b. Research Centre for Applied Science and Technology(RECAST), Kathmandu, Nepal;
c. G B Pant National Institute of Himalayan Environment and Sustainable Development(GBPNIHESD), Sikkim, India;
d. Nature Conservation Division, Department of Forest and Park Services, Thimphu, Bhutan
Plant diversity plays a key role in balancing ecosystems, protecting watersheds, regulating climate, providing habitats to animals and supporting livelihoods by providing food, fibre, medicines, and raw materials for many industries (Díaz et al., 2018). Plant species inventories are vast resources that provide an essential basis for designing conservation interventions at various scales: local, regional, and global (Brooks et al., 2006, Chettri et al., 2001, Basnet and Badola, 2012). Species listings or check lists, which contain primary and/or secondary species data, are critical to obtain information about the baseline condition, understand richness and distribution of species and analyse the pattern and changes in distribution, composition, and diversity. Such data have been used to study the taxonomy and biogeography of species for hundreds of years (Chapman, 2005). Lack of data restricts resource managers and decision makers from making accurate evaluations for interventions for the state of biodiversity of a targeted region or taking scientifically based conservation decisions (Proença et al., 2016). From a biodiversity conservation perspective, the importance of secondary data must not be underestimated, as acquiring new data is costly and time consuming. Using secondary data from the available literature facilitates comparison among and between different studies to reveal landscape-level changes in distribution and population trends. In this context, the importance of plant diversity data for the Kangchenjunga Landscape (KL) - one of the biologically richest landscapes in the Eastern Himalayas - cannot be overemphasized.
The Eastern Himalayas, located at the juncture of the Indo-Malayan, Palearctic and Sino-Japanese realms, stand out as being one of the biologically richest areas on Earth (CEPF, 2005). The considerable climatic variability associated with the topographic complexity and elevational gradient, makes it a repository of wide diversity of flora and fauna. Phytogeographically, the region represents a part of the Eastern Himalayan Province, which appears to be one of the youngest in the Eastern Asiatic Region and contains a number of endemic genera and species (Takhtajan, 1986). The Eastern Himalayas are a part of two biodiversity hotspots – Indo-Burma and Himalayan. Comprising 25 terrestrial ecoregions, the Eastern Himalayas are also known as the 'cradle of flowering plants' and is well known for its rich floral diversity, which includes both high species richness and endemism (Takhtajan, 1969, Olson et al., 2001).
The KL, situated between 26°21′40.49″ to 28°7′51.25″N latitudes and 87°30′30.67″ to 90°24′31.18″E longitudes and with an elevational range of 40 to 8586 m above sea level, is a transboundary landscape in the Eastern Himalayas that is shared by Bhutan, India and Nepal (Yonzon et al., 2000, Chettri et al., 2008, Kandel et al., 2016). Designated through a consultative process and approved by the governments of Bhutan, India and Nepal, the landscape includes an area of over 25, 000 km2 around and to the south of Mount Kangchenjunga, also known as Khangchendzonga in Sikkim, the third highest mountain of the world. Of the total landscape area, the Bhutan portion covers 5834 km2 (23%), the Indian portion covers 14, 062 km2 (56%) and the Nepal portion covers 5190 km2 (21%). The KL is one of 36 'Global Biodiversity Hotspots' (Mittermeier et al., 2004). With 22 Important Bird and Biodiversity Areas (IBAs), 19 protected areas (nine of which are transboundary in nature), one UNESCO World Heritage Site (Khangchendzonga Biosphere Reserve) and one Ramsar site (Mai Pokhari Ramsar site in Ilam, Nepal), the KL is recognized as one of the priority areas for biodiversity conservation in the Himalayan region (CEPF, 2005, ICIMOD et al., 2017, Kandel et al., 2016). Furthermore, the landscape exhibits great cultural diversity with approximately 7.2 million people representing various ethnic and social groups. Some of the ethnic groups that reside in the KL are found nowhere else in the world. They include the Lepcha community of Sikkim and Darjeeling in KL–India, eastern Nepal, and southwestern parts of KL–Bhutan; the Lhop (Doya) community of the Amo Chhu Valley in KL–Bhutan; and the Walungpas of Olangchung Gola of Taplejung district in KL–Nepal (ICIMOD et al., 2017).
The landscape is bestowed with lush vegetation and is a repository of globally significant plant species. It is home to several high-elevation species which are endemic to the region (Manish et al., 2017). Approximately 5200 species of plants, including orchids, rhododendrons, wild edible plants, non-timber forest products (NTFPs), and medicinal plants of high value are recorded from the region. Of particular significance is the occurrence of more than 40 species of rhododendron, which are native to the Eastern Himalayas (ICIMOD et al., 2017). The flora of the KL has been the subject of ecological investigations since the 1840s. Sir J.D. Hooker initiated the botanical exploration of the KL in Eastern Nepal in 1848. Other pioneering contributions have been made by Jacot-Guillarmod in the Yalung Glacier near Kangchenjunga in 1905; W. Griffith, a plant collector in Sikkim, and Smith and Cave who detailed the floral world of the high-elevation zone of KL–India in 1911; M.L. Banerji, between 1948 and 1957 from Koshi Basin, and Darjeeling via Ilam, Taplejung, Topkegola; Hara in 1963; Numata during 1963–1982; and, Grew-Wilson in 1973 and 1981 in Taplejung and adjoining areas (Rajbhandari, 2016). Over the past two decades, many surveys on flora of the KL (e.g., Badola and Pradhan, 2010a, Badola and Pradhan, 2010b, Chettri et al., 2009, Kholia, 2011; Manish and Pandit, 2018; Pala et al., 2019; Pradhan and Badola, 2008, Rai and Sharma, 1995, Saurav and Das, 2014, Shankar, 2001, Singh et al., 2003, Singh and Sundriyal, 2005, Sundriyal and Rai, 1996, Uprety et al., 2016) have been published. These publications have broadened our knowledge on species distribution, composition, abundance, biogeography and traditional knowledge for utilization and conservation of flora of the KL, and the field of botany in the Himalayas in general. The primary objective of many publications, except those pertaining to ecological research (Carpenter, 2005, Ohsawa et al., 1986, Tambe and Rawat, 2010, Pradhan and Badola, 2015; Manish and Pandit, 2018) and new records (Das and Verbeken, 2012, Sharma and Pandit, 2009, Thapa and Lama, 2015), included listing the floral species occurring in some parts of the landscape. However, except for scattered information often severely limited to certain parts of the landscape, no attempt has been made to provide cumulative data on the flora of the entire landscape. For instance, even the basic checklist of species for some areas, including protected areas (e.g., Mahananda Wildlife Sanctuary), are scanty, whereas some areas (e.g., Sikkim and Kanchenjunga Conservation Area) are intensively studied. Furthermore, often this information is not easily accessible because it is either unpublished or archived at various sources such as libraries or private collections. Thus, this paper is an attempt to consolidate the knowledge on flora reported and documented from the landscape, understand research trends and distribution, identify focused research areas, and document conservation and management challenges. The goal is to serve the needs of a range of stakeholders, including students, researchers, conservation institutions as well as the policy- and decision-makers and to provide valuable information on the flora of the KL that may strengthen conservation and development in an important transboundary landscape in the future.
2. Materials and methods 2.1. Study areaThe Kangchenjunga Landscape encompasses eastern Nepal (parts of Taplejung, Panchthar, Ilam and Jhapa districts), Sikkim and North Bengal (Darjeeling and Jalpaiguri, and recently formed Alipurduar and Kalimpong districts) in India, and western Bhutan (portions of Haa, Chukha, Samtse, Dagana and Paro districts). Its elevational range extends from 40 m a.s.l. in Jalthal forest of Nepal to 8586 m a.s.l., the height of Mount Kangchenjunga. Given its extreme elevational variation, it is endowed with varied climatic conditions, complex topography and diverse vegetation. The vegetation of the KL is broadly divisible into the following groups: (1) tropical; (2) subtropical; (3) warm temperate; (4) cool temperate; (5) subalpine; and (6) alpine zones (Chaudhary et al., 2015, Kandel et al., 2016, Uprety et al., 2016). The varied forest types and rich habitat diversity of the KL support many threatened plant and animal species. It provides habitat to many charismatic mammals, including the snow leopard (Panthera uncia), Bengal tiger (Panthera tigris), Asian elephant (Elephas maximus), red panda (Ailurus fulgens), and takin (Budorcas taxicolor)(Chettri et al., 2008, Kandel et al., 2016). The landscape is home to many threatened species of Himalayan birds such as tragopans (e.g. Tragopan satyra) and hornbills (e.g. Aceros nipalensis). Similarly, the landscape is equally rich in flowering plants, including orchids and rhododendrons (Kandel et al., 2016). Several threatened and endangered plant species are recorded from the landscape, including Chiraito (Swertia chirayita, Roxb. ex Fleming, Karsten), Himalayan Yew (Taxus wallichiana Zucc.), Kutki (Neopicrorhiza scrophulariiflora, Hook. F. (Prain)), Marsh Orchid (Dactylorhiza hatagirea (D.Don) Soo) and Himalayan Mayapple (Sinopodophyllum hexandrum (Royle) T.S.Ying) (Uprety et al., 2016, O'Neill et al., 2017).
2.2. Data collection and analysisWe carried out a systematic review of accessible literature relating to the flora of the KL. We used 'Google Scholar' for the web based searches, using specific search terms 'Darjeeling, ' 'Sikkim, ' 'Jalpaiguri, ' 'Kalimpong, ' 'Eastern Nepal, ' 'Western Bhutan, ' 'Eastern Himalaya, ' 'Kangchenjunga Landscape, ' and 'Flora, ' 'Plants, ' 'Vegetation, ' 'Ethnobotany, ' 'NTFPs.' For those publications that did not appear with these search terms, we made additional searches using the name of the protected areas in the landscape. Altogether 314 publications were retrieved, which also included grey literature. However, to increase the practicality and reliability of our survey, we considered only 215 peer-reviewed English language journal articles for analyses. Because the literature searches were done in 2017, our list of publications included literature published until 2016. The list of publications on flora from the KL is provided as a supporting document (S1). Once collected, the peer-reviewed articles were chronologically listed in a Microsoft Excel Spreadsheet. The analyses included a review of publications for the following subcategories: study sites, including the name of countries and districts, publication year and subject focus of the publication.
