b. The Key Laboratory of Ecology and Biological Resources in Yarkand Oasis Under the Department of Education of Xinjiang Uygur Autonomous Region, Kashi University, Kashi, Xinjiang, 844000, PR China;
c. Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical, Garden, Chinese Academy of Sciences, Menglun Town, 666303, PR China;
d. Laboratory of Ecology and Evolutionary Biology, Yunnan University, Kunming, Yunnan, 650091, PR China
Darwin proposed that heterostyly (distylous and tristyly), the condition of having styles of different lengths relative to the stamens in individual plants, promotes outcrossing rather than selffertilization. Nevertheless, some heterostylous species selfpollinate under harsh conditions that prevent effective pollinator activity or reduce plant density (Barrett, 1990; Ferrer et al., 2009). When environmental conditions deviate from those found in the main distributional range of a heterostylous species, a morph can disappear from some populations (Meeus et al., 2012; Weber et al., 2012; Santos-Gally et al., 2013). Among distylous species that have long-styled and short-styled floral morphs (hereafter referred to as L-morphs and S-morphs), low pollinator activity, small population size, and the low frequency of one floral morph might inhibit legitimate pollination and increase illegitimate pollination, especially in harsh environments. The loss of self-incompatibility in distylous species, which include L-morph and S-morph plants (also called pin and thrum plants), might alter morph ratio and/or floral trait evolution (Barrett, 1990; Kohn et al., 1996; Weber et al., 2012; Brys and Jacquemyn, 2015). But high self-compatibility in distylous species affects inbreeding depression and female fitness, and reduces changes in the long- and short-styled floral morph ratio and plant density (Barrett and Eckert, 1990; Sampson and Krebs, 2012; Weber et al., 2013), as variations in style morph frequency in distylous plants are highly sensitive to stochastic natural selection pressures in different populations (Thompson et al., 2003; Santos-Gally et al., 2013; Papuga et al., 2015).
Inbreeding depression is an important selection pressure against the production of self-progeny in cross-pollinated heterostylous species. Self-incompatible distyly within a population might influence the evolution of mating systems (Charlesworth and Charlesworth, 1987; Weber et al., 2012; Delmas et al., 2014). Inbreeding depression increases with the self-fertilization rate at both the population and species levels (Weller et al., 2005; Goodwillie and Knight, 2006). The effects of inbreeding depression have been explored in populations with different style morph frequencies (Weber et al., 2013) and in relation to habitat fragmentation (Nguyen et al., 2015). However, the effects of morph ratio variation within a population on female fitness and inbreeding depression in distylous species remain poorly understood (Endels et al., 2002; Spigler and Chang, 2009; De Vere et al., 2009).
Mating patterns and population characteristics may reduce the compatibility of a plant species with its pollinators, which, in turn, influence the evolution of distyly (Weber et al., 2012). Therefore, the decrease in effective pollinators with increasing elevation (up to the alpine zone) might affect the breeding system and population characteristics of distylous species growing at various elevations (Wirth et al., 2010). Seed susceptibility to predators, morph ratio, and female fitness might mediate variations in elevationdependent environmental factors (temperature or pollinator activity) (Baeten et al., 2010; Straka and Starzomski, 2015). Furthermore, resource availability, flowering time, pollinator preference, abundance or effectiveness of pollinators, and mating system could all influence variation in seed quantity, viability, and fruit abortion rate (Baker, 1972; Forrest and Thomson, 2008; Rafferty and Ives, 2012; Straka and Starzomski, 2015). Reduced pollinator service might reduce offspring quality via inbreeding depression. Therefore, the absence of pollinators in harsh environments could increase selfing rates (Barrett and Eckert, 1990; Van Etten et al., 2015). Consequently, species growing in alpine zones, which are generally characterized by low air temperatures, low species diversity, and short growing seasons (Körner, 1999; Vittoz et al., 2009), might have undergone significant changes in mating type and plant recruitment (Fay and Schultz, 2009; Sun et al., 2014).
Our preliminary observations indicated that, in the distylous species Primula nivalis (Primulaceae), pollinator visitation frequency decreased and L-morph frequency increased with increasing elevation. Therefore, we hypothesized that elevationdependent pollinator limitation and high selfing levels in both sexual morphs affect population characteristics and female offspring fitness. To test this hypothesis, we investigated (1) whether the population characteristics and fruit set of P. nivalis vary at different elevations; (2) the effects of pollination treatment and elevation on seed mass and viability; and (3) the relationship between female fitness and post-dispersal inbreeding depression in the two floral morphs at different elevations.
2. Material and methods 2.1. Material and study siteP. nivalis is a spring-flowering herbaceous perennial distylous species that grows in forest and grassland habitats in northwestern Xinjiang at elevations between 1600 and 3000 m. P. nivalis plant density increases from low to high elevation. In high-elevation grassland populations, the frequency of L-morphs is greater than that of S-morphs; furthermore, at high elevations, the stamenstigma distance of L-morphs is shorter (Abdusalam, 2017). Plant height, length of scapes, and length of leaves all decrease from low to high elevation; in addition, flowering and fruiting occur later in populations at high elevations than in populations at low elevations (Abdusalam and Li, 2018).
