b. Department of Biological Sciences, Jaramogi Oginga Odinga University of Science and Technology, P.O Box 210-40601, Bondo, Kenya;
c. Department of Plant Ecology, University of Bayreuth, D-95440, Bayreuth, Germany
Climate change and land use are modifying plant composition and vegetation cover within East African savannahs (Ssemmanda et al., 2014, Serdeczny et al., 2015). In recent decades, the savannahs have experienced a rise in human settlement. There has also been an increase in land use intensity, especially due to increasing livestock density. These changes are characterized by the conversion of natural ecosystems into agricultural use (Muriuki et al., 2005, K'Otuto et al., 2012, Musau et al., 2016). Climate variability manifested through changing rainfall patterns is ongoing in East African regions (Conway et al., 2005, Serdeczny et al., 2015), where both shorter periods of intense rainfall and longer periods of drought have been recorded (K'Otuto et al., 2012).
Changing rainfall patterns have varying consequences on herbaceous structure and composition (Lovette et al., 2005, Zerbo et al., 2016). Increases in rainfall intensities often result in accelerated runoffs and soil erosion, and destroy shallow-rooted plants (Baudena et al., 2015, Zerbo et al., 2016). On the other hand, reduced rainfall may limit plant physiological functions such as photosynthetic CO2 uptake and fixation, plant growth, and species survival. Drought inhibits seed germination, causes plant mortality and alters community structure (Wang et al., 2010, Western et al., 2015). Depending on the time-span, drought may cause dominant deep-rooted vegetation in the herbaceous layer to replace shallow rooted vegetation (Hoover et al., 2014). Cumulatively, these factors alter herbaceous plant communities, reducing plant cover, and potentially lowering the survival chances of intolerant species (Ji and Peters, 2013). In some instances, such outcomes are short-term, with recovery occurring during subsequent favourable years. In other cases, however, changes in rainfall intensity may stimulate irreversible shifts in species composition in the herbaceous community (van der Plas et al., 2013).
Savannah in East Africa has evolved in conjunction with wild grazingherbivores, which normally occur at ecologically sustainable levels (Kioko et al., 2012, Mureithi et al., 2014). The introduction and expansion of livestock in the savannah, however, poses major challenges, given that the ecosystem is subjected to increased grazing pressure. This potentially alters vegetation structure and function. Overgrazing by livestock may notably reduce plant growth and vigor which in turn may lead to shifts in species composition (Schieltz and Rubenstein, 2016). There is also a reduction in class 1 (palatable species) category plants which are often consumed by livestock before seed dispersal (Rutherford et al., 2012, Koerner and Collins, 2014). Overgrazing also leads to a shift from communities of perennials to those predominately composed of annual vegetation, which exhibit superior growth and seed dispersal abilities, and, therefore, rapidly colonize open, grazed patches (Holmes and Rice, 1996; Bilota et al., 2007; Kioko et al., 2012). Moderate and light grazing, on the other hand, suppress dominant species and promote diversity, especially in productive ecosystems (Koerner and Collins, 2014). This may occur in the savannah in Lambwe, which is relatively productive (K'Otuto et al., 2012). At within patch-scale, grazing at moderate intensity can also promote plant diversity by reducing the intensity of competition for light (Bakker et al., 2003, Deng et al., 2014) and by opening colonizing windows due to gap creation. This enables the regeneration of gap opportunistic plant species (Kikoti et al., 2015).
The ecosystem in Lambwe valley in southwestern Kenya is predominantly savannah. It has experienced significant modification due to changing rainfall patterns and increased livestock grazing intensities (Njoka et al., 2003, Muriuki et al., 2005). The rainy periods have become shorter but more intense, while the dry seasons have become longer, with extended droughts (Njoka et al., 2003, K'Otuto et al., 2012). These changes are likely altering composition and richness of the herbaceous layer community. Because both livestock grazing and rainfall act simultaneously on the vegetation, this study sought to identify changes that occur in the herbaceous layer as a result of shifts in rainfall intensity and increased livestock grazing. We hypothesize that livestock grazing, changing rainfall regimes, and the interaction between these factors decrease the diversity and richness of the savannah herbaceous layer.
2. Materials and methods 2.1. Study siteThis study was conducted in Ruma (00°35′S, 34°12′E), located within the Lambwe valley in Homa Bay County, Suba District, western Kenya from 2014 to 2015. The elevation of the area is around 1300 m above sea level. The site was located on a north-facing slope at the foothills of the Gwasi massif, on land belonging to the Kenya National Youth (NYS). The climate is warm and humid, with a mean (2003–2013) annual air temperature of 22 ℃. In addition to the expansive savannah, with semi-natural vegetation, other land cover types include a conserved area within the Ruma National Park, human settlements, open cattle (cows, sheep, and goats) grazing fields, and seasonally cultivated crop fields (Maitima et al., 2010). The animal stocking rate is at 7.4 animal units ha−1. The mean annual rainfall (1993–2013) is 1100 mm, with a weak bimodal distribution pattern between April–June and September–November. January–March is usually the driest and hottest period of the year. Soils are shallow, stony, red-brown clay loams. The higher elevations support ferruginous tropical soils and holomorphic soils on rocks that are rich in ferromagnesian minerals. Mixed soil formations of red-brown friable clays, grey mottled clays, and gray compacted loamy sands predominate. Towards the valley bottom, the soils are largely black clays, i.e., "black cotton" (Arnhold et al., 2015). Soils here have a high mineral content and tend to be alkaline (Allsop and Baldry, 1972). Measurements were conducted on a 150-ha area of mainly rolling grassland, with tracts of open woodland and thickets dominated by Acacia, Combretum, Bridelia and Rhus and a wide diversity of herbaceous vegetation, dominated by the grasses Hyparrhenia filipendula and Bracharia decumbens. The area has a slight slope (3°).