In addition, for species richness and diversity, we transcribed species data into a working database from various published works (Chaudhary et al., 2015, ICIMOD et al., 2017; Lucksom, 2007; Maiti and Maiti, 2007; Pradhan and Lachungpa, 1990; Uprety et al., 2016; WCD, 2014). After examining the taxonomic information and records, an exhaustive checklist of seed plants (dicots, monocots and gymnosperms) was prepared and verified according to recent nomenclature. We used Angiosperm Phylogeny Group (APG) IV for the classification and analyses of the seed plants (APG IV, 2016). The data were analysed to look into the taxonomic coverage of the floral species, including family, genera and species of the flowering plants, as well as endemic and threatened plants of the KL. The list of species is available on ICIMOD's Regional Database System (http://rds.icimod.org/Home/Data?group=9&&page=2).
3. Results 3.1. Pattern of publicationsOur review included 215 publications related to flora from the KL. It revealed that the earliest studies on flora from the KL were published during the 1840s when the East India Company's regime was expanding across the Indian Subcontinent (Hooker, 1849). The first documented study in the KL is about the traditional knowledge of the Lepchas of Sikkim on plant species, which was authored by Sir Archibald Campbell, the first British army officer to Sikkim and Darjeeling (Campbell, 1840). In 1854, Sir Joseph Dalton Hooker, a notable British naturalist, published an account of his botanical expeditions in the Kangchenjunga region in two volumes entitled Himalayan Journals. Along these lines, much of the early publications from the KL are the records of naturalists during their travel and expeditions around Sikkim and Darjeeling. These include studies by Hooker, 1849, Hooker, 1852, and King and Pantling (1889). The mid-20th century witnessed a few generalist yet notable surveys, including preliminary exploration and surveys of forest vegetation, pteridophytic flora and wild edible plants of Bhutan, Sikkim, Darjeeling and eastern Nepal (Grierson and Long, 1983, Hajra and Chakraborty, 1981, Mehra and Bir, 1964, Numata, 1966, Yoda, 1967). There is a dearth of publications on the flora of the KL for 140 years after the first recorded publication by Campbell in 1840. Approximately 15% of the floristic studies from the KL were published between 1840 and 1990. During the late 20th century, there was an exponential increase in the number of studies across KL, with 85% of the total studies published between 1990 and 2016 (Fig. 1).
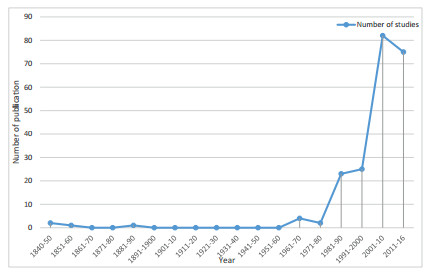
|
| Fig. 1 Temporal pattern of publications on flora of the Kangchenjunga Landscape. |
Based on country-specific documentation, India received the highest survey records (81%), followed by Nepal (13%) and Bhutan (6%) (Fig. 2). Location and state-specific analysis revealed that the majority of studies from India were carried out in the state of Sikkim (55%) followed by Darjeeling, including Kalimpong (33%), and Jalpaiguri (including Alipurduar) district (12%). Of the total studies, 14% were carried out in the protected areas of the KL.
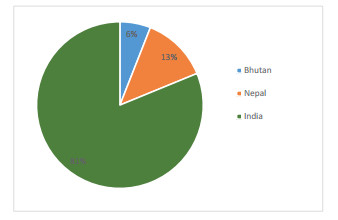
|
| Fig. 2 Country specific distribution of publications in the Kangchenjunga Landscape. |
The majority of the 215 publications on flora from the KL focused on vegetation surveys (43%), followed by ethnobotanical studies (32%), and documentation of NTFPs (25%) (Fig. 3a). Among the vegetation surveys, 53% were related to diversity and distribution of flora, 40% were related to ecological research and 7% were about new records of floral species. Of the total vegetation surveys, only 16% were related to specific species found in the KL, e.g., rhododendrons and orchids (Fig. 3b).
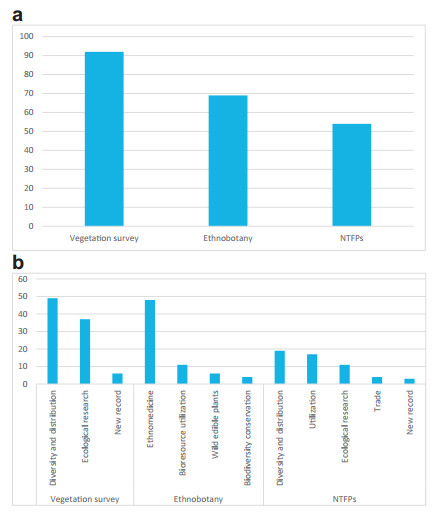
|
| Fig. 3 Subject focus of publications in the Kangchenjunga landscape, with major thematic areas (a) and sub-thematic areas (b). |
Among ethnobotanical studies, 70% were focused on ethnomedicine, of which the majority (71%) was related to use of particular species for specific ailments. Of the studies on traditional knowledge, 16% were related to bioresource utilization, 9% to the utilization of various wild edible plants, and 6% to traditional approaches of biodiversity conservation. Approximately 35% of the total ethnobotanical studies were focused on traditional knowledge of specific ethnic communities, e.g., Lepcha, Rabha, Satar, Limbu, Meche, Rajbanshi and Dhimal communities.
Of the studies on NTFPs in the KL, 35% were related to diversity and distribution, 31% to utilization, 20% to ecological research, 8% to trade, and 6% to new records of NTFP use in the KL.
3.4. Forest types and characteristic speciesThe types of forests in the KL are broadly divisible into the following groups based on the elevational range in which they occur: (ⅰ) tropical (below 1000 m) characterized by tropical moist and dry forests comprising Shorea, Bombax and Cycas species; (ⅱ) subtropical (1000–2000 m a.s.l.) with various broadleaved species such as Schima and Castanopsis; (ⅲ) warm temperate (2000–2500 m a.s.l.) dominated by broadleaved forests of Castanopsis, Quercus, Schima and Ilex; (ⅳ) cool temperate (2500–3000 m a.s.l.) mostly dominated by Rhododendron, Abies and Acer species; (ⅴ) subalpine (3000–4000 m a.s.l.) dominated mostly by dwarf conifers, dwarf species of rhododendrons, Tsuga and Juniperus species; and (ⅵ) alpine (> 4000 m a.s.l.) with sparse vegetation but mostly dominated by Juniperus, Rosa and Rhododendron species (Table 1). Scrutiny of the literature revealed that the forests in the KL have high species diversity (Sundriyal and Sharma, 1996, Sundriyal et al., 1994, Singh and Sundriyal, 2005). Although the KL consists of only a small fraction of the total area of the Himalayas, it contains half of the 10, 000 floral species of the Himalayas (Xu et al., 2019). Similarly, the number is close to the total flora of Nepal, viz. 6653 (Kunwar et al., 2010), that of Arunachal Pradesh of India, viz. 4117 (Arya and Sunny, 2016) and Kinabalu Mountain of Malaysia, viz. 5000 (Ministry of Natural Resources and Environment, 2014). However, overexploitation of the forest resources for fuelwood, fodder and timber has impacted forest structure, composition, species regeneration, and woody biomass productivity (Chettri et al., 2002, Sundriyal and Sharma, 1996, Sundriyal et al., 1994).
| Elevational Zone | Forest type | Characteristic species |
| Tropical (< 1000 m) | - Tropical riverine evergreen/deciduous forest - Tropical moist evergreen/deciduous forest - Tropical moist mixed forest - Tropical dry evergreen/deciduous forest |
Shorea robusta Gaertn, Lagerstroemia parviflora Roxb., Bombax ceiba L., Cycas pectinata Buch.- Ham., Dillenia pentagyna Roxb. |
| Subtropical (1000–2000 m) | - Subtropical riverine evergreen/deciduous forest - Subtropical moist evergreen/deciduous forest - Subtropical moist mixed forest - Subtropical dry evergreen/deciduous forest |
Schima wallichi (DC.) Korth, Castanopsis tribuloides (Sm.) A. DC., Macaranga pustulata King ex Hook. F., Machilus odoratissima Nees |
| Warm temperate (2000–2500 m) | - Warm temperate riverine evergreen/deciduous forest - Warm temperate moist evergreen/deciduous forest - Warm temperate moist mixed forest - Warm temperate dry evergreen/deciduous forest |
Castanopsis tribuloides (Sm.) A. DC., Ilex dipyrena Wall., Quercus lamellosa Sm., Quercus semecarpifolia Sm., Lithocarpus pachyphylla (Kurz) Rehder |
| Cool temperate (2500–3000 m) | - Cool temperate riverine deciduous forest - Cool temperate moist evergreen forest - Cool temperate moist mixed forest - Cool temperate dry evergreen forest |
Abies spectabilis (D.Don) Mirbel, Betula utilis D.Don, Rhododendron arboreum Sm., Acer Campbellii Hook. f. & Thomson. |
| Subalpine (3000–4000 m) | - Subalpine riverine evergreen forest - Subalpine deciduous forest - Subalpine moist evergreen forest - Subalpine moist deciduous forest - Subalpine dry evergreen forest |
Abies spectabilis (D.Don) Mirbel, Tsuga dumosa D.Don, Betula utilis D.Don, Acer sp., Larix griffithiana Carriere, Rhododendron barbatum Wall. ex G. Don, Juniperus indica Bertol. |
| Alpine (> 4000 m) | - Alpine riverine - Alpine meadow - Alpine scrub |
Rhododendron niveum Hook. f., Rosa spp, Juniperus indica Bertol. |
| Adapted from: Kandel et al. (2016). | ||
The systematic review of literature for the detailed inventory of seed plants of the KL revealed the presence of 5198 species (dicotyledons, monocotyledons and gymnosperms) belonging to 1548 genera and 216 families (Table 2). Among the documented species, 3860 are dicots, 1315 monocots and 23 gymnosperms. The ratio of monocots to dicots in respect to families, genera, and species is 1:5.7, 1:3.2 and 1:2.9, respectively. The most diverse families in the KL in terms of species richness include Orchidaceae (704 species in 152 genera), followed by Fabaceae (308 species in 105 genera), Asteraceae (255 species in 100 genera), Poaceae (233 species in 96 genera), and Rubiaceae (147 species in 50 genera). The ten dominant families of flowering plants in the KL are listed in Fig. 4. Our analysis shows that 42% of the total flowering plant species found in the KL belong to these ten major families. Among gymnosperms, six families are recorded in the KL, of which Pinaceae (10 species) and Cupressaceae (9 species) emerged as dominant. The Cycadaceae, Ephedraceae, Podocarpaceae and Taxaceae families are represented by one species each.
