扩展功能
文章信息
- 杜琛, 陈秀娟, 侯石磊, 赵杰
- DU Chen, CHEN Xiujuan, HOU Shilei, ZHAO Jie
- 多囊卵巢综合征患者与正常排卵妇女GV期卵母细胞转录组的比较
- Comparsion of GV oocytes transcriptome between PCOS patients and normal ovulatory women
- 吉林大学学报(医学版), 2018, 44(03): 568-573
- Journal of Jilin University (Medicine Edition), 2018, 44(03): 568-573
- 10.13481/j.1671-587x.20180321
-
文章历史
- 收稿日期: 2017-08-08
多囊卵巢综合征(polycystic ovary syndrome, PCOS)是一种常见的因月经调节机制失常导致的生殖功能障碍性疾病, 是不孕的一种常见病因[1]。通过药物促排卵可增加多胎妊娠率风险至30%, 卵巢过度刺激综合征(ovarian hyper-stimulation syndrome, OHSS)的发生率高达10 %, 严重时甚至会诱发血栓栓塞或肝肾功能衰竭, 危及患者生命[2]。通过实施控制性卵巢刺激(controlled ovarian stimulation, COS)获取未成熟卵母细胞进行体外受精/卵胞浆内单精子显微注射胚胎移植(in vitro fertilization/intracytoplasmic sperm injection-embryo transfer, IVF/ICSI-ET)可治疗不孕。研究[3]显示:从PCOS患者中获取的不成熟卵母细胞数多于对照组, 且受精率较低。卵泡液微环境和颗粒细胞对卵母细胞的成熟发挥一定作用, 但在体外培养条件下去除颗粒细胞后, 卵母细胞同样可以完成减数分裂并进行早期胚胎的卵裂, 说明促进卵母细胞发育以及早期胚胎卵裂的重要遗传信息己经全部存储于卵母细胞之中。因此, 筛选具有优良发育潜能的卵母细胞是人类辅助生殖技术成功的关键。
转录组测序(transcriptome sequencing, RNA-seq)得到的序列中不含非编码和内含子序列, 可在单核苷酸水平研究基因结构, 转录本差异分析, 识别可变剪接位点及预测新的基因功能, 提供全面的转录信息[4-6]。目前微量靶向捕获技术的出现, 使得高通量转录组研究在生殖领域成为主流和趋势, 研究者[7-10]分别利用MethylC-seq、RRBS和RNA-seq高通量测序技术对小鼠GV期、MⅡ期卵母细胞和人类早期胚胎等进行研究。高通量测序技术是转录组学研究的革命性工具。研究者[11-15]在其他非模式生物上分别发现了500个以上的差异表达相关基因, 使得转录组研究取得了重要进展。对于生殖系细胞相关的高通量测序主要受到样品数量少的局限, 对于PCOS患者的GV期卵母细胞转录组的研究尚未见文献报道。
基于转录组学在功能基因组研究中的重要价值, 本研究利用Illumina MiSeq高通量测序技术, 基于GV期卵母细胞基因表达和转录组数据, 筛选候选基因和信号通路网络, 探讨PCOS患者和正常排卵妇女GV期卵母细胞成熟的分子机制。
1 资料与方法 1.1 一般资料选择2015—2016年在内蒙古医科大学附属医院生殖中心接受IVF-ET治疗的不孕患者。PCOS组(3例)纳入标准:参照2003年鹿特丹专家会议提出的PCOS诊断标准[16]。正常排卵妇女(对照组, 3人)的纳入标准:月经规律(月经周期为21~35 d), 基础体温测试证实有正常排卵, 超声证实卵巢形态正常, 雄激素水平正常(< 0.6 μg·L-1), 且基础卵泡刺激素(follicle-stimulating hormone, FSH) < 10 IU·L-1, 体质量指数(body mass index, BMI)为18.0~25.0 kg·cm-2, 因男性不孕因素接受长方案将调。
1.2 COS方案本研究中COS采用标准长方案, 在本次月经周期的第2~3天采用重组人促卵泡素(recombined human follicle-stimulating hormone, rhFSH)启动。当有1个卵泡直径达18 mm或2个卵泡直径达16 mm时, 当日停用rhFSH, 注射人绒毛膜促性腺激素(human chorionic gonadotropin, hCG)10 000 IU, 34~36 h后经阴道超声引导下取卵。
1.3 细胞收集收集经过ICSI之后废弃的未成熟卵母细胞。将收集的卵母细胞在50 μL磷酸盐缓冲液(phosphate-buffered saline solution, PBS)液滴中反复冲洗几次, 移入无RNA酶的EP管并加入50 μL Trizol(Invitrogen公司, 美国), 贮存于-80 ℃冰箱待测。
