b. University of Chinese Academy of Sciences, Beijing, 100049, China;
c. Mid-Florida Research and Education Center, Department of Environmental Horticulture, University of Florida, FL, 32703, USA
Plants experience various kinds of stress during their life cycles, such as drought, salt, and high temperature. With the effects of global warming, high temperature has become an increasingly severe problem for plants (Ding et al., 2018). High temperature adversely affects photosynthesis (Fan et al., 2017), hormone homeostasis (Maestri et al., 2002), and secondary metabolite contents (Wahid et al., 2007). Plants have evolved a number of responses to heat stress, evidence suggests that carotenoid accumulation and ABA and SA signaling appear to mediate plant defense (Wahid et al., 2007; Du et al., 2010). However, understanding how these processes interact to mediate plant defense against heat stress remains a major challenge in botany.
Previous studies have shown that plant stress defense is mediated by carotenoids and enzymes in the carotenoid biosynthetic pathway. Two key enzymes in the carotenoid biosynthetic pathway are β-carotene hydroxylase and β-carotene ketolase. β-carotene hydroxylase, which is common in photosynthetic plants, catalyzes the conversion of β-carotene to zeaxanthin (Davison et al., 2002); β-carotene ketolase, which is present only in some bacteria and green algae, catalyzes the conversion of β-carotene to canthaxanthin (Mann et al., 2000; Huang et al., 2013). Overexpression of these genes has been shown to increase carotenoid levels in tobacco (Hasunuma et al., 2008; Mann et al., 2000), tomato (Huang et al., 2013), maize (Farré et al., 2016), and potato (Morris et al., 2006). Furthermore, previous studies that overexpressed β-carotene hydroxylase in Arabidopsis reported that the resulting plants were more tolerant of high temperature and high light (Davison et al., 2002). Similarly, overexpressing a bacterial β-carotene ketolase in Arabidopsis increased plant tolerance of high light (Zhong et al., 2011). However, few studies have examined whether carotenoids respond to only high temperature stress, and if so, by what mechanism these molecules mediate plant response to high temperatures.
One promising model for examining the mechanisms of heat stress response in plants is the moss Physcomitrella patens. Moss is small, as well as easy to cultivate and propagate (Schaefer, 2001). In addition, moss growth is limited by high-temperature stress. P. patens is the only known plant that can be efficiently genetically modified by homologous recombination (Decker and Reski, 2004), and this high gene-targeting efficiency has made it a model plant for functional genomic studies (Schaefer and Zrÿd, 2001). P. patens is tolerant of a number of abiotic stresses, including drought, salinity (Frank et al., 2005), and cold (Minami et al., 2005). However, few reports have examined its behavior under hightemperature stress.
In this study, we asked whether β-carotene hydroxylase and β-carotene ketolase increase heat stress tolerance in plants and, if so, what molecular mechanisms underlie this response. To answer these questions, we expressed a β-carotene ketolase gene from Chlamydomonas reinhardtii (CrBKT) and a β-carotene hydroxylase gene from Haematococcus pluvialis (HpBHY) in P. patens and subsequently examined plant responses to heat stress.
2. Materials and methods 2.1. Culture conditions and treatmentWild-type P. patens (Gransden 2004) plants (Li et al., 2017) were used for all experiments described in this study. All plants were grown on BCDAT medium, under a light cycle of 16 h light (60-80 μmol photons m-2s-1) and 8 h dark at 25 ℃; 40-day-old colonies were used for all experiments. For high-temperature treatments, 40-day-old plants were transferred to 45 ℃ under normal light intensity for 4 h, then allowed to recover under the normal conditions described above.
2.2. Phylogenetic analyses of BHY and BKTThe amino acid sequences of HpBHY and CrBKT were obtained by translating the coding sequences (CDS) of HpBHY and CrBKT, which were downloaded from the National Center for Biotechnology Information (NCBI) (accession numbers BD250390 and AY860820, respectively), with the translate tool in the ExPASy web server (Artimo et al., 2012) (https://web.expasy.org/translate/). To analyze the relationship of β-carotene hydroxylase and β-carotene ketolase, multiple sequence alignments of β-carotene hydroxylase and β-carotene ketolase amino acid sequences were conducted with the ClustalW program of MEGA6 software, the trees were constructed with MEGA6 software using the neighbor-joining method and the significance of the branching order was tested by bootstrapping (1000 replicates). Trees were visualized with iTOL (Ivica and Peer, 2016).
For β-carotene hydroxylase, we analyzed 30 amino acid sequences from 14 viridiplantae species, including 8 embryophyte and 6 chlorophyte species. For β-carotene ketolase, 16 species were analyzed, including 8 chlorophyte species and 7 marine bacterium species and one fungus.
2.3. Plasmid construction, in vitro translation, and protein importThe coding sequences of HpBHY and CrBKT were synthesized according to sequence information in GenBank (accession numbers BD250390 and AY860820, respectively). The gene sequence encoding a modified Pisum sativum RUBISCO chloroplast transit peptide (tp22) (Shi and Theg, 2013) was added before the sequences of HpBHY and CrBKT for targeting of the expressed proteins to chloroplasts. Then, these sequences were each inserted into the NotI-SalI sites of the vector pPOG1 under the control of the PpEF1-α promoter.
For the moss import assay, chloroplasts were isolated from 7-day-old moss protonema as previously described (Liu and Theg, 2014). In vitro translation was performed in a wheat germ system (Promega or tRNA Probes) in the presence of [3H]-leucine and other non-radiolabeled amino acids as described (Lo and Theg, 2011). For in vitro protein import assays, moss chloroplasts were incubated with the resulting radiolabeled precursor proteins for 30 min in the presence of 3 mM ATP, and the reaction was stopped by adding 600 ml import buffer (330 mM sorbitol, 50 mM Tricine, and 3 mM MgCl2, pH 8.0). The chloroplasts were pelleted by centrifugation at 3000 g for 5 min, resuspended in sample buffer (50 mM TriseHCl at pH 6.8, 2% SDS, 10% glycerol, 1% β-mercaptoethanol, 12.5 mM EDTA, 0.02% bromophenol blue), boiled for 10 min, separated by SDSPAGE, and visualized by fluorography. Thermolysin treatments were performed as previously describe (Lo and Theg, 2011).
