b. Parks and Wildlife Service, Department of Biodiversity, Conservation and Attractions. 120 Albany Highway, Albany, Western Australia, 6330, Australia;
c. Parks and Wildlife Service, Department of Biodiversity, Conservation and Attractions. P.O. Box 100, Narrogin, Western Australia, 6312, Australia;
d. Parks and Wildlife Service, Department of Biodiversity, Conservation and Attractions. P.O. Box 72, Geraldton, Western Australia, 6531, Australia
Globally plant species diversity is under threat, with recent assessments indicating that around a fifth of the estimated 391, 000 plant species worldwide are at risk of extinction in the wild (RBG Kew, 2016; Pimm and Raven, 2017). This problem poses particular challenges for conservation in Mediterranean-type climate regions due to their high plant diversity and endemism. The south-west of Western Australia (herein the Southwest Australian Floristic Region SWAFR; sensu Gioia and Hopper, 2017) (see Fig. 1) is internationally renowned for its remarkable vascular plant diversity and endemism (Coates et al., 2014; Gioia and Hopper, 2017). Some 8379 vascular plant taxa have been described, 49% of which are considered endemic to the region (Gioia and Hopper, 2017), this number increasing with ongoing discovery of new species and taxonomic description of the flora (Wege et al., 2015; Gioia and Hopper, 2017). Like the world's other Mediterranean-type climate regions, the SWAFR has undergone substantial land transformation since European settlement and is recognized as a global biodiversity hotspot - those places with the highest number of endemic species under the most threat (Myers et al., 2000; Mittermeier et al., 2004, 2011). Under IUCN Red List categories (IUCN, 2012), there are 399 taxa listed as Threatened from the SWAFR, with 154 currently ranked as Critically Endangered (Western Australian Herbarium, 2018). In addition, 2076 plant taxa are listed by the Western Australian Government as rare or poorly known in the SWAFR (Smith and Jones, 2018). These are considered to be Near Threatened or Data Deficient under IUCN categories but are termed 'Priority' species in Western Australia. Land clearing for agriculture, urbanisation and the inadvertent human-mediated introduction of the invasive soil-borne plant pathogen Phytophthora cinnamomi have been the primary causes of declines in plant diversity. Other emerging threats are now also evident placing many species at risk from demographic factors associated with small populations and fragmented habitats, and landscape factors such as environmental weeds, grazing by introduced feral herbivores, altered hydrology and modification of historical disturbance regimes (Coates and Atkins, 2001; Burgman et al., 2007). More recently climate change is recognised as a threat, with the region projected to get hotter and drier with changes to rainfall seasonality and an increase in frequency of extreme events (Indian Ocean Climate Change Indian Ocean Climate Initiative, 2002; Intergovernmental Panel on Climate Change, 2013).
|
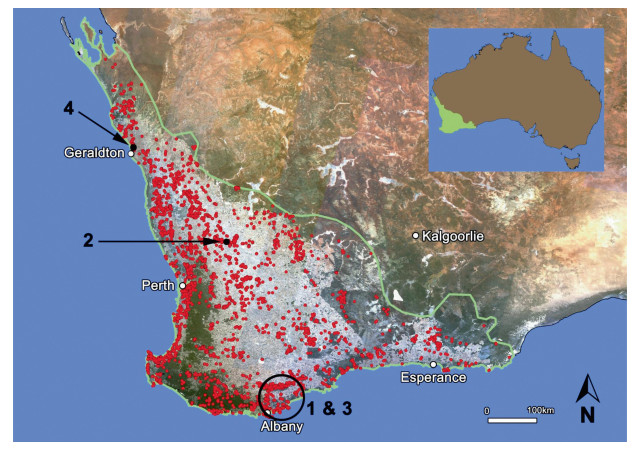
Fig. 1 Threatened flora populations (●) in the Southwest Australian Floristic Region (outlined in green on main map) over satellite imagery of remnant vegetation. Case study locations 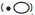 |
While significant advances have been made both in threat mitigation and furthering the knowledge of threatened plant species in the SWAFR, the ongoing discovery of new plant species, many of which are rare, increases the challenges for recovery of threatened species and prioritisation of conservation action. For example, there has been a 10% increase in the number of taxa (some 744 new taxa) in the eleven year period from 2004 to 2015 (Gioia and Hopper, 2017) due to a renewed focus on finding and naming plant species through a strategic taxonomy initiative aimed at expediting the taxonomic description of species of conservation concern (Wege et al., 2015).
Herein we provide an overview of current approaches adopted by our organisation (the Department of Biodiversity, Conservation and Attractions, DBCA) to the conservation of threatened flora in the SWAFR with a focus on active management through recovery and restoration and integration with targeted research. The approach taken by DBCA involves a very strong link between science and on-ground conservation. We draw on the work done by this Department to show how science can support on-ground management. We begin by providing an overview of the DBCA general approach to conservation of threatened plant species in the SWAFR and the strong interdependencies between research and on ground management. We then describe the major threats and scope of interventions for their mitigation. We highlight the integrated approach to management of threats and recovery of species with four case studies of threatened flora recovery projects in the SWAFR. Each of these addresses conservation of threatened species at a different scale and have been chosen as exemplars for illustrating the breadth of interventions ranging from In situ management to conservation reintroductions and restoration of threatened species habitats.
2. Setting priorities and the integrated approach to flora conservation in Western AustraliaIn Western Australia, management and conservation of threatened flora is a primary objective of the Department of Biodiversity, Conservation and Attractions (DBCA). This department has legislative responsibility for conservation under the Biodiversity Conservation Act, 2016, and is undertaken in partnership with other natural resource management and community groups (e.g. Case study 4 Restoration of the Moresby Range). Conservation of the SWAFR's threatened flora utilises a variety of approaches that include establishment of protected areas through a system of conservation reserves and covenants, management of threats in reserves and other government land and private remnant vegetation, protection of flora through legislation, and the recovery and restoration of threatened species and communities (Coates and Atkins, 2001; Coates et al., 2014).
Despite the significant challenges posed by the number of conservation taxa spread across such a large area, much has been accomplished through careful prioritisation and distribution of resources. To date, the process of setting of priorities for conservation of threatened flora has largely focussed on individual taxa at a localised scale, with threat mitigation tackled at both local and landscape scales. Populations, taxa or localised groups of threatened taxa are prioritised for conservation based on the level and immediacy of threatening processes, with resources focussed on those taxa or threatening processes considered to require immediate attention.
Conservation action and prioritisation are based on scientific evidence, particularly knowledge of species' biology and threats. Research into species' biology, ecology and genetics, and threat mitigation strategies supports the implementation of In situ management and restoration of threatened species populations and habitats. Research staff from DBCA, in partnership with on-ground DBCA management staff, work to identify knowledge gaps in species biology/ecology or threat management techniques, undertake research, and implement recovery actions. Regionally based Flora Officers employed by DBCA are responsible for a geographical area and the implementation of recovery actions for individual threatened species or threat mitigation across this area. Support in the recovery process is also guided by regionally based management plans (e.g. Kelly et al., 1990; Hearn et al., 2006), that outline recovery actions for all threatened and poorly known flora in a geographical area, and Recovery Teams, comprised of key stakeholders in the management and recovery of threatened plants in the area covered by the regional recovery plan (e.g. land owners and managers, Natural Resource Management Groups, university researchers and non-government organisations).
2.1. In situ management and threat mitigationThe rich and diverse flora of the SWAFR presents some key challenges to evidence-based management of threatened flora, including the ongoing discovery of new species; the broad range of biological and life history attributes; the diverse evolutionary patterns and genetic systems found in threatened species of the region; and complex interactions with multiple threatening processes (Table 1). Many of these challenges are typical of other Mediterranean-type ecosystems (e.g. Cowling et al., 1999; Vogiatzakis et al., 2006; Underwood et al., 2009; Hopper et al., 2016) and the approaches to threatened species conservation discussed herein are applicable to these areas.
A primary basis for effective threatened plant conservation is the effective identification and description of plant taxa. The rate of discovery of new taxa in the SWAFR remains high, and the proportion of new taxa that are recognised as requiring protection and recovery is increasing (Coates and Atkins, 2001; Wege et al., 2015; Gioia and Hopper, 2017). While taxonomic studies have provided the primary foundation for the delimitation and identification of species, it is also clear from patterns of population genetic variation within taxa that conservation units exist below the taxon level as either individual populations or clusters of populations (see Moritz, 2002; Coates, 2000). This can be important for defining the appropriate geographic scale for management and ensuring the conservation of adequate levels of population-based variation and the genetic diversity it encompasses (see Case Study 1 Banksia brownii).
Understanding ecological factors that limit population persistence, and prioritising and managing threats, are key elements of In situ management and recovery of threatened plant species in this region. The diversity in life history attributes and complex evolutionary history of species in this region means that recovery plans need to cover a broad range of issues and be underpinned by adequate knowledge (Table 1). This highlights the challenge given the number of threatened plant species in this region. While useful generalisations may be made regarding life history attributes, reproductive biology, genetic systems and response to threats, management strategies may still need to be developed that are appropriate only for individual species and their landscape context. Despite this, a significant number of species have been investigated in detail (Table 1) covering a range of research topics. Findings from these studies have been central in developing recovery actions and undertaking threat mitigation.
2.2. Translocations and ex situ conservationOur primary focus of plant recovery is management of natural populations and involves threat abatement guided by research findings (see Table 1). In cases where populations are under imminent threat of destruction or where control of threatening process is not possible or is inadequate, an increased level of intervention may be required. In these cases, In situ management may be complemented by translocation to a new site within the known range and habitat of the species, or indeed translocation well outside the species known range and possibly outside its known habitat to ensure species persistence in the wild (Vallee et al., 2004; Maschinski et al., 2012; Volis, 2017). This has become a major focus for threatened species conservation in the region with 65 threatened species translocated to 110 sites over the last 20 years.
Although In situ conservation is the primary focus for conserving threatened species in the SWAFR, ex situ conservation is playing a critical role by conserving components of plant diversity outside their natural habitats, for example as seed in seed banks. Many situations will justify the need for either long-or short-term ex situ storage of seed resources where the effective management of a threatening process is limited. In these cases, seed storage facilities, primarily DBCA's Threatened Flora Seed Centre, with support from DBCA's Botanic Gardens and Parks Authority and the Royal Botanic Gardens Kew's Millennium Seed Bank, have been established to collect and maintain seeds of rare and threatened species, with a major objective of supporting translocations (Cochrane et al., 2007). To date there are 3526 ex situ seed collections in the Centre from species of conservation concern in the SWAFR. These collections represent 81% of the 399 threatened species and 33% of the 2076 Priority species occurring in the region (Department of Biodiversity, Conservation and Attractions, 2018a). In addition, for those threatened species that are strongly clonal and sterile, or where seeds are very scarce, in vitro micropropagation of shoot tissue and cryogenic storage of culture collections are being used in ex situ conservation (Bunn et al., 2007).
3. Threats and threat mitigation in the SWAFRIdentifying and mitigating threats is essential for recovery of threatened species and the restoration of species and their habitats (Broadhurst and Coates, 2017). In this section we provide a brief review of key threatening processes for plants in the SWAFR and key interventions being undertaken for threatened flora recovery through In situ mitigation of impacts and restoration of species through conservation reintroductions.
