b. School of Life Sciences, Nanjing University, Nanjing, 210023, PR China;
c. Coastal Ecosystems Research Station of the Yangtze River Estuary, Ministry of Education Key Laboratory for Biodiversity Science and Ecological Engineering, Institute of Biodiversity Science, Fudan University, Shanghai, 200438, PR China;
d. Key Laboratory of Vegetation Restoration and Management of Degraded Ecosystems, Chinese Academy of Sciences, South China Botanical Garden, Guangzhou, 510650
Tree transpiration is important to terrestrial water flux (Jasechko et al., 2013). It significantly influences carbon, water, and nutrient cycles, and thus determines biodiversity patterns and ecosystem services (Bernacchi and VanLoocke, 2015). The tree transpiration process involves water uptake by roots, after which it is transported by xylem vessels and/or tracheids to the leaf stomata, and eventually diffuses into the atmosphere through transpirative forces (vapor pressure deficit, D). This process is controlled by plants through the joint regulation of xylem and leaf traits; however, little is known about the extent to which tree transpiration is determined by these tree biological attributes (i.e., xylem and stomatal traits) (Brodribb and McAdam, 2011). Several global-scale estimates of transpiration from climate models at the ecosystem level currently exist; however, these models are potentially inaccurate because they do not consider stomatal behavior (Jasechko et al., 2013).
Water flux can be precisely modeled by compiling in situ transpiration measurements (e.g., sap flow at the forest stand level) upscaled to canopy stomatal conductance (Gs) based on tree biological parameters (Granier et al., 1996). However, canopy biological attributes are rarely measured due to logistical and technical difficulties (Dial et al., 2004). In the past decades, a large body of evidence suggests that there exists empirical relationships between leaf stomatal conductance (gs), CO2 uptake, and stomatal anatomy (Araus et al., 1986; Chen et al., 1990; Dow et al., 2014a, b; Drake et al., 2013; Giday et al., 2013; McElwain et al., 2015; Raven, 2014). However, little is known about the influence of leaf stomata morphology on canopy water flux at the individual tree level. Currently, to our knowledge, no empirical studies have quantified the possible correlation between Gs and stomata morphology.
In the present study, a quantile model was used to investigate the relationship between functional leaf traits and water flux at the tree level (Gao et al., 2015, 2016). Quantile regression is particularly applicable in the study of stomatal behavior, which is constantly influenced by a range of environmental/biological variables (Cade et al., 2005; Gao et al., 2016). In the current study, we compiled fine measurements of the biophysical attributes of seven tree species (Gao et al., 2015), the published Gs, alongside unpublished stomata data. Because stomata are regarded as hydraulically driven valves on the leaf surface, or alternatively the so-called 'Watergates' (Roelfsema and Hedrich, 2005), we hypothesized that canopy water flux and stomatal density are positively correlated, especially for the trees with denser stomata. In addition to stomata, we also hypothesized that xylem functional traits, such as wood density, would be negatively correlated with canopy water flux. A vast body of literature has shown that trees with heavier wood have lower gs and leaf-specific hydraulic conductance than those with lighter wood (Gao et al., 2015; Hacke et al., 2001; Jacobsen et al., 2007).
Therefore, linking the two key components of the hydraulic continuum in trees, the "intermediate path" (i.e., xylem conduits indicated by wood density) and "export" (i.e., leaf stomata), should provide new knowledge regarding the underlying physiological mechanisms that influence tree transpiration. We thus hypothesized that stomatal density might also be a predictor of canopy water flux similar to wood density.
2. Materials and methods 2.1. Site description and tree speciesThree research plots with different tree species were used in this study. All three research sites were located in lower sub-tropical China, where there is a moist climate type. The first plot, in the Huangmian State Forest Farm (109°54'E, 24°46'N), was mainly used for the plantation of Eucalyptus grandis Hill × Eucalyptus urophylla S. T. Blake over the last five years. We selected five trees for measurement of sap flux during the period from 1 October to 31 October 2012. The second research plot was located in Heshan National Field Research Station of Forest Ecosystem, Chinese Academy of Sciences, Guangdong Province (112°54'E, 22°41'N). This plot contained mostly native species, with trees approximately 25 years old. The sap flux measurements were performed in a secondary broadleaved forest that contained Schima superba Gardn. et Champ, Michelia macclurei Dandy, Castanopsis fissa (Champ. ex Benth.) Rehd. et Wils., and Castanopsis hystrix Miq. as the dominant tree species. We selected 3-5 individual trees of each species for sap flux measurement from 1 October to 31 October 2012, which was a period where there was no apparent soil water deficit. The third research plot was located in an Australian Garden of the South China Botanical Garden (113°22'E, 23°11'N). In this garden, species from Australia were introduced, with trees approximately 50 years old. Acacia auriculiformis A. Cunn. ex Benth. and Eucalyptus citriodora Hook. f. were planted in this garden in the 1960s, mainly for appreciation. We selected five individual trees of each species for sap flux measurement from 1 October to 31 October 2013. The characteristics of the biometrics of the studied trees and climate variables are summarized in Table 1 (Gao et al., 2015).
