Reintroduction is widely practiced to conserve critically imperiled species threatened with extinction. If remaining populations are small, unprotected, or immediately threatened by habitat destruction, reintroduction to protected sites can increase the distribution, abundance, and genetic diversity of a species and improve its long-term survival prospects. However, despite over two decades of advancement in plant reintroduction science, determining suitable habitat and locations for species reintroduction in degraded ecosystems remains a fundamental challenge (Falk et al., 1996; Maschinski and Haskins, 2012). Indeed, habitat suitability was considered the leading cause of failure in a global review of 249 rare plant reintroductions (Godefroid et al., 2011). In other cases, however, rare species fail to establish in apparently suitable habitat for reasons that are poorly understood (Bottin et al., 2007; Drayton and Primack, 2012; Holl and Hayes, 2006).
Determining suitable habitat for rare plant reintroductions often focuses on matching the ecological properties of recipient and reference sites based on similarities in community composition (Lawrence and Kaye, 2011; Noël et al., 2011), indicator species (Bontrager et al., 2014; Ren et al., 2010), or habitat features (Maschinski and Wright, 2006). However, several challenges can arise when using these approaches to characterize the niche of a rare species and suitable locations for reintroduction. First, uncertainty in optimal habitat occurs when rare species are known from only a few locations that differ in vegetation structure and composition (e.g., Bontrager et al., 2014; Volis et al., 2011). Second, if rare plants exist today as remnant populations or demographic sinks, then current environmental conditions are suboptimal and can no longer support positive or stable population growth (Falk et al., 1996); for example, disturbance-dependent rare species can become confined to suboptimal habitat as disturbance regimes shift over time (Pavlovic, 1994). Thus, choosing recipient sites based on their ecological similarity to remnant populations may result in reintroductions with limited success. Finally, rare plants often maintain extremely specialized abiotic and biotic requirements, which can sometimes be cryptic (e.g., specialized soil symbionts; Smith et al., 2009) or difficult to quantify (e.g., subtle differences in microsites; Dunwiddie and Martin, 2016). For even the most intensively studied rare species, we often lack the detailed understanding of their biology and microenvironmental requirements that is needed for successful reintroduction (Dunwiddie and Martin, 2016; Holl and Hayes, 2006).
Determining optimal reintroduction sites can be further complicated when herbivores or natural enemies limit the abundance and population growth of rare species. Herbivores can influence lifetime fitness and population growth rates by directly or indirectly impacting plant survival, growth, or reproduction (Maron and Crone, 2006). If herbivore consumption decreases plant growth and causes reduced or delayed reproduction, restored populations can exhibit increased extinction risk, especially when initial populations sizes are small (Knight, 2012). Because herbivores can negatively influence population growth in both low and high quality habitat, determining the causes of failed establishment at restored sites can therefore be challenging without experimentally controlling herbivore access. Hence, herbivore exclusion is a common post-planting management practice when restoring rare species (Guerrant, 2012), with a majority of studies demonstrating that fencing or caging improves demographic rates over the shortterm (Fenu et al., 2016; Godefroid et al., 2011). However, the extent to which herbivores or natural enemies limit longer-term plant reintroduction success remains unknown.
Reintroduction success can also depend on the design of founder populations (Guerrant, 1996; Menges, 2008). In general, reintroductions are more successful in the short-term when founded with greater numbers of individuals and larger propagule stages (Albrecht and Maschinski, 2012; Godefroid et al., 2011), as smaller propagules (e.g., seeds and seedlings) often have narrow niche requirements and high mortality rates (Young et al., 2005). Small founder populations with limited genetic variation can further reduce the odds that genotypes or population sources are appropriately matched to restored sites (Colas et al., 2008; Reckinger et al., 2010). For example, in rare species, some source populations or genotypes exhibit superior performance to others independent of site or environmental conditions (Helenurm, 1998; Peterson et al., 2013). Moreover, increasing genetic diversity in reintroductions by mixing source populations, rather than using a single local source, can sometimes improve population fitness and resiliency over the long-term (Maschinski et al., 2013).
Reintroductions designed as experiments can help determine the habitat requirements of rare species and techniques that maximize reintroduction success (Guerrant and Kaye, 2007; Maschinski et al., 2012a). In particular, using an adaptive management framework, where experiments build on previous knowledge can resolve uncertainties and improve success rates in reintroduction programs (Armstrong et al., 2007; Menges et al., 2016). In this study, we developed a reintroduction experiment that builds upon information learned from failed reintroduction attempts with the federally endangered perennial, Astragalus bibullatus Barneby and Bridges (hereafter, Astragalus). In previous attempts to expand the occupancy of this species to protected habitat, reintroduced populations failed to persist beyond a decade despite several initial indicators of success, including next generation recruitment (Albrecht and McCue, 2010). The reasons for the failed reintroductions are unknown, but the most probable causes include poor habitat suitability, herbivores, or small founder size (Albrecht and McCue, 2010). Because these factors are nonexclusive and understanding their role is crucial for planning future reintroductions, we developed a factorial experiment to disentangle the relative importance of these factors on reintroduction success. We addressed key questions relevant to most reintroduction programs (Armstrong and Seddon, 2008): 1) Can improving habitat quality and increasing the genetic variability and size of founder populations increase demographic rates at sites with historically low rates of persistence? 2) How do herbivores impact demographic vital rates and persistence? and 3) What is the relative importance of habitat quality and herbivores on reintroduction success?
2. Study species and reintroduction programLimestone cedar glades (LCG) are habitat islands of thin rocky soils comprised of small-statured, heliophytic C3 grasses and annual forbs, many of which are rare endemics (Baskin and Baskin, 1999) including the perennial legume Astragalus. Listed as federally endangered under the Endangered Species Act, Astragalus naturally occurs at 8 sites and is known from < 5000 individuals in the wild (USFWS, 2011). Astragalus is a slow-growing, hemicryptophyte and a nitrogen-fixing member of the Fabaceae. Plants flower in April-May prior to deciduous tree leaf-out, and fruits mature in May-June. Seeds are gravity dispersed, exhibit physical dormancy, and can potentially remain viable in the soil for decades (Morris et al., 2002). Vegetative populations exhibit relatively low-levels of genetic differentiation, suggesting historical gene flow among sites (Baskauf and Burke, 2009). However, increased landscape fragmentation has probably reduced gene flow relative to historic levels, placing the species at risk of inbreeding depression (Morris et al., 2002). At present, most natural populations are small with declining growth rates (Albrecht et al., 2016; Bernardo et al., 2016).
