b. University of the Chinese Academy of Sciences, Beijing, 100049, China;
c. Laboratory of Ecology and Evolutionary Biology, Yunnan University, Kunming, Yunnan, 650091, China
Most flowering plants are hermaphroditic, and unisexual individuals (dioecious) have evolved from hermaphroditic ancestors many times (Renner, 2014). Trioecy is an uncommon sexual system in which hermaphrodites, females, and males coexist in some species. Trioecy occurs during the evolutionary transition from hermaphroditism to dioecy (Charlesworth and Charlesworth, 1978; Ross, 1982; Spigler and Ashman, 2012). Trioecy is a stable evolutionary stage under pollen limitation of female seed production because pollen limitation reduces the fitness of females but not self-fertile hermaphrodites, counteracting the seed fertility advantage of females (Maurice and Fleming, 1995). However, previous research has shown that this sexual system is not stable because when the conditions that enable invasion by females into androdioecious populations are the same as those that result in the displacement of hermaphrodites by females, the sexual system will become dioecy (Perry et al., 2012; Wolf and Takebayashi, 2004). Thus, the stable coexistence of hermaphroditic and dioecious plants remains poorly understood.
Ploidy level (diploid and polyploid) and gender polymorphism (dioecy, gynodioecy, subdioecy, and trioecy) are associated in several flowering plant genera (Miller et al., 2016; Miller and Venable, 2000; Pannell et al., 2004; Ramsey and Ramsey, 2014; Spigler and Ashman, 2012). For example, in Lycium, polyploidy is a trigger for the evolution of gender dimorphism in selfincompatible groups (Miller and Venable, 2000, 2002). In Leptinella, gender dimorphism has evolved from monoecy; and, dioecy has been found in two tetraploid species (Himmelreich et al., 2012).
Although the shrubby cinquefoil Dasiphora was formerly a member of Potentilla (Davidson and Lenz, 1989; Elkington, 1969), genetic evidence has shown that Dasiphora is distinct (Eriksson et al., 2003; Zhang et al., 2017). Dasiphora fruticosa is widely distributed in cool temperate and subarctic regions. Each individual dozens of stamens and/or stigmas per flower (Fig. 1A-F). D. fruticosa flowers begin opening in the morning and the flowers are mainly visited by nectarivorous and pollenivorous insects (Denisow et al., 2013; Elkington and Woodell, 1963; VanOverbeke et al., 2007).
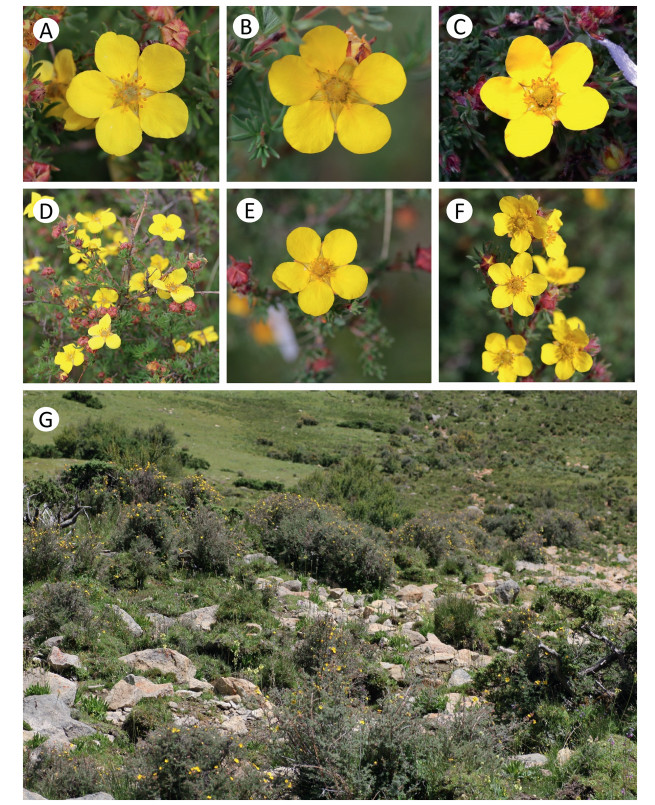
|
| Fig. 1 Three different sexual flowers (A: male flower, B: female flower, C: hermaphrodite flower), inflorescence part of plants (D: males, E: females, F: hermaphrodites), and habitat (G) of the Dasiphora fruticosa population in the Qinghai-Tibet Plateau, China. |
Dasiphora shows considerable variation in ploidy and sexual system among and within species (Ashman et al., 2013; Elkington, 1969; Miller and Venable, 2000). D. fruticosa from northern Europe are often tetraploid and dioecious, whereas southern European and North American individuals are diploid with perfect flowers (Elkington, 1969; Elkington and Woodell, 1963). Klackenberg (1983) noted that two species should be recognized in Europe and North America, a diploid and hermaphrodite species and a tetraploid and in some characteristics, such as morphology, physiology, and life history (Ramsey and Schemske, 2002). For example, diploids have smaller pollen and stomata than tetraploids (Elkington, 1969). These differences play an important role in the establishment of polyploid species in novel ecological settings (Miller et al., 2016). Therefore, polyploidy may be the factor that triggers gender specialization in this genus. Furthermore, D. fruticosa is an excellent model for studying the evolutionary transition from hermaphroditism to dioecy.
