It was recognized as early as the 1990s that legal land protection is only the first step in securing threatened species by removing the immediate and most detrimental threats, but is by no means a guarantee of the long-term species survival (Possiel et al., 1995; Maxted et al., 1997; Dopson et al., 1999). In the last two decades, it became clear that mere designation of protected areas, which has been the primary approach to conserving biodiversity, is not enough to protect biodiversity (e.g. Brashares et al., 2001; Clark et al., 2013; Havens et al., 2014; Bridgewater, 2016; Heywood, 2016, 2017, 2018). No conservationist will cast doubt on vital importance of protected areas in biodiversity conservation. Without protection from logging, grazing, poaching and other factors detrimental to maintaining the effective functioning of natural habitats, the extinction of a large number of species is inevitable. However, reliance on passive conservation through strict area protection that prohibits any modifications of the protected habitat of threatened species or other management interventions to address threats to them is not a viable strategy for several reasons. One reason why passive protection alone will fail to prevent species loss is the ongoing climate change. For species with small populations scattered in fragmented landscapes, occupying rare habitat types or having limited dispersal abilities, the anticipated range shifts as a result of climate change can be lethal. Even if their populations are currently protected, such species are doomed to extinction, unless some additional measures to adjust their range are taken. Another, even more important reason is the wide-scale anthropogenic disturbance that affects virtually all ecosystems, including those in protected areas, and disrupts previously existed species interactions and ecological processes (e.g., Chapman et al., 2010). Habitat alterations and fragmentation that result from this disturbance, can reduce population sizes of many species below the viability threshold or make regeneration impossible. If some individuals of a species still survive in the habitat, this indicates that recovery at that location is possible in principle, but only if the factors contributing to the population's decline and failure to regenerate are identified and addressed. Elimination of these factors in majority of situations is impossible without management interventions. These interventions must either maintain crucial ecosystems' dynamic processes such as succession, or remove the dispersal and establishment limitations responsible for the commonly observed extinction debt phenomenon (see below) in many populations of threatened species, even in strictly protected areas.
Given the lack of regeneration in many populations of threatened species, even in reserves and national parks, passive area protection must be replaced with in situ conservation, including land protection but not limited to it, and having a strong emphasis on interventions that help restore species recruitment. The major step in any species recovery program must be determining which factors, either physical or biological, act as "barriers" as well as those that can be "facilitators" to regeneration. Once these factors are identified, this knowledge can be used for either ⅰ) limiting population augmentation by seeding/planting to the suitable microsites only, thereby greatly reducing the amount of valuable plant material needed and the cost of recovery, or ⅱ) applying the most appropriate treatments that would eliminate the barriers, enforce the facilitating factors, or both.
2. Extinction debt and causes of recruitment failureThe natural regeneration cycle includes a sequence of stages, starting with the flowering of a mature adult and ending with the establishment of a new reproductive individual. Among the events associated with these stages, several are of a decisive importance for long-term population viability. They include processes of pollen production and flower fertilization, seed development, arrival at safe sites and germination of seeds, seedling survival and establishment (Grubb, 1977; Eriksson and Ehrlen, 1992; Fischer et al., 2016). Collectively, species' requirements for successful completion of a regeneration cycle are known as the species' ontogenetic niche. Decoupling the environmental conditions and the species ontogenetic niche can lead to recruitment failure. Restoring recruitment requires removing the obstacles to seed production and their successful germination and growth (e.g., lack of pollinators, pre- or post-dispersal seed predation, lack of germination cues, altered/unfavourable canopy or substrate conditions).
Usually, adverse human impact on an ecosystem starts from the destruction of species regeneration niches, when seed production/ dispersal is no longer possible due to extirpation of pollinators/seed dispersers, seeds do not germinate or seedlings die in altered soil or light conditions. For many plant species, the particular set of conditions favoring seedlings is much narrower than at the adult stage, and this explains why under the altered conditions, many longliving species can temporarily persist as non-recruiting adults ("living dead" sensu Janzen, 2001). This phenomenon when declining populations can temporarily persist but will eventually go extinct in degraded habitats even under no further habitat deterioration has been described as 'extinction debt' (Tilman et al., 1994). This phenomenon occurs not only with long-living plant species but with any species following alteration or fragmentation of its habitat if a threshold in habitat quality, area and connectivity is crossed (Hanski and Ovaskainen, 2002).
Two causes of extinction debt phenomenon, i.e. disrupted recruitment, are (ⅰ) seed limitation, i.e. the failure of seeds to arrive at available sites, or (ⅱ) establishment limitation, i.e the failure of seeds to germinate or to develop into a reproducing individual (Eriksson and Ehrlen, 1992; Clark et al., 1998, 1999). The importance of seed limitation is obvious - natural regeneration can occur only if the seeds are produced, dispersed and able to germinate. If no seedlings are observed in a population, before testing whether the germination conditions are met, we must ensure that the seeds are produced and not consumed by predators before they can germinate, and remain viable at least some time after dispersal. Anthropogenic alterations of habitats can be a cause of seed limitations. A transition of continuously distributed population to spatially isolated small patches of plants may have numerous negative demographic, ecological and genetic consequences. Degradation and fragmentation of habitats may result in reduced density and fecundity of parent trees (Ghazoul et al., 1998), decreased pollinator abundance, visitation rates and pollen deposition (Jennersten, 1988; Aizen and Feinsinger, 1994; Cunningham, 2000; Quesada et al., 2003; Montero-Castano and Vila, 2012), and impeded dispersal following frugivores' decimation (Stoner et al., 2007; Terborgh et al., 2008).
Establishment limitation is another common cause of a recruitment failure. Anthropogenic alterations of habitat physicoechemical parameters, such as soil moisture, nutrients or light availability can cause negative changes in biotic interactions with predators, pathogens or competitors, and disappearance or reduction in suitability of required for seed germination and seedling establishment microsites. Even within a critical habitat, i.e. "the specific areas…on which are found those physical or biological features essential to the conservation of the species" (Endangered Species Act of 1973), degree of habitat suitability at a small scale varies, and only a subset of the microsites available can support germination and plant establishment ("safe sites" sensu Harper et al., 1961; Fowler, 1988). Topography, soil moisture, presence/absence of litter and symbiotic soil biota are the major factors determining microsite suitability. Spatial heterogeneity generated by canopy openings is another important component of species regeneration niche. As a result of degradation/fragmentation many habitats do not have enough microsites that can support natural species regeneration.
3. Two ways of extending recruitment nicheFor species having recruitment problems, there are three possible solutions, as outlined by Young et al. (2005): 1) One can restore the once existing population environmental conditions to repair the lost links in the recruitment chain and give the population a hope of survival (Fig. 1). 2) One can extend the dispersal niche by promoting species' dispersal followed by successful regeneration into the environments where that species had not been previously recorded (Fig. 1). 3) One can create more living dead non-recruiting populations by planting individuals in sites where seeds cannot germinate or seedlings develop but saplings and adults can thrive (Fig. 1) (Young et al. (2005). The first two ways of addressing recruitment problems can be called assisted establishment and assisted colonization, respectively. Unfortunately, in conservation projects creating more living dead is much more common outcome than successful assisted establishment/colonization, and too many introductions of rare and endangered plant species resulted in the establishment of the introduced individuals but without producing new generations (Godefroid et al., 2011; Dalrymple et al., 2012).