| Plant groups | Family | Genus | Species |
| Dicotyledons | 179 | 1175 | 3860 |
| Monocotyledons | 31 | 357 | 1315 |
| Gymnosperms | 6 | 16 | 23 |
| Total | 216 | 1548 | 5198 |

|
| Fig. 4 Dominant families of seed pants with number of genera and species in the Kangchenjunga Landscape. |
The KL hosts at least 44 plant species that are threatened at the global or national level. Of these, one species is considered globally threatened under IUCN Red list status, viz. T. wallichiana (Endangered, IUCN, 2019). There are two species categorized as 'Near Threatened': Abies spectabilis and Juglans regia L. (Table 3). Additionally, the conservation policies of three countries in the KL have provided protection to a number of floral species (Sikkim Biodiversity Action Plan, 2012, ICIMOD et al., 2017). Among nationally threatened plant species of Bhutan, 14 species are found in the KL. Similarly, 20 plant species that are considered threatened by the Government of India and 25 species that are nationally threatened in Nepal are found in the KL.
| Family | Species | IUCNa | CITES | Threatened statusb | ||
| KL–Bhutan | KL–India | KL–Nepal | ||||
| Cycadaceae | Cycas pectinata Buch.- Ham. | √ (V) | ||||
| Pinaceae | Abies spectabilis (D. Don) Mirb. | NT | √ (GoN-Ⅱ) | |||
| Pinaceae | Larix griffithii Hook. f. | LC | √ | √ (R, T) | ||
| Pinaceae | Pinus roxburghii Sarg. | LC | √ (T) | |||
| Taxaceae | Taxus wallichiana Zucc. | E | Ⅱ | √ (V, GoN-Ⅱ) | ||
| Magnoliaceae | Magnolia campbelli Hook.f. & Thomson | Ⅱ | √ | √ (T) | ||
| Magnoliaceae | Magnolia globosa Hook.f. & Thomson | LC | Ⅱ | √ (T) | ||
| Magnoliaceae | Magnolia kisopa (Buch.-Ham. ex DC.) Figlar | |||||
| Magnoliaceae | Magnolia champaca (L.) Baill. ex Pierre | Ⅱ | √ | √ (E) | ||
| Magnoliaceae | Magnolia lanuginosa (Wall.) Figlar & Noot. | Ⅱ | √ (T) | |||
| Lauraceae | Cinnamomum glaucescens (Nees) Hand.-Mazz. | √ (T; GoN-Ⅱ) | ||||
| Dioscoreaceae | Dioscorea deltoidea Wall. ex Griseb. | Ⅱ | √ (T) | |||
| Dioscoreaceae | Dioscorea prazeri Prain & Burkill | Ⅱ | √ (T) | |||
| Melanthiaceae | Paris polyphylla Sm. | √ (V) | ||||
| Orchidaceae | Cymbidium eburneum Lindl. | √ (V) | ||||
| Orchidaceae | Cymbidium hookerianum Rchb.f. | √ (V) | ||||
| Orchidaceae | Cymbidium whiteae King & Pantl. | √ (E) | ||||
| Orchidaceae | Tipularia cunninghamii (King & Prain) S.C.Chen, S.W.Gale & P.J.Cribb | √ (E) | ||||
| Orchidaceae | Paphiopedilum venustum (Wall. ex Sims) Pfitzer | √ (V) | ||||
| Orchidaceae | Zeuxine pulchra King & Pantl. | √ (E) | ||||
| Berberidaceae | Sinopodophyllum hexandrum(Royle) T.S.Ying | √ | √ (V) | |||
| Saxifragaceae | Bergenia ciliata (Haw.) Sternb. | √ (T) | ||||
| Ranunculaceae | Aconitum ferox Wall. ex Ser. | √ | √ (E) | |||
| Ranunculaceae | Aconitum napellus L. | √ | √ (T) | |||
| Trochodendraceae | Tetracentron sinense Oliv. | Ⅲ | √ (T) | |||
| Fagaceae | Lithocarpus fenestratus (Roxb.) Rehder | √ (T) | ||||
| Juglandaceae | Juglans regia L. | NT | √ | √ (GoN-Ⅰ, Ⅲ) | ||
| Anacardiaceae | Choerospondias axillaris (Roxb.) B.L.Burtt & A.W.Hill | √ | √ (V) | |||
| Sapindaceae | Acer calcaratum Gagnep. | √ (E) | ||||
| Sapindaceae | Calamus nambariensis Becc. | √ (E) | ||||
| Polygonaceae | Rheum nobile Hook. f. & Thomson | √ | √ (T) | |||
| Ericaceae | Rhododendron barbatum Wall. ex G. Don | √ | √ (V) | |||
| Ericaceae | Rhododendron niveum Hook. f. | √ (V) | ||||
| Gentianaceae | Swertia chirayita (Roxb.) Buch.-Ham. ex C.B.Clarke | √ | √ (V) | |||
| Apocynaceae | Ceropegia hookerii C.B.Clarke ex Hook.f. | Ⅱ | √ (T) | √ (T) | ||
| Apocynaceae | Ceropegia lucida Wall. | √ (E) | ||||
| Boraginaceae | Maharanga emodi (Wall.) A. DC. | √ (T) | ||||
| Scrophulariaceae | Picrorhiza kurrooa Royle | √ | √ (V) | |||
| Scrophulariaceae | Neopicrorhiza scrophulariiflora(Pennell) D.Y.Hong | Ⅱ | √ (V, GoN-Ⅰ) | |||
| Asteraceae | Tibetoseris depressa (Hook.f. & Thomson) Sennikov | √ (E) | ||||
| Caprifoliaceae | Nardostachys jatamansi (D. Don) DC. | Ⅱ | √ | √ (V) | √ (V) | |
| Araliaceae | Panax pseudoginseng Wall. | √ | √ (E) | |||
| Apiaceae | Pimpinella tongloensis P.K. Mukherjee | √ (E) | ||||
| Apiaceae | Pimpinella wallichiana (Miq.) Gandhi | √ (E) | ||||
| a IUCN Categories: E = Endangered; V = Vulnerable; NT = Near Threatened; LC = Least Concern. b National categories: E = Endangered; V = Vulnerable; T = Threatened; R = Rare. Source: Chaudhary et al., 2015, DPR, 2012, ICIMOD et al., 2017. |
||||||
Based on our review of literature, the KL has 178 species and 4 subspecies of endemic flowering plants (Annex 1). These 182 species, including 4 subspecies, are from 105 genera and 38 families. Altogether, 8 families have one species each, and 6 families have 2 species each. Asteraceae had the highest number of endemic species, with 34 species from 18 genera (Fig. 5).
