b. Key Laboratory of Three Gorges Reservoir Region's Eco-Environment, Ministry of Education, Chongqing University, Chongqing 400045, China;
c. Graduate School of Horticulture, Chiba University, 648 Matsudo, Chiba 271-8510, Japan;
d. Institute of Botany, Yunnan University, Kunming, Yunnan 650091, China;
e. Southern Institute of Ecology, Vietnam Academy of Science and Technology, Ho Chi Minh City, Viet Nam;
f. Research Institute of Resource Insects, Chinese Academy of Forestry, Kunming, Yunnan 650224, China;
g. School of Ecological and Environmental Sciences, East China Normal University, Shanghai 200241, China;
h. Pacific Gas and Electric Company, 3401 Crow Canyon Road, San Ramon, CA 94583, USA;
i. The Academy of Natural Science, 1900 Benjamin Franklin Parkway, Philadelphia, PA 19103, USA;
j. The Nature Conservancy Society of Japan, Mitoyo Bldg.2F, 1-16-10 Shinkawa, Chuo-ku, Tokyo 104-0033, Japan
East Asia harbors an exceptional assemblage of monotypic genera of Paleogene-Neogene and even Cretaceous age relict plants (Qian, 2001, Manchester et al., 2009, Tang et al., 2018). Today, some of these genera are reduced to a single species with small wild populations occupying specific habitats in subtropical China, as exemplified by Ginkgo biloba, Metasequoia glyptostroboides, Glyptostrobus pensilis, Cathaya argyrophylla, Taiwania cryptomerioides, and Davidia involucrata (Tang and Ohsawa, 2002, Tang et al., 2011, Tang et al., 2012, Zheng et al., 2011, Tang, 2015, He et al., 2015, Qian et al., 2016). Habitat loss, degradation, and overexploitation have played significant and substantial roles in the decline of wild plants in China during the 20th and 21st centuries. At least 200 species have become extinct in the last 50 years (Chinese State Report on Biodiversity Editorial Committee, 1998), and about 5000 species are currently threatened or on the verge of extinction, leaving China to occupy one of the highest priorities for global biodiversity conservation (Volis, 2016). The survival of wild populations of such relict species strongly depends on their ability to regenerate in natural habitats (Tang, 2015). Detailed knowledge of relict plants' forest stands and demographic structure is often a pre-requisite to implementing effective conservation and management strategies for the species and habitats (e.g. Tang et al., 2011, Tang et al., 2012, He et al., 2015).
Glyptostrobus pensilis, commonly known as Chinese water pine or Chinese swamp cypress, is a deciduous, monoecious, wind-pollinated conifer of the Cupressaceae (previously Taxodiaceae). The fossil record of Glyptostrobus extends from the Cretaceous to the early Pleistocene (LePage, 2007). The genus was distributed widely in North America and Eurasia during the Eocene and Miocene epochs (Fig. 1A). Increasing global aridity and cooling, as well as landscape stabilization together with increasing competition for resources and habitat by representatives of the Pinaceae, seem to have forced the genus out of North America, Europe, and most of Asia during the Miocene and Pliocene (LePage, 2007). The youngest fossil record of the genus, is from the late early Pleistocene (ca. 1.2 Ma) of central Japan (Momohara, 2011). Today G. pensilis grows in swamps or on waterlogged soils along river banks, streams, floodplains, ponds, paddies, and low-lying wet areas in the subtropics of Fujian, Jiangxi, Guangdong, Hunan, Guangxi, and Yunnan Provinces in southern China, the tropics of central Vietnam and eastern Laos (Li and Xia, 2004, Farjon, 2005, Averyanov et al., 2009). The species resembles Taxodium distichum, which is common throughout the southeastern United States in general appearance, form, and habit, though the max. DBH (120 cm) and height (30 m) of G. pensilis are much smaller (300 cm DBH, 40 m tall for T. distichum) (Earle, 2018). When growing in water, G. pensilis produces knee-like aerial roots (pneumatophores) that are presumed to assist in bringing oxygen to the root system and/or providing stability to the trees growing in a soft substrate.
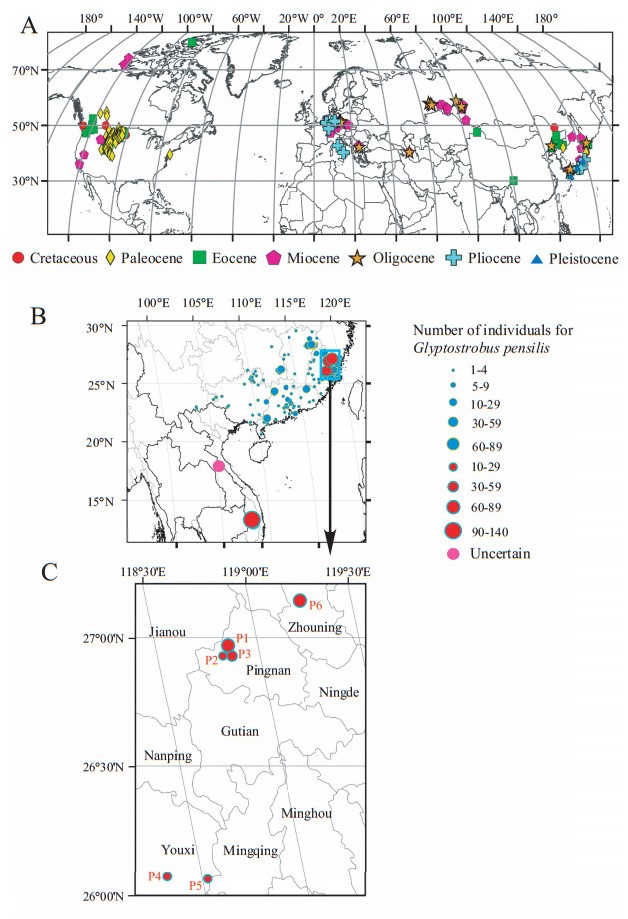
|
| Fig. 1 Distribution of fossil Glyptostrobus and extant Glyptostrobus pensilis (A) Fossil occurrences of Glyptostrobus. (B) Present-day distribution of G. pensilis. (C) The locations of six populations of G. pensilis in Fujian, China. Red circles are the wild populations in Fujian and Vietnam, presented in this study. A pink circle indicates the population size is uncertain. P=Population. Data sources for (A): Miki, 1941, Miki, 1948, Tanai, 1961, Shimazu and Teraoka, 1962, Tanai and Suzuki, 1963, Matsuo, 1967, Ishida, 1970, Tanai, 1970, Hayashi, 1975, WGCPC (Writing Group of Cenozoic Plants of China), 1978, Guo and Li, 1979, Tanai, 1979, Chen et al., 1983, Fujita and Kasama, 1983, Tanai and Uemura, 1983, Hase, 1988, Matthews and Ovenden, 1990, Ina, 1992, Momohara, 1992, Liu et al., 1996, Jin and Sang, 1998, Geng et al., 1999, Uemura et al., 1999, Martinetto, 2001; Guo and Zhang, 2002, Greenwood et al., 2005, Yamakawa et al., 2008, Bozukov and Tsenov, 2012, Mathewes et al., 2016, PALEOBIDB (Paleobiological database), 2017. Data sources for (B): Electronic Supplementary Information S1 |
The plant fossil record indicates the genus was much more widespread from the Cretaceous to the Pliocene, with large areas covered with bottom-land Glyptostrobus forests. In southern China, G. pensilis roots were discovered in a peaty soil over large areas in Guangdong Province. The estimated age of some of these roots is 1000 years, indicating that G. pensilis trees once formed large forest stands (Xu and Li, 1959). Today G. pensilis exists only in small and isolated patches. In eastern Laos, G. pensilis trees are rare and less than 250 mature individuals are estimated to occur in small stands in Borikhamxai and Khammouan Provinces (Averyanov et al., 2014). In Vietnam, two populations are known, 18 trees in Trap K'sor and 140 trees in Ea Ral. In addition, four trees have been identified at three sites near Buon Ho Town and Ea Ho Commune.