For D. fruticosa, Asia is the center of variation in ploidy level (Elkington and Woodell, 1963; Klackenberg, 1983); for example, di-, tri-, tetra-, hexa- and octoploids have been found in cultivated plants belonging to Asian materials (Bowden, 1957). Dioecious and hermaphroditic individuals occur in the Qinghai-Tibet Plateau and dioecious species. Polyploids differ from their diploid progenitors produces hundreds of yellow flowers that have five petals and the Altai Mountains, where diploids, tetraploids, and hexaploids have been recorded from wild populations (Elkington, 1969; Klackenberg, 1983). However, the correlation between ploidy level and sex variation remains inconclusive. Prior work has documented that the reciprocal crosses between the dioecious and hermaphroditic tetraploid forms of D. fruticosa were successful, but hybridization between diploid and tetraploid hermaphrodites was unsuccessful or resulted in sterile triploids (Davidson and Lenz, 1989; Grewal and Ellis, 1972). However, whether polyploidization drives the coexistence of hermaphroditic and dioecious individuals in D. fruticosa remains unclear.
The aim of this study was to test the hypothesis that polyploidization and sexual dimorphism drive the coexistence of hermaphroditic and dioecious individuals in D. fruticosa. To test this hypothesis, we first assessed the DNA ploidy levels of hermaphroditic and dioecious individuals in D. fruticosa. Then, we compared the number of pollen grains and ovules from different sex phenotypes. Finally, we carried out hand-pollination experiments among the three sex phenotypes.
2. Materials and methods 2.1. Study species and siteOur study population (29°41'N, 92°15'E, 4520-4680 m) is near Riduo, Maizhokunggar, Lhasa, China. The mean annual temperature is 3.0 ℃, mean annual precipitation is 409 mm, over 80% of which falls during the summer monsoon. The most common shrub is D. fruticosa (Fig. 1). In this area, the flowering period of D. fruticosa is from the middle of June to early September. Graminoids, such as Kobresia spp., Carex spp., and Poa spp., dominate the plant community. Weed species, including Lancea tibetica, Pedicularis spp., Gentiana spp., are abundant.
2.2. Sex ratio and sex labilityWe scored the sexes of plants from June to July 2015, during the period when most individuals flowered. The sexes of plants were scored while walking transects through the population in July; a total of 578 plants were scored. To assess sex lability, we collected hermaphrodite (N = 8), male (N = 4), and female (N = 5) plants from the Qinghai-Tibet Plateau in 2015, and transplanted them into a mixture of sand and peat (3:1) in 25-cm pots at the Kunming Botanical Garden, Kunming, Yunnan, China. We determined the sexual phenotype of flowering plants over 4 years (2015-2018).
2.3. DNA ploidy levelsTo estimate the difference in DNA ploidy levels in three sexual phenotypes, fresh branches with leaves and buds from each sex type of D. fruticosa were collected and taken back to the laboratory. Because young leaves were few and small, the petals of D. fruticosa and an external standard (Oryza sativa; 1C = 0.4 pg) were separated and crushed in a Petri dish containing 2 mL of pre-chilled lysis buffer. After filtration, centrifugation, re-suspension, the homogenate was storage in the dark at 4 ℃ for 30 min; the resulting cell suspensions were analyzed using a FACS-Vantage flow cytometer following the manufacturer's recommendations (Partec, German). The DNA content was calculated formulas follows: (sample DNA content)/(standard DNA content) = (sample mean fluorescence intensity)/(standard mean fluorescence intensity). The relative DNA ploidy levels of male and female plants of D. fruticosa were estimated by comparing the mean fluorescence intensity of the nuclei of the sample material with that of hermaphrodite plants.
2.4. Pollen and ovule productionTo determine the pollen and ovule production of different sex types, over thirty terminal buds on different plants of each sex type were selected and fixed in standard FAA solution (formalin: acetic acid: 100% ethanol at a ratio of 5:5:90 by volume). In the laboratory, we squashed all anthers and suspended pollen grains in 5 mL of water with three drops of detergent solution for full suspension for perfect and male flowers. Pollen grains were determined by counting the number of pollen grains in 10 drops of pollen solution (1 μL) with a light microscope. Ovule numbers in the perfect and female flowers were counted under a stereoscope. We used oneway ANOVA to determine the differences in pollen number between perfect and male flowers, and ovule number between perfect and female flowers.
