b. Yunnan Key Laboratory for Conservation of Plant Species with An Extremely Small Population Size, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China;
c. Guangnan Forestry and Grassland Bureau, Guangnan 663300, China;
d. The State Phosphorus Resource Development and Utilization Engineering Technology Research Centre, Yunnan Phosphate Chemical Group Co. Ltd, Kunming, PR China
Malania oleifera Chun & S.K. Lee is a tree species in the monotypic genus Malania, family Olacaceae (Qiu and Gilbert, 2003). It is endemic to very restricted areas in western Guangxi Province and southeastern Yunnan Province, China. The tree can grow up to 20 m in height and produces fruits containing a single large seed (ca. 9 g per fresh seed; Lü et al., 2016b). These fruits are highly sought after for medical purposes, as seeds of M. oleifera have high oil content (>60%) and the highest-known proportion of nervonic acid (>55%) (Ma et al., 2004; Tang et al., 2013). Unfortunately, M. oleifera trees are not common in the wild owing to habitat disturbances and overexploitation. M. oleifera has been listed in the IUCN Red List as a vulnerable species (Sun, 1998) and recently assigned as a plant species of extremely small population size with a high priority for conservation (Ma et al., 2013). Efforts have been taken to investigate causes of vulnerability in M. oleifera (Liang et al., 2003; Xiong et al., 2003; Wu et al., 2004; Lai et al., 2008; Xie et al., 2009), and to regenerate seedlings for conservation as well as utilization purposes (Yu, 2013; Lü et al., 2016a; Mao et al., 2018). However, regenerating seedlings remains difficult (Mao et al., 2018; Xu et al., 2018), and many aspects of M. oleifera biology are poorly understood. This greatly hinders the conservation and utilization of this valued tree.
Previous studies have reported that some species of Olacaceae are root hemiparasites (Kuijt, 1969; Werth et al., 1979; Pate et al., 1990), capable of extracting nutrients and water from host plant roots via swelling structures called haustoria while retaining their own photosynthetic capability. Because Olacaceae also consists of non-parasitic members (Qiu and Gilbert, 2003; Malécot and Nickrent, 2008), predicting the occurrence of parasitism in a species without reference or evidence (i.e., the competence to form haustoira; Kuijt, 1969) is challenging. Previous studies on M. oleifera have noted tuberous structures on their roots (Wu, 2002; Lai, 2006), which were assumed to have been microbial infections (Wu, 2002). Thus far, we have been unable to discover any empirical documentation of parasitism for M. oleifera. Based on taxonomic proximity of M. oleifera to other documented root hemiparasites in the family Olacaceae (Malécot and Nickrent, 2008), we hypothesized that those tuberous structures observed on M. oleifera roots may actually be their parasitic organs (i.e. haustoria). If that is the case, root hemiparasitism may at least partially account for the difficulty encountered cultivating M. oleifera. If M. oleifera is a root hemiparasite, specific care must be taken to successfully cultivate these plants, because life strategies of root hemiparasitic plants differ greatly from autotrophic plants.
In this study, we collected root samples of M. oleifera from Guangnan County, Yunnan Province, China. Root hemiparasitic habit of M. oleifera was confirmed based on field observation and anatomical study of the tuberous structures, which turned out to be haustoria, the defining characteristic organs for parasitic plants (Kuijt, 1969). To the best of our knowledge, this is the first empirical evidence of root hemiparasitism in M. oleifera. These findings will directly contribute to both conservation biology and the sustainable utilization of this highly valued tree by providing guidance for regeneration efforts of M. oleifera seedlings.