In China, G. pensilis has suffered heavy losses from over-logging, drainage, and cultivation during the 21st century. Agricultural encroachment and excessive harvest of its decay-resistant wood, which is valuable for making coffins, boats, lifebuoys, bridges, buildings, furniture, and cork all contributed to the accelerated disappearance of this species. The genetic diversity of G. pensilis at both the species level and within populations is low (Li and Xia, 2005). It was once claimed that there were no G. pensilis remaining in the wild in China (Thomas et al., 2011). However, a recent Chinese national project "Investigating Ancient and Big Trees" has led to new discoveries of wild G. pensilis in the Chinese subtropics. These wild populations in Fujian, Jiangxi, Hunan, Guangdong, and Yunnan Provinces (Fig. 1B) are small and scattered, occur in rice paddies, secondary shrub land, planted forests, or human settlements and support the contention that Glyptostrobus is on the verge of extinction. It is listed as a first-grade protected species in the Catalog of the National Protected Key Wild Plants of 1999 and 2011 and, according to the IUCN evaluation criteria, it is categorized as "Critically endangered, " with a decreasing number of individuals (Thomas et al., 2011).
Vertical stratifications of G. pensilis forest stands can provide information of the light condition in the understory for regeneration. Understanding species diversity of the fragmented forest stands is important for evaluation of the local threatened status because species in a community often interact and depend on each other. Clarification of forest stand characteristics, population sizes, and regeneration status provide valuable ecological indicators and inform future restoration activities. Unfortunately, understanding of the vertical stratification and species composition and species diversity of G. pensilis swamp forest stands, as well as the population status and age structure of this endangered species in China, are unavailable, though wild populations of G. pensilis have been discovered in Fujian Province, China (e.g. Zheng et al., 2011, Wu et al., 2008, Huang, 2013, Wu, 2016, Lei, 2017). The surviving G. pensilis populations and forest stands of Vietnam provide an opportunity to compare the status of this species of Fujian Province with that of Vietnam. The comparison would bring insights to evaluation of the current conservation measures. Therefore, the objectives of this paper are (1) to clarify the swamp community characteristics and population structure of wild G. pensilis in Fujian Province; and (2) to compare these Chinese data with those of wild forest stands and populations in Vietnam.
2. Methods 2.1. Study areaWe investigated six natural G. pensilis stands in south-central and southeastern Fujian Province. The study sites are located at 26°03.687′N−27°8.7167′N, 118°19.637′−119°14.6167′E in Shangloucun, Lingfengcun, Lingfenglu in Pingnan County, Dongshancun and Shanlingcun in Youxi County, and Xianfengshan in Zhouning County (Fig. 1B, C). The elevational range of these populations extends from 1025 to 1300 m above sea level (asl) and is characteristic of the subtropical moist evergreen broad-leaved forest zone with a humid, warm climate. Within this zone, natural azonal forest communities of coniferous G. pensilis forest stands are found.
The study area is characterized by a subtropical, humid climate that is largely controlled by the East Asia monsoon. The mean annual temperature ranges from 11 to 19 ℃ with a warm month mean of 24–29 ℃ (July) and a cold month mean of 5–10 ℃ (January). The mean annual precipitation is 1600–1850 mm, of which about 80% falls between March and October. The monthly relative humidity is greater than 83%.
2.2. Data collection and analysisIn July 2015 and August 2016, we sought natural G. pensilis communities in Fujian Province. We found six natural swamp forests dominated by G. pensilis in Pingnan, Youxi, and Zhouning Counties. Local Forestry Bureau staff interviewed elderly residents of local villages and used village records to confirm estimates of the land areas for the six swamp forests and that these swamp forests have never been used. Among these forests, the swamp forest in Shanlingcun, Youxi (Plot 5), which is located in an extremely remote and isolated area, was only discovered by local villages recently, i.e., April, 2014. Experts at the Forestry Bureau of Youxi Country and Fujian Province confirmed the pristine natural conditions of this swamp forest (www.fjyx.gov.cn). In addition, our own field investigations have verified the natural status of these six swamp forests by comparing species composition and diversity of natural G. pensilis swamp forests and the habitats of planted G. pensilis populations. In contrast to the six natural G. pensilis swamp forests, forests of planted G. pensilis have only one tree species in the overstory, i.e., G. pensilis. In the understory of these planted forests, native species are very rare; in some cases, no plants are present in the understory, whereas in other forests, invasive species are present. We obtained 24 increment cores from the trunks of wild trees of varying DBHs. A core was taken from each trunk above ground level at 1.3 m, and the length of time from the position at 1.3 m in height to ground level was estimated to be 10 years (on average) as based on field observations made in past decades. Tree ages were determined using the software WinDENRO tree ring analysis system.
To include all individuals of G. pensilis in each study site, we established plots of various sizes (Plot 1: 4800 m2 in Shangloucun; Plot 2: 1600 m2 in Lingfengcun; and Plot 3: 800 m2 in Lingfenglu, Pingnan County; Plot 4: 3200 m2 in Dongshanchun; Plot 5: 10, 000 m2 in Shanlingcun, Youxi County; and Plot 6: 10, 000 m2 in Xianfengshan, Zhouning County, Fujian Province) in July–August of 2016 and 2017. The plots among Pingnan, Youxi and Zhouning are widely separated from each other; the geographic direct distance among them are from 50 km to 160 km. Though the first three plots are found within Pingnan, they are in fact isolated from each other because they are separated by mountains or valleys. Plots 1, 2 3 are located in different watersheds. In Youxi, Plots 4 and 5 are also in different watersheds and isolated by mountains. Therefore, we consider the six plots to be six populations. We used the relative basal area method (Ohsawa, 1984) for our vegetation sampling. In a community dominated by a single species, its relative dominance may be stated at 100%. If, however, two species share dominance, the relative dominance of each should ideally be 50%, or if there are three co-dominants, 33.3%, and so on. The number of dominant species is that which shows the least deviation between the actual relative dominance values and the expected percent share of the corresponding codominant-number model. The deviation (d) is calculated by the following equation:
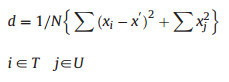
|
where xi is the actual percent share (here relative basal area is adopted) of the top species (T), i.e., in the top dominant in the one-dominant model, or the two top dominants in the two-dominants model, and so on; x' is the ideal percent share based on the model as mentioned above, and xj is the percent share of the remaining species (U). N is total number of species.
For the overstory, a species inventory was performed for all individuals at least 1.3 m in height in each plot. All were tagged, numbered, and identified to species level, whether healthy, unhealthy, or dead. We measured the DBH (diameter at breast height) and height of trees ≥ 1.3 m tall using a height-measuring pole. Tree stems were classified into three groups based on their vertical position, crown position, and height: canopy (at least 18 m tall); sub-canopy (8–18 m tall); and shrub layer (1.3 m and 8 m tall). The level of vitality was assessed by estimating the percent occupied by the living part of the tree canopy. Values ranged from 100% when all branches in the canopy were covered with normal healthy leaves to 0% when the canopy had no leaves (Averyanov et al., 2009). All woody species ranging in height from 5 cm to < 50 cm tall for seedlings and 50 cm to < 130 cm for saplings in the understory of each plot were identified, counted, and measured for height and percent cover. Each plot was divided into 10-m x 10-m subplots. In each subplot, we set up five 1-m x 1-m squares selected for investigation for the herbaceous species. Five 1-m x 1-m squares were respectively located in the four corners and the center of each subplot. Herb taxa were identified and the coverage and number of individuals of each species were recorded. In order to compare the populations of Glyptostrobus in Fujian Province and in Vietnam, in July 2018 we conducted fieldwork for the G. pensilis stands in Vietnam in addition to the work by Averyanov et al. (2009), using the same measurement criteria used in the Fujian Province forest stands.