| Study site | Huangmian Forest Farm, Guangxi Province (GXHM) | Heshan National Field Research Station, Guangdong Province (GDHS) | Botanical Garden, Guangzhou, Guangdong Province (GDGZ) | ||||
| Coordinates | 109°54'E, 24°46'N | 112°54'E, 22°41'N | 113°22'E, 23°11'N | ||||
| aMAT (℃) | 19 | 21.7 | 21.9 | ||||
| bMAP (mm) | 1750 | 1700 | 1696.5 | ||||
| Species | E. grandis × urophylla | S. superba | M. macclurei | C. fissa | C. hystrix | A. auriculiformis | E. citriodora |
| Code | EGU | SS | MM | CF | CH | AA | EC |
| Family | Myrtaceae | Theaceae | Magnoliaceae | Fagaceae | Fagaceae | Fabaceae | Myrtaceae |
| Wood type | Diffuse-porous | Diffuse-porous | Diffuse-porous | Diffuse-porous | Diffuse-porous | Diffuse-porous | Diffuse-porous |
| Leaf habit | Evergreen | Evergreen | Evergreen | Evergreen | Evergreen | Evergreen | Evergreen |
| n | 5 | 5 | 5 | 3 | 3 | 5 | 5 |
| Sapwood width (cm) | 3.96 | 5.34 | 7.05 | 4.22 | 3.85 | 2.05 | 2.33 |
| Sapwood area (cm2) | 69.24 ± 13.57 | 143.43 ± 118.47 | 239.57 ± 81.71 | 162.88 ± 9.28 | 94.05 ± 6.57 | 174.21 ± 42.96 | 191.34 ± 51.57 |
| DBH (cm) | 9.96 ± 1.08 | 13.92 ± 5.32 | 19.54 ± 3.73 | 21.57 ± 0.74 | 12.63 ± 0.48 | 30.84 ± 5.49 | 30.04 ± 4.66 |
| Tree height (m) | 14.97 ± 6.93 | 7.18 ± 1.81 | 12.12 ± 4.04 | 10.05 ± 4.08 | 11.08 ± 1.52 | 19.99 ± 1.73 | 26.52 ± 3.54 |
| cWood density(g · cm-3) | 0.43 ± 0.03e | 0.61 ± 0.03bc | 0.53 ± 0.03d | 0.47 ± 0.07e | 0.59 ± 0.03c | 0.71 ± 0.03a | 0.65 ± 0.07b |
| dGsref | 195.51 ± 37.85a | 145.57 ± 30.71ab | 163.28 ± 43.39ab | 188.21 ± 27.00ab | 194.52 ± 34.04a | 119.55 ± 40.43b | 146.67 ± 74.00ab |
| eSoil property | SPH (3.53), SOM(27.7 g kg-1), TN(1.08 g kg-1), AP(5.59 mg kg-1) | SPH (4.26), SOM (24.2 g kg-1), TN (1.16 g kg-1), AP (2.35 mg kg-1) | SPH (4), SOM (21.7 g kg-1), TN (0.68 g kg-1), AP(5.3 mg kg-1) | ||||
| a: MAT, mean annual temperature; b: MAP, mean annual precipitation; c & d: Different small letters denote significance at 0.05 (P < 0.05). e: soil property of 0-20 cm soil layer. SPH, soil pH; SOM, soil organic matter; TN, total nitrogen; AP, available phosphorous. | |||||||
Granier thermal dissipation probes (TDP) were directly inserted into the xylem of the studied trees to measure the sap flux (Js) (Granier, 1987). Each TDP sensor consisted of a pair of 20-mm long, 2-mm diameter stainless steel probes installed approximately 10e15 cm apart along the axis of the hydroactive xylem. The upper probe was heated by a constant power of 0.2 W with a DC supply of 120 mA, whereas the lower probe remained unheated. An instantaneous temperature difference between the probes could be converted into a voltage value and recorded using a data collection instrument (Gao et al., 2017). The data were measured every 30 s and stored as 10-min averages using a Delta-T logger (DL2e, UK). Finally, the Js (g H2O m-2 s-1) was calculated according to the following formula:
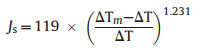
|
(1) |
where △Tm is the temperature difference obtained under zero flux conditions and △T is the instantaneous temperature (Granier, 1987).
Data on photosynthetically active radiation (PAR), air temperature (T) and relative humidity (RH), and wind speed (m s-1) were obtained from a meteorological station of the Heshan National Field Research Station, which was approximately 100 m away from the experimental plot. For Huangmian Forest Farm and Botanical Garden, the meteorological data were directly collected from the observation tower (18-20 m) in the forest. The radiation (LI-COR, Lincoln, USA), temperature, and humidity (Delta-T Devices Ltd. Cambridge, UK) sensors were deployed on the top of the towers. We calculated vapor pressure deficit (D, kPa) by combining the air temperature and RH:
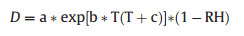
|
(2) |
where a, b, and c are fixed parameters, which are 0.611 kPa, 17.502 (unitless), and 240.97 ℃, respectively.
2.3. Canopy water flux (Gs and Gsref)Owing to the difficulty in obtaining the leaf area data of studied trees, canopy stomatal conductance (Gs) was determined by using sap flux and D in the following equation:
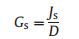
|
(3) |
which assumes that sap flux is equal to canopy transpiration, and stem sap flux is not affected by hydraulic capacitance (Gao et al., 2015). D was standardized by standard atmospheric pressure. Analyses were only conducted on clear days (10:00-16:00) with high PAR and high D (>1 kPa) to minimize the effects of stem water storage; this procedure also minimized the impact of low irradiance on Gs. The three research plots were open and void of canopy closure, thus satisfying our assumption that Tcanopy = Tair. The calculations of sensitivity and reference Gs were based on the model of Oren et al. (1999) as follows:
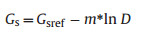
|
(4) |
where m (-dGs/dlnD) is the sensitivity of Gs to D, and Gsref is reference Gs when D = 1 kPa.
