523. DOI:10.1111/boj.12047 |
Leveille, H., 1914. Flore du Kouy-Tchéou. Published by the author, Le Mans, 1915.
|
Lace J.H., 1915. Some new species from Burma. Bull. Misc. Inform. Kew, 1915: 393-407. DOI:10.2307/4113228 |
Lauener L.A., 1970. Catalogue of the name published by Hector Léveillé:Ⅵ. Notes Roy. Bot. Gard. Edinburgh, 30: 239-294. |
Legume Phylogeny Working Group, 2017. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon, 66: 44-77. DOI:10.12705/661.3 |
Li, J., Gao, C., Zheng, F., Ren, H., 1994. Bark quality of Acacia mearnsii provenances from different geographic origins growing in south China. In: Brown, A.G.(Ed.), Australian Tree Species Research in China. Australian Centre for International Agricultural Research, Zhangzhou, Fujian Province, China, pp. 203-213.
|
Li, Z.Y., Fan, X.H., Boufford, D.E., 2015. Naturalized and invasive plants in China. In: Hong, D.Y., Blackmore, S. (Eds.), Plants of China, a Companion to the Flora of China. Science Press & Cambridge University Press, Beijing, pp. 397-404.
|
Linnaeus C., 1753. Species Plantarum 1 ed.. Stockholm: Laurentius Salvius.
|
Liu M., Yang M., Song D., Zhang Z., Ou X., 2016. Invasive Acacia mearnsii De Wilde in Kunming, Yunnan Province, China:a new biogeographic distribution that threatens airport safety. NeoBiota, 29: 53-62. DOI:10.3897/neobiota.29.7230 |
Liu S.L., Zhao J.M., 2009. Higher Plants in Dehong Prefecture, Yunnan. Beijing: Science Press.
|
Loureiro, J., 1790. Flora Cochinchinensis. Typis, et expensis Academicis, Ulyssipone.
|
Luckow, M., Miller, J.T., Murphy, D.J., Livshultz, T., 2003. A phylogenetic analysis of the Mimosoideae (Leguminosae) based on chloroplast DNA sequence data. In: Klitgaard, B.B., Bruneau, A. (Eds.), Advances in Legume Systematics. Royal Botanic Gardens, Kew, London, pp. 197-220.
|
Luque G.M., Bellard C., Bertelsmeier C., Bonnaud E., Genovesi P., Simberloff D., Courchamp F., 2014. The 100th of the world's worst invasive alien species. Biol.Invas, 16: 981-985. DOI:10.1007/s10530-013-0561-5 |
Mabberley D., 2008. Mabberley's Plant-Book third ed. Cambridge: Cambridge University Press.
|
Maiden J.H., 1917. Notes on Acacia, No. 3-extra-tropical Western Australia. With descriptions of new species. J. Proc. Roy. Soc. N. S. W, 51: 238-274. |
Maslin, B.R., 1992. Acacia miscellany 6. A review of Acacia victoriae and related species (Leguminosae: Mimosoideae: section Phyllodineae). Nuytsia 8, 285-309. https://florabase.dpaw.wa.gov.au/nuytsia/article/180.
|
Maslin, B.R., 2001. Acacia holosericea. In: Orchard, A.E., Wilson, A.J.G. (Eds.), Flora of Australia. ABRS/CSIRO Publishing, Melbourne, pp. 173-174.
|
Maslin, B.R., 2012. New combinations in Senegalia (Fabaceae:Mimosoideae) for Australia. Nuytsia 22, 465-468. https://florabase.dpaw.wa.gov.au/nuytsia/article/661.
|
Maslin B.R., 2015. Synoptic overview of Acacia sensu lato (Leguminosae:Mimosoideae) in East and southeast Asia. Gard. Bull. Singapore, 67: 231-250. DOI:10.3850/s2382581215000186 |
Maslin, B.R., McDonald, M.W., 2004. AcaciaSearch: Evaluation of Acacia as a Woody Crop Option for Southern Australia. Rural Industries Research and Development Corporation, Canberra.