Diversity was calculated for each forest stand using species richness (number of species), the reciprocal of the Berger–Parker index (1/d) (Magurran, 1988) and the Shannon–Wiener index (Pielou, 1969). The Sø rensen's similarity index (beta diversity) was used to compare the forest stands of Fujian Province, China with those of Vietnam (Sørensen, 1948).
3. Results 3.1. Community characteristicsSix forest stands dominated by G. pensilis are located in Pingnan (3 stands), Youxi (2 stands), and Zhouning (1 stand) Counties (Fig. 1, Fig. 2). They typically grow in swamps with 15–60 cm of standing water being present throughout much of the year and at elevations that range from 1025 to 1300 m asl.
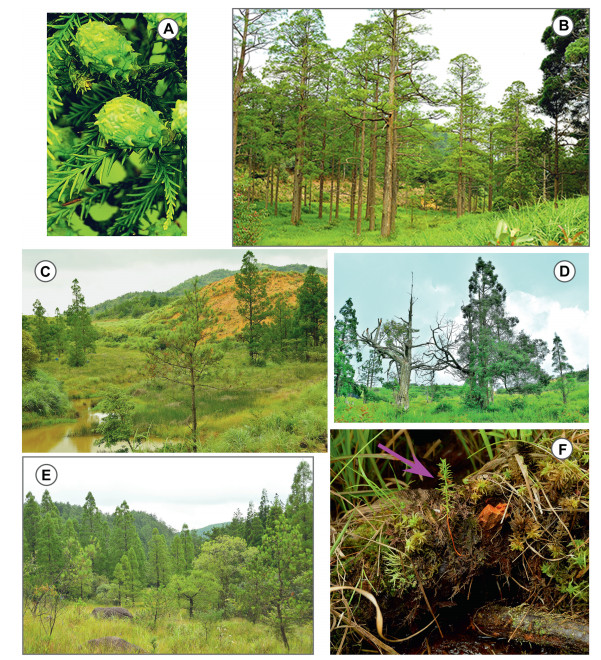
|
| Fig. 2 Glyptostobus pensilis orest stands in Fujian Province (A) Seed cones of G. pensilis in July. (B) A swamp forest stand of G. pensilis in Shangloucun, Pingnan. (C) A swamp forest stand of G. pensilis in Shanlingcun, Youxi. (D) A swamp forest stand of G. pensilis in Dongshancun, Youxi, showing some dead trees. (E) A swamp forest stand of G. pensilis in Xianfengshan, Zhouning. (F) A seedling of G. pensilis growing on a fallen log. Photographs by Cindy Q. Tang. |
The vertical stratifications of the forest stands are shown in Fig. 3A–F. The maximum tree height was 31 m. All six plots were structurally simple and characterized by sparse (15–50%) canopy cover and once the trees reached approximately 8 m in height, the sub-canopies and canopies were dominated almost exclusively by G. pensilis. Cryptomeria japonica var. sinensis (naturally regenerated from planted C. japonica var. sinensis in the surrounding areas) (Plot 1, Fig. 3A) and/or Pinus massoniana (Plots 1, 2, 6, Fig. 3A, B, F) were occasionally present. In the shrubby layer, individuals of Alnus trabeculosa, Hydrangia paniculata, and P. massoniana were sparsely distributed, except A. trabeculosa was abundant in Plot 4 (Fig. 3D). In Pingnan, G. pensilis tree heights ranging from 4 to 8 m were rare. Other small tree and shrub elements that were identified in the 6 plots included Nyssa sinensis, Ilex crenata, Ilex serrata, Viburnum luzonicum, Salix chienii, Lindera aggregata (Table 1). In the herbaceous layer, Molinia hui, Ischaemum anthephoroides, Isachne globosa, Osmundastrum cinnamomeum, Polygonum microcephalum were generally dominant (Table 2). Few seedlings of G. pensilis, C. japonica var. sinensis, Spiraea japonica, or Symplocos paniculata were found on the forest floor. In these stands there were 43 woody species representing 32 genera and 22 families, and 83 herbaceous species representing 70 genera of 36 families (Table 1, Table 2).
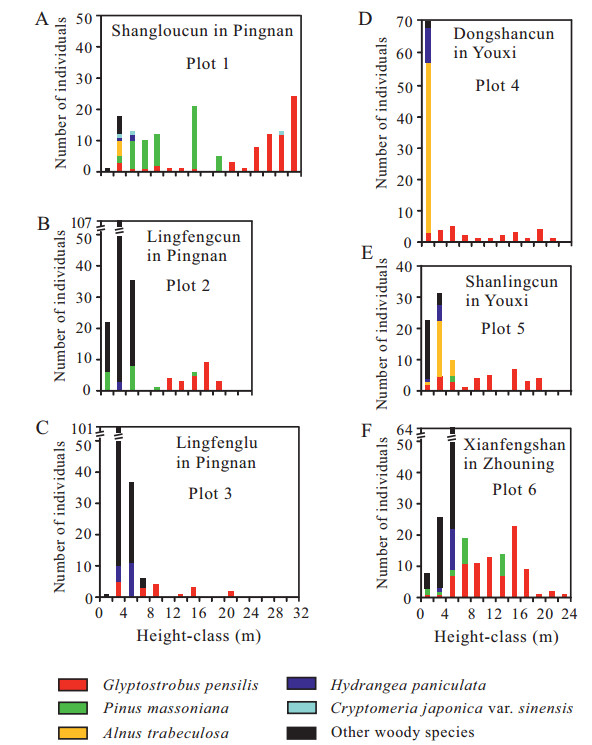
|
| Fig. 3 Frequency distribution of woody species based on height (H ≥ 1.3 m) in the six swamp forest stands in Fujian (A)−(F) |
| Plot | Plot 1 | Plot 2 | Plot 3 | Plot 4 | Plot 5 | Plot 6 |
| Plot area (m2) | 4800 | 1600 | 600 | 2500 | 15, 000 | 5500 |
| Elevation (m) | 1249 | 1158 | 1180 | 1132 | 1025 | 1300 |
| Plot site | Shangloucun | Lingfengcun | Lingfenglu | Dongshancun | Shanlingcun | Xianfengshan |
| in Pingnan | in Pingnan | in Pingnan | in Youxi | in Youxi | in Zhouning | |
| Species | RBA (%) | RBA (%) | RBA (%) | RBA (%) | RBA (%) | RBA (%) |
| Glyptostrobus pensilis | 86.5 | 96.2 | 96.3 | 99.96 | 99.3 | 97.6 |
| Pinus massoniana | 11.6 | 2.7 | 0.1 | 1.2 | ||
| Cryptomeria japonica var. sinensis | 1.9 | |||||
| Toxicodendron succedaneum | 0.02 | |||||
| Sassafras tsumu | 0.01 | |||||
| Alnus trabeculosa | 0.007 | 0.03 | 0.4 | |||
| Euscaphis japonica | 0.004 | |||||
| Hydrangea paniculata | 0.001 | 0.02 | 0.1 | 0.008 | 0.09 | 0.08 |
| Viburnum luzonicum | 0.4 | 0.0005 | ||||
| Rhododendron simsii | 0.2 | 0.3 | ||||
| Salix chienii | 0.2 | 2.3 | 0.8 | |||
| Lindera glauca | 0.07 | 0.003 | 0.004 | |||
| Litsea cubeba | 0.07 | 0.004 | ||||
| Rhododendron henryi | 0.05 | 0.2 | ||||
| Eurya chinensis | 0.04 | |||||
| Ilex serrata | 0.04 | 0.06 | 0.05 | |||
| Adinandra glischroloma | 0.02 | 0.01 | ||||
| Dalbergia mimosoides | 0.004 | |||||
| Rosa laevigata | 0.001 | 0.001 | 0.002 | |||
| Ilex crenata | 0.8 | 0.001 | ||||
| Nyssa sinensis | 0.3 | |||||
| Lindera aggregata | 0.01 | |||||
| Rhamnus crenata | 0.001 | 0.003 | ||||
| Rhaphiolepis indica | 0.0003 | |||||
| Glochidion puberum | 0.004 | |||||
| Symplocos anotiiala | 0.003 | |||||
| Eurya macartneyi | 0.001 |
| Plot | Plot 1 | Plot 2 | Plot 3 | Plot 4 | Plot 5 | Plot 6 |
| Plot area (m2) | 4800 | 1600 | 600 | 2500 | 15, 000 | 5500 |
| Elevation (m) | 1249 | 1158 | 1180 | 1132 | 1025 | 1300 |