2.4. Moss transformation and genotypingThe generation of transgenic plants was performed using the polyethylene glycol (PEG)-mediated transformation method as described by Liu and Vidali (2011).
For genotyping, moss genomic DNA was isolated using a CTAB method. HpBHY and CrBKT coding sequence amplification primers HpBHY-F/R and CrBKT-F/R (listed in Supplementary Table S1) were used for detection of HpBHY and CrBKT genes in P. patens, with 2 × MastTaq (Novoprotein, China) of 35 cycles of 94 ℃ for 20 s, 56 ℃ for 20 s, 72 ℃ for 30 s).
To detect the expression of HpBHY and CrBKT, total RNA was extracted using TRIzol (Takara, Japan). For cDNA synthesis, 1 μg RNA was used following the manufacturer's instructions for the PrimeScriptTMRT reagent Kit (Takara, Japan). The relative expression levels of HpBHY and CrBKT were measured using primers HpBHY-F/R (RT) and CrBKT-F/R (RT) (indicated in Supplementary Table S1) by semiquantitative RT-PCR analysis, which was carried out with 2 × MastTaq (Novoprotein, China); the endogenous moss actin gene served as the reference gene (Vera et al., 2017). The quantification of gel bands was performed with the software ImageJ. The data were plotted in GraphPad Prism 6.
2.5. Pigment analysisPhotosynthetic pigments were monitored by following the method described by Huang et al. (2011) with some modification. Pigments were isolated from the leafy gametophores of 40-day-old P. patens with the extract buffer which contain 50% acetone and 50% ethanol, and measured with an Infinite® 200 PRO (TECAN, Austria) through absorbance at 665, 642.5, 470, 485, and 474 nm. The contents of chlorophyll a, chlorophyll b, carotenoid and lutein were calculated as follows, C(Chlorophyll a) = 9.99 A665 - 0.0872A642.5; C(Chlorophyll b) = 17.7 A642.5-3.04A665; C(Lutein) = 10.2 A470-11.5A485-0.0036[a]-0.652[b]; C(Carotenoid) = 4.92A474-0.0255[a]-0.225[b].[a] in the formula indicates the concentration of chlorophyll a (mg/L), [b] indicates the concentration of chorophyll b (mg/L).
2.6. Measurement of the maximal photochemical efficiency of PSIIThe photochemical efficiency was estimated by measuring the maximal photochemical efficiency of PSII (Fv/Fm) of 40-day-old leafy gametophores with an IMAGING-PAM chlorophyll fluorometer and the Imaging Win software application (Walz, Germany). After dark-adaptation of P. patens for 30 min, the Fv/Fm was measured, where Fv is the variable chlorophyll fluorescence yield and Fm is the maximum chlorophyll fluorescence yield (Camejo et al., 2005).
2.7. Biochemical analysisThe amount of malonyldialdehyde (MDA) in the moss tissue, as an estimate of lipid peroxidation, was measured through a modification of the method of Cui and Wang (2006). About 0.3 g fresh tissue was ground in 3 mL 10% trichloroacetic acid (TCA) using a mortar and pestle. The homogenate was centrifuged at 12, 000×g for 10 min, 2 mL extract and 2 mL thiobarbituric acid (TBA) were mixed as the reaction mixture and was heated at 95 ℃ for 15 min, then cooled on ice quickly and centrifuged at 3000×g for 10 min. The absorbance of the supernatant was determined at 532, 600, and 450 nm, respectively, with an Infinite® 200 PRO (TECAN, Austria). Equation C(MDA) = 6.45 (A532 - A600) - 0.56 A450 was used to calculate the MDA content.
The activities of SOD and POD were measured by the methods of Meloni et al. (2003) and Fu and Huang (2001), respectively, with some modification. The enzymes were extracted with about 0.3 g fresh tissues and the samples were ground in 3 mL 50 mM sodium phosphate buffer containing 1% PVP (polyvinylpyrrolidone) using a mortar and pestle. The homogenate was centrifuged at 12, 000×g for 20 min to get the enzyme supernatant extract. For SOD, the reaction solution (3 mL) contained 0.3 mL of 0.75 mM NBT, 0.3 mL of 0.02 mM riboflavin, 0.3 mL of 0.13 M methionine, 0.3 mL of 0.1 mM EDTA, 1.5 mL of 50 mM sodium phosphate buffer (pH 7.8), 0.05 mL distilled water, and 0.05 mL enzyme extract. Test tubes containing the reaction solution and enzyme extract were irradiated in a light incubator at 4000 lx for 10 min. The absorbances of the irradiated and non-irradiated solutions at 560 nm were determined with an Infinite® 200 PRO (TECAN, Austria). The amount of enzyme that would inhibit 50% of NBT photo-reduction was defined as one unit of SOD activity. For POD, the reaction mixture consisted of 50 mM sodium phosphate buffer (pH 7.0), 1% guaiacol, 0.4% H2O2, and enzyme extract. Increase in the absorbance due to oxidation of guaiacol was measured at 470 nm for 1 min.
2.8. Measurement of plant phytohormonesPhytohormones (SA and ABA) were extracted from 0.3 g of frozen gametophores. Stable isotope-labeled CKs (0.01 ng) and phytohormones (SA 20 ng, ABA 0.6 ng) were added to the sample extraction buffer. Phytohormones were extracted and the content was determined by HPLC using the method described by Lee et al. (2015).
2.9. Statistical analysisAll experiments were performed with three or more biological replicates. Pigment contents and physiological indexes were analyzed by one-way analysis of variance; Tukey's multiple range test was used to identify the means of three or more groups; the significance thresholds were 0.05 (marked by *) and 0.01 (**). All data were analyzed with the software SPSS version 19.0 (International Business Machines Corporation, Armonk, NY, USA).
