With one in five of the world's vascular plants currently threatened with extinction (RBG Kew, 2016), the conservation and restoration of plant diversity is an urgent issue. Whilst the conservation of intact wild populations is vital, habitat degradation and destruction, invasive pests and diseases, climate change and other human-induced impacts on the environment mean the survival of many species is likely to depend on assisted recovery or reintroduction projects (sensu McDonald et al., 2016) and ex situ conservation in botanic gardens and elsewhere (Smith, 2016). A framework for action is provided by the Global Strategy for Plant Conservation (CBD, 2012), which outlines 16 targets including commitments to secure the in situ conservation of least 75% of known threatened species (Target 7) and the ex situ conservation of at least 75% of threatened species, with at least 20% available for recovery or restoration use (Target 8).
Botanic gardens contribute a unique set of skills and resources to delivering the GSPC, including plant identification, collection, plant production, direct management of wild species and habitats, research and public engagement activities (Smith, 2016; Hardwick et al., 2011). The leading role of botanic gardens in ex situ conservation is particularly marked, with at least 30% of plant species diversity held in the living collections or associated seed banks of botanic gardens around the world (Mounce et al., 2017). This represents an exceptional resource for conservation, although the need to assess the size and quality of these collections and fill significant biogeographical and phylogenetic gaps is recognised. It will also be important to build capacity in under-represented tropical areas and provide more effective coordination at an international level, learning from progress made in developing a Global System for the conservation of crop diversity (Mounce et al., 2017; Smith, 2016).
Seed banking has become an increasingly important form of ex situ conservation in botanic gardens, with almost 57,000 taxa conserved in more than 350 institutions around the world, including 37,000 taxa at the Royal Botanic Gardens (RBG) Kew's Millennium Seed Bank (MSB) at Wakehurst Place (O'Donnell and Sharrock, 2017). Seed banking is a practical, cost-effective means of conserving wild plant diversity (Li and Prichard, 2009). Methodologies for the long-term storage of desiccation-tolerant orthodox species are well established, with cryopreservation, micropropagation and other techniques increasingly permitting the storage and subsequent use of desiccation-intolerant and shortlived species (Hay and Probert, 2013; Walters et al., 2013; Li and Prichard, 2009; Walters et al., 2008).
As the science and practice of nature conservation and ecological restoration have grown, so has the call to expand the capacity of seed banks to provide scientifically-robust, practical solutions to seed-related problems, from single-species conservation and small protected sites to complex, dynamic communities at the largest scales (Smith, 2016; Merritt and Dixon, 2011; Miller et al., 2016). In this paper, we discuss the MSB's response to this call in the UK, outlining our programme and providing examples of how our seed collections and associated expertise have contributed to in situ species reintroduction and habitat restoration projects. Significant progress has been made, but the need remains to adapt and respond to changing environmental conditions and approaches to nature conservation, to increase our impact and find new ways to contribute at a landscape scale.
2. The MSB's UK programmeThe MSB's UK programme is currently structured around three projects: the UK Flora project, which continues to make collections across the entire UK flora for conservation at the MSB; the UK National Tree Seed Project (UKNTSP), which is building genetically representative collections of native woody species from across their range in the UK; and the UK Native Seed Hub (UKNSH), which uses the seed collections and associated expertise to increase the quantity, quality and diversity of native plant material available to practitioners in the UK.
2.1. Building diverse, high quality, representative collectionsA primary focus of the UK Flora Project has been to secure at least one collection from the broadest possible range of native and archeophyte species (sensu BSBI, 2007), with priority given to threatened and endemic taxa. The MSB currently conserves 7435 wild-origin and regenerated (cultivated) collections from the UK, comprising 2077 native and archeophyte taxa (Table 1). This represents 75% of the UK's total native and archeophyte flora and 78% of threatened taxa, a high proportion reflecting both the relatively small size of the UK flora and the intensity of collecting effort in recent decades. Work to collect outstanding taxa continues, including very rare or highly specialised species, taxonomicallycomplex microspecies and those that do not reliably produce seed in the UK.
| Collections in MSBa | Taxa in the MSBb | Taxa in UK florab | % of UK flora in MSB | |
| Wild-origin | 6131 | 1904 | 2759 | 69% |
| Regenerated | 1304 | 542 | 2759 | 20% |
| Total | 7435 | 2077 | 2759 | 75% |
| Threatenedc, wild | 925 | 303 | 430 | 70% |
| Threatenedc, regenerated | 415 | 143 | 430 | 33% |
| Total | 1340 | 336 | 430 | 78% |
| a Data for MSB collections were extracted from the MSB's Seed Bank Database on 5th February 2018, comprising angiosperm native and archeophyte taxa collected in the UK, including sub-specific taxa and microspecies. b MSB data were cross-reference with the UK's angiosperm native and archeophyte flora using data from the Botanical Society of Britain and Ireland (BSBI, 2007) incor-porating changes identified in Stace (2010) and additional data from McCosh and Rich (2011). c Threatened taxa identified using IUCN Red List categories EX-VU (INNC, 2018). |
||||
In recent years, an increasing emphasis has been placed on intraspecific sampling depth - making large, multiple-origin collections that capture and conserve genetic diversity within and between populations of a single species across its range in the UK (Willis et al., 2018). This approach has been facilitated by collaboration with conservation geneticists at Kew and elsewhere and is typified by the UKNTSP, which applies a rigorous sampling strategy to capture genetic diversity at national, eco-geographical and individual mother plant scales (Kallow and Trivedi, 2017; Trivedi and Kallow, 2017). Whilst completing this work for the UK's woody flora is a priority (RBG Kew, 2015), in-depth sampling strategies are also being developed for threatened non-woody species and those likely to provide the greatest ecosystem benefits and adaptability to environmental change (Willis et al., 2018).