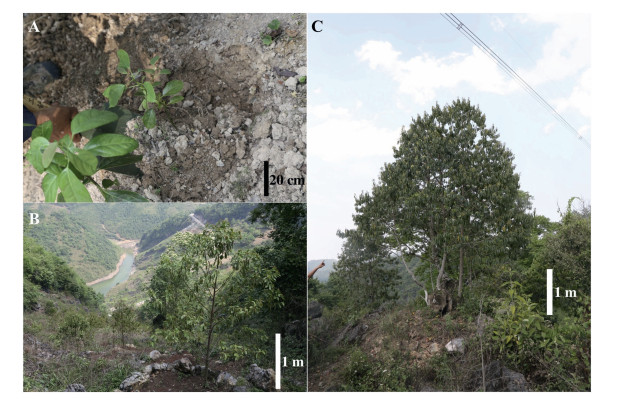
2. Materials and methods 2.1. Root samplingRoot samples were collected from cultivated M. oleifera seedlings as well as wild old trees around Tianfang Village (23°52'17"N, 105°12'14"E; elev. 1327 m), Shuguang Town, Guangnan County, Wenshan Zhuang and Miao Autonomous Prefecture, Yunnan Province, China. In total, 36 M. oleifera plants were sampled, including three cultivated one-year-old seedlings (Fig. 1A), 25 cultivated four-year-old juvenile trees (Fig. 1B), and eight wild, big trees (Fig. 1C). Root excavation involved careful removal of rocks and soil to expose the main roots, which were then traced to lateral roots. During excavation, care was taken to maintain intact connections between M. oleifera roots and those of neighboring plants. M. oleifera roots were easily identified because they are much lighter than the roots of other trees present. Rootlets with tuberous structures were cut off together with attached roots. Sampled rootlets were put into plastic bags with moisture soil immediately after excavation, then stored in 50% ethanol before further analysis.
|
| Fig. 1 Sampled Malania oleifera plants of various ages from different growth conditions. A. Cultivated one-year-old seedlings. B. Cultivated four-year-old juvenile plants. C. Wild, old tree with plenty of flowers. |
Transverse sections of the tuberous structures observed on roots of M. oleifera were cut (ca. 10 mm thickness) with a portable sliding microtome (GSL1, Switzerland) and stained for microscopic observation following a modified protocol from Tennakoon and Cameron (2006). Briefly, samples were stained with Safranine (1% dissolved in reverse osmosis water, w/v) for 2-3 min and Fast Green (0.05% dissolved in 95% ethanol, w/v) for 20 s. Nuclei and lignified and suberised cell walls stain red, and cellulose stains blue-green. Stained sections were washed with 100% ethanol for 2 min and stored in glycerin until microscopic examination. Sections were mounted on a slide using glycerin and a glass cover slip. The slides were then examined using a stereomicroscope (Olympus SZX7, Japan). Photographs were taken with a digital microscope camera (Olympus DP74, Japan).
3. ResultsAll M. oleifera plants sampled in this study were found to form numerous haustoria attached to neighboring roots (Fig. 2A-D). Haustoria were observed on both fine rootlets (Fig. 2C) and thick roots (Fig. 2D). M. oleifera haustoria were found to encircle the host root when attached to a fine rootlet (Fig. 2C), while forming a bell-shaped structure when attached to a thick root (Fig. 2D). Most observed mature haustoria were bell-shaped, but newly initiated haustoria were oval or irregular tuberous swellings. Haustoria size varied, with diameters ranging from two millimetres to three centimeters. Developing and newly matured haustoria were white or light yellow with a fleshy texture (Fig. 2A-C), whereas aged haustoria turned brown or black and were more lignified (Fig. 2D).

|
| Fig. 2 Haustoria (indicated by arrows and asterisks) produced by Malania oleifera plants of various ages from different growth conditions. A. Cultivated one-year-old M. oleifera seedling forming haustoria. B. Close-up of a haustorium formed by the one-year-old seedling. C. Haustoria formed by a cultivated four-year-old M. oleifera plant. D. Haustoia produced by a wild, old flowering M. oleifera tree. |
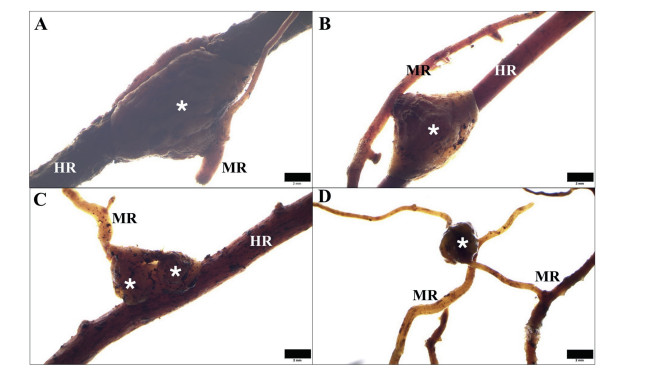
M. oleifera produced haustoria laterally, in most cases by lateral roots (Fig. 3). One bell-shaped haustorium encircling a host root had an obvious merging line (Fig. 3A). On vigorous M. oleifera lateral roots, several haustoria formed in a row (Fig. 3B); in some cases, haustoria were closely adjacent to each other and ended up in a bead-like pattern (Fig. 3C). Haustoria were also found to form between rootlets of the same M. oleifera plant (auto-parasitism) (Fig. 3D).
