Over 20% of forest and agricultural lands in Latin America are degraded: 300 million hectares of the region's forests are considered degraded, and about 350 million hectares are classified as deforested, leaving many remaining forests fragmented (Vergara et al., 2016). Small forest fragments tend to retain a degraded structure (Tabanez and Viana, 2000) as fragmentation promotes a decrease in species richness, a shift in the relative abundance of tree reproductive traits, and a reduction in the functional diversity of tree assemblages in fragmented landscapes (Girão et al., 2007). These effects drive fragments toward early-successional states (Pütz et al., 2011), leading to tree species impoverishment.
Governments in Latin America and the Caribbean have committed to bringing 20 million hectares of degraded land into restoration by 2020 (WRI, 2017). In Brazil targets have been set to restore twelve million hectares of deforested and degraded forest land by 2030 through forest restoration initiatives (WRI, 2016), and forest restoration is mandatory under the Native Vegetation Protection Law of Brazil (Law #12,651/2012). As a result there are a number of state, NGO, and corporate land restoration initiatives underway throughout the country (IUCN, 2016; AFRP, 2016). This level of restoration can provide myriad benefits to degraded land, such as restored biodiversity (including recovery of threatened species), increased ecological functioning, the supply of goods and ecological services, and the amelioration of rural poverty (Lamb et al., 2005).
Land can be restored passively or actively. Passive restoration is the spontaneous recovery of native tree species, whereas active restoration requires planting nursery-grown seedlings, direct seeding, or mimicking disturbance regimes to speed up recovery processes. Although passive regeneration has been demonstrated to promote richer regeneration than active restoration at a fraction of the cost, it is not more effective in highly fragmented areas where population levels are low and species rich communities cannot be naturally recruited (Crouzeilles et al., 2017). Because active restoration is most appropriate for fragmented forests, this paper is limited to the role of nursery-grown seedlings in restoration plantings.
1.2. Species selection in restoration interventionsThere are differing recommendations for the ideal number of native species to be included in restoration plantings, each of which depend on particular restoration objectives. The "Framework Species Approach" recommends 20 to 30 species (Goosem and Tucker, 1995). However, high-diversity plantings, defined by 80 to 90 species per hectare, are preferable to lower-diversity plantings as this number of species represents multiple functional groups that a smaller number of common and fast-growing species lacks (Rodrigues et al., 2009). When restoration includes a limited set of 20 to 30 taxa, the "restored" area cannot achieve maximal functionality; it cannot recruit threatened species under pressures such as lack of seed flow from neighboring locations, small population sizes, competition, and encroachment (Volis, 2016b). Conversely, the more threatened species included, the more representation for taxa with narrow regeneration niches and limited dispersal abilities (Volis, 2016b).
The delivery of high-diversity plantings is a challenge within the restoration industry, as the instability of native species markets and problems with the commercialization of native seedlings usually result in species bottlenecks (Bozzano et al., 2014; Silva et al., 2014). In Mexico 60 to 80 species have been demonstrated to be a financially feasible target number, but due to constraints within the market only 20 to 30 are typically used (Arroyo-Rodríguez et al., 2009).
It is valuable to include threatened species in high-diversity plantings, as they are exceedingly vulnerable in fragmented landscapes. Threatened populations are expected to continue decreasing due to time-lag biological responses even if no further degradation occurs (Metzger et al., 2009), compounded with bottlenecks in genetic diversity (Sork and Smouse, 2006). Furthermore, permanent distortions of species composition in favor of abundant dominant or dispersal-efficient subdominant species in fragmented landscapes makes rare and threatened species disproportionately vulnerable to extinction due to their limited immigration and colonization abilities (Maina and Howe, 2000; Tabarelli et al., 2005). Given the increased vulnerability of rare and threatened species in fragmented landscapes, therefore, their inclusion is an essential strategy to support their in situ conservation, which is a key goal in ongoing restoration projects in the Latin America region (Gill et al., 2017).
1.3. Constraints on species selectionAlthough high-diversity plantings are demonstrably more successful in terms of maximizing representation of functional groups and therefore ecosystem function, there are various practicalities imposing restraints on the ability of restoration actors to use a large number of species-including threatened species-in their plantings. Restoration actors attempting to balance species richness goals with their available resources must consider a multitude of factors in their species selection processes. The scope of this paper focuses on two primary actors in the restoration supply chain: nurseries who grow seedlings for restoration projects, and restoration practitioners who purchase seedlings from nurseries in order to carry out restoration plantings.
Nurseries encounter seed sourcing, collection, production, and storage of species as significant challenges to their use (Jalonen et al., 2017; Ladouceur et al., 2017), as well as adequate information on a wide range of species, preventing their ubiquity in plantings (Hoffmann et al., 2015). Nurseries are restricted by their ability to travel to seed sources and the technical feasibilities of wild seed collection in adequate quantities. Specific barriers include limited number of individuals and populations, difficulty and cost to access these populations, in addition to narrow collection windows, seed crops of mixed maturity, and atypical germination patterns (Broadhurst et al., 2016; Hoffmann et al., 2015). Currently native seed collection is forbidden in Protected Areas, which limits the inclusion of species with higher conservation value in restoration projects, especially in biomes with very low forest cover remaining such as the Atlantic Forest (Silva et al., 2016). Further restraints include low market prices for seedlings, which results in lack of motivation for nurseries to diversify their stock, and relationships with restoration practitioners who request a limited set of species (Jalonen et al., 2017; Volis, 2016a).
Restoration practitioners-those who purchase seedlings from nurseries-must choose species with ecological properties advantageous to plantings, such as high survival and growth rates in degraded sites, dense crowns that shade out herbaceous weeds, provision of resources that attract seed dispersers at early restoration stages, and natural regeneration capacity (Blakesley et al., 2002; Lindell et al., 2013; dos Santos et al., 2008). Restoration practitioners tend to use common and widespread species because they are ubiquitous in nurseries and have high success rates once planted (Aronson et al., 2011). Although the selection of a limited set of species produces successful plantings, it can lead to the homogenization of restored areas with few, widespread species dominating the landscape (Silva et al., 2016).
Given these competing considerations, diversity is seldom prioritized and typically only common or commercially important species are cultivated in large numbers and used for plantings (Jalonen et al., 2017). This leads to a species bias (Broadhurst et al., 2016), in which a few core species that can be reliably and readily sourced, easily stored and germinated are selected by nurseries, and these same reliable species are then purchased by practitioners. Biased selections deliver cost-effective outcomes with low risk to both nurseries and practitioners, but they represent only a fraction of species required to reconstruct diverse and resilient restoration outcomes (Volis, 2016b).
1.4. Restoration of the Araucaria forestThe subtropical Araucaria forest ecosystem in Brazil is a unique case for restoration as so little remains: less than 0.8% of the original forest is extant in advanced successional stages, none of which is considered primary forest (Castella and Britez, 2004). It is a subregion of the Atlantic Forest biome-a global biodiversity hotspot (Myers et al., 2000)-and hosts 352 known tree species (Leite and Klein, 1990). There are currently 71 taxa classified as threatened (39) and rare (32) (Appendix A), 1 which comprises 20% of all taxa in this ecosystem.