To reduce human interference, field experiments were conducted during the flowering seasons of 2014 and 2015 (May to August) in populations of P. nivalis at three different elevations (1650 m, 2403 m, and 2704 m) on the northern slope of the Tianshan Mountains in the Kunas area of northwestern Xinjiang (Fig. 1). These elevations allowed us to evaluate natural populations in forests (1650 m) and grasslands (2403 and 2704 m). Mean annual temperature, precipitation, sunshine, and habit characteristics of each study population are presented in Table 1.
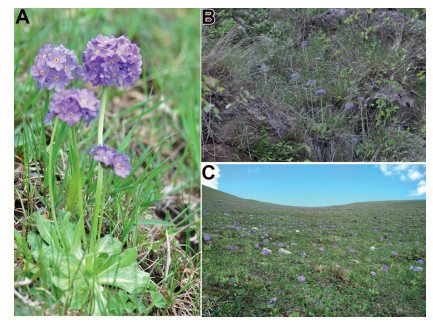
|
| Fig. 1 Plant individuals (A) and different elevation of natural populations of Primula nivalis in forests (1650 m) and grasslands (2704 m) environments (B, C) in northwestern Xinjiang, China. |
| Parameter | Population 1 | Population 2 | Population 3 |
| Elevation (m) | 1657 ± 8 | 2423 ± 11 | 2704 ± 10 |
| Longitude (E) | 84°17'29.05" | 84°22'54.82" | 84°21'22.43" |
| Latitude (N) | 43°16'02.86" | 43°23'52.91" | 43°08'53.00" |
| Habitat | Forest-grassland | Grassland | Grassland |
| Mean temperature (℃) | 0.180 | -3.340 | -4.430 |
| Mean annual precipitation (mm) | 226 | 276 | 289 |
| Annual sunshine (h) | 9.141 | 8.467 | 8.233 |
| Growing season length each year | 130-160 d | 120-155 d | 120-150 d |
| Flowering time | Early May | Late May | Early June |
| Fruit mature time | Middle July | Late July | Early Aug |
| Floral display | 5-6 d | 6-7 d | 6-7 d |
To determine elevational variation in plant density and floral morph ratios, we randomly selected 35 plots, each 1 m × 1 m (selecting 5 plots in each 20-m2 sample), for each of the three different elevation study populations of P. nivalis at the peak flowering period (Fig. 1). The distance between plots was ≥10 m. The total number of L-morph, S-morph, and all flowering plants per plot was determined. The percentage of individuals of each sexual morph per plot was calculated as follows: P=[1-(N/T)]×100, where P is the percentage of L-morph or S-morph individuals per plot, N is the number of L-morph or S-morph plants per plot, and T is the total number of flowering individuals (L-morphs + Smorphs) per plot. Flowering plants of both morphs were marked in each plot, and the fruit set of each individual plant and the number of flowers per inflorescence were determined for each population at fruit maturity.
2.3. Effect of pollination treatment on seed mass and viabilityTo explore the effects of elevation-dependent mating patterns on seed quality, in flowering time, we selected 150 soon-to-open P. nivalis flowers from 50 inflorescences of both L-morph and Smorph individuals at each population and covered them with a paper bag. After all flowers were freshly opened, they were divided into three treatments: (1) open-pollination, (2) intra-morph hand self-pollination (all flowers were hand self-pollinated using pollen from flowers on the same plant and then re-bagged with paper bags), and (3) inter-morph hand cross-pollination (all flowers were covered with paper bags after removal of all stamens, and then flowers were hand cross-pollinated (from 10 m away) with pollen from another plant of the opposing morph and flowers re-bagged with paper bags) in each of the three study populations in 2015. One month after pollination, the fully matured fruits were collected, and the seed set per fruit was determined for all the treatments. Seed quality was assessed from viability and mass. To determine seed viability, three replicates of 30 mature, fully developed seeds from each treatment of both flower types were soaked in water for 24 h at 30 ℃. The seed coat was then opened to expose the embryo. Seeds were placed in 1% TTC (2, 3, 5-triphenyl tetrazolium chloride) and incubated in the dark for 24 h at 30 ℃ (Baskin and Baskin, 2014). If the embryo turned pink, it was scored as viable. No color change indicated that the embryo was nonviable. To determine seed mass, 10 replications of 1000 seeds from each treatment, morph, and elevation were weighed to a precision of ±0.0001 g using a Sartorius BS210S electronic analytical balance (Göttingen, Germany).
2.4. Female fitness and post dispersal inbreeding depressionTo investigate the influence of pollination treatments on female fitness and post-dispersal inbreeding depression, seed germination data for each treatment, morph, and elevation were used. First, eight replicates of 50 seeds generated by each morph in each treatment and elevation were placed in Petri dishes on two sheets of filter paper moistened with distilled water. All seeds were coldstratified with moist air at 0 ℃ in darkness for five weeks (Washitani and Kabaya, 1988). The seeds were then incubated under optimal germination conditions (12 h:12 h fluorescent light:-dark at 15/30 ℃) for four weeks. Germination was scored as a visible shoot protruding from the top of the seed.