3. Results 3.1. HpBHY and CrBKT have special evolutionary positionsTo investigate the evolution of β-carotene hydroxylases and β-carotene ketolases, we first analyzed the evolutionary presence of β-carotene hydroxylases and β-carotene ketolases. β-carotene hydroxylases are ubiquitous among the viridiplantae, we analyzed 8 embryophyte and 6 chlorophyte species, such as Dunaliella salina and Haematococcus pluvialis (Fig. 1A). In contrast, in the 17 viridiplantae species analyzed, β-carotene ketolases are present only in some chlorophytes, marine bacterium, and the fungus Xanthophyllomyces dendrorhous (Fig. 1B), which is widely used for biotechnological production of carotenoid (Ye et al., 2015). Further, β-carotene ketolases are present in single copies in all species.
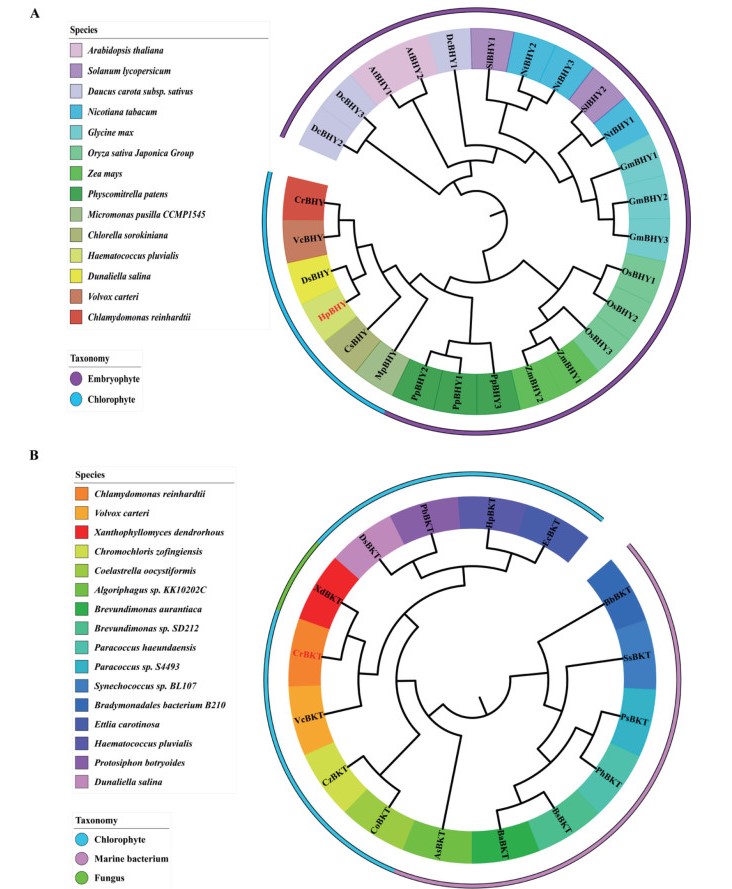
|
| Fig. 1 Phylogeny of β-carotene hydroxylase and β-carotene ketolase. The tree was constructed using the Neighbor-Joining (NJ) method based on the amino acid sequences of bcarotene hydroxylases (BHY; A) and β-carotene ketolase (BKT; B). |
To examine carotenoid biosynthesis in P. patens, we chose to transgenically express β-carotene hydroxylase from H. pluvialis (HpBHY) and β-carotene ketolase from C. reinhardtii (CrBKT). HpBHY is closely related to PpBHY. CrBKT is most similar to the β-carotene ketolase from the fungus X. dendrorhous (XdBKT). In addition, both HpBHY and CrBKT are widely used in the carotenoid biosynthetic industry (Guerin et al., 2003).
3.2. Expression profiling of β-carotene hydroxylase and β-carotene ketolase under stressTo investigate the functions of HpBHY and CrBKT, we first analyzed available gene expression for these genes or their homologs under different abiotic stresses using data from public databases BAR (http://bar.utoronto.ca/) and JGI (https://jgi.doe.gov/). Specifically, we examined the expression of HpBHY homologs in response to various stressors in Oryza sativa (rice; Fig. 2A), Arabidopsis (Fig. 2B), Glycine max (soybean; Fig. 2C), and P. patens (Fig. 2D). In addition, we examined CrBKT expression in response to nitrogen deficiency and hydrogen peroxide treatment (Fig. 2E). The expression profiles of HpBHY homologs in O. sativa, Arabidopsis, G. max, and P. patens changed under abiotic stresses such as drought, cold, and heat (Fig. 2A-D). For example, the expression of the P. patens β-carotene hydroxylase homologs (Pp3c21_16590V3.1 and Pp3c19_16780V3.2) increased between 4.3 and 5.3 times, respectively, in response to heat stress. In response to nitrogen deficiency treatment, CrBKT expression increased almost 2.5 times after 30 min. These results show that β-carotene hydroxylases and β-carotene ketolase play important roles in abiotic stress response.
