b. Steinmann Institute, Bonn University, Nussallee 8, Bonn, D-53115, Germany;
c. Senckenberg Research Institute and Natural History Museum/Biodiversity and Climate Research Centre, Senckenberganlage 25, Frankfurt, D-60325, Germany;
d. Xishuangbanna Tropical Botanical Garden of Chinese Academy of Sciences(CAS), Menglum, Mengla, Yunnan 666303, China
The geological climate and vegetation of Kazakhstan were completely different from present conditions. The present-day flora of Kazakhstan is dominated by steppe and desert vegetation due to a continental and arid climate. Only the northern part of the country has forests, covering less than 2% of the entire area. Until the Neogene, Kazakhstan and adjacent areas were covered by different forest types with a high taxa diversity now known as the Turgai flora. The taxonomic diversity of the Turgai flora was lost, however, due to the evolution of paleogeographical and paleoclimatological conditions (Borsuk, 1935, Kornilova and Lavrov, 1949).
The immediate predecessor of the Turgai type vegetation was the so-called Poltavian type flora. The term was used first by A. N. Kryshtofovich in 1955. This type of flora included the most typical "Poltavian" elements, Dryophyllum spp., which are not found in deposits dated later than the Eocene. Other characteristic taxa included Lauraceae, Sapindaceae, Arecaceae and other subtropical plants (Averyanova, 2012, Budantsev, 2017, Iljinskaja, 1986; Iljinskaja, 1986), sometimes in considerable abundance. From the Eocene - Oligocene boundary, forests composed of temperate broadleaved taxa began replacing ancient subtropical vegetation until the total elimination of the "Poltavian" elements at the end of the Oligocene. These forests generally represented the typical Turgai flora type. This floristic concept was introduced by Kryshtofovich (1955) and primarily implied its geographical distribution. However, in more recent studies, the Turgai flora has been treated as an ecological flora type (Dorofeev, 1963, Akhmetjev, 1985, Akhmetjev and Iljinskaja, 1989, Bruch and Zhilin, 2007; Tarasevich and Tropina, 2017, Popova et al., 2018).
The composition of the Turgai type flora varied over time in different areas. The early Oligocene vegetation of Kazakhstan does not represent typical Turgai flora. During the early Oligocene, thermophilic taxa such as Myrica spp., Pistacia sp., Apocynophyllum helveticum, Macclintokia sp., and taxa with coriaceous evergreen leaves of unclear affinity still appeared in the western part of Kazakhstan (Zhilin, 1984). Oligocene floras of Eastern Kazakhstan still require further investigation. According to Iljinskaya (1991) and Akhmetjev (1985) and Akhmetjev and Iljinskaja (1989), this territory was covered by non-typical Turgai type vegetation in the absence of Taxodiaceae and the presence of species of Cocculus, Comptonia, Pistacia, Platanus, and Eucommia. Nevertheless, typical families of Turgai type such as Betulaceae, Salicaceae, and Fagaceae also existed in Eastern Kazakhstan.
The Turgai type flora became prominent during the Aquitanian in Kazakhstan, the south of Western Siberia, Bashkiriya (Bashkortostan Republic, Russia), and the north-western part of Uzbekistan (Bruch and Zhilin, 2007). Although Turgai type vegetation is characterized by modern genera, their present-day distribution differs from their distribution in the past. For example, the genera composition of the Turgai flora type (Metasequoia, Sequoia, and Taxodium) does not currently grow in the same plant associations. Nevertheless, being displaced during the Neogene by climate change and an increase in continentality by the herbaceous vegetation, the woody components of Turgai flora gave rise to many present taxa of adjacent areas. According to Kryshtofovich, 1946, Kryshtofovich, 1955, these components served as a basis of the modern East Asian flora. Therefore, understanding the evolution of the Turgai type flora is crucial to understanding the modern temperate vegetation of the Northern Hemisphere.
The studies on the Aquitanian floras of Kazakhstan, which were started at the beginning of the 20th century, were prompted by the discovery of a new locality on the Ashutas mountain in the Zaisan depression (Eastern Kazakhstan) by geologist V. V. Rezhnitschenko in 1903. Fossil plants have been collected from the Oshaganda suite (including the Ashutas flora bearing horizons) and were first described by Neiburg (1928). More comprehensive investigations of this collection were carried out by Kryshtofovich et al. (1956). In the early 1960s, many studies on floras of Western Kazakhstan (northern Ustyurt), including early Miocene collections, were conducted. The research of Merklin (1962) and Korobkov (1967) played an important role in adding new knowledge to the paleobotanical history of Kazakhstan. More than 18 local Aquitanian floras were discovered at that time. On the basis of these collections and newly described floras, Zhilin, 1974, Zhilin, 1984 noted key floras of Mynsualmas, Kyntyktsche, and Kinjak localities from Western Kazakhstan and considered these floras as potential standards for dating the Aquitanian period. These floras were related to the Baygubek suite, which was dated by marine biostratigraphy [mollusca, sharks (Orlov, 1939)]. Thus far, most Turgai floras of Kazakhstan have been described based on fossil leaves and pollen records.
In this paper, we aimed to better understand the origin of the temperate Turgai type flora. To achieve this goal, we analyzed the carpology and palynology of the Kumyrtas flora collected from a flora-bearing horizon of the regional coal-bearing Zhilanchik site, which is dated to the Aquitanian. Specifically, we dated and identified species based on pollen, fossil fruits and fossil seeds. We then used pollen records to reconstruct the paleoclimate of the Kumyrtas flora. We also classified Plant Functional Types to characterize palaeovegetation patterns. The carpological data presented in the frame of this study is of special interest as it complements our knowledge on the composition of the studied flora, in particular, with respect to local herbaceous components.
2. Materials and methods 2.1. Stratigraphical position of the Kumyrtas flora and age determinationThe Kumyrtas locality is situated on the Turgai Plateau in the central part of Kazakhstan (Kostanay district, coordinates: 49°47′N 64°59′E, cf. Fig. 1). It was discovered in 1995 during a paleobotanical expedition led by S. Zhilin. The Turgai Plateau is characterized by a wide distribution of Oligocene to Miocene sediments deposited in the Cenozoic Turgai Depression. The source area of these dominantly alluvial deposits are the southern Urals and Mugodzhar, the southern foothills of the Ural Mountains in Kazakhstan. The eastern and western parts of the Turgai Plateau differ in sedimentary facies related to a different tectonic regime and basin subsidence at that time, leading to the deposition of coarser-grained clastics to the west and clays and lignites in the study area to the east (Boytsova et al., 1956).
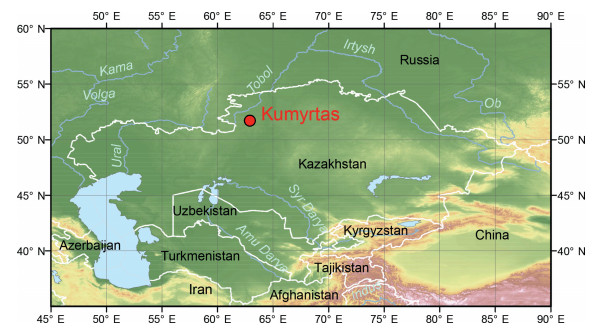
|
| Fig. 1 Study area and position of the Kumyrtas locality. |
The lithological description of the Kumyrtas section is taken from unpublished records made at the site of the paleobotanical excavation.