|
| Fig. 3 Haustoria (indicated by asterisks) of Malania oleifera observed under a stereomicroscope. A. Typical shape of haustoria of M. oleifera attached to a host root. B. Rootlet of M. oleifera with one mature attached haustorium and several developing haustoria. C. Haustoria occurred beside each other in a bead-like pattern. D. One haustorium was observed on another rootlet of the same M. oleifera plant. MR, M. oleifera root; HR, host root. Scale bar = 2 mm. |
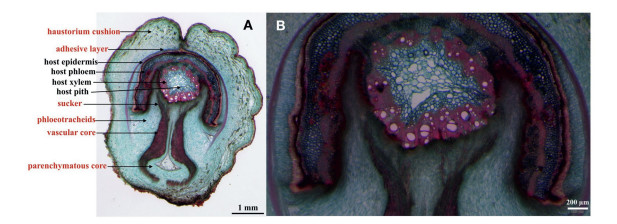
Transverse sections of mature haustoria revealed typical haustorial structures with clear xylem connections between rootlets of M. oleifera and those of a host (Fig. 4). Mature haustoria are composed of two regions: the haustorial cushion that is external to the host root and a central located vascular core that makes initial contact with the host root, penetrating the host tissue (Fig. 4A). The vascular core penetrates the host root cortex and differentiates into suckers that form direct contact with the host xylem (Fig. 4B). The hosteparasite interface is almost entirely composed of parenchymatous tissue. No obvious phloem connections with the host were observed. The host cortex was broken and pushed apart after being penetrated. However, no collapsed host cells were observed.
|
| Fig. 4 Transverse section of a mature haustorium of Malania oleifera attached to an unknown host root. A. Overview of the haustorium transverse section (structures explained in red originate from M. oleifera). B. A close-up showing xylem connections between M. oleifera and its host. |
M. oleifera had a wide range of hosts, forming haustoria on virtually all plant roots with which it came into contact. Host species frequently observed for M. oleifera included Cunninghamia lanceolata, Keteleeria evelyniana, Myrica rubra, Albizia simeonis, Pistacia weinmannifolia, Imperata cylindrica, Erigeron canadensis, Artemisia argyi, Lophatherum gracile, Eupatorium adenophora, Potentilla chinensis, Bidens pilosa, Senecio scandens, Rostellularia procumbens, Eurya groffii, Fallopia multiflora, and Polygonum cuspidatum. In general, haustoria formed on shrubs or trees were larger (one to three centimeters in longer diameter) than those on herbaceous plants (often less than one centimeter in longer diameter). Haustorial morphology and anatomical characters were not different between wild and cultivated M. oleifera plants.
4. DiscussionIn this study, we confirmed hemiparasitism in M. oleifera. M. oleifera haustoria lack phloem connections with hosts, which is consistent with the majority of haustoria for root hemiparasites (Irving and Cameron, 2009). Our study of mature haustoria anatomy in M. oleifera showed that these haustoria are produced laterally, connecting directly to the xylem of compatible hosts. These attributes are typical of the sandalwoods (Santalales) (Werth et al., 1979; Pate et al., 1990; Tennakoon and Cameron, 2006). Previous studies on Santalum album (Sandalwood) haustoria have suggested that host penetration is mediated by cell wall-degrading enzymes, which are indicated by the absence of collapsed host cells (Tennakoon and Cameron, 2006). We found no evidence of collapsed host cells in host tissue penetrated by M. oleifera haustoria, suggesting that M. oleifera haustoria secrete cell walldegrading enzymes.
How important root hemiparasitism is in the growth and development of M. oleifera is a question that cannot be definitely answered at this time, because root hemiparasitic plant dependence on a host varies greatly. Some root hemiparasites undergo a pre-parasitic phase lasting from several hours to several months before attaching to a host (Pate et al., 1990; Seel et al., 1993). Several facultative hemiparasites can complete their life history without a host (Press et al., 1993). Seedlings of M. oleifera have been noted to grow independently for several months, probably due to their large seeds, which have high nutrient reserves, and their slow growth rate at an early stage. The long period of independent growth along with hidden haustorial connections in soil may have partially led to the negligence of root hemiparasitic habit in M. oleifera.