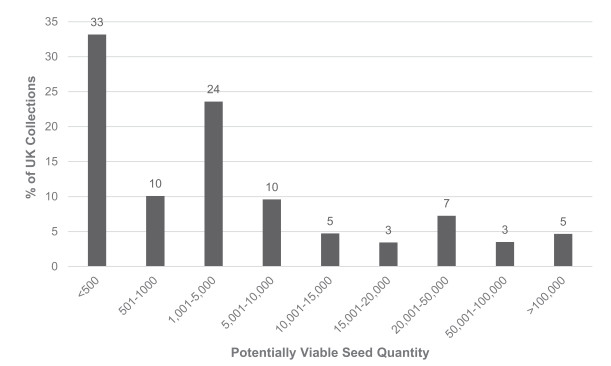
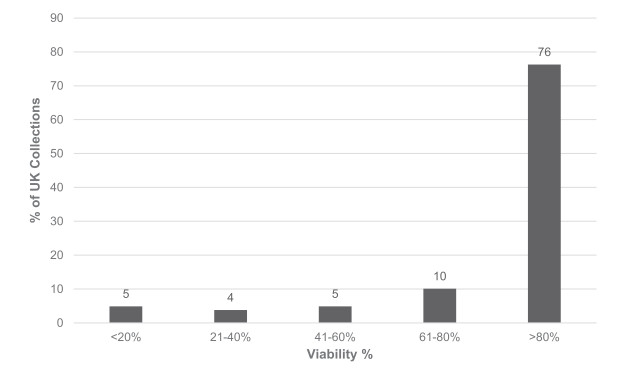
The quality and quantity of seed held in the UK collections is also critical - the collections must be legally acquired, accurately identified, of high viability and large enough to permit routine curation in the seed bank and meaningful conservation or research use (Way, 2003). The MSBP's Seed Conservation Standards (MSBP, 2015) provide a framework for high quality seed collecting and conservation, and partners are provided with training and detailed guidance on sampling techniques, data collection, the preparation of herbarium specimens, drying and packaging, tissue sampling etc. (for example, RBG Kew, 2001; Kallow, 2014). Taxonomic, locality, altitude, habitat, sampling, germination and viability data are available for most UK collections (Fig. 1) and, although many collections are small (Fig. 2), efforts are being focussed on making larger collections where this can be done without compromising the natural regeneration of the donor population. Viability data is available for 55% of UK collections (Fig. 1), with a majority of collections tested displaying high viability of 80% or greater (Fig. 3).

|
| Fig. 1 Availability of key data for UK native and archeophyte collections held in the MSB. Includes collections currently in processing, where data may not be complete. Data extracted from the MSB's Seed Bank Database on 5th February 2018 |
|
| Fig. 2 Potentially viable seed quantity. Percentage of native and archeophyte UK collections in different classes of estimated potentially viable seed quantity, excluding non-viable seed detected by X-ray or cut-testing of dry samples. Collections lacking quantity data - principally those currently being processed - are excluded. Data extracted from the MSB's Seed Bank Database on 5th February 2018 |
|
| Fig. 3 Seed Viability. Percentage of native and archeophyte UK collections in different viability classes, based on the most recent MSB viability test. Collections without viability data are excluded. Data extracted from the MSB's Seed Bank Database on 5th February 2018 |
Whilst the diversity and quality of seed is critical, the conservation value of a seed collection is also a function of how accessible the material is for research, reintroduction, habitat restoration and other uses (Liu et al., 2018). Small samples of MSB seed - typically of 60 seed or less - are available free of charge for non-commercial purposes via the MSB Seed List. Between 2012 and 2017, 1290 samples from the UK collections were dispatched via this list, 930 (72%) for research purposes, 206 (16%) to produce plants for the living collections at RBG Kew and other botanic gardens and 154 (12%) for environmental purposes including regeneration, reintroduction and habitat restoration projects (data extracted from the MSB's Seed Information Database, 31st January 2018). In the UK, the UKNSH has made larger quantities of seed available at costrecovery prices via the UKNSH Seed List and through a range of partnership, project and consultancy work. Between 2011 and 2018, the UKNSH provided plant materials and technical assistance to 57 projects, working with 31 partner or client organisations including the Wildlife Trusts, the National Trust, the South Downs National Park, Natural England, local authorities, ecological consultancy firms, Toyota Motor Manufacturing (UK) Limited and Toyota GB (PLC). Examples of this work are presented in Section 3 and Section 4 of this paper.
Efforts are also underway to enhance access to the data recorded during the collection, processing and ongoing curation of the seed collections. Basic biological data - seed weight, morphology, storage behaviour, germination protocols etc. - are made available via RBG Kew's Seed Information Database (RBG Kew, 2018). We believe this represents a valuable and under-utilised resource for plant conservation and hope to do more to expand and interpret these data for application under nursery and field conditions. Seed collecting data forms that accompany each wild collection are valuable biological records, contributing to distribution data mapped and made available by the Botanical Society of Britain and Ireland (BSBI). A new BSBI mapping tool to overlay MSB collections with species-distribution data is in development, enabling the MSB and collecting partners to assess where new wild collections are required and practitioners to see where seed collections may be available for use. Data-sharing agreements allow more bespoke, detailed use of MSB data for conservation use. Data relating to UKNTSP collections, for example, has been supplied to the Future Trees Trust and Woodland Trust to identify new registered seed sources for Tilia cordata Mill. and Carpinus betulus L.
3. Making seed availableThe availability of high quality, genetically-diverse seed and plants of known native-origin can impose a significant constraint on ecological restoration (Broadhurst et al., 2016; Oldfield and Olwell, 2015; Miller et al., 2016; Nevill et al., 2016), particularly when projects seek to move beyond a relatively narrow range of high demand, easily-cultivated 'workhorse' species (Broadhurst et al., 2016; Ladouceur et al., 2017). Seed banks can provide the seed and associated expertise in seed processing, storage and use to expand this limited restoration species pool (Ladouceur et al., 2017) and make a broader range of biological material available to practitioners.