2.2. MicroclimateDuring the experimental period, weather parameters were continuously monitored using an automatic weather station (AWS-GP1, Delta-T Devices, Cambridge, UK) installed within the study site in an open area to avoid interference from trees. Parameters that were continuously monitored included rainfall and air temperature. Measurements were taken every 5 min, and data averaged and logged half-hourly for a period of 2 years.
2.3. Experimental designThe experiments were set in a split-plot design, with three replicates of grazed and fenced areas as main plots, and rainfall manipulation splits that included ambient rainfall (control), 50% more rainfall, and 50% less rainfall. The split-plots were nested within the land use plots that were respectively grazed by livestock or fenced (2 m high perimeter fence since 2011) to exclude livestock. The grazed plot was an open savannah subjected to year-round livestock grazing since 2005. The rain manipulations were achieved by the construction of rain-out shelters above the herbaceous vegetation canopy. To exclude rainfall, bisections of the rain exclusion split plots were covered with transparent plastic gutters and inclined at 2° downslopes to re-direct 50% of the excluded rainfall to the split plots designated for more rainfall. Control plots received ambient rainfall. Each rainfall manipulation shelter measured 6 m by 3 m and were embedded on land use plots each measuring 70 m by 100 m. The land use plots were either grazed or fenced. Trenches, 30 cm deep, were dug around the plots and plastic gutters vertically inserted into the trenches to prevent surface runoff and lateral movement from the surrounding soil. Rain-out gutters were replaced every six months.
2.4. Soil water contentIn the herbaceous vegetation study plots, gravimetric soil water content(SWC) was determined monthly for a period of 2 years. Soil samples were taken using a 3-cm-diameter corer down to 30 cm. Each sample was immediately weighed before oven drying at 105 ℃ for 48 h and re-weighing. SWC was determined as the relative change in weight between fresh and dry soil samples (Brady and Weil, 2002).
2.5. Plant biomass determinationPlant biomass within the 40 cm by 40 cm frames was harvested monthly for the two-year study period. The harvested biomass was separated into live and dead biomass. Green standing plant material constituted live biomass, whereas brown standing and non-standing (on the ground/litter) plant material constituted dead biomass. The aboveground samples were oven-dried at 80 ℃ for 48 h, before determining their dry weight.
2.6. Vegetation composition and diversitySpecies composition within the herbaceous layer was assessed towards the end of the rainy season (June and November) in 2014 and repeated at the same period in 2015. The sampling time coincided with the floweringperiod of most herbaceous species, making their identification easier. Plant species composition was estimated by randomly establishing 3 separate 40 cm by 40 cm quadrats in each of the treatment plots. All the standing plant materials in the quadrats were identified and recorded. Individual species were further classified in terms of life-forms (i.e. annuals and perennials) and their palatability was determined from the literature (Van Oudtshoorn, 2002, Muyekho et al., 2004). Species counts were then used to calculate percentage cover; herbaceous diversity was determined using species richness (R) and Simpson's index (D) of diversity. Species richness was defined as the total number of species present in a particular sampling plot, which could also be referred to as diversity (Waite, 2000). Simpson's index of diversity was calculated as follows:
(D') = 1-D, where D = ∑(Pi)2 and Pi = ni/N. ni is the number of individuals of species in i and N is the total number of individuals in the sample. Simpson's index of diversity has a range of 0–1 where 1 represents maximum diversity.
2.7. Statistical analysisAll statistical analyses were performed using SAS software (version 9.1, California, USA). One way ANOVA was used to compare herbaceous vegetation variables (palatability and life form) within the research plots. Two-way ANOVA was used to test the effect of grazing and/fencing and rainfall manipulations on plant species diversity, composition, and dominance as measured by the Berger–Parker index. Tukey's t-test was used for mean comparison of the treatment plots. Data on biomass was compared using one-way ANOVA, with site as the fixed effect. Tukey-HSD post hoc tests for pairwise comparison of means was conducted with significance level of P < 0.05 (see Fig. 1).
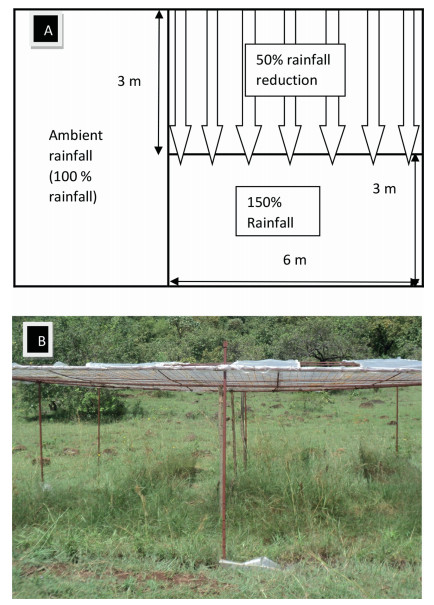
|
| Fig. 1 (A) The layout of rainfall manipulation plots. Parallel arrows show transparent rainout gutters used to simulate rainfall reduction and direction to which excluded rainfall flows (rainfall increment plot/150% rainfall). (B) Photograph of rainfall manipulation plot with a slanting roof partially covered by transparent rainout gutters to exclude 50% of the rainfall and redirect it to the plot designated to receive more rainfall (i.e., 150% rainfall). |
Rainfall was bimodal, occurring from April to June and September to December (Fig. 2). The total rainfall amounts in 2014 and 2015 were 1148.4 mm and 1169.5 mm, respectively. Gravimetric soil water contentwithin the 0–30 cm soil profile followed the rainfall pattern, increasing during the wet months and declining during the dry months (Fig. 2).
