扩展功能
文章信息
- 王丹, 李怡飞, 历春, 董志恒, 董营, 盖晓东
- WANG Dan, LI Yifei, LI Chun, DONG Zhiheng, DONG Ying, GAI Xiaodong
- 结直肠癌组织中B7-H1和B7-H4表达与Foxp3+调节性T细胞浸润的关联性
- Relationships between expressions of B7-H1 and B7-H4 and Foxp3+ regulated T-cell infiltration in colorectal cancer tissue
- 吉林大学学报(医学版), 2018, 44(03): 543-547
- Journal of Jilin University (Medicine Edition), 2018, 44(03): 543-547
- 10.13481/j.1671-587x.20180316
-
文章历史
- 收稿日期: 2017-09-21
2. 吉林省吉林市中心医院骨关节科, 吉林 吉林 132011
2. Department of Orthopaedics, Central Hospital, Jilin City, Jilin Province, Jilin 132011, China
结直肠癌(colorectal cancer, CRC)是世界范围内最常见的恶性肿瘤之一。由于外科技术的进步、放疗和化疗药物的使用,临床上CRC患者的预后有明显的改善,但仍有复发和转移的危险。因此,有必要进一步探讨CRC免疫逃逸机制从而开发新的CRC治疗方法[1]。抗原提呈细胞调控T细胞免疫是通过B7家族中的一类共激活分子完成的。B7分子与T细胞表面相应受体结合后,在抗原特异性免疫应答过程中发挥正性或负性调控作用[2]。B7-H1是B7家族中的负性调控分子,其与受体PD-1的相互作用在肿瘤免疫应答中发挥重要作用[3]。B7-H4是B7家族中新发现的成员,其在卵巢癌、乳腺癌、子宫内膜癌和肺癌等多种肿瘤组织中表达,而在正常组织中几乎检测不到[4]。上述研究结果提示:B7-H1和B7-H4可能作为免疫反应负调节分子,通过不同途径在抗肿瘤免疫中发挥重要的作用[5]。调节性T细胞(Treg)是一个独特的CD4+辅助性T细胞亚群,其特点是CD4+及CD25+阳性表达。叉头转录因子(Foxp3+)是调节T细胞发育及其功能效应的关键因素,也是Treg的特异且可靠的标记[6]。Treg在许多人类肿瘤组织中均有表达,与肿瘤免疫逃避机制有密切关联[7]。研究者[8-9]发现:B7-H1与肿瘤微环境中Treg表达量增加相关,B7-H4对Treg功能具有调控作用。抑制性B7分子在肿瘤细胞中的表达与Treg浸润的相关性目前尚无研究报道。本研究选择56例CRC患者的癌组织作为研究对象,检测B7-H1和B7-H4在CRC组织中的表达及Treg在CRC组织中的浸润性,并且探讨二者之间的相关性,为CRC的诊断和预后判断提供参考。
1 资料与方法 1.1 研究对象收集2007—2009年北华大学附属医院手术切除的经病理诊断为CRC患者的结肠癌标本56例,均取癌组织和癌旁正常组织(大于肿瘤边缘5cm处)。其中男性患者35例,女性患者21例,年龄34~79岁。24例患者有淋巴结转移,32例患者无淋巴结转移。所有患者均无术前放疗或化疗。根据国际癌症控制联盟(UICC 2002)标准进行病理分期。
1.2 免疫组织化学法检测CRC组织和癌旁正常组织中B7-H1和B7-H4的表达将OCT胶包埋好的组织标本采用恒冷冰冻切片机(Leica CM1900,德国莱卡公司)切成5 μm厚度的连续切片,室温干燥后以多聚甲醛固定,PBS冲洗。以下步骤按说明书操作:0.3%过氧化氢室温下阻断内源性过氧化物酶活性,PBS冲洗,山羊血清封闭。切片分别滴加抗B7-H1抗体(1:30稀释)、抗B7-H4抗体(1:500稀释)和抗Foxp3+抗体(1:100稀释),4℃过夜。PBS洗涤切片, 以生物素标记的二抗处理,链霉亲和素-辣根过氧化物酶复合物孵育,DAB显色,切片脱水,清洗和封片。
1.3 结果判定标准采用双盲法评估所有载玻片。高倍镜下(×400)随机取10个视野,每个视野计数100个细胞,以细胞质中有黄至棕褐色颗粒染色者为B7-H1和B7-H4染色阳性。按阳性细胞数计分:0分(无阳性肿瘤细胞),1分(阳性肿瘤细胞率 < 10%),2分(10%≤阳性肿瘤细胞率≤50%),3分(阳性肿瘤细胞率>50%)。按染色强度计分:未着色或与背景一致为0分,浅黄色为1分,黄褐色为2分,褐色为3分。2项指标的乘积分数小于4归类为低表达,其余为高表达。
根据随机选择的高倍视野(HPF)中阳性染色细胞的百分率来评估Treg细胞的表达情况,以细胞核中有黄至棕褐色颗粒者为判定Treg细胞阳性的标准。每张切片随机选择10个高倍视野(×400)计数阳性细胞数, 计算Treg阳性细胞数平均值。Treg阳性细胞数平均值≤20为低浸润,Treg阳性细胞数平均值>20为高浸润。