3.1. Species recovery and reintroductionThe breadth and increasing diversity of collections held in the MSB represent an exceptional resource for species recovery and reintroduction projects, particularly of rare, highly threatened and protected species where appropriate material from other wild or cultivated sources is unlikely to be available. In some cases - Ranunculus ophioglossifolius Vill. and Chenopodium urbicum L., for example - collections held in long-term storage have been used to augment dwindling wild populations or reintroduce otherwise extinct populations to their original growing site. In at least one case, Bromus interruptus (Hack.) Druce, MSB collections have facilitated the reintroduction of an endemic species that has become extinct in the wild.
In some cases, wild collections held in the bank may be large enough to be used directly in small-scale projects. In others, the ability to regenerate small wild collections - growing plants to harvest a greater number of seed - has proved essential. R. ophioglossifolius, for example, is restricted to populations at two sites (Holland et al., 1986), one of which, at Inglestone Common, Gloucestershire, has declined to the brink of extinction. In 2007, a small wild collection was made at the site. In 2015, this collection was regenerated by the UKNSH under carefully controlled conditions mimicking the muddy, seasonally-inundated habitat provided by grazed pool edges. A successful protocol was developed and 124,926 seed were harvested, providing material to propagate 200 plants for planting at Inglestone in 2016. To promote the long-term persistence of this annual species, plants were introduced to the site at the flowering stage to maximise seed production and dispersal. Counts of buds, flowers and fruiting heads in summer 2016 provide an estimate that approximately 60,000 seed entered the system in the first year, with large numbers of seedlings observed in autumn 2016 (Lansdown, 2016). Although most of these plants died during exceptionally dry weather in early 2017, a small number persisted to produce an estimated 600 further seed (Lansdown, 2018), suggesting the introduced population is capable of self-regeneration under both optimal and severely sub-optimal conditions.
Since 2011, the UKNSH has regenerated 49 collections of 44 species, focussing on material that would not be available from any other source, typically rare or difficult species or specific, knownorigin material for a specialist project or use. UKNSH protocols have been designed to minimise losses in wild genetic diversity during regeneration and thereby maximise the adaptive potential and long-term sustainability of reintroduced populations (Schröder and Prasse, 2013; Basey et al., 2015). Where possible, original collecting data is used to inform the selection of the wild seed collection for regeneration. Large systematically-sampled populations from sites providing a good ecological match to the reintroduction site are prioritised. A range of laboratory and nurserybased techniques are employed to promote high germination and establishment rates, with grow-outs that fall below a threshold 50% conversion rate discarded. In seed production, congeners are separated to avoid unintentional hybridisation, harvesting is carried out sequentially throughout the season to retain the varied phenology of the wild population and cultivation is limited to a single generation to avoid losses in genetic variability and minimise adaptation to cultivation conditions.
3.2. Habitat restoration and creationWild and regenerated single-species collections held at the MSB, although small by commercial standards, have proved large enough to create bespoke mixtures for small-scale habitat restoration and creation projects or to supplement bulk-harvested materials to create species-rich mixtures for use at larger scales.
In the UK, landscape partnership projects provide a valuable opportunity to link ex situ seed collections and associated expertise to practical conservation at landscape scales. The South Downs Way Ahead Nature Improvement Area (NIA) brought 29 organisations together to deliver improved management of 42,000 ha of chalk ecosystem between 2012 and 2015 (SDNPA, 2015). Responding to partner concerns about the limited availability of local-origin seed for many calcareous species, the UKNSH regenerated South Downs origin collections of 25 species, making crops of hundreds of thousands or millions of seed available to practitioners. These collections have been used to produce plug plants to enhance existing grassland, for direct sowing to restore grassland following scrub clearance, to reinstate grassland damaged by road construction in protected areas and to provide a bespoke mixture of larval and nectar food plants to create butterfly habitat. Importantly, the NIA also provided a forum for discussion, training and advice on seed sourcing, collecting, processing and use, amplifying the resources of RBG Kew by building awareness and capacity in local authorities, conservation organisations and land managers.
Capturing and using seed to reassemble complex plant communities can benefit from the mixed approach suitably-equipped seed banks are able to provide, including capacity for wild collecting, regeneration, mechanised seed harvest, seed testing and long-term storage. In 2014, for example, the UKNSH worked with ecological consultants RSK-Environment to capture and restore priority habitats damaged by excavations for the construction of underground cabling for the Rampion off-shore wind farm (Gilbey and Chapman, 2016). The objective was to harvest seed from the broadest possible range of species at three priority habitats on the excavation route - two calcareous grassland sites in the South Downs and one species-rich mesotrophic grassland site in the Sussex Weald. Seed was then processed and placed in long-term storage at the MSB, ensuring high quality material was available for post-construction restoration three to five years later. Botanical surveys of each site enabled the identification of target species and collecting strategies, considering how species abundance, phenology and morphology influence collection timing and technique. A bulk matrix of grasses and some forbs was collected by repeated brush harvests using a Logic MSH420 brush harvester, supplemented by hand harvests of species that could not be captured mechanically. Brush harvests were analysed to determine species composition and collections were cleaned, viability tested and stored in the MSB. This technique maximised the species and phenological diversity of the final restoration mixes - at the calcareous grassland site at Tottington Mount, for example, 23 species were captured in over 9 kg of seed material (Tables 2 and 3). In total, 28 kg of brush harvested seed and 42 single-species collections were made available for restoration at the three sites, with the first seed dispatched and sown in autumn 2016.