The Q4 Layer is represented by light gray sandy loam, penetrated by plant roots.
The Q1-3 layer is represented by light gray aleuritic sands with ferruginous spots.
The N2 layer is represented by light brown cross-bedded sand, 2.5–3.5 m in thickness, with up to 20 cm iron-rich interlayers and lenses of white sand. The N1 layer is represented by cross-laminated sands with a thickness of up to 6 m, with alternating layers of light brown and light-yellow sands, and a clay layer at the base of the unit. Approximately 60 cm below the top of this layer, a carbonaceous plant-bearing lens, with a horizontal extension of about 20 m occurs which contains the fossil flora. This coal-bearing horizon refers to the regional Zhilanchik coal-bearing stratum of the Uly-Zhilanchik River Basin (Zhilin, 1974), especially to its upper part, dated to the Aquitanian (Fig. 2). A total of 15 samples were selected from this horizon for carpological and palynological analyses.
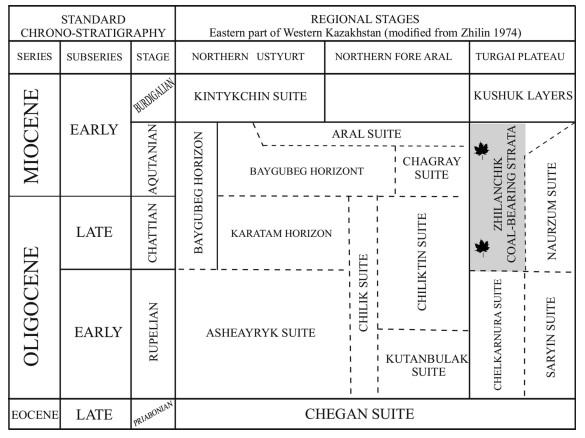
|
| Fig. 2 Regional stratigraphical chart of Kazakhstan; grey color marks the flora-bearing formation from which the studied samples were collected. |
The age determination of the Kumyrtas flora was based on the comparison with fossil plant remains from well-dated palaeofloras of the adjacent areas. The rich and well-dated flora of Nausha locality (Turgai Plateau) (Kornilova, 1960) showed the highest similarity to the Kumyrtas flora. The Nausha flora lays in the Aquitanian part of the Zhilanchik coal-bearing layer (Fig. 2, modified from Zhilin, 1974). The age determination of this flora was also supported by the swamp rhinoceros remains from the upper part of this fauna-bearing horizon (Reshetov, 1971; Zhilin, 1974). This horizon is comparable to the so-called Baigubeck layers of the northern Ustyurt Plateau, which are well-dated by mollusc paleofauna records.
2.2. Palaeobotanical methodsPalynological and carpological analyses were performed for the samples taken from the same layer.
The modernized hydrofluoric method using sieve systems (Pokrovskaya, 1950, Raevskaya and Shurekova, 2011) was carried out for two selected pollen samples. A total of 270 pollen grains were extracted from the studied sample. Pollen analysis was performed in the palynological laboratory of All-Russia Petroleum Research Exploration Institute (VNIGRI) (see Plate 2, Plate 3, Plate 4, Plate 5, Plate 6, Plate 7, Plate 8).
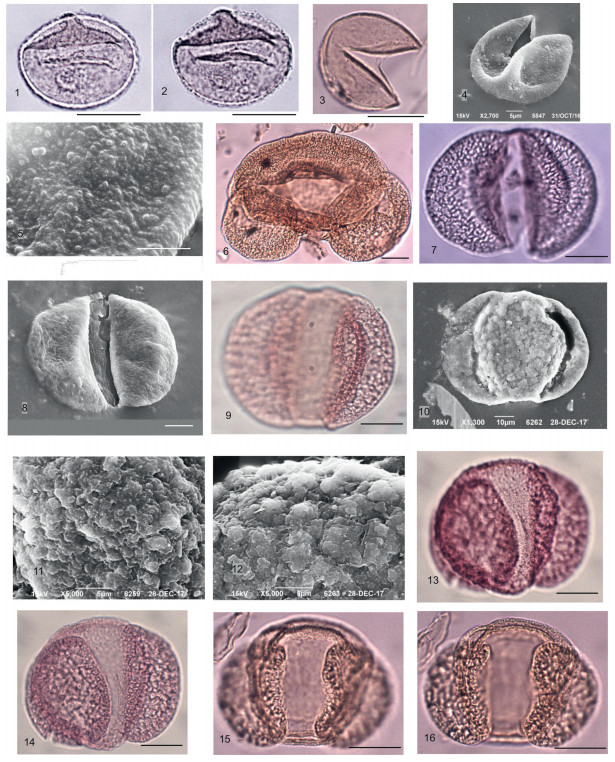
|
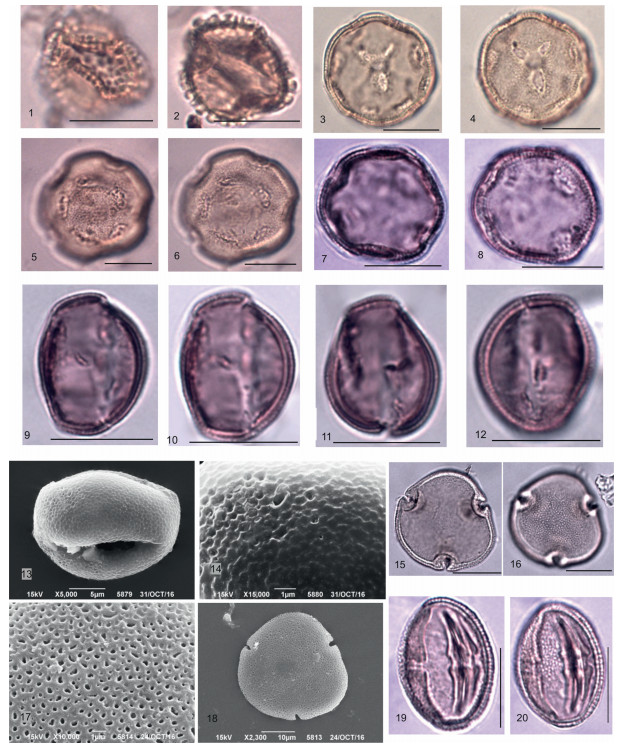
| Plate 2 Scale bar is 10 μm. 1, 2 – Sequoia sp.; 3 – Taxaceae; 4, 5 – Taxodiоideae (Cupressaceae) (4, 5 – microgranulate sculpture SEM); 6 – Abies cristata Anan., CМ; 7, 8 – Сathaya sp. (7 – general view, LM, 8 – distal view, SEM); 9–12 – Pinus bayleianaTrav. (9 – general view, LM; 10 – general view, 11, 12 –distinct coarsely tuberculate sculpture of cappa, SEM); 13–16 – Pinus spр. subgenus Diploxylon, LM. |
|