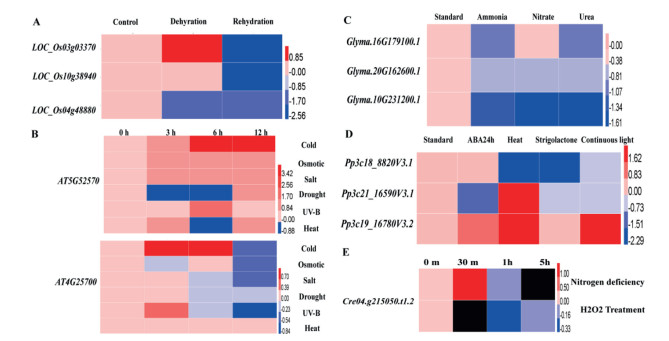
|
| Fig. 2 Heat map of the relative gene expression of various HpBHY homologs and CrBKT under different stress treatments. Heat maps of the relative gene expression values under dehydration and rehydration treatments of three HpBHY homologs in O. sativa (A), two HpBHY homologs in Arabidopsis under cold, osmotic, salt, drought, UV-B, and heat treatments (B), three HpBHY homologs in G. max under ammonia, nitrate, and urea treatments (C), and three HpBHY homologs in P. patens under ABA, strigolactone, heat, and continuous light treatments (D), as well as that of CrBKT under nitrogen deficiency and H2O2 treatment (E). Images were prepared with the HemI software (Deng et al., 2014) with the log2 of relative expression data. |
Before transforming P. patens with HpBHY and CrBKT, we first verified that these genes would be successfully imported into moss chloroplasts. To do this, we synthesized fusion proteins in which the transit peptide of the chloroplast protein RUBISCO was fused to HpBHY and CrBKT, and then compared the sizes of the precursor and mature proteins by SDS-PAGE. As expected, once their transit proteins were removed, mature HpBHY and CrBKT proteins were smaller than precursor proteins. This indicated that precursor proteins translated in vitro were successfully imported into isolated moss chloroplasts under light conditions (Fig. 3).
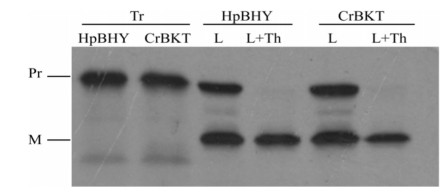
|
| Fig. 3 Import assay of HpBHY and CrBKT precursors into 7-day-old moss chloroplasts. L, import assay in the light; L + Th, import assay in the light followed by thermolysin treatments; Tr, 20% radiolabeled precursor proteins; Pr, precursor protein; M, mature protein. |
To detect the functions of HpBHY and CrBKT in P. patens, we transformed P. patens with expression vectors (Fig. 4A, B). Twenty to thirty independent transgenic lines for the two expression constructs were obtained after genotyping by direct PCR (Fig. 4C), and the presence and expression of HpBHY and CrBKT were confirmed by RT-PCR analysis (Fig. 4D). The transformants Y36 (hereafter, HpBHY-expressing plants) and T25 (hereafter, CrBKT-expressing plants) were used for further analysis.
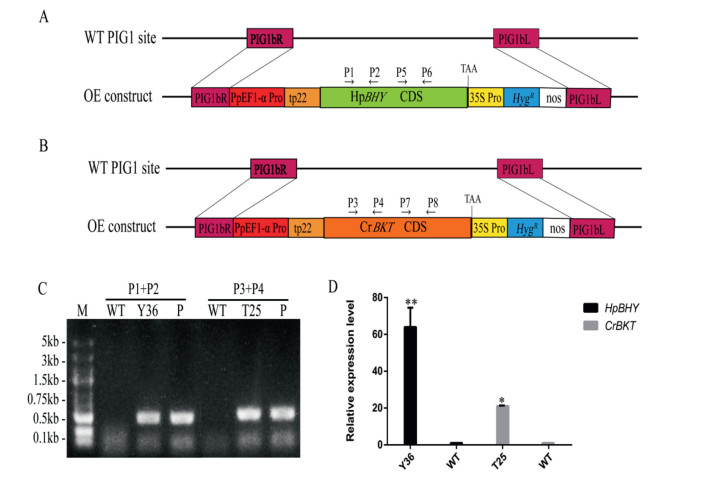
|
| Fig. 4 Recombinant expression of HpBHY and CrBKT in stable transgenic P. patens lines. (A) Schematic representation of the constructs for expression of HpBHY in wild-type plants. (B) Schematic representation of the constructs for expression of CrBKT in wild-type plants. (C) Identification of transformants by PCR analysis of genomic DNA isolated from wildtype and transgenic lines. (D) RT-PCR detection of HpBHY and CrBKT expression in transgenic and wild-type plants. WT, wild type; Y36, HpBHY-expressing plant; T25, CrBKT-expressing plant; P, corresponding plasmid; PIG1bR, right targeting site; PIG1bL, left targeting site; PpEF1-α Pro, the EF1-α promoter from P. patens; tp22, RUBISCO chloroplast transit peptide from Pisum sativum; 35S Pro, the 35S promoter from cauliflower mosaic virus; HygR, a selectable marker cassette hygromycin; nos, nos terminator. The wild-type plant was included as a negative control and the P was included as a positive control. Data are presented as means ± SEM of 3 replicates; t-test was used and asterisk indicates that the value of treatment is different from control (WT) *P < 0.05, **P < 0.01. |
Expression of HpBHY and CrBKT was confirmed in both the HpBHY-expressing moss and the CrBKT-expressing moss, either gene (Fig. 4D).
3.5. Pigment concentrations in transgenic P. patens are alteredTo detect whether HpBHY and CrBKT affect pigment synthesis of P. patens, we measured pigment concentrations in the transgenic plants. The relative abundances of pigments in the transformants were different from those in wild-type plants (Table 1). The transgenic HpBHY-expressing and CrBKT-expressing lines had lower chlorophyll a, chlorophyll b, and total chlorophyll content but higher total carotenoids content than the wild-type plants, indicating that the expression of HpBHY and CrBKT changed the pigment synthesis. Notably, compared to wild-type plants, the HpBHY-expressing and CrBKT-expressing lines accumulated higher amounts of lutein.
| Lines | Pigment (mg/g FW) | ||||
| Chlorophyll a | Chlorophyll b | Total chlorophyll | Carotenoid | Lutein | |
| WT | 1.050 ± 0.011 | 0.297 ± 0.005 | 1.346 ± 0.015 | 0.405 ± 0.003 | 0.187 ± 0.001 |
| Y36 | 0.997 ± 0.013** | 0.287 ± 0.008** | 1.284 ± 0.021** | 0.445 ± 0.005** | 0.205 ± 0.003** |
| T25 | 1.035 ± 0.010 | 0.294 ± 0.004 | 1.329 ± 0.014 | 0.449 ± 0.004** | 0.209 ± 0.003** |
| The data are presented as means ± SEM of 4 replicates; t-test was used and asterisk indicates that the value of treatment is different from control (WT) *P < 0.05, **P < 0.01. FW, fresh weight of tissue; WT, wild type; Y36, HpBHY-expressing plants; T25, CrBKT-expressing plants. | |||||
Lutein is a carotenoid that has been reported to play several important roles in photoprotection. We therefore hypothesized that the higher lutein content in HpBHY-expressing and CrBKT-expressing transformants might lead to higher stress tolerance.