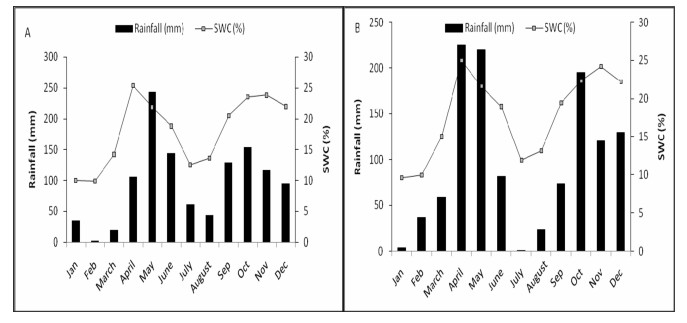
|
| Fig. 2 Mean monthly rainfall amount (mm) and soil water content (%) within 0–30 cm soil profile recorded in 2014 (A) and 2015 (B) when measurements were conducted. |
There were significant differences (P < 0.0001, F = 322.7) in total aboveground biomass between the plots (Table 1). The highest total aboveground biomass (1198.2 ± 78.4 g m−2) was recorded in the fenced plot while the lowest biomass (473.7 ± 23.8 g m−2) was recorded in the grazed plots. The highest standing (green) biomass recorded during the growing period (703.4 ± 50.7 g m−2) was in the fenced plot. A significantly higher (494.8 ± 27.7 g m−2) amount of dead biomass accumulated in the fenced plot compared to the grazed plot.
| Plant Biomass | Grazed plot | Fenced plot | |||||||
| Ambient rainfall | Reduced rainfall | Increased rainfall | Means biomass | Ambient rainfall | Reduced rainfall | Increased rainfall | Mean biomass | ||
| Green (g m−2) | 373.7 ± 51.9a | 210.0 ± 2 1b | 378.0 ± 21a | 320.6 ± 31.3a | 749.19 ± 9.9a | 561.7 ± 10.7b | 799.3 ± 9.9c | 703.4 ± 50.7a | |
| Dead (g m−2) | 196.4 ± 15.2a | 75.2 ± 6.1b | 217.6 ± 1.2b | 163.1 ± 7.5b | 521.1 ± 1.6a | 353.2 ± 2.5b | 610.1 ± 5.9c | 494.8 ± 27.7b | |
| Total (g m−2) | 570.1 ± 67.1a | 285.2 ± 27.1b | 595.6 ± 22.2b | 483.6 ± 38.8c | 1270.29 ± 11.5a | 914.9 ± 13.2b | 1409.4 ± 15.8c | 1198.2 ± 78.4c | |
Herbaceous diversity was high within the plots (Simpson's index 0.63–0.87; 3A and B). The herbaceous diversity in grazed plots was 19.6% higher than fenced plots (p = 0.021; 3A and B). When rainfall was reduced on grazed plots, herbaceous diversity decreased by 13.8% in comparison to ambient conditions (p = 0.079; 3C). The interaction between grazing and rainfall enhancement had no significant (p = 0.075) influence on diversity in comparison to ambient conditions. The interaction of grazing exclusion and rainfall treatments had no significant effects on diversity (p = 0.077; Fig. 3C). Vegetation dominance in fenced plots was 38.9% higher than grazed plots (p < 0.0005; 3 D). However, the interaction of grazing and rainfall manipulation did not have a significant effect on dominance.
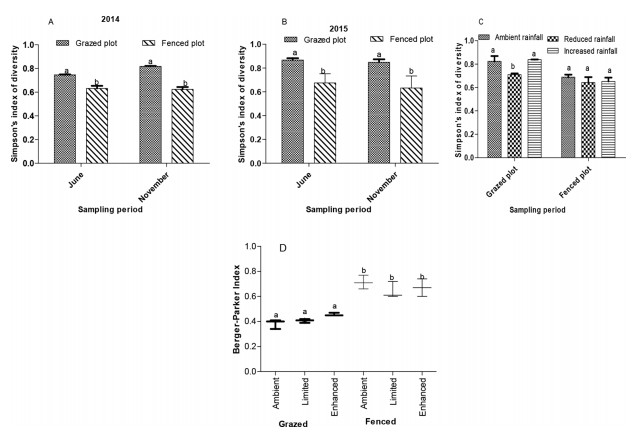
|
| Fig. 3 (A and B) Herbaceous plant diversity within grazed and fenced plots in 2014 and 2015 respectively. Interaction of grazing and rainfall manipulation on herbaceous plant diversity (C) and dominance (D) for the entire study period. Bars are means (±SD). Bars within plots not sharing the same letters are significantly different from each other (Tukey-LSD, p < 0.05). |
All the sites were dominated by perennial herbaceous species with few annuals. Percentage cover of perennials differed significantly (p < 0.05) between grazed (90.72 ± 1.95%) and fenced plots (96.67 ± 1.92%). There was a significant difference (p < 0.05) in the cover of annuals within grazed (9.23 ± 1.95%) and fenced plots (3.33 ± 1.92%). The coverage of palatable herbaceous species was higher in both grazed (87.89 ± 2.87%) and fenced plots (95.06 ± 3.82%), but the differences was not significant (p = 0.057).