| Plate 3 Scale bar is 10 μm. 1–3 – Keteleeria microreticulata Anan. (1 – general view), LM; 2 – general view, SEM; 3 –granulate-tuberculate cappa sculpture (proximal view), merged tubercles with indistinct borders, SEM; 4–8 – Pseudotsuga magna(R.Pot.)Anan. (4, 5 – general view, LM; 6 – general view, 7, 8 – surface covered by orbicules, SEM); 9–12 – Tsuga macroserrata (Wulff)Anan. (9 – general view, LM, 10 – general view, 11– verrucate -rugulate sculpture, 12 – rugulate sculpture, SEM); 13–15 – Tsuga aculeata Anan. (13 – microechinate surface of body, long spines with narrow base, 14 – general view, 15 – echinate sculpture of the frilled area, SEM). |

|
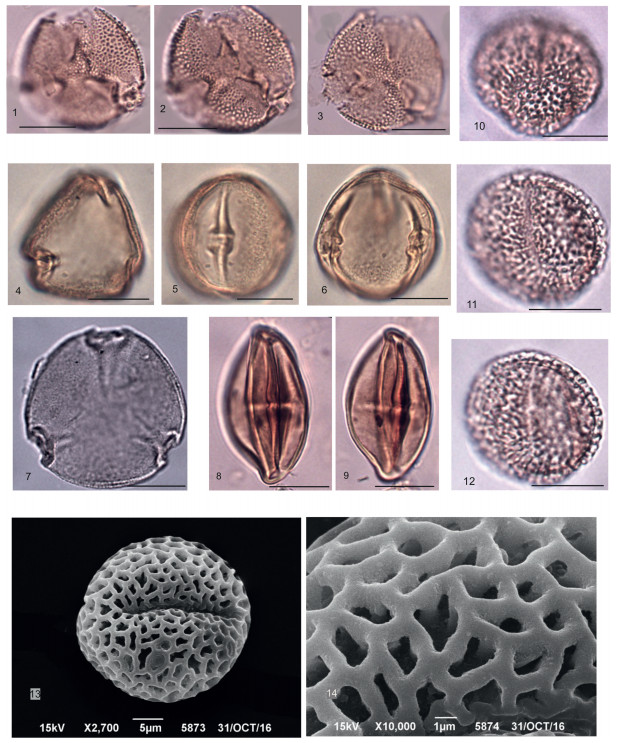
| Plate 4 Scale bar is 10 μm. 1, 2 – Betula sp. polar and equatorial views, LM; 3 – Alnus sp.; 4, 5 – Сorylus sp.; 6, 7, 8, 9 – Fagus sp.; 10 – Castanea sp.; 11, 12, 13 – Quercus sp. (11– general view, LM, 12 – general view, 13 – microtuberculate surface, SEM); 14 – Ulmus sp.; 15, 16 – Zelkova sp.; 17 – Сeltis sp.; 18, 19, 20 – Pterocarya sp. (18 – general view, LM, 19 – microtuberculate sculpture of surface, 20 – general view, SEM). |
|
| Plate 5 Scale bar is 10 μm. 1, 2 – Ilex sp. – general view, LM; 3, 4, 5, 6 – Liquidambar stigmosa (R.Pot.) Rudolph – general view, LM; 7, 8 – Altingia minor Anan. – general view, LM; 9, 10, 11, 12, 13, 14 – Platanus sp. (9–12 – general view, LM; 13 – perforated surface, 14 – general view, SEM); 15–18 – Tilia sp. (15, 16 – general view, LM; 17 – perforated surface, 18 – general view, SEM); 19, 20 – Parthenocissus macroreticulata Taras. |
|
| Plate 6 Scale bar is 10 μm. 1, 2, 3– Staphylea gracilis Taras. – general view, LM; 4, 5, 6, 7 – Nyssa sp. (4, 5, 6 – general view, LM; 7 – general view, SEM); 8, 9 – Diospyros ovalis Taras. – general view, LM; 10–14 – Fraxinus sp. (10–12 – general view, LM; 13 – general view, 14 – reticulate surface, SEM). |
|
| Plate 7 Scale bar is 10 μm. 1, 2, 3 – Jussiea azovica Anan. (1 – general view, LM; 2 – general view, 3 – tuberculate surface, SEM); 4–10 – Nyssa sp.(4, 10 – general view, 5, 6 – perforated-tuberculate surface, SEM); 7, 8, 9 – Equatorial and polar views, LM; 11– 17 – Nelumbo sp. (11 – general view, 12 – ruptured sporoderm, thick perforated tectum, short columellae, and the underlaying layer consisting of foot layer and endexine, SEM; 13, 14, 17 – general view in different planes and magnifications, LM; 15, 16 – exine sculpturing). |

|
| Plate 8 Scale bar is 10 μm. 1–9 – Heptacodium spinosa forma minor Anan. (1, 2, 6–8 – polar view, 3 – equatorial view, LM; 4 – echinate sculpture with distinct high solid and sparsely distributed echini, SEM; 5 – polar view, SEM; 9 – echinate sculpture of the surface covered by orbicules, SEM); 10–12, 17 – H. miconioides Rehder (10, 11 – polar view, 12 – the pores, SEM; 17 – the pores with an annulus, LM); 13, 14 – Osmunda sp. (13 – general view, LM, 14 – general view, SEM); 15, 16 – spore of bryophytes; 18, 19 – Ephedra sp. – general view, LM; 20, 21 – Сhenopodiaceae gen. sp. |
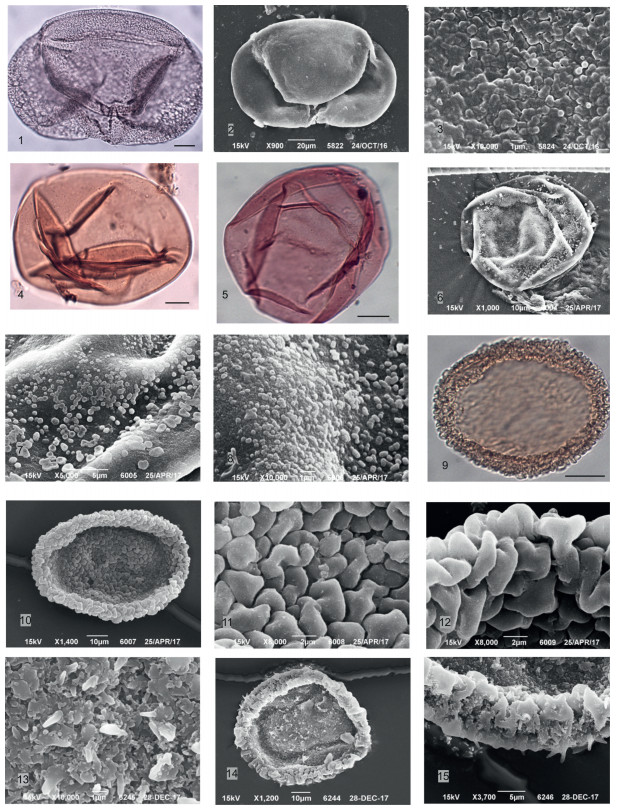
The carpological analysis was carried out for three samples collected from the same layer, using the method described by Nikitin (1969). The laboratory treatment was carried out following the standard procedure: after a 24-h soak with soda ash, the sample was washed on a sieve with a mesh size of 0.25 mm using laboratory equipment at premises of Palaeobotanical Department of Komarov Botanical Institute.