In this study, canopy water flux was characterized by Gsref. Gsref was a good modeling scalar that could be used to compare among different tree species from different sites (Gao et al., 2015).
2.4. Wood and stomatal densityTwo cores (Φ = 5.15 mm) from 6-7 trees per species were sampled using an increment borer (Haglöf, Sweden) in September 2012 wrapped with a wet towel immediately after sampling, and placed in sealed plastic bags. Sampled cores were then transported to the laboratory and weighed on an electronic balance (Shinko, Japan) to an accuracy of 0.0001 g. The wood cores were dried until weights were constant at 80 ℃ to obtain a dry weight value. The wood density was calculated as the ratio of the dry weight to fresh volume.
Three mature, sun-exposed leaves in three trees of each species were selected for the measurement of stomata density in June 2013, which was determined using nail-polish imprints following a modified version of the method of Li and Xing (2007) and Xiong et al. (2014). The number of stomata (# mm-2) was recorded using a light microscopy (Zeiss, Jena, Germany). At least five fields of view on the abaxial surface of leaves were randomly selected for counting. Most of the trees selected were chosen from or at least around the research plots. The leaves were sampled in those shorter trees as surrogates due to the difficulties in sampling very tall trees, e.g., A. auriculiformis and E. citriodora.
2.5. Statistical analysisIn this study, to test the correlations between canopy water flux and wood, stomatal density, we fitted linear models using both ordinary least-squares (OLS) and quantile regressions. Quantile regression seeks to complement classical linear regression analysis to estimate all parts of the response distribution conditional to the predictor variable, thus providing a more comprehensive characterization of the effects than those provided by estimates of the conditional mean made with OLS regression (Cade et al., 2005).
Quantile regression is a non-parametric test that makes no assumptions regarding normality of distribution or variance homogeneity. Thus, quantile regression overcomes various limitations of using OLS regression. For instance, by focusing on the mean, information about the tails of distribution is lost. Additionally, OLS regression is particularly sensitive to extreme outliers, which can significantly distort the results. By contrast, quantile regression reduces outlier effects because it is based on absolute values rather than on squared deviations. We estimated the quantile regression functions of 0.2, 0.4, and 0.6 quantiles using the R package 'quantreg' (Koenker, 2013).
Statistical analysis of stomatal and wood density was performed using one-way ANOVA in the predictive analysis software (PASW, IBM, USA). When the one-way ANOVA results were significant at alpha = 0.05, the differences among the means were then explored using Duncan multiple range test.
3. ResultsSap flux of the seven tree species was measured during the late wet season of October 2012 and 2013. During this period, there was no apparent soil water deficit, thereby aiding the comparison of data collected from three different sites in these lower subtropical forests.
Both stomatal density (Figs. 1 and 2A, F6, 14 = 10.127, P < 0.00001) and wood density (Fig. 2B, F6, 36 = 27.145, p < 0.00001) varied significantly between species. We also found that stomatal density was significantly positively correlated with Gsref at the 0.6 quantile (Fig. 3A and Table 2); however, there was no correlation with Gsref at lower quantiles, i.e., the 0.2 and 0.4 quantiles, which suggests that stomatal density likely has a "threshold effect" on Gsref. We also found that wood density was negatively correlated with Gsref regardless of quantile, but displayed significant correlation at the 0.2 and 0.4 quantiles (Fig. 3B and Table 2).
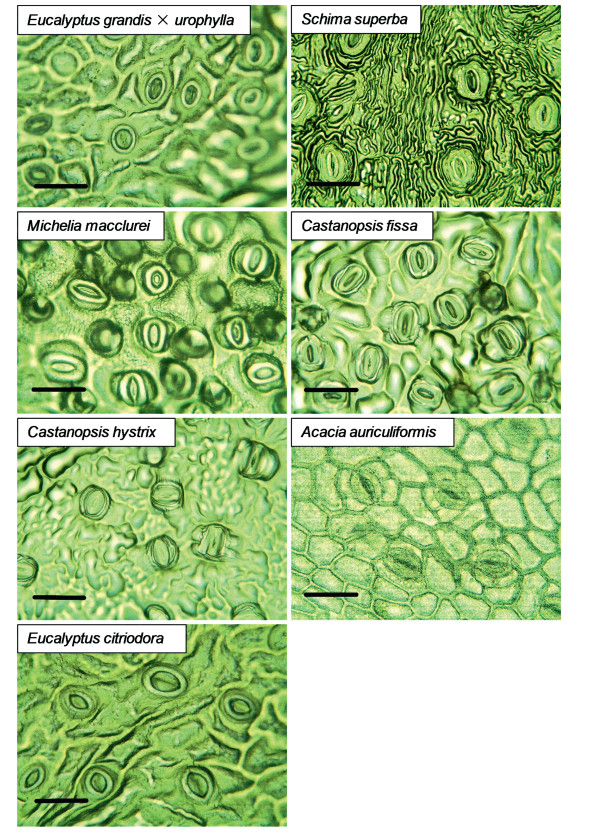