1 This list is adapted from Hoffmann et al. (2015), but this paper advocates for many of the species' threat statuses to be updated, given their observed rarity in the field. It is likely that in actuality their threaten statuses are more severe.
The original extent of the Araucaria forest is an estimated 25,379,300 ha (Ribeiro et al., 2009), yet historic timber exploitation and intensive agriculture has led to large-scale loss of forest habitat. Today the landscape is an environmental agro-mosaic with small patches of edge-affected Araucaria forest remnants < 50 ha (Ranta et al., 1998; Gascon et al., 2000; Ribeiro et al., 2009), early-to-late secondary forest patches recovering from cropland or pasture abandonment (Tabarelli et al., 2010), high-yield agriculture (Fonesca et al., 2009), and ecologically-managed monocultures of Pinus and Eucalyptus timber plantations (Carlos et al., 2009) which have been steadily expanding in the last three decades (Fundação, 2001; Baptista and Rudel, 2006).
As the Araucaria forest is comprised of highly fragmented populations, actors must employ active restoration projects with a diverse species composition that promotes successful recruitment and establishment. Evidence of successful legislative high-diversity minimum requirements in Brazil exist: São Paolo state has the exemplary minimum requirement of 80 native species per hectare (Wuethrich, 2007). Unfortunately, the Brazilian states where the Araucaria forest ecosystem is located do not have such requirements. Although restoration projects in this region must legally be comprised of native species, there is no law specifying which or how many species should be used, and consequently a limited selection of approximately 10 to 20 common species are typically found in plantings (Pablo Hoffmann, 2016; pers. comm., 17 December). Silva et al. (2016) reports that most nurseries do not meet their production capacities, which represents a practical and currently missed opportunity to increase the native seed quantity and diversity within inventories.
1.5. Research objectivesIn support of the in situ conservation of the Araucaria forest generally and threatened species specifically, targeted high-diversity restoration is essential. Given that commitments to restoration are presently underway, optimal strategies may be identified in order to use available resources for the deliberate protection of a wider number of species. To identify logistical opportunities for less species-biased choices, the present study examines drivers of the species-selection process for nurseries and restoration practitioners.
This study interviewed nurseries and restoration practitioner organizations working in the Araucaria forest to (1) identify a baseline sample of what species are produced and planted in restoration projects; and (2) identify which factors are most important in governing species selection. Nurseries and restoration practitioners will hereafter be referred to as Ns and RPs, respectively.
2. Methods 2.1. Study areaThe original extent of the Araucaria forest ranges from 53.95613°W to 48.22327°W (west to east), and from 23.56218°S to 29.74095°S (north to south) throughout Paraná, Santa Catarina, and Rio Grande du Sol states in southern Brazil. Interviews were conducted within the original Araucaria forest extent in Paraná and Santa Catarina (Fig. 1), however due to resource limitations interviews were not conducted in Rio Grande do Sul. According to the AFRP-identified land suitable for restoration (Calmon et al., 2011), Paraná is suitable for the largest area of restoration (2,455,537 ha), followed by Santa Catarina (1,402,183 ha) and Rio Grande du Sol (891,716 ha). Paraná state nurseries are also the main producers for restoration efforts in the Araucaria forest region (Martins et al., 2004), so interviews were prioritized for Paraná and subse-quently Santa Catarina.
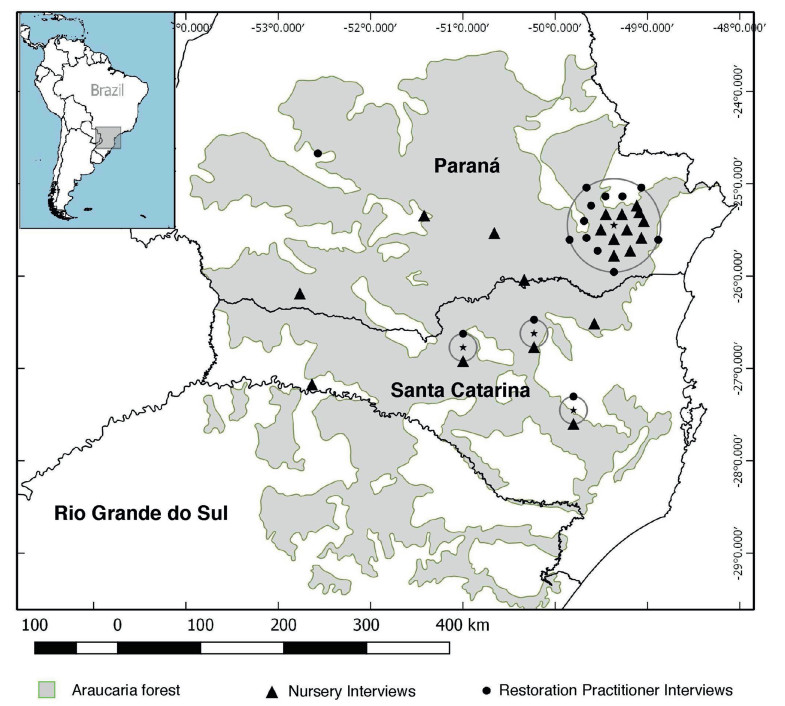
|
| Fig. 1 Original distribution of the Araucaria forest in southern Brazil. Interviews are demarcated by triangles (nurseries) and circles (restoration practitioners) and were conducted from April 25th to June 9th, 2017 |
Ns and RPs were identified from a combination of sources: Diagnosis of the Production of Native Forest Seedlings in Brazil (Silva et al., 2015); Embrapa, an agricultural research institution's nursery list (Embrapa, 2017); the Brazilian Institute of Forestry nursery list (IBF, 2017); Environmental Institute of Paraná registered nursery list (IAP, 2017); contacts from previous nursery research from The Nature Conservancy; and Internet searches. Participants were selected by stratified random sampling method: nurseries were grouped according to municipality and participants within each group were randomly selected and asked via telephone to participate in the study. Those who agreed were scheduled for an in-person interview. The sample represented a gradient of demographic variables such as size and public/private nurseries, and were relatively evenly distributed throughout the study area. RPs were not stratified by location because most had centrally located offices in Curitiba, Paraná's capital city, although they coordinate plantings throughout the entire study area.
2.3. Data collection 2.3.1. Baseline dataStructured interviews (Neuman, 2014) were conducted in Portuguese from April to June 2017. The 36 interviews included 20 Ns (9 public, 11 private) and 16 RPs (11 private consultants, 4 NGOs, 1 government agency). N participants were nursery managers and RP participants were company owners, managers, or high-level staff involved in planning and coordinating restoration projects.