3.5. Herbaceous plant species within grazed and fenced plotsList of herbaceous species and their percentage composition within grazed and fenced plots are shown in Table 2. A total of 29 and 25 species were recorded in the grazed and fenced plots respectively. Fenced plots were dominated by H. filipendula (52%) and Brachiaria decumbens (25%). The dominant vegetation in the grazed plots was Bothriochloa insculpta (28%) (see Table 3).
| Plant attributes | Grazed plots | Fenced plots | |||||
| Ambient rainfall | Reduced rainfall | Increased rainfall | Ambient rainfall | Reduced rainfall | Increased rainfall | ||
| Perennials (%) | 91.5 ± 2.4a | 92.2 ± 2.8a | 88.5 ± 3.8b | 98.5 ± 1.5a | 96.8 ± 4.7a | 94.7 ± 2.0b | |
| Annuals (%) | 8.5 ± 2.4a | 7.8 ± 2.7a | 11.5 ± 3.8b | 1.5 ± 1.5a | 3.2 ± 4.7a | 5.3 ± 2.0b | |
| Palatable (%) | 86.5 ± 11.2a | 91.2 ± 2.9b | 86.0 ± 1.6a | 96.8 ± 3.4a | 97.7 ± 2.3a | 90.67 ± 0.5b | |
| Grazed plots | |||
| Plant species | Life form | Palatability | % composition |
| Bothriochloa insculpta (A. Rich) A. Camus | Perennial | Palatable | 27.8 |
| Paspalum dilatatum Poir | Perennial | Palatable | 18.72 |
| Hyparrhenia fillipendula (Hochst) Stapf. | Perennial | Palatable | 11.83 |
| Sporobolus agrostoides Chiov. | Perennial | Unpalatable | 9.09 |
| Vernonia glabra (Steetz) Vatke | Perennial | Unpalatable | 5.41 |
| Justicia striata Vahl | Annual | Unpalatable | 3.92 |
| Brachiaria decumbens Stapf | Perennial | Palatable | 2.67 |
| Aspilia pluriseta Schweinf | Perennial | Unpalatable | 2.35 |
| Ipomoea tenuirostris Steud ex Choisy | Perennial | Palatable | 1.88 |
| Digitaria sanguinalis (L) Scop | Annual | Palatable | 1.73 |
| Cynodon dactylon (L) Pers | Perennial | Palatable | 1.73 |
| Barleria acanthoides Vahl. | Perennial | Palatable | 1.34 |
| Triumphetta rhomboidea Jacq. | Perennial | Palatable | 1.26 |
| Indigofera arrecta Hochst ex. A. Roch. | Perennial | Palatable | 1.18 |
| Urena lobata L. | Annual | Palatable | 1.1 |
| Hypoestes aristata Soland ex Roem & Schalt | Perennial | Palatable | 1.1 |
| Sida acuta Burm. F | Perennial | Unpalatable | 1.1 |
| Waltheria indica Bak. | Perennial | Unpalatable | 0.94 |
| Striga asiatica (L) Kuntze | Annual | Unpalatable | 0.71 |
| Euphorbia hirta Linn. | Annual | Unpalatable | 0.71 |
| Rhynchosia minima (L.) DC. | Perennial | Palatable | 0.47 |
| Cajanus cajan L. Millsp | Perennial | Palatable | 0.47 |
| Hoslundia opposita Vahl. | Perennial | Unpalatable | 0.47 |
| Sonchus schweinfurthii Oliv. | Perennial | Palatable | 0.47 |
| Sphaeranthus suaveolens (Forsk) DC | Perennial | Palatable | 0.47 |
| Solanum incanum Linn. | Perennial | Unpalatable | 0.47 |
| Desmodium gangeticum (L.) D.C. | Perennial | Palatable | 0.24 |
| Hypoestes forskaolii (Vahl) R.Br. | Annual | Unpalatable | 0.24 |
| Indigofera brevicalyx Bak. | Perennial | Unpalatable | 0.24 |
| Fenced plots | |||
| Hyparrhenia filipendula (Hochst) Stapf. | Perennial | Palatable | 51.97 |
| Brachiaria decumbens Stapf | Perennial | Palatable | 24.92 |
| Aspilia pluriseta Schweinf | Perennial | Unpalatable | 3.11 |
| Triumphetta rhomboidea Jacq. | Perennial | Palatable | 2.42 |
| Justicia striata Vahl | Annual | Unpalatable | 2.35 |
| Vernonia glabra (Steetz) Vatke | Perennial | Unpalatable | 1.73 |
| Ipomoea tenuirostris Steud ex Choisy | Perennial | Palatable | 1.59 |
| Desmodium gangeticum (L.) D.C. | Perennial | Palatable | 1.45 |
| Barleria acanthoides Vahl. | Perennial | Palatable | 1.25 |
| Cajanus cajan L. Millsp | Perennial | Palatable | 1.11 |
| Rhynchosia minima (L.) DC. | Perennial | Palatable | 1.04 |
| Themeda triandra Forssk | Perennial | Palatable | 0.97 |
| Hypoestes aristata Soland ex Roem & Schalt | Perennial | Palatable | 0.76 |
| Urena lobata L. | Annual | Palatable | 0.63 |
| Hoslundia opposita Vahl. | Perennial | Unpalatable | 0.56 |
| Cynodon dactylon (L) Pers | Perennial | Palatable | 0.56 |
| Paspalum dilatatum Poir | Perennial | Palatable | 0.49 |
| Hypoestes forskaolii (Vahl) R.Br. | Annual | Unpalatable | 0.49 |
| Leonotis nepetifolia (L) R.Br. | Annual | Palatable | 0.42 |
| Digitaria sanguinalis (L) Scop | Annual | Palatable | 0.42 |
| Indigofera arrecta Hochst ex. A. Roch. | Perennial | Palatable | 0.42 |
| Ocimum kilimandscharicum Guerke | Perennial | Palatable | 0.42 |
| Bothriochloa insculpta (A. Rich) A. Camus | Perennial | Palatable | 0.35 |
| Panicum maximum Jacq. | Perennial | Palatable | 0.35 |
| Lantana trifolia L. | Annual | Unpalatable | 0.35 |
Higher species diversity in our grazed plots (Fig. 3A and B) is a result of reduced vegetation dominance (Fig. 3D). Decreased dominance of vegetation can be linked to improved availability of resources, such as light, nutrients, and water. With the enriched resources, diversity is often improved through the proliferation of less common species, colonisation of new species and/or a decrease in local species extinctions (Olff and Ritchie, 1998). By feeding and trampling on dominant vegetation, grazers alter the competitive interactions within the grass layer by reducing the vigor and presence of dominant plants, consequently enabling the establishment of less competitive species, which in the long run increases diversity (Pekin et al., 2014). During grazing, cattle introduce new plant propagules in the environment through their droppings; these propagules later grow into new vegetation and improve diversity within the savannah (Fynn et al., 2016). Grazing