3.1. DiseaseA range of native and exotic plant pathogens that threaten the flora, including aerial canker, and several Phytophthora species have already established in the SWAFR. Currently the most damaging plant pathogen is P. cinnamomi, which was introduced into Western Australia after European settlement and has been widely spread through infested soil, construction materials and nursery supplies. Listed as one of the world's most destructive invasive plant pathogens (Lowe et al., 2000), the spread of P. cinnamomi through susceptible plant communities in the SWAFR has, and will continue to have, a significant impact on susceptible species and communities (Shearer et al., 2007; Cahill et al., 2008; see Case studies 1 and 3). Already, it is estimated that there are 2284 species of the region susceptible to the disease (Shearer et al., 2007, 2013). Current broadscale options for the management of P. cinnamomi in the SWAFR have focused on restricting access and ensuring hygiene procedures are consistently implemented when conducting highrisk activities within or adjacent to the highest-priority protection areas. At more localised scales a level of control of P. cinnamomi has been achieved through the application of phosphite to key local areas such as threatened flora populations and threatened ecological communities (Barrett et al., 2003). This has limitations due to the cost and the need for frequent reapplication; however, it has been successful in preventing the extinction of a number of species (Barrett et al., 2008; Barrett and Yates, 2015; see Case study 3). Experimental containment and eradication projects have demonstrated that the spread of the pathogen into healthy native plant communities can be prevented, if new spot infestations are detected early and on-ground controls quickly implemented (Dunne, 2012). Management of threatened flora species that are susceptible to P. cinnamomi also involves collection and storage of seed ex situ (Shearer et al., 2007). In some instances, seed has already been used to establish new populations of susceptible species at sites where the disease is absent (32 new populations of susceptible flora have been established under these circumstances in SWAFR) or to establish seed orchards to increase the number of seed available for recovery and restoration of threatened plant species (17 seed orchard sites have been established in the SWAFR). The seed generated in seed orchards is being collected and stored ex situ and will be available for reintroduction into natural locations of species in the event P. cinnamomi can be eradicated at these sites in the future.
3.2. Fragmentation, demographic and genetic consequences of small and declining population sizeExtensive land clearing in the SWAFR during the 20th century has resulted in the fragmentation of the remaining native vegetation into small, often isolated remnants. These remnants occur across a range of land tenures with some 72% of threatened flora occurring outside the conservation estate in small remnants on private land, other government owned land and along infrastructure corridors (Coates and Atkins, 1997). These infrastructure corridors are regularly maintained, and threatened plant species can be impacted through pruning, grading and other management activities.
Multiple interacting responses to fragmentation have been demonstrated through integrated genetic and ecological studies of a number of threatened species from the SWAFR woodlands and shrublands. The findings from these studies reflect both landscape and biological complexity and highlight a range of effects associated with altered population parameters; such as size, shape and isolation (Krauss et al., 2007; Byrne et al., 2007; Yates et al., 2007a, b; Llorens et al., 2012, 2013); and altered soil nutrients and soil salinity (Lamont et al., 1994a, b; Llorens et al., 2013, 2018). For example, investigation of pollinator behaviour and the mating system in populations of the bird-pollinated shrub Calothamnus quadrifidus subsp. quadrifidis show no effects of population size or isolation on pollinator abundance or levels of pollination. However, they do show an effect of changed pollinator behaviour with greater within-plant visitation in small populations leading to increased inbreeding, reduced seed production and less pollen diversity (Yates et al., 2007a; b). Similarly, in the primarily insectpollinated Eucalyptus wandoo, pollination is high in small populations (Byrne et al., 2008), but seed production is significantly reduced (associated with the Allee effect and inbreeding), although seed germination is high. Trees in more linear populations had higher rates of seedling survival than trees in other shaped remnants. Soil salinity and increased soil phosphorous had a negative effect on fruit production of E. wandoo, however, increased soil phosphorous in small populations increased progeny performance (Llorens et al., 2018).
A number of studies on threatened SWAFR species have also demonstrated adverse mating system effects associated with negative impacts on pollinator behaviour and ecology in areas subject to habitat fragmentation and disturbance. The animalpollinated, relatively long-lived, woody shrubs Banksia cuneata, Banksia oligantha, Lambertia orbifolia (Proteaceae), Verticordia fimbrilepis subsp. fimbrilepis (Myrtaceae) and Acacia sciophanes (Fabaceae), all show a trend towards increased inbreeding, smaller effective sizes of paternal pollen pools, and greater variation in outcrossing among plants as populations become smaller and habitat disturbance increases (Coates et al., 2007, 2013). In the fragmented landscape of the SWAFR, there is evidence that habitat disturbance, along with the loss of many associated plant species important for the maintenance of pollinator populations, has resulted in temporal changes in abundance, foraging rates and behavioural patterns of pollinators, such as birds, mammals and insects (Coates et al., 2007; Coates et al., 2013; Yates et al., 2007a; b; Llorens et al., 2012, 2013; Sampson et al., 2014).
The impact of fragmentation on Banksia sphaerocarpa var. caesia (Llorens et al., 2012, 2013), an important species for autumn nectarfeeding animals, showed a significant influence of population shape on reproduction, fitness and viability of plant populations. Although linear populations have larger plants that produce more inflorescences and cones than patch-shaped populations (most likely due to greater resources), the plants in linear populations produce substantially smaller seeds with lower survival seedling rates (Llorens et al., 2012, 2013).
While negative effects associated with habitat fragmentation have been demonstrated for a number of threatened species, the rare C. quadrifidus subsp. teretifolius showed no effect of population size or isolation on mating system and reproductive output (Gibson et al., 2012; Sampson et al., 2014). In this species low genetic diversity and high differentiation combined with high selfing and high seed production support the theory of a breeding system with a purged genetic load (Sampson et al., 2014). Despite no direct genetic and demographic effects of fragmentation, the associated disturbance has led to high weed densities resulting in a lack of recruitment in populations in degraded remnants (Gibson et al., 2012). In another rare species, Hakea oldfieldii, high outcrossing rates and long distance pollen dispersal appear to maintain connectivity among naturally fragmented populations (Sampson et al., 2016).
These contrasting findings highlight the broad genetic responses found in plant populations where vegetation has been removed and fragmented, and the ongoing need for a better understanding of the diverse genetic systems that characterise the Western Australian flora. These studies demonstrate that despite the often useful generalities that can be made about the genetic consequences of habitat fragmentation, management may still need to be designed around individual species and their landscape context, particularly where mixing of populations for translocations or restoration is envisaged. While mixing populations of a number SWAFR species may result in detrimental effects in some species (for example, hybrid sterility and outbreeding depression associated with chromosomal differences), other plant groups, such as Eucalyptus, Banksia and Acacia have reproductive systems that can effectively reduce the likelihood of significant outbreeding depression when genetically divergent populations are combined (James, 2000).
Beyond the obvious impacts on fragment and landscape parameters, landscape fragmentation and its associated modification can also influence landscape fluxes associated with soils, such as hydrology and nutrients. These in turn affect individual fragments and plant populations (Hobbs and Yates, 2003), yet their impacts are rarely considered in studies of plant reproduction (but see Lamont et al., 1994a, b; Llorens et al., 2013).
A number of options are available for reducing the impacts of habitat fragmentation. Management of remanent vegetation along road and railway verges primarily relies on maintaining relationships with infrastructure managers so that there is awareness of the flora, and in delineating threatened flora populations with permanent recognisable markers so that there is a visual reminder when verge maintenance occurs (Fig. 2). Whilst simple to implement, installation of visible signs has proven to be one of the most effective In situ management actions at preventing accidental damage during maintenance of the verges. For many remnants reversing the effects of fragmentation will involve restoration and revegetation at levels that have not been carried out to date. Increasing fragment and population size, maintaining landscape connectivity through pollen dispersal, and avoiding linear-shaped remnants, are all management opportunities that need to be explored.

|
| Fig. 2 Yellow road markers are used to delineate and alert machine operators of the boundaries of Threatened Flora populations in SWAFR (Photo: Sarah Barrett, DBCA) |
Weed invasion into native vegetation is a significant consequence of the extensive land clearing and habitat fragmentation that occurred across the SWAFR. Most weeds in the SWAFR are plants imported for horticultural or agricultural purposes that have naturalised and expanded into natural areas (Brown and Brooks, 2002; Keighery and Longman, 2004). The dominant weeds of the region are herbs and grasses (Brown and Brooks, 2002; Keighery and Longman, 2004) that typically invade disturbed areas, such as along roads and tracks, in old settlements and throughout agricultural zones.
Weeds often invade areas post disturbance, competing with native species and preventing seedling recruitment (Fisher et al., 2006, 2009). Disturbance in the form of fire is considered a factor facilitating weed invasion but the interactions are complex (Hobbs and Yates, 2003). On severely nutrient-impoverished soils fire is unlikely to promote significant invasion by annual weed species, except at perimeter edges, where higher propagule availability and elevated soil nutrient levels are the main factors promoting weed invasion (Fisher et al., 2006; Gosper et al., 2011). As remnants become smaller, edge effects, and therefore susceptibility to invasion, are likely to increase. Road and railway verges are particularly susceptible to weed invasion due to the high edge to interior ratio. The critically endangered Acacia aprica is an example of a species being significantly impacted by weed invasion in the road verges where it occurs in SWAFR (Yates and Broadhurst, 2002). Weeds were found to negatively impact both seedling emergence and survival, and are likely to be a major contributor to the threatened status of species (Yates and Broadhurst, 2002). Mechanical disturbance, particularly combined with fire, significantly enhances the spread of weeds in low-nutrient soils (Hobbs and Atkins, 1988; Hester and Hobbs, 1992).
Managing weeds in the highly fragmented landscape of the SWAFR is challenging. Effective weed management in species-rich areas starts with knowledge of the diversity and distribution of native and weed species at the site and the patterns of disturbance that have facilitated the invasion (Brown and Brooks, 2002). This is followed by targeted weed control through physical, chemical or biological methods, and in some cases followed up with other restoration activities, such as revegetation with indigenous species (Brown and Brooks, 2002).
In some areas, such as narrow road verges adjoining agricultural land, control of weeds is particularly challenging due to the edge effect and the constant reinvasion of weeds from the farmland and along the road. In these instances, establishment (translocation) of new populations of threatened flora in areas where weeds are absent, or able to be controlled, has been an effective management strategy. To deal with the threat of weeds in natural locations DBCA has established 41 new populations of threatened flora, primarily through planting nursery grown seedlings.
3.4. Altered fire regimesFire is a primary agent of recurrent disturbance in the seasonally dry landscapes of the SWAFR, consuming biomass and interacting with climate and geology to influence plant population dynamics and community assembly (Abbott and Burrows, 2003). As is the case for other fire-prone Mediterranean-type climate regions, plants in the SWAFR's fire-prone ecosystems possess traits that provide resilience to periodic burning (Burrows and WardellJohnson, 2003; Keeley et al., 2012; Gosper et al., 2013; Harvey et al., 2017). Variation in traits relating to survival, reproduction, dispersal, recruitment, competitive ability and longevity contribute to a diversity of responses and makes some species vulnerable to decline under particular fire regimes (Burrows and WardellJohnson, 2003; Gosper et al., 2013; Harvey et al., 2017). Consequently, changes in the fire regime relative to historical variability, brought about by patterns of human settlement and land-use, may result in species decline (Yates and Ladd, 2010). Those species that are killed by fire and rely on seed germination after fire for population persistence are most at risk from fire interval extremes (Bond and van Wilgen, 1996). These obligate-seeding species face two threats in relation to fire interval: immaturity-risk, where intervals are shorter than the time needed to establish a seed bank; and senescence-risk, where intervals are longer than the life span of plants and their seed bank (Gosper et al., 2013; Keeley et al., 2012).