Root hemiparasitism is an adaptive life strategy in the plant kingdom, which evolved to increase the efficiency of nutrient or water uptake under stressful conditions (Press et al., 1999). Attachment to a compatible host often increases growth performance of the root hemiparasite (Irving and Cameron, 2009), which is the case even for facultative hemiparasites that do not require a host to complete their life cycle (Press et al., 1999). Apart from nutritive benefits, root hemiparasitism in some taxa has been reported to confer a competitive advantage over nearby plants (Veenendaal et al., 1996). Because life strategies of root hemiparasitic plants differ greatly from autotrophic plants, cultivation practices suitable for autotrophic plants may be problematic for root hemiparasites, which at least partially explains the low success rate in seedling regeneration for M. oleifera. Although M. oleifera has a wide range of hosts, it may have strong host preferences, as reported for other root hemiparasites (Irving and Cameron, 2009; Tennakoon and Cameron, 2006). Further investigations into root hemiparasitism of this tree may provide valuable insight for successful regeneration, including when young seedlings become parasitic, host preference and host switches between different developmental stages, and how we can manipulate host combinations for optimal M. oleifera growth.
5. ConclusionsThis study provides the first empirical evidence of root hemiparasitism in M. oleifera. This tree exhibits typical morphological and anatomical haustorium attributes of root hemiparasitistic plants. It produces lateral haustoria that connect to the xylem of a compatible host, ranging from herbaceous plants to large trees. In view of different life strategies of root hemiparasitic plants from free-living ones, root hemiparasitic habit should be taken into account in future efforts to regenerate M. oleifera seedlings for either conservation or utilization purposes.
Conflicts of interestNone.
AcknowledgementsMany thanks to Professor Aizhong Liu from Southwest Forestry University for discussion of problems encountered in cultivation of M. oleifera and for mention of the tuberous structures, which inspired this study. The research was financially supported by funding for Airong Li from Yunnan Ten Thousand Talents Plan Young and Elite Talents Project (YNWR-QNBJ-2018-092), Youth Innovation Promotion Association of Chinese Academy of Sciences (2011276), and Young Academic and Technical Leader Raising Foundation of Yunnan Province (2014HB047), funding for Yunju Li from Young Academic and Technical Leader Raising Foundation of Yunnan Province (2019HB060), and Yunnan Science and Technology Innovation Team Program (Grant No. 2019HC015).
Irving L.J., Cameron D.D., 2009. You are what you eat:interactions between root parasitic plants and their hosts. Adv. Bot. Res, 50: 87-138. DOI:10.1016/S0065-2296(08)00803-3 |
Kuijt J., 1969. The Biology of Parasitic Flowering Plants.. Berkeley and Los Angeles: University of California Press.
|
Lai, J.Y., 2006. Study on Conservation Biology of Rare and Precious Plant Malania oleifera. unpublished doctoral dissertation. Sichuan University, Chengdu, China.