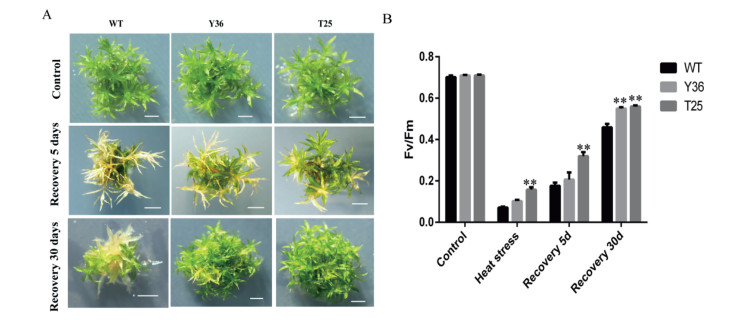
3.6. HpBHY and CrBKT transgenic P. patens are resistant to high temperatureTo detect the stress tolerance of the transgenic P. patens, we treated the wild-type and transgenic lines with different stressors. The result suggested that the transgenic lines had greater tolerance of high-temperature stress. In both wild-type plants and the HpBHY-expressing and CrBKT-expressing transgenic lines, the whole plants showed bleaching and leaf necrosis after a heat treatment of 4 h at 45 ℃ followed by 5 days of recovery at normal temperature. However, the HpBHY-expressing and CrBKT-expressing lines were clearly greener and healthier than wild-type plants, and showed less necrosis. After 30 days of recovery, the gametophytes of the transgenic plants remained healthier than those of the wild-type plants (Fig. 5A). To estimate the damage to PSⅡ, we also analyzed the changes in the maximal efficiency of PSⅡ photochemistry (Fv/Fm). The Fv/Fm values for all plants decreased sharply after high-temperature treatment, but were higher in the transgenic lines than in the wild-type plants (Fig. 5B). After 5 days of recovery, the Fv/Fm values for all plants were partially restored to previous levels, but the values for wild-type plants were still lower than those for HpBHY-expressing and CrBKT-expressing plants. After 30 days of recovery, the Fv/Fm values for all plants had increased considerably, Fv/Fm values for transgenic lines recovered to 0.5-0.6, whereas the Fv/Fm values for wild-type plants were only restored to about 0.4, which were much lower than those under optimal growth conditions (0.75-0.8). Although additional evidence is needed, we concluded that the expression of HpBHY and CrBKT may result in improved high-temperature stress tolerance in plants.
|
| Fig. 5 Effects of high temperature on phenotypes and Fv/Fm in transgenic and wild-type P. patens. (A) Phenotypic comparison of wild type and transformants grown under control conditions (Control), after heat stress treatment for 4 h followed by recovery for 5 days (Recovery 5 days), or after heat stress treatment for 4 h followed by recovery 30 days (Recovery 30 days). Scale bars, 20 mm. (B) Fv/Fm of transgenic and wild-type plants grown under control conditions (Control), after heat stress treatment for 4 h (Heat stress), after heat stress treatment for 4 h followed by recovery for 5 days (Recovery 5 d) and recovery 30 days (Recovery 30 d). Y36, HpBHY-expressing plants; T25, CrBKT-expressing plants; WT, wild type plants. Data are presented as means ± SEM of 5 replicates; t-test was used and asterisk indicates that the value of treatment is different from control (WT) *P < 0.05, **P < 0.01. |
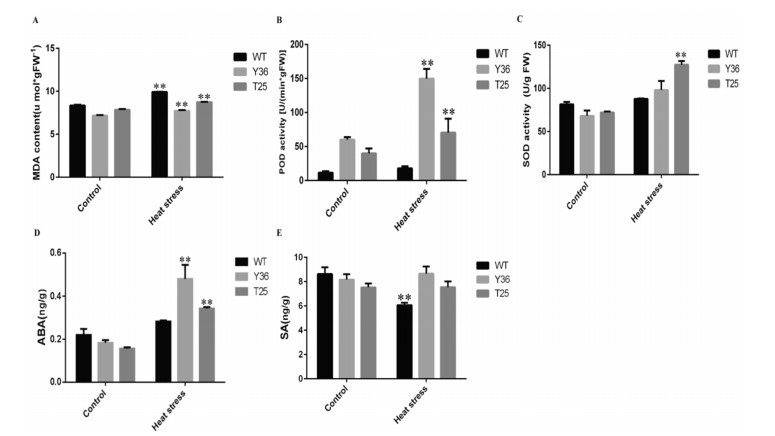
To further investigate the physiological changes that lead to high temperature stress tolerance in transgenic P. patens, we measured three physiological indexes of stress tolerance: malonyldialdehyde (MDA) level, peroxidase (POD) activity and superoxide dismutase (SOD) activity. Under control conditions, the MDA levels of transgenic lines were lower than those of wild-type plants. After heatstress treatment, the MDA levels of the transgenic lines increased less than those of the wild-type plants (HpBHY-expressing plants increased 7.56%, CrBKT-expressing plants increased 11.37%, and wild-type plants increased 18.84%) (Fig. 6A). As shown in Fig. 6B, the POD activities showed no significant difference among all plants under control conditions. After treating plants to hightemperature stress, however, POD activity increased more in the transgenic lines than in wild-type plants (HpBHY-expressing plants increase 149.33%, CrBKT-expressing plants increase 76.22%, and wild-type plants increase 57.77%). Similarly, after treating plants to heat-stress, SOD activity increased significantly more in transgenic lines than in wild-type plants (HpBHY-expressing plants increased 43.72%, CrBKT-expressing plants increased 77.04%, wild-type plants increased 7.67%). Taken together, these results indicate that HpBHY-expressing and CrBKT-expressing transgenic lines have increased antioxidant capacity.