Inappropriate fire regimes are identified as a major threat of growing importance to threatened plant species in the SWAFR (Coates and Atkins, 2001; Burgman et al., 2007; Shedley et al., 2018). The nature of this threat is likely to vary across the landscape because of the influence that population density and patterns of land-use have on ignition and factors which affect flammability such as landscape fuel continuity. For example, in peri-urban landscapes with moderate population densities, increases in ignitions and the frequency of fire at the wildlandeurban interface, may expose threatened plant species to immaturity-risk (Forsyth and van Wilgen, 2008). In contrast, in heavily cleared agricultural landscapes (see above section) with relatively low population densities, many fragments of native vegetation have a considerably lower probability of burning than analogous continuously vegetated landscapes because of low fuel continuity, reduced likelihood of ignitions and fire suppression (Parsons and Gosper, 2011), potentially exposing threatened plant species to increased senescence-risk (Yates and Ladd, 2010).
Shedley et al. (2018) determined the fire response traits of 242 (60%) of the region's 401 extant threatened species. Over half of the 242 species were obligate seeders, and consequently, have population dynamics particularly sensitive to fire interval and frequency. Their study also found differences in fire history across nine bioregions in the SWAFR and identified areas where current fire regimes may be detrimental to threatened flora. The greatest risk appears to be in the highly fragmented agricultural landscapes of the wheatbelt, where the absence of fire may be limiting opportunities for regeneration of obligate seeder species. In contrast, in some areas of continuous vegetation, fires may be too frequent for species with long juvenile periods, increasing the risk of local extinctions.
New approaches to fire management of threatened species based on their fire responses and other critical attributes such a juvenile periods and plant longevity are being developed to use fire specifically to conserve and regenerate threatened flora and their habitats in the SWAFR (Barrett et al., 2009; Gosper et al., 2013; Beecham and Lacey, 2016; see Case study 2 below). Landscape context and the extent of weed invasion are also important considerations when using fire to regenerate threatened species and their habitats (Brown et al., 2016). For example, in highly altered remnants of native vegetation or at the edges of larger remnants where there is nutrient enrichment and weeds are abundant, the introduction of fire will not be appropriate unless there is post-fire control of the weeds that flourish, smother and outcompete seedlings of native species (Yates and Broadhurst, 2002; Brown et al., 2016; see section above on weeds).
3.5. Grazing by introduced and native animalsIntroduced animals (such as sheep, goats, or rabbits) and native animals (such as kangaroos, quokkas or possums) impact on threatened plant populations by damage through trampling and/or reducing plant biomass and plant reproductive ability through grazing. In addition, weed invasion can be facilitated through animal vectors and increased soil nutrients from animal droppings. As habitat is fragmented through land clearing there is a reduction in available habitat for native animals which can result in increased pressure on remaining areas of vegetation from grazing by native animals. Management of threatened flora impacted by grazing is primarily through fencing of sites to exclude herbivores (e.g. Brundrett, 2016; Rathbone and Barrett, 2017).
3.6. Altered hydro-ecologyA consequence of the wide scale land clearing of the SWAFR is alteration of regional hydro-ecology. Vegetation removal at such a significant scale has resulted in saline ground water tables rising and increased salinity of the ground water (salts stored in the soils are dissolved into the ground water as it rises) (Clarke et al., 2002). Water logging and increased soil salinity has impacted on the native vegetation across whole water catchments threatening some otherwise stable species. Large scale revegetation plantings (e.g. Gondwana Link, Bradby et al., 2016) and engineering works to redirect saline water (e.g. Toolibin Lake, Wallace et al., 2011) are required across whole water catchments, to stabilise water tables and reverse hydrological trends, in order to prevent the extinction of many plant species.
3.7. Drought/climate changeSuperimposed on the threats outlined above is anthropogenic climate change. Mean annual temperatures in the SWAFR have increased during the 20th century, and since the 1970s there has been a significant decline in autumn and early winter rainfall. This decline is largely a consequence of changes to synoptic patterns and changes in sea level atmospheric pressure, which are likely to be due at least in part to increases in greenhouse gas emissions from human activities (Bates et al., 2008). The consensus among global climate models is that temperature will continue to increase and rainfall will continue to decline in the SWAFR (Bates et al., 2008). These changes are projected to have a substantial influence on species geographic ranges (Fitzpatrick et al., 2008; Keppel et al., 2017) and impacts may be exacerbated by fragmentation and other threatening processes (Yates et al., 2010). Phylogeographic analysis of both common and rare species indicates that species have persisted In situ through cycles of aridity associated with Quaternary climate change in the region suggesting some level of resilience in the flora (Yates et al., 2007b; Byrne, 2007; Byrne and Hopper, 2008; Byrne, 2008; Tapper et al., 2014a; b). Research on refugia and genetic adaptation are yielding important insights into vulnerability and providing a basis for management (Schut et al., 2014; Steane et al., 2015).
4. Case studies illustrating recovery of threatened speciesSpecies often face multiple interacting threats that require a variety of recovery actions (Burgman et al., 2007; Broadhurst and Coates, 2017). In this section we illustrate where scientifically informed recovery actions have been used to conserve and recover threatened species and restore the vegetation community in which they occur.
4.1. Case study 1: Recovery of the critically endangered Banksia brownii 4.1.1. BackgroundB. brownii is a long-lived, non-lignotuberous shrub or small tree to 4 m. It is killed by fire and regenerates from seed that has been stored in woody fruits in the plant canopy (Fig. 3; George, 1984). Plants are known from three geographically disjunct areas of southwest Western Australia over a range of approximately 90 km: the Stirling Range, the Millbrook-Waychinicup area extending from Millbrook Nature Reserve 40 km east to Cheyne Beach, and at a single site on Vancouver Peninsula near Albany (Fig. 3). The species is ranked as Critically Endangered under World Conservation Union Red List criteria (IUCN, 2012).
|
| Fig. 3 Mean q-matrix membership proportions of Banksia brownii populations (pie charts)when K = 3 from a STRUCTURE analysis (see Coates et al., 2015). The size of pie charts is relative to the level of genetic diversity. Extant populations. Germinated seed from extinct (◆) and extant populations (■) was used to establish two separate translocated populations (T1 and T2) in disease free areas. Translocated population T3 was established with seed from the single Vancouver Peninsula population |
The species is self-compatible, with a mixed mating system indicating significant levels of selfing (Sampson et al., 1994). It produces large, conspicuous inflorescences that are pollinated by a range of nectar feeding birds and small mammals (Day et al., 1997). Seeds are non-dormant once released from woody fruiting cones and germination studies indicate that most collections are highly viable (>80% germination). However, suboptimal temperatures during germination are predicted to impact on recruitment (Cochrane et al., 2011) and projected warming temperatures together with a drying climate are predicted to have a substantial impact on the species' geographic range (Fitzpatrick et al., 2008).
Population genetic studies have demonstrated significant genetic structure within B. brownii corresponding to the three geographically disjunct population groups (Fig. 3). The high differentiation among population groups suggests long-term historical isolation while higher levels of genetic diversity in the Stirling Range populations indicate larger and more stable population sizes over longer timeframes. These historically isolated population groups also display ecological differences occupying contrasting habitats in terms of substrate, associated vegetation and climate, and may be expected to have adaptive differences. The three population groups are therefore considered to be discrete conservation units important for the management and recovery of B. brownii (Coates et al., 2015).
B. brownii is highly susceptible to the introduced soil-borne pathogen P. cinnamomi, which is considered to be the greatest threat to the species' ongoing persistence (Shearer et al., 2013). Of 30 known populations, 10 are now presumed extinct and 13 have less than 100 plants, each as a result of this disease. Genetic diversity studies based on material from extinct (ex situ seed collections) and extant populations indicate that some 38% of total genetic diversity, based on contributions of within population variation and differentiation, has been lost from B. brownii due to P. cinnamomi (Coates et al., 2015).
B. brownii has a long juvenile period of five to six years in lowland populations and more than eight years in upland Stirling Range populations (Gilfillan and Barrett, 2005). This renders the species vulnerable to short fire intervals. Two fires in close succession in 1991 and 2000 in the eastern Stirling Range had a devastating impact with no or minimal regeneration of several populations (Barrett and Yates, 2015). However, the long-term exclusion of fire has led to population senescence and decline, highlighting the importance of appropriate fire management.
4.1.2. Aims of managementManagement of B. brownii is focussed on arresting the significant decline associated with the impact of P. cinnamomi and the establishment of translocated populations in Phytophthora free areas.
4.1.3. Management activitiesSince 1997 populations of B. brownii in the Stirling Range and Milbrook - Waychinicup areas (Fig. 3) have been sprayed with phosphite to limit the impact of P. cinnamomi and minimise any further spread (Gilfillan and Barrett, 2005).
While managing populations of B. brownii through the control of Phytophthora dieback is an important focus for conservation efforts, ex situ conservation through seed storage and the translocation and establishment of new populations into P. cinnamomi free areas have been key management priorities for this species since 2007 (see Barrett et al., 2008; Cochrane et al., 2010). There are now 65 collections of seed from across 24 distinct B. brownii populations. As a risk management strategy, 23 of these collections (~14, 000 seeds) have been duplicated with the Royal Botanic Gardens Kew, in the Millennium Seed Bank. Some 60, 000 seeds held in long-term storage represent the genetic diversity of the species and include seeds from populations now extinct in the wild.
Three new populations have been established covering the three genetically and biogeographically distinct conservation units recognised in B. brownii (Fig. 3). The Vancouver and Millbrook-Waychinicup populations have been translocated to similar habitat while the Stirling Range populations were introduced outside their known habitat. Two translocation sites were established with a proportion of seed collected from now extinct populations. The use of seed from extinct populations highlights the importance of ex situ seed collections, not only for the conservation of this species, but many other threatened species in the SWAFR (Cochrane et al., 2007).
Translocations and associated recovery actions are usually primarily beneficial to the single target threatened species. However, there can be significant benefits for co-dependant species that may also be threatened if management can be coordinated between the host and dependent. B. brownii is the sole host to the Critically Endangered herbivorous plant-louse Trioza barretta. A coordinated approach to prevent co-extinction has recently involved the successful translocation of the plant louse from the Vancouver B. brownii population to the new translocated population (T3, Fig. 3) (Moir et al., 2016).
4.1.4. Current achievements● Phosphite aerial application techniques for managing P. cinnamomi have been successful in preventing further population loss and stabilised population numbers despite the natural populations being infected by the pathogen.
● New populations representing three genetically distinct conservation units have been established through translocations to secure Phytophthora free sites.
● Genetically diverse ex situ seed collections have proven to be critical for the conservation of B. brownii and the establishment of translocations.
4.2. Case study 2: using fire to regenerate declining populations of threatened species in a highly fragmented landscape 4.2.1. BackgroundThe Wongan Hills situated 194 km north-east of Perth in the Western Australian wheatbelt are a series of flat-topped hills occupying an area of approximately 1750 ha (Fig. 1). The hills, covered by surficial laterite and dissected by numerous deep gullies were unsuitable for farming and still support a rich diversity of kwongan heath plant communities that are renowned for their endemic and diverse flora, including many Threatened and Priority plant species (Kenneally, 1977; Johnston and Thomas, 2006).
