b. Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China;
c. School of Life Sciences, Yunnan University, Kunming 650091, China
The existence of heavy metals (HMs) in soil is of great concern because of their potential toxicity (Khan et al., 2015, 2016). Even at low concentrations, HMs may be toxic to plants, animals, and humans (Di Salvatore et al., 2008; Khan et al., 2016). Reducing the risk of HM toxicity to humans requires minimizing HM concentrations in crops and vegetables. Hence, research on soil HMs has focused on reducing the bioavailability of HMs in soils or weakening the HM absorption capacity of plants (Arshad et al., 2016; Che et al., 2016). Conversely, numerous methods have been employed for remediating contaminated soils (Buendia-Gonzalez et al., 2010). Phytoremediation, which is economic and eco-friendly, is considered a promising method for soil remediation (Ehsan et al., 2014; Sarwar et al., 2017). Phytoremediation mainly depends on the HM absorption and translocation capacities of plants; thus, high-HM accumulation species, especially hyperaccumulators, are good contributors in phytoremediation (Gerhardt et al., 2017). However, plant biomass is also an important factor of phytoremediation efficiency. Phytoremediation is generally a slow process because few plants possess a large biomass and also have the capacity to accumulate high levels of HMs. Additionally, single phytoremediation may produce limited effects on contaminated soils with multiple HMs because plants often have distinct HM accumulation capacities (Xie et al., 2016; Zhou et al., 2016). Therefore, in addition to understanding the mechanisms of HM accumulation in plants, it is critical to screen for more efficient high-HMaccumulating plants.
Several recent studies have investigated the soil-root-leaf transfer mechanism of HMs (Yin et al., 2011; Khan et al., 2016). Environmental factors, such as soil properties, have been shown to regulate accumulation levels of HMs in plants (Gustin et al., 2009; Benis et al., 2015; Huang et al., 2016; Velickovic et al., 2016). Specifically, the physicochemical properties of soil, including pH, electrical conductivity (EC), organic matter content, and cation exchange capacity (CEC), have been found to affect the absorption of HMs in plants (Yu et al., 2014). However, the same soil property can have differential effects on different HM-plant systems (Yu et al., 2014). For example, a study reported that the soil CEC and exchangeable calcium (Ca) are important indexes for predicting the critical value of nickel (Ni) to barley and tomato (Rooney et al., 2007), whereas two other reports found that soil pH rather than soil CEC significantly affects the critical value of Ni toxicity in barley or tomato (Zhang et al., 2009; Li et al., 2011). Therefore, analyzing the effect of soil properties on the HM accumulation characteristics of plants for specific HM-plant systems is of great importance in assessing the safety risks of crop and vegetable foodstuffs or phytoremediation efficiency.
Turnip (Brassica rapa var. rapa Lin.) is a cruciferous biennial species that is widely cultivated in Europe, Asia, and America as a local vegetable or fodder. To date, several studies have investigated the turnip accumulation characteristics for various HMs, including cadmium (Cd), lead (Pb), zinc (Zn), copper (Cu), and manganese (Mn) (Parveen et al., 2015; Li et al., 2016). However, these studies have produced inconsistent conclusions about the HM accumulation capacity of turnips for certain HMs (Bingham et al., 1975; Parveen et al., 2013). This might be partially attributed to the diverse soil environment backgrounds. Among the various HMs, Cd accumulation in turnip has attracted significant attention because of its high toxicity, wide distribution and high mobility in soils (Moreno-Caselles et al., 2000; Wei et al., 2006). The turnip has been designated as a high-Cd-accumulating species (Arthur et al., 2000), and Chinese turnips have been recently reported to possess a strong capacity for accumulating Cd under mucky soils (Li et al., 2016, 2017). These findings indicate both the possibility that animals and humans may be at risk of Cd toxicity from turnip foodstuffs and that turnips have a potential role in phytoremediation. However, the HM accumulation characteristics of Chinese turnips for non-Cd HMs are still unknown. It is also unclear whether different soil types affect turnip HM accumulation characteristics.
In this study, we first examined the accumulation and translocation characteristics of four HMs (Cu, Mn, Zn, and Cd) in the Chinese turnip. Then, we analysed whether physicochemical soil properties affect HM accumulation or translocation in Chinese turnip. The results can help us to further assess the risk of HM toxicities to animals and humans via turnip foodstuff or the potential values of turnip for phytoremediation.