| Taxon | % of total by weighta | % viability2 |
| Achillea millefolium L. | 0.07% | not tested |
| Agrostis sp. | 0.27% | 89 |
| Bromus erectus Huds. | 36.37% | 100 |
| Cerastium fontanum Baumg. | 0.04% | not tested |
| Holcus lanatus L. | 0.41% | 100 |
| Linum catharticum L. | 0.02% | not tested |
| Lotus corniculatus L. | 0.35% | not tested |
| Medicago lupulina L. | 3.24% | 100 |
| Phleum pratense L. | 0.13% | 100 |
| Pimpinella saxifraga L. | 4.08% | 76 |
| Plantago lanceolata L. | 1.33% | 71 |
| Trifolium pratense L. | 8.61% | 95 |
| Trifolium repens L. | 0.02% | not tested |
| Other species | 0.90% | not tested |
| Debris | 28.35% | not tested |
| Total Seed Weight, excl. debris (g) | 9315 | |
| a Three one-gram samples were taken using a riffle divider, separated into taxa and non-seed debris and weighed. The weight of each species is expressed as a percentage of the weight of the samples as a whole. Viability tests were carried out on species recorded as 'frequent' at the site using standard MSB methodologies (Davies et al., 2015a). Viability % = (G+F+A)/X x 100, where G = number of germinated seed, F = number of fresh ungerminated seed, A = number of abnormal seedlings, X = number of seed sown (excluding empty and infested seed). | ||
| Date Collected | Taxon | Number of seeda | % viability2 |
| 07/07/2014 | Ranunculus bulbosus L. | 15,261 | 98 |
| 07/07/2014 | Briza media L. | 31,294 | 98 |
| 24/07/2014 | Koeleria macrantha (Ledeb.) Schult. | 1624 | 89 |
| 24/07/2014 | Linum catharticum L. | 13,304 | 68 |
| 24/07/2014 | Anacamptis pyramidalis (L.) Rich. | 306,531 | not tested |
| 22/08/2014 | Lotus corniculatus L. | 15,853 | 100 |
| 22/08/2014 | Centaurea scabiosa L. | 2662 | 96 |
| 22/08/2014 | Blackstonia perfoliata (L.) Huds. | 23,942 | 94 |
| 22/08/2014 | Cirsium acaule Scop. | 626 | 78 |
| 22/08/2014 | Phyteuma orbiculare L. | 847 | 100 |
| 11/09/2014 | Galium verum L. | 7181 | 94 |
| 11/09/2014 | Centaurea nigra L. | 7937 | 94 |
| a Number of seed is the estimated potentially viable seed, excluding non-viable seed detected by X-ray or cut-testing of dry samples. Viability tests were carried out using standard MSB methodologies (Davies et al., 2015a). Viability % = (G+F+A)/X x 100, where G = number of germinated seed, F = number of fresh ungerminated seed, A = number of abnormal seedlings, X = number of seed sown (excluding empty and infested seed). | |||
Where seed can be made available, the failure of seeds to germinate and establish sustainable restored populations places a significant - perhaps the greatest (Miller et al., 2016) - additional constraint on successful ecological restoration. This failure may be due to climate, herbivory or other external factors, but may also be related to the seed itself, including low seed quality (Ryan et al., 2008; Marin et al., 2017); complex and poorly understood dormancy mechanisms (Miller et al., 2016); genetic fitness (Broadhurst et al., 2008); and site preparation, reintroduction and management practices that do not provide appropriate germination and establishment niches (Wagner et al., 2011). The restoration of more challenging species or complex plant communities is consequently inhibited (Broadhurst et al., 2016), large quantities of seed are wasted (Merritt and Dixon, 2011) and the ability of native seed producers to bring a wider range of species into large-scale production is reduced (Tishew et al., 2011; Ladouceur et al., 2017).
Seed banks, particularly those located within botanic gardens, are in a strong position to help overcome these constraints, combining ex situ collections of wild plant material and a range of supporting scientific and technical skills, including germination ecology and propagation, horticulture and conservation genetics (Hardwick et al., 2011).
4.1. Dormancy, germination and plant establishmentMost collections in the MSB are subject to an initial germination test to provide viability data and enable the future propagation and use of the seed (Davies et al., 2015a), employing a range of environmental conditions and dormancy-breaking treatments (Davies et al., 2015b). Very small collections are not routinely tested, although non-destructive X-ray data does provide a guide to seed quality (Terry et al., 2003). Germination data exists for 57% of UK collections (data extracted from the MSB's Seed Bank Database, 9th February 2018), covering 77% of UK species held in the MSB. Globally, germination data exists for 70% of collections held in the MSB (Liu et al., 2018), much of which is made publicly available online via the Seed Information Database (RBG Kew, 2018). These data do not represent proven propagation protocols, but can provide useful clues about germination ecology, including variation within and between collections of the same species.
In some cases, a more focussed research effort is required to understand complex germination requirements, produce practicable germination protocols and permit the production and effective use of seed in conservation projects (Wagner et al., 2011). Attempts to reinforce and reintroduce populations of Critically Endangered (JNNC, 2018) Galeopsis angustifolia Ehrh. ex Hoffm., for example, have been frustrated by very low availability of wild seed and complex dormancy mechanisms. The UKNSH has worked for several years to develop a propagation protocol for this species and, in 2016, successfully applied an embryo-excision technique to propagate 276 seedlings and produce a regenerated collection of 32,000 seed. A long-term 'move along' experiment (sensu Baskin and Baskin, 2003) is also underway, mimicking natural environmental conditions to better understand the germination ecology of G. angustifolia and assist the conservation management of the species. Regenerated collections will be used to reinforce the existing population at Cleeve Common, Gloucestershire, and be sown at new sites by Colour in the Margins, an arable restoration project forming part of the Back from the Brink endangered species programme. The UKNSH is providing regenerated seed of five arable species for Colour in the Margins - G. angustifolia, Adonis annua L., Ranunculus arvensis L., Silene gallica L. and Torilis arvensis (Huds.) Link - and will synthesise MSB and nursery data to develop detailed propagation protocols for a total of ten species.
4.2. Multi-disciplinary approachesMuch of the MSB's work in the UK emphasises the value of applying multiple skill-sets and resources to the conservation or restoration of a species or habitat.