1.4 文库构建采用微量提取试剂盒提取PCOS组和对照组研究对象的总RNA, 其中Total RNA总量≥10 μg, RIN>8。经DNaseⅠ消化后加入打断试剂与Oligo(dT)磁珠富集的mRNA在适温条件下作用一段时间, 以此为模板加入随机引物合成第一链cDNA, 反转录合成双链cDNA, 纯化、末端补平及磷酸化, 末端加“A”及连接测序接头; PCR扩增构建cDNA文库。通过生物信息学软件混合所有读取片段(reads), 使用DAVID软件模块对PCOS患者和正常女性GV期卵母细胞中筛选得到的差异基因进行功能注释与分类, 并利用RT-PCR法对其进行分子生物功能的验证, 筛选关键信号通路网络。
1.5 引物设计和合成以看家基因β-actin为内参, 根据GenBank中登录的人Runt相关转录因子2(Runt-related transcription factor 2, RUNX2)、趋化因子1(chemokine factor 1, CXCL1)、热休克蛋白27(heat shock protein27, Hsp27)、血管内皮生长因子(vascular endothelial growth factor, VEGF)和脂肪酸脱氢酶1(fatty acid dehydrogenase 1, FADS1)基因信息采用Primer 5.0软件设计引物, 引物序列见表 1。引物由上海桑尼公司合成。
| Gene | GenBank accession | Primer sequence | Annealing temperature(θ/℃) |
| RUNX2 | NM_001024630 | F:5′-CCCAAATTTATAGGACAG-3′ R:5′-GAGGGACAAGTATGTAAGTG-3′ |
58.0 |
| CXCL1 | NR_046035 | F:5′-CCTGCACAAAGACAGTGATG-3′ R:5′-TGGCAAAGACAGTGAAAAAG-3′ |
61.0 |
| Hsp27 | AB020027 | F:5′- CCAAGGATGCTGTGTAGATAAG?3′ R:5′-CCAACAGAGGACTCTTGGTCT-3′ |
62.0 |
| VEGF | AF437895 | F:5′-GCCCAGATACCATTCGCACT-3′ R:5′-GCATGGCCCCCTCTCTGATTC-3′ |
58.0 |
| FADS1 | NM_013402 | F:5′-CACAGAGAGAGTCTGGCCACGT-3′ R:5′-CCAACAGAGGACTCTTGGTCT-3′ |
60.0 |
| β-actin | NM_001101 | F:5′-ACAGATGTTGACTTGGTAA-3′ R:5′-ACTTGCGCAGCAGTTGGA-3′ |
59.0 |
采用RT-PCR法分别检测卵母细胞中目的基因RUNX2、CXCL1、Hsp27、VEGF、FADS1和看家基因β-actin的Ct值, 所有实验均重复3次, 采用2-ΔCt法进行相对表达水平分析。
2 结果 2.1 GV期卵母细胞转录组的组装获得RNA-Seq的原始数据后去掉低质量数据, 获得了51 002 482个序列读取片段(reads), 包含8 G碱基序列信息, GC含量均在50%以上, 可为后续的数据分析提供很好的原始数据。见表 2。
| Sample | Reads | Reads mapped(η/%) | GC(η/%) |
| Control1 | 14 214 544 | 85.5 | 51 |
| Control2 | 12 353 592 | 86.3 | 51 |
| Control3 | 13 234 521 | 87.1 | 51 |
| PCOS1 | 11 841 100 | 87.2 | 52 |
| PCOS2 | 14 521 612 | 85.8 | 51 |
| PCOS3 | 13 879 346 | 86.2 | 52 |
通过公式计算PCOS患者和正常排卵女性GV期卵母细胞基因的每百万reads中来自于某基因每千碱基长度的reads数(reads per kilobase per million mapped reads)RPKM值, 大部分基因的RPKM值均小于40, RPKM值为0~10的基因占50.3%, RPKM值大于100的基因占8.2%。筛选到差异表达基因63个, 极显著上调基因有19个, 极显著下调基因有44个。见表 3。
| Group | Gene name | ||||||
| Up-regulation | VEGF | FADS1 | UCLH1 | ING2 | IGFBP3 | BCL2 | |
| DNMT3A | GLG1 | SH3BP4 | UPK3BL | CHA4 | CTCF1 | ||