|
Maslin B.R., Miller J., Seigler D.S., 2003. Overview of the generic status of Acacia(Leguminosae:Mimosoideae). Austral. Syst. Bot, 16: 1-18. DOI:10.1071/SB02008 |
Maslin B.R., Seigler D.S., Ebinger J.E., 2013. New combinations in Senegalia and Vachellia (Leguminosae:Mimosoideae) for Southeast Asia and China. Blumea, 58: 39-44. DOI:10.3767/000651913X669914 |
Maslin B.R., Thomson L.A.J., 1992. Re-appraisal of the taxonomy of Acacia holosericea. Including the description of a new species, A. colei, and the reinstatement of A. neurocarpa. Austral. Syst. Bot, 5: 729-743. DOI:10.1071/SB9920729 |
McDonald M.W., Maslin B.R., 2000. Taxonomic revision of the salwoods:Acacia aulacocarpa Cunn. Ex Benth. And its allies (Leguminosae:Mimosoideae:section Juliflorae). Austral. Syst. Bot, 13: 21-78. DOI:10.1071/SB98031 |
McNeill, J., 2014. Holotype specimens and type citations: general issues. Taxon 63, 1112-1113. https://www.iapt-taxon.org/historic/Congress/IBC_2017/holotype.pdf.
|
McNeill J., Turland N.J., 2011. Major changes to the Code of nomenclature-Melbourne, july 2011. Taxon, 60: 1495-1497. DOI:10.1071/SB01038 |
Merrill E.D., 1910. An enumeration of Philippine Leguminosae, with keys to the genera and species. Philipp. J. Sci, 5: 1-94. |
Merrill E.D., 1923. An enumeration of Philippine flowering plants. Philipp. J. Sci, 2: 530. |
Merrill E.D., 1927. An enumeration of Hainan plants. Lingnan Sci. J, 5: 1-186. |
Merrill E.D., 1935. A commentary on Loureiro's "flora Cochinchinensis". Trans.Amer. Philos. Soc, 24: 1-445. DOI:10.2307/1005470 |
Merrill E.D., Chun W.Y., 1940. Additions to our knowledge of the Hainan Flora, Ⅲ[pro parte]. Sunyatsenia, 5(1-2): 74. |
Midgley, S.J., Beadle, C.L., 2007. Tropical acacias an expanding market for solid wood. In: Beadle, C.L., Brown, A.G. (Eds.), Acacia Utilisation and Management: Adding Value. Rural Industries Research and Development Corporation, Victoria, Austrilia, pp. 40-44.
|
Midgley, S.J., Thomson, L., 2018. Acacia crassicarpa. In: Thomson, L., et al. (Eds.), Trees for Life in Oceania: Conservation and Utilisation of Genetic Diversity.Australian Centre for International Agricultural Research (ACIAR), Canberra, pp. 35-38.
|
Midgley S.J., Turnbull J.W., 2003. Domestication and use of Australian acacias:case studies of five important species. Austral. Syst. Bot, 16: 89-102. DOI:10.1071/SB01038 |
Miller, J.T., Bayer, R.J., 2000. Molecular phylogenetics of Acacia (Fabaceae: Mimosoideae) based on chloroplast TRNK/MATK and nuclear histone H3-D sequences. In: Herendeen, P.S., Bruneau, A. (Eds.), Advances in Legume Systematics. Royal Botanic Gardens, Kew, London, pp. 181-200.
|
Miller J.T., Bayer R.J., 2001. Molecular phylogenetics of Acacia (Fabaceae:Mimosoideae) based on the chloroplast matK coding sequence and flanking trnK intron spacer region. Amer. J. Bot, 88: 697-705. DOI:10.2307/2657071 |
Miller J.T., Grimes J.W., Murphy D.J., Bayer R.J., Ladiges P.Y., 2003. A phylogenetic analysis of the Acacieae and Ingeae (Mimosoideae:Fabaceae) based on trnK, matK, psbA-trnH and trnL/trnF sequence data. Syst. Bot, 28: 558-566. DOI:10.1043/02-48.1 |
Miller J.T., Seigler D.S., 2012. Evolutionary and taxonomic relationships of Acacia s. l.(Leguminosae:Mimosoideae). Austral. Syst. Bot, 25: 217-224. DOI:10.1071/SB11042 |
Miller J.T., Terra V., Riggins C., Ebinger J.E., Seigler D.S., 2017. Molecular phylogenetics of Parasenegalia and Pseudosenegalia (Fabaceae:Mimosoideae). Syst.Bot, 42: 465-469. DOI:10.1600/036364417X696140 |
Newman, M., Svengsuksa, B., Thomas, P., Sengdala, K., Lamxay, V., Armstrong, K., 2007. A Checklist of the Vascular Plants of Lao PDR. Royal Botanic Garden Edinburgh, Edinburgh, Scotland.
