扩展功能
文章信息
- 刘霞, 齐凤杰, 杜晓媛
- LIU Xia, QI Fengjie, DU Xiaoyuan
- 沉默CRAF基因表达对肝癌细胞侵袭和迁移的影响
- Effects of silencing expression of CRAF gene on invasion and metastasis of hepatocellular carcinoma cells
- 吉林大学学报(医学版), 2018, 44(03): 521-525
- Journal of Jilin University (Medicine Edition), 2018, 44(03): 521-525
- 10.13481/j.1671-587x.20180312
-
文章历史
- 收稿日期: 2017-10-31
肝细胞癌(hepatocelluar carcinoma,HCC)是全球最常见的恶性肿瘤之一,具有病死率高、容易复发和转移的特点,在肿瘤相关死亡率中排第3位[1]。目前已知,侵袭和转移是HCC病死率高的最重要原因之一[2],但临床上缺乏有效的方法抑制HCC的侵袭和转移[3],因此对HCC侵袭和转移的机制研究十分必要。侵袭和转移是恶性肿瘤的重要特征,也是恶性肿瘤患者预后不佳的重要原因,抗侵袭和转移治疗一直被认为是提高恶性肿瘤治疗效果的一种重要手段[4]。RAF激酶家族在恶性肿瘤的发生、进展和耐药性形成过程中具有重要作用,其主要由人体内鼠类肉瘤滤过性病毒同源癌基因3611mRNA(ARAF)、原癌基因丝氨酸/苏氨酸蛋白激酶(BRAF)和原癌基因丝氨酸/苏氨酸蛋白激酶(CRAF)组成[5]。研究[6]表明:CRAF通过RAF/MEK/ERK信号通路调节恶性肿瘤的增殖和耐药,但对恶性肿瘤的迁移和侵袭研究较少。基质金属蛋白酶(matrix metalloproteinases,MMPs)是肿瘤侵袭和转移过程中起关键作用的蛋白水解酶[7]。研究[8]表明:MMPs的关键成员MMP-2和MMP-9可以促进肿瘤细胞降解细胞外基质, 促进肿瘤细胞的侵袭和转移。本研究以HCCSMMC7721细胞作为研究对象, 采用shRNA技术沉默SMMC7721细胞中CRAF的表达,探讨CRAF对肝癌细胞迁移和侵袭的作用。
1 材料与方法 1.1 细胞、主要试剂和仪器人HCCSMMC7721、HepG2和QGY7703细胞购于中国科学院上海细胞库。DMEM培养液和胎牛血清购于美国Gibco公司,兔抗人CRAF、MMP-2和MMP-9单克隆抗体购于英国Abcam公司,Lipofectamine2000购于美国Life公司,shCRAF#1、shCRAF#2和对照载体购于上海吉凯公司,Matrigel凝胶购于美国BD公司。酶标仪和SDS-PAGE蛋白电泳仪购于美国Bio-Rad公司,低温高速离心机购于美国Thermo公司。
1.2 细胞培养和转染将人HCCSMMC7721、HepG2和QGY7703细胞培养于含10% FBS和1%青霉素/链霉素的DMEM培养液中,培养条件为37℃、5% CO2。每2~3d更换培养液1次。转染按美国Invitrogen公司的实验指南进行。将细胞接种于6孔细胞培养板中,当细胞密度达到90%时开始转染,转染后48h采用Western blotting法检测转染效率。质粒用量为4μg,转染试剂体积为12μL。shCRAF#1序列为CGGAGATGTTGCAGTAAAGAT,shCRAF#2序列为GAGACATGAAATCCAACAATA。将SMMC7721细胞分为对照组和沉默组,分别对沉默组转染shCRAF#1和shCRAF#2(shCRAF#1转染组和shCRAF#2转染组)。
1.3 Western blotting法检测各组细胞中CRAF表达水平及其沉默效果细胞收样后,RIPA缓冲液裂解细胞,BCA定量。采用10% SDS-PAGE凝胶电泳,电泳结束后转至PVDF膜,1%BSA室温封闭1 h,加入CRAF兔抗人单克隆抗体(1:1 000稀释),4℃孵育过夜。TBST漂洗3次,每次10 min。漂洗结束后加入HRP标记的羊抗兔二抗,室温孵育1 h后,采用TBST漂洗3次,以ECL发光液于凝胶成像系统中检测显影情况。得到CRAF条带,采用凝胶成像系统进行灰度分析。
1.4 细胞划痕法检测细胞创口愈合指数取对数生长期SMMC-7721细胞,调整细胞密度为2×105 mL-1,铺于12孔板,每组设3个复孔。24 h内细胞长至70%后进行转染,细胞汇合成单层细胞进行划痕操作。采用20 μL枪头贴着经消毒处理的直尺于每孔中间轻划1道痕迹,PBS洗涤3次,加入1 mL无血清DMEM培养基继续培养,分别于0和24 h显微镜下观察各组细胞愈合情况,测量创口宽度,记为W0和W24,并计算创口愈合指数。创口愈合指数=(W0-W24)/W0。
1.5 Transwell实验检测穿膜细胞数转染后12 h将细胞用胰酶消化后置于无血清DMEM培养液制成细胞悬液,接种至Matrigel凝胶包被的Transwell培养板的上层,每孔1×104个,体积200 μL。在Transwell培养板的下层加入完全培养液。36 h后取出,4%多聚甲醛固定10 min,采用结晶紫染色20 min,显微镜高倍视野下照相。随机选取3个高倍视野,计数穿膜细胞数。
1.6 Western blotting法检测各组细胞中CRAF、MMP-2和MMP-9表达水平细胞收样后,RIPA缓冲液裂解细胞,BCA定量。采用10% SDS-PAGE凝胶电泳,电泳结束后转至PVDF膜,1%BSA室温封闭1 h,加入CRAF、MMP-2和MMP-9兔抗人单克隆抗体(1:1 000稀释),4℃孵育过夜。TBST漂洗3次,每次10 min。漂洗结束后加入HRP标记的羊抗兔二抗,室温孵育1 h后,采用TBST漂洗3次,以ECL发光液于凝胶成像系统中检测显影,分别得到CRAF、MMP-2和MMP-9条带,采用凝胶成像系统进行灰度分析。