| Plot site | Shangloucun | Lingfengcun | Lingfenglu | Dongshancun | Shanlingcun | Xianfengshan |
| in Pingnan | in Pingnan | in Pingnan | in Youxi | in Youxi | in Zhouning | |
| Coverage | Coverage | Coverage | Coverage | Coverage | Coverage | |
| Species | (%) | (%) | (%) | (%) | (%) | (%) |
| Isachne globosa | 25 | 8 | + | 31 | + | 6 |
| Ischaemum anthephoroides | 22 | 1 | + | 12 | 17 | 1 |
| Molinia hui | 20 | 45 | + | 1 | 15 | |
| Osmundastrum cinnamomeum | 13 | 9 | 24 | + | 17 | + |
| Cyperus haspan | 3 | 3 | + | 1 | 1 | + |
| Juncus bufonius | 3 | + | 1 | 2 | ||
| Carex dickinsii | 2 | + | ||||
| Cryptomeria japonica var. sinensis | + | |||||
| Drosera rotundifolia | + | + | + | + | ||
| Eleocharis tetraquetra | + | + | 7 | + | + | |
| Euphorbia esula | + | + | ||||
| Glyptostrobus pensilis | + | + | + | + | ||
| Hypericum japonicum | + | + | 2 | + | + | |
| Ligularia japonica | + | 15 | ||||
| Lobelia sessilifolia | + | 10 | + | 2 | ||
| Scirpus rosthornii | + | + | + | 1 | ||
| Viola verecunda | + | 1 | + | 5 | + | + |
| Hemerocallis fulva | + | + | ||||
| Miscanthus sacchariflorus | 4 | + | + | 16 | 10 | |
| Ostericum grosseserratum | 4 | + | + | + | ||
| Scirpus juncoides | 3 | |||||
| Nymphaea tetragona | 2 | |||||
| Phragmites karka | 2 | |||||
| Aster ageratoides | 1 | + | + | |||
| Carex glossotigma | 1 | 9 | + | + | ||
| Cicuta virosa | 1 | + | + | |||
| Cirsium chinense | 1 | + | + | + | ||
| Juncus effusus | 1 | 1 | 17 | |||
| Lysimachia fortunei | 1 | + | + | |||
| Polygonum microcephalum | 1 | 30 | 3 | 1 | ||
| Calamagrostis epigeios | + | 3 | 4 | + | ||
| Eurya macartneyi | + | + | ||||
| Cyclosorus parasiticus | + | + | ||||
| Habenaria schindleri | + | + | + | |||
| Osmunda japonica | + | + | + | |||
| Parathelypteris glanduligera | + | 1 | 11 | + | ||
| Pyrrosia lingua | + | + | ||||
| Rabdosia amethystoides | + | |||||
| Rhamnus crenata | + | + | + | |||
| Rhynchospora chinensis | + | 3 | 1 | 19 | ||
| Rosa laevigata | + | |||||
| Rosa lucidissima | + | |||||
| Rosa roxburghii | + | + | ||||
| Rubia cordifolia | + | |||||
| Smilax china | + | + | ||||
| Viburnum luzonicum | + | + | ||||
| Leersia hexandra | 27 | |||||
| Galium trifidum | + | + | ||||
| Houttuynia cordata | + | + | ||||
| Lonicera japonica | + | + | ||||
| Lysimachia heterogenea | + | |||||
| Lythrum sp. | + | |||||
| Neanotis boerhaavioides | + | + | ||||
| Sagittaria sagittifolia | + | |||||
| Triadenum breviflorum | + | |||||
| Eriocaulon faberi | 17 | 3 | ||||
| Alnus trabeculosa | + | + | ||||
| Eriocaulon nipponicum | 3 | + | ||||
| Oenanthe thomsonii subsp. stenophylla | 2 | |||||
| Haloragis micrantha | 1 | + | + | |||
| Isachne nipponensis | 1 | + | ||||
| Polygonum sp. | 1 | |||||
| Isodon sp. | 1 | + | + | |||
| Dryopteris peninsalae | + | |||||
| Emilia sonchifolia | + | + | ||||
| Kalimeris indica | + | |||||
| Lepisorus thunbergianus | + | |||||
| Luzula sp. | + | |||||
| Osbeckia chinensis | + | + | ||||
| Scleria biflora | + | + | 1 | |||
| Eriocaulon buergerianum | 15 | |||||
| Lindera erythrocarpa | + | |||||
| Adinandra glischroloma | + | + | ||||
| Adinandra millettii | + | |||||
| Arundinella hirta | + | |||||
| Caldesia reniformis | + | |||||
| Cephalotaxus fortunei | + | |||||
| Dioscorea japonica | + | |||||
| Fordiophyton fordii | + | + | ||||
| Ilex asprella | + | + | ||||
| Lepisorus lewissi | + | |||||
| Ligustrum retusum | + | |||||
| Lindera glauca | + | |||||
| Rhaphiolepis indica | + | |||||
| Rubus corchorifolius | + | |||||
| Rubus impressinervius | + | |||||
| Scripus triqueter | + | |||||
| Vaccinium carlesii | + | + | ||||
| Eleocharis congesta | 8 | |||||
| Oplismenus undulatifolius | 6 | |||||
| Arisaema sp. | + | |||||
| Athyrium iseanum | + | |||||
| Cerastium sp. | + | |||||
| Dryopteris championii | + | |||||
| Elaeagnus henryi | + | |||||
| Gynura crepidioides | + | |||||
| Ilex crenata | + | |||||
| Sp. of Fabaceae | + | |||||
| Lespedeza formosa | + | |||||
| Melastoma dodecandrum | + | |||||
| Paederia foetida | + | |||||
| Parathelypteris nipponica | + | |||||
| Pellionia brevifolia | + | |||||
| Pseudocystopteris schizochlamys | + | |||||
| Siraitia grosvenorii | + | |||||
| Smilax sp. | + | |||||
| Spiraea japonica | + | |||||
| Symplocos paniculata | + | |||||
| Sp. of Apiaceae | + |
Fig. 4A–C shows the overstory of each stand had very low values in species richness (4–13 species), Reciprocal of Berger–Parker index (1–1.2), and Shannon–Wiener index (0.05–0.65), whereas the understory of each stand had much higher values in species richness (18–62 species), Reciprocal of Berger–Parker Index (2.2–6.6), and Shannon Wiener-Index (1.6–3.9).