With concerns about the small size, restricted distribution, and fragmentation of natural populations, a long-term reintroduction program began in 2000 to increase the abundance of Astragalus in protected areas within the species' historic range. In the first attempt, Astragalus was introduced to five protected LCGs at Stones River National Battlefield (SRNB). However, all populations failed to persist beyond a decade despite several initial indicators of success, including next generation seedling recruitment (Albrecht and McCue, 2010). We developed three interrelated hypotheses for why these restored populations failed to persist.
The habitat suitability hypothesis posits that recipient sites simply did not match the species' ecological niche requirements. At the time, inferring optimal habitat of Astragalus was challenging because the three known natural populations varied in levels of woody succession and vegetation composition. Because wild plants occurred most frequently in partially shaded glade-forest ecotones, this microhabitat type was targeted in previous SRNB reintroductions (Albrecht and McCue, 2010). However, recent studies demonstrated that woody encroachment, especially eastern redcedar (Juniperus virginiana L.), in glade-forest ecotones reduced growth and reproduction of Astragalus, although plants can survive for many years as remnant populations under dense shade (Albrecht et al., 2016). Long-term declines in the abundance of other LCG endemics have similarly been linked to increased woody encroachment (Sutter et al. 2011). Unlike most other LCG endemics, Astragalus rarely occurs in the thin soil of open glades, but instead prefers deeper soil ecotones, which are readily colonized by woody vegetation in the absence of disturbance. Historically, fire and other disturbances in the landscape matrix likely prevented formation of dense woody vegetation around glades, slowing forest development and expansion into deeper soil ecotones (Albrecht et al., 2016). Today, in the absence of fire, ecotones abruptly transition from open glade to dense cedar-hardwood forest. Thus, we predicted that future reintroductions would be most successful in open ecotones with low densities of woody vegetation.
The herbivore hypothesis predicts that top-down processes contributed to the failed reintroductions. Shortly after the spring (2001) outplanting, stems on many transplants were browsed by vertebrate herbivores. In response, managers caged all surviving plants to exclude herbivores; in a second round of transplanting in autumn (2001), all transplants were caged immediately after outplanting. Cages were then removed once transplants established. Although caging probably improved short-term survival of spring transplants, the long-term consequences of the initial browsing event remained unknown, as there were no uncaged controls for comparison. While many transplants resprouted after being browsed, few spring transplants flowered over the course of their lifespan.
The founder size hypothesis predicts that the initially small population size created demographic and genetic barriers to population growth (Albrecht and Maschinski, 2012). The first reintroduction used transplants grown from seed sourced from three natural populations, but initial population sizes (30-40 transplants/site) were below the minimum number (>50 transplants) recommended by best-practice guidelines (Maschinski et al., 2012a). A majority of the transplants were planted in the spring and experienced lower survival and flowering than those outplanted in the autumn, and no additional augmentations were done in subsequent years to bolster population size. Further, genetic data suggests that Astragalus is an obligate outcrosser, making it susceptible to Allee effects and increased extinction risk when reintroduced at low densities (Colas et al., 2008). At one reintroduction site (SRNB G7), a few next generation seedling recruits were observed, but these exhibited very low survival while the founder population dwindled to only a few individuals. Thus, small initial founder size coupled with herbivory could have created very small populations that were susceptible to stochastic extinction (Colas et al., 2008).
3. Methods 3.1. Reintroduction experimentTo disentangle the relative importance of these factors on reintroduction success, we used experiments that controlled herbivore access across three sites that varied in habitat suitability, while transplanting over five times as many individuals as previously failed reintroductions. In 2012, we experimentally reintroduced Astragalus to SRNB and to Couchville Cedar Glade and Barrens State Natural Area (Couchville), another protected conservation area within the species' historical range. There are no historic records documenting the presence of Astragalus at these locations prior to the reintroduction program. Exact locations are withheld for conservation purposes.
SRNB sites are typical of most LCG's where thin-soil, rocky openings are surrounded by dense woody vegetation, which creates shaded, mesic microenvironmental conditions along gladeforest ecotones (hereafter, mesic ecotones). The first site (G7; Fig. 1a) was selected because in the previous reintroduction, transplants reached reproductive maturity, produced viable seed, and recruited new seedlings before going extinct. Thus, we hypothesized that this site could support a persistent reintroduced population of Astragalus if we improved habitat quality, excluded herbivores, and increased founder size. Because reintroduced populations at the four other 2001 reintroduction sites (SRNB) did not meet demographic benchmarks for short-term success (Pavlik, 1996), we assumed these sites could not support Astragalus even with changes in reintroduction technique. The second site at SRNB (G54; Fig. 1b) is located ~0.45 km from G7 and supports a successful reintroduction of the rare endemic Echinacea tennesseensis (Bowen, 2011). G54 is a smaller and shadier glade than G7 (Fig. 1), making it similar to the four reintroduction sites where Astragalus failed to establish in 2001. We selected this site to test whether changes in reintroduction technique could increase population establishment in suboptimal habitat.

|
| Fig. 1 Astragalus bibullatus reintroduction sites in the Tennessee Central Basin: A) Xeric barren, B) Open mesic ecotone, and C) Closed mesic ecotone |
To determine whether planting a greater number of individuals across spatially variable microhabitats could increase reintroduction success over previous SRNB reintroductions, we established at both mesic ecotone sites a transect (8-15 m) in each of three different microhabitats spanning a successional gradient from shallow to deep soils (Quarterman, 1950): glade edge, glade-shrub, and shrubredcedar. To increase light-availability before outplanting, we reduced woody encroachment in each microhabitat by selectively thinning large-diameter (>10 cm) cedars, moving felled trees at least 10 m beyond areas targeted for outplanting. Although we removed nearly twice as many trees at G54 (21 trees) than at G7 (11 trees), canopy openness was 26% greater in the latter site (hereafter, open ecotone) because the former site (hereafter, closed mesic ecotone) contained greater tree densities, especially cedars.