|
Nghiem L.T.P., Tan H.T.W., Corlett R., 2015. Invasive trees in Singapore:are they a threat to native forests?. Trop. Cons. Sci., 8: 201-214. DOI:10.1177/194008291500800116 |
Nielsen, I.C., 1980. Note on Indo-Chinese Mimosaceae. Adansonia sér. 2 19, 339-363.
|
Nielsen, I.C., 1981. Legumineuses-Mimosoidees. In: Aubreville, A., Leroy, J.-F. (Eds.), Flore du Cambodge du Laos et du Viet-nam. Museum National d'Histoire Naturelle, Paris, p. 159.
|
Nielsen, I.C., 1985a. Leguminosae-Mimosoideae. In: Smitinand, T., Larsen, K. (Eds.), Flora of Thailand. Forest Herbarium, Royal Forest Department, Bangkok, pp. 131-222.
|
Nielsen I.C., 1985b. The Malesian species of Acacia and Albizia (Leguminosae-Mimosoideae). Opera Bot, 81: 1-50. |
Nielsen, I.C., 1992. Mimosaceae (Leguminosae-Mimosoideae). In: de Wilde, W.J.J.O., et al. (Eds.), Flora Malesiana. Foundation Flora Malesiana, The Netherlands, pp. 1-226.
|
Orchard A.E., Maslin B.R., 2003. Proposal to conserve the name Acacia (Leguminosae:Mimosoideae) with a conserved type. Taxon, 52: 362-363. DOI:10.2307/3647418 |
Ou Yang C.R., Sun H., Li Z.M., Zhang J.W., 2013. Application of DNA barcoding in Chinese Acacia s. l. (Leguminosae). Pl. Divers. Res, 35: 547-554. |
Pan, Z.G., Yang, M.Q., 1987. Australian acacias in the People's Republic of China. In: Turnbull, J.W. (Ed.), Australian Acacias in Developing Countries. Australian Centre for International Agricultural Research, Gympie, Australia, pp. 136-138.
|
Pan Z.G., You Y.T., 1994. Introduction and Cultivation of Main Exotic Tree Species in China. Beijing: Beijing Science and Technology Press.
|
Parker R.N., 1929. Indian climbing Acacias of the caesia group. Indian Forester, 55: 325-333. |
Pedley L., 1975. Revision of the extra-Australian species of Acacia subg. Heterophyllum. Contr. Queensland Herb, 18: 1-24. |
Pedley, L., 1978. A revision of Acacia mill. In Queensland. Part 1. Austrobaileya 1, 75-234.
|
Pedley L., 1986. Derivation and dispersal of Acacia (Leguminosae), with particular reference to Australia, and the recognition of Senegalia and Racosperma. Bot. J.Linn. Soc, 92: 219-254. DOI:10.1111/j.1095-8339.1986.tb01429.x |
Pedley, L., 1987. Australian acacias; taxonomy and phytogeography. In: Turnbull, J.W. (Ed.), Australian Acacias in Developing Countries. Australian Centre for International Agricultural Research, Gympie, Australia, pp. 11-16.
|
Pedley, L., 2014. New combinations for Senegalia Raf. And Vachellia Wight & Arn.Species (Mimosaceae) that occur in Australia. Austrobaileya 9, 314-315. https://www.jstor.org/stable/43869011.
|
Pei, S.J., Chen, S.Y., Tong, S.Q., 1991. Palmae. In: Pei, S.J., Chen, S.Y. (Eds.), Flora Reipublicae Popularis Sinicae. Science Press, Beijing, pp. 1-154.