2. Materials and methods 2.1. Soil preparationTo investigate the effects of soil properties on HM accumulation in turnip, we prepared four different HM-contaminated soils and measured their physicochemical properties (Table 1). A total of 15 kg of dried soil of each soil type filled uniform watertight boxes (length: 64 cm; width: 44 cm; height: 26 cm).
| Parameter | Unit | Soil types | |||
| Soil A | Soil B | Soil C | Soil D | ||
| pH | / | 7.65 | 7.53 | 7.72 | 7.44 |
| Organic matter | g kg-1 | 112 | 161 | 293 | 330 |
| Humus | g kg-1 | 65.1 | 93.1 | 170 | 191 |
| Total N | g kg-1 | 1.31 | 1.43 | 1.29 | 0.794 |
| Total P | g kg-1 | 0.633 | 0.97 | 0.618 | 0.374 |
| Available K | mg kg-1 | 634 | 547 | 434 | 275 |
| Exchangeable Ca | cmol kg-1 | 21.5 | 19.4 | 15.6 | 12 |
| Exchangeable Mg | cmol kg-1 | 3.57 | 5.59 | 1.21 | 1.18 |
| Available Fe | mg kg-1 | 7.56 | 41.7 | 50 | 131 |
| Available Cu | mg kg-1 | 12 | 11.77 | 10.587 | 10.264 |
| Available Mn | mg kg-1 | 48.1 | 53.1 | 48.1 | 42 |
| Available Zn | mg kg-1 | 17.68 | 13.93 | 12.75 | 11.71 |
| Available Cd | mg kg-1 | 5.387 | 5.32 | 5.245 | 5.367 |
Turnip seeds (Landrace KTRG-B54) were germinated in the greenhouse and grown for five weeks. Then, seedlings that showed consistent growth were transplanted into prepared boxes, three seedlings to a box. The boxes were placed under natural light and temperature, and watered appropriately. After growing for one month, the root and leaf parts of the plants were collected, and the roots were cleaned with ultrapure water. The samples were dried at 80 ℃ for 48 h before being weighed. The root and leaf parts of plants growing in different soil types were then used to measure Cu, Mn, Zn and Cd concentrations.
2.3. Detection of HM concentrationsThe four HM concentrations were determined according Li et al. (2016). Briefly, approximately 0.5-1.0 g of dried samples were added to the polytetrafluoroethylene digestion tanks. Then, 5 mL of HNO3 was injected, and the tanks were left to stand. After the reactions, the tanks were sealed with caps and placed into a microwave digestion instrument (WX-8000, Yi Yao Instrument, Shanghai, CN) for digestion. When the temperature cooled below 50 ℃, the digestion tanks were taken to the fume hood. The digestion solutions were transferred to 50-mL volumetric flasks, and filled to 50 mL by rinsing three times using ultrapure water. The blank control was treated using the same method. The sample solutions were detected using an inductively coupled plasma mass spectrometer (ICP-MS, Thermo Fisher Scientific, USA), and the HM contents were calculated according to the standard curve.
To draw the standard curve, the content of the respective standard solutions (1 mg mL-1) of Cu, Mn, Zn and Cd were diluted with 5% HNO3 to a 20-mg L-1 stock solution. The stock solution was then prepared into 0, 8, 16, 24, 32, 48, and 64 mg L-1 standard solutions for detection. The standard curve was drawn only when the linear correlation coefficient was greater than 0.99.
2.4. Parameter calculationTo understand the absorption and translocation characteristics of turnip of different HMs, two parameters, namely bioconcentration factor (BCF) and translocation factor (TF), were introduced (He, 2013). BCF represents the ratio of plant HM concentration to the soil HM concentration, while TF is the ratio of HM concentration in the leaf to that in the root.
2.5. Statistical analysisOne-way ANOVA was used to analyse the significant differences among multiple samples. Linear regression and Pearson correlation analyses were performed to identify the correlations. These statistical analyses were performed using SPSS version 18.0.
3. Results 3.1. HM accumulation in turnip plants under different soil typesThe concentrations of four HMs in leaves and roots of different samples were measured. The Cu concentrations in turnip roots ranged from 4.90 (Soil C) to 7.70 mg kg-1 DW (Soil D) and 4.22-6.64 mg kg-1 DW in leaves (Fig. 1A). However, no significant differences among the different plants cultivated in four soil types were observed (Fig. 1A). The root Mn concentrations were 36.23, 25.84, 18.23 and 115.75 mg kg-1 DW in turnip plants in Soils A, B, C and D, respectively (Fig. 1B), while the leaf Mn concentrations were 42.04, 83.55, 54.48 and 570.30 mg kg-1 DW, respectively (Fig. 1B). Both the root and leaf Mn concentrations in turnip plants in Soil D were markedly higher than in those cultivated in the other three soil types (P < 0.05) (Fig. 1B). The root Zn concentrations were 47.52, 52.16, 50.26 and 126.00 mg kg-1 DW in turnip plants in Soils A, B, C and D, respectively (Fig. 1C), while the leaf Zn concentrations were 62.39, 91.41, 73.31 and 227.75 mg kg-1 DW, respectively (Fig. 1C). Both the root and leaf Zn concentrations in turnip plants in Soil D were obviously higher than in those cultivated in the other three soil types (Fig. 1C). The root Cd concentrations were 4.94, 1.91, 2.09 and 6.45 mg kg-1 DW in turnip plants in Soils A, B, C and D, respectively (Fig. 1D), while the leaf Cd concentrations were 6.82, 3.76, 5.63 and 18.01 mg kg-1 DW, respectively (Fig. 1D). The leaf Cd concentration in turnip plants in Soil D was significantly higher than in those cultivated in the other three soil types (P < 0.05) (Fig. 1D).
