b. Department of Applied Biology, College of Sciences, University of Sharjah, P. O. Box 27272, Sharjah, United Arab Emirates;
c. Independent Researcher, Selargius, Cagliari, Italy
Seed banking is widely acknowledged as a principal ex situ conservation tool for reliable and efficient storage of wild plant genetic resources (Liu et al., 2018). In a seed bank, it is fundamental to assess and monitor the viability of seeds throughout the storage time (Smith et al., 2003, Fenner and Thompson, 2005, Godefroid et al., 2010). Accordingly, the viability of seed collections stored in seed banks is monitored by a program of germination trials. Information on the viability of stored seeds is important in order to assess their ability to germinate when sown in the field (Al-Turki and Baskin, 2017). Recently, Liu et al. (2018) assessed the conservation value of germplasm stored at the Millennium Seed Bank (MSB) of the Royal Botanic Gardens, Kew (RBG Kew) and highlighted that plant conservation is critical in face of threats to global plant diversity, and seed banks serve as rich biological resources with high quality germplasm, substantial taxonomic diversity, and wide geographic coverage of accessions that represent significant natural capital and population value. Conserving plant diversity efficiently in seed banks requires a comprehensive understanding of seed maturation periods, harvest time, germination requirements, as well as storage and viability. Seed germination is an effective tool for assessing seed viability and determining conservation value of seed bank collections. Specifically, understanding seed germination characteristics has great importance in developing effective procedures for promoting ex situ conservation of rare, threatened, and economically important species stored in the seed banks (Kirmizi et al., 2010). Seed viability and germination responses of stored seed accessions under ex situ seed bank conditions has not received much attention for desert plants from the Arabian Gulf region.
The germination ability of stored seeds has been widely reported to decline gradually as seeds age (Roberts and Ellis, 1982). Baskin and Baskin (1988) suggested that seeds collected for conservation in seed banks must have higher viability when maintained for longer storage period. However, seeds of some species, especially succulent halophytes, lose viability over short periods, even when stored at low temperatures (El-Keblawy, 2014). The International Standards for Gene banks (FAO/IPGRI, 1994) recommends that viability of seeds must be determined before storage in the gene bank and monitored at regular intervals. However, monitoring intervals depend on several factors, such as species, seed viability at harvest and storage conditions. Similarly, the interval between subsequent monitoring and assessment can be determined based on prior experience. For example, seeds of succulent desert halophytes lose viability over a short period, so testing at short intervals of 3–5 years would be appropriate to monitor the trends (El-Keblawy, 2014).
Seed dispersal and germination events are essential parts of complex life history strategies of desert plants that allow them to persist in their arid habitat under unpredictable environmental conditions (Liu et al., 2014). To disperse their seeds over long distances, the common halophytic species of the Arabian deserts, such as Anabasis setifera Haloxylon salicornicum, Salsola drummondii, and Salsola imbricata, have winged structures as a dispersal-enhancing character. Seeds of these species are characterized by very fast germination after dispersal and the formation of a transient soil seed bank (Zaman and Khan, 1992, El-Keblawy, 2013). In general, dispersal units in seeds of plant species are thought to reduce the probability of their offspring survival in unpredictable environments, and promote arrival and colonization of new habitats for seed germination and population regeneration (Gutterman, 1993, Levin et al., 2003, Venable et al., 2008). Other halophytes, such as Arthrocnemum macrostachyum, Halopeplis perfoliata, Halocnemum strobilaceum, Limonium axillare, Suaeda aegyptiaca, and Suaeda vermiculata, produce small seeds and have limited dispersal as their seeds lack dispersal-enhancing characters. For such species, it has been reported that their small un-winged seeds usually develop dormancy and form persistent seed banks (Gulzar and Khan, 2001, Khan and Gul, 2006, Zia and Khan, 2008).
The effect of light on seed germination of halophytes varies between and within species. A survey of 41 halophytes found that in 20 species seed germination increased in the presence of light, whereas, 10 species germinated better in darkness, and 11 species showed no preference (Baskin and Baskin, 1995). More recently, a study has shown that some halophytes fail to germinate in darkness, whereas germination in other halophytes is unaffected by light (Gul et al., 2013). Light is an environmental signal that helps some seeds detect their position in the soil. In general, smaller seeds require light for germination, but larger seeds need darkness or germinate well in light as well in darkness (El-Keblawy, 2017). Lack of germination or lower germination due to dormancy during burial of small seeds would allow them to schedule germination and hence limit seedling mortality (Mapes et al., 1989, Milberg et al., 2000). Different halophytes of the Arabian Gulf region have been shown to vary in respect to germination and salinity tolerance (e.g., Mahmoud et al., 1983, El-Keblawy et al., 2007, El-Keblawy et al., 2013, El-Keblawy et al., 2017a, Bhatt et al., 2016).
The Amaranthaceae (which includes the former Chenopodiaceae) is a relatively large family. It includes 174 genera with about 2050 species, and represents the most species-rich lineage within the Caryophyllales (Slama et al., 2015). The largest number of halophytic species are found in this family worldwide. There are about 26 species of halophytes from this family growing in the United Arab Emirates (Gairola et al., 2019). The present study focuses on eight Amaranthaceae species. The selected halophytes have potential ecological and economic values, including as medicines, sources of nutrition, and in urban landscaping (Jongbloed, 2003, El-Keblawy et al., 2015, Gairola et al., 2015). In addition, some of the selected species have potential to restore degraded salt marshes and/or could be cultivated for their oily seeds in new crops targeting biodiesel (Cybulska et al., 2014). According to Shabala (2013), halophytes are a good experimental system to understand the molecular mechanism of salt tolerance and identify candidate genes for engineering salt tolerance in other conventional crops. Further, halophytes can be either domesticated into new, salt-resistant crops, or used as a source of genes to be introduced into crop species by classical breeding or molecular methods (Abdelly et al., 2006 and references therein). Al-Jaloud and Hussain (2006) also highlighted the importance of ex situ conservation of halophytes of the Gulf region. The evaluation of halophytes for land reclamation and native plant landscaping has gained increasing attention as many halophytes, because of sustained growth under highly brackish water irrigation, can provide useful vegetation cover for landscaping.