Female fitness was determined following the methods of Ferrer et al. (2009), where ff = fdsssv (ff is female fitness, fd is floral display, ss is seed set, and sv is the percentage of seed germination). Floral display (floral longevity) is 6-7 d for both morph flowers and it did not significantly differ among the three elevations (unpublished data). Therefore, any variation in female fitness was attributed to differences in seed set and viability. Post-dispersal inbreeding depression was determined for the seeds of both sex morphs produced by the three pollination treatments at the three elevations. Inbreeding depression (δ) was determined from the relative performance (RP). The inbreeding benefits of flowering plants increased with decreasing RP, calculated as follows: RP = 1-(Wo-Ws)/Wmax, where Wo and Ws represent the fitness of self- and outcrossed progeny, respectively, and Wmax is the larger of the two values (Baskin and Baskin, 2015).
2.5. Data analysisAll data were analyzed using SPSS v. 18.0. Figures were drawn using SigmaPlot v. 12.5. Data were first tested for normality and homogeneity of variances before the analysis. Fruit set, seed viability, seed mass, female fitness, and inbreeding depression data were not normal, so they were arcsine-, log10-, or square root-transformed before analysis to ensure homogeneity of variance. Pre-analysis normality of variance was determined to ensure that the data met the requirements for MANOVA (generalized linear model, GLM) or one-way ANOVA. A generalized linear model was used to evaluate elevation-dependent population density, the percentage of each sexual morph, seed mass, seed viability, female fitness, and inbreeding depression. If ANOVA results indicated significant differences for the same elevation or sexual morph, Tukey's HSD test was performed for multiple comparisons to determine significance (p < 0.05) among treatments.
3. Results 3.1. Population and fruit set characteristicsPopulation characteristics were significantly affected by sexual morph (F1, 180 = 11.589, p < 0.001) and elevation (F2, 180 = 6.059, p < 0.05), but not by the interaction of elevation and sexual morph (F2, 180 = 1.651, p > 0.05). The percentage of L-morph individuals was higher than that of S-morph individuals at high elevation (Fig. 2A). Plant density was significantly affected by sexual morph (F1, 178 = 21.651, p < 0.001), elevation (F2, 172 = 15.171, p < 0.001), and the interaction of elevation and sexual morph (F2, 170 = 5.403, p < 0.01). The density of both sexual morphs increased with increasing elevation; however, the density of the L-morphs was higher in each population than that of S-morphs (Fig. 2B).

|
| Fig. 2 Effect of elevation on the percentage (A) and density (B) of long-styled (L-morph) and short-styled (S-morph) morphs of Primula nivalis in a natural population at three elevations (mean + SE). Bars with different lowercase letters indicate significant differences between the two morphs at the same elevation, and different uppercase letters, significant differences between the same morphs at different elevations. |
The fruit set was significantly affected, both at the individual plant and flower levels, by sexual morph (F1, 234 = 8.059, p < 0.001; F1, 189 = 6.203, p < 0.001), elevation (F2, 235 = 16.660, p < 0.001; F2, 188 = 12.039, p < 0.001), and the interaction of elevation and sexual morph (F1, 234 = 4.213, p < 0.001; F1, 180 = 3.008, p < 0.05). The effect of elevation on fruit set was greater on individual plants of both morphs than on flowers. The fruit set for each individuals and flowers was lower in the Smorph than in the L-morph (Fig. 3).
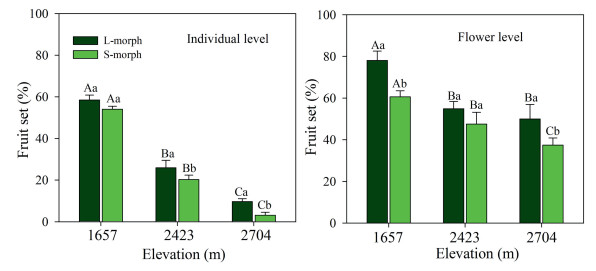
|
| Fig. 3 Individual (A) and flower (B) level fruit set of Primula nivalis plants with long-styled (L-morph) and short-styled (S-morph) morphs in a natural population at three elevations (mean + SE). Bars with different lowercase letters indicate significant differences between the two morphs at the same elevation, and different uppercase letters, significant differences between the same morphs at different elevations. |
Seed mass was significantly influenced by pollination treatment (F2, 102 = 63.745, p < 0.001), elevation (F2, 102 = 18.506, p < 0.001), sexual morph (F1, 102 = 6.118, p < 0.05), the interaction of treatment and elevation (F4, 102 = 14.608, p < 0.001), and the interaction of treatment and sexual morph (F2, 102 = 3.636, p < 0.05), but it was not affected by the interaction of sexual morph and elevation (F2, 102 = 0.118, p > 0.05) or by treatment, sexual morph, and elevation (F4, 102 = 0.482, p > 0.05). However, the seed mass from the three pollination treatments of both sexual morph flowers differed significantly (F2, 102 = 121.482, p < 0.001). The rank order of seed mass was cross-pollination > open-pollination > self-pollination. The seed mass of S-morphs was greater than that of Lmorphs (Fig. 4).