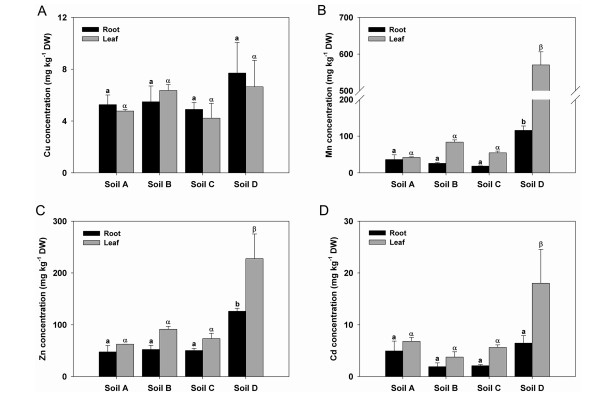
|
| Fig. 1 Heavy metal concentrations in turnip roots and leaves under different soil types. (A) Cu concentrations. (B) Mn concentrations. (C) Zn concentrations. (D) Cd concentrations. Data are means ± standard errors. Different letters labelled on the same colour bars indicate significant differences using Tukey's test (n = 3, P < 0.05; A-D). |
In order to reflect HM absorption and distribution characteristics in turnips under different soil types, BCF and TF parameters were calculated for each sample. The changes in the BCFs of the four HMs under different soil types are shown in Fig. 2A. The Cu BCFs in turnip leaves ranged from 0.40 (Soil A) to 0.65 (Soil D) (Fig. 2A), without significant differences among different plants cultivated in the four soil types (Fig. 2A). The Mn BCFs in turnip leaves were 0.87, 1.57, 1.13 and 13.58 in Soils A, B, C and D, respectively, and the Mn BCF in turnip leaf in Soil D was significantly higher than in those cultivated in the other three soil types (P < 0.05; Fig. 2A). The Zn BCFs in turnip leaves were 3.53, 6.56, 5.75 and 19.45 in Soils A, B, C and D, respectively, and the Zn BCF in turnip leaf in Soil D was significantly higher than in those cultivated in the other three soil types (P < 0.05; Fig. 2A). The Cd BCFs in turnip leaves were 1.27, 0.71, 1.07 and 3.35 in Soils A, B, C, and D, respectively, and the Cd BCF in the turnip leaf in Soil D was significantly higher than in those cultivated in the other three soil types (P < 0.05; Fig. 2A).
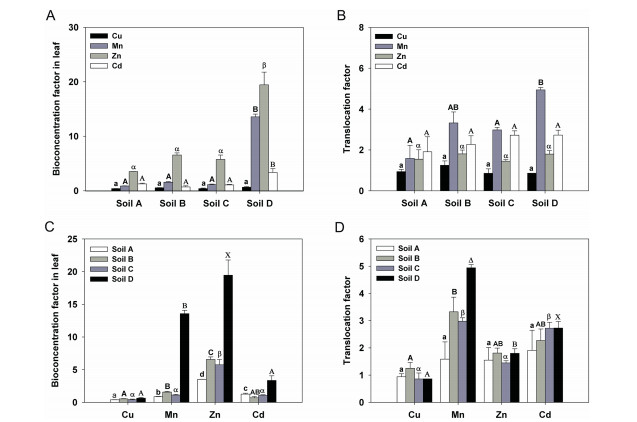
|
| Fig. 2 Bioconcentration Factors (BCFs) and Translocation Factors (TFs) of four heavy metals (HMs) in turnip plants under different soil types. (A) Changes in the BCFs of four HMs under different soil types. (B) Changes in the TFs of four HMs under different soil types. (C) Differences in the BCFs of four HMs under different soil types. (D) Differences in the TFs of four HMs under different soil types. Data are means ± standard errors. Different letters labelled on the same colour bars indicate significant differences using Tukey's test (n = 3, P < 0.05; AeD). |
The changes in the TFs of the four HMs under different soil types are shown in Fig. 2B. The Cu TFs in turnip plants ranged from 0.86 (Soil D) to 1.25 (Soil B) (Fig. 2B), without significant differences among the different plants cultivated in the four soil types (Fig. 2B). Similar results were observed in the Zn and Cd TFs in turnip plants, which ranged from 1.45 (Soil C) to1.81 (Soil B) and from 1.91 (Soil A) to 2.73 (Soil D), respectively (Fig. 2B). The Mn TFs in turnip plants were 1.59, 3.32, 2.98 and 4.94 in Soils A, B, C and D, respectively, and the Mn TF in turnip in Soil D was significantly higher than that cultivated in Soil A (P < 0.05; Fig. 2B).