During each N interview, annual inventory lists were collected to ascertain species occurrence, as defined by which species were present/absent in any given nursery. Abundance, defined by the quantity of present species produced annually, was also recorded. The RP interviews collected similar data, although occurrence is defined by which species were present/absent in any plantings of the past year, and abundance is defined by the quantity of each species planted annually. Species which were only present on one nursery or planting list were noted but excluded from further analysis, as the majority of species were singly occurring and would have skewed the results to disproportionately represent rarely occurring species as commonly present.
2.3.2. QuestionnairesSeparate structured questionnaires were given to Ns (Appendix B) and RPs (Appendix C) to assess the economic, technical, and institutional constraints on species selection. Questionnaires were composed of open-ended, multiple-choice, and Likert-scale response (Likert, 1932) questions. The N questionnaire was composed of 62 questions in the following categories: infrastructure, business objectives, seedling sale, technical knowledge, market and client needs, seed acquisition methods, fluctuations in nursery operational activity, regulations, inventory decisionmaking processes, and incentives for using threatened species. The RP questionnaire was composed of 64 questions in the following categories: project planning, business objectives, nursery selection, species selection, staffing, and the planting process.
3. Results 3.1. Baseline sampleParticipant responses provided baseline data on how many species are present in N and RP inventories (Table 1). In total 139 native species were found to occur in two nurseries or more (Appendix D). Only 25 tree species occurred in nine (median occurrence) or more nurseries. In RP lists, 63 tree species occurred in two or more plantings (Appendix E), although only 18 species occurred in six plantings (median occurrence) or more. The mean number of occurring species (richness) is 34 in nurseries and 21.8 in RP planting lists.
| N/RP | Total No. Species Present (Occurence)a | Total Native Species Present, Single Occurrence Removed | No. Species Occurring > Mean Freq. (over half the lists) | Total Abundance | Threatened Taxa Present (% of Total Occurrence) | No. Threatened Species Occurring > Median Freq. (% of Total Occurrence) | Combined Abundance of Threatened Species (% of Total Abundance) |
| N(n=20) | 354 | 139 | 25 | 12,554,600 | 25(17.9%) | 7(5.0%) | 1,732,535 (13.8%) |
| RP(n=16) | 154 | 62 | 18 | 870,122b | 17 (27.4%) | 3 (4.8%) | 147,050 (16.9%) |
| a Including non-tree and exotic species. b n = 9 (not 16); RP participants were able to tell us which species they used but were unable to provide quantities. |
|||||||
Although 20% of Araucaria forest taxa are threatened, less than 20% of recorded occurrence and abundance are comprised of threatened species. Of the Ns and RPs which had high occurrences of threatened species (defined as > median occurrence), their inventories showed a significantly lower proportion of threatened species than one would expect to occur by chance (N: t (138) = 4.19, p < 0.001; RP: t (61) = 2.92, p < 0.005). Of the taxa commonly occurring in N and RP lists (>median frequency), only 4.6% (N) and 4.7% (RP) are threatened.
3.2. Questionnaire responses 3.2.1. Factors governing species selection in nurseriesN responses indicated that although 40% of nurseries purchase seeds and 15% farm seeds, 100% of nurseries participate in wild seed collection. This practice enables nurseries to acquire seeds for free and they are only limited by the resources (fuel, time, and staff labor) required for travel and seed collection. One hundred percent of Ns reported that on seed collection trips they do not target certain species but rather collect any seeds they happen across. Ninety percent of nurseries reported adding additional species to their inventory in the last year, the primary reason (55% of responses) being opportunistic: they simply found a new species' seeds in sufficient quantity.
Seedlings most commonly occurring in N inventories were common species easily available for seed collection. When asked which species were most easy and inexpensive to acquire, common species were most frequently reported, with the exception of Araucaria angustifolia, which is a flagship species and despite its threatened status is found in every nursery (and therefore is also easy and cheap to acquire). Conversely, when asked what are the most difficult and expensive species to acquire, mostly threatened species were cited (Table 2). "Sporadic seed availability, " "technically advanced seed collection requirements, " and "difficult to access" were cited as primary reasons for difficulties collecting these species.
| Category | Species | Ranking | Threat Status |
| Easy to Acquire | Araucaria angustifolia | 1 | Near Threateneda, Endangeredb, Critically Endangeredd |
| Psidium cattleianum | 2 | - | |
| Eugenia uniflora | 3 | - | |
| Inexpensive to Acquire | Araucaria angustifolia | 1 | Near Threateneda, Endangeredb, Critically Endangeredd |
| Eugenia uniflora | 1 | - | |
| Eugenia involucrata | 2 | Rarec | |
| Psidium cattleianum | 2 | - | |
| Difficult to Acquire | Ocotea odorifa | 1 | Near Threateneda, Endangeredb, Vulnerabled |
| Ocotea porosa | 2 | Near Threateneda, Endangeredb, Vulnerabled | |
| Caesalpinia echinata | 3 | Endangeredd | |
| Cedrella fissillis | 3 | Endangeredd | |
| Expensive to Acquire | Ocotea odorifa | 1 | Near Threateneda, Endangeredb, Vulnerabled |
| Ocotea porosa | 2 | Near Threateneda, Endangeredb, Vulnerabled | |
| Cedrella fissillis | 3 | Endangeredd | |
| Jacaranda puberal | 3 | - | |
| Caesalpinia echinata | 3 | Endangeredd | |
| a SEMA(1995). b MMA (2008). c Hoffmann et al. (2015). d IUCN (2013). |
|||
Nursery participants were asked to score a list of barriers that prevented them from increasing the number of threatened species in their inventory. The highest mean scores were "seeds too far away" (8/10), "difficult to find seeds" (8/10), and "not enough resources to acquire seeds" (7/10) (Fig. 2a). Customers wanting or not wanting the seeds was not highly scored (5.5/10). When N respondents were asked their reasons for the addition of a new species to their inventory, only 35% cite "customer request" as a reason. Seventy percent of nurseries would be willing to add threatened species to their inventory if clients would pay more, but only 25% believe they would. These scores indicate that nurseries do not consider customer demand as a high priority, and that it is not driving their species selection decision-making process.