|
| Fig. 1 Optical images of stomata in the seven studied tree species. Black scale bar = 30 μm. |
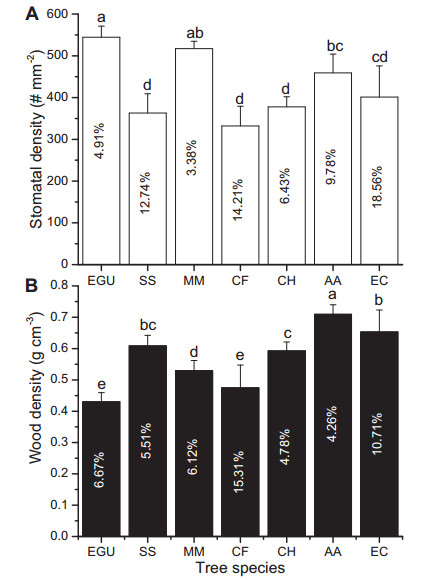
|
| Fig. 2 Stomatal density (A) and wood density (B) of the seven studied tree species. Different small letters denote significance at the 0.05 level. Data are the means ± SD (n = 3-7). The numbers on the column are the coefficients of variation (CV, %). Species notations: EGU, Eucalyptus grandis × urophylla; SS, Schima superba; MM, Michelia macclurei; CF, Castanopsis fissa; CH, Castanopsis hystrix; AA, Acacia auriculiformis; EC, Eucalyptus citriodora. |
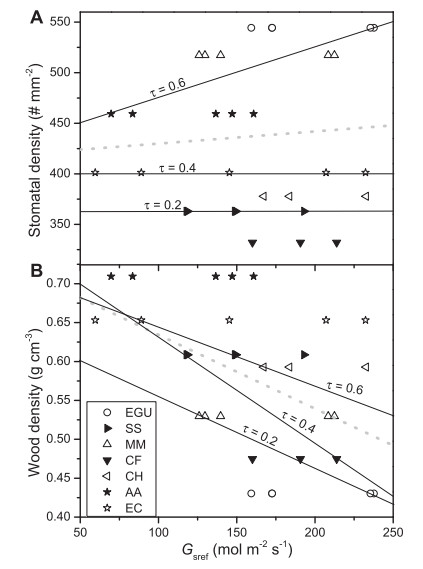
|
| Fig. 3 Relationship of stomatal density (A) and wood density (B) with respect to reference canopy stomatal conductance (Gsref), in which stomatal density was positively correlated with Gsref at the 0.6 quantile (τ=6), and wood density was negatively correlated with Gsref at 0.2 and 0.4 quantiles (τ=2, 0.4). The results of statistical analyses of slopes are presented in Table 2. The thin black lines correspond to the quantiles (0.2, 0.4 and 0.6), and the gray dotted line is the least-square estimate of the conditional mean function. Legend denotes the code indicated in Fig. 2. |
| Stomatal density vs Gsref | Wood density vs Gsref | ||
| Regression type | |||
| OLS | 0.12 (R2 = 0.006) | -0.00095** (R2 = 0.237) | |
| Quantile | τ= 0.2 | 0.000 | -0.00094* |
| τ= 0.4 | 0.000 | -0.0014** | |
| τ= 0.6 | 0.753** | -0.00074 | |
| OLS, ordinary least-squares regression; τ, quantile value; *P < 0.05, **P < 0.01. | |||
We constructed a conceptual model (Fig. 4) illustrating the correlation between stem, leaf traits, and water flux. This model is based on our empirical study and describes four scenarios. Scenario Ⅰ denotes "lighter wood, denser stomata, " indicating a higher sap flow (Gs), and higher sensitivity to D, i.e., higher slope. Scenario Ⅱ denotes "heavier wood, less stomata, " indicating a lower sap flow (Gs), and lesser sensitivity to D, i.e., lower slope. "Light wood, less stomata (lower Gs)" (scenario Ⅲ) and "heavy wood, denser stomata (higher Gs)" (scenario Ⅳ), in addition to further intermediate states or scenarios are between the two extremes, i.e., scenarios Ⅰ and Ⅱ (Fig. 4).
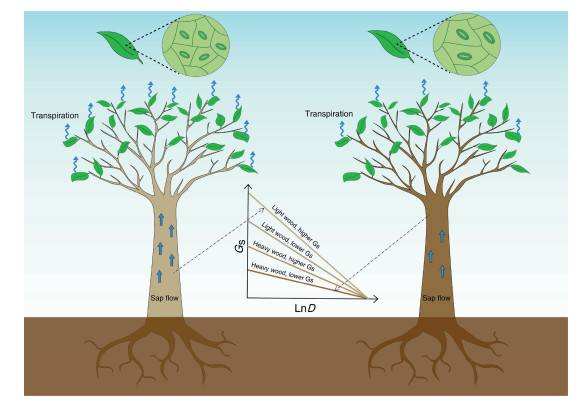
|
| Fig. 4 A conceptual model illustrating the relationship between wood, leaves, and canopy water flux (reference canopy stomatal conductance, Gsref). The left panel denotes "light wood, higher canopy stomatal conductance (Gs), " indicating a higher sap flow and denser stomata, and higher sensitivity to vapor pressure deficit (D), i.e., higher slope (scenario Ⅰ). The right panel denotes "heavy wood, lower Gs, " indicating a lower sap flow and less stomata, and lesser sensitivity to D, i.e., lower slope (scenario Ⅱ). This figure depicts the two extreme scenarios describing the impacts of wood and stomatal density on tree transpiration. "Light wood, lower Gs (less stomata)" (scenario Ⅲ) and "heavy wood, higher Gs (denser stomata)" (scenario Ⅳ), in addition to further intermediate states or scenarios, are not fully depicted. LnD is the natural logarithm of D. |
In the current study, wood density correlated with Gsref at each of the analyzed quantiles, suggesting that there was an inhibited impact of xylem biophysical attributes on canopy water flux, which agrees with other studies (Hacke et al., 2001; Jacobsen et al., 2007). We also found that stomatal density positively correlated with Gsref at a high quantile. Xylem and stomata are two key components of a plant's hydraulic system; their co-ordination or tradeoff determines tree growth, function, and survival (Brodribb et al., 2014; Carlson et al., 2016; Skelton et al., 2015). As is the case for wood density, which is a good predictor of Gsref, this study highlights the potential of using stomatal density as a trait to predict canopy water flux.