|
Philp J., Sherry S.P., 1949. The genetics of hybrids between green wattle (Acacia decurrens) and black wattle (Acacia mollissima). J. S. African Forest. Assoc, 17: 6-58. |
Pinyopusarerk, K., Midgley, S., Thomson, L., 2018. Acacia auriculiformis. In: Thomson, L., et al. (Eds.), Trees for Life in Oceania: Conservation and Utilisation of Genetic Diversity. Australian Centre for International Agricultural Research(ACIAR), Canberra, pp. 28-30.
|
Rafinesque C.S., 1838. Sylva Telluriana. Philadelphia: Printed for the author and publisher.
|
Ragupathy S., Seigler D.S., Ebinger J.E., Maslin B.R., 2014. New combinations in Vachellia and Senegalia (Leguminosae:Mimosoideae) for South and West Asia. Phytotaxa, 162: 174-180. DOI:10.11646/phytotaxa.162.3.6 |
Rehder A., 1932. Notes on the ligneous plants described by Léveillé from eastern Asia. J. Arnold Arbor, 13: 299-322. |
Rico Arce L., Bachman S., 2006. A taxonomic revision of Acaciella (Leguminosae, -Mimosoideae). Anales Jard. Bot. Madrid, 63: 189-244. DOI:10.3989/ajbm.2006.v63.i2.7 |
Roskov, Y.R., Bisby, F.A., Zarucchi, J.L., Schrire, B.D., White, R.J., 2005. International Legume Database and Information Service (ILDIS), World Database of Legumes: Draft Checklist, Version 10. CD ROM, Reading, UK.
|
Ross J.H., 1975. The typification of Mimosa senegal. Bothalia, 11: 449-451. DOI:10.4102/abc.v11i4.1484 |
Ross J.H., 1979. A conspectus of the African Acacia species. Mem. Bot. Surv. South Africa, 44: 1-155. |
Ross J.H., 1981. An analysis of the African Acacia species:their distribution, possible origins and relationships. Bothalia, 13: 389-413. DOI:10.4102/abc.v13i3/4.1326 |
Searle, S., 2000. Black Wattle (Acacia Mearnsii) for Farm Forestry. Department of Natural Resources and Environment, Melbourne.
|
Seigler D.S., 2014. A new Senegalia (Fabaceae, Mimosoideae) from southern Peru. Novon, 23: 90-93. DOI:10.3417/2011100 |
Seigler, D.S., Ebinger, J.E., 2005. New combinations in the genus Vachellia (Fabaceae: Mimosoideae) from the new world. Phytologia 87, 139-178. https://www.biodiversitylibrary.org/page/12995331#page/3/mode/1up.
|
Seigler, D.S., Ebinger, J.E., 2009. New combinations in the genus Senegalia (Fabaceae: Mimosoideae). Phytologia 91, 26-30. https://www.phytologia.org/uploads/2/3/4/2/23422706/91126-30seaglersenegaliarev2-2-09.pdf.
|
Seigler, D.S., Ebinger, J.E., 2010. New combinations in Senegalia and Vachellia(Fabaceae: Mimosoideae). Phytologia 92, 90-93. https://www.biodiversitylibrary.org/page/47217973#page/98/mode/1up.
|
Seigler D.S., Ebinger J.E., Aupic C., Aymonin G.G., Loup C., 2014. Lectotypification in American Acacia species (Fabaceae, Mimosoideae), with clarifications for types at the Museum National d'Histoire Naturelle. Novon, 23: 98-112. DOI:10.3417/2010021 |
Seigler, D.S., Ebinger, J.E., Miller, J.T., 2006a. The genus Senegalia (Fabaceae: Mimosoideae) from the new world. Phytologia 88, 38-93. https://www.phytologia.org/uploads/2/3/4/2/23422706/seigler88138-93.pdf.
|
Seigler D.S., Ebinger J.E., Miller J.T., 2006b. Mariosousa, a new segregate genus from Acacia s. l. (Fabaceae, Mimosoideae) from central and north America. Novon, 16: 413-420. DOI:10.3417/1055-3177(2006)16[413:MANSGF]2.0.CO;2 |
Seigler, D.S., Ebinger, J.E., Ribeiro, P.G., 2012. A previously unrecognized species of Senegalia (Fabaceae) from northeastern Brazil. J. Bot. Res. Inst. Texas 6, 397-401.https://www.jstor.org/stable/41972428.