In common with other fragments of native vegetation in the Wheatbelt, much of the kwongan vegetation has not been burnt in the last 60 years (Phillips et al., 2016), and this may be affecting the composition and structure of plant communities and persistence of threatened species. Anecdotally, land managers often attribute the apparent decline and senescence of some vegetation communities in the Wheatbelt to a lack of fire. Recent research in shrublands, mallee-heaths and kwongan provide some support for this proposition with significant correlations between senescence and structural decline with time since fire (Gosper et al., 2012, 2013). Furthermore, numerous studies have demonstrated that some threatened species are most abundant in the first decade after fire and gradually retreat from the plant community to persist as seeds in the soil until the passage of another fire (Yates and Broadhurst, 2002; Yates et al., 2003; Yates and Ladd, 2005; Nield et al., 2009).
In response to the above issues, a project was initiated to develop ecological fire management guidelines to protect and recover Critically Endangered flora and their associated habitat on the Wongan Hills (Phillips et al., 2016).
4.2.2. Aims of managementThe project aimed to 1) demonstrate that prescribed fire could be used at operational scales to regenerate declining populations of threatened flora and 2) develop operational guidelines for using prescribed fire to manage seven Critically Endangered species and their vegetation habitats.
4.2.3. Management activitiesPrescribed burns were undertaken at two locations in senescent habitat where populations of the Critically Endangered Daviesia euphorbioides and Gastrolobium hamulosum had been recorded but were no longer present, or in the case of D. euphorbioides were still extant in adjacent areas at very low densities (Fig. 4a). The planned burn area for D. euphorbioides was 28.3 hectares (ha) and for G. hamulosum was 51.7 ha. Both species have seeds with thick integuments (hard seeded) and are likely to form long-lived seed banks. It was hypothesised that the passage of fire would break the physical dormancy in seeds of both species and stimulate germination and regeneration of populations. Post-burn monitoring to determine whether the fire treatments were successful was undertaken in subsequent years (Fig. 4b; Phillips et al., 2016).

|
| Fig. 4 a) A prescribed burn on a Nature Reserve in SWAFR to regenerate a population of the Critically Endangered Gastrolobium hamulosum and associated shrubland habitat (Photo: Brett Beecham, DBCA). b) Seedlings of the Critically Endangered Daviesia euphorbioides regenerating following a prescribed burn (Photo: Laura Canackle, DBCA) |
To investigate possible difference in the tolerable fire intervals between threatened and more common species in these habitats, levels of population senescence amongst several serotinous nonsprouting species thought to be sensitive to long fire return intervals were measured using a method modified from Gosper et al. (2012). Transects were established in vegetation types like those supporting populations of the threatened flora across a chronosequence of time since fire sites, and the proportion of standing dead plant material and proportion of live to dead plants in populations of key indicator species measured. The population densities of indicator species were also measured at points along the transect, together with an estimate of their canopy seed bank.
4.2.4. Current achievementsThe prescribed fires resulted in increased plant numbers and population area for both threatened species. Conditions were not optimal for carrying the fire through the planned burn area for D. euphorbioides resulting in only approximately 9 ha burning. Nevertheless, the fire stimulated germination of Daviesia euphorbiodes increasing the area of occupancy for the species by 2.5 ha and producing an estimated 2500 juvenile plants, some of which reached reproductive maturity in the year following the fire. Similarly, for the G. hamulosum fire conditions were not optimal for carrying the fire with only 4.7 ha of vegetation burning, but still resulted in the area of occupancy for the species increasing by 0.42 ha and an estimated 10, 866 plants (Phillips et al., 2016).
Of the 12 transects where vegetation and population senescence were assessed, eight had greater than 25% standing dead vegetation. Gosper et al. (2013) proposed a conservative mortality threshold of 25% as a proxy for the maximum tolerable fire interval, and on this basis, the vegetation at most sites is senescing. Eight out of 12 transects also had at least one indicator species at moderate or high-risk of not persisting in the long-term based on the proportion of live to dead plants. In one kwongan community, Coates (1992) recorded Hakea circumalata and Petrophile ericifolia as commonly occurring species, but in 2015 no living examples of these species were relocated in the same sites (Phillips et al., 2016). Populations of Hakea platysperma, a widespread species that is serotinous and non-sprouting, display high mortality at many sites where the fire interval exceeds 40-45 years. At some locations the only evidence of their occurrence are the remains of their large woody fruit on the ground (Phillips et al., 2016; Beecham, B., unpubl. data). These results suggest that the long-term absence of fire across much of the Wongan Hills may result in the loss of some plant species, particularly serotinous non-sprouters such as these.
Management guidelines were developed that identified the minimum and maximum fire return intervals for each vegetation community within the Wongan Hills using the results from this and other published sources (Phillips et al., 2016). Together with spatial data on the vegetation and fire history, this data will be analysed using the Fire Regime Optimisation Planning System (Beecham and Lacey, 2016) to produce a long-term prescribed burn program and to minimise species loss from the Wongan Hills.
4.3. Case study 3: managing multiple threats in the eastern Stirling Range montane heath and thicket; a critically endangered ecological community 4.3.1. BackgroundOn the high peaks of the eastern Stirling Range in the SWAFR, montane conditions prevail with low temperatures, high humidity and exposure, skeletal soils and snowfalls several times a year. The Range is located within the 115, 900 ha Stirling Range National Park (Fig. 1). On these peaks there is a unique shrub thicket with many endemic species (Diels, 2007; Pignatti et al., 1993; Barrett, 1996; Department of Parks and Wildlife, 2016) that has been subject to a range of threats leading to the thicket being listed by the Australian Government as a Threatened Ecological Community (TEC).
An assessment of the conservation status of the Eastern Stirling Range Montane Heath and Thicket (montane thicket) using the IUCN Red list criteria for ecosystems found the ecosystem to be Critically Endangered. This assessment was based on a significant reduction (≥80%) in the geographic distribution of the ecosystem in the last 50 years, its naturally limited geographic extent and area of occupancy, and continuing decline in environmental quality due to plant disease, fire and grazing (Barrett and Yates, 2015). The foremost threats to the montane thicket are Phytophthora dieback, inappropriate fire regimes, herbivory and climate change.
P. cinnamomi was most likely introduced onto the mountain peaks through infested soil carried on the footwear of hikers. The pathogen subsequently spread down-slope, such that the montane thicket is extensively infested. The composition of the montane thicket has changed dramatically in the last three decades with the decline of obligate seeding species susceptible to P. cinnamomi. Many of the montane thicket's species, especially those from the Proteaceae, Ericaceae and Fabaceae, are highly susceptible to the pathogen which thrives in the montane environment. Less than 14 per cent of the montane thicket retains a representative suite of plant species that were once common, and many Phytophthora dieback-susceptible species have become locally extinct.
Growth rates of the dominant overstorey species such as Kunzea montana are slow and it may take at least 30 years for the montane thicket to recover its maximum height structure. Several species endemic to the montane thicket, such as the Critically Endangered Banksia montana, have very long juvenile periods of eight to 10 years. Major fires occurred in the Stirling Ranges in February 1972, April 1991 and November 2000, and most recently in May 2018; 74% of the montane thicket was burnt in 2000 and 1991, a fire interval of only nine years. Monitoring of population recovery following this latest fire will provide information on the impacts of these fire intervals. The species most susceptible to fire related decline (serotinous, non-sprouting Proteaceae species) are coincidentally the species most susceptible to P. cinnamomi (Barrett and Yates, 2015). Estimates of the density of this group show that when the pathogen is present they are most abundant in areas that have not been burnt since at least 1972 and lowest in areas that have experienced the most fire.
The emerging threat of browsing in the montane thicket became apparent in regenerating vegetation after a wildfire in 2000, with visible impacts and faecal evidence of the native marsupial quokka (Setonix brachyurus) and the feral rabbit (Oryctolagus cuniculus). Analysis of faecal material implicated quokka as responsible for impacts on dicotyledonous species, and in particular those of conservation significance (Rathbone and Barrett, 2017). Montane ecosystems may be particularly vulnerable to browsing due to their naturally slow recovery after disturbance while browsing may also create environmental conditions, such as higher soil temperature, that are more conducive to plant disease.
Climate change is also considered a threat to the montane thicket as projected increases in temperature may negatively affect the persistence of component species. Investigations of temperature and seed germination for selected species from the thicket show a negative relationship between increasing mean temperature and germination for some species (Cochrane et al., 2011).
4.3.2. Aims of managementManagement of the montane thicket community aims to maintain or improve the overall condition of the community and reduce the level of threat. The aims of management of individual threatened species that occur within the montane thicket community are to abate identified threats and improve the conservation status of the species in the wild.
4.3.3. Management activitiesP. cinnamomi management has been undertaken on an annual or biennial basis since 1997. The management program involves the aerial spraying of the fungicide phosphite across key occurrences of the montane thicket (Fig. 5a). Phosphite enhances the ability of susceptible species to survive the impacts of the disease and has successfully prevented the extinction of several montane species (Barrett et al., 2008; Barrett and Yates, 2015).

|
| Fig. 5 a) Aerial application of phosphite in the Montane Heath Thicket Community (Photo: Sarah Barrett, DBCA). b) Exclosures to prevent grazing of Montane Heath Thicket Community (Photo: Sarah Barrett, DBCA) |
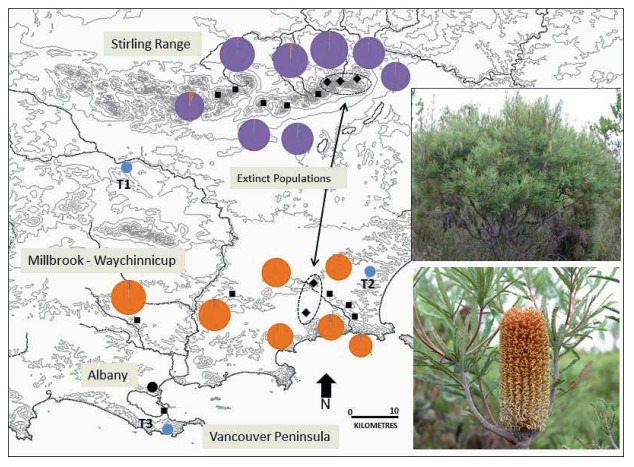
Due to the threat of P. cinnamomi, seed orchards have been established for a range of threatened plant species that occur in the montane thicket. As the pathogen is widespread, sites were found outside the known range of the species, in lowland areas where the disease is not present. Strict hygiene protocols for entry to the orchard sites have maintained the pathogen free status. Three sites each contain a suite of threatened species from the montane thicket (Fig. 6), with a key objective to preserve the genetic diversity of wild populations and ensure adequate seed is banked from each site for future reintroduction attempts back into montane thickets when appropriate.
|
| Fig. 6 Location of Montane Heath Thicket Community relative to the three translocation sites for threatened species from the community. Tables show the species and number of individuals translocated to each translocation site |
Exclosure experiments conducted over one year revealed significant changes in abundance, cover and height of perennial herbs and an increase in growth and/or reproduction of four threatened endemic plants (Fig. 5b; Rathbone and Barrett, 2017). Exclosures have subsequently been retained following the conclusion of the experiment to ensure ongoing protection of the flora.
Fire management is challenging in the montane thickets due to the topography and remoteness, and the need to protect other assets (such as recreation sites) within the Stirling Range National Park. Prescribed burning is strategically used in lowland areas as part of an overall fire mitigation strategy to protect montane thickets. To protect recovering montane thickets from wildfire, their occurrences have been listed on the DBCA's fire Geographic Information System database as high priority for protection in the case of wildfire.