|
| Fig. 1 Ontogenetic niche and the possible effects of restoration activities (modified from Young et al., 2005). The x-axis is a gradient of an environmental parameter that defines niche space. Seeds can disperse to more sites than are suitable for establishment. Conversely, dispersal limitations can prevent arrival of seeds to some suitable sites. Restoration activities may broaden the recruitment niche through assisted establishment (dashed lines) or the dispersal niche through assisted colonization (dotted lines). Assisted colonization can result in establishment with regeneration or without it. In the latter case colonization cannot be considered successful |
The widespread phenomenon of extinction debt has important conservation implications. As long as a species that is predicted to become extinct still persists, we can pay the extinction debt not by allowing the species to go extinct but by restoring the habitat to assist its recruitment, before the species disappears, in such a way that the threshold conditions are met again. On the other hand, introduction of the species into the habitats outside its current range can prevent the species' global extinction even if the local extinction debt is paid. Thus, large-scale restoration of either occupied locations to enable assisted establishment or of potentially suitable but currently not occupied locations to make successful their assisted colonization, should be viewed as two complementary ways to achieve species recovery and prevent biodiversity loss. A goal of any species conservation plan must be determination of the suitable species range and safe sites within it, and, on the other hand, identification of the appropriate population maintenance or habitat restoration interventions that will insure long-term species persistence.
The two major causes of recruitment failure, seed production limitations and low availability of suitable microsites are also the two factors that limit range expansion in plants. According to the literature, up to 50% of species' current potential ranges are not filled due to the factors limiting seed availability (e.g., Primack and Miao, 1992; Ehrlen and Eriksson, 2000; Turnbull et al., 2000; Myers and Harms, 2009), while many species are absent in particular habitats because these habitats have insufficient number of microsites suitable for recruitment (Clark et al., 1999; Caspersen and Saprunoff, 2005). Additional factors that prevent or limit colonization of suitable habitats are low seed dispersal distance and physical barriers for dispersal.
The above reasons suggest that many species would expand their ranges naturally but are unable to do so due to severe constraints, some of which have been created by human-driven impacts (destruction and fragmentation of habitats followed by decreased abundance, disappearance of pollinators or seed dispersers, invasion of predators, pests or competitors).
4. Existing conservation practices and the new challenges of the AnthropoceneThe described above recruitment problems faced by many species, on one hand, and inadequacy of passive protection to address the possible solutions (i.e. assistant establishment and assistant colonization), on the other hand, call for a critical evaluation of the available conservation tools and practices. With respect to conservation of plants, what are these conservation practices? Briefly they include:
1) assessments of biodiversity summarized in IUCN species categorization and creating global, national and regional lists of threatened species;
2) global and regional prioritization of species, habitats and areas for conservation;
3) establishment of protected areas preserving natural habitats and their biota;
4) no intervention in strictly protected areas; minor interventions in less strictly protected areas usually limited to control of invasive species and prescribed burning;
5) preservation of threatened species in ex situ seed banks and botanic garden living collections with minimal coordination between ex situ and in situ actions;
6) reinforcement or reintroduction of endangered species usually conducted at single or very few locations;
7) focus in conservation plans on single species rather than on groups of species or species assemblages.
The practices listed above implicitly assume that the threatened species habitat is either pristine or almost intact. If this was the case, passive protection with minor interventions such as reinforcement/reintroduction would do the job, because the conservation biology research conducted over the last 50 years provided us with quite a good understanding of how natural ecosystems operate under either no or minimal to moderate human impact. However, rates of landscape modification, habitat fragmentation, invasions and species extinctions became so dramatic in the 21st century that few, if any, ecosystems remain untouched by the impact of human activity (Vitousek et al., 1997; Sanderson et al., 2002; Schmitt et al., 2009; Krupnick and Kress, 2003; Dirzo et al., 2014; Pimm et al., 2014). The combined effects of these processes at times exceeded the ability of ecosystems to maintain their structure and function. While the ecosystem effects of individual drivers can usually be predicted, their combinations introduce a lot of uncertainty and complexity. Not surprisingly, historically authentic, co-evolved biotic assemblages rapidly disappear, being replaced by new combinations of species living under environmental conditions that have no historical analogs (Hobbs et al., 2006, 2013).
These reasons explain why the listed above practices did not establish an efficient link between conservation biology conceptual developments and implementation of these developments in today's real-world situations. Witnessing inability of the existing conservation practices to even slow down the steadily accelerating loss of species and natural habitats (Heywood, 2017, 2018) we need a new approach that would reconcile these practicies with the new world realities, when virtually no habitat is intact, and designated for protection habitats are so few, small, poorly connected and degraded that their passive protection can not preserve biodiversity.
Such a creative and flexible approach should allow to deal with not only pristine but altered habitats, and making possible to restore disrupted recruitment. Several authors proposed restoration of the degraded habitats as being crucial for conserving biodiversity in the 21st century (Dobson et al., 1997; Young, 2000; Burney and Burney, 2007). However, up to now, habitat restoration has been mainly considered as a prerequisite of a threatened species recovery (Birkinshaw et al., 2013) rather than an integral part of the recovery program, although examples of the latter do exist (Foin et al., 1998; Chen, 2001).
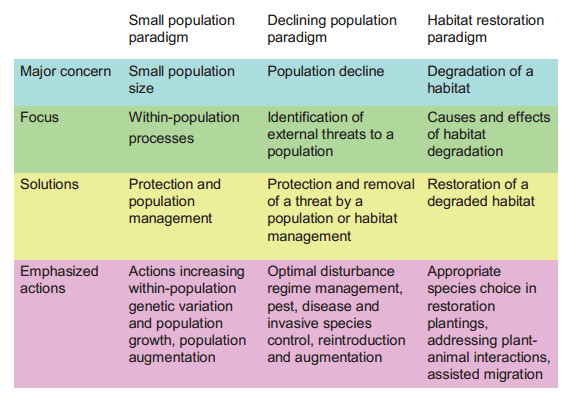
5. Conservation-oriented restorationI propose that efficient conservation of biodiversity can be achieved only by applying interventions to partly degraded habitats designated as target areas for either assisted establishment or assisted colonization. The new concept called conservationoriented restoration (Volis, 2016a, b, 2017) proposes to make habitat restoration (instead of focal species and their populations) a focus of plant conservation. The concept adopts the idea of creating partly novel (i.e. having species compositions that differ from historical analogs) ecosystems, but with a goal of conservation of threatened species and their habitats, and not for improving wellbeing of local communities through improved ecological services. The proposed concept, making ecological restoration an integral part of conservation planning and implementation, and using threatened plant species in habitat restoration, if proved realistic and producing good results, may become a new conservation paradigm (Fig. 2). This concept appears to be adopted in recently proposed creation of "protorefuges" and "protorefugia" - "restoration sites that threatened species can be translocated to, where the restoration design can be specifically adapted to help reduce the decline of threatened species at the leading and trailing edges (respectively) of bioclimatic envelope shifts" (Braidwood et al., 2018). The major principles of conservation-oriented restoration were first outlined in Volis (2016b), and then described in more detail in Volis(2016a, 2017). Below I will summarize in a systematic manner the distinct features of the proposed concept, and place it within an existing framework of approaches dealing with human impacts which adversely affect ecosystems and their biodiversity.