|
| Fig. 6 Effects of high temperature on MDA contents, POD activity, SOD activity, ABA and SA content in transgenic and wild-type Physcomitrella patens. Effect of high temperature on MDA content (A), POD activity (B), SOD activity (C), ABA content (D), and SA content (E). Measurements were taken under control condition (Control) and after direct heat treatment (Heat stress) at 45 ℃ for 4 h. WT, wild type plants; Y36, HpBHY-expressing plants; T25, CrBKT-expressing plants. Data are presented as means ± SEM of 5 replicates; t-test was used and asterisk indicates that the value of treatment is different from control (WT) *P < 0.05, **P < 0.01. |
Endogenous hormone levels are also reported to be unstable under heat stress (Wahid et al., 2007). To investigate whether the endogenous hormone levels of transgenic P. patens change under heat stress, we examined the endogenous levels of plant hormones such as abscisic acid (ABA) and salicylate (SA) in HpBHYexpressing plants and CrBKT-expressing plants and wild-type plants. After heat stress, the ABA levels of transgenic lines increased much more than wild-type plants. (HpBHY-expressing plants increased 162.30% and CrBKT-expressing plants increased 119.08%, whereas wild-type plants increased 28.51%) (Fig. 6D). In contrast, the SA levels in wild-type plants decreased markedly in response to heat stress, whereas SA levels in transgenic lines did not change after heat stress for 4 h. Under control conditions, ABA and SA levels were not significantly different in all plants (Fig. 6E).
Because the two transgenic lines had higher ABA and SA contents in response to high-temperature stress, we speculated that ABA and SA might mediate increased heat resistance by inducing damage-repair pathways.
4. Discussion 4.1. Expression of HpBHY and CrBKT in P. patens increases carotenoids contentsβ-carotene hydroxylase and β-carotene ketolase are key enzymes in the carotenoid biosynthetic pathway that are known to play significant regulatory roles in the biosynthesis of carotenoids (Giuliano, 2014). Overexpressing these two genes has been previously reported to increase carotenoid content in tobacco (Hasunuma et al., 2008; Mann et al., 2000), tomato (Huang et al., 2013), maize (Farré et al., 2016), rice (Du et al., 2010) and potato (Morris et al., 2006). In this study, we investigated the functions of CrBKT and HpBHY in P. patens plants.
After analyzing the phylogenetic position and expression profiles of β-carotene hydroxylase homologs and β-carotene ketolase, we found that abiotic stresses change the expression of these genes in various plant species. Thus, we hypothesized that β-carotene hydroxylase and β-carotene ketolase play significant roles in abiotic stresses response. To test this hypothesis, we constructed P. patens lines heterologously expressing each gene and analyzed the pigment concentration of the resulting transgenic plants.
We found that the total carotenoid level and lutein content increased (Table 1). Lutein is known to play a fundamental role in photoprotection (Jahns and Holzwarth, 2012; Matsubara et al., 2007; Dall"Osto et al., 2006). In our study, transgenic lines that expressed either β-carotene hydroxylase or β-carotene ketolase accumulated higher amounts of lutein than wild-type plants (Table 1). This increase in lutein content contrasts with lutein contents reported previously for various transgenic plants expressing carotenoid-pathway-related genes. For instance, in the transplastomic tobacco plants expressing both β-carotene hydroxylase (CrtZ) and β-carotene ketolase (CrtW) from Brevundimonas sp. SD212, the lutein content was decreased 12.4-fold (Fan et al., 2017), in addition, the lutein content in the transplastomic tomatoes leaves overexpressing CrBKT decreased 4.9-fold (Huang et al., 2013). This difference in how β-carotene hydroxylase and β-carotene ketolase affect lutein content suggests that these genes play distinct functions in the moss P. patens.
4.2. Antioxidant system and ABA and SA signaling are involved in tolerance of P. patens to heat stressPlants have evolved various resistance mechanisms to stress, including adjusting photosynthesis; eliminating active oxygen species through increased activation of antioxidant enzymes and accumulation of some antioxidants; and accumulation of secondary metabolites, such as stress resistance hormones and carotenoids (Wahid and Ghazanfar, 2006). Carotenoids exhibit strong antioxidant properties and are important for plant photoprotection (Wahid et al., 2007); indeed, the levels of plant carotenoids may be related to stress tolerance (Wahid and Ghazanfar, 2006). For example, salt tolerance in mungbean has been are tightly correlated with steady carotenoids levels (Wahid et al., 2004), and sugarcane improves its salinity tolerance through reduced chlorophyll and steady carotenoid levels (Wahid and Ghazanfar, 2006).
To investigate the role of two carotenoid biosynthetic enzymes-β-carotene hydroxylase and β-caroteneketolase — in stress resistance, we treated transgenic P. patens expressing each gene to heat stress (45 ℃ for 4 h). We found that expression of β-carotene hydroxylase or β-carotene ketolase increased heat tolerance in transgenic P. patens plants. Specifically, PSⅡ efficiency in transgenic plants expressing β-carotene hydroxylase or β-carotene ketolase recovered quicker from heat stress thaninwild-typeplants (Fig. 5B). Changes inPSⅡefficiency, measured by chlorophyll fluorescence, Fv/Fm ratio, are considered reliable diagnostic indicators of photoinhibition (Dew et al., 2015). Furthermore, following heat stress, transgenic lines expressing β-carotene hydroxylase and β-carotene ketolase were visibly greener and showed less leaf necrosis than wild-type plants (Fig. 5A). Similar results have been reported in Arabidopsis, for example, β-carotene hydroxylase overexpression in Arabidopsis increased resistance to high-light and high-temperature treatment (Davison et al., 2002) and overexpression CrBKT has been shownincrease tolerance tohigh-light stress (Zhong et al., 2011). Similarly, in rice, overexpression of a putative β-carotene hydroxylase (DSM2) in rice increases the resistance to oxidative stresses and drought significantly (Du et al., 2010). These results suggest that overexpression of β-carotene hydroxylase and β-carotene ketolase can increase plant tolerance of stress and maintain relatively high PSⅡ activity under stress. However, the mechanisms that underlie increased stress tolerance remain unclear. Peroxidative damage in membrane lipids, for which MDA content is a common indicator, is especially evident under stress (Liu and Huang, 2000). Higher activities of antioxidant enzymes, such as SOD and POD, are associated with less peroxidative damage and higher stress tolerance. In this study, the much lower MDA contents (Fig. 6A) and the higher POD (Fig. 6B) and SOD (Fig. 6C) activity indicated that the cell membranes of the transgenic lines were less damaged than those of the wild-type plants.