| EML6 | ANKDD1A | RGMA | CXCL16 | GPHA2 | PIAS1 | MKNK2 | |
| Down-regulation | RUNX2 | ADAMTS | CXCL1 | HSP 27 | AGO4 | COX1 | |
| BAX | NPTX2 | FMNL3 | SMAD | FADS | DHRS3 | ||
| TGFB1 | SFRP4 | ITGB5 | IBGB3 | IGBA5 | IGFBP5 | ||
| LXN | IGFBP7 | SMG1 | MAOB | BCAT1 | ATP2 | ||
| ECM | RPSAP | SOX6 | PRKD1 | MBD2 | BMP1 | ||
| IRF3 | CYP11A1 | INSR | DENND1C | PTGIS | ARL15 | ||
| PRDX4 | GAPDH | TBC1D | PDK1 | LRRC17 | LIMS1 | ||
| HMGA2 | ZFPM1 | ||||||
与对照组GV期卵母细胞比较, 经历卵巢刺激的PCOS组患者未成熟卵母细胞中RUNX2、CXCL1和Hsp27 mRNA表达水平明显降低(P < 0.05), VEGF和FADS1 mRNA表达水平明显升高(P < 0.05)。见图 1。
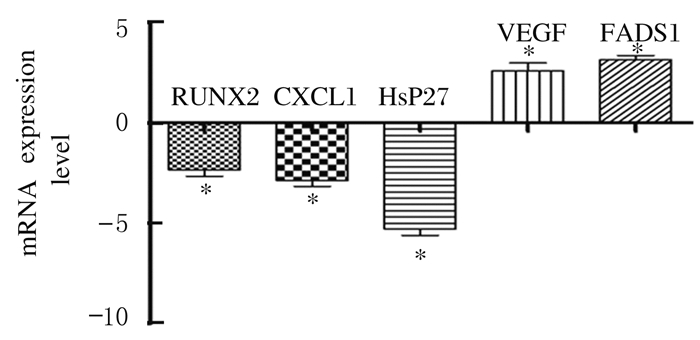
|
| *P < 0.05 vs control group. 图 1 GV期卵母细胞中RUNX2、CXCL1、Hsp27、VEGF和FADS1 mRNA表达水平 Figure 1 Expression levels of RUNX2, CXCL1, Hsp27, VEGF and FADS1 mRNA in GV oocytes |
|
|
GO terms分为生物过程、细胞组分和分子功能3大类。在生物过程中, PCOS组中表达水平上调基因主要参与磷酰化、细胞自噬、透明质酸代谢和转录调控等过程; 下调基因GO terms主要与细胞分化增殖等通路有关。细胞组分中显著表达的差异基因与胞外区有关。分子功能中上调基因GO terms与蛋白酶活性通路有关; 下调基因参与蛋白和细胞因子结合等过程。见图 2。

|
| A:Up-regulation gene ontology; B:Down-regulation gene ontology. 图 2 GV期卵母细胞中差异表达基因GO功能分类图 Figure 2 Diagram of GO function classification of differentially expressed genes in GV oocytes |
|
|
PCOS患者和正常女性GV期卵母细胞中差异表达的基因主要富集在磷脂酰肌醇-3-激酶/蛋白激酶B(PI3K-Akt)、转化生长因子β(TGF-β)、Hippo、p53和过氧化物酶体增殖剂激活受体(PPAR)信号通路上。见表 4。
| Pathway | Dif gene | Gene in pathway | P | FDR | -log2P |
| Focal adhesion | 30 | 207 | 9.294E-6 | 0.002 314 2 | 16.715 257 |
| ECM-receptor interaction | 17 | 89 | 3.637E-5 | 0.003 097 0 | 14.746 969 |
| Proteoglycans in cancer | 30 | 225 | 3.731E-5 | 0.003 097 0 | 14.709 953 |
| PI3K-Akt signaling pathway | 36 | 347 | 0.000 633 3 | 0.039 425 1 | 10.624 745 |
| FoxO signaling pathway | 15 | 133 | 0.010 508 3 | 0.257 138 8 | 6.5723 204 |
| TGF-beta signaling pathway | 10 | 86 | 0.027 217 8 | 0.339 499 7 | 5.1993 069 |
| Hippo signaling pathway | 15 | 153 | 0.029 335 1 | 0.339 499 7 | 5.0912 284 |
| MicroRNAs in cancer | 25 | 296 | 0.030 611 2 | 0.339 499 7 | 5.0297 976 |
| p53 signaling pathway | 8 | 68 | 0.042 716 5 | 0.380 377 5 | 4.5490 622 |