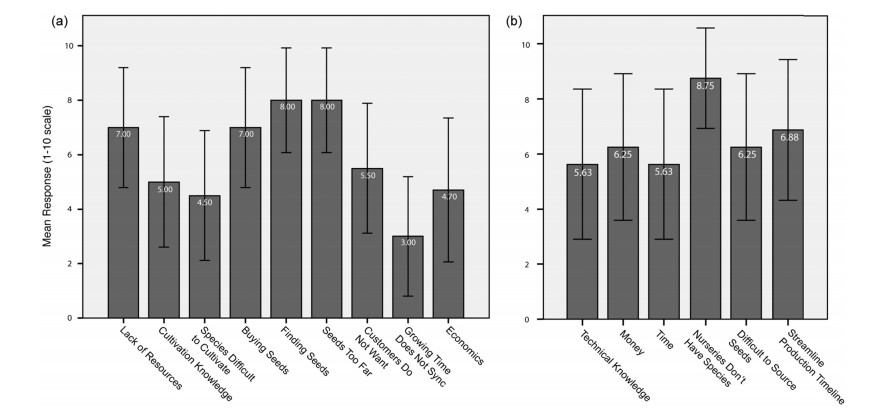
|
| Fig. 2 Mean responses of (a) Nurseries and (b) Restoration Practitioners in Paraná and Santa Catarina states, Brazil (2017) when asked to rate a 1-10 scale (1 = not important, 10 = very important) on various potential barriers to adding threatened species to their inventories. Means are listed in white. Error bars represent 95% CI |
When RPs were asked to cite the greatest barriers to increasing the use of threatened species in their plantings, nurseries simply not carrying those species was the most cited reason (Fig. 2b). When asked to select only one driving reason that would limit their use of threatened species, the majority (62.5% of responses) cited an absence of those species in nursery inventories; the next most cited reason was price (18.75%). Nurseries, regardless of customer demand, are simply not carrying these species, thus eliminating the option for RPs to include them in their plantings.
Another factor which proved important to the decision-making process was willingness of RPs to be flexible with their planting list. Respondents were more likely to adapt their planting list to match what a nursery had on hand than search for another nursery with a more species-diverse stock of seedlings. Of RPs, 81.25% reportedly come to nurseries with pre-defined lists of species, although 75% of RPs were willing to shorten their list if the nurseries did not carry all the species on it. N responses substantiate the RP claims: when a client discusses a species list with a nursery, 30% of Ns report that the clients request specific species, while 70% say their clients are willing to purchase whatever the nursery has in its inventory. Taken together, most RPs are more likely to change their lists than spend time contacting and liaising with multiple nurseries.
Rather than receiving an order with adequate time to grow the requested number of seedlings, nurseries are expected to have large quantities of seedlings in stock at all times, with little or no notice before making a potential sale. The mean advance notice a typical RP gives a nursery prior to transaction is 1.5 days. The mean time RPs plan a planting is 4.1 weeks. As plantings happen relatively quickly, the time it takes participants to find a suitable nursery carrying all the species on their original planting list would consume a considerable amount of their overall planning time, impacting their profit margin.
When asked to rate on a 1-10 scale the different considerations involved in selecting which nursery to purchase seedlings from, "timely delivery" and "price" were rated the most important reasons. When asked to choose one primary reason driving nursery selection, the majority of RPs selected price as the primary factor (43.75%). On average RPs rated 9.77/10 level of interest in increasing their use of threatened species, however because price and time are limiting factors, one can infer that despite a strong reported interest their decisions are ultimately governed by minimizing costs.
The mean number of species RPs used in plantings was 20.73, which is notable given in another question RPs reported a mean number of 30.1 species as "sufficient for a quality planting, " and 73.5 species as an "ideal number for any quality planting." RPs, given their priorities of price and time, are on average knowingly planting fewer species than they consider sufficient or ideal for a quality planting (which supports the difficulties outlined by other authors (Rodrigues et al., 2009)).
4. Discussion 4.1. Seed availabilityThe Southeast region of Brazil has the greatest number of nurseries and produces the largest quantities of seedlings compared to other regions in Brazil, yet has the smallest variation in the number of species produced between nurseries (Silva et al., 2015). While nurseries across the region vary greatly in their capacity to produce a diverse range of native seedlings (Silva et al., 2016), the present study demonstrates that N inventories in the Araucaria forest region focus on a disproportionally low number of threatened species in occurrence (17.9% occurring in two nurseries, 5% occurring in 7 or more) and abundance (comprising 13.8% of total abundance). RPs have lower species occurrences in their plantings than the ideal recommended amount (80-90), instead planting on average 21 species, supporting previous findings which draw the same conclusions (Volis, 2016b; Aronson et al., 2011).
Although consumer demand did not emerge as a crucial driver of species selection for Ns, opportunities for seed acquisition were found to be extremely important. Ns cannot acquire more species without expending considerably more of their resources on accessing new seeds and cultivating them in large enough quantities for an RP to immediately purchase. RPs are unwilling to spend additional back-and-forth time with nurseries in order to request and secure a more species-rich planting, which in turn makes nurseries less likely to carry a wider variety of species in the future.
In both public and private nurseries, the two most highly reported factors impeding the increased use of threatened species are lack of resources and opportunity for seed acquisition. This is a common difficulty in other regions of Brazil (Brancalion et al., 2011) and countries in Europe (Bischoff et al., 2008). If nurseries, therefore, could acquire additional species' seeds at no or minimal extra cost, which would not then be passed on to practitioners, one of the substantial hurdles would be eliminated which could spur increased adoption of threatened species for both N and RP actors.
4.2. Short-term actionable recommendationsAs the existing restoration framework currently does not provide incentives to increase the number of species for Ns and RPs, actionable steps must be outlined to preferentially improve access to currently under-represented and threatened taxa. Results suggest that increasing seed availability is a most crucial factor governing the species selection decision-making process, and is therefore the first step toward increasing diversity in nurseries and subsequent restoration plantings. The following recommendations mirror similar recommendations put forth by other researchers in this field (Silva et al., 2016; Jalonen et al., 2017), and are geared toward increasing seed availability and improving the conditions which would enable actors to broaden the focus of seed collection and restoration efforts to include more (including threatened) species.
4.2.1. Comprehensive species listThe creation of a comprehensive list of species is crucial if restoration actors are to know what variety of species are available to them and appropriate for their site. Paraná-based NGO Sociedade Chauá provides such lists, grouped by region (http://www.sociedadechaua.org/floraparana). They include identifying photos and cultivation information for public use. Comprehensive lists such as these could also provide assessments of the state of wild seed supply for collection, information which would be useful to all nurseries who participate in wild seed collection.
4.2.2. Foster knowledge-sharingAdequate information about each species on a comprehensive list is necessary for actors to successfully use these species. Insufficient knowledge of threatened species' reproductive biology, and lack of efficient propagation and planting methods are primary barriers for their use in restoration projects (Volis, 2016a). Some Araucaria forest species exhibit seasonal fluctuations in phenology, have low levels of fruit production, or produce a high proportion of non-viable seeds (due to maturation and predation complications), hence the timing and ease of seed collection remains a constant challenge (Hoffmann et al., 2015).
In addition to further empirical research on less-studied species, expanding and strengthening a network of stakeholders in public and private forums can provide opportunities for exchange of cultivation and planting knowledge. Policy regulations alone are not sufficient to meet restoration goals (Silva et al., 2016); they must be simultaneously approached from the stakeholder perspective as a sustainable and feasible economic activity (Brancalion et al., 2012). When stakeholders can develop their knowledge base and exchange success stories, confidence and perceived feasibility of adopting a wider variety of species increases. The stronger the network and exchange of knowledge, the more these networks can produce flexible approaches, increased competency of practitioners, and less risk in implementing new strategies (Nyoka et al., 2015).