1.4 统计学分析采用GraphPad Prism 5.0统计软件进行统计学分析。B7-H1和B7-H4表达频数与CRC患者临床病理参数的关系分析采用χ2检验;不同临床病理参数CRC患者Treg阳性细胞数以x±s表示,组间比较采用t检验;B7-H1表达与Treg浸润及B7-H4表达与Treg浸润的关联性分析采用χ2检验及Cramer’ s检验。以P < 0.05表示差异有统计学意义。
2 结果 2.1 CRC患者癌组织和癌旁正常组织中B7-H1和B7-H4的表达B7-H1和B7-H4阳性表达呈棕黄色颗粒,主要定位于肿瘤细胞的细胞质和细胞膜(图 1A和B,见插页四);癌旁正常组织中其为阴性表达(图 1C和D,见插页四)或在肠腺上皮细胞质中呈弱阳性表达。56例癌旁正常组织中B7-H1呈弱阳性表达5例(8.93%),B7-H4呈弱阳性表达4例(7.14%)。CRC组织中B7-H1高表达27例,高表达率为48.21%;B7-H4高表达27例,高表达率为48.21%。CRC组织中B7-H1阳性表达率为89.29%,明显高于癌旁正常组织(8.93%)(χ2= 72.34,P < 0.01);CRC组织中B7-H4阳性表达率为91.07%,明显高于癌旁正常组织(7.14%)(χ2=78.92,P < 0.01)。
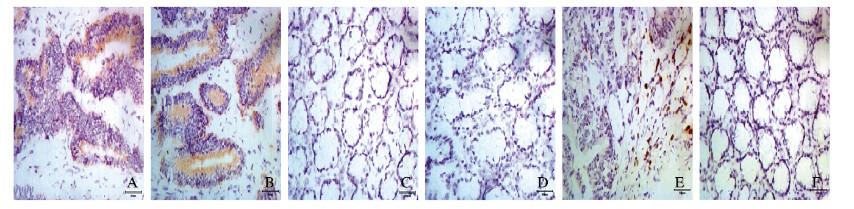
|
| 图 1 CRC和癌旁正常组织中B7-H1、B7-H4和Treg阳性细胞表达(×200) Figure 1 Expressions of B7-H1, B7-H4 and Treg positive cells in CRC and adjacent normal tissues(×200) |
|
|
Treg细胞形态完整, 结构清晰,DAB显色核呈棕黄色,散在分布于CRC组织中,尤其在CRC组织和癌旁正常组织交界处分布密集。本研究56例CRC组织中均有Treg表达, 其阳性细胞数(23.5±7.0)明显高于癌旁正常组织(0.7±1.9)(P < 0.01)。见图 1E和F(插页四)。
2.3 不同临床病理特征CRC患者癌组织中B7-H1、B7-H4和Treg的表达B7-H1表达频数与肿瘤浸润深度有关联(P < 0.01),淋巴结转移阳性者CRC组织中B7-H1高表达频数高于淋巴结转移阴性者(P < 0.01),Duke’ s分期C+D者B7-H1高表达频数高于Duke’ s分期A+B者(P < 0.01)。B7-H1表达频数与患者年龄、性别、肿瘤位置和分化程度无关联(P> 0.05)。见表 1。
| Clinicopathological feature | n | Expression frequency of B7-H1 | Expression frequency of B7-H4 | No.of Treg positive expression cells | P | |||||
| Low | High | P | Low | High | P | |||||
| Gender | ||||||||||
| Men | 35 | 19 | 16 | 0.628 8 | 21 | 14 | 0.112 2 | 21.8±8.0 | 0.312 7 | |
| Women | 21 | 10 | 11 | 8 | 13 | 18.7±6.2 | ||||
| Age(year) | ||||||||||
| ≤60 | 26 | 13 | 13 | 0.803 4 | 15 | 11 | 0.410 2 | 27.1±8.1 | 0.522 9 | |
| >60 | 30 | 16 | 14 | 14 | 16 | 19.9±5.8 | ||||
| Tumor position | ||||||||||
| Colon | 23 | 10 | 13 | 0.299 0 | 14 | 9 | 0.256 1 | 17.7±5.6 | 0.067 2 | |
| Rectum | 33 | 19 | 14 | 15 | 18 | 22.2±6.5 | ||||
| Differentiation degree | ||||||||||
| High | 27 | 16 | 11 | 0.209 7 | 15 | 12 | 0.197 3 | 18.2±4.3 | 0.002 9 | |
| Moderate and low | 24 | 10 | 14 | 9 | 15 | 25.8±6.5 | ||||
| Infiltration depth | ||||||||||
| T1+T2 | 18 | 15 | 3 | 0.003 0 | 14 | 4 | 0.007 0 | 9.7±8.8 | 0.648 5 | |