| PPAR signaling pathway | 8 | 70 | 0.048 561 8 | 0.408 777 8 | 4.3640 345 |
PCOS是女性最常见的内分泌疾病之一, PCOS患者常以月经异常和不孕为主要原因就诊, 排卵障碍是导致PCOS患者不孕的重要原因[17]。在IVF-ET治疗周期中PCOS患者进行促排卵治疗可以产生大量卵子, 但通常质量较差, 且正常受精率较低, 具有较高的周期取消率。一方面, 促排卵药物的使用可能造成卵子成熟的自然选择受到抑制; 另一方面, 患者自身激素合成和糖脂代谢等紊乱导致卵泡微环境改变从而引起卵子发育异常和排卵障碍。既往转录组的研究需要大量细胞, 这就使干细胞、早期胚胎等数目较少的细胞基因表达研究受到限制, 微量靶向捕获技术的出现使基因转录组研究在生殖领域也取得了重要进展[18]。本研究利用高通量转录测序技术对GV期卵母细胞转录本进行量化, 测序共获得了510 024 82个序列读取片段(reads), 包含8 G碱基序列信息。通过分析比较基因表达谱共找到63个差异表达基因, 表达极显著上调的基因有19个, 表达极显著下调基因有44个(Fold Change>4, FDR < 0.01)。这些差异基因主要参与炎症反应、脂类代谢和胰岛素相关的信号通路。采用实时定量技术对生物信息学筛选的差异基因进行分子验证, 两者结果一致, 表明本课题组组装的数据较好, 可进行进一步分析。本研究结果显示:Hsp27在人类卵母细胞中呈高表达, PCOS组患者未成熟卵母细胞中Hsp27 mRNA表达水平明显下调, 这与Cai等[19]研究结果一致。
Hsp27作为抗凋亡因子, 参与卵母细胞的抗凋亡过程, 导致PCOS患者卵母细胞中Hsp27表达水平低于正常人, 同时影响卵母细胞的发育[20]。PCOS患者还表现出卵母细胞内膜及间质的增生和过度血管化等特征, 本研究结果显示在PCOS患者GV期卵母细胞中VEGF表达水平明显高于正常人, 与Tal等[21]和Vural等[22]的研究结果一致。
本研究中PCOS患者和正常排卵女性GV期卵母细胞差异表达基因主要参与PI3K-Akt、TGF-β和Hippo信号通路。研究[23]显示:PI3K-Akt在细胞生长、增殖和分化等过程中起重要作用, 该信号通路的激活不仅常见于各种肿瘤, 还见于子宫内膜异位症和多囊卵巢综合征等妇科疾病。PCOS患者多伴随有胰岛素抵抗(IR), 然而IR的作用是使PI3K/Akt这一胰岛素信号减弱, 造成PCOS患者卵泡成熟障碍。Hippo信号通路是较为保守的通路, 参与维持细胞增殖及肿瘤发生过程。近年研究[24]显示:Hippo信号通路在生殖发育调控中起重要作用, 该通路紊乱将导致卵泡增殖分化的异常。Hippo与TGF-β和PI3K/Akt在不同水平形成交叉网络, 共同在原始卵泡的启动调控中扮演重要角色[25]。
本研究采用高通量RNA-seq技术对PCOS患者和正常排卵女性GV期卵母细胞转录组进行测序和分析揭示了未成熟卵母细胞表达特征, 获得了PCOS患者GV期卵母细胞表达过程中具有重要功能的基因, 通过GO和KEGG注释发现差异表达基因主要富集在PI3K-Akt和Hippo等信号通路上, 针对该信号通路的干扰调控相关药物应用于PCOS患者的治疗中将成为新的研究方向。
| [1] | Kollmann M, Martins WP, Raine-Fenning N. Terms and thresholds for the ultrasound evaluation of the ovaries in women with hyperandrogenic anovulation[J]. Hum Reprod Update, 2014, 20(3): 463–464. DOI:10.1093/humupd/dmu005 |
| [2] | 张宁. PCOS患者卵泡输出率预测IVF结局和脂联素及其受体与胚胎发育潜能的相关性研究[D]. 济南: 山东大学, 2013. |
| [3] | 马俊宇. 不同发育潜能小鼠GV期卵母细胞转录组比较及糖尿病对卵母细胞转录组影响[D]. 哈尔滨: 东北农业大学, 2013. |
| [4] | Scarlet D, Ertl R, Aurich C, et al. The orthology clause in the next generation sequencing era:novel reference genes identified by RNA-seq in humans improve normalization of neonatal equine ovary RT-qPCR data[J]. PLoS One, 2015, 10(11): 540–541. |