4.2.3. Seed sharing programA seed exchange program is an organized group of seed harvesters with training and coordination for native seed production which could distribute seeds throughout a network of nurseries interested in growing a wider range of threatened species. This solution has been piloted elsewhere in the Atlantic forest (São Paolo state), has proven to be an effective support of high-diversity reforestation initiatives (Brancalion et al., 2011), and such decentralization of seedling production has been recommended by leaders in community- and industry-based restoration communities (Merritt and Dixon, 2011; Nevill et al., 2016; Silva et al., 2016). Programs with collaborative participation between independent seed collectors, community-based organizations, and local seed exchange programs have been shown to yield increased restoration diversity-measured by number of species and seed lots-compared to relying on any one strategy alone (Brancalion et al., 2011).
Seed sharing programs which span over 100 km (long-distance germplasm exchange) are particularly advantageous, given that distance still falls within a species' native range. Although local germplasm sourcing is important to maximize local adaptations in plant traits and avoid outbreeding depression (Mijnsbrugge et al., 2010; Edmands, 2006), doing so in highly fragmented landscapes produces poor restoration outcomes, so on balance actors should prioritize high quality and highly diverse seeds (Broadhurst et al., 2008; Bischoff et al., 2008). Mixing provenances of germplasm increases the genetic diversity of seed in addition to enhancing taxonomic diversity. Seeds of mixed provenances within a participating network result in enhanced seed quality (Brancalion et al., 2011) which is critical to successful restoration efforts. Enhanced seed quality reduces germination and cultivation risk for Ns, and reduces risk for RPs who have a vested interest in a high survival rate in their plantings. High seed quality also provides resilience of restored areas to climate change, now an important consideration in any restoration project (Jalonen et al., 2017). Moreover, seed sharing networks will positively feedback on comprehensive species lists, associated collective knowledge, and seed sources of local tree species.
4.2.4. Increased coordination of plantingsIncreasingly linked stakeholder networks can also produce more coordinated plantings within the RP community. As Ns are encouraged to increase the variety of species available in their nurseries, RPs can likewise improve the degree to which they link their plantings to other plantings in the region. Three quarters of RPs reported mean planting areas of 5 ha or less, while three RPs reported plantings of 115,200, and 300 ha, raising the mean planting area to 41.87 ha. Even the largest reported plantings (mean 268.9 ha) are still considered small in terms of forest fragments in the Atlantic forest, which are defined by Oliveira et al. (2008) as < 300 ha and Ribeiro et al. (2009) as < 100 ha. Given that restoration plantings in this region are at best restoring fragments, it is crucial to maximize restoration impact by coordinating plantings occurring in neighboring areas, ideally serving to link and bolster populations of newly added species.
Coordinated restoration efforts across the entire North American Great Lakes region have been shown to be nine times more cost-effective than individual local-scale planning (Neeson et al., 2015). Coordinated efforts also work in direct opposition to habitat fragmentation, one of the leading causes of declining biodiversity and ecosystem services (Fahrig, 2004). Although coordinated plantings at a landscape level have historically been a major challenge for this region (Rodrigues et al., 2009), they are critical to restoring land on a large enough scale to promote a diverse, healthy, ecologically functional ecosystem (Lopes et al., 2009).
Currently RP projects act in isolation of one another and are planting mostly common species which have reliable establishment success rates. Instead of asking individual RPs to add a large quantity of new species (high risk), RPs can coordinate their planting lists to each add a small quantity of new species (low risk), which cumulatively expands the richness of planted species in a given region. Coordination between RPs will serve as a decisionsupport tool for species selection (Beier et al., 2011), and will help actors identify linkage opportunities, which is currently not possible.
4.3. Long-term strategiesWild seed collection requires ethical and genetic considerations, particularly when collecting threatened and rare species (Broadhurst et al., 2016). Seed collection of threatened species should be targeted and limited, where the fewest sufficient number of seeds should be collected under strict ecological criteria, in order to prevent decimating any given population's ability to sustain itself naturally. Extremely rare species or those having small isolated populations require expert and targeted intervention organized by appropriate conservation organizations, meeting minimum collection requirements before removing any seeds from the wild (Jalonen et al., 2017). The global assessment of forest genetic resources adopted by the FAO (2013) calls for policymakers to reinforce national seed programs to provide sufficient quantities of genetically appropriate seeds for restoration so as not to exhaust wild populations. Hence exsitu seed farming programs are an essential long-term component to the conservation of threatened species, as current and future demand for seeds exceed the volume that can be practically and economically sourced from the wild (Nevill et al., 2016).
Intermediate approaches which combine and bridge in- and exsitu strategies exist as long-term methods that can be used for increasing species richness in restoration efforts (Volis, 2017). While botanical gardens and arboreta host small ex situ living collections, opportunities for inter-situ collections exist in areas such as abandoned agricultural lands. These designated areas can exist outside of the current species range but within the past range of a species (Burney and Burney, 2007), and can host a wider range of species in larger numbers than are typically possible in strictly ex situ operations (Volis, 2017). Inter-situ collections can be planted to simultaneously reintroduce a large number of threatened species and restore degraded lands, an ideal conservation solution for highly fragmented regions.
Quasi in situ (Volis and Blecher, 2010) collections are another solution appropriate for a highly fragmented region such as the Araucaria forest, defined as "living collections in protected areas under natural or semi-natural conditions, where site selection accounts for local adaptation, and focuses on preservation and production of plant material." Planting threatened species outside their current natural range can be advantageous in light of anticipated range shifts due to climate change (Vitt et al., 2016; Butterfield et al., 2016), particularly when there are few alternatives as extant populations are so rare and isolated. This method would produce a large quantity of plant material, relieving pressure on nurseries to access and collect rare and threatened samples from the wilds.
Complementing the inter-situ method, the quasi in situ method focuses on preserving locally-adapted genetic variation and producing large quantities of seeds of species that present the greatest challenge in regional restoration projects. In Belgium, seed orchards propagating locally sourced planting stock have successfully demonstrated the ability to preserve local adaptations and a diverse range of native plants in highly fragmented areas (Vander Mijnsbrugge, 2014). Even in extreme cases of critically endangered species of as few as 30 remaining individuals, seed orchards outside a species' current natural range have been demonstrated to increase genetic diversity of future generations, while also creating more planting material ex situ without detracting from the existing population (Ducci, 2014). These newly emerging, adaptive methods are recommended as long-term strategies to increase the use of threatened species in restoration plantings in the Araucaria forest region.
4.4. ConclusionAlthough this study is not exhaustive in sample size or potential decision-making factors to investigate, it provides a baseline sample of what species are commonly used in the restoration industry, and baseline information on N and RP attitudes over a large study area in the Araucaria forest. It provides evidence that some factors such as seed acquisition and financial risk are more important drivers of species selection than others, such as customer demand.