An exemplar of this multi-disciplinary approach is provided by RBG Kew's contribution to the development of a Species Recovery Plan for Nuphar pumila (Timm) DC. a perennial aquatic plant which is Critically Endangered in England (Stroh, 2014) and survives as a single population at Cole Mere, Shropshire. Viability and germination studies at the MSB found that seed produced at Cole Mere displays high viability, but that germination is significantly influenced by seed maturity at the point of collection and the duration of post-harvest ripening (Peach et al., 2017). Propagation protocols from seed and root fragments were developed and, although the seed is recalcitrant and cannot be banked in conventional dry-cold storage, ex situ living collections have been established atWakehurst Place and Kew (Peach et al., 2017). A study was also carried out by RBG Kew's Conservation Genetics team to assess the genetics of the Cole Mere population in comparison with surviving populations in Scotland and putative samples of the hybrid Nuphar × spenneriana Gaudin. (Gargiulo et al., 2017). Although the sample size was limited, this work found no evidence of hybridisation, suggested populations in England and Scotland are not strongly differentiated and made recommendations for the controlled use of Scottish material in reintroduction attempts in England. This work was carried out in collaboration with Richard Lansdown, Chair of the IUCN SSC Freshwater Plant Specialist Group, who also conducted a comprehensive ecological study of the species and conditions at Cole Mere (Lansdown, 2017). Together, this work provides a robust evidence base and practical guidance for the conservation of N. pumila in England.
5. Responding to future challenges e amplifying impact and working at the biggest scalesAs challenges facing the natural environment intensify and evolve, so does the policy, science and practice of nature conservation. In the UK, as elsewhere, the need to understand, restore and reconnect ecosystems on landscape-scales has been well documented (Lawton et al., 2010) and consistently reflected in government policy (Defra, 2011; Defra, 2018). Natural capital approaches, emphasising the value and sustainable management of ecosystems and the services they provide to people, have also become important (Defra, 2018), requiring new insights into ecosystem function, resilience and adaptability (RBG Kew, 2018).
Responding to these challenges provides new and exciting opportunities for the MSB and other seed banks to mobilise their collections, data and expertise. A focus on multiple-origin collections and genetic diversity, for example, will build collections that protect locally-adapted ecotypes (Vander Mijnsbrugge et al., 2010), maximise variation and adaptive potential in restored populations (Broadhurst et al., 2008) and enable adaptive sourcing strategies to cope with changing environmental conditions (Weeks et al., 2011; Breed et al., 2013; Jones, 2013). Ready access to these materials and data can help researchers identify genetic boundaries, assess diversity within and between plant populations and facilitate the identification of adaptive traits, supported by rapid advances in sequencing technology (Nevill et al., 2016). Understanding these traits will, in turn, help model plant responses to environmental change and identify useful characteristics including adaptability to climate change and resistance to invasive pests and diseases (Willis et al., 2018).
New approaches also require significant and sometimes difficult shifts in the focus of seed bank activity. Resources for intensive, comprehensive sampling must be found alongside the continuing need to collect and protect endangered species, often from a single population; collecting towards long-term conservation targets must be balanced against responses to immediate project or partner demand; large collections that maximise genetic variation must sit alongside targeted sampling aimed at capturing specific traits; safeguarding long-term collections in the bank must be balanced against maximising the availability and use of seed. Perhaps the greatest challenge relates to scale. Involvement in larger projects like the South Downs NIA has enabled the MSB to mobilise its seed resources beyond the conservation of a single-species or small, highly protected sites, but stretched the capacity of infrastructure and procedures designed for small wild seed collections. UKNSH regenerated harvests are typically in the range of 1-2 kg of seed, large by MSB standards but well short of delivering the many kilograms or tons of restoration-ready material required at the largest scales (Merritt and Dixon, 2011).
To have the greatest impact, seed banks must apply their resources and expertise to help large-scale producers overcome constraints to the production of a broader, more diverse and higher quality range of native material (Merritt and Dixon, 2011; Tishew et al., 2011; Menz et al., 2013; Nevill et al., 2016). Seed banks are in a strong position to assist (Table 4) particularly those with access to the multi-disciplinary resources of a botanic garden (Hardwick et al., 2011), but must consult and collaborate closely with those who specify, produce and use native seed (Abbandonato et al., 2017; De Vitis et al., 2017). In the UK, the MSB has made insufficient progress in this regard and will need to build new bridges with industry in the years ahead. Initiatives like the Native Seed Science, Technology and Conservation Initial Training Network (www.nasstec.eu), which concluded in 2017, provide a good model, demonstrating the value of bringing researchers, seed banks, seed producers and practitioners together to find and share science-based solutions to practical problems in the production and use of native seed.
| Constraint | Solution |
| The availability of genetically-diverse, known-origin founder stock is limited.a | ● Make seed available from appropriate wild or regenerated collections.
● Develop seed production areas or seed orchards. ● Provide training and resources in wild seed collecting techniques. |
| Dormancy mechanisms or lack of propagation experience inhibit successful germination.a, c, d, e | ● Provide germination data and propagation protocols.
● Research and develop methodologies for seed pre-treatment and priming. ● Provide training and resources for propagation and establishment. |
| The growing requirements of some species or ecotypes are not fully understood.a, d, e | ● Laboratory and field-based studies to develop cultivation protocols for native species.
● Comparative studies to detect intraspecific variation in germination and cultivation requirements. |
| Intraspecific diversity and genetic boundaries are not fully understood for many species, limiting the ability of collectors and producers to identify and fill gaps in provision.a, b | ● Studies of intraspecific diversity and population structure for priority species. |
| Production processes reduce the genetic diversity of regenerated seed.b, c, h, i | ● Genetic studies to identify where diversity is lost in the production process and how this can be mitigated. |
| Production processes reduce the viability of regenerated seed, which may be untested.c, f, g | ● Genetic studies to identify where diversity is lost in the production process and how this can be mitigated. Production processes reduce the viability of regenerated seed, which may be untested.c, f, g ● Training and technical support in seed processing and storage. ● Seed testing services. ● Developing and promoting standards for native seed testing (for example, ISTA, 2017). ● Developing and promoting quality assurance schemes. |
| Market and regulatory conditions do not provide sufficient demand or lead-in time for the production of less commonly-used species or ecotypes.b, d, e, j | ● Provide evidence in support of the use of a broader range of native plant material.