At the third reintroduction site in Couchville, we established a transect in a xeric barren ecotone dominated by C4 perennial grasses (hereafter xeric barren, Fig. 1c). This site is comprised of a rare intact glade-barren mosaic complex underlain by Lebanon limestone, has been under prescribed fire management for over a decade, and is more open and drier compared to the mesic ecotones, as indicated by the lower cover of moss, greater cover of rock and bare ground, and greater canopy openness (see below). A dormant season (March 2012) prescribed burn was conducted prior to our reintroduction in autumn. Given the differences in habitat structure and management history, testing different successional microhabitats was not possible and thinning was unnecessary to improve habitat quality, unlike at SRNB (Fig. 1). We hypothesized that demographic performance would be greater here than the mesic ecotones, because ecological conditions more closely match those that are thought to favor Astragalus (Albrecht et al., 2016).
We propagated Astragalus plants in spring 2012 from ex situ accessions in Missouri Botanical Garden's frozen seed-bank. Accessions ranged in age from 2 to 18 years and represented most extant natural populations (except one) with a majority (58.3%) of the seed being sourced from the largest wild population. Following McCue et al.'s (2001) protocol, seeds were scarified (May 2012), germinated in flats, and seedlings were then transferred to plugs containing a potting medium that mimics well-drained glade soils; plants were grown outdoors in full sun and watered as needed (up to twice per day during hot and dry periods) until outplanting. Because plants had been grown for many months in pots without fertilizer, liquid fertilizer (Peters professional©, 20-20-20, at a rate of 1 tablespoon per gallon water) was applied weekly for the last four weeks of propagation to improve plant health. All plants were treated twice with ultra-fine paraffin oil, to minimize aphid damage.
We reintroduced 168 Astragalus plugs to each of the three habitat types (n = 504 plants in total) in September 2013, since previous reintroduction experiments found autumn was the optimal transplant season (Albrecht and McCue, 2010). At each site, we established paired 1 m2 plots (n = 21 paired plots per site), with a distance of ~0.5-1.5 m between plots in each pair. In each plot, we outplanted four transplants spaced equally apart (n = 8 plants per paired plot and n = 168 plants per site). We marked each transplant with a permanent metal tag and measured initial diameter. To mimic patterns of genetic structure within wild populations (Baskauf and Burke, 2009; Morris et al., 2002), we mixed the six population sources at each site and created genetically standardized populations by ensuring even representation of each source population among the three reintroduction sites (Table 1). At the time of outplanting, vegetation was cleared in each transplant plot to minimize the effects of competition on establishment. Deciduous shrubs and tree seedlings were clipped at ground-level within and around (≤1 m) plots.
| Source population (collection year) | Xeric barren | Mesic ecotone | |
| Open | Closed | ||
| APT(1996) | 33% (70) | 9% (70) | 10% (69) |
| APT (2010) | 35% (17) | 6% (17) | 6% (17) |
| APT (2011) | 33% (46) | 5% (45) | 0% (47) |
| ALX(2011) | 0% (8) | 0% (10) | 0% (10) |
| DVS (1993) | 0% (2) | 0% (3) | 0% (2) |
| DAV(1996) | 75% (4) | 20% (5) | 0% (4) |
| DAV (2011) | 17%(6) | 0% (5) | 0% (6) |
| MAN (2004) | 33% (6) | 0% (5) | 20% (5) |
| OVB (1995) | 56% (9) | 0% (8) | 0% (8) |
Immediately after outplanting, all plants were thoroughly watered and each transplant plot was covered with a 30 × 30 × 20 cm cage (hardware cloth, 1.25 × 1.25 cm mesh) to exclude vertebrate herbivores. Cages were staked into the ground with metal landscape pins to deter burrowing by small rodents; rocky, thin soils prevented us from having a standardized cage burial depth. Because we transplanted during an unusually dry autumn, supplemental watering was provided on a weekly basis during the first two months after outplanting; after which, no supplementary amendments were given. To experimentally test herbivory effects on demographic performance, we removed a cage from each paired plot in May 2013 after the first growing season demographic census. Because the original cages could potentially restrict pollinators and plant growth, we replaced cages with larger, circular wire baskets (56 cm diameter × 20 cm height), which allowed pollinator access and excluded larger vertebrates (e.g., rabbits and deer) but not small rodents. Stems of browsed plants showed a sharp, clean, angled slice indicative of rodents or lagomorphs, although we could not distinguish which species were cutting plants in uncaged plots. In spring 2013, we removed all exotic species and clipped forbs to ground level growing < 1 m around plots at the mesic ecotone sites, because mechanical thinning stimulated a flush of tall ruderal species.
From 2013 to 2017, we assessed transplant survival and stage class (vegetative or reproductive) in April or May. We recorded the total numbers of inflorescences, infructescences, and fruits per reproductive plant. In May 2014, we quantified microhabitat variation by measuring the percent cover of eight variables: forbs, grasses, bryophytes (hereafter moss), woodies, graminoid litter (hereafter thatch), non-graminoid litter, exposed rock/gravel, and bare ground in all 1 m2 plots at each site. In addition, percent canopy openness was calculated above each plot using a convex spherical densiometer following the methods of Lemmon (1956).
3.2. Data analysisAll analyses were conducted in R Statistical package version 3.4.2 (R Development Core Team, 2017). To quantify differences in environmental gradients among habitats, we conducted a Principal components analysis (PCA) in the vegan package (Oksanen et al., 2017) on the nine standardized microhabitat variables. Percent cover data was transformed into Braun-Blanquet cover classes prior to analysis. We used the broken stick method to determine the number of PCs that explained significant ecological variation among habitats (Borcard et al., 2011).
We used generalized linear mixed models (binomial distribution) in the lme4 package (Bates et al., 2015) to determine the effects of habitat, cage, and initial transplant diameter and their interactions on probabilities of transplant survival, flowering, and fruiting. Initial transplant diameter was centered for all analyses to improve interpretation of regression coefficients (Schielzeth, 2010). Due to suspected human disturbance of two plots at Couchville, which impacted plant survival, we excluded those individuals from all analyses. For all analyses, we followed the minimum adequate model approach by using backwards elimination and sequential likelihood ratio tests to compare models with and without a single focal term (Crawley, 2007).