Plant biomass, vegetation productivity, carbon and water exchange, and water use efficiency are mainly determined by stomatal morphology and physiology, i.e., Gs and Gsref, which are widely used scalars for modeling water and carbon balance (Drake et al., 2013; Lawson and Blatt, 2014; McElwain et al., 2015; Wang et al., 2015). Many studies have reported that smaller, denser stomata lead to a greater maximum gs and are more sensitive to environmental variables (Drake et al., 2013; Giday et al., 2013; Raven, 2014), indicating that stomata directly influence water flow. The relationship between gs (transpiration) and stomatal attributes at the leaf level are well characterized (Dow et al., 2014a, b; McElwain et al., 2015). However, the empirical correlation between leaf stomatal traits and water flux at the whole-tree level has been surprisingly rarely investigated.
Following the absorption of water by roots and its transportation through xylem conduits to substomatal cavities, the stomatal size and whether the pore is open or closed determines water flux. During this process, trees maximize carbon assimilation while minimizing water loss (Dow et al., 2014a, b; McElwain et al., 2015). This prioritized water use strategy is often influenced by drought conditions (Carlson et al., 2016; Litvak et al., 2012). For example, some trees close their stomata rapidly in order to prevent water loss before desiccation during early droughts; however, this strategy leads to a higher risk of "carbon starvation." On the other hand, in some trees, the stomata remain open for a longer time to photosynthesize during severe droughts (Franks et al., 2007). The former water use strategy is drought avoidance, whereas the latter is drought resistance. In most cases, under mild water stress, or in mesic environments, the trees that exhibit drought avoidance strategy are more competitive than those trees that exhibit drought resistance, e.g., an isohydric species, E. grandis × urophylla (Gao et al., 2015). By contrast, A. auriculiformis, and E. citriodora, which possess heavier wood (Fig. 2B), exhibit more resistance to embolism in order to avoid hydraulic failure (Gao et al., 2015). The Gs of these trees is not very sensitive to environmental variables, and thus are classified as anisohydric (Domec and Johnson, 2012; Gao et al., 2015; McCulloh and Woodruff, 2012). The co-ordination between stem and leaf is important for tree growth and drought survival (Skelton et al., 2015).
A conceptual model was established to describe the xylem, stomatal traits, and transpiration based on the empirical and theoretical relationships between wood density, stomatal density, and Gsref (Fig. 4). The model demonstrated how Gs was regulated by wood and stomatal traits. The co-ordination between wood and stomata promoted water flux in Scenario Ⅰ ("lighter wood, denser stomata"). Trees with these characteristics were more competitive in mesic environments, similarly to those E. grandis × urophylla trees in this study. However, the trees of Scenario Ⅱ ("heavier wood, less stomata") displayed slower water flux, which allowed higher drought resistance. Scenario Ⅲ ("Lighter wood, less stomata") and Scenario Ⅳ ("heavier wood, denser stomata"), in addition to further intermediate scenarios, should also be included in natural forest ecosystems. The co-ordination between leaf and stem is determined by functional groups, genotypes, ontogenic development and environmental conditions (Apgaua et al., 2015; Arango-Velez et al., 2011; Carlson et al., 2016; Franks et al., 2009; Locosselli and Ceccantini, 2012). For example, the vulnerability segmentation hypothesis that characterized by the co-ordination by xylem and leaves, as well as the maintenance of hydraulic continuum, is determined by the aridity level (Zhu et al., 2016). This indicates that the relationships between xylem and stomata would be diverse (Méndez-Alonzo et al., 2012). Further research is required to elucidate these correlations (Martínez-Vilalta et al., 2014).
Our previous research showed there was no significant correlation between Gsref and tree structure factors (Gao et al., 2015). This indicated that tree biometric parameters exert trivial effects on Gs, although they play a dominant role in whole tree water transport. This was attributed to the following: (1) Gs is mainly the function of environmental variables, i.e., D, radiation, and maximum daily temperature (Aasamaa and Sõber, 2011), and results from passive water transport; and (2) Gs is influenced by biological attributes to a greater extent (e.g., stomatal density in this study). The stomatal density of the seven tree species studied varied significantly (Figs. 1 and 2A), and was positively correlated with Gsref above 450 mm-2, suggesting that there were intrinsic associations between stomatal morphology and tree water use. Recently, a study on three Eucalyptus species showed that there was no significant relationship between stomatal anatomy and whole tree transpiration (Gharun et al., 2015). Similarly, Locosselli and Ceccantini (2012) reported that leaf stomatal distribution and tracheid dimensions in the wood of Podocarpus lambertii were only weakly correlated. However, these studies were qualitative in nature and did not quantify the extent to which whole tree water use, or transpiration, was determined by stomatal density.
Stomata pores of plants respond to the environment by facilitating transpirative cooling for mesophyll enzyme activity, CO2 uptake, and transpirational water loss. On the other hand, for tree stems, which underlie tree height growth, a higher density results in lower hydraulic efficiency during drought-resistance, which is a tradeoff between "efficiency" and "safety" (Brodribb et al., 2014; Meinzer and McCulloh, 2013). Moreover, hydraulic efficiency is determined by stomatal density and size. A recent study by de Boer et al. (2016) revealed a highly negative correlation between stomatal size and density, indicating a tradeoff between stomata size and density. This indicates that there is higher leaf hydraulic conductance, leading to more stringent control of transpiration (Gharun et al., 2015).