The macro- and microfossil plant remains were morphologically examined (Plate 1). To identify and illustrate key taxa, representative fossil seeds and fruits specimens in the collection of the Komarov Botanical Institute of the Russian Academy of Sciences, Saint Petersburg, were examined under a Zeiss Stemi 2000-CS stereomicroscope and imaged using a Lomo-Microsystems MC-6.3 Camera with MS-view software (Plate 2).

|
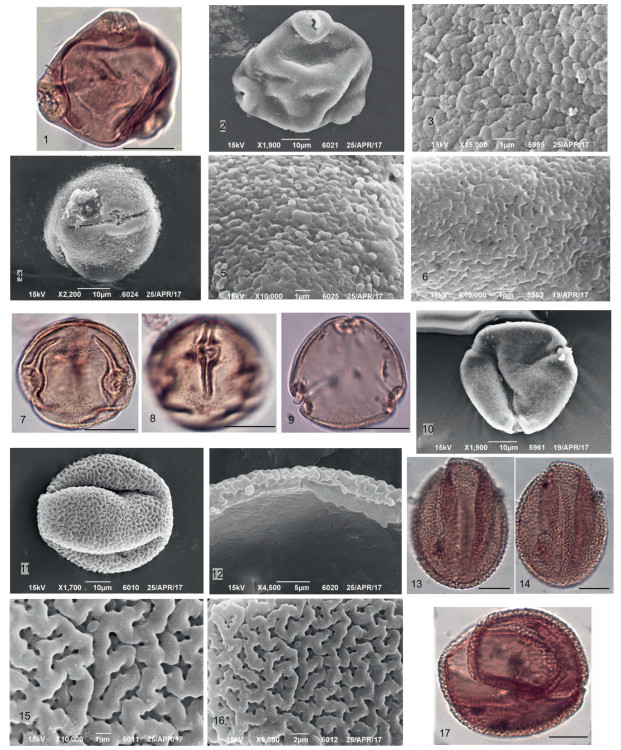
| Plate 1 Key taxa of plant macrofossils of Kumyrtas (scale bars: 1–18, 20, 21.1 mm, 19, 22, 23–25. 200 μm, 24.0, 1 mm, 26. 20 μm, 27. 100 μm, 28. 10 μm). 1 – Alnus sp. coll. 1920 BIN, 2– Cupressaceae gen. sp. coll. 1906 BIN, 3 – Pinaceae gen. sp. coll. 1920 BIN, 4–8 Coniferae gen. sp. coll. 1920 BIN, 9–11 Tubela cf. decipiens P. Dorof. coll. 1920 BIN, 12 – Betula sp. coll. 1900 BIN, 13 - Decodon cf. sibiricus P. Dorof. coll. 1900 BIN, 14 - Nyssa sp. coll. 1915 BIN, 15–16 - Microdiptera panii Arbuzova et Zhilin coll. 1900 BIN, 17 - Rubus sp. coll. 1900 BIN, 18 - Typha pusilla P. Dorof. coll. 1900 BIN, 19 - Saururus bilobatus P. Dorof. coll. 1900 BIN, 20 – Sparganium sp. coll. 1906 BIN, 21 - Viola sp. coll. 1900 BIN, 22 – Cephalanthus kireevskianus (P. Dorof.) P. Dorof. coll. 1900 BIN, 23 – Aldrovanda sp. coll. 1900 BIN, 24 – Selaginella pliocenica coll. 1906 BIN; 25 – Salvinia sp. coll. 1900 BIN, X100 μm, 26 – Microdiptera panii Arbuzova et Zhilin seed coat, SEM, 10 μm, 27 – Cephalanthus kireevskianus (P. Dorof.) P. Dorof., SEM, X100 μm, 28 – Salvinia sp. surface, SEM, X10 μm. |
We quantitatively reconstructed palaeoclimate of the Kumyrtas flora using the Coexistence Approach (CA) (Mosbrugger and Utescher, 1997, Utescher et al., 2014). The CA is applicable on both, macro- and microflora, and provides data for various temperature and precipitation variables. The CA relies on climate requirements identified for the NLRs known for the plant fossil record of a given locality. Three temperature parameters (mean annual temperature – MAT, cold and warm month mean temperature – CMT, WMT) and four precipitation variables (mean annual precipitation – MAP, mean monthly precipitation in the wettest, driest, and warmest months – MPwet, MPdry, MPwarm) were reconstructed. Climate requirements of modern taxa come from the Palaeoflora database (Utescher and Mosbrugger, 2015). Sciadopitys was excluded from the analysis, as suggested by Utescher et al. (2014).
2.4. Vegetation reconstructionThe Plant Functional Type (PFT) approach was used to reconstruct the palaeovegetation of the Kumyrtas flora. The methodology is described in detail in François et al. (2011). In the PFT approach, the plant fossil record is classified into various functional types, based on the available concept of botanical affinity. Ecospectra displaying diversities of PFTs are then interpreted in terms of palaeovegetation. The PFTs are defined using traits and climatic thresholds of key taxa, and comprises species related by morphological and phenological traits. Here we use a PFT classification including 26 herbaceous to arboreal PFTs (Popova et al., 2013, Henrot et al., 2017).
3. Results 3.1. Carpological and palynological diversityThe carpological analysis revealed remains of Cupressaceae(Taxodioideae) (Pl. 1, Fig. 2), needles of Pinaceae (Pl. 1, Fig. 3), megasporesof Salvini a sp. (Pl. 1, Fig. 25), spores of Selaginella pliocenica P. Dorof. (Pl. 1, Fig. 24), and infructescence of Betula sp. (Pl. 1, Fig. 12) and Alnus sp. (Pl. 1, Fig. 1) with pollen in situ. A total of 19 genera of fruits and seeds were identified (Table 1). Aquatic Aldrovanda sp. (Pl. 1, Fig. 23), Sparganium sp. (Pl. 1, Fig. 20), Typha sp. (Pl. 1, Fig. 18), Saururus sp. (Pl. 1, Fig. 19), Stratiotes sp., mesophitic (species of Hypericum, Actinidia, Rubus (Pl. 1, Fig. 17)) and riparian (Decodon cf. tavdensis, Microdiptera cf. tavdensis (Pl. 1, Figs. 15–16)) herbaceous plants dominate in the carpological spectrum. The arboreal component in carpo-spectrum is represented by genera Tubela cf. decipiens P. Dorof. (Pl. 1, Figs. 9–11), as well as Alnus sp. (cones bracts), Nyssa sp. (Pl. 1, Fig. 14), Spirematospermum wetzleri (Heer) Chandl., Cephalanthus sp. (Pl. 1, Fig. 22) and some Coniferae gen. sp. (Pl. 1, Figs. 4–8).