2.5. Pollen removal and depositionTo determine whether perfect and male flowers differed in pollen export, we randomly selected and netted 100 flower buds in each of the two sexual morphs. After flowers opened, we removed the net and collected three anthers that did not dehisce after counting the anther number, then fixed them in standard FAA solution to estimate the total pollen production. The remaining anthers were left for pollinator visitation. After flowers wilted, we collected all remaining anthers and fixed them in FAA solution to examine the total pollen grains kept in each flower. In the laboratory, we split the anthers and suspended all pollen grains in 1.5 mL of water with a drop of detergent solution for full suspension. Total pollen grain production and remaining pollen grains were determined by counting the number of pollen grains in 10 replicates of a 1-μL pollen solution. Then, the proportion of exported pollen grains was calculated.
To determine whether pollen grains deposited on stigmas differed in perfect and female flowers, we selected and fixed 5 stigmas from each flower from 101 female and 100 perfect flowers in standard FAA solution when the flowers wilted. The mean number of pollen grains per flower deposited on the stigma was determined in the laboratory. The stigma was softened in 8 mol/L NaOH for 4 h at room temperature, then re-watered and dyed in aniline blue solution (1%) before being rinsed again in distilled water for 2 h. The stigma was squashed on a slide, and the number of pollen grains on the stigma was counted under the microscope. We used one-way ANOVA to determine the difference in the mean number of pollen grains on stigmas per flower between perfect flowers and female flowers.
2.6. Hand-pollination experimentsTo quantify the effects of pollen source on fruit set and seed production, we conducted experimental hand-pollination in July 2015. A total of 150 terminal buds on different hermaphrodite plants were randomly selected and netted to exclude pollinators after emasculation. These flowers were divided into three groups and subjected to each of the three treatments after flowers opened: (1) hand-selfing with pollen grains from the same plant; (2) handoutcrossing with pollen grains from other hermaphrodites or (3) hand-outcrossing with pollen grains from males. For females, we randomly selected 100 terminal buds on different plants and divided them into two groups. Each group of flowers was subjected to the following treatments after flowers opened: (1) handpollination with pollen grains from hermaphrodites and (2) supplemental hand pollination with pollen grains from males. After hand pollination, flowers were immediately enclosed in gauze bags. In addition, we randomly selected over 110 hermaphrodite and female buds for natural pollination. Because of grazing damage, some labeled flowers were lost and the sample size was reduced for some treatments (Table 1). We define the index of selfcompatibility (ISC) as the ratio of seeds produced by selffertilization in relation to seeds produced by outcrossing (Lloyd and Schoen, 1992; Ramirez and Brito, 1990). ISC can range from 0 to 1. ISC scores of less than 0.3 indicate self-incompatibility (Ramirez and Brito, 1990). One-way ANOVA was performed using a post-hoc least significant difference (LSD) test to reveal the significance of differences in mean seed number.
| Treatments | Hermaphrodites | Females | |||
| N | fruit set (%) | N | fruit set (%) | ||
| Control | 143 | 95.1 | 107 | 24.3 | |
| Hand self-pollination | 37 | 78.38 | na | na | |
| Hand cross-pollination with pollen grains from hermaphrodites | 48 | 95.83 | na | na | |
| Hand cross-pollination with pollen grains from males | 50 | 28 | na | na | |
| Hand-pollination with pollen grains from hermaphrodites | na | na | 50 | 0 | |
| Supplemental pollen grains from males | na | na | 47 | 95.74 | |
| N: number of samples, na: not available. | |||||
The number of hermaphroditic plants was significantly more than that of unisexual plants (χ2 = 670.27, df = 2, P < 0.001). The percentages of males, females, and hermaphrodites were 6.92% (N = 40), 9% (N = 52) and 84.08% (N = 486), respectively. Under favorable glasshouse conditions at the Kunming Botanical Garden, the sexual type of every plant was constant from 2015 to 2018.
3.2. DNA contentsThere was no significant difference in the average DNA contents of separate sexes (1.35 ± 0.13 pg for males, 1.50 ± 0.15 pg for females, t = 2.046, df = 25, P = 0.051). The DNA content of males and females were roughly twice as much as that of hermaphrodites (0.74 ± 0.10 pg, Fig. 2).