4.3.4. Current achievementsRecovery actions to protect the Eastern Stirling Range Montane Heath and Thicket to date include:
● establishment of fenced enclosures within the montane thicket to facilitate a trial re-introduction of a keystone Proteaceous species, Banksia oreophila, with promising results;
● extensive seed collection and ex situ conservation program for threatened species;
● translocation of several threatened species (B. montana, Latrobea colophona, Leucopogon gnaphiliodes, Persoonia micranthera and Lambertia fairallii) to P. cinnamomi-free sites outside of the Stirling Range;
● continued management of P. cinnamomi through aerial application of phosphite;
● management of herbivory in key areas through fencing; and
● management of the fire return interval, which is critical to conserve the remaining values of the montane thicket.
4.4. Case study 4: Restoration of threatened plant species and their habitats in the Moresby Range Conservation Park 4.4.1. BackgroundMoresby Range Conservation Park (CP) is a 747 ha reserve managed by the DBCA. It is located within the Shire of Chapman Valley, 15 km north of the major regional town of Geraldton, on the west coast in Western Australia (Fig. 1).
The vegetation of the Moresby Range CP consists of floristically diverse kwongan vegetation communities and provides habitat for one Threatened bird (Calyptorhynchus latirostris) and five Threatened plants (Caladenia hoffmanii, Drummondita ericoides, Grevillea bracteosa subsp. howatharra, Eucalyptus cuprea and Leucopogon marginatus). There are also twelve Priority Flora species and several potentially new taxa. The vegetation is recognized by the Western Australian Government as the Priority 1 Ecological Community Plant Assemblages of the Moresby Range System. This community is described as the Melaleuca megacephala and Hakea pycnoneura thicket on stony slopes, Verticordia dominated low heath, and Allocasuarina campestris and Melaleuca concreta thicket on superficial laterite, on Moresby Range.
Threats to the vegetation of Moresby Range CP predominantly relate to historical clearing for agriculture and include grazing, weed invasion, lack of supportive habitat and habitat connectivity, small population size and lack of genetic diversity. At Moresby Range CP the vegetation supporting Threatened flora populations is fragmented by large areas of cleared previously cropped land (232 ha within the park). The cleared land within the park has not been cropped or grazed by stock for over 18 years and yet there has been little natural regeneration. It is thought that this could be because of high levels of paddock weeds, grazing by kangaroos (Macropus fuliginosus) and rabbits (O. cuniculus) and the loss of the soil seed bank of native species. Recovery of native vegetation is unlikely without intervention (Yates et al., 2000; Cramer et al., 2008).
4.4.2. Aims of managementThe project aims to restore vegetation in cleared areas of the Moresby Range CP, increase the number of populations of Threatened plants through translocation and improve connectivity between Threatened plant populations.
4.4.3. Management activitiesThe restoration work of the cleared areas within the Morseby Range CP initially started with a survey of 19 reference sites within adjoining vegetation to determine suitable planting composition for the variations in soil and landform. Seed collections were then made from the existing adjoining vegetation and seedlings raised for planting.
Initially, a two-hectare site was fenced to protect seedlings from grazing from rabbits, kangaroos and damage from feral pigs (Fig. 7). Weed control (multiple treatments of broadscale spraying over the two years prior to planting and hand removal) was undertaken throughout the fenced area. The ground within the fenced site was prepared to alleviate compaction, maximise moisture infiltration and minimise erosion. This was followed by the establishment of a population of the Endangered E. cuprea within the fenced area and planting of seedlings of non-threatened indigenous species. Further work two years later expanded the area of management by an additional 16 ha (Fig. 7). Weed control was undertaken across the new area of management followed by deep ripping. Habitat restoration over the 16 ha site included planting of 40, 000 seedlings of 33 different native species. The habitat planting also included three Priority flora species (Acacia guinettii, Eucalyptus blaxellii and Grevillea triloba). An area adjacent to the existing population of the Critically Endangered G. bracteosa subsp. howatharra was fenced and seedlings of the subspecies planted into the fenced area to increase the population size.
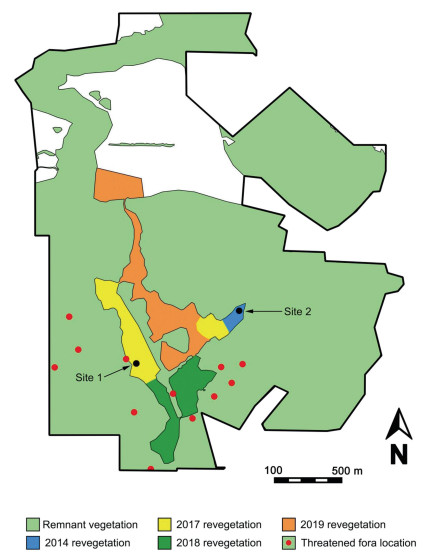
|
| Fig. 7 Restoration map for the Moresby Range Conservation Park. Site 1 contains translocation sites for the Critically Endangered Grevillea bracteosa subsp. howatharra (planted in 2017) and the Endangered Eucalyptus cuprea (to be planted in 2019). Site 2 contains translocation sites for Eucalyptus cuprea (planted in 2015) and Grevillea bracteosa subsp. howatharra (to be planted in 2019) |
Monitoring quadrats are located within the revegetation area to monitor seedling establishment success, weed levels and grazing. The data collected, as well as rainfall observations, are being used to guide management and planning for future planting.
Control of grazing by rabbits, kangaroos and feral pigs is ongoing across the whole restoration site. Work planned for the future includes adding more plants to the current G. bracteosa subsp howatharra site in 2018 and the establishment of further translocation sites for E. cuprea and G. bracteosa subsp. howatharra in 2019. In addition, habitat restoration of an additional 40 ha of the remaining cleared areas adjoining the Threated flora populations will take place in 2018 and 2019 (Fig. 7), and will include several additional species of conservation concern.
4.4.4. Current achievements● establishment of new population of the Endangered E. cuprea;
● augmentation of an existing population of the Critically Endangered G. bracteosa subsp. howatharra and;
● restoration of 18 ha of agricultural land, including, weed control, grazing control, alleviation of soil compaction and planting of 40, 000 seedlings of 33 species (including the Priority flora A. guinettii, E. blaxellii, G. triloba).
5. ConclusionsOur review and case studies emphasise that despite the scale of the challenge, a scientific understanding of threats and their impacts enables effective conservation actions to arrest decline and enhance recovery of threatened species and their habitats. This integrated approach can be implemented in other regions with high flora diversity, such as Mediterranean-type climate ecosystems, to maximise conservation outcomes from evidence-based management. We suggest that future research and management focus on key areas where there are knowledge gaps and opportunities for making progress in conservation of the region's diverse flora. The scale of translocations and habitat restoration required to recover and down list species remains challenging. The significant opportunities presented by large scale restoration programs for threatened species recovery are being realised, as highlighted by the Moresby Range Restoration Program. The use of fire as a tool to maintain and restore populations through stimulation of the soil seed bank is showing promising results, although limited data on soil seed bank longevity remains a major knowledge gap when considering maximum tolerable fire intervals. Phytophthora dieback continues to pose a significant threat to the large number of susceptible species and new innovative approaches are required for understanding mechanisms of resistance, actions of phosphite and control of the pathogen. Projected climate change and its interaction with other threatening processes is an added complexity that will need to be considered more fully in coming decades.
We have no conflicts of interest to declare. All work was carried out with appropriate licenses and approval from the Western Australian Government. Much of the work in Threatened plant management done by the DBCA and its predecessor agencies has been funded by Australian and Western Australian government threatened species and natural resources management initiatives, which we gratefully acknowledge. We also acknowledge the contribution of the many DBCA Flora Conservation Officers, DBCA staff, students and volunteers who have contributed to flora conservation in Western Australia. Case study 4 has benefited from considerable involvement from community and other organisations for both on ground work and funding. These organisations include Geraldton Regional Herbarium Group, Central West College of Technical and Further Education, Northern Agricultural Catchment Council, Western Mulga, Greenough Regional Prison, City of Geraldton Community Nursery, the Department of Environment and Energy's '20 Million Trees Program' and Western Australian State Natural Resource Management Program.
Abbott I., Burrows N., 2003. Fire in Ecosystems of South-west Western Australia:Impacts and Management. Leiden: Backhuys Publishers.
|
Barrett, S., 1996. Biological Survey of Mountains of Southern Western Australia: Report. Department of Conservation and Land Management, Albany, Western Australia.
|
Barrett, S.B.L., Shearer, B.L., Hardy, G.E.St.J., 2003. Control of Phytophthora cinnanmomi by the fungicide phosphite in relation to in planta phosphite concentrations and phytotoxicity in native plant species in Western Australia. In: McComb, J.A., Hardy, G.E.St. J., Tommerup, I.C. (Eds.), Phytophthora in Forests and Natural Ecosystems. 2nd International IUFRO Working Party 7.02.09 Meeting, Albany, Western Australia 30th Sept. - 5th Oct 2001. Murdoch University Print, pp. 139-143.
|
Barrett S., Yates C.J., 2015. Risks to a mountain summit ecosystem with endemic biota in southwestern Australia. Aust. J. Ecol, 40: 42-432. |
Barrett S., Shearer B.L., Crane C.E., Cochrane A., 2008. An extinction-risk assessment tool for flora threatened by Phytophthora cinnamomi. Aust. J. Bot, 56: 477-486. DOI:10.1071/BT07213 |
Barrett, S., Comer, S., McQuoid, N., Porter, M., Tiller, C., Utber, D., 2009. Identification and Conservation of Fire Sensitive Ecosystems and Species of the South Coast Natural Resource Management Region. Department of Environment and Conservation, Western Australia.
|
Bates B.C., Hope P., Ryan B., Smith I., Charles S., 2008. Key findings from the Indian Ocean Climate Initiative and their impact on policy development in Australia. Climatic Change, 89: 339-354. DOI:10.1007/s10584-007-9390-9 |
Beecham, B., Lacey, P., 2016. Planning and Implementing Prescribed Fire to Conserve Wildlife in Reserves and Landscapes: the Fire Regime Optimisation Planning System (FiReOPS), Paper Presented at the 28th Australasian Wildlife Management Society Conference - Wildlife in a Changing Environment. Perth 23-26 November 2015.
|
Binks R.M., Millar M.A., Byrne M., 2015a. Contrasting patterns of clonality and finescale genetic structure in two rare sedges with differing geographic distributions. Heredity, 115: 235-242. DOI:10.1038/hdy.2015.32 |
Binks R.M., O'Brien M., Macdonald B., Maslin B., Byrne M., 2015b. Genetic entities and hybridization within the Acacia microbotrya species complex in Western Australia. Tree Genet. Genomes, 11: 65. DOI:10.1007/s11295-015-0896-4 |
Biodiversity Conservation Act, 2016. www.legislation.wa.gov.au.
|
Bond W.J., van Wilgen B.W., 1996. Fire and Plants. London: Chapman and Hall.