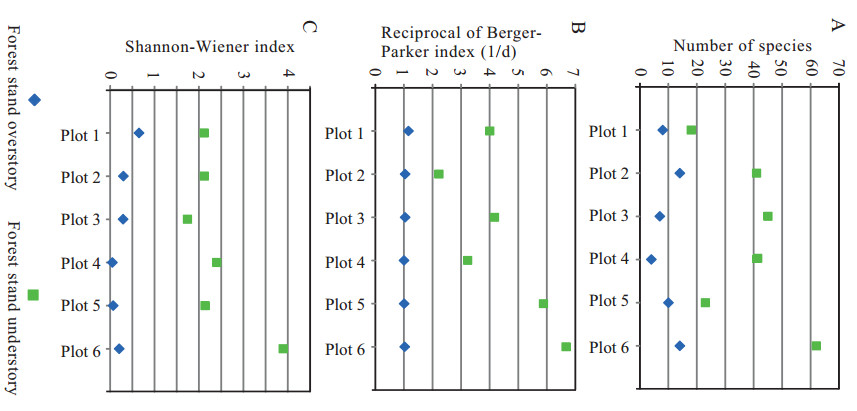
|
| Fig. 4 Species diversity in the forest stand overstory and understory (A) Number of species. (B) Reciprocal Berger–Parker Index (1/d). (C) Shannon–Wiener Index. Locations of each plot: Plot 1 at Shangloucun in Pingnan; Plot 2 at Lingfengcun in Pingnan; Plot 3 at Lingfenglu in Pingnan; Plot 4 in Dongshancun in Youxi; Plot 5 in Shanlingcun in Youxi; Plot 6 in Xianfengshan in Zhouning. |
There is a positive correlation between G. pensilis age and DBH (y = 0.0099x2 + 1.4508x + 12.671, Pearson's r2 = 0.89, p < 0.001) (Fig. 5). The growth ring data indicate the growth rate or amount of wood added to the tree annually was relatively higher when the trees were under 60 years old, though the overall trend of the growth rate decreased with advancing age. The growth ring width varied from 7 mm for trees under 60 years old to 0.89 mm per year for trees older than 100 years.
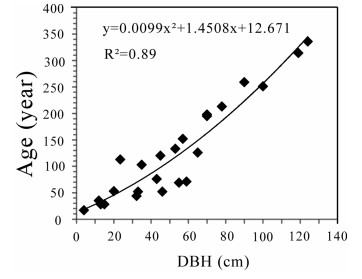
|
| Fig. 5 Relationships between DBH and age for G. pensilis |
The population structure in terms of the DBH and age distribution can provide an indication of the regeneration process of the population. Trees with DBHs between 1.5 and 127 cm ranged in ages from 15 to 357 years (Fig. 6A–L). The DBH and age distribution of each population was multimodal (a type with more than one peak in the size and age classes), indicating that recruitment varied by chance with regeneration depending on habitat and the availability of space. Few individuals with DBHs between 80 and 127 cm were observed and corresponded to ages of 192–357 years in Pingnan (Fig. 6A, C, G, I) and Youxi (Fig. 6D, E, J, K). There were very few G. pensilis trees with DBHs less than 10 cm, corresponding to ages < 28 years, in Plots 1 and 3 in Pingnan (Fig. 6A, C, G, I), Plots 4 and 5 in Youxi (Fig. 6D, E, J, K) and Plot 6 in Zhouning (Fig. 6F, L). G. pensilis trees with DBHs less than 20 cm, i.e., less than 46 years old, were absent in Plot 2 in Pingnan (Fig. 6B, H). Seedlings between 5 cm and 50 cm in height and saplings between 50 cm and 130 cm in height were rare. Only 18 seedlings/samplings were found in all of our plots growing in better drained soil mounds that were located above the groundwater table and fallen logs where more sunlight reached them (Fig. 2F). Among the 18 seedlings/samplings, 14 were found in forest stand gaps without herbaceous coverage (Fig. 7). This suggests the establishment of G. pensilis seedlings require light, moderate disturbance for gap formation, and mesic (not saturated) soil conditions. Although the species is capable of reproducing vegetatively, reproduction is primarily through seeds.
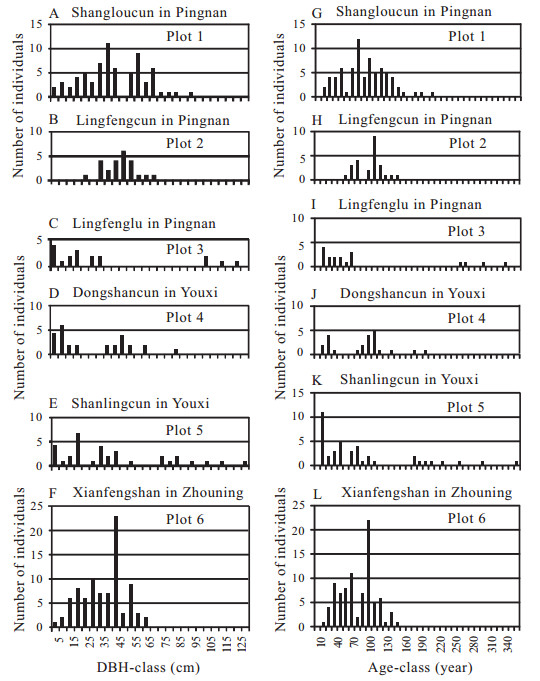
|
| Fig. 6 Frequency distribution in DBH- and age-classes of Glyptostrobus pensilis in six swamp forest stands in Fujian (A)–(F) Frequency distribution in DBH-classes; (G)–(L) Frequency distribution in age-classes |
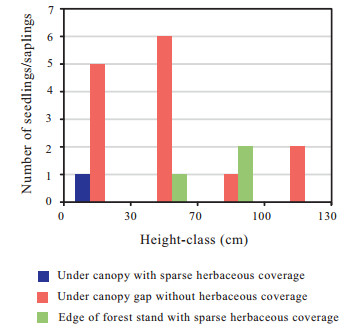
|
| Fig. 7 G. pensilis seedlings/saplings emergence for swamp forest stands of Fujian, 2016 |
Twenty-two withered G. pensilis trees with basal diameters of 26–90 cm and one standing dead tree with a DBH of 100 cm were identified in Plot 4 in Dongshancun in Youxi. The vitality of G. pensilis in the other plots (Plots 1, 2, 3, 5, and 6) was generally healthy.
3.3. A comparison of diversity of taxa between Fujian Province and VietnamThe extant G. pensilis stands in Fujian Province and Vietnam were associated with different species assemblages. In Fujian Province, the forest stands were open, with G. pensilis trees covering 15–50% of the canopy and occasionally accompanied by scattered P. massoniana, and/or C. japonica var. sinensis. In the shrub layer, wet-loving Hydrangea paniculata, A. trabeculosa, and S. chienii were present. Species of Viburnum, Ilex, Nyssa, Lindera, Rhododendron, and Adinandra were components in the shrub layer of some of the swamps. In Vietnam, Averyanov et al. (2009) showed G. pensilis used to provide at least 65–70% coverage in the canopy a few decades ago and was associated with broad-leaved species such as Sterculia pierrei and Litsea longipes. The sub-canopy included Elaeocarpus hygrophilus, an evergreen broad-leaved tree. The shrub layer consisted mainly of Ficus simplicissima, F. formosana, Syzygium formosum, Ilex cymosa, and Glochidion zeylanicum var. tomentosum. On the forest floor, herbacous species and numerous wet-loving riparian and sub-aquatic grasses (Poaceae) and sedges (Cyperaceae) and a number of fern species were found in the two regions. Among the herbaceous flora, the dominant species in Fujian Province included M. hui, I. anthephoroides, I. globosa, O. cinnamomeum, and P. microcephalum, whereas in the Vietnamese stands, Scleria sumatrensis, Leersia hexandra, Nephrolepis biserrata, Asplenium longissimum, and Colocasia esculenta were dominants.