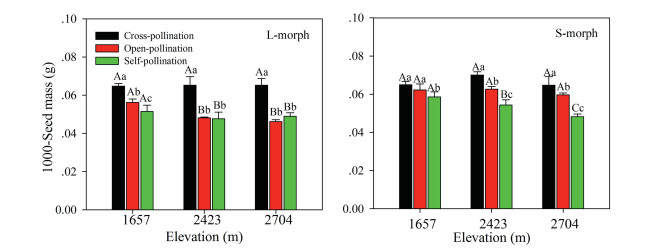
|
| Fig. 4 Mass of seeds resulting from different pollination treatments of long-styled (L-morph) and short-styled (S-morph) morphs of Primula nivalis in a natural population at three elevations (mean + SE). Bars with different lowercase letters indicate significant differences between the two morphs at the same elevation, and different uppercase letters, significant differences between the same morphs at different elevations. |
Seed viability was significantly affected by treatment (F2, 180 = 29.345, p < 0.001), sexual morph (F1, 180 = 6.118, p < 0.05), elevation (F2, 180 = 11.501, p > 0.05), and the interaction of treatment and elevation (F4, 180 = 9.279, p < 0.05), but it was not influenced by the interaction of treatment and sexual morph (F2, 180 = 1.206, p > 0.05); sexual morph and elevation (F2, 180 = 0.378, p > 0.05); or treatment, elevation, and sexual morph (F4, 180 = 0.482, p > 0.05). Viability of seeds from self-pollinated flowers of both sexual morphs was lower than that of seeds from open-pollinated and cross-pollinated flowers, and viability of seeds from self-pollinated L-morph flowers was higher than that of seeds from self-pollinated S-morph flowers (Fig. 5). Viability of seeds from self-pollinated Smorph flowers decreased with increasing elevation, but it was not significantly affected by elevation in L-morph flowers (F2, 30 = 0.462, p > 0.05, Fig. 5). However, seed viability was higher for open-pollinated S-morph flowers than for open-pollinated Lmorph flowers, whereas seed viability of cross-pollinated flowers did not differ significantly for either of the two morphs (F1, 60 = 0.509, p > 0.05, Fig. 5).
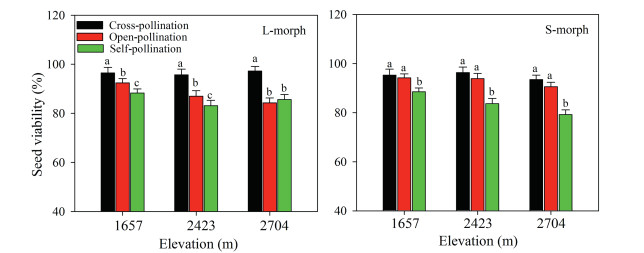
|
| Fig. 5 Viability of seeds resulting from different pollination treatments of long-styled (L-morph) and short-styled (S-morph) morphs of Primula nivalis in a natural population at three elevations (mean + SE). Bars with different lowercase letters indicate significant differences between the same elevations within different treatments flowers. |
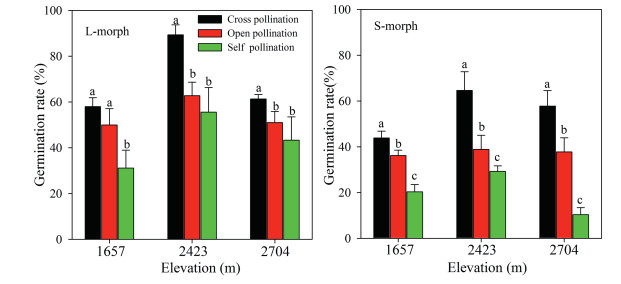
Seed germination was significantly affected by pollination treatment (F2, 54 = 13.977, p < 0.01, Fig. 6), sexual morph (F1, 54 = 5.835, p < 0.05), and the interaction of treatment and sexual morph (F1, 54 = 2.808, p < 0.05). The seed germination rates of crosspollinated flowers were higher than those of self- and openpollinated flowers. The seed germination rate of L-morph flowers was higher than that of S-morph flowers; and the rate of selfpollinated flowers increased with increasing elevation.
|
| Fig. 6 Seed germination rate of different pollination treatments of long-styled (L-morph) and short-styled (S-morph) morphs of Primula nivalis in a natural population at three elevations (mean + SE). Bars with different lowercase letters indicate significant differences between the same elevations within different treatments flowers. |
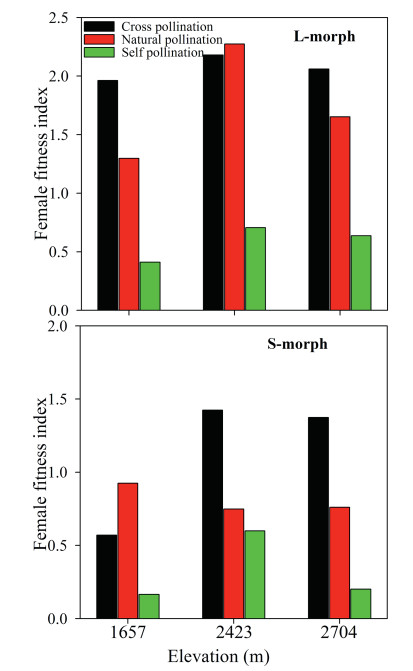
Female fitness was affected by treatment (F2, 18 = 21.645, p < 0.01), sexual morph (F1, 18 = 23.743, p < 0.01), elevation (F2, 18 = 10.780, p < 0.001), and the interaction of sexual morph and treatment (F2, 18 = 5.373, p < 0.05). However, female fitness was not significantly affected by the interactions of sexual morph and elevation (F2, 18 = 0.163, p > 0.05); treatment and elevation (F4, 18 = 0.054, p > 0.05); or treatment, elevation, and sexual morph (F4, 18 = 0.030, p > 0.05, Fig. 7). Interestingly, fitness in the different treatments differed significantly for both sexual morphs (F2, 18 = 13.072, p < 0.01). The rank order of seed fitness was cross- > open- > self-pollination. The fitness of L-morphs was higher in each population than that of S-morphs. Female fitness in the three pollination treatments of L-morphs and in the cross-pollination treatment of S-morphs increased with increasing elevation (Fig. 7), but it did not differ significantly for naturally or selfpollinated S-morphs at any of the three elevations (Fig. 7).