The differences in the BCFs of the four HMs under the same soil type are shown in Fig. 2C. For plants grown in each soil type, the Zn BCF was the highest followed by the Mn BCF (P < 0.05; Fig. 2C). The BCFs of Cu and Cd were similar in Soils B, C and D, whereas the Cd BCF was significantly higher than that of Cu (P < 0.05; Fig. 2C). The differences in the TFs of the four HMs under the same soil type are shown in Fig. 2D. In Soil A, the TFs of the four HMs were similar (Fig. 2D). The Mn TFs were the highest, and the Cu TFs were the lowest in Soils B, C and D (P < 0.05; Fig. 2D). The TFs of Zn and Cd were similar in Soil B, whereas the Cd TFs were much higher than those of Zn in Soils C and D (P < 0.05; Fig. 2D).
3.3. Correlation analysis between the BCFs and the soil propertiesIn order to understand the effects of soil factors on HM accumulation in turnip, we performed correlation analysis between soil factors and the BCFs of four HMs. The Cu BCF in turnip leaf was negatively correlated with soil pH (P < 0.05) (Table 2). However, the Mn, Zn and Cd BCFs in the turnip leaves were significantly affected by various soil properties (P < 0.05) (Table 2); they were significantly negatively correlated with soil pH, the contents of total N, total P, available K, Cu, Mn and Zn (excluding Cd BCF) and exchangeable Ca, whereas they had significantly positive correlations with the contents of organic matter, humus and available Fe (P < 0.05 or P < 0.01) (Table 2).
| Cu bioconcentration factor | Mn bioconcentration factor | Zn bioconcentration factor | Cd bioconcentration factor | ||
| pH | Pearson Correlation | -0.653a | -0.795b | -0.778b | -0.596a |
| Sig. (2-tailed) | 0.021 | 0.002 | 0.003 | 0.041 | |
| Organic matter | Pearson Correlation | 0.313 | 0.680a | 0.706a | 0.582a |
| Sig. (2-tailed) | 0.322 | 0.015 | 0.01 | 0.047 | |
| Humus | Pearson Correlation | 0.31 | 0.679a | 0.704a | 0.581a |
| Sig. (2-tailed) | 0.326 | 0.015 | 0.011 | 0.048 | |
| Total N | Pearson Correlation | -0.465 | -0.963b | -0.901b | -0.886b |
| Sig. (2-tailed) | 0.127 | 0 | 0 | 0 | |
| Total P | Pearson Correlation | -0.172 | -0.717b | -0.631a | -0.760b |
| Sig. (2-tailed) | 0.593 | 0.009 | 0.028 | 0.004 | |
| Available K | Pearson Correlation | -0.463 | -0.854b | -0.863b | -0.717b |
| Sig. (2-tailed) | 0.13 | 0 | 0 | 0.009 | |
| Exchangeable Ca | Pearson Correlation | -0.422 | -0.816b | -0.828b | -0.693a |
| Sig. (2-tailed) | 0.172 | 0.001 | 0.001 | 0.013 | |
| Exchangeable Mg | Pearson Correlation | -0.037 | -0.512 | -0.477 | -0.558 |
| Sig. (2-tailed) | 0.909 | 0.089 | 0.117 | 0.06 | |
| Available Fe | Pearson Correlation | 0.569 | 0.944b | 0.941b | 0.779b |
| Sig. (2-tailed) | 0.053 | 0 | 0 | 0.003 | |
| Available Cu | Pearson Correlation | -0.293 | -0.690a | -0.705a | -0.608a |
| Sig. (2-tailed) | 0.356 | 0.013 | 0.01 | 0.036 | |
| Available Mn | Pearson Correlation | -0.285 | -0.829b | -0.746b | -0.831b |
| Sig. (2-tailed) | 0.368 | 0.001 | 0.005 | 0.001 | |
| Available Zn | Pearson Correlation | -0.42 | -0.612a | -0.681a | -0.433 |
| Sig. (2-tailed) | 0.174 | 0.035 | 0.015 | 0.16 | |
| Available Cd | Pearson Correlation | 0.229 | 0.378 | 0.265 | 0.392 |
| Sig. (2-tailed) | 0.475 | 0.226 | 0.405 | 0.207 | |
| The bold data imply significant correlation. a Correlation is significant at the 0.05 level. b Correlation is significant at the 0.01 level. | |||||
We also performed correlation analysis between soil factors and the TFs of four HMs to understand the effects of soil factors on HM translocation from roots to leaves in turnip. The TFs of Cu, Zn and Cd in the turnip plants had no significant correlations with the soil properties (Table 3). However, the TF of Mn in turnip was significantly negatively correlated with soil pH, the contents of total N, available K, Cu and Zn and exchangeable Ca, whereas it showed significantly positive correlations with the contents of organic matter, humus and available Fe (P < 0.05 or P < 0.01) (Table 3).