| Family/order | Taxa | Microfossil number of grains (%) | Macrofossil |
| Salviniaceae | Salvinia sp. | + | |
| Selaginellaceae | Selaginella pliocenica P.Dorof | + | |
| Osmundaceae | Osmunda sp. | single | |
| Gen. sp. | + | ||
| Sphagnaceae | Sphagnum sp. | single | |
| Bryales | Gen. sp. | single | |
| Polypodiaceae | Gen. sp. | single | |
| Podocarpaceae | Podocarpus sp. | + | |
| Cupressaceae | Sequoia sp. | 2 (0.7) | |
| Cryptomeria sp. | + | ||
| Cupressaceae (Taxodioideae) | Gen. sp. | 25 (9.2) | |
| Pinales (Cupresaceae -Taxaceae) | Gen. sp. | 14 (5.1) | |
| Taxodium sp. | + | ||
| Taxaceae | Gen. sp. | 9 (3.3) | |
| Pinaceae | Gen. sp. | + | |
| Pinus ruthenica Anan. | single | ||
| P. baileyana Trav. | 3 (1.2) | ||
| Pinus sp. | 7 (2.5) | ||
| Picea sp. | single | ||
| Abies cristata Anan. | single | ||
| Keteleeria microreticulata Anan. | single | ||
| Tsuga aculeata Anan. | single | ||
| T. macroserrata (Wolff) Anan. | single | ||
| Cathaya sp. | single | ||
| Pseudotsuga magna (R.Pot.) Anan. | single | ||
| Altingiaceae | Altingia sp. | single | |
| Anacardiaceae | Rhus sp. | single | |
| Aquifoliaceae | Ilex sp. | single | |
| Asteraceae | Centaurea sp. | single | |
| Betulaceae | Tubela cf. decipiens P. Dorof. | + | |
| Betula spp. | 74 (27.9) | + | |
| Alnus spp. | 13 (4.9) | + | |
| Carpinus sp. | 4 (1.3) | ||
| Corylus sp. | 2 (0.6) | ||
| Caprifoliaceae | Heptacodium spinosa Anan. | single | |
| Chenopodiaceae | Gen. sp. | single | |
| Cornaceae | Nyssa spp. | 6 (2.0) | + |
| Droseraceae | Aldrovanda sp. | + | |
| Ephedraceae | Ephedra sp. | single | |
| Eucommiaceae | Eucommia sp. | single | |
| Fagaceae | Fagus spp. | 39 (14.7) | |
| Lithocarpus sp. | single | ||
| Castanea sp. | 9 (3.4) | ||
| Quercus sp. | single | ||
| Juglandaceae | Juglans polyporata Vojc. | 2 (0.7) | |
| Juglans dneprovica Anan. | single | ||
| Carya sp. | 3 (1.1) | ||
| Pterocarya sp. | 20 (7.5) | ||
| Hammamelidaceae | Liquidambar stigmosa(R.Pot.)Rudolph | 5 (2.6) | |
| Hypericaceae | Hypericum sp. | + | |
| Malvaceae | Tilia sp. | single | |
| Nelumbonaceae | Nelumbo sp. | single | |
| Lythraceae | Decodon cf. tavdensis | + | |
| D. cf. sibiricus P. Dorof. | + | ||
| D. gibbosus (E.M.Reid) | + | ||
| Microdiptera panii Arbuzova et Zhilin | + | ||
| Onagraceae | Jussieua azovica Anan. | single | |
| Platanaceae | Platanus sp. | single | |
| Rosaceae | Rubus sp. | + | |
| Potentilla sp. | + | ||
| Rubiaceae | Cephalanthus kireevskianus (P. Dorof.) P. Dorof. | + | |
| Saururaceae | Saururus bilobatus P. Dorof. | + | |
| Sciadopityaceae | Sciadopitys | single | |
| Staphyleaceae | Staphylea gracilis Taras. | single | |
| Ulmaceae | Zelkova sp. | single | |
| Ulmus sp. | 3 (1.0) | ||
| Celtis sp | single | ||
| Vitaceae | Parthenocissus macroreticulata Taras. | single | |
| Violaceae | Viola sp. | + | |
| Typhaceae | Sparganiu m sp. | + | |
| Typha pusilla P. Dorof. | + | ||
| Zingiberaceae | Spirematospermum wetzleri(Heer) Chandl. | + |
Angiosperms are dominant in the pollen spectrum and attain about 73% of the total palynomorph sum (270 grains). Among them, morphotypes of Betulaceae, Fagaceae, and Juglandaceae are most abundant. Betulaceae are mainly represented by Betula spp. (27.9%) (Pl. 4, Figs. 1, 2) and Alnus spp. (4.9%) (Pl. 4, Fig. 3), while Сorylus sp. (Pl. 4, Figs. 4, 5) and Carpinus sp. are rarely encountered. Fagaceae are mostly represented by Fagus sp. (14.7%) (Pl. 4, Figs. 6–9), while Castanea sp. (Pl. 4, Fig. 10) comprises only 3.4%. The Juglandaceae family is mainly represented by the genus Pterocarya (7.5%) (Pl. 4, Figs. 18–20). Juglans sp. and Сarya sp. each have a single occurrence in the spectra. Ulmaceae are represented by Ulmus sp. (Pl. 4, Fig. 14), Zelkova sp. (Pl. 4, Figs. 15, 16) and Сeltis sp. (Pl. 4, Fig. 17). Also, Ilex sp. (Pl. 5, Figs. 1, 2), Rhus sp., Liquidambar stigmosa (R.Pot.) Rudolph (2.6%) (Pl. 5, Figs. 3–6), Altingia minor Anan. (Pl. 5, Figs. 7, 8), Platanus sp. (Pl. 5, Figs. 9–14), Staphylea gracilis Taras. (Pl. 6, Figs. 1–3), Tilia sp. (Pl. 5, Figs. 15–18), Parthenocissus macroreticulata Taras. (Pl. 5, Figs. 19, 20), Diospyros ovalisTaras. (Pl. 6, Figs. 8, 9), Heptacodium spinosa Anan. (Pl. 8, Figs. 1–5) are recorded. Asaccate pollen grains found in Cenozoic sediments and similar in morphology were previously attributed by various authors to different formal genera related to Larix or Pseudotsuga. As described by E.N. Ananova (1974), the diagnostic features of the morphospecies Pseudotsuga magna include large grain size of more than 80 μm and a rather thick exine of about 1.8 μm. This pollen grain is also distinguished by its dark yellow color, in contrast to the pale gray or pale yellow color which characterizes Larix pollen. The fossil pollen of highest interest include those that are morphologically similar to the morphotypes described by Ananova (1974), such as Heptacodium spinosa from younger deposits (early Sarmatian), and, its analog, the modern species Heptacodium mecanoides.
H. spinosa pollen grains are triporate, rather large, oblate, with an equatorial diameter of about 50–40 μm. The pores are rounded, large, about 14 μm in diameter with an annulus of about 3 μm wide. The exine rises in the pore area, forming a protrusion of about 7 μm Сonical echini of about 3–6 μm high are irregularly scattered on the exine surface; there are densely distributed and differently sized granules between the echini clearly seen in SEM. Pollen grains of Heptacodium mecnonoides are also triporate but are characterized by a larger size with the polar axis of 52.5–65.4 μm and the equatorial diameter of (51.2) 66–76.7 μm. The pores are rounded, large, about 17.2–18.9 in diameter. The annulus is 4.8–6.6 μm wide. The sculpture is echinate, the echini are 5–6 μm high; the exine surface is finely perforated. A distinct annulus is seen in SEM (Pl. 7). According to Ananova (1974), the size of pollen grains of the fossil species (Heptacodium spinosа) used as a diagnostic is quite diverse. The diameter of the pollen grain from Kumyrtas is 42 μm and was therefore identified as Heptacodium spinosа forma minor (Pl. 8, Figs. 1–9).