|
Lai J.Y., Shi H.M., Pan C.L., et al, 2008. Pollination biology of rare and endangered species Malania oleifera Chun et Lee. J. Beijing For. Univ, 30: 59-64. |
Liang Y.F., Wu S.G., Li X.D., 2003. Study on the endangered causes for Malania oleifera. Guihaia, 23: 404-407. |
Lü S.H., Huang F.Z., Lu S.H., et al, 2016a. Effects of shrub-grass on direct seedling of Cyclobalanopsis glauca and Malania oleifera in rocky desertification mountains in southwest Guangxi. Plant Sci. J, 34: 38-46. |
Lü S.H., Wei C.Q., Huang F.Z., et al, 2016b. Fruit and seed traits and adaptability to rocky desertification mountain of rare tree species Malania oleifera. Chin. J. Ecol, 35: 57-62. |
Ma B.L., Liang S.F., Zhao D.Y., et al, 2004. Study on plants containing nervonic acid. Acta Bot. Bor. Sinica, 24: 2362-2365. |
Ma Y.P., Chen G., Grumbine E.R., et al, 2013. Conserving plant species with extremely small populations (PSESP) in China. Biodivers. Conserv, 22: 803-809. DOI:10.1007/s10531-013-0434-3 |
Malécot V., Nickrent D.L., 2008. Molecular phylogenetic relationships of Olacaceae and related Santalales. Syst. Bot, 33: 97-106. DOI:10.1600/036364408783887384 |
Mao J.H., Jia D.S., Chen F., et al, 2018. Hypocotyle grafting techniques of rare and endanger plant Malania oleifera. J. West China For. Sci, 47: 39-45. |
Pate J.S., Kuo J., Davidson N.J., 1990. Morphology and anatomy of the haustorium of the root hemiparasite Olax phyllanthi (Olacaceae), with special reference to the haustorial interface. Ann. Bot, 65: 435-436. |
Press M.C., Parsons A.N., Mackay A.W., et al, 1993. Gas-exchange characteristics and nitrogen relations of 2 mediterranean root hemiparasites - Bartsia trixago and Parentucellia viscosa. Oecologia, 95: 145-151. DOI:10.1007/BF00649518 |
Press, M.C., Scholes, J.D., Watling, J.R., 1999. Parasitic plants: physiological and ecological interactions with their hosts. In: Press, M.C., Scholes, J.D., Barker, M.G.(Eds.), Physiological Plant Ecology. The British Ecological Society and Blackwell Science, Oxford, UK, pp. 175-197.
|
Qiu, H.X., Gilbert, M.G., 2003. Olacaceae. In: Wu, Z., Raven, P. (Eds.), Flora of China. Vol. 5 (Ulmaceae through Basellaceae). Science Press, Beijing, pp. 200-204.
|
Seel W.E., Parsons A.N., Press M.C., 1993. Do inorganic solutes limit growth of the facultative hemiparasite Rhinanthus minor L. in the absence of a host. New Phytol, 124: 283-289. DOI:10.1111/j.1469-8137.1993.tb03818.x |
Sun, W., 1998. Malania oleifera. The IUCN red list of threatened species 1998: e.T32361A9701100. Downloaded on. https://doi.org/10.2305/IUCN.UK.1998.RLTS.T32361A9701100.en. (Accessed 13 May 2019).
|
Tang T.F., Liu X.M., Ling M., et al, 2013. Constituents of the essential oil and fatty acid from Malania oleifera. Ind. Crops Prod, 43: 1-5. DOI:10.1016/j.indcrop.2012.07.003 |
Tennakoon K.U., Cameron D.D., 2006. The anatomy of Santalum album (Sandalwood) haustoria. Can. J. Bot, 84: 1608-1616. DOI:10.1139/b06-118 |
Veenendaal E.M., Abebrese I.K., Walsh M.F., Swaine M.D., 1996. Root hemiparasitism in a West African rainforest tree Okoubaka aubrevillei (Santalaceae). New Phytol, 134: 487-493. DOI:10.1111/j.1469-8137.1996.tb04366.x |
Werth C.R., Baird W.V., Musselman L.J., 1979. Root parasitism in Schoepfia Schreb.(Olacaceae). Biotropica, 11: 140-143. DOI:10.2307/2387793 |
Wu, Y.Q., 2002. The Primary Study on the Conservation of Malania oleifera. unpublished master thesis. Guangxi University, Nanning, China.
|
Wu Y., Li X., Hu Y., 2004. Reproductive biology of Malania oeifera. Acta Sci. Nat. Univ. Sunyatseni, 43: 81-83. |
Xie W.D., Chen J.H., Lai J.Y., et al, 2009. Analysis on relationship between geographic distribution of Malania oleifera and hydrothermal factors. J. Trop. Subtrop. Bot, 17: 388-394. |
Xiong Y., Hong L., Li H.L., Li X.D., 2003. Bionomics of the pathogens of Malania oleifera seed rot. For. Pest Dis, 22: 1-4. |
Xu D.B., Chen F., Guo X.C., et al, 2018. Research on the bottleneck of resource protection and industrialization development of rarely endangered Malania oleifera. Iss. For. Econ, 38: 13-19. |
Yu H.R., 2013. Effects of different base fertilizers on the growth of Malania oleifera seedlings. Anhui For. Sci. Tech, 39: 33-35. |