For transplant survival, we developed three different models since all plants remained caged until after the first census (spring 2013). The first model examined the effects of habitat and initial diameter on the first-year survival of caged transplants (2012-2013). In the second model, we tested for the effects of habitat, cage, initial size, and all two-way interactions on cumulative transplant survival (2013-17). In the third model, we tested for the effects of microhabitat, caging, and initial diameter on cumulative transplant survival (2013-17) at the mesic ecotone sites (SRNB G7 and G54). In the second and third models, survival was conditional on surviving to the time when cages were removed. To account for non-independence of transplants within plots, we included plot as a random intercept in all models.
For models that examined flowering and fruiting probabilities, we pooled data from 2014 to 2017 due to low or absent reproduction in some years at mesic ecotone sites. We also combined the two mesic ecotone sites together, because flowering was extremely low or absent in some year by site combinations. Because only one flowering plant produced fruit in uncaged plots over the four year period, we only tested for the main effects of habitat and initial diameter in the model that examined fruiting probabilities. For flowering and fruiting models, we nested individual plants within plots as the random effect to account for repeated sampling.
4. ResultsThe first three PCs explained 75.5% of the environmental variation among habitats. While PC1 explained the greatest variation (42.3%), PC2 (17.4%) and PC3 (15.8%) explained similar levels of variation. However, only eigenvalues from PC1 and PC3 were greater than those generated from a broken stick model (Fig. 2a), thus variables with the highest loadings along these PC axes describe significant ecological variation among habitats. Along PC1, canopy openness, grass cover, and graminoid litter cover (i.e., thatch) had the highest negative loadings (xeric barren), whereas forb and litter cover had the highest positive loadings (mesic ecotones) (Fig. 2b). Canopy openness was greatest in the xeric barren habitat and lowest in the closed mesic ecotone (Fig. 2c), while forb and moss cover were much lower in the xeric barren habitat relative to the mesic ecotones (Fig. 2d).
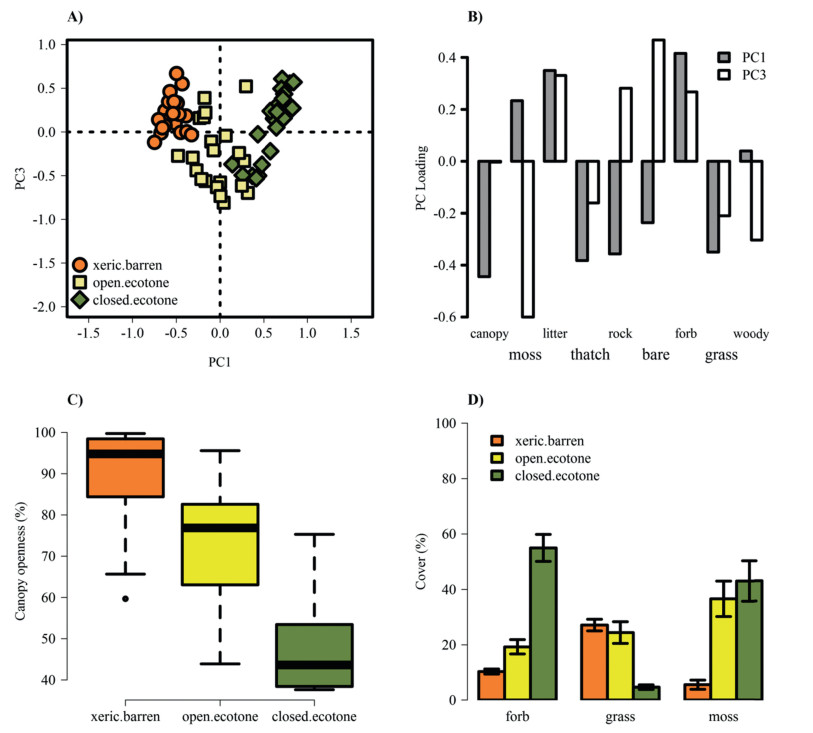
|
| Fig. 2 Microhabitat variation at three Astragalus bibullatus reintroduction sites. A) Principal components analysis (PCA) of microhabitat variation showing plot scores on PC1 and PC3 at each site. B) Factor loadings of nine microhabitat variables on PC1 and PC3. C) Variation in canopy openness. D) Percent cover (mean ± se) of forbs, mosses, and grasses |
The open mesic ecotone maintained similar amounts of grass cover as the xeric barren, but lower levels of canopy openness (Fig. 2c and d). The best-fit model for first-year transplant survival when all plants were caged included site (P < 0.0001), initial transplant diameter (P < 0.0001), and the site × diameter interaction (P = 0.056) (Table 2). Overall, first-year survival was significantly greater in the xeric barren compared to the mesic ecotones (Fig. 3a). Survival probabilities of plants were positively correlated with initial diameter, but the shape of the relationship varied among sites (Fig. 4a). Small diameter plants showed similar survival probabilities across sites while larger diameter plants showed much higher survival probabilities in the xeric barren relative to the mesic ecotones (Fig. 4a). In the mesic ecotones, there was no significant effect of microhabitat (P = 0.72) or interactions between microhabitat and initial diameter (P = 0.86) on transplant survival.