Maintaining seed longevity under storage conditions is crucial for genetic resource preservation. Likewise, an assessment of the effect of storage time on seed germination is important in order to use stored seed lots for various conservation and research purposes. In this study, we evaluated ex situ storage potential of eight native halophytes of the UAE by studying seed germination. Monitoring germination change of stored seeds could provide valuable information for these species ex situ conservation. Therefore, the longevity of seeds should be tested repeatedly.
2. Material and methods 2.1. Collection and storage of seedsMature diaspore unit (seed or fruit) of eight Amaranthaceae species, A. setifera, A. macrostachyum, H. strobilaceum, H. perfoliata, S. drummondii, S. imbricata, S. aegyptiaca, and S. vermiculata, were collected from natural populations to ensure adequate representation of the population's genetic diversity. Herbarium vouchers were collected and deposited at Sharjah Seed Bank and Herbarium (SSBH). The collections were georeferenced, and other relevant information related to the plant population and habitat characteristics was recorded in the field. After collection, seeds of each species were cleaned, checked for physical purity, and then seed number per accession, seed weight, seed morphological structures, etc. were recorded to ensure the traceability of germplasm in the SSBH seed collection database. Once cleaned, the seed collection was divided into two lots. The first lot was tested for germination immediately within 10 days of collection (hereafter referred to as 'fresh seed'). The second lot was progressively dried at 15 ℃ and 15% relative humidity (RH) to 5–8% moisture content, as these conditions are considered optimal for the majority of orthodox seeds. Once dried, seeds were stored long-term at −18 ℃ at the SSBH, United Arab Emirates (UAE). For this study, seeds were removed from −18 ℃ storage and allowed to equilibrate to room temperature for 24 h before bags were opened (hereafter referred to as 'stored seeds'). The same accession was used for the experiment and that the two study parts were conducted three years apart. Information on each taxon's seed dispersal ability, habitat, and adult longevity (annual, biennial, perennial) were retrieved from the literature and the SSBH database, including long-term field observations (Table 1). Seed mass data were obtained by weighing five replicates of 100 seeds on an electronic balance (0.0001 g precision) according to ISTA (2018).
| Species | Code | Habitat | Life span | 100 seed weight (mg ± SE) | RLG | Seed dispersal ability | |
| Fresh | Stored | ||||||
| Anabasis setifera | Anab. seti. | Coastal saline plain | Perennial | 17.47 ± 6.51 | 0.67 | 0.36 | Long distance |
| Arthrocnemum macrostachyum | Arth. macr. | Coastal salt marshes | Perennial | 13.07 ± 1.65 | 0.50 | 0.61 | Limited dispersal |
| Halocnemum strobilaceum | Halo. stro. | Coastal salt marshes | Perennial | 15.26 ± 6.16 | 0.55 | 0.53 | Limited dispersal |
| Halopeplis perfoliata | Halo. perf. | Coastal salt marshes | Perennial | 10.26 ± 0.81 | 0.64 | 0.63 | Limited dispersal |
| Salsola drummondii | Sals. drum. | Saline sandy plain | Perennial | 50.30 ± 8.05 | 0.62 | 0.56 | Long distance |
| Salsola imbricata | Sals. imbr. | Saline sandy area | Perennial | 46.31 ± 2.37 | 0.65 | 0.69 | Long distance |
| Suaeda aegyptiaca | Suae. aegy. | Saline sandy area | Annual/Biennal | 19.24 ± 1.97 | 0.64 | 0.67 | Limited dispersal |
| Suaeda vermiculata | Suae. verm. | Coastal saline plain | Perennial | 23.91 ± 2.78 | 0.57 | 0.54 | Limited dispersal |
Seeds of target species were germinated in a temperature- and light-controlled incubator. The incubator was set at 12/12 h daily thermoperiods of 25/15 ℃ and two photoperiods: 12/12 h light/dark (hereafter referred to as 'light') and in continuous darkness. The incubation temperatures were used to approximate the temperature fluctuations in the natural habitats during seed germination time. Seeds were placed in 9 cm Petri dishes containing two layers of filter paper (Whatman No. 1) moistened with 10 ml of distilled water. Water was added to the dishes as needed to keep the substrate moist. Three replications of 25 seeds per dish were used in each treatment for each species. Darkness was achieved by wrapping the Petri dishes with two layers of aluminium foil. The darkness simulates the conditions when seeds are buried in soils, but light simulates conditions when seeds are located on the soil surface. Petri dishes were randomly distributed in the incubator and their position changed daily. Seeds were checked daily for 10 'd' and the number of germinated seeds was registered. After 10 'd' of incubation, the rate of germination was calculated by using a modified Timson's index of germination velocity: Germination velocity = ΣG/t where, 'G' is the percentage of seed germination at 1 'd' intervals and 't' is the total germination period (Khan and Ungar, 1985). High Timson's index values indicate rapid germination. Germination of the seeds incubated in dark was noted once after 10 'd'. T1 was also calculated to represent the number of days needed to observe the first germinated seed in a replicate. The relative light germination index (RLG) was calculated using the formula: RLG = LG/(LG + DG), where LG is the light germination percentage and DG is the darkness germination percentage (Milberg et al., 2000). The values vary from 0 (germination in dark only) to 1 (germination in light only).
2.3. Statistical analysesGermination data were arcsine transformed and TI and T1 were log10-transformed before statistical analysis. Analyses of Variance (ANOVAs) were used to determine if treatments (species, storage, and photoperiod) had a significant effect on seed parameters. Fisher's Least Significant Differences (LSD) post hoc test was carried out to compare mean values for significant (P < 0.05) difference. Statistical analyses were performed with Statistica 8.0 for Windows (Software Statsoft Release 8) and graphs produced using Sigmaplot 11.0 (Systat Software Inc., London, UK).