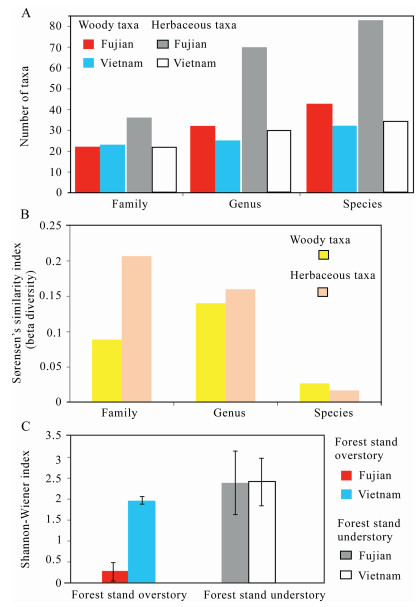
Fig. 8A shows that the number of families of the woody taxa for the forest stands as a whole was about the same (around 22) for Fujian Province and Vietnam, and the number of woody genera and species was higher in Fujian Province (32 genera and 42 species) than in Vietnam (25 genera and 32 species). The number of herbaceous families, genera, and species represented in the forest plots was greater in Fujian Province (36 families, 70 genera, 83 species) compared to those in Vietnam (21 families, 30 genera, 34 species). Fig. 8B indicates that Sørensen's similarity index (beta diversity) between the stands of the two regions was low (0.03–0.14 for woody taxa, 0.02–0.21 for herbaceous taxa) at all taxonomic levels for all classes of vegetation. This demonstrates the taxonomic compositions of the stands in the two regions were different, except that they shared the common dominant species-G. pensilis. The Shannon–Wiener index shows the overstory of each stand had much lower diversity (0.26 on average) in Fujian Province than that (1.97 on average) in Vietnam, whereas the diversity indices for the understories in the two regions were about the same (around 2.41) (Fig. 8C).
|
| Fig. 8 A comparison of diversity of forest stands in Fujian and Vietnam (A) The number of taxa respectively at the family, genus, and species levels for six stands in Fujian and two stands in Vietnam. (B) Sørensen's similarity index (beta diversity) at family, genus, and species levels between the forest stands in Fujian and Vietnam, respectively. (C) Shannon–Wiener index on average with standard deviation (SD) for the overstories and the understories of forest stands in Fujian and Vietnam. |
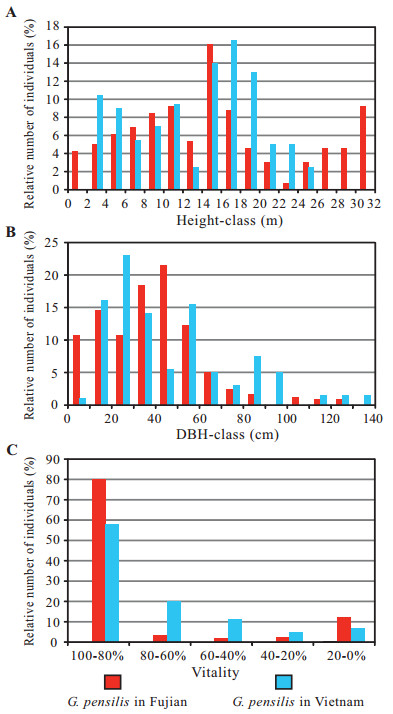
The population sizes of G. pensilis in Fujian Province (261 individuals in the six stands) and Vietnam (158 in the two stands) were small. The frequency distributions for height and DBH-classes in both Fujian Province and Vietnam populations show a multi-model type (Fig. 9A, B). In Fujian Province, the maximum height of G. pensilis was 31 m, whereas in Vietnam it was 25 m. The maximum DBH was 127 cm and 140 cm, with corresponding ages of 357 and 372 years in Fujian Province and Vietnam, respectively. In the Fujian Province populations, 9% of the trees were less than 4 m tall and 11% of the trees had DBHs less than 10 cm. In Vietnam, there were no trees less than 2 m tall and only 1% of the individuals had DBHs less than 10 cm. Regeneration of this species was poor (18 seedlings) in Fujian Province, while there was no natural regeneration occurring in Vietnam (Nguyen et al., 2013; our investigation in 2018).
|
| Fig. 9 A comparison of the population structures of Glyptostrobus pensilis in Fujian (the six stands) and Vietnam (combined the two stands in Trap K'sor and Ea Ral) (A) Frequency distribution of height. (B) Frequency distribution of DBH. (C) Frequency distribution of Vitality. Data for the Vietnamese stands are from Averyanov et al. (2009). |
The proportions of the number of individuals for both the high-vitality (100-80%) and the low-vitality (20-0%) for the Fujian Province populations were greater than those of Vietnam (Fig. 9C). The 22 withered G. pensilis trees in Dongshancun of Youxi, Fujian Province were caused by water loss and water pollution resulting from growing commercial mushrooms at the upper side of the habitat during 2012–2014. This demonstrates how human activities have impacted the habitat, dramatically threatening the survival of wild populations of G. pensilis.
4. DiscussionAs far back as the Cretaceous (100 million years ago), and through to the late Pliocene (2.6 million years ago), ancient wetland forests were co-dominated by Glyptostrobus and Metasequoia (LePage et al., 2005, LePage, 2007, Yamakawa et al., 2017). Today, G. pensilis and M. glyptostroboides are not known to naturally coexist. However, G. pensilis found in the unstable swamp stands (with varied water levels throughout the year) shares ecological traits with many other Tertiary relict plant species, as exemplified by M. glyptostroboides, which is found on steep slopes and stream sides in deep valleys of Lichuan, Hubei (Tang et al., 2011); G. biloba, which favors rock crevices in limestone habitats in the Dalou Mountains, Guizhou (Tang et al., 2012); C. argyrophylla, whichis found on steep slopes surrounded by cliffs on Mt. Jinfo, Chongqing (Qian et al., 2016); Thuja sutchuenensis, which is found on steep limestone slopes in the Daba Mountains, Chongqing (Tang et al., 2015); T. cryptomerioides, which is found on riverbanks in deep valleys, on steep slopes, and on cliffs in the Gaoligong mountains, Yunnan (He et al., 2015); D. involucrata, which is found in scree slops on the Mt. Emei, Sichuan (Tang and Ohsawa, 2002); Tetracentron sinense, which is found in deep ravines and on steep slopes in the Ailao Mountains, Yunnan (Tang et al., 2013). Recruitment of these species is restricted to local habitats that have moderate disturbance regimes where competition usually is less intense and the regeneration potential of non-relict species is lower.
G. pensilis is a heliophilous species that is highly drought sensitive, a poor competitor under dry conditions, and does not tolerate saline soil conditions (Xu and Li, 1959). In our study sites in Fujian Province, the dark red soil is deep, permeable, and has a pH that ranges from 6 to 7 (Zheng et al., 2008; NFGRP, 2018). The soil conditions appear to be suitable for the growth of G. pensilis in Fujian Province. However, the regeneration of this species is extremely poor. The micro-sites of the 18 seedlings found in Fujian Province suggest germination and establishment of G. pensilis requires light, wet but not saturated soil conditions. This observation agrees with experimental results that show germination and seedlings prefer wet sites, but not standing water (Xu and Li, 1959, Chen, 1977).
The population of G. pensilis is declining. The effective conservation and management of this endangered species requires several measures. Because the species shows weak recruitment, we recommend establishing G. pensilis seedling/sapling nurseries in situ. Tall, dense herbaceous vegetation covers most of the understories in Fujian Province forest stands. Thus, to provide well-lit sites for G. pensilis seedling survival, we recommend controlling herbaceous density in the understory. In the two stands in Shangloucun and Xianfengshan, water levels are very low, even intermittent. Thus, we suggest water levels be increased to maintain the present populations. Previous research reported that G. pensilis genetic diversity is low at both the species level and within populations, whereas the genetic differentiation among G. pensilis populations is high (Li and Xia, 2005). Although this study included only one of our six study sites, these findings indicate that it is critical to conserve genetic variability in G. pensilis. Thus, ex situ collections of G. pensilis should include representatives from all extant wild populations.
Conflict of interestNone.