| Term | Chisq | d.f. | P | |
| A) | Survival (2012—13) Habitat | 28.65 | 2 | < 0.0001 |
| Initial diameter (ID) | 17.87 | 1 | < 0.0001 | |
| Habitat × ID | 5.77 | 2 | 0.056 | |
| B) | Conditional survival (2013—17) Habitat | 28.01 | 2 | < 0.0001 |
| Initial diameter (ID) | 7.76 | 1 | 0.005 | |
| Cage | 0.02 | 1 | 0.87 | |
| Cage ×ID | 6.60 | 1 | 0.01 | |
| C) | Flowering (2013—17) Habitat | 7.25 | 1 | < 0.001 |
| Initial diameter (ID) | 5.49 | 1 | 0.02 | |
| Cage | 13.73 | 1 | < 0.0001 | |
| D) | Fruiting (2013—17) Habitat | 2.39 | 1 | 0.12 |
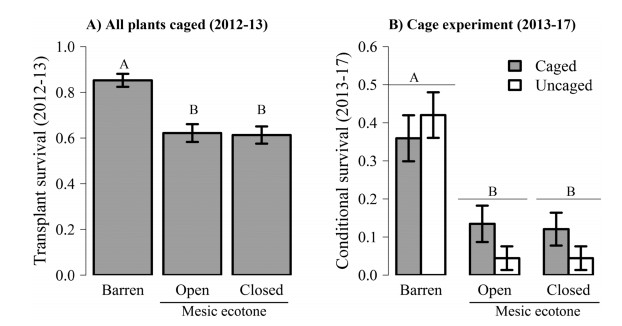
|
| Fig. 3 Survival probabilities (mean ± se) of Astragalus bibullatus transplants among A) sites the first-year after outplanting (all plants caged) and B) sites and caging treatments fouryears after cages were removed from half of the plots. In the caging experiment (B), survival probabilities are conditional on surviving to the end of the first-year before cages were removed |

|
| Fig. 4 Differences in the effects of initial diameter on survival probabilities of Astragalus bibullatus transplants among A) sites the first-year after outplanting (all plants caged) and B) sites and caging treatments four-years after cages were removed from half of the plots. Predicted survival probabilities (±95% confidence interval) are derived from minimum adequate models (Table 2). Open = Mesic open ecotone; Closed = Mesic closed ecotone |
In the herbivore exclusion experiment, conditional survival (2013-17) varied among sites, but there was no significant main effect of caging (Table 2). After five years, cumulative transplant survival in the xeric barren ecotone (41 ± 4%, mean ± se) was significantly greater than in the open (21 ± 3%) and closed (9 ± 2%) mesic ecotones (Fig. 3b). However, the effects of initial size on survival varied among caging treatments, with larger diameter plants having a survival advantage when caged (Fig. 4b). In 2017, the xeric barren maintained genetic representation from seven of the nine original source populations whereas mesic ecotones were represented by ≤4 source populations (Table 1). There was no evidence that individuals from any population source survived at greater rates in the mesic ecotones relative to the xeric barren (Table 1).
Caging, site and initial diameter significantly influenced flowering probabilities (Table 2). Overall, caged transplants had much greater probabilities of flowering than uncaged transplants (Fig. 5a). On average, flowering and fruiting probabilities were two times greater in the xeric barren relative to the mesic ecotones (Fig. 5a and b). Transplants with larger initial diameters had significantly (Table 2) greater probabilities of flowering over the five-year period (2.004 ± 0.856, estimate ± se). Over the five-year period, there were over ten times as many fruits produced in the xeric barren relative to the mesic ecotones (Fig. 5c).

|
| Fig. 5 Cumulative fitness components (2013-17) of Astragalus bibullatus transplants in each habitat. A) Proportion of surviving transplants that flowered in the caging experiment, B) proportion of flowering plants that produce at least one fruit (across caging treatments), and C) cumulative number of fruits (across caging treatments). Across all sites, only a single flowering plant produced fruit outside of cages. Mean flowering probabilities are based on least square mean estimates (±se) from best-fit models. Numbers in parentheses represent the number of plants (A and B) or total number of fruits produced (C) |
We conducted a reintroduction experiment that tested the relative importance of multiple hypotheses to explain the reintroduction success of an endangered plant species. Our results provide strong support for the hypothesis that habitat suitability is the primary diver of success rates in rare plant reintroductions (Godefroid et al., 2011; Knight, 2012; Maschinski et al., 2012b). We observed dramatic differences in demographic performance between mesic ecotones and the xeric barren. In the first year, when all plants were caged, survival rates were much greater in the xeric barren relative to the mesic ecotones, demonstrating that poor habitat suitability rather than herbivores was the primary cause of poor establishment rates in previous reintroductions to mesic ecotones. After five years, cumulative transplant survival probabilities were two times greater and cumulative fruit production was ten times greater in the xeric barren relative to the mesic ecotones. The microhabitat conditions (e.g., dry, rocky, and open) in the xeric barren are consistent with the hypothesized historical reference conditions for Astragalus and other declining LCG endemics (Albrecht et al., 2016). Thus, xeric, high-light microhabitats with low tree, forb, litter, and moss cover should be the targeted habitat for future Astragalus reintroductions.
Although our experiment did not directly test the founder size hypothesis, we found limited evidence that initially small population sizes directly caused the low viability of previous reintroductions. In mesic ecotones, we tripled the number of genetic source populations and created initial population sizes five times that of previous reintroductions, yet demographic vital rates remained depressed with and without herbivore exclusion. If processes associated with small founder size (e.g., Allee effects) or genetic mismatches between source and recipients sites reduced fitness in previous reintroductions, we expected improved vital rates with larger and more genetically variable founder populations (Albrecht and Maschinski, 2012; Guerrant, 1996). Instead, despite caging plants, using large adult transplants, and planting across multiple microhabitats, we still found that cumulative survival and transition rates (5-yr) to reproductive adulthood were similar to or lower than previous attempts at SRNB. For example, only three fruits were produced over five-years in mesic ecotones, which is far below the seed input needed to maintain a stable population of Astragalus (Bernardo et al., 2016). We conclude that reintroduction sites at SRNB poorly match the ecological niche requirements of Astragalus. After multiple reintroduction attempts across space (n = 6 sites) and time (2001 and 2012) with no apparent improvements in population viability when excluding herbivores and manipulating habitat structure, our results do not support future reintroductions of Astragalus to mesic ecotones sites.
Reintroduction experiments spanning longer time scales and multiple sites with varied environmental contexts can provide clues into the ecological niche of rare species (Bowles et al., 2015). In our study, we expected that removing woody encroachment in mesic ecotones would restore the environmental conditions optimal for Astraglus, as interactions between soil depth and tree cover are the presumed drivers of light and soil moisture availability in LCGs (Baskin and Baskin, 1999; Quarterman, 1950). However, although our restoration thinning treatments created canopy openness levels (average: 56%; range: 23-91%) comparable to natural populations (63%; 12-80%), we found little improvement in demographic performance relative to previous reintroductions at SRNB. Given the poor performance of Astragalus reintroductions with and without woody removal across multiple sites at SRNB, we hypothesize that other factors are probably contributing to poor performance. Although we did not quantify soil moisture, anecdotal evidence suggests that soils at SRNB remained moist after precipitation events for longer periods relative to xeric barren and natural Astraglus populations. Soils at SRNB are underlain by thick-bedded Ridley limestone, whereas Couchville and natural populations occur on thin-bedded, Lebanon limestone. Although little is known about how edaphic properties differ among these limestone types, tree species with xeric soil affinities are more frequent in some naturally occurring Astragalus sites relative to SRNB (Adams et al., 2012). In cultivation, Astragalus plants are susceptible to disease and root rot when grown in poorly-drained or consistently moist potting media irrespective of light and nutrient conditions (McCue et al., 2001), suggesting that soil moisture could be a contributing factor to the poor performance in glades at SRNB. Thus, edaphic conditions should be accounted for when selecting future reintroduction sites.