Carotenoids are precursors in ABA and SA biosynthesis (Du et al., 2010). Thus, we suspected that SA and ABA levels in β-carotene hydroxylase- and β-carotene ketolase-expressing transgenic plants may be high under control condition. Interestingly, we found that before heat stress treatment, ABA and SA contents were no significant differences high. However, ABA contents increased and SA levels remained stable in β-carotene hydroxylase- and β-carotene ketolase-expressing transgenic plants under high-temperature treatment in our study (Fig. 6D, E). This finding suggested that ABA and SA play key roles in plants response to high temperature. Moreover, these results suggest that the increased heat-stress tolerance of the transgenic plants may be related to ABA and SA signaling. Taken together, we speculate that overexpression of β-carotene hydroxylase and β-carotene ketolase in P. patens may increase plants heat tolerance because of increased antioxidant capacity and improved damage repair related with ABA and SA signaling. This interpretation of our findings is consistent with previous reports which have shown that ABA and SA as important components of plant thermotolerance (Maestri et al., 2002).
Heterologous expression of HpBHY and CrBKT in P. patens not only increased carotenoid content but also improved heat tolerance in the transgenic plants, showing that these two genes could be valuable genetic resources for researchers seeking to develop plants with higher nutrition and stronger stress resistance. In this study, we confirmed carotenoids play important roles in plant heat stress response, through the antioxidant and damage repair metabolism, which is related to abscisic acid and salicylate signaling. It laid the foundation for further research on the mechanism of plant response to high temperature stress.
Author contributionsLL, HQH, JCH and JFH initiated and designed the research. JFH, PL, LNL and TT performed the experiments. JFH, MXH analyzed the data. LL contributed reagents/materials/analysis tools. JFH wrote the manuscript. LL, JFH and PL revised the manuscript. All authors read and approved the manuscript.
Conflict of interestAll authors declared no conflict of interest.
AcknowledgementsThis work was supported by the CAS Pioneer Hundred Talents Program, the National Natural Science Foundation of China (31571262) and Yunnan Natural Science Foundation (2017FB031). We are grateful to Dr. Mitsuyasu Hasebe for providing moss spores and pPOG1 plasmid.
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2019.04.001.
Artimo P., Jonnalagedda M., Arnold K., et al, 2012. ExPASy:SIB bioinformatics resource portal. Nucleic Acids Res, 40: W603-289. |
Camejo D., Rodrã-Guez P., Morales M.A., et al, 2005. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol, 162: 281-289. DOI:10.1016/j.jplph.2004.07.014 |
Cui Y., Wang Q., 2006. Physiological responses of maize to elemental sulphur and cadmium stress. Plant Soil Environ, 52: 523-529. |
Dall"Osto L., Lico C., Alric J., et al, 2006. Lutein is needed for efficient chlorophyll triplet quenching in the major lhcii antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol, 6: 32-40. DOI:10.1186/1471-2229-6-32 |
Davison P.A., Hunter C.N., Horton P., 2002. Overexpression of β-carotene hydroxylase enhances stress tolerance in arabidopsis. Nature, 418: 203-206. DOI:10.1038/nature00861 |
Decker E.L., Reski R., 2004. The moss bioreactor. Curr. Opin. Plant Biol, 7: 166-170. DOI:10.1016/j.pbi.2004.01.002 |
Deng W., Wang Y., Liu Z., et al, 2014. HemI:a toolkit for illustrating heatmaps. PLoS One, 9: e111988. DOI:10.1371/journal.pone.0111988 |
Dew K.S., Sven B.A., Carl-Otto O., et al, 2015. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plantarum, 153: 284-298. DOI:10.1111/ppl.12245 |
Ding H., He J., Wu Y., et al, 2018. The tomato mitogen-activated protein kinase SlMPK1 is as a negative regulator of the high temperature stress response. Plant Physiol, 177: 633-651. DOI:10.1104/pp.18.00067 |
Du H., Wang N., Cui F., et al, 2010. Characterization of the beta-carotene hydroxylase gene DSM2 conferring drought and oxidative stress resistance by increasing xanthophylls and abscisic acid synthesis in rice. Plant Physiol, 154: 1304-1318. DOI:10.1104/pp.110.163741 |
Fan M., Sun X., Xu N., et al, 2017. Integration of deep transcriptome and proteome analyses of salicylic acid regulation high temperature stress in Ulva prolifera. Sci. Rep, 7: 11052. DOI:10.1038/s41598-017-11449-w |
Farré G., Perez-Fons L., Decourcelle M., et al, 2016. Metabolic engineering of astaxanthin biosynthesis in maize endosperm and characterization of a prototype high oil hybrid. Transgenic Res, 25: 477-489. DOI:10.1007/s11248-016-9943-7 |
Frank W., Ratnadewi D., Reski R., 2005. Physcomitrella patens is highly tolerant against drought, salt and osmotic stress. Planta, 220: 384-394. DOI:10.1007/s00425-004-1351-1 |
Fu J., Huang B., 2001. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot, 45: 105-114. DOI:10.1016/S0098-8472(00)00084-8 |