|
| Fig. 7 Female fitness resulting from self-, open-, and cross-pollination treatments on long-styled (L-morph) and short-styled (S-morph) morphs of Primula nivalis among populations at three elevations (mean +SE). |
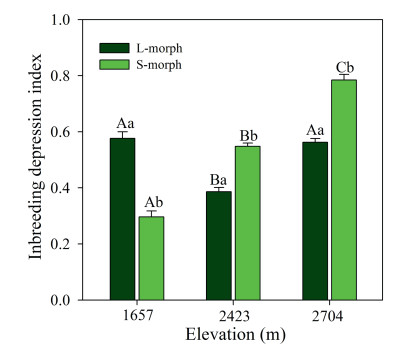
Post-dispersal inbreeding depression was positively influenced by sexual morph (F1, 18 = 17.203, p < 0.001), elevation (F2, 18 = 23.091, p < 0.001), and the interaction between elevation and sexual morph (F2, 18 = 4.048, p < 0.01, Fig. 8). The inbreeding depression index for S-morphs increased with increasing elevation, but was lower in the population at 2423 m than in the populations at 1657 and 2704 m. The inbreeding depression index was higher for S-morphs than for L-morphs in the populations at 2423 and 2704 m, but it was lower for S-morphs than for L-morphs in the population at 1657 m.
|
| Fig. 8 Post-dispersal inbreeding depression of seeds from long-styled (L-morph) and short-styled (S-morph) morphs of Primula nivalis among populations at three elevations (mean + SE). Bars with different lowercase letters indicate significant differences between the two morphs at the same elevation, and different uppercase letters, significant differences between the same morphs at different elevations. |
Both the sexual morph frequency and density of P. nivalis were significantly affected by elevation, with the percentage of L-morph plants being higher than that of S-morph plants in each of the three populations (Fig. 2). Many biotic and abiotic factors influence morph frequency in populations of distylous species (Jacquemyn et al., 2001; Endels et al., 2002), including population size, density of individuals, low pollinator activity, mating type, and inbreeding depression (Kéry et al., 2000, 2003). For instance, in the distylous perennial Primula veris, demographic stochasticity causes the morph ratio to deviate from unity in populations at high elevations (Kéry et al., 2003). In the present study, the percentage of L- morphs was higher than that of the S-morphs, regardless of elevation (Fig. 3A); however, the density of each morph increased with elevation (Fig. 2B). Therefore, in P. nivalis, elevationdependent increases in density in both morphs and the relatively high percentage of L-morphs affect offspring fitness (female fitness and inbreeding depression).
The fruit set of individual plants and flowers of both sexual morphs was affected by elevation, i.e., fruit set decreased with increasing elevation (Fig. 3), which might be due to type of pollination, low pollinator efficiency, and/or low temperature in the harsh alpine environment (Knight et al., 2005; Bernareggi et al., 2015; Qi et al., 2015). In addition, the increase in the percentage of L-morph plants at high elevation had a further negative effect on the fruit set of individual plants and flowers in these pollinatorlimited high-elevation populations. Specifically, the highly compatible individual plants and flowers of L-morphs produced more mature fruits than did the S-morphs. Although the fruit set of open-pollinated flowers decreased with increasing elevation in both morphs, the selfing rate of L-morphs increased with increasing elevation (unpublished data). Our results suggest that elevation-dependent variation in the mating type of both sexual morphs and high plant density cause the level of fruit set to decline in both morphs among high-elevation populations.
Seed mass and viability of P. nivalis individuals were more strongly affected by the type of pollination than by elevation (Figs. 4 and 5), and these values were lower in self-pollinated than in crossand open-pollinated flowers of both sexual morphs. The level of pollination has varying effects on seed quantity (number) and quality (mass) (Agren et al., 2006; Baskin and Baskin, 2014, 2015), while the seed mass of cross-pollinated flowers is greater than that of self-pollinated plants. However, some studies suggest that selfed seeds are larger than crossed seeds (Agren et al., 2006; Susko and Clubb, 2008; Baskin and Baskin, 2015) and may therefore contain more resources and be considered higher in quality. For example, seeds from cross-pollinated flowers of Clarkia tembloriensis (Holtsford and Ellstrand, 1990), Primula farinosa (Galetto et al., 2000), and Erythrina crista-galli (Agren et al., 2006) have greater mass than those from open or self-pollinated flowers. However, seeds from the self-pollinated flowers of Hesperis matronalis have greater mass than those from cross-pollinated flowers (Susko and Clubb, 2008). The mass and viability of seeds from selfed Smorphs and open-pollinated L-morphs decreased significantly with increasing elevation in the present study. Seed mass was mostly affected by the type of pollination at high elevations, where pollinator activity was low (Figs. 4 and 5), because seed viability decreases at high elevation due to inbreeding related to population size (Goodwillie and Knight, 2006; Susko and Clubb, 2008; Weber et al., 2012) in different populations.