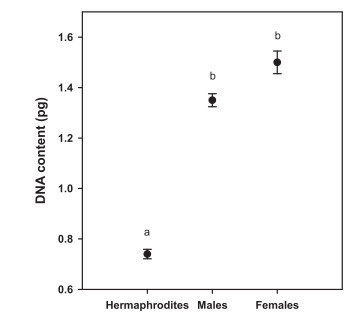
|
| Fig. 2 The DNA contents of combined and separate sexes of Dasiphora fruticosa. The average DNA contents of males (1.35 ± 0.026, N = 5) and females (1.50 ± 0.045, N = 22) was roughly twice that of the hermaphrodites (0.74 ± 0.019, N = 19) (χ2 = 34.142, df = 2, P < 0.001). Different letters beside the bars indicate significant differences at P = 0.05 level. Error bars indicate SE. |
There was a significant difference in the number of pollen grains between male flowers and perfect flowers (F = 219.82, df = 1, P < 0.001, Fig. 3A). Total pollen grains per flower averaged about 3.85 ± 0.36 × 105 in hermaphrodites, whereas males produced an average of 5.35 ± 0.39 × 105 pollen grains. Female flowers produced substantially more ovules than hermaphrodites (F = 120.80, df = 1, P < 0.001, Fig. 3B). Females and hermaphrodites produced an average of 97.58 ± 4.79 and 78.5 ± 3.43 ovules per flower, respectively.

|
| Fig. 3 The pollen number (A) and ovule number (B) per flower of combined and separate sexes of Dasiphora fruticosa. There were more pollen grains in males (5.35 ± 0.39 × 105, N = 32) than in hermaphrodites (3.85 ± 0.36 × 105, N = 36) (F = 219.82, df = 1, P < 0.001). Female flowers (97.58 ± 4.79, N = 40) also produced substantially more ovules than hermaphrodites (78.5 ± 3.43, N = 36) (F = 120.80, df = 1, P < 0.001). Different letters beside the bars indicate significant differences at P = 0.05 level. Error bars indicate SE. |
While pollen grains removal from hermaphrodites was similar to that from males, more pollen grains were deposited on the stigmas of hermaphrodites than of females (Fig. 4). For example, 96.63 ± 0.36% of the pollen grains were removed from hermaphrodites; similarly, 96.91 ± 0.25% were removed from males (F = 0.277, df = 1, P = 0.599, Fig. 4A). The mean numbers of pollen grains deposited on the stigmas were 64.78 ± 3.78 in hermaphrodites and 9.78 ± 1.05 in female (F = 200.187, df = 1, P < 0.001, Fig. 4B).
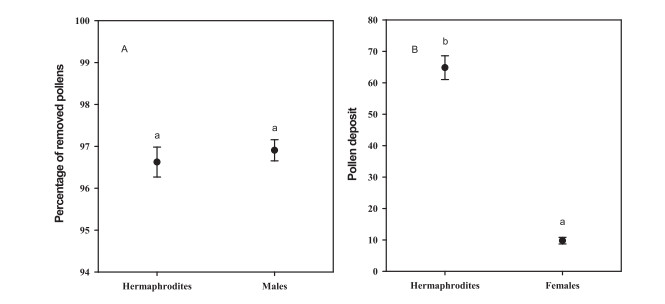
|
| Fig. 4 (A) Percentages of pollen removal during the total male phase in the hermaphrodite (96.63 ± 0.36%, N = 99) and male (96.91 ± 0.25%, N = 100) flowers; there is no significant difference between female flowers and bisexual flowers (F = 0.277, df = 1, P = 0.599). (B) Mean number of pollen grains deposited on the stigmas in natural hermaphrodite flowers (64.78 ± 3.78, N = 100) and female flowers (9.78 ± 1.05, N = 101); the difference between the two sexual morphs was significant (F = 200.187, df = 1, P < 0.001). Different letters beside the bars indicate significant differences at P = 0.05 level. Error bars indicate SE. |
Fruit set and mean seed number of hermaphrodites were significantly higher than those of females in natural treatments (fruit set: 95.1% in hermaphrodites and 24.7% in females; the mean seed number: 35.87 ± 2.30 in hermaphrodites and 0.64 ± 0.18 in females, Table 1 and Fig. 5). Over three-quarters of the flowers developed into fruits and those fruits produced more than twenty seeds in hermaphrodites both self-pollinated and cross-pollinated by hand, and also in female flowers with pollen grains from males (Table 1 and Fig. 5). The index of self-compatibility was 0.21 in hermaphrodites. However, in hermaphrodites, hand-pollination with pollen grains from males produced as low as 28% of fruit set and 7.12 ± 2.34 sterile seeds (Fig. 5A), because none of these seeds developed endosperms or embryos. There was no fruit produced in females with pollen grains from hermaphrodites (Table 1 and Fig. 5B).