Existing theory and models suggest that stomatal density has a significant positive influence on gs (Brown and Escombe, 1900; Franks and Beerling, 2009; Sack and Buckley, 2016). However, in the present study, higher wood density lead to a lower Gsref at each quantile, whereas stomatal density was positively correlated with water flux only after surmounting a high level "threshold, " which was inconsistent with the law of "diminishing returns" applied in plants' water use (Cowan and Farquhar, 1977; Eagleson, 2002; Niklas et al., 2007; Schulze et al., 1994). The observed correlations may be attributed to (1) possible hormonal regulations or heterogeneous canopy nutrients (e.g., nitrogen) in those trees at lower quantiles, i.e., τ = 0.2 and 0.4 (less stomata but higher Gs); (2) low sample size was acknowledged for this kind study of tree physiology. The significant correlation between stomatal density and Gsref at τ = 0.6 was consistent with the study in Eucalytpus globulus, indicating higher gs observed in those trees with higher stomatal density in more favorable environments (Franks et al., 2009). This result indicated that stem and leaf hydraulics are finely tuned to avoid embolism in the xylem (Locosselli and Ceccantini, 2012; Méndez-Alonzo et al., 2012; Nolf et al., 2015). The co-ordination between wood density and stomatal morphology could potentially also be explained by the tradeoff between "efficiency" and "safety." Trees with heavier wood, for example, exhibit low sensitivity to environmental variables (low sap flow); in these trees, the stem and canopy are well-tuned if the leaves atop have denser stomata to relieve the relatively weak transpirative force (Wu et al., 2015). However, if the leaves atop have sparser stomata, the trees are at a growth disadvantage (e.g., Scenario Ⅱ of Fig. 4), but may be more drought tolerant, similar to A. auriculiformis in this study (Figs. 2 and 4). Xylem and stomata are two adjoining parts of the hydraulic system, their relationship determines homeostasis in the hydraulic continuum. The antagonistic effects of xylem and stomata on water flux most likely form the basis of the trees' homeostatic responses to environmental variables (Brodribb et al., 2014).
5. ConclusionsWe demonstrated that the co-determinants of Gsref are wood and stomatal density, i.e., stomata above 450 mm-2 promoted water flux, whereas canopy water flux was decreased with increasing wood density. The conceptual model demonstrated the significance of the hydraulic continuum to tree growth and drought tolerance. Scenario Ⅰ ("lighter wood, denser stomata") emphasized the significance of rapid growth, whereas Scenario Ⅱ ("heavier wood, less stomata") emphasized drought resistance. Scenarios Ⅲ ("Lighter wood, less stomata") and Ⅳ ("heavier wood, denser stomata"), and further intermediate scenarios, were essentially determined by environmental variables, functional groups, and genotypes among others. Understanding the plant-water coordination between stem and canopy may be useful in predicting the impacts of climate change on woody plants. We believe that the contributions of stems and leaves to canopy water flux would be clarified following the collection of data describing in situ whole tree transpiration and tree stomata.
Conflict of interestThe authors declare that they have no conflict of interest.
AcknowledgmentsMany thanks go to Professor Ping Zhao and the graduate student Peiqiang Zhao of South China Botanical Garden (SCBG), CAS for their very early fieldwork. Hui Liu of SCBG provides critical insights into our experimental results. Sincere thanks go to two anonymous reviewers for their constructive comments and encouragements.
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2019.06.003.
Aasamaa K., Sõber A., 2011. Responses of stomatal conductance to simultaneous changes in two environmental factors. Tree Physiol, 31: 855-864. DOI:10.1093/treephys/tpr078 |
Apgaua D.M.G., Ishida F.Y., Tng D.Y.P., Laidlaw M.J., Santos R.M., Rumman R., Eamus D., Holtum J.A., Laurance S.G., 2015. Functional traits and water transport strategies in lowland tropical rainforest trees. PLoS One, 10: e0130799. DOI:10.1371/journal.pone.0130799 |
Arango-Velez A., Zwiazek J.J., Thomas B.R., Tyree M.T., 2011. Stomatal factors and vulnerability of stem xylem to cavitation in poplars. Physiol. Plantarum, 143: 154-165. DOI:10.1111/j.1399-3054.2011.01489.x |
Araus J.L., Alegre L., Tapia L., Calafell R., Serret M.D., 1986. Relationship between photosynthetic capacity and leaf structure in several shade plants. Am. J. Bot, 73: 1760-1770. DOI:10.1002/j.1537-2197.1986.tb09708.x |
Bernacchi C.J., VanLoocke A., 2015. Terrestrial ecosystems in a changing environment:a dominant role for water. Annu. Rev. Plant Biol, 66: 599-622. DOI:10.1146/annurev-arplant-043014-114834 |
Brodribb T.J., McAdam S.A.M., 2011. Stomatal (mis) behavior. Tree Physiol: 1039-1040. |
Brodribb T.J., McAdam S.A., Jordan G.J., Martins S.C., 2014. Conifer species adapt to low-rainfall climates by following one of two divergent pathways. Proc. Natl. Acad. Sci. U.S.A, 7: 14489-11493. |