The "stress tolerance" hypothesis suggests that seed mass increases with increasing elevation, because large seeds have an advantage over small seeds during seedling establishment in stressful high elevation environments (Pluess et al., 2005; Qi et al., 2015). In contrast, the "energy constraint" hypothesis suggests that seed mass is negatively correlated with elevation, because low temperatures and the short growing season at high elevations might reduce photosynthetic rates, thus reducing the energy available for seed development and seed provisioning (Baker, 1972; Guo et al., 2010). In P. nivalis, seed mass and seed viability were affected more by pollination treatment than by other factors, suggesting that these factors are restricted by mating type and by low legitimate pollination, as seed development time increases with elevation. Thus, our data do not support either the stress tolerance or the energy constraint hypothesis.
Seed mass has been shown to play an important role in seed dispersal, germination, and fitness (Pluess et al., 2005; Guo et al., 2010; Baskin and Baskin, 2015). In general, we found that seeds from the cross-pollinated flowers of both sexual morphs at all elevations had more mass and higher viability than those from selfpollinated flowers of both sexual morphs at each elevation (Fig. 6A). In both of the sexual morphs of P. nivalis, the female fitness of offspring from cross-pollinated heavy seeds was higher than that of offspring from self-pollinated seeds (Figs. 6A and 7). Thus, variation in pollination-dependent seed mass between the L-morphs and Smorphs at different elevations might affect seedling establishment in both morphs.
Seed germination rate and female fitness in L-morphs were higher than those in S-morphs at all three elevations (Figs. 6A and 7). Likewise, seeds from L-morphs had a higher mean germination rate than those from S-morphs in Primula cusickiana (Rayburn et al., 2013). Davidson and Wolf (2011) concluded that the major difference between LS and SS flowers (which they describe as pin and thrum flowers, with pin being more prevalent) could be reduced by high levels of legitimate pollination. In the present study, P. nivalis female fitness decreased while inbreeding depression increased in S-morphs at high elevations. The decrease in the mass and viability of seeds from S-morphs could result in the decreased growth of seedling progeny from S-morphs in a population. Furthermore, the lower inbreeding depression of seeds from L-morphs at high elevations, relative to that of seeds from S-morphs, might alter the population characteristics and dynamics of P. nivalis, impacting the breeding system and floral morph ratio.
Reduction in the fitness of progeny due to the type of mating might also be important in the population dynamics of P. nivalis. Regardless of elevation, a bias in the self-compatibility and mating patterns of L-morphs would reduce effective offspring fitness, thus increasing inbreeding depression in S-morphs, which, in turn, would affect the function and structure of populations at different elevations (Fig. 8). Furthermore, natural selection driven by sexual morph frequency and mating patterns in a harsh environment should result in a decrease in legitimate pollination levels and the offspring fitness of S-morph plants in high elevation populations.
5. ConclusionsVariation in elevation-dependent mating might influence the female fitness and inbreeding depression of flowers of both sexual morphs in P. nivalis. Low levels of adaptation of seeds from shortstyled flowers might cause the frequency of individuals with short-styled flowers to decrease at high elevations. The lack of a 1:1 sexual morph frequency at each elevation probably reflects a difference in the natural selection pressure on the two morphs in the different environments, leading to variations in population characteristics and reproductive mechanisms.
Author contributionAysajan A and Qing-jun Li designed experiments; Aysajan A carried out experiments and analyzed experimental results. Aysajan A and Qing-jun Li wrote the manuscript.
Conflicts of interestWe declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
AcknowledgmentsThe authors thank Parhat Sabit and Yusup Alim for providing help in the field. Special thanks are extended to Professor Qin Ying Lan Xishuangbanna Tropical Botanical Garden for their very helpful suggestions about this research. This study was supported in part by the National Natural Science Foundation of China (NSFC 31400279, 31860121) and Funded by the Scientific Research Program of the Higher Education Institution of Xinjiang (XJEDU2016I042) and High-level Scientific Research Foundation of Kashi University (GCCZK-004); China.
Abdusalam A., 2017. Effect of habitat heterogeneity on floral trait differentiation level in distylous species Primula nivalis. Acta Bot. Boreali-Occidentalia Sin, 38: 158-165. |
Abdusalam A., Li Q.J., 2018. Morphological plasticity and adaptation level of distylous Primula nivalis in a heterogeneous alpine environment. Plant Divers, 42: 284-292. |
Agren J., Fortunel C., Ehrlen J., 2006. Selection on floral display in insect-pollinated Primula farinosa:effects of vegetation height and litter accumulation. Oecologia, 150: 225-232. DOI:10.1007/s00442-006-0509-x |
Baeten L., De Frenne P., Verheyen K., et al, 2010. Forest herbs in the face of global change:a single-species-multiple-threats approach for Anemone nemorosa. Plant Ecol. Evol, 143: 19-30. DOI:10.5091/plecevo.2010.414 |
Baker H.G., 1972. Seed weight in relation to environmental conditions in California. Ecology, 53: 997-1010. DOI:10.2307/1935413 |
Barrett, S.C.H., Eckert, C.G., 1990. Variation and evolution in mating systems in seed plants. In: Kawano, S. (Ed.), Biological Approaches and Evolutionary Trends in Plants. Academic Press, London, pp. 229-254.