|
| Fig. 2 Comparison of the proposed concept (habitat restoration paradigm) with the two existing conservation paradigms |
An idea of usage of threatened species in restoration is a cornerstone of the proposed strategy, and it has the following logic. On one hand, many threatened species will have a future only in restored habitats. On the other hand, if they belong to the functionally important plant category, a category needed to restore the ecosystem integrity, why not to use them in a form of seedlings or saplings for ecological restoration? An area to be restored can be a subject to either assisted establishment or assisted colonization depending on whether the species is still present there or has no past records of occurrence. And, to bring the idea that the threatened species can be used in restoration to a practical use, we need clear understanding of which of them, under what circumstances and how can be used for restoration.
Assisted establishment using threatened species is actually augmentation of extant populations. Introduced plants can revitalize an existing population by improving availability of mates and pollinators' visitation or decreasing genetic drift and thus its negative effects on offspring performance. Restoration of recruitment in insect pollinated species exhibiting limited seed set and spatial segregation of reproducing plants requires reinforcement of the remaining wild populations with genetically variable seedlings or saplings, planted at the distance from each other within a migrating distance of the effective pollinators. The introduced groups of plants must be sufficiently large and not too far from each other to enable inter-group gene flow. This consideration must be explicit in projects augmenting existing and especially creating new populations, because in the latter the introduced population sizes are usually small and attritions during the establishment phase inevitable. In addition to increasing availability of mates and releasing seed limitation, the plants introduced into both the currently occupied and unoccupied but suitable habitat can improve the whole ecosystem and conditions for the other species (vegetation cover, canopy structure, trophic web, mutualistic interactions) if the threatened species has a functionally important role in the ecosystem (which many threatened tree and shrub species do have). Decline in abundance/disappearance of species that are more vulnerable to habitat degradation and fragmentation often results in a dominance of just a few species that are most tolerant to anthropogenic disturbances. As dominance is related inversely to species diversity, reduction in numbers of the dominant species in favor of threatened species can be a way to preserve both high species diversity and the threatened species themselves. Liberating suppressed juveniles or replacement planting can be especially relevant for those threatened species that are poor competitors and outcompeted by aggressive fast growing and dispersal-efficient dominant species.
For assisted colonization, the following considerations should be relevant. Many of the currently rare species are likely to be "anthropogenic rarities" (Fiedler and Laven, 1996). These species could have become threatened as a result of higher vulnerability in comparison with other species to alteration of once existing habitats and biotic interactions, with their current range being considerably smaller than one in the past. Species which decline and suffer range contraction due to extrinsic factors (e.g. invasion of non-native species, livestock grazing, fire suppression or land conversion), may turn out to be useful for restoration of altered or partly degraded habitats outside their current range. Moreover, uncertainty about a cause of rareness for many threatened species, but with a high probability that they are anthropogenic rarities, allows substantial broadening of lists of candidate species for habitat restoration with threatened species. Threatened species with small populations, highly fragmented ranges, long generation cycles, limited adaptation and migration capacities, regeneration problems or suffering declines due to introduced pests, diseases or invasive competitors should be first priority candidates for assisted colonization (Gallagher et al., 2015). This can be recommended regardless of "whether this is from the perspective of a habitat being restored or a species being conserved, within and beyond their current known range" (Vitt et al., 2016). Some of these species, after introduction into numerous apparently suitable locations within their potential distribution range, can become common or even dominant species in some of the restored ecosystems.
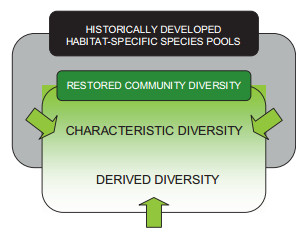
Compiling species lists for conservation-oriented restoration can be based on the concept of characteristic and derived diversity (Helm et al., 2015). According to Helm et al. (2015) the observed in a community diversity represents two species pools of different historical backgrounds. The first one, called characteristic diversity, consists of species belonging to habitat-specific regional species pool, and the second one, called derived diversity, represents species not typical to a given community (region) and whose presence is due to intended or unintended human impact. Although majority of the candidate species will be from the first group, some threatened and functionally important species can be from the second group (Fig. 3). All the species from the regional species pool absent in the characteristic diversity pool can be considered representatives of 'dark diversity' group sensu Partel et al. (2011), the set of species in a region that currently do not inhabit a site due to dispersal or establishment limitations. The latter can be a result of human-caused alteration of abiotic and biotic conditions. Assisted colonization of the species from the regional species pool can convert dark diversity into characteristic diversity.
|
| Fig. 3 A concept of characteristic and derived diversity applied to conservationoriented restoration (modified from Helm et al., 2015). A restored community diversity (smaller square) will consist of characteristic and derived diversity, the former being species that belong to habitat-specific species pool (larger square), and the latter representing species from other species pools. The appropriate candidates from the second group must be threatened and functionally important species able to establish and reproduce under environmental conditions that are typical of the particular habitat |
Comparisons or rare species with congeneric common species show similar fecundity and germination rates (Carlsen et al., 2002; Fu et al., 2009), as well as distributions of individuals among size classes (Byers and Meagher, 1997; Kelly et al., 2001). Available literature shows suitability and similar prospects of the establishment of threatened plant species in comparison with nonthreatened species in restoration projects (Morgan, 1999; Shono et al., 2007; Cordell et al., 2008; Millet et al., 2013; Schneider et al., 2014; Subiakto et al., 2016).
The reasons for limited use of threatened species in restoration projects include a requirement of large seed quantities and a lack of knowledge of the species' reproductive biology and efficient methods of propagation and planting. Even for non threatened species it is often not possible to provide enough seed or other propagules for recovery let alone conservation restoration. And for successful establishment of planted individuals it is vital to have the knowledge about propagation by seeds or cuttings, and ideal individual size and time for planting. The problem of propagule supply can be efficiently solved by the quasi in situ living collections as explained below, and the crucial knowledge about species propagation, although still very limited, is steadily accumulating for rare and endangered species (e.g. Iturriaga et al., 1994; Sakai et al., 2002; Danthu et al., 2008; De Motta, 2010; Herranz et al., 2010; Kay et al., 2011; Ratnamhin et al., 2011; Koch and Kollmann, 2012; Castellanos-Castro and Bonfil, 2013; Gratzfeld et al., 2015; Lu et al., 2016). Once the necessary knowledge is acquired and protocols are available, comparable to those for common species, the cost per seedling will make restoration practitioners more likely to incorporate rare and threatened species into their plans (Rodrigues et al., 2011), because availability of seedlings rather than the cost of a seedling per added species is an obstacle to planting highdiversity species pools (Aronson et al., 2011).