AcknowledgementsWe acknowledge funding by the National Natural Science Foundation of China (31760152 and 31500355), the Key National Research and Development Plan Program of China (2016YFC050310203), the Natural Science Foundation Project of CQ CSTC, China (cstc2016jcyjA0379) and the National Program on Space Science and Technology, Vietnam Academy of Science and Technology, Vietnam (project VT-UD.09/18-20).
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2019.06.007.
Averyanov L.V., Nguyen T.H., Nguyen S.K., Pham V.T., Lamxay V., Bounphanmy S., et al, 2014. Gymnosperms of Laos. Nord. J. Bot, 32(6): 765-805. DOI:10.1111/njb.00498 |
Averyanov, L.V., Phan, K.L., Nguyen, T.H., Nguyen, S.K., Nguyen, T.V., Pham, T.D., 2009. Preliminary observation of native Glyptostrobus pensilis (Taxodiaceae) stands in Vietnam. Taiwania 54(3), 191-212. https://doi.org/10.6165/tai.2009.54(3).191.
|
Bozukov V.S., Tsenov B.V., 2012. Catalogue of the Cenozoic plants of Bulgaria(Eocene to Pliocene). Addendum and Corrigendum. Phytol. Balcan, 18(3): 237-261. |
Chen M.-H., Kong Z.-C., Chen Y., 1983. On the discovery of Palaeogene flora from the western Sichuan Plateau and its significance in Phytogeography. Acta Bot. Sin, 25(5): 482-493. |
Chen, X.-Y., 1977. Ancient tree species-cultivating seedlings of Glyptostrobus pensilis. Forestry Sci. Fujian 03, 19-20.
|
Chinese State Report on Biodiversity Editorial Committee, 1998. Chinese State Report on Biodiversity. Chinese Environmental Science Press, Beijing.
|
Earle, C.J., 2018. The Gymnosperm Database at. http://www.conifers.org/cu/Taxodium_distichum.php.(Accessed 5 March 2018).
|
Farjon, A., 2005. A Monograph of Cupressaceae and Sciadopitys. Royal Bot. Gardens, Kew.
|
Fujita, K., Kasama, T., 1983. Geology of Kobe District. Geol. Surv. Japan, p. 115.
|
Geng B., Manchester S.R., Lu A., 1999. The first discovery of Eucommia fruit fossil in China. Chin. Sci. Bull, 44: 1506-1509. DOI:10.1007/BF03183573 |
Greenwood D.R., Archibald S.B., Mathewes R.W., Moss P.T., 2005. Fossil biotas from the Okanagan Highlands, southern British Columbia and northeastern Washington State:climates and ecosystems across an Eocene landscape. Can. J. Earth Sci, 42(2): 167-185. DOI:10.1139/e04-100 |
Guo S.-X., Li H.-M., 1979. Late Cretaceous flora from Hunchun of Jilin. Acta Palaeontol. Sin, 18(6): 547-564. |
Guo S.-X., Zhang G.-F., 2002. Oligocene Sanhe flora in Longjing county of Jilin, Northeast China. Acta Palaeontol. Sin, 41(2): 193-210. |
Hase Y., 1988. Late Cenozoic history and paleoenvironment of southern Kyushu, Japan, Mem. Fac. General Education. Kumamoto Univ. Nat. Sci, 23: 37-82. |
Hayashi, T., 1975. Fossils from the Chojabaru, Iki Island, Japan. Island Sci. Lab 120.
|
He, L.-Y., Tang, C.Q., Wu, Z.-L., Wang, H.-C., Ohsawa, M., Yan, K., 2015. Forest structure and regeneration of the tertiary relict Taiwania cryptomerioides in the Gaoligong mountains, Yunnan, southwestern China. Phytocoenologia 45, 135-156. https://doi.org/10.1127/phyto/2015/0038.
|
Huang H., 2013. Habitat survey and protection countermeasure of rare Chinese cypress in Youxi Jiufushan Nature Reserve. Anhui Agric. Sci. Bull, 19(15): 109-111. |
Ina H., 1992. Miocene vegetational and climatic history of the eastern part of the Setouchi Geologic Province. J. Earth Planet. Sci. Nagoya Univ, 39: 47-82. |
Ishida S., 1970. The Noroshi flora of Noto Peninsula central Japan. Mem. Fac. Sci., Kyoto Univ., Ser. Geol. Mim, 37: 1-112. |
Jin, J.H., Sang, P., 1998. Discovery of early Tertiary flora in Shenbei coalfield. Acta Sci. Nat. Univ. Sunyatseni 37, 129-130.
|
Lei, X.Y., 2017. The spatial distribution of Glyptostrobus pensilis in provincial forest park in Xianfengshan, Zhouning, Fujian. Forestry Explor. Design 1, 53-57.
|
LePage B.A., 2007. The taxonomy and biogeographic history of Glyptostrobus endlicher (Cupressaceae). Bull. Peabody Mus. Nat. Hist, 48(2): 359-426. DOI:10.3374/0079-032X(2007)48[359:TTABHO]2.0.CO;2 |
LePage, B.A., Yang, H., Matsumoto, M., 2005. The evolution and biogeographic history of Metasequoia. In: LePage, B.A., Williams, C.J., Yang, H. (Eds.), The Geobiology and Ecology of Metasequoia. Springer, New York, pp. 3-114.
|
Li F.-G., Xia N.-H., 2004. The geographical distribution and cause of threat to Glyptostrobus pensilis (Taxodiaceae). J. Trop. Subtrop. Bot, 12(1): 13-20. |
Li F.-G., Xia N.-H., 2005. Population structure and genetic diversity of an endangered species, Glyptostrobus pensilis (Cupressaceae). Bot. Bull. Acad. Sin, 46: 155-162. |
Liu Y.S., Guo S.X., Ferguson D.K., 1996. A catalogue of Cenozoic megafossil plants in China. Palaeontogr. Abt. B Palaeophytol, 238: 141-179. |
Magurran, A.E., 1988. Ecological Diversity and its Measurement. Princeton Univ. Press, Princeton.
|
Manchester S.R., Chen Z.-D., Lu A.-M., Uemura K., 2009. Eastern Asian endemic seed plant genera and their paleogeographic history throughout the Northern Hemisphere. J. Syst. Evol, 47: 1-42. DOI:10.1111/j.1759-6831.2009.00001.x |
Martinetto E., 2001. The role of central Italy as a centre of refuge for thermophilous plants in the Late Cenozoic. Acta Palaeobot, 41: 299-319. |
Mathewes R.W., Greenwood D.R., Archibald S.B., 2016. Paleoenvironment of the Quilchena flora, British Columbia, during the early Eocene climatic optimum. Can. J. Earth Sci, 53: 1-17. DOI:10.1139/cjes-2015-0107 |
Matsuo H., 1967. Palaeogene floras of northwestern Kyushu, part 1:the takashima flora. Ann. Sci. Kanazawa Univ, 4: 15-90. |
Matthews, J.V., Ovenden, L.E., 1990. Late Tertiary plant macrofossils from localities in Arctic/subarctic North America: a review of the data. Arctic 43, 364-392.
|
Miki S., 1948. Floral remains in Kinki and adjacent districts since the Pliocene with description of 8 new species. Miner. Geol, 2: 105-144. |
Miki S., 1941. On the change of flora in Eastern Asia since Tertiary period. I. The clay or lignite beds flora in Japan with special reference to the Pinus trifolia beds in Central Hondo. Jpn. J. Bot, 11: 237-304. |
Momohara, A., 1992. Late Pliocene plant biostratigraphy of the lowermost part of the Osaka Group, southwest Japan, with reference to extinction of plants. Quat. Res. Tokyo 31, 76-88.
|
Momohara A., 2011. Survival and extinction of the taxodiaceae in the quaternary of Japan. Jpn. Hist. Bot, 19: 55-60. |
NFGRP, http://www.nfgrp.cn/data/list/resource_detailp.shtml?kid=4C3A38A8C4016634F7&pingtaiziyuanhao=1111C0003700001895 Retrieved on May 5, 2018.