Giuliano G., 2014. Plant carotenoids:genomics meets multi-gene engineering. Curr. Opin. Plant Biol, 19: 111-117. DOI:10.1016/j.pbi.2014.05.006 |
Guerin M., Huntley M.E., Olaizola M., 2003. Haematococcus astaxanthin:applications for human health and nutrition. Trends Biotechnol, 21: 210-216. DOI:10.1016/S0167-7799(03)00078-7 |
Hasunuma T., Miyazawa S.I., Yoshimura S., et al, 2008. Biosynthesis of astaxanthin in tobacco leaves by transplastomic engineering. Plant J, 55: 857-868. DOI:10.1111/j.1365-313X.2008.03559.x |
Huang J., Zhong Y., Liu J., et al, 2013. Metabolic engineering of tomato for highyield production of astaxanthin. Metab. Eng, 17: 59-67. DOI:10.1016/j.ymben.2013.02.005 |
Huang Q., Wei Y., Wei F., et al, 2011. The comparative analysis of leaf color and carotenoids content of the common apocynaceae plants. J. Anhui Agric. Sci, 39: 15203-15204, 15262. |
Ivica L., Peer B., 2016. Interactive tree of life (iTOL) v3:an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res, 44: W242-W245. DOI:10.1093/nar/gkw290 |
Jahns P., Holzwarth A.R., 2012. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem Ⅱ. Biochim. Biophys. Acta, 1817: 182-193. DOI:10.1016/j.bbabio.2011.04.012 |
Lee S.M., Radhakrishnan R., Kang S.M., et al, 2015. Phytotoxic mechanisms of bur cucumber seed extracts on lettuce with special reference to analysis of chloroplast proteins, phytohormones, and nutritional elements. Ecotoxicol. Environ. Saf, 122: 230-237. DOI:10.1016/j.ecoenv.2015.07.015 |
Liu L., Theg S.M., 2014. ATP requirement for chloroplast protein import is set by the km for ATP hydrolysis of stromal Hsp70 in Physcomitrella patens. Plant Cell, 26: 1246-1255. DOI:10.1105/tpc.113.121822 |
Liu X., Huang B., 2000. Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci, 40: 503-510. DOI:10.2135/cropsci2000.402503x |
Liu Y., Vidali L., 2011. Efficient polyethylene glycol (PEG) mediated transformation of the moss Physcomitrella patens. JoVE (, (50). |
Li C., Sako Y., Imai A., et al, 2017. A Lin 28 homologue reprograms differentiated cells to stem cells in the moss Physcomitrella patens. Nat. Commun, 8: 14242. DOI:10.1038/ncomms14242 |
Lo S.M., Theg S.M., 2011. Protein targeting across and into chloroplast membranes. Methods Mol. Biol, 684: 139-157. |
Maestri E., Klueva N., Perrotta C., et al, 2002. Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Mol. Biol, 48: 667-681. DOI:10.1023/A:1014826730024 |
Mann V., Harker M., Pecker I., et al, 2000. Metabolic engineering of astaxanthin production in tobacco flowers. Nat. Biotechnol, 18: 888-892. DOI:10.1038/78515 |
Matsubara S., Morosinotto T., Osmond C.B., et al, 2007. Short-and long-term operation of the lutein-epoxide cycle in light-harvesting antenna complexes. Plant Physiol, 144: 926-941. DOI:10.1104/pp.107.099077 |
Meloni D.A., Oliva M.A., Martinez C.A., et al, 2003. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot, 49: 69-76. DOI:10.1016/S0098-8472(02)00058-8 |
Minami A., Nagao M., Ikegami K., et al, 2005. Cold acclimation in bryophytes:lowtemperature-induced freezing tolerance in Physcomitrella patens is associated with increases in expression levels of stress-related genes but not with increase in level of endogenous abscisic acid. Planta, 220: 414-423. DOI:10.1007/s00425-004-1361-z |
Morris W.L., Ducreux L.J., Fraser P.D., et al, 2006. Engineering ketocarotenoid biosynthesis in potato tubers. Metab. Eng, 8: 253-263. DOI:10.1016/j.ymben.2006.01.001 |
Schaefer D.G., 2001. Gene targeting in Physcomitrella patens. Curr. Opin. Plant Biol, 4: 143-150. DOI:10.1016/S1369-5266(00)00150-3 |
Schaefer D.G., Zrÿd J.P., 2001. The moss Physcomitrella patens now and then. Plant Physiol, 127: 1430-1438. DOI:10.1104/pp.010786 |
Shi L., Theg S.M., 2013. Energetic cost of protein import across the envelope membranes of chloroplasts. Proc. Natl. Acad. Sci. U. S. A, 110: 930-935. DOI:10.1073/pnas.1115886110 |
Vera V.S., Kenchappa C.S., Landberg K., et al, 2017. Autophagy is required for gamete differentiation in the moss Physcomitrella patens. Autophagy, 13: 1939-1951. DOI:10.1080/15548627.2017.1366406 |
Wahid A., Gelani S., Ashraf M., et al, 2007. Heat tolerance in plants:an overview. Environ. Exp. Bot, 61: 199-223. DOI:10.1016/j.envexpbot.2007.05.011 |
Wahid A., Ghazanfar A., et al, 2006. Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J. Plant Physiol, 163: 723-730. DOI:10.1016/j.jplph.2005.07.007 |
Wahid A., Hameed M., Rasul E., 2004. Salt-induced injury symptoms, changes in nutrient and pigment composition, and yield characteristics of mungbean. Int. J. Agric. Biol, 6: 1143-1152. |
Ye L., Xie W., Zhou P., et al, 2015. Biotechnological production of astaxanthin through metabolic engineering of yeasts. Chembioeng Rev, 2: 107-117. DOI:10.1002/cben.201400023 |
Zhong Y., Huang J., Jin L., et al, 2011. Functional characterization of various algal carotenoid ketolases reveals that ketolating zeaxanthin efficiently is essential for high production of astaxanthin in transgenic Arabidopsis. J. Exp. Bot, 62: 3659-3669. DOI:10.1093/jxb/err070 |