3. Results 3.1. Seed characteristicsFive of the eight species studied have small seeds; average mass of 100 seeds ranged between 10.26 ± 0.81 mg for H. perfoliata and 19.24 ± 1.97 mg for S. aegyptiaca (Table 1). The other three species (S. vermiculata, S. imbricata and S. drummondii) have comparatively larger seeds; the heaviest is S. drummondii (50.30 ± 8.05 mg/100 seeds). Seeds of five species have limited dispersal abilities, but three species have supporting structures (wings) that help in their long-distance dispersal by wind. Species whose seeds having limited dispersal properties lack wings and are therefore lighter (16.35 mg/100 seeds), but seeds of species with distance dispersal have wings and are heavier (38.03 mg/100 seeds) (Table 1).
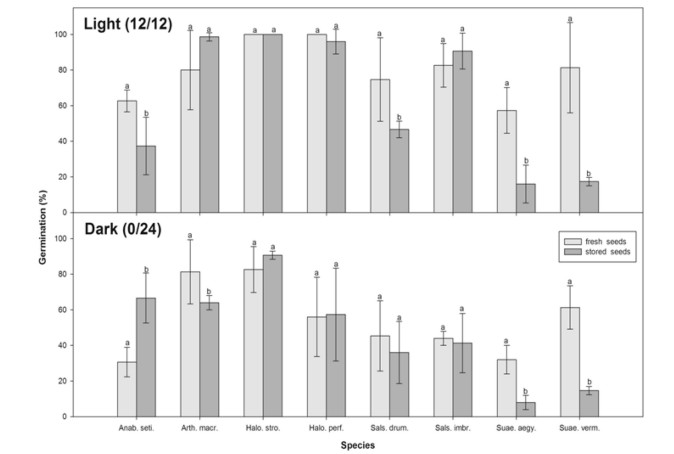
3.2. Effects on final germinationAll factors examined (species, storage and photoperiod) and most interactions had significant effects on germination (p < 0.01; Table 2). Fresh seeds of both A. macrostachyum and H. strobilaceum attained more than 80% germination under both light/dark conditions. In addition, final germination of H. perfoliata, S. imbricata, S. drummondii, and S. vermiculata attained more than 80% in light, but only 30–60% in dark. Both fresh seeds of A. setifera and S. aegyptiaca attained moderate germination in light, but low germination in dark (Fig. 1). Storage resulted in a significant decline in final germination percentage for the two Suaeda species (S. vermiculata and S. aegyptiaca). Interestingly, neither storage nor photoperiod affected germination of H. strobilaceum; both fresh and stored seeds attained > 95% germination in both light and darkness treatments. Storage did not affect the light requirement for germination in A. macrostachyum, H. perfoliata, and S. imbricata; seeds germinated equally well in light and total darkness (Fig. 1). RLG, which gives an indication of light requirement, showed that most species are positively photoblastic (i.e., RLG > 0.5). Fresh seeds were neutrally photoblastic in A. macrostachyum (RLG = 0.5) or slightly positive photoblastic in H. strobilaceum (RLG = 0.55) and S. vermiculata (RLG = 0.57). Storage has increased the light requirement for A. macrostachyum (RLG increased to 0.61), but did not affect that of both H. strobilaceum and S. vermiculata (Table 1). However, storage had reduced the light requirement for A. setifera (RLG for fresh and stored seeds was 0.67 and 0.36, respectively) and S. drummondii (RLG for fresh and stored seeds was 0.62 to 0.56, respectively). Pearson correlation coefficient indicated that the relationship between seed mass and RLG of both fresh and stored seeds was insignificantly positive (r2 = 0.311 and 0.214 for fresh and stored seeds, respectively, Table 1).
| Source of variation | Sum of Squares | Degree of Freedom | Mean Squares | F-value | Probability |
| Species (Sp) | 23244.8 | 7 | 3320.7 | 33.46 | *** |
| Photoperiod (Ph) | 7148.3 | 1 | 7148.3 | 72.02 | *** |
| Storage (St) | 1456.6 | 1 | 1456.6 | 14.68 | *** |
| Sp × Ph | 2982.3 | 7 | 426.0 | 4.29 | *** |
| Sp × St | 4524.3 | 7 | 646.3 | 6.51 | *** |
| Ph × St | 153.9 | 1 | 153.9 | 1.55 | ns |
| Sp × Ph × St | 2158.9 | 7 | 308.4 | 3.11 | ** |
| Error | 6352.4 | 64 | 99.3 |
|
| Fig. 1 Effects of photoperiod, species type and storage on seed germination of eight halophytes. Bars with different lowercase letters indicate significant differences at P < 0.05 (Fisher's Least Significant Difference). Values are means ± SD of three replicates |
The interaction between photoperiod and storage was insignificant in seven species; the only significant effect was in A. setifera (Table 3). Both fresh and stored seeds of H. perfoliata and S. imbricata attained significantly greater germination in light than in darkness. In both stored and fresh seeds of three species (H. strobilaceum, S. drummondii and S. vermiculata) there were no significant differences between germination in light and in dark. In A. setifera, fresh seeds attained significantly greater germination in light compared to in total darkness, but the opposite was true for stored seeds, indicating that storage induced a dark requirement for the germination of A. setifera (Fig. 1).