Brown H.T., Escombe F., 1900. Static diffusion of gases and liquids in relation to the assimilation of carbon and translocation in plants. Philos. Trans. R. Soc. Lond. Ser. B, 193: 223-291. DOI:10.1098/rstb.1900.0014 |
Cade B.S., Noon B.R., Flather C.H., 2005. Quantile regression reveals hidden bias and uncertainty in habitat models. Ecology, 86: 786-800. DOI:10.1890/04-0785 |
Carlson J.E., Adams C.A., Holsinger K.E., 2016. Intraspecific variation in stomatal traits, leaf traits and physiology reflects adaptation along aridity gradients in a South African shrub. Ann. Bot, 117: 195-207. DOI:10.1093/aob/mcv146 |
Chen W.F., Xu Z.J., Zhang L.B., Yang S.R., 1990. Comparative studies on stomatal density and its relations to gas diffusion resistance and net photosynthetic rate in rice leaf. Chin. J. Rice Sci, 4: 163-168. |
Cowan I., Farquhar G.D., 1977. Stomatal function in relation to leaf metabolism and environment. Symp. Soc. Exp. Biol, 31: 471-505. |
de Boer H.J., Price C.A., Wagner-Cremer F., Dekker S.C., Franks P.J., Veneklaas E.J., 2016. Optimal allocation of leaf epidermal area for gas exchange. New Phytol, 210: 1219-1228. DOI:10.1111/nph.13929 |
Dial R.J., Sillett S.C., Antoine M.E., Spickler J.C., 2004. Methods for horizontal movement through forest canopies. Selbyana, 25: 151-163. |
Domec J.C., Johnson D.M., 2012. Does homeostasis or disturbance of homeostasis in minimum leaf water potential explain the isohydric versus anisohydric behavior of Vitis vinifera L. cultivars?. Tree Physiol, 32: 245-248. DOI:10.1093/treephys/tps013 |
Dow G.J., Bergmann D.C., Berry J.A., 2014a. An integrated model of stomatal development and leaf physiology. New Phytol, 201: 1218-1226. DOI:10.1111/nph.12608 |
Dow G.J., Berry J.A., Bergmann D.C., 2014b. The physiological importance of developmental mechanisms that enforce proper stomatal spacing in Arabidopsis thaliana. New Phytol, 201: 1205-1217. DOI:10.1111/nph.12586 |
Drake P.L., Froend R.H., Franks P.J., 2013. Smaller, faster stomata:scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot, 64: 495-505. DOI:10.1093/jxb/ers347 |
Eagleson P.S., 2002. Ecohydrology:Darwinian Expression of Vegetation Form and Function. Cambridge: Cambridge University Press.
|
Franks P.J., Beerling D.J., 2009. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. U.S.A, 106: 10343-10347. DOI:10.1073/pnas.0904209106 |
Franks P.J., Drake P.L., Beerling D.J., 2009. Plasticity in maximum stomatal conductance constrained by negative correlation between stomatal size and density:an analysis using Eucalytpus globulus. Plant Cell Environ, 32: 1737-1748. DOI:10.1111/j.1365-3040.2009.002031.x |
Franks P.J., Drake P.L., Froend R.H., 2007. Anisohydric but isohydrodynamic:seasonally constant plant water potential gradient explained by a stomatal control mechanism incorporating variable plant hydraulic conductance. Plant Cell Environ, 30: 19-30. DOI:10.1111/j.1365-3040.2006.01600.x |
Gao J.G., Zhao P., Shen W.J., Niu J.F., Zhu L.W., Ni G.Y., 2015. Biophysical limits to responses of water flux to vapor pressure deficit in seven tree species with contrasting land use regimes. Agric. For. Meteorol, 200: 258-269. DOI:10.1016/j.agrformet.2014.10.007 |
Gao J.G., Zhao P., Shen W.J., Rao X.Q., Hu Y.T., 2017. Physiological homeostasis and morphological plasticity of two tree species subjected to precipitation seasonal distribution changes. Perspect. Plant Ecol. Evol. Systemat, 25: 1-19. DOI:10.1016/j.ppees.2017.01.002 |
Gao J.G., Zhou J., Sun Z.W., Niu J.F., Zhou C.M., Gu D.X., Huang Y.Q., Zhao P., 2016. Suppression of nighttime sap flux with lower stem photosynthesis in Eucalyptus trees. Int. J. Biometeorol, 60: 545-556. DOI:10.1007/s00484-015-1050-6 |
Gharun M., Turnbull T.L., Pfautsch S., Adams M.A., 2015. Stomatal structure and physiology do not explain differences in water use among montane eucalypts. Oecologia, 177: 1171-1181. DOI:10.1007/s00442-015-3252-3 |
Giday H., Kjaer K.H., Fanourakis D., Ottosen C.O., 2013. Smaller stomata require less severe leaf drying to close:a case study in Rosa hydrida. J. Plant Physiol, 170: 1309-1316. DOI:10.1016/j.jplph.2013.04.007 |
Granier A., 1987. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol, 3: 309-320. DOI:10.1093/treephys/3.4.309 |
Granier A., Biron P., Breda N., Pontailler J.Y., Saugier B., 1996. Transpiration of trees and forest stands:short and long-term monitoring using sapflow methods. Glob. Chang. Biol, 2: 265-274. DOI:10.1111/j.1365-2486.1996.tb00078.x |
Hacke U.G., Sperry J.S., Pockman W.T., Davis S.D., McCulloh K.A., 2001. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia, 126: 457-461. DOI:10.1007/s004420100628 |
Jacobsen A.L., Pratt R.B., Ewers F.W., Davis S.D., 2007. Cavitation resistance among 26 chaparral species of southern California. Ecol. Monogr, 77: 99-115. DOI:10.1890/05-1879 |
Jasechko S., Sharp Z.D., Gibson J.J., Birks S.J., Yi Y., Fawcett P.J., 2013. Terrestrial water fluxes dominated by transpiration. Nature, 496: 347-350. DOI:10.1038/nature11983 |
Koenker, R., 2013. quantreg: quantile regression. R package version 5.05[WWW document] URL. http://cran.r-project.org/web/packages/quantreg/quantreg.pdf.(Accessed 30 July 2015).