|
Seigler D.S., Ebinger J.E., Riggins C.W., Terra V., Miller J.T., 2017. Parasenegalia and Pseudosenegalia (Fabaceae):new genera of the Mimosoideae. Novon, 25: 180-205. DOI:10.3417/2015050 |
Seigler D.S., Morim M.P., Barros M., Ebinger J.E., 2013. A new species of Senegalia(Fabaceae) from Brazil. Phytotaxa, 132: 59-63. DOI:10.11646/phytotaxa.132.1.6 |
Sherry S.P., 1971. The Black Wattle (Acacia Mearnsii De Willd.). Pietermaritzburg, South Africa: University of Natal Press.
|
Srisanga, P., Sasirat, S., 2000. Acacia tonkinensis I.C. Nielsen (Leguminosae-Mimosoideae), a new record for Thailand. Thai Forest Bull. Bot. 28, 25-31. https://www.tci-thaijo.org/index.php/ThaiForestBulletin/article/view/24940.
|
Stafleu, F.A., Cowan, R.S., 1976. Taxonomic Literature[Authors A-G], second ed.Bohn, Scheltema & Holkema, Utrecht.
|
Stafleu, F.A., Cowan, R.S., 1979. Taxonomic Literature[Authors H-Le], second ed.Bohn, Scheltema & Holkema/dr. W. Junk b.v. Publishers, Utrecht/The Hague.
|
Standards Association of Australia, 1983. The Nomenclature of Australian Timbers.Australian Standard AS 2543. Standards Association of Australia, North Sydney.
|
Stirton, C.H., 1978. Plant Invaders: Beautiful, but Dangerous. The Department of Nature and Environmental Conservation of the Cape Provincial Administration, Cape Town.
|
Sun, H., 2006. Acacieae. In: Kunming Institute of Botany Chinese Academy of Sciences (Ed), Flora Yunnanica. Science Press, Beijing, pp. 293-307.
|
Sun H., Chen C., 1990. Taxonomy, distribution and possible floristic origin of the genus Acacia from China. Acta Bot. Yunnan, 12: 255-268 (maps). |
Terra V., Garcia F.P.C., de Queiroz L.P., van der Bank M., Miller J.T., 2017. Phylogenetic relationships in Senegalia (Leguminosae-Mimosoideae) emphasizing the south American lineages. Syst. Bot, 42: 458-464. DOI:10.1600/036364417X696122 |
Tindale, M.D., Kodela, P.G., 2001. Acacia decurrens. In: Orchard, A.E., Wilson, A.J.G.(Eds.), Flora of Australia. Australian Biological Resources Study & CSIRO Publishing, Melbourne, p. 240.
|
Turland N.J., Jarvis C.E., 1997. Typification of Linnaean specific and varietal names in the Leguminosae (Fabaceae). Taxon, 46: 457-485. DOI:10.2307/1224388 |
Turland, N.J., Wiersema, J.H., Barrie, F.R., Greuter, W., Hawksworth, D.L., Herendeen, P.S., Knapp, S., Kusber, W.-H., Li, D.-Z., Marhold, K., May, T.W., McNeill, J., Monro, A.M., Prado, J., Price, M.J., Smith, G.F., 2018. International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017.Regnum Vegetabile 159. Koeltz Botanical Books, Glashütten.
|
Turnbull, J.W. (Ed.), 1986. Multipurpose Australian Trees and Shrubs: Lesser-Known Species for Fuelwood and Agroforestry. Australian Centre for International Agricultural Research, Canberra.
|
Turnbull, J.W., Midgley, S.J., Cossalter, C., 1998. Tropical acacias planted in Asia: an overview. In: Turnbull, J.W., et al. (Eds.), Recent Developments in Acacia Planting. Australian Centre for International Agricultural Research, Hanoi, Vietnam, pp. 14-28.