| Species | Photoperiod (Ph) | Storage (St) | Ph × St |
| Anabasis setifera | * | ** | ** |
| Arthrocnemum macrostachyum | ns | ns | ns |
| Halocnemum strobilaceum | *** | ns | ns |
| Halopeplis perfoliata | *** | ns | ns |
| Salsola drummondii | ns | ns | ns |
| Salsola imbricata | *** | ns | ns |
| Suaeda aegyptiaca | * | *** | ns |
| Suaeda vermiculata | ns | *** | ns |
The germination rate for all the studied halophytic species was very high; seeds germinated within 1–2 days of imbibition. For A. setifera and S. imbricata, the germination velocity did not differ between fresh and stored seeds (Table 4). However, for A. macrostachyum, H. strobilaceum, H. perfoliata, seed storage significantly enhanced germination speed. An opposite trend was observed in S. drummondii, S. aegyptiaca, and S. vermiculata. The onset of germination did not differ among fresh and stored seeds of studied species, except for H. strobilaceum, which demonstrated a quicker germination response after being stored. For both fresh and stored seeds of all species, germination started the day after they were sown, except for stored seeds of H. strobilaceum and S. vermiculata, which germinated on the second day (Table 4).
| Species | Germination velocity | Fresh | Stored | |
| Anabasis setifera | TI | 56.6 ± 3.1 | 96.7 ± 1.7 | *** |
| T1 (days) | 1.00 ± 1.00 | 1.00 ± 1.00 | ns | |
| Arthrocnemum macrostachyum | TI | 60.8 ± 2.1 | 92.2 ± 0.1 | *** |
| T1 (days) | 1.33 ± 0.57 | 1.00 ± 1.00 | ns | |
| Halocnemum strobilaceum | TI | 92.7 ± 2.9 | 90.0 ± 0.0 | ns |
| T1 (days) | 1.00 ± 0 | 2.00 ± 0 | * | |
| Halopeplis perfoliata | TI | 83.7 ± 1.1 | 94.6 ± 0.5 | ** |
| T1 (days) | 1.00 ± 0 | 1.00 ± 0 | ns | |
| Salsola drummondii | TI | 81.3 ± 3.5 | 85.3 ± 4.7 | ns |
| T1 (days) | 1.00 ± 0 | 1.00 ± 0 | ns | |
| Salsola imbricata | TI | 90.0 ± 1.7 | 86.7 ± 0.4 | ns |
| T1 (days) | 1.00 ± 0 | 1.00 ± 0 | ns | |
| Suaeda aegyptiaca | TI | 88.0 ± 2.4 | 69.7 ± 12.9 | ns |
| T1 (days) | 1.00 ± 0 | 1.33 ± 0.57 | ns | |
| Suaeda vermiculata | TI | 86.1 ± 4.7 | 66.5 ± 12.9 | ns |
| T1 (days) | 1.00 ± 0 | 2.00 ± 1.00 | ns | |
The findings of present study demonstrate that ex situ conservation is an effective way to maintain the viability of seeds of the most studied halophytes. Fresh seeds of most species showed high final germination in light; two species attained > 60% germination and the other six species germinated to more than 80%. Similar results have been reported for H. strobilaceum (El-Keblawy and Bhatt, 2015), and for H. perfoliata and S. imbricata (Khan and Weber, 2007, El-Keblawy et al., 2007). In previous studies, seeds of many other halophytes such as Limonium stocksii (Zia and Khan, 2004), Atriplex centralasiatica (Li et al., 2008), Suaeda heterophylla (Hameed et al., 2013) and Zygophyllum propinquum (Manzoor et al., 2017) also germinated to higher percentages in light than in dark conditions. The germination response to light is an important signal to ensure seed germination in the appropriate places (e.g., depth in the soil) and times (e.g., season) that ensure seedling establishment (Baskin and Baskin, 2014, Murru et al., 2015). Higher germination of small seeds in light compared to darkness can be considered an adaptive advantage that helps them acquire light in the shortest time, thus ensuring seedling growth and establishment (Kitajima, 2002; Baraloto and Forget, 2007). This is especially relevant for the desert areas of the Arabia, where salinity, drought and heat stress leaves only a shorter period for the seedlings to establish. In halophyte seeds, the main factors that regulate germination via enforcement of dormancy are salinity, light, and temperature (Pujol et al., 2000, Qu et al., 2007, Gul et al., 2013, Al-Shamsi et al., 2018, Elnaggar et al., 2018).
In unpredictable, heterogeneous environments, such as the saline habitats of arid deserts, plants develop multiple strategies to increase germination, including producing offspring that differ in time and place of germination, and increasing their tolerance to environmental stresses (Baskin and Baskin, 2014). Consequently, fluctuations in these environmental conditions lead to adaptations and changes in plant community structure and composition. Seeds of many halophytic plants have winged perianths that help their dispersal and determine the proper site of seed germination (Wei et al., 2008, Xing et al., 2013). Wind-dispersal allows seeds to increase their chances of finding a suitable location for germination. Several studies on wind-dispersed seeds have indicated that the wings play an important role in controlling seed dormancy and germination requirements, such as light, temperatures and salinity (e.g., El-Keblawy, 2014, Bhatt and Santo, 2018, Bhatt et al., 2019). In our study, seeds of three species (S. imbricata, S. drummondii and A. setifera) have winged perianths that might help them not only to disperse and explore new safe-sites, but also regulate dormancy and soil seed bank dynamics (El-Keblawy, 2013). However, seeds of the other halophytes (H. perfoliata, H. strobilaceum, A. macrostachyum, S. aegyptiaca and S. vermiculata) lack dispersal structures. Instead, they retain seeds within the maternal tissues as an aerial seed bank (bradychory), and have limited chances to become buried in soils. Seeds of these limited dispersed species are more likely to germinate in safe-sites, where maternal plants are established and growing successfully.
There was a positive relationship between seed mass and RLG for both fresh and stored seeds. It has been suggested that light response and smaller seed mass coevolved as an adaptation to ensure germination of small-seeded species only when seeds of these species are close to the soil surface. Typically, smaller seeds germinate successfully when close to the soil surface; consequently, small seeds require light for germination. This light requirement is likely an adaptation to smaller food reserves in these seeds (Milberg et al., 2000). Aud and Ferraz (2012) found that the limited resources available in small seeds are compensated by a requirement for light to aid germination. In our study, most of the investigated species germinated better in light than in dark, but this pattern was not particularly correlated with seed size/mass. For example, some of the comparatively large-seeded species, namely S. drummondii, S. imbricata, and S. vermiculata were positively photoblastic when germinated immediately after harvesting (i.e., fresh seeds). However, these seeds have dispersal wings that ensure that they are stored on the soil surface (S. drummondii, S. imbricata) or on maternal plants (bradychory, S. vermiculata) (authors personal observation). Seed storage on soil surface or within maternal tissue would induce light requirements (Bhatt et al., 2016). Aerial-stored seeds face diurnal fluctuations in temperatures and are exposed to intense light during storage (Zalamea et al., 2015).