|
Barrett S.C.H., 1990. The evolution and adaptive significance of heterostyly. Trends Ecol. Evol, 5: 144-148. DOI:10.1016/0169-5347(90)90220-8 |
Baskin, C.C., Baskin, J.M., 2014. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, second ed. Elsevier/Academic Press, San Diego, USA.
|
Baskin C.C., Baskin J.M., et al, 2015. Inbreeding depression and the cost of inbreeding on seed germination. Seed Sci. Res., 25: 355-385. DOI:10.1017/S096025851500032X |
Bernareggi G., Carbognani M., Petraglia A., et al, 2015. Climate warming could increase seed longevity of alpine snowbed plants. Alpine Bot, 125: 69-78. DOI:10.1007/s00035-015-0156-0 |
Brys R., Jacquemyn H., 2015. Disruption of the distylous syndrome in Primula veris. Ann. Bot. (Lond.), 115: 27-39. DOI:10.1093/aob/mcu211 |
Charlesworth D., Charlesworth B., 1987. Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Systemat, 18: 237-268. DOI:10.1146/annurev.es.18.110187.001321 |
Davidson, J.B., Wolf, P.G., 2011. Natural history of Maguire primrose, Primula cusickiana var. maguirei (Primulaceae). Western N. Ame. Naturalist. 71, 327-337.
|
De Vere N., Jongejans E., Plowman A., et al, 2009. Population size and habitat quality affect genetic diversity and fitness in the clonal herb Cirsium dissectum. Oecologia, 159: 59-68. DOI:10.1007/s00442-008-1203-y |
Delmas C.E.L., Cheptou P.O., Escaravage N., et al, 2014. High lifetime inbreeding depression counteracts the reproductive assurance benefit of selfing in a massflowering shrub. BMC Evol. Biol, 14: 243-249. DOI:10.1186/s12862-014-0243-7 |
Endels P., Jacquemyn H., Brys R., et al, 2002. Changes in pin thrum ratios in populations of the heterostyle Primula vulgaris Huds:does imbalance affect population persistence?. Flora, 197: 326-331. DOI:10.1078/0367-2530-00048 |
Fay P.A., Schultz M.J., 2009. Germination, survival, and growth of grass and forb seedlings:effects of soil moisture variability. Acta Oecol, 35: 679-684. DOI:10.1016/j.actao.2009.06.007 |
Ferrer M.M., Good-Avila S.V., Montan#7863; C., et al, 2009. Effect of variation in selfincompatibility on pollen limitation and inbreeding depression in Flourensia cernua (Asteraceae) scrubs of contrasting density. Ann. Bot. (Lond.), 103: 1077-1089. DOI:10.1093/aob/mcp033 |
Forrest J., Thomson J.D., 2008. Pollen limitation and cleistogamy in subalpine Viola praemorsa. Botany, 86: 511-519. DOI:10.1139/B08-020 |
Galetto L., Bernardello G., IseleI C., et al, 2000. Reproductive biology of Erythrina crista-galli (Fabaceae). Ann. Mo. Bot. Gard, 87: 127-145. DOI:10.2307/2666157 |
Goodwillie C., Knight M.C., 2006. Inbreeding depression and mixed mating in Leptosiphon jepsonii:a comparison of three populations. Ann. Bot. (Lond.), 98: 351-360. DOI:10.1093/aob/mcl105 |
Guo H., Mazer S.J., Du G.Z., 2010. Geographic variation in seed mass within and among nine species of Pedicularis (Orobanchaceae):effects of elevation, plant size and seed number per fruit. J. Ecol, 98: 1232-1242. DOI:10.1111/j.1365-2745.2010.01688.x |
Holtsford T.P., Ellstrand N.C., 1990. Inbreeding effects in Clarkia tembloriensis (Onagraceae) populations with different natural outcrossing rates. Evolution, 44: 2031-2046. DOI:10.1111/j.1558-5646.1990.tb04309.x |
Jacquemyn H., Brys R., Hermy M., 2001. Within and between plant variation in seed number, seed mass and germinability of Primula elatior:effect of population size. Plant Biol, 3: 561-568. DOI:10.1055/s-2001-17728 |
Kéry M., Matthies D., Schmid B., 2003. Demographic stochasticity in population fragments of the declining distylous perennial Primula veris (Primulaceae). Basic Appl. Ecol, 4: 197-206. DOI:10.1078/1439-1791-00142 |
Kéry M., Matthies D., Spillmann H.H., 2000. Reduced fecundity and offspring performance in small populations of the declining grassland plants Primula veris and Gentiana lutea. J. Ecol, 88: 17-30. DOI:10.1046/j.1365-2745.2000.00422.x |
Knight T.M., Steets J.A., Vamosi J.C., et al, 2005. Pollen limitation of plant reproduction:pattern and process. Annu. Rev. Ecol. Systemat, 36: 467-497. DOI:10.1146/annurev.ecolsys.36.102403.115320 |
Kohn J.R., Graham S.W., Morton B., et al, 1996. Reconstruction of the evolution of reproductive characters in Pontederiaceae using phylogenetic evidence from chloroplast DNA restriction-site variation. Evolution, 50: 1454-1469. DOI:10.1111/j.1558-5646.1996.tb03919.x |
Körner C., 1999. Alpine Plant Life:Functional Plant Ecology of High Mountain Ecosystems. Springer-Verlag, Berlin.. Berlin: Springer-Verlag.