5.2. Importance of experimentation in assisted establishment and colonizationAccepting that active intervention is a necessary part of modern plant conservation, we must recognize experimentation as its major tool for a number of reasons.
Experimentation is needed for efficient management of protected areas. For some areas, especially those that harbor critically endangered species with no viable relocation options, it would be desirable to apply a variety of experimental treatments among and within protected areas, so that some areas remain untouched while others are managed. Comparison of the outcomes will make it possible to identify the best treatment to facilitate the transition of ecosystems along desired trajectories (Radeloff et al., 2015).
Management of protected areas may include creation of favorable microsites for the target species. Some habitats, e.g. calcareous grasslands or desertified shrublands suffer from micro-site limitation (Shachak et al., 1998; Wagner et al., 2016). However, artificial creation of such micro-sites has a lot of uncertainty because the optimal levels of required intervention differ among species. Some species prefer drier micro-sites with strongly reduced competition, whereas others, whose seedlings are more vulnerable to desiccation, may prefer wetter sites. Thus different types of disturbance of varying intensity need to be tested for working out the optimal ones required to create suitable conditions for the establishment of introduced species.
Experimentation is also needed for mitigating climate change effects. Potential species niche can be determined through species distribution modeling and used to predict the anticipated range shifts. However, only experimentation will allow identifying those locations where the species can establish viable populations because no modeling can predict presence of suitable microsites, mutualistic biota or detrimental herbivores. Because responses to climate are usually species-specific, climatic changes will result in complex and difficult-to-predict novel species combinations. As a result of range shifts and competition, species with previously non-overlapping ranges under new conditions will reassemble into presently not existing communities and ecosystems (Williams and Jackson, 2007; Hobbs et al., 2009; Gilman et al., 2010). While many of these new ecosystems will be unsuitable for imperiled species, in some of them they may find a new home. For those threatened species for which the once existing habitat has been altered throughout the species range and therefore the reference habitat does not exist, and whose abiotic/biotic requirements are poorly understood, there is no alternative to limited scale translocation trials (Roncal et al., 2012; Wendelberger and Maschinski, 2016; Menges et al., 2016; Volis, 2016d; Vitt et al. 2016). Such introduction over a range of environments rather than within the historical distribution or single "preferred habitat" should increase chances of successful establishment.
Large-scale restoration projects, particularly when using rare and threatened species with limited seed availability, should always be preceded by experiments investigating species- and treatment-specific responses (Cabin et al., 2002; Brudvig et al., 2017). This can be done by establishing mosaics of replicated treatments within mosaics of habitats (Howe and Martinez-Garza, 2014). This approach is an alternative to the commonly applied 'best available practice' of establishing a single combination of a limited number of plant species. Created in this way, plant communities are expected to differ in species composition among the introduction micro-sites, mostly in presence and abundance of rare species, and be potential sources of colonization for each other. The experimental approach is highly relevant to restoration whose goal is to rehabilitate existing habitats or create new habitats for threatened species because introductions of such species in general have a low chance of success (Maunder, 1992; Seddon et al., 2007; Godefroid et al., 2011; Dalrymple et al., 2012; Drayton and Primack, 2012). Thus, broadening the list of species introduced in different combinations and treatments (Howe and Martinez-Garza, 2014), and replicating introduced populations over time and space (Guerrant, 1996; Dani Sanchez et al., 2018) is a way to maximize likelihood of reintroduction success.
5.3. Integration of ex situ and in situ strategiesNot only assisted colonization but in many cases also assisted establishment require introducing plant material, predominantly in a form of seedlings or saplings. Plant germplasm maintained and propagated ex situ can be used for this purpose. The potential of ex situ collections for in situ actions via storing and propagating plant material has been recognized for a long time (Cugnac, 1953; Raven, 1981) but obvious space and other limitations of ex situ collections for achieving this are also well known (Simmons et al., 1976; Hamilton, 1994 Schoen and Brown, 2001; Maunder et al., 2004; Volis and Blecher, 2010). This motivated search for an approach bridging ex situ and in situ through creating living collections of needed capacity outside botanic gardens and arboreta. For example, the idea of establishing botanic garden facilities within protected areas was briefly mentioned in Maunder et al. (2001) but not explained. There are many examples of botanic gardens established in natural vegetation communities in different biogeographic zones, some in protected areas (Heywood, 2015), but their role is mainly limited to study, cultivation and display of the local flora including rare and endangered plant species, i.e. to ex situ conservation per se.
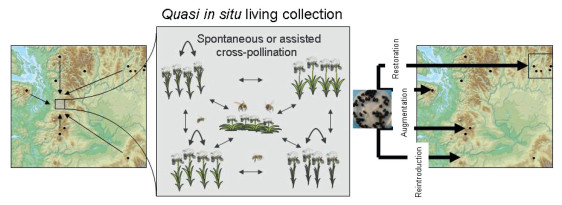
More recently, a concept called quasi in situ has been introduced. This concept proposes the creation of depositories of genetically variable source material for species of conservation concern as living plants maintained under natural or close to natural conditions (Volis and Blecher, 2010; Volis, 2016d) (Fig. 4). The quasi in situ site maintains a living collection of individuals from populations sharing the same climatic zone and biotic/abiotic environment, has natural or semi-natural conditions and is legally protected. Besides preserving species genetic variation, quasi in situ living collections can be a reliable source of seeds for in situ conservation and restoration projects. Seed banks cannot fulfill this task due to space limitations and problems with storing non-orthodox seeds, while collecting large quantities of seeds in natural populations is undesirable because of negative impacts of seed harvesting on local population dynamics (Broadhurst et al., 2008). It is often a problem for the nurseries producing seedlings of rare and threatened species to plan the numbers of seedlings that might be produced with certainty because of low predictability of seed production in many such species (Shaw, 2018). For the species which seed output is low, varies greatly between years, or reproducing adults in natural populations are very few and far apart, quasi in situ collections can be a solution because in these collections cross-pollination of plants will result in production of healthy, showing good performance offspring (Volis, 2016c). These offspring can be produced at no or very low cost and collected in large quantities needed for reintroduction or conservation-oriented restoration to be successful.
|
| Fig. 4 Role of quasi in situ living collections in conservation-oriented restoration. A quasi in situ collection (grey rectangle) is established in a protected location having the same environmental conditions with the extant species populations (circles in red). Seeds that result from spontaneous or assisted cross-pollination within a quasi in situ site are used to produce the outplants for in situ actions. |
Habitat protection has a crucial role in plant conservation because legal protection prohibits activities that can damage, destroy or modify the natural habitats. However, protection does not guarantee a halt to further degradation of the habitat and species loss. The latter often is impossible without well-organized interventions and clear recovery criteria to follow. These interventions are possible only if they are allowed by the protection status of the target site. However, many of the interventions required (e.g. introduction of a suite of functionally important for the ecosystem species, creation of deadwood, thinning of pioneer in favor of late-successional tree species, liberation of juveniles of threatened species from competing vegetation, and various forms of translocation) are not allowed in strictly protected areas (Categories Ⅰ-Ⅱ) (Dudley, 2008).