|
| Fig. 5 The mean seed numbers of control and hand-pollination treatments of hermaphrodites and females. (A) Hermaphrodite plants: Control (HC), Hand self-pollination (HSP), Hand cross-pollination (HCP), Hand cross-pollination with pollen grains from males (HCPM); (B) Female plants: Control (FC), Hand-pollination with pollen grains from hermaphrodites (FPH), Supplemental hand-pollination with pollen grains from males (FM). Different letters beside the bars indicate significant differences at P = 0.05 level. Error bars indicate SE. |
The biological species concept stipulates that species are actually or potentially interbreeding natural populations, which are reproductively isolated from other such groups (de Queiroz, 2005). In this scenario, new species are established when they are reproductively isolated. However, two or more distinct species may be 'cryptic' because they are morphologically indistinguishable (Grundt et al., 2006; Paterson et al., 2016), leading to an underestimation of biodiversity. The current study found D. fruticosa was self-incompatible in hermaphrodites (ISC = 0.21), and male and female individuals produced substantially more pollen and ovule per flower than hermaphrodite individuals (Fig. 3). The DNA content of males and females were twice that of hermaphrodites (Fig. 2). Hand-pollinated treatments showed that unisexual flowers were fully sterile in one sexual function and bisexual flowers were fully fertile for both functions; however, hybridization between unisexual and bisexual flowers produced sterile seeds (Fig. 5). The results support the hypothesis that polyploidy contributes to the coexistence of hermaphroditic and dioecious individuals, and two cryptic biological species (hermaphroditic species and dioecious species) exist in D. fruticosa.
Several studies have shown that the combined appearance of polyploidy and consequent loss of self-incompatibility play a role in the evolution of gender dimorphism (Glick et al., 2016; Miller and Venable, 2000; Sutherland et al., 2018). For example, in most Lycium species, polyploidy has been shown to disrupt the selfincompatibility system, which subsequently led to the evolution of gender dimorphism (Miller et al., 2016; Miller and Venable, 2002). In the Campanula rotundifolia polyploid complex, loss of self-incompatibility is associated with genome duplication and increases with increased ploidy (Sutherland et al., 2018). According to our findings, polyploidy is associated with gender dimorphism in D. fruticosa and supports the evolutionary transition from hermaphroditism to dioecy.
Polyploidization causes considerable cytological and morphological changes, which means that ecological requirements may differ substantially between diploids and their polyploid derivatives (Ramsey and Schemske, 2002; Soltis et al., 2014). For instance, polyploids generally have a higher stress tolerance than diploids, which is supported by the observation that present-day polyploids often occur at increased frequencies in newly-created, disrupted, or harsh environments (Guo et al., 2016; Ramsey, 2011; Rice et al., 2019; Van de Peer et al., 2017). Because of the advantages of polyploidy, polyploids may occupy newly available niches or harsher environments created by recent uplifts and the climatic oscillations of the Qinghai-Tibet Plateau (Ma et al., 2014; Mangla et al., 2019). The Qinghai-Tibet Plateau has the highest and most extreme environment on earth; furthermore, repeated glaciation events since the Quaternary have created new habitats for polyploid colonization (Wen et al., 2014). Many studies have shown that under cold treatments and in harsh and fluctuating environments the frequency of unreduced games increases (Ramsey and Schemske, 1998; Van de Peer et al., 2017), which is known to facilitate polyploid formation (Bretagnolle and Thompson, 1995). Polyploids may occur more frequently in vacant and disturbed habitats because of high niche availability and lower competition (Brochmann et al., 2004; Parisod et al., 2010). In formerly glaciated regions, hybridizations between divergent allopatric populations may have led to the formation and establishment of new allopolyploid lineages for secondary contact (Rice et al., 2019; Stebbins, 1984). In Dasiphora fruticosa, the frequent appearance of polyploids and the rapid range expansion across the whole Plateau may have led to high genetic diversity and increased divergence. These factors may explain why high ploidy dioecious plants occupy higher elevations than low ploidy hermaphroditic plants in the QinghaiTibet Plateau (personal observation).