Overcoming limitations at various stages of the restoration process will improve the likelihood of increased species use, including threatened species. A multifaceted approach would maximize the ability for restoration actors to increase species richness: (1) seed acquisition support enabling nurseries to carry more species in adequate quantities on hand; (2) increased knowledge of threatened species so restoration practitioners can make informed decisions on which species they can confidently add without depressing status quo survival rates; (3) increasing opportunities for Ns and RPs to create stakeholder networks where knowledge, seeds, and landscape-level plans can be shared between actors; and (4) use of long-term inter-situ and quasi in situ conservation strategies, which simultaneously provide long-term preservation of genetic diversity and increase seed production of target species. With a balance of practical considerations, it is possible for restoration plantings in the Araucaria forest region to be species-rich, representing an increased number of functional groups and targeted for the conservation of threatened species at risk of extinction.
I am grateful to all the nursery employees and restoration practitioners who agreed to participate in this research. Thank you to Valmir Campolino Lorenzi, without whose contributions in the interview process this research would not have been possible. A sincere thanks to Pablo Hoffmann, Marilia Borgo, and all the staff at Sociedade Chauá for their hospitality and support. Thank you to David Gill for your valuable comments in the writing process. I would also like to express my gratitude to Global Trees Campaign, Flora and Fauna International, University of East Anglia, and the Sir Philip Reckitt Educational Trust for funding this research.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.pld.2018.08.002.
AFRP-Atlantic Forest Restoration Pact, 2016. Actions and Projects. Retrieved from. http://www.pactomataatlantica.org.br/actions-and-projects on 28/02/2018.
|
Aronson J., Brancalion P.H.S., Durigan G., Rodrigues R.R., Enge V.V., Tabarelli M., et al, 2011. What role should government regulation play in ecological restoration?. Ongoing debate in Sao Paulo state, Brazil. Restor. Ecol, 19: 690-695. DOI:10.1111/rec.2011.19.issue-6 |
Arroyo-Rodríguez V., Pineda E., Escobar F., Benítez-Maldivo J., 2009. Value of small patches in the conservation of plant-species diversity in highly fragmented rainforest. Conserv. Biol, 23: 729-739. DOI:10.1111/cbi.2009.23.issue-3 |
Baptista S.R., Rudel T.K., 2006. A re-emerging Atlantic Forest? Urbanization, industrialization and the forest transition in Santa Catarina, southern Brazil. Environ. Conserv, 33: 195-202. DOI:10.1017/S0376892906003134 |
Beier P., Spencer W., Baldwin R.F., McRae B.H., 2011. Toward best practices for developing regional connectivity maps. Conserv. Biol, 25(5): 879-892. DOI:10.1111/j.1523-1739.2011.01716.x |
Bischoff A., Steinger T., Müller-Schärer H., 2008. The important of plant provenance and genotypic diversity of seed material used for ecological restoration. Restor. Ecol, 18(3): 338-348. DOI:10.1111/rec.2010.18.issue-3 |
Blakesley D., Elliott S., Kuarak Cl, Navakitbumrung P., Zangkum C., Anusarnsunthorn V., 2002. Propagating framework tree species to restore season dry tropical forest:implications of season seed dispersal and dormancy. For. Ecol. Manag, 164: 31-38. DOI:10.1016/S0378-1127(01)00609-0 |
Bozzano, M., Jalonen, R., Thomas, E., Boshier, D., Gallo, L., Cavers, S., Bordács, S., Smith, P., Loo, J. (Eds.), 2014. Genetic Considerations in Ecosystem Restoration Using Native Tree Species. State of the World's Forest Genetic Resources-Thematic Study. FAO and Biodiversity International, Rome.
|
Brancalion P.H.S., Viani R.A.G., Aronson J., Rodrigues R.R., Nave A.G., 2011. Improving planting stocks for the Brazilian Atlantic Forest restoration through community-based seed harvesting strategies. Restor. Ecol, 20: 704-711. |
Brancalion, P.H., Castro, P., Rodrigues, R.R., Aronson, J., Calmon, M., 2012. In: The Atlantic Forest Restoration Pact-a Major Effort by Brazilian Society to Restore and Transform its Most Threatened Biome. 18th Annual Conference of the International Society of Tropical Foresters, Yale Chapter. Yale University, New Haven.
|
Broadhurst L.M., Lowe A., Coates D.J., Cunningham S.A., McDonald M., Vesk P.A., et al, 2008. Seed supply for broad scale restoration:maximizing evolutionary potential. Evolut. Appl, 1(4): 587-597. |
Broadhurst L.M., Jones T.A., Forrest S.S., North T., Guja L., 2016. Maximizing seed resources for restoration in an uncertain future. Bioscience, 66(1): 77-79. |
Burney D.A., Burney L.P., 2007. Paleoecology and "inter-situ" restoration on Kauai, Hawaii. Front. Ecol. Environ, 5: 483-490. DOI:10.1890/070051 |
Butterfield B.J., Copeland S.M., Munson S.M., Roybal C.M., Wood T.E., 2016. Prestoration:using species in restoration that will persist now and into the future. Restor. Ecol, 25(S2): S155-S163. |
Calmon M., Brancalion P.H.S., Paese A., Aronson J., Castro P., da Silva S.C., Rodrigues R.R., 2011. Emerging threats and opportunities for large-scale ecological restoration in the Atlantic forest of Brazil. Restor. Ecol, 19(2): 154-158. DOI:10.1111/rec.2011.19.issue-2 |
Carlos R.F., Ganade G., Baldissera R., Becker C.G., Boelter C.R., Brescovit A.D., et al, 2009. Towards an ecologically-sustainable forestry in the Atlantic Forest. Biol. Conserv, 142: 1209-1219. DOI:10.1016/j.biocon.2009.02.017 |
Castella, P.R., Britez, R.M., 2004. A Floresta Com Araucária No Estado Do Paraná. Ministério do Meio Ambiente, Fundação de Pesquisas Florestais do Paraná, Brasília, Brazil.
|
Crouzeilles R., Ferreira M.S., Chazdon R.L., Lindenmayer D.B., Sansevero J.B.B., Monteiro L., et al, 2017. Ecological restoration success is higher for natural regeneration than for active restoration in tropical forests. Sci. Adv, 3(11): e1701345. DOI:10.1126/sciadv.1701345 |
dos Santos R., Citadini-Zanette V., Leal-Filho S., Hennies W.T., 2008. Spontaneous vegetation on overburden piles in the coal basin of Santa Catarina, Brazil. Restor. Ecol, 16: 444-452. DOI:10.1111/rec.2008.16.issue-3 |
Ducci, F., 2014. Species restoration through dynamic ex situ conservation: Abies nebrodensis as a model. In: Bozzano, M., Jalonen, R., Thomas, E., Boshier, D., Gallo, L., Cavers, S., Bordács, S., Smith, P., Loo, J. (Eds.), Genetic Considerations in Ecosystem Restoration Using Native Tree Species. State of the World's Forest Genetic Resources-Thematic Study Rome. FAO/Bioversity International, pp. 224-233.