disturbance of herbaceous canopies likely increase plant diversity by promoting colonization of ruderal species (Huston, 1979, Bakker et al., 2006). Grazing also promotes the growth of forb species leading to a relatively high species-rich community in semiarid savannahs (Jacobs and Naiman, 2008). Our finding that there is a positive relationship between livestock grazing and plant diversity is in agreement with those of Hanke et al. (2014). Our results, however, contradict previous studies that examined less productive savannahs with mean annual precipitations less than 600 mm (Lechmer-Oertel et al., 2005, Rutherford et al., 2012, Scott-Shaw and Morris, 2015). One possible interpretation of these contradictory results is that grazing has a positive influence on diversity only in productive ecosystems with mean annual rainfalls of greater than 600 mm. For example, the Lambwe savannah plots we studied lie on a humid savannah with a mean annual rainfall of about 1100 mm. In unproductive ecosystems with limited soil resources, grazing reduces plant diversity by eliminating rare palatable species and trampling on plants, which often do not recover from such impacts (Lezama et al., 2014).
In this experiment, fencing reduced grazing disturbance and allowed a few species to develop large local populations with more biomass (Table 1 and Fig. 3D), i.e., become dominant. The dominant vegetation in the fenced plots out-competed other less colonising species for canopy resources (i.e light) (Thirgood, 2009). A considerable number of species with lower competitive abilities reduce their density or diminish in plant communities because of competition for light resources and nutrients (Grime, 1998; der Wal et al., 2004). This is in agreement with competitive exclusion theory which states that at high levels of biomass, dominant species tend to outcompete other species for resources (Grime, 1973; Abrams, 1995 and Jacobs and Naiman, 2008). Competitive exclusion leads to the dominance of a few species and causes an increase in spatial homogeneity and a decrease in species diversity. Additionally, higher dead biomass accumulation in the fenced plots (Table 1) may be the reason for lower diversity in the plots. Accumulated litter may limit seedling emergence and growth with regard to forming a mechanical barrier, reducing the light radiation to the soil surface or possibly releasing toxic secondary metabolites that ultimately lower diversity (Zhu et al., 2012, Xiong et al., 2016). These results are in agreement with a number of field studies in African savannahs, which have found that exclusion of grazing has negative impacts on herbaceous plant diversity through the build-up in biomass and promotion of coloniser or competitive species (Jacobs and Neiman, 2008, Hanke et al., 2014, Van Coller and Siebert, 2014).
4.2. Interaction of grazing and rainfall treatment on plant diversityGrazing and water availability are likely the key drivers of species change in grazed savannahs and other similar ecosystems (Bat-Oyun et al., 2016). In this study, we expected that the interaction of grazing and reduced rainfall would decrease species diversity (Fig 3C). These results maybe a consequence of direct and indirect effects of both the abiotic (rainfall manipulation, i.e. reducing ambient rainfall) and biotic (grazing) factors. As reduced rainfall treatment decreased rather than increased resources, we could expect a reduction in diversity, consistent with observations in low productivity ecosystems (Ren et al., 2012). Grazing in water-limited environments tends to increase plant mortality and ultimately decrease species richness and diversity (Proulx and Mazumder, 1998, Fynn andO'Connor, 2000). Grazing reduces vegetative biomass and exposes soil to direct radiation and therefore warming which is further aggravated by the reduction in rainfall which would otherwise dampen the increased soil temperatures. Such warming negatively impacts diversity either directly through species-specific physiological responses, such as heat stress, or through ecological factors such as altered species interactions (Farrer et al., 2014). At lower soil moisture levels, stimulated by reducing ambient rainfall in grazed plots, biodiversity was predicted to decrease due to herbivore grazing and an increase in dominance by drought tolerant species; together, these two factors may reduce colonisation rates or enhance extinction of species which are less tolerant to grazing and low moisture levels (Olff and Ritchie, 1998, Bat-Oyan et al., 2016). The interaction between fencing that excluded grazing and either increased or decreased ambient rainfall did not have a significant effect on species diversity (Fig. 3C). This response points to the fact that the altered ambient rainfall in our fenced plots did not significantly change soil moisture levels to the extent that it would elicit change in plant diversity. Fencing improves soil macro-aggregation, which prevents water loss and ensures adequate soil moisture supply irrespective of the rainfall treatment. One possible explanation of our results is that species in our fenced plots are inherently less sensitive to the rainfall manipulations as used in this study. The mechanisms of species change might be largely site-specific. Furthermore, they may vary significantly in water-limited ecosystems similar to our grazed plots but dissimilar to our fenced plots.