| [5] | Wang Z, Gerstein M, Snyder M. RNA-Seq:a revolutionary tool for transcriptomics[J]. Nat Rev Genet, 2009, 10(1): 57–63. DOI:10.1038/nrg2484 |
| [6] | Mortazavi A, Williams BA, McCue K, et al. Mapping and quantifying mammalian transcriptomes byRNA-Seq[J]. Nat Methods, 2008, 5(7): 621. DOI:10.1038/nmeth.1226 |
| [7] | Kobayashi H, Sakurai T, Imai M, et al. Contribution of intragenic DNA methylation in mouse gametic DNA methylomes to establish oocyte-specific heritable marks[J]. PLoS Genet, 2012, 8(1). |
| [8] | Smallwood SA, Tomizawa S, Krueger F, et al. Dynamic CpG islandmethylation landscape in oocytes and preimplantation embryos[J]. Nat Genet, 2011, 43(8): 811–814. DOI:10.1038/ng.864 |
| [9] | Reyes JM, Silva E, Chitwood JL, et al. Differing molecular response of young and advanced maternal age human oocytes to IVM[J]. Hum Reprod, 2017, 32(11): 2199. DOI:10.1093/humrep/dex284 |
| [10] | Smith ZD, Chan MM, Mikkelsen TS, et al. A unique regulatory phase of DNA methylation in the early mammalian embryo[J]. Nature, 2012, 484(7394): 339–344. DOI:10.1038/nature10960 |
| [11] | Ma JY, Li M, Ge ZJ, et al. Whole transcriptome analysis of the effects of type, Ⅰ, diabetes on mouse oocytes[J]. PLoS One, 2012, 7(7): e41981. DOI:10.1371/journal.pone.0041981 |
| [12] | Wu Y, Lin J, Li X. Transcriptome profile of one-month-old lambs' granulosa cells after superstimulation[J]. Asian Austral J Anim, 2016, 30(1): 20–33. DOI:10.5713/ajas.15.0999 |
| [13] | Labrecque R, Fournier E, Sirard MA. Transcriptome analysis of bovine oocytes from distinct follicle sizes:Insights from correlation network analysis[J]. Mol Reprod Dev, 2016, 83(6): 558–569. DOI:10.1002/mrd.22651 |
| [14] | Kate-Jaffe MG, Mccallie BR, Preis KA, et al. Transcriptome analysis of in vivo and in vitro matured bovine MⅡoocytes[J]. Theriogenology, 2009, 71(6): 939–946. DOI:10.1016/j.theriogenology.2008.10.024 |
| [15] | Khan DR, Landry DA, Fournier É, et al. Transcriptome meta-analysis of three follicular compartments and its correlation with ovarian follicle maturity and oocyte developmental competence in cows[J]. Physiol Genomics, 2016, 48(8): 633–643. DOI:10.1152/physiolgenomics.00050.2016 |