| Cu bioconcentration factor | Mn bioconcentration factor | Zn bioconcentration factor | Cd bioconcentration factor | ||
| pH | Pearson Correlation | -0.139 | -0.684a | -0.366 | -0.093 |
| Sig. (2-tailed) | 0.667 | 0.014 | 0.242 | 0.773 | |
| Organic matter | Pearson Correlation | -0.33 | 0.710b | 0.02 | 0.457 |
| Sig. (2-tailed) | 0.295 | 0.01 | 0.95 | 0.135 | |
| Humus | Pearson Correlation | -0.331 | 0.708a | 0.019 | 0.457 |
| Sig. (2-tailed) | 0.293 | 0.01 | 0.954 | 0.135 | |
| Total N | Pearson Correlation | 0.347 | -0.672a | -0.133 | -0.265 |
| Sig. (2-tailed) | 0.269 | 0.017 | 0.679 | 0.405 | |
| Total P | Pearson Correlation | 0.521 | -0.358 | 0.053 | -0.217 |
| Sig. (2-tailed) | 0.082 | 0.253 | 0.87 | 0.499 | |
| Available K | Pearson Correlation | 0.276 | -0.817b | -0.118 | -0.425 |
| Sig. (2-tailed) | 0.385 | 0.001 | 0.714 | 0.168 | |
| Exchangeable Ca | Pearson Correlation | 0.301 | -0.790b | -0.089 | -0.436 |
| Sig. (2-tailed) | 0.342 | 0.002 | 0.782 | 0.157 | |
| Exchangeable Mg | Pearson Correlation | 0.539 | -0.343 | 0.15 | -0.333 |
| Sig. (2-tailed) | 0.071 | 0.275 | 0.641 | 0.29 | |
| Available Fe | Pearson Correlation | -0.197 | 0.860b | 0.203 | 0.366 |
| Sig. (2-tailed) | 0.539 | 0 | 0.527 | 0.241 | |
| Available Cu | Pearson Correlation | 0.367 | -0.683a | -0.003 | -0.448 |
| Sig. (2-tailed) | 0.24 | 0.014 | 0.992 | 0.144 | |
| Available Mn | Pearson Correlation | 0.47 | -0.475 | -0.017 | -0.23 |
| Sig. (2-tailed) | 0.123 | 0.119 | 0.959 | 0.471 | |
| Available Zn | Pearson Correlation | 0.099 | -0.801b | -0.128 | -0.45 |
| Sig. (2-tailed) | 0.759 | 0.002 | 0.693 | 0.143 | |
| Available Cd | Pearson Correlation | 0.014 | -0.034 | 0.173 | -0.263 |
| Sig. (2-tailed) | 0.966 | 0.917 | 0.591 | 0.41 | |
| The bold data imply significant correlation. a Correlation is significant at the 0.05 level. b Correlation is significant at the 0.01 level. | |||||
In this study, we show that (1) Chinese turnips have a strong ability to accumulate and transport various HMs, and that (2) the accumulation and transport of different HMs are affected by diverse soil factors. The ability of Chinese turnips to accumulate and transport HMs was shown by our calculation of bio-concentration factors and translocation factors. The BCF values show that Chinese turnips accumulate Mn, Zn, and Cd at much higher levels than they accumulate Cu. When we calculated translocation factors, we found that Chinese turnips have a strong ability to transfer Mn, Zn, and Cd from root to leaf. Both the strong ability of turnips to accumulate and transport HMs is consistent with previous studies on Cd accumulation in turnips (Li et al., 2016, 2017).
These results suggest that Chinese turnips may act as hyperaccumulators. Hyperaccumulators, which are plants suitable for use in phytoremediation, are defined as plants with BCF and TF values higher than one (He, 2013). Previous studies have shown that some Chinese turnip landraces are Cd hyperaccumulators (Li et al., 2016). Our BCF and TF values for Mn and Zn in Chinese turnips also satisfy the standard of hyperaccumulators. However, the maximum concentrations of Mn and Zn in turnips are still unknown, and it remains unclear whether turnips can serve as Mn or Zn hyperaccumulators.
Our findings also suggest that in addition to being a candidate plant for phytoremediation of either single or multiple HM pollution, Chinese turnips may be able to play a role in the intake of mineral nutrition, including Cu, Mn, and Zn (see Ma et al., 2016). Functional studies on the regulatory genes of the various HM accumulation characteristics in Chinese turnips are critical, as understanding the molecular mechanisms underlying HM accumulation is a promising method for effectively dealing with HM pollution and reducing the HM intake risk of crop plants; alternatively, this understanding may increase the efficiency of phytoremediation of HM-contaminated soils through the genetic engineering technology (Liu et al., 2017; Luo et al., 2018).