4.3. Characteristics of herbaceous vegetation within grazed and rainfall treatment plotsThe composition of herbaceous vegetation varied considerably within our study sites irrespective of rainfall treatment, which characterises the heterogeneity of Lambwe valley (Allsopp and Baldry, 1972). Spatial variation in the composition of herbaceous species may be attributed to the ability of individual species to adapt to local and edaphic conditions (Silva et al., 2013, Augustine, 2003), which are different within our sites (Otieno et al., 2011). It is possible that the lower vegetation dominance in the grazed plots (Fig. 3D) was as a result of reduced competition for light, allowing different species to flourish. Grazing at moderate intensities depresses the vigor and presence of dominant species, enabling colonisation by less competitive species with an overall increase in diversity (Kikoti and Mlingo, 2015). These results are similar to the findings of Zerbo et al. (2016) who linked grazing to the reduction in vegetation dominance in West African savannah ecosystems. Our results reveal an abundance of palatable species (Table 2). Moreover, perennialspecies dominated all plots irrespective of the rainfall treatment (Table 2). The higher dominance of palatable species within our plots can be attributed to a decline in selective grazing which minimises overconsumption of palatable plants. Since the abundance of palatable species appears to be an indicator of moderate grazing (Ren et al., 2012), we can conclude that grazing within our plots is within a sustainable range that does not necessarily lead to ecosystem degradation. The interaction of grazing and rainfall manipulation is complex and requires additional survey campaigns to create a complete picture of its implications on savannah structure and composition.
None declared.
We thank the Kenya National Youth Service (NYS) Lambwe Unit for allowing us to conduct experiments on their land. We are grateful to International Foundation for Research Fund (IFS) under grant number I-1-D-6174-1 and National Research Fund, Kenya (NRF; 2016/17 FY) for funding this research.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2019.04.005.
Abrams A., 1995. Monotonic or unimodal diversity-productivity gradient:What does competition theory predict?. Ecology, 76(7): 2019-2027. DOI:10.2307/1941677 |
Allsopp R., Baldry T., 1972. A general description of the Lambwe valley area of south Nyanza district, Kenya. World Health Organisat. Bull, 47: 691-697. |
Arnhold S., Otieno D., Onyango J., Koeller T., Huwe B., Tenhunen J., 2015. Soil properties along a gradient from hillslopes to the savanna plains in the Lambwe Valley, Kenya. Soil Tillage Res, 154: 75-83. DOI:10.1016/j.still.2015.06.021 |
Augustine J., 2003. Spatial heterogeneity of herbaceous layer of a semi-arid savannah ecosystem. Plant Ecol, 167: 319-332. DOI:10.1023/A:1023927512590 |
Bakker C., Blair J., Knnap A., 2003. Does resource availability, resource heterogeneity or species turnover mediate changes in plant species richness in grazed grassland?. Oecologia, 137: 385-391. DOI:10.1007/s00442-003-1360-y |
Bakker E., Ritchie M., Olff H., Milchunas D., Knops J., 2006. Herbivore impact on grassland plant diversity depends on habitat productivity and herbivore size. Ecol. Lett, 9: 780-788. DOI:10.1111/j.1461-0248.2006.00925.x |
Bat-Oyan T., Shinoda M., Cheng Y., Purevdorj Y., 2016. Effect of grazing and precipitation variability on vegetation dynamics in a Mongolian dry steppe. J. Plant Ecol, 9(5): 508-519. DOI:10.1093/jpe/rtv083 |
Baudena M., Dekker S., Bodegom P., Cuesta B., Higgins S., Lehsten V., Reick C., Rietterk M., Yin Z., Zavala M., Brovkin V., 2015. Forests, savannas, grasslands:bridging the knowledge gap between ecology and Dynamic Vegetation Models. Biogeosciences, 12: 1833-1848. DOI:10.5194/bg-12-1833-2015 |
Bilota G., Brazier R., Haygarth P., 2007. The impact of grazing animals on the quality of soils, vegetation, and surface waters in intensively managed grasslands. Adv. Agron, 94: 237-280. DOI:10.1016/S0065-2113(06)94006-1 |
Brady, N., Weil, R., 2002. The Nature and Properties of Soils, 13th Ed. Prentice-Hall Inc, New Jersey, USA, p. 960.