| T3+T4 | 38 | 14 | 24 | 15 | 23 | 21.0±7.5 | ||||
| Lymphnode metastasis | ||||||||||
| Negative | 32 | 22 | 10 | 0.003 4 | 21 | 11 | 0.016 7 | 18.9±7.5 | 0.006 5 | |
| Positive | 24 | 7 | 17 | 8 | 16 | 24.4±6.4 | ||||
| Duke’s stage | ||||||||||
| A+B | 30 | 21 | 9 | 0.003 4 | 19 | 11 | 0.063 2 | 19.3±7.6 | 0.162 8 | |
| C+D | 26 | 8 | 18 | 10 | 16 | 23.3±7.0 | ||||
B7-H4的表达频数与肿瘤浸润深度有关联(P < 0.05),淋巴结转移阳性者CRC组织中B7-H4高表达频数高于转移阴性者(P < 0.05)。B7-H4的表达频数与患者性别、年龄、肿瘤位置、分化程度和Duke’ s分期无关联(P> 0.05)。Treg阳性细胞数与肿瘤分化程度有关联(P < 0.01),淋巴结转移阳性者CRC组织中Treg阳性细胞数高于转移阴性者(P < 0.01)。Treg阳性细胞数与患者性别、年龄、肿瘤位置、分化程度和Duke’ s分期无关联(P> 0.05)。见表 1。
2.4 CRC患者癌组织中B7-H1、B7-H4表达与Treg浸润程度的关系本研究56例CRC组织中Treg分布情况:Treg低浸润30例,Treg高浸润26例。Treg高浸润组B7-H1和B7-H4的高表达频数明显高于低浸润组,CRC组织中B7-H1表达与Treg有相关性(P < 0.01,Cramer’ s=0.463),CRC组织中B7-H4表达与Treg有相关性(P < 0.05,Cramer’ s=0.320)。见表 2。
| Treg | n | B7-H1 | χ2 | P | Cramer’s | B7-H4 | χ2 | P | Cramer’s | ||
| Low | High | Low | High | ||||||||
| Low infiltration High infiltration | 30 | 22 | 8 | 12.020 | 0.000 5 | 0.463 | 20 | 10 | 5.731 | 0.016 7 | 0.320 |
| High infiltration | 26 | 7 | 19 | 20 9 | 10 17 | ||||||
肿瘤细胞可以被抗原特异性T细胞免疫应答识别和攻击。B7家族在抗原特异性T细胞介导的正性和负性免疫调控作用中起核心作用。负性共刺激分子B7表达上调能通过抑制T细胞功能负性调控T细胞的免疫应答,从而促进肿瘤免疫逃避[10]。本研究结果显示:负性共刺激分子B7-H1和B7-H4在CRC组织中均呈异常高表达,并且B7-H1在CRC组织中的高表达与浸润深度、淋巴结是否转移及Duke’ s分期有关联;B7-H4高表达频数随着肿瘤浸润深度、淋巴结转移也出现出升高趋势。但B7-H1和B7-H4与CRC患者其他病理特征(如性别、年龄、部位和分化程度等)无关联,提示B7-H1和B7-H4在CRC的转移和进展中起重要作用,但CRC进程的具体机制尚需进一步探讨。
Treg通过在肿瘤微环境中发挥肿瘤特异性免疫抑制功能而促进肿瘤生长,对肿瘤的发生发展有重要意义[11-12]。本研究探讨了CRC患者肿瘤组织中Treg细胞分布情况, 发现肿瘤组织中Treg表达水平明显高于相应的癌旁正常组织。对Treg浸润与临床病理特征关系分析结果显示:中-低分化患者CRC组织中Treg阳性细胞数明显高于高分化者, 肿瘤的异型性越大,Treg阳性细胞数越多, 恶性程度越高。这些结果提示Treg在CRC肿瘤免疫中有重要作用,Treg可能影响了肿瘤的分化,Treg的高表达可能是影响CRC预后不良的因素之一。
目前,在肿瘤微环境中如何趋化Treg细胞并维持Treg细胞的生存和功能机制尚不清楚。负性共刺激分子B7-H1和B7-H4能增加肿瘤特异性抗原激活的T细胞凋亡、抑制特异性T细胞免疫应答、减少白细胞介素2(IL-2)、白细胞介素10(IL-10)和干扰素γ(IFN-γ)的分泌,以及阻碍细胞周期的进程[13-15]。研究[16-17]显示:B7-H1在调节诱导性Treg中起关键作用,通过抑制活化T细胞中的细胞外信号调节蛋白激酶、C-Jun N端激酶、丝裂原激活蛋白激酶P38和AKT信号通路,以影响T细胞的增殖[18]。体内实验结果显示:抑制B7-H1信号通路可抑制肿瘤特异性抗原激活的Treg生成。Treg可诱导人卵巢癌细胞表达B7-H4,从而抑制抗原提呈细胞的活性[19],表明B7-H4对Treg功能具有调控作用[20]。本研究结果显示:Treg高浸润组B7-H1和B7-H4高表达频数明显高于低浸润组,B7-H1和B7-H4在CRC组织中的表达与Treg有关联性。随着CRC的发生发展、浸润及转移,肿瘤细胞增殖活性呈递增趋势。