Gymnosperms comprise about 18.3% and are mainly represented by Taxaceae and Cupressaceae (Taxodioidae). Pinaceae are less frequent (about 7%). Picea sp., Pinus baileyana Trav. (Pl. 2, Figs. 9–12), Pinus ruthenica Anan., Pinus spp., Abies cristata Anan. (Pl. 2, Fig. 6), Tsuga spp. (Pl. 3, Figs. 9–15), Pseudotsuga sp. (Pl. 3, Figs. 4–8), Cathaya sp. (Pl. 2, Figs. 7, 8) have single occurrences. Both spore plants and herbs are very rare in the spectrum. Pollen analysis revealed 32 genera related to arboreal components such as trees and shrubs [192 grains (98.3%)]; four genera are herbs and prostrate shrubs [5 (1, 5%)] and about four genera are ferns and bryophytes [4 (0.5%)].
3.2. Palaeoclimate of the Kumyrtas floraThe results obtained from the CA analysis are provided in Table 2 and detailed diagrams with climatic ranges of a single NLR taxon are given in the Supplement.
| Flora | No. of taxa (with climate data) | MAT (℃) | MAP (mm) | CMT (t) | WMT (t) | MPWET (mm) | MPDRY (mm) |
| Kumyrtas pollen | 33 | 14–18.6 | 1300–1613 | 3.4–3.7 | 23–28.4 ℃ | 180–245 mm | 25–43 |
| Kumyrtas carpo | 12 | 6.9–14.7 | 843–1613 | −6.4–6.8 | 21.6–24.6 ℃ | 103–187 mm | 41–75 mm |
With 33 mainly zonal taxa, the Kumyrtas pollen flora provides a better climatic resolution in CA analysis, compared to the carpological record. The MAT at 15.3–18.6 ℃ (limits set by Altingia/Heptacodium), CMT at 3.4–8.1 ℃ (limits set by Altingia/Heptacodium) and WMT at 23–28.4 ℃ [limits set by Heptacodium/Nelumbo nucifera (Pl. 10, Figs. 1-8)] indicate a warm temperate, frost-free climate. When excluding the monotypic Heptacodium, presently endemic to China and rarely encountered in our record, somewhat wider temperature intervals are obtained (MAT 15.3–21.8 ℃ (Altingia/Cryptomeria); CMT 3.4–12 ℃ (Altingia/Staphylea); WMT 21.1–28.4 ℃ (Glyptostrobus/N. nucifera)). A MAP of 1090–1613 mm (limits set by Altingia/Rhus, Sequoia) suggests overall humid conditions. However, the study area may have received rainfall up to ten times higher in the wet season (MPwet 180–245 mm) when compared to the dry season (MPdry 17–43 mm). Moreover, our data point to humid summers (MPwarm 132–189 mm).
For the carpoflora, only 12 taxa were available for CA analysis, therefore the results might be less precise. The carpoflora points to somewhat cooler MAT (6.9–14.7 ℃; limits set by Saururus cernuus/Stratiotes aloides), while CMT (−6.4–6.8 ℃) and WMT (21.6–24.6 ℃) overlap with the values obtained from the microflora. The precipitation data largely overlap with those obtained for the microflora for MAP (843–1613 mm) and MPwet (103–187 mm), while macrofossils-based MPdry is at the wet end of the microfloral data (41–75 mm; dry limit set by S. cernuus), and MPwarm is significantly drier (84–95 mm; limits set by S. cernuus/S. aloides), thus indicating a lower seasonality compared to the microflora.
3.3. PalaeovegetationPollen-based PFT spectra better reflect the zonal vegetation. In the spectrum of the Kumyrtas pollen flora ca. 45% of diversity relates to the arboreal broadleaved deciduous components (mainly PFTs 23, 24, supplementary data). Conifer trees represent ca. 35% of the total diversity (PFTs 13, 15, 16). Broadleaved evergreens make a very minor contribution to the diversity.
The PFT spectrum of the Kumyrtas carpoflora has a very significant edaphic signal, namely a high diversity proportion of aquatic plants (PFT 27), and diverse shrub layer (PFTs 5, 6; Popova et al., 2013).
Conifer PFTs and Broadleaved Evergreen PFTs are not recorded. Thus, the eco-spectrum of the macrofossil flora primarily reflects local, riverine vegetation.
4. DiscussionQuantitative climate parameters (MAT, CMT, WMT, MAP, MPWet, MPDry) were calculated based on 33 taxa for the Kumyrtas palynoflora and on taxa for the carpological record, respectively. Interestingly, both data sets possibly indicate different paleoclimatic conditions. The carpoflora reveals a MAT ranging from 6.9 to 14.7 ℃ and, which, for taphonomical reasons, more closely reflects the local climate. The pollenrecord suggests a general arboreal vegetation pattern with a warmer MAT interval ranging from 14 to 18.6 ℃, and showing only a small overlap with the carpological data. The higher proportion of boreal shrubs in the macrofossils (PFT 5) is consistent with a CMT that ranges from −6.4–6.8 ℃; in contrast, the pollen data suggest that the CMT may have been lower (3.4–3.7 ℃). The WMTs of both floras do not show any significant difference. Unlike macrofloras, pollen and spores provide information on regional climate by integrating various local conditions. In this context, it must be stated that long-distance transport and the inclusion of taxa with single grain records may bias the microflora-based reconstruction. Uncertainties caused by mixing elements that originate from different altitudes may play a minor role because the study site is located on the Russian Plateau, which has no significant palaeoelevation nearby. As regards evidence from single grains, more samples are currently under study to improve the representativeness of the regional pollen record.
Reconstructions of the Early Miocene climate of Kazakhstan based on leaf floras (Bruch and Zhilin, 2007) and the given Kumyrtas flora (pollen, fruit, and seed records) can be compared with analysis based on carpological data from Western Siberia and the Russian Far East (Popova, 2012) and Northern China (Liu et al., 2011). This comparison shows an evident gradient to warmer and wetter conditions from Western Siberia/Far East towards the south and southeast (Popova, 2012). The MATs of the floras from the Far East and Western Siberia range from about 10 ℃ to 13 ℃, while the Kazakhstan MATs calculated from leaf floras are warmer by 5–6 ℃. Floras of Northern China suggest MATs warmer by 7–9 ℃ when compared to the Far East and Western Siberia. This gradient is also supported by the results obtained from the Kumyrtas palynoflora, but not necessarily from the carpological record. CMT and WMT reconstructed for Western Siberian carpofloras show that conditions were cooler by about 3 ℃ than for leaf floras in Kazakhstan (CMT: 1 ℃, WMT: 5 ℃). Northern China had a MAT of 15.6–18.4 ℃, CMT of 5–12.5 ℃, and WMT of 24.7–27.9 ℃. When pollen has been used to reconstruct the CMT of the Kumyrtas flora, the values are within the temperature intervals calculated for other Kazakhstan floras (Bruch and Zhilin, 2007) fruit and seed data do not exclude cooler conditions (Table 2), but, due to species deficits, these data indicate an overall low climatic resolution. Drier conditions existed in Western and Eastern Siberia and in the Russian Far East, with MAPs at 994 mm and 896 mm, respectively, whereas wetter conditions were obtained for Kazakhstan (1077 mm) reconstructed with leaf floras (Bruch and Zhilin, 2007) and the values may have reached almost 1600 mm according to the pollen and carpological data reconstructed for the Kumyrtas palaeofloras; the floras in Northern and Western China range from 1173 mm to 1111 mm.