● Develop and promote best-practice guidance for those who specify and use seed. ● Provide evidence and technical assistance in the development of quality assurance, accreditation |
|
a MacIntyre (2017). b Nevill et al. (2016). c Miller et al. (2016). d Ladouceur et al. (2017). e Tishew et al. (2011). f Ryan et al. (2008). g Marin et al. (2017). h Basey et al. (2015). i Schröder and Prasse (2013). j Abbandonato et al. (2017). |
|
Ex situ plant conservation through seed banking is a crucial complement to the in situ conservation and restoration of species and habitats. Banked seed collections can provide the biological materials for reintroduction and restoration programmes, either directly as seed or plants, or indirectly via regenerated collections. The data, expertise and specialist facilities that accompany collections are valuable too, helping overcome constraints to the collecting, production and use of seed in the landscape. Adaptability is key - to ensure ex situ collections protect the species and genetic diversity that will enable plants to adapt to a changing environment, and to ensure seed banks themselves find new ways to apply their resources to the requirements of 21st century conservation. Advances in ex situ conservation mean there is now no technological reason why any plant species should become extinct (Smith et al., 2011) - a strong imperative to make the best possible use of the opportunity this represents.
The authors are indebted to the many colleagues and partners who have contributed to the work of the MSB in the UK. Thanks to Janet Terry, Rachael Davies and Udayangani Liu for providing data and advice. The restoration of R. ophioglossifolius was undertaken in partnership with the Freshwater Habitats Trust, Bristol Zoological Society, South Gloucestershire Biodiversity Action Group, Gloucestershire County Council and Richard Lansdown. Thanks to Ellie Philips, Cleeve Common Conservators, for donations of G. angustifolia seed for experimental and regeneration use. The provision of seed for the construction of butterfly banks was carried out in partnership with Dan Danahar and the Dorothy Stringer School, who also provided funding for this work. Work on N. pumila was commissioned and funded by Natural England, with thanks to Mags Cousins for leading this project, Jennifer Peach, Joanna Walmisley, Roberta Gargiulo, Mike Fay and Richard Lansdown. The UK Flora Programme and UK Native Seed Bank are funded by the Esmée Fairbairn Foundation, the UK Native Tree Seed Project is funded by The People's Postcode Lottery. We are also grateful to the reviewers who provided valuable constructive comments on this paper.
Abbandonato H., Pedrini S., Pritchard H.W., De Vitis M., Bonomi C., 2017. Native seed trade of herbaceous species for restoration:a European policy perspective with global implications. Restor. Ecol. DOI:10.1111/rec.12641 |
Basey A.C., Fant J.B., Kramer A.T., 2015. Producing native plant materials for restoration:10 rules to collect and maintain genetic diversity. Native Plants J, 16: 37-53. DOI:10.3368/npj.16.1.37 |
Baskin C.C., Baskin J.M., 2003. When breaking dormancy is a problem, try a move-along experiment. Native Plants J, 4: 17-21. DOI:10.3368/npj.4.1.17 |
Breed M.F., Stead M.G., Ottewell K.M., Gardner M.G., Lowe A.J., 2013. Which provenance and where?. Seed sourcing strategies for revegetation in a changing environment. Conserv. Genet, 14: 1-10. |
Broadhurst L.M., Lowe A., Coates D.J., Cunningham S.A., McDonald M., Vesk P.A., Yates C., 2008. Seed supply for broad scale restoration:maximizing evolutionary potential. Evol. Appl, 1: 587-597. |
Broadhurst L.M., Jones T.A., Smith F.S., North T., Guja L., 2016. Maximizing seed resources for restoration in an uncertain future. BioScience, 66: 73-79. DOI:10.1093/biosci/biv155 |
BSBI, 2007. List of Accepted Plant Names. Available at: https://bsbi.org/resources (Accessed 5 February 2018).
|
CBD, 2012. Global Strategy for Plant Conservation: 2011-2020. Botanic Gardens Conservation International, Richmond.
|
Davies, R., Di Sacco, A., Newton, 2015a. Technical Information Sheet 13a: Germination Testing: Procedures and Evaluation. Royal Botanic Gardens, Kew, Richmond. Available at: https://www.kew.org/science/collections/seed-collection/millennium-seed-bank-resources.
|
Davies, R., Di Sacco, A., Newton, 2015b. Technical Information Sheet 13b: Germination Testing: Environmental Factors and Dormancy-breaking Treatments. Royal Botanic Gardens, Kew, Richmond. Available at: https://www.kew.org/science/collections/seed-collection/millennium-seed-bank-resources.
|
De Vitis M., Abbandonato H., Dixon K.W., Laverack G., Bonomi C., Pedrini S., 2017. The European Native Seed Industry:characterization and perspectives in grassland restoration. Sustainability, 9: 1682. DOI:10.3390/su9101682 |
Defra, 2011. Biodiversity 2020: a Strategy for England's Wildlife and Ecosystem Services. Department for the Environment, Food and Rural Affairs, London. Available at: https://www.gov.uk/government/publications/biodiversity-2020-a-strategy-for-england-s-wildlife-and-ecosystem-services.
|
Defra, 2018. A Green Future: Our 25 Year Plan to Improve the Environment. Department for Environment, Food and Rural Affairs, London. Available at: https://www.gov.uk/government/publications/25-year-environment-plan.
|
Gargiulo, R., Lansdown, R.V., Fay, M.F., 2017. Evaluation of Genetic Diversity and Admixture in the Only English Population of Nuphar Pumila. Natural England Commissioned Report, Number 245. York. Available at: http://nepubprod.appspot.com/file/4984406162276352.
|
Gilbey, V., Chapman, T., 2016. Workshop Session: mitigating impacts on sensitive habitats using seed harvesting and turf translocation. In: CIEEM (Chartered Institute of Ecology and Environmental Management) 2016 Summer Conference, Linear Infrastructure and Biodiversity: Impacts and Opportunities. Birmingham, UK, 28 June 2016. Available at: https://www.cieem.net/previous-conferences-2016-spring-conference-772.