|
Meeus S., Honnay O., Jacquemyn H., 2012. Strong differences in genetic structure across disjunct, edge, and core populations of the distylous forest herb Pulmonaria officinalis (Boraginaceae). Am. J. Bot, 99: 1809-1818. DOI:10.3732/ajb.1200223 |
Nguyen D.Q., Hoa Phan T.P., Dao V.T., 2015. Effect of storage time and pretreatment on seed germination of the threatened coniferous species Fokienia hodginsii. Plant Species Biol, 30: 291-296. DOI:10.1111/1442-1984.12062 |
Papuga G., Gauthier P., Ramos J., et al, 2015. Range-wide variation in the ecological niche and floral polymorphism of the western Mediterranean geophyte Narcissus dubius Gouan. Int. J. Plant Sci, 176: 724-738. DOI:10.1086/683010 |
Pluess A.R., Schütz W., Stöcklin J., 2005. Seed weight increases with altitude in the Swiss Alps between related species but not among populations of individual species. Oecologia, 144: 55-61. DOI:10.1007/s00442-005-0047-y |
Qi W., Bu H.Y., Cornelissen J.H.C., et al, 2015. Untangling interacting mechanisms of seed mass variation with elevation:insights from the comparison of interspecific and intra-specific studies on eastern Tibetan angiosperm species. Plant Ecol, 216: 283-292. DOI:10.1007/s11258-014-0435-7 |
Rafferty N.E., Ives A.R., 2012. Pollinator effectiveness varies with experimental shifts in flowering time. Ecology, 93: 803-814. DOI:10.1890/11-0967.1 |
Rayburn A.A.P., Davidson J.B., Schupp E.W., 2013. Effect of storage time, site and floral morph on seed germination of the threatened distylous primrose Primula cusickiana var. maguirei. Plant Species Biol, 28: 101-108. DOI:10.1111/j.1442-1984.2011.00362.x |
Sampson D.A., Krebs R.A., 2012. Quantitative evaluation of reciprocal herkogamy in the distylous species, Hedyotis caerulea (Rubiaceae). Plant Systemat. Evol, 298: 1361-1370. DOI:10.1007/s00606-012-0642-4 |
Santos-Gally R., Pérez-Barrales R., Simón V.I., et al, 2013. The role of short-tongued insects in floral variation across the range of a style-dimorphic plant. Ann. Bot.(Lond.), 111: 317-328. DOI:10.1093/aob/mcs261 |
Spigler R.B., Chang S.M., 2009. Pollen limitation and reproduction varies with population size in experimental populations of Sabatia angularis (Gentianaceae). Botany, 87: 330-338. DOI:10.1139/B08-146 |
Straka J.R., Starzomski B.M., 2015. Fruitful factors:what limits seed production of flowering plants in the alpine?. Oecologia, 178: 249-260. DOI:10.1007/s00442-014-3169-2 |
Sun H., Niu Y., Chen Y.S., et al, 2014. Survival and reproduction of plant species in the QinghaieTibet plateau. J. Syst. Evol, 52: 378-396. DOI:10.1111/jse.12092 |
Susko D.J., Clubb M., 2008. Pollination effects on patterns of ovule fate in Hesperis matronalis (Brassicaceae). Botany, 86: 466-474. DOI:10.1139/B08-017 |
Thompson K., Ceriani R.M., Bakker J.P., et al, 2003. Are seed dormancy and persistence in soil related? Seed Sci. Res, 13: 97-100. |
Van Etten M.L., Tate J.A., Anderson S.H., et al, 2015. The compounding effects of high pollen limitation, selfing rates and inbreeding depression leave a New Zealand tree with few viable offspring. Ann. Bot. (Lond.), 116: 833-843. DOI:10.1093/aob/mcv118 |
Vittoz P., Dussex N., Wassef J., et al, 2009. Diaspore traits discriminate good from weak colonisers on high-elevation summits. Basic Appl. Ecol, 10: 508-515. DOI:10.1016/j.baae.2009.02.001 |
Washitani I., Kabaya H., 1988. Germination responses to temperature responsible for the seedling emergence seasonality of Primula sieboldii E. Morren in its natural habitat. Ecol. Res, 3: 9-20. |
Weber J.J., Weller S.G., Sakai A., et al, 2012. Purging of inbreeding depression within a population of Oxalis alpina (Oxalidaceae). Am. J. Bot, 99: 923-932. DOI:10.3732/ajb.1100383 |
Weber J.J., Weller S.G., Sakai A.K., et al, 2013. Breeding system evolution in Oxalis alpina:asymmetrical expression of tristylous incompatibility. Int. J. Plant Sci, 174: 179-188. DOI:10.1086/668786 |
Weller S.G., Sakai A.K., Thai D.A., et al, 2005. Inbreeding depression and heterosis in populations of Schiedea viscosa, a highly selfing species. J. Evol. Biol, 18: 1434-1444. DOI:10.1111/j.1420-9101.2005.00965.x |
Wirth L.R., Graf R., Gugerli F., et al, 2010. Lower selfing rate at higher altitudes in the alpine plant Eritrichium nanum (Boraginaceae). Am. J. Bot, 97: 899-901. DOI:10.3732/ajb.0900297 |