Previous studies have shown that during the Oligocene and Early Miocene, the Kazakhstan area in Central Eurasia was covered by forest with warm-temperate deciduous trees and shrubs of the Turgai type flora (Bruch and Zhilin, 2007). This finding was described mostly based on the analysis of fossil leaf floras. Our study of pollen, in addition to fruit and seed records from the Kumyrtas flora, supports this conclusion. The pollen spectrum of Kumyrtas is dominated by angiosperms (73%). Broadleaved taxa are represented more diversely than others, which occurred in lower abundance, such as Rhus sp., L. stigmosa, A. minor, Platanus sp., Nyssa sp., Tilia sp., S. gracilis, P. macroreticulata, D. ovalis, Fraxinus sp. (Pl. 8, Figs. 10–14), and H. spinosa. The dominance of Betula sp. (28%) and Pterocarya sp. (7.7%) pollen in the spectrum is comparable to the composition of another Miocene complex from the south-eastern part of Kazakhstan and floras of the southern part of the Russian and Oka-Don Plains. Because pollen flora better reflects the zonal vegetation, we conclude that this represents a general vegetation type in the early Miocene of Kazakhstan. The PFT data also support the high diversity of both broadleaved deciduous and conifer taxa, characteristic for Turgai type vegetation.
The carpoflora has a significant edaphic signal with a high proportion of aquatics, herbaceous plants (PFT 27), and shrubs (supplementary data). These data indicate a riverine vegetation with broadleaved summer-green trees and mesophytic herbs.
5. ConclusionThree different fossil flora types (pollen, seeds and fruits, and leaves) were used to reconstruct paleovegetation in central Kazakhstan with each data type providing additional information and certainty to the analyses. Pollen floras provide the most complete picture with respect to all vegetation levels and best describe zonal vegetation, unlike carpofloras, which are commonly characterized by an over-representation of herbaceous plants including aquatics. and mainly reflect the local edaphic conditions (Vassio and Martinetto, 2012, Popova et al., 2017, Teodoridis et al., 2017).
This comprehensive and complementary study of the Kumyrtas flora allows us to compare results with previously published Kazakhstan Aquitanian fossil floras (Zhilin, 1974). Pollen spectra are dominated by angiosperms pollen (73%) and have a high diversity of broadleaved summer-green plants. The carpological analysis reveals 19 taxa in total characterizing the floral composition of the Turgai area, including taxa that are described for the first time for this region (species of Tubela, Alnus, Actinidia, Rubus). The fruits and seeds represent aquatics, mesophitic herbaceous plants, and shrubs. The ecological profile of the Kumyrtas flora indicates typical Turgai flora conditions. However, intense weathering under arid climate conditions, together with erosional processes may have affected the preservation of fossils adversely, and thus additional sampling is required.
Palaeoclimate reconstructed based on the Kumyrtas pollen taxa shows similar temperature and precipitation patterns as previous results obtained for Aquitanian flora of Kazakhstan (Bruch and Zhilin, 2007). Moreover, a gradient to warmer and wetter conditions from Western Siberia/Far East towards the south and southeast is evident when comparing our data with early Miocene records from other Asian regions.
We declare that there is no conflict of interest.
This study was carried out as part of the institutional research project of the Botanical Institute of the Russian Academy of Sciences (Saint Petersburg, Russia) № AAAA-A19-119021190031-8. It was done using the equipment of the Core Facility Center "Cell and Molecular Technologies in Plant Science" at this institute. Our study was funded by RFBR and NSFC according to the research project No 19-55-53010 and Pioneer Hundred Talents Program of the Chinese Academy of Sciences No. 419 115 30 105 to Y.W. Xing and the Chinese Academy of Sciences 135 program (No. 2017XTBG- F01); the results of this study are also a contribution to NECLIME. We are grateful to Dr. Elena Herzog and Dr. Maria Tekleva for critical reading of the manuscript.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2019.04.002.
Akhmetjev M.A., 1985. The floras of Zaisan depression on the eocene-oligocene border. Bull. Acad. Sci. USSR Ser. Geol, 11: 76-85. |
Akhmetjev M.A., Iljinskaja I.A., 1989. The species of Anacardiaceae from the lower Oligocene deposits of Kiin-Kerish ridge of Zaisan depression. Paleontol. J, 2: 79-88. |
Ananova, E.N., 1974. Pollen in Neogene Deposites of South Part of Russian Plate.Leningr. st. uni of Zhdanov, Leningrad (In Russian).
|
Averyanova, A., 2012 (Unpublished Doctoral Dissertation). Late Eocene Flora of Zaisan Depression (Eastern Kazakhstan), vol. 24. Komarov Botanical Institute of RAS, Saint Petersburg, Russia (In Russian).
|
Borsuk, M.I., 1935. By study of Turgai tertiary flora. Proc. CNIGRI 37 (In Russian).
|
Boytsova, E.P., Gladkova, A.N., Zauer, V.V., Krutschinina, N.V., Malyasova, E.S., Moreva, V.A., Pokrovskaya, I.M., Romanovskaya, G.M., Sedova, M.A., Sigova, N.N., 1956. Atlas of Miocene spores-pollen complexes of different regions of USSR. In: Pokrovskaya, I.M. (Ed.), Proceedings of A.P, vol. 13. Karpinsky Russian Geological Research Institute (VSEGEI), pp. 77-83.
|
Bruch A.A., Zhilin S.G., 2007. Early Miocene climate of central Eurasia d evidence from Aquitanian floras of Kazakhstan. Palaeogeogr. Palaeoclimatol. Palaeoecol, 248: 32-48. DOI:10.1016/j.palaeo.2006.11.014 |
Budantsev, L.Y., 2017. The comparison of the Oligocene flora of the northern fore aral sea region with the age-related floras of northern Asia. In: Budantsev, A.L.(Ed.), Selected Works. KMK Scientific Press Ltd, Moscow, pp. 295-306.
|
Dorofeev P.I., 1963. Tertiary plants of Kazakhstan. Bot. J, 48(2): 171-181. |
François L., Utescher T., Favre E., Henrot A.J., Warnant P., Micheels A., Erdei B., Suc J.P., Cheddadi R., Mosbrugger V., 2011. Modelling late Miocene vegetation in Europe:results of the CARAIB model and comparison with palaeovegetation data. Palaeogeogr. Palaeoclimatol. Palaeoecol, 304: 359-378. DOI:10.1016/j.palaeo.2011.01.012 |
Henrot A.-J., Utescher T., Erdei B., Dury M., Hamon N., Ramstein G., Krapp M., Herold N., Goldner A., Favre E., Munhoven G., FranScois L., 2017. Middle Miocene climate and vegetation models and their validation with proxy data. Palaeogeogr. Palaeoclimatol. Palaeoecol, 467: 95-119. DOI:10.1016/j.palaeo.2016.05.026 |
Iljinskaja, I.A., 1986. The Changes of the Flora of Zaisan Depression from the Cretaceous up to Miocene.[Problems of Paleobotany]. Leningrad, pp. 84-112 (in Russ.).