To survive in newly available niches or harsher environments, newly formed polyploids need to overcome minority cytotype exclusion. Specifically, polyploids must find polyploid partners to mate with in a community in which diploids are dominant and suitable polyploid mating partners are scarce (Van de Peer et al., 2017). We found that the percentage of unisexual individuals in the population was less than 16%, and females only produced a few fruits and seeds (Table 1 and Fig. 5). Both male and perfect flowers have more than 96% of pollen grains removed by pollinators (Fig. 4A), and the number of pollen grains on the stigma is far more than the number of ovules in natural pollination (Fig. 4B). These results indicate that seed production is limited not by pollen quantity but by pollen quality (Figs. 4B and 5). This can be explained by the transfer of pollen grains between low ploidy hermaphroditic plants and high ploidy dioecious plants of D. fruticosa. The flowers of D. fruticosa are radially symmetrical and can attract several pollinators (Denisow et al., 2013; VanOverbeke et al., 2007). Generalist pollinators such as flies and bees transfer pollen grains from hermaphroditic plants to females, or from males to hermaphrodites because of the higher density of hermaphroditic plants (Ehlers and Bataillon, 2007; Yakimowski and Barrett, 2016). Pollinators frequently have been observed to move between low ploidy hermaphroditic plants and high ploidy dioecious plants in D. fruticosa (personal observation). Thus, heteroploidy pollen transfer may result in reproductive isolation of the interacting plants (Fang and Huang, 2016a; Flanagan et al., 2009). Another explanation relies on interspecific pollen transfer. Heterospecific pollen can interfere with conspecific pollen deposition, germination, pollen tube growth, ovule fertilization, and seed development (Caruso and Alfaro, 2000; Galen and Gregory, 1989; Morales and Traveset, 2008; Wilcock and Neiland, 2002). In natural communities, generalist flowering plants often share pollinators and receive pollen from various species (Fang and Huang, 2016a; Memmott, 1999). In the Qinghai-Tibet Plateau, bumblebees and flies are the generalist pollinators and pollinate many plant species (Fang and Huang, 2016a, 2016b), which may explain why, in our experiments, the fruit set of hermaphrodites was high but the seed number in the control treatment was low. Because hermaphrodites of D. fruticosa are self-incompatible (Davidson and Lenz, 1989; Denisow et al., 2013) and produce hundreds of flowers per plant (Fig. 1), pollen transfer within flowers or plants may occur. Those self-pollinated flowers might set few seeds because of the selfincompatibility mechanism.
Conflict of interestThe authors declare that they have no competing interests.
AcknowledgmentsWe thank Ms. Min Qian for her help in the lab. This work was supported by the Natural Science Foundation of China (31570385).
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2019.06.002.
Ashman T.L., Kwok A., Husband B.C., 2013. Revisiting the dioecy-polyploidy association:alternate pathways and research opportunities. Cytogenet. Genome Res, 140: 241-255. DOI:10.1159/000353306 |
Bowden W.M., 1957. Cytotaxonomy of Potentilla fruticosa allied species and cultivars. J. Arnold. Arb, 38: 381-388. |
Bretagnolle F., Thompson J.D., 1995. Gametes with the somatic chromosome number:mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytol, 129: 1-22. DOI:10.1111/j.1469-8137.1995.tb03005.x |
Brochmann C., Brysting A.K., Alsos I.G., Borgen L., Grundt H.H., Scheen A.C., Elven R., 2004. Polyploidy in arctic plants. Biol. J. Linn. Soc, 82: 521-536. DOI:10.1111/j.1095-8312.2004.00337.x |
Caruso C.M., Alfaro M., 2000. Interspecific pollen transfer as a mechanism of competition:effect of Castilleja linariaefolia pollen on seed set of Ipomopsis aggregata. Can. J. Bot, 78: 600-606. |
Charlesworth B., Charlesworth D., 1978. A model for the evolution of dioecy and gynodioecy. Am. Nat, 112: 975-997. DOI:10.1086/283342 |
Davidson C.G., Lenz L.M., 1989. Experimental taxonomy of Potentilla fruticosa. Can. J. Bot, 67: 3520-3528. DOI:10.1139/b89-433 |
de Queiroz K., 2005. Ernst Mayr and the modern concept of species. Proc. Natl. Acad. Sci. U.S.A, 102: 6600-6607. DOI:10.1073/pnas.0502030102 |