Evidence that the accumulation and transport of different HMs are affected by diverse soil factors has been demonstrated in three ways. First, soil pH was negatively correlated with accumulation of all four HMs (Zn, Mn, Cd, Cu) examined in turnips leaves. These results, which are similar to previous studies (Zhang et al., 2009; Li et al., 2011; Liao et al., 2013; Yu et al., 2014), might be explained by the fact that (1) these metal ions, including Zn and Cd, are more mobile in acidic soil (Velickovic et al., 2016), and (2) the bioavailability of some HMs, such as Cd, decreases as the pH value increases under environments with pH > 6 (Liao et al., 1999).
Second, we found that other soil components showed either positive (e.g. organic matter, humus or available Fe) or negative (e.g. total N or P, available K and exchangeable Ca) correlations with accumulation of Mn, Zn and Cd, but not Cu, in turnip leaves. For example, we found that several exchangeable or available metals can disturb the accumulation of Mn, Zn, and Cd. This may be attributed to ion competition, as many studies have shown that metal transporters may have multiple metal substrates (Migocka et al., 2015). Our results are partly consistent with previous studies (Sloan et al., 1997; Brown et al., 1999; Liao et al., 2013). However, it must be acknowledged that soil material contents may have opposing effects on HM accumulation in different soil-plant systems. For example, our results support the findings of the finding of Sloan et al. (1997) that adding organic matter in soils improves HM accumulations in plants, but contradict the work of Brown et al. (1999), who reached the opposite conclusion. One possible explanation for these contradictory conclusions might be the types of organic matter in the soil, i.e., soluble or solid macromolecule organic matter. Different views also exist on the effects of N or P on HM bioavailability (Li et al., 2000).
Third, in contrast to our findings on the relationship between soil conditions and HM accumulation, we detected a weak correlation between soil conditions and HM transport in turnips. The transfer of Mn from roots to leaves in turnips was the only HM transport that was either negatively or positively affected by soil pH and various soil components. Although several studies have reported that soil environment can affect the transport of metal ions in plants (Huang et al., 2012), the underlying reasons for this effect are unclear. One potential explanation for our findings is that a special Mn transport mechanism exists in turnip.
In summary, the present study indicates that Chinese turnips have a relatively strong ability to absorb various HMs and transport them to leaves, and that soil conditions affect the accumulation and transport of these HMs. These findings have several potential applications, including the use of turnips in phytoremediation, intake of mineral nutrition, and food safety. Furthermore, understanding the molecular mechanisms underlying the HM accumulation characteristics of these plants may be useful for genetic engineering technology. Importantly, the assessment of these practical applications should be conducted with a full knowledge of plantesoil parameters. Although the effect of each soil factor on HM accumulation requires further verification, some agricultural measures may be used to regulate the HM accumulation in turnips. For example, N, P, K and Ca fertilizers may help reduce HM accumulation in turnips, whereas organic matter and Fe fertilizer may have the opposite effects.
Author contributionsX. Li designed the experiments; B.Q. Li, D. Chen, and X. Li performed the experiments; X. Li analyzed the data and wrote the manuscript; Y. P. Yang revised the manuscript.
Conflict of interestThe authors declare no potential conflict of interest.
AcknowledgmentsThis work was financially supported by the Western Youth Project B of the "Light of West China" Program of Chinese Academy of Sciences (Y7260411W1) and the Yunnan Applied Basic Research Projects (2018FB068).
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2019.06.006.