Herbivores reduced flowering across all reintroduction sites, but only in the high-quality xeric barren habitat were they the primary factor limiting population viability of Astragalus. Due to their high nitrogen content and nutritious forage, legumes are often preferred by vertebrate herbivores over other forbs and grasses (Hulme, 1996; Zorn-Arnold et al., 2006). Although we did not quantify community-level herbivory, we observed little evidence of browse on neighboring heterospecifics, suggesting herbivores selectively targeted Astragalus. By altering plant size, vertebrate herbivores can directly impact fitness in perennial plants and therefore population growth rate (Maron and Crone, 2006). In uncaged plots, initially larger Astragalus plants exhibited lower survival probabilities than smaller plants, perhaps because herbivores selectively browsed larger plants, as reported in other perennial forbs (Knight et al., 2009). While herbivores did occasionally clip racemes on flowering adults, we found that vegetative stems were clipped both before and after the reproductive period, and on some plants repeatedly. In turn, uncaged plots maintained a population structure over the four-year experiment that was skewed towards smaller plants with extremely low flowering probabilities, with only a single plant producing fruit in the xeric barren. However, in caged plots, we observed the opposite relationship between plant size and survival, with over 20% of surviving plants transitioning to flowering adults over the four-year experiment.
Given the long time-lags that can occur between reproductive maturity and next generation recruitment, reintroductions with perennials often require a decade or more to accurately assess their viability (Bell et al., 2003). We have yet to observe next generation seedling recruits in the xeric barren habitat. In previous Astragalus reintroductions, next generation seedlings were not observed until five years after outplanting, since Astragalus seeds can remain dormant in the soil for many years and require specialized conditions to germinate (Albrecht and Penagos, 2012). Thus, several more years may pass before the seeds germinate and we are able to evaluate long-term viability of herbivore exclusion in the xeric barren.
Our study illustrates the importance of designing reintroductions as experiments within an adaptive management framework. By incorporating knowledge (e.g., transplant season) from previous reintroductions with ongoing research on the biology of the species, we were able to determine suitable habitat (xeric rocky barren) and techniques (herbivore exclusion) that maximize demographic performance in reintroductions of Astragalus. Had we not followed-up with a second reintroduction experiment at SRNB, key questions would remain regarding the relative role of habitat suitability and herbivores in driving reintroduction outcomes. Our study illustrated the challenges of determining suitable habitat for rare species known from only a few sites in a fragmented ecosystem where reference conditions are poorly understood. The fact that mesic ecotones sites cannot support viable populations, even after habitat restoration and herbivore exclusion, has important implications for choosing future reintroduction sites and ensuring that additional effort is not misappropriated at sites with low habitat quality. Our study revealed that xeric barrens underlain by Lebanon limestone represent important priorities for future habitat protection, management, and reintroduction of Astragalus. Our results reinforce previous recommendations that reintroductions are best approached as long-term, iterative experiments with careful monitoring, so that failures can provide important learning opportunities that inform future reintroduction and recovery efforts with rare species (Menges, 2008; Pavlik, 1996).
MAA conceived the research and acquired funding; MAA and QGL designed the experiment and conducted field-work; MAA analyzed data; MAA and QGL wrote paper.
We thank A. Bishop, T. Morris, T. Hogan, G. Backlund, D. Adams, D. Lincicome, G. Call, B. Bowen, J. Allen, T. Hall, G. Allington, S. Woodbury, and T. Brandt for logistical, propagation, and field support. We thank the many students and volunteers that helped with the project. We thank H. Bernardo, R. Becknell, J. Lucas, and two anonymous reviewers for providing helpful comments on the manuscript. This research was supported by the United States Department of Interior National Park Service Challenge Cost-Share Program (Grant: H55901000010) and the Tennessee Department of Environment and Conservation-Division of Natural Areas (Grants: 32701-00385, 32701-0899, and 32701-01236).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2018.09.004.
Adams D.A., Walck J.L., Howard R.S., Milberg P., 2012. Forest composition and structure on glade-forming limestones in middle Tennessee. Castanea, 77: 335-347. DOI:10.2179/12-010 |
Albrecht M.A., Becknell R.E., Long Q., 2016. Habitat change in insular grasslands:woody encroachment alters the population dynamics of a rare ecotonal plant. Biol. Conserv, 196: 93-102. DOI:10.1016/j.biocon.2016.01.032 |
Albrecht, M.A., Maschinski, J., 2012. Influence of founder population size, propagule stages, and life history on the survival of reintroduced plant populations. In: Maschinski, J., Haskins, K.E. (Eds.), Plant Reintroduction in a Changing Climate: Promises and Perils. Island Press, Washington, DC, pp. 171-188.
|
Albrecht M.A., McCue K.A., 2010. Changes in demographic processes over long time scales reveal the challenge of restoring an endangered plant. Restor. Ecol, 18: 235-243. |
Albrecht M.A., Penagos J.C., 2012. Seed germination ecology of three imperiled plants of rock outcrops in the southeastern United States. J. Torrey Bot. Soc, 139: 86-95. DOI:10.3159/TORREY-D-11-00066.1 |
Armstrong D.P., Castro I., Griffiths R., 2007. Using adaptive management to determine requirements of re-introduced populations:the case of the New Zealand hihi. J. Appl. Ecol, 44: 953-962. DOI:10.1111/jpe.2007.44.issue-5 |
Armstrong D.P., Seddon P.J., 2008. Directions in reintroduction biology. Trends Ecol. Evol, 23: 20-25. DOI:10.1016/j.tree.2007.10.003 |
Baskauf C.J., Burke J.M., 2009. Population genetics of Astragalus bibullatus (Faba-ceae) using AFLPs. J. Hered, 100: 424-431. DOI:10.1093/jhered/esp033 |
Baskin, J.M., Baskin, C.C., 1999. Cedar glades of the southeastern United States. In: Fralish, J.S., Baskin, J.M., Anderson, R.C. (Eds.), Savannas, Barrens, and Rock Outcrop Plant Communities of North America. Cambridge University Press, Cambridge, pp. 206-219.