|
Vassal J., 1972. Apport des recherches ontog#233;niques et séminologiques à l'étude morphologique taxonomique et phylogénique du genre Acacia. Bull. Soc. Hist.Nat. Toulouse, 108: 105-247. |
Verdcourt, B., 1979. A Manual of New Guinea Legumes. Office of Forests Division of Botany, Lae, Papua New Guinea.
|
Walker E.H., 1976. Flora of Okanawa and the Southern Ryuku Islands. Washington: Smithsonian Institution Press.
|
Wang, H., Fang, Y., 1991. The history of Acacia introductions to China. In: Turnbull, J.W. (Ed.), Advances in Tropical Acacia Research. Australian Centre for International Agricultural Research, Bankok, Thailand, pp. 64-66.
|
Wang H.R., Jiang J.M., Zhong C.L., 2017. A Guide to Introduction of Australian Trees to china. Beijing: Science Press.
|
Wang, H.R., Jiang, Z.P., Yan, H., 1994. Australian trees grown in China. In: Brown, A.G.(Ed.), Australian Tree Species Research in China. Australian Centre for International Agricultural Research, Zhangzhou, Fujian Province, China, pp. 19-25.
|
Wang, Y.Z., 2004. A brief history of plant collection (before 1949). In: Zeng, J.F., Huo, C.Y. (Eds.), Flora Reipublicae Popularis Sinicae. Science Press, Beijing, pp. 658-704.
|
Wiersum, K.F., 1991. Acacia mearnsii de Willd. In: Lemmens, R.H.M.J., WulijarniSoetjipto, N. (Eds.), Plant Resources of South-East Asia No. 3. Dye and TanninProducing Plants. Wageningen, The Netherlands, pp. 41-45.
|
Wu, T.L., 1982. Acacia. In: How, K.C. (Ed.), A Dictionary of the Families and Genera of Chinese Seed Plants, second ed. Science Press, Beijing, p. 2.
|
Wu, T.L., 1988. Mimosoideae. In: Chen, T.C. (Ed.), Flora Reipublicae Popularis Sinicae.Science Press, Beijing, pp. 1-74.
|
Wu, T.L., 2008. Mimosaceae. In: Hu, Q.M., Wu, T.L. (Eds.), Flora of Hong Kong.Agriculture, Fisheries and Conservation. Department, Government of the Hong Kong Special Administrative Region, Hong Kong, pp. 36-46.
|
Wu, T.L., Nielsen, I.C., 2010. Acacieae. In: Wu, Z.Y., et al. (Eds.), Flora of China. Science Press & Missouri Botanical Garden Press, Beijing & St Louis, pp. 55-59.
|
Wu Z.L., Pan Z.G., Ding Z.K., 1983. Introduction to Exotic Trees in China. Beijing: Science Press.
|
Xing, F.W., 2005. Flora of Macao. In: Xing, F.W. (Ed.), Dept. of Gardens & Green Areas, Civic and Municipal Affairs Bureau of Macao Special Administrative Region, South China Botanical Garden, Chinese Academy of Sciences, China, p. 328.
|
Ye, H., Peng, S., 2006. Plant Diversity Inventory of Guangdong. World Book Inc., Guangdong, Shanghai, Xi'an & Beijing.
|
Ye H., Zeng F., Ye Y., Liu N., 2013. Medicinal Plants of South China. Wuhan: Huazhong University of Science & Technology Press.
|
Zhang, F., Searle, S., Chen, Z., 2004. Acacia melanoxylon-provenance and family variation in survival, height and stem number at 14 months in Guangdong province China. In: Brown, A.G. (Ed.), Blackwood Management: Learning from New Zealand. Proceedings of an International Workshop. RIRDC Publication No 04/086, Rotorua, New Zealand, pp. 63-70.
|
Zhang, F.Q., Chen, Z.X., Searle, S.D., Li, X.M., Zhou, J.J., Li, Q., 1998. Temperate Australian Acacia species elimination trials in southern China. In: Turnbull, J.W., et al. (Eds.), Recent Developments in Acacia Planting. Australian Centre for International Agricultural Research, Hanoi, Vietnam, pp. 36-44.
|
Zheng, H., Yang, Z., 1993. Acacias for rural industrial and environmental development in southern China. In: Awang, K., Taylor, D.A. (Eds.), The Second Meeting of the Consultative Group for Research and Development of Acacias (COGREDA).Winrock International Institute for Agricultural Research, and FAO, Udorn Thani, Thailand, pp. 15-20.