综上所述,CRC患者癌组织中B7-H1、B7-H4和Treg高表达可能促进肿瘤的浸润和转移,并在CRC的发生发展中起抑制作用,B7-H1和B7-H4表达可能是诱导机体产生Foxp3+ Treg的机制之一。因此,联合检测B7-H1、B7-H4和Treg的表达对CRC预后评估有较高的临床参考价值。
| [1] | Koudougou C, Bonneville M, Matysiak-Budnik T, et al. Review article:antitumoural immunity in colorectal cancer-current and potential future implications in clinical practice[J]. Aliment Pharmacol Ther, 2013, 38(1): 3–15. DOI:10.1111/apt.2013.38.issue-1 |
| [2] | Ichikawa M, Chen L. Role of B7-H1 and B7-H4 molecules in down-regulating effector phase of T-cell immunity:novel cancer escaping mechanisms[J]. Front Biosci, 2005, 10: 2856–2860. DOI:10.2741/1742 |
| [3] | Mirza N, Duque MA, Dominguez AL, et al. B7-H1 expression on old CD8+ T cells negatively regulates the activation of immune responses in aged animals[J]. J Immunol, 2010, 184(10): 5466–5474. DOI:10.4049/jimmunol.0903561 |
| [4] | Zang X, Thompson RH, Al-Ahmadie HA, et al. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome[J]. Proc Natl Acad Sci USA, 2007, 104(49): 19458–19463. DOI:10.1073/pnas.0709802104 |
| [5] | Janakiram M, Abadi YM, Sparano JA, et al. T cell coinhibition and immunotherapy in human breast cancer[J]. Discov Med, 2012, 14(77): 229–236. |
| [6] | Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3[J]. Science, 2003, 299(5609): 1057–1061. DOI:10.1126/science.1079490 |
| [7] | Nomura T, Sakaguchi S. Naturally arising CD4+ CD25+ regulartory T cells in tumor immunity[J]. Curr Top Microbiol Immunol, 2005, 293: 287–302. |
| [8] | Ni XY, Sui HX, Liu Y, et al. TGF-β of lung cancer microenvironment upregulates B7H1 and GITRL expression in dendritic cells and is associated with regulatory T cell generation[J]. Oncol Rep, 2012, 28(2): 615–621. DOI:10.3892/or.2012.1822 |
| [9] | Kristensen NN, Schmidt EG, Rasmussen S, et al. B7-H4-Ig treatment of normal mice changes lymphocyte homeostasis and increases the potential of regulatory T cells[J]. Immunopharmacol Immunotoxicol, 2013, 35(4): 505–513. DOI:10.3109/08923973.2013.810642 |