| Family | Species | KL-Bhutan | KL-India | KL-Nepal |
| Cupressaceae | Juniperus indica Bertol | √ | ||
| Cupressaceae | Juniperus recurva Ham. | √ | ||
| Annonaceae | Uvaria lurida var. sikkimensis | |||
| Orchidaceae | Ponerorchis chusua (D. Don) Soo. | √ | ||
| Orchidaceae | Bhutanthera himalayana Renz | √ | ||
| Orchidaceae | Coelogyne treutleri Hook. F. | √ | ||
| Amaryllidaceae | Allium macranthum Baker. | √ | ||
| Amaryllidaceae | Allium sikkimense Baker | √ | ||
| Arecaceae | Calamus inermis T. Anderson | √ | ||
| Eriocaulaceae | Eriocaulon exsertum Satake, in H. Hara, Fl. E. Himal. 2: 156, f. 9 (1971). | √ | ||
| Eriocaulaceae | Eriocaulon obclavatum Satake | √ | ||
| Eriocaulaceae | Eriocaulon trisectoides Satake | √ | ||
| Cyperaceae | Carex kingiana Levl. & Vant. | √ | ||
| Cyperaceae | Rhynchospora rugosa subsp. browni (Roem. & Schult.) T.Koyama | √ | ||
| Papaveraceae | Corydalis staintonii Ludlow | √ | ||
| Papaveraceae | Corydalis cashmeriana Royle. | √ | ||
| Papaveraceae | Meconopsis horridula Hook. f. & Thomson | √ | ||
| Papaveraceae | Meconopsis simplicifolia Walp. | √ | ||
| Papaveraceae | Meconopsis superba King ex Prain | √ | ||
| Berberidaceae | Berberis angulosa Wall. | √ | ||
| Berberidaceae | Berberis concinna Hook. f. & Thomson | √ | ||
| Berberidaceae | Berberis mucrifolia Ahrendt, J. RHS. 81: 135 (1956). | √ | ||
| Berberidaceae | Berberis sikkimensis (C.K.Schneid.) Ahrendt 1942 | √ | ||
| Berberidaceae | Mahonia sikkimensis Takeda | √ | ||
| Berberidaceae | Podophyllum sikkimensis Chatterjee & Mukharjee | √ | ||
| Ranunculaceae | Anemone demissa Hook. f. & Thomson | √ | ||
| Ranunculaceae | Caltha scaposa Hook.f. | √ | ||
| Ranunculaceae | Delphinium caeruleum Jacq. | √ | ||
| Ranunculaceae | Delphinium glaciale Hook.f. | √ | ||
| Ranunculaceae | Thalictrum alpinum Linn. | √ | ||
| Ranunculaceae | Aconitum staintonii Lauener | √ | ||
| Ranunculaceae | Ranunculus sikkimensis Hand.-Mazz. | √ | ||
| Saxifragaceae | Saxifraga aristulata Hook. f. & Thomson | √ | ||
| Saxifragaceae | Saxifraga flagellaris Willd. | √ | ||
| Saxifragaceae | Saxifraga hemisphaerica Hook. f. & Thomson | √ | ||
| Saxifragaceae | Saxifraga hirculus Linn. | √ | ||
| Saxifragaceae | Saxifraga jacquemontiana Dene. | √ | ||
| Saxifragaceae | Saxifraga lychinits aff. | √ | ||
| Saxifragaceae | Saxifraga pallida Wall. | √ | ||
| Saxifragaceae | Saxifraga ramulosa Wall. | √ | ||
| Saxifragaceae | Saxifraga saginoides J. D. Hooker & Thomson | √ | ||
| Crassulaceae | Rhodiola bupleuroides Wallich ex Hook.f. & Thomson | √ | ||
| Crassulaceae | Sedum fischeri R.Hamet. | √ | ||
| Vitaceae | Cissus spectabilis Planch. | √ | ||
| Fabaceae | Astragalus zemuensis W.W. Sm. | √ | ||
| Fabaceae | Caragana spinifera Kom. | √ | ||
| Fabaceae | Astragalus confertus Benth | √ | ||
| Fabaceae | Hedysarum sikkimense Benth. | √ | ||
| Fabaceae | Stracheya tibetica Benth. | √ | ||
| Fabaceae | Oxytropis tartarica Jacq. | √ | ||
| Rosaceae | Cotoneaster microphylla Wall. | √ | ||
| Rosaceae | Fragaria daltoniana J. Gay | √ | ||
| Rosaceae | Potentilla fruticose L. | √ | ||
| Rosaceae | Potentilla microphylla D. Don | √ | ||
| Rosaceae | Potentilla sinonivea Hult,n | √ | ||
| Rosaceae | Sibbaldia purpurea Hook.f. | √ | ||
| Rosaceae | Cotoneaster staintonii Klotz | √ | ||
| Rosaceae | Cotoneaster simonsi Hort. ex Baker | √ | √ | |
| Rosaceae | Potentilla monanthes var. alata Sojak | √ | ||
| Begoniaceae | Begonia dolichoptera S. Rajbhandary & KK Shrestha | √ | ||
| Begoniaceae | Begonia leptoptera H. Hara | √ | ||
| Begoniaceae | Begonia panchtharensis S Rajbhandary | √ | ||
| Violaceae | Viola biflora Linn. | √ | ||
| Violaceae | Viola bhutanica Hara | √ | ||
| Salicaceae | Salix plectilis Kimura | √ | ||
| Salicaeae | Salix calyculata Hook.f. | √ | ||
| Salicaeae | Salix lindlyeana Wall. | √ | ||
| Euphorbiaceae | Euphorbia stracheyi Boiss | √ | ||
| Euphorbiaceae | Glochidion metanubigenum Hurus. & Ya. Tanaka | √ | ||
| Geraniaceae | Geranium collinum M.Bieb. | √ | ||
| Brassicaceae | Erysimum deflexum Hook. f. & Thomson | √ | ||
| Brassicaceae | Lepidium capitatum Hook.f.& Thomson | √ | ||
| Brassicaceae | Microgynaecium tibeticum Hook.f. | √ | ||
| Brassicaceae | Thlaspi alpestre Linn. | √ | ||
| Polygonaceae | Aconogonon hookeri | √ | ||
| Polygonaceae | Polygonum sibiricum Laxm. | √ | ||
| Polygonaceae | Rheum nobile Hook.f. & Thomson | √ | ||
| Polygonaceae | Rheum spiciforme Royle | √ | ||
| Polygonaceae | Bistorta diopetes H. Ohba & S. Akiyama | √ | ||
| Caryophyllaceae | Arenaria ciliolata Edgew. | √ | ||
| Caryophyllaceae | Arenaria densissima Wall. | √ | ||
| Caryophyllaceae | Arenaria glanduligera Edgew | √ | ||
| Caryophyllaceae | Arenaria monticola | √ | ||
| Caryophyllaceae | Arenaria melandroyoides Edgew. | √ | ||
| Caryophyllaceae | Arenaria musciformis Wall. | √ | ||
| Caryophyllaceae | Arenaria polytrichoides Edgew | √ | ||
| Caryophyllaceae | Arenaria thangoensis W.W. Sm. | √ | ||
| Caryophyllaceae | Silene apetala Willd. | √ | ||
| Caryophyllaceae | Silene caespitella F. N. Williams | √ | ||
| Caryophyllaceae | Silene nigrescens (Edgeworth) Majumdar | √ | ||
| Caryophyllaceae | Stellaria decumbens Edgew. | √ | ||
| Hydrangeaceae | Ribes luridum Hook. f. & Thomson | √ | ||
| Primulaceae | Androsace selago Klatt. | √ | ||
| Primulaceae | Primula sikkimensis Hook.f. | √ | ||
| Primulaceae | Primula tibetica Watt | √ | ||
| Primulaceae | Bryocarpum himalaicum Hook. f. & Thomson | √ | ||
| Ericaceae | Cassiope fastigata (Wallich).D.Don. | √ | ||
| Ericaceae | Cassiope selaginoides Hook. f. & Thomson | √ | ||
| Ericaceae | Gaultheria trichophylla Royle. | √ | ||
| Ericaceae | Rhododendron nivale Hook.f. | √ | ||
| Ericaceae | Rhdododendron sikkimense Pradhan & Lachungpa | √ | ||
| Ericaceae | Rhododendron campanulatum subsp. aeruginosum (Hook.f.) Chamb. | √ | ||
| Ericaceae | Rhododendron dalhousiae subsp. tashi Pradhan & Lachungpa | √ | ||
| Ericaceae | Rhododendron niveum Hook.f. | √ | ||
| Gentianaceae | Gentiana detonsa Fries | √ | ||
| Gentianaceae | Gentiana ornata (G.Don).Griesb | √ | ||
| Gentianaceae | Gentiana robusta King. | √ | ||
| Gentianaceae | Swertia multicaulis Don. | √ | ||
| Boraginaceae | Eritrichium pustulosum C. B. Clarke | √ | ||
| Boraginaceae | Eritrichium pygmaeum Clarke | √ | ||
| Boraginaceae | Microula pustulosa (C. B. Clarke) Duthie | √ | ||
| Boraginaceae | Onosoma hookeri Clarke | √ | ||
| Boraginaceae | Maharanga verruculosa (I. M. Johnst.) I. M. Johnst | √ | ||
| Plantaginaceae | Lagotis glauca Gaertn. | √ | ||
| Scrophulariaceae | Euphrasia officinalis Linn. | √ | ||
| Scrophulariaceae | Lancea tibetica Hook. f. & Thomson | √ | ||
| Scrophulariaceae | Oreosolen wattii Hook.f. | √ | ||
| Scrophulariaceae | Parnassia nubicola Wall. | √ | ||
| Acanthaceae | Justicia tukuchensis V. A. W. Graham | √ | ||
| Lamiaceae | Dracocephalum heterophyllum Benth | √ | ||
| Lamiaceae | Elsholtzia eriostachya Benth. | √ | ||
| Lamiaceae | Nepeta discolor Royle. ex Bentham | √ | ||
| Lamiaceae | Phlomis rotata Bentham ex J.D. Hooker | √ | ||
| Orobanchaceae | Pedicularis integrifolia Hook.f. | √ | ||
| Orobanchaceae | Pedicularis lachnoglossa Hook.f. | √ | ||
| Orobanchaceae | Pedicularis longifolia Rudolph | √ | ||
| Orobanchaceae | Pedicularis roylei Maxim. | √ | ||
| Orobanchaceae | Pedicularis trichoglossa Hook. f. | √ | ||
| Orobanchaceae | Pedicularis anserantha T. Yamaz. | √ | ||
| Orobanchaceae | Pedicularis ingentoides T. Yamaz. | √ | ||
| Orobanchaceae | Pedicularis terrenoflora T. Yamaz. | √ | ||
| Campanulaceae | Campanula aristata Wall. | √ | ||
| Campanulaceae | Campanula immodesta Hook. f. & Thomson | √ | ||
| Campanulaceae | Codonopsis foetens Hook. f. & Thomson | √ | ||
| Campanulaceae | Codonopsis thalictrifolia Wall. | √ | ||
| Campanulaceae | Cyananthus incanus Hook. f. & Thomson | √ | ||
| Campanulaceae | Codonopsis affinis Hook. f. & Thomson | √ | ||
| Asteraceae | Anaphalis xylorhiza Schultz-Bip | √ | ||
| Asteraceae | Artemisia biennis Willd. | √ | ||
| Asteraceae | Artemisia campbelli Hook. f. & Thomson | √ | ||
| Asteraceae | Artemsia salsoloides Willd. | √ | ||
| Asteraceae | Aster diplostephoides Benth. | √ | ||
| Asteraceae | Chionocharis hookeri (C. B. Clarke) I. M. Johnston | √ | ||
| Asteraceae | Cremanthodium decaisnei Clarke | √ | ||
| Asteraceae | Cremanthodium oblongatum Clarke | √ | ||
| Asteraceae | Cremanthodium reniforme Benth | √ | ||
| Asteraceae | Hippolytia gossypina Hook. f. & Thomson | √ | ||
| Asteraceae | Leontopodium haastioides Hand.-Mazz | √ | ||
| Asteraceae | Saussurea gossypiphora Don. | √ | ||
| Asteraceae | Saussurea heiracioides Hook.f | √ | ||
| Asteraceae | Saussurea katochaete | √ | ||
| Asteraceae | Saussurea leontodontoides (DC.) | √ | ||
| Asteraceae | Saussurea stella Maxim. | √ | ||
| Asteraceae | Saussurea tridactyla Clarke | √ | ||
| Asteraceae | Saussurea uniflora Wall. | √ | ||
| Asteraceae | Saussurea werneroides Hook.f. | √ | ||
| Asteraceae | Soroseris glomerata (Decne.) Stebb | √ | ||
| Asteraceae | Waldheimia tridactylites | √ | ||
| Asteraceae | Youngia depressa Hook. f. & Thomson | √ | ||
| Asteraceae | Youngia gracilipes Hook.f. | √ | ||
| Asteraceae | Anaphalis cavei Chatterjee | √ | ||
| Asteraceae | Anaphalis hookeri C.B. Clarke | √ | ||
| Asteraceae | Cacalia chola W. W. Sm. | √ | ||
| Asteraceae | Cremanthodium palmatum subsp. benthamii | √ | ||