1.7 统计学分析采用SPSS 20.0统计软件进行统计学分析。各组SMMC7721细胞中CRAF表达水平、各组细胞创口愈合指数和Transwell穿膜细胞数均以x±s表示,计量资料数据符合正态分布,组间比较用单因素方差分析。以P < 0.05为差异有统计学意义。
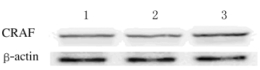
2 结果 2.1 肝癌细胞的选择采用Western blotting法检测HepG2、QGY-7703和SMMC7721细胞中CRAF表达情况结果显示:CRAF在这3种细胞中均表达,其中SMMC7721细胞中表达水平最高(图 1),因此选定SMMC7721细胞用于后续研究。
|
| Lane 1: HepG2 cells; Lane 2: QGY-7703 cells; Lane 3: SMMC-7721cells. 图 1 Western blotting法检测HepG2、QGY-7703和SMMC-7721细胞中CRAF表达电泳图 Figure 1 Electrophoregram of expressions of CRAF in HepG2, QGY-7703, and SMMC7721 cells detected by Western blotting method |
|
|
与对照组(0.90±0.11)比较,shCRAF#1转染组和shCRAF#2转染组SMMC7721细胞中CRAF表达水平(0.63±0.22和0.55±0.27)明显降低(P < 0.05)。见图 2。

|
| Lane 1: Control group; Lane 2: ShCRAF#1 transfection group; Lane 3: ShCRAF#2 transfection group. 图 2 Western blotting法检测各组SMMC7721细胞中CRAF表达电泳图 Figure 2 Electrophoregram of expressions of CRAF in SMMC7721 cells in various groups detected by Western blotting method |
|
|
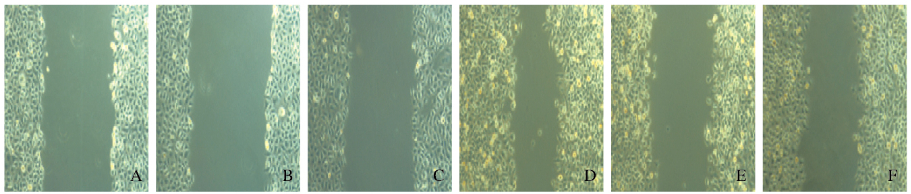
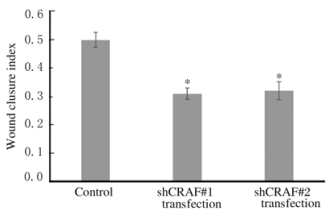
细胞划痕检测结果显示:与对照组比较,转染shCRAF#1和shCRAF#2组SMMC7721细胞的创口愈合速度明显降低(图 3,见插页四)。对照组细胞的创口愈合指数为(0.50±0.03),shCRAF#1和shCRAF#2转染组细胞的创口愈合指数分别为(0.31±0.02)和(0.32±0.03),组间比较差异有统计学意义(P < 0.05)。见图 4。
|
| A-C:0 h; D-F:24 h; A, D:Control group; B, E:shCRAF#1 transfection group; C, F:shCRAF#2 tansfection group. 图 3 划痕愈合分析法检测各组SMMC7721细胞迁移 Figure 3 Migration of SMMC7721 cells in various groups detected by wound healing analysis(seen on page 523in paragraph) |
|
|
|
| *P < 0.05 vs control group. 图 4 细胞划痕法检测各组SMMC7721细胞的创口愈合指数 Figure 4 Wound closure indexes of SMMC7721 cells in various groups detected by wound healing analysis |
|
|
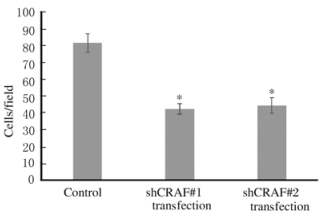
与对照组比较,shCRAF#1转染和shCRAF#2转染组SMMC7721细胞穿膜细胞数明显降低,组间比较差异有统计学意义(P < 0.05)。见图 5(插页四)和图 6。

|
| A:Control group; B:shCRAF#1 transfection group; C:shCRAF#2 transfection group. 图 5 Transwell实验检测各组SMMC7721细胞侵袭能力 Figure 5 Invasion abilities of SMMC7721 cells in various groups detected by Transwell assay(seen on page 523in paragraph) |
|
|
|
| *P < 0.05 vs control group. 图 6 Transwell实验检测各组SMMC7721细胞穿膜细胞数 Figure 6 Number of transmembrane cells of SMMC7721 cells in various groups detected by Transwell assay |
|
|
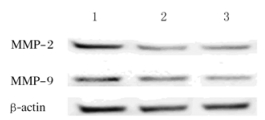
与对照组(1.13±0.02和1.00±0.07)比较,shCRAF#1转染组(0.51±0.03和0.49±0.11)和shCRAF#2转染组(0.46±0.05和0.47±0.05)SMMC7721细胞中MMP-2和MMP-9表达水平明显降低,差异有统计学意义(P < 0.05)。见图 7。
|