Denisow B., Anton S., Szymczak G., 2013. The flowering, pollen production, and insect visitors in the ornamental shrub Potentilla fruticosa L. (Rosaceae). J. Apic. Sci, 57: 95-106. |
Ehlers B.K., Bataillon T., 2007. 'Inconstant males' and the maintenance of labile sex expression in subdioecious plants. New Phytol, 174: 194-211. DOI:10.1111/j.1469-8137.2007.01975.x |
Elkington T.T., 1969. Cytotaxonomic variation in Potentilla fruticosa L. New Phytol, 68: 151-160. DOI:10.1111/j.1469-8137.1969.tb06428.x |
Elkington T.T., Woodell S.R.J., 1963. Potentilla fruticosa L (Dasiphora fruticosa(L) Rydb). J. Ecol, 51: 769-781. DOI:10.2307/2257763 |
Eriksson T., Hibbs M.S., Yoder A.D., Delwiche C.F., Donoghue M.J., 2003. The phylogeny of Rosoideae (Rosaceae) based on sequences of the internal transcribed spacers (ITS) of nuclear ribosomal DNA and the trnL/F region of chloroplast DNA. Int. J. Plant Sci, 164: 197-211. DOI:10.1086/346163 |
Fang Q., Huang S.-Q., 2016a. A paradoxical mismatch between interspecific pollinator moves and heterospecific pollen receipt in a natural community. Ecology, 97: 1970-1978. DOI:10.1002/ecy.1433 |
Fang Q., Huang S.Q., 2016b. Plant-pollinator interactions in a biodiverse meadow are rather stable and tight for 3 consecutive years. Integr. Zool, 11: 199-206. DOI:10.1111/1749-4877.12190 |
Flanagan R.J., Mitchell R.J., Knutowski D., Karron J.D., 2009. Interspecific pollinator movements reduce pollen deposition and seed production in Mimulus ringens(Phrymaceae). Am. J. Bot, 96: 809-815. DOI:10.3732/ajb.0800317 |
Galen C., Gregory T., 1989. Interspecific pollen transfer as a mechanism of competition:consequences of foreign pollen contamination for seed set in the alpine wildflower, Polemonium-viscosum. Oecologia, 81: 120-123. DOI:10.1007/BF00377020 |
Glick L., Sabath N., Ashman T.L., Goldberg E., Mayrose I., 2016. Polyploidy and sexual system in angiosperms:is there an association? Am. J. Bot, 103: 1223-1235. DOI:10.3732/ajb.1500424 |
Grewal M.S., Ellis J.R., 1972. Sex determination in Potentilla fruticosa. Heredity, 29: 359-362. DOI:10.1038/hdy.1972.99 |
Grundt H.H., Kjolner S., Borgen L., Rieseberg L.H., Brochmann C., 2006. High biological species diversity in the arctic flora. Proc. Natl. Acad. Sci. U.S.A, 103: 972-975. DOI:10.1073/pnas.0510270103 |
Guo W., Yang J., Sun X.D., Chen G.J., Yang Y.P., Duan Y.W., 2016. Divergence in ecophysiological responses to drought mirrors the distinct distribution of Chamerion angustifolium cytotypes in the Himalaya-Hengduan Mountains region. Front. Plant Sci, 7: 1329. |
Himmelreich S., Breitwieser I., Oberprieler C., 2012. Phylogeny, biogeography, and evolution of sex expression in the southern hemisphere genus Leptinella(Compositae, Anthemideae). Mol. Phylogenet. Evol, 65: 464-481. DOI:10.1016/j.ympev.2012.07.001 |
Klackenberg L., 1983. The holarctic complex Potentilla fruticosa (Rosaceae). Nord. J. Bot, 3: 181-191. DOI:10.1111/j.1756-1051.1983.tb01061.x |
Lloyd D.G., Schoen D.J., 1992. Self- and cross-fertilization in plants. Ⅰ. functional dimensions. Int. J. Plant Sci, 153: 358-369. |
Ma Y.Z., Li Z.H., Wang X., Shang B.L., Wu G.L., Wang Y.J., 2014. Phylogeography of the genus Dasiphora (Rosaceae) in the Qinghai-Tibetan plateau:divergence blurred by expansion. Biol. J. Linn. Soc, 111: 777-788. DOI:10.1111/bij.12246 |
Mangla Y., Das K., Bali S., Ambreen H., Raina S.N., Tandon R., Goel S., 2019. Occurrence of subdioecy and scarcity of gender-specific markers reveal an ongoing transition to dioecy in Himalayan seabuckthorn (Hippophae rhamnoides ssp. turkestanica). Heredity, 122: 120-132. DOI:10.1038/s41437-018-0084-z |
Maurice S., Fleming T.H., 1995. The effect of pollen limitation on plant reproductive systems and the maintenance of sexual polymorphisms. Oikos, 74: 55-60. DOI:10.2307/3545674 |
Memmott J., 1999. The structure of a plant-pollinator food web. Ecol. Lett, 2: 276-280. DOI:10.1046/j.1461-0248.1999.00087.x |
Miller J.S., Kamath A., Husband B.C., Levin R.A., 2016. Correlated polymorphism in cytotype and sexual system within a monophyletic species, Lycium californicum. Ann. Bot, 117: 307-317. |
Miller J.S., Venable D.L., 2000. Polyploidy and the evolution of gender dimorphism in plants. Science, 289: 2335-2338. DOI:10.1126/science.289.5488.2335 |