Arshad M., Ali S., Noman A., Ali Q., Rizwan M., Farid M., Irshad M.K., 2016. Phosphorus amendment decreased cadmium (Cd) uptake and ameliorates chlorophyll contents, gas exchange attributes, antioxidants, and mineral nutrients in wheat (Triticum aestivum L.) under Cd stress. Arch. Agron Soil Sci, 62: 533-546. DOI:10.1080/03650340.2015.1064903 |
Arthur E., Crews H., Morgan C., 2000. Optimizing plant genetic strategies for minimizing environmental contamination in the food chain. Int. J. Phytoremediation, 2: 1-21. DOI:10.1080/15226510008500027 |
Benis M.R.S., Hassani A.H., Nouri J., Mehregan I., Moattar F., 2015. The effect of soil properties and plant species on the absorption of heavy metals in industrial sewage contaminated soil:a case study of Eshtehard Industrial Park. Bulg. Chem. Commun, 47: 211-219. |
Bingham F.T., Page A.L., Mahler R.J., Ganje T.J., 1975. Growth and cadmium accumulation of plants grown on a soil treated with a cadmium-enriched sewage sludge. J. Environ. Qual, 4: 207-211. |
Brown G.E., Foster A.L., Ostergren J.D., 1999. Mineral surfaces and bioavailability of heavy metals:a molecular-scale perspective. Proc. Natl. Acad. Sci. U.S.A, 96: 3388-3395. DOI:10.1073/pnas.96.7.3388 |
Buendia-Gonzalez L., Orozco-Villafuerte J., Cruz-Sosa F., Barrera-Diaz C.E., VernonCarter E.J., 2010. Prosopis laevigata a potential chromium (Ⅵ) and cadmium (Ⅱ) hyperaccumulator desert plant. Bioresour. Technol, 101: 5862-5867. DOI:10.1016/j.biortech.2010.03.027 |
Che J., Yamaji N., Shao J.F., Ma J.F., Shen R.F., 2016. Silicon decreases both uptake and root-to-shoot translocation of manganese in rice. J. Exp. Bot, 67: 1535-1544. DOI:10.1093/jxb/erv545 |
Di Salvatore M., Carafa A.M., Carratu G., 2008. Assessment of heavy metals phytotoxicity using seed germination and root elongation tests:a comparison of two growth substrates. Chemosphere, 73: 1461-1464. DOI:10.1016/j.chemosphere.2008.07.061 |
Ehsan S., Ali S., Noureen S., Mahmood K., Farid M., Ishaque W., Shakoor M.B., Rizwan M., 2014. Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol. Environ. Saf, 106: 164-172. DOI:10.1016/j.ecoenv.2014.03.007 |
Gerhardt K.E., Gerwing P.D., Greenberg B.M., 2017. Opinion:taking phytoremediation from proven technology to accepted practice. Plant Sci, 256: 170-185. DOI:10.1016/j.plantsci.2016.11.016 |
Gustin J.L., Loureiro M.E., Kim D., Na G., Tikhonova M., Salt D.E., 2009. MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. Plant J, 57: 1116-1127. DOI:10.1111/j.1365-313X.2008.03754.x |
He Q.X., 2013. Research progress of screening cadmium hyperaccumulators. Environ. Prot. Circ. Econ, 33: 46-49. |
Huang H.Y., Xu J., Bai Y., Zhang W.Q., Zhu F., Li T., Wang X.Y., An C.H., 2012. Enrichment of heavy metals in Saccharum arundinaceum (Retz.) Jeswiet in different soil habitats. Chin. J. Ecol, 31: 961-966. |
Huang X.Y., Deng F.L., Yamaji N., Pinson S.R.M., Fujii-Kashino M., Danku J., Douglas A., Guerinot M.L., Salt D.E., Ma J.F., 2016. A heavy metal P-type ATPase OsHMA4 prevents copper accumulation in rice grain. Nat. Commun: 7. |
Khan A., Khan S., Alam M., Khan M.A., Aamir M., Qamar Z., Rehman Z.U., Perveen S., 2016. Toxic metal interactions affect the bioaccumulation and dietary intake of macro- and micro-nutrients. Chemosphere, 146: 121-128. DOI:10.1016/j.chemosphere.2015.12.014 |
Khan A., Khan S., Khan M.A., Qamar Z., Waqas M., 2015. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk:a review. Environ. Sci. Pollut. Control Ser, 22: 13772-13799. |
Li B., Qing C.L., Zhou Z.B., Yang Q.M., 2000. Effects of nitrogen, phosphorus and organic matter on heavy metal behavior in soils and its application of controlling pollution. Agro-environ. Prot, 19: 375-377. |
Li B., Zhang H.T., Ma Y.B., Mclaughlin M.J., 2011. Influences of soil properties and leaching on nickel toxicity to barley root elongation. Ecotoxicol. Environ. Saf, 74: 459-466. DOI:10.1016/j.ecoenv.2010.10.021 |
Li X., Zhang X.M., Li B.Q., Wu Y.S., Sun H., Yang Y.P., 2017. Cadmium phytoremediation potential of turnip compared with three common high Cdaccumulating plants. Environ. Sci. Pollut. Res, 24: 21660-201670. DOI:10.1007/s11356-017-9781-z |
Li X., Zhang X.M., Yang Y., Li B.Q., Wu Y.S., Sun H., Yang Y.P., 2016. Cadmium accumulation characteristics in turnip landraces from China and assessment of their phytoremediation potential for contaminated soils. Front. Plant Sci, 7: 1862. |