| Asteraceae | Crepis atropappa Babcock | √ | ||
| Asteraceae | Jurinea cooperi J. Anthony | √ | ||
| Asteraceae | Ligularia kingiana (W. W. Smith) R. Mathur | √ | ||
| Asteraceae | Ligularia yakla (C. B. Clarke) V. Singh & P. Singh | √ | ||
| Asteraceae | Saussurea topkegolensis H. Ohba & S. Akiyama | √ | ||
| Asteraceae | Senecio brunneo-villosus Kitam. | √ | ||
| Asteraceae | Senecio topkegolensis Kitam. | √ | ||
| Caprifoliaceae | Lonicera hispida Poll. | √ | ||
| Caprifoliaceae | Pterocephalus hookeri (C. B. Clarke) Diels | √ | ||
| Caprifoliaceae | Morina nepalensis D. Don. | √ | ||
| Caprifoliaceae | Nardostachys jatamansi (D. Don) DC. | √ | ||
| Apiaceae | Chamaesium novem-jugum | √ | ||
| Apiaceae | Cortia hookeri Clarke | √ | ||
| Apiaceae | Cortiella lamondiana Fullarton & M.F. Watson | √ | ||
| Apiaceae | Acronema pseudotenera P.K. Mukh. | √ | ||
| Apiaceae | Angelica harae Pimenov | √ | ||
| Apiaceae | Angelica nubigena P.K. Mukh. | √ | ||
| Sources: Rajbhandari and Adhikari (2009), Rajbhandari and Dhungana(2010, 2011), Rajbhandary et al. (2010), Telwala et al. (2013), GBPNIHESD (2015), NCD (2015), ICIMOD et al. (2017), ENVIS Centre Sikkim (2019). | ||||
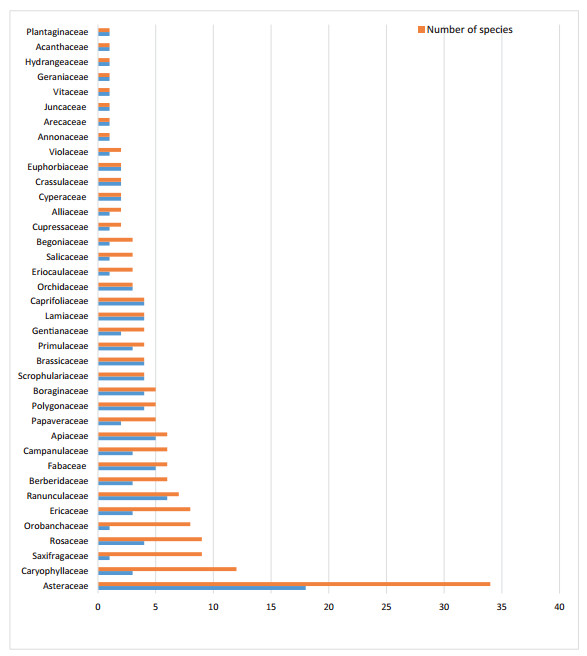
|
| Fig. 5 Number of endemic genera and species per family in the Kangchenjunga Landscape. |
Preliminary assessment of the landscape through literature review (Majumdar et al., 1984, Shrestha and Ghimire, 1996, Maiti and Maiti, 2007, O'Neill et al., 2017) and analyses indicate that the KL harbours more than 5100 species of flora, which includes 700 + orchid species, 40 + rhododendron species and 700 + medicinal and aromatic plants. A substantial number of these plants are either endemic to the region or threatened nationally and globally.
Despite being an area of interest to botanists since the 19th Century, many parts of the KL still remain inadequately explored. Biological surveys are extremely difficult in many remote and inaccessible mountainous parts of the landscape. Thus, many floral taxa are inadequately studied and the degree of their richness is underestimated. Species richness in the KL is likely to increase with a systematic biodiversity inventory and scientific research on distribution patterns, especially in the remote and less explored areas. For instance, in the KL–Nepal, in the Kangchenjunga-Singhalila ridge bordering India, a preliminary survey of vascular plants recorded 598 species of flowering plants, of which 12 species were reported as being new to Nepal, and two species of Begonia (Begonia dolichoptera S. Rajbhandary & K.K. Shrestha, and Begonia panchtharensis S. Rajbhandary) were reported as being new to science (Shrestha et al., 2008). Similarly, in Sikkim alone in the KL–India, flowering plants are represented by about 4500 species belonging to 1371 genera of 197 families (Bhutia et al., 2002, Subba, 2002, Badola and Subba, 2012). The Darjeeling hills also have high floral diversity, representing an estimated one-seventh of the flora of India with about 4000 species of flowering plants under 160 families (Bhujel, 1996). Similarly, out of 736 recorded plant species in the KL–Bhutan, there are 427 species of flora under 115 families in the Jigme Khesar Strict Nature Reserve alone (Rai et al., 2008). This further underscores the importance of the KL in terms of biodiversity.
Our analysis indicates that most survey efforts have been in the KL–India. This could be attributed to the fact that the largest part of the KL is covered by the KL–India as compared to Nepal and Bhutan and also because 17 of the 19 protected areas of the KL are located in the KL–India, where, during the colonial period, a fairly substantial number of surveys were carried out. Furthermore, in Nepal botanical explorations have been slowed because foreigners were not allowed to travel outside of Kathmandu until after 1949 (Rajbhandari, 2016). Also, significant contributions to research in the KL-India have been expedited by the presence of two state level universities, i.e., North Bengal University and Sikkim University, and research institutes such as the Botanical Survey of India and the Govin Ballabh Pant National Institute of Himalayan Environment and Sustainable Development (Kandel et al., 2016). However, only 14% of the publications are from protected areas of the KL. Although protected areas cover 30% of the total landscape, comprehensive documentation of the flora of these protected areas is scarce. Indeed, many protected areas lack even basic information such as species checklists. Moreover, in cases in which documentation is available, access to that information is limited.
Our analyses also reveal that the majority of publications are focused on vegetation survey followed by ethonobotanical studies and documentation of NTFPs. Among the vegetation surveys, the majority are related to diversity and distribution of floral species. This could be credited to the exploratory studies carried out in Sikkim and Darjeeling that are basically the checklists of flora prepared during excursions in the state's hills (For instance, Hooker, 1849, King and Pantling, 1889, Matthew, 1971, Mehra and Bir, 1964). More recently, ecological research is gaining momentum in the KL. Some representative ecological research on the flora of the KL include those by Acharya et al., 2010, Chettri et al., 2002, Dey et al., 2015, Moktan et al., 2009, Singh and Sundriyal, 2005, Subba et al., 2015, Pradhan and Badola, 2015, Tambe and Rawat, 2010. A small number of studies are related to new records of floral species (for instance, Chettri et al., 2012, Chettri et al., 2012, Chowdhury et al., 2013, Thapa and Lama, 2015) and exploration of newer populations of rare rhododendrons (Badola and Pradhan, 2010a, Badola and Pradhan, 2010b) and micro-habitat diversity and conservation aspects of threatened species (Pradhan and Badola, 2015). This indicates the high biodiversity value of the KL in terms of species richness, which may increase with more intensive and systematic exploration in the less explored areas. A substantial number of studies have focused on ethnobotanical aspects (Pradhan and Badola, 2008, Uprety et al., 2016, O'Neill et al., 2017), which indicates that the KL is rich in traditional knowledge and local people are not only aware of the valuable attributes of these species but also the community ecology and life histories of diverse floral species.
The substantial number of surveys on NTFPs indicates that they are particularly important in the KL because they contribute greatly to the livelihood and economy of a large proportion of rural communities (Badola and Pradhan, 2013, O'Neill et al., 2017, Uprety et al., 2016). Most commonly used NTFPs include medicinal plants and wild edibles (Chettri et al., 2005). Publications indicate that the domestic as well as cross-border trade of NTFPs is prevalent in the region since the historical times and trades are both legal and illegal (Chaudhary et al., 2015, Choudhary, 2008). Medicinal plants are the most traded NTFPs, followed by some wild edible plants and fibre-yielding plants. As previously alluded to, a substantial number of floral species in the KL are endemic and threatened nationally and globally. The number of endemic species from the region has been widely cited and reported (Myers, 1988, CEPF, 2005, CFPF, 2007; Chettri et al., 2010). The list prepared for this review exceeds the number recorded for the Eastern Himalayas by Behera et al. (2002). Myers (1988) has already reported that about 60% of the flora from Sikkim are endemic. Likewise, Bhutan and Nepal as expected to have 15% and 8% endemics, respectively (Anonymous, 1992). The high floristic diversity of the KL is attributed to contributions from the surrounding floristic regions such as Chinese, Malayan and Siberian elements (Hooker, 1904).
Like many biodiversity-rich landscapes in the world, the KL is also faced with numerous local and transboundary challenges, both natural and anthropogenic, which threaten its rich biological diversity. The evaluation of threats being faced by the biodiversity of the KL shows that rapid population growth has led to widespread logging, habitat fragmentation and extensive clearing of forests, and grasslands for cultivation and large scale infrastructural development, including construction of highways and large dams (Gurung, 2006, Grumbine and Pandit, 2013, Krishna et al., 2002, Pandit and Grumbine, 2012). Only one study in the KL assessed the disturbance indices of threatened herbs in Sikkim, which highlighted human movement and NTFP collection as top conservation threats (Pradhan and Badola, 2015). The geospatial analyses of land cover in the KL reveals the conversion of forests and rangeland to other land uses, particularly transformation of pristine habitats into extensive monocultures of tea and cardamom, which may greatly reduce the species richness of the landscape. Additional major threats to floral diversity throughout the KL include invasive species, livestock overgrazing, erosions and landslides caused by deforestation of extremely steep slopes, and unsustainable and/or illegal extraction and utilization of economically important species (Chaudhary et al., 2015, ICIMOD et al., 2017, Pradhan and Badola, 2015, Uprety et al., 2016, O'Neill et al., 2017).