Miller J.S., Venable D.L., 2002. The transition to gender dimorphism on an evolutionary background of self-incompatibility:an example from Lycium (Solanaceae). Am. J. Bot, 89: 1907-1915. DOI:10.3732/ajb.89.12.1907 |
Morales C.L., Traveset A., 2008. Interspecific pollen transfer:magnitude, prevalence and consequences for plant fitness. Crit. Rev. Plant Sci, 27: 221-238. DOI:10.1080/07352680802205631 |
Pannell J.R., Obbard D.J., Buggs R.J.A., 2004. Polyploidy and the sexual system:what can we learn from Mercurialis annua? Biol. J. Linn. Soc, 82: 547-560. DOI:10.1111/j.1095-8312.2004.00340.x |
Parisod C., Holderegger R., Brochmann C., 2010. Evolutionary consequences of autopolyploidy. New Phytol, 186: 5-17. DOI:10.1111/j.1469-8137.2009.03142.x |
Paterson I.D., Mangan R., Downie D.A., Coetzee J.A., Hill M.P., Burke A.M., Downey P.O., Henry T.J., Compton S.G., 2016. Two in one:cryptic species discovered in biological control agent populations using molecular data and crossbreeding experiments. Ecol. Evol, 6: 6139-6150. DOI:10.1002/ece3.2297 |
Perry L.E., Pannell J.R., Dorken M.E., 2012. Two's company, three's a crowd:experimental evaluation of the evolutionary maintenance of trioecy in Mercurialis annua (Euphorbiaceae). PLoS One, 7: e35597. DOI:10.1371/journal.pone.0035597 |
Ramirez N., Brito Y., 1990. Reproductive biology of a tropical palm swamp community in the Venezuelan Llanos. Am. J. Bot, 77: 1260-1271. DOI:10.1002/j.1537-2197.1990.tb11378.x |
Ramsey J., 2011. Polyploidy and ecological adaptation in wild yarrow. Proc. Natl. Acad. Sci. U.S.A, 108: 7096-7101. DOI:10.1073/pnas.1016631108 |
Ramsey J., Ramsey T.S., 2014. Ecological studies of polyploidy in the 100 years following its discovery. Philos. Trans. R. Soc. Lond. B Biol. Sci, 369: 1-20. |
Ramsey J., Schemske D.W., 1998. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu. Rev. Ecol. Syst, 29: 467-501. DOI:10.1146/annurev.ecolsys.29.1.467 |
Ramsey J., Schemske D.W., 2002. Neopolyploidy in flowering plants. Annu. Rev. Ecol. Syst, 33: 589-639. DOI:10.1146/annurev.ecolsys.33.010802.150437 |
Renner S.S., 2014. The relative and absolute frequencies of angiosperm sexual systems:dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot, 101: 1588-1596. DOI:10.3732/ajb.1400196 |
Rice A., Smarda P., Novosolov M., Drori M., Glick L., Sabath N., Meiri S., Belmaker J., Mayrose I., 2019. The global biogeography of polyploid plants. Nat. Ecol. Evol, 3: 265-273. DOI:10.1038/s41559-018-0787-9 |
Ross M.D., 1982. Five evolutionary pathways to subdioecy. Am. Nat, 119: 297-318. DOI:10.1086/283911 |
Soltis P.S., Liu X., Marchant D.B., Visger C.J., Soltis D.E., 2014. Polyploidy and novelty:Gottlieb's legacy. Philos. Trans. R. Soc. Lond. B Biol. Sci, 369: 20130351. DOI:10.1098/rstb.2013.0351 |
Spigler R.B., Ashman T.L., 2012. Gynodioecy to dioecy:are we there yet? Ann. Bot, 109: 531-543. DOI:10.1093/aob/mcr170 |
Stebbins G.L., 1984. Polyploidy and the distribution of the arctic-alpine flora:new evidence and a new approach. Bot. Helv, 94: 1-13. |
Sutherland B.L., Quarles B.M., Galloway L.F., 2018. Intercontinental dispersal and whole-genome duplication contribute to loss of self-incompatibility in a polyploid complex. Am. J. Bot, 105: 249-256. DOI:10.1002/ajb2.1027 |
Van de Peer Y., Mizrachi E., Marchal K., 2017. The evolutionary significance of polyploidy. Nat. Rev. Genet, 18: 411-424. DOI:10.1038/nrg.2017.26 |
VanOverbeke D.R., Neff P.K.K., Fettig S.M., 2007. Potentilla fruticosa (Rosaceae) as a nectar plant for butterflies. J. Lepid. Soc, 61: 222-227. |
Wen J., Zhang J.Q., Nie Z.L., Zhong Y., Sun H., 2014. Evolutionary diversifications of plants on the Qinghai-Tibetan plateau. Front. Genet, 5: 4. |
Wilcock C., Neiland R., 2002. Pollination failure in plants:why it happens and when it matters. Trends Plant Sci, 7: 270-277. DOI:10.1016/S1360-1385(02)02258-6 |
Wolf D.E., Takebayashi N., 2004. Pollen limitation and the evolution of androdioecy from dioecy. Am. Nat, 163: 122-137. DOI:10.1086/380493 |
Yakimowski S.B., Barrett S.C., 2016. The role of hybridization in the evolution of sexual system diversity in a clonal, aquatic plant. Evolution, 70: 1200-1211. DOI:10.1111/evo.12941 |
Zhang S.D., Jin J.J., Chen S.Y., Chase M.W., Soltis D.E., Li H.T., Yang J.B., Li D.Z., Yi T.S., 2017. Diversification of rosaceae since the late cretaceous based on plastid phylogenomics. New Phytol, 214: 1355-1367. DOI:10.1111/nph.14461 |