Liao M.A., Huang C.Y., Xie Z.M., 1999. Effect of pH on transport and transformation of cadmium in soil-water system. Acta Sci. Circumstantiae, 19: 81-86. |
Liao Q.L., Liu C., Cai Y.M., Zhu B.W., Wang C., Hua M., Jin Y., 2013. A preliminary study of element bioconcentration factors within milled rice and wheatmeal in some typical areas of Jiangsu Province. Chin. Geol, 40: 331-340. |
Liu H., Zhao H.X., Wu L.H., Liu A.N., Zhao F.J., Xu W.H., 2017. Heavy metal ATPase 3(HMA3) confers cadmium hypertolerance on the cadmium/zinc hyperaccumulator Sedum plumbizincicola. New Phytol, 215: 687-698. DOI:10.1111/nph.14622 |
Luo J.S., Huang J., Zeng D.L., Peng J.S., Zhang G.B., Ma H.L., Guan Y., Yi H.Y., Fu Y.L., Han B., Lin H.X., Qian Q., Gong J.M., 2018. A defensin-like protein drives cadmium efflux and allocation in rice. Nat. Commun, 9: 645. DOI:10.1038/s41467-018-03088-0 |
Ma G.C., Wang Y.R., Xuan Z.Y., 2016. Analysis and comparison of nutritional compositions in Xinjiang turnip (Brassica rapa L. ). Sci. Technol. Food Ind, 37: 360-364. |
Migocka M., Papierniak A., Kosieradzka A., Posyniak E., MaciaszczykDziubinska E., Biskup R., Garbiec A., Marchewka T., 2015. Cucumber metal tolerance protein CsMTP9 is a plasma membrane H+-coupled antiporter involved in the Mn2+ and Cd2+ efflux from root cells. Plant J, 84: 1045-1058. DOI:10.1111/tpj.13056 |
Moreno-Caselles J., Moral R., Perez-Espinosa A., Perez-Murcia M.D., 2000. Cadmium accumulation and distribution in cucumber plant. J. Plant Nutr, 23: 243-250. DOI:10.1080/01904160009382011 |
Parveen T., Hussain A., Rao M.S., 2015. Growth and accumulation of heavy metals in turnip (Brassica rapa) irrigated with different concentrations of treated municipal wastewater. Nord. Hydrol, 46: 60-71. DOI:10.2166/nh.2014.140 |
Parveen T., Inam A., Mehrotra I., 2013. Treated municipal wastewater for irrigation:effect on turnip (Brassica rapa). Desalination Water Treat, 51: 5430-5443. DOI:10.1080/19443994.2013.797109 |
Rooney C.P., Zhao F.J., Mcgrath S.P., 2007. Phytotoxicity of nickel in a range of European soils:influence of soil properties, Ni solubility and speciation. Environ. Pollut, 145: 596-605. DOI:10.1016/j.envpol.2006.04.008 |
Sarwar N., Imran M., Shaheen M.R., Ishaque W., Kamran M.A., Matloob A., Rehim A., Hussain S., 2017. Phytoremediation strategies for soils contaminated with heavy metals:modifications and future perspectives. Chemosphere, 171: 710-721. DOI:10.1016/j.chemosphere.2016.12.116 |
Sloan J.J., Dowdy R.H., Dolan M.S., Linden D.R., 1997. Long-term effects of biosolids applications on heavy metal bioavailability in agricultural soils. J. Environ. Qual, 26: 966-974. |
Velickovic Z., Ivankovic N., Strikovic V., Karkalic R., Jovanovic D., Bajic Z., Bogdanov J., 2016. Investigation of soil properties influence on the heavy metals sorption by plants and possibilities for prediction of their bioaccumulation by response surface methodology. J. Serb. Chem. Soc, 81: 947-958. DOI:10.2298/JSC151130045V |
Wei S.H., Zhou Q.X., Koval P.V., 2006. Flowering stage characteristics of cadmium hyperaccumulator Solanum nigrum L. and their significance to phytoremediation. Sci. Total Environ, 369: 441-446. |
Xie W.J., Che L., Zhou G.Y., Yang L.N., Hu M.Y., 2016. The bioconcentration ability of heavy metal research for 50 kinds of rice under the same test conditions. Environ. Monit. Assess, 188: 675. DOI:10.1007/s10661-016-5660-1 |
Yin L.Y., Cheng Y.W., Espinasse B., Colman B.P., Auffan M., Wiesner M., Rose J., Liu J., Bernhardt E.S., 2011. More than the ions:the effects of silver nanoparticles on Lolium multiflorum. Environ. Sci. Technol, 45: 2360-2367. DOI:10.1021/es103995x |
Yu S.J., Gao S.F., Qu Y.M., Chen Y.H., Wang G., 2014. Toxicity and its threshold of cadmium to tomato roots in different soils. J. Agro-Environ. Sci, 33: 640-646. |
Zhang H.T., Li B., Liu J.F., Ma Y.B., Wei D.P., 2009. Major soil factors controlling nickel toxicity to tomato in a wide range of Chinese soils and the predictable models. Asian J. Ecotoxicol, 4: 569-576. |
Zhou H., Yang W.T., Zhou X., Liu L., Gu J.F., Wang W.L., Zou J.L., Tian T., Peng P.Q., Liao B.H., 2016. Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. J.Environ. Res. Public Health, 13: 289. DOI:10.3390/ijerph13030289 |