In order to address various conservation and development opportunities and challenges that lie within this landscape, several conservation efforts are being made at the national and transboundary levels. The governments of Bhutan, India and Nepal have jointly initiated the Kangchenjunga Landscape Conservation and Development Initiative (KLCDI) which adopts transboundary ecosystem management approaches to ensure biodiversity conservation and sustainable development in the region (ICIMOD et al., 2017). Nine out of 19 protected areas in the KL are transboundary in nature (see Kandel et al., 2016 for details of the protected areas). Except for two protected areas (one in the KL–Bhutan and the other in the KL–Nepal), all other protected areas are located in KL–India. These protected areas are inhabited by many charismatic floral species. The Khangchendzonga Biosphere Reserve has recently been designated as a UNESCO World Heritage Site for its outstanding natural/biodiversity and cultural values, becoming the first 'Mixed Heritage' site in India. There is only one Ramsar site, the Mai Pokhari in the KL, which was established on 28 October 2008. With the catchment area of around 12 ha, it is situated at an elevation of 2100 m a.s.l. in Ilam District, Nepal. It is located in the center of the Kangchenjunga-Singhalila Biodiversity Landscape Complex, a biological corridor that forms a part of the Himalayan biodiversity hotspot, which is also recognized as an endemic bird area and a center for plant species dispersal and diversity (CEPF, 2005). The dominant vegetation of this site is cloud forest and East-Himalayan oak-laurel forest. A total of 231 plant species belonging to 185 genera and 78 families has been recorded in this area. Of the recorded species, 62 are medicinal and aromatic plants, 17 are orchids, five rhododendrons and three magnolias. Among these, 22 species of flowering plants are globally threatened and are listed in the IUCN Red List and/or in CITES appendix (DFO, 2012).
In addition, eleven Important Plant Areas (IPAs) for medicinal plants have been identified in the KL (Hamilton and Radford, 2007, GWB, 2010) – 2 in KL–Bhutan, 7 in KL–India, and 18 in KL–Nepal. In KL–Bhutan, the IPAs are Chele La (2500–4000 m a.s.l.) and Toorsa (1600–3000 m a.s.l.). In KL–India, the IPAs are Dzongri-Phedang-Sandakphu (3600–4000 m a.s.l.); Lachen and Lachung (2750–3000 m a.s.l.); North Rajabhatkhawa in Buxa Tiger Reserve, Jalpaiguri; Sursuti in Jalpaiguri; and Dhotrey, North Sevoke and Tonglu in Darjeeling. In KL–Nepal, the IPAs are Yamphudin-Hellok, Gyapla-Ghunsa, Ghunsa-Khangbachen, SarjuPokhari- Olangchung Gola, Dorangdin-Ramje, and Chairam-Yalung in Taplejung District; Timbung Pokhari, Lam Pokhari- Suke Pokhari-Ose, Bhaise Pokhari, Mejartham-Chiwabhanjyang, and Tinsimana-Gorkhepani-Fokte in Panchthar District; Hangetham, Kala Pokhari, Chintapu, Sandakphu, and Dhupi-Guranse in Ilam District; and Ghorwa-Sanischare, Gauriganj-Kathgara, and Jalthal Forest in Jhapa District (ICIMOD et al., 2017). A rich diversity of floral species, many of which are of high botanical value, thrive in these natural and semi-natural sites.
5. ConclusionThe Kangchenjunga Landscape has been an area of interest to botanists since the mid-19th century and since then it has continued to gain attention from researchers around the world. One of the richest biodiversity repositories in the Eastern Himalayas, the landscape is home to many charismatic floral species of high botanical value. Our review shows that there are many species that are threatened at national and global levels and are endemic to the landscape. Furthermore, the unique ethnic and social groups residing in the landscape have rich traditional knowledge regarding utilization and conservation of these valuable species. Yet, conservation of biodiversity in the Kangchenjunga landscape faces many threats and challenges. In this regard, the KLCDI, which was established by India, Nepal, and Bhutan to conserve and sustainably develop the Kangchenjunga Landscape, is very timely and progressive.
Based on our findings, we recommend scientific and comprehensive floral surveys, particularly in the less explored areas. For instance, some protected areas (e.g., Mahananda Wildlife Sanctuary) and western Bhutan – excluding Jigme Khesar Strict Nature Reserve – have been inadequately studied. Although numerous scattered and one-time studies of the Kangchenjunga landscape exist, long-term data and monitoring are needed, especially to understand how natural and anthropogenic drivers cause changes in the landscape that affect abundance, phenology, and distribution of species. Comprehensive and systematic studies in the protected areas of the Kangchenjunga Landscape would provide important insights into the rich floral diversity of the region. Proper management of Important Plant Areas could provide a safety net for many threatened floral species in the landscape. We recommend that current policies and sustainable conservation interventions adopt transboundary landscape conservation programmes and joint activities that involve long-term regional cooperation, knowledge, and data sharing. Importantly, to bridge the existing gaps in our knowledge regarding biodiversity in the Kangchenjunga Landscape, conservation and development organizations, government agencies, academic institutions, and the private sector must join their efforts.
All the authors declare that there is no conflict of interest.
The authors would like to express their gratitude to Dr David Molden, Director General of ICIMOD, for his inspiration and support. The authors would like to acknowledge the support provided by Mr Kabir Uddin, GIS and Remote Sensing Specialist, ICIMOD for providing the map used in the article. The continuous support and commitment from ICIMOD's eight regional member countries is also acknowledged, as is the support of the Austrian Development Agency (ADA) and the German Federal Ministry for Economic Cooperation and Development through its German Agency for International Cooperation (GIZ), which made this publication possible. The authors would also like to thank Thomas Samuel Moloppomannil, Senior Editor, ICIMOD and Raymond Porter for English language editorial inputs. We are highly indebted to the two anonymous reviewers for their critical review and constructive suggestions to bring the paper in the present form. The views and interpretations in this publication are those of the authors and are not necessarily attributable to the affiliated organizations.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2019.04.006.
Acharya J., Acharya J., Mukharjee A., 2010. A contribution to the study of the Caprifoliaceae jussieu in West Bengal and Sikkim, India. Pleione, 4(1): 42-47. |
Anonymous, 1992. World Conservation Monitoring Centre. Global Biodiversity.Chapman and Hall, London.
|
Arya S.C., Sunny N., 2016. Assessment of tree diversity and resource use pattern in bath Putu forest, Itanagar, Arunachal Pradesh. Int. J. Environ. Sci, 5(3): 166-172. |
APG, 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG Ⅳ. Bot. J. Linnean Soc. 181, 1-20.https://doi.org/10.1111/boj.12385.
|
Badola H.K., Pradhan B.K., 2010a. Population exploration of Rhododendron maddenii in Sikkim, bordering Khangchendzonga biosphere reserve-questioning rarity and endangerment. NeBIO, 1: 1-9. |
Badola H.K., Pradhan B.K., 2010b. Discovery of new populations of a rare species Rhododendron niveum in Khangchendzonga National Park, Sikkim. Rhododendron, 50: 41-49. |
Badola H.K., Pradhan B.K., 2013. Plants used in healthcare practices by Limboo tribe in south ewest of Khangchendzonga Biosphere Reserve, Sikkim, India. Indian J.Trad. Knowl, 12(3): 355-369. |
Badola, H.K., Subba, J.B., 2012. Khangchendzonga Biosphere Reserve (Sikkim). Pp. 133-142. In: Palni, L.M.S., Rawal, R.S., Rai, R.K., Reddy, S.V. (Eds.), Compendium on Indian Biosphere Reserves: Progression during Two Decades of Conservation, GBPIHED, Kosi-Almora and Ministry of Environment & Forests (Govt of India).
|
Basnet K., Badola H.K., 2012. Birds of Fambonglho Wildlife Sanctuary, Sikkim, India:a baseline survey for conservation and area management. NeBIO, 3(2): 1-12. |
Behera M.D., Kushwaha S.P.S., Roy P.S., 2002. High plant endemism in an Indian hotspoteeastern Himalaya. Biodivers. Conserv, 11(4): 669-682. DOI:10.1023/A:1015596309833 |
Bhujel, R.B., 1996. Studies on the Dicotyledonous Flora of Darjeeling District (PhD Thesis). North Bengal University, Darjeeling.
|
Bhutia, C., Chettri, N., Tambe, S., 2002. Khangchendzonga, the Sacred Mountain: A Biodiversity Handbook. Khangchendzonga Conservation Committee, Yuksam, Sikkim.
|
Brooks T.M., Mittermeier R.A., da Fonseca G.A., Gerlach J., Hoffmann M., Lamoreux J.F., Mittermeier C.G., Pilgrim J.D., Rodrigues A.S., 2006. Global biodiversity conservation priorities. Science, 313(5783): 58-61. DOI:10.1126/science.1127609 |
Campbell A., 1840. Note on the Lepchas of Sikkim, with a vocabulary of their language. J. Asiat. Soc. Bengal, 9: 379-393. |
Carpenter C., 2005. The environmental control of plant species density on a Himalayan elevation gradient. J. Biogeogr, 32: 999-1018. DOI:10.1111/j.1365-2699.2005.01249.x |
CEPF, 2005. Ecosystem Profile: Indo-Burman Hotspot, Eastern Himalayan Region.WWF, US-Asian Programme/Critical Ecosystem Partnership Fund, Kathmandu.
|
CFPF, 2007. Ecosystem Profile: Indo-Burma Hotspot, Indo-China Region. WWF USAsian Program.
|
Chapman, A.D., 2005. Uses of Primary Species-Occurrence Data Version 1.0. Report for the Global Biodiversity Information Facility, Copenhagen. Available at: http://www.niobioinformatics.in/books/Uses%20of%20Primary%20Data.pdf.Accessed on 12 May 2008.
|
Chaudhary, R.P., Uprety, Y., Joshi, S.P., Basnet, K., Basnet, G., Shrestha, K.R., Bhatta, K.P., Acharya, K.P., Chettri, N., 2015. Kangchenjunga Landscape Nepal: from Conservation and Development Perspectives. Kathmandu: Ministry of Forests and Soil Conservation (MoFSC). Government of Nepal; Research Centre for Applied Science and Technology (RECAST), Tribhuvan University; and International Centre for Integrated Mountain Development (ICIMOD).