To make implementation of conservation-oriented restoration possible, the current definitions of these categories must be changed to permit management through active interventions while forbidding any unauthorized activities. Second, introduction of critically endangered species based on predictions of species distribution modeling, into these areas should be allowed even if there are no records of their past occurrence.
5.5. Conservation-oriented restoration vs. species-targeted conservation and traditional restorationA focus on improving a habitat in which degradation is a cause of the population decline rather than on a population itself through various forms of interventions distinguishes the proposed concept from conservation which focusers on particular species (speciestargeted conservation), and makes it similar to a particular form of ecological restoration called 'restoration sensu stricto' (Aronson et al., 1993; Jackson et al., 1995). The aim of the latter is not reclamation of severely or completely degraded habitats when the whole ecosystem must be recreated or altered to a desirable state but restoring desired species composition, structure and processes in a degraded but still functioning ecosystem. What makes the proposed concept different from 'restoration sensu stricto' is the wide utilization of threatened plant species in restoration, as discussed above.
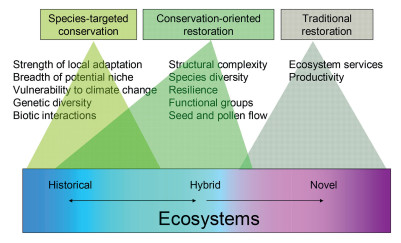
The new concept is called conservation-oriented restoration to distinguish it from both, having more utilitarian goals traditional restoration and focusing on particular species species-targeted conservation. Conservation-oriented restoration should not be seen as an alternative to them but as a complement because these three approaches differ not only in broadly defined goals and attributes of their targets, but also in the types of ecosystems they are applicable to (Fig. 5).
|
| Fig. 5 Three approaches to conservation of species and ecosystems, attributes of their targets on which the approach actions are focused, and the range of ecosystem degradation they can be successfully applied to (terms are sensu Hobbs et al., 2013) |
Hobbs et al. (2013) distinguished historical ecosystems, which remain within their historical range of variability, hybrid ecosystems, where the changes are reversible, and novel ecosystems, where the changes are irreversible. Despite some overlap (Fig. 5), traditional restoration predominantly focuses on novel ecosystems, while species-targeted conservation focuses on historical ecosystems, and conservation-oriented restoration on hybrid ones. The explicit conservation goals of conservation-oriented restoration are to boost the naturally occurring succession, restore some missing compositional or functional elements and bring the ecosystem back to the historical state. Therefore it can be applied to the hybrid but not the novel ecosystem because only the former can be returned to the historical state. The historical state does not mean a single set of abiotic and biotic conditions which in restoration is called a reference. Rather it is a range of variation in such conditions accounting for patch dynamics and physical site heterogeneity (Clewell and Rieger, 1997; Landres et al., 1999), and multiple possible trajectories (Holl and Cairns, 2002; Suding et al., 2004; Falk et al., 2006; Balaguer et al., 2014).
While in traditional restoration the priority areas for restoration are the most degraded ones, in conservation-oriented restoration it is just the opposite - the least altered habitats, which still need interventions to restore altered system properties but have a reasonable chance of approaching a historical ecosystem. In these areas, removal of threats (e.g. prohibition of grazing and logging) can lead to spontaneous recovery of a degraded system, but, as a rule, a successional change will be too slow or not to a desired state without crucial interventions (e.g. thinning or plant/animal introductions) (Benayas et al., 2008). Restoration of these systems' structure and missing functions is often impossible without enrichment with latesuccessional species and absent functional groups, for example species with diverse flower and fruit types that support diverse pollinator and frugivorous fauna. Instead of using widespread, often exotic and even invasive species, as usually is done in traditional restoration, conservation-oriented restoration should utilize functionally equivalent threatened species that became rare due to disappearance and fragmentation of once existing habitats.
Other features that make a site an appropriate target for conservation-oriented restoration and distinguish it from traditional restoration are high total forest cover remaining in the landscape and legal protection status. If the habitat is unprotected or represents a small habitat fragment surrounded by hostile human-utilized environment the interventions will make little sense. The restored but unprotected and easily access habitat will almost certainly become once again a victim of human destructive utilization.
The major features of the proposed concept presented and discussed above will be developed further and more in depth from the results of studies applying this concept in real settings.
There is no conflict of interests.
No grant supported this study. I am grateful to David Gill and Vernon Heywood for constructive comments on an earlier version of the manuscript.
Aizen M.A., Feinsinger P., 1994. Habitat fragmentation, native insect pollinators, and feral honey bees in Argentine "Chaco Serrano". Ecol. Appl, 4: 378-392. DOI:10.2307/1941941 |
Aronson J., et al, 2011. What role should government regulation play in ecological restoration? Ongoing debate in Sao Paulo state, Brazil. Restor. Ecol, 19: 690-695. DOI:10.1111/rec.2011.19.issue-6 |
Aronson J., et al, 1993. Restoration and rehabilitation of degraded ecosystems in arid and semi-arid lands. I. A view from the south. Restor. Ecol, 1: 8-17. DOI:10.1111/rec.1993.1.issue-1 |
Balaguer L., et al, 2014. The historical reference in restoration ecology:Re-defining a cornerstone concept. Biol. Conserv, 176: 12-20. DOI:10.1016/j.biocon.2014.05.007 |
Benayas J.M.R., et al, 2008. Creating woodland islets to reconcile ecological restoration, conservation, and agricultural land use. Front. Ecol. Environ, 6: 329-336. DOI:10.1890/070057 |
Birkinshaw C., et al, 2013. Supporting target 4 of the global strategy for plant conservation by integrating ecological restoration into the Missouri Botanical Garden's conservation program in Madagascar. Ann. Mo. Bot. Gard, 99: 139-146. DOI:10.3417/2012002 |
Braidwood D.W., et al, 2018. Translocations, conservation, and climate change:use of restoration sites as protorefuges and protorefugia. Restor. Ecol, 26: 20-28. DOI:10.1111/rec.12642 |