All the studied species showed moderate to very high germination of fresh seeds (Fig. 1). High germination of fresh seeds has also been reported in other halophytes, such as Salsola species, Salsola affinis (Wei et al., 2008), Salsola nitraria (Chang et al., 2008), Salsola ferganica (Wang et al., 2013), and Salsola iberica (Khan et al., 2002). The ability of halophyte seeds to germinate immediately after maturation could be an important adaptation to coincide with typical rainfall patterns in the arid deserts of the Gulf region. Most of the studied species produce an abundance of ready-to-germinate seeds early in the growing season (November–December), with rainfall typically occurring during December to February, which is concurrent with the time of fruit dispersal of most of the studies halophytes (El-Keblawy, 2013). Such rainfall could dilute soil salinity and consequently increase soil water potential, allowing seedling establishment before soil salinity increases once more. In this study, storage resulted in significant declines in germination percentage of the two Suaeda species. However, these same species have been known to survive for several years at room temperature (A. El-Keblawy pers. obs.). Consequently, storage conditions affected germination. However, for most species, fresh and stored seeds showed no difference in germination. Interestingly, these same species have been shown to lose viability under natural habitats in a period of only a few months (El-Keblawy, 2013). Interestingly, the storage induced a dark requirement for seed germination in A. setifera as there was significant increase in percentage and rate of germination. Such result indicates that storage did not affect seed viability of this species, but might changed phytochrome sensitivity in dark (Casal and Sánchez, 1998, El-Keblawy et al., 2017b).
Seeds of most of the studied halophytes showed fast germination; most germination occurred within two days after sowing. This is a common phenomenon observed for many halophytes, such as Arthrocnemum indicum (Saeed et al., 2011), L. stocksii (Zia and Khan, 2004), and Aeluropus lagopoides (Khan and Gulzar, 2003). Early germination within a season can also confer a competitive advantage over seedlings that germinate later (Gul et al., 2013). Faster emergence usually produces vigorous seedlings due to increased resource interception that are more competitive than seedlings that emerged slowly (El-Keblawy et al., 2016). This might have important consequences for survival and reproductive success, which are important components of plant fitness under stressful environments.
In 25 weed species with contrasted seed characteristics, Gardarin et al. (2011) showed that the dormancy level was positively correlated with the speed of germination parameters, indicating that the final germination is negatively correlated with germination speed. Similarly, Thomson and El-Kassaby (1993) investigated the relationship between seed viability and germination speed in 19 seedlots of Pseudotsuga menziesii and found that fast germination was associated with low viability, but slow germination occurred in seed lots with high viability. Our study revealed a negative correlation between GRI and final germination in five species (A. setifera, S. imbricata, H. perfoliata, S. drummondii and H. strobilaceum). However, this correlation was only significant in A. setifera. This relationship needs to be further examined for individual species that have different levels of dormancy in different habitat types or produce seeds in different seasons.
5. Conservation implicationsIt is a well-established fact that ex situ conservation is becoming increasingly important in conservation of plants in today's changing environments (Pečnikar et al., 2018). Halophytic plants have evolved various morphological and physiological traits that enable them to naturally tolerate multiple types of abiotic stresses and grow successfully on saline substrates. Due to their unique abilities to thrive under stressful environmental conditions, halophytes have gained increasing attention from researchers, focused on among other things, biofuel production, rehabilitation of salt-affected lands, and phytoremediation of polluted soils (Sharma et al., 2016 and references therein). Duarte et al. (2013) indicated that the coastal ecosystems that are per se fragile and particularly threatened by anthropogenic disturbances, are also vulnerable to some of the consequences of predicted changes in the climate associated with rising levels of atmospheric CO2. The increasing salinization due to sea level rise is irreversible in arid regions because water is not available to leach the accumulated salts out of the soil, thereby threatening halophyte diversity mainly by shrinking their population sizes and reducing their genetic resources. Thus, wild plant populations may have difficulties thriving in their current range; strategies must be put in place to deal with this situation.
Understanding the germination characteristics of seed collections is critical for seed banks. Thus, collecting, banking, and testing seeds of native plants will ensure that species are available for future research, conservation, and restoration efforts. Our study highlight the utility of germination studies on seeds of important halophytes under seedbank conditions. Halophyte study species vary in their germination pattern; generally, most of them maintain seed viability under long-term storage, thus increasing the likelihood of seeding success for restoration.
6. ConclusionResults of the present study indicate that most of the studied halophytes maintained germination after storage at −18 ℃ for three years. Such results demonstrate that ex situ conservation using seed banks is a feasible method for maintaining the viability of seeds. The results of our study are encouraging for the ex situ conservation of halophytes, as this information will contribute to defining storage conditions for halophyte species, and help develop germination protocols useful in the restoration of salt-damaged habitats. For some species, especially those that showed high germination after the storage period, it may facilitate to establish plants from stored seeds for possible re-introduction into the wild and restoration ecology of salt-damaged habitats. In contrast, species with low germination after storage will require further studies to develop better storage conditions. Failing this, regular seed collections may be necessary, although this would prove costly and time consuming. Long-term viability is of crucial importance for halophytes, since seeds of these plants are considered short-lived. Above all, seed conservation helps to store, preserve, and make available genetic diversity to be used for many purposes, including for scientific research, propagation, plant breeding, restoration and re-introduction.
Conflict of interestThe authors declare that they have no conflict of interest.