|
Chettri A., Barik S.K., Lyngdoh M.K., Pandey H.N., 2009. Notes on geographic distribution. Plantae, magnoliophyta, gentianales, apocynaceae, asclepiadoideae, Ceropegia hookeri:distribution and rediscovery in eastern Himalayas, Sikkim, India. Check List, 5(3): 695-698. DOI:10.15560/5.3.695 |
Chettri A., Barik S.K., Singh B., Adhikari D., Lyngdoh M.K., 2012. Cornus kousa F. Buerger ex Hance subsp. Kousa (Cornaceae):a new record from India. Taiwania, 57(1): 77-81. |
Chettri, N., Sharma, E., Shakya, B., Thapa, R., Bajracharya, B., Uddin, K., Oli, K.P., Choudhary, D., 2010. Biodiversity in the Eastern Himalayas: Status, Trends and Vulnerability to Climate Change. ICIMOD, Kathmandu, Nepal.
|
Chettri, N., Shakya, B., Sharma, E., 2008. Biodiversity Conservation in the Kangchenjunga Landscape. ICIMOD, Kathmandu.
|
Chettri N., Sharma E., Deb D.C., 2001. Bird community structure along a trekking corridor of Sikkim Himalaya:a conservation perspective. Biol. Conserv, 102(1): 1-16. |
Chettri N., Sharma E., Deb D.C., Sundriyal R.C., 2002. Effect of firewood extraction on tree structure, regeneration, and woody biomass productivity in a trekking corridor of the Sikkim Himalaya. Mt. Res. Dev, 22(2): 150-158. DOI:10.1659/0276-4741(2002)022[0150:IOFEOT]2.0.CO;2 |
Chettri N., Sharma E., Lama S.D., 2005. Non-timber forest produces utilization, distribution and status in a trekking corridor of Sikkim, India. Lyonia, 8(1): 89-101. |
Choudhary, D., 2008. Potential micro-enterprises and income generating activities in the Kangchenjunga Landscape. In: Chettri, N., Shakya, B., Sharma, E. (Eds.), Biodiversity Conservation in the Kangchenjunga Landscape. ICIMOD, Kathmandu, pp. 133-140.
|
Chowdhury A., Chowdhury M., Choudhury D., Das A.P., 2013. Ludwigia peruviana (Linnaeus) H. Hara [Onagraceae]:a new record for West Bengal, India. Pleione, 7(1): 286-289. |
Díaz S., Pascual U., Stenseke M., Martín-Lopez B. Watson R.T., Molnáar Z., et al, 2018. Assessing nature's contributions to people. Science, 359(6373): 270-272. DOI:10.1126/science.aap8826 |
Das K., Verbeken A., 2012. New species of Lactarius Subg. Plinthogalus and new records of Lactifluus Subg. Gerardii (Russulaceae) from Sikkim, India. Taiwania, 57(1): 37-48. |
Dey S.B., Ghosh R., Shekhar M., Mukherjee B., Bera S., 2015. What drives elevational pattern of phytolith diversity in Thysanolaena maxima (Roxb.) O. Ktze? A study from the Darjeeling Himalayas. Flora (JENA), 211: 51-61. |
DFO, 2012. Management Plan of Mai Pokhari Ramsar Site, Ilam, Nepal. District Forest Office, Ilam, Nepal.
|
DPR, 2012. Plants of Nepal: Fact Sheet. Kathmandu. Department of Plant Resources, Ministry of Forests and Soil Conservation, Kathmandu, Nepal.
|
ENVIS Centre Sikkim, 2019. Endemic Plants. Forest, Environment and Wildlife Management Department, Government of Sikkim, Gangtok, India. Downloaded from: http://sikenvis.nic.in/WriteReadData/UserFiles/file/List%20of%20Endemic%20Plant%202015.pdf. Accessed on: 20 January 2019.
|
GBPNIHESD, 2015. Feasibility Assessment Report-Kangchenjunga Landscape-India.G B Pant National Institute of Himalayan Environmental and Sustainable development, Sikkim.
|
Grierson, A.J., Long, D.G., 1983. Flora of Bhutan, Including a Record of Plants from Sikkim, vol. 1. Royal Botanic Gardens Edinburgh, United Kingdom parts 1 and 2.
|
Grumbine R.E., Pandit M.K., 2013. Threats from India's Himalaya dams. Science, 339(6115): 36-37. DOI:10.1126/science.1227211 |
Gurung, G.S., 2006. Reconciling Biodiversity Conservation Priorities with Livelihood Needs in Kangchenjunga Conservation Area. Human Geography Series. University of Zurich, Switzerland.
|
GWB, 2010. State Report on National Programme on Promoting Medicinal Plants Conservation and Traditional Knowledge for Enhancing Health and Livelihood Security for West Bengal (UNDP-CCF-Ⅱ Project No. 13047). Research Circle, Directorate of Forests, Government of West Bengal, Kolkata. http://www.westbengalforest.gov.in/publication_pdf/UNDP%20CCF%20II%20Report%20WBengal.pdf. Accessed on 17 May 2015.
|
Hajra P.K., Chakraborty P., 1981. A survey of wild plants sold in the Lal market of Gangtok. Ind. J. For, 4(3): 217-220. |
Hamilton, A.C., Radford, E.A., 2007. Identification and Conservation of Important Plant Areas for Medicinal Plants in the Himalaya. Salisbury: Plantlife International and Kathmandu. Ethnobotanical Society of Nepal.
|
Hooker J.D., 1852. On the climate and vegetation of the temperate and cool regions of East Nepal and the Sikkim Himalayan mountains. J. Hort. Soc. Lond, 7: 69-131. |
Hooker, S.J.D., 1849. Notes Chiefly Botanical, Made during an Excursion from Darjeeling to Tonglo: A Lofty Mountain on the Confines of Sikkim and Nepal. J.Thomas at the Baptist Mission Press.
|
Hooker, J.D., 1904. A Sketch of the Flora of British India. Oxford.
|
ICIMOD, WCD, GBPNIHESD, RECAST, 2017. Kangchenjunga Landscape Conservation and Development Initiative Feasibility Assessment Report e Regional Synthesis.ICIMOD Working Paper 2017/9. ICIMOD, Kathmandu.
|
IUCN, 2019. IUCN red list. Downloaded from. https://www.iucnredlist.org/species/46171879/9730085 on 15 January 2019.
|
Kandel P., Gurung J., Chettri N., Ning W., Sharma E., 2016. Biodiversity research trends and gap analysis from a transboundary landscape, Eastern Himalayas. J. Asia Pac. Biodiv, 9(1): 1-10. DOI:10.1016/j.japb.2015.11.002 |
Kholia, B.S., 2011. Pteridophytic wealth of Sikkim Himalaya, pp43-65. In: Arrawatia, M.L., Tambe, S. (Eds.), Biodiversity of Sikkim Exploring and Conserving a Global Hotspot. Information and Public Relations Department, Gangtok.
|
King G., Pantling R., 1889. The orchids of the Sikkim Himalaya. Ann. Roy. Bot. Gard.Calcutta, 8(2-4): 1-448. |
Krishna A.P., Chhetri S., Singh K.K., 2002. Human dimensions of conservation in the Khangchendzonga Biosphere Reserve:the need for conflict prevention. Mt.Res. Dev, 22(4): 328-331. DOI:10.1659/0276-4741(2002)022[0328:HDOCIT]2.0.CO;2 |
Kunwar R.M., Shrestha K., Dhungana S.K., 2010. Floral biodiversity of Nepal:an update. J. Nat. Hist. Mus, 25: 295-311. |
Lucksom, S.Z., 2007. The Orchids of Sikkim and North East Himalaya: Development Area. Jiwan Thing Marg, Gangtok, East Sikkim, p. 984.
|
Maiti, D., Maiti, G.G., 2007. Diversity of vascular plants of Kanchenjunga Biosphere Reserve, Sikkim, and its conservation. Ind. For. 1416-1436.
|
Majumdar N.C., Krishna B., Biswas M.C., 1984. Vegetation of Neora valley and adjacent regions in Kalimpong forest division, West Bengal. J. Econ. Taxon. Bot, 5(5): 1013-1025. |
Manish K., Pandit M.K., 2018. Phylogenetic diversity, structure and diversification patterns of endemic plants along the elevational gradient in the Eastern Himalaya. Plant Ecol. Divers, 11(4): 501-513. DOI:10.1080/17550874.2018.1534147 |
Manish K., Pandit M.K., Telwala Y., Nautiyal D.C., Koh L.P., Tiwari S., 2017. Elevational plant species richness patterns and their drivers across non-endemics, endemics and growth forms in the Eastern Himalaya. J. Plant Res, 130(5): 829-844. DOI:10.1007/s10265-017-0946-0 |
Matthew K.M., 1971. The pteridophytes of the Darjeeling district. Bull. Bot. Soc.Bengal, 25: 97-102. |
Mehra P.N., Bir S.S., 1964. Pteridophytic flora of Darjeeling and Sikkim Himalayas. Resour. Bull Punjab Univ, 15(1-2): 69-181. |
Mittermeier, R.A., Gils, P.R., Hoffman, M., Pilgrim, J., Brooks, T., Mittermeier, C.G., et al., 2004. Hotspots Eevisited. Earth's Biologically Richest and Most Endangered Terrestrial Ecoregions. CEMEX/Agrupacion Sierra Madre, Mexico City.
|
Ministry of Natural Resources and Environment, 2014. Fifth National Report to the Convention on Biological Diversity, Malaysia. https://www.cbd.int/doc/world/my/my-nr-05-en.pdf. (Accessed 13 February 2019).
|
Moktan M.R., Gratzer G., Richards W.H., Rai T.B., Dukpa D., 2009. Regeneration and structure of mixed conifer forests under single-tree harvest management in the western Bhutan Himalayas. For. Ecol. Manag, 258(3): 243-255. DOI:10.1016/j.foreco.2009.04.013 |
Myers N., 1988. Threatened biotas:'hotspots' in tropical forestry. Environmentalist, 8: 1-20. DOI:10.1007/BF02240305 |
NCD, 2015. Feasibility Assessment Report-Kangchenjunga Landscape-Bhutan. Nature Conservation Division, Department of Forest and Park Services, Thimphu.