Brashares J.S., et al, 2001. Human demography and reserve size predict wildlife extinction in West Africa. Proc. R. Soc. Lond. Ser. B, B 268: 1-6. |
Bridgewater P., 2016. The Anthropocene biosphere:do threatened species, Red Lists, and protected areas have a future role in nature conservation? Biodivers. Conserv, 25: 603-607. |
Broadhurst L.M., et al, 2008. Seed supply for broadscale restoration:maximizing evolutionary potential. Evol. Appl, 1: 587-597. |
Brudvig L.A., et al, 2017. Interpreting variation to advance predictive restoration science. J. Appl. Ecol, 54: 1018-1027. DOI:10.1111/jpe.2017.54.issue-4 |
Burney D.A., Burney L.P., 2007. Paleoecology and "inter-situ" restoration on Kauai, Hawaii. Front. Ecol. Environ, 5: 483-490. DOI:10.1890/070051 |
Byers D.L., Meagher T.R., 1997. A comparison of demographic characteristics in a rare and a common species of Eupatorium. Ecol. Appl, 7: 519-530. DOI:10.1890/1051-0761(1997)007[0519:ACODCI]2.0.CO;2 |
Cabin R.J., et al, 2002. Effects of light, alien grass, and native species additions on Hawaiian dry forest restoration. Ecol. Appl, 12: 1595-1610. DOI:10.1890/1051-0761(2002)012[1595:EOLAGA]2.0.CO;2 |
Carlsen T.M., et al, 2002. Reproductive ecology and the persistence of an endangered plant. Biodivers. Conserv, 11: 1247-1268. DOI:10.1023/A:1016066618824 |
Caspersen J.P., Saprunoff M., 2005. Seedling recruitment in a northern temperate forest:the relative importance of supply and establishment limitation. Can. J.For. Res, 35: 978-989. DOI:10.1139/x05-024 |
Castellanos-Castro C., Bonfil C., 2013. Propagation of three Bursera species from cuttings. Bot. Sci, 91: 217-224. |
Chapman C.A., et al, 2010. Tropical tree community shifts:implications for wildlife conservation. Biol. Conserv, 143: 366-374. DOI:10.1016/j.biocon.2009.10.023 |
Chen L.Y., 2001. Cost savings from properly managing endangered species habitats. Nat. Area J, 21: 197-203. |
Clark J.S., et al, 1999. Interpreting recruitment limitation in forests. Am. J. Bot, 86: 1-16. DOI:10.2307/2656950 |
Clark J.S., et al, 1998. Stages and spatial scales of recruitment limitation in southern Appalachian forests. Ecol. Monogr, 68: 213-235. DOI:10.1890/0012-9615(1998)068[0213:SASSOR]2.0.CO;2 |
Clark N.E., et al, 2013. Protected areas in South Asia have not prevented habitat loss:a study using historical models of land-use change. PLoS One, 8: e65298. DOI:10.1371/journal.pone.0065298 |
Clewell A., Rieger J.P., 1997. What practitioners need from restoration ecologists. Restor. Ecol, 5: 350-354. DOI:10.1046/j.1526-100X.1997.00548.x |
Cordell S., et al, 2008. Towards restoration of Hawaiian tropical dry forests:the Kaupulehu outplanting programme. Pac. Conserv. Biol, 14: 279-284. DOI:10.1071/PC080279 |
Cugnac A.d., 1953. Le role des jardines botaniques pour la conservation des especes menacees de disparition ou d'alteration. Annales de Biologie, 29: 361-367. |
Cunningham S.A., 2000. Depressed pollination in habitat fragments causes low fruit set. Proc. Biol. Sci, 267: 1149-1152. DOI:10.1098/rspb.2000.1121 |
Dalrymple, S.E., et al., 2012. A meta-analysis of threatened plant reintroductions from across the globe. In: Maschinski, J., Haskins, E.H. (Eds.), Plant Reintroduction in a Changing Climate: Promises and Perils. Island Press, Washington, D.C., pp. 31-50
|
Dani Sanchez M., et al, 2018. Conserving and restoring the Caicos pine forests-the first decade. Plant Divers, 41: 75-83. |
Danthu P., et al, 2008. Seasonal dependence of rooting success in cuttings from natural forest trees in Madagascar. Agrofor. Syst, 73: 47-53. DOI:10.1007/s10457-008-9116-7 |
De Motta M.J., 2010. A history of Hawaiian plant propagation. Sibbaldia, 8: 31-43. |
Dirzo R., et al, 2014. Defaunation in the anthropocene. Science, 345: 401-406. DOI:10.1126/science.1251817 |
Dobson A.P., et al, 1997. Hopes for the future:restoration ecology and conservation biology. Science, 277: 515-522. DOI:10.1126/science.277.5325.515 |
Dopson, S.R., et al., 1999. The Conservation Requirements of New Zealand's Nationally Threatened Vascular Plants. Threatened Species Occasional Publication No. 13. New Zealand Biodiversity Recovery Unit Department of Conservation, Wellington.
|
Drayton B., Primack R.B., 2012. Success rates for reintroductions of eight perennial plant species after 15 years. Restor. Ecol, 20: 299-303. DOI:10.1111/rec.2012.20.issue-3 |
Dudley, N., 2008. Guidelines for applying protected area management categories.IUCN, Gland, Switzerland.
|
Ehrlen J., Eriksson O., 2000. Dispersal limitation and patch occupancy in forest herbs. Ecology, 81: 1667-1674. DOI:10.1890/0012-9658(2000)081[1667:DLAPOI]2.0.CO;2 |
Eriksson O., Ehrlen J., 1992. Seed and microsite limitation of recruitment in plant populations. Oecologia, 91: 360-364. DOI:10.1007/BF00317624 |
Falk, D.A., et al. (Eds.), 2006. Foundations of Restoration Ecology. Island Press, Washington, DC.
|
Fiedler, P.L., Laven, R.D., 1996. Selecting reintroduction sites. In: Falk, D.A., et al.(Eds.), Restoring Diversity: Strategies for Reintroduction of Endangered Plants.Island Press, Washington, DC, pp. 157-170.
|
Fischer, H., et al., 2016. Developing restoration strategies for temperate forests using natural regeneration processes. In: Stanturf, J.A. (Ed.), Restoration of Boreal and Temperate Forests, second ed. CRC Press, Florida, Estados Unidos, pp. 103-164.
|
Foin T.C., et al, 1998. Improving recovery planning for threatened and endangered species. Bioscience, 48: 177-184. DOI:10.2307/1313263 |
Fowler N.L., 1988. What is a safe site-neighbor, litter, germination date, and patch effects. Ecology, 69: 947-961. DOI:10.2307/1941250 |
Fu Y., et al, 2009. Comparison of seed modality and germination charateristics of compare of Manglietia hookeri and endangered M. grandis. J. NW. For. Coll, 24: 33-37. |
Gallagher R.V., et al, 2015. Assisted colonization as a climate change adaptation tool. Austral Ecol, 40: 12-20. DOI:10.1111/aec.2015.40.issue-1 |
Ghazoul J., et al, 1998. Disturbance-induced density-dependent seed set in Shorea siamensis (Dipterocarpaceae), a tropical forest tree. J. Ecol, 86: 462-473. DOI:10.1046/j.1365-2745.1998.00270.x |
Gilman S.E., et al, 2010. A framework for community interactions under climate change. Trends Ecol. Evol, 25: 325-331. DOI:10.1016/j.tree.2010.03.002 |
Godefroid S., et al, 2011. How successful are plant species reintroductions? Biol. Conserv, 144: 672-682. DOI:10.1016/j.biocon.2010.10.003 |
Gratzfeld J., et al, 2015. Whither rare relict trees in climate of rapid change?. BG J, 1, 2: 21-25. |
Grubb P.J., 1977. Maintenance of species-richness in plant communities - importance of regeneration niche. Biol. Rev. Camb. Philos. Soc, 52: 107-145. DOI:10.1111/brv.1977.52.issue-1 |
Guerrant, E.O.J., 1996. Designing populations: demographic, genetic and horticultural dimensions. In: Falk, D.A., et al. (Eds.), Restoring Diversity. Strategies for Reintroduction of Endangered Plants. Island Press, Washington, pp. 171-207.