AcknowledgementsAuthors would like to thank Dr. Amr Abdel-Hamid, Director General of the Sharjah Research Academy for encouragement and support. Assistance provided by SSBH team members at various stages of this study is greatly acknowledged. We thank Dr. Dave Aplin for his critical revision of the earlier version of this manuscript.
Abdelly, C., Barhoumi, Z., Ghnaya, T., Debez, A., Hamed, K.B., Ksouri, R., Talbi, O., Zribi, F., Ouerghi, Z., Smaoui, A., Huchzermeyer, B., 2006. Potential utilization of halophytes for the rehabilitation and valorization of salt-affected areas in Tunisia. In: Öztürk, M., Waisel, Y., Khan, M.A., Görk, G. (Eds.), Biosaline Agri-culture and Salinity Tolerance in Plants. Birkhäuser Verlag, Switzerland, pp. 163-172.
|
Al-Jaloud, A.A., Hussain, G., 2006. Sabkha ecosystem and halophyte plant communities in Saudi Arabia. In: Khan, M.A., Böer, B., Kust, G.S., Barth, H.J. (Eds.), Sabkha Ecosystems: Volume Ⅱ, West and Central Asia. Dordrecht, Springer, Netherlands, pp. 1-7.
|
Al-Shamsi N., El-Keblawy A., Mosa K.A., Navarro T., 2018. Drought tolerance and germination response to light and temperature for seeds of saline and nonsaline habitats of the habitat-indifferent desert halophyte Suaeda vermiculata. Acta Physiol. Plant, 40: 200. DOI:10.1007/s11738-018-2771-z |
Al-Turki T.A., Baskin C.C., 2017. Determination of seed viability of eight wild Saudi Arabian species by germination and X-ray tests. Saudi J. Biol. Sci, 24: 822-829. DOI:10.1016/j.sjbs.2016.06.009 |
Aud F.F., Ferraz I.D., 2012. Seed size influence on germination responses to light and temperature of seven pioneer tree species from the Central Amazon. Ann. Acad. Bras. Ciênc, 84: 759-766. DOI:10.1590/S0001-37652012000300018 |
Baraloto, C., Forget, P.M., 2007. Seed size, seedling morphology, and response to deep shade and damage in neotropical rain forest trees. Am. J. Bot 94, 901-911.
|
Baskin C.C., Baskin J.M., 1988. Germination ecophysiology of herbaceous plant species in a temperate region. Am. J. Bot, 1: 286-305. |
Baskin, C.C., Baskin, J.M., 1995. Dormancy types and dormancy-breaking and germination requirements in seeds of halophytes. In: Khan, M.A., Ungar, I.A.(Eds.), Biology of Salt Tolerant Plants. University of Karachi Press, Karachi, Pakistan, pp. 23-30.
|
Baskin, C.C., Baskin, J.M., 2014. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination. Academic Press, San Diego, CA, USA.
|
Bhatt A., Santo A., 2018. Does perianth colour affect the seed germination of two desert shrubs under different storage periods and conditions? Nord. J. Bot, 36. |
Bhatt, A., Bhat, N.R., Murru, V., Santo, A., 2019. Eco-physiological studies on desert plants: germination of Halothamnus iraqensis Botsch. seeds under different conditions. J. Arid Land 11, 75-85.
|
Bhatt A., Pérez-García F., Mercedes C.M., Gallacher D., 2016. Germination response of Salsola schweinfurthii (Chenopodiaceae) to salinity and winged perianth removal. Seed Sci. Technol, 44: 428-434. DOI:10.15258/sst.2016.44.2.14 |
Casal J.J., Sanchez R.A., 1998. Phytochromes and seed germination. Seed Sci. Res, 8: 317-329. DOI:10.1017/S0960258500004256 |
Chang S.J., Zuo B., Wang X.W., Huang J.H., 2008. Influence of light, temperature and salt on the germination of Salsola nitraria Pall. Arid. Land Geogr, 31: 897-903. |
Cybulska, I., Brudecki, G., Alassali, A., Thomsen, M., Brown, J.J., 2014. Phytochemical composition of some common coastal halophytes of the United Arab Emirates. Emir. J. Food Agric. 26, 1046-1056.
|
Duarte B., Santos D., Marques J.C., Caçador I., 2013. Ecophysiological adaptations of two halophytes to salt stress:photosynthesis PS Ⅱ photochemistry and antioxidant feedback-implications for resilience in climate change. Plant Physiol. Biochem, 67: 178-188. DOI:10.1016/j.plaphy.2013.03.004 |
El-Keblawy, A., Bhatt, A., Gairola, S., 2013. Perianth colour affects germination behaviour in wind-pollinated Salsola rubescens in Arabian deserts. Botany 92, 69-75.
|
El-Keblawy A., 2013. Effects of seed storage on germination of two succulent desert halophytes with little dormancy and transient seed bank. Acta Ecol. Sin, 33: 338-343. DOI:10.1016/j.chnaes.2013.09.008 |
El-Keblawy, A., 2014. Effects of seed storage on germination of desert halophytes with transient seed bank. In: Khan, M.A., Böer, B., Öztürk, M., Al Abdessalaam, T.Z., Clüsener-Godt, M., Gul, B. (Eds.), Sabkha Ecosystems, Tasks for Vegetation Science, vol. 47. Springer, Dordrecht, pp. 93-103.
|
El-Keblawy A., 2017. Light and temperature requirements during germination of potential perennial grasses for rehabilitation of degraded sandy Arabian Deserts. Land Degrad. Dev, 28: 1687-1695. DOI:10.1002/ldr.2700 |
El-Keblawy A., Al-Ansari F., Hassan N., Al-Shamsi N., 2007. Salinity, temperature and light affect germination of Salsola imbricata. Seed Sci. Technol, 35: 272-281. DOI:10.15258/sst.2007.35.2.03 |
El-Keblawy A., Bhatt A., 2015. Aerial seed bank affects germination in two smallseeded halophytes in Arab Gulf desert. J. Arid Environ, 117: 10-17. DOI:10.1016/j.jaridenv.2015.02.001 |
El-Keblawy A., Bhatt A., Gairola S., 2015. Developing sea watered landscapes:a potential way to reduce stress on fresh water resources. Curr. Sci, 108: 1773-1774. |
El-Keblawy, A., Gairola, S., Bhatt, A., 2016. Maternal salinity environment affects salt tolerance during germination in Anabasis setifera: a facultative desert halophyte. J. Arid Land 8, 254-263.