|
Iljinskaja, I.A., 1991. Interrelations of the early oligocene flora of the Kiin-Kerish mountain with the modern flora. In: Formation of the Eocene-Miocene Flora of Kazakhstan and the Russian Plain. Proceedings of Kryshtofovich readings, vol. 2, pp. 15-36.
|
Kornilova V.S., 1960. New data regarding flora of Indricoterium layers of Turgai(In Russian). Izvestiya AN Kaz. SSR, seriya botanicheskaya, 5: 61-67. |
Kornilova V.S., Lavrov V.V., 1949. About tertiary xeric flora findings in Turgai and its stratigraphical position. Vestnik Akademii Nauk Kazakhstan ASSR, 50: 104-107. |
Kryshtofovich A.N., 1946. The evolution of vegetation in the geological past and its main factors. Materialy po istorii flory I rastitelnosti SSSR, 2: 21-86. |
Kryshtofovich A.N., 1955. Development of botanical and geographical regions of the northern hemisphere since the beginning of the Tertiary period. Voprosy geologii Asii, 2: 824-844. |
Kryshtofovich A.N., Palibin I.V., Shaparenko K.K., Yarmolenko A.V., Baikovskaya T.N., Grubov V.I., Iljinskaja I.A., 1956. The Oligocene flora of Ashutas in Kazakhstan. Pap. Bot. Inst. Acad. Sci. USSR, 8: 1-180. |
Korobkov A.I., 1967. Oligocene deposits of northern Ustyurt as the basis for separation of Oligocene deposits of Kazakhstan and central Asia. Bulletin nauchnotechnitscheskikh inform. Seria geologiya mestorozhd. I poleznykh iskopaemykh, 7: 87-94. |
Liu, Y.C., Utescher, T., Zhou, Z., Sun, B., 2011. The evolution of miocene climates in North China: preliminary results of quantitative reconstruction from plant fossil records. In: Utescher, T., Boehme, M., Mosbrugger, V. (Eds.), The Neogene of Eurasia: Spatial Gradients and Temporal Trends - the Second Synthesis of NECLIME. Palaeogeography, Palaeoclimatology, Palaeoecology, vol. 304, pp. 308-317.
|
Merklin R.L., 1962. The Horizons of middle and upper Oligocene sediments in the south of the USSR. Doklady AN SSSR, 144(2): 420-423. |
Mosbrugger V., Utescher T., 1997. The coexistence approach-a method for quantitative reconstructions of Tertiary terrestrial palaeoclimate data using plant fossils. Palaeogeogr. Palaeoclimatol. Palaeoecol, 134: 61-86. DOI:10.1016/S0031-0182(96)00154-X |
Neiburg, M.F., 1928. About the Materials of the Ashutas Expedition of the Geological Museum of Academy of Sciences, vol. 8. Doklady AN SSSR, pp. 445-448.
|
Nikitin, V.P., 1969. Palaeocarpologycal Method. Izd. TGU, Tomsk (In Russian).
|
Orlov, Yu A., 1939. Locality of Tertiary Mammals Near the Aral Sea. Nature, 5, vol. 97.Academy of science USSR. P, Leningrad.
|
Pokrovskaya, L.M., 1950. Pollen Analysis. Gosgeolitizdat, Moscow (in Russian).
|
Popova S., Utescher T., Gromyko D.V., Bruch A.A., Mosbrugger V., 2012. Palaeoclimate evolution in Siberia and the Russian far East from the oligocene to pliocene e Evidence from fruit and seed floras. Turkish J. Earth Sci, 21: 315-334. |
Popova S., Utescher T., Gromyko D.V., Mosbrugger V., Herzog E., François L., 2013. Vegetation change in Siberia and the northeast of Russia during the Cenozoic cooling:a study based on diversity of plant functional types. Palaios, 28: 418-432. DOI:10.2110/palo.2012.p12-096r |
Popova S., Utescher T., Gromyko D.V., Bruch A.A., Henrot A.-J., Mosbrugger V., 2017. Cenozoic vegetation gradients in the mid-and higher latitudes of Central Eurasia and climatic implications. Palaeogeogr. Palaeoclimatol. Palaeoecol, 467: 69-82. DOI:10.1016/j.palaeo.2016.09.016 |
Popova, S., Utescher, T., Gromyko, D.V., Mosbrugger, V., François, L., 2018. Dynamics and evolution of Turgay-type vegetation in Western Siberia throughout the early Oligocene to earliest Miocene e a study based on diversity of plant functional types in the carpological record. J. Syst. Evol. https://doi.org/10.1111/jse.12420.
|
Raevskaya, E.G., Shurekova, O.V., 2011. Modern technologies and equipment in the processing of carbonate-terrigenous rocks for palynological analysis. Problems of modern palynology. In: Proceedings XⅢ Palynological Conference. Syktyvkar, vol. 1, pp. 103-107.
|
Tarasevich, V.F., Tropina, P., 2017. Miocene flora of Kumyrtas investigated by pollen and spores and carpological methods. Actual problems of palynology. In: Proceedings of ⅩⅣ All-Russian palynological conference dedicated of memory of Grichuk Vladimir. Moscow, pp. 344-347.
|
Teodoridis V., Bruch A.A., Vassio E., Martinetto E., Kvaček Z., Stuchlik L., 2017. Plio-Pleistocene floras of the Vildštejn formation in the Cheb basin Czech Republic - a floristic and palaeoenvironmental review. Palaeogeogr. Palaeoclimatol. Palaeoecol, 467: 166-190. DOI:10.1016/j.palaeo.2015.09.038 |
Utescher T., Bruch A.A., Erdei B., FranScois L., Ivanov D., Jacques F., Kern A., Liu Y.-S., Mosbrugger V., Spicer R.A., 2014. The Coexistence approach:theoretical background and practical considerations of using plant fossils for climate quantification. Palaeogeogr. Palaeoclimatol. Palaeoecol, 410: 58-73. DOI:10.1016/j.palaeo.2014.05.031 |
Utescher, T., Mosbrugger, V., 2015. The Palaeoflora Database. http://www.geologie.unibonn.de/Palaeoflora.
|
Vassio E., Martinetto E., 2012. Biases in the frequency of fruits and seeds in modern fluvial sediments in northwestern Italy:the key to interpreting analogous fossil assemblages. Palaios, 27: 779-797. DOI:10.2110/palo.2012.p12-050r |
Zhilin, S.G., 1974. Tertiary Floras of the Ustyurt Plateau (Trans Caspian). Nauka, Leningrad (in Russian).
|
Zhilin, S.G., 1984. The Main Stages of Formation of Temperate Forest Flora in Oligocene-Early Miocene. Nauka, Leningrad (in Russian).
|