|
Hardwick K.A., Fiedler P., Lee L.C., Pavlik B., Hobbs R.J., Aronson J., Bidartondo M., Black E., Coates D., Daws M.I., Dixon K., Elliott S., Ewing K., Gann G., Gibbons D., Gratzfeld J., Hamilton M., Hardman D., Harris J., Holmes P.M., Jones M., Mabberley D., Mackenzie A., Magdalena C., Marrs R., Milliken W., Mills A., Lughadha E.N., Ramsay M., Smith P., Taylor N., Trivedi C., Way M., Whaley O., Hopper S.D., 2011. The role of botanic gardens in the science and practice of ecological restoration. Conserv. Biol, 25(2): 265-275. |
Hay F.R., Probert R.J., 2013. Advances in seed conservation of wild plant species:a review of recent research. Conserv. Physiol, 1: 1-11. |
Holland, S.C., Caddick, H.M., Dudley-Smith, D.S., 1986. Supplement to the Flora of Gloucestershire. Grenfell Publishing, Bristol.
|
JNNC, 2018. Conservation Designations Spreadsheet. Available at: http://jncc.defra.gov.uk/page-3408 (Accessed 5 February 2018).
|
Jones T.A., 2013. When local isn't best. Evol. Appl, 6: 1109-1118. |
Kallow, S., 2014. UK National Tree Seed Project Seed Collecting Manual. Royal Botanic Gardens, Kew, Richmond. Available at: https://www.kew.org/science/collections/seed-collection/millennium-seed-bank-resources.
|
Kallow, S., Trivedi, C., 2017. Collecting genetic variation on a small Island. In: Sniezko, R.A., Man, G., Hipkins, V., Woeste, K., Gwaze, D., Kliejunas, J.T., McTeague, B.A. (Eds.), Gene Conservation of Tree Species-Banking on the Future. Proceedings of a Workshop. US Forest Service, Portland. Available at: https://www.fs.usda.gov/treesearch/pubs/55062.
|
RBG Kew, 2001. A Field Manual for Seed Collectors. Royal Botanic Gardens, Kew, Richmond. Available at: https://www.kew.org/science/collections/seed-collection/millennium-seed-bank-resources.
|
RBG Kew, 2015. A Global Resource for Plant and Fungal Knowledge. Science Strategy 2015-2020. Royal Botanic Gardens, Kew, Richmond.
|
RBG Kew, 2016. State of the World's Plants. Royal Botanic Gardens, Kew, Richmond.
|
RBG Kew, 2018. Seed Information Database (SID). Version 7.1. Royal Botanic Gardens, Kew, Richmond. Available at: http://data.kew.org/sid/.
|
Ladouceur E., Jiménez-Alfaro B., Marin M., De Vitis M., Abbandonato H., Iannetta P.P.M., Bonomi C., Pritchard H.W., 2017. Native seed supply and the restoration species pool. Conserv. Lett. DOI:10.1111/conl.12381 |
Lansdown, R.V., 2016. Report on Site Preparation and Reintroduction of Adder's-tongue Spearwort to Inglestone Common (Unpublished).
|
Lansdown, R.V., 2017. Development of a Conservation Plan for Least Water-lily (Nuphar Pumila) in England. Natural England Commissioned Reports, Number 243. York. Available at: http://publications.naturalengland.org.uk/file/5299384836685824.
|
Lansdown, R.V., 2018. Discussion About Restored Ranunculus Ophioglossifolius Populations at Inglestone Common[Email]. Personal communication, 7 February 2018.
|
Lawton, J.H., Brotherton, P.N.M., Brown, V.K., Elphick, C., Fitter, A.H., Forshaw, J., Haddow, R.W., Hilborne, S., Leafe, R.N., Mace, G.M., Southgate, M.P., Sutherland, W.J., Tew, T.E., Varley, J., Wynne, G.R., 2010. Making Space for Nature: a Review of England's Wildlife Sites and Ecological Network. Report to Defra. Available at: https://www.researchgate.net/publication/268279426.
|
Li D., Prichard H.W., 2009. The science and economics of ex situ plant conservation. Trends Plant Sci, 14(11): 614-621. DOI:10.1016/j.tplants.2009.09.005 |
Liu U., Breman E., Cossu T.A., Kenney S., 2018. The conservation value of germ-plasm stored at the Millennium Seed Bank, Royal Botanic Gardens, Kew, UK. Biodivers. Conserv, 10. DOI:10.1007/s10531-018-1497-y |
MacIntyre, D., 2017. Discussion Between RBG Kew and Emorsgate Seeds[Meeting]. Personal communication, 23 November 2018.
|
Marin M., Toorop P., Powell A.A., Laverack G., 2017. Tetrazolium staining predicts germination of commercial seed lots of European native species differing in seed quality. Seed Sci. Technol, 45(1): 1-16. DOI:10.15258/sst.2017.45.1.05 |
McCosh, D., Rich, T., 2011. Atlas of British and Irish Hawkweeds (Pilosella L And Hieracium L.). Botanical Society of Britain and Ireland, London.
|
McDonald, T., Gann, G.D., Jonson, J., Dixon, K.W., 2016. International Standards for the Practice of Ecological Restoration-Including Principles and Key Concepts. Society for Ecological Restoration, Washington, D.C.