|
Conway D., Allison E., Felstead R., Goulden M., 2005. Rainfall variation in East Africa:implication for natural resources management and livelihood. Phil.Trans, 363: 49-54. DOI:10.1098/rsta.2004.1475 |
Deng L., Sweeney S., Shangguan Z., 2014. Grassland responses to grazing disturbance:plant diversity changes with grazing intensity in a desert steppe. Grass Forage Sci, 69: 524-533. DOI:10.1111/gfs.12065 |
Farret E., Ashton J., Suding N., 2014. Separating direct and indirect effects of global change:a population dynamic modelling approach using readily available field data. Glob. Chang. Biol, 20: 1238-1250. DOI:10.1111/gcb.12401 |
Fynn R., O'Connor T., 2000. Effect of stocking rate and rainfall on rangeland dynamics and cattle performance in a semiarid savannah, South Africa. J. Appl.Ecol, 37: 491-507. DOI:10.1046/j.1365-2664.2000.00513.x |
Fynn S., Augustine J., Peel S., de Garine-Wichatitsky Michael, 2016. Strategic management of livestock to improve biodiversity conservation in African savannahs:a conceptual basis for wildlife-livestock coexistence-A review. J. Appl. Ecol, 53: 388-397. DOI:10.1111/1365-2664.12591 |
Grime J., 1973. Control of species density in a herbaceous vegetation. J. Environ.Manag, 1: 151-167. |
Grime J., 1998. Benefits of plant diversity to ecosystems:immediate, filter and founder effects. J. Ecol, 86: 902-910. DOI:10.1046/j.1365-2745.1998.00306.x |
Hanke W., Bohner J., Dreber N., Jurgen N., Schmiedel U., Wesuls D., Dengler J., 2014. The impact of livestock grazing on plant diversity:an analysis across dryland ecosystems and scales in South Africa. Ecol. Appl, 24(5): 1188-1203. DOI:10.1890/13-0377.1 |
Holmes T., Rice K., 1996. Patterns of growth and soil-water utilization in some exotic and native perennial bunch grasses of California. Ann. Bot, 78: 233-243. DOI:10.1006/anbo.1996.0117 |
Hoover D., Knapp K., Smith D., 2014. Resistance and resilience of grassland ecosystem to climate extremes. Ecology, 95: 2646-2656. DOI:10.1890/13-2186.1 |
Huston M., 1979. A general hypothesis of species diversity. Am. Nat, 113: 81-101. DOI:10.1086/283366 |
Jacobs S., Neiman R., 2008. Large African herbivores decrease herbaceous plant biomass while increasing plant species richness in a semiarid savannah toposequence. J. Arid Environ, 72: 891-903. DOI:10.1016/j.jaridenv.2007.11.015 |
Ji L., Peters A., 2013. Assessing vegetation response to drought in the northern Great Plains using vegetation and drought indices. Remote sensing environments. Elsevier, 87(1): 1555-1570. |
K'Otuto G., Otieno D., Seo B., Ogindo H., Onyango J., 2012. Carbon dioxide exchange and biomass productivity of the herbaceous layer of a managed tropical savanna ecosystem in western Kenya. J. Plant Ecol, 6(4): 286-297. |
Kikoti A., Mlingo C., 2015. Impact of livestock grazing on plant species composition in a montane forest of the northern slope of Mount Kilimanjaro, Tanzania. Int. J.Biodivers. Sci. Ecosyst. Serv. Manag, 11: 114-127. DOI:10.1080/21513732.2015.1031179 |
Kikoti A., Mligo C., Kilemo B., 2015. The impact of grazing on plant natural regeneration in Northern Slopes of Mount Kilimanjaro, Tanzania. Open J. Ecol, 5: 266-273. DOI:10.4236/oje.2015.56021 |
Kioko J., Kiringe J., Seno S., 2012. Impact of livestock grazing on savanna grassland in Kenya. J. Arid Land, 4(1): 29-35. DOI:10.3724/SP.J.1227.2012.00029 |
Koerner S., Collins S., 2014. Interactive effects of grazing, drought and fire on grassland plant communities in North America and South Africa. Ecology, 95: 98-109. DOI:10.1890/13-0526.1 |
Lechmer-Oertel R., Kerley G., Cowling R., 2005. Patterns and implications of transformation in semi-arid succulent thicket, South Africa. J. Arid Environ, 63: 459-474. |
Lezama F., Baeza S., Altesor A., Cesa A., Chaneton J., Paruelo M., 2014. Variation of grazing induced vegetation changes across a large-scale productivity gradient. J. Veg. Sci, 25: 8-21. DOI:10.1111/jvs.12053 |
Lovette J., Midgley P., Barnard B., 2005. Climate change and ecology in Africa. Afr. J.Ecol, 43: 279-281. DOI:10.1111/j.1365-2028.2005.00608.x |
Maitama J., Oslon J., Mugatha S., Mugisha S., Mutie I., 2010. Land use changes, impacts and options for sustaining productivity and livelihoods in the basin of Lake Victoria. J. Sustain. Dev. Afr, 12: 189-206. |
Mureithi S., Verdoodt A., Njoka T., Gachene K., Warinwa F., Ranst E., 2014. Impact of community conservation management on herbaceous layer and soil nutrients in a Kenyan semi-arid savannah. Land Degrad. Dev, 10: 1002-1015. |
Muriuki G., Njoka T., Reid R., Nyariki D., 2005. Tsetse control and landuse change in Lambwe valley, south western Kenya. Agric. Ecosyst. Environ, 106: 99-107. DOI:10.1016/j.agee.2004.04.005 |
Musau J., Patil S., Sheffield J., Marshall M., 2016. Spatio-temporal vegetation dynamics and relationship with climate over East Africa. Hydrol. Earth Syst, 10: 502-519. |
Muyekho, F., Barrison, A., Khan, Z., 2004. A Primer on Grass Identification and their uses in Kenya. Communication Limited, Nairobi, Kenya.