|
Lawson T., Blatt M., 2014. Stomatal size, speed and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol, 164: 1556-1570. DOI:10.1104/pp.114.237107 |
Li B.J., Xing S.Y., 2007. Anatomical structure and stomatal characteristics on the leaf of Ginkgo biloba var. epiphylla. Sci. Silvae Sin, 43: 34-39. |
Litvak E., McCarthy H.R., Pataki D.E., 2012. Transpiration sensitivity of urban trees in a semi-arid climate is constrained by xylem vulnerability to cavitation. Tree Physiol, 32: 373-388. DOI:10.1093/treephys/tps015 |
Locosselli G.M., Ceccantini G., 2012. Plasticity of stomatal distribution pattern and stem tracheid dimensions in Podocarpus lambertii:an ecological study. Ann. Bot, 110: 1057-1066. DOI:10.1093/aob/mcs179 |
Martínez-Vilalta J., Poyatos R., Aguadé D., Retana J., Mencuccini M., 2014. A new look at water transport regulation in plants. New Phytol, 204: 105-115. DOI:10.1111/nph.12912 |
McCulloh K.A., Woodruff D.R., 2012. Linking stomatal sensitivity and whole-tree hydraulic architecture. Tree Physiol, 32: 369-372. DOI:10.1093/treephys/tps036 |
McElwain J.C., Yiotis C., Lawson T., 2015. Using modern plant trait relationships between observed and theoretical maximum stomatal conductance and vein density to examine patterns of plant macroevolution. New Phytol, 209: 94-103. |
Meinzer F.C., McCulloh K.A., 2013. Xylem recovery from drought-induced embolism:where is the hydraulic point of no return?. Tree Physiol, 3(3): 331-334. |
Méndez-Alonzo R. Paz H., Cruz-Zuluaga R., Rosell J.A., Olson M.E., 2012. Coor-dinated evolution of leaf and stem economics in tropical dry forest trees. Ecology, 93: 2397-2406. DOI:10.1890/11-1213.1 |
Niklas K.J., Cobb E.D., Niinemets U., Reich P.B., Sellin A., Shipley B., Wright I.J., 2007. "Diminishing returns" in the scaling of functional leaf traits across and within species groups. Proc. Natl. Acad. Sci. U.S.A, 104: 8891-8896. DOI:10.1073/pnas.0701135104 |
Nolf M., Creek D., Duursma R., Holtum J., Mayr S., Choat B., 2015. Stem and leaf hydraulic properties are finely coordinated in three tropical rain forest tree species. Plant Cell Environ, 38: 2652-2661. DOI:10.1111/pce.12581 |
Oren R., Sperry J.S., Katul G.G., Pataki D.E., Ewers B.E., Phillips N., Schäfer K.V.R., 1999. Survey and synthesis of intra-and interspecific variation in stomatal sensitivity to vapor pressure deficit. Plant Cell Environ, 22: 1515-1526. DOI:10.1046/j.1365-3040.1999.00513.x |
Raven J.A., 2014. Speedy small stomata? J. Exp. Bot, 65: 1415-1424. DOI:10.1093/jxb/eru032 |
Roelfsema M.R., Hedrich R., 2005. In the light of stomatal opening:new insights into 'the Watergate'. New Phytol, 167: 665-691. DOI:10.1111/j.1469-8137.2005.01460.x |
Sack L., Buckley T.N., 2016. The developmental basis of stomatal density and flux. Plant Physiol, 171: 2358-2363. |
Schulze E.D., Kelliher F., Körner C., Lloyd J., Leuning R., 1994. Relationships among maximum stomatal conductance, ecosystem surface conductance, carbon assimilation rate, and plant nitrogen nutrition:a global ecology scaling exercise. Annu. Rev. Ecol. Systemat, 25: 629-662. DOI:10.1146/annurev.es.25.110194.003213 |
Skelton R.P., West A.G., Dawson T.E., 2015. Predicting plant vulnerability to drought in biodiverse regions using functional traits. Proc. Natl. Acad. Sci. U.S.A, 112: 5744-5749. DOI:10.1073/pnas.1503376112 |
Wang R.L., Yu G.R., He N.P., Wang Q.F., Zhao N., Xu Z.W., Ge J.P., 2015. Latitudinal variation of leaf stomatal traits from species to community level in forests:linkage with ecosystem productivity. Sci. Rep, Rep, 5: 14454. |
Wu L.J., Li Z.H., Yang M.H., Wang P.L., 2015. Response of leaf anatomical characteristics of Cyclobalanopsis gilva seedlings to drought stress. Chin. J. Appl. Ecol, 26: 3619-3626. |
Xiong H., Ma C.E., Li L., Zeng H., Guo D.L., 2014. Stomatal characteristics of ferns and angiosperms and their responses to changing light intensity at different habitats. Chin. J. Plant Ecol, 38: 868-877. DOI:10.3724/SP.J.1258.2014.00081 |
Zhu S.D., Liu H., Xu Q.Y., Cao K.F., Ye Q., 2016. Are leaves more vulnerable to cavitation than branches?. Funct. Ecol, 30: 1740-1744. DOI:10.1111/1365-2435.12656 |