|
El-Keblawy A., Gairola S., Bhatt A., Mahmoud T., 2017a. Effects of maternal salinity on salt tolerance during germination of Suaeda aegyptiaca, a facultative halophyte in the Arab Gulf desert. Plant Species Biol, 32: 45-53. DOI:10.1111/1442-1984.12127 |
El-Keblawy A., Shabana H.A., Navarro T., Soliman S., 2017b. Effect of maturation time on dormancy and germination of Citrullus colocynthis (Cucurbitaceae) seeds from the Arabian hyper-arid deserts. BMC Plant Biol, 17: 263. DOI:10.1186/s12870-017-1209-x |
Elnaggar, A., El-Keblawy, A., Mosa, K.A., Navarro, T., 2018. Adaptive drought tolerance during germination of Salsola drummondii seeds from saline and nonsaline habitats of the arid Arabian deserts. Botany 97, 123-133.
|
FAO/IPGRI, 1994. Genebank Standards. FAO/IPGRI, Rome.
|
Fenner, M., Thompson, K., 2005. The Ecology of Seeds. Cambridge University Press.
|
Gairola, S., Bhatt, A., El-Keblawy, A., 2015. A perspective on potential use of halophytes for reclamation of salt-affected lands. Wulfenia 22, 88-97.
|
Gairola, S., Mahmoud, T., Shabana, H.A., 2019. Diversity and distribution of salttolerant plants of the United Arab Emirates: perspectives for sustainable utilization and future research. In: Gul, B., Boer, B., Khan, M.A., Clüsener-Godt, M., Hameed, A. (Eds.), Sabkha Ecosystems, Tasks for Vegetation Science VI. https://doi.org/10.1007/978-3-030-04417-6_21.
|
Gardarin A., D#252;rr C. Colbach N., 2011. Prediction of germination rates of weed species:relationships between germination speed parameters and species traits. Ecol. Model, 222: 626-636. DOI:10.1016/j.ecolmodel.2010.10.005 |
Godefroid S., Van de Vyver A., Vanderborght T., 2010. Germination capacity and viability of threatened species collections in seed banks. Biodivers. Conserv, 19: 1365-1383. DOI:10.1007/s10531-009-9767-3 |
Gul B., Ansari R., Flowers T.J., Khan M.A., 2013. Germination strategies of halophyte seeds under salinity. Environ. Exp. Bot, 92: 4-18. DOI:10.1016/j.envexpbot.2012.11.006 |
Gulzar S., Khan M.A., 2001. Seed germination of a halophytic grass Aeluropus lagopoides. Ann. Bot, 87: 319-324. DOI:10.1006/anbo.2000.1336 |
Gutterman, Y., 1993. Seed Germination in Desert Plants (Adaptation of Desert Organisms). Springer-Verlag, Germany.
|
Hameed A., Ahmed M.Z., Gulzar S., Gul B., Alam J., Hegazy A.K., Alatar A.R., Khan M.A., 2013. Seed germination and recovery responses of Suaeda heterophylla to abiotic stresses. Pak. J. Bot, 45: 1649-1656. |
ISTA, 2018. International Rules for Seed Testing. International Seed Testing Association, Bassersdorf, Switzerland.
|
Jongbloed, M., 2003. The Comprehensive Guide to the Wild Flowers of the United Arab Emirates. Environmental Research and Wildlife Development Agency, Abu Dhabi, UAE.
|
Khan M.A., Gul B., Weber D.J., 2002. Seed germination in the great basin halophyte Salsola iberica. Can. J. Bot, 80: 650-655. DOI:10.1139/b02-046 |
Khan M.A., Weber D.J., 2007. Dormancy, germination and viability of Salsola imbricata seeds in relation to light, temperature and salinity. Seed Sci. Technol, 35: 595-606. DOI:10.15258/sst.2007.35.3.07 |
Khan M.A., Gulzar S., 2003. Germination responses of Sporobolus ioclados:a saline desert grass. J. Arid Environ, 53: 387-394. DOI:10.1006/jare.2002.1045 |
Khan, M.A., Gul, B., 2006. Halophyte seed germination. In: Khan, M.A., Weber, D.J.(Eds.), Ecophysiology of High Salinity Tolerant Plants. Springer, Dordrecht, pp. 11-30.
|
Khan, M.A., Ungar, I.A., 1985. The role of hormones in regulating the germination of polymorphic seeds and early seedling growth of Atriplex triangularis under saline conditions. Physiol. Plant 63, 109-113.
|
Kirmizi S., Gueleryuez G., Arslan H., Sakar F.S., 2010. Effects of moist chilling, gibberellic acid, and scarification on seed dormancy in the rare endemic Pedicularis olympica (Scrophulariaceae). Turk. J. Bot, 34: 225-232. |
Kitajima K., 2002. Do shade-tolerant tropical tree seedlings depend longer on seed reserves? functional growth analysis of three Bignoniaceae species. Funct. Ecol, 16: 433-444. DOI:10.1046/j.1365-2435.2002.00641.x |
Levin S.A., Muller-Landau H.C., Nathan R., Chave J., 2003. The ecology and evolution of seed dispersal:a theoretical perspective. Annu. Rev. Ecol. Evol. Syst, 34: 575-604. DOI:10.1146/annurev.ecolsys.34.011802.132428 |
Li W., An P., Liu X., Khan M.A., Tsuji W., Tanaka K., 2008. The effect of light, temperature and bracteoles on germination of polymorphic seeds of Atriplex centralasiatica Iljin under saline conditions. Seed Sci. Technol, 36: 325-338. DOI:10.15258/sst.2008.36.2.06 |
Liu, H., Zhang, D., Yang, X., Huang, Z., Duan, S., Wang, X., 2014. Seed dispersal and germination traits of 70 plant species inhabiting the Gurbantunggut desert in northwest China. Sci. World J. 1-12. https://doi.org/10.1155/2014/346405.