|
Bates D., Mächler M., Bolker B., Walker S., 2015. Fitting linear mixed-effects models using lme4. J. Stat. Software, 67: 48. |
Bell, T.J., Bowles, M., McEachern, A.K., 2003. Projecting the success ofplant population restoration with viability analysis. In: Brigham, C.A., Schwartz, M.W. (Eds.), Population Viability in Plants. Springer-Verlag, Berlin, Germany, pp. 313-348.
|
Bernardo H.L., Albrecht M.A., Knight T.M., 2016. Increased drought frequency alters the optimal management strategy of an endangered plant. Biol. Conserv, 203: 243-251. DOI:10.1016/j.biocon.2016.09.030 |
Bontrager M., Webster K., Elvin M., Parker I., 2014. The effects of habitat and competitive/facilitative interactions on reintroduction success of the endangered wetland herb, Arenaria paludicola. Plant Ecol, 215: 467-478. DOI:10.1007/s11258-014-0317-z |
Borcard, D., Gillet, F., Legendre, P., 2011. Numerical Ecology with R. Springer, New York.
|
Bottin L., Le Cadre S., Quilichini A., Bardin P., Moret J., Machon N., 2007. Reestablishment trials in endangered plants:a review and the example of Arenaria grandiflora, a species on the brink of extinction in the Parisian region (France). Ecoscience, 14: 410-419. DOI:10.2980/1195-6860(2007)14[410:RTIEPA]2.0.CO;2 |
Bowen B., 2011. Natural areas protection at its best:protecting the Tennessee Purple Cone flower (Echinacea tennesseensis). Nat. Areas J, 31: 326-330. DOI:10.3375/043.031.0402 |
Bowles M.L., McBride J.L., Bell T.J., 2015. Long-term processes affecting restoration and viability of the federal threatened Mead's milkweed (Asclepias meadii). Ecosphere, 6: art11. DOI:10.1890/ES14-00240.1 |
Colas B., Kirchner F., Riba M., Olivieri I., Mignot A., Imbert E., Beltrame C., Carbonell D., Freville H., 2008. Restoration demography:a 10-year demographic comparison between introduced and natural populations of endemic Centaurea corymbosa (Asteraceae). J. Appl. Ecol, 45: 1468-1476. DOI:10.1111/jpe.2008.45.issue-5 |
Crawley, M.J., 2007. The R Book. John Wiley & Sons, London.
|
Drayton B., Primack R.B., 2012. Success rates for reintroductions of eight perennial plant species after 15 years. Restor. Ecol, 20: 299-303. DOI:10.1111/rec.2012.20.issue-3 |
Dunwiddie P.W., Martin R.A., 2016. Microsites matter:improving the success of rare species reintroductions. PLoS One, 11: e0150417. DOI:10.1371/journal.pone.0150417 |
Falk, D.A., Millar, C.I., Olwell, M., 1996. Restoring Diversity: Strategies for Reintroduction of Endangered Plants. Island Press, Washington, DC.
|
Fenu G., Cogoni D., Bacchetta G., 2016. The role of fencing in the success of threatened plant species translocation. Plant Ecol, 217: 207-217. DOI:10.1007/s11258-015-0517-1 |
Godefroid S., Piazza C., Rossi G., Buord S., Stevens A.-D., Aguraiuja R., Cowell C., Weekley C.W., Vogg G., Iriondo J.M., Johnson I., Dixon B., Gordon D., Magnanon S., Valentin B., Bjureke K., Koopman R., Vicens M., Virevaire M., Vanderborght T., 2011. How successful are plant species reintroductions?. Biol. Conserv, 144: 672-682. DOI:10.1016/j.biocon.2010.10.003 |
Guerrant Jr., E.O., 1996. Designing populations: demographic, genetic, and horticultural dimensions. In: Falk, D.A., Millar, C.I., Olwell, M. (Eds.), Restoring Diversity: Strategies for Reintroduction of Endangered Plants. Island Press, Washington, DC, pp. 171-208.
|
Guerrant Jr., E.O., 2012. Characterizing two decades of rare plant reintroductions. In: Maschinski, J., Haskins, K.E. (Eds.), Plant Reintroduction in a Changing Climate: Promises and Perils. Island Press, Washington, DC.
|
Guerrant Jr. E. O., Kaye T.N., 2007. Reintroduction of rare and endangered plants:common factors, questions, and approaches. Aust. J. Bot, 55: 362-370. DOI:10.1071/BT06033 |
Helenurm K., 1998. Outplanting and differential source population success in Lupinus guadalupensis. Conserv. Biol, 12: 118-127. DOI:10.1046/j.1523-1739.1998.96316.x |
Holl K.D., Hayes G.F., 2006. Challenges to introducing and managing disturbance regimes for Holocarpha macradenia, an endangered annual grassland forb. Conserv. Biol, 20: 1121-1131. DOI:10.1111/j.1523-1739.2006.00416.x |
Hulme P.E., 1996. Herbivores and the performance of grassland plants:a comparison of arthropod, mollusc and rodent herbivory. J. Ecol, 84: 43-51. DOI:10.2307/2261698 |
Knight, T.M., 2012. Using population viability analysis to plan reintroductions. In: Maschinski, J., Haskins, K.E. (Eds.), Plant Reintroduction in Changing Climate. Island Press, Washington, DC, pp. 155-169.
|
Knight T.M., Caswell H., Kalisz S., 2009. Population growth rate of a common understory herb decreases non-linearly across a gradient of deer herbivory. For. Ecol. Manag, 257: 1095-1103. DOI:10.1016/j.foreco.2008.11.018 |
Lawrence B.A., Kaye T.N., 2011. Reintroduction of Castilleja levisecta:effects of ecological similarity, source population genetics, and habitat quality. Restor. Ecol, 19: 166-176. DOI:10.1111/rec.2011.19.issue-2 |
Lemmon P.E., 1956. A spherical densiometer for estimating forest overstory density. For. Sci, 2: 314-320. |
Maron J.L., Crone E., 2006. Herbivory:effects on plant abundance, distribution and population growth. Proc. Roy. Soc. Biol. Sci, 273: 2575-2584. DOI:10.1098/rspb.2006.3587 |
Maschinski, J., Albrecht, M.A., Monks, L.T., Haskins, K.E., 2012a. Center for plant conservation best reintroduction practice guidelines. In: Maschinski, J., Haskins, K.E. (Eds.), Plant Reintroduction in a Changing Climate. Island Press, Washington, DC, pp. 277-306.