|
Edmands S., 2006. Between a rock and a hard place:evaluating the relative risks of inbreeding and outbreeding for conservation and management. Mol. Ecol, 16(3): 463-475. DOI:10.1111/mec.2007.16.issue-3 |
Embrapa, 2017. Seedlings and Seeds: Nurseries. Retrieved from. https://www.embrapa.br/codigo-florestal/viveiros-de-especies-florestais on 06/04/2017.
|
Fahrig L., 2004. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst, 34(1): 487-515. |
FAO-Food and Agriculture Organization of the United National, 2013. Global Plan of Action for the Conservation, Sustainable Use and Development of Forest Genetic Resources. Retrieved from. http://www.fao.org/3/a-i3849e.pdf on 19/08/2017.
|
Fonesca C.R., Ganade G., Baldissera R., Becker C.G., Boelter C.R., Brescovit A.D., et al, 2009. Towards an ecologically-sustainable forestry in the Atlantic forest. Biol. Conserv, 142: 1209-1219. DOI:10.1016/j.biocon.2009.02.017 |
Fundação SOS Mata Atlântica, INPE-Instituto Nacional de Pesquisas Espaciais, 2001. Atlas dos remanescentes florestais da Mata Atlântica e ecossistemas associados no periodo de. Fundação SOS Mata Atlântica, São Paolo, and INPE, São José dos Campos, Brasil, pp. 1995-2000.
|
Gascon C., Williamson G.B., da Fonseca G.A.B., 2000. Receding forest edges and vanishing reserves. Science, 288: 1356-1358. DOI:10.1126/science.288.5470.1356 |
Gill, D.J.C., Bannister, J.R., Blum, C.T., Echeverria, C., Fernandez, G.M., González, et al., 2017. ¿Cómo se pueden incluir más especies arbóreas amenazadas en proyectos de restauración? (Simposio). In: Zuleta, G.A., Rovere, A.E., Mollard, F.P.O. (Eds.), SIACRE-2015: Aportes y Conclusiones. Tomando decisiones para revertir la degradación ambiental. Vázquez Mazzini Editores, Buenos Aires, pp. 139-146.
|
Girão L.C., Lopes A.V., Tabarelli M., Bruna E.M., 2007. Changes in tree reproductive traits reduce functional diversity in a fragmented Atlantic forest landscape. PLoS One, 2(9): e908. DOI:10.1371/journal.pone.0000908 |
Goosem, S.P., Tucker, N.I., 1995. Repairing the Rainforest-theory and Practice of Rainforest Re-establishment in North Queensland's Wet Tropics. Wet Tropics Management Authority, Cairns.
|
Hoffmann P.M., Blum C.T., Velazco S.J.E., Gill D.J.C., Borgo M., 2015. Identifying target species and seed sources for the restoration of threatened trees in southern Brazil. Oryx, 49(3): 425-430. DOI:10.1017/S0030605314001069 |
IAP-Environmental Institute of Paraná, 2017. Information of IAP's Nurseries. Retrieved from. http://www.iap.pr.gov.br/modules/conteudo/conteudo.php?conteudo=1354 on 05/04/2017.
|
IBF-Instituto Brasiliero de Florestas, 2017. List of Nurseries. Retrieved from. http://www.ibflorestas.org.br/lista-de-viveiros-de-mudas-florestais-nativas.html on 05/04/2017.
|
IUCN-International Union for Conservation of Nature, 2016. Combined Effort Amplifies Restoration in Brazil's Atlantic Forest. Retrieved from. https://www.iucn.org/news/forests/201611/combined-effort-amplifies-restoration-brazil%E2%80%99s-atlantic-forest on 28/02/2018.
|
Jalonen R., Valette M., Boshier D., Duminil J., Thomas E., 2017. Forest and landscape restoration severely constrained by a lack of attention to the quantity and quality of tree seed:insights from a global survey. Conservat. Lett: 27. DOI:10.1111/conl.12424 |
Ladouceur E., Jiménez-Alfaro B., Marin M., De Vitis M., Abbandonato H., Iannetta P.P.M., et al, 2017. Native seed supply and the restoration species pool. Conservat. Lett, 0: 1-9. |
Lamb D., Erskine P.D., Parrotta J.A., 2005. Restoration of degraded tropical forest landscapes. Science, 310(5754): 1628-1632. DOI:10.1126/science.1111773 |
Leite, P., Klein, R.M., 1990. Vegetačão. In: Geografia do Brasil: Região Sul, vol. 2. Instituto Brasileiro de Geografia e Estatistica, Rio de Janeiro, Brazil, pp. 113-150.
|
Likert R., 1932. A technique for the measurement of attitudes. Arch. Psychol, 140: 1-55. |
Lindell C.A., Reid J.L., Cole R.J., 2013. Planting design effects on avian seed dispersers in a tropical forest restoration experiment. Restor. Ecol, 21: 515-522. DOI:10.1111/rec.2013.21.issue-4 |
Lopes A.V., Luciana C.G., Santoa B.A., Peres C.A., Tabarelli M., 2009. Long-term erosion of tree reproductive train diversity in edge-dominated Atlantic forest fragments. Biol. Conserv, 142: 1154-1165. DOI:10.1016/j.biocon.2009.01.007 |
Maina G.G., Howe H.F., 2000. Inherent rarity in community restoration. Conserv. Biol, 15(5): 1335-1340. |
Martins, S.S., Silva, I.C., Bortolo, L., Nepomuceno, A.N., 2004. Fundação de mudas de espécies florestais nos viveiros do Instituto Ambiental do Paraná. Clichetec, Maringá, Brazil.
|
Merritt D.J., Dixon K.W., 2011. Restoration seed banks-a matter of scale. Science, 332: 424-425. DOI:10.1126/science.1203083 |
Metzger J.P., Martensen A.C., Dixo M., Bernacci L.C., Riberio M.C., Teixeira A.M.G., Pardini R., 2009. Time-lag in biological responses to landscape changes in a highly dynamic Atlantic forest region. Biol. Conserv, 142: 1166-1177. DOI:10.1016/j.biocon.2009.01.033 |
Mijnsbrugge K.V., Bischoff A., Smith B., 2010. A question oforigin:where and how to collect seed for ecological restoration. Basic Appl. Ecol, 11(4): 300-311. DOI:10.1016/j.baae.2009.09.002 |
MMA-Ministro do Meio Ambiente, 2008. Lista Oficial das Espécies da Flora Bra-sileira Ameaçadas de Extinção. Instrução Normativa No. 6, 2008. Retrieved from. http://www.mma.gov.br/estruturas/ascom_boletins/_arquivos/83_19092008034949.pdf. on 02/08/2017.
|
Myers N., Mittermeier R.A., Mittermeier C.G., Fonseca G.A.B., Kent J., 2000. Biodiversity hotspots for conservation priorities. Nature, 403: 853-858. DOI:10.1038/35002501 |
Neeson T.M., Ferris M.C., Diebel M.W., Doran P.J., O'Hanley J.R., McIntyre P.B., 2015. Enhancing ecosystem restoration efficiency through spatial and temporal coordination. Proc. Natl. Acad. Sci, 11(19): 6236-6241. |
Neuman, W.L., 2014. Basics of Social Research: Qualitative & Quantitative Approaches. Pearson Education, Harlow.