|
Menz M.H.M., Dixon K.W., Hobbs R.J., 2013. Hurdles and opportunities for landscape-scale restoration. Science, 339: 526-527. DOI:10.1126/science.1228334 |
Merritt D.J., Dixon K.W., 2011. Restoration seed banks-a matter of scale. Science, 332: 424-425. DOI:10.1126/science.1203083 |
Miller B.P., Sinclair E.A., Menz M.H.M., Elliott C.P., Bunn E., Commander L.E., Dalziell E., David E., Davis B., Erickson T.E., Golos P.J., Krauss S.L., Lewandrowski W., Mayence C.E., Merino-Martín L., Merritt D.J., Nevill P.G., Phillips R.D., Ritchie A.L., Ruoss S., Stevens J.C., 2016. A framework for the practical science necessary to restore sustainable, resilient, and biodiverse ecosystems. Restor. Ecol, 25: 605-617. |
Mounce R., Smith P., Brockington S.F., 2017. Ex situ conservation of plant diversity in the world's botanic gardens. Nat. Plants, 3(10): 795-802. DOI:10.1038/s41477-017-0019-3 |
MSBP, 2015. Seed Conservation Standards for MSB Partnership Collections. Millennium Seed Bank Partnership.
|
Nevill P.G., Tomlinson S., Elliott C.P., Espeland E.K., Dixon K.W., Merritt D.J., 2016. Seed production areas for the global restoration challenge. Ecol. Evol, 6: 7490-7497. DOI:10.1002/ece3.2455 |
Oldfield S., Olwell P., 2015. The right seed in the right place at the right time. BioScience, 65: 955-956. DOI:10.1093/biosci/biv127 |
O'Donnell K., Sharrock S., 2017. The Contribution of Botanic Gardens to Ex Situ Conservation through Seed Banking. Plant Divers, 39: 373-378. DOI:10.1016/j.pld.2017.11.005 |
Peach, J., Davies, R., Walmisley, J., Chapman, T., 2017. An Assessment of Seed Viability, Germination and Vegetative Propagation Requirements for Nuphar Pumila. Natural England Commissioned Report, Number 244. York. Available at: http://nepubprod.appspot.com/file/6034091874451456.
|
Ryan N., Laverack G., Powell A., 2008. Establishing quality control in UK wild-flower seed production. Seed Test. Int, 135: 49-53. |
Schroder R., Prasse R., 2013. From nursery into nature:a study on performance of cultivated varieties of native plants used in re-vegetation, their wild relatives and evolving wild x cultivar hybrids. Ecol. Eng, 60: 428-437. DOI:10.1016/j.ecoleng.2013.09.036 |
SDNPA, 2015. Case Study: South Downs Way Ahead Nature Improvement Area. South Downs National Park Authority, Midhurst. Available at: https://www.southdowns.gov.uk/wp-content/uploads/2016/02/Case-Study-South-Downs-Way-Ahead-NIA.pdf.
|
Smith P., 2016. Guest essay:building a global system for the conservation of all plant diversity:a vision for botanic gardens and Botanic Gardens Conservation International. Sibbaldia, 14: 5-13. |
Smith P., Dickie J., Linington S., Probert R., Way M., 2011. Making the case for plant diversity. Seed Sci. Res, 21(1): 1-4. DOI:10.1017/S0960258510000309 |
Stace, C., 2010. New Flora of the British Isles, third ed. Cambridge University Press, Cambridge.
|
Stroh, P.A., 2014. A Vascular Plant Red List for England. Botanical Society of Britain and Ireland, Bristol.
|
Terry, J., Probert, R.J., Linington, S.H., 2003. Processing and maintenance of the Millennium seed bank collections. In: Smith, R.D., Dickie, J.B., Linington, S.H., Pritchard, H.W., Probert, R.J. (Eds.), Seed Conservation: Turning Science into Practice. Royal Botanic Gardens, Kew, Richmond, p. 17.
|
Tishew S., Youtie B., Kirmer A., Shaw N., 2011. Farming for restoration:building bridges for native seeds. Ecol. Restor, 29: 219-222. DOI:10.3368/er.29.3.219 |
Trivedi, C., Kallow, S., 2017. Benefits and challenges for gene conservation: a view from the UK National Tree Seed project. In: Sniezko, R.A., Man, G., Hipkins, V., Woeste, K., Gwaze, D., Kliejunas, J.T., McTeague, B.A. (Eds.), Gene Conservation of Tree Species-Banking on the Future. Proceedings of a Workshop. US Forest Service, Portland. Available at: https://www.fs.usda.gov/treesearch/pubs/55062.
|
Vander Mijnsbrugge K., Bischoff A., Smith B.A., 2010. A question of origin:where and how to collect seed for ecological restoration. Basic Appl. Ecol, 11: 300-311. DOI:10.1016/j.baae.2009.09.002 |
Wagner M., Pywell R.F., Knopp T., Bullock J.M., Heard M.S., 2011. The germination niches of grassland species targeted for restoration:effects of seed pre-treatments. Seed Sci. Res, 21: 117-131. DOI:10.1017/S0960258510000450 |
Walters, C., Wesley-Smith, J., Crane, J., Hill, L.M., Chmielarz, P., Pammenter, N.W., Berjak, P., 2008. Cryopreservation of recalcitrant (i.e. desiccation-sensitive) seeds. In: Reed, B.M. (Ed.), Plant Cryopreservation: a Practical Guide. Springer, New York. Ch. 18.
|
Walters C., Berjak P., Pammenter N., Kennedy K., Raven P., 2013. Preservation of recalcitrant seeds. Science, 339: 915-916. DOI:10.1126/science.1230935 |
Way, M.J., 2003. Collecting seeds from non-domesticated plants for long-term conservation. In: Smith, R.D., Dickie, J.B., Linington, S.H., Pritchard, H.W., Probert, R.J. (Eds.), Seed Conservation: Turning Science into Practice. Royal Botanic Gardens, Kew, Richmond. Ch. 3.
|
Weeks A.R., Sgro C.M., Young A.G., Frankham R., Mitchell N.J., Miller K.A., Byrne M., Coates D.J., Eldridge M.D.B., Sunnucks P., Breed M.F., James E.A., Hoffmann A.A., 2011. Assessing the benefits and risks of translocations in changing environments:a genetic perspective. Evol. Appl, 4: 709-725. DOI:10.1111/j.1752-4571.2011.00192.x |
Willis, K.J., Paton, A.J., Smith, R.J., 2018. Science Collections Strategy 2018-2028. Royal Botanic Gardens, Kew, Richmond.
|