|
Maschinski, J., Falk, D.A., Wright, S.J., Possley, J., Roncal, J., Wendelberger, K.S., 2012b. Optimal locations for plant reintroductions in a changing world. In: Maschinski, J., Haskins, K.E. (Eds.), Plant Reintroduction in a Changing Climate. Island Press, Washington, DC, pp. 109-129.
|
Maschinski, J., Haskins, K.E., 2012. Plant Reintroduction in a Changing Climate: Promises and Perils. Island Press, Washington, DC.
|
Maschinski J., Wright S.J., 2006. Using ecological theory to plan restorations of the endangered Beach jacquemontia (Convolvulaceae) in fragmented habitats. J. Nat. Conserv, 14: 180-189. DOI:10.1016/j.jnc.2006.05.003 |
Maschinski J., Wright S.J., Koptur S., Pinto-Torres E.C., 2013. When is local the best paradigm? Breeding history influences conservation reintroduction survival and population trajectories in times of extreme climate events. Biol. Conserv, 159: 277-284. DOI:10.1016/j.biocon.2012.10.022 |
McCue K.A., Belt E., Yurlina M., 2001. Propagation protocol for Astragalus bibullatus. Nat. Plants J, 2: 131-132. DOI:10.3368/npj.2.2.131 |
Menges E.S., 2008. Restoration demography and genetics of plants:when is a translocation successful? . Aust. J. Bot, 56: 187-196. |
Menges E.S., Smith S.A., Weekley C.W., 2016. Adaptive introductions:how multiple experiments and comparisons to wild populations provide insights into requirements for long-term introduction success of an endangered shrub. Plant Diversity, 38: 238-246. DOI:10.1016/j.pld.2016.09.004 |
Morris A.B., Baucom R.S., Cruzan M.B., 2002. Stratified analysis of the soil seed bank in the cedar glade endemic Astragalus bibullatus:evidence for historical changes in genetic structure. Am. J. Bot, 89: 29-36. DOI:10.3732/ajb.89.1.29 |
Noël F., Prati D., van Kleunen M., Gygax A., Moser D., Fischer M., 2011. Establishment success of 25 rare wetland species introduced into restored habitats is best predicted by ecological distance to source habitats. Biol. Conserv, 144: 602-609. DOI:10.1016/j.biocon.2010.11.001 |
Oksanen, J., Guillaume Blanchet, F., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchin, P.R., O'Hara, R.B., Simpson, G.L., Solymos, P., Stevens, M.H.H., Szoecs, E., Wagner, H., 2017. Vegan: Community Ecology Package, R Package Version 2.4-4.
|
Pavlik, B.M., 1996. Defining and measuring success. In: Falk, D.A., Millar, C.I., Olwell, M. (Eds.), Restoring Diversity: Strategies for the Reintroduction of Endangered Species. Island Press, Washington, DC, pp. 127-156.
|
Pavlovic, N.B., 1994. Disturbance-dependent persistence of rare plants: anthropogenic impacts and restoration implications. In: Bowles, M.L., Whelan, C.J. (Eds.), Restoration of Endangered Species: Conceptual Issues, Planning and Implementation. Cambridge University Press, New York, pp. 159-193.
|
Peterson C.L., Kaufmann G.S., Vandello C., Richardson M.L., 2013. Parent genotype and environmental factors influence introduction success of the critically endangered Savannas Mint (Dicerandra immaculata var. savannarum). PLoS One, 8: e61429. DOI:10.1371/journal.pone.0061429 |
Quarterman E., 1950. Major plant communities of Tennessee cedar glades. Ecology, 31: 234-254. DOI:10.2307/1932389 |
R Development Core Team, 2017. R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, pp. 20172009-2028.
|
Reckinger C., Colling G., Matthies D., 2010. Restoring populations of the endangered plant Scorzonera humilis:influence of site conditions, seed source, and plant stage. Restor. Ecol, 18: 904-913. DOI:10.1111/j.1526-100X.2009.00522.x |
Ren H., Ma G., Zhang Q., Guo Q., Wang J., Wang Z., 2010. Moss is a key nurse plant for reintroduction of the endangered herb, Primulina tabacum Hance. Plant Ecol, 209: 313-320. DOI:10.1007/s11258-010-9754-5 |
Schielzeth H., 2010. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol, 1: 103-113. DOI:10.1111/mee3.2010.1.issue-2 |
Smith Z.F., James E.A., McDonnell M.J., McLean C.B., 2009. Planting conditions improve translocation success of the endangered terrestrial orchid Diuris fra-grantissima (Orchidaceae). Aust. J. Bot, 57: 200-209. DOI:10.1071/BT09072 |
Sutter, R.D., Govus, T.E., Smyth, R.L., Nordman, C., Pyne, M., Hogan, T., 2011. Monitoring change in a Central U.S. calcareous glade: resampling transects established in 1993. Nat. Area J. 31,163-172.
|
USFWS, 2011. Recovery Plan for Astragalus bibullatus (Pyne's Ground-plum). USFWS, Atlanta, Georgia: p, 43. |
Volis S., Dorman M., Blecher M., Sapir Y., Burdeniy L., 2011. Variation partitioning in canonical ordination reveals no effect of soil but an effect of co-occurring species on translocation success in Iris atrofusca. J. Appl. Ecol, 48: 265-273. DOI:10.1111/j.1365-2664.2010.01898.x |
Young T.P., Petersen D.A., Clary J.J., 2005. The ecology of restoration:historical links, emerging issues, and unexplored realms. Ecol. Lett, 8: 662-673. DOI:10.1111/ele.2008.8.issue-6 |
Zorn Arnold B., Brown J.S., Howe H.F., 2006. Obvious and cryptic vole suppression of a prairie legume in experimental restorations. Int. J. Plant Sci, 167: 961-968. DOI:10.1086/505719 |