|
Bradby K., Keesing A., Wardell-Johnson G., 2016. Gondwana Link:connecting people, landscapes, and livelihoods across southwestern Australia. Restor. Ecol, 24(6): 827-835. DOI:10.1111/rec.2016.24.issue-6 |
Bradbury D., Grayling P., Macdonald B., Hankinson M., Byrne M., 2016. Clonality, interspecific hybridisation and inbreeding in a rare mallee eucalypt, Eucalyptus absita (Myrtaceae) and implications for conservation. Conserv. Genet, 17: 193-205. DOI:10.1007/s10592-015-0771-8 |
Broadhurst L., Byrne M., Craven L., Lepschi B., 2004. Genetic congruence with new species boundaries in the Melaleuca uncinata complex (Myrtaceae). Aust. J. Bot, 52: 729-737. DOI:10.1071/BT04073 |
Broadhurst L., Coates D., 2017. Plant conservation in Australia:current directions and future challenges. Plant Divers, 39: 348-356. DOI:10.1016/j.pld.2017.09.005 |
Brown K., Brooks K., 2002. Bushland Weeds. Australia: Environmental Weeds Action Network (Inc), Greenwood.
|
Brown K., Paczkowski G., Gibson N., 2016. Mitigating impacts of weeds and kangaroo grazing following prescribed burn in a Banksia woodland. Ecol. Manag.Restor, 17: 133-139. DOI:10.1111/emr.12208 |
Brundrett M., 2016. Using vital statistics and core habitat maps to manage critically endangered orchids in the West Australian wheatbelt. Aust. J. Bot, 64: 51-64. DOI:10.1071/BT15087 |
Burgman M.A., Keith D., Hopper S.D., Widyatmoko D., Drill C., 2007. Threat syndromes and conservation of the Australian flora. Biol. Conserv, 134: 73-82. DOI:10.1016/j.biocon.2006.08.005 |
Bunn E., Turner S., Panaia M., Dixon K., 2007. The contribution of in vitro technology and cryogenic storage to conservation of indigenous plants. Aust. J. Bot, 55: 345-355. DOI:10.1071/BT06065 |
Burne H.M., Yates C.J., Ladd P.G., 2003. Comparative population structure and reproductive biology of the critically endangered shrub Grevillea althoferorum and two closely related more common congeners. Biol. Conserv, 114: 53-65. DOI:10.1016/S0006-3207(02)00420-2 |
Burrows, N., Wardell-Johnson, G., 2003. Fire and plant interactions in forested ecosystems of south-west Western Australia. In: Abbott, I., Burrows, N. (Eds.), Fire in Ecosystems of South-west Western Australia: Impacts and Management.Backhuys Publishers, Leiden, pp. 395-420.
|
Butcher R., Byrne M., Crayn D., 2007. Evidence for convergent evolution among phylogenetically distant rare species Tetratheca (Elaeocarpaceae, formerly Tremandraceae). Aust. Syst. Bot, 20: 126-138. DOI:10.1071/SB06017 |
Byrne M., 2003. Phylogenetics and the conservation of a diverse and ancient flora. Comptes Rendus Biol, 326: S73-S79. DOI:10.1016/S1631-0691(03)00041-6 |
Byrne M., 2007. Phylogeography provides an evolutionary context for the conservation of a diverse and ancient flora. Aust. J. Bot, 55: 316-325. DOI:10.1071/BT06072 |
Byrne M., 2008. Evidence for multiple refugia at different time scales during Pleistocene climatic oscillations in southern Australia inferred from phylogeography. Quat. Sci. Rev, 27: 2576-2585. DOI:10.1016/j.quascirev.2008.08.032 |
Byrne M., Hopper S.D., 2008. Granite outcrops as ancient islands in old landscapes:evidence from the phylogeography and population genetics of Eucalyptus caesia in Western Australia. Biol. J. Linn. Soc, 93: 177-188. |
Byrne, M., Coates, D.J., Forest, F., Hopper, S.D., Krauss, S.L., Sniderman, J.M.K., Thiele, K., 2014. A diverse flora-species and genetic relationships. In: Lambers, H. (Ed.), Plant Life on the Sandplains in Southwest Australia, a Global Biodiversity Hotspot.University of Western Australia Publishing, Crawley, Australia, pp. 81-99.
|
Byrne M., Elliott C.P., Yates C., Coates D.J., 2007. Extensive pollen dispersal in a bird-pollinated shrub, Calothamnus quadrifidus, in a fragmented landscape. Mol.Ecol, 16: 1303-1314. DOI:10.1111/j.1365-294X.2006.03204.x |
Byrne M., Elliott C., Yates C., Coates D., 2008. Maintenance of high pollen dispersal in Eucalyptus wandoo, a dominant tree of the fragmented agricultural region in Western Australia. Conserv. Genet, 9: 97-105. DOI:10.1007/s10592-007-9311-5 |
Byrne M., Tischler G., McComb J., Coates D., 2001. Phylogenetic relationship between two rare Acacia species and their wide spread relatives. Conserv. Genet, 2: 157-166. DOI:10.1023/A:1011826214278 |
Cahill D.M., Rookes J.E., Wilson B.A., Gibson L., McDougal l K.L., 2008. Phytophthora cinnamomi and Australia's biodiversity:impacts, predictions and progress towards control. Aust. J. Bot, 56: 279-310. DOI:10.1071/BT07159 |
Clarke C.J., George R.J., Bell R.W., Hatton T.J., 2002. Dryland salinity in southwestern Australia:its origins, remedies, and future research directions. Soil Res, 40: 93-113. DOI:10.1071/SR01028 |
Coates, A., 1992. Vegetation Survey of Reserve No. 16418 and Part Reserve No. 18672 Wongan Hills Area. Department of Conservation and Land Management. Como, Western Australia.
|
Coates D.J., 2000. Defining conservation units in a rich and fragmented flora:implications for the management of genetic resources and evolutionary processes in south-west Australian plants. Aust. J. Bot, 48: 329-339. DOI:10.1071/BT99018 |
Coates, D.J., Atkins, K.A., 1997. Threatened flora of Western Australia: a focus for conservation outside reserves. In: Hale, P., Lamb, D. (Eds.), Conservation outside Nature Reserves. The Centre for Conservation Biology. University of Queensland., Brisbane, Australia, pp. 432-441.
|
Coates D.J., Atkins K.A., 2001. Priority setting and the conservation of Western Australia's diverse and highly endemic flora. Biol. Conserv, 97: 251-263. DOI:10.1016/S0006-3207(00)00123-3 |
Coates, D.J., Byrne, M., Cochrane, A., Dunne, C., Gibson, N., Keighery, G., Lambers, H., Monks, L., Thiele, K., Yates, C., 2014. Conservation of the kwongan flora: threats and challenges. In: Lambers, H. (Ed.), Plant Life on the Sandplains in Southwest Australia, a Global Biodiversity Hotspot. University of Western Australia Publishing, Crawley, Australia, pp. 263-284.
|
Coates D.J., McArthur S.L., Byrne M., 2015. Significant genetic diversity loss following pathogen driven population extinction in the rare endemic Banksia brownii (Proteaceae). Biol. Conserv, 192: 353-360. DOI:10.1016/j.biocon.2015.10.013 |
Coates D.J., Sampson J.F., Yates C.J., 2007. Plant mating systems and assessing population persistence in fragmented landscapes. Aust. J. Bot, 55: 239-249. DOI:10.1071/BT06142 |
Coates D.J., Williams M.R., Madden S., 2013. Temporal and spatial mating-system variation in fragmented populations of Banksia cuneata, a rare bird-pollinated long-lived plant. Aust. J. Bot, 61: 235-242. DOI:10.1071/BT12244 |
Cochrane A., 2016. Can sensitivity to temperature during germination help predict global warming vulnerability?. Seed Sci. Res, 26: 14-29. DOI:10.1017/S0960258515000355 |
Cochrane J., Barrett S., Monks L., Dillon R., 2010. Partnering conservation actions Inter-situ solutions to recover threatened species in South West Western Australia. Kew Bull, 65: 655-662. DOI:10.1007/s12225-010-9233-0 |
Cochrane A., Brown K., Kelly A., 2002. Low temperature and low moisture storage of seed of the endemic Western Australian genus Eremophila (R.Br.) (Myoporaceae). J. Roy. Soc. West Aust, 85: 31-35. |
Cochrane J.A., Crawford A.D., Monks L.T., 2007. The significance of ex-situ seed conservation to reintroduction of threatened plants. Aust. J. Bot, 55: 356-361. DOI:10.1071/BT06173 |
Cochrane A., Daws M.I., Hay F.R., 2011. Seed-based approach for identifying flora at risk from climate warming. Austral Ecol, 36: 923-935. DOI:10.1111/aec.2011.36.issue-8 |
Cowling R.M., Pressey R.L., Lombard A.T., Desmet P.G., Ellis A.G., 1999. From representation to persistence:requirements for a sustainable system of conservation areas in the mediterranean-climate desert of southern Africa. Divers.Distrib, 5: 51-71. DOI:10.1046/j.1472-4642.1999.00038.x |
Cramer V.A., Hobbs R.J., Standish R.S., 2008. What's new about old fields? Land abandonment and ecosystem assembly. Trends Ecol. Evol, 23: 104-112. DOI:10.1016/j.tree.2007.10.005 |
Crawford A.D., Steadman K.J., Plummer J.A., Cochrane A., Probert R.J., 2007. Analysis of seed-bank data confirms suitability of international seed-storage standards for the Australian flora. Aust. J. Bot, 55(1): 18-29. |
Day D.A., Collins B.G., Rees R.G., 1997. Reproductive biology of the rare and endangered Banksia brownii Baxter ex R. Br. (Proteaceae). Aust. J. Ecol, 22: 307-315. DOI:10.1111/aec.1997.22.issue-3 |
Department of Biodiversity, Conservation and Attractions, 2018a. WASeed - Threatened Flora Seed Centre Database. (Accessed 9 February 2018).
|
Department of Biodiversity, Conservation and Attractions, 2018b. Threatened and Priority Flora Database. (Accessed 7 February 2018).
|
Department of Parks and Wildlife, 2016. Montane Heath and Thicket of the South West Botanical Province, above approximately 900 m above sea level (Eastern Stirling Range Montane Heath and Thicket Community). Interim Recovery Plan 2016-2021 for Interim Recovery Plan No. 370. Perth.
|
Diels L., 2007. The plant life of western Australia, south of the Tropics. Conserv. Sci.West Aust, 6: 1-373. |
Dillon R.A., Monks L.T., Coates D.J., 2018. Establishment success and persistence of threatened plant translocations:an experimental approach. Aust. J. Bot, 66: 338-346. DOI:10.1071/BT17187 |
Dunne, C.P., 2012. Prevention, Containment and Eradication of Phytophthora cinnamomi Infestations in the National Parks from the South Coast of Western Australia. Specific Nature Conservation Project's Final Report. Department of Environment and Conservation, Perth, Western Australia.
|
Fisher J.L., Veneklaas E.J., Lambers H., Loneragan W.A., 2006. Enhanced soil and leaf nutrient status of a Western Australian Banksia woodland community invaded by Ehrharta calycina and Pelargonium capitatum. Plant Soil, 284: 253-264. DOI:10.1007/s11104-006-0042-z |
Fisher J.L., Loneragan W.A., Dixon K., Delaney J., Veneklaas E.J., 2009. Altered vegetation structure and composition linked to fire frequency and plant invasion in a biodiverse woodland. Biol. Conserv, 142: 2270-2281. DOI:10.1016/j.biocon.2009.05.001 |
Fitzpatrick M.C., Gove A.D., Sanders N.J., Dunn R.R., 2008. Climate change, plant migration, and range collapse in a global biodiversity hotspot:the Banksia(Proteaceae) of Western Australia. Global Change Biol, 14: 1337-1352. DOI:10.1111/j.1365-2486.2008.01559.x |
Forsyth G.G., van Wilgen B.W., 2008. The recent fire history of the Table Mountain National Park and implications for fire management. Koedoe, 50: 3-9. |
George A.S., 1984. The Banksia Book. Sydney: Kangaroo Press.