|
Hamilton M.B., 1994. Ex situ conservation of wild plant species:time to reassess the genetic assumptions and implications of seed banks. Conserv. Biol, 8: 39-49. DOI:10.1046/j.1523-1739.1994.08010039.x |
Hanski I., Ovaskainen O., 2002. Extinction debt at extinction threshold. Conserv.Biol, 16: 666-673. DOI:10.1046/j.1523-1739.2002.00342.x |
Harper J.L., et al, 1961. Evolution and ecology of closely related species living in same area. Evolution, 15: 209. DOI:10.1111/evo.1961.15.issue-2 |
Havens K., et al, 2014. Getting plant conservation right (or not):the case of the United States. Int. J. Plant Sci, 175: 3-10. DOI:10.1086/674103 |
Helm A., et al, 2015. Characteristic and derived diversity:implementing the species pool concept to quantify conservation condition of habitats. Divers. Distrib, 21: 711-721. DOI:10.1111/ddi.2015.21.issue-6 |
Herranz J.M., et al, 2010. Seed germination ecology of the threatened endemic Iberian Delphinium fissum subsp sordidum (Ranunculaceae). Plant Ecol, 211: 89-106. DOI:10.1007/s11258-010-9775-0 |
Heywood V.H., 2015. Mediterranean botanic gardens and the introduction and conservation of plant diversity. Flora Mediterranea, 25: 103-114. |
Heywood V.H., 2016. In situ conservation of plant species - an unattainable goal?. Isr. J. Plant Sci, 63: 211-231. |
Heywood V.H., 2017. Plant conservation in the Anthropocene - challenges and future prospects. Plant Divers, 39: 314-330. DOI:10.1016/j.pld.2017.10.004 |
Heywood V.H., 2018. Conserving plants within and beyond protected areas-still problematic and future uncertain. Plant Divers, 41: 36-49. |
Hobbs R.J., et al, 2006. Novel ecosystems:theoretical and management aspects of the new ecological world order. Glob. Ecol. Biogeogr, 15: 1-7. DOI:10.1111/geb.2006.15.issue-1 |
Hobbs R.J., et al, 2009. Novel ecosystems:implications for conservation and restoration. Trends Ecol. Evol, 24: 599-605. DOI:10.1016/j.tree.2009.05.012 |
Hobbs, R.J., et al. (Eds.), 2013. Novel Ecosystems: Intervening in the New Ecological World Order. Wiley-Blackwell, Oxford, UK.
|
Holl, K.D., Cairns, J., 2002. Monitoring and appraisal. In: Perrow, M.R., Davy, A.J.(Eds.), Handbook of Ecological Restoration. Principles of Restoration. Cambridge University Press, Cambridge, pp. 409-432.
|
Howe H.F., Martinez-Garza C., 2014. Restoration as experiment. Bot. Sci, 92: 459-468. DOI:10.17129/botsci.146 |
Iturriaga L., et al, 1994. In vitro culture of Sophora toromiro (Papilionaceae), an endangered species. Plant Cell Tissue Organ Cult, 37: 201-204. DOI:10.1007/BF00043617 |
Jackson L.L., et al, 1995. Ecological restoration:a definition and options. Restor.Ecol, 3: 71-75. DOI:10.1111/rec.1995.3.issue-2 |
Janzen, D.H., 2001. Latent extinctions: the living dead. In: Levin, S.A. (Ed.), Encyclopedia of Biodiversity. Academic Press, NY, USA, pp. 689-699.
|
Jennersten O., 1988. Pollination in Dianthus deltoides (Caryophyllaceae):effect of habitat fragmentation on visitation and seed set. Conserv. Biol, 2: 359-366. DOI:10.1111/cbi.1988.2.issue-4 |
Kay J., et al, 2011. Palma corcho:a case study in botanic garden conservation horticulture and economics. HortTechnology, 21: 474-481. |
Kelly C.K., et al, 2001. Investigations in commonness and rarity:a comparative analysis of co-occurring, congeneric Mexican trees. Ecol. Lett, 4: 618-627. DOI:10.1046/j.1461-0248.2001.00278.x |
Koch C., Kollmann J., 2012. Clonal re-introduction of endangered plant species:the case of German false tamarisk in pre-Alpine rivers. Environ. Manag, 50: 217-225. DOI:10.1007/s00267-012-9880-z |
Krupnick G.A., Kress W.J., 2003. Hotspots and ecoregions:a test of conservation priorities using taxonomic data. Biodivers. Conserv, 12: 2237-2253. DOI:10.1023/A:1024582529645 |
Landres P.B., et al, 1999. Overview of the use of natural variability concepts in managing ecological systems. Ecol. Appl, 9: 1179-1188. |
Lu Y., et al, 2016. Propagation of native tree species to restore subtropical evergreen broad-leaved forests in SW China. Forests, 7. |
Maunder M., 1992. Plant reintroduction - an overview. Biodivers. Conserv, 1: 51-61. DOI:10.1007/BF00700250 |
Maunder M., et al, 2001. The effectiveness of botanic garden collections in supporting plant conservation:a European case study. Biodivers. Conserv, 10: 383-401. DOI:10.1023/A:1016666526878 |
Maunder, M., et al., 2004. Hybridization in ex situ plant collections: conservation concerns, liabilities, and opportunities. In: Guerrant, E.O.J., et al. (Eds.), Ex Situ Plant Conservation: Supporting Species Survival in the Wild. Island Press, Washington, pp. 325-364.
|
Maxted, N., et al., 1997. Complementary conservation strategies. In: Maxted, N., et al. (Eds.), Plant Genetic Conservation, the in Situ Approach. Chapman and Hall, London.
|
Menges E.S., et al, 2016. Adaptive introductions:how multiple experiments and comparisons to wild populations provide insights into requirements for longterm introduction success of an endangered shrub. Plant Divers, 38: 238-246. DOI:10.1016/j.pld.2016.09.004 |
Millet J., et al, 2013. Enrichment planting of native species for biodiversity conservation in a logged tree plantation in Vietnam. N. For, 44: 369-383. |
Montero-Castano A., Vila M., 2012. Impact of landscape alteration and invasions on pollinators:a meta-analysis. J. Ecol, 100: 884-893. DOI:10.1111/jec.2012.100.issue-4 |
Morgan J.W., 1999. Have tubestock plantings successfully established populations of rare grassland species into reintroduction sites in western Victoria?. Biol. Conserv, 89: 235-243. DOI:10.1016/S0006-3207(99)00014-2 |
Myers J.A., Harms K.E., 2009. Seed arrival, ecological filters, and plant species richness:a meta-analysis. Ecol. Lett, 12: 1250-1260. DOI:10.1111/ele.2009.12.issue-11 |
Partel M., et al, 2011. Dark diversity:shedding light on absent species. Trends Ecol.Evol, 26: 124-128. DOI:10.1016/j.tree.2010.12.004 |
Pimm S.L., et al, 2014. The biodiversity of species and their rates of extinction, distribution, and protection. Science, 344: 1246752. DOI:10.1126/science.1246752 |
Possiel, W., et al., 1995. Chapter 2 — in-situ conservation of biodiversity. In: Saunier, R.E., Meganck, R.A. (Eds.), Conservation of Biodiversity and the New Regional Planning. Organization of American States and the IUCN — the World Conservation Union. Department of Regional Development and Environment Executive Secretariat for Economic and Social Affairs General Secretariat, Organization of American States.