|
Nevill P.G., Tomlinson S., Elliot C.P., Espeland E.K., Dixon K.W., Merritt D.J., 2016. Seed production areas for the global restoration challenge. Ecol. Evol, 6: 7490-7497. DOI:10.1002/ece3.2455 |
Nyoka B.I., Roshetko J., Jamnadass R., Muriuki J., Kalinganire A., Lillesø J.B., Beedy T., Cornelius J., 2015. Tree seed and seedling supply systems:a review of the Asia, Africa and Latin America models. Smallscale Forest, 14(2): 171-191. |
Oliveira M.A., Santos A.M.M., Tabarelli M., 2008. Profound impoverishment of the large-tree stand in a hyper-fragmented landscape of the Atlantic forest. For. Ecol. Manag, 256: 1910-1917. DOI:10.1016/j.foreco.2008.07.014 |
Putz S., Groeneveld J., Alves L.F., Metzger J.P., Huth A., 2011. Fragmentation drives tropical forest fragments to early successional states:a modelling study for Brazilian Atlantic forests. Ecol. Model, 222: 1986-1997. DOI:10.1016/j.ecolmodel.2011.03.038 |
Ranta P., Blom T., Niemela J., Joensuu E., Siitonen M., 1998. The Fragmented Atlantic rain forest of Brazil:size, shape, and distribution of forest fragments. Biodivers. Conserv, 7: 385-403. DOI:10.1023/A:1008885813543 |
Ribeiro M.C., Metzger J.P., Martensen A.C., Ponzoni F., Hirota M., 2009. Brazilian Atlantic forest:how much is left and how is the remaining forest distributed?. Implications for conservation. Biol. Conserv, 142: 1141-1153. DOI:10.1016/j.biocon.2009.02.021 |
Rodrigues R.R., Lima R.A.F., Gandolfi S., Nave A.G., 2009. On the restoration of high diversity forests:30 years of experience in the Brazilian Atlantic forest. Biol. Conserv, 142: 1242-1251. DOI:10.1016/j.biocon.2008.12.008 |
SEMA, 1995. Lista vermelha de plantas ameaçadas de extinção no estado do Paraná. SEMA/GTZ, Curitiba, Brazil.
|
Silva, A.P.M., Marques, H.R., Luciano, M.S.F., Sambuichi, R.H.R., 2014. Challenges of the forestry restoration chain for the implementation of Law 12,651/2012 in Brazil. In: Monasterio, L.M., Neri, M.C., Soares, S.S.D. (Eds.), Brasil em desen-volvimento 2014: estado, planejamento e politicas publicas. Ipea, Brasília, pp. 85-102.
|
Silva, A.P.M., Marques, H.R., Nascente dos Santos, T.V.M., Teixeira, A.M.C., Luciano, M.S.F., Sambuichi, R.H.R., 2015. Diagnóstico da Produção de Mudas Florestais Nativas no Brasil: Relatóario de Pesquisa. Federal District: Instituto de Pesquisa Econômica Aplicada (IPEA), Brasília.
|
Silva A.P.M., Schweizer D., Marques H.R., Cordeiro Teixeira A.M., Nascente dos Santos T.V.M., Sambuichi R.H.R., et al, 2016. Can current native tree seedling production and infrastructure meet an increasing forest restoration demand in Brazil?. Restor. Ecol, 25(4): 509-515. |
Sork V.L., Smouse P.E., 2006. Genetic analysis of landscape connectivity in tree populations. Landsc. Ecol, 21: 821-836. DOI:10.1007/s10980-005-5415-9 |
Tabanez A.A.J., Viana V.M., 2000. Patch structure within Brazilian Atlantic Forest fragments and implications for conservation. Biotropica, 32: 925-933. DOI:10.1111/btp.2000.32.issue-4b |
Tabarelli M., Pinto L.P., Silva J.M.C., Hirota M., Bedê L., 2005. Challenges and opportunities for biodiversity conservation in the brazilian atlantic forest. Con-serv. Biol, 19: 695-700. DOI:10.1111/cbi.2005.19.issue-3 |
Tabarelli M., Aguiar A.V., Ribeiro M.C., Metzger J.P., Peres C.A., 2010. Prospects for biodiversity conservation in the Atlantic forest:lessons from aging human-modified landscapes. Biol. Conserv, 143: 2328-2340. DOI:10.1016/j.biocon.2010.02.005 |
Vander Mijnsbrugge, K., 2014. Continuity of local genetic diversity as an alternative to importing foreign provenances. In: Bozzano, M., Jalonen, R., Thomas, E., Boshier, D., Gallo, L., Cavers, S., Bordács, S., Smith, P., Loo, J. (Eds.), Genetic Considerations in Ecosystem Restoration Using Native Tree Species. State of the World's Forest Genetic Resources-Thematic Study Rome. FAO/Bioversity International, pp. 38-46.
|
Vergara, W., Lomeli, L.G., Rios, A.R., Isbell, P., Prager, S., Camino, R.D., 2016. The Economic Case for Landscape Restoration in Latin America. World Resources Institute, Washington, D.C.
|
Vitt P., Belmaric P.N., Book R., Curran M., 2016. Assisted migration as a climate change adaptation strategy:lessons from restoration and plant reintroductions. Isr.J. Plant Sci, 63(4): 250-261. DOI:10.1080/07929978.2016.1258258 |
Volis S., 2016a. Conservation meets restoration-rescuing threatened plant species by restoring their environments and restoring environments using threatened plant species. Isr. J. Plant Sci, 63: 262-275. DOI:10.1080/07929978.2016.1255021 |
Volis S., 2016b. Conservation-oriented restoration-how to make it a success?. Isr. J. Plant Sci, 63: 276-296. |
Volis S., 2017. Complementarities of two existing intermediate conservation approaches. Plant Divers, 39: 379-382. DOI:10.1016/j.pld.2017.10.005 |
Volis S., Blecher M., 2010. Quasi in situ:a bridge between ex situ and in situ conservation of plants. Biodivers. Conserv, 19: 2441-2454. DOI:10.1007/s10531-010-9849-2 |
WRI-World Resources Institute, 2016. STATEMENT: Brazil Announces Goal of Restoring 22 Million Hectares ofDegraded Land by 2030. Retrieved from. http://www.wri.org/news/2016/12/statement-brazil-announces-goal-restoring-22-million-hectares-degraded-land-2030 on 28/02/2018.
|
WRI-World Resources Institute, 2017. Initiative 20x20. Retrieved from. https://www.wri.org/our-work/project/initiative-20x20 on 18/08/2017.
|
Wuethrich B., 2007. Reconstructing Brazil's Atlantic rainforest. Science, 315: 1070-1072. DOI:10.1126/science.315.5815.1070 |