|
Numata M., 1966. Vegetation and conservation in eastern Nepal. J. Coll. Arts Sci.Chiba Univ. Nat. Sci, 4: 559-569. |
O'Neill A.R., Badola H.K., Dhyani P.P., Rana S.K., 2017. Integrating ethnobiological knowledge into biodiversity conservation in the Eastern Himalayas. J. Ethnobiol.Ethnomed, 13(1): 21. DOI:10.1186/s13002-017-0148-9 |
Ohsawa M., Shakya P.R., Numata M., 1986. Distribution and succession of west Himalayan forest types in the eastern part of the Nepal Himalaya. Mt. Res. Dev: 143-157. |
Olson M., Dinerstein E., Wikramanayake E.D., Burgess N.D., Powell G.V.N., Underwood E.C., D'Amico J.A., Itoua I., Strand H.E., Morrison J.C., Loucks C.J., Allnutt T.F., Ricketts T.H., Kura Y., Lamoreux J.F., Wettengel W.W., Hedao P., Kassem K., 2001. Terrestrial ecoregions of the world:a new map of life on earth. Biol. Sci, 51(11): 933-938. |
Pala N.A., Sarkar B.C., Shukla G., Chettri N., Deb S., Bhat J.A., Chakravarty S., 2019. Floristic composition and utilization of ethnomedicinal plant species in home gardens of the Eastern Himalaya. J. Ethnobiol. Ethnomed, 15(1): 14. DOI:10.1186/s13002-019-0293-4 |
Pandit M.K., Grumbine R.E., 2012. Potential effects of ongoing and proposed hydropower development on terrestrial biological diversity in the Indian Himalaya. Conserv. Biol, 26(6): 1061-1071. DOI:10.1111/j.1523-1739.2012.01918.x |
Pradhan B.K., Badola H.K., 2015. Swertia chirayta, a threatened high-value medicinal herb:Microhabitats and conservation challenges in Sikkim Himalaya, India. Mt. Res. Dev, 35(4): 374-381. DOI:10.1659/MRD-JOURNAL-D-14-00034.1 |
Pradhan B.K., Badola H.K., 2008. Ethnomedicinal plant use by Lepcha tribe of Dzongu valley bordering Khangchendzonga Biosphere Reserve, in North Sikkim, India. J. Ethnobiol. Ethnomed, 4: 22. DOI:10.1186/1746-4269-4-22 |
Pradhan, K.C., Lachungpa, S.T., 1990. Sikkim Himalayan Rhododendrons. Primulaceae Books, Kalimpong.
|
Proença, V., Martin, L.J., Pereira, H.M., Fernandez, M., McRae, L., Belnap, J., Böhm, M., Brummitt, N., García-Moreno, J., Gregory, R.D., Honrado, J.P., 2016. Global biodiversity monitoring: from data sources to essential biodiversity variables.Biol. Conserv. https://doi.org/10.1016/j.biocon.2016.07.014.
|
Rai, D.S., Tshering, K., Gyeltshen, K., Norbu, N., Sherub, Ngawang, R., Wangchuk, S., 2008. Biodiversity of Toorsa Strict nature Reserve e Jigme Dorji national Park proposed conservation corridor, western Bhutan. In: Chettri, N., Shakya, B., Sharma, E. (Eds.), Biodiversity Conservation in the Kangchenjunga Landscape.ICIMOD, Kathmandu, pp. 39-56.
|
Rai, L.K., Sharma, E., 1995. Medicinal Plants of Sikkim Himalaya: Status, Uses and Potential. Bishen Singh, Mahendra Pal Singh, Dehra Dun, India.
|
Rajbhandari, K.R., 2016. History of botanical exploration in Nepal: 1802-2015. In: Jha, P.K., Siwakoti, M., Rajbhandari, S. (Eds.), Frontiers of Botany. Central Department of Botany, Tribuvan University, Kirtipur, pp. 1-99.
|
Rajbhandari, K.R., Dhungana, S.K., 2010. Endemic Flowering Plants of Nepal Part 2.Department of Plant Resources Bulletin-Special Publication No. 2. Department of Plant Resources, Kathmandu.
|
Rajbhandari, K.R., Dhungana, S.K., 2011. Endemic Flowering Plants of Nepal Part 3.Department of Plant Resources Bulletin-Special Publication No. 3. Department of Plant Resources, Kathmandu.
|
Rajbhandari, K.R., Adhikari, M.K., 2009. Endemic Flowering Plants of Nepal Part I.Department of Plant Resources Bulletin-Special Publication No. 1. Department of Plant Resources, Kathmandu.
|
Rajbhandary S., Hughes M., Shrestha K.K., 2010. Three new species of Begonia Sect. Platycentrum from Nepal. Gard. Bull. (Singap.), 62(1): 151-162. |
Saurav M., Das A.P., 2014. Plant species richness and phytosociological attributes of the vegetation in the cold temperate zone of Darjiling Himalaya, India. Int. Res.J. Environ. Sci, 3(10): 14-19. |
Shankar U., 2001. A case of high tree diversity in a sal (Shorea robusta)-dominated lowland forest of Eastern Himalaya:floristic composition, regeneration and conservation. Curr. Sci, 81(7): 776-786. |
Sharma S.K., Pandit M.K., 2009. A new species of Panax L. (Araliaceae) from Sikkim Himalaya. India. Syst. Bot, 34(2): 434-438. DOI:10.1600/036364409788606235 |
Shrestha, K.K., Ghimire, S.K., 1996. Plant Diversity Inventory of the Proposed Kangchenjunga Conservation Area (Ghunsa and Simbua Khola Valleys). WWF Nepal, Kathmandu.
|
Shrestha K.K., Kunwar R.M., Dhamala M.K., Humagain K., Pandey J., Khatri N.B., 2008. Conservation of plant resources in Kangchenjungha-Singhalila ridge, eastern Nepal. J. Plant Sci, 2: 62-68. |
Sikkim Biodiversity Action Plan, 2012. Sikkim Biodiversity Action Plan. Published by Sikkim Biodiversity Conservation and Forest Management Project. FEWMD, Government of Sikkim, Printer at Concept, India, p. 44.
|
Singh K.K., Kumar S., Rai L.K., Krishna A.P., 2003. Rhododendron conservation in Sikkim Himalayas. Curr. Sci, 85(5): 602-606. |
Singh H.B., Sundriyal S.C., 2005. Composition, economic use, and nutrient contents of alpine vegetation in the Khangchendzonga Biosphere Reserve, Sikkim Himalaya, India. Arctic Antarct. Alpine Res, 37: 591-601. DOI:10.1657/1523-0430(2005)037[0591:CEUANC]2.0.CO;2 |
Subba, J.R., 2002. Biodiversity of Sikkim Himalayas. Ambica Printers, New Delhi, India.
|
Subba S.J., Lachungpa D., Subba S., Nepal S., 2015. Analysis of vegetation in a representative temperate plant community in Lachung range of the Sikkim Himalaya. Int. J. Environ. Biodiv, 6(3): 18-24. |
Sundriyal M., Rai L.K., 1996. Wild edible plants of Sikkim Himalaya. J. Hill Res, 9: 267-278. |
Sundriyal R.C., Sharma E., Rai L.K., Rai S.C., 1994. Tree structure, regeneration and woody biomass removal in a sub-tropical forest of Mamlay watershed in the Sikkim Himalaya. Plant Ecol, 113(1): 53-63. DOI:10.1007/BF00045463 |
Sundriyal R.C., Sharma D., 1996. Anthropogenic pressure on tree structure and biomass in the temperate forest of Mamlay watershed in Sikkim. For. Ecol.Manag, 81(1): 113-134. |
Takhtajan, A.L., 1986. Floristic Regions of the World. University of California Press, USA.
|
Takhtajan, A., 1969. Flowering Plants: Origin and Dispersal. Oliver & Boyd, Edinburgh and London.
|
Tambe S., Rawat G.S., 2010. The alpine vegetation of the Khangchendzonga landscape, Sikkim Himalaya:community characteristics, diversity, and aspects of ecology. Mt. Res. Dev, 30(3): 266-274. DOI:10.1659/MRD-JOURNAL-D-09-00058.1 |
Telwala Y., Brook B.W., Manish K., Pandit M.K., 2013. Climate-induced elevational range shifts and increase in plant species richness in a Himalayan biodiversity epicentre. PLoS One, 8(2): e57103. DOI:10.1371/journal.pone.0057103 |
Thapa N., Lama D., 2015. Occurrence of Pteris austrosinica (ching) ching [Pterideaceae] in Darjiling hills:a new record of endemic Chinese element in Indian sub-continent. Researcher, 7(5): 36-38. |
Uprety Y., Poudel R.C., Gurung J., Chettri N., Chaudhary R.P., 2016. Traditional use and management of NTFPs in Kangchenjunga Landscape:Implications for conservation and livelihoods. J. Ethnobiol. Ethnomed, 12(1): 1-59. DOI:10.1186/s13002-015-0076-5 |
WCD, 2014. Feasibility assessment report. Wildlife Conservation Division, Department of Forests and Park Services, Ministry of Agriculture and Forests. Royal Government of Bhutan, KL-Bhutan.
|
Xu, J., Badola, R., Chettri, N., Chaudhary, R.P., Zomer, R., Pokhrel, B., Hussain, S.A., Pradhan, S., Pradhan, R., 2019. Sustaining biodiversity and ecosystem Services in the Hindu Kush Himalaya. Chapter 5. In: Wester, P., Mishra, A., Mukherji, A., Shrestha, A.B. (Eds.), The Hindu Kush Himalaya AssessmentdMountains, Climate Change, Sustainability and People. Springer Nature Switzerland, AG, Cham.
|
Yoda K., 1967. A preliminary survey of the forest vegetation of eastern Nepal. Ⅱ. General description, structure and floristic composition of sample plots chosen from different vegetation zones. J. Coll. Arts Sci. Chiba Univ. Natl. Sci. Ser, 5: 99-140. |
Yonzon, P.B., Pradhan, S., Bhujel, R., Khaling, S., Lachungpa, U., Lachungpa, C., 2000.Kangchenjunga Mountain Complex: Biodiversity Assessment and Conservation Planning. WWF Nepal, Kathmandu.
|