|
Zhu X.Y., Chen Z.D., Liu B., 2015. Species Catalogue of China Volume 1 Plants Spermatophytes (Ⅳ). Beijing: Angiosperms Science Press.
|
Web references
|
WorldWideWattle: Info Gallery (Species numbers). http://www.worldwidewattle.com/infogallery/species/ (Accessed October 2019).
|
WorldWideWattle: Info Gallery (Distribution). http://worldwidewattle.com/infogallery/distribution/ (Accessed October 2019).
|
WorldWideWattle: Info Gallery (Acacia Name Issue). http://www.worldwidewattle.com/infogallery/nameissue/index.php (Accessed October 2019).
|
WorldWideWattle: Species Gallery (Advanced Search). http://worldwidewattle.com/speciesgallery/search.php (Accessed October 2019).
|
Catalogue of Life: http://www.catalogueoflife.org/col/search/all (Accessed October 2019).
|
Thiers, B.[continuously updated]. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium. http://sweetgum.nybg.org/ih/) (Accessed October 2019).
|
IPNI (The International Plant Name Index): http://www.ipni.org/index.html (Accessed June 2019).
|
Collections & Collectors, a history in Thailand. Office of the Forest Herbarium (BKF), Bangkok. http://www.dnp.go.th/botany/Botany_Eng/Herbarium/herbariumCollectionCollector_Eng.html (Accessed October 2019).
|
Bretschneider, E., 1896. Map of China and Surrounding Regions.[Showing Localities Referred to in Bretschneider (1898a, b)]. http://www.swaen.com/zoomV2.php?id=24380&referer=antique-map-of.php (Accessed October 2019).
|
Vachellia nilotica on WorldWideWattle website. http://worldwidewattle.com/speciesgallery/species-intro.php?id=-3484 (Accessed October 2019).
|
Senegalia senegal on WorldWideWattle website. http://worldwidewattle.com/speciesgallery/species-intro.php?id=-671 (Accessed October 2019).
|
Kodela, P.G. & Tindale, M.D.[continuously updated]. Acacia elata. In Flora of Australia [Online]. Australian Biological Resources Study, Department of the Environment and Energy, Canberra. https://profiles.ala.org.au/opus/foa/profile/Acacia%20elata (Accessed October 2019).
|
Acacia auriculiformis on WorldWideWattle website. http://worldwidewattle.com/speciesgallery/species-intro.php?id=44543 (Accessed October 2019).
|
Kodela, P.G. & Tindale, M.D.[continuously updated]. Acacia auriculiformis. In Flora of Australia [Online]. Australian Biological Resources Study, Department of the Environment and Energy, Canberra. https://profiles.ala.org.au/opus/foa/profile/Acacia%20auriculiformis (Accessed October 2019).
|
Kodela, P.G. & Tindale, M.D.[continuously updated]. Acacia cincinnata. In Flora of Australia [Online]. Australian Biological Resources Study, Department of the Environment and Energy, Canberra. https://profiles.ala.org.au/opus/foa/profile/Acacia%20cincinnata (Accessed June 2019).
|
Pelser, P.B., 2015. Senegalia. In: Pelser, P.B., Barcelona, J.F., Nickrent, D.L. (Eds.), Co's Digital Flora of the Philippines. https://www.philippineplants.org/Families/FabaceaeMimosoideae.html (Accessed October 2019).
|
Acacia confusa on WorldWideWattle website. http://worldwidewattle.com/speciesgallery/species-intro.php?id=-425 (Accessed October 2019).
|
Kodela, P.G. et al.[continuously updated]. Acacia crassicarpa. In Flora of Australia [Online]. Australian Biological Resources Study, Department of the Environment and Energy, Canberra. https://profiles.ala.org.au/opus/foa/profile/Acacia%20crassicarpa (Accessed October 2019).
|
Acacia dealbata on WorldWideWattle website. http://worldwidewattle.com/speciesgallery/species-intro.php?id=17858 (Accessed October 2019).