|
Nguyen M.T., Vu D.D., Bui T.T.X., Nguyen M.D., 2013. Genetic variation and population structure in Chinese water pine (Glyptostrobus pensilis):a threatened species. Indian J. Biotechnol, 12: 499-503. |
Ohsawa, M., 1984. Differentiation of vegetation zones and species strategies in the subalpine region of Mt. Fuji. Vegetatio 57, 15-52.
|
PALEOBIDB (Paleobiological database), 2017. www.paleobidb.org.(Accessed 8 October 2017).
|
Pielou, E.C., 1969. An Introduction to Mathematical Ecology. Wiley, New York.
|
Qian H., 2001. A comparison of generic endemism of vascular plants between East Asia and North America. Int. J. Plant Sci, 162: 191-199. DOI:10.1086/317909 |
Qian S., Yang Y., Tang C.Q., Momohara A., Yi S., Ohsawa M., 2016. Effective conservation measures are needed for wild Cathaya argyrophylla populations in China:insights from the population structure and regeneration characteristics. For. Ecol. Manag, 361: 358-367. DOI:10.1016/j.foreco.2015.11.041 |
Shimazu, M., Teraoka, Y., 1962. Explanatory Test of Thegological Map of Japan. Rikuchu-Noda. Geological Survey of Japan, p. 53.
|
Sørensen T.A., 1948. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. K. Dan. Vidensk. Selsk. Biol. Skr, 5: 1-34. |
Tanai T., 1961. Neogene floral change in Japan. J. Fac. Sci. Hokkaido Univ. Ser. IV Geol. Min, 11: 119-398. |
Tanai T., 1970. The Oligocene floras from the Kushiro coal field, Hokkaido, Japan. J. Fac. Sci. Hokkaido Univ. Ser. IV Geol. Min, 14: 383-514. |
Tanai T., 1979. Late Cretaceous floras from the Kuji district, l northeastern Honshu, Japan. Jour. Fac. Sci. Hokkaido Univ. Ser, 4(19): 75-136. |
Tanai, T., Suzuki, N., 1963. Miocene floras of southwestern Hokkaido, Japan. Tertiary floras of Japan, Miocene floras. In: Collaborating Association to Commemorate the 80th Anniversary of the Geological Survey of Japan, vol. 1, pp. 9-149.
|
Tanai T., Uemura K., 1983. Engelhardia fruits from the tertiary of Japan. J. Fac. Sci. Hokkaido Univ. Ser, 4(20): 249-260. |
Tang, C.Q., 2015. The Subtropical Vegetation of Southwestern China: Plant Distribution, Diversity and Ecology. Plants and Vegetation, vol. 11. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9741-2.
|
Tang, C.Q., Matsui, T., Ohashi, H., Dong, Y.-F., Momohara, A., Herrando-Moraira, S., et al., 2018. Identifying long-term stable refugia for relict plant species in East Asia. Nat. Commun. 9, 4488. https://doi.org/10.1038/s41467-018-06837-3.
|
Tang C.Q., Ohsawa M., 2002. Tertiary relic deciduous forests on a subtropical mountain, Mt. Emei, Sichuan, China. Folia Geobot, 37: 93-106. DOI:10.1007/BF02803193 |
Tang C.Q., Peng M.-C., He L.-Y., Ohsawa M., Wang C.-Y., Xie T.-H., Li W.-S., Li J.-P., Zhang H.-Y., Li Y., Yang X.-M., Li G.-S., 2013. Population persistence of a tertiary relict tree Tetracentron sinense on the Ailao mountains, Yunnan, China. J. Plant Res, 126(5): 651-659. DOI:10.1007/s10265-013-0559-1 |
Tang C.Q., Yang Y., Ohsawa M., Momohara A., Hara M., Cheng S., Fan S., 2011. Population structure of relict Metasequoia glyptostroboides and its habitat fragmentation and degradation in south-central China. Biol. Conserv, 144: 279-289. DOI:10.1016/j.biocon.2010.09.003 |
Tang, C.Q., Yang, Y., Ohsawa, M., Momohara, A., Yi, S.-R., Robertson, K., Song, K., Zhang, S., He, L.-Y., 2015. Community structure and survival of Tertiary relict species Thuja sutchuenensis (Cupressaceae) in the subtropical Daba Mountains, southwestern China. PLoS One 10(4), e0125307. https://doi.org/10.1371/journal.pone.0125307.
|
Tang C.Q., Yang Y., Ohsawa M., Yi S.-R., Momohara A., Su W.-H., et al, 2012. Evidence for the persistence of wild Ginkgo biloba (Ginkgoaceae) populations in the Dalou Mountains, southwestern China. Am. J. Bot, 99(8): 1408-1414. DOI:10.3732/ajb.1200168 |
Thomas, P., Yang, Y., Farjon, A., Nguyen, D., Liao, W., 2011. "Glyptostrobus pensilis". The IUCN Red List of Threatened Species. IUCN. 2011: e.T32312A9695181. https://doi.org/10.2305/IUCN.UK.20112. RLTS.T32312A9695181.en Retrieved 31 March 2018. Database entry includes justification for why this species is critically endangered.
|
Uemura K., Doi E., Takahashi F., 1999. Plant megafossil assemblage from the Kiwado formation (Oligocene) from Ouchiyama-kami in Yamaguchi Pref. , western Honshu, Japan. Bull. Mine City Mus, 15: 1-59. |
Volis, S., 2016. How to conserve threatened Chinese plant species with extremely small populations? Plant Divers. 38, 45-52. https://doi.org/10.1016/j.pld.2016.05.003.
|
WGCPC (Writing Group of Cenozoic Plants of China), 1978. Cenozoic Plants from China. Fossil Plants of China, vol. 3. Science Press, Beijing, p. 232.
|
Wu B., 2016. An analysis of the community structure of Chinese cypress forests in Shanling village of Tangchuan Town of Youxi county, East China. For. Manag, 30(2): 55-58. |
Wu Z.-Y., Liu J.-F., Hong W., Wang Z.-J., Lu J.-C., Fu D.-L., He Z.-S., 2008. The spatial distribution pattern of the population of relict plant Glyptostrobus pensilis in Pingnan. Subtrop. Agric. Res, 4(1): 36-39. |
Xu X.-H., Li M.-P., 1959. The ecology and geographical distribution of Glyptostrobus pensilis. South China Normal Univ. Nat. Sci, 3: 84-99. |
Yamakawa C., Momohara A., Nunotani T., Matsumoto M., Watano Y., 2008. Paleovegetation reconstruction of fossil forests dominated by Metasequoia and Glyptostrobus from the late Pliocene Kobiwako group, central Japan. Paleontl. Res, 12: 167-180. DOI:10.2517/1342-8144(2008)12[167:PROFFD]2.0.CO;2 |
Yamakawa C., Momohara A., Saito T., Nunotani T., 2017. Composition and paleoenvironment of wetland forests dominated by Glyptostrobus and Metasequoia in the latest Pliocene (2.6Ma) in central Japan. Palaeogeogr. Palaeoclimatol. Palaeoecol, 467: 191-210. DOI:10.1016/j.palaeo.2015.12.004 |
Zheng, S.-Q., Liu, J.-F., Wu, Z.-Y., Fu, D.-L., He, Z.-S., Dai, L.-C., Li, J.-C., 2008. The interspecific competition of main population in Glyptostrobus pensilis natural forest in Pingnan County. J. Fujian College Forestry 28(3), 216-219.
|
Zheng S.-Q., Wu Z.-Y., Liu J.-F., Hong W., He Z.-S., Xu D.-W., 2011. The endangering causes and protection strategies for Glyptostrobus pensilis, an endemic relict plant in China. Subtrop. Agric. Res, 7(4): 217-220. |