| Lane 1:Control group; Lane 2:ShCRAF#1 transfection group; Lane 3: ShCRAF#2 transfection group. 图 7 Western blotting法检测各组SMMC-7721细胞中MMP-2和MMP-9表达电泳图 Figure 7 Electrophoregram of expressions of MMP-2 and MMP-9 in SMMC7721 cells in various groups detected by Western blotting method |
|
|
侵袭和转移是恶性肿瘤的重要生物学特征,是一个复杂的、严格受控的过程。HCC细胞具有极强的侵袭能力,易于通过血液、淋巴系统发生侵袭和转移,是HCC患者预后不佳的重要原因[9]。尽管抗侵袭转移是肝癌治疗的重要手段,可以提高HCC治疗的效果,但目前临床上缺乏有效的抑制侵袭和转移的方法[10],主要是因为对HCC侵袭和转移的机制认识不够深刻。
CRAF是RAF激酶家族的重要成员[11-13],可通过激活下游的MEK/ERK信号通路调节细胞增殖[14-16],但其对HCC侵袭和转移是否具有调节作用目前报道较少。本研究检测CRAF在人HCCHepG2、QGY7703和SMMC-7721细胞中表达结果显示:尽管CRAF在这3种人HCC细胞中均有表达,但其在SMMC-7721细胞中表达水平较高。本研究采用shRNA技术下调SMMC-7721细胞中CRAF表达,探讨其对SMMC7721细胞侵袭和迁移能力影响的结果显示:下调CRAF可以有效抑制细胞侵袭和迁移,提示沉默CRAF可以有效抑制HCC的侵袭和转移。
MMP-2和MMP-9是MMPs家族中降解Ⅳ型胶原的主要酶[17-18],参与细胞外基质和基底膜降解过程,在肿瘤侵袭和转移中发挥重要作用[19-20]。MMP-2和MMP-9表达水平是衡量肿瘤侵袭和转移能力的重要指标[21]。本研究结果显示:在SMMC7721细胞中沉默CRAF可以降低MMP-2和MMP-9表达水平。
综上所述,沉默CRAF可以有效地抑制HCC细胞的侵袭和转移,而这种抑制作用通过降低MMP-2和MMP-9表达水平实现。CRAF可以作为抗HCC侵袭和转移的有效靶点,本研究结果为临床上抑制HCC的侵袭和转移提供新的线索。
| [1] | Wong R, Frenette C. Updates in the management of hepatocellular carcinoma[J]. Gastroenterol Hepatol(N Y), 2011, 7(1): 16–24. |
| [2] | Zhang S, Li J, Liu PG, et al. Pygopus-2 promotes invasion and metastasis of hepatic carcinoma cell by decreasing E-cadherin expression[J]. Oncotarget, 2015, 6(13): 11074–11086. |
| [3] | Chen X, Song B, Lin Y, et al. PTK6 promotes hepatocellular carcinoma cell proliferation and invasion[J]. Am J Transl Res, 2016, 8(10): 4354–4361. |
| [4] | 甘声通, 胡向阳, 陈若冰, 等. 肝细胞生长因子/c-Met信号通路在肝癌恶性行为中的作用[J]. 生命的化学, 2017, 37(3): 349–354. |
| [5] | Mitra S, Ghosh B, Gayen N, et al. Bipartite role of heat shock protein 90(Hsp90) keeps CRAF kinase poised for activation[J]. J Biol Chem, 2016, 291(47): 24579–24593. DOI:10.1074/jbc.M116.746420 |
| [6] | Atefi M, Titz B, Tsoi J, et al. CRAF R391W is a melanoma driver oncogene[J]. Sci Rep, 2016, 6: 27454. DOI:10.1038/srep27454 |
| [7] | Cepeda MA, Evered CL, Pelling JJH, et al. Inhibition of MT1-MMP proteolytic function and ERK1/2 signalling influences cell migration and invasion through changes in MMP-2 and MMP-9 levels[J]. J Cell Commun Signal, 2017, 11(2): 167–179. DOI:10.1007/s12079-016-0373-3 |
| [8] | Chen L, Li M, Li Q, et al. DKK1 promotes hepatocellular carcinoma cell migration and invasion through β-catenin/MMP7 signaling pathway[J]. Mol Cancer, 2013, 12: 157. DOI:10.1186/1476-4598-12-157 |
| [9] | Yu Y, Song J, Zhang R, et al. Preoperative neutrophil-to-lymphocyte ratio and tumor-related factors to predict microvascular invasion in patients with hepatocellular carcinoma[J]. Oncotarget, 2017, 8(45): 79722–79730. |