|
Kodela, P.G. & Tindale, M.D.[continuously updated]. Acacia dealbata. In Flora of Australia [Online]. Australian Biological Resources Study, Department of the Environment and Energy, Canberra. https://profiles.ala.org.au/opus/foa/profile/Acacia%20dealbata (Accessed October 2019).
|
Acacia decurrens on WorldWideWattle website. http://worldwidewattle.com/speciesgallery/species-intro.php?id=16975 (Accessed October 2019).
|
Kodela, P.G. & Tindale, M.D.[continuously updated]. Acacia decurrens. In Flora of Australia [Online]. Australian Biological Resources Study, Department of the Environment and Energy, Canberra. https://profiles.ala.org.au/opus/foa/profile/Acacia%20decurrens (Accessed October 2019).
|
Maslin, B.R.[continuously updated]. Acacia holosericea. In Flora of Australia [Online]. Australian Biological Resources Study, Department of the Environment and Energy, Canberra. https://profiles.ala.org.au/opus/foa/profile/Acacia%20holosericea (Accessed October 2019).
|
Cowan, R.S. & Maslin, B.R.[continuously updated]. Acacia implexa. In Flora of Australia [Online]. Australian Biological Resources Study, Department of the Environment and Energy, Canberra. https://profiles.ala.org.au/opus/foa/profile/Acacia%20implexa (Accessed October 2019).
|
Kodela, P.G. & Tindale, M.D.[continuously updated]. Acacia mangium. In Flora of Australia [Online]. Australian Biological Resources Study, Department of the Environment and Energy, Canberra. https://profiles.ala.org.au/opus/foa/profile/Acacia%20mangium (Accessed June 2019)
|
Acacia mearnsii on WorldWideWattle website http://worldwidewattle.com/speciesgallery/species-intro.php?id=17958 (Accessed October 2019).
|
Kodela, P.G. & Tindale, M.D.[continuously updated]. Acacia mearnsii. In Flora of Australia [Online]. Australian Biological Resources Study, Department of the Environment and Energy, Canberra. https://profiles.ala.org.au/opus/foa/profile/Acacia%20mearnsii (Accessed October 2019).
|
Cowan, R.S. & Maslin, B.R.[continuously updated]. Acacia melanoxylon. In Flora of Australia [Online]. Australian Biological Resources Study, Department of the Environment and Energy, Canberra. https://profiles.ala.org.au/opus/foa/profile/Acacia%20melanoxylon (Accessed October 2019).
|
Acacia podalyriifolia on WorldWideWattle website. http://worldwidewattle.com/speciesgallery/species-intro.php?id=17860 (Accessed October 2019).
|
Maslin, B.R.[continuously updated]. Acacia podalyriifolia. In Flora of Australia [Online]. Australian Biological Resources Study, Department of the Environment and Energy, Canberra. https://profiles.ala.org.au/opus/foa/profile/Acacia%20podalyriifolia (Accessed October 2019).
|
Acaciella glauca on WorldWideWattle website. http://worldwidewattle.com/speciesgallery/species-intro.php?id=-810 (Accessed June 2019).
|
Invasive plant risk assessment: Redwood, Acaciella glauca. (Biosecurity Queensland, Department of Agriculture and Fisheries). https://www.daf.qld.gov.au/__data/assets/pdf_file/0008/52667/IPA-Redwood-Risk-Assessment.pdf (Accessed October 2019).
|
Senegalia catechu on WorldWideWattle website. http://worldwidewattle.com/speciesgallery/species-intro.php?id=-3475 (Accessed October 2019).
|
Vachellia farnesiana on WorldWideWattle website. http://worldwidewattle.com/speciesgallery/species-intro.php?id=-172 (Accessed October 2019).
|
Kodela, P.G.[continuously updated]. Acacia farnesiana. In Flora of Australia [Online]. Australian Biological Resources Study, Department of the Environment and Energy, Canberra. https://profiles.ala.org.au/opus/foa/profile/Vachellia%20farnesiana (Accessed October 2019).
|
Biodiversity of the Hengduan Mountains and adjacent areas of southcentral China. http://hengduan.huh.harvard.edu/fieldnotes (Accessed October 2019)
|