| [10] | Huang H, Li C, Ren G. Clinical significance of the B7-H4 as a novel prognostic marker in breast cancer[J]. Gene, 2017, 623: 24–28. DOI:10.1016/j.gene.2017.04.003 |
| [11] | Park JJ, Omiya R, Matsumura Y, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance[J]. Blood, 2010, 116(8): 1291–1298. DOI:10.1182/blood-2010-01-265975 |
| [12] | Akimova T, Levine MH, Beier UH, et al. Standardization, evaluation, and area-under-curve analysis of human and murine Treg suppressive function[J]. Methods Mol Biol, 2016, 1371(1): 43–78. |
| [13] | Zhao LW, Li C, Zhang RL, et al. B7-H1andB7-H4expression in colorectal carcinoma:correlation with tumor Foxp3+ regulatory T-cell infiltration[J]. Acta Histochem, 2014, 116(7): 1163–1168. DOI:10.1016/j.acthis.2014.06.003 |
| [14] | Xu M, Zhang B, Zhang M, et al. Clinical relevance of expression of B7-H1 and B7-H4 in ovarian cancer[J]. Oncol Lett, 2015, 11(4): 2815–2819. |
| [15] | Wang X, Hao J, Metzger DL, et al. B7-H4 treatment of T cells inhibits ERK, JNK, p38, and AKT activation[J]. PLoS One, 2012, 7(1): e28232. DOI:10.1371/journal.pone.0028232 |
| [16] | Parsa AT, Bloch O, Crane C, et al. Gliomas promote induction of b7-h1/pd-l1 expression on monocytes:clinical evidence of an immunosuppressive mechanism that can be targeted with antibody blockade[J]. Neuro Oncol, 2014, 16: iii41. |
| [17] | Zhang C, Li Y, Wang Y. Diagnostic value of serum B7-H4 for hepatocellular carcinoma[J]. J Surg Res, 2015, 197(2): 301–306. DOI:10.1016/j.jss.2015.04.034 |
| [18] | Groeger S, Jarzina F, Mamat U, et al. Induction of B7-H1 receptor by bacterial cells fractions of Porphyromonas gingivalis on human oral epithelial cells[J]. Immunobiology, 2017, 222(2): 137–147. DOI:10.1016/j.imbio.2016.10.011 |
| [19] | Hirunwidchayarat W, Furusawa E, Kang S, et al. Site-specific regulation of oral mucosa-recruiting CD8+ T cells in a mouse contact allergy model[J]. Biochem Biophys Res Commun, 2017, 490(4): 1294–1300. DOI:10.1016/j.bbrc.2017.07.012 |
| [20] | Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment[J]. Nat Rev Immunol, 2008, 8(6): 467–477. DOI:10.1038/nri2326 |
 2018, Vol. 44
2018, Vol. 44