| [10] | 李雅睿, 王梦瑶, 卢桂芳, 等. DNMT3B基因在肝细胞癌中的表达及其对肝癌细胞增殖和侵袭迁移的影响[J]. 西安交通大学学报:医学版, 2017, 38(3): 380–385. |
| [11] | Chatelle CV, Hövermann D, Müller A, et al. Optogenetically controlled RAF tocharacterize BRAF and CRAF protein kinase inhibitors[J]. Sci Rep, 2016, 6(3): 23713. |
| [12] | Kamata T, Hussain J, Giblett S, et al. BRAF inactivation drives aneuploidy by deregulating CRAF[J]. Cancer Res, 2010, 70(21): 8475–8486. DOI:10.1158/0008-5472.CAN-10-0603 |
| [13] | Montagut C, Sharma SV, Shioda T, et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma[J]. Cancer Res, 2008, 68(12): 4853–4861. DOI:10.1158/0008-5472.CAN-07-6787 |
| [14] | Mielgo A, Seguin L, Huang M, et al. A MEK-independent role for CRAF in mitosis and tumor progression[J]. Nat Med, 2011, 17(12): 1641–1645. DOI:10.1038/nm.2464 |
| [15] | Sun X, Li J, Sun Y, et al. miR-7 reverses the resistance to BRAFi in melanoma by targeting EGFR/IGF-1R/CRAF and inhibiting the MAPK and PI3K/AKT signaling pathways[J]. Oncotarget, 2016, 7(33): 53558–53570. |
| [16] | Lochhead PA, Clark J, Wang LZ, et al. Tumor cells with KRAS or BRAF mutations or ERK5/MAPK7 amplification are not addicted to ERK5 activity for cell proliferation[J]. Cell Cycle, 2016, 15(4): 506–518. DOI:10.1080/15384101.2015.1120915 |
| [17] | Kuliczkowski W, Radomski M, Gąsior M, et al. MMP-2, MMP-9, and TIMP-4 and response to aspirin in diabetic and nondiabetic patients with stable coronary artery disease:A pilot study[J]. Biomed Res Int, 2017, 2017: 9352015. |
| [18] | Webb AH, Gao BT, Goldsmith ZK, et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in vitro models of retinoblastoma[J]. BMC Cancer, 2017, 17(1): 434. DOI:10.1186/s12885-017-3418-y |
| [19] | Shi WZ, Ju JY, Xiao HJ, et al. Dynamics of MMP-9, MMP-2 and TIMP-1 in a rat model of brain injury combined with traumatic heterotopic ossification[J]. Mol Med Rep, 2017, 15(4): 2129–2135. DOI:10.3892/mmr.2017.6275 |
| [20] | Zhang QY, Li R, Zeng GF, et al. Dihydromyricetin inhibits migration and invasion of hepatoma cells through regulation of MMP-9 expression[J]. World J Gastroenterol, 2014, 20(29): 10082–10093. DOI:10.3748/wjg.v20.i29.10082 |
| [21] | Roomi MW, Kalinovsky T, Rath M, et al. In vitro modulation of MMP-2 and MMP-9 in pediatric human sarcoma cell lines by cytokines, inducers and inhibitors[J]. Int J Oncol, 2014, 44(1): 27–34. DOI:10.3892/ijo.2013.2159 |
 2018, Vol. 44
2018, Vol. 44