|
Njoka T., Muriuki G., Reid R., Nyariki D., 2003. The use of sociological methods to asses land use change:a case study of Lambwe valley, Kenya. Int. J. Sociol, 7(3): 181-185. |
Olff H., Ritchie M., 1998. Effect of herbivores on grassland plant biodiversity. Trends Ecol. Evol, 13: 261-265. DOI:10.1016/S0169-5347(98)01364-0 |
Otieno D., K'Otuto G., Jakli B., Maina J., Jung E., Onyango J., 2011. Spatial heterogeneity in ecosystem structure and productivity in a moist Kenyan savanna. Plant Ecol, 212: 769-789. DOI:10.1007/s11258-010-9863-1 |
Pekin, B., Wisdom, M., Endress, B., Neylor, B., Parks, C., 2014. Ungulate browsing maintains shrub diversity in the absence of episodic disturbance in seasonallyarid conifer forest. PLoS One 9, e86288. https://doi.org/10.1371/journal.Prone.0086288.
|
Proulx M., Mazumder A., 1998. Reversal of grazing impact on plant species richness in nutrient-poor vs nutrient rich ecosystems. Ecology, 79: 2581-2591. DOI:10.1890/0012-9658(1998)079[2581:ROGIOP]2.0.CO;2 |
Ren H., Schonbach P., Wan H., Gierus M., Taube, 2012. Effect of grazing intensity and environmental factors on species composition and diversity in Typical Steppe of Inner Mongolia, China. PLoS One, 7(12): E52180. DOI:10.1371/journal.pone.0052180 |
Rutherford M., Powrie L., Husted L., 2012. Herbivore-driven land degradation:consequences for plant diversity and soil in arid subtropical thicket in south eastern African savannah. Nat. Lett, 438: 846-849. |
Schieltz M., Rubenstein I., 2016. Evidence based review:positive versus negative effects of livestock grazing on wildlife. What do we really know? Environ. Res.Lett, 11: 113003. |
Scott-Shaw Morris, 2015. Grazing depletes forbs species diversity in mesic grasslands of KwaZulu-Natal, South Africa. Afr. J. Range Forage Sci, 32: 21-31. DOI:10.2989/10220119.2014.901418 |
Serdeczny O., Adams S., Baarsch F., Coumou D., Robinson A., Hare W., Schaeffer M., Perrette M., 2015. Climate change impact in Sub-Saharan Africa:from physical changes to their social repercussions. Region. Clim. Change, 15: 8-21. |
Silva K., Santos J., Santos D., Ferraz E., Aruaj E., 2013. Spatial variation in structure and composition of the herbaceous community in a semiarid region of northeastern Brazil. Braz. J. Biol, 73: 135-148. DOI:10.1590/S1519-69842013000100015 |
Ssemmanda I., Gelorini V., Verschuren D., 2014. Sensitivity of East African savannah vegetation to historical moisture-balance variation. Clim. Past, 10: 2067-2080. DOI:10.5194/cp-10-2067-2014 |
Thirgood S., 2009. Effect of fencing and grazing on a kobresia-dominated meadow in the Quinghai Plateau. Plant Soil, 319(1-2): 115-126. DOI:10.1007/s11104-008-9854-3 |
Van Coller Siebert F., 2014. Herbaceous biomass-species diversity relationship in nutrient hotspots of a semi-arid African riparian ecosystem. Afr. J. Range Forage Sci, 119: 1-11. |
Van der Plas A., Zeinstar P., Veldhuis M., Fokkema R., Tielens E., Howinson R., Olff H., 2013. Response of savannah lawn and bunch grasses to water limitation. Plant Ecol, 214(9): 1157-1168. DOI:10.1007/s11258-013-0240-8 |
Van Oudtshoorn, F., 2002. Guide to Grasses of South Africa. BRIZA Publication, Pretoria, South Africa.
|
Waite, S., 2000. Statistical Ecology in Practice; A Guide to Analysing Environmental and Ecological Field Data. Prentice Hall, England.
|
Wang W., Wang J., Li S., Wu H., Liu T., 2010. The impact of sustained drought on vegetation ecosystem in South West China based on remote sensing. Proc.Environ. Sci, 2: 1679-1691. DOI:10.1016/j.proenv.2010.10.179 |
Western D., Mose V., Worden J., Maitumo D., 2015. Predicting extreme drought in savannah Africa:a comparison of proxy and direct measures in detecting biomass fluctuations, trends and their causes. PLoS One, 10(8): 1371-1384. |
Xiong D., Shi P., Zhang X., Zou B., 2016. Effects of grazing exclusion on carbon sequestration and plant diversity in grassland of China. A meta-analysis. Ecol.Eng, 94: 647-655. |
Zerbo I., Markus B., Ouedraogo O., Hahn K., Thiombiano A., 2016. Effects of climate and landuse on herbaceous species richness and vegetation composition in West Africa Savanna Ecosystem. J. Bot, 10: 1155-1166. |
Zhu X., Jia H., Jiang P., Zhao C., Hu Y., Cao Y., 2012. Effects of enclosure on plant diversity and community characteristics of pasture of Middle Tianshan Mountain. Pratacult. Sci, 29: 989-992. |