|
Liu U., Breman E., Cossu T.A., Kenney S., 2018. The conservation value of germplasm stored at the Millennium Seed Bank, Royal Botanic Gardens, Kew, UK. Biodivers. Conserv, 27: 1347-1386. DOI:10.1007/s10531-018-1497-y |
Mahmoud A., El Sheikh A.M., Abdul B.S., 1983. Germination of two halophytes:Halopeplis perfoliata and Limonium axillare from Saudi Arabia. J. Arid Environ, 6: 87-98. DOI:10.1016/S0140-1963(18)31520-9 |
Manzoor, S., Hameed, A., Khan, M.A., Gul, B., 2017. Seed germination ecology of a medicinal halophyte Zygophyllum propinquum: responses to abiotic factors. Flora 233, 163-170.
|
Mapes, G.G., Rothwell, W., Haworth, M.T., 1989. Evolution of seed dormancy. Nature 337, 645-646.
|
Milberg P., Andersson L., Thompson K., 2000. Large-seeded spices are less dependent on light for germination than small-seeded ones. Seed Sci. Res, 10: 99-104. DOI:10.1017/S0960258500000118 |
Murru, V., Santo, A., Piazza, C., Hugot, L., Bacchetta, G., 2015. Seed germination, saltstress tolerance, and the effect of nitrate on three Tyrrhenian coastal species of the Silene mollissima aggregate (Caryophyllaceae). Botany 93, 881-892.
|
Pečnikar, Z.F., Balant, M., Glasnović, P., Surina, B., 2018. Seed dormancy and germination of the rare, high elevation Balkan endemic Cerastium dinaricum(Caryophyllaceae). Biologia 73, 937-943.
|
Pujol J.A., Calvo J.F., Ramirez-Diaz L., 2000. Recovery of germination from different osmotic conditions by four halophytes from southeastern Spain. Ann. Bot, 85: 279-286. DOI:10.1006/anbo.1999.1028 |
Qu X.X., Huang Z.Y., Baskin J.M., Baskin C.C., 2007. Effect of temperature, light and salinity on seed germination and radicle growth of the geographically widespread halophyte shrub Halocnemum strobilaceum. Ann. Bot, 101: 293-299. DOI:10.1093/aob/mcm047 |
Roberts, E.H., Ellis, R.H., 1982. Physiological, ultrastructural and metabolic aspects of seed viability. In: Khan, A.A. (Ed.), The Physiology and Biochemistry of Seed Development, Dormancy and Germination. Elsevier Biomedical Press, Amsterdam, pp. 465-485.
|
Saeed S., Gul B., Khan M.A., 2011. Comparative effects of NaCl and sea salt on seed germination of Arthrocnemum indicum. Pak. J. Bot, 43: 2-14. |
Shabala S., 2013. Learning from halophytes:physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot, 112: 1209-1221. DOI:10.1093/aob/mct205 |
Sharma R., Wungrampha S., Singh V., Pareek A., Sharma M.K., 2016. Halophytes as bioenergy crops. Front. Plant Sci, 7: 372. |
Slama I., Abdelly C., Bouchereau A., Flowers T., Savouré A., 2015. Diversity dis-tribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot, 115: 433-447. DOI:10.1093/aob/mcu239 |
Smith, R.D., Dickie, J.B., Linington, S.H., Pritchard, H.W., Probert, R.J., 2003. Seed Conservation: Turning Science into Practice. Royal Botanic Gardens, Kew, UK.
|
Thomson A.J., El-Kassaby Y.A., 1993. Interpretation of seed-germination parameters. N. For, 7: 123-132. |
Venable, D.L., Flores-Martinez, A., Muller-Landau, H.C., Barron-Gafford, G., Becerra, J.X., 2008. Seed dispersal of desert annuals. Ecology 89, 2218-2227.
|
Wang Y., Jiang G.Q., Han Y.N., Liu M.M., 2013. Effects of salt, alkali and saltealkali mixed stresses on seed germination of the halophyte Salsola ferganica (Chenopodiaceae). Acta Ecol. Sin, 33: 354-360. DOI:10.1016/j.chnaes.2013.09.010 |
Wei, Y., Dong, M., Huang, Z.Y., Tan, D.Y., 2008. Factors influencing seed germination of Salsola affinis (Chenopodiaceae), a dominant annual halophyte inhabiting the deserts of Xinjiang, China. Flora 203, 134-140.
|
Xing J., Cai M., Chen S., Chen L., Lan H., 2013. Seed germination, plant growth and physiological responses of Salsola ikonnikovii to short-term NaCl stress. Plant Biosyst, 147: 285-297. DOI:10.1080/11263504.2012.731017 |
Zalamea P.C., Sarmiento C., Arnold A.E., Davis A.S., Dalling J.W., 2015. Do soil microbes and abrasion by soil particles influence persistence and loss of physical dormancy in seeds of tropical pioneers? Front. Plant Sci, 5: 1-14. |
Zaman A.U., Khan M.A., 1992. The role of buried viable seeds in saline desert plant community. Bangladesh J. Bot, 21: 1-10. |
Zia S., Khan M.A., 2004. Effect of light, salinity, and temperature on seed germination of Limonium stocksii. Can. J. Bot, 82: 151-157. DOI:10.1139/b03-118 |
Zia S., Khan M.A., 2008. Seed germination of Limonium stocksii under saline conditions. Pak. J. Bot, 40: 683-695. |