|
Primack R.B., Miao S.L., 1992. Dispersal can limit local plant distribution. Conserv.Biol, 6: 513-519. DOI:10.1046/j.1523-1739.1992.06040513.x |
Quesada M., et al, 2003. Effects of habitat disruption on the activity of nectarivorous bats (Chiroptera:Phyllostomidae) in a dry tropical forest:implications for the reproductive success of the neotropical tree Ceiba grandiflora. Oecologia, 135: 400-406. DOI:10.1007/s00442-003-1234-3 |
Radeloff V.C., et al, 2015. The rise of novelty in ecosystems. Ecol. Appl, 25: 2051-2068. DOI:10.1890/14-1781.1 |
Ratnamhin A., et al, 2011. Vegetative propagation of rare tree species for forest restoration. Chiang Mai J. Sci, 38: 306-310. |
Raven P.H., 1981. Research in botanical gardens. Botanische Jahrbücher fur Systematik. Pflanzengeschichte und Pflanzengeographie, 102: 53-72. |
Rodrigues R.R., et al, 2011. Large-scale ecological restoration of high-diversity tropical forests in SE Brazil. For. Ecol. Manag, 261: 1605-1613. DOI:10.1016/j.foreco.2010.07.005 |
Roncal J., et al, 2012. Testing appropriate habitat outside of historic range:the case of Amorpha herbacea var. crenulata (Fabaceae). J. Nat. Conserv, 20: 109-116. DOI:10.1016/j.jnc.2011.09.003 |
Sakai C., et al, 2002. Mass propagation method from the cutting of three dipterocarp species. J. For. Res, 7: 73-80. DOI:10.1007/BF02762511 |
Sanderson E.W., et al, 2002. The human footprint and the last of the wild. Bioscience, 52: 891-904. DOI:10.1641/0006-3568(2002)052[0891:THFATL]2.0.CO;2 |
Schmitt C.B., et al, 2009. Global analysis of the protection status of the world's forests. Biol. Conserv, 142: 2122-2130. DOI:10.1016/j.biocon.2009.04.012 |
Schneider T., et al, 2014. Growth performance of sixty tree species in smallholder reforestation trials on Leyte, Philippines. N. For, 45: 83-96. |
Schoen D.J., Brown A.D.H., 2001. The conservation of wild plant species in seed banks. Bioscience, 51: 960-966. DOI:10.1641/0006-3568(2001)051[0960:TCOWPS]2.0.CO;2 |
Seddon P.J., et al, 2007. Developing the science of reintroduction biology. Conserv.Biol, 21: 303-312. DOI:10.1111/cbi.2007.21.issue-2 |
Shachak M., et al, 1998. Ecosystem management of desertified shrublands in Israel. Ecosystems, 1: 475-483. DOI:10.1007/s100219900043 |
Shaw T., 2018. Species diversity in restoration plantings:important factors for increasing the diversity of threatened tree species in the restoration of the Araucaria forest ecosystem. Plant Divers, 41: 84-93. |
Shono K., et al, 2007. Performance of 45 native tree species on degraded lands in Singapore. J. Trop. For. Sci, 19: 25-34. |
Simmons, J.B., et al. (Eds.), 1976. Conservation of Threatened Plants. Plenum Pub Corp, New York and London.
|
Stoner K.E., et al, 2007. Hunting and plant community dynamics in tropical forests:a synthesis and future directions. Biotropica, 39: 385-392. DOI:10.1111/btp.2007.39.issue-3 |
Subiakto A., et al, 2016. Choosing native tree species for establishing man-made forest:a new perspective for sustainable forest management in changing world. Biodiversitas. J. Biol. Divers, 17. |
Suding K.N., et al, 2004. Alternative states and positive feedbacks in restoration ecology. Trends Ecol. Evol, 19: 46-53. DOI:10.1016/j.tree.2003.10.005 |
Terborgh J., et al, 2008. Tree recruitment in an empty forest. Ecology, 89: 1757-1768. DOI:10.1890/07-0479.1 |
Tilman D., et al, 1994. Habitat destruction and the extinction debt. Nature, 371: 65-66. DOI:10.1038/371065a0 |
Turnbull L.A., et al, 2000. Are plant populations seed-limited? A review of seed sowing experiments. Oikos, 88: 225-238. DOI:10.1034/j.1600-0706.2000.880201.x |
Vitousek P.M., et al, 1997. Human domination of Earth's ecosystems. Science, 277: 494-499. DOI:10.1126/science.277.5325.494 |
Vitt P., et al, 2016. Assisted migration as a climate change adaptation strategy:lessons from restoration and plant reintroductions. Isr. J. Plant Sci, 63: 250-261. DOI:10.1080/07929978.2016.1258258 |
Volis S., 2016a. Conservation-oriented restoration-how to make it a success? Isr. J.Plant Sci, 63: 276-296. |
Volis S., 2016b. Conservation meets restoration-rescuing threatened plant species by restoring their environments and restoring environments using threatened plant species. Isr. J. Plant Sci, 63: 262-275. DOI:10.1080/07929978.2016.1255021 |
Volis S., 2016c. How to conserve threatened Chinese species with extremely small populations?. Plant Divers, 38: 53-62. DOI:10.1016/j.pld.2016.05.004 |
Volis S., 2016d. Species-targeted plant conservation:time for conceptual integration. Isr. J. Plant Sci, 63: 232-249. |
Volis, S., 2017. Plant Conservation in the Anthropocene: Definitely Not WineWin but Maybe Not LoseeLose?, Reference Module in Earth Systems and Environmental Sciences. Elsevier.
|
Volis S., Blecher M., 2010. Quasi in situ - a bridge between ex situ and in situ conservation of plants. Biodivers. Conserv, 19: 2441-2454. DOI:10.1007/s10531-010-9849-2 |
Wagner M., et al, 2016. Creation of micro-topographic features:a new tool for introducing specialist species of calcareous grassland to restored sites?. Appl. Veg. Sci, 19: 89-100. DOI:10.1111/avsc.12198 |
Wendelberger, K.S., Maschinski, J., 2016. Assessing microsite and regeneration niche preferences through experimental reintroduction of the rare plant Tephrosia angustissima var. corallicola. Plant Ecol. 217, 155-167. http://www.researchgate.net/publication/282268648_Assessing_microsite_and_regeneration_niche_preferences_through_experimental_reintroduction_of_the_rare_plant_Tephrosia_angustissima_var._corallicola
|
Williams J.W., Jackson S.T., 2007. Novel climates, no-analog communities, and ecological surprises. Front. Ecol. Environ, 5: 475-482. DOI:10.1890/070037 |
Young T.P., 2000. Restoration ecology and conservation biology. Biol. Conserv, 92: 73-83. DOI:10.1016/S0006-3207(99)00057-9 |
Young T.P., et al, 2005. The ecology of restoration:historical links, emerging issues and unexplored realms. Ecol. Lett, 8: 662-673. DOI:10.1111/ele.2008.8.issue-6 |