|
Gibson N., Yates C., Byrne M., Langley M., Thavornkanlapachai R., 2012. The importance of recruitment patterns versus reproductive output in the persistence of a short range endemic shrub in a highly fragmented landscape of south western Australia. Aust. J. Bot, 60: 643-649. DOI:10.1071/BT12194 |
Gilfillan, S., Barrett, S., 2005. Interim Recovery Plan No 210 Feather-leaved Banksia(Banksia brownii) 2005-2010. Department of Environment and Conservation, Perth, Western Australia.
|
Gioia P., Hopper S.D., 2017. A new phytogeographic map for the Southwest Australian Floristic Region after an exceptional decade of collection and discovery. Bot. J. Linn. Soc, 184: 1-15. DOI:10.1093/botlinnean/box010 |
Gosper C.R., Yates C.J., Prober S.M., Williams M.R., 2011. Fire does not facilitate invasion by alien annual grasses in an infertile Australian agricultural landscape. Biol. Invasions, 13: 533-544. DOI:10.1007/s10530-010-9847-z |
Gosper C.R., Yates C.J., Prober S.M., Parsons B.C., 2012. Contrasting changes in vegetation structure and diversity with time since fire in two Australian Mediterranean-climate plant communities. Austral Ecol, 37: 164-174. DOI:10.1111/j.1442-9993.2011.02259.x |
Gosper C.R., Prober S.M., Yates C.J., 2013. Estimating fire interval bounds using vital attributes:implications of uncertainty and among-population variability. Ecol.Appl, 23: 924-935. DOI:10.1890/12-0621.1 |
Harvey J.M., Hopkins A.J.M., Langley M.A., Gosper C.R., Williams M., Yates C.J., 2017. Long term studies of post fire reproduction in an Australian Shrubland and Woodland. Aust. J. Bot, 65: 339-347. DOI:10.1071/BT17011 |
Hearn, R.W., Meissner, R., Brown, A.P., Macfarlane, T.D., Annels, T.R., 2006. Declared Rare and Poorly Known Flora in the Warren Region Western Australian Wildlife Management Program No. 40. Department of Conservation and Land Management, Perth, Western Australia.
|
Hester A.J., Hobbs R.J., 1992. Influence of fire and soil nutrients on native and nonnative annuals at remnant vegetation edges in the Western Australian wheatbelt. J. Veg. Sci, 3: 101-108. DOI:10.2307/3236003 |
Hobbs R.J., Atkins L., 1988. Effect of disturbance and nutrient addition on native and introduced annuals in plant communities in the Western Australian wheatbelt. Austral Ecol, 13: 171-179. DOI:10.1111/aec.1988.13.issue-2 |
Hobbs R.J., Yates C.J., 2003. Turner review. No. 7, impacts of ecosystem fragmentation on plant populations:generalising the idiosyncratic. Aust. J. Bot, 51: 471-488. DOI:10.1071/BT03037 |
Hopper S.D., Silveira F.A.O., Fiedler P.L., 2016. Biodiversity hotspots and Ocbil theory. Plant Soil, 403: 167-216. DOI:10.1007/s11104-015-2764-2 |
Indian Ocean Climate Initiative, 2002. Climate Variability and Change in South West Western Australia State Panel. Indian Ocean Climate Initiative Panel.
|
Intergovernmental Panel on Climate Change, 2013. In: Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M. (Eds.), Climate Change 2013: the Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
|
IUCN, 2012. IUCN Red List Categories and Criteria: Version 3.1, second ed. IUCN, Gland, Switzerland and Cambridge, UK. iv+32pp.
|
James S.H., 2000. Genetic systems in the south-west flora:implications for conservation strategies for Australian plant species. Aust. J. Bot, 48: 341-347. DOI:10.1071/BT99016 |
Johnston, W., Thomas, S., 2006. Threatened, Poorly Known and Other Flora of Wongan-ballidu. Department of Environment and Conservation, Kensington, Western Australia.
|
Joyce E.M., Butcher R.M., Byrne M., Grierson P.F., Thiele K.R., 2017. Taxonomic resolution of the Tetratheca hirsuta (Elaeocarpaceae) species complex using an integrative approach. Aust. Syst. Bot, 30: 1-25. DOI:10.1071/SB16040 |
Keeley J.E., Bond W.J., Bradstock R.A., Pausas J.G., Rundel P.W., 2012. Fire in Mediterranean Ecosystems:Ecology, Evolution and Management. New York: Cambridge University Press.
|
Keighery G., Longman V., 2004. The naturalized vascular plants of Western Australia. I, checklist, environmental weeds and distribution in IBRA regions. Pl.Prot. Quart, 19: 12-32. |
Kelly, A.E., Coates, D.J., Herford, I., Hopper, S.D., O'Donoghue, M., Robson, L., 1990. Declared Rare Flora and Other Plants in Need of Special Protection in the Northern Forest Region. Wildlife Management Program No. 5. Department of Conservation and Land Management, Perth, Western Australia.
|
Kenneally, K.F., 1977. The flora. In: Kenneally, K.F. (Ed.), The Natural History of the Wongan Hills Handbook. Western Australian Naturalists' Club, Perth, Western Australia, pp. 7-38.
|
Keppel G., Robinson T.P., Wardell-Johnson G.W., Yates C.J., van Niel K., Byrne M., Schut A.G.T., 2017. A low altitude mountain range as an important refugium for two narrow endemics in the Southwest Australian Floristic Region biodiversity hotspot. Ann. Bot. London, 119: 289-300. DOI:10.1093/aob/mcw182 |
Krauss S.L., Dixon B., Dixon K.W., 2002. Rapid genetic decline in a translocated population of the endangered plant Grevillea scapigera. Conserv. Biol, 16: 986-994. DOI:10.1046/j.1523-1739.2002.01105.x |
Krauss S.L., Hermanutz L., Hopper S.D., Coates D.J., 2007. Population-size effects on seeds and seedlings from fragmented eucalypt populations:implications for seed sourcing for ecological restoration. Aust. J. Bot, 55: 390-399. DOI:10.1071/BT06141 |
Lamont B.B., Rees R.G., Witkowski E.T.F., Whitten V.A., 1994a. Comparative size, fecundity and ecophysiology of roadside plants of Banksia hookeriana. J.App.Ecol, 31: 137-144. DOI:10.2307/2404606 |
Lamont B.B., Written V.A., Witkowski E.T.F., Rees R.G., Enright N.J., 1994b. Regional and local (road verge) effects on size and fecundity in Banksia menziesii. Austral Ecol, 19: 197-205. |
Llorens T.M., Byrne M., Coates D.J., Macdonald B., McArthur S., 2015. Disjunct, highly divergent genetic lineages within two rare Eremophila (Scrophulariaceae:Myoporeae) species:implications for taxonomy and conservation. Bot. J.Linn. Soc, 177: 96-111. DOI:10.1111/boj.2015.177.issue-1 |
Llorens T.M., Byrne M., Yates C.J., Nistelberger H.M., Coates D.J., 2012. Evaluating the influence of different aspects of habitat fragmentation on mating patterns and pollen dispersal in the bird pollinated Banksia sphaerocarpa var. caesia. Mol.Ecol, 21: 314-328. DOI:10.1111/j.1365-294X.2011.05396.x |
Llorens T.M., Yates C.J., Byrne M., Nistelberger N.M., Williams M.R., Coates D.J., 2013. Complex interactions between remnant shape and the mating system strongly influence reproductive output and progeny performance in fragmented populations of a bird-pollinated shrub. Biol. Conserv, 164: 129-139. DOI:10.1016/j.biocon.2013.05.011 |
Llorens T.M., Yates C.J., Byrne M., Elliott C.P., Sampson J., Fairman R., Macdonald B., Coates D.J., 2018. Altered soil properties inhibit fruit set but increase progeny performance for a foundation tree in a highly fragmented landscape. Front Ecol Evol, 6: 39. DOI:10.3389/fevo.2018.00039 |
Lowe, S., Browne, M., Boudjelas, S., De Poorter, M., 2000. 100 of the World's Worst Invasive Alien Species a Selection from the Global Invasive Species Database.Published by the Invasive Species Specialist Group (ISSG)-a Specialist Group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN), p. 12.
|
Maschinski, J., Albrecht, M.A., Monks, L., Haskins, K.E., 2012. Center for Plant Conservation best reintroduction practice guidelines. In: Maschinski, J., Haskins, K.E. (Eds.), Plant Reintroduction in a Changing Climate: Promises and Perils. Island Press, Washington, pp. 277-306.
|
Millar M.A., Byrne M., 2013. Cryptic divergent lineages of Pultenaea pauciflora M.B.Scott (Fabaceae, Mirbelieae) exhibit different patterns of evolutionary history. Biol. J. Linn. Soc, 108: 871-881. DOI:10.1111/bij.2013.108.issue-4 |
Millar M.A., Byrne M., Coates D., 2010. The maintenance of disparate levels of clonality, genetic diversity and genetic differentiation in disjunct subspecies of the rare Banksia ionthocarpa. Mol. Ecol, 19: 4217-4227. DOI:10.1111/mec.2010.19.issue-19 |
Millar M.A., Coates D.J., Byrne M., 2013. Genetic connectivity and diversity in inselberg populations of Acacia woodmaniorum, a rare endemic plant of the Yilgarn Craton Banded Iron Formations. Heredity, 111: 437-444. DOI:10.1038/hdy.2013.66 |
Millar M.A., Coates D.J., Byrne M., 2014. Extensive long-distance pollen dispersal and highly outcrossed mating in historically small and disjunct populations of Acacia woodmaniorum (Fabaceae), a rare banded iron formation endemic. Ann.Bot, 114: 961-971. DOI:10.1093/aob/mcu167 |
Mittermeier, R.A., Gil, P.R., Hoffman, M., Pilgrim, J., Brooks, T., Mittermeier, C.G., Lamoreux, J., da Fonseca, G.A.B., 2004. Hotspots Revisited. Earth's Biologically Richest and Most Endangered Terrestrial Ecoregions. Cemex, pp. 29-33.
|
Mittermeier, R.A., Turner, W.R., Larsen, F.W., Brooks, T.M., Gascon, C., 2011. Global biodiversity conservation: the critical role of hotspots. In: Zachos, E., Habel, J.C.(Eds.), Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas. Springer, Berlin, pp. 3-22.
|
Moir M.L., Coates D.J., Kensington W.J., Barrett S., Taylor G.S., 2016. Concordance in evolutionary history of threatened plant and insect populations warrant unified conservation management approaches. Biol. Conserv, 198: 135-144. DOI:10.1016/j.biocon.2016.04.012 |
Monks L., Coates D., 2002. The translocation of two critically endangered Acacia species. Conserv. Sci. West Aust, 4: 54-61. |
Monks, L., Coates, D., Bell, T., Bowles, M., 2012. Determining success criteria for reintroductions of threatened long-lived plants. In: Maschinski, J., Haskins, K.E.(Eds.), Plant Reintroduction in a Changing Climate: Promises and Perils. Island Press, Washington, pp. 189-208.