| [16] | Group ESPCW. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome[J]. Fertil Steril, 2004, 81(1): 19–25. DOI:10.1016/j.fertnstert.2003.10.004 |
| [17] | Rahsepar M, Mahjoub S, Esmaelzadeh S, et al. Evaluation of vitamin D status and its correlation with oxidative stress markers in women with polycystic ovary syndrome[J]. Int J Reprod Biomed(Yazd), 2017, 15(6): 345–350. DOI:10.29252/ijrm.15.6.345 |
| [18] | Li L, Dong J, Yan L, et al. Single-cell RNA-Seq analysis maps development of human germline cells and gonadal niche interactions[J]. Cell Stem Cell, 2017, 20(6): 858–873. DOI:10.1016/j.stem.2017.03.007 |
| [19] | Cai L, Ma X, Liu S, et al. Effects of upregulation of Hsp27 expression on oocyte development and maturation derived from polycystic ovary syndrome[J]. PLoS One, 2013, 8(12): e83402. DOI:10.1371/journal.pone.0083402 |
| [20] | Charette SJ, Lavoie JN, Lambert H, et al. Inhibition of Daxx-mediated apoptosis by heat shock protein 27[J]. Mol Cell Biol, 2010, 20(20): 7602–7612. |
| [21] | Tal R, Seifer DB, Grazi RV, et al. Follicular fluid placental growth factor is increased in polycystic ovarian syndrome:correlation with ovarian stimulation[J]. Reprod Biol Endocrinol, 2014, 12(1): 82. DOI:10.1186/1477-7827-12-82 |
| [22] | Vural F, Vural B, Doger E, et al. Perifollicular blood flow and its relationship with endometrial vascularity, follicular fluid EG-VEGF, IGF-1, and inhibin-a levels and IVF outcomes[J]. J Assist Reprod Genet, 2016, 33(10): 1355–1362. DOI:10.1007/s10815-016-0780-7 |
| [23] | 刘俐伶, 植枝福. PI3K/Akt/mTOR信号通路在常见妇科疾病中的研究进展[J]. 中国现代医学杂志, 2016, 26(9): 59–62. |
| [24] | Yu JZ, John P, Huang YC, et al. The hippo pathway promotes Notch signaling in regulation of cell differentiation, proliferation, and oocyte polarity[J]. PLoS One, 2008, 3(3): e1761. DOI:10.1371/journal.pone.0001761 |
| [25] | Sasaki H. Roles and regulations of Hippo signaling during preimplantation mouse development[J]. Develop Growth Differ, 2017, 59(1): 12–20. DOI:10.1111/dgd.2017.59.issue-1 |
 2018, Vol. 44
2018, Vol. 44