|
Moritz C., 2002. Strategies to protect biological diversity and the evolutionary processes that sustain it. Syst. Biol, 51: 238-254. DOI:10.1080/10635150252899752 |
Myers N., Mittermeier R.A., Mittermeier C.G., Da Fonseca G.A.B., Kent J., 2000. Biodiversity hotspots for conservation priorities. Nature, 403: 853-858. DOI:10.1038/35002501 |
Nield A.P., Ladd P.G., Yates C.J., 2009. Reproductive biology, post-fire succession dynamics and population viability analysis of the critically endangered Western Australian shrub Calytrix breviseta subsp. breviseta (Myrtaceae). Aust. J. Bot, 57: 451-464. DOI:10.1071/BT09043 |
Nistelberger H.M., Byrne M., Coates D., Roberts J.D., 2015. Phylogeography and population differentiation in terrestrial island populations of Banksia arborea(Proteaceae). Biol. J. Linn. Soc, 114: 860-872. DOI:10.1111/bij.2015.114.issue-4 |
Nistelberger N., Gibson N., Macdonald B., Tapper S.-L., Byrne M., 2014. Phylogeographic evidence for two mesic refugia in a biodiversity hotspot. Heredity, 113: 454-463. DOI:10.1038/hdy.2014.46 |
Obbens, F., Coates, D., 1998. Critically Endangered W.A. Flora: Monitoring and Weed Control Research: ESP Project Number 564: Progress Report, March 1998. Department of Conservation and Land Management. Como, Western Australia.
|
Parsons B.C., Gosper C.R., 2011. Contemporary fire regimes in a fragmented and an unfragmented landscape:implications for vegetation structure and persistence of the fire-sensitive malleefowl. Int. J. Wildland Fire, 20: 184-194. DOI:10.1071/WF09099 |
Phillips, B., Beecham, B., Jolliffe, D., Crawford, A., 2016. Fire and the Wongan Hills: Determining Fire Return Intervals for Vegetation and Critically Endangered Flora. A State Natural Resource Management-funded Project. Department of Parks and Wildlife, Kensington, Western Australia.
|
Pignatti E., Pignatti S., Lucchese F., 1993. Plant communities of the Stirling range, western Australia. J. Veg. Sci, 4: 477-488. DOI:10.2307/3236075 |
Pimm S.L., Raven P.H., 2017. The fate of the world's plants. Trends Ecol. Evol, 32(5): 317-320. DOI:10.1016/j.tree.2017.02.014 |
Rathbone D.A., Barrett S., 2017. Vertebrate browsing impacts in a threatened montane plant community and implications for management. Ecol. Manag.Restor, 18(2): 164-171. DOI:10.1111/emr.2017.18.issue-2 |
RBG Kew, 2016. The State of the World's Plants Report - 2016. Royal Botanic Gardens, Kew.
|
Sampson J.F., Collins B.G., Coates D.J., 1994. Mixed mating in Banksia brownii Baxter ex R. Br. (Proteaceae). Aust. J. Bot, 42: 103-111. DOI:10.1071/BT9940103 |
Sampson J.F., Byrne M., 2016. Assessing genetic structure in a rare clonal eucalypt as a basis for augmentation and introduction translocations. Conserv. Genet, 17: 293-304. DOI:10.1007/s10592-015-0781-6 |
Sampson J.F., Byrne M., Gibson N., Yates C., 2016. Limited inbreeding in disjunct isolated populations of a woody shrub. Ecol. Evol, 6: 5867-5880. DOI:10.1002/ece3.2016.6.issue-16 |
Sampson J.F., Byrne M., Yates C.J., Gibson N., Thavornkanlapachai R., Stankowski S., Macdonald B., Bennett I., 2014. Contemporary pollen-mediated gene immigration reflects the historical isolation of a rare shrub in a fragmented landscape. Heredity, 112: 172-181. DOI:10.1038/hdy.2013.89 |
Sampson J.F., Hankinson M., McArthur S., Tapper S., Gibson N., Yates C., Byrne M., 2015. Long-term 'islands' in the landscape:low gene flow, effective population size and genetic divergence in the shrub Hakea oldfieldii Benth. Bot. J. Linn. Soc, 179: 319-334. DOI:10.1111/boj.12322 |
Schut A.G.T., Wardell-Johnson G.W., Yates C.J., Keppel G., Franklin S.E., Hopper S.D., Van Niel K., Mucina L., Byrne M., 2014. Rapid characterization of vegetation structure to predict refugia and climate change impacts across a biodiversity hotspot. PLoS One, 9: e82778. DOI:10.1371/journal.pone.0082778 |
Shearer B.L., Crane C.E., Cochrane A., 2004. Quantification of the susceptibility of the native flora of the South-west Botanical Province, western Australia, to Phytophthora cinnamomi. Aust. J. Bot, 52: 435-443. DOI:10.1071/BT03131 |
Shearer B.L., Crane C.E., Barrett S., Cochrane A., 2007. Phytophthora cinnamomi invasion, a major threatening process to conservation of flora diversity in the South-west. Botanical Province of Western Australia. Aust. J. Bot, 55: 225-238. DOI:10.1071/BT06019 |
Shearer B.L., Crane C.E., Cochrane J.A., 2013. Variation in susceptibility of Banksia(including Dryandra) to Phytophthora cinnamomi. Australas. Plant Pathol, 42: 351-361. |
Shedley E., Burrows N., Yates C.J., Coates D.J., 2018. Using bioregional variations in fire history, fire responses and vital attributes as a basis for managing threatened flora in a fire-prone. Mediterranean climate biodiversity hotspot. Aust. J.Bot, 66: 134-143. DOI:10.1071/BT17176 |
Shepherd K.A., Perkins A., Collins J., Byrne M., Thiele K.R., 2013. Morphological and molecular evidence supports the recognition of a new subspecies of the critically endangered Pityrodia scabra (Lamiaceae). Aust. Syst.Bot, 26: 1-12. DOI:10.1071/SB12009 |
Shepherd K.A., Thiele K.R., Sampson J., Coates D., Byrne M., 2015. A rare, new species of Atriplex (Chenopodiaceae) comprising two genetically distinct but morphologically cryptic populations in arid Western Australia:implications for taxonomy and conservation. Aust. Syst. Bot, 28: 234-245. DOI:10.1071/SB15029 |
Smith, M., Jones, A., 2018. Department of Biodiversity, Conservation and Attractions Threatened and Priority Flora List 16 January 2018. Perth, Western Australia.
|
Steane D.A., Potts B.M., Mclean E., Collins L., Prober S.M., Stock W.D., Vaillancourt R.E., Byrne M., 2015. Genome-wide scans reveal cryptic population structure in a dry-adapted eucalypt. Tree Genet. Genomes, 11: 33. DOI:10.1007/s11295-015-0864-z |
Tapper S.-L., Byrne M., Yates C.J., Keppel G., Hopper S.D., Van Niel K., Schut A.G.T., Mucina L., Wardell-Johnson G.W., 2014a. Isolated with persistence or dynamically connected? Genetic patterns in a common granite outcrop endemic. Divers. Distrib, 20: 987-1001. DOI:10.1111/ddi.2014.20.issue-9 |
Tapper S.-L., Byrne M., Yates C.J., Keppel G., Hopper S.D., Van Niel K., Schut A.G.T., Mucina L., Wardell-Johnson G.W., 2014b. Prolonged isolation and persistence of a common endemic on granite outcrops in both mesic and semi-arid environments in south-western Australia. J. Biogeogr, 41: 2032-2044. DOI:10.1111/jbi.2014.41.issue-11 |
Underwood E.C., Viers J.H., Klausmeyer K.R., Cox R.L., Shaw M.R., 2009. Threats and biodiversity in the mediterranean Biome. Divers. Distrib, 15: 188-197. DOI:10.1111/ddi.2009.15.issue-2 |
Vallee, L., Hogbin, T., Monks, L., Makinson, B., Matthes, M., Rossetto, M., 2004. Guidelines for the Translocation of Threatened Plants in Australia, second ed.Australian Network for Plant Conservation, Canberra, Australia.
|
Vogiatzakis I.N., Mannion A.M., Griffiths G.H., 2006. Mediterranean ecosystems:problems and tools for conservation. Prog. Phys. Geogr, 30: 175-200. DOI:10.1191/0309133306pp472ra |
Volis S., 2017. Complementaries of two existing intermediate conservation approaches. Plant Divers, 39: 379-382. DOI:10.1016/j.pld.2017.10.005 |
Walker E., Byrne M., Macdonald B., Nicolle D., McComb J., 2009. Clonality and hybrid origin of the rare Eucalyptus bennettiae (Myrtaceae) in Western Australia. Aust. J. Bot, 57: 180-188. DOI:10.1071/BT08148 |
Wallace, K., Connell, K., Vogwill, R., Edgely, M., Hearn, R., Huston, R., Lacey, P., Massenbauer, T., Mullan, G., Nicholson, N., 2011. Natural Diversity Recovery Catchment Program: 2010 Review. Department of Environment and Conservation, Perth, Western Australia.
|
Wege J.A., Thiele K.R., Shepherd K.A., Butcher R., Macfarlane T.D., Coates D.J., 2015. Strategic taxonomy in a biodiverse landscape:a novel approach to maximizing conservation outcomes for rare and poorly known flora. Biodivers.Conserv, 24: 17-32. DOI:10.1007/s10531-014-0785-4 |
Western Australian Herbarium, 2018. Western Australian Herbarium Database, Department of Biodiversity, Conservation and Attractions. (Accessed 6 February 2018).
|
Yates C.J., Broadhurst L.M., 2002. Assessing limitations on population growth in two critically endangered Acacia taxa. Biol. Conserv, 108: 13-26. DOI:10.1016/S0006-3207(02)00084-8 |
Yates C.J., Ladd P.G., 2005. Relative importance of reproductive biology and establishment ecology for persistence of a rare shrub in a fragmented landscape. Conserv. Biol, 19: 239-249. DOI:10.1111/cbi.2005.19.issue-1 |
Yates C.J., Ladd P.G., 2010. Using population viability analysis to predict the effect of fire on the extinction risk of an endangered shrub Verticordia fimbrilepis subsp. fimbrilepis in a fragmented landscape. Plant Ecol, 211: 305-319. DOI:10.1007/s11258-010-9791-0 |
Yates C.J., Hobbs R.J., Atkins L., 2000. Establishment of perennial shrub and tree species in degraded Eucalyptus salmonophloia (Salmon Gum) remnant woodlands:effects of restoration treatment. Restor. Ecol, 8: 135-143. DOI:10.1046/j.1526-100x.2000.80020.x |
Yates, C.J., Abbott, I., Hopper, S.D., Coates, D.J., 2003. Fire as a determinant of rarity in the south-west Western Australian global biodiversity hotspot. In: Abbott, I., Burrows, N. (Eds.), Fire in Ecosystems of South-west Western Australia: Impacts and Management. Backhuys Publishers, Leiden, pp. 395-420.
|
Yates C.J., Coates D.J., Elliott C., Byrne M., 2007a. Composition of the pollinator community, pollination and the mating system variation for a shrub in fragments of species rich kwongon in south-west Western Australia. Biodivers.Conserv, 16: 1379-1395. DOI:10.1007/s10531-006-6736-y |
Yates C.J., Ladd P.G., Coates D.J., McArthur S., 2007b. Hierarchies of cause:understanding rarity in an endemic shrub Verticordia staminosa (Myrtaceae) with a highly restricted distribution. Aust. J. Bot, 55: 194-205. DOI:10.1071/BT06032 |
Yates C.J., McNeill A., Elith J., Midgley G.F., 2010. Assessing the impacts of climate change and land transformation on Banksia in the Southwest Australian floristic region. Divers. Distrib, 16: 187-201. DOI:10.1111/j.1472-4642.2009.00623.x |



